Introduction
Pulmonary arterial hypertension (PAH) is one of the
most severe types of chronic cardiopulmonary disorder with an
incidence of 15–50/million, high mortality and poor prognosis, and
the 5-year survival rate is 38%. The PAH is caused by a multitude
of diseases and diverse pathogenic mechanisms (such as left heart
failure, Interstitial lung disease with hypoxemia) and
characterized by progressive pulmonary artery remodeling, resulting
in elevated mean pulmonary arterial pressure (mPAP) >25 mmHg at
rest and pulmonary vascular resistance, culminating in right heart
failure and mortality (1). At
present, the pathogenesis of PAH has not been completely
elucidated. However, PAH pulmonary vascular remodeling is
histopathologically similar to malignant tumors, including cell
proliferation and glucose metabolism pathway abnormalities
(2). These findings have led to
PAH being denoted as the ‘cancer of the cardiovascular world’. Like
cancer, PAH is marked by autonomous growth signaling, resistance to
antiproliferative cues, evasion of programmed cell death and
persistent neovascularization (3).
Patients with PAH exhibit inactivated or deleted oncogenes,
including protein tyrosine phosphatase, large tumor suppressor
kinase 1 gene, forkhead box O1 and phosphoprotein p53, alongside an
increased expression of telomerase reverse transcriptase in
pulmonary arterial smooth muscle cells (PASMCs) (4).
The pathological characteristics of both cancer and
PAH can be regarded as a manifestation of glucometabolic
reprogramming resulting from cell proliferative disorder (5). Previous studies have demonstrated
that dysregulated activation of hypoxia-inducible factor-1α
(HIF-1α) influences the activity of a number of aerobic glycolytic
enzymes, including glucose transporter 1 (Glut1), hexokinase 2
(HK2), pyruvate dehydrogenase kinase 1(PDK1), and lactate
dehydrogenase (LDH), which redirects ATP generation from
mitochondria to the cytosolic space, a phenomenon that can
transpire under the circumstance of sufficient oxygen availability
and is commonly referred to as aerobic glycolysis or the Warburg
effect (6,7). These changes potentiate uptake of
glucose in PASMCs, suppress the activity of the tricarboxylic acid
(TCA) cycle and increase rapid cell proliferation by the
upregulation of the pentose phosphate pathway in synthesis of
purine nucleotides (8).
Dichloroacetate (DCA), a PDK1 inhibitor, inhibits pulmonary artery
remodeling and decreases pulmonary vascular resistance by promoting
oxidative phosphorylation of glucose and facilitating the TCA
metabolic pathway (9). Typically,
mitochondrial oxidative phosphorylation is the primary source of
energy in differentiated cells, whereas a number of tumor cells
predominantly rely on aerobic glycolysis, commonly referred to as
the Warburg effect (10). Despite
the low efficiency of ATP production by aerobic glycolysis, it
provides raw materials for tumor cell proliferation and promotes
tumor cell metastasis (11). In
summary, inhibition of the Warburg effect by targeting regulation
of enzymes associated with to glucose metabolism could impede
pulmonary vascular remodeling and decrease pulmonary circulation
resistance.
Oroxylin A (OA), a flavonoid derivative of
Scutellaria baicalensis, possesses therapeutic properties
against numerous types of malignant tumors (12). OA has been demonstrated as a
therapeutic candidate for breast cancer by decreasing HK2
expression, leading to a substantial decrease in proliferation of
MDA-MB-231 and MCF-7 breast cancer cell lines (13). Moreover, OA inhibits HIF-1α and
glycolysis via the downregulation of aerobic glycolytic enzymes,
including HK2, LDH and PDK1. It also decreases the levels of
complex III in the electron transport chain. These mechanisms
contribute to decreased lactate production in hypoxic HepG2
hepatocellular carcinoma cells (14,15).
Overall, OA as an anticancer drug achieves therapeutic effects by
inhibiting cell proliferation and glycolysis, which suggests
potential use of OA in PAH.
The present study aimed to assess therapeutic
potential of OA in a monocrotaline (MCT)-induced PAH rat model to
evaluate underlying mechanisms of the beneficial effects of OA in
PAH, particularly through mitigating Warburg effect.
Materials and methods
Animals and ethics approval
A total of 48 adult male Sprague-Dawley rats [age, 3
months; weight, 250–280 g; specific-pathogen-free (SPF)-grade;
certificate no. SCXK 2019–0004] was purchased from Hunan Slake
Jingda Experimental Animal Co., Ltd.). The rats were housed in an
SPF-grade animal facility of Zunyi Medical University [Zunyi,
China; certificate no. SYXK (QIAN) 2021–0003] and had ad
libitum access to food and water, 12/12- h alternation of day
and night at 20–24°C and 50–60% humidity. Standard feed and water
were provided for 1 week as acclimatization. The general condition
of rats was observed every day during the experiment The study was
approved by the Experimental Animal Ethics Committee of Zunyi
Medical University (approval no. ZMU11-2203-273).
Grouping and drug administration
Sprague-Dawley rats were allocated randomly into
five groups as follows: Control (n=6); PAH (n=12); OA 40 and 80
mg/kg/day (both n=10) and the 100 mg/kg/day DCA (n=10). The rats
were administered 55 mg/kg MCT (InvivoChem LLC) or an equal volume
of normal saline as a control via intraperitoneal injection
(16). The rats in OA40, OA80 and
DCA groups were treated with 40 or 80 mg/kg OA (Jiangsu Yongjian
Pharmaceutical Technology Co., Ltd.) (17,18)
or DCA (100 mg/kg; InvivoChem LLC) by intragastric administration
for 2 weeks, respectively. The control and PAH groups were
administered an equivalent volume of normal saline via the same
route. Observe the rats daily, record their weight.
Measurement of mPAP by right heart
catheterization
Rats were anesthetized by intraperitoneal injection
of pentobarbital sodium solution (50 mg/kg). The measurement of
mPAP was performed using the central venous catheter technique, as
previously reported (19). A
central venous catheter (Secalon, 16 G/1.6×400.0 mm, Viggo
products) was positioned into the right subclavian vein, extending
through the superior vena cava, right atrium, right ventricle and
into the pulmonary artery. The catheter was linked to a PowerLab
physiological recording system (ADInstruments Pty Ltd.) and
pressure transducer for the real-time display of pulmonary artery
pressure. The recorded data were analyzed to determine the mPAP.
After the pressure measurement, rats were anesthetized by
intraperitoneal injection of 1% sodium pentobarbital (130 mg/kg).
After anesthesia, the rats were sacrificed by cervical dislocation,
and the heart and lung tissues of the rats were collected. The
humane endpoints were as follows: Inability to eat or drink without
anesthesia or sedation or stand for up to 24 h; poor condition
including hypothermia with a body temperature <37°C in the
absence of anesthesia/sedation and central nervous system
depression, tremor, paralysis or pain that does not respond to
analgesics. A total of two rats in the PAH and one each in the OA40
and OA80 group were euthanized in compliance with the humane
endpoints.
