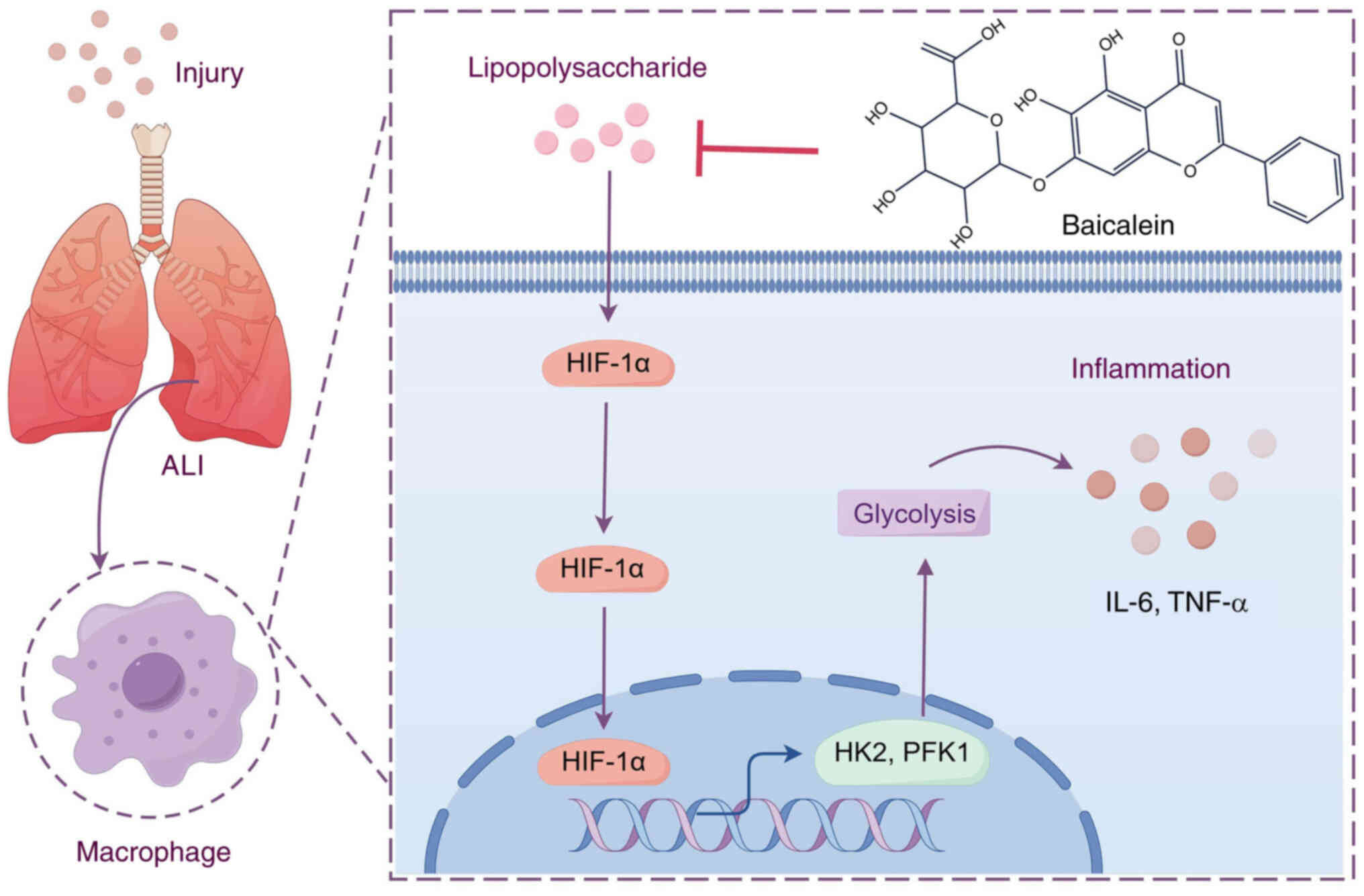
Introduction
Acute lung injury (ALI) is characterized by injury
of the alveolar epithelium and capillary endothelium, eventually
resulting in reduced lung volume and compliance,
ventilation/perfusion mismatch, intrapulmonary and alveolar edema,
and even acute hypoxemic respiratory failure (1,2). The
most common treatment approaches for ALI include treating the
primary disease and respiratory support (3). Antibiotics and corticosteroids used
in the clinical treatment of ALI are not recommended for long-term
use, due to their significant adverse reactions and the risk of
drug dependency (4). Therefore,
investigating the mechanisms underlying the development of ALI, and
identifying novel drugs for the prevention and treatment of ALI,
are of significant importance.
