Introduction
Bones provide structural support for the body and
protect soft and vulnerable tissues and organs (1). With a rapidly aging population, the
incidence of orthopedic clinical cases involving bone healing has
been steadily increasing (2).
Treating bone defects often involves nonunion, which seriously
affects the patient's quality of life.
An increasing number of researchers have focused on
tissue engineering for bone regeneration (3,4).
While chitosan and other bioscaffolds have achieved considerable
success in bone regeneration, the seed cells remain paramount
(5,6). Mesenchymal stem cells (MSCs) possess
the capacity for multidirectional differentiation and self-renewal,
making them excellent candidate cells (7). MSCs may exert their effects through
direct differentiation into bone cells or creating a regenerative
environment through paracrine mechanisms (8–10).
Ectodermal mesenchymal stem cells (EMSCs) represent a distinctive
subset of MSCs originating from the neural crest stem cell
(11). The neural crest stem cell
plays a pivotal role in skull formation during early embryonic
development (12,13). The majority of craniofacial bones
are derived from the ectodermal germ layer, which is contributed by
neural crest stem cell. These bones are notably different from the
long bones derived from the mesoderm (14–16).
Scientists have emphasized the osteogenic qualities of EMSCs and
bone marrow mesenchymal stem cells (BMSCs). Noteworthy is their
discovery that EMSCs exhibit superior proliferation characteristics
when grown on three-dimensional scaffolds, highlighting the
promising potential of EMSCs in tissue engineering endeavors
(16). Prior investigations
conducted in our laboratory also have demonstrated that the
administration of EMSCs facilitates bone defect repair (17,18).
However, the current inadequacy in the osteogenic differentiation
capacity of EMSCs hinders their clinical application. Thus, there
is a need for the development of more efficient and simplified
therapeutic approaches to enhance the osteogenic differentiation
potential of EMSCs.
The differentiation of MSCs into mature, functional
osteoblasts represents a complex process intricately regulated and
influenced by a number of factors. Alterations in the extracellular
milieu can modulate cellular stress, consequently affecting the
differentiation trajectory of MSCs (19). As an anti-inflammatory factor,
IL-10 has recently been proved to have properties in regulating the
osteogenic orientation of MSCs (20). Various inflammatory pretreatment
methods applied to MSCs revealed clues suggesting enhancement of
their immunological capabilities (21,22).
Though the enhanced immune effects remain complex,
it is possible that IL-10 may emerge as a significant contributor
to inflammatory preconditioning. Based on these foundations, it was
hypothesized that our approach to inflammatory adaptation could
augment the paracrine capabilities of EMSCs, leading to an
upregulation in IL-10 levels and thereby facilitating significant
osteogenic differentiation.
The present study aimed to determine whether the
activation of EMSCs by lipopolysaccharide (LPS) enhances the
process of osteogenesis and to delve deeper into the underlying
mechanisms involved in this differentiation process. To achieve
this objective, the present study conducted osteogenic
differentiation assays to compare the behavior of EMSCs with that
of LPS-activated EMSCs. Additionally, the expression of IL-10 in
LPS-activated EMSCs was examined and IL-10 used as a positive
control to assess its specific role. Previous studies have reported
the osteogenic effect of IL-10 on MSCs (23,24).
The aim of the present study was to ascertain whether LPS promoted
osteogenic differentiation of ecto-MSCs by upregulating IL-10 and
to compare the difference of EMSCs between the LPS and the IL-10
group. The findings carry implications for evaluating the
osteogenic potential of EMSCs and underscore the significance of
inflammatory adaption in the efficacy of MSC-based bone tissue
engineering therapies.
Materials and methods
Cell culture
The human samples were obtained from anonymous
healthy donors. Between March 2021 and December 2022, 10 volunteers
ranging in age from 18–60 were recruited, maintaining an equal sex
ratio of 1:1. All samples were collected with the donor's informed
written consent. Permission for obtaining the samples was granted
by the Affiliated Hospital of Jiangsu University (Zhenjiang, China;
approval no. SWYXLI20190225-2). EMSCs were isolated from human
nasal mucosa following previously established protocols with slight
modifications. Briefly, mucosal tissues obtained from biopsies were
finely minced and cultured in Dulbecco's modified Eagle's
medium/nutrient mixture F12 (DMEM/F12) supplemented with 10% fetal
bovine serum (FBS; HyClone; Cytiva). The tissues were maintained in
a humidified incubator at 37°C with 5% CO2 and the
medium was refreshed every 4 days. Upon outgrowth of cells, EMSCs
were harvested using 0.25% trypsin and subcultured until reaching
85% confluence. The identity and purity of the cells were confirmed
by immunofluorescence staining for nestin (Wuhan Boster Biological
Technology, Ltd.), SRY-related HMG box-containing 9 (Sox9; Wuhan
Boster Biological Technology, Ltd.) and vimentin (Wuhan Boster
Biological Technology, Ltd.).
A total of 3,000 cells were evenly distributed into
six-well plates and subjected to continuous culture until the
emergence of discernible cell colonies. Following this incubation
period, the plates underwent fixation at 4°C using a 4%
paraformaldehyde solution for 15 min, followed by PBS washes.
Subsequently, specimens were treated with a 0.1% crystal violet
staining solution at room temperature, allowing for a 15-min
staining period, after which they were rinsed with running water
and images captured.
Inflammatory training of EMSCs
Cells were uniformly seeded in culture dishes at a
density of 50% while in a favorable growth state. Prior to
osteogenic induction, an inflammatory acclimation procedure was
conducted. Briefly, EMSCs were cultured with low concentrations of
LPS and incubated at 37°C in an incubator for five consecutive
days. The stimulated medium was replaced every two days until the
procedure was finished.
Calcein-AM/propidium iodide (PI)
staining
The Calcein-AM/PI staining method was employed to
distinguish between living and dead cells. Calcein-AM specifically
stains living cells due to its ability to efficiently penetrate
intact cell membranes, whereas PI selectively stains dead cells as
it cannot traverse the intact cell membrane of living cells. The
Calcein-AM/PI staining kit (Beijing Solarbio Science &
Technology Co., Ltd.) was used following the manufacturer's
protocol to assess the viability of EMSCs following LPS treatment.
A solution containing 2 µM Calcein-AM and 1 µM PI was applied to
the cells for 30 min at 37°C in a dark environment. Images were
captured at 10× magnification using a fluorescence microscope.
Osteogenic differentiation of EMSCs in
vitro
For osteogenic differentiation, EMSCs were cultured
in osteogenic induction medium consisting of culture medium
supplemented with 0.1 µM dexamethasone, 50 µg/ml ascorbic acid and
10 mM β-glycerophosphate. The medium was refreshed every 3 days
over a 14-day period. Then, cells were fixed with 4%
paraformaldehyde at RT for 10 min and subsequently stained with a
2% solution of Alizarin red S (Mackin Biochemical Co., Ltd.) for 10
min at room temperature. Meanwhile, alkaline phosphatase (ALP)
staining was applied to EMSCs undergoing osteogenic differentiation
to visualize the presence of osteoblasts. ALP activity was examined
using an ALP staining kit (Beijing Solarbio Science &
Technology Co., Ltd.) according to the manufacturer's protocol. The
cells were stained for 30 min at RT and then imaged at 10×
magnification with a light microscope, capturing five random fields
of view.
