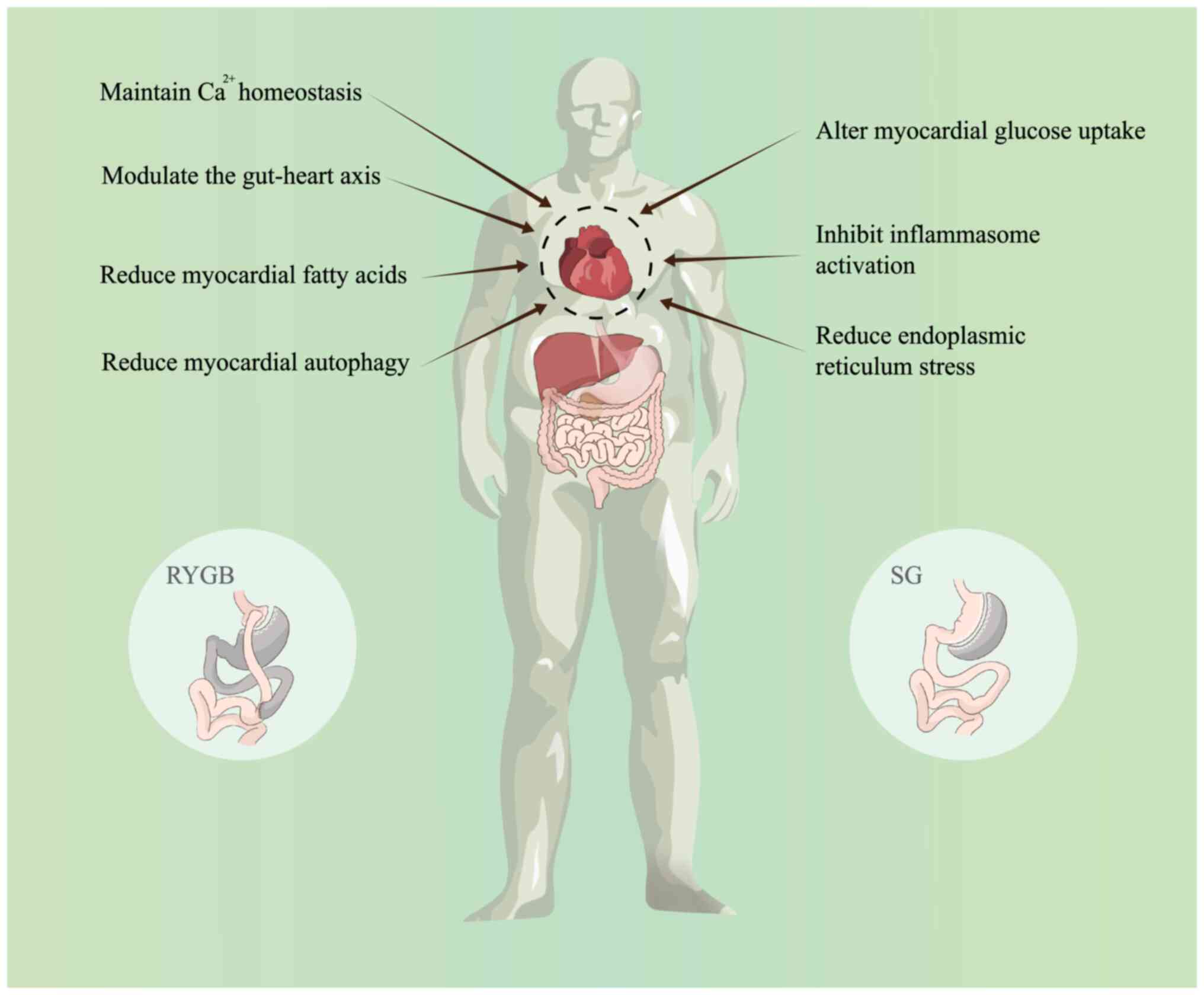
Diabetes mellitus, an age-related metabolic disorder
of escalating global prevalence, is a formidable clinical challenge
to public health, with the number of affected individuals expected
to reach 783 million by 2045 (1).
The chronic nature of diabetes, with the associated insulin
resistance and hyperinsulinemia, can precipitate a distinct form of
cardiomyopathy, diabetic cardiomyopathy (DCM), that develops
independently of traditional risk factors such as coronary artery
disease and hypertension (2). As a
serious and under-recognized complication of diabetes, the
pathogenesis of DCM is complex and multifactorial (3,4). DCM
is characterized by an initial phase of myocardial fibrosis and
diastolic dysfunction, which may evolve into progressive systolic
impairment and, ultimately, heart failure (5,6).
Advanced cardiac dysfunction in DCM is a principal determinant of
mortality among diabetic patients (7–9).
Bariatric surgery, a transformative metabolic
intervention, is a pivotal treatment for severe obesity, which is
uniquely capable of inducing sustained weight loss and
significantly ameliorating complications (10). This surgical approach surpasses
conventional pharmacotherapy in its ability to enhance insulin
sensitivity, stabilize blood glucose and lipid levels, and
ameliorate diabetes-related complications (2). Mingrone et al (11) found that Roux-en-Y gastric bypass
(RYGB) and biliopancreatic diversion could effectively alleviate
DCM. English and Williams (12)
found that numerous patients with type 2 diabetes mellitus
complicated with other cardiovascular diseases can reduce or
completely stop cardiovascular medications after undergoing
bariatric surgery. Improvement in left ventricular structure and
function, visceral fat and reverse myocardial remodeling after
bariatric surgery may be beneficial for the recovery of DCM
(13,14). The present review will discuss
recent research advances in bariatric surgery to improve DCM
(Fig. 1), with the aim to aid the
understanding of the pathogenesis of DCM, explore new therapeutic
targets and develop more targeted drugs.
In addition to treating obesity, bariatric surgery
is more advantageous than pharmacological treatment alone in terms
of glycemic control and reduction of cardiovascular risk factors
(21). Moreover, its mechanism is
not only limited to the simple reduction of body mass but also
includes the improvement of enteric insulin levels, insulin
secretion and insulin sensitivity (22,23).
Kopp et al (24) found that
C-reactive protein and interleukin-6 circulating levels decrease
almost immediately after RYGB or sleeve gastrectomy (SG), while
insulin sensitivity improves. Changes in cardiac structure and
function also occur in the months and years after surgery,
including mainly a reduction in left ventricular mass. However,
this may be unrelated to a decrease in blood pressure (25–30).
Rider et al (26) performed
MRI examinations on 30 obese individuals without cardiac risk
factors at baseline and one year after weight loss (bariatric
surgery or dieting). Among them, 13 obese patients with left
ventricular ejection fraction (LVEF) exceeding 40% showed
regression of subclinical abnormalities of myocardial deformability
within 6–24 months after bariatric surgery (31). At present, the mechanisms by which
bariatric surgery regulates metabolism to improve metabolic
diseases is a topic of significant research interest.
