Introduction
The incidence rate of obesity has been increasing
rapidly in the past few years and obesity has become a crucial
health issue and the most serious epidemic threatening human health
(1). Chronic kidney disease (CKD)
and cardiovascular illnesses are both significantly affected by
obesity (2). Studies have shown
that metabolic syndrome, obesity and being overweight are important
separate indicators of risk for CKD and end-stage renal disease
(ESRD) (1,3). The dominant pathological
characteristic of CKD is renal fibrosis, which is accompanied by
sparse blood vessels and morphological manifestations such as
tubular atrophy, glomerulosclerosis and chronic interstitial
inflammation (4). Although the
precise mechanisms underlying the promotion of renal fibrosis by
obesity, metabolic problems and inflammatory responses resulting
from a high-fat diet (HFD) remain unclear, numerous investigations
have supported this finding (5–7).
One of the most vital organelles in the cell, the
endoplasmic reticulum (ER), is responsible for a number of vital
processes, including protein synthesis, folding, maturation and
Ca2+ storage. It also greatly affects the movement of
materials inside cells (8).
Misfolded and unfolded proteins may accumulate as a result of ER
malfunction, which can initiate the unfolded protein response (UPR)
and endoplasmic reticulum stress (ERS) (9). ERS is an important self-defense
mechanism of the body that aims to maintain the stability of the ER
environment to ensure its normal physiological functions (10). However, excessive UPR activity can
harm the ER and cause impaired protein function or cell death
through the accumulation of a number of misfolded proteins in the
ER (11).
A crucial aspect of the lipid metabolism problem is
ERS. Numerous investigations conducted in the last few years have
demonstrated a strong correlation between ERS and the development
of hyperlipidemic complications (12–14).
However, whether ERS plays a role in kidney damage caused by HFD
and its exact molecular mechanisms are unclear. The present study
verified that ERS promoted renal fibrosis in HFD-fed mice and
revealed that ERS may play an important role in regulating the
TGF-β/SMAD signaling pathway.
Materials and methods
Animals
A total of 30 3-week-old C57BL/6J male mice
(certificate no: SCXK 2019-0010) weighing 10–12 g were obtained
from SPF (Beijing) Biotechnology Co. Animal institutions housed
mice free of viruses and parasites. A 12-h light/dark cycle with an
ambient environment of 24–26°C and a humidity level of 50–65% was
used for the mice. Before the experiment, all the mice were
acclimated and fed for one week. A total of 10 mice per group were
assigned randomly to the control, the HFD and the HFD + Sal groups.
The National Experimental Animal Feeding Guidelines were followed
and the Dali University Animal Ethics Committee approved all animal
uses (approval no. 2023-PZ-278).
Establishment of the animal model and
specimen harvesting
Control mice were fed a regular chow diet and HFD
mice were fed a high-fat diet with a fat-to-energy ratio of 60%.
The mice in the HFD group weighed 20% more than those in the
control group, indicating successful modeling (15). The HFD + Sal group received a daily
intraperitoneal injection of 1 mg/kg of salubrinal solution
(16). The mice were observed at a
fixed time every day for diet, water intake, body posture, behavior
and response to external stimuli. The body weights of the mice were
measured each week. Animals were humanely euthanized if they
reached the humane endpoint [>15% weight loss or >20% overall
weight loss within 1–2 days or showing obvious signs of distress
(lethargy, squinting, dehydration, hunching)]. However, no such
cases were observed in the present experiment. The mice were all
kept under standard conditions for 10 weeks and then harvested. The
mice were passively fasted for 12–14 h before surgery without
access to drinking water. Mice were anesthetized by intraperitoneal
injection of sodium pentobarbital solution 50 mg/kg and 0.5–1 ml
blood was collected by cardiac puncture and sacrificed by cervical
dislocation. The blood was centrifuged at 4°C for 10 min at 1,000 ×
g. The serum was separated and subjected to biochemical analysis.
After blood collection, the kidneys were removed, rinsed and
rapidly weighed. The left kidney of mice were submerged in liquid
nitrogen at −80°C for later experiments. The right kidney was then
preserved in a 4% paraformaldehyde solution at room temperature for
24 h for histological analysis.
Blood biochemistry analysis
The serum-related indicators of the mice were
detected via test kits for total cholesterol (TC; cat. no.
A111-1-1), triglyceride (TG; cat. no. A110-1-1), low-density
lipoprotein (LDL; cat. no. A113-1-1) and high-density lipoprotein
(HDL; cat. no. A112-1-1) to assess whether the obese model was
successfully established. The renal function of the mice was
assessed using a test kit for creatinine (Cr; cat. no. C011-2-1),
blood urea nitrogen (BUN; cat. no. C013-2-1) and uric acid (UA;
cat. no. C012-2-1). Superoxide dismutase (SOD; cat. no. A001-3) and
malondialdehyde (MDA; cat. no. A003-2) were detected to analyze
oxidative stress. The kits used were from Nanjing Jiancheng
Bioengineering Institute.
Hematoxylin and eosin (H&E)
staining
After being cleaned with regular saline, the kidney
tissue was preserved with 4% paraformaldehyde at room temperature
and subjected to gradient dehydration in ethanol. The tissue blocks
were placed in molds containing wax solution and left to cool and
solidify on a freezer table for 10 min before removal. The embedded
tissue was continuously sectioned with a microtome at a thickness
of 4–5 µm. The sections were stained using hematoxylin for 8 min
and eosin for 5 min at room temperature. The renal morphology of
each group was examined microscopically. A total of three kidneys
were selected from each group and 10 non-overlapping regions were
randomly selected from each kidney at ×40 magnification.
Masson staining
The Masson three-color staining procedure was used
to assess the severity of renal fibrosis. Kidney samples were
extracted as soon as possible after death and stored in 4%
paraformaldehyde at room temperature. After rinsing the kidney
tissues with PBS, complete dehydration was performed at 50, 70, 80,
95 and 100% starting with 30% ethanol. The tissue blocks were
placed in molds filled with wax solution, cooled and solidified on
a freezer table for 10 min, and then removed for sectioning. At
room temperature, sections were stained with hematoxylin staining
solution for 5 min, Ponceau acid fuchsin staining solution for 10
min, and finally stained with bright green staining solution for 1
min. The degree of collagen accumulation in renal interstitial
fibrosis was observed via Masson's trichrome staining. The blue
linear or granular deposits were positive for collagen. Sections
were observed under light microscopy and analyzed
semi-quantitatively using ImageJ v1.8.0 software (National
Institutes of Health).
Immunohistochemistry
Paraffin sections of 4 µm renal tissue were
deparaffinized with xylene at 50°C for 3 min, hydrated by graded
ethanol series and then incubated in 3% hydrogen peroxide at room
temperature for 15 min to inactivate endogenous peroxidase. After
washing with PBS, sections were blocked with 5% goat serum
(Beyotime Institute of Biotechnology) for 60 min. Sections were
incubated overnight at 4°C with primary antibodies as follows:
Grp78 (Proteintech Group, Inc.; cat. no. 11587-1-AP; 1:200), CHOP
(Wuhan Servicebio Technology Co., Ltd.; cat. no. GB11204; 1:500),
α-SMA (Cell Signaling Technology, Inc.; cat. no. 19245; 1:400),
collagen I (Chengdu Zen-Bioscience Co., Ltd.; cat. no. 343277;
1:100), TGF-β1 (Wuhan Boster Biological Technology, Ltd.; cat. no.
BA0290; 1:100), Phospho-SMAD2/3 (Affinity Biosciences; cat. no.
AF3367; 1:100) and SMAD7 rabbit pAb (ABclonal Biotech Co., Ltd.;
cat. no. A12343; 1:100). Sections were incubated with secondary
antibody (Wuhan Servicebio Technology Co., Ltd.; cat. no. G1214;
1:200) for 1 h at room temperature and stained with
3,3′-diaminobenzidine (DAB) for 5 min at room temperature and
counterstained with hematoxylin for 60 sec at room temperature.
