Introduction
Systemic lupus erythematosus (SLE) is a systemic
autoimmune disease that affects multiple organs and is more
pronounced in female patients, with a female to male ratio of ~10:1
(1). The incidence ranges from
0.3–31.5 cases per 100,000 individuals per year and has increased
over the past 40 years, probably due to recognition of milder cases
(2). Global adjusted prevalence
rates approach or exceed 50-100 cases per 100,000 adults (1). The pathogenesis of SLE is closely
related to the overactivation of different immune cells (such as T
cells, B cells and monocytes) (3),
and the complex interaction with cytokines can lead to various
clinical manifestations and even threaten life. This clinical
heterogeneity is most likely caused by complex immune
dysregulation, such as loss of immune tolerance to autoantigens and
production of multiple autoantibodies (4). The immune complex formed by the
combination of autoantibody and intracellular autoantigen is
deposited in various tissues and organs. As the disease progresses,
these immune complexes cause tissue damage and various diseases or
manifestations, such as facial rashes, joint pain, nephritis and
cardiovascular disease (2,5).
The complexity of SLE's clinical features indicates
that SLE has multiple subtypes and a potentially unique combination
of disease pathways, genes and environmental factors. Therefore,
management and treatment options for SLE remain challenging for
clinicians. Treatment is mainly based on non-steroidal
anti-inflammatory drugs, corticosteroids, antimalarial drugs,
immunosuppressants (such as cyclophosphamide, tacrolimus and
mycophenolate) and biologics (6–8).
Surprisingly, only three drug treatments related to SLE have been
approved by the Food and Drug Administration in the past decade
(corticosteroids, hydroxychloroquine and beliuzumab). However,
traditional treatments can have a variety of side effects and ideal
treatment options are few. The anti-inflammatory effects of these
drugs are often accompanied by some adverse reactions, including
eye lesions, severe osteoporosis, cardiovascular injury, severe
infection, malignant tumors (2,9,10),
and in severe cases, other organ damage and even death. In
addition, patients with SLE are occassionally resistant to these
drugs (11), therefore it is
urgent to find a drug with significant efficacy and few side
effects.
Resveratrol (RSV) is a natural plant antitoxin found
in grapes, mulberries, peanuts, rhubarb and other plants (8). RSV exists in two isomeric forms,
cis-trans and trans-trans, but the trans-form is the main form,
which has the most effective therapeutic benefits due to its lower
steric hindrance of the side chain and becomes the more
biologically active form due to its higher stability (12–14).
Regarding the immune system, RSV has been proposed for numerous
years as an immunomodulator capable of regulating innate and
adaptive immune responses by interacting with a variety of
molecular pathways, such as macrophages, T cells and B cells. RSV
can also participate in the inhibitory function of CD4+
CD25+ regulatory cell subsets (15,16)
and affect B cell proliferation and autoantibody production
(17,18). Its role as an immunomodulator has
been demonstrated in various animal models and different cell
lines. In addition, RSV has been reported to slow the progression
of autoimmune diseases, such as rheumatoid arthritis (RA),
psoriasis (PsO) and inflammatory bowel disease (IBD) (19). In addition, RSV exerts
anti-inflammatory effects by inhibiting the activation of NF-κB in
immune cells and reducing the levels of tumor necrosis factor-α
(TNF-α), interleukin-1β, IL-6, transforming growth factor-β (TGF-β)
and TNF (20,21). It suggests that RSV can be used as
a potential treatment for SLE.
An increasing number of studies is currently focused
on exploring new drugs and therapies, and certain natural products
have been found to offer significant therapeutic promise in the
treatment of SLE. In the present review, the authors focused on the
protective effects and potential mechanisms of RSV against SLE. At
the same time, the present review summarizes the progress of RSV in
the treatment of SLE and its associated adverse reactions,
providing new insights into the treatment options for SLE.
Related pathogenesis of SLE
The pathogenesis of SLE is closely related to the
overactivation of different immune cells (Fig. 1). In innate immunity, type I
interferon (IFN)-α gene expression was detected in peripheral blood
mononuclear cells in >50% of patients with SLE (22,23).
There is a close relationship between IFN and SLE, especially
IFN-α. IFN-α can promote the transformation of monocytes into
dendritic cells (DC), which recognize antigens and continuously
produce IFN-α, thus further activating lymphocytes and natural
killer (NK) cells, thereby breaking autoimmune tolerance (24). In SLE, the increase of apoptotic
rate or the clearance of obstacles may increase the
autoantigen-antibody complexes, which are endogenous IFN inducers
and can continue to produce IFN, forming a vicious cycle (24). In addition, IFN-α can promote the
activation of helper T cells (Th), improve the ability of DC
antigen presentation, and then induce the production of
interleukin-1, IL-2, IL-4 and other cytokines, promote Th17
differentiation, activate B cells and produce autoantibodies
(24).
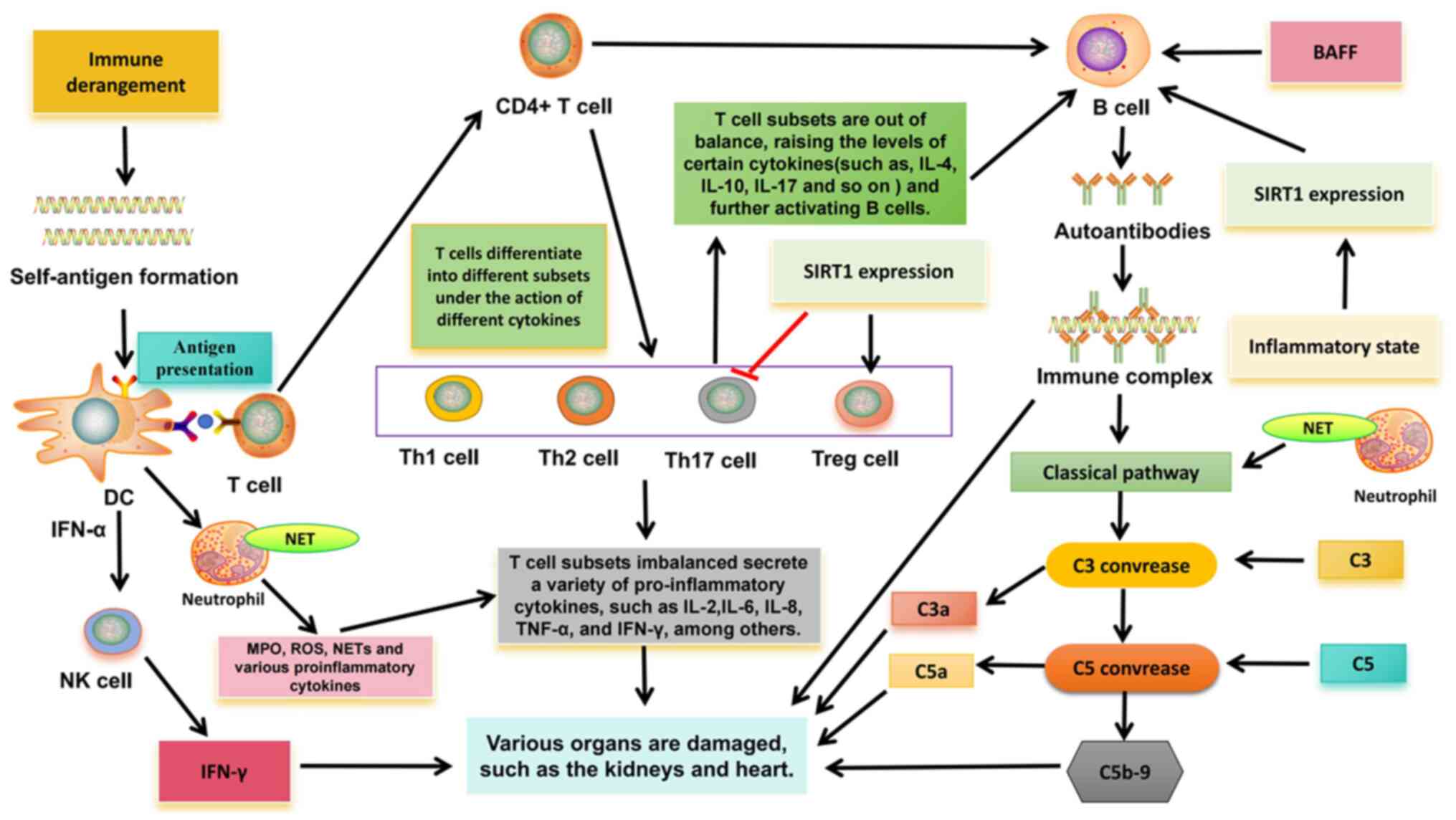 | Figure 1.Related pathogenesis of systemic
lupus erythematosus. DC, dendritic cell; IL, interleukin; MPO,
myeloperoxidase; TNF-α, tumor necrosis factor alpha; IFN-γ,
interferon gamma; ROS, reactive oxygen species; IFN, interferon;
SIRT1, Sirtuin-1; BAFF, B-cell activating factor from the tumor
necrosis factor family; C, complement; NK cell, natural killer
cell; Th, T-helper; Treg, regulatory T cell; NET, neutrophil
extracellular trap. |
In addition, in SLE, overactivation of neutrophils
can release tissue damage factors, such as myeloperoxidase (MPO),
reactive oxygen species (ROS) and a large number of cytokines,
resulting in immune regulation disorders and further tissue damage
(25). Especially neutrophil
extracellular traps (NETs). Obstruction of clearance and/or
excessive formation of NET can externalize self-antigens, thus
inducing IFN synthesis and endothelial damage, which is related to
the pathogenesis of SLE (26). NET
fragments are taken up by plasmacytoid dendritic cells and
presented to autoreactive B cells and T cells, thereby producing
more IFN-α and autoantibodies (27). NETs bind to complement 1q, activate
the classical pathway of complement, consume a large amount of
complement (C3 and C4) and activate other inflammatory complement
fragments (C3a and C5a), and activated complement fragments can
inhibit the degradation of NETs and further aggravate autoimmunity
(24).
