Introduction
Cancer is a major global concern, causing millions
of deaths each year and imposing a significant economic burden to
society. In 2012, 8.2 million individuals died of cancer worldwide,
and in 2020, >600,000 individuals in the United States succumbed
to cancer (1). The disease is
complex, involving tumour cells and a large number of noncancerous
cells within the extracellular matrix. Any cell type can
potentially transform into a tumour cell, which exhibits
characteristics such as continuous self-proliferation, immune
evasion, metabolic abnormalities, metastasis and invasion, and
stimulation of angiogenesis (2).
As healthcare technology advances, human life expectancy and the
elderly population have increased, cancer deaths have also
increased (3). Most cancers occur
in individuals >60 years, making cancer a major public health
issue that will increase in severity in the future (4). In China, rapid economic development,
improved living standards, an aging population and lifestyle
changes have led to a rapid rise in cancer morbidity and mortality
(5). However, early diagnosis and
effective treatment of malignant tumours remain challenging, and
the overall survival rate of patients with malignant tumours is
still low. For instance, pancreatic ductal adenocarcinoma (PDAC) is
the third-leading cause of cancer-related mortality with a 5-year
survival rate of <12% (6,7).
Therefore, there is a need for further exploration of novel
biomarkers and therapeutic targets to improve cancer diagnosis,
treatment and prognosis. Recently, Traditional Chinese Medicine
research has shed light on its potential in cancer treatment,
particularly in inhibiting cancer progression (8). Cordyceps sinensis is a
valuable medicinal compound widely used in north-western China that
has garnered attention for its potential in cancer treatment
(9). Further investigation into
the function and mechanism of action of Cordyceps may lead to new
opportunities for cancer treatment.
Introduction to cordycepin
Cordyceps sinensis is an entomopathogenic
fungus belonging to the Ascomycetes family that is distributed
mainly in China, Nepal, Tibet and the Himalayas region of India at
an altitude of ~14,000 ft (10).
It has been a commonly used drug in Traditional Chinese Medicine
for the past 300 years (11).
Cordyceps is considered to be one of the largest fungal genera and
contains ~500 species, such as Cordyceps sinensis, Cordyceps
sobolifera, Cordyceps cicadicola, Cordyceps liangshanesis,
Cordyceps ophioglossoides and Cordyceps militaris
(12). Cordyceps are difficult to
harvest and are unevenly distributed, but they are rich in natural
bioactive components, such as proteins, fats, carbohydrates,
polysaccharides, cordycepin, phenolic compounds, cordycepin and
adenosine (11). Additionally,
they have strong biological activity, and nutritional and medicinal
value (13). Cordycepin is the
main active ingredient in Cordyceps sinensis; it is a
nucleoside molecule with a structure similar to adenosine but lacks
the 3′-hydroxyl group in the ribose molecule (14). Its chemical structure is
3′-deoxyadenosine, which consists of a purine molecule and a ribose
molecule (15). The determination
of the chemical structure of cordycepin has enabled its chemical
synthesis. In 1976, the biosynthetic pathway of cordycepin in C.
militaris was studied using adenosine and ribose. The results
showed that cordycepin is directly biosynthesized by converting
adenosine to 3′-deoxyadenosine without hydrolysing the N-nucleotide
bond (16). The results also
showed that adenine or adenosine, precursors of cordycepin, could
lead to an increase in cordycepin in C. militaris (16). However, due to the lack of genome
sequence information, the biosynthetic pathway of this fungus took
much longer to be elucidated. In 2011, whole-genome sequencing was
performed for C. militaris and it was found that most of the
genes related to C. militaris are also required for adenine
and adenosine metabolism (17).
They constructed the biosynthetic pathway of deoxyadenosine based
on the existing purine and adenosine metabolic pathways (18). Adenosine is synthesized by
three-step enzymatic reactions using inosine monophosphate and then
phosphorylated by nucleoside/nucleotide kinases to
adenosine-3′-monophosphate. Cordycepin is ultimately synthesized
through distribution reactions using 3′-adenosine monophosphate as
the precursor, which includes oxidoreductase/dehydrogenase and
metal-dependent phosphohydrolase (19). In addition, some studies produced
cordycepin from lipo-glucose (20,21).
A recent study examined the molecular interaction
between cordycepin and the spike protein of SARS-CoV-2 and showed
that cordycepin can inhibit virus entry and replication in the
host, demonstrating its therapeutic potential against this disease
(22). An increasing number of
studies on the potential application of cordycepin in medicine have
focused on its stable and effective production (19–21).
However, the production of cordycepin is challenging due to various
disadvantages, such as low yield, cost issues and poor extraction
processes (23). Therefore, more
effective strategies for cordycepin production need to be
developed.
In vivo and in vitro antitumour effects of
cordycepin
Numerous studies have shown that cordycepin can
penetrate cells and be converted to the phosphoric form,
competitively inhibiting the synthesis and metabolism of DNA and
RNA, post-transcriptionally processing heterogeneous nuclear RNA,
activating adenylate cyclase, inhibiting macrophage lineage and
chemotaxis, and specifically promoting protein synthesis (24–26).
Additionally, cordycepin has antitumour effects on some cell lines,
enhances cell differentiation, inhibits the growth and metastasis
of tumour cells during cell cycle arrest, and induces apoptosis in
cancer cells and inhibits angiogenesis (27,28).
Cordycepin can also regulate cell function through adenosine
receptors, death receptors or epidermal growth factor receptors,
inhibit tumour proliferation, migration, invasion and the cell
cycle, and regulate chemotherapy resistance of gastric cancer,
liver cancer, kidney cancer, bladder cancer and testicular cancer
cells (29).
The latest research shows that cordycepin may
participate in antitumour mechanisms through mitogen-activated
protein kinase, NF-κB, caspase, serine/threonine kinase (Akt) and
JNK-MAPK pathways (28). In
addition, it was reported that cordycepin can activate the caspase
cascade and increase intracellular reactive oxygen species (ROS)
levels, thereby eliminating human tongue cancer cells, testicular
tumour cells and human OEC-M1 oral cancer cells (30). The aforementioned results indicate
that cordycepin can activate different cell signalling pathways and
play a role in tumour progression. Cordycepin also induced a
systemic antitumour immune response in a subcutaneous tumour model
in colon cancer mice and inhibited tumour growth (31). In addition, cordycepin promoted the
antitumour function of immune cells by upregulating the immune
response and downregulating immunosuppression in the tumour
microenvironment and resetting the immune cell phenotype (30).
Previous studies have shown that cordycepin has
tumour treatment efficacy both in vitro and in vivo.
Table I summarizes the studies on
the antitumour effects of cordycepin on various tumour
diseases.
 | Table I.Summary of antitumour mechanism of
cordycepin on various tumour diseases. |
Table I.
Summary of antitumour mechanism of
cordycepin on various tumour diseases.
| Tumour type | Cell lines | Tumour xenograft
model | Effects and related
pathways | (Refs.) |
|---|
| Nasopharyngeal | C666-1 | - | Activation of
MAPK/ERK and β-catenin | (40) |
| carcinoma |
|
| signalling
pathways |
|
| Oesophageal
cancer | ECA109, TE-1 | Four- to
six-week- | Influence on cell
proliferation, apoptosis and | (41) |
|
|
| old male nude | cell cycle arrest
via MEK/ERK pathway |
|
|
|
| mice |
|
|
|
| HK, K180, K70, | Four-week- | Induction of
activation of AMPK and | (106) |
|
| ECA109 | old female
nude | inhibition of AKT
signalling pathway enhances |
|
|
|
| mice | chemoresistance to
cisplatin in cancer cells |
|
| Pancreatic
cancer | BxPC-3, AsPC-1 | Female BALB/cA | Induction of
apoptosis and blockade of FGFR/ | (42) |
|
|
| nu/nu mice | Ras/ERK signalling
causing cell cycle arrest |
|
| Bladder cancer | T24 | - | Activation of
exogenous and endogenous | (50) |
|
|
|
| apoptotic pathways
and ROS-dependent |
|
|
|
|
| inactivation of
