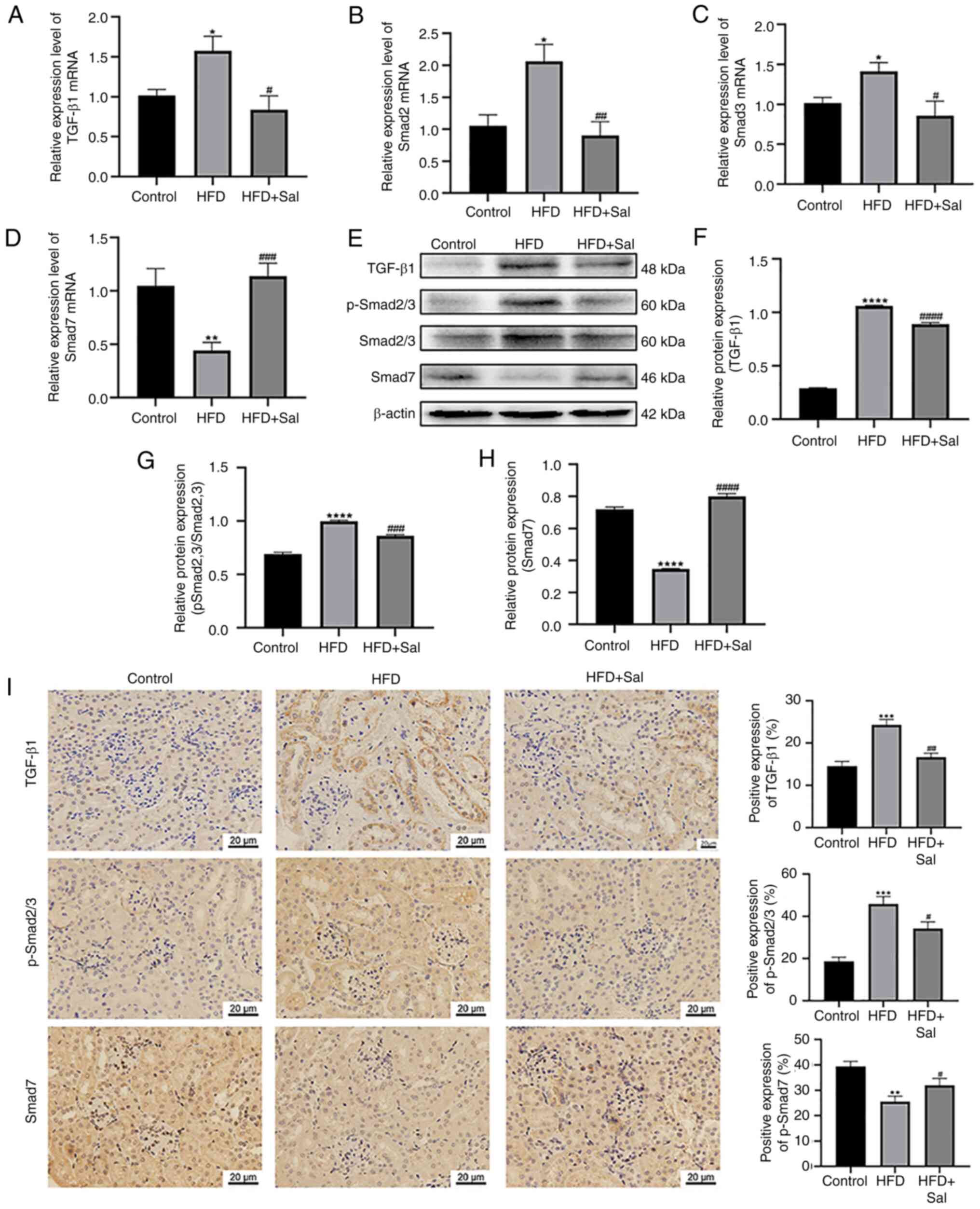
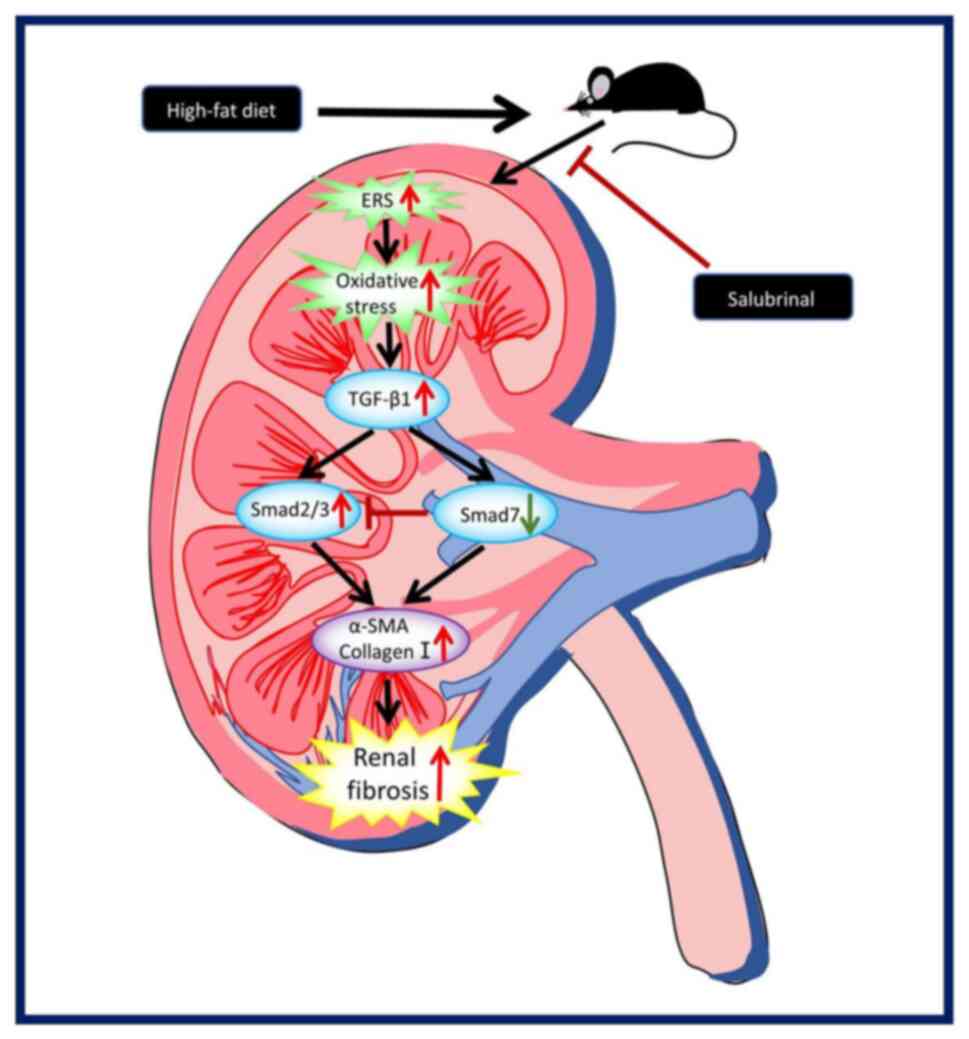
|
1
|
Jiang Z, Wang Y, Zhao X, Cui H, Han M, Ren
X, Gang X and Wang G: Obesity and chronic kidney disease. Am J
Physiol Endocrinol Metab. 324:E24–E41. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stasi A, Cosola C, Caggiano G, Cimmarusti
MT, Palieri R, Acquaviva PM, Rana G and Gesualdo L: Obesity-related
chronic kidney disease: Principal mechanisms and new approaches in
nutritional management. Front Nutr. 9:9256192022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alizadeh S, Ahmadi M, Ghorbani Nejad B,
Djazayeri A and Shab-Bidar S: Metabolic syndrome and its components
are associated with increased chronic kidney disease risk: Evidence
from a meta-analysis on 11 109 003 participants from 66 studies.
