Introduction
Cinnamon is widely used because of its culinary
uses. The medicinal value of cinnamon has attracted the attention
of more and more researchers (1).
Cinnamaldehyde (CA) is a main ingredient extracted from the bark of
the cinnamon tree (2), with a
broad range of pharmacological effects, including anti-inflammatory
(3), antioxidant (4), antiviral (5), anti-bacterial (6), antithrombic (7), hypoglycemic (8), hepatoprotective (9), anti-diabetic (10), neuroprotective (11) and anticancer effects (12), which largely contribute to the
prevention and treatment of various diseases such as inflammatory
diseases, neurodegenerative diseases, cardiovascular disease,
diabetes mellitus and cancer. Advancements in cancer research have
highlighted the promising potential of CA in restricting the growth
of cancer cells (3–12). As demonstrated in a previous study,
CA has shown a marked ability to impede cancer cell proliferation
(13), prompting a surge in
scientific interest in exploring its potential role in cancer
therapy. Furthermore, to address issues such as the poor targeting
and high toxicity of anticancer drugs, targeted formulations based
on CA are also under constant research. These can enhance the
effectiveness of anticancer drugs and ensure patient safety.
Therefore, researchers utilize techniques such as structural
modification and nano-carriers to optimize the performance of CA,
aiming to improve its efficacy and safety in targeted cancer
therapy (14–18). This progress lays the foundation
for further investigation into the effects of CA in cancer
prevention and therapy to identify potential effective and targeted
treatment options in the future.
Therefore, the relevant literature in the PubMed
(https://pubmed.ncbi.nlm.nih.gov/), Web
of Science (https://www.webofscience.com/), Science Direct
(https://www.sciencedirect.com/) and
China National Knowledge Infrastructure (https://www.cnki.net/) databases was searched using
the main keywords ‘cinnamon’, ‘CA’, ‘antitumor’, ‘pharmacological
activity’, ‘pharmacokinetics’ and ‘toxicity’, and their
combinations. The present study systematically reviews the
pharmacokinetics, antitumor activity and toxicity of CA, which
provides a theoretical basis and direction for further research and
clinical expansion.
Physicochemical and pharmacokinetic
characteristics of CA
Physical and chemical properties of
CA
The physicochemical characteristics of CA have been
extensively studied (19–22). Peters and Caldwell (19) demonstrated that CA naturally exists
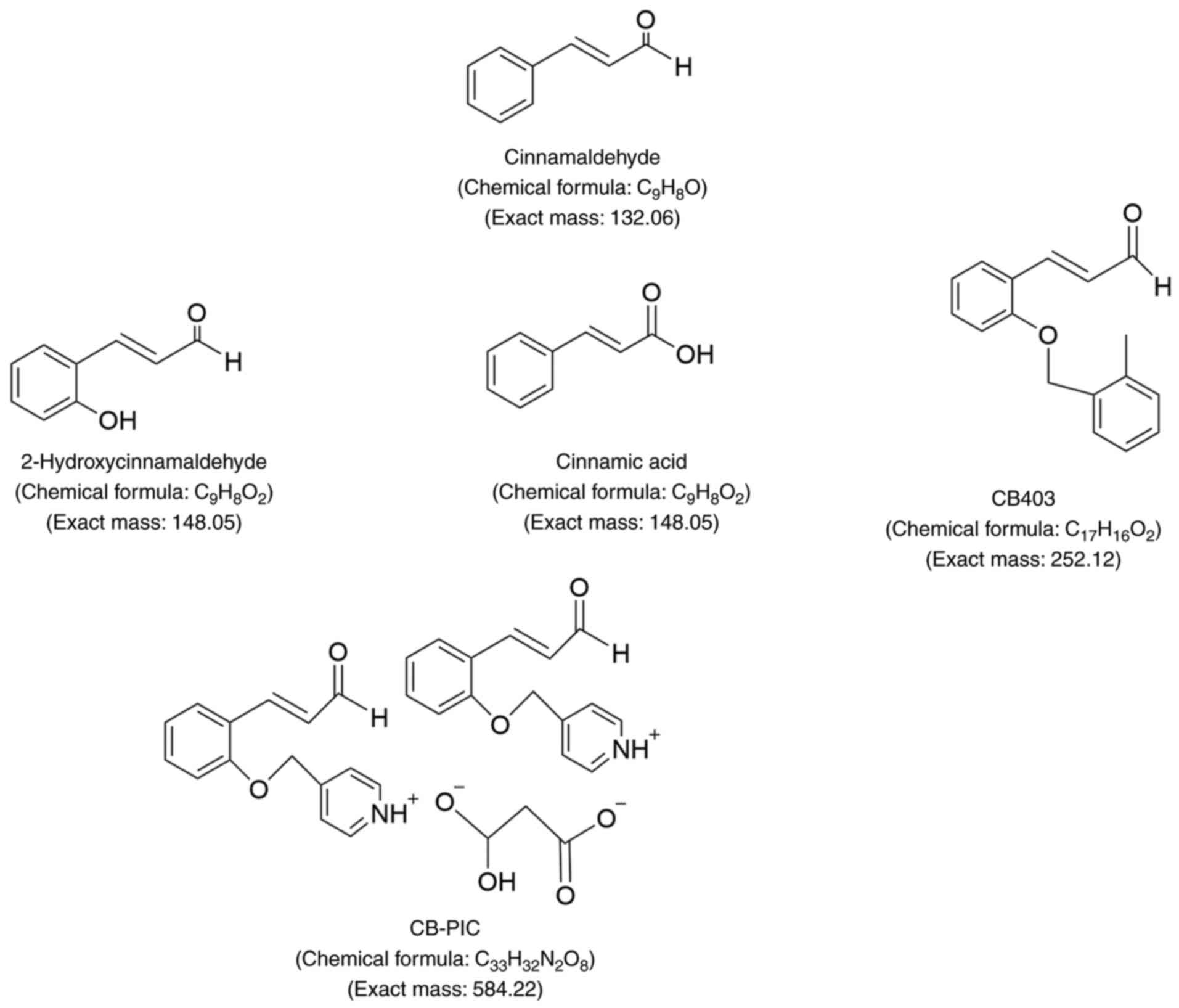
in the form of trans-CA. CA (C9H8O; Fig. 1) is also known as cinnamon
aldehyde, 3-phenyl-2-propenalin and trans-CA (20). CA is a yellow oily liquid with low
solubility in water, and soluble in ethanol and chloroform
(21). Due to its aldehyde
structure, when CA comes into contact with air and light, it
gradually oxidizes into cinnamic acid (21). Zinn et al (22) demonstrated that there may be four
possible stereoisomers of CA.
Research on the pharmacokinetics of
CA
To comprehend the mechanism of action of the drug
and provide guidance for clinical practice, it is crucial to
investigate pharmacokinetic parameters. Furthermore, ensuring the
safety and effectiveness of the drug in clinical settings is
imperative.
Bickers et al (23) revealed that CA is an active
aldehyde that can be converted to cinnamyl alcohol. As a result, CA
is unstable in the body and has the potential to be metabolized to
cinnamic acid and converted to cinnamyl alcohol (23). In addition, Vasconcelos et
al (24) demonstrated that,
in vivo, it is possible that trans-CA decomposes to cinnamic
acid by enzyme catalysis before it can elicit its antibacterial
activity, and thus, could be considered unstable in blood. In a
study by Zhao et al (25),
the pharmacokinetics of CA in rats were assessed using a highly
sensitive gas chromatography-mass spectrometry technique. The rats
in the experiment received CA orally at a dose of 500 mg/kg and
intravenously at a dose of 20 mg/kg. The results indicated that the
bioavailability of intravenous administration of CA was superior to
that of oral administration (25).
In another study, the researchers utilized gas chromatography-mass
spectrometry to measure the concentration of CA and its metabolite
cinnamyl alcohol in rat tissues at the same time and investigated
their distribution patterns. According to the study findings, the
spleen exhibited the highest concentrations of both CA and cinnamyl
alcohol among the major organs of rats, including the heart, liver,
spleen, lungs, kidneys and brain. Additionally, there was no
detectable long-term buildup of CA in the rat tissues (26).
To improve the stability and bioavailability of CA,
researchers have designed a series of new dosage forms (27–32).
For example, Zhao et al (27) developed a novel intravenous
submicron CA (SME-CA) emulsion that not only successfully improved
the solubility and absorption of CA, but also had lower toxicity
and higher antitumor effects. Furthermore, SME-CA improved the
tissue distribution in the kidney, liver, spleen and brain, and a
27% higher concentration was found in the brain compared with CA
(27).
The advantages of convenience and good adherence
make oral administration the preferred route for drug delivery
(28). Researchers have mainly
considered oral administration when studying CA dosage forms. For
example, Wu et al (29)
made CA into CA solid lipid nanoparticles, which increased the oral
bioavailability of CA by >1.69 times. Furthermore, CA-solid
lipid nanoparticles had a higher absorption rate under intestinal
pH conditions compared with CA (29). Liu et al (30) developed a self-emulsifying drug
delivery system (SEDDS) containing CA to overcome the shortcomings
of poor solubility and limited absorption of CA. Compared with the
free CA group, the CA-SEDDS group exhibited higher accumulation of
CA and cinnamic acid in various tissues, especially in the kidney
(30). In addition, Cai et
al (31) investigated the
ability of SEDDS to deliver lipophilic aldehyde CA-SEDDS in rat
mucus, mucin solution, and Caco-2 and Caco-2/HT29 co-culture
monolayers. The results of the study showed that CA-SEDDS exhibited
excellent mucus permeability in mucus and mucin solutions, which
was 5.1- and 2.8-fold higher, respectively, than that in the free
CA group. CA-SEDDS penetration increased by 2.5-fold compared with
that of free CA when using the mucus-secreting co-culture cell
model as a barrier. The relative oral bioavailability of CA-SEDDS
was 242% compared with CA (31).