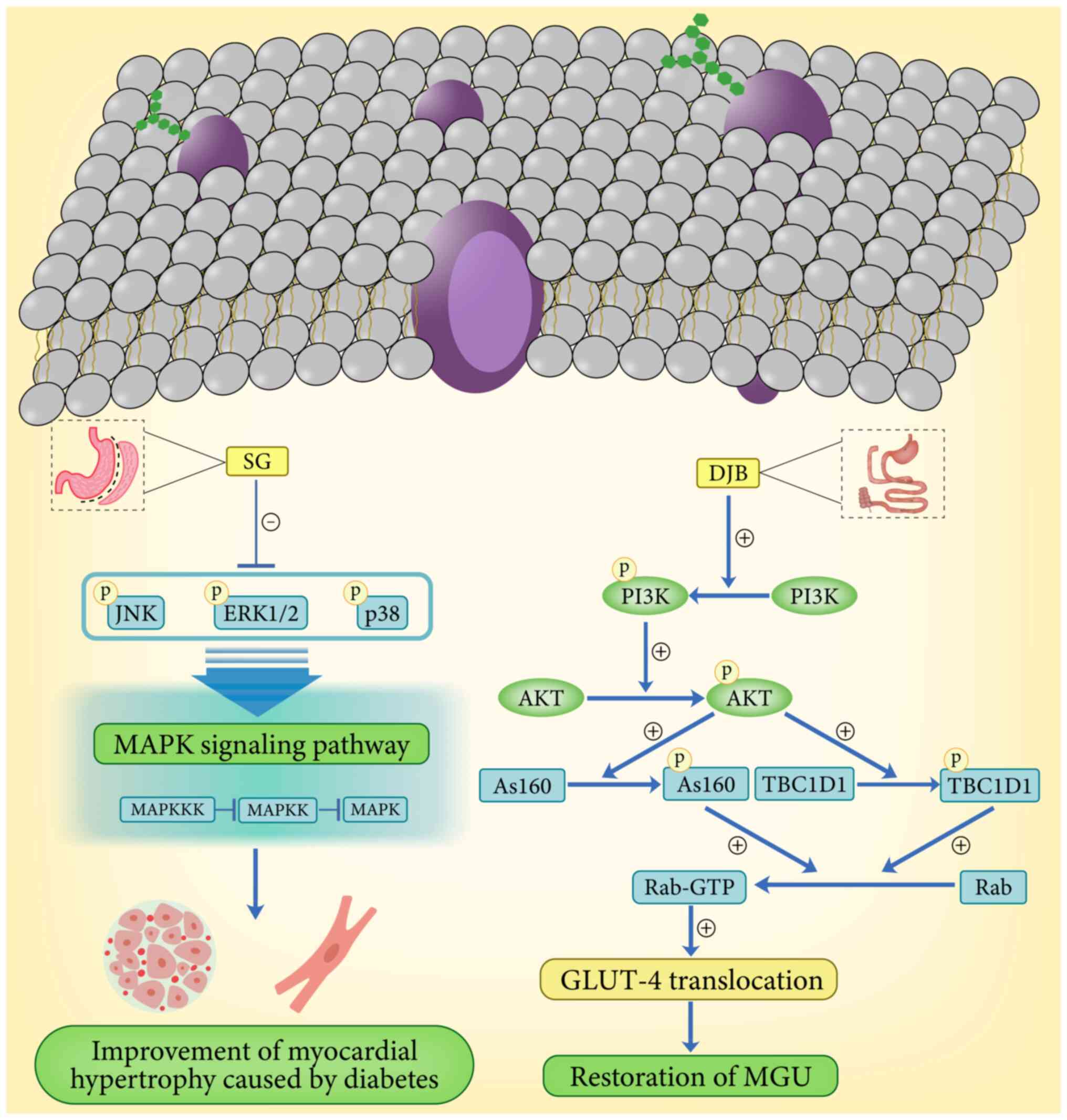
Insulin resistance is one of the major pathogenic
factors in DCM, and diabetes results in impaired insulin-mediated
myocardial glucose uptake (MGU) and glucose utilization, and
promotes a shift in substrate toward non-esterified fatty acid
oxidation, which can lead to cardiac damage (2,37–39).
PI3K/AKT is the central hub of signal transduction in the
myocardial insulin signaling pathway (40,41),
which receives upstream signals from the insulin substrate receptor
family and plays a central role in promoting glucose transporter 4
(GLUT-4) translocation (42).
There is still a gap between upstream insulin signaling and
downstream GLUT-4 translocation (43,44).
However, two novel AKT substrates, glucose metabolism regulator
protein 160 and TBC1 domain family member 1 (TBC1D1), are prime
candidates for potentially bridging this gap (45). These substrates essentially act as
brakes on the cytosolic action of GLUT-4 vesicles and are
phosphorylated by AKT in response to insulin, leading to the
conversion of some downstream Rab proteins to active GTP-bound
forms, thereby triggering GLUT-4 translocation to the cell membrane
(45,46). However, studies on the roles of
glucose metabolism regulatory proteins 160 and TBC1D1 in DCM are
more limited. In a study addressing GLUT-4 translocation, Huang
et al (37) found that
after duodenal-jejunal bypass (DJB), MGU recovered and was involved
in the remission of DCM after DJB by promoting GLUT-4
translocation. Furthermore, by assessing the uptake of myocardial
energy substrates after bariatric surgery, DJB was found to restore
MGU by promoting myocardial GLUT-4 translocation in diabetic rats,
and phosphorylation and activation of glucose metabolism-regulating
protein 160 was restored after DJB (37). This suggests that DJB alters the
activity of the PI3K/AKT pathway by modulating the expression of
glucose metabolism-regulating protein 160, thereby playing a key
role in the recovery of MGUs (37). The study by Huang et al
demonstrated that the improvement of MGU defects in diabetic rats
by DJB was associated with the promotion of myocardial insulin
signaling and GLUT-4 translocation. In addition, Xu et al
(47) similarly found an
improvement in MGU after SG surgery in Wistar rats in which DCM had
been induced. This alteration was associated with a significant
down-regulation of three MAPKs (phosphorylated p38, phosphorylated
c-Jun N-terminal kinase and phosphorylated extracellular
signal-regulated kinase 1/2) in the myocardial tissues of Wistar
rats (Fig. 2). Although
restoration of MGU and improvement of cardiac metabolic homeostasis
were shown to be effective in reversing DCM (48), a gap still exists between increased
myocardial GLUT-4 at the cell membrane and eventual improvement in
cardiac function, and more studies are required to explore the
association between the two.
In the healthy heart, 50–70% of ATP is produced from
fatty acids, however this percentage is higher in patients with
obesity or diabetes, reaching 80–90% (49). Although the availability of fatty
acids is essential for maintaining cardiac function, this greater
reliance on fatty acids may also lead to myocardial lipotoxicity
that can eventually result in myocardial dysfunction (49–51).
Studies on animal models have shown that obesity increases
myocardial fatty acid metabolism and oxygen consumption, leading to
increased oxidative stress, cardiac dysfunction and increased
apoptosis (52–55). Owing to disturbed lipid metabolism,
excessive fat deposition in the heart creates a lipotoxic
environment and induces insulin resistance, which not only impairs
pancreatic β-cell function, but also increases myocardial uptake
and utilization of fatty acids (56,57).
Lin et al (58) measured a
decrease in myocardial total fatty acid utilization in individuals
with a body mass index of >30 kg/m2 who underwent
RYGB and decreased post-surgical left ventricular mass and
relatively decreased myocardial total fatty acid oxidation. In
addition, reduction in the left ventricular mass was an independent
predictor of improvement in myocardial diastolic function, with a
significant reduction in left ventricular end-diastolic volume and
significant improvement in cardiac function in patients with
reduced body mass. Carreau et al (59) found that bariatric surgery reduced
cardiac fatty acid utilization and enhanced left ventricular
function. Existing studies have reported both increases and
decreases in fatty acid utilization after bariatric surgery
(60,61), with a trend toward decreased fatty
acid utilization in the short-term postoperative period (62). In addition, the effect of bariatric
surgery on cardiac fatty acid partitioning in patients with type 2
diabetes has also been demonstrated (59), which provides strong evidence for
improvement in postoperative DCM. However, more studies are needed
to determine the effects of altered fatty acid utilization on
cardiac structure and function after bariatric surgery and to
determine whether these changes persist over time.
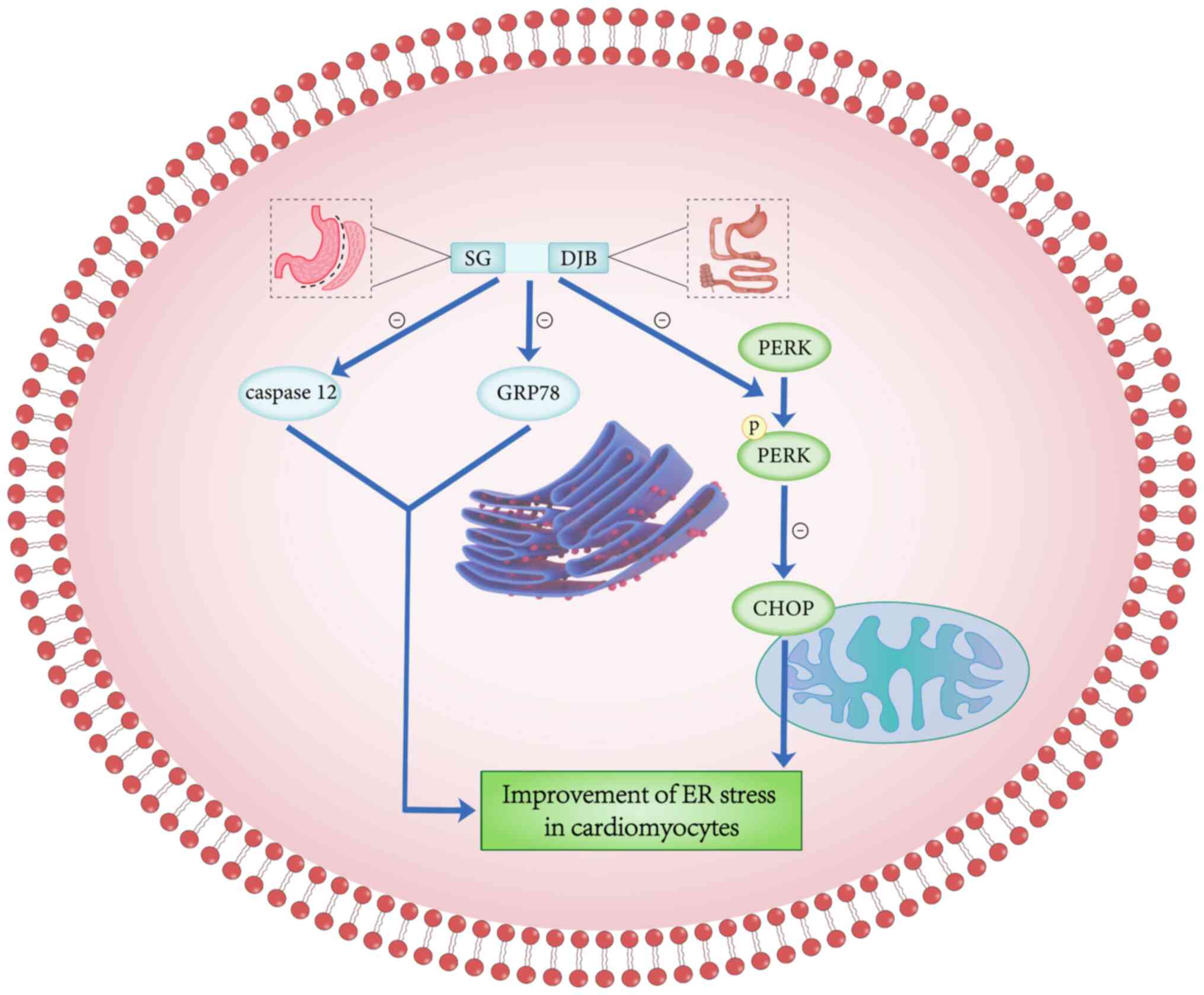
The ER controls the proper folding of polypeptides
and proteins through various chaperones and enzymes within the ER
organelles (63). The ER folding
process is disturbed when the overburdened protein folding exceeds
the ER processing capacity, resulting in the accumulation of
misfolded/unfolded proteins in the lumen of the ER, a state known
as ER stress (63). ER stress
plays an important role in the pathogenesis of DCM; furthermore, in
diabetic patients, hyperglycemia and insulin resistance lead to an
increase in intracellular ER stress (64). Lakshmanan et al (65) found that ER stress can be induced
by a variety of pathological conditions such as ischemia, oxidative
stress, hypoxia, hyperglycemia and hyperlipidemia.