Int J Clin Pract. May 23–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang R, Fu P and Ma L: Kidney fibrosis:
From mechanisms to therapeutic medicines. Signal Transduct Target
Ther. 8:1292023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu S, Fu S, Jin Y, Geng R, Li Y, Zhang Y,
Liu J and Guo W: Tartary buckwheat flavonoids alleviates high-fat
diet induced kidney fibrosis in mice by inhibiting MAPK and
TGF-β1/Smad signaling pathway. Chem Biol Interact. 379:1105332023.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu X, Si F, Hao R, Zheng J and Zhang C:
Nuciferine protects against obesity-induced nephrotoxicity through
its hypolipidemic, anti-inflammatory, and antioxidant effects. J
Agric Food Chem. 71:18769–18779. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee LE, Doke T, Mukhi D and Susztak K: The
key role of altered tubule cell lipid metabolism in kidney disease
development. Kidney Int. 106:24–34. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang SX, Wang JJ, Starr CR, Lee EJ, Park
KS, Zhylkibayev A, Medina A, Lin JH and Gorbatyuk M: The
endoplasmic reticulum: Homeostasis and crosstalk in retinal health
and disease. Prog Retin Eye Res. 98:1012312024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen X and Cubillos-Ruiz JR: Endoplasmic
reticulum stress signals in the tumour and its microenvironment.
Nat Rev Cancer. 21:71–88. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Metcalf MG, Higuchi-Sanabria R, Garcia G,
Tsui CK and Dillin A: Beyond the cell factory: Homeostatic
regulation of and by the UPRER. Sci Adv. 6:eabb96142020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Merighi A and Lossi L: Endoplasmic
reticulum stress signaling and neuronal cell death. Int J Mol Sci.
23:151862022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma K, Zhang Y, Zhao J, Zhou L and Li M:
Endoplasmic reticulum stress: Bridging inflammation and
obesity-associated adipose tissue. Front Immunol. 15:13812272024.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carnuta MG, Deleanu M, Barbalata T, Toma
L, Raileanu M, Sima AV and Stancu CS: Zingiber officinale extract
administration diminishes steroyl-CoA desaturase gene expression
and activity in hyperlipidemic hamster liver by reducing the
oxidative and endoplasmic reticulum stress. Phytomedicine.
48:62–69. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu L, Guo T, Deng R, Liu L and Yu Y:
Apigenin ameliorates insulin resistance and lipid accumulation by
endoplasmic reticulum stress and SREBP-1c/SREBP-2 pathway in
palmitate-induced HepG2 cells and high-fat diet-fed mice. J
Pharmacol Exp Ther. 377:146–156. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paik J, Fierce Y, Drivdahl R, Treuting PM,
Seamons A, Brabb T and Maggio-Price L: Effects of murine norovirus
infection on a mouse model of diet-induced obesity and insulin
resistance. Comp Med. 60:189–195. 2010.PubMed/NCBI
|
|
16
|
Tian RD, Chen YQ, He YH, Tang YJ, Chen GM,
Yang FW, Li Y, Huang WG, Chen H, Liu X and Lin SD: Phosphorylation
of eIF2α mitigates endoplasmic reticulum stress and hepatocyte
necroptosis in acute liver injury. Ann Hepatol. 19:79–87. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang WB, Ling GH, Sun L and Liu FY: Smad
anchor for receptor activation (SARA) in TGF-beta signaling. Front
Biosci (Elite Ed). 2:857–860. 2010.PubMed/NCBI
|
|
19
|
Hojs R, Ekart R, Bevc S and Vodošek Hojs
N: Chronic kidney disease and obesity. Nephron. 147:660–664. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsu CY, Mcculloch CE, Iribarren C,
Darbinian J and Go AS: Body mass index and risk for end-stage renal
disease. Ann Intern Med. 144:21–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iseki K, Ikemiya Y, Kinjo K, Inoue T,
Iseki C and Takishita S: Body mass index and the risk of
development of end-stage renal disease in a screened cohort. Kidney
Int. 65:1870–1876. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou S, Wu Q, Lin X, Ling X, Miao J, Liu
X, Hu C, Zhang Y, Jia N, Hou FF, et al: Cannabinoid receptor type 2
promotes kidney fibrosis through orchestrating β-catenin signaling.