Histopathological assessment
A total of five rats from each group were randomly
chosen. The lower right lobe of the lung was excised and fixed in
10% neutral formaldehyde at room temperature for 24 h, followed by
dehydration through a series of graded alcohol concentrations. The
tissues were embedded in paraffin and sectioned at 4 µm thickness,
followed by staining steps at room temperature. Hematoxylin and
eosin (HE) staining was used to examine pulmonary artery
remodeling. The tissue sections were immersed in hematoxylin
staining solution for 7 min and rinsed with running water for 15
sec. Next, the sections were soaked in 1% hydrochloric ethanol,
differentiated for 3 sec, and rinsed with running water for 15 sec.
Finally, the tissue sections were immersed in eosin staining
solution, stained for 3 min, and rinsed with running water for 15
sec. Masson's trichrome staining was applied to assess pulmonary
fibrosis. The sections were immersed in Bouin solution (60°C, 30
min) and rinsed with running water for 15 sec. Next, the sections
were soaked in azure blue, stained for 3 min, and rinsed with
running water for a few seconds. After that, the tissue sections
were immersed in Mayer hematoxylin, stained for 3 min, and rinsed
with running water for 15 sec. The sections were immersed in 1%
hydrochloric ethanol, differentiated for 3 sec, and rinsed with
running water for 15 sec. The tissue sections were further immersed
in Ponceau magenta solution, stained for 10 min, and rinsed with
running water for 15 sec. The tissue sections were then immersed in
phosphomolybdic acid solution for 10 min. Next, the tissue sections
were directly immersed in aniline blue solution and stained for 8
min. The final treatment was carried out with a weak acid solution
for 2 min. All tissue sections were subjected to gradient
dehydration using alcohol at the end of staining and were sealed
and preserved by dropping neutral gum. Digital photographs were
captured by the light microscope (Olympus Corporation; cat. no.
BX43; magnification, ×400). The cross-sectional dimensions and wall
area (WA) of all small arteries were quantified using Image-Pro
Plus software version 50.100 (Media Cybernetics, Inc.). Pulmonary
vessel WA was calculated as a percentage of the vessel
cross-sectional area as follows: WA=(vessel WA/vessel
cross-sectional area) ×100. Fibrosis was measured using Image-Pro
Plus 6.0 (Media Cybernetics, Inc.) and the percentage of pulmonary
fibrosis (area ratio) was calculated as follows: Area ratio=(blue
collagen fibrosis staining area/total test area) ×100.
Untargeted metabolomics
assessment
A total of five rats were randomly selected from
each group for untargeted metabolomics assessment. A sample of 25
mg rat lung tissue was combined with 500 µl extraction solution,
consisting of a 2:2:1 ratio of acetonitrile, methanol and water,
supplemented with isotopically-labelled internal standard mixture
(Shanghai Zhenzhun Biotechnology Co., LTD, Merck Serono). Then,
lung tissue samples were ground for 4 min at a frequency of 35 Hz
and subjected to ultrasonic processing at a frequency of 40 kHz in
ice-water (ultrasound 5 sec/interval 5 sec, 30 times, total time 5
min). The grinding and ultrasonic treatment were repeated three
times. After incubation at −40°C for 1 h, samples were centrifuged
at 13,800 × g at 4°C for 15 min. The supernatant was transferred
into sample vials for analysis. Equal volumes of supernatant from
all samples were combined to create a quality control (QC) sample
for instrument testing.
LC-MS/MS analyses were performed using an UHPLC
system (Vanquish; Thermo Fisher Scientific, Inc.) with a UPLC BEH
Amide column (2.1×100 mm, 1.7 µm) coupled to Orbitrap Exploris 120
mass spectrometer (Orbitrap MS, Thermo). Chromatographic
conditions: Waters ACQUITY UPLC BEH Amide (2.1×100 mm, 1.7 µm)
column, mobile phase A:25 mmol/l ammonium acetate and 25 mmol/l
ammonia water/1 l ultrapure water, mobile phase B: acetonitrile.
Gradient elution (0–0.5 min, 95% B; 0.5–7 min, 95–65% B; 7–8 min,
65–40% B B; 8 to 9 min, 40% B; 9–9.1 min, 40–95% B; 9.1–12 min, 95%
B), and column temperature 30°C, sample room temperature of 4°C,
the flow rate of 0.5 ml/min, 2 µl sample quantity.
Orbitrap Exploris 120 mass spectrometer (room
temperature, nebulizer pressure, 87 psi, flow rate 0.3 l/min),
controlled by the acquisition software (Xcalibur 4.4; Thermo Fisher
Scientific, Inc.), was used due to its ability of acquiring tandem
mass spectrometry (MS) spectra in information-dependent acquisition
mode. Under this operational setting, the software consistently
evaluates the complete full scan MS spectrum. The electrospray
ionization source parameters were as follows: Sheath gas flow rate,
50 arbitrary units (Arb); auxiliary gas flow rate, 15 Arb;
capillary temperature, 320°C; full MS resolution, 60,000; MS/MS
resolution, 15,000; collision energy in Normalized Collision Energy
mode, 10–60 units and spray voltage, 3.8 or −3.4 kV for positive
and negative ionization polarity, respectively.
The raw data was converted into mzXML format using
the ProteoWizard 3.0 (http://proteowizard.sourceforge.net/) software suite
and subjected to a custom processing pipeline (20) developed using R version 4.3.3 (R
Core Team, R-project.org/) built upon the XCMS framework (21). This proprietary program used peak
detection, extraction, alignment and integration. Subsequently, an
internally constructed MS2 database was used for metabolite
annotation purposes, with the threshold for annotation established
at 0.3. Principal component analysis (PCA) and Orthogonal
Projections to Latent Structures-Discriminant Analysis (OPLS-DA)
were performed using SIMCA software (V16.0.2, Sartorius Stedim Data
Analytics AB) Logarithmic (LOG) and centralized (CTR) format, then
automatic modeling analysis. In order to verify the quality of the
model, we used 7-fold cross validation to test. Then R2Y
(interpretability of the model to categorical variables Y) and
Q2 (predictability of the model) obtained after
cross-validation were used to evaluate the effectiveness of the
model. Finally, through permutation test, the permutation order of
categorical variable Y is changed at random to obtain different
random Q values, and the validity of the model is further tested.
By mapping the differential metabolites to authoritative metabolite
databases such as KEGG and PubChem (22) (kegg.jp/, we obtained matching
information for the differential metabolites and then searched and
analyzed metabolic pathways for the corresponding species Rattus
norvegicus (rat). Regarding the hierarchical clustering analysis of
differential metabolites, the Euclidean distance matrix of
quantitative values of differential metabolites is calculated, and
the differential metabolites are clustered using the complete
linkage method.
Western blotting
A total of five rats were randomly selected from
each group for western blotting to determine protein expression
levels. Frozen lung tissue (−80°C) samples were homogenized and
immersed in RIPA lysis buffer supplemented with 1 nM PMSF (both
Beijing Solarbio Science & Technology Co., Ltd.). The samples
were homogenized and centrifuged at 12,000 × g at 4°C for 10 min.