Sections were observed under a light microscope at 40×
magnification and analyzed semi-quantitatively using ImageJ v1.8.0
software (National Institutes of Health).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was isolated from kidney Tissue (20 mg)
using the Fast pure® Cell/Tissue Total RNA Isolation Kit
(Vazyme, China). Then, the RNA was reverse transcribed into cDNA
using the HiScript®III RT SuperMix for qPCR kit
(Vazyme). The qPCR reaction system was then prepared using 2×ChamQ
Universal SYBR qPCR Master Mix (Vazyme, China). RNA extraction,
cDNA synthesis and qPCR were performed according to the
manufacturer's protocol. The Step One Real-time PCR apparatus
(StepOnePlus; Applied Biosystems; Thermo Fisher Scientific, Inc.)
was subsequently used to conduct RT-qPCR. Thermocycling conditions
were as follows: 95°C for 30 sec, followed by 40 cycles at 95°C for
30 sec and 60°C for 30 sec. Finally, the reaction was completed at
95°C for 15 sec, 60°C for 60 sec, and 95°C for 15 sec. The reaction
volume was 10 µl. The relative expression of each gene was
determined by the 2−ΔΔCq method (17). These experiments were repeated
three times. Table I details PCR
primers for C/EBP-homologous protein (CHOP), glucose-regulated
protein 78 (GRP78), α-smooth muscle actin (α-SMA), Collagen I,
TGF-βl, SMAD2, SMAD3 and SMAD7.
 | Table I.PCR primer nucleotide sequences used
during the research. |
Table I.
PCR primer nucleotide sequences used
during the research.
| Gene | Forward primer | Reverse primer |
|---|
| CHOP |
CCAGGAAACGAAGAGGAAGAAT |
CACTGACCACTCTGTTTCCGTTT |
| GRP78 |
CGCACTTGGAATGACCCTT |
CATCTTTGGTTGCTTGTCGC |
| α-SMA |
TCAGGGAGTAATGGTTGGAATG |
CCAGAGTCCAGCACAATACCAG |
| Collagen
I |
GGTCCTGCTGGTCCTGCTG |
GAGAAGCCACGATGACCCTTTATG |
| TGF-β1 |
GCTGAACCAAGGAGACGGAATA |
GGCTGATCCCGTTGATTTCC |
| Smad2 |
TCGTCCATCTTGCCATTCACTCC |
CCATTCTGCTCTCCACCACCTG |
| Smad3 |
AGCCCCAGAGCAATATTCCAG |
GACATCGGATTCGGGGAGAG |
| Smad7 |
AGCCGCCCTCGTCCTACTC |
GATTCACAGCAACACAGCCTCTTG |
| GAPDH |
GGCAAATTCAACGGCACAGTCAAG |
TCGCTCCTGGAAGATGGTGATGG |
Western blot analyses
Western blotting was used to assess the expression
of ERS-associated proteins (GRP78 and CHOP), α-SMA, collagen I and
pathway-associated proteins. Renal tissues from the mice in each
experimental group were placed in precooled RIPA lysis buffer
(Beyotime Institute of Biotechnology), homogenized with an electric
homogenizer and then centrifuged at 4°C. The BCA quantitative
measurement method was used to assess the protein concentration of
the supernatant. Sample buffer and RIPA buffer were used to prepare
the samples to ensure that the amounts of the three sets of protein
samples were equal. The loading volume of each group was 5 µl and
the proteins were separated via 10% SDS-PAGE. A Bio-Rad rotary
membrane device (Bio-Rad Laboratories, Inc.) was then used to
transfer the proteins from the gel onto the PVDF membrane. The PVDF
membranes were incubated with primary antibodies overnight at 4°C
after being blocked for 30 min at ambient temperature via a quick
blocking solution (Shanghai share-bio Biotechnology Co., Ltd.). The
antibody dilutions used were as follows: β Actin monoclonal
antibody (Proteintech Group, Inc.; cat. no. 66009-1-lg; 1:6,000),
GRP78 rabbit polyclonal antibody (Proteintech Group, Inc.; cat. no.
11587-1-AP; 1:3,000), CHOP/GADD153 monoclonal antibody (Proteintech
Group, Inc.; cat. no. 66741-1-lg; 1:3,000), anti-TGF-β1 (Wuhan
Boster Biological Technology, Ltd.; cat. no. BA0290; 1:1,000),
SMAD2/3 rabbit mAb (Cell Signaling Technology, Inc.; cat. no.8685;
1:1,000), phosphorylated (p-)SMAD2/SMAD3 rabbit mAb (Cell Signaling
Technology, Inc.; cat. no.8828; 1:1,000), SMAD7 rabbit pAb
(ABclonal Biotech Co., Ltd.; cat. no. A12343; 1:1,000), α-SMA (Cell
Signaling Technology, Inc.; cat. no.19245; 1:1,000) and collagen I
(Chengdu Zen-Bioscience Co., Ltd.; cat. no. 343277; 1:1,000). The
membranes were incubated with secondary antibodies (Proteintech
Group, Inc.; cat. no. SA00001-2; 1:6,000) for 90 min the following
day. The images were developed with a sensitive ECL
chemiluminescence kit, exposed and scanned with a chemiluminescence
imager. For proteins with the same molecular weight as the internal
reference, an antibody was applied to the target protein first,
then developed with a luminescent kit and then eluted with an
antibody eluent (which removes the antibody bound to the membrane
without affecting the antigen protein). Upon completion, the
membrane was developed again to ensure that it has been thoroughly
eluted, then the developer was washed with TBST (0.1% Tween-20) and
finally the β-actin antibody was applied and developed again.
ImageJ v1.8.0 software (National Institutes of Health) was used for
semiquantitative analysis, with β-actin used as an internal
reference.
Statistical analysis
The expression values for all the experimental data
were presented as the means ± standard deviation (n=5). One-way
analysis of variance (ANOVA) was used to compare three or more
groups with Tukey's post hoc test. For comparisons, unpaired t-test
was used to compare two groups. Statistics were analyzed with
GraphPad Prism 9.0 software (Dotmatics). P<0.05 was considered
to indicate a statistically significant difference.
