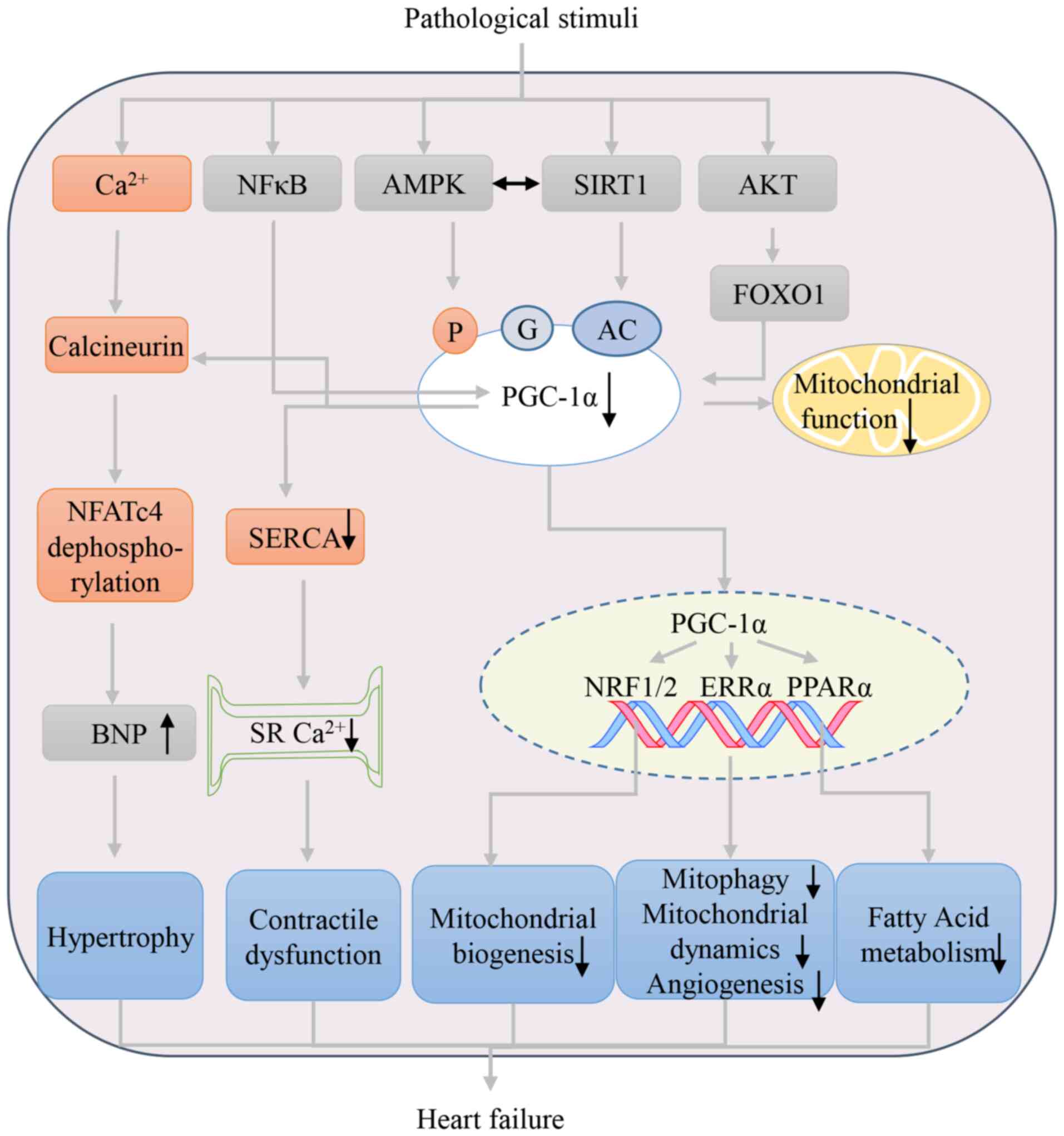
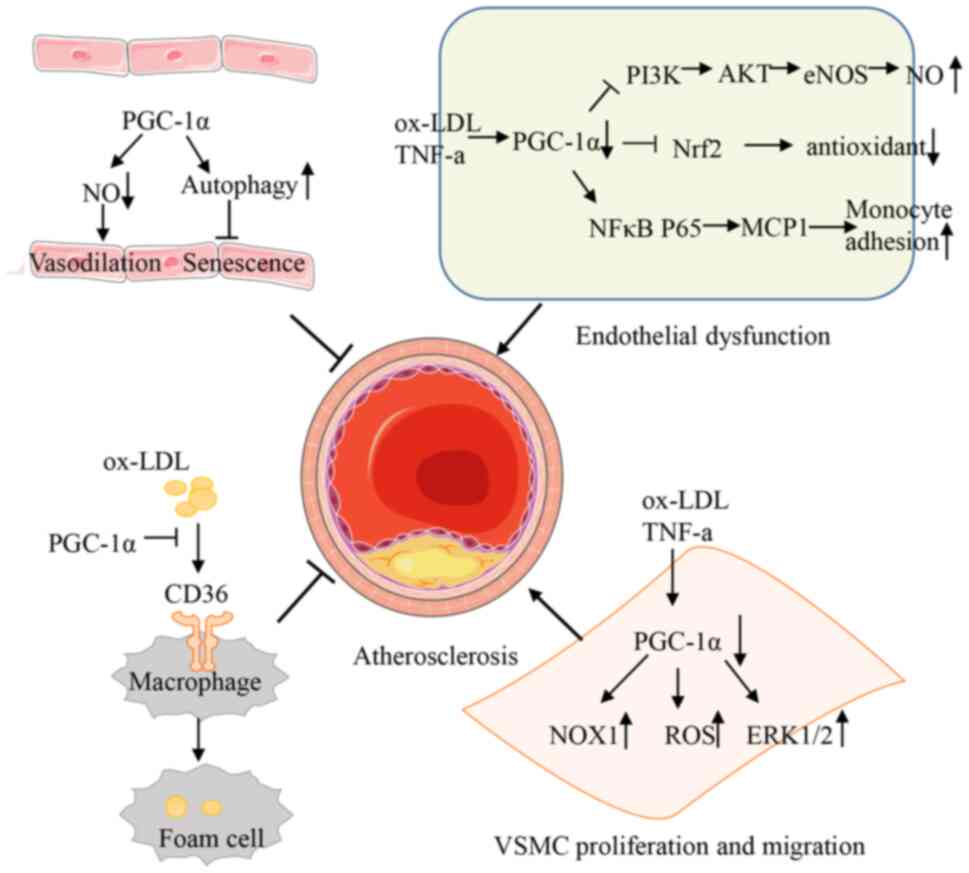
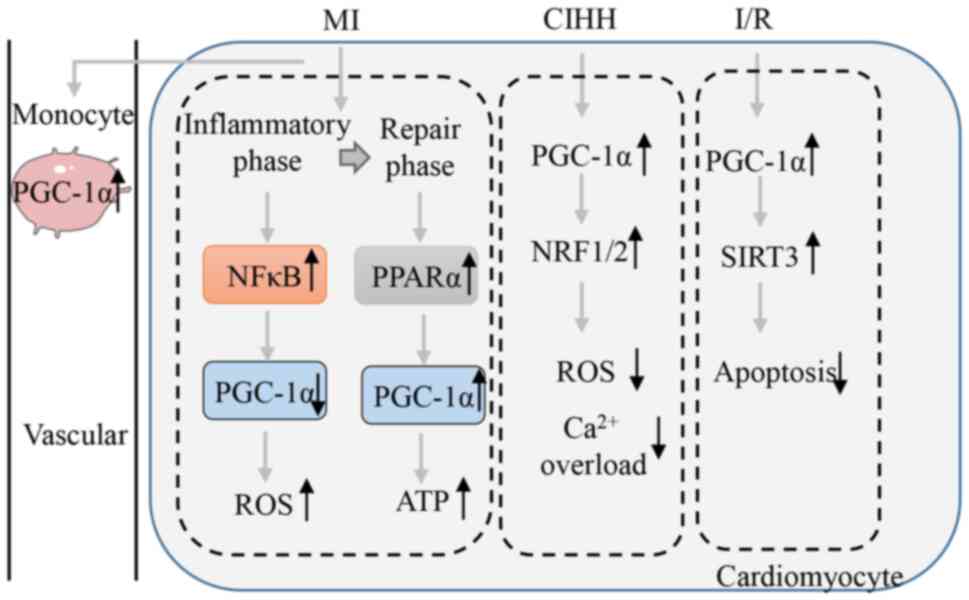
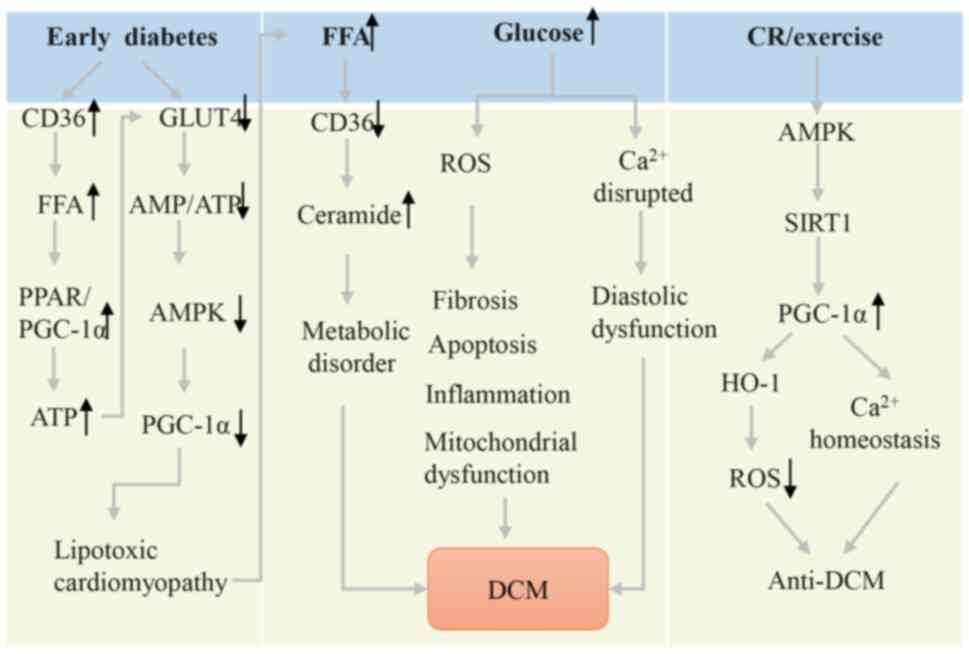
|
1
|
Harrington JS, Ryter SW, Plataki M, Price
DR and Choi AMK: Mitochondria in health, disease and aging. Physiol
Rev. 103:2349–2422. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jannig PR, Dumesic PA, Spiegelman BM and
Ruas JL: SnapShot: Regulation and biology of PGC-1α. Cell.
185:1444.e12022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Andrulionyte L, Peltola P, Chiasson JL and
Laakso M; STOP-NIDDM Study Group, : Single nucleotide polymorphisms
of PPARD in combination with the Gly482Ser substitution of PGC-1A
and the Pro12Ala substitution of PPARG2 predict the conversion from