Furthermore, Dong et al (32) examined the oral bioavailability of
CA from the perspective of a microemulsion-mucus system. CA
microemulsion (CA-ME) was prepared, and the results demonstrated
that CA-ME had the highest absorption in the ileum compared with CA
solution. Pharmacokinetic experiments indicated that the relative
bioavailability of CA-ME was 2.5 times higher than that of CA
solution (32).
Overall, these studies (29–32)
have demonstrated the potential of various drug delivery systems,
such as solid lipid nanoparticles, SEDDSs and microemulsions, to
enhance the oral bioavailability and absorption of cinnamic
acid.
Antitumor effects of CA in different types
of cancer
According to the latest Global Cancer Statistics
report released in 2022, there were nearly 20 million new cancer
cases globally, with 9.7 million associated deaths in this year
(33). According to forecasts,
cancer is expected to surpass cardiovascular disease as the leading
cause of premature death in most countries (34). In 2022, the five main types of
cancer diagnosed in China were lung, colorectal, stomach, liver and
breast cancer (35).
Application of CA in lung cancer
In 2022 globally, there were nearly 2.5 million new
cases and over 1.8 million deaths from lung cancer (33). By 2022, lung cancer had become a
leading cause of both incidence and mortality (33). The global burden of lung cancer is
increasing. By 2035, China will become the country with the highest
number of new cases (36).
Therefore, in addition to controlling the incidence factors of lung
cancer, finding novel chemotherapy drugs is also the key to solving
the problem.
Using a
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induced rasH2 mouse
lung cancer model, it was demonstrated that CA reduced the combined
incidence of lung adenocarcinoma and cancer. Specifically, in male
rasH2 mice, the incidence decreased from 86 to 31%. The underlying
mechanism may be to reduce the proliferation of tumor-initiating
cells (37).
A previous study (38) provided evidence that suggested a
combination of berberine and CA could reduce the susceptibility of
mice to ammonia-induced lung cancer. The combined treatment
activated AMP-activated protein kinase (AMPK), and inhibited the
proliferation and growth of tumor cells in mice with
methane-induced lung cancer. Additionally, the combined treatment
effectively targeted the mTOR signaling pathway, which is a
critical signaling pathway for cell proliferation and survival,
thereby blocking tumor cell proliferation and survival (38). Furthermore, it has been observed
that the combination of berberine and CA induced apoptosis of A549
cells, and inhibited cell proliferation, autophagy and wound
healing, while upregulating AMPK and downregulating aquaporin 1
in vitro (38). A549 and
NCI-H460 lung cancer cell lines were found to respond well to CA
treatment. Additionally, CA treatment led to the induction of
apoptosis in these cells, with the degree of induction being
dependent on the concentration of CA used. Notably, the researchers
observed a substantial increase in the expression levels of
circular RNA hsa_circ_0043256 following CA treatment (39). This upregulation was found to serve
a crucial role in triggering apoptosis in the cells (39). Furthermore, CA has the potential to
disrupt abnormal cell growth, promote apoptosis and effectively
hinder the advancement of lung cancer cells by interfering with the
Wnt/β-catenin signaling pathway (40). Another study explored the effects
of combining CA with hyperthermia on non-small cell lung cancer
cells, specifically A549 cells. The research results indicate that
the combination therapy of CA and hyperthermia could inhibit the
growth and proliferation of A549 cells, and induce cell apoptosis
by regulating the activity of reactive oxygen species (ROS) and the
mitogen-activated protein kinase family. Especially when combined
with hyperthermia therapy at 42°C and 43°C, CA could inhibit cell
proliferation (41). Furthermore,
CA induces apoptosis in non-small cell lung cancer cells by
regulating Janus kinase/STAT, the NF-κB signaling pathway and RNA
degradation (42).
Overall, these findings (37–42)
suggest that CA possesses chemo-preventive properties and may have
potential therapeutic benefits in lung cancer treatment. However,
these studies were conducted in vitro or on animal models,
and further clinical trials are required to validate the
effectiveness and safety of these treatments in humans.
Application of CA in colorectal cancer
(CRC)
In 2022, there were over 1.9 million new cases of
colorectal cancer (including anal cancer) and 904,000 associated
deaths globally (33). Surgery for
patients with CRC is considered to be the most effective approach,
but postoperative complications can affect the quality of life to a
certain extent (43). In addition,
Sargent et al (44)
demonstrated that patients with colon cancer still have relatively
low 5-year survival rates in chemotherapy, with high recurrence
rates. The 1–5 year recurrence rates are 12, 14, 8, 5 and 3%,
respectively. The median time from recurrence to death is 12 months
(44). Therefore, in addition to
controlling the factors of direct bowel cancer incidence, research
and development of novel chemotherapy drugs is also the key to
solving this problem.
CB403 (Fig. 1) is a
cinnamaldehyde derivative that inhibits the activity of
cyclin-dependent kinases (CDKs), particularly CDK1, CDK2 and CDK4,
thereby halting cell cycle progression. Simultaneously, CB403 also
suppresses the expression of cyclin D1, exerting antitumor effects
(45). In addition, Lee et
al (46) demonstrated that
2-hydroxycinnamaldehyde (HCA; Fig.
1) inhibits the growth of SW620 colon cancer cells by reducing
the expression of c-Jun and c-Fos, inhibiting the DNA binding
activity of activator protein 1, and inducing cell apoptosis
(46). The CA derivative CB-PIC
(Fig. 1) has marked cytotoxicity
and induces apoptosis in SW620 human colon cancer cells by
activating the AMPKα and ERK signaling pathways (47). Furthermore, CB-PIC is able to
overcome drug resistance in chemotherapy cancer cells by inhibiting
multidrug resistance protein 1 and its upstream STAT3 and AKT
signaling pathways (48). At the
same time, combining CA with chemotherapy drugs has shown promise
in enhancing the sensitivity of cancer cells to these drugs. For
instance, when CA is combined with 5-fluorouracil (5-FU), CA
increases the sensitivity of CRC cells to 5-FU by reducing the
expression of thymidylate synthase, ERCC1, DNA topoisomerase 1 and
BRCA1, increasing the percentage of apoptotic cells to 92.7%
(49). This finding suggests that
utilizing CA as an adjunct therapy with 5-FU may lead to improved
treatment outcomes for patients with CRC.
Research has indicated that CA exerts its antitumor
effects by activating nuclear factor erythroid 2-related factor
(Nrf2) (50). A study has found
that inhibition of the PI3K/AKT signaling pathway can inhibit tumor
cell proliferation and promote apoptosis (51). For example, researchers have found
that CA can induce apoptosis in colon cancer cells by inhibiting
the PI3K/Akt signaling pathway. Additionally, CA upregulates the
expression of E-cadherin while downregulating the expression of
matrix metalloproteinase-2 (MMP2) and MMP9 (52). Furthermore, Zhang et al
(53) demonstrated that CA induced
cell apoptosis by inhibiting the PI3K/Akt signaling pathway. In
addition, the study revealed a decrease in Ki-67 expression in the
CA group, along with the accumulation of numerous apoptotic cells
(53). Nguyen and Kim (54) reported that HCA, a derivative of
CA, induced apoptosis in colon cancer cells via heat shock
transcription factor 1-mediated BAG cochaperone 3 expression. An
inhibitory effect of CA on the hypoxia-activated Wnt/β-catenin
pathway has been observed, leading to an augmented sensitivity of
CRC cells to oxaliplatin, and enhancing the apoptosis of cancer
cells (55). The presence of
Escherichia coli has been linked to the advancement of colon
cancer. A study conducted by Kosari et al (56) revealed that CA exhibited regulatory
effects on the expression of the clbB gene, thereby mitigating the
biofilm-forming capability of E. coli. CA (75 µM) treatment
could induce apoptosis, necrosis and cell cycle slowing in Caco-2
and SW-620 cells after 72 h of treatment (57). Nile et al (13) revealed that, after
cinnamaldehyde-rich cinnamon extract treatment, the number of
HCT116 and HT-29 cells in the G1 phase was decreased,
the number of cells in the sub-G1 phase was increased,
and the number of cells in the G2 phase was stagnant
compared with the number of untreated cells. In addition, CA was
also able to induce apoptosis in cancer cells by increasing
intracellular ROS levels (13).
These findings suggest the potential of CA and its
derivatives to inhibit colon cancer growth and promote apoptosis of
colon cancer cells.
Application of CA in breast
cancer
In 2022, there were ~2.3 million new cases of breast
cancer in women globally, with 666,000 associated deaths (33).
