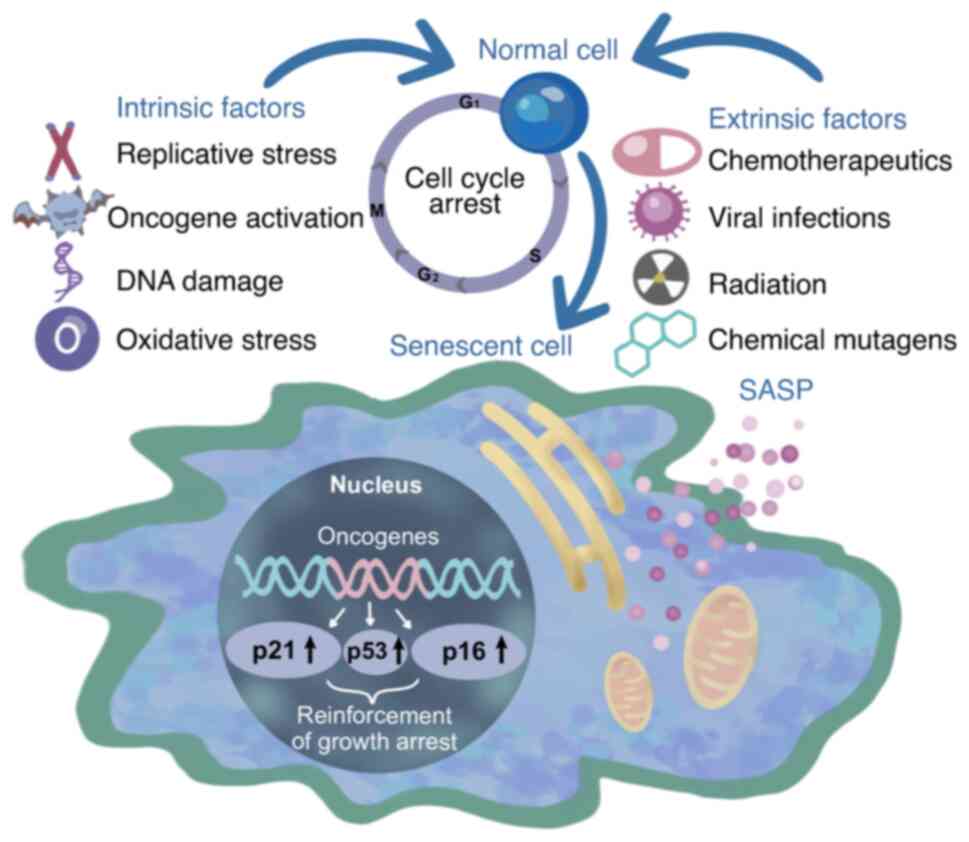
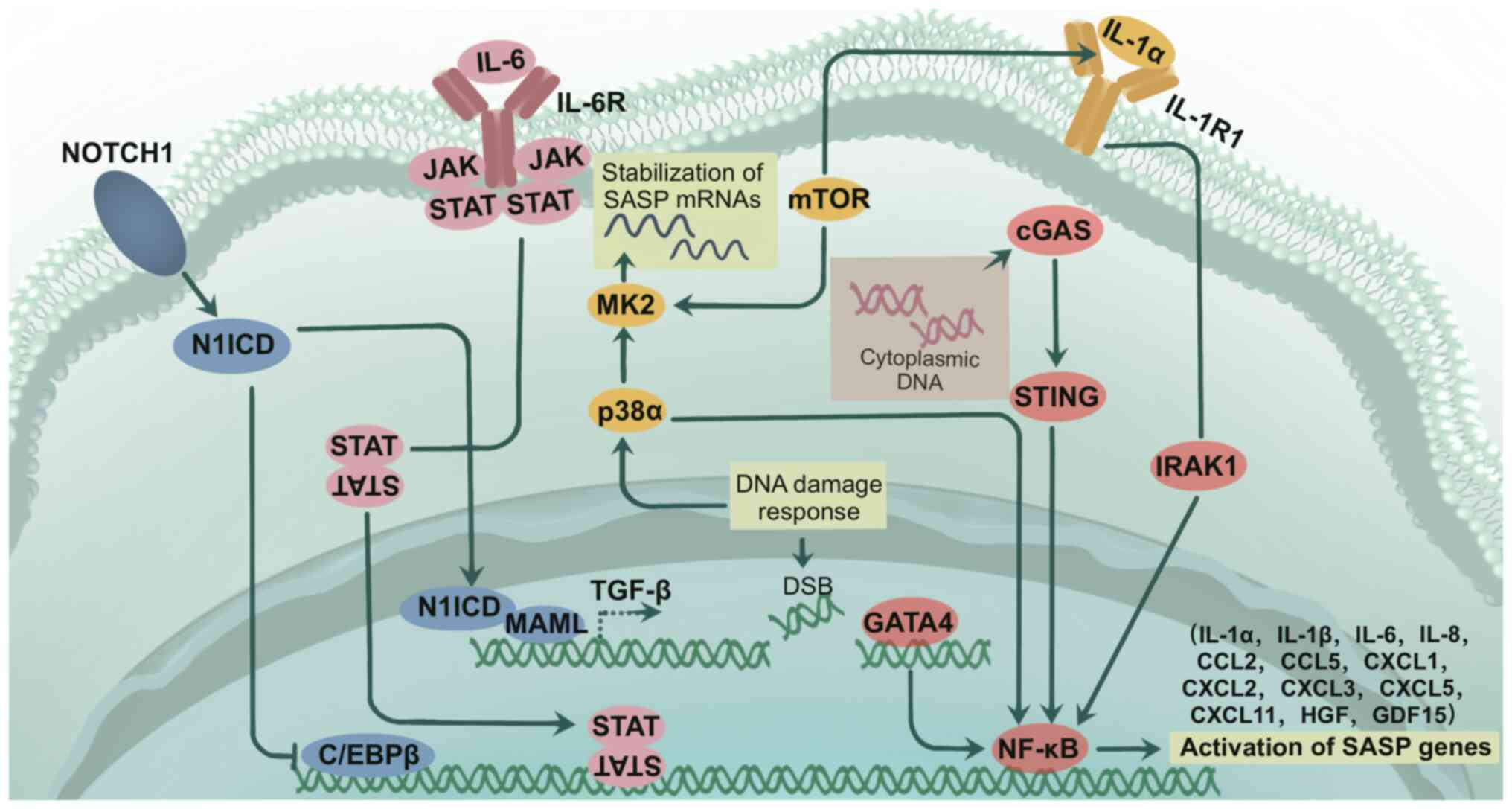
Prostate cancer (PCa) is a type of malignant tumor
that originates in the epithelium of the prostate gland. According
to the 2023 American Cancer Society report, PCa is ranked as the
second most common type of cancer in men worldwide and is a leading
cause of cancer-related death in the male population (1,2).
Although radical prostatectomy is an effective strategy in treating
early-stage PCa, a number of patients with PCa present with distant
metastases at diagnosis (3).
Surgical removal of the tumor, or chemical and surgical castration,
are the standard treatments for patients with advanced PCa.
However, a large proportion of patients with PCa that are initially
responsive to endocrine therapy progress to chemoresistant PCa
(CRPC) in 18–24 months (4).
Patients with CRPC typically develop metastases; bone and lymph
node metastases are the most common types of metastases in these
patients (5). Furthermore, a
number of patients with metastatic PCa present with resistance to
established treatment modalities, notably androgen deprivation
therapy (ADT), which results in suboptimal therapeutic outcomes
(6). Therefore, understanding the
molecular mechanisms involved in the transition from localized to
metastatic PCa is crucial for the development of more effective
treatments for patients with metastatic PCa.
A large proportion of the available PCa treatments
are associated with the induction of cellular senescence.
Senescence, while effectively arresting the cell cycle, has a dual
role in tumor treatment. Although senescence imparts a steady state
of cell cycle arrest, it also increases the potential of tumor cell
invasion and lymphangiogenesis, which promotes PCa metastasis
(7). This phenomenon is suggested
to be associated with the secretome produced by senescent cells,
which includes cytokines, chemokines, inflammatory factors and
proteases, collectively referred to as the senescence-associated
secretory phenotype (SASP) (8).
The SASP can promote tumor cell epithelial-mesenchymal transition
(EMT) and angiogenesis, which are both pivotal in tumor metastasis
(9). It is considered a challenge
to effectively harness the antitumor effects of the SASP, while
avoiding its potential pro-tumor effects in current drug
development. However, few studies have summarized the role of
senescence and its associated phenotype in promoting PCa metastasis
and its relevance to therapeutic mechanisms (10–12).
In the present review, the complex relationship between PCa
metastasis, the SASP and the potential of senescence-targeting
therapies as an opportunity for treatment of metastatic PCa is
discussed, with the aim of advancing research on senotherapeutic
drugs.
Recent studies have suggested that the induction of
cellular senescence as a treatment for PCa may yield certain
benefits to patients, such as improved immune surveillance and
inhibition of tumor growth (11,20).
However, ~1 year following senescence-inducing treatment, patients
with PCa were reported to develop resistance to the treatment and
progress to CRPC (21). These
contradictory outcomes can be attributed to the SASP.
The SASP serves a dual role in tumorigenesis and
progression. Positive regulatory effects involve some SASP factors,
such as IL-6 and IL-8, which increase the immunosurveillance of
senescent cells. This stimulation prompts the immune system to
eliminate precancerous senescent cells and promotes tissue repair
(33). By contrast, negative
regulation involves the secretion of soluble factors, such as ILs,
chemokines, growth factors and degradative enzymes, including
matrix metalloproteinases (MMPs), as well as insoluble
proteins/extracellular matrix (ECM) components of the SASP. These
factors can influence the tumor microenvironment (TME) to promote
tumor progression (34). The TME
is a complex, dynamic environment formed from the interactions of
tumor cells with immune cells, mesenchymal stromal cells and the
extracellular environment, such as the ECM and soluble biomolecules
secreted by tumor cells (35). The
effects of the SASP on the PCa microenvironment are predominantly
in the form of immune reactivity and matrix or vascular remodeling
(36). IL-6 can recruit
myeloid-derived suppressor cells into the TME; these cells can
block IL-1-mediated cellular senescence and reduce immune
surveillance by inhibiting antitumor cells, such as CD8+
T cells and natural killer (NK) cells (37). Furthermore, in addition to direct
immunomodulation through the release of cytokines and chemokines,
the SASP may indirectly affect immunoreactivity by affecting other
stromal cells in the TME, particularly endothelial cells (38). Senescent cells produce high levels
of proangiogenic SASP factors from the VEGF, platelet-derived
growth factor and fibroblast growth factor families, which mediate
neointimal formation and vascular remodeling, thereby promoting
cancer development (39).
