The liver is the organ that interacts most with the
digestive system. As such, the liver is exposed to numerous gut
microbiota (GM). The GM is a diverse ecosystem that contains
bacteria, protozoa, archaea, fungi and viruses. There are
>1×1014 species of microorganisms in the human
gastrointestinal tract, including ~1×104 species of
bacteria (1). The unique
microenvironment and physicochemical barriers of each region of the
digestive system determine the growth of specific microbiota. It is
widely recognized that transgenes serve a role in the physiological
and pathological aspects of human health, particularly liver health
(2,3).
Previous studies have reported the role of the GM in
occurrence and development of a number of liver diseases (such as
hepatitis, alcoholic liver disease (ALD), non-alcoholic liver
disease (NAFLD), liver fibrosis, cirrhosis, and liver cancer, etc.)
(4,5). In recent years, researchers have
assessed the gut-liver axis (6–10).
For example, Pseudomonas aeruginosa can significantly
inhibit NAFLD-HCC progression by secreting acetate salts (7). The present review aimed to assess the
previous research to provide a broader understanding of this axis
and discuss the mechanism of the gut-liver axis in a number of
common types of liver diseases, highlighting the potential drugs
and treatment methods targeting GM in clinical treatment of liver
disease.
The term ‘liver disease’ covers a range of
illnesses, including acute problems caused by harmful agents such
as viruses, poisons, alcohol and pharmaceutical agents, as well as
chronic liver disease, which that may result in cirrhosis. Any type
of cirrhosis increases the risk of developing hepatic cell
carcinoma (HCC), a primary liver cancer; this risk is higher if the
cirrhosis is caused by hepatitis B (HBV) or hepatitis C (HCV)
infection (11,12). Most types of liver diseases (such
as hepatitis, ALD, NAFLD, focal liver disease, and some types of
liver cancer) (13–18) can be conservatively treated with
non-surgical treatment methods), including targeted therapy
(19,20), immunotherapy (21), radiotherapy (22), etc. Acute liver failure is
associated with rapid and massive injury to hepatocytes, which is
rare, but has a high incidence and mortality rate (23). HCV infection causes ~290,000 deaths
worldwide each year and is the primary cause of liver cirrhosis and
associated complications (i.e. decompensated cirrhosis) (24). Other data indicate that globally,
the number of HBV-related deaths is expected to reach 1,109,500 by
2030 (25). ALD is the most common
cause of liver cirrhosis worldwide. According to WHO data in 2018,
the global prevalence of alcohol use disorders was 5.1% (26). A modeling study suggests that if
current drinking trends are not controlled for, the age-specific
mortality rate (ASDR) associated with ALD in the United States is
expected to increase from 8.2 deaths per 100,000 patients per year
in 2019 to 15.2 deaths per 100,000 patients per year in 2040
(27). Other data indicate that
globally, the number of HBV-related deaths is expected to reach
1,109,500 by 2030 (25). ALD is
the most common cause of liver cirrhosis worldwide. According to
WHO data in 2018, the global prevalence of alcohol use disorders
was 5.1% (26). Primary liver
cancer is the seventh most common cancer in the world and the
second most common cause of cancer death. HCC is the main type of
liver cancer worldwide, accounting for approximately 75% of the
total. It is the primary cause of diagnosis and death in liver
cancer cases (28).
The gut and liver communicate with each other. The
liver can regulate gut function through the bile ducts. The
intestine can regulate liver function through the portal vein. In
addition, these two organs can indirectly affect each other's
function through the whole body blood circulation. The liver
delivers bile salts and antimicrobial molecules such as
immunoglobulin A and angiopoietin to the gut via the biliary tract.
This maintains the GM by modulatin microbiota growth (29). Bile acids (BAs) exert direct
antibacterial effects by disrupting the cell membranes of
intestinal bacteria and causing membrane protein degradation
(30). Moreover, BAs indirectly
regulate the composition of the GM by activating BA receptors in
the intestine, particularly farnesoid X receptor (FXR) encoded by
Nuclear Receptor Subfamily 1 Group H Member 4 (31). Liu et al (32) reported that activated FXR is
involved in the expression of gut tight junction markers (claudin1
and zonula occludens-1), maintaining BA homeostasis and inhibiting
the expression of inflammatory factors, thereby inhibiting
bacterial overgrowth and mucosal damage in the ileum. Mice lacking
FXR exhibit an increase in harmful bacteria in the ileum and damage
to the epithelial barrier. BAs also regulate hepatic BA synthesis,
glucose and lipid metabolism and dietary energy use via nuclear
receptors such as the FXR and the G protein-coupled BA receptor
(TGR5). FXR receptors inhibit the expression of BA synthase by
binding to endogenous BAs, which provides negative feedback to
regulate BA synthesis (33). For
example, cholesterol 7-α hydroxylase 1 and cytochrome P450 Family
27 Subfamily A Member 1 are enzymes required for the synthesis of
BAs, with their expression significantly reduced upon FXR
stimulation (34). BAs can
interact with nuclear transcription factors in the promoter region
of gluconeogenesis-associated genes via the FXR-small heterodimer
partner-dependent pathway and inhibit their expression (35). TGR5 belongs to the G
protein-coupled receptor superfamily. The activated TGR5 receptor
is associated with energy expenditure of the body. According to
Watanabe's research report, treating brown adipocytes and human
skeletal muscle cells with BA can increase the activity of TGR5 in
cells, thereby upregulating the expression of cAMP-dependent type 2
deiodinase (DIO2). This enzyme catalyzes the deiodination of
prothyroid hormones to triiodothyronine (T3), thereby enhancing
oxygen and energy consumption in key thermogenic tissues such as
brown adipose tissue and skeletal muscle (36,37).
In terms of regulating blood sugar, previous studies have reported
that the liver decreases hepatic gluconeogenesis through the BA FXR
signal, which induces hepatic glycogen synthesis to regulate blood
glucose levels (33). The GM and
its metabolites, such as lipopolysaccharides (LPS), short-chain
fatty acids (SCFAs), and tryptophan metabolites, are transported to
the liver via the portal vein, inducing a local inflammatory
response and exacerbating hepatic necrosis (38–40).
In addition, metabolites produced by the liver, such as free fatty
acids (FFAs), inflammatory factors, choline metabolites, and
ethanol metabolites, can enter the systemic circulation, thereby
prolonging the gut-liver axis and exerting systemic effects on
multiple organs throughout the body, including the gut. For
example, butyrate in the blood can enhance gut barrier function and
reduce the translocation of gut microbial toxic metabolites to
extraintestinal sites (41,42).
Ethanol and acetaldehyde can increase intracellular calcium ion
(Ca2+) concentration and disrupt the integrity of gut
epithelial tight junctions (43).
The human microbiota, which includes bacteria,
fungi, viruses, archaea and protozoa, is a collection of
microorganisms that live in humans. The term ‘human microbiome’
refers to genes carried by these microbes and the surrounding
environment in which they live and interact (44). The human microbiota, especially in
the gastrointestinal tract, serves a role in human health. As the
gastrointestinal tract makes direct contact with the liver through
the portal vein, GM can directly affect liver (45).
GM are concentrated within the lumen of the gut and
adhere to the mucosal surface. The location and diameter of the gut
lumen vary, and the types and abundance of GM present also vary.
The bacterial population density in the jejunum and ileum is higher
than that in the stomach cavity and duodenum. However, the most
densely populated area is the colon, which contains ~1,000
colony-forming units per milliliter/ml and is mainly composed of
anaerobic bacteria such as Bacteroides, Porphyromonas,
Bifidobacterium, Lactobacillus and Clostridium. In the
colon, the ratio of anaerobic to aerobic bacteria is 100:1-1,000:1.
This is influenced by changes in optimal growth conditions for
these bacteria, which are caused by local colonic lesions (48–50).
Microbes are concentrated in the lumen of the gut wall or adhere to
the surface of the mucous membrane.
The gastrointestinal tract contains a large number
of microorganisms, particularly bacteria, which are a source of
pathogen-associated molecular patterns (PAMPs) and metabolites
(51). Under normal conditions,
small amounts of GM and metabolites enter the liver and are rapidly
cleared. However, when the normal gut barrier permeability is
increased, as in gut ecological dysbiosis, large amounts of GM and
GM metabolites enter the liver, leading to activation of the immune
cascade in the liver and production of pro-inflammatory cytokines
(52,53). Increased gut permeability is
attributed to tight junction disruption, potentially from the
pathological change in the composition of GM and its metabolites or
their induced immune cascade and inflammatory response (54–57).
Dendritic cells form an extensive network under the gut epithelium.
Dysregulated GM stimulates immature dendritic cells to produce
IL-23, which promotes the secretion of cytokines IL-17A and IL-22
by interacting with surface receptors on activated CD4+
T cells, thereby inducing a local gut inflammatory response
(58,59). Moreover, dendritic cells and
macrophages produce cytokines including IL-1β, IL-6, IL-18 and TNF
to exacerbate the inflammatory response (60). Large amounts of pro-inflammatory
cytokines affect tight junctions between gut epithelial cells, in
addition to increasing the inflammatory burden. IL-1β recruits
granulocytes to infiltrate foci of infection and directly disrupt
the junctions and tightness of gut epithelial cells (61). TNF-α promotes myosin light chain
kinase (MLCK) protein expression level in the gut epithelium
(62). Previous studies have shown
that MLCK triggers perijunction actinosin ring (PAMR) contraction,
leading to increased permeability of tight junctions adjacent to
gut epithelial cells (63).
Moreover, TNF-α and IL-1β induce endoplasmic reticulum stress,
which affects gut epithelial cells and alters proteins in the
apical and basal lateral membranes, including E-calmodulin. This
further disrupts the tight junctions of the gut epithelium
(64). Release of these cytokines
can activate natural killer cells, which bind to epithelial cells,
releasing toxic particles (such as perforin, and granzymes)
(65,66) and inducing apoptosis in epithelial
cells. Furthermore, dendritic cells phagocytose antigens, which
activate T cells and promote the differentiation of T helper 0
(Th0) cells into Th1, Th2 and Th17 cells. Th1 cells induce
cytotoxic T cells to activate, proliferate and attack infected gut
epithelial cells. IL-18 is a pro-inflammatory cytokine that promote
the secretion of significant amounts of interferon-γ (IFN-γ) by Th1
cells. IFN-γ induces apoptosis in gut epithelial cells, thereby
compromising the integrity of the gut epithelium (67–69).
Th2 cells activate B cells, causing B cell proliferation and
differentiation into plasma cells that secrete IL-4, IL-5 and IL-13
(70). These factors are believed
to be associated with local eosinophil and mononuclear
infiltration, increased mucus production, and epithelial cell
proliferation and hypertrophy in the gastrointestinal tract
(71). In addition, IL-13
activates STAT6 in epithelial cells and affects tight junctions in
gut epithelium (72). IL-13 can
also induce apoptosis of gut epithelial cells and further increase
gut permeability (73,74). Th17 cells secrete IL-17A to mediate
inflammatory responses (75).
Large amounts of pro-inflammatory cytokines affect tight junctions
between gut epithelial cells, in addition to increasing the
inflammatory burden.
Increased gut permeability results in a heightened
passage of GM and metabolites, such as LPS and endotoxins, into the
portal circulation (76).