PI3K/AKT signalling |
|
|
| T24 | - | Inhibition of
Est-1-dependent MDR1 | (104) |
|
|
|
| transcription
enhances cisplatin sensitivity in |
|
|
|
|
| cancer cells |
|
| Testicular
cancer | MA-10 | - | Activation of AKT
and MAPK pathways, | (51) |
|
|
|
| upregulation of ERK
and JNK and |
|
|
|
|
| downregulation of
p38 |
|
|
| MA-10 | - | Inhibition of
ERK1/2, Rb/E2FR1, cell cycle | (81) |
|
|
|
| pathway and FGFR1-4
protein expression |
|
|
|
|
| inhibits tumour
growth |
|
|
| MA-10 | Male 5- to | Cell cycle arrest,
cysteine asparaginase | (101) |
|
|
| 7-week- | pathway and
endoplasmic reticulum stress |
|
|
|
| old C57BL/6 | enhance
radiosensitivity and induce apoptosis |
|
|
|
| mice | in cancer
cells |
|
|
| MA-10 | Male 4 to | Induction of cancer
cell death via ROS | (102) |
|
|
| 5-week-old | accumulation and
DNA damage in combination |
|
|
|
| C57BL/6 mice | with
radiotherapy |
|
|
Cholangiocarcinoma | HuCCT1, | Four-week-old | Reprogramming lipid
metabolism to inhibit | (52) |
|
| QBC939, RBE | BALB/c nude | metastasis and EMT
via ERO1A/mTOR/ |
|
|
|
| female mice | SREBP1 |
|
|
| KKU-213A, | - | Increased TRAILR
expression enhances cancer | (84) |
|
| KKU-100, |
| cell sensitivity to
NK-92 cells |
|
|
| KKU-055 |
|
|
|
| Lung cancer | A549 | BALB/c nude | Activation of AMPK
and inhibition of AKT | (59) |
|
|
| mice | signalling pathway
reverses cisplatin resistance |
|
|
|
|
| in non-small cell
lung cancer |
|
|
| H1957, PC9, | Nude mice | Activation of AMPK
signalling pathway | (60) |
|
|
|
| inhibits
progression of drug-resistant non-small |
|
|
|
|
| cell lung
cancer |
|
|
| A549, PC9 | - | Down-regulation of
VEGF/PI3K/AKT | (109) |
|
|
|
| signalling pathway
in combination with apatinib |
|
|
|
|
| synergistically
inhibits cancer cell growth |
|
| Ovarian cancer | A2780, OVCAR3 | 4-week-old | Through
ENT1-mediated transport, | (61) |
|
|
| BALB/c nude | induction of AMPK
signalling and cellular |
|
|
|
| mice | autophagy |
|
|
| SKOV-3, | - | Induction of
autophagy and apoptosis through | (75) |
|
| OVCAR3 |
| Dickkopf-related
protein 1/β-catenin signalling |
|
| Breast cancer | MAD-MB-231, | - | Induction of
apoptosis and inhibition of metastasis | (65) |
|
| MAD- MB-468, |
| in breast cancer
cells by inhibiting the hedgehog |
|
|
| MCF-7 |
| pathway |
|
|
| - | BALB/c nude | Modulation of the
hedgehog pathway inhibits | (66) |
|
|
| mice | growth and
metastasis of xenograft tumours in |
|
|
|
|
| nude mice |
|
|
| BT549 | Female BALB/c | Regulation of
EMT-TFs SLUG, TWIST1, | (27) |
|
|
| mice | SNAIL1 and ZEB1
inhibits cancer cell |
|
|
|
|
| migration and
invasion |
|
|
| MCF-7, MAD- | - | Increased
Nrf2-related ROS sensitise cancer | (87) |
|
| MB-231 |
| cells to
radiation |
|
| Leukaemia | AC133-MUTZ-2 | - | Induction of
leukaemia cell death through re- | (71) |
|
|
|
| expression of WIF
and down-regulation of MYC |
|
|
| U937 | - | Regulation of the
MAPK pathway enhances | (80) |
|
|
|
|
thermotherapy-induced apoptosis and cell
cycle |
|
|
|
|
| arrest |
|
| Oral squamous
cell | HSC-4 | - | Attenuation of
cancer cell migration and | (74) |
| carcinoma |
|
| invasion through
autophagy-dependent |
|
|
|
|
| FAK/AKT and
MMP2/MMP9 inhibition |
|
|
| SAS, OC-3 | Nude mice | Induces DNA damage
repair and enhances | (103) |
|
|
|
|
radiosensitivity |
|
| Colorectal
cancer | HCT116 | - | Inducing
Bax-dependent apoptosis | (76) |
|
| CT26, SW480, | 6-8weeks | Inhibition of the
expression of the phagocytic | (84) |
|
| CCD841, CoN | old BALB/c | immune checkpoint
CD47 enhances anti-tumour |
|
|
|
| nude mice | immunity |
|
|
| MC38, CT26 | - | Enhancement of CD8+
T cell mediated anti- | (31) |
|
|
|
| tumour
immunity |
|
|
| MC38, CT26 | C57BL/6J mice | Significant
enhancement of tumour suppression | (86) |
|
|
|
| by combination
therapy with anti-CD47 antibody |
|
|
| HCT-116,
Caco-2 | - | Inhibition of MYC
expression suppresses cancer | (92) |
|
|
|
| cell
proliferation |
|
|
| HT-29 | - | Cordycepin-coated
liposomes effectively increase | (99) |
|
|
|
| apoptosis and
inhibit growth of cancer cells |
|
| Tongue cancer | CAL-27 | Male nude | Induces
apoptosis | (104) |
|
|
| BALB/c mice |
|
|
| Retinoblastoma | - | Nude mice | Regulation of
c-Myc/cell cycle protein D1 | (78) |
|
|
|
| pathway inhibits
cancer cell proliferation, |
|
|
|
|
| migration invasion
and lung metastasis |
|
| Cervical
cancer- | - | - | Downregulation of
CDK-2 interferes with the | (82) |
|
|
|
| cell cycle and
increases ROS to increase apoptosis |
|
|
| - | C57BL/6J mice | Lactobacillus
plantarum CQPC02 prevents | (88) |
|
|
|
| obesity in mice
through PPAR-α signalling pathway |
|
| Liver cancer | HUVECs, | C57BL/6 | Regulation of
adhesion spot kinase and p53 | (89) |
|
| HCAECs, | and BALB/c | inhibits
endothelial cell proliferation, migration, |
|
|
| HPAECs | nude mice | angiogenesis and
tumour growth |
|
|
| HepG2, Huh7 | - | Downregulation of
CXCR4 expression inhibits | (90) |
|
|
|
| migration and
invasion of hepatocellular |
|
|
|
|
| carcinoma
cells |
|
| Stomach cancer | MGC-803, | - | Inhibition of lipid
metabolism through AMPK | (91) |
|
| HGC-27 |
| and MAPK activation
inhibits cancer cell |
|
|
|
|
| proliferation and
migration |
|
| Uveal melanoma | 92.1, Omm1, | Female nude
mice | Inhibition of heat
shock protein 90 function | (93) |
|
| Mel202, |
| targets tumour
growth in an adenosine |
|
|
| Omm2.3, |
|
deamination-dependent manner |
|
|
| Omm2.5, MP46, |
|
|
|
|
| MM28 |
|
|
|
|
Choriocarcinoma | JAR | - | Disruption of
centrosome homeostasis inhibits | (94) |
|
|
|
| cancer cell
growth |
|
| Endometrial
cancer | Ishikawa | - | Induction of
apoptosis in cancer cells | (98) |
| Osteosarcoma | U2OS, SAOS2, | Female BALB/c | Activation of AMPK
and inhibition of the AKT | (105) |
|
| MNNGHOS, | nude mice | signalling pathway
enhances the |
|
|
| 143B, MG63, | (4–5 weeks
old) | chemosensitivity of
cancer cells to cisplatin |
|
|
| SJSA1 |
|
|
|
| Glioblastoma | LN-299 | - | Down-regulation of
MYC improves the | (107) |
|
|
|
| sensitivity of
cancer cells to temozolomide |
|
|
| LN-229, U251, | - | Modulation of EMT
in combination with | (108) |
|
| T98G |
| doxorubicin
inhibits cell migration and invasion |
|
Cordycepin inhibits tumour growth through
various pathways
Direct antitumour effect
Cordycepin may affect tumour cells through a variety
of mechanisms, including promoting tumour cell apoptosis,
inhibiting tumour cell proliferation, invasion and metastasis, and
regulating the expression of tumour-related genes. Additionally, in
animal models, cordycepin also inhibited tumour growth (28). Fig.
1 shows a diagram of the antitumour mechanism of
cordycepin.
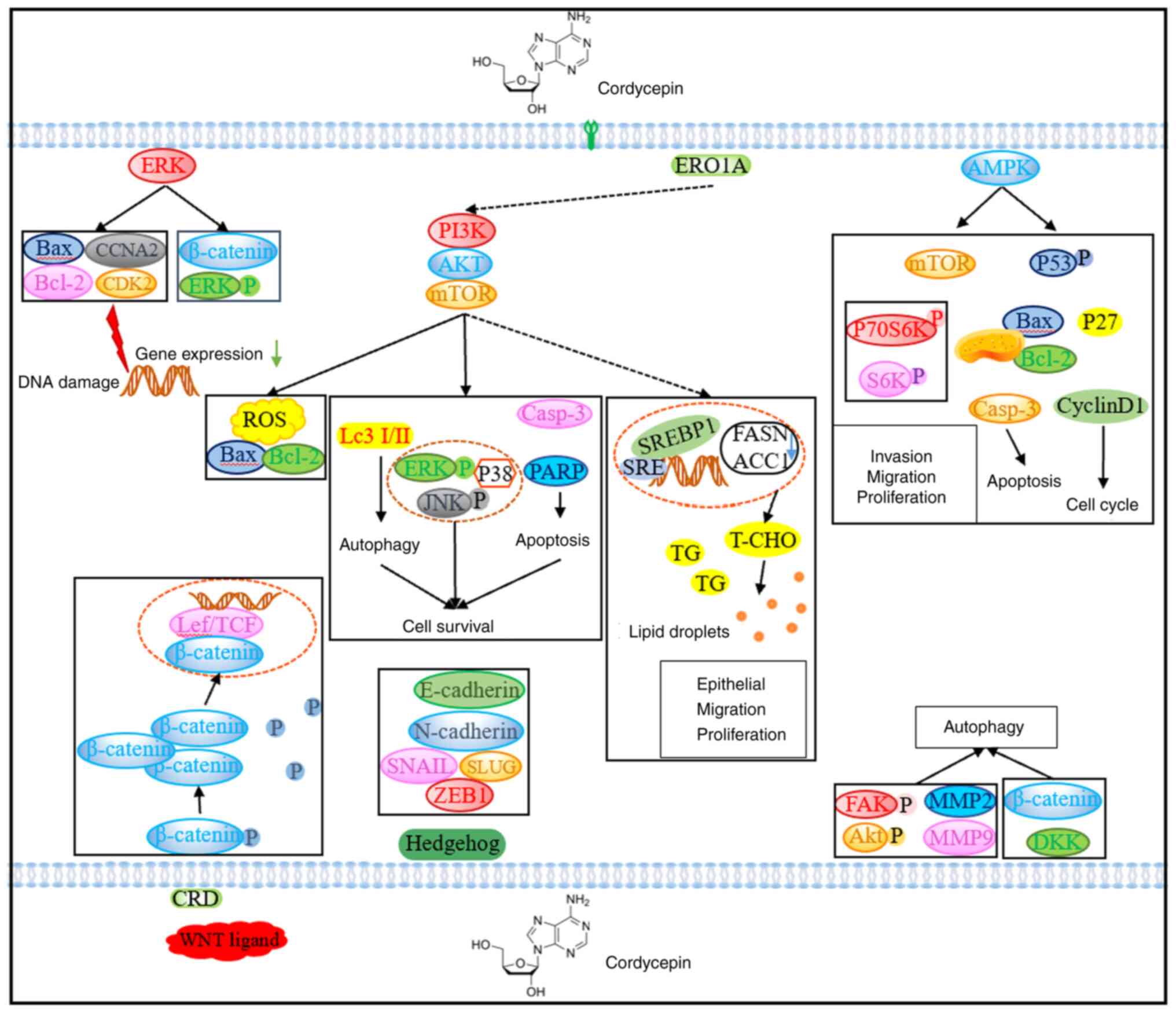 | Figure 1.Schematic diagram of the antitumour
mechanism of cordyceps. Cordycepin affects tumour growth and
proliferation through various signalling pathways such as ERK,
PI3K, AMPK, Hedgehog, ROS and autophagy, and exerts antitumour
effects in cancer. ERO1A, endoplasmic reticulum oxidoreductase 1α;
CCNA2, cyclin A2; CDK2, cyclin-dependent kinase 2; ROS, reactive
oxygen species; Casp-3, caspase 3; PARP, poly(ADP-ribose)
polymerase; S6K, ribosomal protein s6 kinase; P70S6K, p70 ribosomal
S6 protein kinase; DKK, Dickkopf; FAK, protein tyrosine kinase 2;
MMP, matrix metalloproteinase; T-CHO, total cholesterol; TG,
triglyceride; SRE, sterol regulatory element; SREBP1, SRE binding
transcription factor 1; FASN, fatty acid synthase; ACC1, acetyl-CoA
carboxylase alpha; LeF, anthrax toxin lethal factor; CRD, Retinitis
pigmentosa GTPase regulator; TCF, expressed protein. |
Signalling pathway inhibition
MAPK/ERK signalling pathway
The MAPK/ERK pathway plays an important role in
cancer development. This pathway is involved in biological
processes such as cell proliferation, survival, differentiation and
metastasis (32). Abnormal
MAPK/ERK pathway activation is closely associated with the
development and progression of various cancers (33), such as lung cancer, stomach cancer,
ovarian cancer (34–36). Therefore, this pathway has become
an important target for cancer treatment. Currently, several
inhibitors of the MAPK/ERK pathway have been developed and used to
treat several cancers (37). These
inhibitors can reduce the proliferation and metastasis of cancer
cells by inhibiting the MAPK/ERK pathway, thereby achieving
therapeutic effects (38). In a
study on oral cancer, it showed that the small molecule inhibitor
OTX008 induced the MAPK signaling pathway early (39).