In addition, new evidence suggests that NK cells may
be involved in SLE. A number of studies have found that the number
of circulating NK cells in patients with SLE is reduced, which is
not only related to clinical manifestations and disease activity,
but also related to increased serum IFN-α level (28–30).
In addition, previous studies have shown that NK cells can promote
the production of IFN-α by DC (30,31),
and the immune complex mediation of the production of IFN-α by DC
is a characteristic of SLE; thus, there may be an association
between the two. However, the interaction between NK cells and DC
in SLE needs to be further explored. In addition to reduced
numbers, NK cell production of IFN-γ to various stimuli is
significantly increased in patients with active SLE, and NK
cell-derived IFN-γ has been shown to associate with serum IFN-α
levels (32). In mouse animal
models, sustained high levels of IFN-γ in serum can trigger SLE
like syndrome, and the expression of IFN-γ is related to the
formation of anti-double stranded-DNA (anti-DS-DNA) antibodies and
anti-Smith antibodies (33,34),
indicating that IFN-γ is the main effector molecule in the
pathogenesis of SLE. It also suggests that NK cells may play an
important role in the pathogenesis of SLE.
In adaptive immunity, T cells play an important role
in the destruction of immune tolerance in SLE. Autoreactive T cells
are present in both human and mouse SLE, indicating an imbalance in
T cell subsets, that is, between pathogenic T cells and regulatory
T (Treg) cells (35). The
imbalance of Th1/Th2 cells is considered to be closely related to
the occurrence and development of SLE. At present, most scholars
consider that SLE is characterized by decreased Th1 function and
hyper Th2 function. Cytokines secreted by Th1 cells (TNF-α, IL-2
and IFN-γ) are involved in the activation of macrophages and
CD8+ T cells. Th2 cytokines (such as IL-4 and IL-10) can
cause excessive activation of B cells, produce autoantibodies and
cause tissue damage (24,36). In addition, the imbalance of Th17
cells and Treg cells is also associated with SLE. Previous studies
have shown that in SLE, Th17 cells enter inflammatory tissues, such
as the kidney, and promote inflammation by increasing the
production of cytokines (IL-17), which in turn can activate the
production of B-cell antibodies (24). Numerous studies have demonstrated a
reduction in the number and function of Tregs, as well as an
increase in Th17 cells, in patients with SLE (37,38),
indicating a disruption in the dynamic balance between Th17 cells
and Treg cells. Sirtuin-1 (SIRT1), an NAD+-dependent
histone deacetylase, has been shown to be a key regulator of
various physiological processes, including cell differentiation,
immune response and more. Recent studies have provided evidence
that SIRT1 may be a regulatory element in the immune system, and
the imbalance of T cell subsets caused by changes in its function
may be related to the development of SLE (39). For example, the downregulation of
SIRT1 expression can promote the activation of CD4+ T
cells and the secretion of IFN-γ, indicating that the
downregulation of SIRT1 expression can promote T cells to
differentiate Th1 and produce IFN-γ (40). Aromatics receptor (AhR) is a
transcription factor involved in autoimmune diseases, and this
ligand is required for CD4+ T cells to differentiate and
mature into Th17 or Tregs cells. Activation of AhR in peripheral
blood is associated with lupus activity (41). SIRT1 activation has been shown to
reverse AhR-induced imbalances between Th17 and Treg populations
and to upregulate IL-17A and IL-22 levels in CD4+ T
cells (42,43). In T cells, FOXP3 can be directly
deacylated by SIRT1, thereby inhibiting proliferation of Tregs
(39). Downregulation of SIRT1
expression can inhibit Th17 cell differentiation (39). Therefore, SIRT1 is important in
maintaining the balance between Treg cells and Th17 cells, and may
be involved in the pathogenesis of SLE, but there is no direct
evidence to clarify it.
A large number of autoreactive B cells in patients
with SLE produce multiple autoantibodies, which are associated with
the pathogenesis. Through classical T-B cell interactions, B cells
are activated, and activated B cells interact with CD4+
T cells through TCR and co-stimulators. Activated B cells can
secrete cytokines IL-6, TNF, IFN-γ and IL-10 (44). Activated CD4+ T cells
produce IL-21 and T follicular helper (Tfh) cells in response to
IFN-γ. Tfh cells promote the proliferation and differentiation of B
cells through the production of IL-21 and produce IgG and IgA
(24). A number of studies have
revealed that the increase of Tfh cells is not only related to SLE
disease activity, but also positively associated with the level of
autoantibody titers (45,46). In addition, B cell activating
factor (BAFF) is also involved in T-B cell interactions.
Overexpression of BAFF may lead to defects in the survival rate of
autoreactive B cells, and at the same time promote the
proliferation of B cells to continuously form autoantibodies, such
as anti-DS-DNA antibodies, and various serum immunoglobulins (IgM,
IgA, IgE and IgG), while immune complexes are deposited in kidney
tissue (47). Regarding SIRT1,
SIRT1 deficiency was found to enhance T cell-dependent antibody
response. Thus, the differentiation and activation of B cells into
plasma cells is inhibited, the secretion of pro-inflammatory
cytokines of B cells is enhanced, and the production of
autoantibodies is promoted (39),
which may be a possible cause of autoimmune diseases. A previous
study demonstrated that SIRT1 deficient mice develop lupus
nephritis (48). In SLE,
overexpression of SIRT1 can significantly improve the activity of B
cells, inhibit cell apoptosis and upregulate pro-inflammatory
cytokines (IL-1, IL-6 and TNF-α) by regulating the nuclear factor
kappa-B pathway (49), indicating
its potential function in the pathogenesis of SLE.
Effects of RSV on immune cells
RSV can enter cells via passive diffusion, mediated
endocytosis, or via transporters to bind to specific receptors,
such as the integrin receptor αvβ3 (50,51).
It helps regulate innate and adaptive immunity, such as regulating
the activity of mononuclear/macrophages, T cells, B cells and NK
cells. At the same time, because RSV has the ability to activate
SIRT1, it may be able to mitigate the progression of autoimmune
diseases.
RSV and innate immunity
RSV revealed anti-inflammatory effects in
monocytes/macrophages and DCs (52). Macrophages are derived from blood
monocytes and are involved in innate and adaptive immunity. RSV
controls macrophage overactivation by inhibiting
lipopolysaccharide, toll-like receptor 4 signaling and other immune
activators (17). For example, it
inhibits the activation of NF-κB pathway, COX-2 pathway, and
inflammatory body containing the pyridine domain 3 of the NLR
family (17), thereby inhibiting
the secretion of cytokines, such as TNF-α and IL-6. This is
consistent with the findings of Schwager et al (53), in which the authors found that
although RSV was found to enhance the expression of IL-1β and IL-6
in peripheral blood lymphocytes, it had the opposite effect in
macrophages. In addition, it can also alter the expression of
maturation markers on the DC surface and the production of
pro-inflammatory cytokines such as IFN. Since RSV has numerous
molecular targets, numerous of which are related to optimal
maturation of DC, RSV appears to play a more effective
immunosuppressive role during DC differentiation, e.g., DC can
secrete more IL-10 and play an immunosuppressive role (54).
In addition, activation of neutrophils is associated
with organ damage in SLE, such as accumulation in the kidney, which
can cause kidney damage. A previous study revealed that oxidative
stress may also be associated with glomerular damage caused by a
range of pro-inflammatory mediators, including cytokines and
chemical factors, leading to ROS production (55). RSV has a wide range of antioxidant
and anti-inflammatory effects in numerous biological reactions
(56–58). RSV can inhibit neutrophil
activation, downregulate the release of pro-inflammatory cytokines,
such as IL-1β, IL-6, TNF-α, IFN, MPO, reduce the release of NETs,
control inflammatory response and play an important role in
regulating renal blood flow and glomerular filtration function by
upregulating nitric oxide levels (59–61).
These results indicated that RSV had a protective effect on renal
involvement in SLE.
Regarding NK cells, in inflammatory diseases, levels
of inflammatory cytokines such as TNF-α, IL-1β, IL-6, IL-8 and
IFN-γ are elevated, which in turn activate circulating white blood
cells, such as NK cells (62).