Overall, the production of SASP factors is diverse
and dynamic. A number of SASP factors exhibit distinct functions,
although even the same SASP factors can exert opposing effects on
tumors at different stages. Resolving the contradictory mechanisms
between the SASP and PCa treatment in important to advance research
on the therapeutic strategies for metastatic PCa in the future.
In the early-stage of PCa progression,
treatment-induced SASP, such as that caused by chemotherapy and
radiotherapy, evolves from an ASAP, which typically appears 5–8
days after the initiation of PCa treatment (11). Core factors of the SASP include
IL-6, IL-8, colony stimulating factor 1 and chemokine (C-C motif)
ligand 2. The p53 and NF-κB signaling pathways can synergistically
regulate these SASP factors, activate macrophages to form an
oncogenic microenvironment, which promotes senescence of tumor
cells, prevent the proliferation and division of senescent tumor
cells and increase the oncogenic effect of PCa therapeutic drugs
(40).
Existing PCa treatments, such as ADT, radiotherapy
and chemotherapy, have been shown to induce senescence of PCa
cells, a phenomenon termed treatment-induced senescence (TIS)
(41). TIS can lead to reduced or
enhanced tumor growth, and investigation of its role in a number of
treatments could help to address drug resistance in PCa therapy
(42). Initially, advanced stage
PCa can be effectively treated with ADT, which rapidly diminishes
serum testosterone levels through the reduction of testicular
androgen production or inhibition of the androgen receptor (AR).
This is achieved by the modulation of luteinizing hormone-releasing
hormone (LHRH) production or activity using LHRH agonists, such as
goserelin, leuprolide and trenbolone, or LHRH antagonists, such as
degareli (43). Gilbert et
al (44) demonstrated an
association between the tumor suppressor mechanism of ADT and the
induction of senescence in an IKKε-deficient PC-3 cell line and a
xenograft PCa mouse model. Pernicová et al (45) reported that ADT may regulate the
tissue microenvironment through senescent cells, which potentially
promote the development of androgen-independent PCa. Mirzakhani
et al (20) demonstrated
the effects of the interaction between the AR and long non-coding
(lnc)RNASAT1 on chromatin level using RNA-chromatin
immunoprecipitation experiments, and identified a novel
AR-lncRNASAT1-AKT-p15INK4b signaling axis that may
mediate supraphysiological androgen level-induced cellular
senescence. Coppé et al (22) analyzed biopsy samples from patients
with PCa who were treated with the antitumor drug mitoxantrone and
demonstrated upregulation of the SASP factors IL-6 and IL-8. In
addition, Blute et al (46)
demonstrated that in patients with PCa treated with chemotherapy
and ADT, the reactive oxygen species-ERK-ETS-p16INK4a
and p27Kip1-retinoblastoma protein pathways were
activated to induce senescence. This highlights the variation in
intracellular signaling within PCa cells that can lead to the
induction of TIS.
In addition to the diverse aforementioned actions of
the SASP, senescent cells also exert their tumor-suppressive
effects through the activation of immune surveillance pathways.
Oncogene-induced cellular senescence (OIS) represents a specific
type of senescence mechanism during tumorigenesis, which inhibits
the oncogenic transformation of tumors, such as prostate, ovarian
and colorectal cancer, and serves as an initial barrier to cancer
development in vivo (47–49).
OIS is triggered by the activation of oncogenes, such as Ras and
BRAF, or the inactivation of tumor suppressor genes, such as PTEN
(50). In OIS, oncogene activation
induces DNA damage, which in turn activates p53 and leads to
senescence. By contrast, PTEN deletion-induced cellular senescence
lacks apparent DNA damage and, in mouse models and human tumor
xenograft models, p53 may be activated via the PI3K/AKT/mTOR
pathway after PTEN deletion (51).
The tumor suppressor gene PTEN is frequently absent or mutated in
human PCa, which leads to the activation of the PI3K/AKT signaling
pathway and tumorigenesis (52).
In addition, PTEN has an AKT-independent function and directly
interacts with p53 to regulate its transcriptional activity and
stability (53). Parisotto et
al (54) demonstrated that
PTEN deficiency in adult mouse prostate luminal epithelial cells
stimulated PCa epithelial proliferation, followed by progressive
growth arrest characterized by cellular senescence. In a PCa mouse
model, Chen et al (55)
demonstrated that double mutant mice with specific inactivation of
PTEN and p53 in the prostate epithelium consistently developed PCa
and died by 7 months of age. This was attributed to the
acceleration of tumor progression in PTEN-deficient mice through
promoting hyperproliferation and transformation, and eliminating
tumor cell senescence.
Overall, PTEN loss-induced progression of PCa
intraepithelial neoplasia is counteracted by cellular senescence in
mouse models of PCa. However, due to the replicative stress
associated with PTEN deletion, strategies to promote PTEN
loss-induced senescence are high-risk in cancer prevention and
treatment (54). The
aforementioned studies provide in vivo evidence to support
the role of OIS as a key ‘brake’ on prostate tumorigenesis. At the
same time, senescent cells can induce senescence in neighboring
cells through the SASP and direct cell-to-cell interactions, which
limit the proliferation of nearby precancerous or fully malignant
cells that have not yet undergone senescence and enhances the tumor
suppressor function of senescent cells (56). In certain instances, SASP factors
in the TME recruit a variety of immune cells to eliminate tumor
cells undergoing senescence, which inhibit tumor cell growth. For
example, senescent tumor cells secrete IL-6, IL-8 and insulin-like
growth factor binding protein 7 into the TME, which initiates a
proinflammatory response. These factors recruit immune cells, such
as T lymphocytes, to the site of tumorigenesis, where these immune
cells recognize and eliminate senescent and tumor cells, inhibit
tumor growth and impede tumor progression (57). In summary, although the molecular
pathways that induce cell cycle arrest can vary among different
therapies, their tumor suppression mechanisms are generally related
to senescence.
In the later stages of PCa, radiotherapy,
chemotherapy and targeted therapies result in a large number of
senescent cells remaining in the body; the continuous accumulation
of senescent cells promotes the formation of a senescent
microenvironment, which enhances SASP secretion and senescence of
immune cells, which can lead to metastasis and drug resistance of
PCa (11). Notably, there is
experimental evidence on the role of SASP factor components in
promoting PCa proliferation. In the present review, the SASP
regulatory and effector factors associated with PCa metastasis
pathways, the targeting of these pathways and the impact of the
regulation of SASP factors on metastatic PCa treatment is
discussed.
IL-6 and IL-8 are two proinflammatory cytokines, and
the main components of the SASP in human senescent fibroblasts,
which have a notable impact on PCa metastasis (58). High levels of IL-6 and IL-8 have
been indicated as biomarkers for metastatic PCa, which promote
angiogenesis and are closely related to the colonization of
metastatic PCa (59).
González-Ochoa et al (60)
demonstrated a positive association between the secretion levels of
IL-8 and IL-6, and the invasiveness and metastatic potential of PCa
using the DU145 PCa cell line. In addition, IL-6 was shown to
promote PCa metastasis by facilitating tumor cell EMT.
Méndez-Clemente et al (61)
demonstrated that IL-6 receptor (IL-6R)-mediated IL-6-dependent
STAT3 activation, through the formation of an IL-6/IL-6R/STAT3
feedback loop, could promote EMT in PCa cells. In summary, IL-6 and
IL-8 are two marked contributors to PCa metastasis.
Members of the MMP superfamily are a component of
the SASP. MMPs, secreted by stromal cells, serve an integral role
in the progression of PCa and are closely associated with bone
metastasis of PCa (62). Park
et al (63) reported that
MMPs, such as MMP2 and MMP9, can mediate the degradation of the
ECM. This process can increase the protein expression levels of
MMPs and VEGF to promote angiogenesis and thus tumor growth
(64). MT1-MMP, a transmembrane
member of the MMP family, is upregulated as PCa progresses from
normal progression to prostatic intraepithelial neoplasia to
invasive cancer, which indicates its role in the invasive process
of prostate adenocarcinoma (65).
Wei et al (66) reported
that MMP activity, particularly through MMP2 and MMP9, is
associated with the invasive and metastatic potential of cancer
cells. It was reported that osteonectin induces MMP activity, which
led to the degradation of the ECM and enabling the invasion of
cancer cells. In cell specimens obtained from metastatic lesions in
the bone marrow of patients with PCa, Miftakhov et al
(67) demonstrated that MMP
production in bone marrow could create a suitable microenvironment
for the metastatic growth of PCa cells. In a previous study, it was
revealed that Timp1 deletion led to the activation of downstream
MMPs, which reprogrammed and initiated the SASP in PTEN-deficient
cells; furthermore, PTEN, and TIMP1-knockout cells could promote
the migration of non-senescent mouse and human tumor cells by
paracrine means (68). Therefore,
TIS should potentially be used with caution in patients with PCa
and a background of TIMP1 gene deletion, to spare a number of
patients from the toxicity of chemotherapy. The aforementioned
studies demonstrate that MMPs may influence PCa metastasis and
further studies should be conducted in the future to identify the
specific pathways involved in the metastatic transformation of
primary tumors, which could serve to develop strategies to prevent
the development of metastatic PCa.
As an additional crucial regulator of the SASP, mTOR
serves a pivotal role in promoting the SASP through its involvement
in translation. A previous study demonstrated that both mTOR
complex (mTORC)1 and mTORC2 are frequently overactivated in PCa
cells, and are associated with the process of cancer metastasis
(75). The oncogenic role of
PI3K-AKT-mTOR signaling and the common genetic alterations in this
pathway are well established (76). Shi et al (77) identified a novel circular
(circ)RNA, circMBOAT29, the overexpression of which led to the
activation of the PI3K/Akt pathway through the upregulation of
mTOR, which promoted the metastasis of PCa. Since PCa is a highly
heterogeneous tumor and its biological behavior may vary within the
same stage of disease, the efficacy of these compounds may also
vary. Based on the expression characteristics and mechanisms of
circRNAs in PCa, it may be possible to utilize precision therapy
for tumor treatment and develop personalized treatment plans for
individual patients (78).