The study by Zhou et al (40) explored the anticancer potential and
mechanism of cordycepin in nasopharyngeal carcinoma. RNA sequencing
(RNA-seq) combined with in vitro experiments revealed that
the expression levels of ERK1/2, phosphorylated ERK1/2 and
β-catenin were significantly decreased after cordycepin treatment.
This suggests that cordycepin may be a novel drug candidate for
nasopharyngeal cancer treatment. In addition, in oesophageal
cancer, cordycepin induces chromatin condensation, activates the
caspase cascade, induces apoptotic signalling, regulates Bcl-2
family members to increase cell apoptosis and alters
cyclin-dependent kinases 1 and 2. The expression of cyclin B1 led
to G2/M phase arrest (41). A mechanistic study revealed that
inactivation of the ERK pathway was involved in the antitumour
effect of cordycepin and the same result was also observed in
vivo (41). Moreover,
cordycepin was confirmed to activate caspase-3, caspase-9 and
cytochrome C to induce apoptosis, activate the checkpoint kinase 2
pathway and downregulate cyclin A2 and cyclin-dependent kinase 2
(CDK2) phosphorylation-related genes, S-phase arrest and DNA damage
(42). Furthermore, the same study
showed that cordycepin blocks the MAPK pathway by inhibiting the
expression of Ras and the phosphorylation of ERK, and the blockade
of the Ras/ERK pathway with fibroblast growth factor receptor 2
effectively inhibited the growth of pancreatic cancer cells
(42).
PI3K/AKT/mTOR signalling pathway
Under normal conditions, the phosphatidylinositol
3-kinase (PI3K/AKT/mTOR) signalling pathway regulates cell
proliferation and survival through a series of signal transduction
and phosphorylation cascades (43). As an initiation factor of the
pathway, PI3K is activated under the signal stimulation of
molecules, including growth factors and cytokines, to catalyse the
conversion of phosphatidylinositol diphosphate into
phosphatidylinositol triphosphate (PIP3) (44). PIP3 activates AKT, which affects
downstream activity, including that of FOXO transcription factors
and mTOR proteins, to regulate cell proliferation and activity
(45). In ovarian, stomach and
breast cancer, abnormal activation of the PI3K/AKT/mTOR signalling
pathway may be due to PIK3CA mutations such as deletion of the
phosphatase and tensin homolog gene, overexpression of AKT and an
increase in mTOR activity (46–48).
Abnormally activated PI3K/AKT/mTOR signalling promotes the abnormal
proliferation, survival and metastasis of cancer cells (49).
In a concentration-dependent manner, treatment of
bladder cancer cells with cordycepin significantly reduced the
survival rate of the cells and activated exogenous and endogenous
cell apoptosis pathways, increased the caspase effect and resulted
in polymerase cleavage (50). By
increasing the ratio of Bax/Bcl-2, disrupting the integrity of
mitochondria and promoting the release of cytochrome C, mechanistic
study showed that cordycepin induces cell apoptosis by activating
exogenous and endogenous apoptotic pathways and ROS-dependent
inactivation of the PI3K/AKT signalling pathway in human bladder
cancer T24 cells (50). In another
study, cluster analysis of mRNA expression profiling and western
blot analysis was performed on cordycepin-treated testicular cancer
cells. The results showed that cordycepin regulates the
FOXO/P15/P27, PERK-eIF2α and IRE1-XBP1UPR pathways. This study also
found that activation of the AKT and MAPK pathways may lead to
cancer cell resistance to cordycepin (51). In addition, cordycepin has a strong
hypolipidemic effect. Therefore, it has been found that
cordycepin-mediated endoplasmic reticulum oxidoreductase
1α/mTOR/SRE binding transcription factor 1 axis inhibits lipid
metabolism and transfer in CCA cells in vivo and in
vitro. After treating CCA cells with simvastatin and exogenous
cholesterol, the same results were obtained, further confirming
that cordycepin inhibits CCA migration through the regulation of
lipid metabolism (52). These data
indicate that cordycepin could be used as a novel drug for the
clinical treatment of CCA and may improve the prognosis of patients
with CCA.
AMPK signalling pathway
In cells, the AMP-activated protein kinase (AMPK)
signalling pathway inhibits the growth and proliferation of cancer
cells while inducing apoptosis (53). It also inhibits the synthesis and
secretion of vascular endothelial growth factor (VEGF) to prevent
angiogenesis, thereby inhibiting tumour growth and metastasis
(54). In addition, activation of
the AMPK pathway can also inhibit autophagy in cancer cells,
thereby reducing the growth rate of tumour cells (55). In terms of metabolism, activation
of the AMPK pathway can promote the metabolism of glucose and fat,
thereby reducing blood glucose and blood lipid levels while
increasing intracellular ATP production (56). The AMPK pathway has been confirmed
to be associated with the development of cancer (57). Since tumour cells usually depend on
glycolysis to generate energy, activation of the AMPK pathway is
considered to interfere with the metabolism of cancer cells
(58). In conclusion, the AMPK
signalling pathway plays an important role in cancer.
In a study on non-small cell lung cancer (NSCLC)
cells, compared with cisplatin alone, cordycepin significantly
inhibited the effect of cisplatin on cell proliferation and the
promotion of cell apoptosis. Moreover, reversing cisplatin
resistance in NSCLC through the AMPK and AKT signalling pathways
could lead to the development of a potential treatment strategy for
overcoming cisplatin resistance in patients with NSCLC (59). In another NSCLC study, cells with
EGFR mutations were more sensitive to cordycepin treatment than the
control cells (60). In ovarian
cancer, cordycepin was found to activate AMPK signalling to
transduce downstream target proteins to induce autophagy-dependent
cell death (61). Based on the
aforementioned results, the present authors considered that
cordycepin alone or in combination with currently available
targeted therapies might be an additional option for the treatment
of lung cancer, especially for patients with EGFR-mutant lung
cancer.
Hedgehog (Hh) signalling pathway
The Hh pathway is composed mainly of the Hh ligand,
the intracellular protein patched (Ptch) and the signal
transduction protein GLI family zinc finger (GLI). In the inactive
state, Hh ligands bind to the Ptch protein on the cell surface.
When Hh signalling is activated, the Hh ligand is released to
dissociate from the Ptch protein (62,63).
Persistent activation of the Hh signalling pathway is associated
with the development of a variety of tumours, such as skin, brain
and pancreatic cancer (64).
Therefore, studying the Hh pathway is highly important for
understanding the underlying mechanisms of cancers and identifying
new therapeutic targets.
For the first time, Liu et al (65) studied the function of the Hh
pathway in the effect of cordycepin on human breast cancer cells
and the results showed that cell apoptosis induced by cordycepin
led to an increase in the p53-upregulated modulator of apoptosis,
Cytochrome C, Fas cell surface death receptor, death receptors 4/5
and caspase-3 and also led to the inhibition of Bcl-2, X-linked
inhibitor of apoptosis protein and PDGFR-α. It also inhibits the
expression of the Hh pathway and the transcriptional activity of
GLI (65). The blockage of
cordycepin-mediated cell apoptosis, epithelial-mesenchymal
transition (EMT) and the Notch pathway after GIL knockout plays an
important role in the effect of cordycepin on breast cancer.
Further in vivo studies revealed that cordycepin reduced the
volume and weight of xenograft tumours, affected proliferation,
apoptosis and EMT, and affected the expression of matrix
metalloproteinase-related proteins in cancer cells but had no side
effects (65). Analysis of RNA-seq
data showed that the Hh pathway was the most enriched in breast
cancer tissues, and by analysing Hh pathway markers and assessing
changes in expression in xenografts, the Hh pathway was shown to
play an important role in the anti-breast cancer effect of
cordycepin (66).
Wnt signalling pathway
The Wnt signalling pathway completes signal
transduction through the extracellular Wnt protein and
intracellular receptors, ligands and transcription regulators
(67). The specific cascades
involved include the Wnt/β-catenin, the canonical Wnt signalling,
the Wnt-planar cell polarity and the Wnt-Ca2+ signalling
pathways (68). In tumours,
abnormal activation of the Wnt signalling pathway is common, as
activation of the Wnt signalling pathway maintains stem cell
properties and promotes tumour development (69). Therefore, the Wnt signalling
pathway is an important target in tumour treatment.