The supernatant was harvested, total protein content was quantified
using a BCA Protein Assay kit (Beijing Solarbio Science &
Technology Co., Ltd.). According to the molecular weight of the
protein, different concentrations of SDS-PAGE (8–10%) were used,
and the protein loading volume was 30 µg. The separated proteins
were transferred onto polyvinylidene fluoride membranes (0.45 µm;
MilliporeSigma) and blocked with 5% skimmed milk solution (Beijing
Solarbio Science & Technology Co., Ltd.) in 1X TBST buffer for
2 h at room temperature. The membranes were incubated overnight at
4°C with primary antibodies against Glut1 (1:1,000; Abcam;
ab115730), HK2 (1:1,000; Abcam; ab209847), pyruvate kinase (PK)
(1:1,000; Cell Signaling Technology; #3186), PDK1 (1:1,000; Abcam;
ab110025), LDH (1:1,000; Proteintech; 19987-1-AP), isocitrate
dehydrogenase 2 (IDH2; 1:1,000; Proteintech; 15932-1-AP), β-tubulin
(1:1,000; Proteintech; 66031-1-Ig) and β-actin (1:10,000;
Proteintech; 66009-1-lg). Subsequently, membranes were incubated
with a horseradish peroxidase-conjugated secondary antibody
(1:5,000; Proteintech; RGAR001) for 1 h at room temperature under
gentle agitation. Protein bands were visualized using enhanced
chemiluminescence substrate (Melunbio; MA0186) and protein
expression was quantified using Quantity One software (version
1.4.6; Bio-Rad Laboratories, Inc.).
Statistical analysis
All data were evaluated using SPSS Statistics
Professional software (version 29.0; IBM Corp.) and presented as
mean ± SEM (n=5). The data was evaluated for normal distribution
using Kolmogorov-Smirnov test. For data that follow a normal
distribution with homogeneous variance, statistical analysis was
performed using one-way ANOVA with Tukey's post hoc test. For data
that did not conform to a normal distribution or had uneven
variance, Welch ANOVA with Tamhane T2 test was used. P<0.05 was
considered to indicate a statistically significant difference.
Results
OA reduces mPAP and inhibits pulmonary
vascular remodeling and vascular fibrosis in PAH model rats
To assess the therapeutic effect of OA on PAH, OA
was administered to MCT-induced PAH rats at doses of 40 and 80
mg/kg/d for 14 days (Fig. 1A).
MCT-induced PAH model in rats is characterized by late-stage
complications including heart failure, hepatic congestion and
ascites, which culminate in a notable mortality rate (23). The mortality of these rats was
associated with the toxic properties of MCT. MCT administration
reduced the growth rate, while OA (40 mg/kg) increased the growth
rate (Fig. 1C).
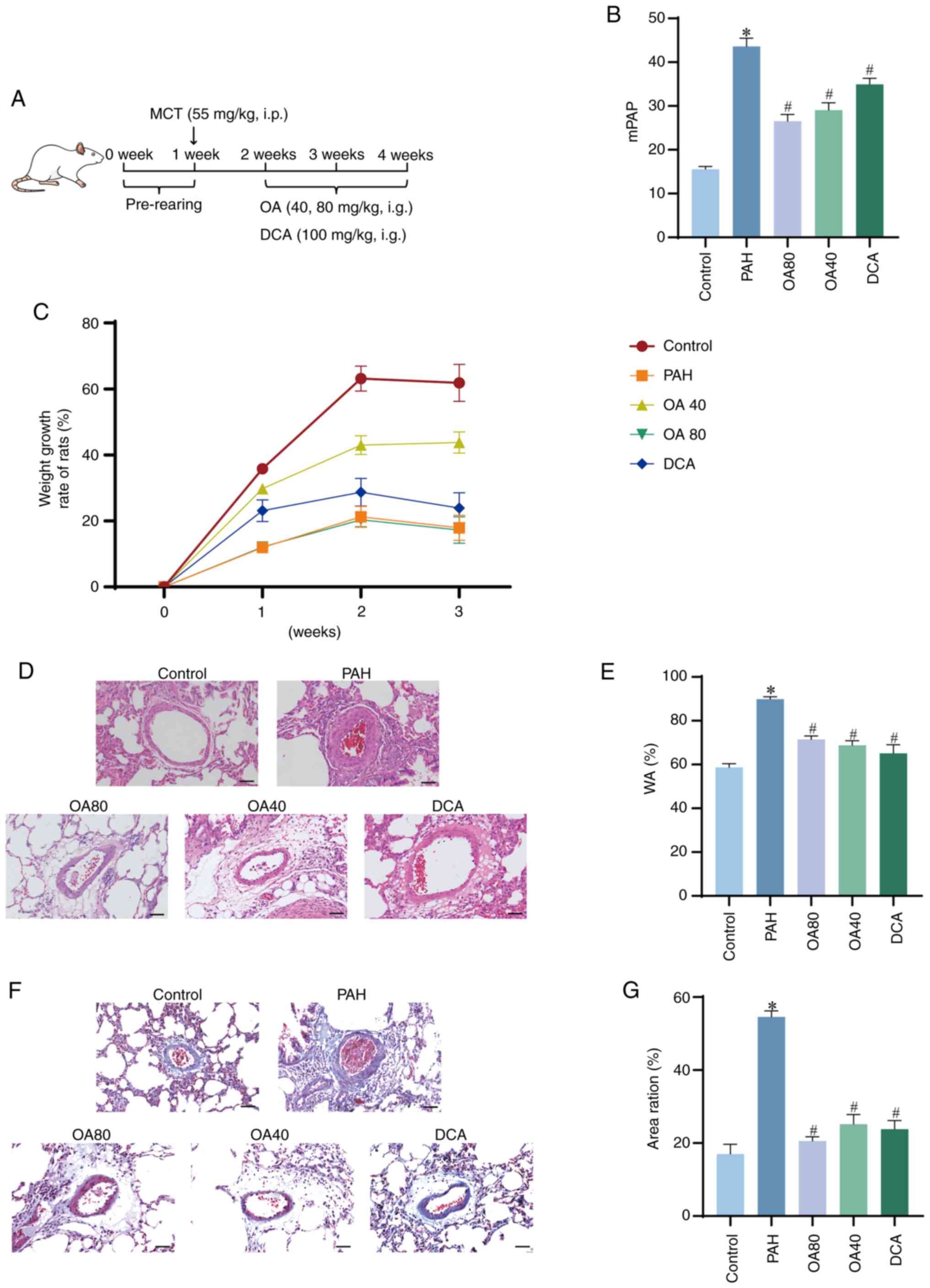 | Figure 1.OA alleviated the development of
MCT-induced PAH in rats. (A) Timeline of OA treatment. (B) mPAP
quantitative analysis. (C) Growth rate of rats. (D) Representative
hematoxylin and eosin staining of pulmonary artery (scale bar, 50
µm). (E) Quantitative analysis of vascular remodeling. (F)
Representative pulmonary artery after Masson staining (scale bar,
50 µm). (G) Quantitative analysis of vascular fibrosis. *P<0.05
vs. control. #P<0.05 vs. PAH, OA, oroxylin A; PAH,
pulmonary arterial hypertension; mPAP, pulmonary arterial pressure;
MCT, monocrotaline; DCA, dichloroacetate; w, week; WA, wall
area. |
In the PAH model group, the mPAP value was 43.6
mmHg, which was significantly increased compared with the control
group at 15.5 mmHg. Furthermore, the OA40 and OA80 groups had a
significantly lower mPAP compared with the PAH group, with a
reduction of 33.4 and 39.2%, respectively, demonstrating a
dose-dependent response. Likewise, the DCA group also had a
significant decrease of 19.9% in mPAP value compared with the PAH
group (Fig. 1B). These results
confirmed establishment of the PAH model and the efficacy of OA in
decreasing mPAP.