impaired glucose tolerance to type 2 diabetes: The STOP-NIDDM
trial. Diabetes. 55:2148–2152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yongsakulchai P, Settasatian C,
Settasatian N, Komanasin N, Kukongwiriyapan U, Cote ML,
Intharapetch P and Senthong V: Association of combined genetic
variations in PPARγ, PGC-1α and LXRα with coronary artery disease
and severity in Thai population. Atherosclerosis. 248:140–148.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rojek A, Cielecka-Prynda M,
Przewlocka-Kosmala M, Laczmanski L, Mysiak A and Kosmala W: Impact
of the PPARGC1A Gly482Ser polymorphism on left ventricular
structural and functional abnormalities in patients with
hypertension. J Hum Hypertens. 28:557–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yao Y, Chen T, Wu H, Yang N and Xu S:
Melatonin attenuates bisphenol A-induced colon injury by dual
targeting mitochondrial dynamics and Nrf2 antioxidant system via
activation of SIRT1/PGC-1α signaling pathway. Free Radic Biol Med.
195:13–22. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kärkkäinen O, Tuomainen T, Mutikainen M,
Lehtonen M, Ruas JL, Hanhineva K and Tavi P: Heart specific PGC-1α
deletion identifies metabolome of cardiac restricted metabolic
heart failure. Cardiovasc Res. 115:107–118. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rowe GC, Jiang A and Arany Z: PGC-1
coactivators in cardiac development and disease. Circ Res.