Secreted pro-inflammatory cytokines and activated immune cells
cause damage to endothelial cells and can affect important organs
(62). RSV can inhibit the
aforementioned inflammatory cytokines, thereby reducing the
activation of NK cells. In addition, previous studies have
evaluated the properties of RSV on human NK cells. RSV was found to
have a concentration-dependent biphasic effect on NK cells. At high
concentrations (50 µM), RSV inhibited NK cell activity and promoted
apoptosis, which may affect inflammatory signaling pathways
(63). However, when the
concentration range was reduced from 3.13 to 1.56 µM, RSV showed a
positive effect on NK cells by increasing the cytotoxicity of NK
cells through upregulation of IFN-γ expression (mRNA and protein
levels) (63). Therefore, more
studies are needed to confirm the relationship between RSV and NK
cells.
RSV and adaptive immunity
RSV effectively controls inflammation by targeting T
cells, regulating T cell differentiation and inhibiting the release
of pro-inflammatory cytokines and other inflammatory mediators
(17). RSV, as an excitant of
SIRT1, can induce the deacetylation of STAT3 and inhibit its
migration into the nucleus, thus interrupting the activation of
retinoic acid orphan receptor γt and inhibiting the differentiation
of T cells to Th1 (41). A
previous study found that RSV can reduce the proliferation of
CD4+ T cells and the expression levels of CD69 and CD71,
thus triggering the apoptosis of CD4+ T cells, in
addition, the ratio of CD4 IFN-γ+ Th1 cells and Th1/Th2
cells is reduced (64). RSV can
regulate Th1/Th2 balance. In addition, Th17 cells are also one of
the important T cell subsets targeted by RSV. One mode of action of
RSV involves AhR activation, which inhibits Th17 cell activity
(41). Another way is to activate
SIRT1 and block the production of IL-17 (41). The aforementioned findings
suggested that RSV can tilt the balance in favor of the
anti-inflammatory Th2 and Treg cell subsets.
RSV has been shown to have inhibitory effects on B
cells and plasma cells, as well as significant effects on the
production of autoantibodies, which play a role in the pathogenesis
of lupus (17). A previous study
revealed that treatment of norphytane-induced lupus mice with RSV
may inhibit CD4+ T cells by triggering SIRT1,
controlling B cell proliferation and autoantibody production
(64). In addition, RSV can also
increase the expression of Fc γ receptor IIb (FCGR2B) in B cells of
MRL/lpr mice, leading to apoptosis, and a large decrease in the
activation and number of B cells/plasma cells in spleen and bone
marrow, thereby reducing serum autoantibody titers (18). RSV can also promote autophagy and
autophagy flux by inhibiting the Akt/mTOR pathway, thereby impeding
the proliferation and survival of BAFF stimulated B cells (65). The aforementioned findings
indicated that RSV may be a promising drug for the prevention and
treatment of aggressive B-cell diseases and autoimmune diseases
caused by BAFF.
RSV and autoimmune diseases
Autoimmune diseases are mainly caused by the
disorder of immune cells in the body, which leads to excessive
activation of immune cells and the production of a large number of
inflammatory cytokines, such as TNF-α, IFN-γ and IL-1β and so on
(19,66–75).
A strong immune response can attack different organs or tissues at
the same time, resulting in local or systemic immune responses that
damage one or more body tissues or organs in the human body,
including type 1 diabetes, autoimmune hepatitis, RA, amyotrophic
lateral sclerosis, PsO and IBD (66–98).
Natural products have been extensively studied in the treatment and
prevention of various chronic diseases. RSV, a molecule derived
from natural products, is a well-studied substance known for its
effects and therapeutic effects on a variety of chronic diseases,
such as antioxidant, anti-inflammatory and immune regulation
(15–19). In addition to SLE, the autoimmune
diseases that have been studied more can be observed in Table I.
 | Table I.Role of RSV in different rheumatic
diseases. |
Table I.
Role of RSV in different rheumatic
diseases.
| Disease | Disease
mechanism | The therapeutic
mechanism of RSV | Dose of RSV | Efficacy |
|---|
| T1DM | Islet resident DC
uptake of beta cell antigens, presenting to naïve T cells and
promoting Th1 differentiation will activate B lymphocytes that will
produce autoantibodies against beta cells. Th1 will also activate
macrophage and neutrophil migrations to the islet that will promote
beta cell destruction by increasing ROS (19,66–69). | RSV acts via SIRT1
to inhibit apoptotic cell injury during oxidative stress and
increases antioxidant capacity by reducing ROS. In addition, it
plays a role in restoring beta cells in the islets (19,70). | Orally 250 mg/kg,
orubcutaneous injection 25 mg/kg or 10 mg/kg intraperitoneally
(71,72). | Reversing higher
stages of insulitis in the islets of Langerhans. Decrement in
glycosuria (71,72). |
| IBD | The activation of
neutrophils and macrophages in the epithelium leads to the
production of inflammatory mediators, such as ROS and TNF-α.
Antigen recognition by naïve T lymphocytes induces the
differentiation to Th1 and Th17 profiles in Crohn's disease, and to
Th2 and Th17 profiles in ulcerative colitis, with the release of
inflammatory cytokines, especially TNF-α (19,71–75). | RSV is capable of
acting on the inhibition of inflammatory cytokines and neutralizing
ROS (19). | Orally 20 mg/kg or
500 mg/day (74,75). | NF-κB activity,
plasma levels of inflammatory factors and highly sensitive
C-reactive protein were reduced (76,77). |
| PsO | The immune complex
activates resident DC cells and then releases IL-23 to activate T
lymphocytes, promoting differentiation into Th17. IL-16 promotes
differentiation into Th1. These cells produce three major
cytokines, IL-17, IL-22 and IFN, which promote the proliferation of
keratinocytes (78–80). | Inhibiting the
production of IL-17 and directly inhibiting the proliferation of
keratinocytes (19). | Orally 400
mg/kg/day (81). | Reduces the
thickness of the animal's skin (81). |
| RA | T cells secrete
cytokines to activate B cells, and autoantibody production
increases. Th17 response increased with the increase of the
pro-inflammatory cytokine IL-17. In addition, TNF-α and IL-1
production increased, stimulating synovial cells. Synovial
fibroblasts at the site of inflammation increased COX2/PGE2 and
decreased SIRT1. Macrophages increase the recruitment of
neutrophils at the site of inflammation through increased ROS
production and activation of MPO and NF-κB (19,82–85). | RSV is able to act
by reducing the production of autoantibodies, Th17 population,
oxidative stress and NF-κB activation and reduces COX2 and PGE2
expression and activates SIRT1 (86–89). | Orally 20 mg/kg
(91). | Inhibits levels of
pro-inflammatory cytokines such as IFN-γ, TNF-α, IL-6, IL-1, MPO
and IL-4. It also inhibited NF-κB activation. Reduce the production
of autoantibodies (89,90). |
| ALS | TAR DNA-binding
protein 43 accumulation in the motor neuron cytoplasm deregulates
mitochondrial biogenesis and SOD1 function, increasing glutamate
and free radicals in the cytosol. Microglia detect an abnormal cell
and activate naïve T-cell differentiation in the Th1 pattern that
releases cytokines (TNF-α, INF, IL-1, IL-2, IL-6, and IL-7)
(91,92). | RSV acts by
activating SIRT1 and regulates its substrate expression, increases
the SOD1 useful life, reduces ROS, and acts in mitochondrial
biogenesis as an antioxidant and antiapoptotic (93). | Orally high fat
diet containing 4 g resveratrol per kg diet (93). | It can reduce motor
neuron degeneration and delay muscle atrophy (19,93). |
| AIH | Dense lymphoplasmic
inflammatory infiltration occurs in the portal vein bundle
(94,95). Activation and clonal expansion of T
cells lead to the release of autoantibodies and pro-inflammatory
cytokines by B cells (96). | RSV against
concanavalin-A-(ConA-) induced liver injury by significantly
inhibiting IL-2, IL-6, TNF-α (97). | Orally 30 mg/kg
(98). | Inflammatory
cytokines and infiltration of macrophages, neutrophils and T cells
in the mouse liver were significantly reduced, and ConA-mediated
downregulation of SIRT1 in the liver was reversed (98). |
RSV and SLE
SLE can affect all organs of the body, such as the
heart, lungs, kidneys and nervous system (1–3).
Cardiovascular complications and kidney damage are common in
patients with SLE, often leading to disability and death (1,2). RSV
is used to treat SLE mainly through its effect on immune cells, the
potential mechanism of which is illustrated in Fig. 2. At present, the existing studies
mainly focus on the effects of RSV on kidney damage and
cardiovascular complications in SLE, and a small number also
involve the nervous system (97–100). Therefore, a corresponding
discussion on this is included.