Metabolites produced by GM, including trimethylamine and alcohol,
exert direct toxic effects on the liver, while PAMPs- the
distinctive molecular structures of GM-induce liver injury through
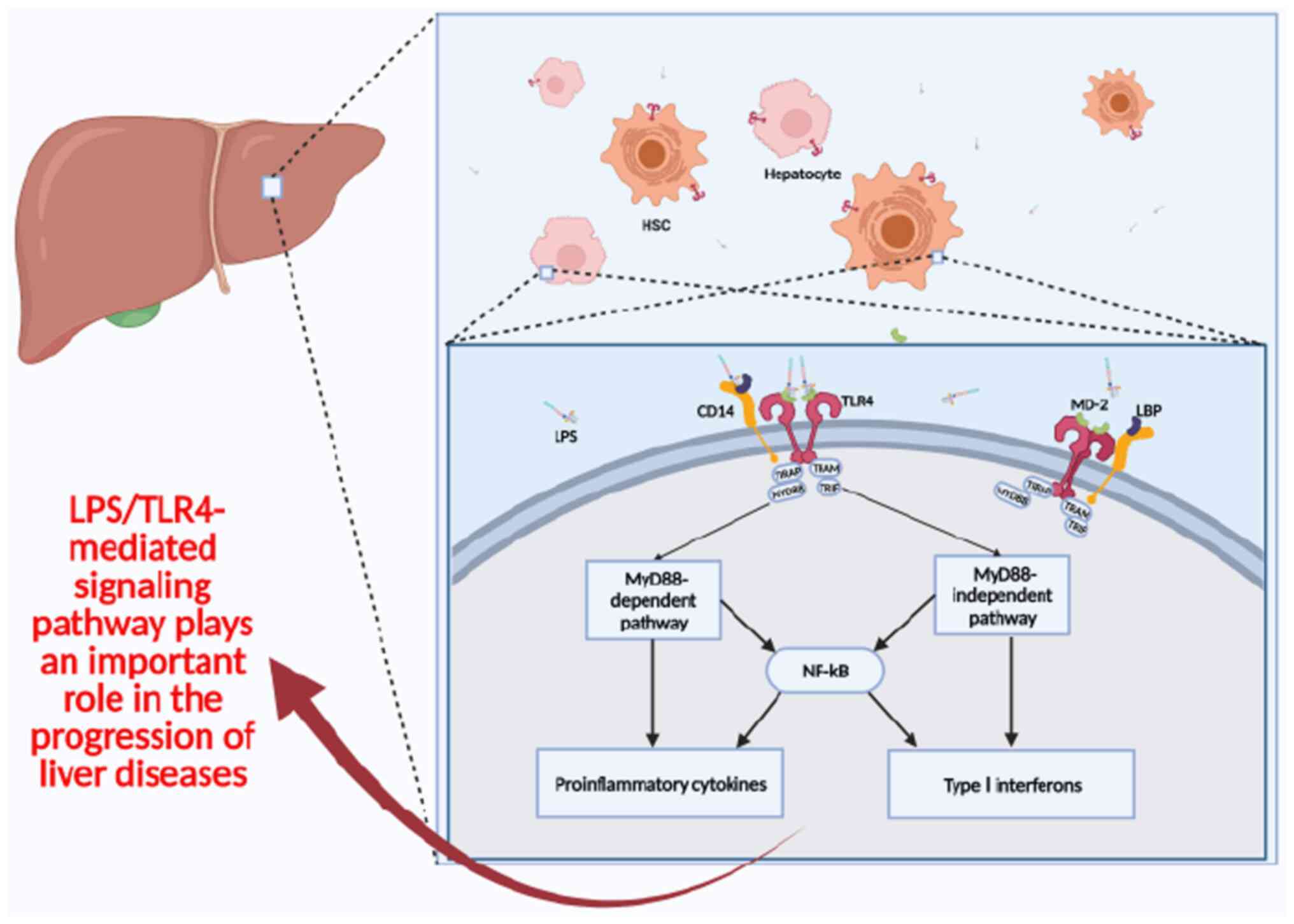
the activation of the innate immune system (77). GM is detected by pathogen
recognition receptors in the liver, encompassing toll-like
receptors (TLRs) and inflammasomes. TLRs are present on hepatic
sinusoidal cells, such as Kupffer cells and hepatic stellate cells,
and they identify PAMPs located on cell membranes (78,79).
TLR-mediated signaling pathways lead to sustained production of
inflammatory cytokines, which cause or exacerbate liver injury
(80) (Fig. 1).
The ‘biological clock’ is an intrinsic rhythm formed
by organisms to adapt to changes in the surrounding environment.
The ability of the circadian clock to persist in the absence of
environmental cues provides internal temporal organization,
allowing rhythmic activities to occur at characteristic times
during the circadian cycle (81).
The mammalian biological clock is composed of a number of core
transcriptional regulators, including Brain and muscle arnt-like
protein 1, Clock Circadian Regulator (CLOCK), period and
cryptochrome. Disturbances in the biological clock are associated
with the progression of a number of diseases, including fatty liver
disease, heart disease, diabetes and cancer (82–85).
However, the specific pathological mechanisms have not been fully
identified. Previous studies have suggested that the GM may serve a
link between circadian rhythm disorders and disease progression,
which may be associated with the role of GM on host immune system
function and metabolism (54,86,87).
Although GM is not directly affected by external
environmental, self-regulation of the biological clock, host
activity, and metabolic patterns, particularly changes in eating
patterns, can induce rhythmic oscillations in the abundance of GM
and GM metabolite. For example, Thaiss et al (88) reported rhythmic changes in GM
composition over a 24-h period by analyzing fecal microbiota of
mice. The abundance of Lactobacillus reuteri in the mouse
gut increased during the light and decreased during the dark
period. By contrast Per1/2−/− mice, which lack a
functional host biological clock, exhibit almost complete loss of
this GM abundance variation. GMs with different compositions
secrete different metabolites. GM affects host metabolism and
energy homeostasis by metabolite signaling. Previous studies have
reported an increase in body fat percentage and insulin resistance
in mice fed without transgenic genes under the same feeding
conditions compared to normal mice (89,90).
Further research reports indicate that differences exist in the
composition of GM between obese and normal mice (91,92).
For example, compared with the normal feed group, the high-fat feed
group had higher abundance of Lachnospiraceae and
Blautia in the gut of mice, while the abundance of
Lactobacillus, Faecalibaculum, Lachnoclostridium,
Bacteroides and Desulfovibrio was lower (92). The aforementioned research
indicates that GM is an important environmental factor affecting
energy collection and storage in the host. Turnbaugh et al
(93) reported that the increased
ability of the GM of obese mice to obtain energy from the diet is
associated with a reduced abundance of Bacteroidetes and an
increased abundance of Firmicutes in the GM. This change has
also been confirmed in humans (94). Akkermansia muciniphila,
Bifidobacterium longum, Clostridium leptum group,
Faecalibacterium prausnitzii and Faecalibacterium and
Dorea were suggested to serve a role in the regulation of
blood glucose; changes in the abundance of these genera can lead to
dysregulation of glucose metabolism and the progression of type 2
diabetes in humans (95).
Viral hepatitis is the most common type of hepatitis
worldwide. Hepatitis is defined as inflammation of the liver
tissue. More than 300 million people worldwide are affected by
viral hepatitis infections, which has a notable negative impact on
public health and the economy and leads to high mortality (96). Hepatitis A, B, C, D and E are the
five most common types of viral hepatitis. HBV and HCV often lead
to chronic infections, and in severe cases, may lead to cirrhosis
and liver cancer, affecting 257 million and 71 million people
worldwide, respectively (97,98).
Chou et al (99) reported
that GM serves a role in age-dependent immunity of mice against HBV
infection. After 6 weeks of infection, normal adult mice with
mature GM completely eliminate HBV, but young mice without GM
remain positive for HBV. Following clearance of the GM of adult
mice, their resistance to HBV decreased. Another study reported
that after treatment with fecal microbiota transplantation (FMT),
patients with hepatitis B e-antigen (HBeAg) positivity showed a
significant decrease in their blood HBeAg levels, indicating a
weakened viral replication activity (100).
Previous studies have reported that the GM
composition of patients with hepatitis changes compared with
healthy individuals (101–103).
For example, the levels of Bacteroides in the GM of patients
with HBV-related cirrhosis is low, at 4 vs. 53% in healthy patients
and the level of Proteus is high at 43 vs. 4% for healthy
patients (104). Furthermore,
Bajaj et al (105)
reported the unique composition of GM in patients with HCV. GM of
these patients predominantly comprises Enterobacteriaceae,
Clostridium and Ruminococcaceae genera. A previous study
compared the GM of stage 4 HCV patients with that of healthy
individuals, revealing that the relative abundance of Bacteroides
in the GM of HCV patients increased, whereas the abundance of
Firmicutes decreased (106).
Using high-throughput 16S rRNA gene sequencing, Aly et al
(106) reported that the GM of
patients with HCV exhibits high level of Proctor and levels
of Acinetobacter, Vibrio and Lactobacillus were also
increased compared with healthy patients. However, the probiotic
genus, Bifidobacterium were found exclusively in the GM of
HCV patients, while no Bifidobacterium were detected in the
GM of healthy individuals. In conclusion, because viral hepatitis
is associated with GM composition, GM could be targeted in the
development of novel hepatitis treatment strategies in future.
ALD is the most prevalent form of chronic liver
disease, affecting 150 million people worldwide (107). Alcoholic steatohepatitis (ASH),
which is characterized by hepatic inflammation, may develop from
alcoholic fatty liver. ASH is characterized by acute inflammatory
response with neutrophils and hepatocellular damage, whereas
cirrhosis involves chronic architectural remodeling with fibrosis
and regenerative nodules (108).
Chronic ASH can lead to fibrosis and cirrhosis. In 2019, ~371,964
people exhibited alcoholic cirrhosis-related mortality, accounting
for 25% of all liver cirrhosis-associated mortalities. There were
90,741 mortalities due to alcohol-related HCC (109). Moreover, ASH can directly lead to
liver failure, and severe ASH may lead to high mortality rates
(110).
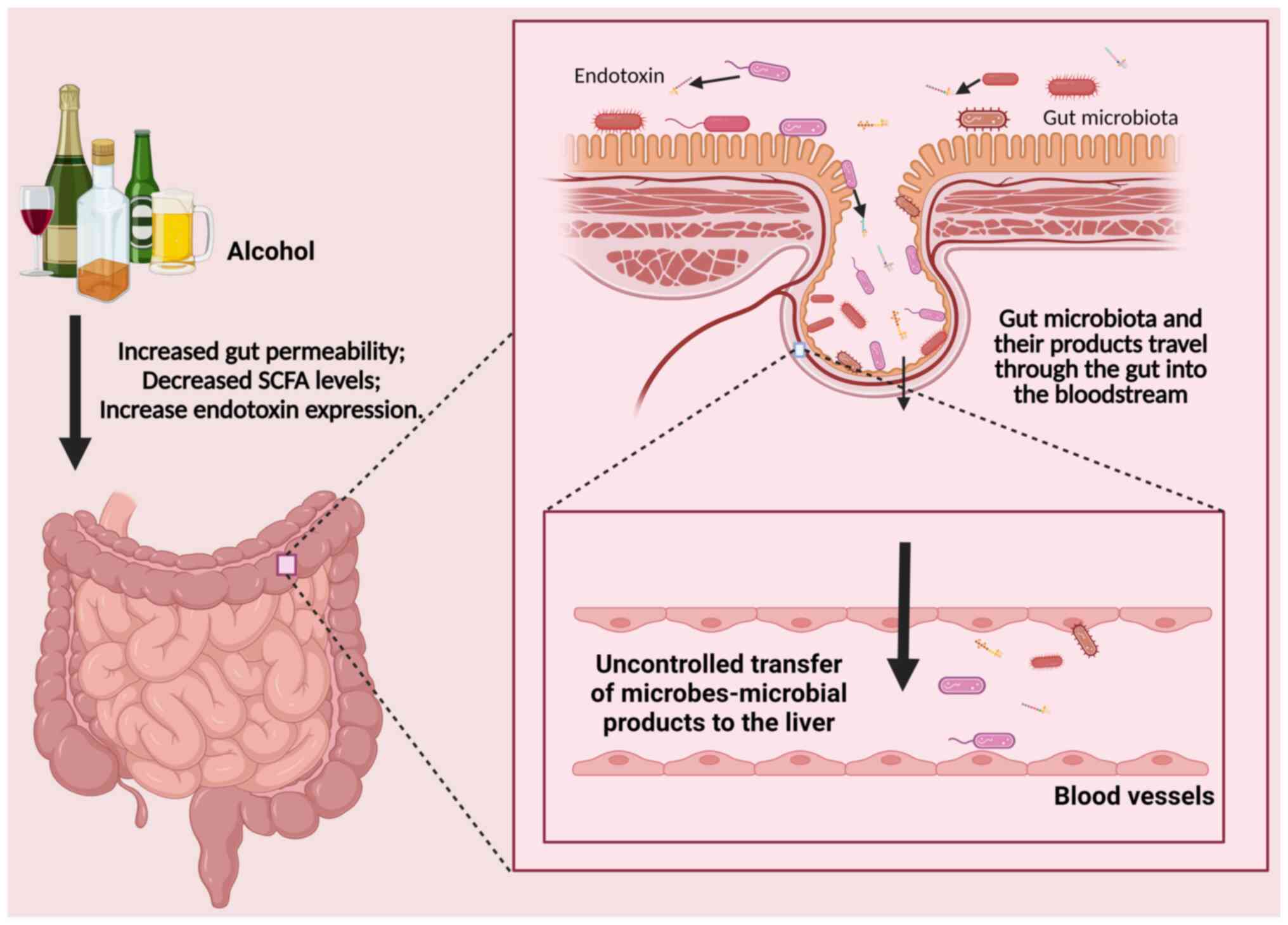
Chronic alcohol consumption directly or indirectly
alters composition of GM and leads to changes in human and animal
gut development (111). Chronic
ethanol feeding leads to a decrease in abundance of
Bacteroidetes and Firmicutes phyla in the GM and an
increase in the proportion of Gram-negative Proteobacteria
and Gram-positive Actinobacteria phyla (112). Alcohol can directly and
indirectly increase the permeability of the gut wall, as
dysfunctional GM and its metabolites enter the liver and activate
Kupffer cells by binding with TLR4 or TLR9, inducing Kupffer cells
to secrete pro-inflammatory cytokines. The GM of healthy
individuals produces SCFAs by breaking down dietary fiber and
resistant starch in the gastrointestinal tract. SCFA plays a role
in gastrointestinal physiology, immune function, and host
metabolism (113). Alcohol use is
associated with lower levels of gut SCFA, suggesting that GM may
serve a role in development and progression of liver disease
(114–116) (Fig.
