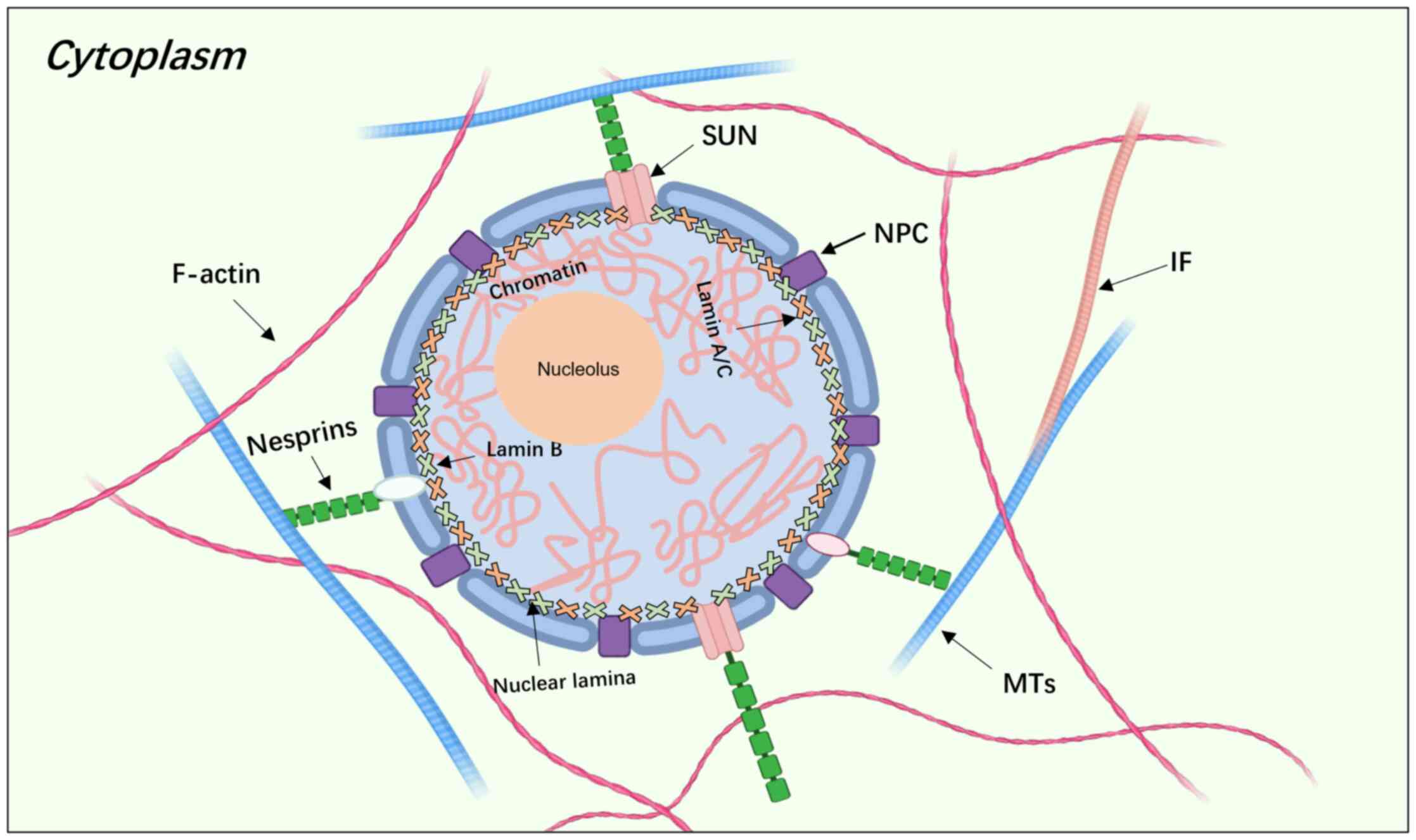
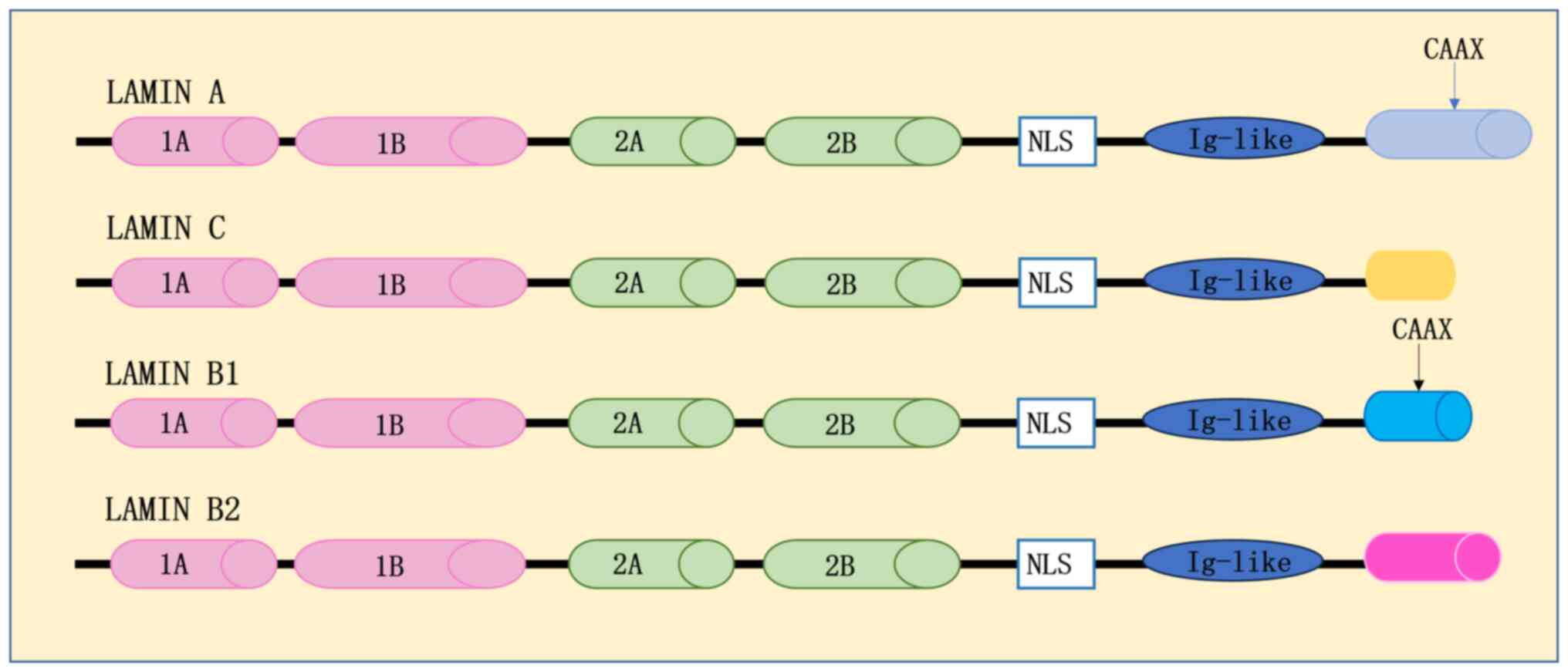
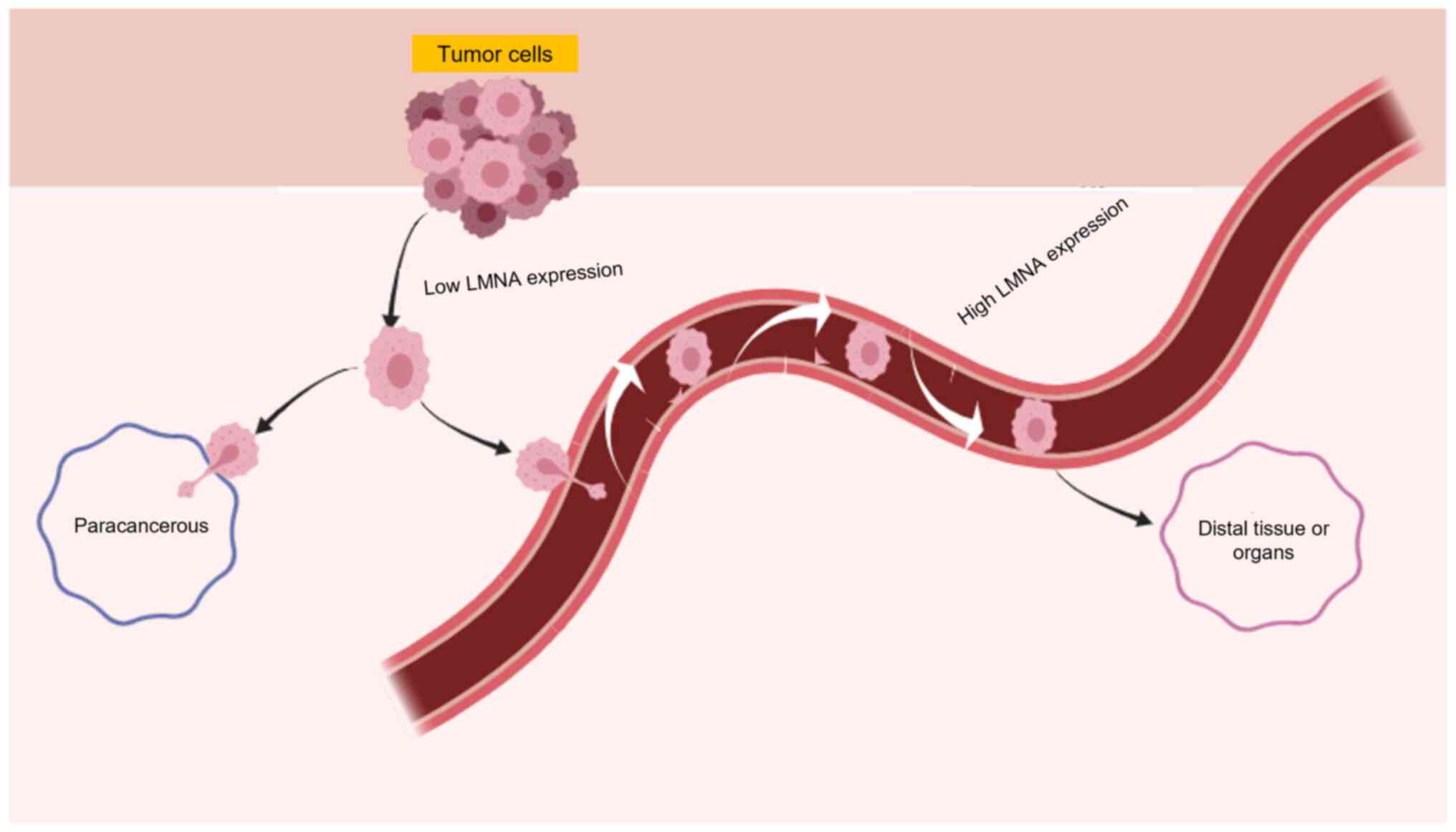
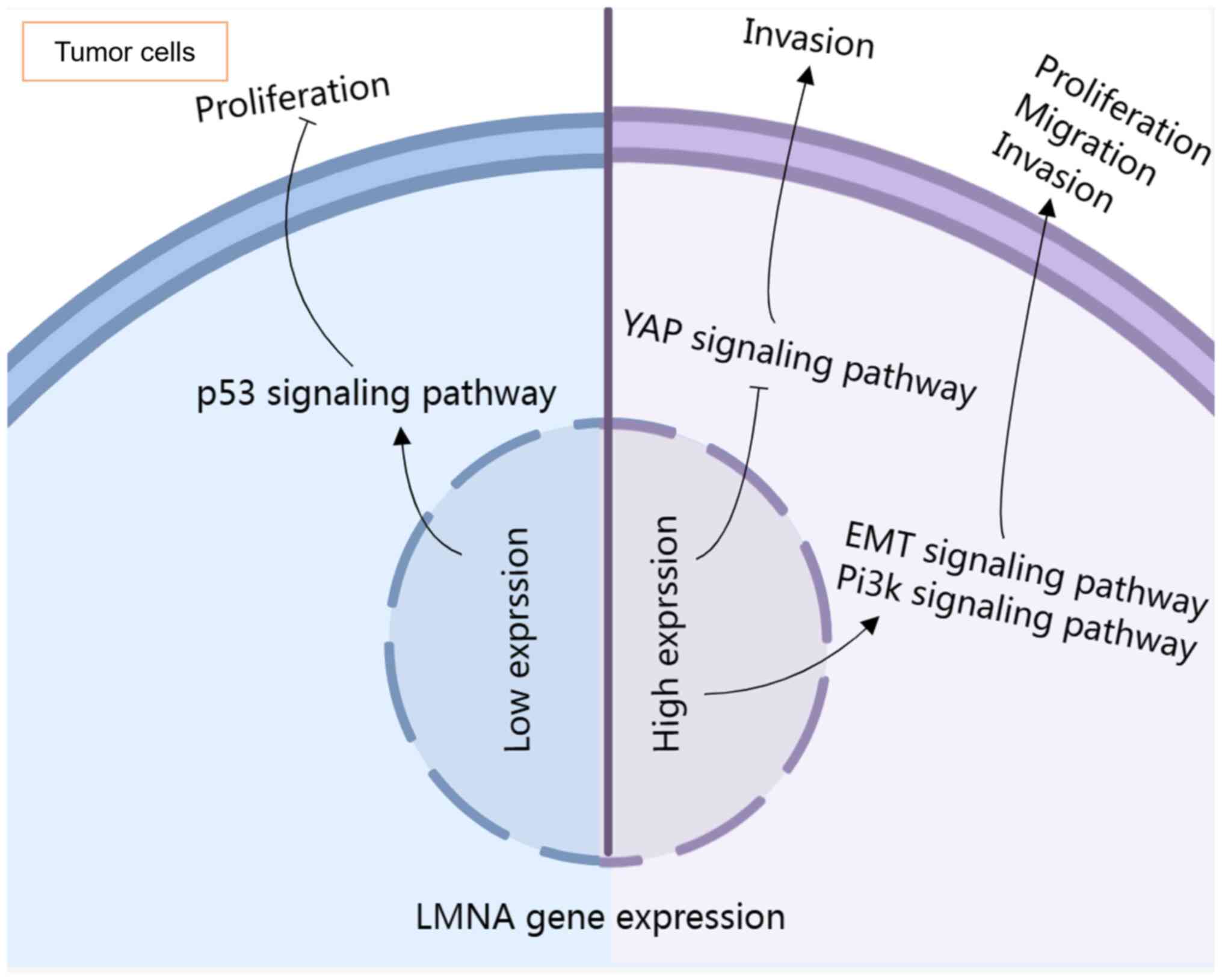
|
1
|
Donnaloja F, Carnevali F, Jacchetti E and
Raimondi MT: Lamin A/C mechanotransduction in laminopathies. Cells.
9:13062020. View Article : Google Scholar
|
|
2
|
Burke B and Stewart CL: The nuclear
lamins: Flexibility in function. Nat Rev Mol Cell Biol. 14:13–24.
2013. View
Article : Google Scholar
|
|
3
|
Xie W and Burke B: Lamins. Curr Biol.
26:R348–R350. 2016. View Article : Google Scholar
|
|
4
|
Dittmer TA and Misteli T: The lamin
protein family. Genome Biol. 12:2222011. View Article : Google Scholar
|
|
5
|
Naetar N, Ferraioli S and Foisner R:
Lamins in the nuclear interior-life outside the lamina. J Cell Sci.
130:2087–2096. 2017. View Article : Google Scholar
|
|
6
|
Tajik A, Zhang Y, Wei F, Sun J, Jia Q,
Zhou W, Singh R, Khanna N, Belmont AS and Wang N: Transcription
upregulation via force-induced direct stretching of chromatin. Nat