Immunofluorescence staining
A total of 1×104 cells were seeded into
24-well plates and cultured in DMEM/F12 medium supplemented with
10% FBS (HyClone, Cytiva). For immunofluorescence staining, cells
were fixed in 4% polyformaldehyde at 4°C overnight and then rinsed
three times with PBS. Cells were permeabilized and blocked by a
mixture of 0.1% Triton X-100 and 3% bovine serum albumin (BSA) for
30 min at RT. Following PBS washing, cells were incubated with
primary antibodies targeting nestin, (1:100; Wuhan Boster
Biological Technology, Ltd.) Sox9 (1:100; Wuhan Boster Biological
Technology, Ltd.), Vimentin (1:100; Wuhan Boster Biological
Technology, Ltd.), IL-10 (1:1,000; Wuhan Proteintech
Biotechnology), Sonic hedgehog (Shh) (1:100; Wuhan Boster
Biological Technology, Ltd.), Gli family zinc finger 1 (Gli1) and
osteopontin (OPN) (1:100; Wuhan ProteinTech Biological Technology,
Ltd.) at 4°C overnight. After PBS washing, cells were incubated
with Cy3-conjugated secondary antibodies at 37°C for 1 h. Nuclear
staining was performed by incubating the cells with DAPI at room
temperature for 10 min and observation was conducted using
fluorescence microscopy at 10× magnification.
Western blotting
Cell protein was extracted with RIPA lysis buffer
(Biosharp Life Sciences) supplemented with protease inhibitors
(Wuhan Boster Biological Technology, Ltd.). The protein
concentration of the samples was measured using the BCA assay. A
total of 3 µg protein was loaded into each lane, separated via 10%
SDS-PAGE) and transferred onto a polyvinylidene fluoride membrane.
Following a 1 h blocking step with 5% BSA (Biosharp, China) at RT,
the membranes were incubated with primary antibodies against Shh
(1:1,000; BA2171; Wuhan Boster Biological Technology, Ltd.), IL-10
(1:1,000; 60269-1-Ig; Wuhan Proteintech Biotechnology), Osteocalcin
(OCN; 1:1,000; 20277-1-AP; Wuhan ProteinTech Biotechnology),
Runt-related transcription factor 2 (1: 1,000; 20700-1-AP; Wuhan
Proteintech Biotechnology) and Actin (1:1,000; bsm-33036M; BIOSS)
for an hour at RT. Subsequently, HRP-conjugated goat anti-rabbit
IgG (1:5,000; BA1058; Wuhan Boster Biological Technology, Ltd.) was
applied and incubated with the membrane for 1 h at 37°C.
Immunoreactive bands were visualized using enhanced
chemiluminescence reagents (Millipore; Sigma). The bands was
quantified by ImageJ (version 1.8.0; NIH) software.
ELISA
Cell supernatant was harvested, followed by
centrifugation at 2,500 × g at room temperature for 10 min to
remove residual cells and debris. An IL-10 ELISA kit purchased from
Boster (Cat. no. EK0416) was used to analyze the sample following
the instructions and its absorbance at 450 nm was quantified using
a microplate reader.
Statistical analysis
Statistical analyses were performed using GraphPad
software (version 8.0.2; Dotmatics). All data are presented as the
mean ± standard deviation. The significance of differences between
groups was assessed using two-tailed unpaired Student's t-test or
one-way analysis of variance with Bonferroni method. P<0.05 was
considered to indicate a statistically significant difference.
Results
EMSCs isolation and
characterization
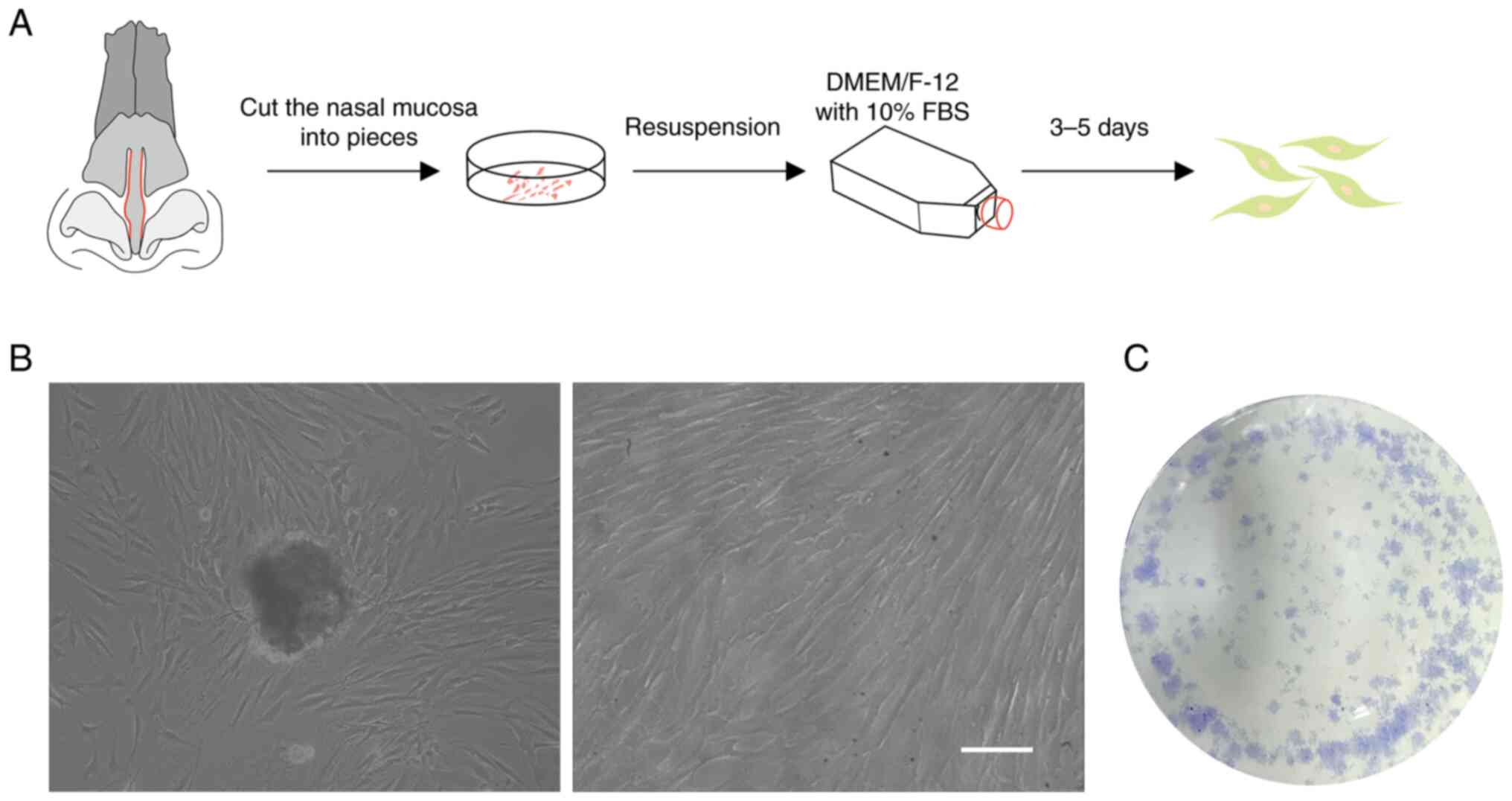
The nasal musca were cut into pieces (1
mm2) and cultured into plates (Fig. 1A). Initially, it was observed that
EMSCs migrated from the tissue (Fig.