Results
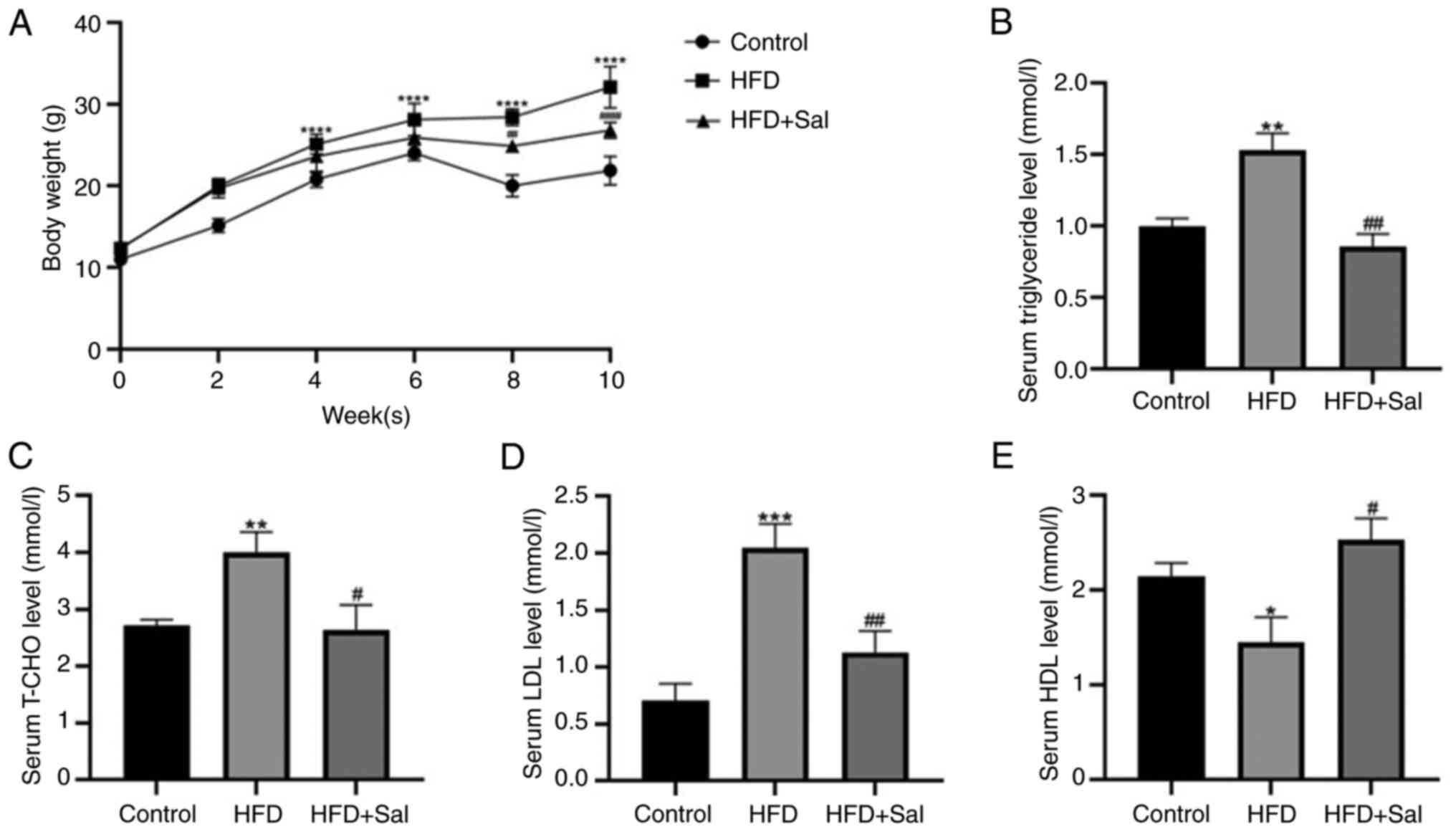
Change in weight and blood lipid
levels in mice
To determine whether the obesity model had been
successfully established, the following indicators were first
examined. Compared with those in the control group, the body
weights of the model group mice increased significantly
(P<0.0001), whereas following Sal intervention, the body weights
of the model group mice decreased (P<0.01; Fig. 1A). Compared with those in the
control group, the blood levels of TG, TC and LDL in the model
group were significantly greater (P<0.01), whereas the HDL level
in the model group was lower (P<0.05). Compared with the model
group, serum levels of TG, TC and LDL in HFD + Sal group were
decreased (P<0.05), while HDL levels were increased (P<0.05;
Fig. 1B-E). These findings
demonstrated that the present study successfully established a
mouse obesity model and that the abnormal lipid metabolism induced
by HFD in mice could be ameliorated by Sal treatment.
Changes in renal function and
morphology in mice
Next, blood biochemical markers were assessed to
determine the renal function of the mice and the level of kidney
damage was observed via HE staining. As shown in Fig. 2A, the renal body ratio of the model
mice was lower than those of the control mice (P<0.05) and it
increased in the model mice following Sal intervention, but the
difference was not statistically significant. Fig. 2B-D shows that the serum levels of
Cr, BUN and UA in the model group were greater than those in the
control group (P<0.05), indicating that renal function was
impaired. However, the levels of these markers significantly
decreased in the model group after they received Sal (P<0.05),
indicating that Sal potentially improved renal function in HFD-fed
mice. Next, the degree of oxidative stress in the three groups of
mice was assessed. The model group had lower levels of the
oxidative stress indicator SOD (P<0.001) and greater amounts of
MDA (P<0.05) than did the control group. Sal decreased oxidative
stress in HFD-fed mice, as evidenced by the fact that Sal
intervention increased SOD (P<0.01) and decreased MDA
(P<0.0001) in the model group (Fig.
2E-F). HE staining revealed that the control group had a
regular renal tissue structure. Compared with those in the control
group, the glomeruli in the HFD group grew, the mesangium increased
and the brush border of the proximal tubules was more severely
damaged, indicating vacuolization. Moreover, Sal considerably
decreased the aforementioned pathological alterations (Fig. 2G). These findings indicate that HFD
exacerbates renal dysfunction and kidney damage in mice, both of
which are partially repaired by Sal.
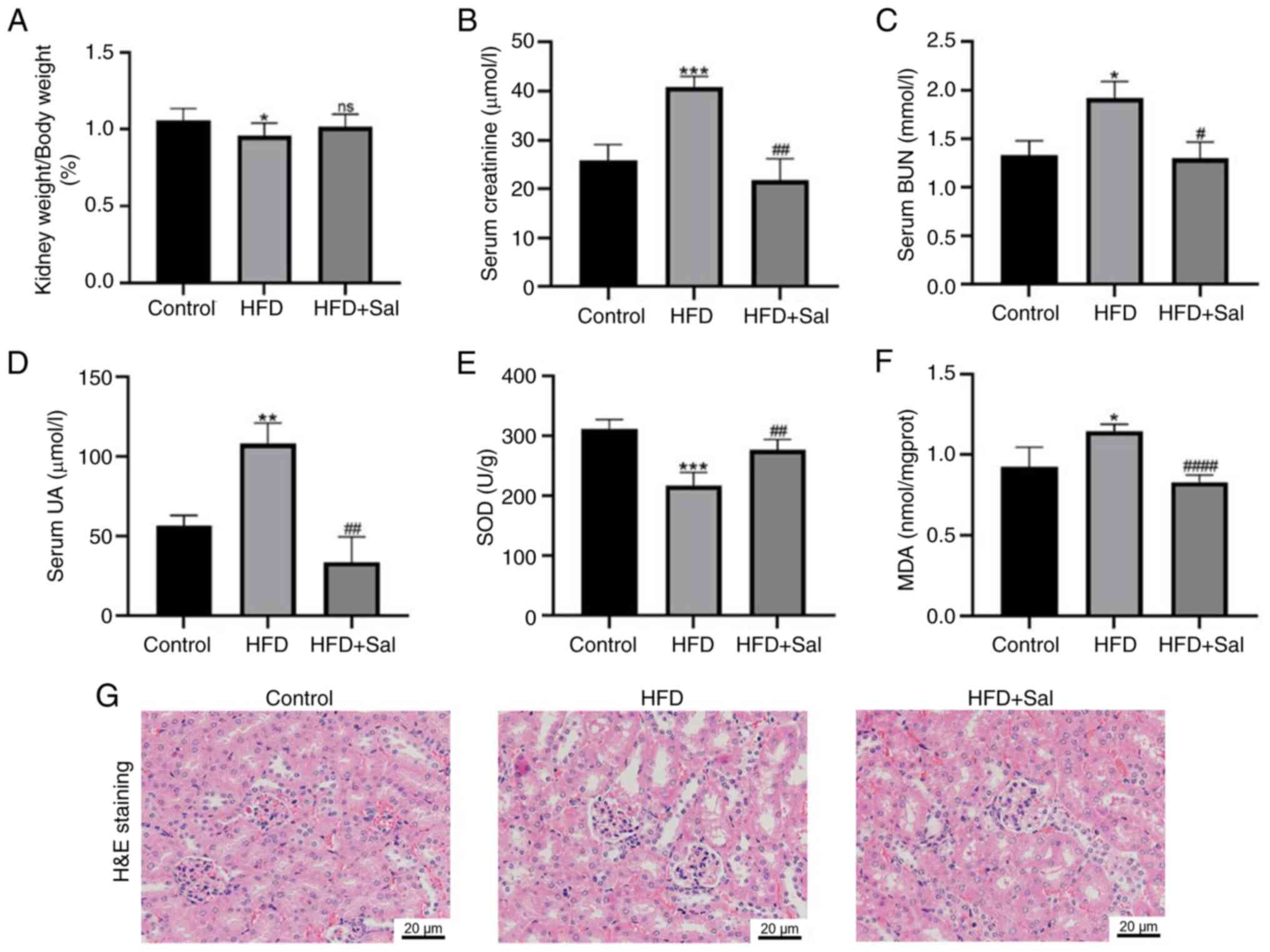 | Figure 2.Changes in renal function and
morphology in mice. (A) The renal-to-body weight ratio in the mice.