Mater. 15:1287–1296. 2016. View
Article : Google Scholar
|
|
7
|
Ramdas NM and Shivashankar GV:
Cytoskeletal control of nuclear morphology and chromatin
organization. J Mol Biol. 427:695–706. 2015. View Article : Google Scholar
|
|
8
|
Davidson PM and Lammerding J: Broken
nuclei-lamins, nuclear mechanics, and disease. Trends Cell Biol.
24:247–256. 2014. View Article : Google Scholar
|
|
9
|
Lee JSH, Hale CM, Panorchan P, Khatau SB,
George JP, Tseng Y, Stewart CL, Hodzic D and Wirtz D: Nuclear lamin
A/C deficiency induces defects in cell mechanics, polarization, and
migration. Biophys J. 93:2542–2552. 2007. View Article : Google Scholar
|
|
10
|
Dahl KN, Kahn SM, Wilson KL and Discher
DE: The nuclear envelope lamina network has elasticity and a
compressibility limit suggestive of a molecular shock absorber. J
Cell Sci. 117:4779–4786. 2004. View Article : Google Scholar
|
|
11
|
Wang X, Zabell A, Koh W and Tang WH: Lamin
A/C cardiomyopathies: Current understanding and novel treatment
strategies. Curr Treat Options Cardiovasc Med. 19:212017.
View Article : Google Scholar
|
|
12
|
Chen SN, Sbaizero O, Taylor MRG and
Mestroni L: Lamin A/C cardiomyopathy: Implications for treatment.
Curr Cardiol Rep. 21:1602019. View Article : Google Scholar
|
|
13
|
Dubik N and Mai S: Lamin A/C: Function in
normal and tumor cells. Cancers (Basel). 12:36882020. View Article : Google Scholar
|
|
14
|
Lund E, Oldenburg AR, Delbarre E, Freberg
CT, Duband-Goulet I, Eskeland R, Buendia B and Collas P: Lamin
A/C-promoter interactions specify chromatin state-dependent
transcription outcomes. Genome Res. 23:1580–1589. 2013. View Article : Google Scholar
|
|
15
|
Nmezi B, Xu J, Fu R, Armiger TJ,
Rodriguez-Bey G, Powell JS, Ma H, Sullivan M, Tu Y, Chen NY, et al:
Concentric organization of A- and B-type lamins predicts their
distinct roles in the spatial organization and stability of the
nuclear lamina. Proc Natl Acad Sci USA. 116:4307–4315. 2019.