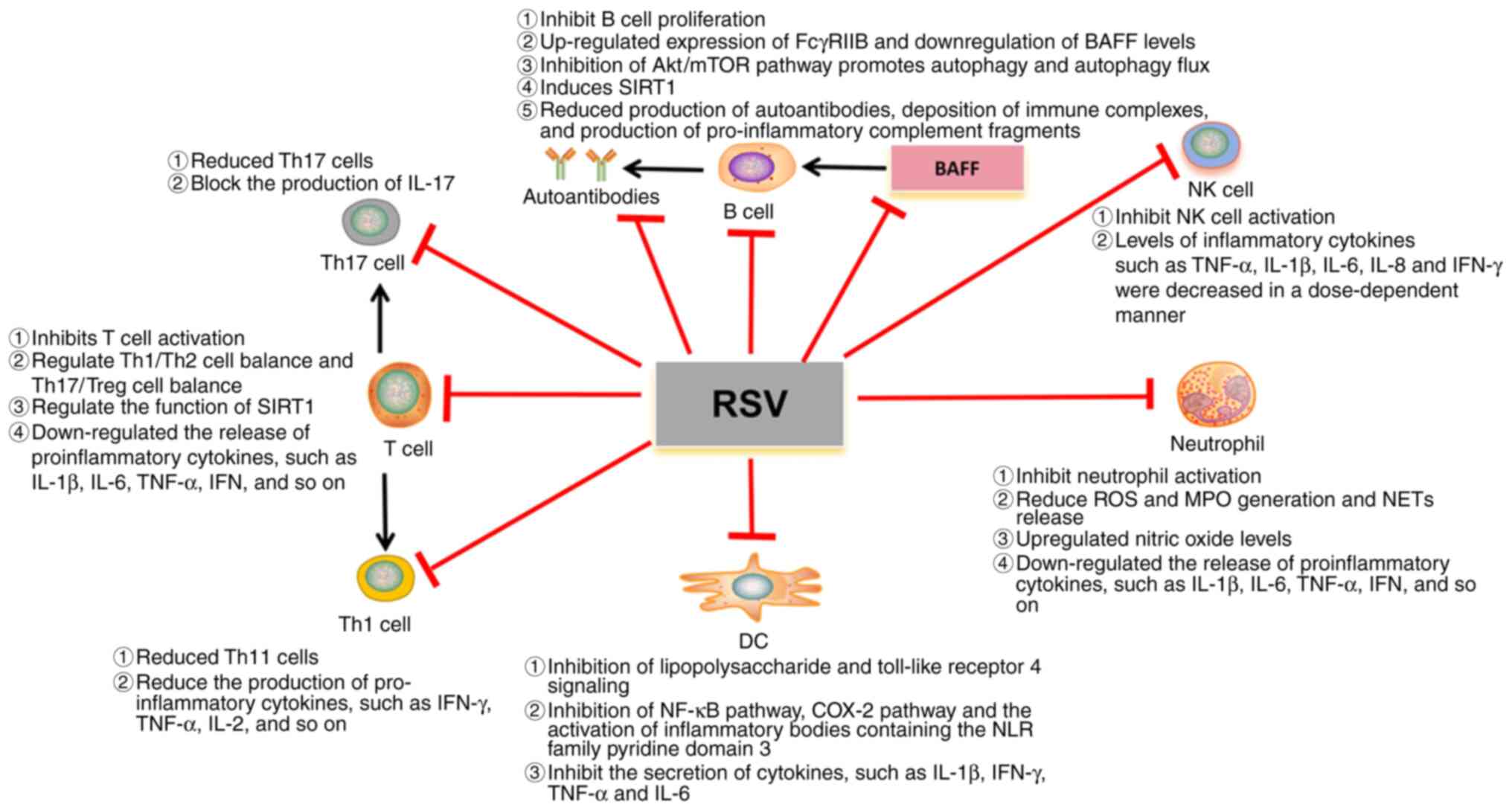 | Figure 2.Potential mechanism of RSV in SLE.
RSV, resveratrol; DC, dendritic cell; IL, interleukin; MPO,
myeloperoxidase; TNF-α, tumor necrosis factor alpha; IFN-γ,
interferon gamma; ROS, reactive oxygen species; IFN, interferon;
SIRT1, Sirtuin-1; BAFF, B cell activating factor from the tumor
necrosis factor family; C, complement; NK cell, natural killer
cell; Th, T-helper; Treg, regulatory T cell; NET, neutrophil
extracellular trap; COX, cyclooxygenase. |
In vitro study
In vitro studies have shown that RSV can
inhibit the activation of CD4+ T cells and B cells,
thereby affecting the proliferation of B cells and antibody
production (64). One of the
functions of IL-10 is to inhibit excessive pro-inflammatory effects
that may lead to tissue damage. It has been shown that IL-10 may
play multiple and opposite roles in mouse lupus (97). IL-10 can promote the proliferation
of autoreactive B cells, Ig class switching and antibody secretion
in SLE, thereby promoting disease progression (98,99).
A different study reported that monocytes in the blood of patients
with active SLE can significantly reduce the level of IL-10 in the
cell culture supernatant after co-culture with RSV (100). The inhibitory effect of RSV on
IL-10 synthesis suggests that it may be useful in the treatment of
SLE. In another study, it was found that the levels of ATP-binding
box transporters A1 and G1 were significantly reduced in cells
treated with plasma from 10% of patients with SLE, which are not
only involved in reverse cholesterol transport, but also enhance
cholesterol efflux from macrophages (101). When SLE plasma is co-incubated
with RSV, efflux protein can be restored to the cellular level of
healthy patients (102),
indicating that RSV can affect cholesterol transport and reduce the
level of oxidized low-density lipoprotein, thus having potential
therapeutic activity for atherosclerotic cardiovascular disease in
SLE.
Animal model
With regard to lupus nephritis, it has been revealed
that different doses of RSV reduced proteinuria, renal
immunoglobulin deposition, glomerulonephritis and serum Ig levels.
One study found that in a mouse model of lupus BBA/C induced by
prostin (102), the mice were
injected with 0.5 ml of Pristine for 7 consecutive months, along
with RSV treatment (50 and 75 mg/kg/day), and were assessed for
autoantibody levels and kidney damage (64). The authors found that RSV can
reduce urinary protein, reduce the deposition of IgM and IgG in the
kidney and reduce the degree of renal histological injury. RSV has
a protective effect on mice with lupus, especially on the kidney.
In a recent study, MRL/lpr mice treated with RSV (50 mg/kg) for 8
weeks were found to have upregulated SIRT1 expression in the
kidneys (103). Upregulation of
SIRT1 interferes with NF-κB expression, transcription and
expression of inflammatory cytokines (103). The second signal is blocked by
SIRT1 regulating ROS production and TRPM2-mediated calcium
perfusion to inhibit the NLRP3 inflammation, thereby slowing down
the progression of lupus nephritis (103). This also suggests that SIRT1 may
be a potential therapeutic target for RSV treatment of SLE. In
addition, Pannu and Bhatnagar (104) studied the combined effects of RSV
(25 and 50 mg/kg) and piperidine in a mouse model of lupus. The
investigators revealed that the combination of RSV and piperidine
successfully alleviated renal manifestations (decreased urinary
protein and serum creatinine), and the combination of other
medications reduced the dose of RSV. The authors also found that
unlike RSV combined with piperine treatment, prophylactic treatment
was more beneficial in reducing lupus-like manifestations such as
lupus nephritis when RSV was used alone (105).
With regard to lupus cardiovascular disease, in
vivo studies revealed that RSV has a therapeutic effect on
atherosclerotic cardiovascular disease in SLE due to its
antioxidant properties (101,106). These results were confirmed in
vivo in a double-knockout ApoE−/− FAS−/−
SLE mouse model (106). The
authors found that mice in the RSV-treated group had fewer
atherosclerotic plaques than those in the untreated group (not
statistically significant), and that 43% of the RSV-treated animals
had no plaques (106). In a
different study, the authors evaluated the progression of aortic
atherosclerosis in mice with SLE associated atherosclerosis after
10 weeks of oral treatment with RSV 0.3–0.4 mg/day in the treatment
group (101). RSV was found to
counteract the effect of SLE on atherosclerosis by preventing lipid
excess by increasing cholesterol efflux (101). These findings suggest that RSV
therapy may provide a novel approach to reduce the atherosclerotic
cardiovascular effects of SLE.
In addition, a recent study also found that in lupus
mice treated with water containing 0.01% RSV, RSV increased the
level of SIRT1 in the hippocampus and decreased the level of
vascular endothelial growth factor and C-X3-C motif chemokine
ligand 1 (CX3CL1) by activating adenosine A2A receptors, but it was
not statistically significant. It also showed a tendency to improve
motor coordination in arteriosclerosis-prone lupus mice (107). This finding indicated that RSV
may be a potential therapeutic candidate for the regulation of
cognitive dysfunction in neuropsychiatric lupus, especially in
motor disorders.