(B) Serum creatinine, (C) BUN and (D) UA levels. Kidney (E) SOD and
(F) MDA levels. *P<0.05, **P<0.01, ***P<0.001 vs. the
control group; #P<0.05, ##P<0.01,
####P<0.0001 vs. the HFD group; ns, not significant.
(G) Hematoxylin and eosin staining for pathological changes in
renal tissue. Scale bar, 20 µm; magnification, ×400. BUN, blood
urea nitrogen; UA SOD, superoxide dismutase; MDA, malondialdehyde;
HFD, high-fat diet; Sal, salubrinal. |
Activation of ERS in mice
To determine ERS activation in mice, the levels of
the ERS-related proteins CHOP and GRP78 were examined. RT-qPCR and
western blotting were employed to assess gene and protein levels in
renal tissues. CHOP and GRP78 gene and protein levels in the model
group were significantly higher than those in the control group
(P<0.01). In the model group, the CHOP and GRP78 gene and
protein levels were decreased by Sal intervention (P<0.01;
Fig. 3A-E). Immunohistochemistry
was used to detect the expression and location of these two
proteins. The positive expression of CHOP and GRP78 in the model
group was significantly higher than those in the control group
(P<0.05). Sal partially reversed the HFD-induced increases in
GRP78 and CHOP (P<0.05; Fig.
2F). According to the aforementioned findings, HFD-fed mice
have markedly triggered ERS.
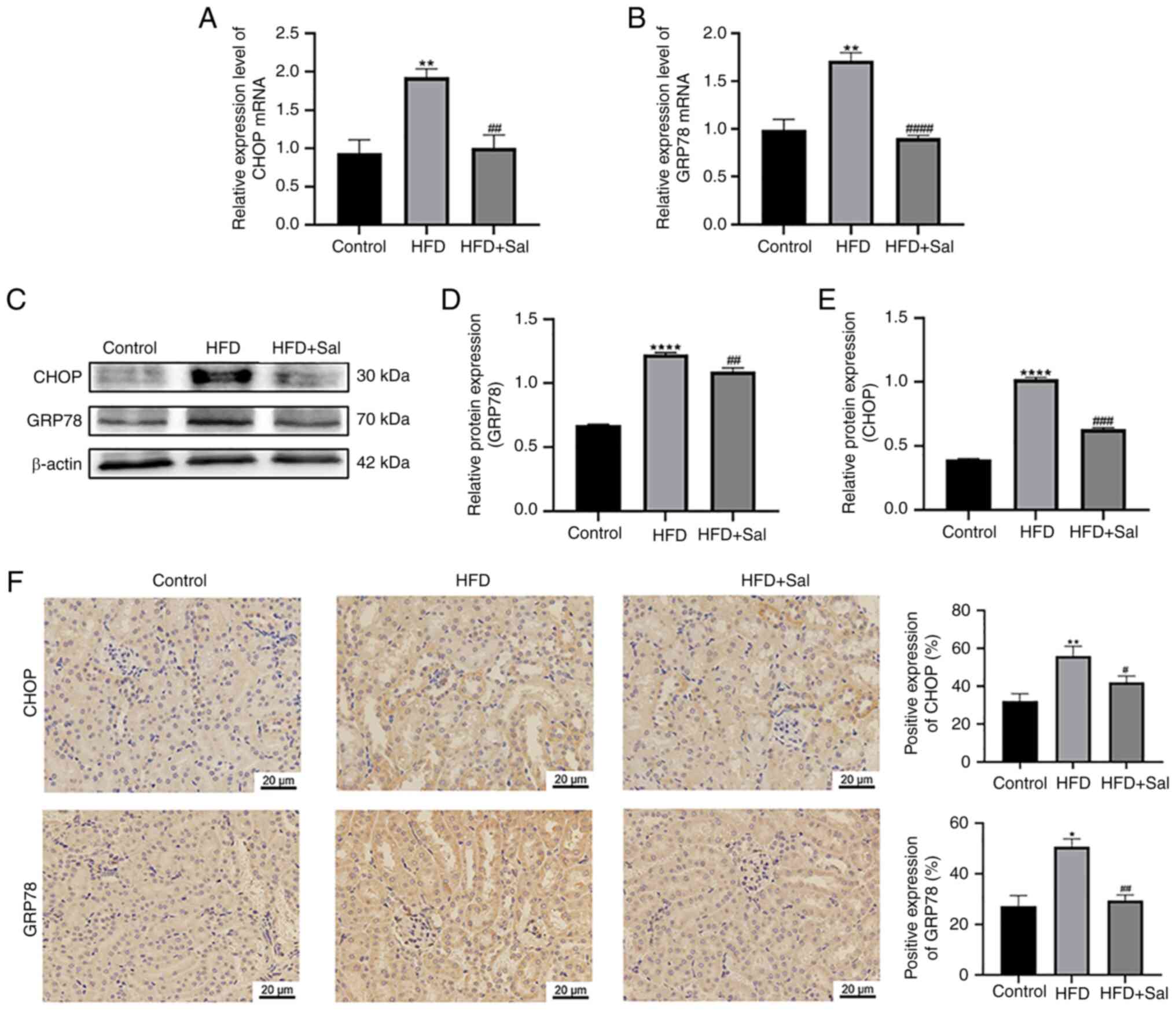 | Figure 3.Activation of ERS in mice. The mRNA
levels of (A) CHOP and (B) GRP78. (C-E) Western blotting for the
levels of proteins, including CHOP and GRP78. **P<0.01,
****P<0.0001 vs. the control group; ##P<0.01,
###P<0.001, ####P<0.0001 vs. the HFD
group. (F) Kidney tissues were immunohistochemically stained for
CHOP and GRP78. Scale bar, 20 µm; magnification, ×400; n=3.
*P<0.05, **P<0.01 vs. the control group;
#P<0.05, ##P<0.01 vs. the HFD group.
ERS, endoplasmic reticulum stress; CHOP, C/EBP-homologous protein;
GRP78, glucose-regulated protein 78; HFD, high-fat diet; Sal,
salubrinal. |
Degree of renal fibrosis in mice
Masson staining revealed the production and
deposition of collagen in the renal tissue. As demonstrated by the
results of Masson's trichrome staining, the renal tissue of the
control group contained normal glomeruli and tubules with minimal
blue staining. The model group displayed a substantial amount of
collagen fiber stripes in their renal interstitium. These stripes
were stained blue, indicating a clear case of renal interstitial
fibrosis. Compared with the model group, the Sal group presented a
partial reduction in the area of blue-positive staining, as well as
a decrease in collagen accumulation in renal tissue and a reduction
in renal interstitial fibrosis (Fig.
4A). Furthermore, Fig. 4B and
C shows that the renal tissue of the model group expressed
higher α-SMA and collagen I gene levels than did the control group
(P<0.05). Sal significantly decreased the α-SMA and collagen I
gene levels that were elevated by HFD (P<0.01). As depicted in
Fig. 4D-F, the renal tissue levels
of the α-SMA and collagen I proteins in the model mice were
considerably greater than those in the control mice (P<0.001),
whereas following Sal treatment, the α-SMA and collagen I protein
levels in the HFD-fed mice were significantly lower (P<0.001).