1B) and that they proliferated rapidly after several subculture
passages (Fig. 1C).
Identification of surface markers of
EMSCs
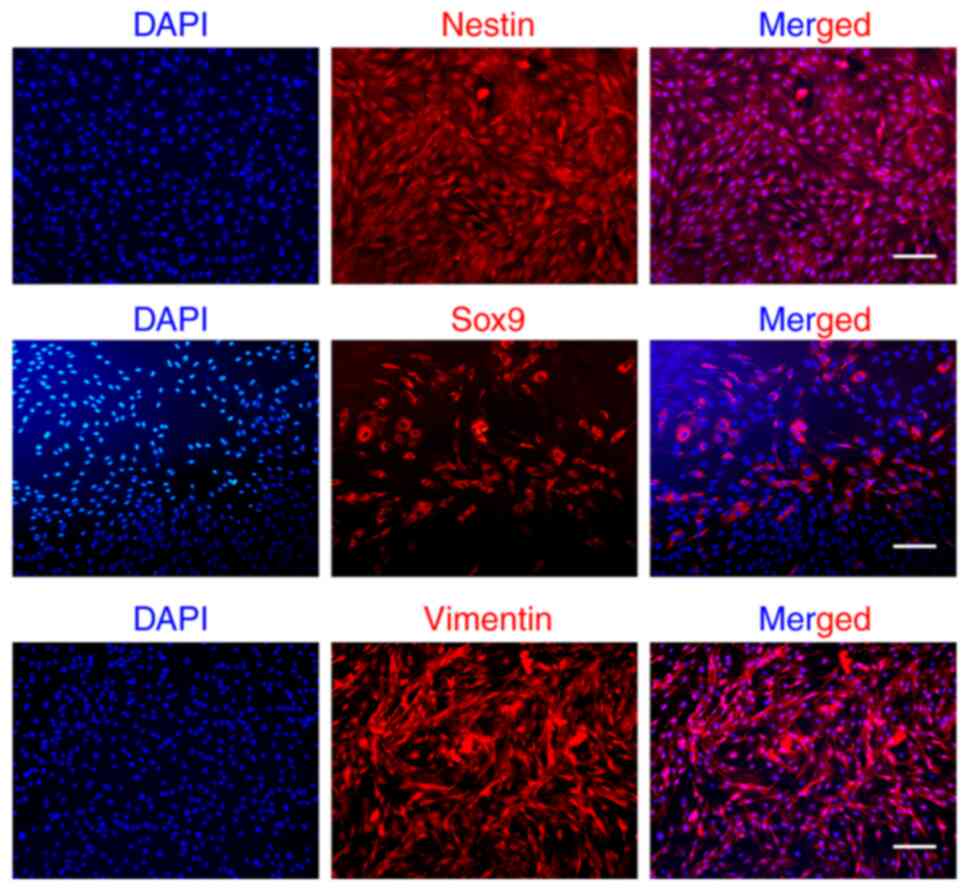
Next, the expression of EMSCs markers (nestin, Sox9,
vimentin) was evaluated through immunofluorescence staining. As
illustrated in Fig. 2,
neuroectodermal lineage marker (nestin), MSCs marker (vimentin) and
neural crest-related marker (Sox9) were detected, which is
consistent with our previous findings (25,26).
Influence of inflammatory adaptation
on EMSCs
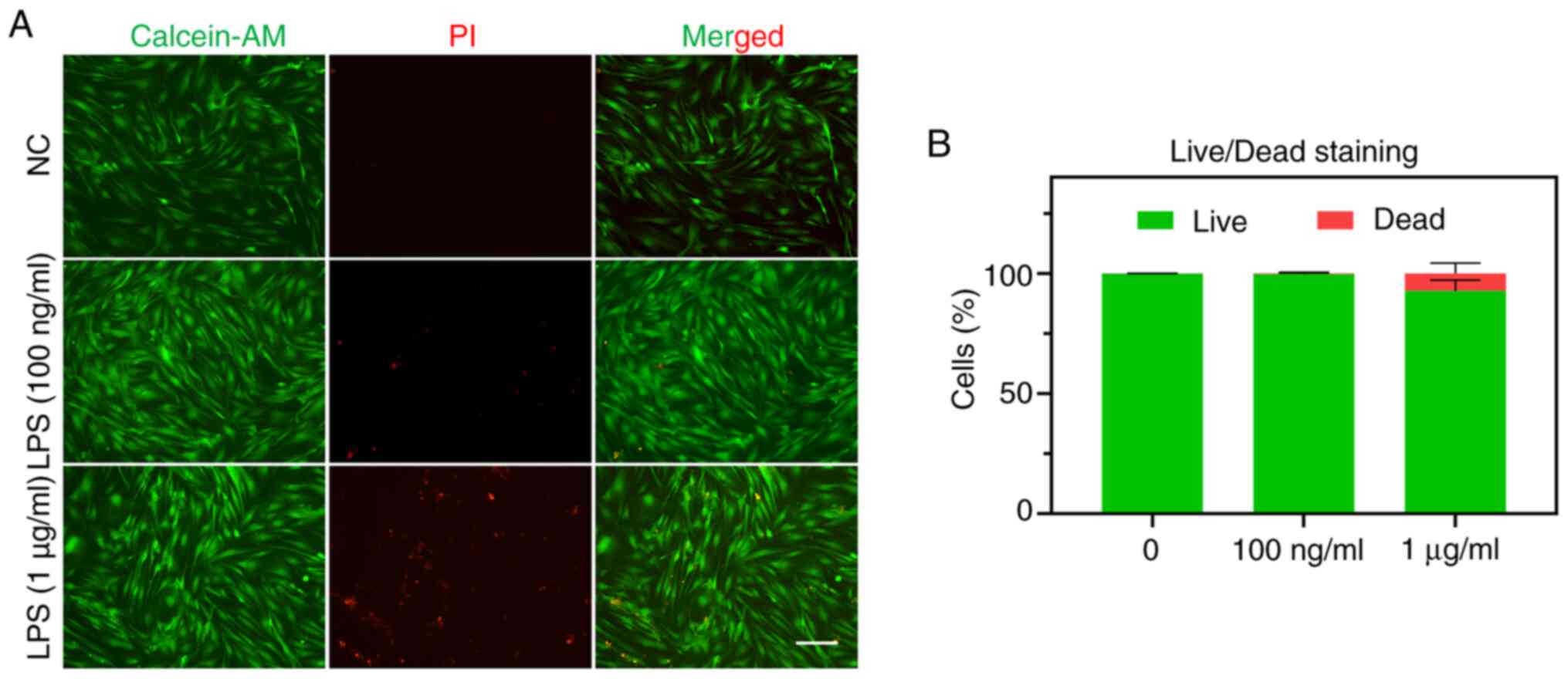
To evaluate whether inflammatory adaptation affects
the survival of EMSCs, live (green)/dead (red) cell staining was
performed. The results indicated that the five consecutive training
days with varying concentrations of LPS resulted in distinct
performances regarding the cell survival rate. Specifically,
exposure to 100 ng/ml LPS appeared to have no detrimental effect on
EMSCs, whereas exposure to 1 µg/ml LPS induced certain damage
(Fig. 3).