2). SCFA in the mouse gut helps alleviate the progression of
ALD, which may be related to the regulatory effect of SCFA on the
liver immune microenvironment (114).
Furthermore, prevalence of NAFLD increases with
obesity and has replaced alcoholic hepatitis as the most common
type of chronic liver disease worldwide (117). Obesity is associated with
dysbiosis of the GM and GM modulation has potential for prevention
and treatment of NAFLD (118).
According to mouse studies and fecal transplantation trials, GM
serves a key role in the development of NAFLD (119,120). Ecological dysbiosis of GM and its
metabolites promotes the signal cascade reaction in the liver
during translocation (such as activation of TLR and NLRP3 signaling
pathway) promotes the secretion of cytokines such as TNF-α and
IL-1β, and leads to steatosis and inflammation in the liver of
susceptible mice (121).
Regardless of the amount of alcohol consumed, GM produce endogenous
ethanol, particularly when sugar-rich foods are consumed (122). Ethanol synthesized by GM
activates TLRs in the liver, promoting cytokine synthesis and
secretion and altering BA profile in obese humans and mice
(123–125).
In conclusion, as GM contributes to the development
of ALD and NAFLD, the GM may be a potential therapeutic target for
ALD and NAFLD. Using MIYAIRI 588, a butyrate-producing probiotic,
to treat NAFLD in rats significantly improves liver lipid
deposition, insulin resistance, serum endotoxin levels, and the
liver inflammation index (126).
Additionally, a meta-analysis indicated that probiotic therapy
significantly reduces blood ALT, AST, total cholesterol (T-chol),
high-density lipoprotein (HDL), and TNF-α levels in NAFLD patients,
while also improving insulin resistance (127). Kirpich et al (128) showed that short-term oral
supplementation with Bifidobacterium and Lactobacillus
plantarum 8PA3 can improve the dysbiosis of GM in patients with
ALD, and reduce serum levels of ALT, AST, gamma-glutamyl
transpeptidase (GGT), lactate dehydrogenase, and total
bilirubin.
Cirrhosis is histological development of
regenerative nodules surrounded by fibrous bands, which arises from
chronic liver injury and leads to portal hypertension and end-stage
liver disease (129). The
mechanism underlying cirrhosis has been extensively studied
(130–132). Cirrhosis is caused by abnormal
accumulation of the extracellular matrix (ECM) (mainly composed of
fibrous collagen, elastin, and matrix proteins) under chronic
(133). Excessive deposition of
ECM can lead to the replacement of the normal structure of liver
lobules with fibrous tissue, resulting in the destruction of the
normal liver architecture and the formation of fibrous septa and
nodules (133). It is
hypothesized that the first step of cirrhosis involves the
production of oxygen-free radicals and inflammatory substances that
damage liver cells and recruitment of Kupffer and inflammatory
cells. Additionally, elevated levels of oxygen free radicals and
inflammatory substances in liver tissue can cause hepatic stellate
cells (HSC) to differentiate into myofibroblasts, the primary
source of ECM (134). The most
common causes of cirrhosis are viral hepatitis, NASH and ALD
(135). According to a 2023
survey, cirrhosis is frequently attributed to alcohol use disorders
(~45% of cases), HCV (41%), and NAFLD (26%) (136). The 2019 Global Burden of Disease
Study estimated global deaths related to cirrhosis as follows:
395,000 from HCV-associated cirrhosis, 331,000 from HBV-related
cirrhosis, 372,000 from alcohol-related cirrhosis, and 134,000 from
NASH-related cirrhosis (137).
Cirrhosis is the 11th most common cause of mortality and the third
most common cause of mortality among people aged 45–64 years.
Together with liver cancer, cirrhosis accounts for 3.5% of all
deaths worldwide (138). To date,
there is no consensus regarding the treatment of cirrhosis, with
current methods limited to controlling symptoms and complications,
as well slowing the progression of cirrhosis. If the liver is
severely damaged, liver transplantation may be the only treatment
option (139,140).
The gut-liver axis serves a role in progression of
cirrhosis. The gut epithelium is a single-cell layer that serves as
a selective permeation barrier, facilitating the absorption of
nutrients, electrolytes, and water, while effectively defending
against intracavitary toxins, antigens, and GM (141). Previous studies have demonstrated
that gut permeability in patients with cirrhosis is increased,
which has been reported to be related to the degree of endotoxemia
(142,143). A prospective study demonstrated
higher serum endotoxin levels in patients with cirrhosis compared
to healthy individuals (144).
Subsequent studies have indicated that increased gut permeability
facilitates the translocation of intestinal-derived endotoxins
(LPS) into the bloodstream (145). In addition, the increase in gut
permeability promotes pathological translocation of GM and its
metabolites into liver, leading to the activation of numerous
inflammatory cytokine signaling pathways in the liver, driving
immune dysfunction associated with inflammation and cirrhosis
(146). Moreover, cytokines
secreted by immune cells can both reduce (i.e. TNFα and IFNγ) gut
barrier function and enhance (i.e. TGFβ and IL-10) gut barrier
function. The immune response may lead to ecological imbalance or
microbial changes in feces, intestinal mucosa, ascites, liver,
serum and saliva (147).
Ecological imbalance is related to gut barrier dysfunction as GM
and its products regulate the barrier function by affecting
epithelial inflammatory reaction and mucosal repair functions
(148). Further research has
shown that gut permeability can be directly regulated by GM through
the release of soluble peptides or toxins, which in turn regulate
the expression of gut tight junction proteins, including integrated
membrane proteins, junction complex proteins, and cytoskeletal
structural proteins (149).
Additionally, other metabolites of GM, such as SCFAs, BA
metabolites, conjugated FAs, indole derivatives, and polyamines,
can regulate the expression of gut tight junction proteins by
binding to receptors such as FXR, G protein-coupled bile acid
receptor (TGR), and aromatic hydrogen receptor (AHR) on the surface
of gut epithelial cells (150).
Small intestinal bacterial overgrowth (SIBO) is
frequently observed in patients with liver cirrhosis and is more
prevalent in those with advanced cirrhosis. Spontaneous bacterial
peritonitis (SBP) and hepatic encephalopathy (HE) are two frequent
complications of liver cirrhosis, both closely associated with
increased patient mortality (136). Increasing evidence suggests that
the imbalance of intestinal ecology in patients with liver
cirrhosis is closely related to disease progression (143,151). Chang et al (152) reported that 70% of patients with
SBP cirrhosis exhibit SIBO, whereas only 20% of patients with
non-SBP cirrhosis had SIBO. In patients with a history of SBP,
intestinal peristalsis is impaired and may contribute to
development of SIBO. Corradi et al demonstrated that the
control of SIBO with antibiotic treatment may mitigate the
progression of spontaneous bacterial peritonitis in cirrhotic rats
(153). HE is associated with the
GM (154). A randomized
controlled trial demonstrated that FMT improved the dysbiosis of GM
in patients with liver cirrhosis and delayed the progression of HE
(155). Bajaj et al
(156) suggested that the sigmoid
microbiota of patients with cirrhosis exhibits a low abundance of
autochthonous genera including Dorea, Subdoligranulum, and
Incertae Sedis other and a high abundance of potentially
pathogenic bacteria, including Enterococcus, Burkholderia,
Proteus and Clostridium. Other studies have reported
that the abundance of Veillonella, Megasphaera, Dialister,
Atopobium and Prevotella increased in the GM of patients
with cirrhosis (143,157). Changes in fungi in GM have also
been reported in alcohol-related cirrhosis (158). Compared with healthy individuals,
the diversity of gut fungi in patients with ALD is decreased,
demonstrated by the overgrowth of Candida (74). Candida hemolysin is a peptide toxin
secreted by Candida that can transfer from the gut to the
bloodstream and translocate to the liver, causing direct damage to
liver cells. β-glucan is a cell wall component of many symbiotic
fungi. Serum levels of β-glucan were significantly elevated in
alcohol-fed mice. Concentration of serum β-glucan is closely
related to gut integrity, inflammation, and the severity of liver
disease (159). β-glucan can
stimulate immune cells to produce a strong immune response, which
is an important cause of disease exacerbation (160). By analyzing the composition and
abundance of GM, the etiology of cirrhosis can be effectively
determined, guiding treatment (161). Controlling the abnormal growth of
GM can effectively manage the progression of cirrhosis. Oral
probiotic lactobacilli can regulate the decrease in
Enterococcal abundance, which is associated with reducing
the severity of liver injury (162).
Primary liver cancer is the sixth most common
malignancy and the fourth highest cause of cancer-associated
mortality worldwide. The most common type of primary liver cancer
is HCC, according to the World Health Organization (163). A number of risk factors are
associated with liver cancer, notably HBV, HCV, alcohol abuse and
aflatoxins in the diet (164).
Based on studies in human and animal models, GM may
contribute to development of HCC (91,101,120,143,157,165–190)(Table
I). Patients with HCC and mice treated with diethylnitrosamine
exhibit dysbiosis of the GM. The reduction of Lactobacillus,
Bifidobacterium and Enterococcus in the gut of rats with
liver cancer, as well as proliferation of Escherichia coli,
suggests an imbalance in composition of GM in liver cancer
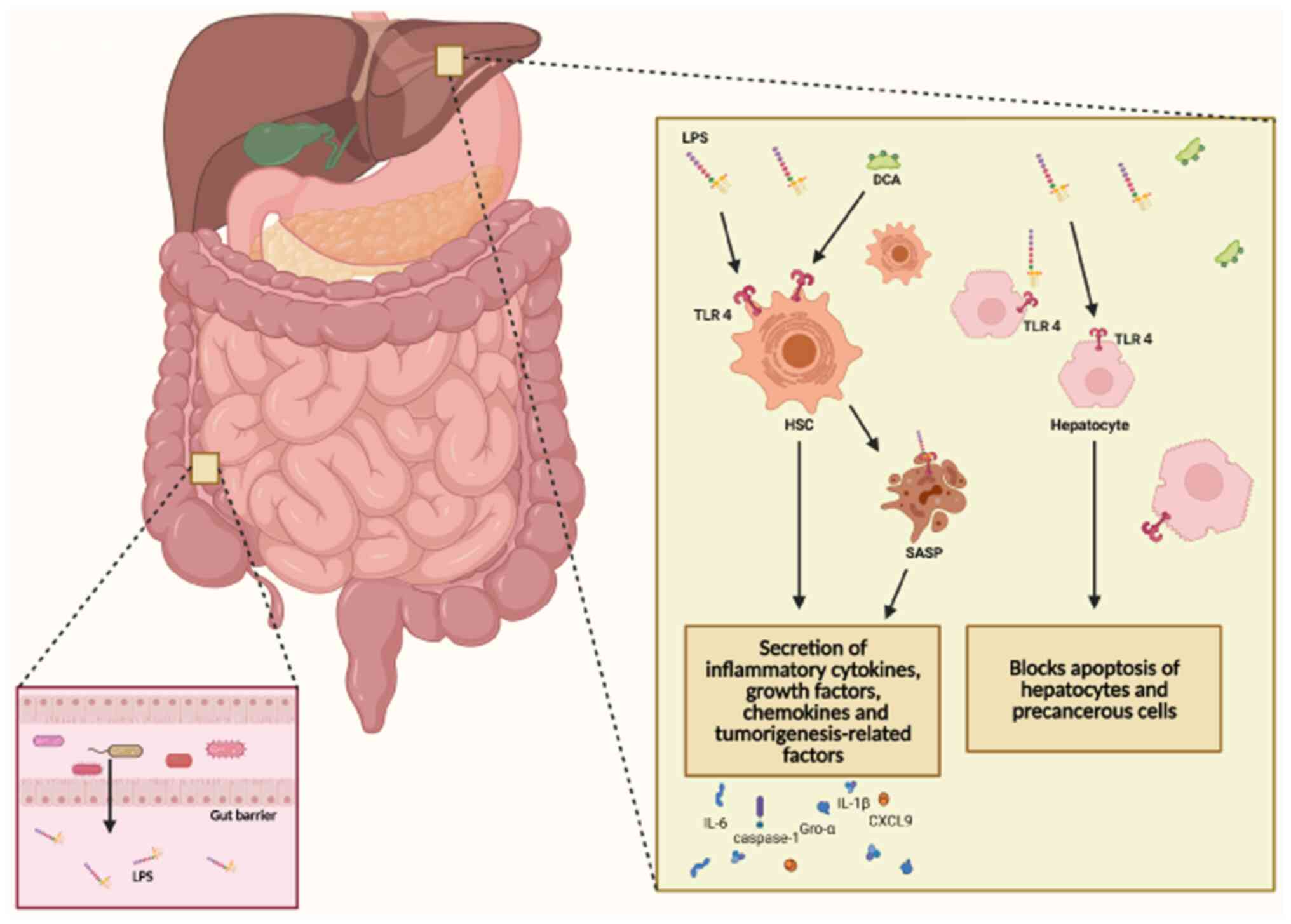
(191). Lipopolysaccharide (LPS),
a metabolite of the GM, binds to TLR4 on HSCs, triggering the
activation of HSCs, which leads to the development of liver
fibrosis and cirrhosis (Fig. 3).