View Article : Google Scholar
|
|
16
|
Kang SM, Yoon MH and Park BJ:
Laminopathies; mutations on single gene and various human genetic
diseases. BMB Rep. 51:327–337. 2018. View Article : Google Scholar
|
|
17
|
Sakthivel KM and Sehgal P: A novel role of
lamins from genetic disease to cancer biomarkers. Oncol Rev.
10:3092016.
|
|
18
|
Foster CR, Przyborski SA, Wilson RG and
Hutchison CJ: Lamins as cancer biomarkers. Biochem Soc Trans.
38:297–300. 2010. View Article : Google Scholar
|
|
19
|
Wang AS, Kozlov SV, Stewart CL and Horn
HF: Tissue specific loss of A-type lamins in the gastrointestinal
epithelium can enhance polyp size. Differentiation. 89:11–21. 2015.
View Article : Google Scholar
|
|
20
|
Fisher DZ, Chaudhary N and Blobel G: cDNA
sequencing of nuclear lamins A and C reveals primary and secondary
structural homology to intermediate filament proteins. Proc Natl
Acad Sci USA. 83:6450–6454. 1986. View Article : Google Scholar
|
|
21
|
Ishikawa H, Bischoff R and Holtzer H:
Mitosis and intermediate-sized filaments in developing skeletal
muscle. J Cell Biol. 38:538–555. 1968. View Article : Google Scholar
|
|
22
|
Lin F and Worman HJ: Structural
organization of the human gene encoding nuclear lamin A and nuclear
lamin C. J Biol Chem. 268:16321–16326. 1993. View Article : Google Scholar
|
|
23
|
Zwerger M and Medalia O: From lamins to
lamina: A structural perspective. Histochem Cell Biol. 140:3–12.
2013. View Article : Google Scholar
|
|
24
|
de Leeuw R, Gruenbaum Y and Medalia O:
Nuclear lamins: Thin filaments with major functions. Trends Cell
Biol. 28:34–45. 2018. View Article : Google Scholar
|
|
25
|
Gruenbaum Y and Medalia O: Lamins: The
structure and protein complexes. Curr Opin Cell Biol. 32:7–12.
2015. View Article : Google Scholar
|
|
26
|
Turgay Y, Eibauer M, Goldman AE, Shimi T,
Khayat M, Ben-Harush K, Dubrovsky-Gaupp A, Sapra KT, Goldman RD and
Medalia O: The molecular architecture of lamins in somatic cells.
Nature. 543:261–264. 2017. View Article : Google Scholar
|
|
27
|
Zwerger M, Roschitzki-Voser H, Zbinden R,
Denais C, Herrmann H, Lammerding J, Grütter MG and Medalia O:
Altering lamina assembly reveals lamina-dependent and -independent
functions for A-type lamins. J Cell Sci. 128:3607–3620. 2015.
|
|
28
|
Broers JLV, Peeters EAG, Kuijpers HJH,
Endert J, Bouten CVC, Oomens CWJ, Baaijens FPT and Ramaekers FCS:
Decreased mechanical stiffness in LMNA-/- cells is caused by
defective nucleo-cytoskeletal integrity: Implications for the
development of laminopathies. Hum Mol Genet. 13:2567–2580. 2004.
View Article : Google Scholar
|
|
29
|
Chiarini F, Evangelisti C, Cenni V, Fazio
A, Paganelli F, Martelli AM and Lattanzi G: The cutting edge: The
role of mTOR signaling in laminopathies. Int J Mol Sci. 20:8472019.
View Article : Google Scholar
|
|
30
|
Stick R: The gene structure of Xenopus
nuclear lamin A: A model for the evolution of A-type from B-type
lamins by exon shuffling. Chromosoma. 101:566–574. 1992. View Article : Google Scholar
|
|
31
|
Hanif M, Rosengardten Y, Sagelius H,
Rozell B and Eriksson M: Differential expression of A-type and
B-type lamins during hair cycling. PLoS One. 4:e41142009.
View Article : Google Scholar
|
|
32
|
Kim Y, Sharov AA, McDole K, Cheng M, Hao
H, Fan CM, Gaiano N, Ko MS and Zheng Y: Mouse B-type lamins are
required for proper organogenesis but not by embryonic stem cells.
Science. 334:1706–1710. 2011. View Article : Google Scholar
|
|
33
|
Al-Saaidi R and Bross P: Do lamin A and
lamin C have unique roles? Chromosoma. 124:1–12. 2015. View Article : Google Scholar
|
|
34
|
Gruenbaum Y and Foisner R: Lamins: Nuclear
intermediate filament proteins with fundamental functions in
nuclear mechanics and genome regulation. Annu Rev Biochem.
84:131–164. 2015. View Article : Google Scholar
|
|
35
|
Peter M, Nakagawa J, Dorée M, Labbé JC and
Nigg EA: In vitro disassembly of the nuclear lamina and M
phase-specific phosphorylation of lamins by cdc2 kinase. Cell.
61:591–602. 1990. View Article : Google Scholar
|
|
36
|
Collas P, Thompson L, Fields AP, Poccia DL
and Courvalin JC: Protein kinase C-mediated interphase lamin B
phosphorylation and solubilization. J Biol Chem. 272:21274–21280.
1997. View Article : Google Scholar
|
|
37
|
Molloy S and Little M: p34cdc2
kinase-mediated release of lamins from nuclear ghosts is inhibited
by cAMP-dependent protein kinase. Exp Cell Res. 201:494–499. 1992.
View Article : Google Scholar
|
|
38
|
Zhang YQ and Sarge KD: Sumoylation
regulates lamin A function and is lost in lamin A mutants
associated with familial cardiomyopathies. J Cell Biol. 182:35–39.
2008. View Article : Google Scholar
|
|
39
|
Shimi T, Kittisopikul M, Tran J, Goldman
AE, Adam SA, Zheng Y, Jaqaman K and Goldman RD: Structural
organization of nuclear lamins A, C, B1, and B2 revealed by
superresolution microscopy. Mol Biol Cell. 26:4075–4086. 2015.