Inflammatory adaptation procedure
improves IL-10 expression of EMSCs
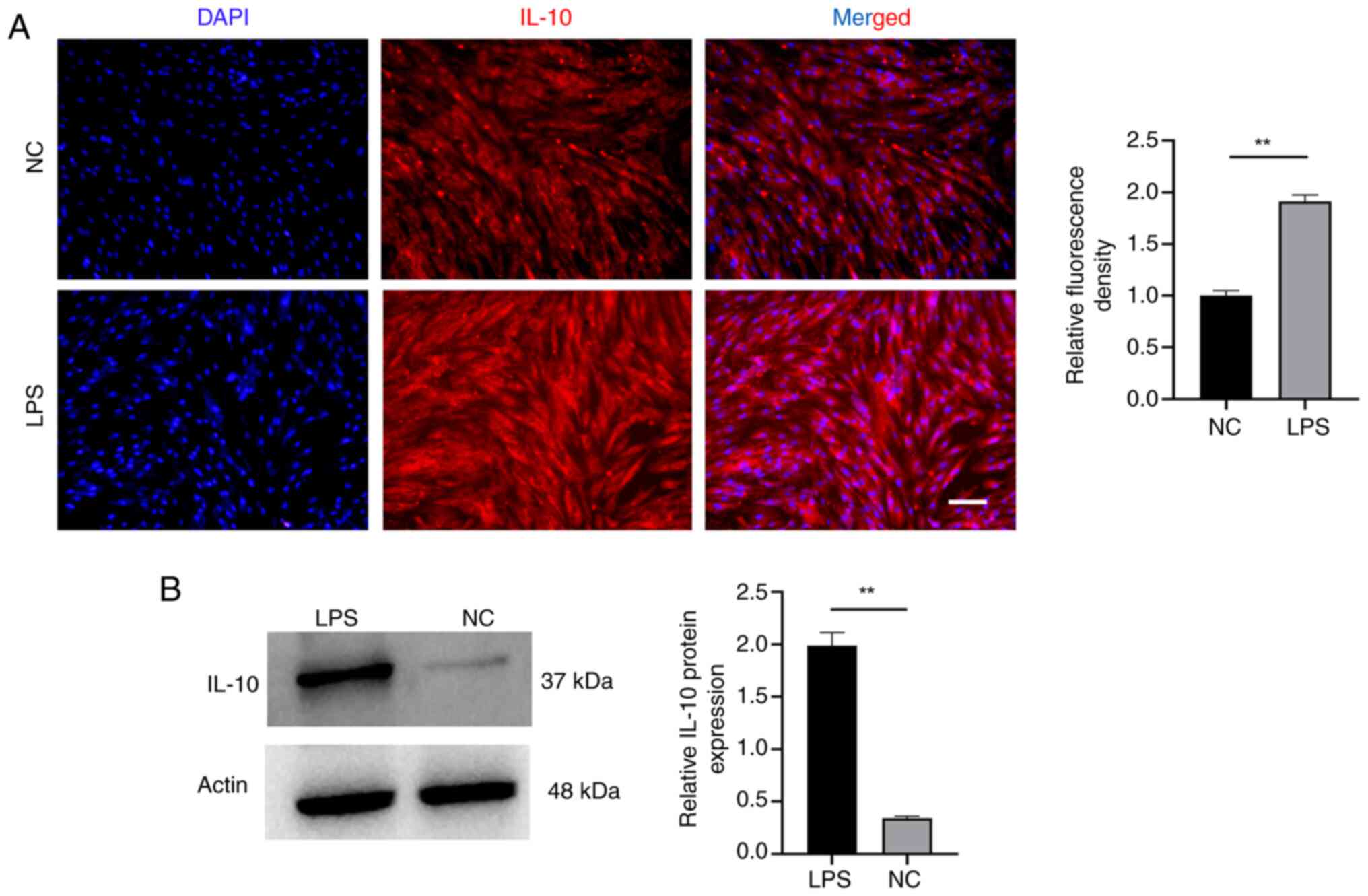
Next, immunofluorescence and western blotting were
conducted to assess whether inflammatory adaptation procedure would
elevate the levels of IL-10 in EMSCs (Fig. 4). The findings indicated a notable
increase in IL-10 expression in EMSCs, demonstrating significant
differences. Immunofluorescence analysis also revealed an enhanced
IL-10 fluorescence signal in the LPS group. During domestication,
the dynamic alternation of IL-10 in EMSCs indicated a positive
outlook for anti-inflammatory therapy and hinted at an increased
potential for osteogenic differentiation (20,27).
Inflammatory adaptation of EMSCs
enhances osteogenic differentiation
Based on the aforementioned studies, it was
investigated whether the elevated levels of IL-10 affected
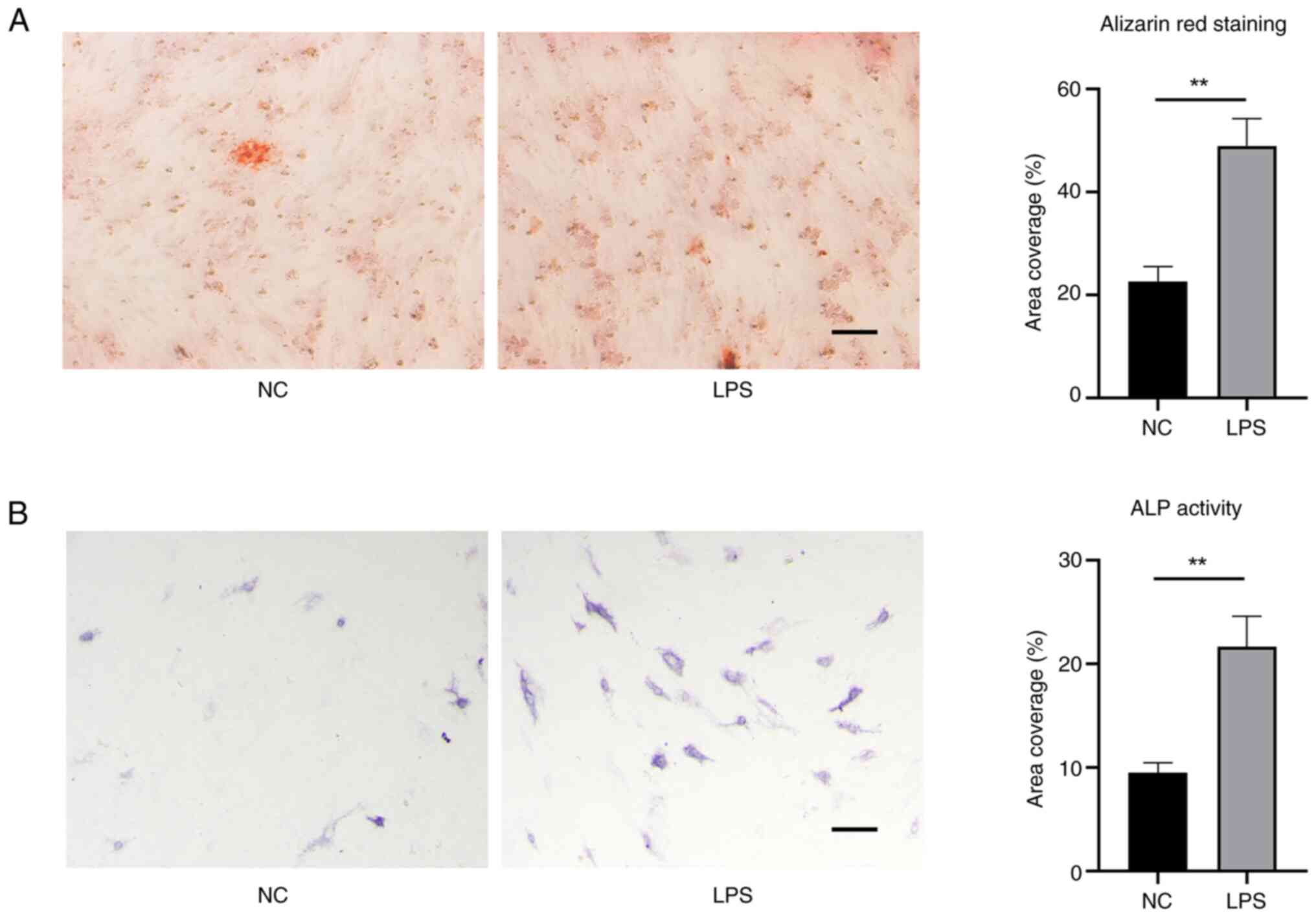
osteogenic differentiation. Alizarin red staining can detect
calcium deposits as a marker to confirm successful osteoblastic
differentiation of stem cells. EMSCs domesticated by LPS obtained
an enhanced osteogenic characteristic (Fig. 5A). The ALP staining results also
corroborated this observation (Fig.