Dapito et al (192)
reported that activation of TLR4 accelerates HCC progression by
promoting cell proliferation and inhibiting apoptosis. Furthermore,
Mou et al (193) reported
the role of the LPS/TLR4 signaling pathway in regulating liver
fibrosis progression in rats. Neomycin is an antibiotic that
inhibit the overgrowth of GM. Yu et al found that the
accumulation of LPS and the expression of TLR4 were reduced in the
liver of liver cancer mice treated with neomycin (194). Further studies using antibiotics
in mouse models are needed to understand the disease progression in
the absence of GM. Dapito et al (192) demonstrated that removal of the GM
by antibiotics protected mice from liver fibrosis and HCC. This may
be due to antibiotics reducing the quantity of GM, decreasing the
secretion of toxic metabolites such as LPS, thereby lessening the
degree of liver damage.
FMT is an increasingly popular method of altering
GM composition during disease. FMT involves transplantation of GM
obtained from the stool of a healthy donor into the
gastrointestinal tract of a patient (197–199). In most cases, this therapy is
used to treat gastrointestinal diseases caused by activity of
pathogenic or conditionally pathogenic microorganisms (200). Recent studies have reported that
this approach has potential for clinical application in treatment
of liver disease (201,202).
FMT can be administered through three channels:
Oral, through the upper gastric portion; nasal, via nasogastric
tubes; and rectal through colonoscopy or enema (203). Fecal suspension perfusion in the
rectum using colonoscopy is considered to be the best method for
FMT (204). By contrast, FMT
using the upper gastric route, including nasogastric tube,
nasogastric or upper endoscopy exposes the entire gastrointestinal
tract to donor stool and can lead to pulmonary or gastrointestinal
complications due to the presence of large numbers of pathogenic
bacteria in the upper GI and respiratory tracts (205). The use of fecal microbiota in
oral capsules has also been reported (206). In a randomized controlled study
of 22 obese patients (206), FMT
capsules exhibited no significant side effects and significantly
improved composition of the GM of patients and decreased metabolic
levels of taurocholic, which cause damage to the liver (207,208) and BAs.
Phages are viruses that specifically infect
bacteria. In the early days of phage therapy application,
infectious phage agents were commonly used to treat diseases caused
by bacterial infections including Staphylococcus, Streptococcus,
Vibrio, Klebsiella, Enterobacter, Shigella, Escherichia,
Pseudomonas and Providencia, which have the advantage
over antibiotics of targeting specific bacterial species or strains
while self-replicating and spreading to infect other target
bacterial cells (209). Notably,
phage therapy has the ability to edit the GM. In two randomized
placebo-controlled trials (study nos. NCT03269617 and NCT04511221),
phages improved the GM profile by targeting specific bacterial
genera, modulated the overall metabolism and reduced the incidence
and severity of gastrointestinal discomfort (210–212).
Specific GM serve a role in the pathogenesis of a
number of types of liver diseases; therefore, phage therapy capable
of eliminating specific GM has potential value in treatment of
liver disease. In humanized mice colonized with bacteria from the
feces of patients with alcoholic hepatitis, phage therapy targeting
lysogenic Enterococcus faecalis decreases mortality as well
as ethanol-induced liver injury, steatosis, inflammation and
fibrosis (213). However, to the
best of our knowledge, there are no clinical trials to validate the
safety and efficacy of phage therapy in treatment of human liver
disease. Despite this, phage therapy has been suggested as
potential novel therapy for the treatment of liver disease
(214).
Bacteria can acquire the ability to transcribe and
translate various genes through gene editing technology. Through
oral administration and other delivery methods, these genetically
modified bacteria can reach the human intestine and colonize it.
These genetically engineered bacteria can express specific enzymes,
thereby promoting the conversion of toxic metabolites in the
intestine to non-toxic products (215). Hyperammonemia is associated with
liver disease, and the intestine is the primary source of systemic
ammonia (NH3) (216). Kurtz et
al (217) developed an
engineered bacterium called SYNB1020 that can colonize the
intestine via oral administration. This bacterium can convert NH3
in the intestine into l-arginine, thereby reducing blood ammonia
levels. In a mouse model of hyperammonemia, SYNB1020
treatment increases survival rate. Moreover, SYNB1020 has
good tolerability in a phase I clinical trial of hyperammonemia
disease (217). Thus,
SYNB1020 warrants further clinical development.
As the most proximal gut segment, the duodenal
mucosa has a unique chemosensory capacity to detect luminal
contents and rapidly release bioactive mediators and hormones with
local and systemic effects. These bioactive compounds include GM
and GM metabolites, which are recognized by metabolite-sensing
receptors (220). Intestinal
luminal chemosensing involves the regulation of gut function and
the systemic regulation of metabolism, energy balance, and food
intake (221). Pharmacological
modulation targeting the duodenum can maintain metabolic
homeostasis in obesity, diabetes and NAFLD (222,223). Duodenal mucosal surface
reconstruction (DMR) is a novel surgical procedure that, under
endoscopic guidance, involves the introduction of a catheter with a
balloon into the duodenum. The balloon is then expanded to segment
the duodenum, and the duodenal mucosa is separated by injecting
saline into the submucosal layer, followed by mucosal ablation
using circulating hot and cold water. The ablation range covers all
duodenal mucosa from 1 cm distal to the main papilla to the
ligament of Treitz. After mucosal regeneration, the formation of
new gut cells and the re-establishment of a healthy neuroendocrine
axis can restore gut function and provide a healthy gut environment
(224–226). In a randomized controlled
clinical trial (227), DMR
reported safety and efficacy in glycemic control and liver fat
content in type 2 diabetes. However, in another clinical trial, DMR
did not improve NASH (228).
The gut-liver axis underscores the connection
between gut health and liver function. Numerous types of liver
diseases, including NAFLD, ALD, HE and HCC, are influenced by
changes in GM. This axis is a target for clinical applications,
aiding in diagnosis, prognosis and the development of treatment. GM
analysis offers insights into the mechanisms and phenotypes of
liver disease. Modulating BA signaling and fecal transplantation or
probiotics from human sources show potential as treatments.
However, safety evaluation data for these methods are still
insufficient. Further research is needed to ensure their efficacy
and safety, bridging the gap between animal models and clinical
practice to prevent progression of early liver disease.
In summary, the correlation between the gut and
liver serves as a pathway for exploring the clinical treatment of
liver disease. The function of a complete gut barrier, GM, and
their associated metabolites communicates complex host-microbial
interactions that can maintain health or promote disease. However,
more research is needed to elucidate the changes in gut microbiota
abundance in patients with different types of liver diseases, as
well as the specific signaling pathways involved, and how to
regulate GM in a way that minimizes side effects, to develop better
clinical treatments for liver diseases. However, the present review
has limitations. The impact of environmental exposure and lifestyle
factors on the occurrence and development of liver disease was not
discussed. Finally, GM could serve as a diagnostic tool and
therapeutic target in patients with liver disease.
Not applicable.
The present study was supported by the National University
Student Innovation Training Program of the Ministry of Education of
the People's Republic of China (grant no. 202110366008) and and
Basic and Clinical Cooperative Research Promotion Program of Anhui
Medical University (grant no. 2023×kiT024).
Not applicable.
JW and SC conceived the study and drafted the
manuscript. XW constructed the figures and table and revised the
manuscript. EZ and BC revised the manuscript. All authors have read
and approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Adak A and Khan MR: An insight into gut
microbiota and its functionalities. Cell Mol Life Sci. 76:473–493.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Woodruff AW, Salih SY, de Savigny D, Baya
EI, Shah AI and Dafalla AA: Toxocariasis in the Sudan. Ann Trop Med
Parasitol. 75:559–561. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan Y and Pedersen O: Gut microbiota in
human metabolic health and disease. Nat Rev Microbiol. 19:55–71.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wiest R, Albillos A, Trauner M, Bajaj JS
and Jalan R: Targeting the gut-liver axis in liver disease. J
Hepatol. 67:1084–1103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Milosevic I, Vujovic A, Barac A, Djelic M,
Korac M, Radovanovic Spurnic A, Gmizic I, Stevanovic O, Djordjevic
V, Lekic N, et al: Gut-Liver axis, gut microbiota, and its
modulation in the management of liver diseases: A review of the
literature. Int J Mol Sci. 20:3952019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim ER, Park JS, Kim JH, Oh JY, Oh IJ,
Choi DH, Lee YS, Park IS, Kim S, Lee DH, et al: A GLP-1/GLP-2
receptor dual agonist to treat NASH: Targeting the gut-liver axis
and microbiome. Hepatology. 75:1523–1538. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song Q, Zhang X, Liu W, Wei H, Liang W,
Zhou Y, Ding Y, Ji F, Ho-Kwan Cheung A, Wong N and Yu J:
Bifidobacterium pseudolongum-generated acetate suppresses
non-alcoholic fatty liver disease-associated hepatocellular
carcinoma. J Hepatol. 79:1352–1365. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bertocchi A, Carloni S, Ravenda PS,
Bertalot G, Spadoni I, Lo Cascio A, Gandini S, Lizier M, Braga D,
Asnicar F, et al: Gut vascular barrier impairment leads to
intestinal bacteria dissemination and colorectal cancer metastasis
to liver. Cancer Cell. 39:708–724.e11. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu X, Chen F, Jia L, Long A, Peng Y, Li X,
Huang J, Wei X, Fang X, Gao Z, et al: A gut-derived hormone
regulates cholesterol metabolism. Cell. 187:1685–700.e18. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuang J, Wang J, Li Y, Li M, Zhao M, Ge K,
Zheng D, Cheung KCP, Liao B, Wang S, et al: Hyodeoxycholic acid
alleviates non-alcoholic fatty liver disease through modulating the
gut-liver axis. Cell Metab. 35:1752–1766.e8. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sheng Z, Xu J, Li F, Yuan Y, Peng X, Chen
S, Zhou R and Huang W: The RING-domain E3 ubiquitin ligase RNF146
promotes cardiac hypertrophy by suppressing the LKB1/AMPK signaling
pathway. Exp Cell Res. 410:1129542022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goto J, Otaki Y, Watanabe T, Kobayashi Y,
Aono T, Watanabe K, Wanezaki M, Kutsuzawa D, Kato S, Tamura H, et
al: HECT (Homologous to the E6-AP Carboxyl Terminus)-Type ubiquitin
E3 ligase ITCH attenuates cardiac hypertrophy by suppressing the
Wnt/β-catenin signaling pathway. Hypertension. 76:1868–1878. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Broquetas T and Carrion JA: Past, present,
and future of long-term treatment for hepatitis B virus. World J
Gastroenterol. 29:3964–3983. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Frenette C, Mendiratta-Lala M, Salgia R,
Wong RJ, Sauer BG and Pillai A: ACG clinical guideline: Focal liver
lesions. Am J Gastroenterol. 119:1235–1271. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
European Association for the Study of the
Liver (EASL); European Association for the Study of Diabetes
(EASD); European Association for the Study of Obesity (EASO) and
the European Association for the Study of the Liver (EASL), .
EASL-EASD-EASO Clinical Practice Guidelines on the management of
metabolic dysfunction-associated steatotic liver disease (MASLD). J
Hepatol. 81:492–542. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Forrest EH, Atkinson SR, Richardson P,
Masson S, Ryder S, Thursz MR and Allison M: ACG clinical guideline
for alcoholic liver disease: The MELD threshold for corticosteroid
treatment has yet to be established. Am J Gastroenterol.