View Article : Google Scholar
|
|
40
|
Grossman E, Dahan I, Stick R, Goldberg MW,
Gruenbaum Y and Medalia O: Filaments assembly of ectopically
expressed Caenorhabditis elegans lamin within Xenopus oocytes. J
Struct Biol. 177:113–118. 2012. View Article : Google Scholar
|
|
41
|
Kapinos LE, Schumacher J, Mücke N,
Machaidze G, Burkhard P, Aebi U, Strelkov SV and Herrmann H:
Characterization of the head-to-tail overlap complexes formed by
human lamin A, B1 and B2 ‘half-minilamin’ dimers. J Mol Biol.
396:719–731. 2010. View Article : Google Scholar
|
|
42
|
Goldberg MW, Huttenlauch I, Hutchison CJ
and Stick R: Filaments made from A- and B-type lamins differ in
structure and organization. J Cell Sci. 121:215–225. 2008.
View Article : Google Scholar
|
|
43
|
Schirmer EC and Gerace L: The stability of
the nuclear lamina polymer changes with the composition of lamin
subtypes according to their individual binding strengths. J Biol
Chem. 279:42811–42817. 2004. View Article : Google Scholar
|
|
44
|
Schirmer EC, Guan T and Gerace L:
Involvement of the lamin rod domain in heterotypic lamin
interactions important for nuclear organization. J Cell Biol.
153:479–489. 2001. View Article : Google Scholar
|
|
45
|
Moir RD, Yoon M, Khuon S and Goldman RD:
Nuclear lamins A and B1: Different pathways of assembly during
nuclear envelope formation in living cells. J Cell Biol.
151:1155–1168. 2000. View Article : Google Scholar
|
|
46
|
Fawcett DW: On the occurrence of a fibrous
lamina on the inner aspect of the nuclear envelope in certain cells
of vertebrates. Am J Anat. 119:129–145. 1966. View Article : Google Scholar
|
|
47
|
Belmont AS, Zhai Y and Thilenius A: Lamin
B distribution and association with peripheral chromatin revealed
by optical sectioning and electron microscopy tomography. J Cell
Biol. 123:1671–1685. 1993. View Article : Google Scholar
|
|
48
|
Taniura H, Glass C and Gerace L: A
chromatin binding site in the tail domain of nuclear lamins that
interacts with core histones. J Cell Biol. 131:33–44. 1995.
View Article : Google Scholar
|
|
49
|
Bruston F, Delbarre E, Ostlund C, Worman
HJ, Buendia B and Duband-Goulet I: Loss of a DNA binding site
within the tail of prelamin A contributes to altered
heterochromatin anchorage by progerin. FEBS Lett. 584:2999–3004.
2010. View Article : Google Scholar
|
|
50
|
Guelen L, Pagie L, Brasset E, Meuleman W,
Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W and
van Steensel B: Domain organization of human chromosomes revealed
by mapping of nuclear lamina interactions. Nature. 453:948–951.
2008. View Article : Google Scholar
|
|
51
|
Stewart CL, Kozlov S, Fong LG and Young
SG: Mouse models of the laminopathies. Exp Cell Res. 313:2144–2156.
2007. View Article : Google Scholar
|
|
52
|
Tesson F, Saj M, Uvaize MM, Nicolas H,
Płoski R and Bilińska Z: Lamin A/C mutations in dilated
cardiomyopathy. Cardiol J. 21:331–342. 2014. View Article : Google Scholar
|
|
53
|
Fatkin D, MacRae C, Sasaki T, Wolff MR,
Porcu M, Frenneaux M, Atherton J, Vidaillet HJ Jr, Spudich S, De
Girolami U, et al: Missense mutations in the rod domain of the
lamin A/C gene as causes of dilated cardiomyopathy and
conduction-system disease. N Engl J Med. 341:1715–1724. 1999.
View Article : Google Scholar
|
|
54
|
van Tintelen JP, Tio RA,
Kerstjens-Frederikse WS, van Berlo JH, Boven LG, Suurmeijer AJ,
White SJ, den Dunnen JT, te Meerman GJ, Vos YJ, et al: Severe
myocardial fibrosis caused by a deletion of the 5′ end of the lamin
A/C gene. J Am Coll Cardiol. 49:2430–2439. 2007. View Article : Google Scholar
|
|
55
|
Tiwari V, Alam MJ, Bhatia M, Navya M and
Banerjee SK: The structure and function of lamin A/C: Special focus
on cardiomyopathy and therapeutic interventions. Life Sci.
341:1224892024. View Article : Google Scholar
|
|
56
|
Kim Y, Bayona PW, Kim M, Chang J, Hong S,
Park Y, Budiman A, Kim YJ, Choi CY, Kim WS, et al: Macrophage Lamin
A/C regulates inflammation and the development of obesity-induced
insulin resistance. Front Immunol. 9:6962018. View Article : Google Scholar
|
|
57
|
Vigouroux C, Guénantin AC, Vatier C, Capel
E, Le Dour C, Afonso P, Bidault G, Béréziat V, Lascols O, Capeau J,
et al: Lipodystrophic syndromes due to LMNA mutations: recent
developments on biomolecular aspects, pathophysiological hypotheses
and therapeutic perspectives. Nucleus. 9:235–248. 2018. View Article : Google Scholar
|
|
58
|
Cao H and Hegele RA: Nuclear lamin A/C
R482Q mutation in canadian kindreds with Dunnigan-type familial
partial lipodystrophy. Hum Mol Genet. 9:109–112. 2000. View Article : Google Scholar
|
|
59
|
De Sandre-Giovannoli A, Bernard R, Cau P,
Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart CL, Munnich A,
Le Merrer M and Lévy N: Lamin a truncation in Hutchinson-Gilford
progeria. Science. 300:20552003. View Article : Google Scholar
|
|
60
|
Eriksson M, Brown WT, Gordon LB, Glynn MW,
Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, et
al: Recurrent de novo point mutations in lamin A cause
Hutchinson-Gilford progeria syndrome. Nature. 423:293–298. 2003.