Wnt/β-catenin signalling is required for the
development and maintenance of leukaemia stem cells (LSCs) in acute
myeloid leukaemia (AML) (70).
Cordycepin downregulates the expression of the Wnt target genes MYC
and prominin-1 (key factors for the maintenance of stem cells)
through Wnt inhibitory factor 1 and Dickkopf-1 (Dkk1). These
results provide new insights into the involvement of
cordycepin-mediated molecular circuits in pharmacological
inhibition and enhanced targeting (71). Wnt/β-catenin and its regulatory
complex in AML treated with cordycepin can potentially target LSCs
by reducing cell viability and stimulating LSC apoptosis, which
supports the potential use of cordycepin as an adjuvant in the
treatment of AML (70). Therefore,
cordycepin may be effective in the treatment of AML.
Cell cycle and apoptosis regulation
Cell apoptosis
Cordycepin inhibits the growth of colorectal cancer
(CRC) cells in vitro and may accelerate cancer cell
apoptosis by inducing Bax translocation to the mitochondrial
membrane (72). The same study
also found that cordycepin-induced Bax translocation-induced cell
apoptosis and reintroduction of Bax expression could restore the
ability of cells to induce apoptosis. These results indicate that
cordycepin may be a new agent for the treatment of Bax-deficient
cancers (72). Cordycepin
significantly inhibited the proliferation of tongue cancer cells in
a dose-dependent manner and further induced the upregulation of
Bax, caspase-3, caspase-9 and caspase-12 at the mRNA and protein
levels while downregulating the increase in the level of the
antiapoptotic gene Bcl-2. In addition, cordycepin effectively
inhibited the growth of tongue cancer tumours in a mouse xenograft
model (73). For the first time,
Fong et al (74) confirmed
the cytotoxic effect of cordycepin on endometrial cancer cells. The
authors observed cell cycle arrest at the
G0/G1 phase in cells treated with different
concentrations of cordycepin, cisplatin or a combination of the two
agents. In addition, the antitumour effect of the combination
treatment was improved compared with that of a single compound,
with fewer adverse drug reactions.
Cell cycle
A previous study showed that C-Myc is a downstream
target of cordycepin and is positively associated with cell cycle
pathways. In retinoblastoma (RB), cordycepin was found to inhibit
the expression of cyclin D1 and overexpression of c-Myc could
reverse this effect, thereby inhibiting the malignant biological
behaviour of RB cells (75). In
leukaemia, after hyperthermia and cordycepin combined treatment,
the MAPK pathway significantly increased cell apoptosis, the level
of matrix metalloproteinases also significantly decreased and ROS
generation increased. Combination treatment also downregulates the
expression of cyclin-dependent kinase 1 and cyclin B1 proteins and
induces cell cycle arrest in the G2/M phase (76). Cordycepin treatment inhibited the
growth of cervical cancer cells, increased apoptosis and interfered
with the cell cycle, especially through prolongation of the S
phase. After cordycepin treatment, the mRNA levels of the cell
cyclins CDK2, Cyclin-A2 and Cyclin-E1 were downregulated, but the
expression of apoptosis-related proteins did not significantly
change. The aforementioned results indicate that cordycepin is
effective at treating cervical cancer cells (77). Microarray analysis of
cordycepin-treated MA-10 mouse stromal tumour cells revealed that
cordycepin downregulated the expression levels of FGF9, FGF18,
FGFR2 and FGFR3 in MA-10 cells. Moreover, the pathway prediction
results showed that the inhibition of ERK1/2 and retinoblastoma
protein/E2F transcription factor 1 pathways, the cell cycle pathway
and the expression of the FGFR1-4 protein might inhibit the growth
of FGF9-induced testicular tumours (78). In a mouse allograft model, the
amount of FGF9-induced tumour growth in the cordycepin treatment
group was significantly lower than that in the PBS treatment group
(78). In summary, cordycepin
could be used as a new anticancer drug for tumours and play a role
in cancer treatment.
Autophagy regulation
Autophagy is an important biochemical process in
cells. Its main function is to maintain cell homeostasis and
vitality by decomposing and recycling damaged proteins, organelles
and other cellular components (79). Autophagy mainly includes
microautophagy, macroautophagy and chaperone-mediated autophagy,
and among these processes, macroautophagy is the most extensively
studied and understood (80).
Autophagy has dual roles in tumours. In the early stage, autophagy
may suppress tumour formation; after tumour progression to a
certain stage, autophagy may switch to a role in promoting tumour
survival and progression (81).
Therefore, in tumour treatment, the regulatory and intervention
strategies for autophagy need to be precisely adjusted according to
the specific situation to achieve the best therapeutic effect.
In oral squamous cell carcinoma cells, the
cordycepin-mediated inhibition of cancer cell migration and
invasion was also altered when autophagy was inhibited by
chloroquine, indicating that the migration and invasion inhibited
by cordycepin may be mediated by autophagy (82). After cordycepin treatment,
autophagy was induced, the fluorescence intensity of
monodansylcadaverine fluorescence intensity (MDC)- and MDC-positive
cells increased, and the expression level of the LC3 gene
increased. Mechanistic study have shown that high doses of
cordycepin inhibit cell death and invasion by inducing stress,
while low doses inhibit invasion through autophagy-dependent focal
adhesion kinase (FAK)/Akt/MMP2 and MMP9 pathways (82). Cordycepin can increase the level of
Dkk1 and inhibit β-catenin signalling in human ovarian cancer
cells. These results indicate that cordycepin may promote the
cleavage of caspase-3 and inhibit the growth of ovarian cancer
cells by coordinating autophagy and Dkk1/β-catenin signalling.
Taken together, these data indicate that cordycepin may inhibit the
growth of ovarian cancer cells through coordinated autophagy and
Dkk1/β-catenin signalling (83).
Cordycepin activates DNA-dependent protein kinase and ERK and
triggers centrosome expansion to inhibit cell proliferation and
destroy the cytoskeleton but does not affect the growth of normal
placental cells (84). In
addition, cordycepin treatment activated autophagy, and the
inhibition of cordycepin-induced cell death by chloroquine
prevented cordycepin-induced cell death, revealing the potential
therapeutic effect of cordycepin.
Immune regulation
Cordycepin inhibited the growth and migration of and
promoted the apoptosis of CRC cells in a dose-dependent manner,
increased the infiltration of CD4+ T cells,
CD8+ T cells, M1 macrophages and NK cells in the tumour
immune microenvironment and inhibited the expression of trisodium
phosphate in tumour cells (85).
Reducing the binding of thrombospondin to CD47 allows more T cells
to infiltrate tumours and inhibits the growth of CRC in a mouse
tumour model (31). These results
indicate that the adjuvant of cordycepin with other antitumour
drugs can further enhance antitumour immunity and inhibit the
growth of CRC and is a potential adjuvant drug for the
immunotherapy of CRC. For the first time, Panwong et al
(86) reported the ability of
cordycepin to sensitize NK cells to toxicity in CCA cells. Compared
with cordycepin or NK treatment alone, cordycepin treatment
significantly increased the expression of the tumour necrosis
factor-related apoptosis-inducing ligand receptor (TRAILR) in
KKU-4A cells. The increased expression of the TRAILR may help
cordycepin regulate immune activity. However, how cordycepin
promotes NK cell activation remains unclear. These results indicate
that cordycepin can be further developed as a substitute
immunomodulatory drug in adoptive NK cell therapy.
In a previous study, combination treatment with
cordycepin and an anti-CD47 antibody significantly inhibited tumour
growth and prolonged the survival of tumour-bearing mice. The flow
cytometry results showed that when cordycepin was used in
combination with the cytotoxic T-lymphocyte antigen 4 (CTLA-4)
blocker, the proportion of M1 and M2 macrophages was decreased, the
presence of tumour-infiltrating CD8+ T cells was
significantly increased, and the proportion of M2 macrophages was
decreased (27). Reducing the
number of Foxp3 Tregs in the tumour microenvironment significantly
inhibited tumour growth and prolonged the survival of
tumour-bearing mice (27). In
summary, the results of this study indicate that the combination of
cordycepin and a CTLA-4 blockade can change the effector and
exhaustion states of CD8+ T cells, thereby enhancing
CD8+ T cell-mediated antitumour immunity in the tumor
microenvironment (TME). According to the results of another
single-cell RNA-seq study, the combination of cordycepin and a CD47
antibody could reactivate macrophages, reverse polarization,
increase the proportion of M1 macrophages, decrease the proportion
of M2 macrophages, regulate the proportion of CD8+ T
cells, and prolong the progression-free survival of patients with
malignant tumours (87). In
summary, these results prove that the use of cordycepin may enhance
the function of immune cells, which is valuable for the treatment
of various types of cancer.
Other
A study on triple-negative breast cancer showed that
cordycepin can inhibit the expression of twist-related protein 1
and snail homolog 2 to inhibit the EMT signalling pathway. IT was
also reported that combination therapy with cordycepin and
thymoquinone had synergistic effects on the inhibition of tumour
metastasis (88). Cordycepin
significantly decreased FAK expression and induced p53 and p21
expression in endothelial cell (EC) to impair angiogenesis and
tumour growth and reduce hepatocellular carcinoma (HCC) tumour
growth in a xenograft model. The present authors further examined
the number of ECs in the tumour area in tumour-bearing mice.
However, due to severe necrosis in the tumour area, the number of
ECs or blood vessels could not be quantified (89). In another HCC study, cordycepin
significantly inhibited IκBα phosphorylation, limited the nuclear
translocation of P65 and activated tissue transcription factors,
resulting in the downregulation of CXCR4 expression and the
inhibition of migration and invasion in HCC cells. JSH-23 (an NF-κB
pathway inhibitor) can inhibit the migration of liver cancer cells
and was found to exert a synergistic effect when combined with
cordycepin (90). These findings
indicate that cordycepin can be used as a potential adjuvant in
cancer treatment and may prevent HCC metastasis when it is used in
combination with other therapeutic compounds.