Existing mTORC1 inhibitors are effective in the treatment of PCa
(79). mTORC1 inhibitors, such as
temsirolimus and everolimus, are U.S. Food and Drug Agency
(FDA)-approved for phase III clinical trials in kidney cancer,
neuroendocrine tumors and metastatic breast cancer (80). Ongoing clinical trials
investigating the combination of mTORC1 inhibitors with standard
anticancer therapies, such as chemotherapy and ADT, in PCa
treatment have achieved improved efficacy compared to the therapies
alone (81–83). Rapamycin, a selective inhibitor of
mTORC1, is well tolerated in patients with PCa according to
preliminary analysis from a number of clinical trials, as mTORC1
displays sensitivity to rapamycin treatment (84,85).
The therapeutic benefit of the mTORC1/2 inhibitor rapalink-1 has
been reported in patient-derived organoid and xenograft models of
bone metastatic PCa, which supports the involvement of the mTOR
pathway in bone metastatic cancers (86). In addition, mTORC2 is required for
PTEN loss-induced PCa development in mice and serves a central role
in mediating PI3K-dependent carcinogenesis (87). mTORC2 signaling promotes the growth
of PTEN-deficient PCa, which indicates that mTORC2 inhibition may
be clinically effective in PTEN-deficient PCa (88). This suggests that targeting mTORC2
in patients with PTEN-deficient PCa may potentially be able to
provide novel therapeutic strategies for those patients with PCa.
To the best of our knowledge, there is no effective treatment for
metastatic PCa, in particular for PCa cases where hormonal ablation
therapy has failed, and highly selective drugs that target the
PI3K-AKT-mTOR pathway have a more favorable therapeutic index in
such cases (89). However,
off-target effects of the dual inhibition of PI3K and mTOR pathways
may cause unacceptable toxicity with dose-dependent adverse effects
and drugs with more favorable therapeutic indexes, as well as the
efficacy and appropriate dosage of different drug combinations,
should be explored in future research.
NF-κB is required to achieve full transcriptional
activation of the SASP. Activation of the NF-κB pathway results in
enhanced secretion of proinflammatory cytokines and chemokines from
senescent cells, resulting in the SASP (90). Li et al (91) identified that the IKKβ/AT-rich
interaction domain 1A/NF-κB feedback axis integrates inflammation
and immunosuppression to promote PCa progression, which supports
the suggestion that anti-NF-κB antibodies or targeting IL-8
receptor b, in combination with immune checkpoint blockade
therapies, such as blockade of PD-1 binding to PD-L1, might serve
as a therapeutic strategy for advanced PCa. Previous studies have
demonstrated that NF-κB expression not only promotes the expression
of cell adhesion molecules, but also promotes the expression of
molecules that facilitate tumor metastasis, which results in
increased resistance to tumor therapy (92). A recent study demonstrated that
NF-κB activation is required for tumor cell EMT, the process
through which tumor epithelial cells acquire mesenchymal features,
and become highly invasive and metastatic (93). Inhibition of NF-κB signaling
prevents EMT, which reduces the metastatic potential of PCa. In
addition, NF-κB regulates metastatic activity by controlling the
transcriptional activity of MMPs and angiogenic enzymes (94). Ayala et al (95) conducted a neoadjuvant clinical
trial of bortezomib, an NF-κB inhibitor, in male patients with PCa
classed as having a high risk of recurrence and demonstrated that
bortezomib was generally safe preoperatively and that, in
vitro, the combination of bortezomib and perifosine, an AKT
inhibitor, was more effective compared with either therapy alone.
It was previously reported that the FDA-approved therapeutic
combinations of artesunate (AS) or NF-κB inhibitors with AR
antagonists may improve the clinical efficacy of treatment in
patients with CRPC (96). Future
clinical trials using AS and AR antagonist therapies in patients
with CRPC are needed for further research advances. In summary, the
activation of the NF-κB pathway may promote PCa metastasis through
a number of mechanisms, such as enhancing immunosuppression,
expression of tumor metastatic molecules and the promotion of
angiogenesis by facilitating the development of EMT.
In addition to cytokines, sEVs have emerged as
contributors to PCa metastasis, as part of the SASP (97). sEVs constitute a diverse population
of membrane-secreting vesicles containing exosomes (98). PCa cell-derived sEVs contain
cytokines that stimulate the differentiation of bone marrow
mesenchymal stem cells into myofibroblasts (99). These sEVs release elevated
expression levels of VEGF-A, hepatocyte growth factor and MMPs that
possess proangiogenic properties, which increase tumor cell
proliferation and metastasis (100). Ma et al (101) analyzed the microRNA (miR)
profiles of tumor-derived sEVs and osteoclasts together and
reported that miR-152-3p, carried by PCa-derived sEVs, could
transmit osteolytic signals from tumor cells to osteoclasts; this
process may promote osteolysis in bone metastasis. sEVs may serve
as biomarkers of PCa progression, and represent potential
therapeutic targets in the prediction and prevention of PCa
metastasis.
The use of senescence-inducing therapy as a
standalone treatment for tumors is currently controversial. A
number of studies have shown that the SASP can exhibit a dual
effect: i) Inhibition of the tumor growth by inducing paracrine
senescence in tumor cells through inflammatory mediator-containing
extracellular vesicle-mediated mechanisms; and ii) influencing the
TME through the secretion of inflammatory cytokines and chemokines,
which potentially promotes the progression of tumors. In recent
years, research has been devoted to the emerging concept of
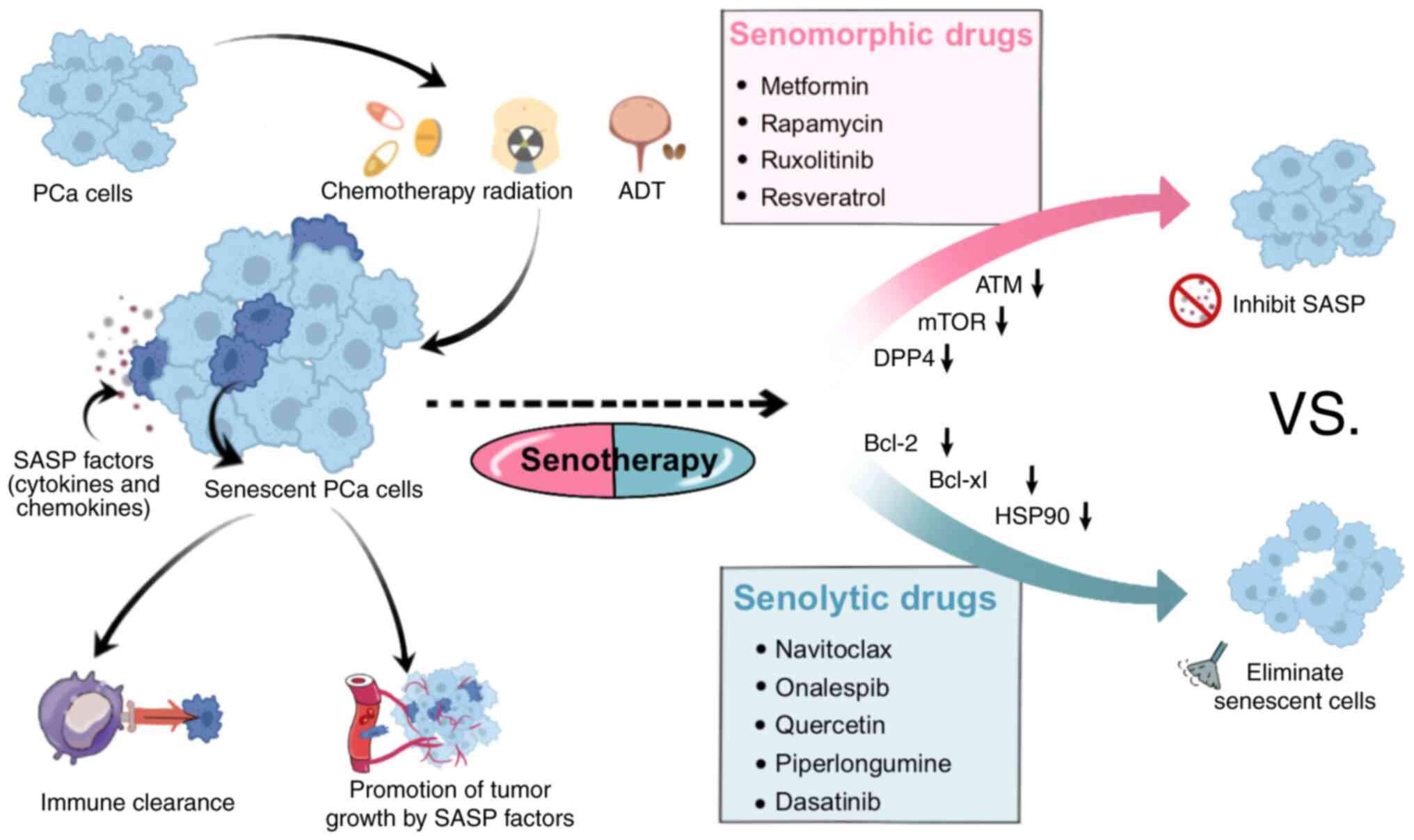
‘senotherapy’ (102–104) (Fig.
3). Senotherapy involves initially inducing senescence in tumor
cells through radiotherapy and chemotherapy to exert
tumor-suppressing effects. Subsequently, adjuvant drugs are
employed to eliminate senescent cells, preventing the release of
SASP factors. This dual-phase strategy aims to avoid the possible
tumor-promoting effects of senescent cells (105). Through the use of adjuvant drugs
to eliminate senescent cells and inhibit the release of SASP
factors from these cells, the potential tumor-promoting effect of
senescent cells is effectively avoided (106). Current therapeutic approaches for
PCa have been expanded to include the use of senomorphic drugs to
mitigate SASP activity and senolytic drugs to eliminate senescent
cells (107). Contemporary
senotherapeutic drugs not only inhibit the SASP, but also leverage
the characteristics of senescent cells to enhance the ability of
the immune system to clear senescent cells. For example, the
application of chimeric antigen receptor-T targeting antigens
specific to senescent cells allows for selective removal of these
senescent cells (108). While
clinical trials validating the efficacy of chimeric antigen
receptor-T targeting antigens specific to senescent cells in PCa
treatment are still lacking, the results of the aforementioned
preclinical studies provide support for the integration of
senotherapeutic drugs with conventional radiotherapy in the
management of metastatic PCa (105). The range of drugs used for the
treatment of senescent cells are listed in Table I (109–129), providing an overview of the
current drug mechanisms of action in the context of PCa
treatment.