Jeong et al (45) demonstrated that CB403, a derivative
of CA, arrested breast cancer cells in mitosis by increasing the
expression levels of cyclin B1. In addition, CB403 did not affect
mouse body weight, while inhibiting tumor growth (45). A research team has synthesized
biocompatible CA functionalized magnetic nanoparticles (CPGF NPs),
which inhibit the proliferation of breast cancer cells by inducing
apoptosis. The IC50 of CPGF NPs was found to be 0.363
and 0.368 µM in MDA-MB-231 and MCF7 cells, respectively, while the
IC50 of free CA for MDA-MB-231 and MCF7 cells was
192.3-fold and 773.6-fold higher than that of CPGF NPs. This
indicated that the CPGF NPs formulation of CA was substantially
more effective in inhibiting the growth of breast cancer cells
compared with free CA alone (58).
In a study by Rad et al (59), it was demonstrated that cinnamon
extract induced apoptosis in MCF7 and MDA-MB-231 cell lines by
modulating antioxidant enzyme activity and activating the caspase
pathway. Compared with healthy individuals, patients with breast
cancer exhibit visibly elevated plasma visfatin concentrations, and
lower survival rates are observed in patients with increased
visfatin gene expression levels (60). However, the promotional effects of
visfatin on breast cancer can be curtailed by the inhibitory
actions of CA (60). By conducting
experiments on breast cancer cells, researchers have demonstrated
that CA stimulated the apoptosis of cancer cells by inhibiting
their proliferation, invasion and migration (61). Through in vitro experiments,
researchers revealed that cinnamon bark extract could inhibit the
proliferation of breast cancer cells and induce apoptosis (62). Researchers have designed a
reasonable co-loading drug formulation, using simple but practical
graphene oxide to encapsulate mesoporous silica nanoparticles,
modify hyaluronic acid (HA), and realize the co-delivery of CA and
doxorubicin (DOX) to enhance their combined therapeutic effect on
tumor cells and reduce their application defects (63). The combined use of CA and DOX
exhibited higher cytotoxicity against MCF7 human breast cancer
cells, which was related to CA-induced activation of the intrinsic
apoptotic pathway in MCF7 cells (63). Through cell cycle analysis, it was
found that the combined treatment of measles virus with baicalein
or CA can induce apoptosis in breast cancer cells, thereby further
enhancing therapeutic efficacy (64). Compared with monotherapy,
combination therapy has a stronger inhibitory effect on breast
cancer cells (63). Schuster et
al (65) revealed that CA in
combination with chlorogenic acid could disrupt the mitochondrial
integrity of breast cancer cells, thereby promoting breast cancer
cell death. At the same time, it did not affect the growth of
normal breast epithelial cells (65). In one study, docetaxel
(DTX)/arginine-glycine-aspartic nanoparticles were prepared by
nanoprecipitation/self-assembly using CA-Oxi-αCD material as a
carrier (66). Through the
endogenous ROS and acidic environmental stimulation of
nanoparticles, the acetal bond between CA and αCD in the
nanoparticles is broken to achieve the efficient release of the
drug DTX. The selective and complete release of the drug is
realized, and the accumulation and therapeutic effect of the drug
in the tumor site are improved (66).
Research indicates that CA holds promise for the
treatment of breast cancer. It has the potential to impede the
growth and survival of breast cancer cells, and induce apoptosis
through various mechanisms. Additional research and clinical trials
are needed to establish the exact role and effectiveness of CA in
breast cancer treatment and advance its development as a potential
therapeutic option.
Application of CA in liver cancer
In 2022, liver cancer claimed the lives of
>750,000 individuals worldwide, ranking it as the third highest
cause of cancer-related death (33). Natural compounds have fewer side
effects and lower toxicity than traditional chemotherapy drugs and
are expected to be a potential treatment option for liver cancer
(67).
CA promotes the apoptosis of cancer cells. CA
induces cell apoptosis by upregulating Bax expression, and
downregulating Bcl-2 and X-linked inhibitor of apoptosis (XIAP)
expression (68). However, when CA
is combined with vitamin E, the promoting effect of CA on the
release of apoptotic factors in the mitochondria of hepatocellular
carcinoma cells can be inhibited by vitamin E, thereby inhibiting
apoptosis (68). A study has
indicated that 2′-benzoyloxycinnamaldehyde and HCA, derivatives of
CA, inhibit the activity of farnesyl transferase, thereby delaying
the onset of liver cancer (69).
There is evidence to suggest that CA activated the ERK1/2, Akt and
JNK signaling pathways, which in turn led to Nrf2 nuclear
translocation, which ultimately increased the expression of phase
II enzymes, making them exert effective chemoprevention effects
(70). CA induces apoptosis in
HepG2 cells by downregulating the expression levels of Bcl-XL, and
upregulating the expression levels of CD95 (apolipoprotein A-I),
p53 and Bax proteins (71).
Researchers have identified that CA instigated apoptosis in human
hepatocellular carcinoma cells by triggering the mitochondrial
death pathway. Following CA treatment, there was a decrease in the
protein levels of anti-apoptotic factors XIAP and Bcl-2, while the
protein levels of the pro-apoptotic factor Bax were elevated
(72). 2-Methoxycinnamaldehyde
inhibits the activity of DNA topoisomerases I and II, thereby
inhibiting the proliferation of Hep 3B cells. In addition, it can
also induce lysosomal vacuolization, increase the volume of acidic
organelles and promote apoptosis of cancer cells (73). A study has shown that cinnamon oil
could reduce the incidence of hepatocellular carcinoma, and reduce
liver damage and tumor growth (74). A derivative of CA, known as CB-PIC,
can hinder the phosphorylation of STAT3 and diminish the expression
of genes associated with STAT3. This process subsequently induces
apoptosis in hepatocellular carcinoma cells (75).
In summary, CA and its derivatives may have
potential anti-proliferative and apoptosis-inducing effects on
hepatocellular carcinoma cells. However, further research is
required to fully understand the mechanisms involved and to
determine the therapeutic potential of these compounds in the
treatment of hepatocellular carcinoma.
Application of CA in prostate
cancer
In 2022, there were ~1.4 million new cases of
prostate cancer globally, with ~375,000 associated deaths (33).
CA prompts apoptosis in cancer-associated
fibroblasts (CAFs) by reducing the mitochondrial membrane
potential, while simultaneously increasing the levels of endogenous
ROS within CAFs and activating caspase-9 and caspase-3 (76). Mei et al (77) also studied prostate CAFs and found
that CA acted on CAFs via a Toll-like receptor 4-dependent
signaling pathway and regulated their function so that they no
longer inhibit the proliferation of T cells, thus CA plays a
certain role in the treatment of tumors. The proteasome is an
anticancer target, and proteasome inhibition can promote apoptosis
and inhibit tumor growth (78,79).
Gopalakrishnan and Ismail (80)
found that cinnamon compounds can inhibit the activity of the
proteasome, leading to the accumulation of p27 protein, thereby
inhibiting the proliferation of prostate cancer cells. Meanwhile,
cinnamon compounds also lead to downregulation of vascular
endothelial growth factor A (VEGFA) and VEGF receptor, thereby
inhibiting the angiogenic capability of tumor cells. Gopalakrishnan
et al (81) revealed that
cinnamon and its active compounds enhanced the activity of
apoptotic markers caspase-8 and caspase-3, leading to the promotion
of cancer cell death. This provides a scientific basis for cinnamon
as a potential chemoprevention agent for prostate cancer (81).
Current research on using CA for prostate cancer
treatment is still limited and further experiments and clinical
trials are required to ascertain its specific role and
effectiveness. However, the initial results present an encouraging
outlook for CA as a prospective therapeutic approach and provide
valuable guidance for further investigations related to prostate
cancer treatment.
Application of CA in leukemia
As early as 1983, Moon and Pack (82) observed the cytotoxic effect of CA
on L1210 mouse leukemia cells and found that the aldehyde group of
the CA molecule directly reacted with amino acids containing thiol
groups in the cell, thereby blocking the utilization of amino acids
contained in the thiol group in the cell and blocking protein
synthesis, resulting in the inhibition of L1210 cell growth
(82). In a previous study,
researchers found that CA was an effective inducer of cell
apoptosis, inducing white blood cell apoptosis through ROS-mediated
mitochondrial permeability transition and cytochrome c release, as
well as activating cascading reactions of cysteine protease-9 and
cysteine protease-3 (83). In
addition, CA can also induce apoptosis in leukemia K562 cells by
reducing the mitochondrial transmembrane potential via
mitochondrial-mediated pathways (84). Furthermore, CA can also inhibit
cell proliferation by affecting the cell cycle. Water extract of
cinnamon activates p38 MAPK kinase, reduces the expression of
cyclin B1 protein and induces G2/M blockade, and thus,
affects the proliferation of cell lines (85). CA exerts its anti-leukemic effect
by downregulating the transcription levels of the BCR-ABL gene and
reducing the expression of the C-MYC protein (86). There has also been a study
indicating that HCA interferes with the growth and transformation
process of leukemia cells by inhibiting the activity of Pim-1, thus
having an anti-leukemia effect (87). To summarize, while CA shows
potential for leukemia treatment, more extensive research is still
needed. These findings provide a direction for future research
regarding leukemia treatments.