The expression and localization results of the two proteins shown
by immunohistochemistry in Fig. 4G
revealed that, compared with those in the control group, α-SMA
expression was significantly greater in the renal
tubulointerstitium and collagen I was significantly expressed at
the intersection of the renal cortex and medulla in HFD-fed mice
(P<0.05). Notably, a substantial decrease in the α-SMA and
collagen I levels was detected in the Sal group compared with the
model group (P<0.05). These findings suggested that HFD-induced
ERS exacerbated renal fibrosis in mice and that this is mitigated
by Sal.
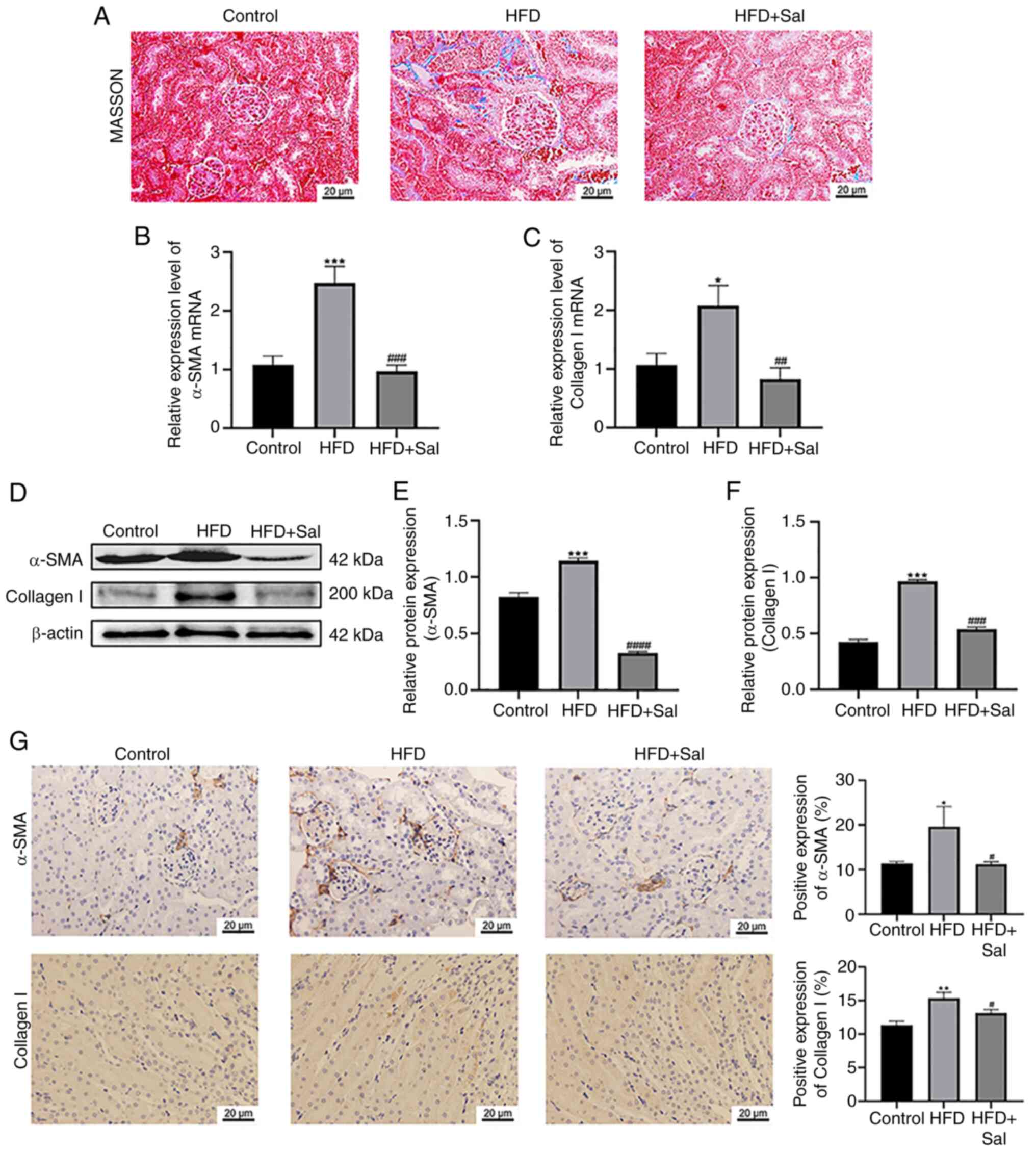 | Figure 4.Degree of renal fibrosis in mice. (A)
Representative sections from the three groups are shown. Scale bar,
20 µm; magnification, ×400. The mRNA levels of (B) α-SMA and (C)
collagen I. (D-F) Western blotting for the levels of proteins,
including α-SMA and collagen I. *P<0.05, ***P<0.001 vs. the
control group; ##P<0.01, ###P<0.001,
####P<0.0001 vs. the HFD group. (G) Kidney tissues
immunohistochemically stained for α-SMA and collagen I. Scale bar,
20 µm; magnification, ×400; n=3. *P<0.05, **P<0.01 vs. the
control group; #P<0.05 vs. the HFD group. α-SMA,
α-smooth muscle actin; HFD, high-fat diet; Sal, salubrinal. |
Activation of the TGF-β/SMAD
pathway
Sal was previously shown to ameliorate HFD-induced
renal fibrosis in mice. However, it is uncertain what molecular
mechanism is responsible for this improvement. Earlier work has
indicated that TGF-β/SMAD signaling, particularly SMAD2/3 and
SMAD7, is involved in the growth and progression of fibrosis
(18). Consequently, the
TGF-β/SMAD pathway-associated protein expression levels was
examined in the three different groups. Using RT-qPCR, the present
study identified the mRNA levels of TGF-β1, SMAD2, SMAD3 and SMAD7.
Fig. 5A-D shows that in the renal
tissue of the model group, the TGF-β1, SMAD2 and SMAD3 gene levels
were greater (P<0.05) and the SMAD7 gene level was lower than
those in the control group (P<0.01). Sal intervention resulted
in a decrease in TGF-β1, SMAD2 and SMAD3 gene expression
(P<0.05) and an increase in SMAD7 gene expression in renal
tissue (P<0.001). The protein expression of these genes was
determined via western blot analysis. Fig. 5E-H shows higher levels of TGF-β1
and p-SMAD2/3 expression in the model mice compared with the
controls (P<0.0001), whereas the SMAD7 level was lower
(P<0.0001). Sal markedly decreased the levels of these two
proteins in HFD-fed mice (P<0.001) and increased the SMAD7 level
(P<0.0001). As shown in Fig.
5I, immunohistochemical staining of renal tissues revealed
that, compared with the control group, the model group markedly
increased the TGF-β1 and p-SMAD2/3 levels (P<0.0001) but
significantly downregulated the expression of SMAD7 (P<0.01). In
addition, Sal partly reversed the increases in the TGF-β1 and
p-SMAD2/3 levels (P<0.01) and the decrease in the SMAD7 level
caused by HFD (P<0.05). In summary, Sal regulated the TGF-β/SMAD
pathway to diminish renal fibrosis in HFD-fed mice and ERS probably
involved the same mechanism to worsen renal fibrosis in HFD-fed
animals.