View Article : Google Scholar
|
|
61
|
Wong X, Melendez-Perez AJ and Reddy KL:
The nuclear lamina. Cold Spring Harb Perspect Biol. 14:a0401132022.
View Article : Google Scholar
|
|
62
|
Dechat T, Shimi T, Adam SA, Rusinol AE,
Andres DA, Spielmann HP, Sinensky MS and Goldman RD: Alterations in
mitosis and cell cycle progression caused by a mutant lamin A known
to accelerate human aging. Proc Natl Acad Sci USA. 104:4955–4960.
2007. View Article : Google Scholar
|
|
63
|
Ragnauth CD, Warren DT, Liu Y, McNair R,
Tajsic T, Figg N, Shroff R, Skepper J and Shanahan CM: Prelamin A
acts to accelerate smooth muscle cell senescence and is a novel
biomarker of human vascular aging. Circulation. 121:2200–2210.
2010. View Article : Google Scholar
|
|
64
|
Kirkland NJ, Skalak SH, Whitehead AJ,
Hocker JD, Beri P, Vogler G, Hum B, Wang M, Lakatta EG, Ren B, et
al: Age-dependent Lamin changes induce cardiac dysfunction via
dysregulation of cardiac transcriptional programs. Nat Aging.
3:17–33. 2023. View Article : Google Scholar
|
|
65
|
Maynard S, Hall A, Galanos P, Rizza S,
Yamamoto T, Gram HH, Munk SHN, Shoaib M, Sørensen CS, Bohr VA, et
al: Lamin A/C impairments cause mitochondrial dysfunction by
attenuating PGC1α and the NAMPT-NAD+ pathway. Nucleic Acids Res.
50:9948–9965. 2022. View Article : Google Scholar
|
|
66
|
Simon M, Yang J, Gigas J, Earley EJ,
Hillpot E, Zhang L, Zagorulya M, Tombline G, Gilbert M, Yuen SL, et
al: A rare human centenarian variant of SIRT6 enhances genome
stability and interaction with Lamin A. EMBO J. 41:e1103932022.
View Article : Google Scholar
|
|
67
|
Yoon MH, Kang SM, Lee SJ, Woo TG, Oh AY,
Park S, Ha NC and Park BJ: p53 induces senescence through Lamin A/C
stabilization-mediated nuclear deformation. Cell Death Dis.
10:1072019. View Article : Google Scholar
|
|
68
|
Lochs SJA, Kefalopoulou S and Kind J:
Lamina associated domains and gene regulation in development and
cancer. Cells. 8:2712019. View Article : Google Scholar
|
|
69
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar
|
|
70
|
Broers JLV and Ramaekers FCS: The role of
the nuclear lamina in cancer and apoptosis. Adv Exp Med Biol.
773:27–48. 2014. View Article : Google Scholar
|
|
71
|
Broers JL, Machiels BM, Kuijpers HJ,
Smedts F, van den Kieboom R, Raymond Y and Ramaekers FC: A- and
B-type lamins are differentially expressed in normal human tissues.
Histochem Cell Biol. 107:505–517. 1997. View Article : Google Scholar
|
|
72
|
Kaufmann SH, Mabry M, Jasti R and Shaper
JH: Differential expression of nuclear envelope lamins A and C in
human lung cancer cell lines. Cancer Res. 51:581–566. 1991.
|
|
73
|
Guinde J, Frankel D, Perrin S, Delecourt
V, Lévy N, Barlesi F, Astoul P, Roll P and Kaspi E: Lamins in lung
cancer: Biomarkers and Key factors for disease progression through
miR-9 regulation? Cells. 7:782018. View Article : Google Scholar
|
|
74
|
Machiels BM, Broers JL, Raymond Y, de Ley
L, Kuijpers HJ, Caberg NE and Ramaekers FC: Abnormal A-type lamin
organization in a human lung carcinoma cell line. Eur J Cell Biol.
67:328–335. 1995.
|
|
75
|
Capo-chichi CD, Cai KQ, Smedberg J,
Ganjei-Azar P, Godwin AK and Xu XX: Loss of A-type lamin expression
compromises nuclear envelope integrity in breast cancer. Chin J
Cancer. 30:415–425. 2011. View Article : Google Scholar
|
|
76
|
Harris H: Concerning the origin of
malignant tumours by Theodor Boveri. Translated and annotated by
Henry Harris. Preface. J Cell Sci. 121 (Suppl 1):S5–S6. 2008.
|
|
77
|
Holland AJ and Cleveland DW: Boveri
revisited: Chromosomal instability, aneuploidy and tumorigenesis.
Nat Rev Mol Cell Biol. 10:478–487. 2009. View Article : Google Scholar
|
|
78
|
Roschke AV, Tonon G, Gehlhaus KS, McTyre
N, Bussey KJ, Lababidi S, Scudiero DA, Weinstein JN and Kirsch IR:
Karyotypic complexity of the NCI-60 drug-screening panel. Cancer
Res. 63:8634–8647. 2003.
|
|
79
|
Thompson SL, Bakhoum SF and Compton DA:
Mechanisms of chromosomal instability. Curr Biol. 20:R285–R295.
2010. View Article : Google Scholar
|
|
80
|
Sen S: Aneuploidy and cancer. Curr Opin
Oncol. 12:82–88. 2000. View Article : Google Scholar
|
|
81
|
Ozols RF, Bookman MA, Connolly DC, Daly
MB, Godwin AK, Schilder RJ, Xu X and Hamilton TC: Focus on
epithelial ovarian cancer. Cancer Cell. 5:19–24. 2004. View Article : Google Scholar
|
|
82
|
Agarwal R and Kaye SB: Ovarian cancer:
Strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View Article : Google Scholar
|
|
83
|
Capo-Chichi CD, Cai KQ and Xu XX:
Overexpression and cytoplasmic localization of caspase-6 is
associated with lamin A degradation in set of ovarian cancers.