Summary
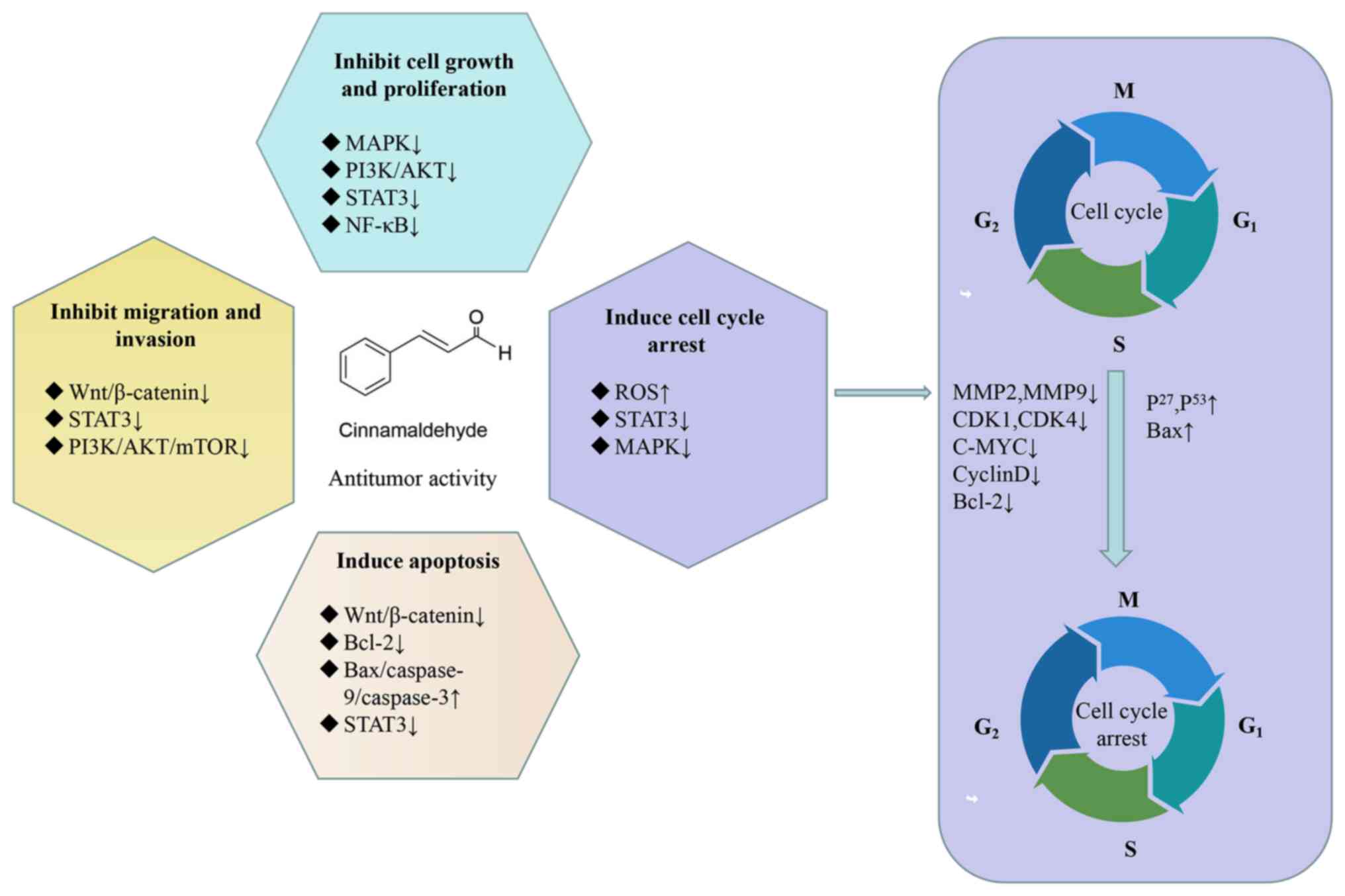
CA exhibits antitumor efficacy against various types
of tumors, such as non-small cell lung cancer (41), colon cancer (45), breast cancer (58), liver cancer (73), prostate cancer (78) and leukemia (84). Extensive research has confirmed
that the antitumor effects of CA are primarily achieved through the
following mechanisms: Inhibiting cell growth and proliferation
(37), arresting the cell cycle
(45), inducing apoptosis
(46), and inhibiting cell
migration and invasion (63). A
summary of the major cellular signaling pathways involved in the
anticancer activity of CA is shown in Fig. 2. Table
I shows the antitumor effects of cinnamaldehyde in different
types of cancer.
 | Table I.Antitumor effects of cinnamaldehyde
in different types of cancer. |
Table I.
Antitumor effects of cinnamaldehyde
in different types of cancer.
| A, Lung cancer |
|---|
|
|---|
| First author/s,
year | In vivo | In
vitro | Mechanisms | Methods | (Refs.) |
|---|
| Imai et al,
2002 | Mouse model of lung
cancer induced by
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone | - | Reduces the
proliferation of tumor-initiating cells | - | (37) |
| Meng et al,
2017 | Urethane to induce
lung adenocarcinoma model | A549 cells | ↑AMPK; ↓AQP-1;
↓mTOR | Western blot
analysis | (38) |
| Tian et al,
2017 | Nude mouse model
induced by NCI-H460 cells | A549 and NCI-H460
cells | ↑Circular RNA
hsa_circ_0043256 | - | (39) |
| Wu et al,
2017 | Mouse lung cancer
model induced by A549 cells | A549 cells | ↓Wnt/β-catenin
pathway | RT-qPCR analysis;
western blot analysis | (40) |
| Park and Baek,
2020 | - | A549 cells | ↑ROS; ↓MAPK | Western blot
analysis | (41) |
| Chen et al,
2020 | Mouse lung cancer
model induced by A549 cells | A549, NCI-H1650,
SK-MES-1 and NCI-H226 cells | ↓JAK/STAT3;
↓NF-κB | RT-qPCR analysis;
western blot analysis | (42) |
|
| B, CRC |
|
| Jeong et al,
2003 | Mouse colon cancer
model induced by SW620 cells | SW620 and MCF7
cells | ↓Cyclin D1 | Western blot
analysis | (45) |
| Lee et al,
2007 | - | SW620 cells | ↓AP-1 | Western blot
analysis | (46) |
| Cho et al,
2013 | - | H460/PT, HCT15/cos
and MCF7/Adr cells | ↑AMPK; ↑ERK | Western blot
analysis | (47) |
| Yun et al,
2015 | - | HCT15/cos
cells | ↓MDR1; ↓STAT3;
↓AKT | RT-PCR analysis;
western blot analysis | (48) |
| Yu et al,
2014 | - | LoVo and HT-29
cells | Induces apoptosis
in tumor cells | RT-qPCR
analysis | (49) |
| Long et al,
2015 | Mouse colon cancer
model induced by azoxymethane/dextran sulfate sodium | HCT116 cells | Promotes the
expression of Nrf2 target genes | PCR analysis | (50) |
| Li et al,
2016 | - | LoVo, SW480, and
HCT116 cells human CRC cell lines | ↓PI3K/Akt | Western blot
analysis | (52) |
| Zhang et al,
2023 | Mouse colon cancer
model induced by HCT116 cells | HCT116 cells | ↓PI3K/Akt;
↓Ki67 | Western blot
analysis | (53) |
| Nguyen and Kim,
2017 | - | SW480 and SW620
cells | ↑BAG3 | RT-PCR analysis;
western blot analysis | (54) |
| Wu et al,
2019 | Mouse colon cancer
model induced by HCT116 cells CRC cell lines | HCT116 and SW480
human | ↓Wnt/β-catenin
pathway | RT-qPCR analysis;
western blot analysis | (55) |
| Kosari et
al, 2020 | - | E. coli | ↓clbB gene | RT-qPCR
analysis | (56) |
| Petrocelli et
al, 2021 | - | NCM-460, Caco-2 and
SW620 cells | Induces apoptosis
in tumor cells | - | (57) |
| Nile et al,
2023 | - | HCT116 and HT-29
cells | ↑ROS | - | (13) |
|
| C, Breast
cancer |
|
| Wani et al,
2014 | - | MDA-MB-231 and MCF7
cells | ↑VEGF;
↑caspase-3 | Western blot
analysis | (58) |
| Rad et al,
2015 | - | MDA-MB-231 and MCF8
cells | ↑Caspase-8 | RT-qPCR analysis;
western blot analysis | (59) |
| Chiang et
al, 2019 | Mouse model of
breast cancer induced by MDA-MB-231-GFP cells | MDA-MB-231-GFP
cells | ↓Visfatin | Western blot
analysis | (60) |
| Liu et al,
2020 | - | MDA-MB-231
cells | Induces apoptosis
in tumor cells | - | (61) |
| Kubatka et
al, 2020 |
N-nitroso-N-methylurea-induced rat breast
cancer model | MDA-MB-231 and MCF7
cells | Inhibits tumor cell
proliferation | - | (62) |
| Dong et al,
2020 | - | H9c2 and MCF7
cells | Induces apoptosis
in tumor cells | - | (63) |
| Kuo et al,
2021 | - | MCF7 cells | Induces apoptosis
in tumor cells | - | (64) |
| Schuster et
al, 2022 | - | MCF7, MDA-MB-231
and HCC1419 cells | Prompts cancer cell
apoptosis | - | (65) |
| Yao et al,
2023 | 4T1 breast cancer
model mice | MDA-MB-231
cells | ↑ROS | - | (66) |
|
| D, Liver
cancer |
|
| Wu et al,
2004 | - | PLC/PRF/5
cells | ↑Bax; ↓Bcl-2; ↓
XIAP | Western blot
analysis | (68) |
| Moon et al,
2006 | H-ras12V transgenic
mouse model | - | ↓Farnesyl
transferase | - | (69) |
| Huang et al,
2011 | - | HepG2 cells | ↑ERK1/2; ↑Akt;
↑JNK; ↑Nrf2 | Western blot
analysis | (70) |
| Ng and Wu,
2011 | - | HepG2 cells | ↓Bcl-XL; ↑CD95
(APO-1); ↑p53; ↑Bax | Western blot
analysis | (71) |
| Lin et al,
2013 | - | PLC/PRF/5
cells | ↓XIAP; ↓Bcl-2;
↑Bax | Western blot
analysis | (72) |
| Perng et al,
2016 | Mouse liver cancer
model induced by Hep 3B cells | Hep 3B cells | ↓DNA topoisomerases
I and II | Western blot
analysis | (73) |
| Aly et al,
2019 | Male albino
rats | - | Reduces tumor
growth | PCR analysis | (74) |
| Kim et al,
2022 | - | Huh7 and HepG2
cells | ↓STAT3 | RT-qPCR analysis;
western blot analysis | (75) |
|
| E, Prostate
cancer |
|
| Han et al,
2020 | - | Prostate
cancer-associated fibroblasts | ↓∆Mψ; ↑ROS; ↓Bcl-2;
↑Bax | Western blot
analysis | (76) |
| Mei et al,
2020 | C57 mice | Prostate
cancer-associated fibroblasts | ↑TLR4 | Western blot
analysis | (77) |
| Gopalakrishnan and
Ismail, 2021 | - | LNCaP, PC3 |
↑P27 | RT-qPCR analysis;
western blot analysis | (80) |
| Gopalakrishnan
et al, 2023 | Mouse prostate
cancer model induced by testosterone propionate | - | ↑Caspase-8;
↑caspase-3 | RT-qPCR analysis;
western blot analysis | (81) |
|
| F,
Leukemia |
|
| Moon and Pack,
1983 | - | L1210 cells | Inhibits tumor cell
growth | - | (82) |
| Ka et al,
2003 | - | HL-60 cells | ↑ROS | - | (83) |
| Zhang et al,
2010 | - | K562 cells | ↓ΔΨm | - | (84) |
| Schoene et
al, 2009 | - | CD45 Jurkat clone,
Wurzburg cells | ↑p38; ↑MAPK;
↓cyclin B1 | Western blot
analysis | (85) |
| Liu et al,
2001 | - | K562 cells |
↓BCR-ABL;↓C-MYC | RT-qPCR analysis;
western blot analysis | (86) |
| Kim et al,
2015 | Mouse tumor model
induced by HEL cells | HEL, HaCaT and A431
cells | ↓Pim-1 | - | (87) |
CA preparations in cancer
The first objective for cancer treatment is to
achieve high treatment outcomes and reduce side effects. Therefore,
with advancements in targeted therapy and immunotherapy, the
clinical treatment of patients with cancer has improved (88).
Nanodrug particles have low systemic toxicity in
vivo and do not cause significant damage to normal tissues
(89). Therefore, researchers have
linked 5-FU and CA through acetal and ester bonds to prepare