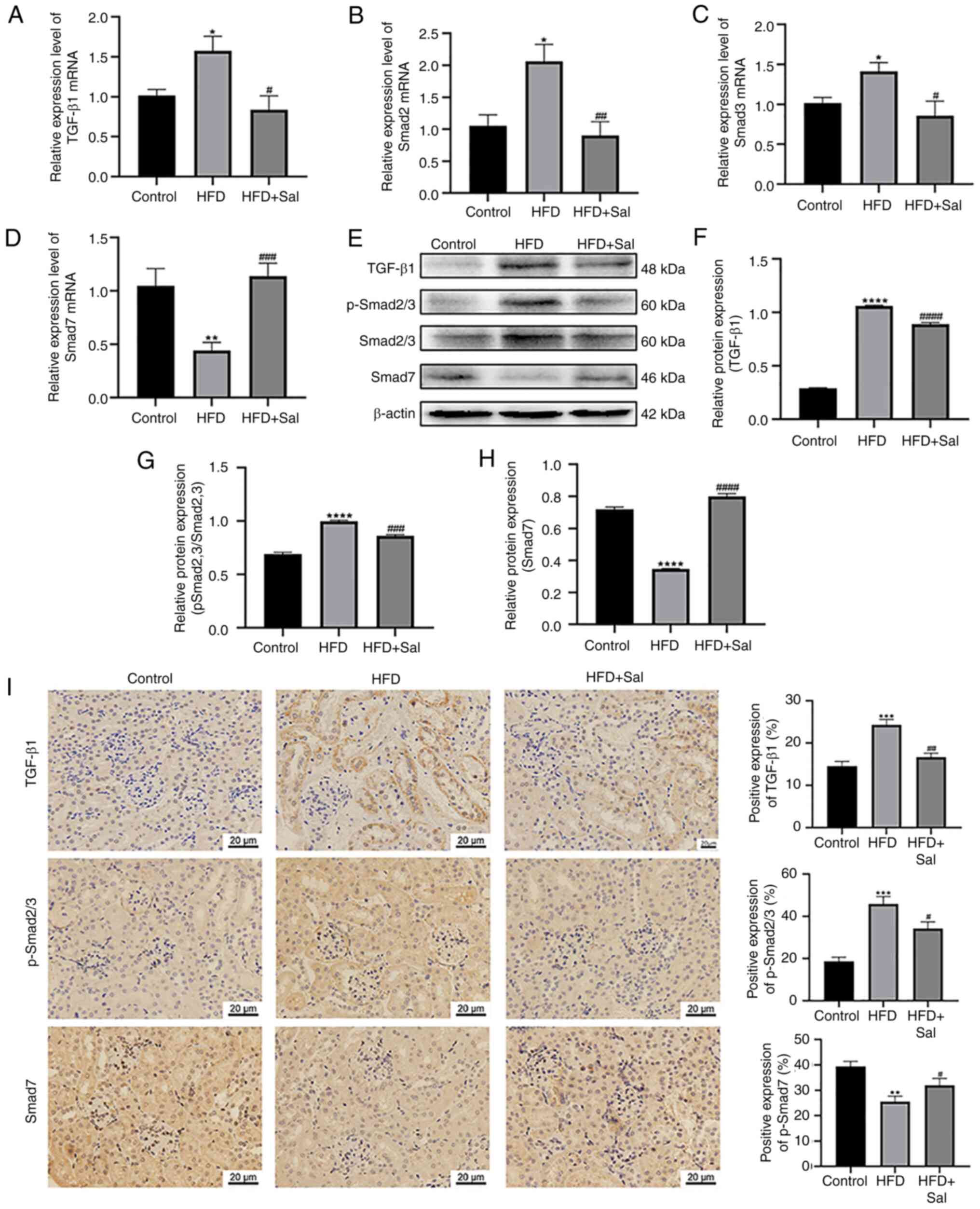 | Figure 5.Activation of the TGF-β/SMAD pathway.
The mRNA levels of (A) TGF-β1, (B) SMAD2, (C) SMAD3 and (D) SMAD7
in the kidney. (E-H) Western blotting was used to measure the
levels of proteins, including those involved in the TGF-β/SMAD
pathway. *P<0.05, **P<0.01, ****P<0.0001 vs. the control
group; #P<0.05, ##P<0.01,
###P<0.001, ####P<0.0001 vs. the HFD
group. (I) Kidney tissues were immunohistochemically stained for
TGF-β1, p-SMAD2/3 and SMAD7. Scale bars are 20 µm; magnification,
×400; n=3. **P<0.01, ***P<0.001 vs. the control group;
#P<0.05, ##P<0.01 vs. the HFD group.
HFD, high-fat diet; p-, phosphorylated; Sal, salubrinal. |
Discussion
Obesity has emerged as a prominent independent
danger marker for CKD and ESRD in the age of the obesity epidemic
(19). Hsu et al (20) report that being overweight or obese
is a risk factor associated with CKD in a sizable cohort consisting
of adults in northern California; Iseki et al (21) arrives at the same conclusion,
demonstrating that obesity markedly increases the relative risk of
CKD in older individuals. These results suggest that obesity is
closely related to CKD. One prevalent pathological characteristic
of CKD is renal fibrosis, which is a predictor of progression to
ESRD (22). HFD is closely related
to renal fibrosis. HFD feeding stimulates lipogenic enzymes in the
fatty acid synthesis pathway but inhibits lipolysis, which
subsequently drives the excessive accumulation of lipids in the
kidney. Altered lipid metabolism ultimately leads to kidney injury,
including glomerulosclerosis, interstitial fibrosis and proteinuria
(23). One study reveals that mice
fed HFD for 12 weeks develop obesity, insulin resistance and
oxidative stress, leading to liver and kidney fibrosis (5). The present study confirmed the
successful establishment of an obese mouse model in which the mice
in the HFD group had significantly greater body weights and
elevated serum TG and TC levels compared with those in the control
group, suggesting that the mice in the HFD group had severe fat
accumulation and were unable to carry out normal lipid metabolism.
Masson's trichrome staining revealed that the mice in the HFD group
presented increased deposition of collagen fibers, suggesting that
renal fibrosis was severe in this group. Moreover,
epithelial-mesenchymal transition (EMT) is an important cause of
renal interstitial fibrosis (24).
It can lead to the upregulation of fibronectin and fibroblast
marker (α-SMA) expression (25).
The findings of the present study also confirmed that the
expression of α-SMA and collagen I was elevated in the HFD group
compared with the control group. In addition, the content of the
lipid peroxide MDA was elevated in the renal tissues of the mice in
the HFD group, whereas the content of the antioxidant enzyme SOD
was decreased, suggesting that excessive lipid accumulation induced
oxidative stress and consequently damage to renal tissues.
Therefore, it was hypothesized that HFD-fed mice accumulated
lipids, leading to disorders of lipid metabolism, which in turn
promoted renal fibrosis, which may also be related to responses
such as the inflammatory response, insulin resistance and oxidative
stress.
The ER is the main location for the synthesis of
secretory and transmembrane proteins, folding and maturation,
Ca2+ storage and lipid biosynthesis (26). Various factors, such as genetic
mutations, hypoxia, malnutrition and oxidative stress, can cause
ERS. As a result of this response, the ER lumen is overloaded with
unfolded and incorrectly folded proteins. Further activation of the
UPR contributes to a reduction in protein function and even cell
death (27). Obesity is a chronic
pathological stimulus associated with insulin resistance
characterized by a state of low-grade inflammation (28), the main features of which include
altered inflammatory signaling in adipocytes (29) and infiltration of immune cells into
adipose tissue (30). Pathological
expansion of adipose tissue leads to abnormal hypertrophy and
thickening of adipocytes, resulting in adipocyte hypoxia, chronic
low-grade inflammation, decreased vascularization, decreased
reactive oxygen species production and ERS (31). Together with the aforementioned
findings, it was hypothesized that adipose tissue dysfunction may
be a common mechanism for HFD-induced renal fibrosis and
stimulation of ERS in mice. It has been shown that HFD-fed rats
exhibit hypothalamic ERS and the modulation of hypothalamic GRP78
activity is associated with white adipose tissue browning (32). Xie et al (33) report that HFD and STZ-induced
diabetes can lead to nephropathy and that the mechanism of injury
may involve ERS and CHOP. Although there is much evidence that ERS
plays a role in HFD-induced inflammation and tissue damage, there
is little evidence that ERS plays a role in HFD-induced renal
fibrosis. Therefore, ERS activity in the HFD and control groups was
evaluated. The results revealed that the expression of ERS markers,
such as GRP78 and CHOP, was increased in the kidney tissues of the
mice in the HFD group compared with those in the control group,
suggesting that ERS may be involved in HFD-induced kidney injury.