Biomarker Res. 6:302018. View Article : Google Scholar
|
|
84
|
Agrelo R, Setien F, Espada J, Artiga MJ,
Rodriguez M, Pérez-Rosado A, Sanchez-Aguilera A, Fraga MF, Piris MA
and Esteller M: Inactivation of the lamin A/C gene by CpG island
promoter hypermethylation in hematologic malignancies, and its
association with poor survival in nodal diffuse large B-cell
lymphoma. J Clin Oncol. 23:3940–3947. 2005. View Article : Google Scholar
|
|
85
|
Maresca G, Natoli M, Nardella M, Arisi I,
Trisciuoglio D, Desideri M, Brandi R, D'Aguanno S, Nicotra MR,
D'Onofrio M, et al: LMNA knock-down affects differentiation and
progression of human neuroblastoma cells. PLoS One. 7:e455132012.
View Article : Google Scholar
|
|
86
|
Venables RS, McLean S, Luny D, Moteleb E,
Morley S, Quinlan RA, Lane EB and Hutchison CJ: Expression of
individual lamins in basal cell carcinomas of the skin. Br J
Cancer. 84:512–519. 2001. View Article : Google Scholar
|
|
87
|
Foster CR, Robson JL, Simon WJ, Twigg J,
Cruikshank D, Wilson RG and Hutchison CJ: The role of Lamin A in
cytoskeleton organization in colorectal cancer cells: A proteomic
investigation. Nucleus. 2:434–243. 2011. View Article : Google Scholar
|
|
88
|
Kong L, Schäfer G, Bu H, Zhang Y, Zhang Y
and Klocker H: Lamin A/C protein is overexpressed in
tissue-invading prostate cancer and promotes prostate cancer cell
growth, migration and invasion through the PI3K/AKT/PTEN pathway.
Carcinogenesis. 33:751–759. 2012. View Article : Google Scholar
|
|
89
|
Bell ES, Shah P, Zuela-Sopilniak N, Kim D,
Varlet AA, Morival JLP, McGregor AL, Isermann P, Davidson PM,
Elacqua JJ, et al: Low lamin A levels enhance confined cell
migration and metastatic capacity in breast cancer. Oncogene.
41:4211–4230. 2022. View Article : Google Scholar
|
|
90
|
Wang Y, Jiang J, He L, Gong G and Wu X:
Effect of lamin-A expression on migration and nuclear stability of
ovarian cancer cells. Gynecol Oncol. 152:166–176. 2019. View Article : Google Scholar
|
|
91
|
Miles FL, Pruitt FL, van Golen KL and
Cooper CR: Stepping out of the flow: Capillary extravasation in
cancer metastasis. Clin Exp Metastasis. 25:305–324. 2008.
View Article : Google Scholar
|
|
92
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar
|
|
93
|
Roncato F, Regev O, Feigelson SW, Yadav
SK, Kaczmarczyk L, Levi N, Drago-Garcia D, Ovadia S, Kizner M,
Addadi Y, et al: Reduced Lamin A/C does not facilitate cancer cell
transendothelial migration but compromises lung metastasis. Cancers
(Basel). 13:23832021. View Article : Google Scholar
|
|
94
|
Mitchell MJ, Denais C, Chan MF, Wang Z,
Lammerding J and King MR: Lamin A/C deficiency reduces circulating
tumor cell resistance to fluid shear stress. Am J Physiol Cell
Physiol. 309:C736–C746. 2015. View Article : Google Scholar
|
|
95
|
Ferrari R, Infante E and Chavrier P:
Nucleus-invadopodia duo during cancer invasion. Trends Cell Biol.
29:93–96. 2019. View Article : Google Scholar
|
|
96
|
Osmanagic-Myers S, Dechat T and Foisner R:
Lamins at the crossroads of mechanosignaling. Genes Dev.
29:225–237. 2015. View Article : Google Scholar
|
|
97
|
Kaspi E, Frankel D, Guinde J, Perrin S,
Laroumagne S, Robaglia-Schlupp A, Ostacolo K, Harhouri K,
Tazi-Mezalek R, Micallef J, et al: Low lamin A expression in lung
adenocarcinoma cells from pleural effusions is a pejorative factor
associated with high number of metastatic sites and poor
performance status. PLoS One. 12:e01831362017. View Article : Google Scholar
|
|
98
|
Willis ND, Cox TR, Rahman-Casañs SF, Smits
K, Przyborski SA, van den Brandt P, van Engeland M, Weijenberg M,
Wilson RG, de Bruïne A and Hutchison CJ: Lamin A/C is a risk
biomarker in colorectal cancer. PLoS One. 3:e29882008. View Article : Google Scholar
|
|
99
|
Setti Boubaker N, Gurtner A, Trabelsi N,
Manni I, Ayed H, Saadi A, Zaghbib S, Naimi Z, Sahraoui G, Zouari S,
et al: The diagnostic applicability of A-type Lamin in non-muscle
invasive bladder cancer. Ann Diagn Pathol. 54:1518082021.
View Article : Google Scholar
|
|
100
|
Wazir U, Ahmed MH, Bridger JM, Harvey A,
Jiang WG, Sharma AK and Mokbel K: The clinicopathological
significance of lamin A/C, lamin B1 and lamin B receptor mRNA
expression in human breast cancer. Cell Mol Biol Lett. 18:595–611.
2013. View Article : Google Scholar
|
|
101
|
Alhudiri IM, Nolan CC, Ellis IO, Elzagheid
A, Rakha EA, Green AR and Chapman CJ: Expression of Lamin A/C in
early-stage breast cancer and its prognostic value. Breast Cancer
Res Treat. 174:661–668. 2019. View Article : Google Scholar
|
|
102
|
Smith ER, George SH, Kobetz E and Xu XX:
New biological research and understanding of Papanicolaou's test.
Diagn Cytopathol. 46:507–515. 2018. View Article : Google Scholar
|
|
103
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar
|
|
104
|
Thompson SL and Compton DA: Proliferation
of aneuploid human cells is limited by a p53-dependent mechanism. J
Cell Biol. 188:369–381. 2010. View Article : Google Scholar
|
|
105
|
Saarinen I, Mirtti T, Seikkula H, Boström
PJ and Taimen P: Differential predictive roles of A- and B-type
nuclear lamins in prostate cancer progression. PLoS One.