Hyperglycemia-induced ER stress has been shown to play a major role
in the pathology of cardiac dysfunction (66). ER stress-induced C/EBP homologous
protein (CHOP) plays an important role in the apoptosis-promoting
executive pathway, which is the most described and characterized
pathway in ER stress-induced cell death, as well as the downstream
signaling pathway of protein kinase R-like endoplasmic reticulum
kinase (PERK) (67). The PERK
downstream signaling pathway, which produces phosphorylated PERK
when phosphorylated, triggers CHOP-induced apoptosis (68). In addition, caspase-12, another
apoptotic signaling pathway in the cystatinase cascade reaction, is
also closely associated to CHOP (69,70).
Zhang et al (71) found
that, compared with the sham surgery group, the expression of
GRP78, PERK, phosphorylated PERK, CHOP and caspase-12 was
positively expressed in the bariatric surgery group, indicating
that bariatric surgery could alleviate ER stress by significantly
inhibiting CHOP and caspase-12 apoptotic signaling pathways
(Fig. 3). ER protein homeostasis
is controlled by the unfolded protein response (UPR), which is a
signaling pathway that regulates the protein-folding ability of
cells to maintain cellular secretory function (72,73).
When the adaptive UPR fails to maintain ER homeostasis, maladaptive
or terminal UPR is engaged, leading to disruption of the ER
integrity and apoptosis (74).
Glucose-regulated protein 78 kD (GRP78) is a protective molecular
chaperone that binds to the UPR during initial ER stress, and GRP78
is a negative regulator of the UPR in a variety of models (75,76).
Zhang et al (71) found
that compared with the sham surgery group, the expression of GRP78
in the bariatric surgery group was significantly decreased,
confirming that bariatric surgery could reduce ER stress in
cardiomyocytes.
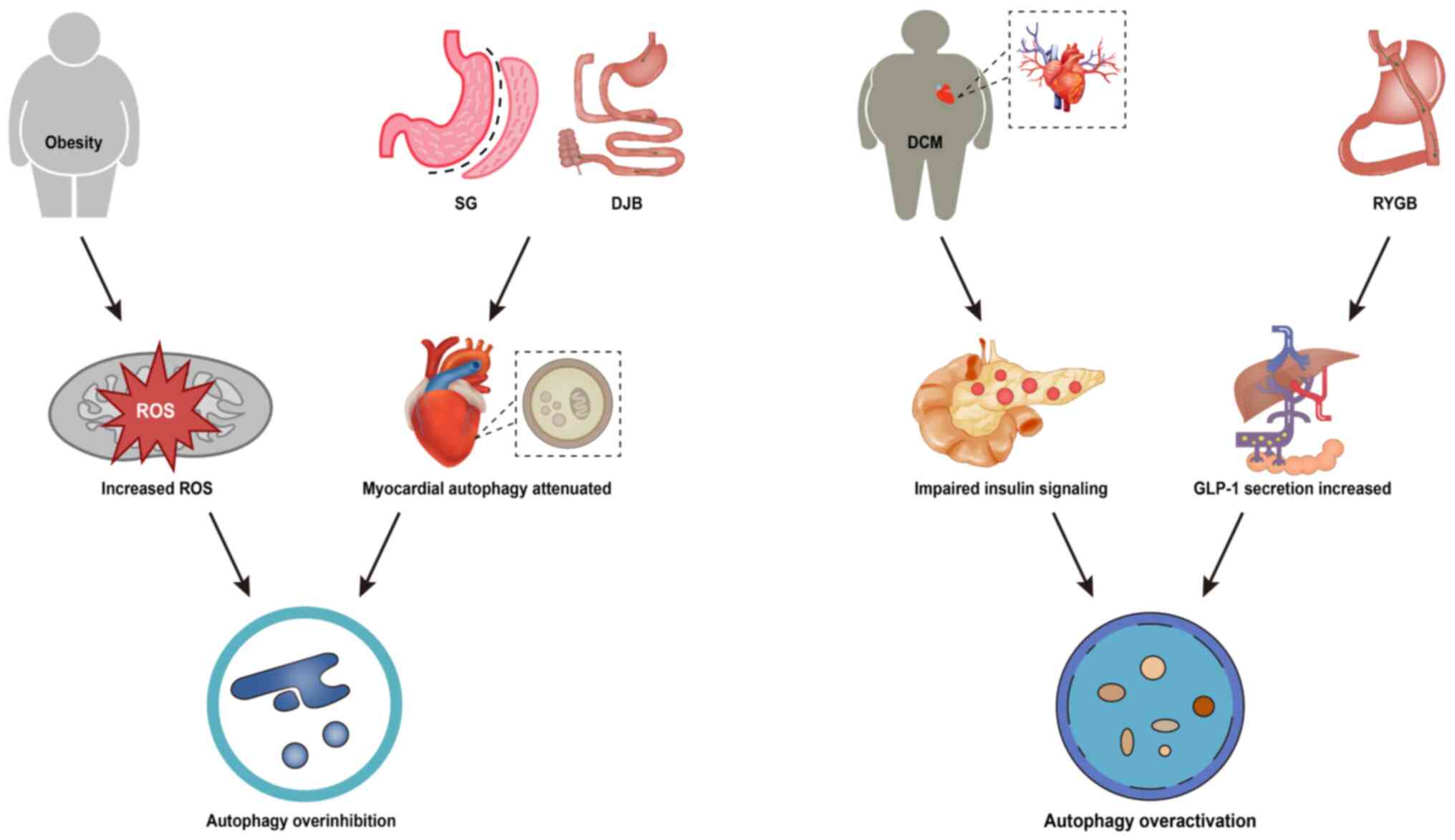
Autophagy is a tightly regulated lysosomal
degradation mechanism that plays an important role in maintaining
intracellular homeostasis as well as coping with intracellular
stress (77). During the
development of diabetes mellitus, intracellular stress (such as ER
stress) can activate autophagy, and the overactivation of autophagy
in DCM cardiomyocytes can lead to self-digestion and increased
reactive oxygen species generation (78). Huang et al (79) used chloroquine to determine
myocardial autophagic flux through the expression of
autophagy-related proteins. The results showed that autophagosome
formation was weakened after SG and DJB, and that cardiomyocyte
hypertrophy in the rats of the SG and DJB groups was also
significantly ameliorated, and the degree of interstitial and
perivascular fibrosis was lower than that of the sham-operated
group. However, obesity in turn inhibits autophagy activation
(80), and the effect of reduced
fat load on autophagy after bariatric surgery should not be
ignored. It has been reported that RYGB significantly activates
hepatic autophagy and may be associated with altered glucagon-like
peptide-1 (GLP-1) levels after surgery (81). Similarly, in cardiomyocytes,
enhanced autophagy contributes to the amelioration of
diabetes-induced cardiac injury (82,83)
(Fig. 4). In summary, autophagy
plays a dual role in DCM, and both inhibition and over-activation
of myocardial autophagy can have pathological effects on DCM.