The pathogenesis of ALI is relatively complex. ALI
is caused either by direct lung injury or by acute systemic
inflammation, and is closely associated with the release of
inflammatory signals (5). The
inflammatory process is one of the core pathophysiological
processes of ALI. During this process, inflammatory cells, such as
macrophages (5), T lymphocytes
(6) and neutrophils (7), are activated and trigger the release
of various inflammatory mediators, including cytokines, chemokines,
reactive oxygen species and proteolytic enzymes. In turn, the
aforementioned inflammatory mediators can directly damage alveolar
epithelial and capillary endothelial cells, thus leading to
increased permeability of the alveolar-capillary barrier, pulmonary
edema and severe impairment of lung function (5,8).
Among these inflammatory cells, macrophages serve a key role in the
inflammatory response, acting as both initiators and regulators.
Recent studies have emphasized the significance of alveolar
macrophages in the development of ALI and their potential as
therapeutic targets (9–11).
Previous studies have also indicated that the
glycolytic pathway could be involved in inflammatory responses and
ALI (12,13). Glycolysis is a metabolic pathway
that mediates the conversion of glucose into pyruvate, and it is
upregulated under hypoxic conditions or in response to
inflammation. As an intracellular enzyme, lactate dehydrogenase
(LDH) is involved in enhanced aerobic glycolysis via catalyzing the
reversible transformation of pyruvate to lactate, reflecting the
metabolic changes and cellular damage associated with the
inflammatory process in ALI (14).
Under inflammatory conditions, there is a switch from cell
metabolism towards increased glycolysis, known as aerobic
glycolysis. This metabolic reprogramming supports the energy and
biosynthetic demands of inflammatory cells, eventually facilitating
the production and release of inflammatory mediators (15). In this process, inflammatory cells
produce large amounts of lactate through aerobic glycolysis, which
not only alters the intracellular and extracellular pH, but also
intensifies the inflammatory response and tissue damage. The
presence of cytokines, such as tumor necrosis factor-α (TNF-α) and
IL-6, during inflammation can lead to cell damage and alterations
in LDH activity, thereby affecting lactate production and its
subsequent effects on the inflammatory process (16). Therefore, investigating glycolysis
for the management and prognosis of patients with ALI is of
clinical significance. Hypoxia-inducible factor-1α (HIF-1α) is a
transcription factor that becomes stabilized and active at low
oxygen levels (17). It has been
reported that HIF-1α not only promotes glycolysis via inducing the
expression of genes encoding glycolytic enzymes and glucose
transporters, but also serves a key role in the inflammatory
response (18,19).
Baicalein, a flavonoid monomer compound isolated
from the dried root of the traditional Chinese herb Scutellaria
baicalensis, has a distinct structure characterized by a
keto-enol tautomeric system (20).
Baicalein has several roles in various processes, including
scavenging of oxygen free radicals (21), anti-inflammatory action (22), inhibition of angiogenesis (23), antitumor activities (24), and antimicrobial and antiviral
properties (25). However, whether
baicalein can alleviate ALI via regulating glycolysis in
macrophages remains to be elucidated. Therefore, the present study
aimed to evaluate the potential therapeutic effects of baicalein
via establishing models of ALI and macrophage inflammatory
response. Additionally, bioinformatics network analysis was
performed to identify the mechanism underlying the effects of
baicalein on ALI.
Materials and methods
Chemicals
Baicalein (CAS no. 491-67-8) was purchased from
Chengdu Must Bio-Technology Co., Ltd. Lipopolysaccharide (LPS) was
obtained from MilliporeSigma and dexamethasone (DEX; CAS no.
50-02-2) from Shanghai Yuan Ye Bio-Technology Co., Ltd. The
antibodies against hexokinase 2 (HK2; cat. no. 66974-1-Ig),
phosphofructokinase-1 (PFK1; cat. no. 55028-1-AP), pyruvate kinase
M2 (PKM2; cat. no. 10078-2-AP) and HIF1α (cat. no. 20960-1-AP) were
purchased from Proteintech Group, Inc.