RSV bioavailability and associated
toxicity
The main problem RSV faces in the treatment of
diseases is its low bioavailability. In order to fully study the
bioavailability of RSV, two maximum spikes in plasma levels
occurred in healthy subjects after oral RSV: The first peak
occurred 30-60 min after ingestion and the second peak occurred 6 h
later (108). It was found that
the RSV dose was 25 mg/day and the plasma peak concentration was 10
ng/ml (109). However, a previous
study of high doses (500 mg/day) also demonstrated low plasma
concentrations of ~71.2 ng/ml (110). After ingestion of RSV, although
it is well absorbed orally and has a bioavailability of ~70%, the
bioavailability of RSV itself is close to zero due to extensive
metabolism in the liver and intestine, including glucoaldehyde and
sulfation, which produce metabolites with low bioavailability
(108). In addition, the low
water solubility of RSV (~0.03 mg/ml) is another significant
problem which severely affects the absorption and bioavailability
of the compound (111). The
solubility of RSV is strongly influenced by pH and temperature
(112). A previous study revealed
that RSV solubility is 64 µg/ml at pH 1.2, but becomes 61 and 50
µg/ml at pH 6.8 and above pH 7.4, respectively (112). RSV, when dissolved in water, is
stable only at room or body temperature and under acidic conditions
(113). Therefore, further
research is needed to overcome these problems. In addition, certain
factors may also influence the bioavailability and physiological
response to RSV, such as variability of the human gut microbiome,
genetic polymorphisms, age, sex, ethnicity, diet and exercise
habits (113).
Regarding toxicity, in animal models, extensive
studies using RSV supplements or for a range of diseases have shown
that there are some side effects of the treatment, mainly that high
doses may lead to cardiac inflammation, renal tubule dilation,
nipple necrosis, acute inflammation of the pelvic region and severe
kidney disease, leading to death (114). For instance, one study found that
when animals were treated with successive doses of 0, 300, 1,000
and 3,000 mg/kg/day, no adverse reactions occurred at doses up to
300 mg/kg/day, while 1,000 and 3,000 mg/kg/day caused
nephrotoxicity along with abnormal expression of liver genes. Serum
liver enzyme and bilirubin levels were significantly increased
(114). In addition, some studies
have also observed different degrees of dehydration, dyspnea and
other symptoms in animals (114–116). In mice with renal fibrosis,
low-dose RSV (≤25 mg/kg) can partially improve renal function in
mice with renal injury due to unilateral ureteral obstruction
(UUO). Large doses of RSV (≥50 mg/kg) aggravate renal fibrosis,
indicating that large doses of RSV lose its anti-fibrotic effect.
Notably, mice with UUO-induced kidney damage were more susceptible
to high-dose RSV-induced kidney damage than normal mice (117).
In human trials, RSV is generally well tolerated and
a dose of 450 mg per day of RSV is safe for a person of 60 kg
(118). However, some side
effects have been reported, including cardiac and renal toxicity
and gastrointestinal problems (119,120). Previous studies have found that
high doses of RSV (1,000 mg/day) can increase biomarkers of
cardiovascular disease risk such as oxidized low-density
lipoprotein, soluble E-selectin 1, soluble intercellular adhesion
molecule-1 and soluble vascular cell adhesion molecule-1 (121). RSV appears to have a negative
effect on metabolic status, endothelial health, inflammation and
cardiovascular markers in human patients (122). In a recent meta-analysis,
patients experienced adverse reactions after treatment with high
doses of RSV (123), with a
maximum dose of 1,000 mg per day, 3 times per day. Adverse events
occurred in 3 cases. These adverse events include mild elevation of
alanine aminotransferase, diarrhea, mild indigestion, mild
hypoglycemia and infection (patients with mild cellulitis at the
biopsy site) (124,125). Although RSV is generally safe,
there have been incidents of adverse effects; therefore, more
animal and related human studies are needed to confirm its efficacy
and safety.
Conclusions
SLE is an autoimmune disease that can accumulate in
all organs of the body, therefore it is one of the most important
factors that directly affect the quality of life of patients. The
pathogenesis of SLE is very complex and is associated with
autoimmune disorders, but the exact mechanism remains unclear.
Glucocorticoids and immunosuppressants are still the treatment of
choice, but long-term use of these drugs can lead to numerous
unavoidable side effects. Bioactive natural ingredients derived
from natural herbs may provide additional benefits in the
prevention and treatment of SLE and represent an important source
of new drug screening and development. RSV has a powerful
anti-inflammatory effect by inhibiting the overactivation of immune
cells, reducing the level of procytokines and the production of
autoantibodies. Therefore, RSV is a potential and beneficial
candidate for the treatment of SLE.
However, the current oral bioavailability of RSV is
very low, with a maximum oral bioavailability of only 20%.
Therefore, targeted delivery of RSV to desired tissues or increased
stability of RSV in vivo through the development of
sustained-release systems are critical to improving
bioavailability. With respect to RSV for SLE, the dosage used in
different studies has varied, thus the drug dosage remains unclear.
If future studies can further explore the effect of different drug
doses of RSV on treatment and the deeper mechanism, it will open a
new window for the treatment of SLE. However, further studies in
animals and humans are still needed to widely evaluate the
biological activity, efficacy, safety and appropriate dosage of
RSV, so as to provide a new way of thinking for clinicians to treat
SLE.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
RXH and YTY wrote the manuscript. XCH, DLM and RJH
acquired and interpreted the data. YY, YJH, XZ and JYL
conceptualised and designed the study. CCW and XXH modified the
manuscript. All authors read and approved the final manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gergianaki I, Fanouriakis A, Repa A,
Tzanakakis M, Adamichou C, Pompieri A, Spirou G, Bertsias A,
Kabouraki E, Tzanakis I, et al: Epidemiology and burden of systemic
lupus erythematosus in a Southern European population: Data from
the community-based lupus registry of Crete, Greece. Ann Rheum Dis.
76:1992–2000. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fanouriakis A, Tziolos N, Bertsias G and
Boumpas DT: Update οn the diagnosis and management of systemic
lupus erythematosus. Ann Rheum Dis. 80:14–25. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fang Q, Li T, Chen P, Wu Y, Wang T, Mo L,
Ou J and Nandakumar KS: Comparative Analysis on Abnormal methylome
of differentially expressed genes and disease pathways in the
immune cells of RA and SLE. Front Immunol. 12:6680072021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bentham J, Morris DL, Graham DSC, Pinder
CL, Tombleson P, Behrens TW, Martín J, Fairfax BP, Knight JC, Chen
L, et al: Genetic association analyses implicate aberrant
regulation of innate and adaptive immunity genes in the
pathogenesis of systemic lupus erythematosus. Nat Genet.
47:1457–1464. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fava A and Petri M: Systemic lupus
erythematosus: Diagnosis and clinical management. J Autoimmun.
96:1–13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fanouriakis A, Kostopoulou M, Alunno A,
Aringer M, Bajema I, Boletis JN, Cervera R, Doria A, Gordon C,
Govoni M, et al: 2019 update of the EULAR recommendations for the
management of systemic lupus erythematosus. Ann Rheum Dis.
78:736–745. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M,
Vidal X, Mitjavila F, Castro Salomó A, Cuquet Pedragosa J,
Ortiz-Santamaria V, Mauri Plana M and Cortés-Hernández J:
Enteric-coated mycophenolate sodium versus azathioprine in patients
with active systemic lupus erythematosus: A randomised clinical
trial. Ann Rheum Dis. 76:1575–1582. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Navarra SV, Guzmán RM, Gallacher AE, Hall
S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, León MG, et al:
Efficacy and safety of belimumab in patients with active systemic
lupus erythematosus: A randomised, placebo-controlled, phase 3
trial. Lancet. 377:721–731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balasubramanian A, Wade SW, Adler RA, Lin
CJF, Maricic M, O'Malley CD, Saag K and Curtis JR: Glucocorticoid
exposure and fracture risk in patients with new-onset rheumatoid
arthritis. Osteoporos Int. 27:3239–3249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh BK and Singh S: Systemic lupus
erythematosus and infections. Reumatismo. 72:154–169. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi H, Gudjonsson JE and Kahlenberg JM:
Treatment of cutaneous lupus erythematosus: Current approaches and
future strategies. Curr Opin Rheumatol. 32:208–214. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cardile V, Chillemi R, Lombardo L, Sciuto
S, Spatafora C and Tringali C: Antiproliferative activity of
methylated analogues of E- and Z-resveratrol. Z Naturforsch C J
Biosci. 62:189–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weiskirchen S and Weiskirchen R:
Resveratrol: How much wine do you have to drink to stay healthy?
Adv Nutr. 7:706–718. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raj P, Thandapilly SJ, Wigle J, Zieroth S
and Netticadan T: A comprehensive analysis of the efficacy of
resveratrol in atherosclerotic cardiovascular disease, myocardial
infarction and heart failure. Molecules. 26:66002021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Švajger U and Jeras M: Anti-inflammatory
effects of resveratrol and its potential use in therapy of
immune-mediated diseases. Int Rev Immunol. 31:202–222. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Y, Paik JH, Cho D, Cho JA and Kim CW:
Resveratrol induces the suppression of tumor-derived CD4+CD25+
regulatory T cells. Int Immunopharmacol. 8:542–547. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malaguarnera L: Influence of resveratrol
on the immune response. Nutrients. 11:9462019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jhou JP, Chen SJ, Huang HY, Lin WW, Huang
DY and Tzeng SJ: Upregulation of FcγRIIB by resveratrol via NF-κB
activation reduces B-cell numbers and ameliorates lupus. Exp Mol
Med. 49:e3812017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oliveira ALB, Monteiro VVS,
Navegantes-Lima KC, Reis JF, Gomes RS, Rodrigues DVS, Gaspar SLF
and Monteiro MC: Resveratrol role in autoimmune disease-a
mini-review. Nutrients. 9:13062017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fu Y, Yan M, Xie C, Hu J, Zeng X and Hu Q:
Polydatin relieves paraquat-induced human MRC-5 fibroblast injury
through inhibiting the activation of the NLRP3 inflammasome. Ann
Transl Med. 8:7652020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yahfoufi N, Alsadi N, Jambi M and Matar C:
The immunomodulatory and anti-inflammatory role of polyphenols.