Evidence from several studies supports that the
NLRP3 inflammasome is closely associated with the development of
DCM (84–88). Bariatric surgery has been found to
inhibit NLRP3 activation in pancreatic islets, hepatocytes and
adipose tissue, and consequently exert anti-apoptotic and
anti-inflammatory effects (89–92).
Recently, Li et al (93)
observed NLRP3 inflammatory vesicle-mediated inactivation of
cardiomyocyte pyroptosis in SG mice. Reactive oxygen species play
an important role in the pathogenesis of type 2 diabetes mellitus,
and the overproduction of reactive oxygen species is considered to
be a mechanism involved in the activation of NLRP3 inflammatory
vesicles (84). Thus, the use of
reactive oxygen species scavengers significantly reduced the
expression of NLRP3. Reactive oxygen species are important
regulators of NLRP3 inflammatory vesicles in cardiomyocytes
(93). It has been shown that
chloride efflux acts downstream of mitochondrial reactive oxygen
species production and activates the NLRP3 inflammatory vesicles in
macrophages (93). In addition,
inhibition of volume-sensitive chloride currents reduces cell death
and reverses the contractile dysfunction in cardiomyopathy
(94). Myocardial NLRP3-mediated
pyroptosis restored by high glucose stimulation was observed after
administration of chloride channel blockers to SG rats, suggesting
that chloride efflux may act as a messenger to regulate the NLRP3
assembly and activation, either directly or indirectly (93). It is therefore clear that cardiac
remodeling in DCM rats can be significantly reversed by reducing
reactive oxygen/chlorine ion efflux-mediated NLRP3 inflammatory
vesicle activation after SG. However, Yang et al (95) found that metformin could inhibit
the expression of the NLRP3 inflammasome by activating autophagy in
DCM cardiomyocytes, which seemed to be in contrast to the results
of Huang et al (79) in
terms of exerting cardioprotective function. It is evident that
bariatric surgery still has a great potential to be investigated in
terms of the regulation of myocardial autophagy.
Mitochondrial homeostasis is important for
maintaining cellular metabolism and function (96). Calcium ions play an important role
in mitochondrial synthesis (97).
In diabetic cardiomyocytes, the decline in cardiomyocyte function
is partly mediated by abnormal mitochondrial calcium handling and
decreased free matrix calcium levels (98). The diminished mitochondrial
capacity for Ca2+ uptake leads to reduced ATP production
(99–101) and favors reactive oxygen species
generation (102). Therefore,
improper mitochondrial Ca2+ handling is considered a key
factor in DCM cell dysfunction (103). Huang et al (79) performed SG, DJB and sham operations
on male Sprague-Dawley rats that had been induced with DCM, and
subsequently assessed the ventricular diastolic function and
Ca2+ homeostasis by echocardiography and calcium
fluorescent probe, respectively. The results showed that both
systolic and diastolic functions of the heart were improved in the
SG and DJB groups of rats after surgery, as well as myoplasmic
reticulum Ca2+ release and Ca2+ decay. In
addition, mitochondrial dysfunction was also improved by
inactivation of nuclear receptor family group 4A member 1 (NR4A1)
(104). NR4A1 causes disruption
of mitochondrial homeostasis by promoting mitochondrial rupture and
decreasing the mitochondrial membrane potential (105,106). The inactivation of NR4A1 is
closely associated with the AMPK pathway (107). It has been found that SG
activates the AMPK signaling pathway, inhibits NR4A1 and corrects
mitochondrial dysfunction in vivo, thus contributing to the
improvement of DCM in terms of morphology and cardiac function
(108). Under the pathological
conditions of DCM, activation of silent information regulator 1
(SIRT1) and phosphorylation of AMPK can promote the clearance of
dysfunctional mitochondria and peroxisomal enzymes to reverse
cardiomyopathy development (109).
There is a bidirectional communication network
between the gut and the heart, known as the ‘gut-heart axis’
(110). GLP-1 is secreted after
meals, lowering glucose levels by enhancing insulin secretion and
inhibiting glucagon release (111). Bariatric surgery not only
increases postprandial GLP-1 release but also alters the
gastrointestinal microbiota and bile acid profile cycle with
beneficial effects (112,113). Bariatric surgery may alter the
gut-heart axis through one or more mechanisms to obtain benefits in
terms of improved cardiac function (114). GLP-1 analogs may exert
cardiovascular protection by reducing inflammation (115), and increased levels of GLP-1
after bariatric surgery may reverse endothelial dysfunction and
restore the endothelial-protective properties of high-density
lipoproteins (116). Bile acids
are considered to be important regulators of systemic metabolism,
producing effects on obesity prevention and improving insulin
resistance and hyperglycemia (117,118). Thus, alterations in bile acid
levels and composition after gastric bypass may help to improve
glucose and lipid metabolism in patients, which in turn modulates
the gut-heart axis, protects the myocardium and improves myocardial
fibrosis in terms of decreasing apoptosis, increasing glucose
uptake, and reversing DCM (119).
Alterations in gut flora after bariatric surgery have also been
repeatedly reported in recent years, indicating that changes in
intestinal flora after bariatric surgery may be related to
improvements in glucose tolerance and insulin sensitivity, and may
also regulate non-alcoholic fatty liver disease from multiple
aspects such as the production of short-chain fatty acids and the
regulation of one-carbon metabolism (120–125). Chaudhari et al (126) found that changes in the
composition of the intestinal microbial community in mice after
bariatric surgery may up-regulate the expression of bile
acid-7-sulphate, thereby regulating the gut-heart axis. As the
intestinal flora and its metabolites play an important regulatory
role in cardiovascular disease, imbalance of the intestinal flora
has been suggested to be an important pathological mechanism in the
development of cardiovascular diseases (127,128). In summary, changes in GLP-1
release, bile acid levels and gut flora composition after bariatric
surgery may facilitate improvements in DCM by exerting an effect on
the gut-heart axis.
Researchers have explored the molecular mechanisms
underlying the improvements in DCM after bariatric surgery, but
some potential therapeutic targets need further investigation. CD36
is a fatty acid transporter that is related to cardiac fatty acid
uptake (129). Wang et al
(130) hypothesized that the loss
of the Takeda G protein-coupled receptor 5 (TGR5) promoted the
localization of CD36 on the plasma membrane through
aspartate-histidine-histidine-cysteine4 (DHHC4)-mediated CD36
palmitoylation, resulting in enhanced cardiac fatty acid uptake and
lipid accumulation, indicating that the TGR5-DHHC4 pathway
regulates cardiac fatty acid uptake and may be a potential target
for the treatment of DCM. Ion channels play an important role in
the pathogenesis of DCM, including changes in cation channels such
as calcium, potassium and sodium, as well as anion channels
(131). Studying the functional
changes of calcium channels, sodium channels and potassium channels
may provide new strategies for the treatment of DCM. In addition,
based on the aforementioned effects of NR4A1 on mitochondria,
studies have found that AMPK can further downregulate NR4A1 by
activating SIRT1, thereby correcting mitochondrial dysfunction and
enhancing myocardial energy production, and improving myocardial
remodeling (132,133). Therefore, bariatric surgery
restores mitochondrial homeostasis and alleviates DCM
morphologically and functionally by maintaining myocardial
Ca2+ homeostasis and downregulating NR4A1 expression
(79,108). The decline in cardiomyocyte
function is partly mediated by abnormal mitochondrial calcium
handling and decreased free matrix calcium levels, which may be a
good target for new therapeutic interventions (98,99,103).
DCM is one of the most serious complications of
diabetes mellitus, and while its etiology involves the synergistic
effect of multiple molecular mechanisms, the specific underlying
pathogenesis is still unclear (101). Hence, the lack of specificity in
DCM treatment is one of the reasons why a clinical cure is
challenging. Currently, the mainstream treatment includes intensive
glucose control, traditional Chinese medicine intervention and
corresponding symptomatic treatment, but none of these approaches
can further improve patient prognosis (134). Therefore, it is particularly
important to explore new treatment modalities. Relevant basic
experiments have proved that bariatric surgery can effectively
alleviate or even reverse DCM-induced cardiomyopathy, but the
specific mechanism of the therapeutic effect of bariatric surgery
has not been fully elucidated (37,47,71).
The present review summarized the latest advances in the treatment
of DCM with bariatric surgery. It is critical to study the
underlying mechanisms of cardiac microstructural changes after
bariatric surgery to develop more effective therapeutic strategies.
Furthermore, research should focus on the association between
signaling pathways, and studies with clear experimental results
should be shifted to clinical studies to guide the use of future
medication, research and the development of new drugs. By
elucidating the molecular and physiological responses to bariatric
surgery, the present review aimed to enhance the understanding of
DCM, identify new targets for intervention and advance the
development of more efficacious and personalized treatment options.