Cordycepin activates the AMPK and MAPK signalling
pathways, inhibits lipid metabolism and EMT processes, and
significantly inhibits the proliferation, colony formation and
migration of gastric cancer cells (91). Cancer cell proliferation is
inhibited by downregulating MYC mRNA and protein expression and
upregulating miR-2a in CRC cells. Furthermore, MYC overexpression
inhibited the expression of miR-26a, while miR-26a expression was
restored after cordycepin treatment. These results indicated that
the inhibition of CRC cell proliferation by cordycepin might be
mediated by the MYC/miR-26a pathway (92).
Cordycepin can slow the migration, growth and
clonality of uveal melanoma and other invasive malignant tumours
with low adenosine deaminase (ADA) levels, indicating that ADA may
be a predictive biomarker of the cordycepin response and that
tumours that are resistant to monotherapy may be sensitive to
combination therapy. The present authors also found that cordycepin
inhibited heat shock protein 90, thereby destroying its function
and leading to the degradation of client proteins such as
hypoxia-inducible factor, protein kinase B, extracellular
signal-regulated kinase and EGFR, resulting in the activation of
proteins in oncogenic signalling pathways, and reported a new
mechanism of action of cordycepin (93). However, only in vivo studies
were performed and the effect of cordycepin on the growth of
orthotopic xenografts in other types of tumours needs to be
evaluated.
Based on a combination of theoretical and
experimental studies, the design and preparation of
cordycepin-encapsulating liposomes were performed. Molecular
dynamics simulations and free energy calculations showed that the
phosphatidylcholine lipid environment was conducive to the
adsorption of cordycepin. Cordycepin passively permeates into the
PC lipid bilayer without causing damage to the membrane and tightly
binds to the polar group of lipids by flipping its deoxyribose
sugar toward the centre of the bilayer. In an in vitro study
of colon cancer cell lines, cordycepin-encapsulated liposomes
enhanced the anticancer activity of cordycepin (94). The study indicated that
cordycepin-encapsulated liposomes may be effective drug candidates
for the treatment of CRC.
Cordycepin in combination with
chemotherapies and radiotherapy
In combination with radiotherapy
Radiation therapy uses radiation energy to damage
the DNA of cancer cells, prevent their ability to proliferate and
divide, and shrink or even eliminate tumours, and can be used to
treat breast cancer, lung cancer, lymphoma, head and neck cancer
and other tumor (95–98). A study showed that in mouse stromal
tumour cells, the combination of cordycepin and radiotherapy has
synergistic effects on the inhibition of cancer cell viability by
activating exogenous and endogenous caspase pathways, cell cycle
arrest and endoplasmic reticulum stress. Moreover, in animal
experiments, it also reduced the tumour mass of stromal tumours
(99). Subsequently, after
combination treatment, ROS accumulated, heme oxygenase-1 protein
levels decreased in mouse mesenchymal tumour cells and DNA
damage-related signalling pathways were activated, including the
ataxia-telangiectasia mutation induced by double-strand and
single-strand breaks/checkpoint kinase 2 (Chk2),
ataxia-telangiectasia mutations and Rad3 related (ATR)/Chk1
signaling axes were identified. The tumour volume, size and weight
were reduced, and high expression of γ-H2AX was observed in in
vivo tumour tissues after combination therapy (100). Network pharmacology analysis
revealed the systemic mechanism underlying the inhibitory effect of
cordycepin on the proliferation of breast cancer cells. Several
studies showed that after radiation exposure, breast cancer cells
cultured with cordycepin increased the levels of intracellular ROS
and γ-H2AX lesions. However, the expression levels of nuclear
factor erythrocyte 2-related factors and a series of downstream
genes were downregulated after cordycepin treatment, sensitizing
breast cancer cells to radiation through the Nrf2/HO-1/ROS axis. In
addition, cordycepin promoted G2/M arrest and apoptosis
in breast cancer cells, thereby inhibiting cell proliferation in
vitro and in vivo after radiation. Therefore, these
study results indicate that cordycepin may be used as a
radiosensitizer during clinical breast cancer radiotherapy
(101,102). Another study showed that
cordycepin can repair DNA damage in oral squamous cell carcinoma
cells through Chk1 phosphorylation and prolong radiotherapy-induced
G2/M phase arrest. According to in vivo
experiments, the growth inhibitory effect of the combination of
cordycepin and radiotherapy on xenografts was greater than that of
radiotherapy alone, and no excessive toxicity was observed
(103). However, the clinical
application of cordycepin in enhancing radiosensitivity needs
further verification.
Cordycepin in combination with
chemotherapy
Treatment with cordycepin or cisplatin alone failed
to induce cell death in bladder cancer cells, but these two drugs
in combination induced mitochondrial membrane depolarization,
decreased the expression of antiapoptotic proteins and increased
the expression of proapoptotic proteins. A high expression level of
multidrug resistance protein 1 (MDR1) in bladder cancer results in
cisplatin resistance. After cordycepin treatment, the inhibition of
MDR1 promoter activity reduces MDR1 expression and induces
resensitization of cancer cells to cisplatin (104). The combination of cordycepin and
cisplatin enhances the sensitivity of osteosarcoma cells to
cisplatin, increases apoptosis and inhibits the growth and invasion
of osteosarcoma cells by activating AMPK and inhibiting the
AKT/mTOR signalling pathway (105). RNA-seq analysis revealed 72 genes
whose expression was significantly different from that of the other
genes and in which different signalling pathways may be regulated
by cordycepin; in addition, the expression levels of ERK1/2,
phosphorylated ERK1/2 and β-catenin were significantly
downregulated (40). A relevant
study confirmed that cordycepin enhances the chemosensitivity of
oesophageal cancer cells to cisplatin through the activation of
AMPK and the inhibition of the AKT signalling pathway, and in
vivo and in vitro experiments demonstrated the
synergistic effects of these two drugs (106). In clinical practice, combination
therapy comprising cordycepin and cisplatin may be a potential
treatment method for improving the treatment of oesophageal cancer
(106). In addition,
sensitization to other chemotherapeutic drugs was also found when
other chemotherapeutic drugs were used in combination. Cordycepin
combined with temozolomide inhibits EMT and regulates the
proliferation, migration and apoptosis of glioblastoma cells
(107). Cordycepin combined with
doxorubicin regulates the EMT of tumour cells to inhibit cell
invasion and migration, and this synergistic effect was proven
through network pharmacology and in vitro experiments
(108). Cordycepin and apatinib
inhibited the VEGF/PI3K/AKT pathway, reduced cell proliferation,
inhibited cell migration and invasion, altered the cell cycle to
increase cell apoptosis, and exerted synergistic anticancer effects
(109).
Therefore, cordycepin may be a novel drug candidate
for cancer treatment and a candidate for combination therapy with
other drugs, but the clinical feasibility of combination treatment
still needs to be comprehensively evaluated in future studies,
which could provide potential insights for anticancer drug design
targets.
Advantages and challenges associated with
cordycepin
Cordycepin is a natural medicinal ingredient
extracted from Cordyceps sinensis that is widely used in
health products and pharmaceuticals and has good safety and
biological activity (11).
Cordycepin has a variety of advantages in disease treatment,
including the following: i) An immunomodulatory effect, whereby
cordycepin can increase the activity of immune cells and promote
the production and function of immune cells, thereby helping the
body resist the invasion of diseases and pathogens (110); ii) an antioxidant effect that can
neutralize free radicals, reduce oxidative damage to cells and
delay the cell aging process; iii) an anti-inflammatory effect,
which includes a reduction in the release of inflammatory mediators
and tissue damage caused by inflammation, and inhibition of the
occurrence and development of inflammation (111,112); iv) an antitumour effect, whereby
cordycepin inhibits the growth and spread of tumour cells, induces
tumour cell apoptosis and reduces tumour malignancy (113); and v) an improvement in the
efficacy of radiotherapy and chemotherapy, whereby cordycepin can
enhance the efficacy of chemotherapeutic drugs, reduce the side
effects of chemotherapy, reduce the side effects of radiotherapy,
enhance radiosensitivity, promote the repair of damaged tissues,
improve the effect of radiotherapy and enhance the efficacy of
patients, can reduce the recovery time after chemotherapy and
improve the quality of life (114).
Long-term cordycepin use may cause chronic toxicity
to organs such as the liver and kidney. when the intake of
cordycepin exceeds the metabolic capacity of the human body, the
surplus may accumulate in the liver, inducing a hepatotoxic
response (115,116). In addition, cordycepin has poor
stability and low solubility in water, which results in the loss of
biological activity. Cordycepin is a bioactive compound extracted
from Cordyceps sinensis. As a natural antibiotic, cordycepin
has a variety of pharmacological effects. It was shown that
cordycepin is rapidly deaminated by ADA, thereby shortening the
half-life of the drug and reducing its bioavailability (117,118).
In conclusion, there are several problems associated
with the medicinal use of cordycepin, which may differ among
different cancer types and individuals. However, if managed
according to the specific conditions of the patient, its
pharmacological effects and functions can also achieve maximum
efficacy, bringing additional benefits to the treatment of human
diseases.
Discussion and future perspectives
Due to the clinical success of artemisinin, several
researchers have focused on natural extracts (119). Artemisinin has been widely
studied in the treatment of malaria and cancer (120,121). An increasing number of
researchers are studying the safety and long-term use of natural
extracts, as well as exploring the molecular mechanisms of their
activities in various pathways. As an active component of
cordyceps, cordycepin has been used in the fields of traditional
Chinese medicine and health care products (11,13).