Pathological changes in pulmonary arteries in the
PAH group included notable narrowing of vessel lumens and
intima-media thickening along with marked arterial remodeling
(Fig. 1D). WA of the PAH group was
significantly increased by 34.6% compared with the control.
Moreover, OA40, OA80 and DCA groups had a significantly decreased
WA compared with the PAH group (Fig.
1E). Masson's staining demonstrated pulmonary vascular fibrosis
surrounded by a large number of collagen fibrosis in the PAH group
(Fig. 1F). Area ratio was
significantly higher in the PAH compared with Control group. OA at
different doses significantly improved pulmonary arteriolar
fibrosis and OA40 mg/kg had an improvement comparable to DCA
(Fig. 1F and G).
OA alters the metabolite profile of
lung tissue in PAH based on untargeted metabolomics analysis
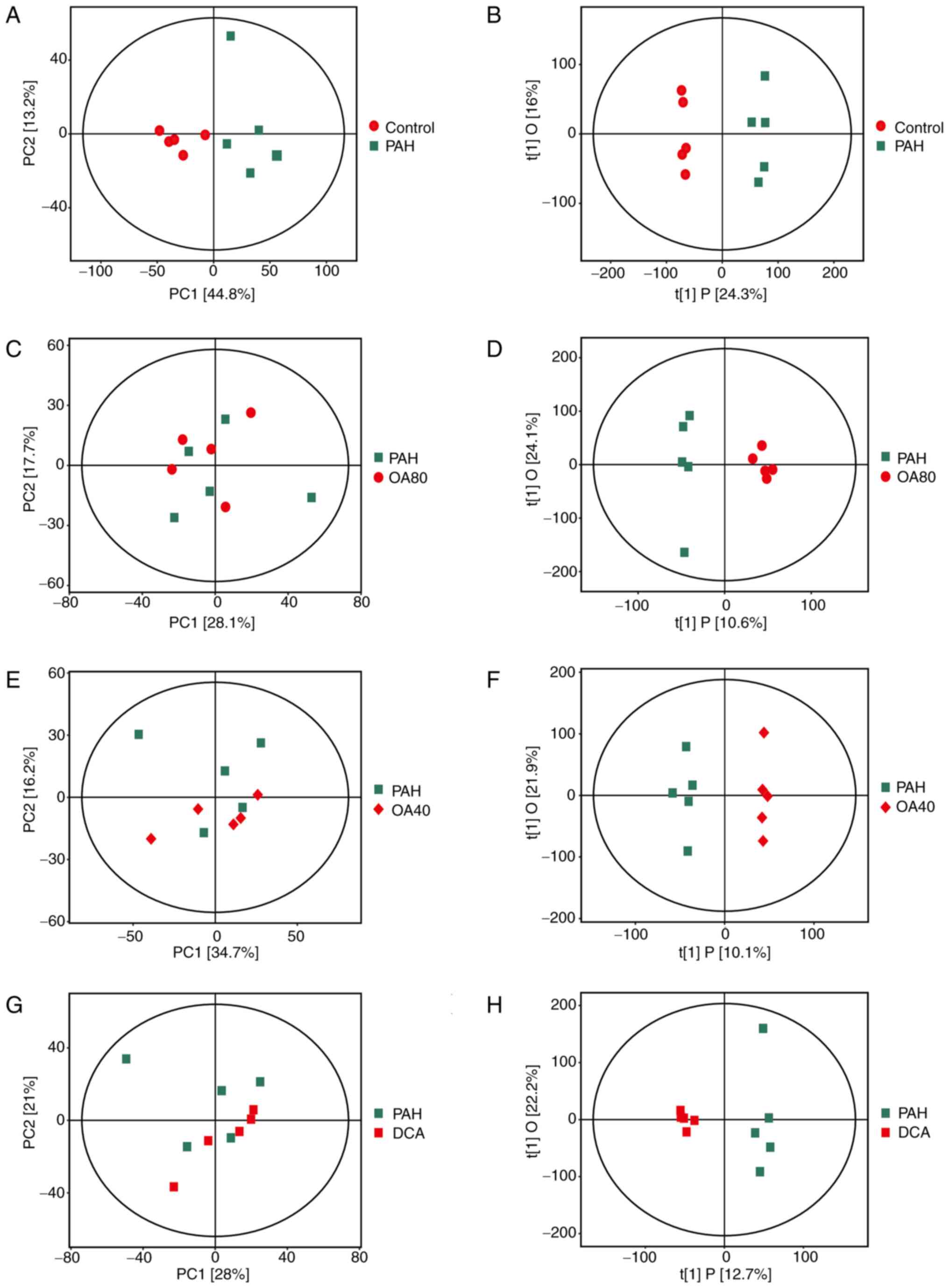
PCA and OPLS-DA scoring plots of the metabolic
profiles of lung tissues were calculated (Fig 2); PCA score plot shows that the PAH
group is initially separated from the Control group, indicating
that the PAH model induced by MCT was successfully established
(Fig. 2A, B). The initial
separation results of the lung tissue metabolome spectra in the
OA40 and 80 groups and the DCA group are not obvious (Fig. 2C, E, G). Therefore, OPLS-DA model
was established for greater differentiation between treatment
groups. Screening of effective differential metabolites was
performed. The predictive capacity of the OPLS-DA model was
satisfactory, effectively distinguishing between the groups
(Fig. 2B, D, F and H). These
findings indicated that OA exerts an impact on the metabolic levels
of PAH rats.