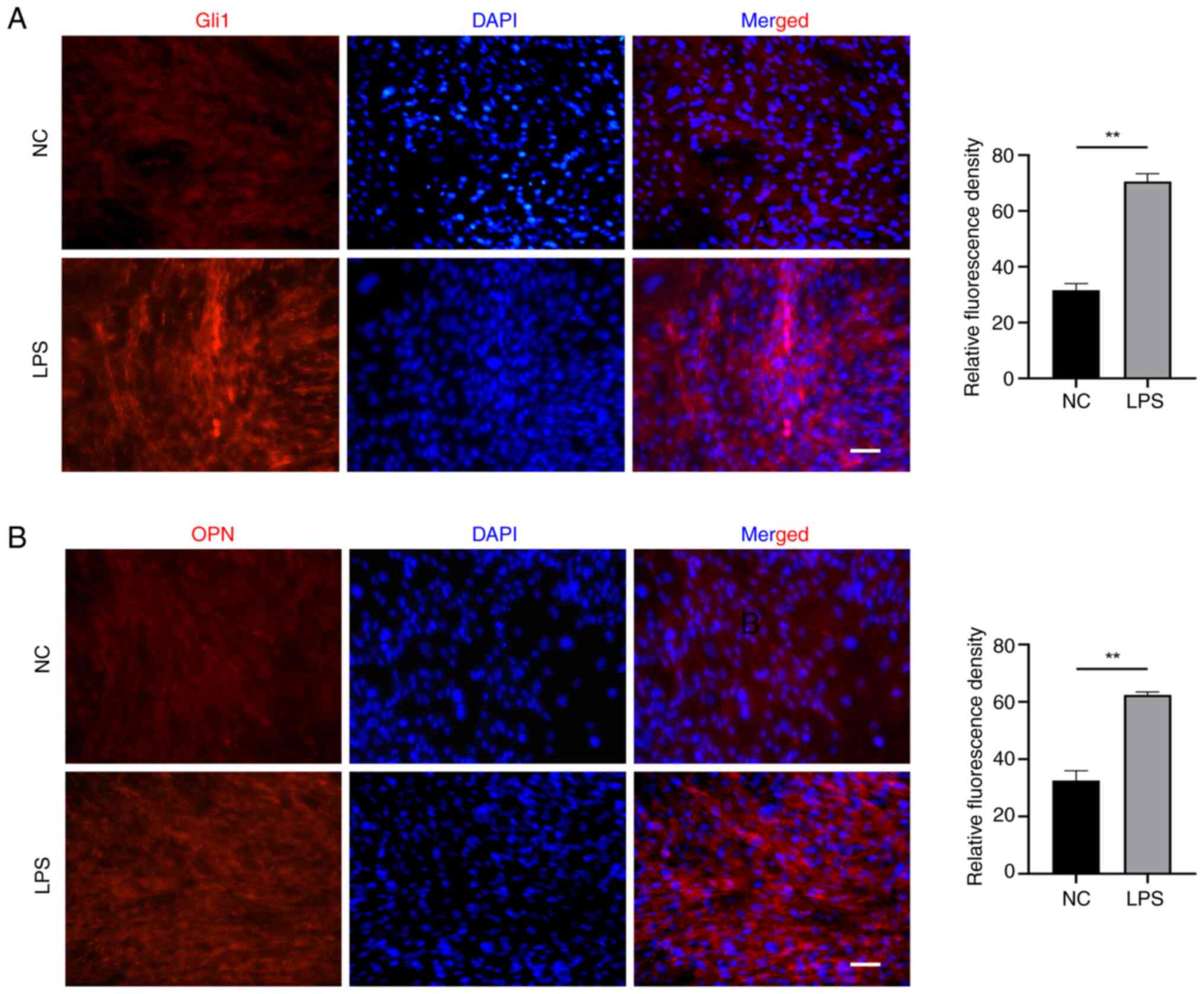
5B). Meanwhile, the expression of Gli1 and OPN as indicators of
osteogenic-related proteins was test. As shown in Fig. 6, EMSCs exposed to LPS exhibited
high levels of expression of (A) Gli1 and (B) OPN.