carrier-free nanodrug particles, and a synergistic effect of
chemotherapy drugs was observed, the antitumor effect was improved
and the systemic toxicity was reduced, indicating good application
prospects (15). To enhance the
permeability of the blood-brain barrier and mitigate drug toxicity,
researchers combined trypsin (Try) and CA through an imine
condensation reaction to form a novel small molecule nano prodrug
and emulsified it into nanoparticles (Try-CA-NPs). Try-CA-NPs could
achieve specific uptake of glioma cells by specifically binding to
upregulated 5-hydroxytryptamine receptors (5-hydroxytryptamine
receptor 1A and 5-hydroxytryptamine receptor 2) and improve
cytotoxicity through endosomal escape, efficient drug release, and
synergistic effects between Try and CA (16).
ROS levels are one of the unique hallmarks of
cancer, and the levels of ROS in cancer cells are much higher than
those in normal tissues (90). A
study has shown that apoptosis and necrosis of cancer cells occur
when ROS levels exceed the tolerance threshold of cancer cells
(91). CA directly kills tumor
cells by producing ROS (83).
Zhou et al (14) utilized cinnamaldehyde-modified
chitosan hybrid nanoparticles for delivering the chemotherapy drug
DOX. Cinnamaldehyde can generate ROS to directly kill tumor cells,
thereby synergizing with DOX to exert antitumor effects (14). To improve the preparation process
of nanomedicines, researchers have prepared CA-copper-polydopamine
(CA-Cu-PDA) nanomedicines through a simple one-step polymerization
reaction. The experimental results showed that CA-Cu-PDA was able
to release copper ions and CA in tumor cells, and weakened the
antioxidant system by binding to glutathione (GSH), which in turn
produced additional ROS, thereby inducing enhanced oxidative stress
effects (17). In addition,
researchers have produced a self-amplifying degradable polymer
composed of ROS-responsive thioacetal groups and CA, which could
not only achieve sustained drug release but could also trigger
immunogenic cell death in cancerous cells (18).
A previous study demonstrated that excess GSH
promotes tumor progression (92).
Researchers have synthesized a tumor-targeted oxidative stress
nanoamplifier using CA as the ROS generator, β-phenethyl
isothiocyanate as the GSH scavenger and HA as the carrier for
targeting tumors. This could synergistically enhance oxidative
stress and suppress tumor growth, and exhibited favorable
biological safety (93). In
addition, researchers have synthesized Fc-CA-PCN-HA nanoparticles
coated with sodium hyaluronate, which not only have improved
biocompatibility and targeting but can also incrementally
H2O2 levels (94). In vivo experiments in nude
mice revealed that sodium hyaluronate-coated Fc-CA-PCN-HA
nanoparticles had antitumor effects under the synergistic effect of
photodynamic therapy and chemodynamic therapy, and had no obvious
toxic side effects on the overall health of nude mice (94).
Despite being in the early stages of research as a
targeted agent, CA has shown some promising results. Future studies
will continue exploring its potential, and optimizing its
pharmacological properties and therapeutic effects to better
address the challenges in treating diseases, particularly
cancer.
Safety of CA
Data have validated the safety of CA, demonstrating
its non-carcinogenicity even at the highest exposure level of 4,100
ppm over an extended period (95).
In a study spanning 3 months to 2 years utilizing microencapsulated
trans-CA in both male and female F344/N rats and B6C3F1
mice, no tumors linked to its exposure were observed in either
species (96). Oral administration
of CA in various animals has been proven to be safe, exemplified by
its median lethal dose values of 2,220 mg/kg in rats (20) and 2,301 mg/kg in mice (7). Notably, Anand et al (97) revealed that even at 20 times the
effective dose (20 mg/kg), CA did not induce significant
abnormalities in physiological parameters. Consistent with these
findings, another study has demonstrated that CA does not exhibit
genotoxic or carcinogenic effects on the body (98).
CA is widely acknowledged for its exceptional safety
profile, and research advancements indicate that CA possesses the
ability to mitigate the toxic side effects of chemotherapy drugs
(99,100). Specifically, CA has demonstrated
a capacity to alleviate cardiotoxicity induced by DOX (99), and exhibits cytoprotective effects,
safeguarding against cardiorenal toxicity triggered by
cyclophosphamide (100).
Conclusions
There has been a steady rise in the occurrence of
cancer, posing great risks and challenges to human survival. With
the continuous development and utilization of natural products,
they occupy an increasingly important position as anticancer drugs.
CA is an active ingredient found in the natural medicine cinnamon.
There is evidence that CA and its derivatives not only have a
positive effect on cancer prevention and treatment but can also
produce synergistic anticancer effects when used in combination
with different chemotherapy drugs, and alleviate the adverse
effects of chemotherapy drugs (38). A large number of studies have
demonstrated that CA and its derivatives exert their antitumor
activity by inhibiting cell growth and proliferation (37), arresting the cell cycle (13), inducing apoptosis (57), inhibiting cell migration and
invasion (52), and inhibiting
angiogenesis (101). Although CA
is not soluble in water, recent studies on various nanomedicine
delivery systems for CA have effectively improved drug stability,
targeting capability and bioavailability (18,90–92).
Although studies have hinted at the potential of CA
as an anticancer agent, particularly in suppressing tumor growth
and metastasis (38,39,41),
its mechanisms of action during tumor initiation, progression and
treatment remain largely unexplored. A crucial step forward lies in
elucidating its interactions with tumor cell signaling pathways and
the impact on gene expression. Furthermore, research should uncover
novel therapeutic targets for CA in cancer therapy, coupled with
advancements in drug optimization and synthesis techniques to yield
more potent and safer derivatives. Chromatin immunoprecipitation
(ChIP) cDNA expression chip [or similar ChIP technologies such as
ChIP-chip or ChIP sequencing (seq)] and assay for
transposase-accessible chromatin with sequencing (ATAC seq) can
provide in-depth research on the mechanisms of genomic regulation
and expression of drugs (102–105). However, to the best of our
knowledge, there are no reports of results related to the
aforementioned technologies for CA. Therefore, in future research,
techniques such as ChIP cDNA expression chip (or similar ChIP
technologies such as ChIP-chip or ChIP seq) and ATAC seq can be
used to further investigate the antitumor effects of CA.