107:825–838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garcia D and Shaw RJ: AMPK: Mechanisms of
cellular energy sensing and restoration of metabolic balance. Mol
Cell. 66:789–800. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tian R, Musi N, D'Agostino J, Hirshman MF
and Goodyear LJ: Increased adenosine monophosphate-activated
protein kinase activity in rat hearts with pressure-overload
hypertrophy. Circulation. 104:1664–1669. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishino Y, Miura T, Miki T, Sakamoto J,
Nakamura Y, Ikeda Y, Kobayashi H and Shimamoto K: Ischemic
preconditioning activates AMPK in a PKC-dependent manner and
induces GLUT4 up-regulation in the late phase of cardioprotection.
Cardiovasc Res. 61:610–619. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tong D, Schiattarella GG, Jiang N, Daou D,
Luo Y, Link MS, Lavandero S, Gillette TG and Hill JA: Impaired
AMP-Activated protein kinase signaling in heart failure with
preserved ejection Fraction-associated atrial fibrillation.
Circulation. 146:73–76. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang T, Xu L, Guo X, Tao H, Liu Y, Liu X,
Zhang Y and Meng X: The potential of herbal drugs to treat heart
failure: The roles of Sirt1/AMPK. J Pharm Anal. 14:157–176. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jäger S, Handschin C, St-Pierre J and
Spiegelman BM: AMP-activated protein kinase (AMPK) action in
skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl
Acad Sci USA. 104:12017–12022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Li X, Guo Y, Chan L and Guan X:
Alpha-Lipoic acid increases energy expenditure by enhancing
adenosine monophosphate-activated protein kinase-peroxisome
proliferator-activated receptor-gamma coactivator-1alpha signaling
in the skeletal muscle of aged mice. Metabolism. 59:967–976. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Malik N, Ferreira BI, Hollstein PE, Curtis
SD, Trefts E, Weiser Novak S, Yu J, Gilson R, Hellberg K, Fang L,
et al: Induction of lysosomal and mitochondrial biogenesis by AMPK
phosphorylation of FNIP1. Science. 380:eabj55592023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu CQ, Li J, Liang ZQ, Zhong YL, Zhang ZH,
Hu XQ, Cao YB and Chen J: Sirtuins in macrophage immune metabolism:
A novel target for cardiovascular disorders. Int J Biol Macromol.
256:1282702024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Komen JC and Thorburn DR: Turn up the
power-pharmacological activation of mitochondrial biogenesis in
mouse models. Br J Pharmacol. 171:1818–1836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang L, Quan N, Sun W, Chen X, Cates C,
Rousselle T, Zhou X, Zhao X and Li J: Cardiomyocyte-specific
deletion of Sirt1 gene sensitizes myocardium to ischaemia and
reperfusion injury. Cardiovasc Res. 114:805–821. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Q and Lesnefsky EJ: A new strategy to
decrease cardiac injury in aged heart following
Ischaemia-reperfusion: Enhancement of the interaction between AMPK
and SIRT1. Cardiovasc Res. 114:771–772. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bugga P, Alam MJ, Kumar R, Pal S,
Chattopadyay N and Banerjee SK: Sirt3 ameliorates mitochondrial
dysfunction and oxidative stress through regulating mitochondrial
bioge-nesis and dynamics in cardiomyoblast. Cell Signal.
94:1103092022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Capece D, Verzella D, Flati I, Arboretto
P, Cornice J and Franzoso G: NF-κB: Blending metabolism, immunity
and inflammation. Trends Immunol. 43:757–775. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bertero E, Dudek J, Cochain C, Delgobo M,
Ramos G, Gerull B, Higuchi T, Vaeth M, Zernecke A, Frantz S, et al:
Immuno-metabolic interfaces in cardiac disease and failure.
Cardiovasc Res. 118:37–52. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rabinovich-Nikitin I, Blant A, Dhingra R,
Kirshenbaum LA and Czubryt MP: NF-κB p65 attenuates cardiomyocyte
PGC-1α expression in hypoxia. Cells. 11:21932022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao MM, Xu MJ, Cai Y, Zhao G, Guan Y,
Kong W, Tang C and Wang X: Mitochondrial reactive oxygen species
promote p65 nuclear translocation mediating high-phosphate-induced
vascular calcification in vitro and in vivo. Kidney Int.
79:1071–1079. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang P, Xu S, Xu J, Xin Y, Lu Y, Zhang H,
Zhou B, Xu H, Sheu SS, Tian R and Wang W: Elevated MCU expression
by CaMKIIδB limits pathological cardiac remodeling. Circulation.
145:1067–1083. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wright DC, Geiger PC, Han DH, Jones TE and
Holloszy JO: Calcium induces increases in peroxisome
proliferator-activated receptor gamma coactivator-1alpha and
mitochondrial biogenesis by a pathway leading to p38
mitogen-activated protein kinase activation. J Biol Chem.