Unraveling factors beyond IL-10 in
promoting EMSCs osteogenic differentiation during inflammatory
adaptation
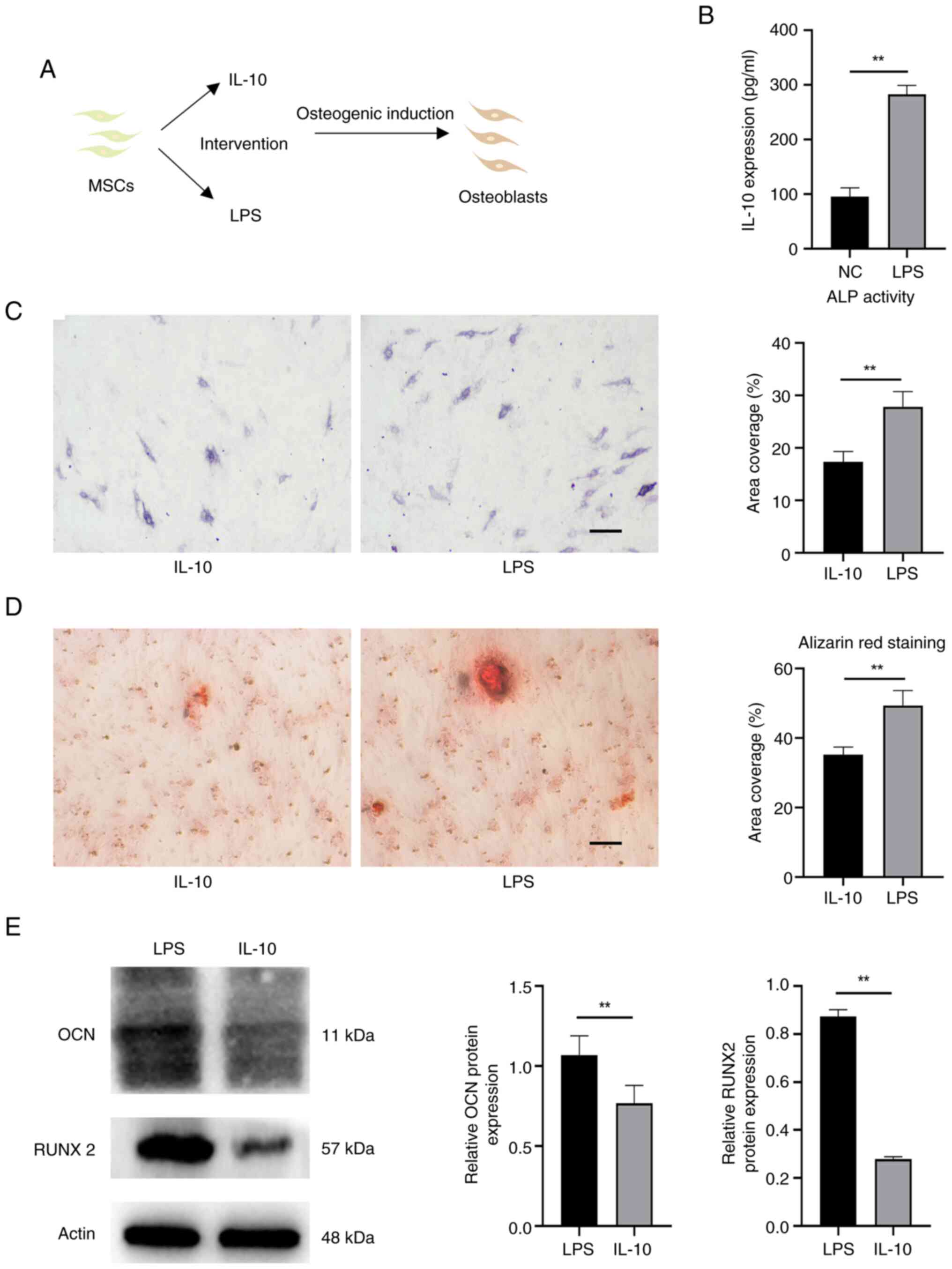
IL-10 can enhance the osteogenic differentiation of
MSCs (23). In order to
investigate whether the heightened osteogenic induction following
inflammatory acclimation was primarily attributed to the increase
in IL-10, cells treated solely with IL-10 were allocated into a
control group (Fig. 7A). Briefly,
IL-10 expression was measured in the inflammatory adaptation of
EMSCs by ELISA (Fig. 7B). The
IL-10 level acted as a benchmark, prompting the introduction of the
corresponding IL-10 factor for intervention. Fig. 7C showed that ALP activity in the
LPS group was also higher compared with the IL-10 group. The LPS
group exhibited a higher prevalence of calcium deposits (Fig. 7D). The high expression of proteins,
such as OCN and RUNX2, further confirmed these phenomena (Fig. 7E).
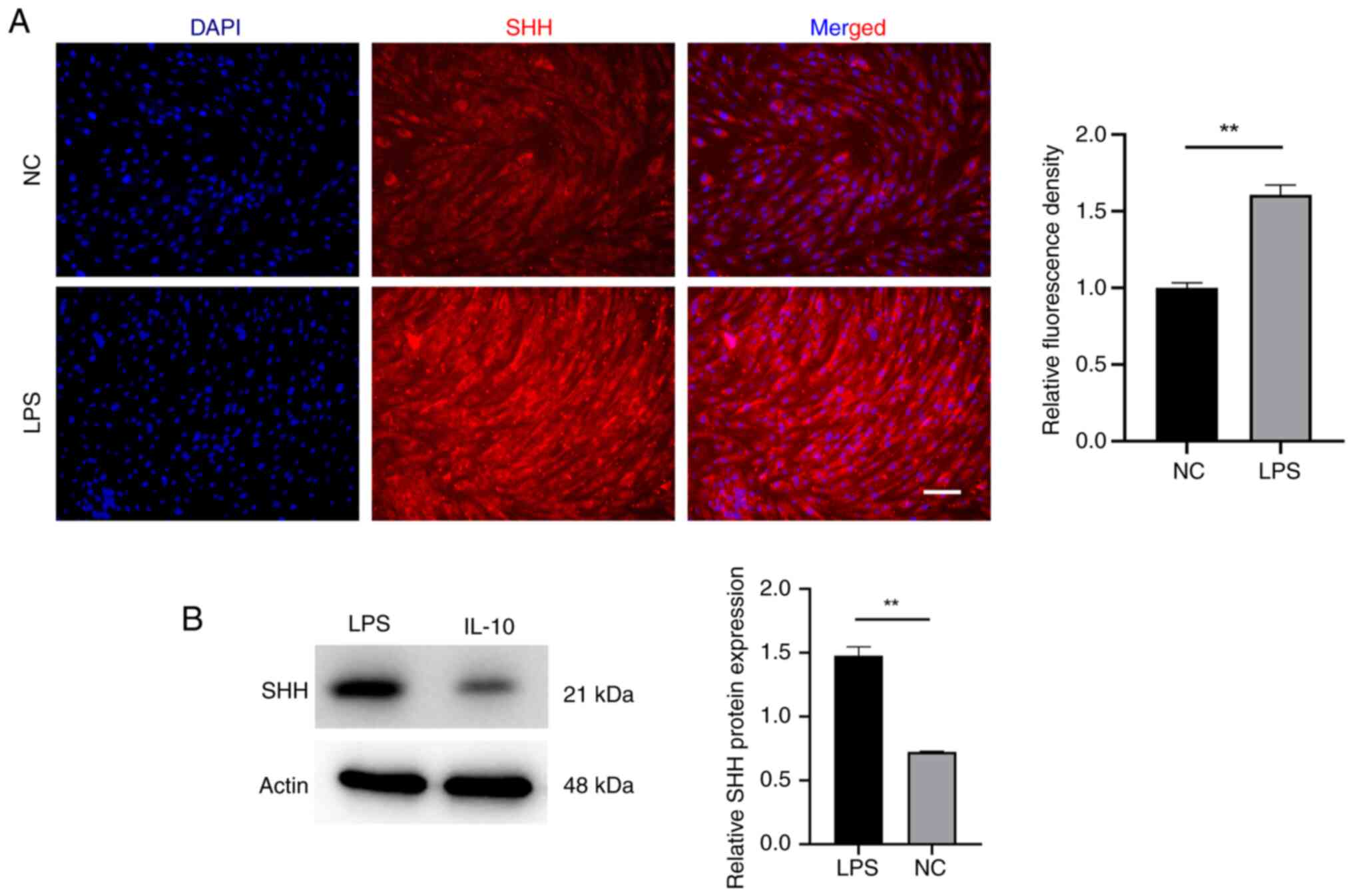
Shh contributes to EMSCs osteogenic
differentiation during inflammatory adaptation
The question is what leads to the outstanding
osteogenic differentiation potential observed in
inflammatory-acclimated EMSCs? Is it simply the upregulation of
IL-10? Shh serves as a morphogen regulating skeletal and vascular
development in embryos (28).
Research has documented its beneficial impact on fostering
osteogenic differentiation (29).
For this reason, the expression of Shh in EMSCs under the
inflammatory adaptation was further detected. Notably, the results
showed that the evaluated expression of Shh was also triggered by
LPS (Fig. 8). Domestication is a
complex process influenced by various factors, including cellular
stress. This discovery suggested that Shh could act as an
additional facilitator in osteogenic differentiation, highlighting
the need for further investigation into other contributing
factors.