Senolytics are a class of drugs designed to induce
apoptosis specifically in senescent cells, and have potential as a
treatment for PCa (130). It has
been suggested that the potential benefits of senolytic therapy are
through the intrinsic anti-aging system present in organisms, i.e.
the immune surveillance of senescent cells (131). Transgenic animal models for in
vivo senescence studies mainly include INK-ATTAC, p16-3MR,
p16-Cre and p21-ATD mice (132–135), and have shown that multiple
components of the immune system, including NK cells, T cells and
macrophages, are involved in controlling and eliminating the
presence of senescent cells in tissues (26). Pathways implicated in this process
include activation of the PI3K/AKT and/or Bcl-2/Bcl-xl pathways
(11). Zhan et al (136) reported evidence from two cell
models with deletions in the ATRX gene (a common molecular marker
for glioma) suggesting the efficacy of senolytic drugs in targeting
senescent tumor cells and precancerous cells. Arai et al
(109) demonstrated the potential
of BH3 mimetics, a novel class of antitumor drugs that targets
Bcl-2 family proteins, as a monotherapy or in combination with
other agents for the treatment of PCa cells. A combination of
navitoclax, a BH3 mimetic, with taxane-based chemotherapy, such as
docetaxel and paclitaxel, increased the rate of apoptotic cell
death in human PCa cells compared with the drug alone. In a murine
model of PCa with PTEN deficiency, Guccini et al (68) demonstrated that the absence of
TIMP1 shifted senescence from a tumor-suppressive process to a
metastasis-promoting process. Furthermore, the elimination of
senescent cells using senolytic Bcl-2 inhibitors attenuated this
transition. Mechanistically, the loss of TIMP1 reprograms the SASP
of senescent tumor cells by activation of MMPs (68). The deletion of TIMP1, either alone
or in PTEN and TIMP1 double deletions, is common in PCa and is
associated with docetaxel resistance (137). A major limitation of navitoclax
identified in early senolytic experiments was the risk of
thrombocytopenia when used in excess, which limited its use
(138). However, it has been
demonstrated that the platelet toxicity can be reduced by
converting navitoclax to PZ15227, with the use of protein
hydrolysis-targeted chimerism technology, through a conversion
process that reduces its toxicity and improves its effectiveness
against senescent cells, which results in the regeneration of
tissue stem and progenitor cells in senescent mice (139). This further supports the future
feasibility of navitoclax in combination with conventional
radiotherapy agents for the treatment of metastatic PCa (140). Bioinformatics analysis of whole
transcriptome RNA sequencing data performed by Ferraldeschi et
al (141) confirmed that the
second-generation heat shock protein 90 (HSP90) inhibitor,
onalespib, altered the splicing of ≥557 genes, including AR, in PCa
cells, which may be beneficial for PCa and suggests HSP90
inhibitors as a class of drugs that could potentially be evaluated
further in metastatic PCa in the future. Lu et al (113) demonstrated that the HSP90
inhibitor quercetin could reverse proliferation, colony formation,
migration, invasion and apoptosis resistance to doxorubicin in PCa,
using doxorubicin-resistant cells (LNCaP/R, PC-3/R) established
from doxorubicin-sensitive cell lines (LNCaP, PC-3). A recent study
reported that the retinoic acid receptor agonist adapalene can be
used as a new senolytic agent, and that in a preclinical mouse
model of PCa, the combination of adapalene and docetaxel could
promote a tumor-suppressive SASP, which prompts NK cells to mediate
tumor clearance more effectively than either drug alone (142). This supports the therapeutic
potential of senolytic therapy in PCa and provide insights into the
mitigation of the side effects associated with senolytic
therapy.
Currently, there are >20 clinical trials related
to senolytics. Due to a limited understanding of the side effects
of senolytics in humans, a large proportion of clinical trials have
been conducted in patients with serious health conditions, which
aimed to optimize the benefit-risk ratio. A number of studies have
suggested that senolytics contribute positively to the improvement
of somatic function, which reduces senescent cellular load and
ameliorates inflammatory states (143–146); however, a number of studies have
reported unsuccessful outcomes (147,148). For example, Spetsieris et
al (149) conducted a phase
II clinical trial of abiraterone followed by randomized assignment
to the addition of dasatinib or sunitinib, for the treatment of
patients with metastatic desmoplasia-resistant PCa. No difference
in overall survival or time to treatment failure between dasatinib
and sunitinib in combination with abiraterone for the treatment of
patients with metastatic CRPC in the bone was found. In the future,
preclinical studies are needed to further elucidate the markers and
mechanisms of action of senescence in patients with PCa, and to
perform more extensive validation of senolytic drugs, to prioritize
disease-specific drug candidates for clinical use.
Senomorphic compounds, also referred to as SASP
inhibitors, can modulate the senescence phenotype and inhibit
generation of the SASP without eliminating senescent cells
(150). Representative drugs
categorized as senomorphic compounds include rapamycin and
metformin, which can directly or indirectly attenuate the SASP in
senescent cells by inhibiting NF-κB, the JAK-STAT signaling pathway
and the serine/threonine protein kinase mTOR (151). Currently, a large proportion of
the data on the efficacy of senotherapeutic drugs come from in
vitro experiments using human cell cultures. Studies have shown
that the use of metformin after ADT induces apoptosis, attenuates
mTOR activation and reduces the number of senescent cells in PCa
in vitro and in vivo (122). Several preclinical studies have
also indicated that the mTOR inhibitor rapamycin can inhibit the
androgen-dependent growth of human PCa cells by downregulating the
expression levels of AR-activated downstream genes (100–102). The findings from the
aforementioned trials suggest that senescence therapies may provide
novel leads for the treatment of metastatic PCa and
desmoplasia-resistant PCa. In addition, specific JAK/STAT small
molecule inhibitors, such as ruxolitinib and fludarabine, have
demonstrated efficacy in the treatment of both curative and
desmoplasia-resistant PCa (127).
In general, senolytic therapies may offer additional
advantages compared with senomorphic therapies. Senolytic drugs are
not only effective, but because they administered intermittently,
the side effects are effectively controlled and they are considered
to be safe (152). Unlike
senolytic drugs that are administered intermittently, a large
proportion of SASP inhibitors require continuous treatment to
maintain SASP inhibition, which potentially increases the
occurrence of side effects associated with the drug (153). Secondly, the complete removal of
all senescent cells or total inhibition of the SASP may be
detrimental in a number of cases (154,155), as it can cause chronic
inflammation, immunosuppression, stimulate the EMT, and even
promote tumor migration and metastasis (156). By contrast, senolytic drugs have
the advantage of specifically targeting senescent cells at the site
of the lesion and can induce apoptosis in these cells. Future drug
studies should prioritize intervening in senescent cells
consistently expressing tissue-damaging SASP, and aim to develop
drugs with enhanced therapeutic potential and minimized off-target
effects. Novel therapeutic options could potentially be based on
the expression characteristics and mechanisms of circRNAs in PCa,
thus bringing PCa tumor treatment into a precision therapy
paradigm. Rigorous clinical trials are necessary to demonstrate the
safety and efficacy of senolytics or SASP inhibitors in PCa;
despite currently available preclinical data, advancements in
relevant research in this field are challenging.
In summary, the microenvironment of senescence can
markedly influence prostate carcinogenesis and metastasis, which
highlights the importance of cellular senescence in PCa treatment.
The intricate composition of SASP factors contributes to the dual
impact of senescence, which acts as both a tumor suppressor and
promoter. Effectively harnessing the tumor-suppressive effects of
SASP, while mitigating its tumor-promoting effects, remains a
challenge in this field. Currently, the main obstacle in the
treatment of metastatic PCa is the development of resistance to
existing therapies and progression to an incurable state. Future
research should focus on identifying the intricate associations
among cellular senescence, the SASP and the TME. A comprehensive
exploration into the types of PCa cell senescence induced by drug
treatments, along with the investigation of specific molecular
mechanisms and their interactions with the immune microenvironment
could potentially advance the current understanding of PCa, and has
ramifications for the precise treatment of metastatic PCa,
alleviation of chemotherapy resistance, minimization of the toxic
side effects of chemotherapy drugs and enhancement of therapeutic
outcomes for metastatic PCa in the future.
Not applicable.
The present review was supported by the College Students'
Innovation and Entrepreneurship Training Program (grant no.
S202210660072) and the Undergraduate Teaching Research Project of
Guizhou Medical University (grant no. JG2023046). Partial support
was provided by the Natural Science Foundation Project of Guizhou
Provincial Science and Technology Department (grant no. Qiankehe
Foundation-ZK 2024 General 182).
Not applicable.
CJ, SL and GL wrote the original draft and prepared
the figures. DY and GG wrote, reviewed and edited the manuscript.
BG, ZY and JX edited the review and were responsible for editing
the references. All authors have read and approved the final
version of the manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Xie J, Xiao X, Dong Z and Wang Q: The
systemic inflammation score is associated with the survival of
patients with prostate cancer. J Inflamm Res. 16:963–975. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan Y, Wang L, Du Y, Liu X, Chen Z, Weng
X, Guo J, Chen H, Wang M and Wang X: Inhibition of BRD4 suppresses
tumor growth in prostate cancer via the enhancement of FOXO1
expression. Int J Oncol. 53:2503–2517. 2018.PubMed/NCBI
|
|
4
|
Dong D, Zhang L, Bai C, Ma N, Ji W, Jia L,
Zhang A, Zhang P, Ren L and Zhou Y: UNC5D, suppressed by promoter
hypermethylation, inhibits cell metastasis by activating
death-associated protein kinase 1 in prostate cancer. Cancer Sci.