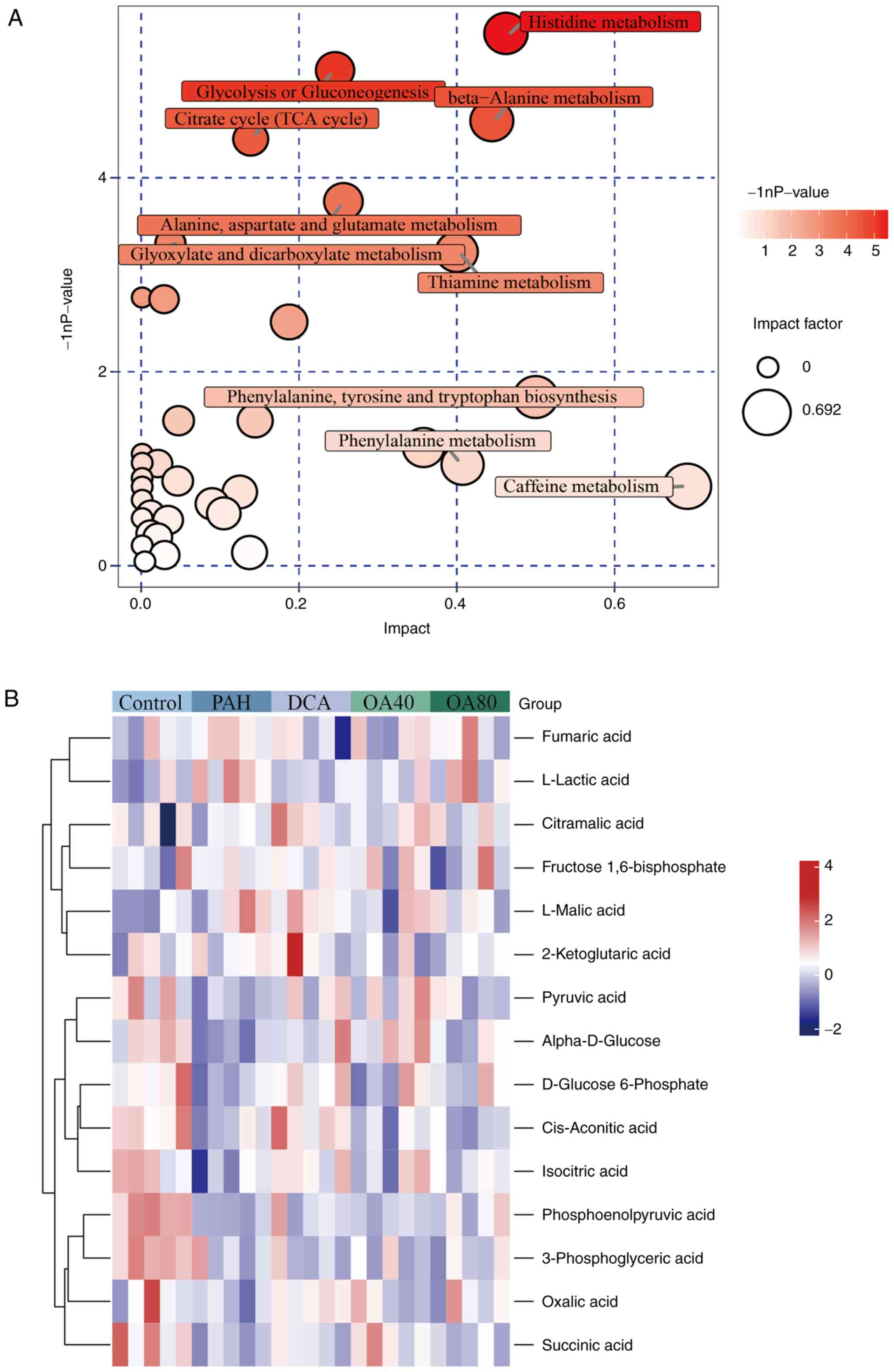
Metabolic pathway analysis and
association with pathway metabolites
To assess the metabolic pathways involved in PAH,
metabolites exhibiting differences between the PAH and control
groups were analyzed using R. This analysis focused on pathways in
which these differentially expressed metabolites were involved,
identifying enrichment in the ‘glycolysis or gluconeogenesis’ and
‘citrate cycle (TCA cycle)’ pathways (Fig. 3A). To assess the association
between metabolites and the Warburg effect in PAH and OA-treated
groups, a Euclidean distance matrix was created based on relative
levels of metabolites within the pathway and data were normalized
using Z-scores. The heatmap indicated that the majority of
metabolites varied across the five groups (Fig. 3B). Further evaluation of these
metabolites aimed to elucidate their association with PAH in the
context of Warburg effect. Notably, eight types of metabolites
demonstrated notable variation between the PAH and control groups
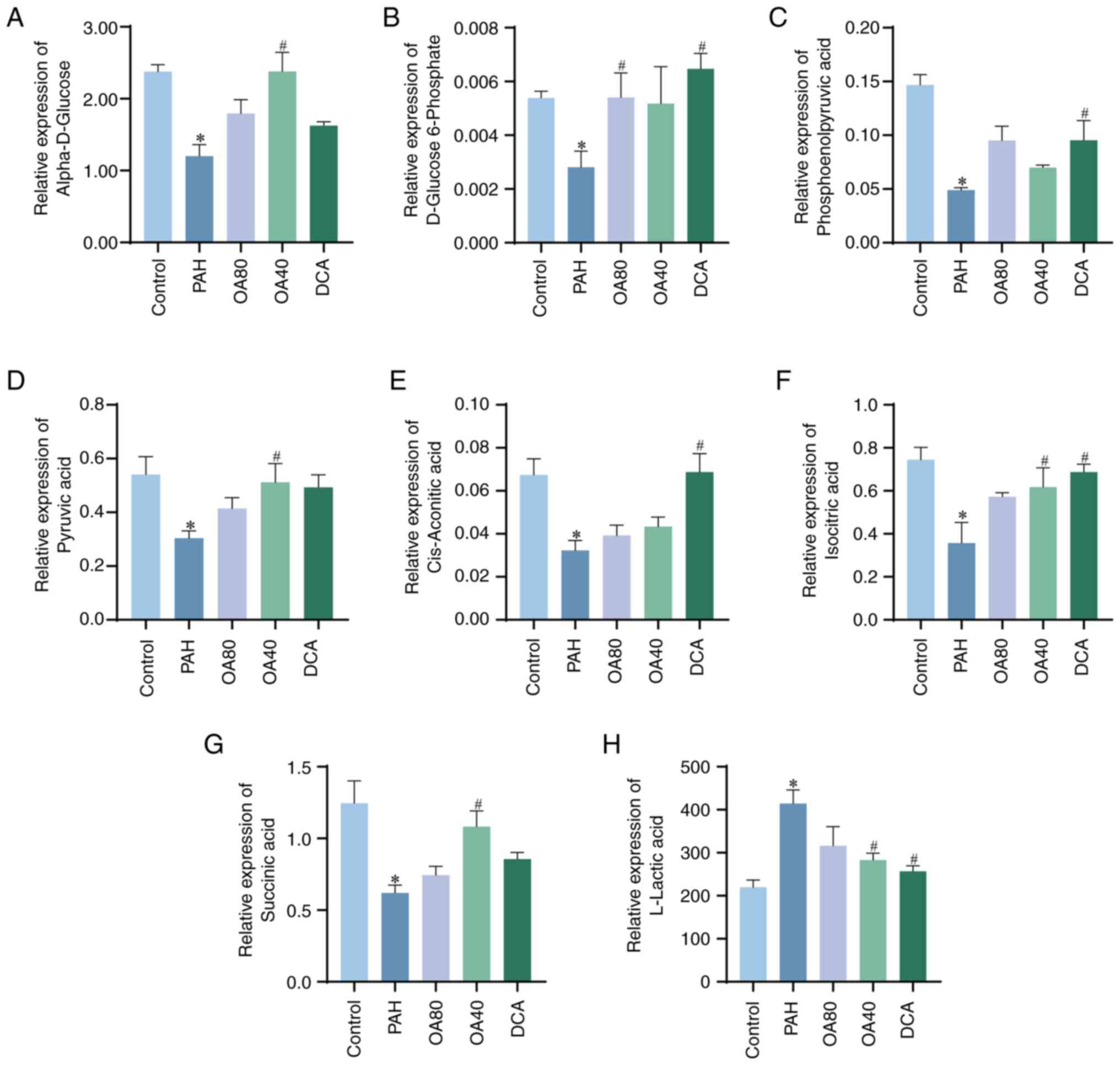
(Fig. 3B). Specifically, levels of
α-D-glucose, D-glucose 6-phosphate, phosphoenolpyruvic acid,
pyruvic acid, cis-aconitic acid, isocitric acid and succinic acid
were significantly lower in the PAH compared with the control
group, whereas L-lactic acid levels were higher in the PAH compared
with the control group. However, after administration of OA and
DCA, it promoted the generation of metabolites in the TCA cycle and
inhibited the generation of L-lactic acid. Preliminary evidence
suggests that Warburg effect occurred in MCT-PAH model rats, and
administration of OA can effectively inhibit the occurrence of
Warburg effect and promote normal glucose metabolism in rats
(Fig. 4).