Discussion
The process of domesticating EMSCs, a novel and
intriguing approach, has the potential to alter numerous properties
of EMSCs. Inflammatory adaptation, a unique model of adaptation
characterized by its heightened anti-inflammatory attributes,
raises new considerations. However, the reported absence of
osteogenic induction in these domesticated MSCs, with the
underlying mechanisms of induction remaining unclear, presents a
significant gap in our understanding. The present study aimed to
fill this gap by exploring the effect of inflammatory adaptation on
MSC differentiation into osteoblasts and elucidating potential
underlying mechanisms.
LPS can trigger inflammation in MSCs, elevate levels
of oxidative stress, induce ROS generation, disrupt mitochondrial
function and induce various metabolic changes. These detrimental
effects impede the osteogenic differentiation process of MSCs
(30,31). However, the varying outcome
primarily relies on the concentration of LPS and the specific type
of cell affected. A previous investigation, with LPS concentrations
reaching 1 µg/ml, led to potential harm to MSCs, ultimately
impeding their osteogenic differentiation (32). The present study also confirmed
that exposure to LPS at a concentration of 1µg/ml could harm EMSCs,
prompting the selection of a lower concentration of LPS. Thus, a
unique approach to acclimate to the low-concentration stimulation
pattern over time was implemented, diverging from prior research
methodologies. Notably, the present study indicated that EMSCs
osteogenesis is expedited by inflammatory adaptation. Meanwhile, it
was discovered that the osteogenic capability was initiated by the
heightened secretion of IL-10, a cytokine abundantly expressed in
the acclimated EMSCs, thereby enhancing the osteogenic
differentiation of EMSCs. IL-10 is crucial as an immunomodulatory
agent and an osteoblastogenic cytokine. The elevation of IL-10
undoubtedly hastens the osteogenesis process. Hence, the process of
inflammatory adaptation not only enables EMSCs to acquire
immunomodulatory properties but also facilitates their osteogenic
potential. Further investigation is needed to determine if
endoplasmic reticulum stress triggered by LPS is the most plausible
explanation for this phenomenon (33).
Furthermore, the present study employed an
equivalent concentration of IL-10 to stimulate osteogenesis, yet it
failed to yield the marked osteogenic outcomes observed with EMSCs
post-inflammatory adaptation. It was hypothesized that the
inflammatory adaptation process imbued EMSCs with a complex network
of osteogenic factors, albeit poorly elucidated. Shh, a
morphogenetic factor, frequently influences the osteogenic
differentiation of stem cells through its expression (34,35).
The present study conducted a preliminary examination of Shh
expression in EMSCs acclimated by LPS and the results also
indicated an observed increase in expression in EMSCs. It was
provisionally verified that the inflammatory adaptation of EMSCs
induced alterations in factor metabolism levels and these
modifications within the factor network facilitated the transition
of EMSCs into osteoblasts. However, the present study only
demonstrated the positive involvement of IL-10 and Shh in promoting
osteogenic differentiation in EMSCs under inflammatory adaption,
while a number of other factors contributing to osteogenic
differentiation remain unexplored. It is necessary to further
investigate the effect of inflammatory adaptation on EMSCs and
elucidate the signaling pathways through which these effects
promote osteogenesis. Biological scaffolds also play a pivotal role
in bone regeneration (36,37). Fully understanding tissue
engineering requires the close integration of cells with scaffolds.
Research interest in chitosan and its derivatives has surged
because of their remarkable biocompatibility and biodegradability.
Chitosan has been proved to be a highly effective scaffold material
in numerous applications within the realm of bone regeneration,
demonstrating notable success (38,39).
Integrating bioengineered scaffolds such as chitosan with MSCs may
also provide a promising perspective for advancement.
In summary, the present study indicated that EMSCs
developed a multifactorial network through inflammatory adaptation,
highlighted by IL-10 and Shh, which enhanced their osteogenic
capabilities. This significant finding not only deepens our
understanding of the osteogenic differentiation process but also
opens up new avenues for the development of novel approaches to
treat bone defects in the future, potentially revolutionizing the
field of regenerative medicine. However, the present study also
encountered some new challenging questions, including what
concentration of LPS can break the adaptive changes and turn into
toxic damage and whether these cell-level changes are caused by ER
stress. These intriguing inquiries require further examination.
Acknowledgements
Not applicable.
Funding
The present study received support from the Scientific research
project of Health Commission of Jiangsu, China (grant no.
H2023141).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Author's contributions
DL was responsible for conceptualization, design,
operation and drafting and revising the manuscript. DL, ZL and BL
revised the manuscript. BL, QZ and ZZ analyzed and interpreted
data. SC and YX were responsible for organization,
conceptualization, analysis and revision. ZL collected human
samples and validated data sets to guarantee their integrity and
accuracy. All authors reviewed and approved the final manuscript.
DL and BL confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The experimental protocol was approved by the
Ethical Committee of the Affiliated Hospital of Jiangsu University
(Jiangsu, China; approval. SWYXLI20190225-2).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rayat Pisheh H, Ansari M and Eslami H: How
is mechanobiology involved in bone regenerative medicine? Tissue
Cell. 76:1018212022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dec P, Modrzejewski A and Pawlik A:
Existing and novel biomaterials for bone tissue engineering. Int J
Mol Sci. 24:5292022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li S, Liu J, Liu S, Jiao W and Wang X:
Chitosan oligosaccharides packaged into rat adipose mesenchymal
stem cells-derived extracellular vesicles facilitating cartilage
injury repair and alleviating osteoarthritis. J Nanobiotechnology.
19:3432021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li S, Tian X, Fan J, Tong H, Ao Q and Wang
X: Chitosans for tissue repair and organ three-dimensional (3D)
bioprinting. Micromachines (Basel). 10:7652019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan G, Li Z, Lin X, Li N and Xu R: New
perspective of skeletal stem cells. Biomater Transl. 3:280–294.
2022.PubMed/NCBI
|
|
6
|
Benayahu D: Mesenchymal stem cell
differentiation and usage for biotechnology applications: Tissue
engineering and food manufacturing. Biomater Transl. 3:17–23.
2022.PubMed/NCBI
|
|
7
|
Charbord P: Bone marrow mesenchymal stem
cells: Historical overview and concepts. Hum Gene Ther.
21:1045–1056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang D, Cao H, Hua W, Gao L, Yuan Y, Zhou
X and Zeng Z: Mesenchymal stem cell-derived extracellular vesicles
for bone defect repair. membranes (basel). 12:7162022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li S, Liu J, Liu S, Jiao W and Wang X:
Mesenchymal stem cell-derived extracellular vesicles prevent the
development of osteoarthritis via the circHIPK3/miR-124-3p/MYH9
axis. J Nanobiotechnology. 19:1942021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin H, Sohn J, Shen H, Langhans MT and
Tuan RS: Bone marrow mesenchymal stem cells: Aging and tissue
engineering applications to enhance bone healing. Biomaterials.