110:1244–1255. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Belmonte M, Saia G, Zugni F, Alessi S,
Colombo A, Summers PE, Luzzago S, Marvaso G, Musi G, De Cobelli O,
et al: The role of MRI in the management of a prostate cancer
patient with bone and lymph nodes metastases. A case report. Acta
Biomed. 92:e20212142021.PubMed/NCBI
|
|
6
|
Witt K, Evans-Axelsson S, Lundqvist A,
Johansson M, Bjartell A and Hellsten R: Inhibition of STAT3
augments antitumor efficacy of anti-CTLA-4 treatment against
prostate cancer. Cancer Immunol Immunother. 70:3155–3166. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun CY, Talukder M, Cao D and Chen CW:
Gilteritinib enhances Anti-tumor efficacy of CDK4/6 inhibitor,
abemaciclib in lung cancer cells. Front Pharmacol. 13:8297592022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rysanek D, Vasicova P, Kolla JN, Sedlak D,
Andera L, Bartek J and Hodny Z: Synergism of BCL-2 family
inhibitors facilitates selective elimination of senescent cells.
Aging. 14:6381–6414. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rhinn M, Zapata-Bodalo I, Klein A, Plassat
JL, Knauer-Meyer T and Keyes WM: Aberrant induction of
p19Arf-mediated cellular senescence contributes to
neurodevelopmental defects. PLoS Biol. 20:e30016642022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alessio N, Aprile D, Squillaro T, Di
Bernardo G, Finicelli M, Melone MA, Peluso G and Galderisi U: The
senescence-associated secretory phenotype (SASP) from mesenchymal
stromal cells impairs growth of immortalized prostate cells but has
no effect on metastatic prostatic cancer cells. Aging (Albany NY).
11:5817–5828. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kallenbach J, Atri Roozbahani G, Heidari
Horestani M and Baniahmad A: Distinct mechanisms mediating
therapy-induced cellular senescence in prostate cancer. Cell
Biosci. 12:2002022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mori JO, Elhussin I, Brennen WN, Graham
MK, Lotan TL, Yates CC, De Marzo AM, Denmeade SR, Yegnasubramanian
S, Nelson WG, et al: Prognostic and therapeutic potential of
senescent stromal fibroblasts in prostate cancer. Nat Rev Urol.
21:258–273. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takemoto K, Kobatake K, Miura K, Fukushima
T, Babasaki T, Miyamoto S, Sekino Y, Kitano H, Goto K, Ikeda K, et
al: BACH1 promotes clear cell renal cell carcinoma progression by
upregulating oxidative stress-related tumorigenicity. Cancer Sci.
114:436–448. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayflick L and Moorhead PS: The serial
cultivation of human diploid cell strains. Exp Cell Res.
25:585–621. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Y, Ge MX, Li YH, Li JL, Yu Q, Xiao
FH, Ao HS, Yang LQ, Li J, He Y and Kong QP: Longevity-associated
transcription factor ATF7 promotes Healthspan by suppressing
cellular senescence and systematic inflammation. Aging Dis.
14:1374–1389. 2023.PubMed/NCBI
|
|
16
|
Ngoi NY, Liew AQx, Chong SJF, Davids MS,
Clement MV and Pervaiz S: The redox-senescence axis and its
therapeutic targeting. Redox Biol. 45:1020322021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park SS, Choi YW, Kim JH, Kim HS and Park
TJ: Senescent tumor cells: An overlooked adversary in the battle
against cancer. Exp Mol Med. 53:1834–1841. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alhaddad L, Nofal Z, Pustovalova M, Osipov
AN and Leonov S: Long-term cultured human glioblastoma multiforme
cells demonstrate increased radiosensitivity and
senescence-associated secretory phenotype in response to
irradiation. Int J Mol Sci. 24:20022023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Z, Uhl B, Gires O and Reichel CA: A
transcriptomic pan-cancer signature for survival prognostication
and prediction of immunotherapy response based on endothelial
senescence. J Biomed Sci. 30:212023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mirzakhani K, Kallenbach J, Rasa SMM,
Ribaudo F, Ungelenk M, Ehsani M, Gong W, Gassler N, Leeder M, Grimm
MO, et al: The androgen receptor-lncRNASAT1-AKT-p15 axis mediates
androgen-induced cellular senescence in prostate cancer cells.
Oncogene. 41:943–959. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Birch J and Gil J: Senescence and the
SASP: Many therapeutic avenues. Genes Dev. 34:1565–1576. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz
DP, Goldstein J, Nelson PS, Desprez PY and Campisi J:
Senescence-associated secretory phenotypes reveal
cell-nonautonomous functions of oncogenic RAS and the p53 tumor
suppressor. PLoS Biol. 6:2853–2868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Georgilis A, Klotz S, Hanley CJ, Herranz
N, Weirich B, Morancho B, Leote AC, D'Artista L, Gallage S,
Seehawer M, et al: PTBP1-mediated alternative splicing regulates
the inflammatory Secretome and the Pro-tumorigenic effects of
senescent cells. Cancer Cell. 34:85–102.e9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yue Z, Nie L, Zhao P, Ji N, Liao G and
Wang Q: Senescence-associated secretory phenotype and its impact on
oral immune homeostasis. Front Immunol. 13:10193132022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu L, Yue X, Sun Z, Hambright WS, Wei J,
Li Y, Matre P, Cui Y, Wang Z, Rodney G, et al: Reduction of
senescent fibro-adipogenic progenitors in progeria-aged muscle by
senolytics rescues the function of muscle stem cells. J Cachexia
Sarcopenia Muscle. 13:3137–3148. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khalil R, Diab-Assaf M and Lemaitre JM:
Emerging therapeutic approaches to target the dark side of
senescent cells: New hopes to treat aging as a disease and to delay
age-related pathologies. Cells. 12:9152023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Pitcher LE, Yousefzadeh MJ,
Niedernhofer LJ, Robbins PD and Zhu Y: Cellular senescence: A key
therapeutic target in aging and diseases. J Clin Invest.
132:e1584502022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duan D, Shang M, Han Y, Liu J, Liu J, Kong
SH, Hou J, Huang B, Lu J and Zhang Y: EZH2-CCF-cGAS axis promotes
breast cancer metastasis. Int J Mol Sci. 23:17882022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang M, Cha Z, Liu R, Lin M, Gafoor NA,
Kong T, Ge F and Chen W: Enhancing immunotherapy outcomes by
targeted remodeling of the tumor microenvironment via combined
cGAS-STING pathway strategies. Front Immunol. 15:13999262024.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee KS, Lin S, Copland DA, Dick AD and Liu
J: Cellular senescence in the aging retina and developments of
senotherapies for age-related macular degeneration. J
Neuroinflammation. 18:322021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Toso A, Revandkar A, Di Mitri D, Guccini
I, Proietti M, Sarti M, Pinton S, Zhang J, Kalathur M, Civenni G,
et al: Enhancing chemotherapy efficacy in Pten-Deficient prostate
tumors by activating the senescence-associated antitumor immunity.
Cell Rep. 9:75–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parry AJ, Hoare M, Bihary D,
Hänsel-Hertsch R, Smith S, Tomimatsu K, Mannion E, Smith A,
D'Santos P, Russell IA, et al: NOTCH-mediated non-cell autonomous
regulation of chromatin structure during senescence. Nat Commun.
9:18402018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tan SYX, Zhang J and Tee WW: Epigenetic
regulation of inflammatory signaling and inflammation-induced
cancer. Front Cell Dev Biol. 10:9314932022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chandrasekaran A, Idelchik MDPS and
Melendez JA: Redox control of senescence and age-related disease.
Redox Biol. 11:91–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Takasugi M, Yoshida Y and Ohtani N:
Cellular senescence and the tumour microenvironment. Mol Oncol.
16:3333–3351. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wan R, Long S, Ma S, Yan P, Li Z, Xu K,
Lian H, Li W, Duan Y, Zhu M, et al: NR2F2 alleviates pulmonary
fibrosis by inhibition of epithelial cell senescence. Respir Res.
25:1542024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao B, Wu B, Feng N, Zhang X, Zhang X,
Wei Y and Zhang W: Aging microenvironment and antitumor immunity
for geriatric oncology: the landscape and future implications. J
Hematol OncolJ Hematol Oncol. 16:282023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hwang HJ, Lee YR, Kang D, Lee HC, Seo HR,
Ryu JK, Kim YN, Ko YG, Park HJ and Lee JS: Endothelial cells under
therapy-induced senescence secrete CXCL11, which increases
aggressiveness of breast cancer cells. Cancer Lett. 490:100–110.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chibaya L, Snyder J and Ruscetti M:
Senescence and the tumor-immune landscape: Implications for cancer
immunotherapy. Semin Cancer Biol. 86:827–845. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Volonte D and Galbiati F: Caveolin-1, a
master regulator of cellular senescence. Cancer Metastasis Rev.
39:397–414. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pardella E, Pranzini E, Nesi I, Parri M,
Spatafora P, Torre E, Muccilli A, Castiglione F, Fambrini M, Sorbi
F, et al: Therapy-induced stromal senescence promoting
aggressiveness of prostate and ovarian cancer. Cells. 11:40262022.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu MY, Xia ZY, Sun JX, Liu CQ, An Y, Xu
JZ, Zhang SH, Zhong XY, Zeng N, Ma SY, et al: A new perspective on
prostate cancer treatment: The interplay between cellular
senescence and treatment resistance. Front Immunol. 15:13950472024.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Meng F, Han X, Min Z, He X and Zhu S:
Prognostic signatures associated with high infiltration of Tregs in
bone metastatic prostate cancer. Aging. 13:17442–17461. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gilbert S, Péant B, Malaquin N, Tu V,
Fleury H, Leclerc-Desaulniers K, Rodier F, Mes-Masson AM and Saad
F: Targeting IKKε in androgen-independent prostate cancer causes
phenotypic senescence and genomic instability. Mol Cancer Ther.
21:407–418. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pernicová Z, Slabáková E, Kharaishvili G,
Bouchal J, Král M, Kunická Z, Machala M, Kozubík A and Souček K:
Androgen depletion induces senescence in prostate cancer cells
through down-regulation of Skp2. Neoplasia. 13:526–536. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Blute ML, Damaschke N, Wagner J, Yang B,
Gleave M, Fazli L, Shi F, Abel EJ, Downs TM, Huang W and Jarrard
DF: Persistence of senescent prostate cancer cells following
prolonged neoadjuvant androgen deprivation therapy. PLoS One.