114:175–176. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai JJ, Zhang YF and Zhang ZH: Global
trends and hotspots of treatment for nonalcoholic fatty liver
disease: A bibliometric and visualization analysis (2010–2023).
World J Gastroenterol. 29:5339–5360. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suddle A, Reeves H, Hubner R, Marshall A,
Rowe I, Tiniakos D, Hubscher S, Callaway M, Sharma D, See TC, et
al: British Society of Gastroenterology guidelines for the
management of hepatocellular carcinoma in adults. Gut.
73:1235–1268. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Q, Li B and Li J: DLL4-Notch
signalling in acute-on-chronic liver failure: State of the art and
perspectives. Life Sci. 317:1214382023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Conde de la Rosa L, Garcia-Ruiz C, Vallejo
C, Baulies A, Nuñez S, Monte MJ, Marin JJG, Baila-Rueda L, Cenarro
A, Civeira F, et al: STARD1 promotes NASH-driven HCC by sustaining
the generation of bile acids through the alternative mitochondrial
pathway. J Hepatol. 74:1429–1441. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bernsmeier C, Singanayagam A, Patel VC,
Wendon J and Antoniades CG: Immunotherapy in the treatment and
prevention of infection in acute-on-chronic liver failure.
Immunotherapy. 7:641–654. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wulf J, Guckenberger M, Haedinger U,
Oppitz U, Mueller G, Baier K and Flentje M: Stereotactic
radiotherapy of primary liver cancer and hepatic metastases. Acta
Oncol. 45:838–847. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Devarbhavi H, Asrani SK, Arab JP, Nartey
YA, Pose E and Kamath PS: Global burden of liver disease: 2023
update. J Hepatol. 79:516–537. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Taha G, Ezra L and Abu-Freha N: Hepatitis
C elimination: Opportunities and challenges in 2023. Viruses.
15:14132023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsu YC, Huang DQ and Nguyen MH: Global
burden of hepatitis B virus: Current status, missed opportunities
and a call for action. Nat Rev Gastroenterol Hepatol. 20:524–537.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hernandez-Evole H, Jimenez-Esquivel N,
Pose E and Bataller R: Alcohol-associated liver disease:
Epidemiology and management. Ann Hepatol. 29:1011622024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Julien J, Ayer T, Bethea ED, Tapper EB and
Chhatwal J: Projected prevalence and mortality associated with
alcohol-related liver disease in the USA, 2019–40: A modelling
study. Lancet Public Health. 5:e316–e23. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McGlynn KA, Petrick JL and El-Serag HB:
Epidemiology of hepatocellular carcinoma. Hepatology. 73 (Suppl
1):S4–S13. 2021. View Article : Google Scholar
|
|
29
|
Collins SL, Stine JG, Bisanz JE, Okafor CD
and Patterson AD: Bile acids and the gut microbiota: Metabolic
interactions and impacts on disease. Nat Rev Microbiol. 21:236–247.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taranto MP, Perez-Martinez G and Font de
Valdez G: Effect of bile acid on the cell membrane functionality of
lactic acid bacteria for oral administration. Res Microbiol.
157:720–725. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han B, Lv X, Liu G, Li S, Fan J, Chen L,
Huang Z, Lin G, Xu X, Huang Z, et al: Gut microbiota-related bile
acid metabolism-FXR/TGR5 axis impacts the response to
anti-α4β7-integrin therapy in humanized mice with colitis. Gut
Microbes. 15:22321432023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu HM, Chang ZY, Yang CW, Chang HH and
Lee TY: Farnesoid X receptor agonist GW4064 protects
lipopolysaccharide-induced intestinal epithelial barrier function
and colorectal tumorigenesis signaling through the
αKlotho/βKlotho/FGFs pathways in mice. Int J Mol Sci. 24:169322023.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ploton M, Mazuy C, Gheeraert C, Dubois V,
Berthier A, Dubois-Chevalier J, Maréchal X, Bantubungi K, Diemer H,
Cianférani S, et al: The nuclear bile acid receptor FXR is a PKA-
and FOXA2-sensitive activator of fasting hepatic gluconeogenesis. J
Hepatol. 69:1099–1109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cao Y, Xiao Y, Zhou K, Yan J, Wang P, Yan
W and Cai W: FXR agonist GW4064 improves liver and intestinal
pathology and alters bile acid metabolism in rats undergoing small
intestinal resection. Am J Physiol Gastrointest Liver Physiol.
317:G108–G115. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yan Y, Sha Y, Huang X, Yuan W, Wu F, Hong
J, Fang S, Huang B, Hu C, Wang B and Zhang X: Roux-en-Y gastric
bypass improves metabolic conditions in association with increased
serum bile acids level and hepatic Farnesoid X receptor expression
in a T2DM rat model. Obes Surg. 29:2912–2922. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Watanabe M, Houten SM, Mataki C,
Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O,
Kodama T, et al: Bile acids induce energy expenditure by promoting
intracellular thyroid hormone activation. Nature. 439:484–489.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zietak M and Kozak LP: Bile acids induce
uncoupling protein 1-dependent thermogenesis and stimulate energy
expenditure at thermoneutrality in mice. Am J Physiol Endocrinol
Metab. 310:E346–E354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Van Treuren W and Dodd D: Microbial
contribution to the human metabolome: Implications for health and
disease. Annu Rev Pathol. 15:345–369. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cornell RP: Restriction of gut-derived
endotoxin impairs DNA synthesis for liver regeneration. Am J
Physiol. 249:R563–R569. 1985.PubMed/NCBI
|
|
40
|
Nolan JP: The role of intestinal endotoxin
in liver injury: A long and evolving history. Hepatology.
52:1829–1835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu H, Xi Q, Tan S, Qu Y, Meng Q, Zhang Y,
Cheng Y and Wu G: The metabolite butyrate produced by gut
microbiota inhibits cachexia-associated skeletal muscle atrophy by
regulating intestinal barrier function and macrophage polarization.
Int Immunopharmacol. 124:1110012023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tang G, Du Y, Guan H, Jia J, Zhu N, Shi Y,
Rong S and Yuan W: Butyrate ameliorates skeletal muscle atrophy in
diabetic nephropathy by enhancing gut barrier function and
FFA2-mediated PI3K/Akt/mTOR signals. Br J Pharmacol. 179:159–178.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Meena AS, Shukla PK, Bell B, Giorgianni F,
Caires R, Fernández-Peña C, Beranova S, Aihara E, Montrose MH,
Chaib M, et al: TRPV6 channel mediates alcohol-induced gut barrier
dysfunction and systemic response. Cell Rep. 39:1109372022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dominguez-Bello MG, Godoy-Vitorino F,
Knight R and Blaser MJ: Role of the microbiome in human
development. Gut. 68:1108–1114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Han YH, Onufer EJ, Huang LH, Sprung RW,
Davidson WS, Czepielewski RS, Wohltmann M, Sorci-Thomas MG, Warner
BW and Randolph GJ: Enterically derived high-density lipoprotein
restrains liver injury through the portal vein. Science.
373:eabe67292021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gomaa EZ: Human gut microbiota/microbiome
in health and diseases: A review. Antonie Van Leeuwenhoek.
113:2019–2040. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Robles-Alonso V and Guarner F: Progress in
the knowledge of the intestinal human microbiota. Nutr Hosp.
28:553–557. 2013.(In Spanish). PubMed/NCBI
|
|
48
|
Charlet R, Bortolus C, Barbet M, Sendid B
and Jawhara S: A decrease in anaerobic bacteria promotes Candida
glabrata overgrowth while β-glucan treatment restores the gut
microbiota and attenuates colitis. Gut Pathog. 10:502018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Charlet R, Pruvost Y, Tumba G, Istel F,
Poulain D, Kuchler K, Sendid B and Jawhara S: Remodeling of the
Candida glabrata cell wall in the gastrointestinal tract affects
the gut microbiota and the immune response. Sci Rep. 8:33162018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bertin Y, Girardeau JP, Chaucheyras-Durand
F, Lyan B, Pujos-Guillot E, Harel J and Martin C:
Enterohaemorrhagic Escherichia coli gains a competitive advantage
by using ethanolamine as a nitrogen source in the bovine intestinal
content. Environ Microbiol. 13:365–377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ma HD, Zhao ZB, Ma WT, Liu QZ, Gao CY, Li
L, Wang J, Tsuneyama K, Liu B, Zhang W, et al: Gut microbiota
translocation promotes autoimmune cholangitis. J Autoimmun.
95:47–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shao T, Zhao C, Li F, Gu Z, Liu L, Zhang
L, Wang Y, He L, Liu Y, Liu Q, et al: Intestinal HIF-1α deletion
exacerbates alcoholic liver disease by inducing intestinal
dysbiosis and barrier dysfunction. J Hepatol. 69:886–895. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Giuffre M, Campigotto M, Campisciano G,
Comar M and Croce LS: A story of liver and gut microbes: How does
the intestinal flora affect liver disease? A review of the
literature. Am J Physiol Gastrointest Liver Physiol. 318:G889–G906.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bellot P, Frances R and Such J:
Pathological bacterial translocation in cirrhosis: Pathophysiology,
diagnosis and clinical implications. Liver Int. 33:31–39. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Giorgio V, Miele L, Principessa L,
Ferretti F, Villa MP, Negro V, Grieco A, Alisi A and Nobili V:
Intestinal permeability is increased in children with non-alcoholic
fatty liver disease, and correlates with liver disease severity.
Dig Liver Dis. 46:556–560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Miele L, Valenza V, La Torre G, Montalto
M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML,
Perotti G, et al: Increased intestinal permeability and tight
junction alterations in nonalcoholic fatty liver disease.
Hepatology. 49:1877–1887. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rao R: Endotoxemia and gut barrier
dysfunction in alcoholic liver disease. Hepatology. 50:638–644.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sewell GW and Kaser A: Interleukin-23 in
the pathogenesis of inflammatory bowel disease and implications for
therapeutic intervention. J Crohns Colitis. 16 (Suppl 2):ii3–ii19.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sokol H, Leducq V, Aschard H, Pham HP,
Jegou S, Landman C, Cohen D, Liguori G, Bourrier A, Nion-Larmurier
I, et al: Fungal microbiota dysbiosis in IBD. Gut. 66:1039–1048.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ng SC, Benjamin JL, McCarthy NE, Hedin CR,
Koutsoumpas A, Plamondon S, Price CL, Hart AL, Kamm MA, Forbes A,
et al: Relationship between human intestinal dendritic cells, gut
microbiota, and disease activity in Crohn's disease. Inflamm Bowel
Dis. 17:2027–2037. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Coccia M, Harrison OJ, Schiering C,
Asquith MJ, Becher B, Powrie F and Maloy KJ: IL-1β mediates chronic
intestinal inflammation by promoting the accumulation of IL-17A
secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med.
209:1595–1609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ma TY, Boivin MA, Ye D, Pedram A and Said
HM: Mechanism of TNF-{alpha} modulation of Caco-2 intestinal
epithelial tight junction barrier: Role of myosin light-chain
kinase protein expression. Am J Physiol Gastrointest Liver Physiol.
288:G422–G430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
He WQ, Wang J, Sheng JY, Zha JM, Graham WV
and Turner JR: Contributions of myosin light chain kinase to
regulation of epithelial paracellular permeability and mucosal
homeostasis. Int J Mol Sci. 21:9932020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chotikatum S, Naim HY and El-Najjar N:
Inflammation induced ER stress affects absorptive intestinal
epithelial cells function and integrity. Int Immunopharmacol.
55:336–344. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kinoshita N, Hiroi T, Ohta N, Fukuyama S,
Park EJ and Kiyono H: Autocrine IL-15 mediates intestinal
epithelial cell death via the activation of neighboring
intraepithelial NK cells. J Immunol. 169:6187–6192. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rohr M, Narasimhulu CA, Keewan E, Hamid S
and Parthasarathy S: The dietary peroxidized lipid, 13-HPODE,
promotes intestinal inflammation by mediating granzyme B secretion
from natural killer cells. Food Funct. 11:9526–9534. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yasuda K, Nakanishi K and Tsutsui H:
Interleukin-18 in health and disease. Int J Mol Sci. 19:6492019.