OA decreases the function of Warburg
effect in PAH model rats
To assess the potential association between the
protective action of OA against MCT-induced PAH and the Warburg
effect, the protein expression of Glut1, HK2, PK, PDK1, LDH and
IDH2 was evaluated. These proteins exhibited significant
differences between the control and PAH groups (Fig. 5). Increased protein expression of
Glut1 and HK2 in the PAH group was indicative of enhanced glucose
uptake. Conversely, downregulation of PK and IDH2, accompanied by
the upregulation of LDH and PDK1, indicates that the normal glucose
metabolism pathway is inhibited, and more pyruvate reacts to
produce lactate under the action of LDH, enhancing the Warburg
effect. Compared with the PAH group, OA (40 mg/kg/d) or DCA
treatment can inhibit the Warburg effect by altering enzymes
related to glucose metabolism, promoting normal energy metabolism
conversion of glucose. These findings indicate that the protective
effect of OA in the MCT induced PAH model is related to its ability
to regulate the Warburg effect.
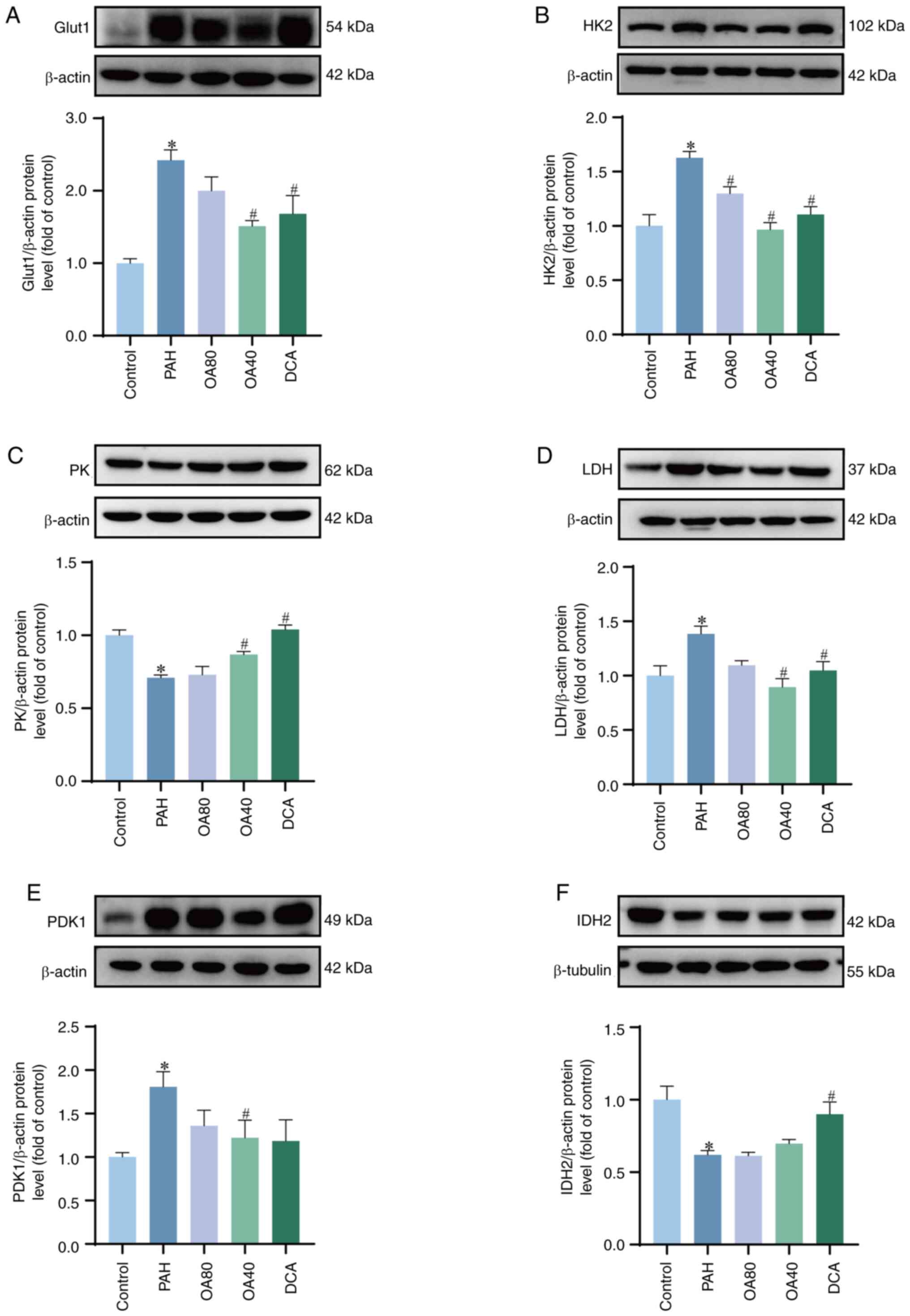 | Figure 5.Expression of glycolysis-associated
proteins in lung tissue from PAH model rats treated with OA.
Representative western blotting and quantitative analysis of (A)
Glut1, (B) HK2, (C) PK, (D) LDH, (E) PDK1 and (F) IDH2. *P<0.05
vs. control. #P<0.05 vs. PAH. OA, Oroxylin A; PAH,
pulmonary arterial hypertension; DCA, dichloroacetate; Glut1,
glucose transporter 1; HK2, Hexokinase 2; PK. Pyruvate kinase; LDH,
Lactate dehydrogenase; PDK1, Pyruvate dehydrogenase kinase 1; IDH2,
Isocitrate dehydrogenase 2. |
Discussion
To the best of our knowledge, the present study is
the first to assess the protective properties of OA against PAH and
its underlying mechanisms. OA exerted a protective influence on
mPAP and attenuated pulmonary vascular remodeling in the
MCT-induced PAH rat model. The mechanism of action of OA may be due
to the blockade of Warburg effect, suggesting that OA has potential
application prospects in treatment of PAH.
OA is a naturally derived flavonoid with potential
therapeutic applications in inhibiting abnormal glycolysis,
angiogenesis, invasion, metastasis, and anti-tumor effects
(24,25). In PAH, PASMC, as an effector cell
of pulmonary vasoconstriction, exhibits many characteristics of
cancer cells under the influence of factors such as inflammatory
factors, growth factors, and vasoactive substances. Its
proliferation and synthesis abilities are enhanced, and apoptosis
is hindered, accompanied by upregulation of oncogene expression
such as p53 and c-Myc or expression of cancer markers. Over
proliferating PASMC is the main cellular component of pulmonary
artery remodeling (26). Based on
the inhibitory effect of OA on cancer cell proliferation, it was
hypothesized that OA may have protective effects in PAH (27). To assess this, a MCT-induced PAH
rat model was used to evaluate the effects of OA. Previous studies
have reported that in rats, subcutaneous injection of 60 mg/kg MCT
for 5 weeks results in a mortality rate of up to 35%, with the
right ventricular systolic pressure reaching 80 mmHg, leading to
severe pulmonary hypertension (16,28).