This research direction is pivotal for the advancement of clinical
strategies that can effectively address the multifaceted challenges
posed by DCM, ultimately improving patient care and outcomes.
Not applicable.
This work was supported by The National Natural Science
Foundation of China (grant no. 82370601) and The Northern Sichuan
Medical College Clinical School Affiliated Hospital Scientific
Research Development Plan Project (grant no. 2022JC002).
Not applicable.
KS and YR conceived the subject of the review,
performed the investigation, and wrote and edited the original
draft. DL, DX and AK wrote, reviewed, and edited the manuscript and
contributed to the figures. All authors have read and approved the
final version of the manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Ahmad E, Lim S, Lamptey R, Webb DR and
Davies MJ: Type 2 diabetes. Lancet. 400:1803–1820. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jia G, DeMarco VG and Sowers JR: Insulin
resistance and hyperinsulinemia in diabetic cardiomyopathy. Nat Rev
Endocrinol. 12:144–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qiu Y, Buffonge S, Ramnath R, Jenner S,
Fawaz S, Arkill KP, Neal C, Verkade P, White SJ, Hezzell M, et al:
Endothelial glycocalyx is damaged in diabetic cardiomyopathy:
Angiopoietin 1 restores glycocalyx and improves diastolic function
in mice. Diabetologia. 65:879–894. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khokhlova A, Myachina T, Volzhaninov D,
Butova X, Kochurova A, Berg V, Gette I, Moroz G, Klinova S,
Minigalieva I, et al: Type 1 diabetes impairs cardiomyocyte
contractility in the left and right ventricular free walls but
preserves it in the interventricular septum. Int J Mol Sci.
23:17192022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan Y, Zhang Z, Zheng C, Wintergerst KA,
Keller BB and Cai L: Mechanisms of diabetic cardiomyopathy and
potential therapeutic strategies: Preclinical and clinical
evidence. Nat Rev Cardiol. 17:585–607. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marfella R, Sardu C, Mansueto G, Napoli C
and Paolisso G: Evidence for human diabetic cardiomyopathy. Acta
Diabetol. 58:983–988. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cosentino F, Grant PJ, Aboyans V, Bailey
CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE,
Hansen TB, et al: 2019 ESC guidelines on diabetes, pre-diabetes,
and cardiovascular diseases developed in collaboration with the
EASD. Eur Heart J. 41:255–323. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bellary S, Kyrou I, Brown JE and Bailey
CJ: Type 2 diabetes mellitus in older adults: Clinical
considerations and management. Nat Rev Endocrinol. 17:534–548.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kelsey MD, Nelson AJ, Green JB, Granger
CB, Peterson ED, McGuire DK and Pagidipati NJ: Guidelines for
cardiovascular risk reduction in patients with type 2 diabetes:
JACC guideline comparison. J Am Coll Cardiol. 79:1849–1857. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arterburn DE, Telem DA, Kushner RF and
Courcoulas AP: Benefits risks of bariatric surgery in adults: A
review. JAMA. 324:879–887. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mingrone G, Panunzi S, De Gaetano A,
Guidone C, Iaconelli A, Nanni G, Castagneto M, Bornstein S and
Rubino F: Bariatric-metabolic surgery versus conventional medical
treatment in obese patients with type 2 diabetes: 5 Year follow-up
of an open-label, single-centre, randomised controlled trial.
Lancet. 386:964–973. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
English WJ and Williams DB: Metabolic,
bariatric surgery: An effective treatment option for obesity and
cardiovascular disease. Prog Cardiovasc Dis. 61:253–269. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sorimachi H, Obokata M, Omote K, Reddy
YNV, Takahashi N, Koepp KE, Ng ACT, Rider OJ and Borlaug BA:
Long-term changes in cardiac structure and function following
bariatric surgery. J Am Coll Cardiol. 80:1501–1512. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heidenreich P: Weight loss and cardiac
reverse remodeling. J Am Coll Cardiol. 80:1513–1515. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Pu Y, Chen J, Tong W, Cui Y, Sun
F, Zheng Z, Li Q, Yang T, Meng C, et al: Gastrointestinal
intervention ameliorates high blood pressure through antagonizing
overdrive of the sympathetic nerve in hypertensive patients and
rats. J Am Heart Assoc. 3:e0009292014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao L, Qin X, Peterson MR, Haller SE,
Wilson KA, Hu N, Lin X, Nair S, Ren J and He G: CARD9 knockout
ameliorates myocardial dysfunction associated with high fat
diet-induced obesity. J Mol Cell Cardiol. 92:185–195. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martin M, Beekley A, Kjorstad R and
Sebesta J: Socioeconomic disparities in eligibility and access to
bariatric surgery: A national population-based analysis. Surg Obes
Relat Dis. 6:8–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nguyen N, Champion JK, Ponce J, Quebbemann
B, Patterson E, Pham B, Raum W, Buchwald JN, Segato G and Favretti
F: A review of unmet needs in obesity management. Obes Surg.
22:956–966. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pories WJ, Swanson MS, MacDonald KG, Long
SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal
JM, et al: Who would have thought it? An operation proves to be the
most effective therapy for adult-onset diabetes mellitus. Ann Surg.
222:339–352. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Phillips BT and Shikora SA: The history of
metabolic and bariatric surgery: Development of standards for
patient safety and efficacy. Metabolism. 79:97–107. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schauer PR, Kashyap SR, Wolski K,
Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE
and Bhatt DL: Bariatric surgery versus intensive medical therapy in
obese patients with diabetes. N Engl J Med. 366:1567–1576. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pareek M, Schauer PR, Kaplan LM, Leiter
LA, Rubino F and Bhatt DL: Metabolic surgery: Weight loss,
diabetes, and beyond. J Am Coll Cardiol. 71:670–687. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferraz-Bannitz R, Kashyap S and Patti ME:
Bariatric surgery: It's not just incretins! J Clin Endocrinol
Metab. 107:e883–e885. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kopp HP, Kopp CW, Festa A, Krzyzanowska K,
Kriwanek S, Minar E, Roka R and Schernthaner G: Impact of weight
loss on inflammatory proteins and their association with the
insulin resistance syndrome in morbidly obese patients.
Arterioscler Thromb Vasc Biol. 23:1042–1047. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leichman JG, Aguilar D, King TM, Mehta S,
Majka C, Scarborough T, Wilson EB and Taegtmeyer H: Improvements in
systemic metabolism, anthropometrics, and left ventricular geometry
3 months after bariatric surgery. Surg Obes Relat Dis. 2:592–599.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rider OJ, Francis JM, Ali MK, Petersen SE,
Robinson M, Robson MD, Byrne JP, Clarke K and Neubauer S:
Beneficial cardiovascular effects of bariatric surgical and dietary
weight loss in obesity. J Am Coll Cardiol. 54:718–726. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ikonomidis I, Mazarakis A, Papadopoulos C,
Patsouras N, Kalfarentzos F, Lekakis J, Kremastinos DT and
Alexopoulos D: Weight loss after bariatric surgery improves aortic
elastic properties and left ventricular function in individuals
with morbid obesity: A 3-year follow-up study. J Hypertens.
25:439–447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Willens HJ, Chakko SC, Byers P, Chirinos
JA, Labrador E, Castrillon JC and Lowery MH: Effects of weight loss
after gastric bypass on right and left ventricular function
assessed by tissue Doppler imaging. Am J Cardiol. 95:1521–1524.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garza CA, Pellikka PA, Somers VK, Sarr MG,
Collazo-Clavell ML, Korenfeld Y and Lopez-Jimenez F: Structural and
functional changes in left and right ventricles after major weight
loss following bariatric surgery for morbid obesity. Am J Cardiol.
105:550–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shah RV, Murthy VL, Abbasi SA, Eng J, Wu
C, Ouyang P, Kwong RY, Goldfine A, Bluemke DA, Lima J and
Jerosch-Herold M: Weight loss and progressive left ventricular
remodelling: The multi-ethnic study of atherosclerosis (MESA). Eur
J Prev Cardiol. 22:1408–1418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Di Bello V, Santini F, Di Cori A, Pucci A,
Talini E, Palagi C, Delle Donne MG, Marsili A, Fierabracci P,
Valeriano R, et al: Effects of bariatric surgery on early
myocardial alterations in adult severely obese subjects.
Cardiology. 109:241–248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rubler S, Dlugash J, Yuceoglu YZ, Kumral
T, Branwood AW and Grishman A: New type of cardiomyopathy
associated with diabetic glomerulosclerosis. Am J Cardiol.
30:595–602. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Avagimyan A, Popov S and Shalnova S: The
pathophysiological basis of diabetic cardiomyopathy development.