View Article : Google Scholar
|
|
68
|
Woznicki JA, Saini N, Flood P, Rajaram S,
Lee CM, Stamou P, Skowyra A, Bustamante-Garrido M, Regazzoni K,
Crawford N, et al: TNF-α synergises with IFN-γ to induce
caspase-8-JAK1/2-STAT1-dependent death of intestinal epithelial
cells. Cell Death Dis. 12:8642021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chang JT: Pathophysiology of inflammatory
bowel diseases. N Engl J Med. 383:2652–2664. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Romagnani S: Lymphokine production by
human T cells in disease states. Annu Rev Immunol. 12:227–257.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Fort MM, Cheung J, Yen D, Li J, Zurawski
SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, et al: IL-25
induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in
vivo. Immunity. 15:985–995. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lin Y, Li B, Yang X, Liu T, Shi T, Deng B,
Zhang Y, Jia L, Jiang Z and He R: Non-hematopoietic STAT6 induces
epithelial tight junction dysfunction and promotes intestinal
inflammation and tumorigenesis. Mucosal Immunol. 12:1304–1315.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ceponis PJ, Botelho F, Richards CD and
McKay DM: Interleukins 4 and 13 increase intestinal epithelial
permeability by a phosphatidylinositol 3-kinase pathway. Lack of
evidence for STAT 6 involvement. J Biol Chem. 275:29132–29137.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
He L, Liu T, Shi Y, Tian F, Hu H, Deb DK,
Bissonnette M and Li YC: Gut epithelial Vitamin D receptor
regulates Microbiota-dependent mucosal inflammation by suppressing
intestinal epithelial cell apoptosis. Endocrinology. 159:967–979.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lee SH, Kwon JE and Cho ML: Immunological
pathogenesis of inflammatory bowel disease. Intest Res. 16:26–42.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang X, Ni J, You Y, Feng G, Zhang S, Bao
W, Hou H, Li H, Liu L, Zheng M, et al: SNX10-mediated LPS sensing
causes intestinal barrier dysfunction via a caspase-5-dependent
signaling cascade. EMBO J. 40:e1080802021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li Q, Rempel JD, Yang J and Minuk GY: The
effects of Pathogen-associated molecular patterns on peripheral
blood monocytes in patients with Non-alcoholic fatty liver disease.
J Clin Exp Hepatol. 12:808–817. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Nakamoto N and Kanai T: Role of toll-like
receptors in immune activation and tolerance in the liver. Front
Immunol. 5:2212014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Szabo G, Dolganiuc A and Mandrekar P:
Pattern recognition receptors: A contemporary view on liver
diseases. Hepatology. 44:287–298. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kesar V and Odin JA: Toll-like receptors
and liver disease. Liver Int. 34:184–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hardin PE: From biological clock to
biological rhythms. Genome Biol. 1:REVIEWS10232000. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jouffe C, Weger BD, Martin E, Atger F,
Weger M, Gobet C, Ramnath D, Charpagne A, Morin-Rivron D, Powell
EE, et al: Disruption of the circadian clock component BMAL1
elicits an endocrine adaption impacting on insulin sensitivity and
liver disease. Proc Natl Acad Sci USA. 119:e22000831192022.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kinouchi K and Sassone-Corsi P: Metabolic
rivalry: Circadian homeostasis and tumorigenesis. Nat Rev Cancer.
20:645–661. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Nassan M and Videnovic A: Circadian
rhythms in neurodegenerative disorders. Nat Rev Neurol. 18:7–24.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Song S, Tien CL, Cui H, Basil P, Zhu N,
Gong Y, Li W, Li H, Fan Q, Min Choi J, et al: Myocardial
Rev-erb-mediated diurnal metabolic rhythm and obesity paradox.
Circulation. 145:448–464. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Choi H, Rao MC and Chang EB: Gut
microbiota as a transducer of dietary cues to regulate host
circadian rhythms and metabolism. Nat Rev Gastroenterol Hepatol.
18:679–689. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Heddes M, Altaha B, Niu Y, Reitmeier S,
Kleigrewe K, Haller D and Kiessling S: The intestinal clock drives
the microbiome to maintain gastrointestinal homeostasis. Nat
Commun. 13:60682022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Thaiss CA, Zeevi D, Levy M,
Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN,
Korem T, Zmora N, et al: Transkingdom control of microbiota diurnal
oscillations promotes metabolic homeostasis. Cell. 159:514–529.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Backhed F, Ding H, Wang T, Hooper LV, Koh
GY, Nagy A, Semenkovich CF and Gordon JI: The gut microbiota as an
environmental factor that regulates fat storage. Proc Natl Acad Sci
USA. 101:15718–15723. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Rabot S, Membrez M, Bruneau A, Gerard P,
Harach T, Moser M, Raymond F, Mansourian R and Chou CJ: Germ-free
C57BL/6J mice are resistant to high-fat-diet-induced insulin
resistance and have altered cholesterol metabolism. FASEB J.
24:4948–4959. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang X, Coker OO, Chu ES, Fu K, Lau HCH,
Wang YX, Chan AWH, Wei H, Yang X, Sung JJY and Yu J: Dietary
cholesterol drives fatty liver-associated liver cancer by
modulating gut microbiota and metabolites. Gut. 70:761–774. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang B, Kong Q, Li X, Zhao J, Zhang H,
Chen W and Wang G: A High-fat diet increases gut microbiota
biodiversity and energy expenditure due to nutrient difference.
Nutrients. 12:31972020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini
V, Mardis ER and Gordon JI: An Obesity-associated gut microbiome
with increased capacity for energy harvest. Nature. 444:1027–1031.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Riva A, Borgo F, Lassandro C, Verduci E,
Morace G, Borghi E and Berry D: Pediatric obesity is associated
with an altered gut microbiota and discordant shifts in Firmicutes
populations. Environ Microbiol. 19:95–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Palmnas-Bedard MSA, Costabile G, Vetrani
C, Aberg S, Hjalmarsson Y, Dicksved J, Riccardi G and Landberg R:
The human gut microbiota and glucose metabolism: A scoping review
of key bacteria and the potential role of SCFAs. Am J Clin Nutr.
116:862–874. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Dunn R, Wetten A, McPherson S and Donnelly
MC: Viral hepatitis in 2021: The challenges remaining and how we
should tackle them. World J Gastroenterol. 28:76–95. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhao X and Guo S: Methods for visualizing
intracellular organelles. J Vis Exp. Mar 3–2023.doi:
10.3791/64966.
|
|
98
|
Zhao W, Ma L, Cai C and Gong X: Caffeine
Inhibits NLRP3 inflammasome activation by suppressing MAPK/NF-κB
and A2aR signaling in LPS-induced THP-1 macrophages. Int J Biol
Sci. 15:1571–1581. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chou HH, Chien WH, Wu LL, Cheng CH, Chung
CH, Horng JH, Ni YH, Tseng HT, Wu D, Lu X, et al: Age-related
immune clearance of hepatitis B virus infection requires the
establishment of gut microbiota. Proc Natl Acad Sci USA.
112:2175–2180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chauhan A, Kumar R, Sharma S, Mahanta M,
Vayuuru SK, Nayak B and Kumar S: Shalimar: Fecal microbiota
transplantation in Hepatitis B e antigen-positive chronic Hepatitis
B patients: A pilot study. Dig Dis Sci. 66:873–880. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Huang H, Ren Z, Gao X, Hu X, Zhou Y, Jiang
J, Lu H, Yin S, Ji J, Zhou L and Zheng S: Integrated analysis of
microbiome and host transcriptome reveals correlations between gut
microbiota and clinical outcomes in HBV-related hepatocellular
carcinoma. Genome Med. 12:1022020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Preveden T, Scarpellini E, Milic N, Luzza
F and Abenavoli L: Gut microbiota changes and chronic hepatitis C
virus infection. Expert Rev Gastroenterol Hepatol. 11:813–819.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Shen Y, Wu SD, Chen Y, Li XY, Zhu Q,
Nakayama K, Zhang WQ, Weng CZ, Zhang J, Wang HK, et al: Alterations
in gut microbiome and metabolomics in chronic hepatitis B
infection-associated liver disease and their impact on peripheral
immune response. Gut Microbes. 15:21550182023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wei X, Yan X, Zou D, Yang Z, Wang X, Liu
W, Wang S, Li X, Han J, Huang L and Yuan J: Abnormal fecal
microbiota community and functions in patients with hepatitis B
liver cirrhosis as revealed by a metagenomic approach. BMC
Gastroenterol. 13:1752013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Bajaj JS, Liu EJ, Kheradman R, Fagan A,
Heuman DM, White M, Gavis EA, Hylemon P, Sikaroodi M and Gillevet
PM: Fungal dysbiosis in cirrhosis. Gut. 67:1146–1154. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Aly AM, Adel A, El-Gendy AO, Essam TM and
Aziz RK: Gut microbiome alterations in patients with stage 4
hepatitis C. Gut Pathog. 8:422016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Luther J, Khan S, Gala MK, Kedrin D,
Sridharan G, Goodman RP, Garber JJ, Masia R, Diagacomo E, Adams D,
et al: Hepatic gap junctions amplify alcohol liver injury by
propagating cGAS-mediated IRF3 activation. Proc Natl Acad Sci USA.
117:11667–11673. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Jophlin LL, Singal AK, Bataller R, Wong
RJ, Sauer BG, Terrault NA and Shah VH: ACG clinical guideline:
Alcohol-associated liver disease. Am J Gastroenterol. 119:30–54.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Huang DQ, Mathurin P, Cortez-Pinto H and
Loomba R: Global epidemiology of alcohol-associated cirrhosis and
HCC: Trends, projections and risk factors. Nat Rev Gastroenterol
Hepatol. 20:37–49. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Singal AK, Bataller R, Ahn J, Kamath PS
and Shah VH: ACG clinical guideline: Alcoholic liver disease. Am J
Gastroenterol. 113:175–194. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Acharya C and Bajaj JS: Gut Microbiota and
complications of liver disease. Gastroenterol Clin North Am.
46:155–169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Dubinkina VB, Tyakht AV, Odintsova VY,
Yarygin KS, Kovarsky BA, Pavlenko AV, Ischenko DS, Popenko AS,
Alexeev DG, Taraskina AY, et al: Links of gut microbiota
composition with alcohol dependence syndrome and alcoholic liver
disease. Microbiome. 5:1412017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Blaak EE, Canfora EE, Theis S, Frost G,
Groen AK, Mithieux G, Nauta A, Scott K, Stahl B, van Harsselaar J,
et al: Short chain fatty acids in human gut and metabolic health.
Benef Microbes. 11:411–455. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wang Z, Zhang X, Zhu L, Yang X, He F, Wang
T, Bao T, Lu H, Wang H and Yang S: Inulin alleviates inflammation
of alcoholic liver disease via SCFAs-inducing suppression of M1 and
facilitation of M2 macrophages in mice. Int Immunopharmacol.
78:1060622020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Yang X, He F, Zhang Y, Xue J, Li K, Zhang
X, Zhu L, Wang Z, Wang H and Yang S: Inulin ameliorates alcoholic
liver disease via suppressing LPS-TLR4-mpsi axis and modulating gut
microbiota in mice. Alcohol Clin Exp Res. 43:411–424. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Deng M, Qu F, Chen L, Liu C, Zhang M, Ren
F, Guo H, Zhang H, Ge S, Wu C and Zhao L: SCFAs alleviated
steatosis and inflammation in mice with NASH induced by MCD. J
Endocrinol. 245:425–437. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Mundi MS, Velapati S, Patel J, Kellogg TA,
Abu Dayyeh BK and Hurt RT: Evolution of NAFLD and its management.
Nutr Clin Pract. 35:72–84. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp
M and Clement K: Metabolism and metabolic disorders and the
microbiome: The intestinal microbiota associated with obesity,
lipid metabolism, and metabolic health-pathophysiology and
therapeutic strategies. Gastroenterology. 160:573–599. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Canfora EE, Meex RCR, Venema K and Blaak
EE: Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev
Endocrinol. 15:261–273. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Kolodziejczyk AA, Zheng D, Shibolet O and
Elinav E: The role of the microbiome in NAFLD and NASH. EMBO Mol
Med. 11:e93022019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Henao-Mejia J, Elinav E, Jin C, Hao L,
Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ,
et al: Inflammasome-mediated dysbiosis regulates progression of
NAFLD and obesity. Nature. 482:179–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhong S, Li L, Liang N, Zhang L, Xu X,
Chen S and Yin H: Acetaldehyde dehydrogenase 2 regulates HMG-CoA
reductase stability and cholesterol synthesis in the liver. Redox
Biol. 41:1019192021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Inokuchi S, Tsukamoto H, Park E, Liu ZX,
Brenner DA and Seki E: Toll-like receptor 4 mediates
alcohol-induced steatohepatitis through bone marrow-derived and
endogenous liver cells in mice. Alcohol Clin Exp Res. 35:1509–1518.