Animal studies
A total of 30 male C57BL/6 mice (weight, 18–22 g;
age, 6–8 weeks) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. In the present study, animal welfare
considerations included all possible efforts to minimize suffering
and distress, as well as the use of anesthetics and controlled
housing conditions. For example, mice were housed in a
temperature-controlled (23.0±1.0°C) and humidity-controlled
(40–60%) cage under a 12-h light/dark cycle and with free access to
food and water. The bedding material was composed of fine and
softwood chips. To maintain a hygienic environment, the bedding
material was changed at 2-day intervals. Animal health and behavior
were monitored every day, and body weights were assessed weekly
over the course of the study. Mice were randomly divided into the
following five groups (n=6 mice/group): Normal, LPS, 10 mg/kg
baicalein, 20 mg/kg baicalein and DEX groups. All groups received
one-time tracheal instillation of LPS, with the exception of the
normal group. A total of 7 days before modeling, the groups
designated to receive medication were administered baicalein and
DEX (2 mg/kg) by gavage. The ALI mouse model was established by a
one-time tracheal instillation of LPS (5 mg/kg), which was a
non-invasive procedure. Firstly, mice were anesthetized via
intraperitoneal injection of pentobarbital sodium (50 mg/kg;
MilliporeSigma). Subsequently, the mice were suspended on the
experimental operating table, and their tongues were pulled to the
side to expose the tracheal opening. A catheter was then inserted
into the trachea along the opening. The needle core was immediately
removed, and the corresponding LPS and saline solution was injected
into the catheter using a 1-ml syringe. The normal group of mice
was only injected with the corresponding volume of saline solution.
Finally, the mice were placed under room temperature to recover
from anesthesia before they were returned to their cages. At 24 h
after modeling, the mice were euthanized by cervical dislocation,
and bronchoalveolar lavage fluid (BALF), serum and lung tissue
samples were collected. In addition, 0.3 ml blood was collected
from the retro-orbital vein just before sacrifice and serum was
obtained by centrifuging the blood at 1,500 × g for 15 min at 4°C.
The death of the mice was verified by lack of respiration,
heartbeat and corneal reflex. The duration of the experiment was 15
days, including 7 days of adaptation, 7 days of dosing and 1 day of
modeling. Animal health and behavior were monitored every day and
no spontaneous death occurred during the present study. The present
study was approved by the Ethics Committee of the Zhumadian
Hospital of Traditional Chinese Medicine (approval no. 2024104001;
Zhumadian, China). Mice that reached the humane endpoints
(including but not limited to >15% weight loss, inability to eat
or drink, and signs of extreme distress or pain) or completed the
experiments were euthanized. In the present study, no mice reached
the predefined humane endpoints before the end of the
experiments.
Cell culture
MH-S mouse alveolar macrophages (cat. no. GNM43; The
Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences) were cultured in RPMI 1640 complete medium (Beijing
Solarbio Science & Technology Co., Ltd.) supplemented with 10%
fetal bovine serum (Lonsera; Shanghai Shuangru Biotechnology Co.,
Ltd.). The RPMI 1640 medium used in the experiment contains 1%
penicillin and streptomycin. The cells were divided into the
following five groups: Control group; model group (100 ng/ml LPS);
and baicalein groups (40, 20 and 10 µM). With the exception of the
control group, the other groups were stimulated with LPS. Cells
were stimulated with LPS (100 ng/ml) and baicalein (40, 20 and 10
µM) in an incubator at 37°C and 5% CO2. After 6 h of
treatment, cells were harvested for testing.
Wet/dry (W/D) lung weight ratio
The left lungs obtained from the mice were washed
with saline and dried, and the wet weight of the lungs was then
measured. Subsequently, the left lungs were dried overnight in an
oven at 65°C and the dry weight was measured. The W/D ratio was
calculated based on the wet and dry weights.
BALF collection and protein assay
The left lungs were lavaged three times with 0.6 ml
PBS to collect BALF, and the protein concentration in the BALF was
then determined using a BCA kit. In addition, BALF was centrifuged
at 1,000 × g for 10 min at 4°C, and the cell pellet and supernatant
of BALF were collected separately. Subsequently, the cell pellet
was resuspended in PBS and total cells were counted using a
hemocytometer.
Evaluation of pathological changes in
lung tissues
The upper lobes of the left lungs were fixed in 4%
paraformaldehyde for 48 h at room temperature, embedded in
paraffin, sectioned (4 µm) and stained with 1% hematoxylin for 10
min at room temperature and 1% eosin for 1 min at room temperature.