Curr Probl Cardiol. 47:1011562022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jia G, Whaley-Connell A and Sowers JR:
Diabetic cardiomyopathy: A hyperglycaemia- and
insulin-resistance-induced heart disease. Diabetologia. 61:21–28.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pappachan JM, Varughese GI, Sriraman R and
Arunagirinathan G: Diabetic cardiomyopathy: Pathophysiology,
diagnostic evaluation and management. World J Diabetes. 4:177–189.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakamura K, Miyoshi T, Yoshida M, Akagi S,
Saito Y, Ejiri K, Matsuo N, Ichikawa K, Iwasaki K, Naito T, et al:
Pathophysiology and treatment of diabetic cardiomyopathy and heart
failure in patients with diabetes mellitus. Int J Mol Sci.
23:35872022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang X, Wu D, Cheng Y, Zhang X, Liu T,
Liu Q, Xia P, Zhang G, Hu S and Liu S: Restoration of myocardial
glucose uptake with facilitated myocardial glucose transporter 4
translocation contributes to alleviation of diabetic cardiomyopathy
in rats after duodenal-jejunal bypass. J Diabetes Investig.
10:626–638. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bugger H and Abel ED: Molecular mechanisms
of diabetic cardiomyopathy. Diabetologia. 57:660–671. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou Z, Mahdi A, Tratsiakovich Y, Zahorán
S, Kövamees O, Nordin F, Uribe Gonzalez AE, Alvarsson M, Östenson
CG, Andersson DC, et al: Erythrocytes from patients with type 2
diabetes induce endothelial dysfunction via arginase I. J Am Coll
Cardiol. 72:769–780. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiao H, Sun X, Lin Z, Yang Y, Zhang M, Xu
Z, Liu P, Liu Z and Huang H: Gentiopicroside targets PAQR3 to
activate the PI3K/AKT signaling pathway and ameliorate disordered
glucose and lipid metabolism. Acta Pharm Sin B. 12:2887–2904. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alaaeldin R, Abdel-Rahman IAM, Hassan HA,
Youssef N, Allam AE, Abdelwahab SF, Zhao QL and Fathy M:
Carpachromene ameliorates insulin resistance in HepG2 cells via
modulating IR/IRS1/PI3k/Akt/GSK3/FoxO1 pathway. Molecules.
26:76292021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang N, Liu X, Zhuang L, Liu X, Zhao H,
Shan Y, Liu Z, Li F, Wang Y and Fang J: Berberine decreases insulin
resistance in a PCOS rats by improving GLUT4: Dual regulation of
the PI3K/AKT and MAPK pathways. Regul Toxicol Pharmacol.
110:1045442020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ruze R, Xu Q, Liu G, Li Y, Chen W, Cheng
Z, Xiong Y, Liu S, Zhang G, Hu S and Yan Z: Central GLP-1
contributes to improved cognitive function and brain glucose uptake
after duodenum-jejunum bypass on obese and diabetic rats. Am J
Physiol Endocrinol Metab. 321:E392–E409. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang N, Zhang S, Yuan Y, Xu H, Defossa E,
Matter H, Besenius M, Derdau V, Dreyer M, Halland N, et al:
Molecular basis for inhibiting human glucose transporters by
exofacial inhibitors. Nat Commun. 13:26322022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mafakheri S, Chadt A and Al-Hasani H:
Regulation of RabGAPs involved in insulin action. Biochem Soc
Trans. 46:683–690. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee KD, Ilavenil S, Karnan M, Yang CJ, Kim
D and Choi KC: Novel bacillus ginsengihumi CMRO6 inhibits
adipogenesis via p38MAPK/Erk44/42 and stimulates glucose uptake in
3T3-L1 pre-adipocytes through Akt/AS160 signaling. Int J Mol Sci.
23:47272022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu Q, Ding H, Li S, Dong S, Li L, Shi B,
Zhong M and Zhang G: Sleeve gastrectomy ameliorates
diabetes-induced cardiac hypertrophy correlates with the MAPK
signaling pathway. Front Physiol. 12:7857992021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Belke DD, Larsen TS, Gibbs EM and Severson
DL: Altered metabolism causes cardiac dysfunction in perfused
hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab.
279:E1104–E1113. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lopaschuk GD, Ussher JR, Folmes CD, Jaswal
JS and Stanley WC: Myocardial fatty acid metabolism in health and
disease. Physiol Rev. 90:207–258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lopaschuk GD and Ussher JR: Evolving
concepts of myocardial energy metabolism: More than just fats and
carbohydrates. Circ Res. 119:1173–1176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Carpentier AC: Abnormal myocardial dietary
fatty acid metabolism and diabetic cardiomyopathy. Can J Cardiol.
34:605–614. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou YT, Grayburn P, Karim A, Shimabukuro
M, Higa M, Baetens D, Orci L and Unger RH: Lipotoxic heart disease
in obese rats: Implications for human obesity. Proc Natl Acad Sci
USA. 97:1784–1789. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Listenberger LL, Ory DS and Schaffer JE:
Palmitate-induced apoptosis can occur through a
ceramide-independent pathway. J Biol Chem. 276:14890–14895. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Aasum E, Hafstad AD, Severson DL and
Larsen TS: Age-dependent changes in metabolism, contractile
function, and ischemic sensitivity in hearts from db/db mice.
Diabetes. 52:434–441. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Vincent HK, Powers SK, Dirks AJ and
Scarpace PJ: Mechanism for obesity-induced increase in myocardial
lipid peroxidation. Int J Obes Relat Metab Disord. 25:378–388.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lopaschuk GD, Karwi QG, Tian R, Wende AR
and Abel ED: Cardiac energy metabolism in heart failure. Circ Res.
128:1487–1513. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wali JA, Jarzebska N, Raubenheimer D,
Simpson SJ, Rodionov RN and O'Sullivan JF: Cardio-metabolic effects
of high-fat diets and their underlying mechanisms-a narrative
review. Nutrients. 12:15052020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lin CH, Kurup S, Herrero P, Schechtman KB,
Eagon JC, Klein S, Dávila-Román VG, Stein RI, Dorn GW II, Gropler
RJ, et al: Myocardial oxygen consumption change predicts left
ventricular relaxation improvement in obese humans after weight
loss. Obesity (Silver Spring). 19:1804–1812. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Carreau AM, Noll C, Blondin DP, Frisch F,
Nadeau M, Pelletier M, Phoenix S, Cunnane SC, Guérin B, Turcotte
EE, et al: Bariatric surgery rapidly decreases cardiac dietary
fatty acid partitioning and hepatic insulin resistance through
increased intra-abdominal adipose tissue storage and reduced
spillover in type 2 diabetes. Diabetes. 69:567–577. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Middleton ALO, Byrne JP and Calder PC: The
influence of bariatric (metabolic) surgery on blood polyunsaturated
fatty acids: A systematic review. Clin Nutr ESPEN. 48:121–140.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Moreland AM, Santa Ana CA, Asplin JR, Kuhn
JA, Holmes RP, Cole JA, Odstrcil EA, Van Dinter TG Jr, Martinez JG
and Fordtran JS: Steatorrhea and hyperoxaluria in severely obese
patients before and after Roux-en-Y gastric bypass.
Gastroenterology. 152:1055–1067.e3. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Verna EC and Berk PD: Role of fatty acids
in the pathogenesis of obesity and fatty liver: Impact of bariatric
surgery. Semin Liver Dis. 28:407–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ajoolabady A, Lebeaupin C, Wu NN, Kaufman
RJ and Ren J: ER stress and inflammation crosstalk in obesity. Med
Res Rev. 43:5–30. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liang B, Chen SW, Li YY, Zhang SX and
Zhang Y: Comprehensive analysis of endoplasmic reticulum
stress-related mechanisms in type 2 diabetes mellitus. World J
Diabetes. 14:820–845. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lakshmanan AP, Harima M, Suzuki K,
Soetikno V, Nagata M, Nakamura T, Takahashi T, Sone H, Kawachi H
and Watanabe K: The hyperglycemia stimulated myocardial endoplasmic
reticulum (ER) stress contributes to diabetic cardiomyopathy in the
transgenic non-obese type 2 diabetic rats: A differential role of
unfolded protein response (UPR) signaling proteins. Int J Biochem
Cell Biol. 45:438–447. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yu H, Zhen J, Yang Y, Gu J, Wu S and Liu
Q: Ginsenoside Rg1 ameliorates diabetic cardiomyopathy by
inhibiting endoplasmic reticulum stress-induced apoptosis in a
streptozotocin-induced diabetes rat model. J Cell Mol Med.