12:e01720482017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang X, Peng Y, Yuan Y, Gao Y, Hu F, Wang
J, Zhu X, Feng X, Cheng Y, Wei Y, et al: Histone methyltransferase
SET8 is regulated by miR-192/215 and induces oncogene-induced
senescence via p53-dependent DNA damage in human gastric carcinoma
cells. Cell Death Dis. 11:9372020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tao YP, Zhu HY, Shi QY, Wang CX, Hua YX,
Hu HY, Zhou QY, Zhou ZL, Sun Y, Wang XM, et al: S1PR1 regulates
ovarian cancer cell senescence through the PDK1-LATS1/2-YAP
pathway. Oncogene. 42:3491–3502. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fang L, Li D, Yin J, Pan H, Ye H, Bowman
J, Capaldo B and Kelly K: TMPRSS2-ERG promotes the initiation of
prostate cancer by suppressing oncogene-induced senescence. Cancer
Gene Ther. 29:1463–1476. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Saleh T, Khasawneh AI, Himsawi N,
Abu-Raideh J, Ejeilat V, Elshazly AM and Gewirtz DA: Senolytic
therapy: A potential approach for the elimination of
oncogene-induced senescent HPV-positive cells. Int J Mol Sci.
23:155122022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ye M, Huang X, Wu Q and Liu F: Senescent
stromal cells in the tumor microenvironment: Victims or
accomplices? Cancers. 15:19272023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Brandmaier A, Hou SQ and Shen WH: Cell
cycle control by PTEN. J Mol Biol. 429:2265–2277. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhou X, Yang X, Sun X, Xu X, Li X, Guo Y,
Wang J, Li X, Yao L, Wang H and Shen L: Effect of PTEN loss on
metabolic reprogramming in prostate cancer cells. Oncol Lett.
17:2856–2866. 2019.PubMed/NCBI
|
|
54
|
Parisotto M, Grelet E, El Bizri R, Dai Y,
Terzic J, Eckert D, Gargowitsch L, Bornert JM and Metzger D: PTEN
deletion in luminal cells of mature prostate induces replication
stress and senescence in vivo. J Exp Med. 215:1749–1763. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen Z, Carracedo A, Lin HK, Koutcher JA,
Behrendt N, Egia A, Alimonti A, Carver BS, Gerald W,
Teruya-Feldstein J, et al: Differential p53-independent outcomes of
p19(Arf) loss in oncogenesis. Sci Signal. 2:ra442009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Guo J, Huang X, Dou L, Yan M, Shen T, Tang
W and Li J: Aging and aging-related diseases: From molecular
mechanisms to interventions and treatments. Signal Transduct Target
Ther. 7:3912022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hua H, Zheng C, Fan J, Li X, Xie W, Chen J
and Yu C: The senescence-related signature predicts prognosis and
characterization of tumor microenvironment infiltration in
pancreatic cancer. BioMed Res Int. 2022:1–28. 2022. View Article : Google Scholar
|
|
58
|
Dyachkova U, Vigovskiy M, Basalova N,
Efimenko A and Grigorieva O: M2-Macrophage-induced chronic
inflammation promotes reversible mesenchymal stromal cell
senescence and reduces their anti-fibrotic properties. Int J Mol
Sci. 24:170892023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Stanojković TP, Matić IZ, Petrović N,
Stanković V, Kopčalić K, Besu I, Đorđić Crnogorac M, Mališić E,
Mirjačić-Martinović K, Vuletić A, et al: Evaluation of cytokine
expression and circulating immune cell subsets as potential
parameters of acute radiation toxicity in prostate cancer patients.
Sci Rep. 10:190022020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
González-Ochoa S, Tellez-Bañuelos MC,
Méndez-Clemente AS, Bravo-Cuellar A, Hernández Flores G,
Palafox-Mariscal LA, Haramati J, Pedraza-Brindis EJ, Sánchez-Reyes
K and Ortiz-Lazareno PC: Combination blockade of the IL6R/STAT-3
Axis with TIGIT and its impact on the functional activity of NK
cells against prostate cancer cells. J Immunol Res.
2022:18108042022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Méndez-Clemente A, Bravo-Cuellar A,
González-Ochoa S, Santiago-Mercado M, Palafox-Mariscal L,
Jave-Suárez L, Solorzano-Ibarra F, Villaseñor-García M,
Ortiz-Lazareno P and Hernández-Flores G: Dual STAT-3 and IL-6R
inhibition with stattic and tocilizumab decreases migration,
invasion and proliferation of prostate cancer cells by targeting
the IL-6/IL-6R/STAT-3 axis. Oncol Rep. 48:1382022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Silk N, Reich J, Sinha R, Chawla S, Geary
K and Zhang D: The effects of resveratrol on prostate cancer
through targeting the tumor microenvironment. J Xenobiotics.
11:16–32. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Park SY, Cui Z, Kim B, Park G and Choi YW:
Treatment with gold nanoparticles using cudrania tricuspidata root
extract induced downregulation of MMP-2/-9 and PLD1 and inhibited
the invasiveness of human U87 Glioblastoma cells. Int J Mol Sci.
21:12822020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fahs A, Hussein N, Zalzali H, Ramadan F,
Ghamloush F, Tamim H, El Homsi M, Badran B, Boulos F, Tawil A, et
al: CD147 promotes tumorigenesis via Exosome-mediated signaling in
rhabdomyosarcoma. Cells. 11:22672022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bair EL, Chen ML, McDaniel K, Sekiguchi K,
Cress AE, Nagle RB and Bowden GT: Membrane type 1 Matrix
Metalloprotease cleaves Laminin-10 and promotes prostate cancer
cell migration. Neoplasia. 7:380–389. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wei R, Wong JPC, Lyu P, Xi X, Tong O,
Zhang SD, Yuen HF, Shirasawa S and Kwok HF: In vitro and clinical
data analysis of Osteopontin as a prognostic indicator in
colorectal cancer. J Cell Mol Med. 22:4097–4105. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Miftakhova R, Hedblom A, Semenas J,
Robinson B, Simoulis A, Malm J, Rizvanov A, Heery DM, Mongan NP,
Maitland NJ, et al: Cyclin A1 and P450 aromatase promote metastatic
homing and growth of Stem-like prostate cancer cells in the bone
marrow. Cancer Res. 76:2453–2464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Guccini I, Revandkar A, D'Ambrosio M,
Colucci M, Pasquini E, Mosole S, Troiani M, Brina D,
Sheibani-Tezerji R, Elia AR, et al: Senescence reprogramming by
TIMP1 deficiency promotes prostate cancer metastasis. Cancer Cell.
39:68–82.e9. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Rodier F, Coppé JP, Patil CK, Hoeijmakers
WA, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR and Campisi
J: Persistent DNA damage signalling triggers senescence-associated
inflammatory cytokine secretion. Nat Cell Biol. 11:973–979. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
van Dessel LF, van Riet J, Smits M, Zhu Y,
Hamberg P, van der Heijden MS, Bergman AM, van Oort IM, de Wit R,
Voest EE, et al: The genomic landscape of metastatic
castration-resistant prostate cancers reveals multiple distinct
genotypes with potential clinical impact. Nat Commun. 10:52512019.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Aggarwal M, Saxena R, Asif N, Sinclair E,
Tan J, Cruz I, Berry D, Kallakury B, Pham Q, Wang TTY and Chung FL:
p53 mutant-type in human prostate cancer cells determines the
sensitivity to phenethyl isothiocyanate induced growth inhibition.
J Exp Clin Cancer Res. 38:3072019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wanjala J, Taylor BS, Chapinski C,
Hieronymus H, Wongvipat J, Chen Y, Nanjangud GJ, Schultz N, Xie Y,
Liu S, et al: Identifying actionable targets through integrative
analyses of GEM model and human prostate cancer genomic profiling.
Mol Cancer Ther. 14:278–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Haffner MC, Mosbruger T, Esopi DM, Fedor
H, Heaphy CM, Walker DA, Adejola N, Gürel M, Hicks J, Meeker AK, et
al: Tracking the clonal origin of lethal prostate cancer. J Clin
Invest. 123:4918–4922. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ding D, Blee AM, Zhang J, Pan Y, Becker
NA, Maher LJ III, Jimenez R, Wang L and Huang H: Gain-of-function
mutant p53 together with ERG proto-oncogene drive prostate cancer
by beta-catenin activation and pyrimidine synthesis. Nat Commun.
14:46712023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jiang SJ and Wang S: Dual targeting of
mTORC1 and mTORC2 by INK-128 potently inhibits human prostate
cancer cell growth in vitro and in vivo. Tumour Biol. 36:8177–8184.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shorning BY, Dass MS, Smalley MJ and
Pearson HB: The PI3K-AKT-mTOR pathway and prostate cancer: At the
crossroads of AR, MAPK, and WNT signaling. Int J Mol Sci.
21:45072020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Shi J, Liu C, Chen C, Guo K, Tang Z, Luo
Y, Chen L, Su Y and Xu K: Circular RNA circMBOAT2 promotes prostate
cancer progression via a miR-1271-5p/mTOR axis. Aging (Albany NY).
12:13255–13280. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li Y, Fan A, Zhang Y, Guo Z, Meng W, Pan
W, Ma Z and Chen W: Cellular senescence: A potential mode of
circular RNAs regulating prostate cancer. MedComm-Oncol. 2:e612023.
View Article : Google Scholar
|
|
79
|
Ellis L, Lehet K, Ramakrishnan S, Adelaiye
R, Miles KM, Wang D, Liu S, Atadja P, Carducci MA and Pili R:
Concurrent HDAC and mTORC1 inhibition attenuate androgen receptor
and hypoxia signaling associated with alterations in microRNA
expression. PLoS One. 6:e271782011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Park H, Williams K, Trikalinos NA, Larson
S, Tan B, Waqar S, Suresh R, Morgensztern D, Van Tine BA, Govindan
R, et al: A phase I trial of temsirolimus and erlotinib in patients
with refractory solid tumors. Cancer Chemother Pharmacol.
87:337–347. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bendell JC, Kurkjian C, Infante JR, Bauer
TM, Burris HA III, Greco FA, Shih KC, Thompson DS, Lane CM, Finney
LH and Jones SF: A phase 1 study of the sachet formulation of the
oral dual PI3K/mTOR inhibitor BEZ235 given twice daily (BID) in
patients with advanced solid tumors. Invest New Drugs. 33:463–471.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li S, Sheng J, Liu Z, Fan Y, Zhang C, Lv
T, Hu S, Jin J, Yu W and Song Y: Potent antitumour of the mTORC1/2
dual inhibitor AZD2014 in docetaxel-sensitive and
docetaxel-resistant castration-resistant prostate cancer cells. J
Cell Mol Med. 25:2436–2449. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Jin Y, Qu S, Tesikova M, Wang L, Kristian
A, Mælandsmo GM, Kong H, Zhang T, Jerónimo C, Teixeira MR, et al:
Molecular circuit involving KLK4 integrates androgen and mTOR
signaling in prostate cancer. Proc Natl Acad Sci USA.