In the study of pulmonary hypertension model, the dose of MCT
usually 50~60 mg/kg. Considering the efficacy and toxicity of MCT,
we select 55 mg/kg dose in the model. In the present 55 mg/kg
MCT-induced PAH rat model, pulmonary artery remodeling was
accompanied by right ventricular hypertrophy. It was hypothesized
that pulmonary artery remodeling was the primary cause of
progressive mPAP increase. OA effectively reduced the MCT-induced
mPAP increase, pulmonary artery wall thickening and the progression
of pulmonary fibrosis. These changes suggested that OA can serve a
protective role in PAH.
To the best of our knowledge there are no studies
using OA in the treatment of PAH. The OA doses of 40 and 80 mg/kg
referred to the dose of OA in numerous types of cancer (29,30).
However, further research is needed to determine whether 40 mg/kg
is close to the minimum effective dose. Similarly, the role of OA
in reducing mPAP through concentration gradient remains to be
further studied. But we still found for the first time that OA has
a therapeutic effect on PAH.
Metabolomics has been used in examination of
metabolic perturbations associated with PAH (31). The discovery of metabolic changes
in PAH may provide new avenues for its treatment. Some literature
suggests that several main metabolic pathways are related to PAH,
including glucose and fatty acid oxidation, glutamine breakdown,
arginine metabolism, one carbon metabolism, TCA, electron transfer
chain, calcium homeostasis, and glycine metabolism (32,33).
However, further research is required to assess how OA modulates
these metabolic changes in PAH. The present study used DCA as a
positive control and a metabolomics approach to study the impact of
OA on metabolic profiling in PAH model rats. Untargeted
metabolomics strategy was used to identify and screen
differentially expressed metabolites. It was demonstrated that most
of these metabolites belonged to the ‘citrate cycle (TCA cycle)’
and ‘glycolysis or gluconeogenesis’ pathways. Glucose metabolism
provides energy for cell proliferation. The phenomenon of increased
glycolysis promoting lactate production in abnormal glucose
metabolism is known as the Warburg effect, which can also be
observed in patients with cancer patients. Its characteristic is
that under normal oxygen supply conditions, the main form of energy
generation in tumor cells ranges from mitochondrial oxidative
phosphorylation to lower efficiency aerobic glycolysis (34,35).
In PAH, the Warburg effect of glucose metabolism
transformation can promote the proliferation of PASMC, and HIF-1 α
activation of glycolytic genes is considered key to metabolic
adaptation to hypoxia, by increasing the conversion of glucose to
pyruvate and subsequently to lactate (36,37).
DCA is a potent metabolic drug for the treatment of PAH that acts
by inhibiting PDK1. DCA promotes glycolysis by inhibiting
phosphorylation of pyruvate dehydrogenase, which converts pyruvate
to acetyl-CoA into the TCA cycle (38). The present study used metabolomics
methods to accurately reveal whether glycolytic metabolic pathways
contribute to the protective effect of OA on PAH. The data shows
that the relative content of pyruvate and some TCA cycle products
in PAH rats decreases, while the relative content of lactate
increases. The OA and DCA treatment groups can change this
phenomenon. Western blot was used to detect expression level of
glucose metabolism related proteins. The results showed significant
changes in the expression levels of glucose transporter Glut1,
enzymes in the glycolytic pathway (HK2, PK, LDH), and enzymes in
TCA (PDK1, IDH2). In the PAH model, expression of Glut1, HK2, PDK1,
and LDH was upregulated, indicating enhanced glycolysis in animal
models; Meanwhile, downregulation of PK and IDH2 expression
indicates inhibition of normal glucose metabolism pathways, with
more pyruvate reacting with LDH to generate lactate, enhancing the
Warburg effect. OA administration can significantly alter this
change, indicating that abnormal glucose metabolism in PAH is
related to the Warburg effect. Inhibiting the Warburg effect is key
to reversing pulmonary artery remodeling.
In summary, OA may decrease PAH by modulating the
Warburg effect. However, the specific mechanism by which OA
ameliorates this is not known. Therefore, future studies should
assess therapeutic targets for OA to treat PAH by targeting the
Warburg effect.
In conclusion, OA has a protective effect on PAH and
can disrupt endogenous metabolic disorders in PAH rats by
regulating the Warburg effect. The results of this study contribute
to understanding the mechanism by which OA reduces PAH and provide
a new approach for further developing drugs for treating PAH.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural Science
Foundation of China (grant nos. 82460780, 82260780 and U1812403),
Guizhou Provincial Science and Technology Plan Project [Qiankehe
Foundation-ZK (2023) General 500] and Zunyi Medical University
Postgraduate Research Fund Projects (grant no. ZYK196).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article. The data generated in
the present study may be found in the CNGB Sequence Archive (CNSA)
of China National GeneBank DataBase (CNGBdb) under the accession
number (CNP0006089) using the following URL: https://db.cngb.org/.
Authors' contribution
YW, YF and YZ performed experiments and wrote the
article. TC and JL analyzed and interpreted data. SX and LL
conceived and designed the study and revised the manuscript. All
authors have read and approved the final manuscript. YW and LL
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Ethical Guidelines for the Welfare of Laboratory Animals of the
Zunyi Medical University (approval no. ZMU21-2203-622).
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Humbert M, Kovacs G, Hoeper MM,
Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS,
Escribano-Subias P, Ferrari P, et al: 2022 ESC/ERS Guidelines for
the diagnosis and treatment of pulmonary hypertension. Eur Heart J.
43:3618–3731. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Culley MK and Chan SY: Mitochondrial
metabolism in pulmonary hypertension: Beyond mountains there are
mountains. J Clin Invest. 128:3704–3715. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakao S and Tatsumi K: Vascular remodeling
in pulmonary arterial hypertension: Multiple cancer-like pathways
and possible treatment modalities. Int J Cardiol. 147:4–12. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spiekerkoetter E, Goncharova EA,
Guignabert C, Stenmark K, Kwapiszewska G, Rabinovitch M, Voelkel N,
Bogaard HJ, Graham B, Pullamsetti SS and Kuebler WM: Hot topics in
the mechanisms of pulmonary arterial hypertension disease:
Cancer-like pathobiology, the role of the adventitia, systemic
involvement and right ventricular failure. Pulm Circ.
9:20458940198897752019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han S and Chandel NS: Lessons from cancer
metabolism for pulmonary arterial hypertension and fibrosis. Am J
Respir Cell Mol Biol. 65:134–145. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu X, Zhang L and Zhang W: Metabolic
reprogramming: A novel metabolic model for pulmonary hypertension.
Front Cardiovasc Med. 9:9575242022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arai MA, Sakuraba K, Makita Y, Hara Y and
Ishibashi M: Evaluation of naturally occurring HIF-1 inhibitors for
pulmonary arterial hypertension. Chembiochem. 22:2799–2804. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reiter RJ, Sharma R and Rosales-Corral S:
Anti-warburg effect of melatonin: A proposed mechanism to explain
its inhibition of multiple diseases. Int J Mol Sci. 22:7642021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dewachter L, Dewachter C and Naeije R: New
therapies for pulmonary arterial hypertension: An update on current
bench to bedside translation. Expert Opin Investig Drugs.