Nutrients. 10:16182018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han GM, Chen SL, Shen N, Ye S, Bao CD and
Gu YY: Analysis of gene expression profiles in human systemic lupus
erythematosus using oligonucleotide microarray. Genes Immun.
4:177–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishii T, Onda H, Tanigawa A, Ohshima S,
Fujiwara H, Mima T, Katada Y, Deguchi H, Suemura M, Miyake T, et
al: Isolation and expression profiling of genes upregulated in the
peripheral blood cells of systemic lupus erythematosus patients.
DNA Res. 12:429–439. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan L, Lu MP, Wang JH, Xu M and Yang SR:
Immunological pathogenesis and treatment of systemic lupus
erythematosus. World J Pediatr. 16:19–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Smith CK and Kaplan MJ: The role of
neutrophils in the pathogenesis of systemic lupus erythematosus.
Curr Opin Rheumatol. 27:448–453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Berthelot JM, Le Goff B, Neel A, Maugars Y
and Hamidou M: NETosis: At the crossroads of rheumatoid arthritis,
lupus, and vasculitis. Joint Bone Spine. 84:255–262. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fresneda Alarcon M, McLaren Z and Wright
HL: Neutrophils in the pathogenesis of rheumatoid arthritis and
systemic lupus erythematosus: Same Foe Different M.O. Front
immunol. 12:6496932021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park YW, Kee SJ, Cho YN, Lee EH, Lee HY,
Kim EM, Shin MH, Park JJ, Kim TJ, Lee SS, et al: Impaired
differentiation and cytotoxicity of natural killer cells in
systemic lupus erythematosus. Arthritis Rheum. 60:1753–1763. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Riccieri V, Spadaro A, Parisi G, Taccari
E, Moretti T, Bernardini G, Favaroni M and Strom R: Down-regulation
of natural killer cells and of gamma/delta T cells in systemic
lupus erythematosus. Does it correlate to autoimmunity and to
laboratory indices of disease activity? Lupus. 9:333–337.
2000.PubMed/NCBI
|
|
30
|
Huang Z, Fu B, Zheng SG, Li X, Sun R, Tian
Z and Wei H: Involvement of CD226+ NK cells in immunopathogenesis
of systemic lupus erythematosus. J Immunol. 186:3421–3431. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eloranta ML, Lövgren T, Finke D, Mathsson
L, Rönnelid J, Kastner B, Alm GV and Rönnblom L: Regulation of the
interferon-alpha production induced by RNA-containing immune
complexes in plasmacytoid dendritic cells. Arthritis Rheum.
60:2418–2427. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hervier B, Beziat V, Haroche J, Mathian A,
Lebon P, Ghillani-Dalbin P, Musset L, Debré P, Amoura Z and
Vieillard V: Phenotype and function of natural killer cells in
systemic lupus erythematosus: Excess interferon-γ production in
patients with active disease. Arthritis Rheum. 63:1698–1706. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hodge DL, Berthet C, Coppola V,
Kastenmüller W, Buschman MD, Schaughency PM, Shirota H, Scarzello
AJ, Subleski JJ, Anver MR, et al: IFN-gamma AU-rich element removal
promotes chronic IFN-gamma expression and autoimmunity in mice. J
Autoimmun. 53:33–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu M, Liu J, Hao S, Wu P, Zhang X, Xiao
Y, Jiang G and Huang X: Higher activation of the interferon-gamma
signaling pathway in systemic lupus erythematosus patients with a
high type I IFN score: Relation to disease activity. Clin
Rheumatol. 37:2675–2684. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nandakumar KS and Nündel K: Editorial:
Systemic lupus erythematosus-predisposition factors, pathogenesis,
diagnosis, treatment and disease models. Front Immunol.
13:11181802022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Horwitz DA, Gray JD, Behrendsen SC, Kubin
M, Rengaraju M, Ohtsuka K and Trinchieri G: Decreased production of
interleukin-12 and other Th1-type cytokines in patients with
recent-onset systemic lupus erythematosus. Arthritis Rheum.
41:838–844. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee HY, Hong YK, Yun HJ, Kim YM, Kim JR
and Yoo WH: Altered frequency and migration capacity of CD4+CD25+
regulatory T cells in systemic lupus erythematosus. Rheumatology
(Oxford). 47:789–794. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma J, Yu J, Tao X, Cai L, Wang J and Zheng
SG: The imbalance between regulatory and IL-17-secreting CD4+ T
cells in lupus patients. Clin Rheumatol. 29:1251–1258. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qiu Y, Zhou X, Liu Y, Tan S and Li Y: The
role of sirtuin-1 in immune response and systemic lupus
erythematosus. Front Immunol. 12:6323832021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen X, Zhang XL, Zhang GH and Gao YF:
Artesunate promotes Th1 differentiation from CD4+ T cells to
enhance cell apoptosis in ovarian cancer via miR-142. Braz J Med
Biol Res. 52:e79922019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dorgham K, Amoura Z, Parizot C, Arnaud L,
Frances C, Pionneau C, Devilliers H, Pinto S, Zoorob R, Miyara M,
et al: Ultraviolet light converts propranolol, a nonselective
β-blocker and potential lupus-inducing drug, into a proinflammatory
AhR ligand. Eur J Immunol. 45:3174–3187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo NH, Fu X, Zi FM, Song Y, Wang S and
Cheng J: The potential therapeutic benefit of resveratrol on
Th17/Treg imbalance in immune thrombocytopenic purpura. Int
Immunopharmacol. 73:181–192. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Delmas D, Limagne E, Ghiringhelli F and
Aires V: Immune Th17 lymphocytes play a critical role in the
multiple beneficial properties of resveratrol. Food Chem Toxicol.
137:1110912020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fillatreau S, Sweenie CH, McGeachy MJ,
Gray D and Anderton SM: B cells regulate autoimmunity by provision
of IL-10. Nat Immunol. 3:944–950. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Choi JY, Ho JHE, Pasoto SG, Bunin V, Kim
ST, Carrasco S, Borba EF, Gonçalves CR, Costa PR, Kallas EG, et al:
Circulating follicular helper-like T cells in systemic lupus
erythematosus: Association with disease activity. Arthritis
Rheumatol. 67:988–999. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
He J, Tsai LM, Leong YA, Hu X, Ma CS,
Chevalier N, Sun X, Vandenberg K, Rockman S, Ding Y, et al:
Circulating precursor CCR7(lo)PD-1(hi) CXCR5+
CD4+ T cells indicate Tfh cell activity and promote
antibody responses upon antigen reexposure. Immunity. 39:770–781.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Groom JR, Fletcher CA, Walters SN, Grey
ST, Watt SV, Sweet MJ, Smyth MJ, Mackay CR and Mackay F: BAFF and
MyD88 signals promote a lupuslike disease independent of T cells. J
Exp Med. 204:1959–1971. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sequeira J, Boily G, Bazinet S, Saliba S,
He X, Jardine K, Kennedy C, Staines W, Rousseaux C, Mueller R and
McBurney MW: sirt1-null mice develop an autoimmune-like condition.
Exp Cell Res. 314:3069–3074. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang Q, Yan C, Xin M, Han L, Zhang Y and
Sun M: Sirtuin 1 (Sirt1) overexpression in BaF3 cells contributes
to cell proliferation promotion, apoptosis resistance and
pro-inflammatory cytokine production. Med Sci Monit. 23:1477–1482.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Delmas D and Lin HY: Role of membrane
dynamics processes and exogenous molecules in cellular resveratrol
uptake: Consequences in bioavailability and activities. Mol Nutr
Food Res. 55:1142–1153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ho Y, Li ZL, Shih YJ, Chen YR, Wang K,
Whang-Peng J, Lin HY and Davis PJ: Integrin αvβ3 in the mediating
effects of dihydrotestosterone and resveratrol on breast cancer
cell proliferation. Int J Mol Sci. 21:29062020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bonizzi G and Karin M: The two NF-kappaB
activation pathways and their role in innate and adaptive immunity.