110:E2572–E2581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Pan HY and Valapala M: Regulation of
autophagy by the glycogen synthase Kinase-3 (GSK-3) signaling
pathway. Int J Mol Sci. 23:17092022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sun Y, Li Z and Song K: AR-mTOR-SRF axis
regulates HMMR expression in human prostate cancer cells. Biomol
Ther. 29:667–677. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Valenti MT, Mottes M, Dalle Carbonare L
and Feron O: Editorial: Bone metastases. Front Oncol.
11:7415152021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Tanaka K, Babic I, Nathanson D, Akhavan D,
Guo D, Gini B, Dang J, Zhu S, Yang H, De Jesus J, et al: Oncogenic
EGFR signaling activates an mTORC2-NF-κB pathway that promotes
chemotherapy resistance. Cancer Discov. 1:524–538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang Q, Tang Y, Yu H, Yin Q, Li M, Shi L,
Zhang W, Li D and Li L: CCL18 from tumor-cells promotes epithelial
ovarian cancer metastasis via mTOR signaling pathway. Mol Carcinog.
55:1688–1699. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wei XX, Hsieh AC, Kim W, Friedlander T,
Lin AM, Louttit M and Ryan CJ: A phase I study of abiraterone
acetate combined with BEZ235, a dual PI3K/mTOR inhibitor, in
metastatic castration resistant prostate cancer. Oncologist.
22:503–e43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Raynard C, Ma X, Huna A, Tessier N,
Massemin A, Zhu K, Flaman JM, Moulin F, Goehrig D, Medard JJ, et
al: NF-κB-dependent secretome of senescent cells can trigger
neuroendocrine transdifferentiation of breast cancer cells. Aging
Cell. 21:e136322022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Li N, Liu Q, Han Y, Pei S, Cheng B, Xu J,
Miao X, Pan Q, Wang H, Guo J, et al: ARID1A loss induces
polymorphonuclear myeloid-derived suppressor cell chemotaxis and
promotes prostate cancer progression. Nat Commun. 13:72812022.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Dushyanthen S, Cossigny DAF and Quan GMY:
The osteoblastic and osteoclastic interactions in spinal metastases
secondary to prostate cancer. Cancer Growth Metastasis. 6:61–80.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chen Q, Du X, Hu S and Huang Q:
NF-κB-related metabolic gene signature predicts the prognosis and
immunotherapy response in gastric cancer. Biomed Res Int.
2022:50925052022.PubMed/NCBI
|
|
94
|
Dewdney B, Jenkins MR, Best SA, Freytag S,
Prasad K, Holst J, Endersby R and Johns TG: From signalling
pathways to targeted therapies: Unravelling glioblastoma's secrets
and harnessing two decades of progress. Signal Transduct Target
Ther. 8:4002023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ayala G, Yan J, Li R, Ding Y, Thompson TC,
Mims MP, Hayes TG, MacDonnell V, Lynch RG, Frolov A, et al:
Bortezomib-mediated inhibition of steroid receptor coactivator-3
degradation leads to activated Akt. Clin Cancer Res. 14:7511–7518.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Nunes JJ, Pandey SK, Yadav A, Goel S and
Ateeq B: Targeting NF-kappa B signaling by artesunate restores
sensitivity of castrate-resistant prostate cancer cells to
antiandrogens. Neoplasia. 19:333–345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chen H, Pang B, Zhou C, Han M, Gong J, Li
Y and Jiang J: Prostate cancer-derived small extracellular vesicle
proteins: The hope in diagnosis, prognosis, and therapeutics. J
Nanobiotechnology. 21:4802023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Rickard BP, Overchuk M, Chappell VA, Kemal
Ruhi M, Sinawang PD, Nguyen Hoang TT, Akin D, Demirci U, Franco W,
Fenton SE, et al: Methods to evaluate changes in mitochondrial
structure and function in cancer. Cancers (Basel). 15:25642023.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Gong L, Chen B, Zhang J, Sun Y, Yuan J,
Niu X, Hu G, Chen Y, Xie Z, Deng Z, et al: Human ESC-sEVs alleviate
age-related bone loss by rejuvenating senescent bone marrow-derived
mesenchymal stem cells. J Extracell Vesicles. 9:18009712020.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Martinez-Vidal L, Murdica V, Venegoni C,
Pederzoli F, Bandini M, Necchi A, Salonia A and Alfano M: Causal
contributors to tissue stiffness and clinical relevance in urology.
Commun Biol. 4:10112021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ma Q, Liang M, Wu Y, Dou C, Xu J, Dong S
and Luo F: Small extracellular vesicles deliver osteolytic
effectors and mediate cancer-induced osteolysis in bone metastatic
niche. J Extracell Vesicles. 10:e120682021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Mongelli A, Atlante S, Barbi V, Bachetti
T, Martelli F, Farsetti A and Gaetano C: Treating senescence like
cancer: Novel perspectives in senotherapy of chronic diseases. Int
J Mol Sci. 21:79842020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Gazzillo A, Volponi C, Soldani C, Polidoro
MA, Franceschini B, Lleo A, Bonavita E and Donadon M: Cellular
senescence in liver cancer: How dying cells become ‘Zombie’
enemies. Biomedicines. 12:262023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Du D, Tang X, Li Y, Gao Y, Chen R, Chen Q,
Wen J, Wu T, Zhang Y, Lu H, et al: Senotherapy protects against
Cisplatin-induced ovarian injury by removing senescent cells and
alleviating DNA damage. Oxid Med Cell Longev. 2022:91446442022.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Gasek NS, Kuchel GA, Kirkland JL and Xu M:
Strategies for targeting senescent cells in human disease. Nat
Aging. 1:870–879. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Liu Y, Zhang Q, Ni W, Ji G and Xu H: A
strategy for the treatment of gastrointestinal cancer: Targeting
tumor senescent cells. Front Mol Biosci. 10:11398402023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ramírez R, Ceprian N, Figuer A, Valera G,
Bodega G, Alique M and Carracedo J: Endothelial senescence and the
chronic vascular diseases: Challenges and therapeutic opportunities
in atherosclerosis. J Pers Med. 12:2152022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Fedorov VD, Themeli M and Sadelain M:
PD-1- and CTLA-4-based inhibitory chimeric antigen receptors
(iCARs) divert off-target immunotherapy responses. Sci Transl Med.
5:215ra1722013. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Arai S, Varkaris A, Nouri M, Chen S, Xie L
and Balk SP: MARCH5 mediates NOXA-dependent MCL1 degradation driven
by kinase inhibitors and integrated stress response activation.
eLife. 9:e549542020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Arai S, Jonas O, Whitman MA, Corey E, Balk
SP and Chen S: Tyrosine kinase inhibitors increase MCL1 degradation
and in combination with BCLXL/BCL2 inhibitors drive prostate cancer
apoptosis. Clin Cancer Res. 24:5458–5470. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ferraldeschi R, Welti J, Powers MV, Yuan
W, Smyth T, Seed G, Riisnaes R, Hedayat S, Wang H, Crespo M, et al:
Second-generation HSP90 inhibitor Onalespib blocks mRNA splicing of
androgen receptor variant 7 in prostate cancer cells. Cancer Res.
76:2731–2742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Slovin S, Hussain S, Saad F, Garcia J,
Picus J, Ferraldeschi R, Crespo M, Flohr P, Riisnaes R, Lin C, et
al: Pharmacodynamic and clinical results from a phase I/II study of
the HSP90 Inhibitor Onalespib in combination with abiraterone
acetate in prostate cancer. Clin Cancer Res. 25:4624–4633. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lu X, Yang F, Chen D, Zhao Q, Chen D, Ping
H and Xing N: Quercetin reverses docetaxel resistance in prostate
cancer via androgen receptor and PI3K/Akt signaling pathways. Int J
Biol Sci. 16:1121–1134. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Ward AB, Mir H, Kapur N, Gales DN,
Carriere PP and Singh S: Quercetin inhibits prostate cancer by
attenuating cell survival and inhibiting anti-apoptotic pathways.
World J Surg Oncol. 16:1082018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Pratheeshkumar P, Budhraja A, Son YO, Wang
X, Zhang Z, Ding S, Wang L, Hitron A, Lee JC, Xu M, et al:
Quercetin inhibits angiogenesis mediated human prostate tumor
growth by targeting VEGFR-2 regulated AKT/mTOR/P70S6K signaling
pathways. PLoS One. 7:e475162012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang DF, Yang ZC, Chen JQ, Jin XX, Qiu
YD, Chen XJ, Shi HY, Liu ZG, Wang MS, Liang G and Zheng XH:
Piperlongumine inhibits migration and proliferation of
castration-resistant prostate cancer cells via triggering
persistent DNA damage. BMC Complement Med Ther. 21:1952021.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Makhov P, Golovine K, Teper E, Kutikov A,
Mehrazin R, Corcoran A, Tulin A, Uzzo RG and Kolenko VM:
Piperlongumine promotes autophagy via inhibition of Akt/mTOR
signalling and mediates cancer cell death. Br J Cancer.
110:899–907. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Golovine KV, Makhov PB, Teper E, Kutikov
A, Canter D, Uzzo RG and Kolenko VM: Piperlongumine induces rapid
depletion of the androgen receptor in human prostate cancer cells.
Prostate. 73:23–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Liu G, Jin Z and Lu X: Differential
targeting of Gr-MDSCs, T cells and prostate cancer cells by
dactolisib and dasatinib. Int J Mol Sci. 21:23372020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Araujo JC, Poblenz A, Corn P, Parikh NU,
Starbuck MW, Thompson JT, Lee F, Logothetis CJ and Darnay BG:
Dasatinib inhibits both osteoclast activation and prostate cancer
PC-3-cell-induced osteoclast formation. Cancer Biol Ther.