20:623–631. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hu H, Tian M, Ding C and Yu S: The C/EBP
homologous protein (CHOP) transcription factor functions in
endoplasmic reticulum stress-induced apoptosis and microbial
infection. Front Immunol. 9:30832018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
B'Chir W, Maurin AC, Carraro V, Averous J,
Jousse C, Muranishi Y, Parry L, Stepien G, Fafournoux P and Bruhat
A: The eIF2α/ATF4 pathway is essential for stress-induced autophagy
gene expression. Nucleic Acids Res. 41:7683–7699. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Belali OM, Ahmed MM, Mohany M, Belali TM,
Alotaibi MM, Al-Hoshani A and Al-Rejaie SS: LCZ696 Protects against
diabetic cardiomyopathy-induced myocardial inflammation, ER stress,
and apoptosis through inhibiting AGEs/NF-κB and PERK/CHOP signaling
pathways. Int J Mol Sci. 23:12882022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Meng Y, Xu X, Niu D, Xu Y, Qiu Y, Zhu Z,
Zhang H and Yin D: Organophosphate flame retardants induce
oxidative stress and Chop/Caspase 3-related apoptosis via
Sod1/p53/Map3k6/Fkbp5 in NCI-1975 cells. Sci Total Environ.
819:1531602022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang X, Liu S, Zhang G, Zhong M, Liu T,
Wei M, Wu D, Huang X, Cheng Y, Wu Q and Hu S: Bariatric surgery
ameliorates diabetic cardiac dysfunction by inhibiting ER stress in
a diabetic rat model. Obes Surg. 27:1324–1334. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hetz C, Zhang K and Kaufman RJ:
Mechanisms, regulation and functions of the unfolded protein
response. Nat Rev Mol Cell Biol. 21:421–438. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–5. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ren J, Bi Y, Sowers JR, Hetz C and Zhang
Y: Endoplasmic reticulum stress and unfolded protein response in
cardiovascular diseases. Nat Rev Cardiol. 18:499–521. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhu G and Lee AS: Role of the unfolded
protein response, GRP78 and GRP94 in organ homeostasis. J Cell
Physiol. 230:1413–1420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Elfiky AA, Baghdady AM, Ali SA and Ahmed
MI: GRP78 targeting: Hitting two birds with a stone. Life Sci.
260:1183172020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kitada M and Koya D: Autophagy in
metabolic disease and ageing. Nat Rev Endocrinol. 17:647–661. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Dewanjee S, Vallamkondu J, Kalra RS, John
A, Reddy PH and Kandimalla R: Autophagy in the diabetic heart: A
potential pharmacotherapeutic target in diabetic cardiomyopathy.
Ageing Res Rev. 68:1013382021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Huang X, Liu S, Wu D, Cheng Y, Han H, Wang
K, Zhang G and Hu S: Facilitated Ca2+ homeostasis and
attenuated myocardial autophagy contribute to alleviation of
diabetic cardiomyopathy after bariatric surgery. Am J Physiol Heart
Circ Physiol. 315:H1258–H1268. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Packer M: SGLT2 inhibitors produce
cardiorenal benefits by promoting adaptive cellular reprogramming
to induce a state of fasting mimicry: A paradigm shift in
understanding their mechanism of action. Diabetes Care. 43:508–511.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
He B, Liu L, Yu C, Wang Y and Han P:
Roux-en-Y gastric bypass reduces lipid overaccumulation in liver by
upregulating hepatic autophagy in obese diabetic rats. Obes Surg.
25:109–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Rodríguez-Hernández A, Cordero MD,
Salviati L, Artuch R, Pineda M, Briones P, Gómez Izquierdo L, Cotán
D, Navas P and Sánchez-Alcázar JA: Coenzyme Q deficiency triggers
mitochondria degradation by mitophagy. Autophagy. 5:19–32. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Russo SB, Baicu CF, Van Laer A, Geng T,
Kasiganesan H, Zile MR and Cowart LA: Ceramide synthase 5 mediates
lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin
Invest. 122:3919–3930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sun Y and Ding S: NLRP3 inflammasome in
diabetic cardiomyopathy and exercise intervention. Int J Mol Sci.
22:132282021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zheng Y, Xu L, Dong N and Li F: NLRP3
inflammasome: The rising star in cardiovascular diseases. Front
Cardiovasc Med. 9:9270612022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhang L, Ai C, Bai M, Niu J and Zhang Z:
NLRP3 inflammasome/pyroptosis: A key driving force in diabetic
cardiomyopathy. Int J Mol Sci. 23:106322022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ding K, Song C, Hu H, Yin K, Huang H and
Tang H: The Role of NLRP3 inflammasome in diabetic cardiomyopathy
and its therapeutic implications. Oxid Med Cell Longev.
2022:37907212022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sun X, Sun X, Meng H, Wu J, Guo X, Du L
and Wu H: Krill oil inhibits NLRP3 inflammasome activation in the
prevention of the pathological injuries of diabetic cardiomyopathy.
Nutrients. 14:3682022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mocanu AO, Mulya A, Huang H, Dan O,
Schauer PR, Dinischiotu A, Brethauer SA and Kirwan JP: Effect of
Roux-en-Y gastric bypass on the NLRP3 inflammasome in pancreatic
islets from zucker diabetic fatty rats. Obes Surg. 26:3076–3081.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Mocanu AO, Mulya A, Huang H, Dan O,
Shimizu H, Batayyah E, Brethauer SA, Dinischiotu A and Kirwan JP:
Effect of Roux-en-Y gastric bypass on the NLRP3 inflammasome in
adipose tissue from obese rats. PLoS One. 10:e01397642015.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Sun K, Wang J, Lan Z, Li L, Wang Y, Li A,
Liu S and Li Y: Sleeve gastroplasty combined with the NLRP3
inflammasome inhibitor CY-09 reduces body weight, improves insulin
resistance and alleviates hepatic steatosis in mouse model. Obes
Surg. 30:3435–3443. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wu D, Yan ZB, Cheng YG, Zhong MW, Liu SZ,
Zhang GY and Hu SY: Deactivation of the NLRP3 inflammasome in
infiltrating macrophages by duodenal-jejunal bypass surgery
mediates improvement of beta cell function in type 2 diabetes.
Metabolism. 81:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Li S, Dong S, Shi B, Xu Q, Li L, Wang S,
Zhang W, Zhong M, Zhu J, Cheng Y, et al: Attenuation of
ROS/chloride efflux-mediated NLRP3 inflammasome activation
contributes to alleviation of diabetic cardiomyopathy in rats after
sleeve gastrectomy. Oxid Med Cell Longev.
2022:46089142022.PubMed/NCBI
|
|
94
|
Zhou R, Yazdi AS, Menu P and Tschopp J: A
role for mitochondria in NLRP3 inflammasome activation. Nature.
469:221–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yang F, Qin Y, Wang Y, Meng S, Xian H, Che
H, Lv J, Li Y, Yu Y, Bai Y and Wang L: Metformin inhibits the NLRP3
inflammasome via AMPK/mTOR-dependent effects in diabetic
cardiomyopathy. Int J Biol Sci. 15:1010–1019. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Li R and Chen J: Salidroside protects
dopaminergic neurons by enhancing PINK1/parkin-mediated mitophagy.
Oxid Med Cell Longev. 2019:93410182019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yang Y, Zhao J, Qiu J, Li J, Liang X,
Zhang Z, Zhang X, Fu H, Korantzopoulos P, Letsas KP, et al:
Xanthine oxidase inhibitor allopurinol prevents oxidative
stress-mediated atrial remodeling in alloxan-induced diabetes
mellitus rabbits. J Am Heart Assoc. 7:e0088072018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhang N, Yu H, Liu T, Zhou Z, Feng B, Wang
Y, Qian Z, Hou X and Zou J: Bmal1 downregulation leads to diabetic
cardiomyopathy by promoting Bcl2/IP3R-mediated mitochondrial
Ca2+ overload. Redox Biol. 64:1027882023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Gutiérrez T, Parra V, Troncoso R, Pennanen
C, Contreras-Ferrat A, Vasquez-Trincado C, Morales PE,
Lopez-Crisosto C, Sotomayor-Flores C, Chiong M, et al: Alteration
in mitochondrial Ca(2+) uptake disrupts insulin signaling in
hypertrophic cardiomyocytes. Cell Commun Signal. 12:682014.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Luptak I, Sverdlov AL, Panagia M, Qin F,
Pimentel DR, Croteau D, Siwik DA, Ingwall JS, Bachschmid MM,
Balschi JA and Colucci WS: Decreased ATP production and myocardial
contractile reserve in metabolic heart disease. J Mol Cell Cardiol.
116:106–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Dillmann WH: Diabetic cardiomyopathy. Circ
Res. 124:1160–1162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zamora M and Villena JA: Contribution of
impaired insulin signaling to the pathogenesis of diabetic
cardiomyopathy. Int J Mol Sci. 20:28332019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Dia M, Gomez L, Thibault H, Tessier N,
Leon C, Chouabe C, Ducreux S, Gallo-Bona N, Tubbs E, Bendridi N, et
al: Reduced reticulum-mitochondria Ca2+ transfer is an
early and reversible trigger of mitochondrial dysfunctions in
diabetic cardiomyopathy. Basic Res Cardiol. 115:742020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Mohan HM, Aherne CM, Rogers AC, Baird AW,
Winter DC and Murphy EP: Molecular pathways: The role of NR4A
orphan nuclear receptors in cancer. Clin Cancer Res. 18:3223–3228.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhou H, Wang J, Zhu P, Zhu H, Toan S, Hu
S, Ren J and Chen Y: NR4A1 aggravates the cardiac microvascular
ischemia reperfusion injury through suppressing FUNDC1-mediated
mitophagy and promoting Mff-required mitochondrial fission by CK2α.