282:18793–18799. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim HK, Ko TH, Song IS, Jeong YJ, Heo HJ,
Jeong SH, Kim M, Park NM, Seo DY, Kha PT, et al: BH4 activates
CaMKK2 and rescues the cardiomyopathic phenotype in rodent models
of diabetes. Life Sci Alliance. 3:e2019006192020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watanabe S, Horie T, Nagao K, Kuwabara Y,
Baba O, Nishi H, Sowa N, Narazaki M, Matsuda T, Takemura G, et al:
Cardiac-specific inhibition of kinase activity in
calcium/calmodulin-dependent protein kinase kinase-β leads to
accelerated left ventricular remodeling and heart failure after
transverse aortic constriction in mice. PLoS One. 9:e1082012014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gill JF, Delezie J, Santos G, McGuirk S,
Schnyder S, Frank S, Rausch M, St-Pierre J and Handschin C:
Peroxisome proliferator-activated receptor γ coactivator 1α
regulates mitochondrial calcium homeostasis, sarcoplasmic reticulum
stress and cell death to mitigate skeletal muscle aging. Aging
Cell. 18:e129932019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oldfield CJ, Duhamel TA and Dhalla NS:
Mechanisms for the transition from physiological to pathological
cardiac hypertrophy. Can J Physiol Pharmacol. 98:74–84. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brainard RE and Facundo HT: Cardiac
hypertrophy drives PGC-1α suppression associated with enhanced
O-glycosylation. Biochim Biophys Acta Mol Basis Dis.
1867:1660802021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu Z, Li M, Lyu D, Xiao H, Li S, Li Z, Li
M, Xiao J and Huang H: Cinnamaldehyde activates AMPK/PGC-1α pathway
via targeting GRK2 to ameliorate heart failure. Phytomedicine.
133:1558942024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu X, Xu X, Huang Y, Fassett J, Flagg TP,
Zhang Y, Nichols CG, Bache RJ and Chen Y: Disruption of sarcolemmal
ATP-sensitive potassium channel activity impairs the cardiac
response to systolic overload. Circ Res. 103:1009–1017. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhuang L, Jia K, Chen C, Li Z, Zhao J, Hu
J, Zhang H, Fan Q, Huang C, Xie H, et al: DYRK1B-STAT3 drives
cardiac hypertrophy and heart failure by impairing mitochondrial
bioenergetics. Circulation. 145:829–846. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang S, Tang F, Yang Y, Lu M, Luan A,
Zhang J, Yang J and Wang H: Astragaloside IV protects against
isoproterenol-induced cardiac hypertrophy by regulating
NF-κB/PGC-1α signaling mediated energy biosynthesis. PLoS One.
10:e01187592015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Planavila A, Iglesias R, Giralt M and
Villarroya F: Sirt1 acts in association with PPARα to protect the
heart from hypertrophy, metabolic dysregulation and inflammation.
Cardiovasc Res. 90:276–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu XP, Gao H, Huang XY, Chen YF, Feng XJ,
He YH, Li ZM and Liu PQ: Peroxisome proliferator-activated receptor
gamma coactivator 1 alpha protects cardiomyocytes from hypertrophy
by suppressing calcineurin-nuclear factor of activated T cells c4
signaling pathway. Transl Res. 166:459–473.e3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pereira RO, Wende AR, Crum A, Hunter D,
Olsen CD, Rawlings T, Riehle C, Ward WF and Abel ED: Maintaining
PGC-1α expression following pressure overload-induced cardiac
hypertrophy preserves angiogenesis but not contractile or
mitochondrial function. FASEB J. 28:3691–3702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bhat S, Chin A, Shirakabe A, Ikeda Y,
Ikeda S, Zhai P, Hsu CP, Sayed D, Abdellatif M, Byun J, et al:
Recruitment of RNA polymerase II to metabolic gene promoters is
inhibited in the failing heart possibly through PGC-1α (Peroxisome
proliferator-activated Receptor-γ coactivator-1α) Dysregulation.
Circ Heart Fail. 12:e0055292019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Naumenko N, Mutikainen M, Holappa L, Ruas
JL, Tuomainen T and Tavi P: PGC-1α deficiency reveals sex-specific
links between cardiac energy metabolism and EC-coupling during
development of heart failure in mice. Cardiovasc Res.