10:e01406712015. View Article : Google Scholar
|
|
106
|
Meaburn KJ and Misteli T: Assessment of
the utility of gene positioning biomarkers in the stratification of
prostate cancers. Front Genet. 10:10292019. View Article : Google Scholar
|
|
107
|
Grozescu T and Popa F: Prostate cancer
between prognosis and adequate/proper therapy. J Med Life. 10:5–12.
2017.
|
|
108
|
Setijono SR, Park M, Kim G, Kim Y, Cho KW
and Song SJ: miR-218 and miR-129 regulate breast cancer progression
by targeting Lamins. Biochem Biophys Res Commun. 496:826–833. 2018.
View Article : Google Scholar
|
|
109
|
Chiarini F, Paganelli F, Balestra T,
Capanni C, Fazio A, Manara MC, Landuzzi L, Petrini S, Evangelisti
C, Lollini PL, et al: Lamin A and the LINC complex act as potential
tumor suppressors in Ewing Sarcoma. Cell Death Dis. 13:3462022.
View Article : Google Scholar
|
|
110
|
Snigireva AV, Vrublevskaya VV, Skarga YY,
Evdokimovskaya YV and Morenkov OS: Effect of heat shock protein 90
(Hsp90) on migration and invasion of human cancer cells in vitro.
Bull Exp Biol Med. 157:476–478. 2014. View Article : Google Scholar
|
|
111
|
Meng X, Cao J, Zheng H, Ma X, Wang Y, Tong
Y, Xie S, Lu R and Guo L: TPX2 promotes ovarian tumorigenesis by
interacting with Lamin A/C and affecting its stability. Cancer Med.
12:9738–9748. 2023. View Article : Google Scholar
|
|
112
|
Sidera K and Patsavoudi E: HSP90
inhibitors: Current development and potential in cancer therapy.
Recent Pat Anticancer Drug Discov. 9:1–20. 2014. View Article : Google Scholar
|
|
113
|
Wang Y, Chen Q, Wu D, Chen Q, Gong G, He L
and Wu X: Lamin-A interacting protein Hsp90 is required for DNA
damage repair and chemoresistance of ovarian cancer cells. Cell
Death Dis. 12:7862021. View Article : Google Scholar
|
|
114
|
Shao X, Tarnasky HA, Lee JP, Oko R and van
der Hoorn FA: Spag4, a novel sperm protein, binds outer dense-fiber
protein Odf1 and localizes to microtubules of manchette and
axoneme. Dev Biol. 211:109–123. 1999. View Article : Google Scholar
|
|
115
|
Shoji K, Murayama T, Mimura I, Wada T,
Kume H, Goto A, Ohse T, Tanaka T, Inagi R, van der Hoorn FA, et al:
Sperm-associated antigen 4, a novel hypoxia-inducible factor 1
target, regulates cytokinesis, and its expression correlates with
the prognosis of renal cell carcinoma. Am J Pathol. 182:2191–2203.
2013. View Article : Google Scholar
|
|
116
|
Elhanati S, Kanfi Y, Varvak A, Roichman A,
Carmel-Gross I, Barth S, Gibor G and Cohen HY: Multiple regulatory
layers of SREBP1/2 by SIRT6. Cell Rep. 4:905–912. 2013. View Article : Google Scholar
|
|
117
|
Ghosh S, Liu B, Wang Y, Hao Q and Zhou Z:
Lamin A is an endogenous SIRT6 activator and promotes
SIRT6-mediated DNA repair. Cell Rep. 13:1396–1406. 2015. View Article : Google Scholar
|
|
118
|
Li H, Ge C, Zhao F, Yan M, Hu C, Jia D,
Tian H, Zhu M, Chen T, Jiang G, et al: Hypoxia-inducible factor 1
alpha-activated angiopoietin-like protein 4 contributes to tumor
metastasis via vascular cell adhesion molecule-1/integrin β1
signaling in human hepatocellular carcinoma. Hepatology.
54:910–919. 2011. View Article : Google Scholar
|
|
119
|
Zhao J, Liu B, Yang JA, Tang D, Wang X and
Chen Q: Human sperm-associated antigen 4 as a potential biomarker
of glioblastoma progression and prognosis. Neuroreport. 30:446–451.
2019. View Article : Google Scholar
|
|
120
|
Liu T, Yu J, Ge C, Zhao F, Chen J, Miao C,
Jin W, Zhou Q, Geng Q, Lin H, et al: Sperm associated antigen 4
promotes SREBP1-mediated de novo lipogenesis via interaction with
lamin A/C and contributes to tumor progression in hepatocellular
carcinoma. Cancer Lett. 536:2156422022. View Article : Google Scholar
|
|
121
|
Eferl R and Wagner EF: AP-1: A
double-edged sword in tumorigenesis. Nat Rev Cancer. 3:859–868.
2003. View Article : Google Scholar
|
|
122
|
Shaulian E and Karin M: AP-1 as a
regulator of cell life and death. Nat Cell Biol. 4:E131–E136. 2002.
View Article : Google Scholar
|
|
123
|
Ding Y, Hao K, Li Z, Ma R, Zhou Y, Zhou Z,
Wei M, Liao Y, Dai Y, Yang Y, et al: c-Fos separation from Lamin
A/C by GDF15 promotes colon cancer invasion and metastasis in
inflammatory microenvironment. J Cell Physiol. 235:4407–4421. 2020.
View Article : Google Scholar
|
|
124
|
Sharma P and Kuehn MR: SENP1-modulated
sumoylation regulates retinoblastoma protein (RB) and Lamin A/C
interaction and stabilization. Oncogene. 35:6429–6438. 2016.
View Article : Google Scholar
|
|
125
|
Elenbaas JS, Bragazzi Cunha J,
Azuero-Dajud R, Nelson B, Oral EA, Williams JA, Stewart CL and
Omary MB: Lamin A/C maintains exocrine pancreas homeostasis by
regulating stability of RB and activity of E2F. Gastroenterology.
154:1625–1629.e8. 2018. View Article : Google Scholar
|