Additionally, emphasis should be placed on
investigating combination therapy strategies, utilizing CA
alongside other anticancer agents to enhance therapeutic efficacy
and minimize drug resistance. As clinical trials advance and
translational research progresses, CA may emerge as a pivotal
component in future cancer therapeutics, offering patients more
effective treatment options and improved quality of life.
Acknowledgements
Not applicable.
Funding
The present study was funded by WU JIEPING Medical Foundation
(grant no. 320. 6750. 2021-10-18), TCM Science and Technology
Project of Shandong Province (grant no. Q-2023105), and Science and
Technology Project of Binzhou Medical University (grant no.
BY2021KJ44).
Availability of data and materials
Not applicable.
Authors' contributions
RH and XL wrote the original draft of the
manuscript. GL and XG reviewed and edited the manuscript. Data
authentication is not applicable. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dorri M, Hashemitabar S and Hosseinzadeh
H: Cinnamon (Cinnamomum zeylanicum) as an antidote or a
protective agent against natural or chemical toxicities: A review.
Drug Chem Toxicol. 41:338–351. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mishra A, Bhatti R, Singh A and Singh
Ishar MP: Ameliorative effect of the cinnamon oil from
Cinnamomum zeylanicum upon early stage diabetic nephropathy.
Planta medica. 76:412–417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ustaoglu E, Turkoglu Z, Ulgen OA, Caytemel
C and Agirgol S: Anti-inflammatory effect of cinnamaldehyde in a
mouse model of 2,4-dinitrofluorobenzene-induced atopic dermatitis.
Indian J Dermatol. 68:170–177. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tanaka Y, Uchi H and Furue M: Antioxidant
cinnamaldehyde attenuates UVB-induced photoaging. J Dermatol Sci.
96:151–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ding Y, Qiu L, Zhao G, Xu J and Wang S:
Influence of cinnamaldehyde on viral myocarditis in mice. Am J Med
Sci. 340:114–120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Friedman M: Chemistry, antimicrobial
mechanisms, and antibiotic activities of cinnamaldehyde against
pathogenic bacteria in animal feeds and human foods. J Agric Food
Chem. 65:10406–10423. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang J, Wang S, Luo X, Xie Y and Shi X:
Cinnamaldehyde reduction of platelet aggregation and thrombosis in
rodents. Thromb Res. 119:337–342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Subash Babu P, Prabuseenivasan S and
Ignacimuthu S: Cinnamaldehyde-a potential antidiabetic agent.
Phytomedicine. 14:15–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tung YT, Huang CC, Ho ST, Kuo YH, Lin CC,
Lin CT and Wu JH: Bioactive phytochemicals of leaf essential oils
of Cinnamomum osmophloeum prevent
lipopolysaccharide/D-galactosamine (LPS/D-GalN)-induced acute
hepatitis in mice. J Agric Food Chem. 59:8117–8123. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo X, Sun W, Huang L, Wu L, Hou Y, Qin L
and Liu T: Effect of cinnamaldehyde on glucose metabolism and
vessel function. Med Sci Monit. 23:3844–3853. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuru Bektaşoğlu P, Koyuncuoğlu T, Demir D,
Sucu G, Akakın D, Peker Eyüboğlu İ, Yüksel M, Çelikoğlu E, Yeğen BÇ
and Gürer B: Neuroprotective effect of cinnamaldehyde on secondary
brain injury after traumatic brain injury in a rat model. World
Neurosurg. 153:e392–e402. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kwon HK, Hwang JS, So JS, Lee CG, Sahoo A,
Ryu JH, Jeon WK, Ko BS, Lee SH, Park ZY and Im SH: Cinnamon extract
induces tumor cell death through inhibition of NFkappaB and AP1.
BMC Cancer. 10:3922010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nile A, Shin J, Shin J, Park GS, Lee S,
Lee JH, Lee KW, Kim BG, Han SG, Saini RK and Oh JW:
Cinnamaldehyde-Rich cinnamon extract induces cell death in colon
cancer cell lines HCT 116 and HT-29. Int J Mol Sci. 24:81912023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Z, Wang C, Bai J, Zeng Z, Yang X, Wei
B and Yang Z: Cinnamaldehyde-modified chitosan hybrid nanoparticles
for DOX delivering to produce synergistic anti-tumor effects. Front
Bioeng Biotechnol. 10:9680652022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang Q, Xu X, Yang L, Xue Y, Cheng X, Wang
X and Tang R: Self-assembled 5-fluorouracil-cinnamaldehyde
nanodrugs for greatly improved chemotherapy in vivo. J Biomater
Appl. 36:592–604. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Z, Yao J, Guan Z, Wu H, Cheng H, Yan
G and Tang R: pH-triggered small molecule Nano-prodrugs emulsified
from tryptamine-cinnamaldehyde twin drug for targeted synergistic
glioma therapy. Colloids Surf B Biointerfaces. 207:1120522021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Q, Jia X, Li X, He M, Hao JN, Guan M,
Mao Y, Cao Y, Dai B and Li Y: One-pot fabrication of a
polydopamine-based nanoplatform for GSH triggered trimodal
ROS-amplification for cancer therapy. Biomater Sci. 10:4208–4217.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tu Y, Xiao X, Dong Y, Li J, Liu Y, Zong Q
and Yuan Y: Cinnamaldehyde-based poly(thioacetal): A ROS-awakened
self-amplifying degradable polymer for enhanced cancer
immunotherapy. Biomaterials. 289:1217952022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peters MM and Caldwell J: Studies on
trans-cinnamaldehyde. 1. The influence of dose size and sex on its
disposition in the rat and mouse. Food Chem Toxicol. 32:869–876.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong SH, Ismail IA, Kang SM, Han DC and
Kwon BM: Cinnamaldehydes in cancer chemotherapy. Phytother Res.
30:754–767. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang LQ, Zhang ZG, Fu Y and Xu Y:
Research progress of trans-cinnamaldehyde pharmacological effects.
Zhongguo Zhong Yao Za Zhi. 40:4568–4572. 2015.(In Chinese).
PubMed/NCBI
|
|
22
|
Zinn S, Betz T, Medcraft C and Schnell M:
Structure determination of trans-cinnamaldehyde by broadband
microwave spectroscopy. Phys Chem Chem Phys. 17:16080–16085. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bickers D, Calow P, Greim H, Hanifin JM,
Rogers AE, Saurat JH, Sipes IG, Smith RL and Tagami H; RIFM expert
panel, : A toxicologic and dermatologic assessment of cinnamyl
alcohol, cinnamaldehyde and cinnamic acid when used as fragrance
ingredients. Food Chem Toxicol. 43:799–836. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vasconcelos NG, Croda J and Simionatto S:
Antibacterial mechanisms of cinnamon and its constituents: A
review. Microb Pathog. 120:198–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao H, Xie Y, Yang Q, Cao Y, Tu H, Cao W
and Wang S: Pharmacokinetic study of cinnamaldehyde in rats by
GC-MS after oral and intravenous administration. J Pharm Biomed
Anal. 89:150–157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao H, Yang Q, Xie Y, Sun J, Tu H, Cao W
and Wang S: Simultaneous determination of cinnamaldehyde and its
metabolite in rat tissues by gas chromatography-mass spectrometry.
Biomed Chromatogr. 29:182–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao H, Yuan J, Yang Q, Xie Y, Cao W and
Wang S: Cinnamaldehyde in a novel intravenous submicrometer
emulsion: Pharmacokinetics, tissue distribution, antitumor
efficacy, and toxicity. J Agric Food Chem. 63:6386–6392. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alqahtani MS, Kazi M, Alsenaidy MA and
Ahmad MZ: Advances in oral drug delivery. Front Pharmacol.
12:6184112021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu L, Meng Y, Xu Y and Chu X: Improved
uptake and bioavailability of cinnamaldehyde via solid lipid
nanoparticles for oral delivery. Pharm Dev Technol. 27:1038–1048.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu L, Cao W, Xia M, Tian C, Wu W, Cai Y
and Chu X: Self-Emulsifying drug delivery system enhances tissue
distribution of cinnamaldehyde by altering the properties of the
mucus layer. AAPS PharmSciTech. 23:2612022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cai Y, Liu L, Xia M, Tian C, Wu W, Dong B
and Chu X: SEDDS facilitate cinnamaldehyde crossing the mucus
barrier: The perspective of mucus and Caco-2/HT29 co-culture
models. Int J Pharm. 614:1214612022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong B, Chen J, Cai Y, Wu W and Chu X: In
vitro and in vivo evaluation of cinnamaldehyde Microemulsion-Mucus
interaction. J Food Biochem. 46:e143072022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bray F, Laversanne M, Weiderpass E and
Soerjomataram I: The ever-increasing importance of cancer as a
leading cause of premature death worldwide. Cancer. 127:3029–3030.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng RS, Chen R, Han BF, Wang SM, Li L,
Sun KX, Zeng HM, Wei WW and He J: Cancer incidence and mortality in
China, 2022. Zhonghua Zhong Liu Za Zhi. 46:221–231. 2024.(In
Chinese). PubMed/NCBI
|
|
36
|
Luo G, Zhang Y, Etxeberria J, Arnold M,
Cai X, Hao Y and Zou H: Projections of lung cancer incidence by
2035 in 40 countries worldwide: Population-based study. JMIR Public
Health Surveill. 9:e436512023. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Imai T, Yasuhara K, Tamura T, Ueda M,
Hirose M and Mitsumori K: Inhibitory effects of cinnamaldehyde on
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung
carcinogenesis in rasH2 mice. Cancer Lett. 175:9–16. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meng M, Geng S, Du Z, Yao J, Zheng Y, Li
Z, Zhang Z, Li J, Duan Y and Du G: Berberine and cinnamaldehyde
together prevent lung carcinogenesis. Oncotarget. 8:76385–76397.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tian F, Yu CT, Ye WD and Wang Q:
Cinnamaldehyde induces cell apoptosis mediated by a novel circular
RNA hsa_circ_0043256 in non-small cell lung cancer. Biochem Biophys
Res Commun. 493:1260–1266. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu C, Zhuang Y, Jiang S, Tian F, Teng Y,
Chen X, Zheng P, Liu S, Zhou J, Wu J, et al: Cinnamaldehyde induces
apoptosis and reverses epithelial-mesenchymal transition through
inhibition of Wnt/β-catenin pathway in non-small cell lung cancer.