Subsequently, pathological changes in the lung tissues were
observed under a light microscope. The lung tissues stained with
hematoxylin and eosin (H&E) were analyzed based on the Szapiel
score (26).
Measurement of lactic acid
The levels of lactic acid in lung tissues and cell
supernatants were measured using a lactate colorimetric assay kit
(cat. no. E-BC-K044-M; Elabscience®; Elabscience
Bionovation Inc.), according to the manufacturer's instructions.
The absorbance was then measured at 530 nm and lactic acid levels
in the intervention groups were normalized to those of the control
group, as previously described (27).
Immunohistochemistry (IHC)
The aforementioned paraffin-embedded tissue sections
from the upper lobes of the left lungs were deparaffinized with
xylene and rehydrated in a graded alcohol series (100, 90 and 70%).
The sections were then heated in 10 mM sodium citrate buffer (pH
6.0), for 15 min in a 95°C water bath for antigen retrieval. The
sections were then washed with PBS three times (3 min each).
Endogenous peroxidase activity was blocked by incubation with 3%
H2O2 for 15 min at room temperature, and
nonspecific immunoreactions were blocked using 5% inactivated goat
serum (Beijing Solarbio Science & Technology Co., Ltd.) in PBS
for 30 min at room temperature. The sections were then incubated
with HK2 (1:200), PFK1 (1:200), PKM2 (1:400) and HIF-1α (1:200)
antibodies overnight at 4°C. After washing with PBS, the sections
were incubated with goat anti-rabbit IgG-HRP secondary antibody
(1:500; cat. no. sc-2030; Santa Cruz Biotechnology, Inc.) for 1 h
at room temperature. After counterstaining with hematoxylin for 5
min at room temperature, images of the sections were captured using
the light microscope (Carl Zeiss AG).
Immunofluorescence staining
The paraffin-embedded lung tissue was cut in 4-µm
sections. After dewaxing and rehydrating, the slides were subjected
to antigen retrieval, by immersing in 10 mM sodium citrate (pH 6.0)
and microwaving at 1,000 W for 30 min. The sections were then
blocked with 5% inactivated goat serum at room temperature for 30
min. Subsequently, the sections were incubated with an antibody
against HIF-1α (1:400) at 4°C overnight. Subsequently, Alexa
Fluor® 488-conjugated Goat Anti-Rabbit IgG H&L
(1:1,000; cat. no. 4412; Cell Signaling Technology, Inc.) was added
and incubated at 37°C for 1 h. The sections were incubated with
DAPI (cat. no. 4083; Cell Signaling Technology, Inc.) in the dark
at room temperature for 5 min for nuclear counterstaining. The
collected images were captured under a laser confocal microscope
(LSM700; Carl Zeiss AG).
Enzyme-linked immunosorbent assay
(ELISA)
The concentrations of TNF-α and IL-6 in mouse BALF,
serum and cell supernatants were measured using the TNF-α ELISA kit
(cat. no. E-EL-M3063; Elabscience®; Elabscience
Bionovation Inc.) and the IL-6 ELISA kit (cat. no. E-EL-M0044;
Elabscience®; Elabscience Bionovation Inc.), according
to the manufacturer's instructions and previous studies (28).
Cell viability assays
Cell viability was detected using the Cell Counting
Kit-8 (CCK-8) assay kit (Dojindo Molecular Technologies, Inc.).
Briefly, cells (3×103 cells/well) were seeded into
96-well plates and incubated at 37°C with 5% CO2. After
incubation with baicalein at varying concentrations (40, 20, 10, 5
and 2.5 µM) for 6 h, 10 µl CCK-8 solution was added to each well
and incubated for an additional 4 h at 37°C. Absorbance was
subsequently measured at a wavelength of 450 nm.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and was reverse-transcribed into cDNA using a
HiScript® II 1st Strand cDNA Synthesis Kit (cat. no.
R211-01; Vazyme Biotech Co., Ltd.), according to the manufacturer's
instructions. The mRNA expression levels of HK2, PFK1, PKM2 and
HIF-1α were detected using AceQ® qPCR SYBR®
Green Master Mix (cat. no. Q111-02; Vazyme Biotech Co., Ltd.). mRNA
expression levels were normalized to ACTB and calculated using the
2−ΔΔCq method (29).