203:96–110. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Delorme B, Nivet E, Gaillard J, Häupl T,
Ringe J, Devèze A, Magnan J, Sohier J, Khrestchatisky M, Roman FS,
et al: The human nose harbors a niche of olfactory ectomesenchymal
stem cells displaying neurogenic and osteogenic properties. Stem
Cells Dev. 19:853–866. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patthey C, Schlosser G and Shimeld SM: The
evolutionary history of vertebrate cranial placodes-I: Cell type
evolution. Dev Biol. 389:82–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Diogo R, Kelly RG, Christiaen L, Levine M,
Ziermann JM, Molnar JL, Noden DM and Tzahor E: A new heart for a
new head in vertebrate cardiopharyngeal evolution. Nature.
520:466–473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Achilleos A and Trainor PA: Neural crest
stem cells: Discovery, properties and potential for therapy. Cell
Res. 22:288–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trainor PA: Craniofacial birth defects:
The role of neural crest cells in the etiology and pathogenesis of
Treacher Collins syndrome and the potential for prevention. Am J
Med Genet A. 52A:2984–2994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Srinivasan A, Teo N, Poon KJ, Tiwari P,
Ravichandran A, Wen F, Teoh SH, Lim TC and Toh YC: Comparative
craniofacial bone regeneration capacities of mesenchymal stem cells
derived from human neural crest stem cells and bone marrow. ACS
Biomater Sci Eng. 7:207–221. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi W, Zhang X, Bian L, Dai Y, Wang Z,
Zhou Y, Yu S, Zhang Z, Zhao P, Tang H, et al: Alendronate
crosslinked chitosan/polycaprolactone scaffold for bone defects
repairing. Int J Biol Macromol. 204:441–456. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi W, Bian L, Wu Y, Wang Z, Dai Y, Zhou
Y, Meng P, Wang Q, Zhang Z, Zhao X, et al: Enhanced bone
regeneration using a ZIF-8-Loaded fibrin composite scaffold.
Macromol Biosci. 22:e21004162022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li S, Wang J, Han Y, Li X, Liu C, Lv Z,
Wang X, Tang X and Wang Z: Carbenoxolone inhibits mechanical
stress-induced osteogenic differentiation of mesenchymal stem cells
by regulating p38 MAPK phosphorylation. Exp Ther Med. 15:2798–2803.
2018.PubMed/NCBI
|
|
20
|
Yuan L, You H, Qin N and Zuo W:
Interleukin-10 modulates the metabolism and osteogenesis of human
dental pulp stem cells. Cell Reprogram. 23:270–276. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su W, Wan Q, Huang J, Han L, Chen X, Chen
G, Olsen N, Zheng SG and Liang D: Culture medium from
TNF-α-stimulated mesenchymal stem cells attenuates allergic
conjunctivitis through multiple antiallergic mechanisms. J Allergy
Clin Immunol. 136:423–432.e8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu H, Zhu X, Cao X, Chi A, Dai J, Wang Z,
Deng C and Zhang M: IL-1β-primed mesenchymal stromal cells exert
enhanced therapeutic effects to alleviate Chronic
Prostatitis/Chronic Pelvic Pain Syndrome through systemic immunity.
Stem Cell Res Ther. 12:5142021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vallés G, Bensiamar F, Maestro-Paramio L,
García-Rey E, Vilaboa N and Saldaña L: Influence of inflammatory
conditions provided by macrophages on osteogenic ability of
mesenchymal stem cells. Stem Cell Res Ther. 11:572020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mahon OR, Browe DC, Gonzalez-Fernandez T,
Pitacco P, Whelan IT, Von Euw S, Hobbs C, Nicolosi V, Cunningham
KT, Mills KHG, et al: Nano-particle mediated M2 macrophage
polarization enhances bone formation and MSC osteogenesis in an
IL-10 dependent manner. Biomaterials. 239:1198332020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi W, Que Y, Lv D, Bi S, Xu Z, Wang D and
Zhang Z: Overexpression of TG2 enhances the differentiation of
ectomesenchymal stem cells into neuron-like cells and promotes
functional recovery in adult rats following spinal cord injury. Mol
Med Rep. 20:2763–2773. 2019.PubMed/NCBI
|
|
26
|
Shi W, Bian L, Lv D, Bi S, Dai Y, Yang K,
Lu H, Zhou H, Que Y, Wang D, et al: Enhanced neural differentiation
of neural stem cells by sustained release of Shh from TG2
gene-modified EMSC co-culture in vitro. Amino Acids. 53:11–22.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ouyang W, Rutz S, Crellin NK, Valdez PA
and Hymowitz SG: Regulation and functions of the IL-10 family of
cytokines in inflammation and disease. Annu Rev Immunol. 29:71–109.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fuchs S, Dohle E and Kirkpatrick CJ: Sonic
Hedgehog-mediated synergistic effects guiding angiogenesis and
osteogenesis. Vitam Horm. 88:491–506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma D, Yu H, Xu S, Wang H, Zhang X, Ning T
and Wu B: Stathmin inhibits proliferation and differentiation of
dental pulp stem cells via sonic hedgehog/Gli. J Cell Mol Med.
22:3442–3451. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bai Y, Zhang W, Hao L, Zhao Y, Tsai IC, Qi
Y and Xu Q: Acetyl-CoA-dependent ac4C acetylation promotes the
osteogenic differentiation of LPS-stimulated BMSCs. Int
Immunopharmacol. 133:1121242024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang Z, Chen G, Wu H, Huang X, Xu R, Deng
F and Li Y: Ebselen restores peri-implantitis-induced osteogenic
inhibition via suppressing BMSCs ferroptosis. Exp Cell Res.
427:1136122023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amarasekara DS, Kim S and Rho J:
Regulation of osteoblast differentiation by cytokine networks. Int
J Mol Sci. 22:28512021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang SX, Wang JJ, Starr CR, Lee EJ, Park
KS, Zhylkibayev A, Medina A, Lin JH and Gorbatyuk M: The
endoplasmic reticulum: Homeostasis and crosstalk in retinal health
and disease. Prog Retin Eye Res. 98:1012312024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takebe H, Shalehin N, Hosoya A, Shimo T
and Irie K: Sonic hedgehog regulates bone fracture healing. Int J
Mol Sci. 21:6772020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guan CC, Yan M, Jiang XQ, Zhang P, Zhang
XL, Li J, Ye DX and Zhang FQ: Sonic hedgehog alleviates the
inhibitory effects of high glucose on the osteoblastic
differentiation of bone marrow stromal cells. Bone. 45:1146–1152.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Y, Zhang H, Hu Y, Jing Y, Geng Z and
Su J: Bone repair biomaterials: A perspective from
immunomodulation. Adv Funct Mater. 32:22086392022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang F, Gu Z, Yin Z, Zhang W, Bai L and Su
J: Cell unit-inspired natural nano-based biomaterials as versatile
building blocks for bone/cartilage regeneration. J
Nanobiotechnology. 21:2932023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang S, Zhao G, Mahotra M, Ma S, Li W,
Lee HW, Yu H, Sampathkumar K, Xie D, Guo J and Loo SCJ: Chitosan
nanofibrous scaffold with graded and controlled release of
ciprofloxacin and BMP-2 nanoparticles for the conception of bone
regeneration. Int J Biol Macromol. 254((Pt 2)): 1279122024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu G, Ma M, Yang H, He W, Xie Y, Li J, Li
J, Zhao F and Zheng Y: Chitosan/polydopamine/octacalcium phosphate
composite microcarrier simulates natural bone components to induce
osteogenic differentiation of stem cells. Biomater Adv.
154:2136422023. View Article : Google Scholar : PubMed/NCBI
|