Int J Biochem Cell Biol. 84:58–74. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park J and Baek SH: Combination therapy
with cinnamaldehyde and hyperthermia induces apoptosis of A549
Non-Small cell lung carcinoma cells via regulation of reactive
oxygen species and mitogen-activated protein kinase family. Int J
Mol Sci. 21:62292020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen R, Wu J, Lu C, Yan T, Qian Y, Shen H,
Zhao Y, Wang J, Kong P and Zhang X: Systematic Transcriptome
analysis reveals the inhibitory function of cinnamaldehyde in
non-small cell lung cancer. Front Pharmacol. 11:6110602020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qu R, Ma Y, Zhang Z and Fu W: Increasing
burden of colorectal cancer in China. Lancet Gastroenterol Hepatol.
7:7002022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sargent DJ, Wieand HS, Haller DG, Gray R,
Benedetti JK, Buyse M, Labianca R, Seitz JF, O'Callaghan CJ,
Francini G, et al: Disease-free survival versus overall survival as
a primary end point for adjuvant colon cancer studies: Individual
patient data from 20,898 patients on 18 randomized trials. J Clin
Oncol. 23:8664–8670. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jeong HW, Han DC, Son KH, Han MY, Lim JS,
Ha JH, Lee CW, Kim HM, Kim HC and Kwon BM: Antitumor effect of the
cinnamaldehyde derivative CB403 through the arrest of cell cycle
progression in the G2/M phase. Biochem Pharmacol. 65:1343–1350.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee CW, Lee SH, Lee JW, Ban JO, Lee SY,
Yoo HS, Jung JK, Moon DC, Oh KW and Hong JT:
2-hydroxycinnamaldehyde inhibits SW620 colon cancer cell growth
through AP-1 inactivation. J Pharmacol Sci. 104:19–28. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cho SY, Lee HJ, Lee HJ, Jung DB, Kim H,
Sohn EJ, Kim B, Jung JH, Kwon BM and Kim SH: Activation of
AMP-Activated protein kinase α and extracelluar signal-regulated
kinase mediates CB-PIC-Induced apoptosis in hypoxic SW620
colorectal cancer cells. Evid Based Complement Alternat Med.
2013:9743132013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yun M, Lee D, Park MN, Kim EO, Sohn EJ,
Kwon BM and Kim SH: Cinnamaldehyde derivative (CB-PIC) sensitizes
chemo-resistant cancer cells to drug-induced apoptosis via
suppression of MDR1 and its upstream STAT3 and AKT signalling. Cell
Physiol Biochem. 35:1821–1830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yu C, Liu SL, Qi MH and Zou X:
Cinnamaldehyde/chemotherapeutic Agents interaction and
drug-metabolizing genes in colorectal cancer. Mol Med Rep.
9:669–676. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Long M, Tao S, Rojo de la Vega M, Jiang T,
Wen Q, Park SL, Zhang DD and Wondrak GT: Nrf2-dependent suppression
of azoxymethane/dextran sulfate sodium-induced colon carcinogenesis
by the cinnamon-derived dietary factor cinnamaldehyde. Cancer Prev
Res (Phila). 8:444–454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dong P, Konno Y, Watari H, Hosaka M,
Noguchi M and Sakuragi N: The impact of microRNA-mediated PI3K/AKT
signaling on epithelial-mesenchymal transition and cancer stemness
in endometrial cancer. J Transl Med. 12:2312014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li J, Teng Y, Liu S, Wang Z, Chen Y, Zhang
Y, Xi S, Xu S, Wang R and Zou X: Cinnamaldehyde affects the
biological behavior of human colorectal cancer cells and induces
apoptosis via inhibition of the PI3K/Akt signaling pathway. Oncol
Rep. 35:1501–1510. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang W, Lei W, Shen F, Wang M, Li L and
Chang J: Cinnamaldehyde induces apoptosis and enhances
anti-colorectal cancer activity via covalent binding to HSPD1.
Phytother Res. Apr 22–2023.doi: 10.1002/ptr.7840 (Epub ahead of
print). View Article : Google Scholar
|
|
54
|
Nguyen HA and Kim SA:
2′-Hydroxycinnamaldehyde induces apoptosis through HSF1-mediated
BAG3 expression. Int J Oncol. 50:283–289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wu CE, Zhuang YW, Zhou JY, Liu SL, Wang RP
and Shu P: Cinnamaldehyde enhances apoptotic effect of oxaliplatin
and reverses epithelial-mesenchymal transition and stemnness in
hypoxic colorectal cancer cells. Exp Cell Res. 383:1115002019.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kosari F, Taheri M, Moradi A, Hakimi Alni
R and Alikhani MY: Evaluation of cinnamon extract effects on clbB
gene expression and biofilm formation in Escherichia coli strains
isolated from colon cancer patients. BMC Cancer. 20:2672020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Petrocelli G, Farabegoli F, Valerii MC,
Giovannini C, Sardo A and Spisni E: Molecules present in plant
essential oils for prevention and treatment of colorectal cancer
(CRC). Molecules. 26:8852021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wani KD, Kadu BS, Mansara P, Gupta P,
Deore AV, Chikate RC, Poddar P, Dhole SD and Kaul-Ghanekar R:
Synthesis, characterization and in vitro study of biocompatible
cinnamaldehyde functionalized magnetite nanoparticles (CPGF Nps)
for hyperthermia and drug delivery applications in breast cancer.
PLoS One. 9:e1073152014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rad SK, Kanthimathi MS, Abd Malek SN, Lee
GS, Looi CY and Wong WF: Cinnamomum cassia suppresses Caspase-9
through stimulation of AKT1 in MCF-7 cells but not in MDA-MB-231
cells. PLoS One. 10:e01452162015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chiang YF, Chen HY, Huang KC, Lin PH and
Hsia SM: Dietary antioxidant trans-cinnamaldehyde reduced
Visfatin-induced breast cancer progression: In vivo and in vitro
study. Antioxidants (Basel, Switzerland). 8:6252019.PubMed/NCBI
|
|
61
|
Liu Y, An T, Wan D, Yu B, Fan Y and Pei X:
Targets and mechanism used by cinnamaldehyde, the main active
ingredient in cinnamon, in the treatment of breast cancer. Front
Pharmacol. 11:5827192020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kubatka P, Kello M, Kajo K, Samec M, Jasek
K, Vybohova D, Uramova S, Liskova A, Sadlonova V, Koklesova L, et
al: Chemopreventive and therapeutic efficacy of Cinnamomum
zeylanicum L. bark in experimental breast carcinoma:
Mechanistic in vivo and in vitro analyses. Molecules. 25:13992020.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dong K, Zhao ZZ, Kang J, Lin LR, Chen WT,
Liu JX, Wu XL and Lu TL: Cinnamaldehyde and Doxorubicin Co-Loaded
graphene oxide wrapped mesoporous silica nanoparticles for enhanced
MCF-7 cell apoptosis. Int J Nanomedicine. 15:10285–10304. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kuo YT, Liu CH, Wong SH, Pan YC and Lin
LT: Small molecules baicalein and cinnamaldehyde are potentiators
of measles virus-induced breast cancer oncolysis. Phytomedicine.
89:1536112021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Schuster C, Wolpert N, Moustaid-Moussa N
and Gollahon LS: Combinatorial effects of the natural products
arctigenin, chlorogenic acid, and cinnamaldehyde commit oxidation
assassination on breast cancer cells. Antioxidants (Basel).