Kidney Int. 99:364–381. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kume S, Uzu T, Araki S, Sugimoto T,
Isshiki K, Chin-Kanasaki M, Sakaguchi M, Kubota N, Terauchi Y,
Kadowaki T, et al: Role of altered renal lipid metabolism in the
development of renal injury induced by a high-fat diet. J Am Soc
Nephrol. 18:2715–2723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stone RC, Pastar I, Ojeh N, Chen V, Liu S,
Garzon KI and Tomic-Canic M: Epithelial-mesenchymal transition in
tissue repair and fibrosis. Cell Tissue Res. 365:495–506. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng B, Yang W, Wang D, Cheng L, Bu L, Rao
J, Zhang J, Xie J and Zhang B: Peptide DR8 suppresses
epithelial-to-mesenchymal transition via the TGF-β/MAPK signaling
pathway in renal fibrosis. Life Sci. 261:1184652020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li YE, Sowers JR, Hetz C and Ren J: Cell
death regulation by MAMs: From molecular mechanisms to therapeutic
implications in cardiovascular diseases. Cell Death Dis.
13:5042022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sims SG, Cisney RN, Lipscomb MM and Meares
GP: The role of endoplasmic reticulum stress in astrocytes. Glia.
70:5–19. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Engin A: The pathogenesis of
obesity-associated adipose tissue inflammation. Adv Exp Med Biol.
960:221–245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lumeng CN and Saltiel AR: Inflammatory
links between obesity and metabolic disease. J Clin Invest.
121:2111–2117. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Olefsky JM and Glass CK: Macrophages,
inflammation, and insulin resistance. Annu Rev Physiol. 72:219–246.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun K, Kusminski CM and Scherer PE:
Adipose tissue remodeling and obesity. J Clin Invest.
121:2094–2101. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Contreras C, González-García I,
Seoane-Collazo P, Martínez-Sánchez N, Liñares-Pose L, Rial-Pensado
E, Fernø J, Tena-Sempere M, Casals N, Diéguez C, et al: Reduction
of hypothalamic endoplasmic reticulum stress activates browning of
white fat and ameliorates obesity. Diabetes. 66:87–99. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie H, Huang L, Li Y, Zhang H and Liu H:
Endoplasmic reticulum stress and renal lesion in mice with
combination of high-fat diet and streptozotocin-induced diabetes.
Acta Cir Bras. 31:150–155. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Wang HL, Liu TT and Lan HY:
TGF-beta as a master regulator of diabetic nephropathy. Int J Mol
Sci. 22:78812021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye Z and Hu Y: TGF-β1: Gentlemanly
orchestrator in idiopathic pulmonary fibrosis (review). Int J Mol
Med. 48:1322021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ahmed H, Umar MI, Imran S, Javaid F, Syed
SK, Riaz R and Hassan W: TGF-β1 signaling can worsen NAFLD with
liver fibrosis backdrop. Exp Mol Pathol. 124:1047332022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peng D, Fu M, Wang M, Wei Y and Wei X:
Targeting TGF-β signal transduction for fibrosis and cancer
therapy. Mol Cancer. 21:1042022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gifford CC, Tang J, Costello A, Khakoo NS,
Nguyen TQ, Goldschmeding R, Higgins PJ and Samarakoon R: Negative
regulators of TGF-β1 signaling in renal fibrosis; pathological
mechanisms and novel therapeutic opportunities. Clin Sci (Lond).
135:275–303. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Antar SA, Ashour NA, Marawan ME and
Al-Karmalawy AA: Fibrosis: Types, effects, markers, mechanisms for
disease progression, and its relation with oxidative stress,
immunity, and inflammation. Int J Mol Sci. 24:40042023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
de Ceuninck van Capelle C, Spit M and Ten
Dijke P: Current perspectives on inhibitory SMAD7 in health and
disease. Crit Rev Biochem Mol Biol. 55:691–715. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gu YY, Liu XS, Huang XR, Yu XQ and Lan HY:
Diverse role of TGF-β in kidney disease. Front Cell Dev Biol.
8:1232020. View Article : Google Scholar : PubMed/NCBI
|