118:1520–1534. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen L, Qin Y, Liu B, Gao M, Li A, Li X
and Gong G: PGC-1α-mediated mitochondrial quality control:
Molecular mechanisms and implications for heart failure. Front Cell
Dev Biol. 10:8713572022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hausenloy DJ and Yellon DM: Ischaemic
conditioning and reperfusion injury. Nature reviews. Cardiology.
13:193–209. 2016.PubMed/NCBI
|
|
44
|
Kadlec AO, Chabowski DS, Ait-Aissa K and
Gutterman DD: Role of PGC-1α in Vascular Regulation: Implications
for Atherosclerosis. Arterioscler Thromb Vasc Biol. 36:1467–1474.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang ZC, Niu KM, Wu YJ, Du KR, Qi LW, Zhou
YB and Sun HJ: A dual Keap1 and p47phox inhibitor Ginsenoside Rb1
ameliorates high glucose/ox-LDL-induced endothelial cell injury and
atherosclerosis. Cell Death Dis. 13:8242022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim HJ, Park KG, Yoo EK, Kim YH, Kim YN,
Kim HS, Kim HT, Park JY, Lee KU, Jang WG, et al: Effects of
PGC-1alpha on TNF-alpha-induced MCP-1 and VCAM-1 expression and
NF-kappaB activation in human aortic smooth muscle and endothelial
cells. Antioxid Redox Signal. 9:301–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
McCarthy C, Lieggi NT, Barry D, Mooney D,
de Gaetano M, James WG, McClelland S, Barry MC, Escoubet-Lozach L,
Li AC, et al: Macrophage PPAR gamma Co-activator-1 alpha
participates in repressing foam cell formation and atherosclerosis
in response to conjugated linoleic acid. EMBO Mol Med. 5:1443–1457.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li YQ, Jiao Y, Liu YN, Fu JY, Sun LK and
Su J: PGC-1α protects from myocardial ischaemia-reperfusion injury
by regulating mitonuclear communication. J Cell Mol Med.
26:593–600. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen Y, Wang Y, Chen J, Chen X, Cao W,
Chen S, Xu S, Huang H and Liu P: Roles of transcriptional
corepressor RIP140 and coactivator PGC-1α in energy state of
chronically infarcted rat hearts and mitochondrial function of
cardiomyocytes. Mol Cell Endocrinol. 362:11–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Caligiuri G: Mechanotransduction,
immunoregulation and metabolic functions of CD31 in cardiovascular
pathophysiology. Cardiovasc Res. 115:1425–1434. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kadlec AO, Chabowski DS, Ait-Aissa K,
Hockenberry JC, Otterson MF, Durand MJ, Freed JK, Beyer AM and
Gutterman DD: PGC-1α (Peroxisome proliferator-activated receptor γ
coactivator 1-α) overexpression in coronary artery disease recruits
NO and hydrogen peroxide during flow-mediated dilation and protects
against increased intraluminal pressure. Hypertension. 70:166–173.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu S, Ilyas I, Little PJ, Li H, Kamato D,
Zheng X, Luo S, Li Z, Liu P, Han J, et al: Endothelial dysfunction
in atherosclerotic cardiovascular diseases and beyond: From
mechanism to pharmacotherapies. Pharmacol Rev. 73:924–967. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li J, Geng XY and Cong XL: PGC-1α
ameliorates Angiotensin II-induced eNOS dysfunction in human aortic
endothelial cells. Vascul Pharmacol. 83:90–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
García-Quintans N, Prieto I, Sánchez-Ramos
C, Luque A, Arza E, Olmos Y and Monsalve M: Regulation of
endothelial dynamics by PGC-1α relies on ROS control of VEGF-A
signaling. Free Radical Biol Med. 93:41–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Moore KJ, Koplev S, Fisher EA, Tabas I,
Björkegren JLM, Doran AC and Kovacic JC: Macrophage trafficking,
inflammatory resolution and genomics in atherosclerosis: JACC
Macrophage in CVD Series (Part 2). J Am Coll Cardiol. 72:2181–2197.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Minsky N and Roeder RG: Inhibition of
adhesion molecule gene expression and cell adhesion by the
metabolic regulator PGC-1α. PLoS One. 11:e01655982016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Qu A, Jiang C, Xu M, Zhang Y, Zhu Y, Xu Q,
Zhang C and Wang X: PGC-1alpha attenuates neointimal formation via
inhibition of vascular smooth muscle cell migration in the injured
rat carotid artery. Am J Physiol Cell Physiol. 297:C645–C653. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhu L, Sun G, Zhang H, Zhang Y, Chen X,
Jiang X, Jiang X, Krauss S, Zhang J, Xiang Y and Zhang CY:
PGC-1alpha is a key regulator of glucose-induced proliferation and
migration in vascular smooth muscle cells. PLoS One. 4:e41822009.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhao Q, Zhang J and Wang H: PGC-1α limits
angiotensin II-induced rat vascular smooth muscle cells
proliferation via attenuating NOX1-mediated generation of reactive
oxygen species. Biosci Rep. 35:e002522015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nah DY and Rhee MY: The inflammatory
response and cardiac repair after myocardial infarction. Korean
Circ J. 39:393–398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Günthel M, van Duijvenboden K, de Bakker
DEM, Hooijkaas IB, Bakkers J, Barnett P and Christoffels VM:
Epigenetic state changes underlie metabolic switch in mouse
post-infarction border zone cardiomyocytes. J Cardiovasc Dev Dis.