2011.PubMed/NCBI
|
|
124
|
Bogatyrev SR, Rolando JC and Ismagilov RF:
Self-reinoculation with fecal flora changes microbiota density and
composition leading to an altered bile-acid profile in the mouse
small intestine. Microbiome. 8:192020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Ma C, Han M, Heinrich B, Fu Q, Zhang Q,
Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, et al: Gut
microbiome-mediated bile acid metabolism regulates liver cancer via
NKT cells. Science. 360:eaan59312018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Endo H, Niioka M, Kobayashi N, Tanaka M
and Watanabe T: Butyrate-producing probiotics reduce nonalcoholic
fatty liver disease progression in rats: New insight into the
probiotics for the gut-liver axis. PLoS One. 8:e633882013.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ma YY, Li L, Yu CH, Shen Z, Chen LH and Li
YM: Effects of probiotics on nonalcoholic fatty liver disease: A
meta-analysis. World J Gastroenterol. 19:6911–6918. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kirpich IA, Solovieva NV, Leikhter SN,
Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG,
Barve SS, McClain CJ and Cave M: Probiotics restore bowel flora and
improve liver enzymes in human alcohol-induced liver injury: A
pilot study. Alcohol. 42:675–682. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Schuppan D and Afdhal NH: Liver cirrhosis.
Lancet. 371:838–851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Quiroz-Aldave JE, Gamarra-Osorio ER,
Durand-Vasquez MDC, Rafael-Robles LDP, Gonzales-Yovera JG,
Quispe-Flores MA, Concepción-Urteaga LA, Román-González A,
Paz-Ibarra J and Concepción-Zavaleta MJ: From liver to hormones:
The endocrine consequences of cirrhosis. World J Gastroenterol.
30:1073–1095. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Horn P and Tacke F: Metabolic
reprogramming in liver fibrosis. Cell Metab. 36:1439–1455. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Yang X, Li Q, Liu W, Zong C, Wei L, Shi Y
and Han Z: Mesenchymal stromal cells in hepatic fibrosis/cirrhosis:
From pathogenesis to treatment. Cell Mol Immunol. 20:583–599. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Iredale JP, Thompson A and Henderson NC:
Extracellular matrix degradation in liver fibrosis: Biochemistry
and regulation. Biochim Biophys Acta. 1832:876–883. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Smith A, Baumgartner K and Bositis C:
Cirrhosis: Diagnosis and management. Am Fam Physician. 100:759–770.
2019.PubMed/NCBI
|
|
136
|
Tapper EB and Parikh ND: Diagnosis and
management of cirrhosis and its complications: A review. JAMA.
329:1589–1602. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
GBD 2019 Diseases and Injuries
Collaborators: Global burden of 369 diseases and injuries in 204
countries and territories, 1990–2019: A systematic analysis for the
Global Burden of Disease Study 2019. Lancet. 396:1204–1222. 2020.
View Article : Google Scholar
|
|
138
|
Asrani SK, Devarbhavi H, Eaton J and
Kamath PS: Burden of liver diseases in the world. J Hepatol.
70:151–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
European Association for the Study of the
Liver. Electronic address, . simpleeasloffice@easloffice.eu
and European Association for the Study of the Liver: EASL clinical
practice guidelines on Acute-on-chronic liver failure. J Hepatol.
79:461–491. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Gines P, Krag A, Abraldes JG, Sola E,
Fabrellas N and Kamath PS: Liver cirrhosis. Lancet. 398:1359–1376.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Groschwitz KR and Hogan SP: Intestinal
barrier function: Molecular regulation and disease pathogenesis. J
Allergy Clin Immunol. 124:3–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Nishimura N, Kaji K, Kitagawa K, Sawada Y,
Furukawa M, Ozutsumi T, Fujinaga Y, Tsuji Y, Takaya H, Kawaratani
H, et al: Intestinal permeability is a mechanical rheostat in the
pathogenesis of liver cirrhosis. Int J Mol Sci. 22:69212021.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Trebicka J, Macnaughtan J, Schnabl B,
Shawcross DL and Bajaj JS: The microbiota in cirrhosis and its role
in hepatic decompensation. J Hepatol. 75 (Suppl 1):S67–S81. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Benjamin J, Singla V, Arora I, Sood S and
Joshi YK: Intestinal permeability and complications in liver
cirrhosis: A prospective cohort study. Hepatol Res. 43:200–207.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Shibamoto A, Kaji K, Nishimura N, Kubo T,
Iwai S, Tomooka F, Suzuki J, Tsuji Y, Fujinaga Y, Kawaratani H, et
al: Vitamin D deficiency exacerbates alcohol-related liver injury
via gut barrier disruption and hepatic overload of endotoxin. J
Nutr Biochem. 122:1094502023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Suk KT and Kim DJ: Gut microbiota: Novel
therapeutic target for nonalcoholic fatty liver disease. Expert Rev
Gastroenterol Hepatol. 13:193–204. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Albillos A, Lario M and Alvarez-Mon M:
Cirrhosis-associated immune dysfunction: Distinctive features and
clinical relevance. J Hepatol. 61:1385–1396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Fukui H: Role of gut dysbiosis in liver
diseases: What have we learned so far? Diseases. 7:582019.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Camilleri M, Madsen K, Spiller R,
Greenwood-Van Meerveld B and Verne GN: Intestinal barrier function
in health and gastrointestinal disease. Neurogastroenterol Motil.
24:503–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Ghosh S, Whitley CS, Haribabu B and Jala
VR: Regulation of intestinal barrier function by microbial
metabolites. Cell Mol Gastroenterol Hepatol. 11:1463–1482. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Oh TG, Kim SM, Caussy C, Fu T, Guo J,
Bassirian S, Singh S, Madamba EV, Bettencourt R, Richards L, et al:
A universal Gut-microbiome-derived signature predicts cirrhosis.
Cell Metab. 32:878–888.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Chang CS, Chen GH, Lien HC and Yeh HZ:
Small intestine dysmotility and bacterial overgrowth in cirrhotic
patients with spontaneous bacterial peritonitis. Hepatology.
28:1187–1190. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Corradi F, Brusasco C, Fernandez J, Vila
J, Ramirez MJ, Seva-Pereira T, Fernández-Varo G, Mosbah IB, Acevedo
J, Silva A, et al: Effects of pentoxifylline on intestinal
bacterial overgrowth, bacterial translocation and spontaneous
bacterial peritonitis in cirrhotic rats with ascites. Dig Liver
Dis. 44:239–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Bajaj JS, Heuman DM, Hylemon PB, Sanyal
AJ, Puri P, Sterling RK, Luketic V, Stravitz RT, Siddiqui MS, Fuchs
M, et al: Randomised clinical trial: Lactobacillus GG modulates gut
microbiome, metabolome and endotoxemia in patients with cirrhosis.
Aliment Pharmacol Ther. 39:1113–1125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu
E, Cox IJ, Kheradman R, Heuman D, Wang J, Gurry T, et al: Fecal
microbiota transplant from a rational stool donor improves hepatic
encephalopathy: A randomized clinical trial. Hepatology.
66:1727–1738. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Bajaj JS, Hylemon PB, Ridlon JM, Heuman
DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M and
Gillevet PM: Colonic mucosal microbiome differs from stool
microbiome in cirrhosis and hepatic encephalopathy and is linked to
cognition and inflammation. Am J Physiol Gastrointest Liver
Physiol. 303:G675–G685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei
D, Wang Y, Zhu B and Li L: Characterization of fecal microbial
communities in patients with liver cirrhosis. Hepatology.
54:562–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Shen TD, Daniel SG, Patel S, Kaplan E,
Phung L, Lemelle-Thomas K, Chau L, Herman L, Trisolini C, Stonelake
A, et al: The Mucosally-adherent rectal microbiota contains
features unique to alcohol-related cirrhosis. Gut Microbes.
13:19877812021. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Egger M, Horvath A, Pruller F, Fickert P,
Finkelman M, Kriegl L, Grønbaek H, Møller HJ, Prattes J, Krause R,
et al: Fungal translocation measured by serum 1,3-β-D-glucan
correlates with severity and outcome of liver cirrhosis-A pilot
study. Liver Int. 43:1975–1983. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Saaoud F, Liu L, Xu K, Cueto R, Shao Y, Lu
Y, Sun Y, Snyder NW, Wu S, Yang L, et al: Aorta- and
liver-generated TMAO enhances trained immunity for increased
inflammation via ER stress/mitochondrial ROS/glycolysis pathways.
JCI Insight. 8:e1581832023. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Pleguezuelo M, Benitez JM, Jurado J,
Montero JL and De la Mata M: Diagnosis and management of bacterial
infections in decompensated cirrhosis. World J Hepatol. 5:16–25.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Kim J, Ahn SW, Kim JY, Whon TW, Lim SK,
Ryu BH, Han NS, Choi HJ, Roh SW and Lee SH: Probiotic
Lactobacilli ameliorate alcohol-induced hepatic damage via
gut microbial alteration. Front Microbiol. 13:8692502022.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Llovet JM, Pinyol R, Yarchoan M, Singal
AG, Marron TU, Schwartz M, Pikarsky E, Kudo M and Finn RS: Adjuvant
and neoadjuvant immunotherapies in hepatocellular carcinoma. Nat
Rev Clin Oncol. 21:294–311. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Ding JH, Jin Z, Yang XX, Lou J, Shan WX,
Hu YX, Du Q, Liao QS, Xie R and Xu JY: Role of gut microbiota via
the gut-liver-brain axis in digestive diseases. World J
Gastroenterol. 26:6141–6162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Queipo-Ortuño MI, Boto-Ordóñez M, Murri M,
Gomez-Zumaquero JM, Clemente-Postigo M, Estruch R, Cardona Diaz F,
Andrés-Lacueva C and Tinahones FJ: Influence of red wine
polyphenols and ethanol on the gut microbiota ecology and
biochemical biomarkers. Am J Clin Nutr. 95:1323–1334. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Mutlu E, Keshavarzian A, Engen P, Forsyth
CB, Sikaroodi M and Gillevet P: Intestinal dysbiosis: A possible
mechanism of alcohol-induced endotoxemia and alcoholic
steatohepatitis in rats. Alcohol Clin Exp Res. 33:1836–1846. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Yan AW, Fouts DE, Brandl J, Starkel P,
Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA and
Schnabl B: Enteric dysbiosis associated with a mouse model of
alcoholic liver disease. Hepatology. 53:96–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Mutlu EA, Gillevet PM, Rangwala H,
Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK and Keshavarzian
A: Colonic microbiome is altered in alcoholism. Am J Physiol
Gastrointest Liver Physiol. 302:G966–G978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Boursier J, Mueller O, Barret M, Machado
M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA,
et al: The severity of nonalcoholic fatty liver disease is
associated with gut dysbiosis and shift in the metabolic function
of the gut microbiota. Hepatology. 63:764–775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Wang B, Jiang X, Cao M, Ge J, Bao Q, Tang
L, Chen Y and Li L: Altered fecal microbiota correlates with liver
biochemistry in nonobese patients with Non-alcoholic fatty liver
disease. Sci Rep. 6:320022016. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Singh DP, Khare P, Bijalwan V, Baboota RK,
Singh J, Kondepudi KK, Chopra K and Bishnoi M: Coadministration of
isomalto-oligosaccharides augments metabolic health benefits of
cinnamaldehyde in high fat diet fed mice. Biofactors. 43:821–835.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Ponziani FR, Bhoori S, Castelli C,
Putignani L, Rivoltini L, Del Chierico F, Sanguinetti M, Morelli D,
Paroni Sterbini F, Petito V, et al: Hepatocellular carcinoma is
associated with gut microbiota profile and inflammation in
nonalcoholic fatty liver disease. Hepatology. 69:107–120. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Sarangi AN, Goel A, Singh A, Sasi A and
Aggarwal R: Faecal bacterial microbiota in patients with cirrhosis
and the effect of lactulose administration. BMC Gastroenterol.
17:1252017. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H,
Xie H, Chen X, Shao L, Zhang R, et al: Gut microbiome analysis as a
tool towards targeted non-invasive biomarkers for early
hepatocellular carcinoma. Gut. 68:1014–1023. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Schneider KM, Mohs A, Gui W, Galvez EJC,
Candels LS, Hoenicke L, Muthukumarasamy U, Holland CH, Elfers C,
Kilic K, et al: Imbalanced gut microbiota fuels hepatocellular
carcinoma development by shaping the hepatic inflammatory
microenvironment. Nat Commun. 13:39642022. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Li Z, Zhang Y, Hong W, Wang B, Chen Y,
Yang P, Zhou J, Fan J, Zeng Z and Du S: Gut microbiota modulate
radiotherapy-associated antitumor immune responses against
hepatocellular carcinoma Via STING signaling. Gut Microbes.