In addition, the kidneys of the mice in the HFD group presented
significant oxidative stress injury, suggesting that HFD induced
oxidative stress. This is consistent with previous finding that HFD
can cause ERS and oxidative stress (31).
TGF-β1 is a versatile cytokine that regulates
several cellular processes and extracellular matrix (ECM)
components. It also plays an essential role in fibrogenesis and
EMT. TGF-β1 specifically regulates the upstream EMT molecules
SMAD2, SMAD3, SMAD4 and SMAD7, the last of which inhibits SMAD3
expression (34). Furthermore,
TGF-β1 expression is upregulated during the fibrotic process in the
lung, liver, kidney and other organs (34–36).
Numerous investigations have demonstrated the strong relationships
among ECM gene regulation, fibrogenesis and the TGF-β pathway
(37). Inflammatory cytokines,
such as elements of the TGF-β superfamily, different interleukins,
oxidative damage and inflammation, are the major factors that
govern fibrosis (38,39). Among these variables, TGF-β is a
critical modulator of fibrogenesis. Renal fibrosis and kidney
disorders are regulated by the TGF-β/SMAD pathway (38). The activity of the TGF-β1 receptor
causes SMAD7 to separate from the receptor, activating and
phosphorylating SMAD2 and SMAD3 (40,41).
In the present study, mice in the HFD group presented increased
expression of TGF-β1 and p-SMAD2/3 and decreased expression of
SMAD7, suggesting that HFD may promote fibrosis in mice through
activation of the TGF-β/SMAD pathway, a process that may be related
to ERS.
For further validation, treatment with the ERS
inhibitor Sal decreased the expression of TGF-β1 and p-SMAD2/3 and
increased the expression of SMAD7. These findings suggested that
Sal protects against renal fibrosis in HFD-fed mice through this
pathway, which may be a possible route by which ERS induces renal
fibrosis in HFD-fed mice. HFD triggers this pathway via ERS, which
promotes the expression of TGF-β1 and SMAD2/3 while decreasing the
expression of SMAD7. This leads to increased ECM deposition, which
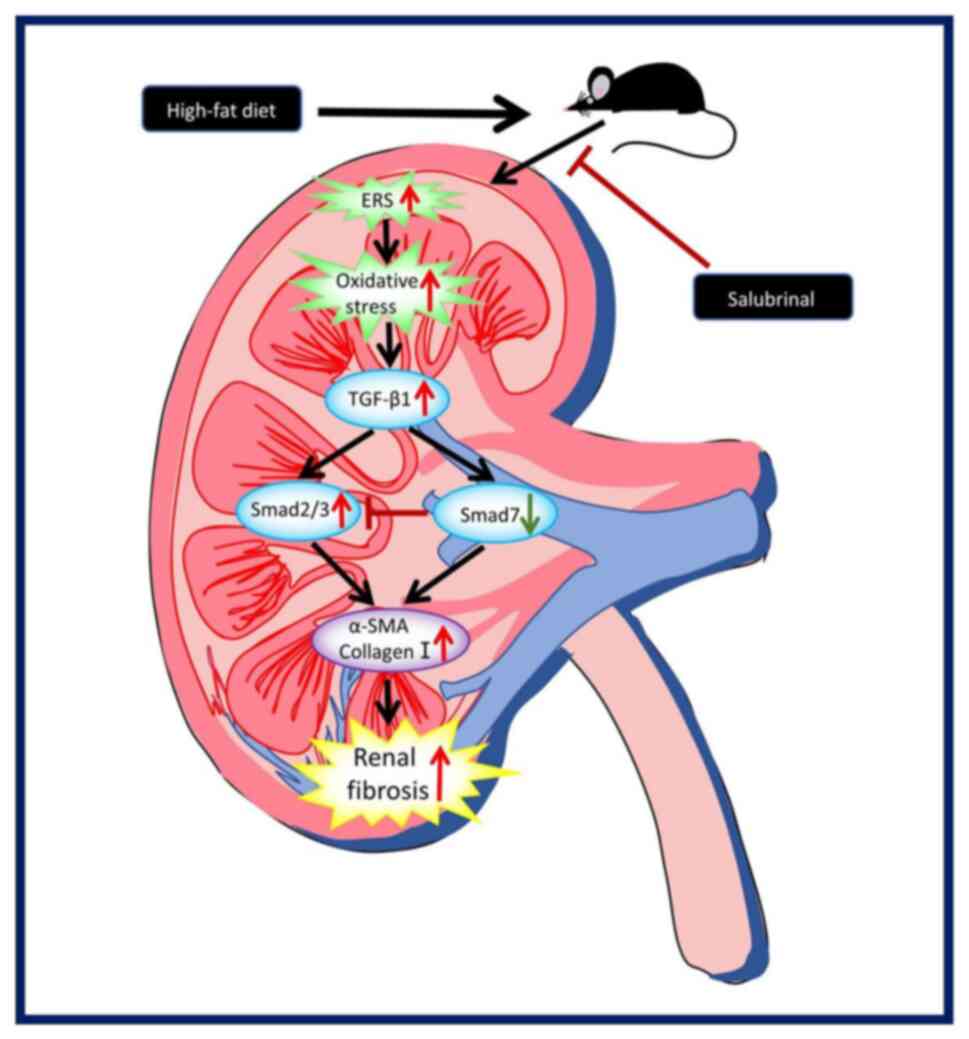
ultimately leads to renal fibrosis in mice (Fig. 6).
In conclusion, the present study revealed that renal
fibrosis in HFD-fed mice is induced by ERS activation, which
upregulates the TGF-β/SMAD pathway. These results provide important
new insights into the mechanism of HFD-induced kidney injury and
point to the possible therapeutic potential of inhibiting ERS
regulation of renal fibrosis in the treatment of obese mice. The
present study laid the groundwork for future directions, such as
studies on important sites associated with HFD-induced obesity
(brain and liver), as well as the extension of the experiments to
in vitro to study the proliferation of fibroblasts and what
causes them to proliferate and the factors that influence them.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Special Basic Cooperative
Research Programs of Yunnan Provincial Undergraduate Universities
Association under (grant no. 202101BA070001-106).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZM and MC performed the research, established the
obesity model, collected kidney specimens, analyzed the data and
wrote the article. CL and BL designed and assisted with the
experiments. JS and WG designed the experiments, provided overall
guidance and helped with the manuscript. ZM and JS confirm the
authenticity of all the raw data. All the authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The experimental protocol of the present study was
performed in accordance with the Guide for the Care and Use of
Laboratory Animals and approved by the Ethical Committee of Dali
University (Yunnan, China; approval no. 2023-PZ-278).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jiang Z, Wang Y, Zhao X, Cui H, Han M, Ren
X, Gang X and Wang G: Obesity and chronic kidney disease. Am J
Physiol Endocrinol Metab. 324:E24–E41. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stasi A, Cosola C, Caggiano G, Cimmarusti
MT, Palieri R, Acquaviva PM, Rana G and Gesualdo L: Obesity-related
chronic kidney disease: Principal mechanisms and new approaches in
nutritional management. Front Nutr. 9:9256192022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alizadeh S, Ahmadi M, Ghorbani Nejad B,
Djazayeri A and Shab-Bidar S: Metabolic syndrome and its components
are associated with increased chronic kidney disease risk: Evidence
from a meta-analysis on 11 109 003 participants from 66 studies.