The main mechanism of action of cordycepin is to fight cancer by
regulating the human immune system and exerting antioxidative
effects, which stimulate the immune system to enhance its ability
to clear cancer cells and limit the growth and spread of tumours by
inhibiting the formation and spread of the tumour microvasculature
(110). Cordycepin also has
antioxidant effects and can reduce oxidative damage caused by free
radicals, protect the liver, lungs and other vital organs, and
improve the anticancer ability of the body (111,112). The anticancer effect of
cordycepin has been confirmed in several experimental studies. For
instance, previous studies showed that cordycepin has the potential
to prevent and treat various cancer types, such as colon (31,85,92,94),
liver (90), breast (65,88,101) and prostate cancers (53). However, cordycepin cannot
completely replace traditional tumour treatment methods and it
still needs to be combined with other treatment methods, such as
surgery, radiotherapy and chemotherapy, to increase the therapeutic
effect. With the development of precision medicine, cordycepin may
become a part of individualized drug treatment. The analysis of
genomic information, genetic variation and tumour characteristics
of patients could allow identification of which patients will
respond best to cordycepin treatment and provide additional
individualized treatment options. Nevertheless, more research and
clinical trials are needed to ensure its safety and efficacy.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National Natural Science
Foundation of China (grant no. 82260555) and Gansu Provincial
Science and Technology Major Project (grant no. 22ZD6FA021-4).
Availability of data and materials
Not applicable.
Authors' contributions
RH wrote and revised the manuscript; and WZ reviewed
and edited the project. Both authors read and approved the final
version of the manuscript. Data authentication not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu Y, Chen L, Tang Q, Wei W, Cao Y, Xie J
and Ji J: Pan-cancer analysis revealed the significance of the
GTPBP family in cancer. Aging (Albany NY). 14((6)): 2558–2573.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang C, Azizi P, Vazirzadeh M,
Aghaei-Zarch SM, Aghaei-Zarch F, Ghanavi J and Farnia P: Non-coding
RNAs/DNMT3B axis in human cancers: From pathogenesis to clinical
significance. J Transl Med. 21:6212023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sepp T, Ujvari B, Ewald PW, Thomas F and
Giraudeau M: Urban environment and cancer in wildlife: Available
evidence and future research avenues. Proc Biol Sci.
286:201824342019.PubMed/NCBI
|
|
4
|
Fane M and Weeraratna AT: How the ageing
microenvironment influences tumour progression. Nat Rev Cancer.
20:89–106. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maomao C, He L, Dianqin S, Siyi H, Xinxin
Y, Fan Y, Shaoli Z, Changfa X, Lin L, Ji P, et al: Current cancer
burden in China: epidemiology, etiology, and prevention. Cancer
Biol Med. 19:1121–1138. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chi Y, Wang D, Wang J, Yu W and Yang J:
Long Non-Coding RNA in the pathogenesis of cancers. Cells.
8:10152019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miao K, Liu W, Xu J, Qian Z and Zhang Q:
Harnessing the power of traditional Chinese medicine monomers and
compound prescriptions to boost cancer immunotherapy. Front
Immunol. 14:12772432023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YC, Chen YH, Pan BS, Chang MM and
Huang BM: Functional study of Cordyceps sinensis and cordycepin in
male reproduction: A review. J Food Drug Anal. 25:197–205. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tuli HS, Sharma AK, Sandhu SS and Kashyap
D: Cordycepin: A bioactive metabolite with therapeutic potential.
Life Sci. 93:863–869. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yue K, Ye M, Zhou Z, Sun W and Lin X: The
genus Cordyceps: A chemical and pharmacological review. J Pharm
Pharmacol. 65:474–493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kontogiannatos D, Koutrotsios G, Xekalaki
S and Zervakis GI: Biomass and cordycepin production by the
medicinal mushroom Cordyceps militaris-A review of various aspects
and recent trends towards the exploitation of a valuable fungus. J
Fungi (Basel). 7:9862021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khan M, Tania M, Zhang D and Chen H:
Cordyceps Mushroom: A Potent Anticancer Nutraceutical. Open
Nutraceuticals J. 3:179–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang S, Liu H, Sun Y, Chen J, Li X, Xu J,
Hu Y, Li Y, Deng Z and Zhong S: An effective and convenient
synthesis of cordycepin from adenosine. Chem Pap. 72:149–160. 2018.
View Article : Google Scholar
|
|
15
|
Ashraf SA, Elkhalifa AEO, Siddiqui AJ,
Patel M, Awadelkareem AM, Snoussi M, Ashraf MS, Adnan M and Hadi S:
Cordycepin for health and wellbeing: A potent bioactive metabolite
of an entomopathogenic cordyceps medicinal fungus and its
nutraceutical and therapeutic potential. Molecules. 25:27352020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Yan H, Zeng B and Hu Z: Research
progress on cordycepin synthesis and methods for enhancement of
cordycepin production in cordyceps militaris. Bioengineering
(Basel). 9:692022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng P, Xia Y, Xiao G, Xiong C, Hu X,
Zhang S, Zheng H, Huang Y, Zhou Y, Wang S, et al: Genome sequence
of the insect pathogenic fungus Cordyceps militaris, a valued
traditional Chinese medicine. Genome Biol. 12:R1162011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jędrejko KJ, Lazur J and Muszyńska B:
Cordyceps militaris: An overview of its chemical constituents in
relation to biological activity. Foods. 10:26342021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xia Y, Luo F, Shang Y, Chen P, Lu Y and
Wang C: Fungal cordycepin biosynthesis is coupled with the
production of the safeguard molecule pentostatin. Cell Chem Biol.
24:1479–1489.e4. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Guo ZJ and Zhou XW: Chinese
cordyceps: Bioactive components, antitumor effects and underlying
mechanism-a review. Molecules. 27:65762022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma YC, Huang P, Wang XL and Liu GQ:
Multi-omics analysis unravels positive effect of rotenone on the
cordycepin biosynthesis in submerged fermentation of Cordyceps
militaris. Bioresour Technol. 373:1287052023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Raethong N, Thananusak R, Cheawchanlertfa
P, Prabhakaran P, Rattanaporn K, Laoteng K, Koffas M and
Vongsangnak W: Functional genomics and systems biology of Cordyceps
species for biotechnological applications. Curr Opin Biotechnol.
81:1029392023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu X, Wu T, Huang A, Shen Y, Zhang X, Song
W, Wang S and Ruan H: New insights into the biosynthesis of typical
bioactive components in the traditional Chinese medicinal fungus
cordyceps militaris. Front Bioeng Biotechnol. 9:8017212021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Cheng J, Su Y, Li M, Wen J and Li
S: Cordycepin induces M1/M2 macrophage polarization to attenuate
the liver and lung damage and immunodeficiency in immature mice
with sepsis via NF-κB/p65 inhibition. J Pharm Pharmacol.
74:227–235. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang R, Wang X, Xi D, Mo J, Wang K, Luo S,
Wei J, Ren Z, Pang H and Luo Y: Cordycepin Attenuates IFN-γ-Induced
Macrophage IP-10 and Mig Expressions by Inhibiting STAT1 Activity
in CFA-Induced Inflammation Mice Model. Inflammation. 43:752–764.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu S, Yang L and Fu J, Li T, Zhou B, Wang
K, Wei C and Fu J: Comprehensive analysis, immune, and cordycepin
regulation for SOX9 expression in pan-cancers and the matched
healthy tissues. Front Immunol. 14:11499862023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen L, Zheng X, Huang H, Feng C, Wu S,
Chen R, Jiang H, Yuan M, Fu Y, Ying H, Zhou J and Jiang J:
Cordycepin synergizes with CTLA-4 blockade to remodel the tumor
microenvironment for enhanced cancer immunotherapy. Int
Immunopharmacol. 124((Pt A)): 1107862023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Khan MA and Tania M: Cordycepin and kinase
inhibition in cancer. Drug Discov Today. 28:1034812023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao C, Yang S and Zhou Z: The potential
application of Cordyceps in metabolic-related disorders. Phytother
Res. 34:295–305. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen YY, Chen CH, Lin WC, Tung CW, Chen
YC, Yang SH, Huang BM and Chen RJ: The role of autophagy in
anti-cancer and health promoting effects of cordycepin. Molecules.
26:49542021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deng Q, Li X, Fang C, Li X, Zhang J, Xi Q,
Li Y and Zhang R: Cordycepin enhances anti-tumor immunity in colon
cancer by inhibiting phagocytosis immune checkpoint CD47
expression. Int Immunopharmacol. 107:1086952022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Asl ER, Amini M, Najafi S, Mansoori B,
Mokhtarzadeh A, Mohammadi A, Lotfinejad P, Bagheri M, Shirjang S,
Lotfi Z, et al: Interplay between MAPK/ERK signaling pathway and
MicroRNAs: A crucial mechanism regulating cancer cell metabolism
and tumor progression. Life Sci. 278:1194992021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ullah R, Yin Q, Snell AH and Wan L:
RAF-MEK-ERK pathway in cancer evolution and treatment. Semin Cancer
Biol. 85:123–154. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li K, Liu Y, Ding Y, Zhang Z, Feng J, Hu
J, Chen J, Lian Z, Chen Y, Hu K, et al: BCL6 is regulated by the
MAPK/ELK1 axis and promotes KRAS-driven lung cancer. J Clin Invest.
132:e1613082022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Q, Wang X, Cao S, Sun Y, He X, Jiang
B, Yu Y, Duan J, Qiu F and Kang N: Berberine represses human
gastric cancer cell growth in vitro and in vivo by inducing
cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt
signaling pathways. Biomed Pharmacother. 128:1102452020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma H, Qi G, Han F, Gai P, Peng J and Kong
B: HMGB3 promotes the malignant phenotypes and stemness of
epithelial ovarian cancer through the MAPK/ERK signaling pathway.
Cell Commun Signal. 21:1442023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yuan J, Dong X, Yap J and Hu J: The MAPK
and AMPK signalings: Interplay and implication in targeted cancer
therapy. J Hematol Oncol. 13:1132020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Barbosa R, Acevedo LA and Marmorstein R:
The MEK/ERK network as a therapeutic target in human cancer. Mol
Cancer Res. 19:361–374. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Greer PFC, Rich A and Coates DE: Effects
of galectin-1 inhibitor OTX008 on oral squamous cell carcinoma
cells in vitro and the role of AP-1 and the MAPK/ERK pathway. Arch
Oral Biol. 134:1053352022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou Y, Mei X, Li Y, Yang W, Su X and Hu
H: Cordycepin inhibits the proliferation and progression of NPC by
targeting the MAPK/ERK and β-catenin pathways. Oncol Lett.