8:2153–2159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Cuyàs E, Verdura S, Llorach-Pares L,
Fernández-Arroyo S, Luciano-Mateo F, Cabré N, Stursa J, Werner L,
Martin-Castillo B, Viollet B, et al: Metformin directly targets the
H3K27me3 demethylase KDM6A/UTX. Aging Cell. 17:e127722018.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Hua Y, Zheng Y, Yao Y, Jia R, Ge S and
Zhuang A: Metformin and cancer hallmarks: Shedding new lights on
therapeutic repurposing. J Transl Med. 21:4032023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wang ZS, Huang HR, Zhang LY, Kim S, He Y,
Li DL, Farischon C, Zhang K, Zheng X, Du ZY and Goodin S:
Mechanistic study of inhibitory effects of metformin and
atorvastatin in combination on prostate cancer cells in vitro and
in vivo. Biol Pharm Bull. 40:1247–1254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Wang J, Lu Y, Wang J, Koch AE, Zhang J and
Taichman RS: Retraction: CXCR6 Induces prostate cancer progression
by the AKT/mammalian target of rapamycin signaling pathway. Cancer
Res. 82:3406. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhang J, Wu D, He Y, Li L, Liu S, Lu J,
Gui H, Wang Y, Tao Y, Wang H, et al: Rapamycin inhibits AR
signaling pathway in prostate cancer by interacting with the FK1
domain of FKBP51. Biochem Biophys Rep. 23:1007782020.PubMed/NCBI
|
|
126
|
Shorning BY, Dass MS, Smalley MJ and
Pearson HB: The PI3K-AKT-mTOR pathway and prostate cancer: At the
crossroads of AR, MAPK, and WNT signaling. Int J Mol Sci.
21:45072020. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Lo U, Chen Y, Cen J, Deng S, Luo J, Zhau
H, Ho L, Lai CH, Mu P, Chung LWK and Hsieh JT: The driver role of
JAK-STAT signalling in cancer stemness capabilities leading to new
therapeutic strategies for therapy- and castration-resistant
prostate cancer. Clin Transl Med. 12:e9782022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Sheth S, Jajoo S, Kaur T, Mukherjea D,
Sheehan K, Rybak LP and Ramkumar V: Resveratrol reduces prostate
cancer growth and metastasis by inhibiting the Akt/MicroRNA-21
pathway. PLoS One. 7:e516552012. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Fenner A: Prostate cancer: Resveratrol
inhibits the AR. Nat Rev Urol. 14:642. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Hickson LJ, Langhi Prata LGP, Bobart SA,
Evans TK, Giorgadze N, Hashmi SK, Herrmann SM, Jensen MD, Jia Q,
Jordan KL, et al: Senolytics decrease senescent cells in humans:
Preliminary report from a clinical trial of Dasatinib plus
Quercetin in individuals with diabetic kidney disease.
EBioMedicine. 47:446–456. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Di Micco R, Krizhanovsky V, Baker D and
d'Adda di Fagagna F: Cellular senescence in ageing: From mechanisms
to therapeutic opportunities. Nat Rev Mol Cell Biol. 22:75–95.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Kaur G, Sundar IK and Rahman I: p16-3MR: A
novel model to study cellular senescence in cigarette smoke-induced
lung injuries. Int J Mol Sci. 22:48342021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Baker DJ, Wijshake T, Tchkonia T,
LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL and van
Deursen JM: Clearance of p16Ink4a-positive senescent cells delays
ageing-associated disorders. Nature. 479:232–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Song P, Duan JL, Ding J, Liu JJ, Fang ZQ,
Xu H, Li ZW, Du W, Xu M, Ling YW, et al: Cellular senescence primes
liver fibrosis regression through Notch-EZH2. MedComm (2020).
4:e3462023.PubMed/NCBI
|
|
135
|
Chen M, Wu G, Lu Y, Sun S, Yu Z, Pan X,
Chen W, Xu H, Qiu H, He W, et al: A p21-ATD mouse model for
monitoring and eliminating senescent cells and its application in
liver regeneration post injury. Mol Ther. Apr 6–2024.(Epub ahead of
print). View Article : Google Scholar
|
|
136
|
Zhan D, Ma D, Wei S, Lal B, Fu Y, Eberhart
C, Laterra J, Ying M, Li Y, Meeker A, et al: Monoallelic IDH1 R132H
mutation mediates glioma cell response to anticancer therapies via
induction of senescence. Mol Cancer Res. 19:1878–1888. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Chen WC, Chang TC, Chou HH, Cheng MH, Hong
JJ, Hsieh YS and Cheng CM: Peritoneal fluid analysis of advanced
ovarian cancers after hyperthermic intraperitoneal chemotherapy.
Int J Mol Sci. 24:97482023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
D'Aguanno S and Del Bufalo D: Inhibition
of Anti-apoptotic Bcl-2 proteins in preclinical and clinical
studies: Current overview in cancer. Cells. 9:12872020. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Harrison CN, Garcia JS, Somervaille TCP,
Foran JM, Verstovsek S, Jamieson C, Mesa R, Ritchie EK, Tantravahi
SK, Vachhani P, et al: Addition of Navitoclax to ongoing
Ruxolitinib therapy for patients with myelofibrosis with
progression or suboptimal response: Phase II safety and efficacy. J
Clin Oncol. 40:1671–1680. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
He Y, Zhang X, Chang J, Kim HN, Zhang P,
Wang Y, Khan S, Liu X, Zhang X, Lv D, et al: Using
proteolysis-targeting chimera technology to reduce navitoclax
platelet toxicity and improve its senolytic activity. Nat Commun.
11:19962020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Ferraldeschi R, Welti J, Powers MV, Yuan
W, Smyth T, Seed G, Riisnaes R, Hedayat S, Wang H, Crespo M, et al:
Second-generation HSP90 inhibitor Onalespib blocks mRNA splicing of
androgen receptor variant 7 in prostate cancer cells. Cancer Res.
76:2731–2742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Colucci M, Zumerle S, Bressan S, Gianfanti
F, Troiani M, Valdata A, D'Ambrosio M, Pasquini E, Varesi A, Cogo
F, et al: Retinoic acid receptor activation reprograms senescence
response and enhances anti-tumor activity of natural killer cells.
Cancer Cell. 42:646–661.e9. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Chi KN, Gleave ME, Klasa R, Murray N,
Bryce C, Lopes de Menezes DE, D'Aloisio S and Tolcher AW: A phase I
dose-finding study of combined treatment with an antisense Bcl-2
oligonucleotide (Genasense) and mitoxantrone in patients with
metastatic hormone-refractory prostate cancer. Clin Cancer Res.
7:3920–3927. 2001.PubMed/NCBI
|
|
144
|
Tolcher AW, Kuhn J, Schwartz G, Patnaik A,
Hammond LA, Thompson I, Fingert H, Bushnell D, Malik S, Kreisberg
J, et al: A Phase I pharmacokinetic and biological correlative
study of oblimersen sodium (genasense, g3139), an antisense
oligonucleotide to the bcl-2 mRNA, and of docetaxel in patients
with hormone-refractory prostate cancer. Clin Cancer Res.
10:5048–5057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Stein MN, Goodin S, Gounder M, Gibbon D,
Moss R, Portal D, Lindquist D, Zhao Y, Takebe N, Tan A, et al: A
phase I study of AT-101, a BH3 mimetic, in combination with
paclitaxel and carboplatin in solid tumors. Invest New Drugs.
38:855–865. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Osada T, Crosby EJ, Kaneko K, Snyder JC,
Ginzel JD, Acharya CR, Yang XY, Polascik TJ, Spasojevic I, Nelson
RC, et al: HSP90-specific nIR Probe identifies aggressive prostate
cancers: Translation from preclinical models to a human phase I
study. Mol Cancer Ther. 21:217–226. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Suh GA, Lodise TP, Tamma PD, Knisely JM,
Alexander J, Aslam S, Barton KD, Bizzell E, Totten KMC, Campbell
JL, et al: Considerations for the use of phage therapy in clinical
practice. Antimicrob Agents Chemother. 66:e02071212022. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Kolodkin-Gal D, Roitman L, Ovadya Y,
Azazmeh N, Assouline B, Schlesinger Y, Kalifa R, Horwitz S,
Khalatnik Y, Hochner-Ger A, et al: Senolytic elimination of
Cox2-expressing senescent cells inhibits the growth of premalignant
pancreatic lesions. Gut. 71:345–355. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Spetsieris N, Boukovala M, Weldon JA,
Tsikkinis A, Hoang A, Aparicio A, Tu SM, Araujo JC, Zurita AJ, Corn
PG, et al: A Phase 2 trial of abiraterone followed by randomization
to addition of dasatinib or sunitinib in men with metastatic
castration-resistant prostate cancer. Clin Genitourin Cancer.
19:22–31.e5. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Rossi M, Anerillas C, Idda ML, Munk R,
Shin CH, Donega S, Tsitsipatis D, Herman AB, Martindale JL, Yang X,
et al: Pleiotropic effects of BAFF on the senescence-associated
secretome and growth arrest. Elife. 12:e842382023. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Chaib S, Tchkonia T and Kirkland JL:
Cellular senescence and senolytics: The path to the clinic. Nat
Med. 28:1556–1568. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Kirkland JL, Tchkonia T, Zhu Y,
Niedernhofer LJ and Robbins PD: The clinical potential of senolytic
drugs. J Am Geriatr Soc. 65:2297–2301. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Ji S, Xiong M, Chen H, Liu Y, Zhou L, Hong
Y, Wang M, Wang C, Fu X and Sun X: Cellular rejuvenation: Molecular
mechanisms and potential therapeutic interventions for diseases.
Signal Transduct Target Ther. 8:1162023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Kreienkamp R, Graziano S, Coll-Bonfill N,
Bedia-Diaz G, Cybulla E, Vindigni A, Dorsett D, Kubben N, Batista
LFZ and Gonzalo S: A cell-intrinsic interferon-like response links
replication stress to cellular aging caused by progerin. Cell Rep.
22:2006–2015. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Krtolica A, Parrinello S, Lockett S,
Desprez PY and Campisi J: Senescent fibroblasts promote epithelial
cell growth and tumorigenesis: A link between cancer and aging.
Proc Natl Acad Sci USA. 98:12072–12077. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Wang L, Jin H, Jochems F, Wang S, Lieftink
C, Martinez IM, De Conti G, Edwards F, de Oliveira RL, Schepers A,
et al: cFLIP suppression and DR5 activation sensitize senescent
cancer cells to senolysis. Nat Cancer. 3:1284–1299. 2022.
View Article : Google Scholar : PubMed/NCBI
|