19:469–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng H, Xiao Y, Deng X, Luo J, Hong C and
Qin X: The warburg effect: A new story in pulmonary arterial
hypertension. Clin Chim Acta. 461:53–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo L, Wu J, Lin T, Lian G, Wang H, Gao G
and Xie L: Influence of atorvastatin on metabolic pattern of rats
with pulmonary hypertension. Aging (Albany NY). 13:11954–11968.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sajeev A, Hegde M, Girisa S, Devanarayanan
TN, Alqahtani MS, Abbas M, Sil SK, Sethi G, Chen JT and
Kunnumakkara AB: Oroxylin A: A promising flavonoid for prevention
and treatment of chronic diseases. Biomolecules. 12:11852022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei L, Zhou Y, Dai Q, Qiao C, Zhao L, Hui
H, Lu N and Guo QL: Oroxylin A induces dissociation of hexokinase
II from the mitochondria and inhibits glycolysis by SIRT3-mediated
deacetylation of cyclophilin D in breast carcinoma. Cell Death Dis.
4:e6012013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei L, Zhou Y, Qiao C, Ni T, Li Z, You Q,
Guo Q and Lu N: Oroxylin A inhibits glycolysis-dependent
proliferation of human breast cancer via promoting SIRT3-mediated
SOD2 transcription and HIF1α destabilization. Cell Death Dis.
6:e17142015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dai Q, Yin Q, Wei L, Zhou Y, Qiao C, Guo
Y, Wang X, Ma S and Lu N: Oroxylin A regulates glucose metabolism
in response to hypoxic stress with the involvement of
hypoxia-inducible factor-1 in human hepatoma HepG2 cells. Mol
Carcinog. 55:1275–1289. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng W, Hu Y, An N, Feng Z, Liu J, Mou J,
Hu T, Guan H, Zhang D and Mao Y: Alginate oligosaccharide
alleviates monocrotaline-induced pulmonary hypertension via
anti-oxidant and anti-inflammation pathways in rats. Int Heart J.
61:160–168. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Tian C, Zhang J, Zhang J and Wu Z:
Oroxylin A ameliorates isoproterenol-induced heart failure model in
rats through promoting myocardial autophagy. J China Pharm Univ.
49:731–738. 2018.(In Chinese).
|
|
18
|
Zhang WB, Zheng YF and Wu YG: Protective
effects of oroxylin A against doxorubicin-induced cardiotoxicity
via the activation of Sirt1 in mice. Oxid Med Cell Longev.
2021:66105432021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li LS, Luo YM, Liu J, Zhang Y, Fu XX and
Yang DL: Icariin inhibits pulmonary hypertension induced by
monocrotaline through enhancement of NO/cGMP signaling pathway in
rats. Evid Based Complement Alternat Med. 2016:79154152016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chambers MC, Maclean B, Burke R, Amodei D,
Ruderman DL, Neumann S, Gatto L, Fischer B, Pratt B, Egertson J, et
al: A cross-platform toolkit for mass spectrometry and proteomics.
Nat Biotechnol. 30:918–920. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nenni M, Çelebier M, Maçin S, Örsten S,
Yabanoğlu-Çiftçi S and Baysal İ: Untargeted metabolomics to
discriminate liver and lung hydatid cysts: Importance of
metabolites involved in the immune response. Vet Parasitol.
328:1101802024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanehisa M, Sato Y, Kawashima M, Furumichi
M and Tanabe M: KEGG as a reference resource for gene and protein
annotation. Nucleic Acids Res. 44:D457–D462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong L, Chen P and Cheng J: Experimental
study on different doses of monocrotaline-induced pulmonary
hypertension in rats. Chin J Integr Med Cardio-Cerebrovasc Dis.
13:1172–1175. 2015.(In Chinese).
|
|
24
|
Sajeev A, Hegde M, Daimary UD, Kumar A,
Girisa S, Sethi G and Kunnumakkara AB: Modulation of diverse
oncogenic signaling pathways by oroxylin A: An important strategy
for both cancer prevention and treatment. Phytomedicine.
105:1543692022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huo TX, Wang XP, Yu Z, Kong B, He Y, Guo
QL, Zhang XB and Qiang L: Oroxylin A inhibits the migration of
hepatocellular carcinoma cells by inducing NAG-1 expression. Acta
Pharmacol Sin. 43:724–734. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boucherat O, Vitry G, Trinh I, Paulin R,
Provencher S and Bonnet S: The cancer theory of pulmonary arterial
hypertension. Pulm Circ. 7:285–299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Naeije R, Richter MJ and Rubin LJ: The
physiological basis of pulmonary arterial hypertension. Eur Respir
J. 59:21023342022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bueno-Beti C, Sassi Y, Hajjar RJ and Hadri
L: Pulmonary artery hypertension model in rats by monocrotaline
administration. Methods Mol Biol. 1816:233–241. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu R, Chen N, Yao J, Zhao Q, Zhang F, Li
ZY, You QD and Guo QL: The role of Nrf2 and apoptotic signaling
pathways in oroxylin A-mediated responses in HCT-116 colorectal
adenocarcinoma cells and xenograft tumors. Anticancer Drugs.
23:651–658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ni T, He Z, Dai Y, Yao J, Guo Q and Wei L:
Oroxylin A suppresses the development and growth of colorectal
cancer through reprogram of HIF1α-modulated fatty acid metabolism.
Cell Death Dis. 8:e28652017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muthubharathi BC, Gowripriya T and
Balamurugan K: Metabolomics: Small molecules that matter more. Mol
Omics. 17:210–229. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu W, Comhair S, Chen R, Hu B, Hou Y, Zhou
Y, Mavrakis LA, Janocha AJ, Li L, Zhang D, et al: Integrative
proteomics and phosphoproteomics in pulmonary arterial
hypertension. Sci Rep. 9:186232019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kao CC, Wedes SH, Hsu JW, Bohren KM,
Comhair SA, Jahoor F and Erzurum SC: Arginine metabolic endotypes
in pulmonary arterial hypertension. Pulm Circ. 5:124–134. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, Yang Y, Zhang B, Lin X, Fu X, An Y,
Zou Y, Wang JX, Wang Z and Yu T: Lactate metabolism in human health
and disease. Signal Transduct Target Ther. 7:3052022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Breault NM, Wu D, Dasgupta A, Chen KH and
Archer SL: Acquired disorders of mitochondrial metabolism and
dynamics in pulmonary arterial hypertension. Front Cell Dev Biol.
11:11055652023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rafikova O, Meadows ML, Kinchen JM, Mohney
RP, Maltepe E, Desai AA, Yuan JX, Garcia JG, Fineman JR, Rafikov R
and Black SM: Metabolic changes precede the development of
pulmonary hypertension in the monocrotaline exposed rat lung. PLoS
One. 11:e1504802016. View Article : Google Scholar
|
|
37
|
Liang S, Yegambaram M, Wang T, Wang J,
Black SM and Tang H: Mitochondrial metabolism, redox and calcium
homeostasis in pulmonary arterial hypertension. Biomedicines.
10:3412022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li T, Li S, Feng Y, Zeng X, Dong S, Li J,
Zha L, Luoh, Zhao L, Liu B, et al: Combination of dichloroacetate
and atorvastatin regulates excessive proliferation and oxidative
stress in pulmonary arterial hypertension development via p38
signaling. Oxid Med Cell Longev. 2020:69736362020.PubMed/NCBI
|