The primer sequences used are listed in Table I. The following thermocycling
conditions were used for qPCR: 95°C for 30 sec, followed by 40
cycles at 95°C for 10 sec and 60°C for 30 sec, 95°C for 15 sec, and
final extension at 60°C for 60 sec and 95°C for 15 sec.
 | Table I.Primers used in the present
study. |
Table I.
Primers used in the present
study.
| Gene | Forward, 5′-3′ | Reverse, 5′-3′ |
|---|
| ACTB |
GTGACGTTGACATCCGTAAAGA |
GCCGGACTCATCGTACTCC |
| HIF-1α |
TCTCGGCGAAGCAAAGAGTC |
AGCCATCTAGGGCTTTCAGATAA |
| HK2 |
TGATCGCCTGCTTATTCACGG |
AACCGCCTAGAAATCTCCAGA |
| PFK1 |
GGAGGCGAGAACATCAAGCC |
CGGCCTTCCCTCGTAGTGA |
| PKM2 |
GCCGCCTGGACATTGACTC |
CCATGAGAGAAATTCAGCCGAG |
| IL-6 |
CTGCAAGAGACTTCCATCCAG |
AGTGGTATAGACAGGTCTGTTGG |
| TNF-α |
CTGAACTTCGGGGTGATCGG |
GGCTTGTCACTCGAATTTTGAGA |
Acquisition of ALI-related
targets
ALI-associated genes were identified using the
GeneCards (https://www.genecards.org) database
with ‘acute lung injury’ used as the key word. The targets of
baicalein were obtained through screening in the
SwissTargetPrediction database (http://old.swisstargetprediction.ch).
Protein-protein interaction (PPI)
network construction and core target screening
The overlapping targets between baicalein and ALI
were imported into the STRING v11.5 database (https://string-db.org/) and a PPI network was
constructed using Cytoscape software (version 3.7.1; http://cytoscape.org/). The top 100 genes were
identified as core targets using the Cytohubba (https://apps.cytoscape.org/apps/cytohubba) (30), a plugin in Cytoscape software.
Gene Ontology (GO) term and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway enrichment
analyses
The overlapping targets were analyzed for GO term