23:202022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu JC, Zhou XP, Wang XA, Xu MD, Chen T,
Chen TY, Zhou PH and Zhang YQ: Cordycepin Induces Apoptosis and
G2/M Phase Arrest through the ERK pathways in esophageal cancer
cells. J Cancer. 10:2415–2424. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li XY, Tao H, Jin C, DU ZY, Liao WF, Tang
QJ and Ding K: Cordycepin inhibits pancreatic cancer cell growth in
vitro and in vivo via targeting FGFR2 and blocking ERK signaling.
Chin J Nat Med. 18:345–355. 2020.PubMed/NCBI
|
|
43
|
Tewari D, Patni P and Bishayee A, Sah AN
and Bishayee A: Natural products targeting the PI3K-Akt-mTOR
signaling pathway in cancer: A novel therapeutic strategy. Semin
Cancer Biol. 80:1–17. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu L, Wei J and Liu P: Attacking the
PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment
in human cancer. Semin Cancer Biol. 85:69–94. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu F, Na L, Li Y and Chen L: Roles of the
PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and
tumours. Cell Biosci. 10:542020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li Q, Li Z, Luo T and Shi H: Targeting the
PI3K/AKT/mTOR and RAF/MEK/ERK pathways for cancer therapy. Mol
Biomed. 3:472022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ediriweera MK, Tennekoon KH and Samarakoon
SR: Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer:
Biological and therapeutic significance. Semin Cancer Biol.
59:147–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fattahi S, Amjadi-Moheb F, Tabaripour R,
Ashrafi GH and Akhavan-Niaki H: PI3K/AKT/mTOR signaling in gastric
cancer: Epigenetics and beyond. Life Sci. 262:1185132020.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Alves CL and Ditzel HJ: Drugging the
PI3K/AKT/mTOR Pathway in ER+ Breast Cancer. Int J Mol Sci.
24:45222023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim SO, Cha HJ, Park C, Lee H, Hong SH,
Jeong SJ, Park SH, Kim GY, Leem SH, Jin CY, et al: Cordycepin
induces apoptosis in human bladder cancer T24 cells through
ROS-dependent inhibition of the PI3K/Akt signaling pathway. Biosci
Trends. 13:324–333. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chang MM, Pan BS, Wang CY and Huang BM:
Cordycepin-induced unfolded protein response-dependent cell death,
and AKT/MAPK-mediated drug resistance in mouse testicular tumor
cells. Cancer Med. 8:3949–3964. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou X, Li Y, Yang C, Chen D, Wang T, Liu
T, Yan W, Su Z, Peng B and Ren X: Cordycepin reprogramming lipid
metabolism to block metastasis and EMT via ERO1A/mTOR/SREBP1 axis
in cholangiocarcinoma. Life Sci. 327:1216982023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yuan H, Han Y, Wang X, Li N, Liu Q, Yin Y,
Wang H, Pan L, Li L, Song K, et al: SETD2 restricts prostate cancer
metastasis by integrating EZH2 and AMPK signaling pathways. Cancer
Cell. 38:350–365.e7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rodríguez C, Muñoz M, Contreras C and
Prieto D: AMPK, metabolism, and vascular function. FEBS J.
288:3746–3771. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang S, Li H, Yuan M, Fan H and Cai Z:
Role of AMPK in autophagy. Front Physiol. 13:10155002022.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Steinberg GR and Hardie DG: New insights
into activation and function of the AMPK. Nat Rev Mol Cell Biol.
24:255–272. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Keerthana CK, Rayginia TP, Shifana SC,
Anto NP, Kalimuthu K, Isakov N and Anto RJ: The role of AMPK in
cancer metabolism and its impact on the immunomodulation of the
tumor microenvironment. Front Immunol. 14:11145822023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hsu CC, Peng D, Cai Z and Lin HK: AMPK
signaling and its targeting in cancer progression and treatment.
Semin Cancer Biol. 85:52–68. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liao XZ, Gao Y, Zhao HW, Zhou M, Chen DL,
Tao LT, Guo W, Sun LL, Gu CY, Chen HR, et al: Cordycepin Reverses
cisplatin resistance in non-small cell lung cancer by activating
AMPK and Inhibiting AKT signaling pathway. Front Cell Dev Biol.
8:6092852021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wei C, Yao X, Jiang Z, Wang Y, Zhang D,
Chen X, Fan X, Xie C, Cheng J, Fu J and Leung EL: Cordycepin
inhibits drug-resistance non-small cell lung cancer progression by
activating AMPK signaling pathway. Pharmacol Res. 144:79–89. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yoon SY, Lindroth AM, Kwon S, Park SJ and
Park YJ: Adenosine derivatives from Cordyceps exert antitumor
effects against ovarian cancer cells through ENT1-mediated
transport, induction of AMPK signaling, and consequent autophagic
cell death. Biomed Pharmacother. 153:1134912022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang Y and Beachy PA: Cellular and
molecular mechanisms of Hedgehog signalling. Nat Rev Mol Cell Biol.
24:668–687. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sigafoos AN, Paradise BD and
Fernandez-Zapico ME: Hedgehog/GLI Signaling Pathway: Transduction,
regulation, and implications for disease. Cancers (Basel).
13:34102021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xia R, Xu M, Yang J and Ma X: The role of
Hedgehog and Notch signaling pathway in cancer. Mol Biomed.
3:442022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu C, Qi M, Li L, Yuan Y, Wu X and Fu J:
Natural cordycepin induces apoptosis and suppresses metastasis in
breast cancer cells by inhibiting the Hedgehog pathway. Food Funct.
11:2107–2116. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wu W, Li X, Qi M, Hu X, Cao F, Wu X and Fu
J: Cordycepin inhibits growth and metastasis formation of
MDA-MB-231 ×enografts in nude mice by modulating the hedgehog
pathway. Int J Mol Sci. 23:103622022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Albrecht LV, Tejeda-Muñoz N and De
Robertis EM: Cell biology of canonical Wnt signaling. Annu Rev Cell
Dev Biol. 37:369–389. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhao H, Ming T, Tang S, Ren S, Yang H, Liu
M, Tao Q and Xu H: Wnt signaling in colorectal cancer: Pathogenic
role and therapeutic target. Mol Cancer. 21:1442022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Parsons MJ, Tammela T and Dow LE: WNT as a
driver and dependency in cancer. Cancer Discov. 11:2413–2429. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gruszka AM, Valli D and Alcalay M: Wnt
Signalling in Acute Myeloid Leukaemia. Cells. 8:14032019.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Abazari N, Stefanucci MR, Bossi LE,
Trojani A, Cairoli R and Beghini A: Cordycepin (3′dA) Induces Cell
Death of AC133+ Leukemia Cells via Re-Expression of WIF1 and
Down-Modulation of MYC. Cancers (Basel). 15:39312023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li SZ, Ren JW, Fei J, Zhang XD and Du RL:
Cordycepin induces Bax dependent apoptosis in colorectal cancer
cells. Mol Med Rep. 19:901–908. 2019.PubMed/NCBI
|
|
73
|
Zheng Q, Sun J, Li W, Li S and Zhang K:
Cordycepin induces apoptosis in human tongue cancer cells in vitro
and has antitumor effects in vivo. Arch Oral Biol. 118:1048462020.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fong P, Ao CN, Tou KI, Huang KM, Cheong CC
and Meng LR: Experimental and In Silico Analysis of Cordycepin and
its Derivatives as Endometrial Cancer Treatment. Oncol Res.
27:237–251. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Min Y, Ding Y, Huang Q, Xu Y and Li J:
Cordycepin inhibited the retinoblastoma cell proliferation,
migration, and invasion as well as lung metastasis via modulating
c-Myc/cyclin D1 pathway. Chem Biol Drug Des. 101:605–613. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shi L, Cao H, Fu S, Jia Z, Lu X, Cui Z and
Yu D: Cordycepin enhances hyperthermia-induced apoptosis and cell
cycle arrest by modulating the MAPK pathway in human lymphoma U937
cells. Mol Biol Rep. 49:8673–8683. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tania M, Shawon J, Saif K, Kiefer R,
Khorram MS, Halim MA and Khan MA: Cordycepin Downregulates Cdk-2 to
Interfere with Cell Cycle and Increases Apoptosis by Generating ROS
in Cervical Cancer Cells: In vitro and in silico Study. Curr Cancer
Drug Targets. 19:152–159. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chang MM, Hong SY, Yang SH, Wu CC, Wang CY
and Huang BM: Anti-Cancer Effect of Cordycepin on FGF9-Induced
Testicular Tumorigenesis. Int J Mol Sci. 21:83362020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu S, Yao S, Yang H, Liu S and Wang Y:
Autophagy: Regulator of cell death. Cell Death Dis. 14:6482023.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Klionsky DJ, Petroni G, Amaravadi RK,
Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cadwell K,
Cecconi F, Choi AMK, et al: Autophagy in major human diseases. EMBO
J. 40:e1088632021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Debnath J, Gammoh N and Ryan KM: Autophagy
and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol.
24:560–575. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Binlateh T, Uppatcha N, Thepchai J,
Pleungtuk Y, Noisa P, Hutamekalin P and Jitprasertwong P:
Cordycepin attenuates migration and invasion of HSC-4 oral squamous
carcinoma cells through autophagy-dependent FAK/Akt and MMP2/MMP9
suppression. J Dent Sci. 17:1677–1688. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Jang HJ, Yang KE, Hwang IH, Huh YH, Kim
DJ, Yoo HS, Park SJ and Jang IS: Cordycepin inhibits human ovarian
cancer by inducing autophagy and apoptosis through Dickkopf-related
protein 1/β-catenin signaling. Am J Transl Res. 11:6890–6906.
2019.PubMed/NCBI
|
|
84
|
Wang CY, Tsai SW, Chien HH, Chen TY, Sheu
SY, So EC and Huang BM: Cordycepin inhibits human gestational
choriocarcinoma cell growth by disrupting centrosome homeostasis.