8:1342021.PubMed/NCBI
|
|
62
|
Oehler D, Spychala A, Gödecke A, Lang A,
Gerdes N, Ruas J, Kelm M, Szendroedi J and Westenfeld R:
Full-length transcriptomic analysis in murine and human heart
reveals diversity of PGC-1α promoters and isoforms regulated
distinctly in myocardial ischemia and obesity. BMC Biol.
20:1692022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lou PH, Zhang L, Lucchinetti E, Heck M,
Affolter A, Gandhi M, Kienesberger PC, Hersberger M, Clanachan AS
and Zaugg M: Infarct-remodelled hearts with limited oxidative
capacity boost fatty acid oxidation after conditioning against
ischaemia/reperfusion injury. Cardiovasc Res. 97:251–261. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bugger H and Pfeil K: Mitochondrial ROS in
myocardial ischemia reperfusion and remodeling. Biochim Biophys
Acta Mol Basis Dis. 1866:1657682020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gu S, Hua H, Guo X, Jia Z, Zhang Y, Maslov
LN, Zhang X and Ma H: PGC-1α participates in the protective effect
of chronic intermittent hypobaric hypoxia on cardiomyocytes. Cell
Physiol Biochem. 50:1891–1902. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Papatheodorou I, Makrecka-Kuka M, Kuka J,
Liepinsh E, Dambrova M and Lazou A: Pharmacological activation of
PPARβ/δ preserves mitochondrial respiratory function in
ischemia/reperfusion via stimulation of fatty acid oxidation-linked
respiration and PGC-1α/NRF-1 signaling. Front Endocrinol.
13:9418222022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yu LM, Dong X, Xue XD, Zhang J, Li Z, Wu
HJ, Yang ZL, Yang Y and Wang HS: Naringenin improves mitochondrial
function and reduces cardiac damage following ischemia-reperfusion
injury: The role of the AMPK-SIRT3 signaling pathway. Food
Function. 10:2752–2765. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lindberg S, Jensen JS, Pedersen SH,
Galatius S, Frystyk J, Flyvbjerg A, Bjerre M and Mogelvang R: Low
adiponectin levels and increased risk of type 2 diabetes in
patients with myocardial infarction. Diabetes Care. 37:3003–3008.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xia Y, Zhang F, Zhao S, Li Y, Chen X, Gao
E, Xu X, Xiong Z, Zhang X, Zhang J, et al: Adiponectin determines
farnesoid X receptor agonism-mediated cardioprotection against
post-infarction remodelling and dysfunction. Cardiovasc Res.
114:1335–1349. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Monsalve M: Induction of PGC-1α expression
can be detected in blood samples of patients with ST-segment
elevation acute myocardial infarction. PLoS One. 6:e269132011.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Duncan JG, Fong JL, Medeiros DM, Finck BN
and Kelly DP: Insulin-resistant heart exhibits a mitochondrial
biogenic response driven by the peroxisome proliferator-activated
receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation.
115:909–917. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kim Y, Lim JH, Kim EN, Hong YA, Park HJ,
Chung S, Choi BS, Kim YS, Park JY, Kim HW and Park CW: Adiponectin
receptor agonist ameliorates cardiac lipotoxicity via enhancing
ceramide metabolism in type 2 diabetic mice. Cell Death Disease.