Trends Immunol. 25:280–288. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Schwager J, Richard N, Widmer F and
Raederstorff D: Resveratrol distinctively modulates the
inflammatory profiles of immune and endothelial cells. BMC
Complement Altern Med. 17:3092017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Svajger U, Obermajer N and Jeras M:
Dendritic cells treated with resveratrol during differentiation
from monocytes gain substantial tolerogenic properties upon
activation. Immunology. 129:525–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sener G, Tuğtepe H, Yüksel M, Cetinel S,
Gedik N and Yeğen BC: Resveratrol improves
ischemia/reperfusion-induced oxidative renal injury in rats. Arch
Med Res. 37:822–829. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang W, Sun L, Zhang P, Song J and Liu W:
An anti-inflammatory cell-free collagen/resveratrol scaffold for
repairing osteochondral defects in rabbits. Acta Biomater.
10:4983–4995. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Orsu P, Murthy BVSN and Akula A:
Cerebroprotective potential of resveratrol through anti-oxidant and
anti-inflammatory mechanisms in rats. J Neural Transm (Vienna).
120:1217–1223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bo S, Ciccone G, Castiglione A, Gambino R,
De Michieli F, Villois P, Durazzo M, Cavallo-Perin P and Cassader
M: Anti-inflammatory and antioxidant effects of resveratrol in
healthy smokers a randomized, double-blind, placebo-controlled,
cross-over trial. Curr Med Chem. 20:1323–1331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Balkrishna A, Sinha S, Kumar A, Arya V,
Gautam AK, Valis M, Kuca K, Kumar D and Amarowicz R:
Sepsis-mediated renal dysfunction: Pathophysiology, biomarkers and
role of phytoconstituents in its management. Biomed Pharmacother.
165:1151832023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu FC, Tsai HI and Yu HP:
Organ-protective effects of red wine extract, resveratrol, in
oxidative stress-mediated reperfusion injury. Oxid Med Cell Longev.
2015:5686342015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
de Souza Andrade MM, Leal VNC, Fernandes
IG, Gozzi-Silva SC, Beserra DR, Oliveira EA, Teixeira FME, Yendo
TM, Sousa MDGT, Teodoro WR, et al: Resveratrol downmodulates
neutrophil extracellular trap (NET) generation by neutrophils in
patients with severe COVID-19. Antioxidants (Basel). 11:16902022.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Rieder SA, Nagarkatti P and Nagarkatti M:
Multiple anti-inflammatory pathways triggered by resveratrol lead
to amelioration of staphylococcal enterotoxin B-induced lung
injury. Br J Pharmacol. 167:1244–1258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li Q, Huyan T, Ye LJ, Li J, Shi JL and
Huang QS: Concentration-dependent biphasic effects of resveratrol
on human natural killer cells in vitro. J Agric Food Chem.
62:10928–10935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang ZL, Luo XF, Li MT, Xu D, Zhou S, Chen
HZ, Gao N, Chen Z, Zhang LL and Zeng XF: Resveratrol possesses
protective effects in a pristane-induced lupus mouse model. PLoS
One. 9:e1147922014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yao Y, Zhu J, Qin S, Zhou Z, Zeng Q, Long
R, Mao Z, Dong X, Zhao R, Zhang R, et al: Resveratrol induces
autophagy impeding BAFF-stimulated B-cell proliferation and
survival by inhibiting the Akt/mTOR pathway. Biochem Pharmacol.
202:1151392022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wållberg M and Cooke A: Immune mechanisms
in type 1 diabetes. Trends Immunol. 34:583–591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Battaglia M: Neutrophils and type 1
autoimmune diabetes. Curr Opin Hematol. 21:8–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kaur G, Padiya R, Adela R, Putcha UK,
Reddy GS, Reddy BR, Kumar KP, Chakravarty S and Banerjee SK: Garlic
and resveratrol attenuate diabetic complications, loss of β-cells,
pancreatic and hepatic oxidative stress in streptozotocin-induced
diabetic rats. Front Pharmacol. 7:3602016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lee SM, Yang H, Tartar DM, Gao B, Luo X,
Ye SQ, Zaghouani H and Fang D: Prevention and treatment of diabetes
with resveratrol in a non-obese mouse model of type 1 diabetes.
Diabetologia. 54:1136–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yonamine CY, Pinheiro-Machado E, Michalani
ML, Freitas HS, Okamoto MM, Corrêa-Giannella ML and Machado UF:
Resveratrol improves glycemic control in insulin-treated diabetic
rats: Participation of the hepatic territory. Nutr Metab (Lond).
13:442016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Boirivant M and Cossu A: Inflammatory
bowel disease. Oral Dis. 18:1–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Singh UP, Singh NP, Busbee B, Guan H,
Singh B, Price RL, Taub DD, Mishra MK, Nagarkatti M and Nagarkatti
PS: Alternative medicines as emerging therapies for inflammatory
bowel diseases. Int Rev Immunol. 31:66–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tian T, Wang Z and Zhang J:
Pathomechanisms of oxidative stress in inflammatory bowel disease
and potential antioxidant therapies. Oxid Med Cell Longev.
2017:45351942017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sánchez-Fidalgo S, Cárdeno A, Villegas I,
Talero E and de la Lastra CA: Dietary supplementation of
resveratrol attenuates chronic colonic inflammation in mice. Eur J
Pharmacol. 633:78–84. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Samsami-Kor M, Daryani NE, Asl PR and
Hekmatdoost A: Anti-inflammatory effects of resveratrol in patients
with ulcerative colitis: A randomized, double-blind,
placebo-controlled pilot study. Arch Med Res. 46:280–285. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hänsel A, Günther C, Ingwersen J, Starke
J, Schmitz M, Bachmann M, Meurer M, Rieber EP and Schäkel K: Human
slan (6-sulfo LacNAc) dendritic cells are inflammatory dermal
dendritic cells in psoriasis and drive strong TH17/TH1 T-cell
responses. J Allergy Clin Immunol. 127:787–794.e1-e9. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lowes MA, Suárez-Fariñas M and Krueger JG:
Immunology of psoriasis. Annu Rev Immunol. 32:227–255. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lynde CW, Poulin Y, Vender R, Bourcier M
and Khalil S: Interleukin 17A: Toward a new understanding of
psoriasis pathogenesis. J Am Acad Dermatol. 71:141–150. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kjær TN, Thorsen K, Jessen N, Stenderup K
and Pedersen SB: Resveratrol ameliorates imiquimod-induced
psoriasis-like skin inflammation in mice. PLoS One.
10:e01265992015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Navegantes KC, de Souza Gomes R, Pereira
PAT, Czaikoski PG, Azevedo CHM and Monteiro MC: Immune modulation
of some autoimmune diseases: The critical role of macrophages and
neutrophils in the innate and adaptive immunity. J Transl Med.
15:362017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tanaka T, Hishitani Y and Ogata A:
Monoclonal antibodies in rheumatoid arthritis: comparative
effectiveness of tocilizumab with tumor necrosis factor inhibitors.
Biologics. 8:141–153. 2014.PubMed/NCBI
|
|
82
|
Brzustewicz E and Bryl E: The role of
cytokines in the pathogenesis of rheumatoid arthritis-Practical and
potential application of cytokines as biomarkers and targets of
personalized therapy. Cytokine. 76:527–536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Engler A, Tange C, Frank-Bertoncelj M, Gay
RE, Gay S and Ospelt C: Regulation and function of SIRT1 in
rheumatoid arthritis synovial fibroblasts. J Mol Med (Berl).
94:173–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ma C, Wang Y, Dong L, Li M and Cai W:
Anti-inflammatory effect of resveratrol through the suppression of
NF-κB and JAK/STAT signaling pathways. Acta Biochim Biophys Sin
(Shanghai). 47:207–213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lee SJ, Thien Quach CH, Jung KH, Paik JY,
Lee JH, Park JW and Lee KH: Oxidized low-density lipoprotein
stimulates macrophage 18F-FDG uptake via hypoxia-inducible
factor-1α activation through Nox2-dependent reactive oxygen species
generation. J Nucl Med. 55:1699–1705. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Tsai MH, Hsu LF, Lee CW, Chiang YC, Lee
MH, How JM, Wu CM, Huang CL and Lee IT: Resveratrol inhibits urban
particulate matter-induced COX-2/PGE2 release in human
fibroblast-like synoviocytes via the inhibition of activation of
NADPH oxidase/ROS/NF-κB. Int J Biochem Cell Biol. 88:113–123. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xuzhu G, Komai-Koma M, Leung BP, Howe HS,
McSharry C, McInnes IB and Xu D: Resveratrol modulates murine
collagen-induced arthritis by inhibiting Th17 and B-cell function.
Ann Rheum Dis. 71:129–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wahba MGF, Messiha BAS and Abo-Saif AA:
Protective effects of fenofibrate and resveratrol in an aggressive
model of rheumatoid arthritis in rats. Pharm Biol. 54:1705–1715.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Avendaño-Vázquez SE, Dhir A, Bembich S,
Buratti E, Proudfoot N and Baralle FE: Autoregulation of TDP-43
mRNA levels involves interplay between transcription, splicing, and
alternative polyA site selection. Genes Dev. 26:1679–1684. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Malaspina A, Puentes F and Amor S: Disease
origin and progression in amyotrophic lateral sclerosis: An
immunology perspective. Int Immunol. 27:117–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Higashida K, Kim SH, Jung SR, Asaka M,
Holloszy JO and Han DH: Effects of resveratrol and SIRT1 on PGC-1α
activity and mitochondrial biogenesis: A reevaluation. PLoS Biol.