Drug Des Devel Ther. 14:2987–3000. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chen R, Feng C, Chen L, Zheng X, Fang W,
Wu S, Gao X, Chen C, Yang J, Wu Y, et al: Single-cell RNA
sequencing indicates cordycepin remodels the tumor immune
microenvironment to enhance TIGIT blockade's anti-tumor effect in
colon cancer. Int Immunopharmacol. 126:1112682024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Panwong S, Wathikthinnakon M, Kaewkod T,
Sawasdee N, Tragoolpua Y, Yenchitsomanus PT and Panya A: Cordycepin
sensitizes cholangiocarcinoma cells to be killed by natural
killer-92 (NK-92) cells. Molecules. 26:59732021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Feng C, Chen R, Fang W, Gao X, Ying H,
Zheng X, Chen L and Jiang J: Synergistic effect of CD47 blockade in
combination with cordycepin treatment against cancer. Front
Pharmacol. 14:11443302023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wei C, Khan MA, Du J, Cheng J, Tania M,
Leung EL and Fu J: Cordycepin inhibits triple-negative breast
cancer cell migration and invasion by regulating EMT-TFs SLUG,
TWIST1, SNAIL1, and ZEB1. Front Oncol. 12:8985832022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lin YT, Liang SM, Wu YJ, Wu YJ, Lu YJ, Jan
YJ, Ko BS, Chuang YJ, Shyue SK, Kuo CC and Liou JY: Cordycepin
Suppresses endothelial cell proliferation, migration, angiogenesis,
and tumor growth by regulating focal adhesion kinase and p53.
Cancers (Basel). 11:1682019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Guo Z, Chen W, Dai G and Huang Y:
Cordycepin suppresses the migration and invasion of human liver
cancer cells by downregulating the expression of CXCR4. Int J Mol
Med. 45:141–150. 2020.PubMed/NCBI
|
|
91
|
Zhang X, Zhou X, Gao M, Lyu Y, Wang Y,
Yang C, Piao Y and Ren X: Cordycepin inhibits the proliferation and
migration of human gastric cancer cells by suppressing lipid
metabolism via AMPK and MAPK activation. Xi Bao Yu Fen Zi Mian Yi
Xue Za Zhi. 38:513–521. 2022.(In Chineae). PubMed/NCBI
|
|
92
|
Zhang Z, Li K, Zheng Z and Liu Y:
Cordycepin inhibits colon cancer proliferation by suppressing MYC
expression. BMC Pharmacol Toxicol. 23:122022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lee SC, Alaali L, Kwon H, Rigi M and
Eberhart CG: Cordycepin (3′-Deoxyadenosine) suppresses heat shock
protein 90 function and targets tumor growth in an adenosine
deaminase-dependent manner. Cancers (Basel). 14:31222022.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Khuntawee W, Amornloetwattana R,
Vongsangnak W, Namdee K, Yata T, Karttunen M and Wong-Ekkabut J: In
silico and in vitro design of cordycepin encapsulation in liposomes
for colon cancer treatment. RSC Adv. 11:8475–8484. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Levy A, Mercier O and Le Péchoux C:
Indications and parameters around postoperative radiation therapy
for lung cancer. J Clin Oncol. 40:556–566. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wirth A, Mikhaeel NG, Aleman BMP, Pinnix
CC, Constine LS, Ricardi U, Illidge TM, Eich HT, Hoppe BS, Dabaja
B, et al: Involved Site Radiation Therapy in Adult Lymphomas: An
Overview of International Lymphoma Radiation Oncology Group
Guidelines. Int J Radiat Oncol Biol Phys. 107:909–933. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Verma S, Young S, Boldt G, Blanchette P,
Lock M, Helou J and Raphael J: Immunotherapy and radiation therapy
sequencing in breast cancer: A systematic review. Int J Radiat
Oncol Biol Phys. 118:1422–1434. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Huang R and Zhou PK: DNA damage repair:
historical perspectives, mechanistic pathways and clinical
translation for targeted cancer therapy. Signal Transduct Target
Ther. 6:2542021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lee YP, Huang WR, Wu WS, Wu YH, Ho SY,
Wang YJ and Huang BM: Cordycepin enhances radiosensitivity to
induce apoptosis through cell cycle arrest, caspase pathway and ER
stress in MA-10 mouse Leydig tumor cells. Am J Cancer Res.
12:3601–3624. 2022.PubMed/NCBI
|
|
100
|
Lee YP, Lin CR, Chen SS, Chen RJ, Wu YH,
Chen YH and Huang BM: Combination treatment of cordycepin and
radiation induces MA-10 mouse Leydig tumor cell death via ROS
accumulation and DNA damage. Am J Cancer Res. 13:1329–1346.
2023.PubMed/NCBI
|
|
101
|
Dong J, Li Y, Xiao H, Luo D, Zhang S, Zhu
C, Jiang M, Cui M, Lu L and Fan S: Cordycepin sensitizes breast
cancer cells toward irradiation through elevating ROS production
involving Nrf2. Toxicol Appl Pharmacol. 364:12–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lee D, Lee WY, Jung K, Kwon YS, Kim D,
Hwang GS, Kim CE, Lee S and Kang KS: The inhibitory effect of
cordycepin on the proliferation of MCF-7 breast cancer cells, and
its mechanism: An investigation using network pharmacology-based
analysis. Biomolecules. 9:4142019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Su NW, Wu SH, Chi CW, Tsai TH and Chen YJ:
Cordycepin, isolated from medicinal fungus Cordyceps sinensis,
enhances radiosensitivity of oral cancer associated with modulation
of DNA damage repair. Food Chem Toxicol. 124:400–410. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Oh SS, Lee KW, Madhi H, Jeong JW, Park S,
Kim M, Lee Y, Han HT, Hwangbo C, Yoo J and Kim KD: Cordycepin
Resensitizes T24R2 cisplatin-resistant human bladder cancer cells
to cisplatin by inactivating Ets-1 Dependent MDR1 transcription.
Int J Mol Sci. 21:17102020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Li HB, Chen JK, Su ZX, Jin QL, Deng LW,
Huang G and Shen JN: Cordycepin augments the chemosensitivity of
osteosarcoma to cisplatin by activating AMPK and suppressing the
AKT signaling pathway. Cancer Cell Int. 21:7062021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gao Y, Chen DL, Zhou M, Zheng ZS, He MF,
Huang S, Liao XZ and Zhang JX: Cordycepin enhances the
chemosensitivity of esophageal cancer cells to cisplatin by
inducing the activation of AMPK and suppressing the AKT signaling
pathway. Cell Death Dis. 11:8662020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zheng SX, Chen J, Zhuang BB, Zhang Q, Shi
SS and Zhang GL: Cordycepin improves sensitivity to temozolomide in
glioblastoma cells by down-regulating MYC. J Cancer Res Clin Oncol.
149:16055–16067. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Chen J, Zhuang YD, Zhang Q, Liu S, Zhuang
BB, Wang CH and Liang RS: Exploring the mechanism of cordycepin
combined with doxorubicin in treating glioblastoma based on network
pharmacology and biological verification. PeerJ. 10:e129422022.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Liao X, Tao L, Guo W, Wu ZX, Du H, Wang J,
Zhang J, Chen H, Chen ZS, Lin L and Sun L: Combination of
cordycepin and apatinib synergistically inhibits NSCLC Cells by
Down-Regulating VEGF/PI3K/Akt signaling pathway. Front Oncol.
10:17322020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Woolley VC, Teakle GR, Prince G, de Moor
CH and Chandler D: Cordycepin, a metabolite of Cordyceps militaris,
reduces immune-related gene expression in insects. J Invertebr
Pathol. 177:1074802020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Lan T, Yu Y, Zhang J, Li H, Weng Q, Jiang
S, Tian S, Xu T, Hu S, Yang G, et al: Cordycepin ameliorates
nonalcoholic steatohepatitis by activation of the AMP-Activated
protein kinase signaling pathway. Hepatology. 74:686–703. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Tan L, Song X, Ren Y, Wang M, Guo C, Guo
D, Gu Y, Li Y, Cao Z and Deng Y: Anti-inflammatory effects of
cordycepin: A review. Phytother Res. Oct 8–2020.(Epub ahead of
print).
|
|
113
|
Khan MA and Tania M: Cordycepin in
anticancer research: Molecular mechanism of therapeutic effects.
Curr Med Chem. 27:983–996. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Seong da B, Hong S, Muthusami S, Kim WD,
Yu JR and Park WY: Cordycepin increases radiosensitivity in
cervical cancer cells by overriding or prolonging radiation-induced
G2/M arrest. Eur J Pharmacol. 771:77–83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Dalla Rosa L, da Silva AS, Gressler LT,
Oliveira CB, Dambrós MG, Miletti LC, França RT, Lopes ST, Samara
YN, da Veiga ML and Monteiro SG: Cordycepin (3′-deoxyadenosine)
pentostatin (deoxycoformycin) combination treatment of mice
experimentally infected with Trypanosoma evansi. Parasitology.
140:663–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Qin P, Li X, Yang H, Wang ZY and Lu D:
Therapeutic potential and biological applications of cordycepin and
metabolic mechanisms in cordycepin-producing fungi. Molecules.
24:22312019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chen M, Luo J, Jiang W, Chen L, Miao L and
Han C: Cordycepin: A review of strategies to improve the
bioavailability and efficacy. Phytother Res. 37:3839–3858. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Lee JB, Adrower C, Qin C, Fischer PM, de
Moor CH and Gershkovich P: Development of cordycepin formulations
for preclinical and clinical studies. AAPS PharmSciTech.
18:3219–3226. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Posadino AM, Giordo R, Pintus G, Mohammed
SA, Orhan IE, Fokou PVT, Sharopov F, Adetunji CO,
Gulsunoglu-Konuskan Z, Ydyrys A, et al: Medicinal and mechanistic
overview of artemisinin in the treatment of human diseases. Biomed
Pharmacother. 163:1148662023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Talman AM, Clain J, Duval R, Ménard R and
Ariey F: Artemisinin bioactivity and resistance in malaria
parasites. Trends Parasitol. 35:953–963. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Chen GQ, Benthani FA, Wu J, Liang D, Bian
ZX and Jiang X: Artemisinin compounds sensitize cancer cells to
ferroptosis by regulating iron homeostasis. Cell Death Differ.
27:242–254. 2020. View Article : Google Scholar : PubMed/NCBI
|