and KEGG pathway enrichment using the DAVID database (https://david.ncifcrf.gov). GO terms and KEGG pathways
with P<0.05 were considered significantly enriched. Notably, no
specific gene count thresholds were applied in the analysis.
Component and target molecular docking were performed using
AutoDock Tools 1.5.6 software (The Scripps Research Institute, La
Jolla, CA, USA).
Statistical analysis
The data from cell experiments are from three
independent experiments consisting of three replicates per
experiment. All experimental data were analyzed using SPSS 23.0
software (IBM Corp.). Data are presented as the mean ± SEM.
Non-parametric data were analyzed using the Kruskal-Wallis test and
Dunn's multiple comparison test. The differences among multiple
groups were analyzed by one-way ANOVA, followed by Dunnett's T3 for
pairwise comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baicalein improves LPS-induced ALI in
mice
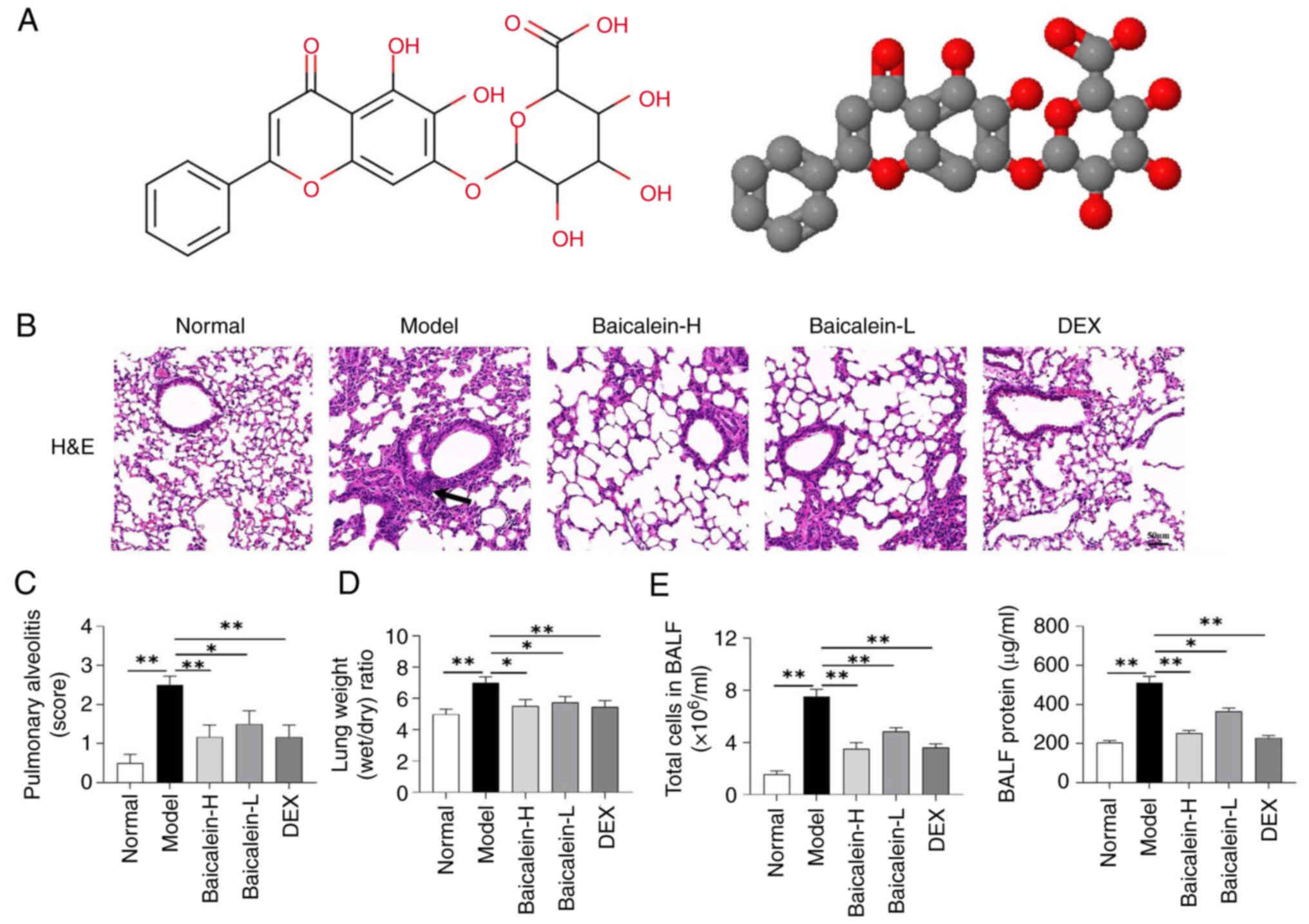
To evaluate the therapeutic effects of baicalein
(Fig. 1A) on ALI, lung tissue
pathology, W/D weight ratio, the number of cells and the total
protein concentration in the BALF of mice were assessed. As a
well-accepted drug in treating lung injury with strong clinical
evidence, DEX was used as a positive control. H&E staining
showed that baicalein could markedly inhibit LPS-induced
inflammatory cell infiltration, alveolar wall edema and thickening
(Fig. 1B and C). Furthermore,
baicalein reduced the lung W/D weight ratio (Fig. 1D), the total number of cells and
the total protein concentration in the BALF of mice (Fig. 1E) compared with those in the model
group. These results suggested that baicalein could improve
LPS-induced lung injury, alveolar-capillary barrier dysfunction and
pulmonary edema.