11:e10016032013. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhao W, Varghese M, Yemul S, Pan Y, Cheng
A, Marano P, Hassan S, Vempati P, Chen F, Qian X and Pasinetti GM:
Peroxisome proliferator activator receptor gamma coactivator-1alpha
(PGC-1α) improves motor performance and survival in a mouse model
of amyotrophic lateral sclerosis. Mol Neurodegener. 6:512011.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
McFarlane IG: Pathogenesis of autoimmune
hepatitis. Biomed Pharmacother. 53:255–263. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ichiki Y, Aoki CA, Bowlus CL, Shimoda S,
Ishibashi H and Gershwin ME: T cell immunity in autoimmune
hepatitis. Autoimmun Rev. 4:315–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Gianchecchi E and Fierabracci A: Insights
on the effects of resveratrol and some of its derivatives in cancer
and autoimmunity: A molecule with a dual activity. Antioxidants
(Basel). 9:912020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Huang TH, Chen CC, Liu HM, Lee TY and
Shieh SH: Resveratrol pretreatment attenuates concanavalin
a-induced hepatitis through reverse of aberration in the immune
response and regenerative capacity in aged mice. Sci Rep.
7:27052017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Biswas S, Bieber K and Manz RA: IL-10
revisited in systemic lupus erythematosus. Front Immunol.
13:9709062022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Facciotti F, Larghi P, Bosotti R, Vasco C,
Gagliani N, Cordiglieri C, Mazzara S, Ranzani V, Rottoli E, Curti
S, et al: Evidence for a pathogenic role of extrafollicular,
IL-10-producing CCR6+B helper T cells in systemic lupus
erythematosus. Proc Natl Acad Sci USA. 117:7305–7316. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Caielli S, Veiga DT, Balasubramanian P,
Athale S, Domic B, Murat E, Banchereau R, Xu Z, Chandra M, Chung
CH, et al: A CD4+ T cell population expanded in lupus
blood provides B cell help through interleukin-10 and succinate.
Nat Med. 25:75–81. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Klonowska-Szymczyk A, Kulczycka-Siennicka
L, Robak T, Smolewski P, Cebula-Obrzut B and Robak E: The impact of
agonists and antagonists of TLR3 and TLR9 on concentrations of
IL-6, IL10 and sIL-2R in culture supernatants of peripheral blood
mononuclear cells derived from patients with systemic lupus
erythematosus. Postepy Hig Med Dosw (Online). 71:867–875. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Voloshyna I, Teboul I, Littlefield MJ,
Siegart NM, Turi GK, Fazzari MJ, Carsons SE, DeLeon J and Reiss AB:
Resveratrol counters systemic lupus erythematosus-associated
atherogenicity by normalizing cholesterol efflux. Exp Biol Med
(Maywood). 241:1611–1619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Satoh M and Reeves WH: Induction of
lupus-associated autoantibodies in BALB/c mice by intraperitoneal
injection of pristane. J Exp Med. 180:2341–2346. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Tian J, Huang T, Chen J, Wang J, Chang S,
Xu H, Zhou X, Yang J, Xue Y, Zhang T, et al: SIRT1 slows the
progression of lupus nephritis by regulating the NLRP3 inflammasome
through ROS/TRPM2/Ca2+ channel. Clin Exp Med.
23:3465–3478. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Pannu N and Bhatnagar A: Combinatorial
therapeutic effect of resveratrol and piperine on murine model of
systemic lupus erythematosus. Inflammopharmacology. 28:401–424.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Pannu N and Bhatnagar A: Prophylactic
effect of resveratrol and piperine on pristane-induced murine model
of lupus-like disease. Inflammopharmacology. 28:719–735. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Feng X, Li H, Rumbin AA, Wang X, La Cava
A, Brechtelsbauer K, Castellani LW, Witztum JL, Lusis AJ and Tsao
BP: ApoE-/-Fas-/-C57BL/6 mice: A novel murine model simultaneously
exhibits lupus nephritis, atherosclerosis, and osteopenia. J Lipid
Res. 48:794–805. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kasselman LJ, Renna HA, Voloshyna I,
Pinkhasov A, Gomolin IH, Teboul I, De Leon J, Carsons SE and Reiss
AB: Cognitive changes mediated by adenosine receptor blockade in a
resveratrol-treated atherosclerosis-prone lupus mouse model. J
Tradit Complement Med. 12:447–454. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Li C, Wang Z, Lei H and Zhang D: Recent
progress in nanotechnology-based drug carriers for resveratrol
delivery. Drug Deliv. 30:21742062023. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Walle T, Hsieh F, DeLegge MH, Oatis JE Jr
and Walle UK: High absorption but very low bioavailability of oral
resveratrol in humans. Drug Metab Dispos. 32:1377–1382. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Sergides C, Chirilă M, Silvestro L, Pitta
D and Pittas A: Bioavailability and safety study of resveratrol 500
mg tablets in healthy male and female volunteers. Exp Ther Med.
11:164–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Summerlin N, Soo E, Thakur S, Qu Z,
Jambhrunkar S and Popat A: Resveratrol nanoformulations: Challenges
and opportunities. Int J Pharm. 479:282–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zupančič Š, Lavrič Z and Kristl J:
Stability and solubility of trans-resveratrol are strongly
influenced by pH and temperature. Eur J Pharm Biopharm. 93:196–204.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Novelle MG, Wahl D, Diéguez C, Bernier M
and de Cabo R: Resveratrol supplementation: Where are we now and
where should we go? Ageing Res Rev. 21:1–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Shaito A, Posadino AM, Younes N, Hasan H,
Halabi S, Alhababi D, Al-Mohannadi A, Abdel-Rahman WM, Eid AH,
Nasrallah GK and Pintus G: Potential adverse effects of
resveratrol: A literature review. Int J Mol Sci. 21:20842020.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Hebbar V, Shen G, Hu R, Kim BR, Chen C,
Korytko PJ, Crowell JA, Levine BS and Kong AN: Toxicogenomics of
resveratrol in rat liver. Life Sci. 76:2299–2314. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Crowell JA, Korytko PJ, Morrissey RL,
Booth TD and Levine BS: Resveratrol-associated renal toxicity.
Toxicol Sci. 82:614–619. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Liu S, Zhao M, Zhou Y, Wang C, Yuan Y, Li
L, Bresette W, Chen Y, Cheng J, Lu Y and Liu J: Resveratrol exerts
dose-dependent anti-fibrotic or pro-fibrotic effects in kidneys: A
potential risk to individuals with impaired kidney function.
Phytomedicine. 57:223–235. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and beta-catenin signalling: Diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Howells LM, Berry DP, Elliott PJ, Jacobson
EW, Hoffmann E, Hegarty B, Brown K, Steward WP and Gescher AJ:
Phase I randomized, double-blind pilot study of micronized
resveratrol (SRT501) in patients with hepatic metastases-safety,
pharmacokinetics, and pharmacodynamics. Cancer Prev Res (Phila).
4:1419–1425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Poulsen MM, Vestergaard PF, Clasen BF,
Radko Y, Christensen LP, Stødkilde-Jørgensen H, Møller N, Jessen N,
Pedersen SB and Jørgensen JO: High-dose resveratrol supplementation
in obese men: An investigator-initiated, randomized,
placebo-controlled clinical trial of substrate metabolism, insulin
sensitivity, and body composition. Diabetes. 62:1186–1195. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Mankowski RT, You L, Buford TW,
Leeuwenburgh C, Manini TM, Schneider S, Qiu P and Anton SD: Higher
dose of resveratrol elevated cardiovascular disease risk biomarker
levels in overweight older adults-A pilot study. Exp Gerontol.
131:1108212020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Ramírez-Garza SL, Laveriano-Santos EP,
Marhuenda-Muñoz M, Storniolo CE, Tresserra-Rimbau A,
Vallverdú-Queralt A and Lamuela-Raventós RM: Health effects of
resveratrol: Results from human intervention trials. Nutrients.
10:18922018. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhang T, He Q, Liu Y, Chen Z and Hu H:
Efficacy and safety of resveratrol supplements on blood lipid and
blood glucose control in patients with type 2 diabetes: A
systematic review and meta-analysis of randomized controlled
trials. Evid Based Complement Alternat Med.
2021:56441712021.PubMed/NCBI
|
|
124
|
Goh KP, Lee HY, Lau DP, Supaat W, Chan YH
and Koh AFY: Effects of resveratrol in patients with type 2
diabetes mellitus on skeletal muscle SIRT1 expression and energy
expenditure. Int J Sport Nutr Exerc Metab. 24:2–13. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Sattarinezhad A, Roozbeh J, Shirazi
Yeganeh B, Omrani GR and Shams M: Resveratrol reduces albuminuria
in diabetic nephropathy: A randomized double-blind
placebo-controlled clinical trial. Diabetes Metab. 45:53–59. 2019.
View Article : Google Scholar : PubMed/NCBI
|
