13:2822022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bekhite M, González-Delgado A, Hübner S,
Haxhikadrija P, Kretzschmar T, Müller T, Wu JMF, Bekfani T, Franz
M, Wartenberg M, et al: The role of ceramide accumulation in human
induced pluripotent stem cell-derived cardiomyocytes on
mitochondrial oxidative stress and mitophagy. Free Radical Biol
Med. 167:66–80. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Waldman M, Arad M, Abraham NG and
Hochhauser E: The peroxisome Proliferator-activated receptor-gamma
coactivator-1α-heme oxygenase 1 axis a powerful antioxidative
pathway with potential to attenuate diabetic cardiomyopathy.
Antioxid Redox Signal. 32:1273–1290. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang SY, Zhu S, Wu J, Zhang M, Xu Y, Xu W,
Cui J, Yu B, Cao W and Liu J: Exercise enhances cardiac function by
improving mitochondrial dysfunction and maintaining energy
homoeostasis in the development of diabetic cardiomyopathy. J Mol
Med (Berl). 98:245–261. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Waldman M, Cohen K, Yadin D, Nudelman V,
Gorfil D, Laniado-Schwartzman M, Kornwoski R, Aravot D, Abraham NG,
Arad M and Hochhauser E: Regulation of diabetic cardiomyopathy by
caloric restriction is mediated by intracellular signaling pathways
involving ‘SIRT1 and PGC-1α’. Cardiovasc Diabetol. 17:1112018.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Whitehead N, Gill JF, Brink M and
Handschin C: Moderate modulation of cardiac PGC-1α expression
partially affects age-associated transcriptional remodeling of the
heart. Front Physiol. 9:2422018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mamoshina P, Rodriguez B and Bueno-Orovio
A: Toward a broader view of mechanisms of drug cardiotoxicity. Cell
Rep Med. 2:1002162021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhao X, Tian Z, Sun M and Dong D: Nrf2: A
dark horse in doxorubicin-induced cardiotoxicity. Cell Death
Discov. 9:2612023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Song JH, Kim MS, Lee SH, Hwang JT, Park
SH, Park SW, Jeon SB, Lee RR, Lee J and Choi HK: Hydroethanolic
extract of Cirsium setidens ameliorates doxorubicin-induced
cardiotoxicity by AMPK-PGC-1α-SOD-mediated mitochondrial
protection. Phytomedicine. 129:1556332024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yin L, Yuan L, Tang Y, Luo Z, Lin X, Wang
S, Liang P and Jiang B: Nucleolin promotes autophagy through PGC-1α
In LPS-induced myocardial injury. Shock. 60:227–237. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Pharoah BM, Zhang C, Khodade VS, Keceli G,
McGinity C, Paolocci N and Toscano JP: Hydropersulfides (RSSH)
attenuate doxorubicin-induced cardiotoxicity while boosting its
anticancer action. Redox Biol. 60:1026252023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Terrar DA: Timing mechanisms to control
heart rhythm and initiate arrhythmias: Roles for intracellular
organelles, signalling pathways and subsarcolemmal Ca2.
Philos Trans R Soc Lond B Biol Sci. 378:202201702023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chadda KR, Edling CE, Valli H, Ahmad S,
Huang CL and Jeevaratnam K: Gene and protein expression profile of
selected molecular targets mediating electrophysiological function
in Pgc-1α deficient murine atria. Int J Mol Sci. 19:34502018.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Saadeh K, Chadda KR, Ahmad S, Valli H,
Nanthakumar N, Fazmin IT, Edling CE, Huang CL and Jeevaratnam K:
Molecular basis of ventricular arrhythmogenicity in a Pgc-1α
deficient murine model. Mol Genet Metab Rep. 27:1007532021.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Liu GZ, Hou TT, Yuan Y, Hang PZ, Zhao JJ,
Sun L, Zhao GQ, Zhao J, Dong JM, Wang XB, et al: Fenofibrate
inhibits atrial metabolic remodelling in atrial fibrillation
through PPAR-α/sirtuin 1/PGC-1α pathway. Br J Pharmacol.
173:1095–1109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lehman JJ, Barger PM, Kovacs A, Saffitz
JE, Medeiros DM and Kelly DP: Peroxisome proliferator-activated
receptor gamma coactivator-1 promotes cardiac mitochondrial
biogenesis. J Clin Invest. 106:847–856. 2000. View Article : Google Scholar : PubMed/NCBI
|