Potential target screening and
analysis of baicalein in ALI
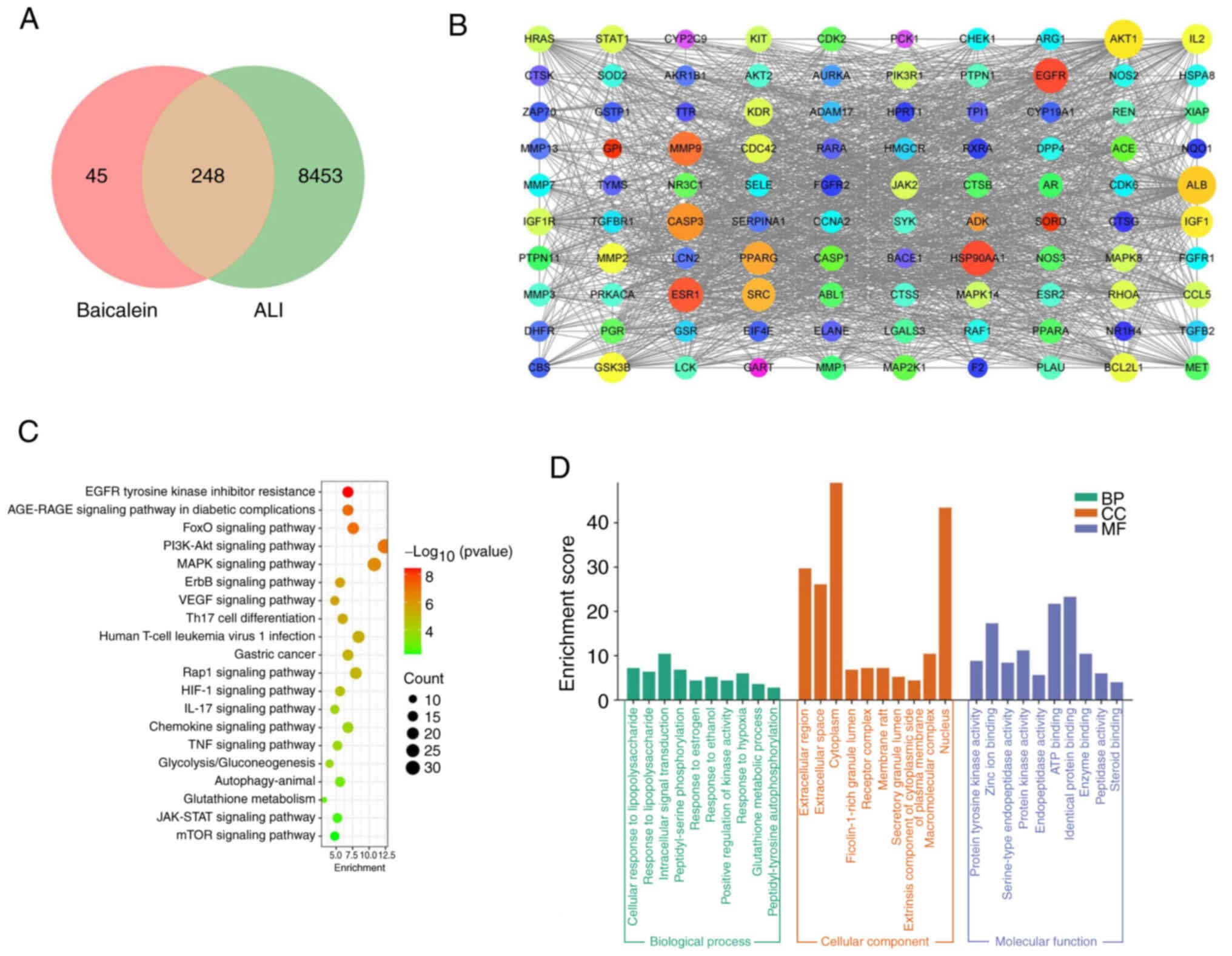
Bioinformatics analysis using the
SwissTargetPrediction and GeneCards databases predicted 293 targets
of baicalein and 8,701 ALI-related genes, respectively. The
intersection of baicalein-related targets and ALI-related genes
revealed a total of 248 overlapping targets (Fig. 2A). The PPI of the top 100
overlapping targets was analyzed using PPI network analysis
(Fig. 2B). KEGG enrichment
analysis with P<0.05 showed that the aforementioned overlapping
targets were enriched in ‘MAPK signaling pathway’, ‘HIF-1 signaling
pathway’ and ‘Glycolysis/Gluconeogenesis’ (Fig. 2C). The GO functional enrichment
analysis results with P<0.05 indicated that the overlapping
targets were associated with ‘cellular response to
lipopolysaccharide’ and ‘response to lipopolysaccharide’ (Fig. 2D).