14:21190552022. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Thoen RU, Longo L, Leonhardt LC, Pereira
MHM, Rampelotto PH, Cerski CTS and Álvares-da-Silva MR: Alcoholic
liver disease and intestinal microbiota in an experimental model:
Biochemical, inflammatory, and histologic parameters. Nutrition.
106:1118882023. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
McMahan RH, Hulsebus HJ, Najarro KM, Giesy
LE, Frank DN and Kovacs EJ: Changes in gut microbiome correlate
with intestinal barrier dysfunction and inflammation following a
3-day ethanol exposure in aged mice. Alcohol. 107:136–143. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Sangineto M, Grander C, Grabherr F, Mayr
L, Enrich B, Schwärzler J, Dallio M, Bukke VN, Moola A, Moschetta
A, et al: Recovery of Bacteroides thetaiotaomicron
ameliorates hepatic steatosis in experimental alcohol-related liver
disease. Gut Microbes. 14:20890062022. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Day AW and Kumamoto CA: Gut microbiome
dysbiosis in alcoholism: Consequences for health and recovery.
Front Cell Infect Microbiol. 12:8401642022. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Wang W, Li Q, Chai W, Sun C, Zhang T, Zhao
C, Yuan Y, Wang X, Liu H and Ye H: Lactobacillus paracasei
Jlus66 extenuate oxidative stress and inflammation via regulation
of intestinal flora in rats with non alcoholic fatty liver disease.
Food Sci Nutr. 7:2636–2646. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Safari Z and Gerard P: The links between
the gut microbiome and non-alcoholic fatty liver disease (NAFLD).
Cell Mol Life Sci. 76:1541–1558. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Ji Y, Yin Y, Li Z and Zhang W: Gut
Microbiota-derived components and metabolites in the progression of
non-alcoholic fatty liver disease (NAFLD). Nutrients. 11:17122019.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Fang J, Yu CH, Li XJ, Yao JM, Fang ZY,
Yoon SH and Yu WY: Gut dysbiosis in nonalcoholic fatty liver
disease: Pathogenesis, diagnosis, and therapeutic implications.
Front Cell Infect Microbiol. 12:9970182022. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Liu S and Yang X: Intestinal flora plays a
role in the progression of hepatitis-cirrhosis-liver cancer. Front
Cell Infect Microbiol. 13:11401262023. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Lee NY and Suk KT: The role of the gut
microbiome in liver cirrhosis treatment. Int J Mol Sci. 22:1992020.
View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Qin N, Yang F, Li A, Prifti E, Chen Y,
Shao L, Guo J, Le Chatelier E, Yao J, Wu L, et al: Alterations of
the human gut microbiome in liver cirrhosis. Nature. 513:59–64.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Akkiz H: The gut microbiome and
hepatocellular carcinoma. J Gastrointest Cancer. 52:1314–1319.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Schwabe RF and Greten TF: Gut microbiome
in HCC-mechanisms, diagnosis and therapy. J Hepatol. 72:230–238.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Zhang S, Hou L and Sun Q: Correlation
analysis of intestinal flora and immune function in patients with
primary hepatocellular carcinoma. J Gastrointest Oncol.
13:1308–1316. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Zhang HL, Yu LX, Yang W, Tang L, Lin Y, Wu
H, Zhai B, Tan YX, Shan L, Liu Q, et al: Profound impact of gut
homeostasis on chemically-induced pro-tumorigenic inflammation and
hepatocarcinogenesis in rats. J Hepatol. 57:803–812. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Dapito DH, Mencin A, Gwak GY, Pradere JP,
Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A,
Bataller R, et al: Promotion of hepatocellular carcinoma by the
intestinal microbiota and TLR4. Cancer Cell. 21:504–516. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Mou WL, Chen SR, Wu ZT, Hu LH, Zhang JY,
Chang HJ, Zhou H and Liu Y: LPS-TLR4/MD-2-TNF-α signaling mediates
alcohol-induced liver fibrosis in rats. J Toxicol Pathol.
35:193–203. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong
W, Tang L, Lin Y, He YQ, Zou SS, et al: Endotoxin accumulation
prevents carcinogen-induced apoptosis and promotes liver
tumorigenesis in rodents. Hepatology. 52:1322–1333. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Yoshimoto S, Loo TM, Atarashi K, Kanda H,
Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et
al: Obesity-induced gut microbial metabolite promotes liver cancer
through senescence secretome. Nature. 499:97–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Nguyen PT, Kanno K, Pham QT, Kikuchi Y,
Kakimoto M, Kobayashi T, Otani Y, Kishikawa N, Miyauchi M, Arihiro
K, et al: Senescent hepatic stellate cells caused by deoxycholic
acid modulates malignant behavior of hepatocellular carcinoma. J
Cancer Res Clin Oncol. 146:3255–3268. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Vaughn BP, Rank KM and Khoruts A: Fecal
microbiota transplantation: Current status in treatment of gi and
liver disease. Clin Gastroenterol Hepatol. 17:353–361. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Routy B, Lenehan JG, Miller WH Jr, Jamal
R, Messaoudene M, Daisley BA, Hes C, Al KF, Martinez-Gili L,
Punčochář M, et al: Fecal microbiota transplantation plus anti-PD-1
immunotherapy in advanced melanoma: A phase I trial. Nat Med.
29:2121–2132. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Belvoncikova P, Maronek M and Gardlik R:
Gut dysbiosis and fecal microbiota transplantation in autoimmune
diseases. Int J Mol Sci. 23:107292022. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Borody TJ, Eslick GD and Clancy RL: Fecal
microbiota transplantation as a new therapy: From Clostridioides
difficile infection to inflammatory bowel disease, irritable bowel
syndrome, and colon cancer. Curr Opin Pharmacol. 49:43–51. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Burz SD, Monnoye M, Philippe C, Farin W,
Ratziu V, Strozzi F, Paillarse JM, Chêne L, Blottière HM and Gérard
P: Fecal microbiota transplant from human to mice gives insights
into the role of the gut microbiota in non-alcoholic fatty liver
disease (NAFLD). Microorganisms. 9:1992021. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Purohit A, Alam MJ, Kandiyal B, Shalimar
Das B and Banerjee SK: Gut microbiome and non-alcoholic fatty liver
disease. Prog Mol Biol Transl Sci. 191:187–206. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Brandt LJ and Aroniadis OC: An overview of
fecal microbiota transplantation: Techniques, indications, and
outcomes. Gastrointest Endosc. 78:240–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Persky SE and Brandt LJ: Treatment of
recurrent Clostridium difficile-associated diarrhea by
administration of donated stool directly through a colonoscope. Am
J Gastroenterol. 95:3283–3285. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Michailidis L, Currier AC, Le M and
Flomenhoft DR: Adverse events of fecal microbiota transplantation:
A meta-analysis of high-quality studies. Ann Gastroenterol.
34:802–814. 2021.PubMed/NCBI
|
|
206
|
Allegretti JR, Kassam Z, Mullish BH,
Chiang A, Carrellas M, Hurtado J, Marchesi JR, McDonald JAK,
Pechlivanis A, Barker GF, et al: Effects of fecal microbiota
transplantation with oral capsules in obese patients. Clin
Gastroenterol Hepatol. 18:855–863.e2. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Yang J, Tang X, Liang Z, Chen M and Sun L:
Taurocholic acid promotes hepatic stellate cell activation via
S1PR2/p38 MAPK/YAP signaling under cholestatic conditions. Clin Mol
Hepatol. 29:465–481. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Mancinelli R, Ceci L, Kennedy L, Francis
H, Meadows V, Chen L, Carpino G, Kyritsi K, Wu N, Zhou T, et al:
The effects of Taurocholic acid on biliary damage and liver
fibrosis are mediated by calcitonin-gene-related peptide signaling.
Cells. 11:15912022. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Uyttebroek S, Chen B, Onsea J, Ruythooren
F, Debaveye Y, Devolder D, Spriet I, Depypere M, Wagemans J,
Lavigne R, et al: Safety and efficacy of phage therapy in
difficult-to-treat infections: A systematic review. Lancet Infect
Dis. 22:e208–e220. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Federici S, Kredo-Russo S, Valdes-Mas R,
Kviatcovsky D, Weinstock E, Matiuhin Y, Silberberg Y, Atarashi K,
Furuichi M, Oka A, et al: Targeted suppression of human
IBD-associated gut microbiota commensals by phage consortia for
treatment of intestinal inflammation. Cell. 185:2879–2898.e24.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Duan Y, Young R and Schnabl B:
Bacteriophages and their potential for treatment of
gastrointestinal diseases. Nat Rev Gastroenterol Hepatol.
19:135–144. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Shuwen H and Kefeng D: Intestinal phages
interact with bacteria and are involved in human diseases. Gut
Microbes. 14:21137172022. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Duan Y, Llorente C, Lang S, Brandl K, Chu
H, Jiang L, White RC, Clarke TH, Nguyen K, Torralba M, et al:
Bacteriophage targeting of gut bacterium attenuates alcoholic liver
disease. Nature. 575:505–511. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Fujiki J and Schnabl B: Phage therapy:
Targeting intestinal bacterial microbiota for the treatment of
liver diseases. JHEP Rep. 5:1009092023. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Gong X, Geng H, Yang Y, Zhang S, He Z, Fan
Y, Yin F, Zhang Z and Chen GQ: Metabolic engineering of commensal
bacteria for gut butyrate delivery and dissection of host-microbe
interaction. Metab Eng. 80:94–106. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Anand AC and Acharya SK: The story of
ammonia in liver disease: An unraveling continuum. J Clin Exp
Hepatol. 14:1013612024. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Kurtz CB, Millet YA, Puurunen MK,
Perreault M, Charbonneau MR, Isabella VM, Kotula JW, Antipov E,
Dagon Y, Denney WS, et al: An engineered E. coli Nissle
improves hyperammonemia and survival in mice and shows
dose-dependent exposure in healthy humans. Sci Transl Med.
11:eaau79752019. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Yu M, Hu S, Tang B, Yang H and Sun D:
Engineering Escherichia coli Nissle 1917 as a microbial
chassis for therapeutic and industrial applications. Biotechnol
Adv. 67:1082022023. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Lynch JP, Goers L and Lesser CF: Emerging
strategies for engineering Escherichia coli Nissle
1917-based therapeutics. Trends Pharmacol Sci. 43:772–786. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Husted AS, Trauelsen M, Rudenko O, Hjorth
SA and Schwartz TW: GPCR-mediated signaling of metabolites. Cell
Metab. 25:777–796. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Akiba Y and Kaunitz JD: Duodenal luminal
chemosensing; acid, ATP, and nutrients. Curr Pharm Des.
20:2760–2765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Kokorovic A, Cheung GW, Breen DM, Chari M,
Lam CK and Lam TK: Duodenal mucosal protein kinase C-δ regulates
glucose production in rats. Gastroenterology. 141:1720–1727. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
223
|
van Baar ACG, Beuers U, Wong K, Haidry R,
Costamagna G, Hafedi A, Deviere J, Ghosh SS, Lopez-Talavera JC,
Rodriguez L, et al: Endoscopic duodenal mucosal resurfacing
improves glycaemic and hepatic indices in type 2 diabetes: 6-month
multicentre results. JHEP Rep. 1:429–437. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
de Oliveira GHP, de Moura DTH, Funari MP,
McCarty TR, Ribeiro IB, Bernardo WM, Sagae VMT, Freitas JR Jr,
Souza GMV and de Moura EGH: Metabolic effects of endoscopic
duodenal mucosal resurfacing: A systematic review and
Meta-analysis. Obes Surg. 31:1304–1312. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Shamseddeen H, Vuppalanchi R and Gromski
MA: Duodenal mucosal resurfacing for nonalcoholic fatty liver
disease. Clin Liver Dis (Hoboken). 20:166–169. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Condello G and Chen CY: Minireview:
Current status of endoscopic duodenal mucosal resurfacing. Obes Res
Clin Pract. 14:504–507. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Mingrone G, van Baar AC, Deviere J,
Hopkins D, Moura E, Cercato C, Rajagopalan H, Lopez-Talavera JC,
White K, Bhambhani V, et al: Safety and efficacy of hydrothermal
duodenal mucosal resurfacing in patients with type 2 diabetes: The
randomised, double-blind, sham-controlled, multicentre REVITA-2
feasibility trial. Gut. 71:254–264. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Hadefi A, Verset L, Pezzullo M, Rosewick
N, Degre D, Gustot T, Moreno C, Devière J and Trépo E: Endoscopic
duodenal mucosal resurfacing for nonalcoholic steatohepatitis
(NASH): A pilot study. Endosc Int Open. 9:E1792–E1800. 2021.
View Article : Google Scholar : PubMed/NCBI
|