Int J Clin Pract. May 23–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang R, Fu P and Ma L: Kidney fibrosis:
From mechanisms to therapeutic medicines. Signal Transduct Target
Ther. 8:1292023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu S, Fu S, Jin Y, Geng R, Li Y, Zhang Y,
Liu J and Guo W: Tartary buckwheat flavonoids alleviates high-fat
diet induced kidney fibrosis in mice by inhibiting MAPK and
TGF-β1/Smad signaling pathway. Chem Biol Interact. 379:1105332023.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu X, Si F, Hao R, Zheng J and Zhang C:
Nuciferine protects against obesity-induced nephrotoxicity through
its hypolipidemic, anti-inflammatory, and antioxidant effects. J
Agric Food Chem. 71:18769–18779. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee LE, Doke T, Mukhi D and Susztak K: The
key role of altered tubule cell lipid metabolism in kidney disease
development. Kidney Int. 106:24–34. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang SX, Wang JJ, Starr CR, Lee EJ, Park
KS, Zhylkibayev A, Medina A, Lin JH and Gorbatyuk M: The
endoplasmic reticulum: Homeostasis and crosstalk in retinal health
and disease. Prog Retin Eye Res. 98:1012312024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen X and Cubillos-Ruiz JR: Endoplasmic
reticulum stress signals in the tumour and its microenvironment.
Nat Rev Cancer. 21:71–88. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Metcalf MG, Higuchi-Sanabria R, Garcia G,
Tsui CK and Dillin A: Beyond the cell factory: Homeostatic
regulation of and by the UPRER. Sci Adv. 6:eabb96142020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Merighi A and Lossi L: Endoplasmic
reticulum stress signaling and neuronal cell death. Int J Mol Sci.
23:151862022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma K, Zhang Y, Zhao J, Zhou L and Li M:
Endoplasmic reticulum stress: Bridging inflammation and
obesity-associated adipose tissue. Front Immunol. 15:13812272024.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carnuta MG, Deleanu M, Barbalata T, Toma
L, Raileanu M, Sima AV and Stancu CS: Zingiber officinale extract
administration diminishes steroyl-CoA desaturase gene expression
and activity in hyperlipidemic hamster liver by reducing the
oxidative and endoplasmic reticulum stress. Phytomedicine.
48:62–69. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu L, Guo T, Deng R, Liu L and Yu Y:
Apigenin ameliorates insulin resistance and lipid accumulation by
endoplasmic reticulum stress and SREBP-1c/SREBP-2 pathway in
palmitate-induced HepG2 cells and high-fat diet-fed mice. J
Pharmacol Exp Ther. 377:146–156. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paik J, Fierce Y, Drivdahl R, Treuting PM,
Seamons A, Brabb T and Maggio-Price L: Effects of murine norovirus
infection on a mouse model of diet-induced obesity and insulin
resistance. Comp Med. 60:189–195. 2010.PubMed/NCBI
|
|
16
|
Tian RD, Chen YQ, He YH, Tang YJ, Chen GM,
Yang FW, Li Y, Huang WG, Chen H, Liu X and Lin SD: Phosphorylation
of eIF2α mitigates endoplasmic reticulum stress and hepatocyte
necroptosis in acute liver injury. Ann Hepatol. 19:79–87. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang WB, Ling GH, Sun L and Liu FY: Smad
anchor for receptor activation (SARA) in TGF-beta signaling. Front
Biosci (Elite Ed). 2:857–860. 2010.PubMed/NCBI
|
|
19
|
Hojs R, Ekart R, Bevc S and Vodošek Hojs
N: Chronic kidney disease and obesity. Nephron. 147:660–664. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsu CY, Mcculloch CE, Iribarren C,
Darbinian J and Go AS: Body mass index and risk for end-stage renal
disease. Ann Intern Med. 144:21–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iseki K, Ikemiya Y, Kinjo K, Inoue T,
Iseki C and Takishita S: Body mass index and the risk of
development of end-stage renal disease in a screened cohort. Kidney
Int. 65:1870–1876. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou S, Wu Q, Lin X, Ling X, Miao J, Liu
X, Hu C, Zhang Y, Jia N, Hou FF, et al: Cannabinoid receptor type 2
promotes kidney fibrosis through orchestrating β-catenin signaling.
Kidney Int. 99:364–381. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kume S, Uzu T, Araki S, Sugimoto T,
Isshiki K, Chin-Kanasaki M, Sakaguchi M, Kubota N, Terauchi Y,
Kadowaki T, et al: Role of altered renal lipid metabolism in the
development of renal injury induced by a high-fat diet. J Am Soc
Nephrol. 18:2715–2723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stone RC, Pastar I, Ojeh N, Chen V, Liu S,
Garzon KI and Tomic-Canic M: Epithelial-mesenchymal transition in
tissue repair and fibrosis. Cell Tissue Res. 365:495–506. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng B, Yang W, Wang D, Cheng L, Bu L, Rao
J, Zhang J, Xie J and Zhang B: Peptide DR8 suppresses
epithelial-to-mesenchymal transition via the TGF-β/MAPK signaling
pathway in renal fibrosis. Life Sci. 261:1184652020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li YE, Sowers JR, Hetz C and Ren J: Cell
death regulation by MAMs: From molecular mechanisms to therapeutic
implications in cardiovascular diseases. Cell Death Dis.
13:5042022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sims SG, Cisney RN, Lipscomb MM and Meares
GP: The role of endoplasmic reticulum stress in astrocytes. Glia.
70:5–19. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Engin A: The pathogenesis of
obesity-associated adipose tissue inflammation. Adv Exp Med Biol.
960:221–245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lumeng CN and Saltiel AR: Inflammatory
links between obesity and metabolic disease. J Clin Invest.
121:2111–2117. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Olefsky JM and Glass CK: Macrophages,
inflammation, and insulin resistance. Annu Rev Physiol. 72:219–246.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun K, Kusminski CM and Scherer PE:
Adipose tissue remodeling and obesity. J Clin Invest.
121:2094–2101. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Contreras C, González-García I,
Seoane-Collazo P, Martínez-Sánchez N, Liñares-Pose L, Rial-Pensado
E, Fernø J, Tena-Sempere M, Casals N, Diéguez C, et al: Reduction
of hypothalamic endoplasmic reticulum stress activates browning of
white fat and ameliorates obesity. Diabetes. 66:87–99. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie H, Huang L, Li Y, Zhang H and Liu H:
Endoplasmic reticulum stress and renal lesion in mice with
combination of high-fat diet and streptozotocin-induced diabetes.
Acta Cir Bras. 31:150–155. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Wang HL, Liu TT and Lan HY:
TGF-beta as a master regulator of diabetic nephropathy. Int J Mol
Sci. 22:78812021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye Z and Hu Y: TGF-β1: Gentlemanly
orchestrator in idiopathic pulmonary fibrosis (review). Int J Mol
Med. 48:1322021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ahmed H, Umar MI, Imran S, Javaid F, Syed
SK, Riaz R and Hassan W: TGF-β1 signaling can worsen NAFLD with
liver fibrosis backdrop. Exp Mol Pathol. 124:1047332022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peng D, Fu M, Wang M, Wei Y and Wei X:
Targeting TGF-β signal transduction for fibrosis and cancer
therapy. Mol Cancer. 21:1042022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gifford CC, Tang J, Costello A, Khakoo NS,
Nguyen TQ, Goldschmeding R, Higgins PJ and Samarakoon R: Negative
regulators of TGF-β1 signaling in renal fibrosis; pathological
mechanisms and novel therapeutic opportunities. Clin Sci (Lond).
135:275–303. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Antar SA, Ashour NA, Marawan ME and
Al-Karmalawy AA: Fibrosis: Types, effects, markers, mechanisms for
disease progression, and its relation with oxidative stress,
immunity, and inflammation. Int J Mol Sci. 24:40042023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
de Ceuninck van Capelle C, Spit M and Ten
Dijke P: Current perspectives on inhibitory SMAD7 in health and
disease. Crit Rev Biochem Mol Biol. 55:691–715. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gu YY, Liu XS, Huang XR, Yu XQ and Lan HY:
Diverse role of TGF-β in kidney disease. Front Cell Dev Biol.
8:1232020. View Article : Google Scholar : PubMed/NCBI
|