Basic Res Cardiol. 113:232018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wang D, Yin Y, Wang S, Zhao T, Gong F,
Zhao Y, Wang B, Huang Y, Cheng Z, Zhu G, et al: FGF1ΔHBS
prevents diabetic cardiomyopathy by maintaining mitochondrial
homeostasis and reducing oxidative stress via AMPK/Nur77
suppression. Signal Transduct Target Ther. 6:1332021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zheng Y, Tao Y, Zhan X and Wu Q: Nuclear
receptor 4A1 (NR4A1) silencing protects hepatocyte against
hypoxia-reperfusion injury in vitro by activating liver kinase B1
(LKB1)/AMP-activated protein kinase (AMPK) signaling.
Bioengineered. 13:8349–8359. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Li S, Dong S, Xu Q, Shi B, Li L, Zhang W,
Zhu J, Cheng Y, Zhang G and Zhong M: Sleeve gastrectomy-induced
AMPK activation attenuates diabetic cardiomyopathy by maintaining
mitochondrial homeostasis via NR4A1 suppression in rats. Front
Physiol. 13:8377982022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Meier JJ: GLP-1 receptor agonists for
individualized treatment of type 2 diabetes mellitus. Nat Rev
Endocrinol. 8:728–742. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Du Z, Wang J, Lu Y, Ma X, Wen R, Lin J,
Zhou C, Song Z, Li J, Tu P and Jiang Y: The cardiac protection of
Baoyuan decoction via gut-heart axis metabolic pathway.
Phytomedicine. 79:1533222020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Chambers AP, Jessen L, Ryan KK, Sisley S,
Wilson-Pérez HE, Stefater MA, Gaitonde SG, Sorrell JE, Toure M,
Berger J, et al: Weight-independent changes in blood glucose
homeostasis after gastric bypass or vertical sleeve gastrectomy in
rats. Gastroenterology. 141:950–958. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ryan KK, Tremaroli V, Clemmensen C,
Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE,
Sandoval DA, Kohli R, Bäckhed F and Seeley RJ: FXR is a molecular
target for the effects of vertical sleeve gastrectomy. Nature.
509:183–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ding H, Zhang Y, Ma X, Zhang Z, Xu Q, Liu
C, Li B, Dong S, Li L, Zhu J, et al: Bariatric surgery for diabetic
comorbidities: A focus on hepatic, cardiac and renal fibrosis.
Front Pharmacol. 13:10166352022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Helmstädter J, Frenis K, Filippou K, Grill
A, Dib M, Kalinovic S, Pawelke F, Kus K, Kröller-Schön S, Oelze M,
et al: Endothelial GLP-1 (glucagon-like peptide-1) receptor
mediates cardiovascular protection by liraglutide in mice with
experimental arterial hypertension. Arterioscler Thromb Vasc Biol.
40:145–158. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Osto E, Doytcheva P, Corteville C, Bueter
M, Dörig C, Stivala S, Buhmann H, Colin S, Rohrer L, Hasballa R, et
al: Rapid and body weight-independent improvement of endothelial
and high-density lipoprotein function after Roux-en-Y gastric
bypass: Role of glucagon-like peptide-1. Circulation. 131:871–881.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Lee CJ, Sears CL and Maruthur N: Gut
microbiome and its role in obesity and insulin resistance. Ann NY
Acad Sci. 1461:37–52. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Castellanos-Jankiewicz A, Guzmán-Quevedo
O, Fénelon VS, Zizzari P, Quarta C, Bellocchio L, Tailleux A,
Charton J, Fernandois D, Henricsson M, et al: Hypothalamic bile
acid-TGR5 signaling protects from obesity. Cell Metab.
33:1483–1492.e10. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Fuchs CD and Trauner M: Role of bile acids
and their receptors in gastrointestinal and hepatic
pathophysiology. Nat Rev Gastroenterol Hepatol. 19:432–450. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Tu J, Wang Y, Jin L and Huang W: Bile
acids, gut microbiota and metabolic surgery. Front Endocrinol
(Lausanne). 13:9295302022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Stefura T, Zapała B, Gosiewski T,
Krzysztofik M, Skomarovska O and Major P: Relationship between
bariatric surgery outcomes and the preoperative gastrointestinal
microbiota: a cohort study. Surg Obes Relat Dis. 17:889–899. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Coimbra VOR, Crovesy L, Ribeiro-Alves M,
Faller ALK, Mattos F and Rosado EL: Gut microbiota profile in
adults undergoing bariatric surgery: A systematic review.
Nutrients. 14:49792022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Anhê FF, Zlitni S, Zhang SY, Choi BS, Chen
CY, Foley KP, Barra NG, Surette MG, Biertho L, Richard D, et al:
Human gut microbiota after bariatric surgery alters intestinal
morphology and glucose absorption in mice independently of obesity.
Gut. 72:460–471. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Martínez-Montoro JI, Kuchay MS,
Balaguer-Román A, Martínez-Sánchez MA, Frutos MD, Fernández-García
JC and Ramos-Molina B: Gut microbiota and related metabolites in
the pathogenesis of nonalcoholic steatohepatitis and its resolution
after bariatric surgery. Obes Rev. 23:e133672022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Debédat J, Le Roy T, Voland L, Belda E,
Alili R, Adriouch S, Bel Lassen P, Kasahara K, Hutchison E, Genser
L, et al: The human gut microbiota contributes to type-2 diabetes
non-resolution 5-years after Roux-en-Y gastric bypass. Gut
Microbes. 14:20506352022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Gutiérrez-Repiso C, Moreno-Indias I,
Martín-Núñez GM, Ho-Plagaro A, Ocaña-Wilhelmi L, Fernández García
D, Gonzalo Marín M, Moreno-Ruiz FJ, García-Fuentes E and Tinahones
FJ: Influence of factors altering gastric microbiota on bariatric
surgery metabolic outcomes. Microbiol Spectr. 9:e00535212021.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Chaudhari SN, Luo JN, Harris DA,
Aliakbarian H, Yao L, Paik D, Subramaniam R, Adhikari AA, Vernon
AH, Kiliç A, et al: A microbial metabolite remodels the gut-liver
axis following bariatric surgery. Cell Host Microbe. 29:408–424.e7.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Wang J, Chen P, Cao Q, Wang W and Chang X:
Traditional Chinese medicine ginseng dingzhi decoction ameliorates
myocardial fibrosis and high glucose-induced cardiomyocyte injury
by regulating intestinal flora and mitochondrial dysfunction. Oxid
Med Cell Longev. 2022:92059082022.PubMed/NCBI
|
|
128
|
Bastin M and Andreelli F: The gut
microbiota and diabetic cardiomyopathy in humans. Diabetes Metab.
46:197–202. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Shu H, Peng Y, Hang W, Nie J, Zhou N and
Wang DW: The role of CD36 in cardiovascular disease. Cardiovasc
Res. 118:115–129. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wang H, Wang J, Cui H, Fan C, Xue Y, Liu
H, Li H, Li J, Li H, Sun Y, et al: Inhibition of fatty acid uptake
by TGR5 prevents diabetic cardiomyopathy. Nat Metab. 6:1161–1177.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Cesario DA, Brar R and Shivkumar K:
Alterations in ion channel physiology in diabetic cardiomyopathy.
Endocrinol Metab Clin North Am. 35:601–610. ix–x. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Ming Y, Yin Y and Sun Z: Interaction of
nuclear receptor subfamily 4 group A member 1 (Nr4a1) and liver
linase B1 (LKB1) mitigates type 2 diabetes mellitus by activating
monophosphate-activated protein kinase (AMPK)/sirtuin 1 (SIRT1)
axis and inhibiting nuclear factor-kappa B (NF-κB) activation. Med
Sci Monit. 26:e9202782020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Liu M, Chen H, Dai H, Wang Y, Li J, Tian
F, Li Z and Ge RS: Effects of bis (2-butoxyethyl) phthalate on
adrenocortical function in male rats in puberty partially via
down-regulating NR5A1/NR4A1/NR4A2 pathways. Environ Toxicol.
37:2419–2433. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Huynh K, Bernardo BC, McMullen JR and
Ritchie RH: Diabetic cardiomyopathy: Mechanisms and new treatment
strategies targeting antioxidant signaling pathways. Pharmacol
Ther. 142:375–415. 2014. View Article : Google Scholar : PubMed/NCBI
|