11:5912022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yao P, Wang X, Wang Q, Dai Q, Peng Y, Yuan
Q, Mou N, Lv S, Weng B, Wang Y and Sun F: Cyclic RGD-functionalized
pH/ROS Dual-responsive nanoparticle for targeted breast cancer
therapy. Pharmaceutics. 15:18272023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Taniguchi H: Liver cancer 2.0. Int J Mol
Sci. 24:172752023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wu SJ, Ng LT and Lin CC: Effects of
vitamin E on the cinnamaldehyde-induced apoptotic mechanism in
human PLC/PRF/5 cells. Clin Exp Pharmacol Physiol. 31:770–776.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Moon EY, Lee MR, Wang AG, Lee JH, Kim HC,
Kim HM, Kim JM, Kwon BM and Yu DY: Delayed occurrence of
H-ras12V-induced hepatocellular carcinoma with long-term treatment
with cinnamaldehydes. Eur J Pharmacol. 530:270–275. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Huang TC, Chung YL, Wu ML and Chuang SM:
Cinnamaldehyde enhances Nrf2 nuclear translocation to upregulate
phase II detoxifying enzyme expression in HepG2 cells. J Agric Food
Chem. 59:5164–5171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ng LT and Wu SJ: Antiproliferative
activity of cinnamomum cassia constituents and effects of
pifithrin-alpha on their apoptotic signaling pathways in Hep G2
cells. Evid Based Complement Alternat Med. 2011:4921482011.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lin LT, Tai CJ, Chang SP, Chen JL, Wu SJ
and Lin CC: Cinnamaldehyde-induced apoptosis in human hepatoma
PLC/PRF/5 cells involves the mitochondrial death pathway and is
sensitive to inhibition by cyclosporin A and z-VAD-fmk. Anticancer
Agents Med Chem. 13:1565–1574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Perng DS, Tsai YH, Cherng J, Kuo CW, Shiao
CC and Cherng JM: Discovery of a novel anti-cancer agent targeting
both topoisomerase I and II in hepatocellular carcinoma Hep 3B
cells in vitro and in vivo: Cinnamomum verum component
2-methoxycinnamaldehyde. J Drug Target. 24:624–634. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Aly SM, Fetaih HA, Hassanin AAI,
Abomughaid MM and Ismail AA: Protective effects of garlic and
cinnamon oils on hepatocellular carcinoma in albino rats. Anal Cell
Pathol (Amst). 2019:98954852019.PubMed/NCBI
|
|
75
|
Kim H, Lee HJ, Sim DY, Park JE, Ahn CH,
Park SY, Jang E, Kim B and Kim SH: The antitumor effect of
cinnamaldehyde derivative CB-PIC in hepatocellular carcinoma cells
via inhibition of pyruvate and STAT3 signaling. Int J Mol Sci.
23:64612022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Han L, Mei J, Ma J, Wang F, Gu Z, Li J,
Zhang Z, Zeng Y, Lou X, Yao X, et al: Cinnamaldehyde induces
endogenous apoptosis of the prostate cancer-associated fibroblasts
via interfering the Glutathione-associated mitochondria function.
Med Oncol. 37:912020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mei J, Ma J, Xu Y, Wang Y, Hu M, Ma F, Qin
Z, Xue R and Tao N: Cinnamaldehyde treatment of prostate
cancer-associated fibroblasts prevents their inhibitory effect on T
cells through Toll-Like receptor 4. Drug Des Devel Ther.
14:3363–3372. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhang X, Linder S and Bazzaro M: Drug
development targeting the ubiquitin-proteasome system (UPS) for the
treatment of human cancers. Cancers (Basel). 12:9022020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Concannon CG, Koehler BF, Reimertz C,
Murphy BM, Bonner C, Thurow N, Ward MW, Villunger A, Strasser A,
Kögel D and Prehn JH: Apoptosis induced by proteasome inhibition in
cancer cells: Predominant role of the p53/PUMA pathway. Oncogene.
26:1681–1692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Gopalakrishnan S and Ismail A: Aromatic
monophenols from cinnamon bark act as proteasome inhibitors by
upregulating ER stress, suppressing FoxM1 expression, and inducing
apoptosis in prostate cancer cells. Phytother Res. 35:5781–5794.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gopalakrishnan S, Dhaware M, Sudharma AA,
Mullapudi SV, Siginam SR, Gogulothu R, Mir IA and Ismail A:
Chemopreventive effect of cinnamon and its bioactive compounds in a
rat model of premalignant prostate carcinogenesis. Cancer Prev Res
(Phila). 16:139–151. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Moon KH and Pack MY: Cytotoxicity of
cinnamic aldehyde on leukemia L1210 cells. Drug Chem Toxicol.
6:521–535. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ka H, Park HJ, Jung HJ, Choi JW, Cho KS,
Ha J and Lee KT: Cinnamaldehyde induces apoptosis by ROS-mediated
mitochondrial permeability transition in human promyelocytic
leukemia HL-60 cells. Cancer Lett. 196:143–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang JH, Liu LQ, He YL, Kong WJ and Huang
SA: Cytotoxic effect of trans-cinnamaldehyde on human leukemia K562
cells. Acta Pharmacol Sin. 31:861–866. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Schoene NW, Kelly MA, Polansky MM and
Anderson RA: A polyphenol mixture from cinnamon targets p38 MAP
kinase-regulated signaling pathways to produce G2/M arrest. J Nutr
Biochem. 20:614–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Liu LQ, Liu ZL, Wang X, Cui HY, Jin MD,
Wang DY and Huang SA: Mechanism of cinnamic aldehyde-inducing
apoptosis of chronic myeloid Leukemic cells in vitro. Zhongguo Shi
Yan Xue Ye Xue Za Zhi. 19:617–620. 2011.(In Chinese). PubMed/NCBI
|
|
87
|
Kim JE, Son JE, Jeong H, Joon Kim D, Seo
SK, Lee E, Lim TG, Kim JR, Chen H, Bode AM, et al: A Novel
Cinnamon-Related natural product with Pim-1 inhibitory activity
inhibits leukemia and skin cancer. Cancer Res. 75:2716–2728. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cui Q, Wang JQ, Assaraf YG, Ren L, Gupta
P, Wei L, Ashby CR Jr, Yang DH and Chen ZS: Modulating ROS to
overcome multidrug resistance in cancer. Drug Resist Updat.
41:1–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Farokhzad OC and Langer R: Impact of
nanotechnology on drug delivery. ACS Nano. 3:16–20. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Dong K, Yang C, Yan Y, Wang P, Sun Y, Wang
K, Lu T, Chen Q, Zhang Y, Xing J and Dong Y: Investigation of the
intracellular oxidative stress amplification, safety and anti-tumor
effect of a kind of novel redox-responsive micelle. J Mater Chem B.
6:1105–1117. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Bansal A and Simon MC: Glutathione
metabolism in cancer progression and treatment resistance. J Cell
Biol. 217:2291–2298. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liu Q, Ding X, Xu X, Lai H, Zeng Z, Shan
T, Zhang T, Chen M, Huang Y, Huang Z, et al: Tumor-targeted
hyaluronic acid-based oxidative stress nanoamplifier with ROS
generation and GSH depletion for antitumor therapy. Int J Biol
Macromol. 207:771–783. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Bai Y, Wang R, Wang X, Duan X, Yan X, Liu
C and Tian W: Hyaluronic acid coated Nano-particles for
H2O2-elevation augmented Photo-/Chemodynamic
therapy. Int J Biol Macromol. 245:1255232023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
National Toxicology Program, . NTP
toxicology and carcinogenesis studies of trans-cinnamaldehyde (CAS
No. 14371-10-9) in F344/N rats and B6C3F1 mice (feed studies). Natl
Toxicol Program Tech Rep Ser. 2004:1–281. 2004.PubMed/NCBI
|
|
96
|
Hooth MJ, Sills RC, Burka LT, Haseman JK,
Witt KL, Orzech DP, Fuciarelli AF, Graves SW, Johnson JD and Bucher
JR: Toxicology and carcinogenesis studies of microencapsulated
trans-cinnamaldehyde in rats and mice. Food Chem Toxicol.
42:1757–1768. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Anand P, Murali KY, Tandon V, Murthy PS
and Chandra R: Insulinotropic effect of cinnamaldehyde on
transcriptional regulation of pyruvate kinase, phosphoenolpyruvate
carboxykinase, and GLUT4 translocation in experimental diabetic
rats. Chem Biol Interact. 186:72–81. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kiwamoto R, Ploeg D, Rietjens IM and Punt
A: Dose-dependent DNA adduct formation by cinnamaldehyde and other
food-borne α,β-unsaturated aldehydes predicted by physiologically
based in silico modelling. Toxicol In Vitro. 31:114–125. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Mao M, Zheng W, Deng B, Wang Y, Zhou D,
Shen L, Niku W and Zhang N: Cinnamaldehyde alleviates
doxorubicin-induced cardiotoxicity by decreasing oxidative stress
and ferroptosis in cardiomyocytes. PLoS One. 18:e02921242023.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Abd El Salam ASG, Samaha MM and Abd
Elrazik NA: Cytoprotective effects of cinnamaldehyde and adipoRon
against cyclophosphamide-induced cardio-renal toxicity in rats:
Insights into oxidative stress, inflammation, and apoptosis. Int
Immunopharmacol. 124:1110442023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Bae WY, Choi JS, Kim JE and Jeong JW:
Cinnamic aldehyde suppresses hypoxia-induced angiogenesis via
inhibition of hypoxia-inducible factor-1α expression during tumor
progression. Biochem Pharmacol. 98:41–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
DeCaprio J and Kohl TO: Chromatin
Immunoprecipitation. Cold Spring Harbor Protocols. 2020:0986652020.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Nakato R and Sakata T: Methods for
ChIP-seq analysis: A practical workflow and advanced applications.
Methods. 187:44–53. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hino S, Sato T and Nakao M: Chromatin
immunoprecipitation sequencing (ChIP-seq) for detecting histone
modifications and modifiers. Methods Mol Biol. 2577:55–64. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kumar P, Kiran S, Saha S, Su Z, Paulsen T,
Chatrath A, Shibata Y, Shibata E and Dutta A: ATAC-seq identifies
thousands of extrachromosomal circular DNA in cancer and cell
lines. Sci Adv. 6:eaba24892020. View Article : Google Scholar : PubMed/NCBI
|