Baicalein inhibits the inflammatory
response in LPS-induced macrophages and mice with LPS-induced
ALI
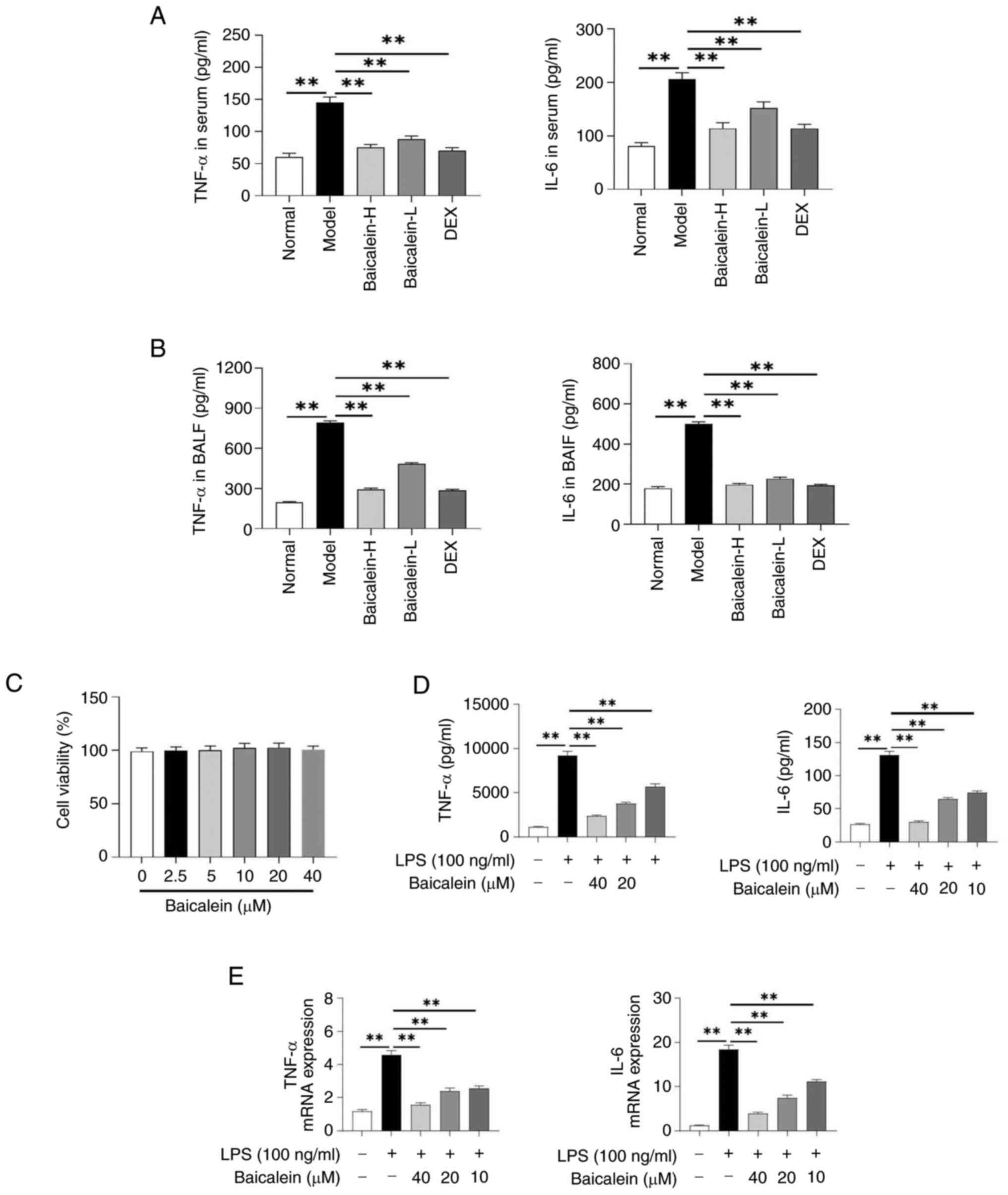
The inflammatory response is a key pathological
process in ALI; therefore, the level of inflammation in mice with
ALI was first evaluated. The results showed that baicalein
significantly reduced the levels of IL-6 and TNF-α in the serum and
BALF of mice compared with those in the model group mice (Fig. 3A and B). Additionally, cell
viability was measured to assess the cytotoxicity of baicalein on
MH-S cells. The result showed that the cell viability was not
significantly affected by baicalein treatment (Fig. 3C). Furthermore, baicalein
significantly inhibited the levels of IL-6 and TNF-α in LPS-induced
MH-S cells (Fig. 3D and E).
Baicalein inhibits glycolysis in
LPS-induced macrophages and in the lung tissues of mice with
LPS-induced ALI
Glycolysis serves a significant role in the
inflammatory response; therefore, the present study aimed to
explore whether baicalein could attenuate the inflammatory response
via inhibiting glycolysis. The results showed that baicalein could
inhibit the expression of key glycolysis-related enzymes (HK2, PFK1
and PKM2) in the lungs of mice with LPS-induced ALI and in
LPS-induced macrophages (Fig. 4A-D and
F). In addition, baicalein reduced lactate content in the lung
tissues of LPS-induced ALI mice and in LPS-induced macrophages
(Fig. 4E and G).
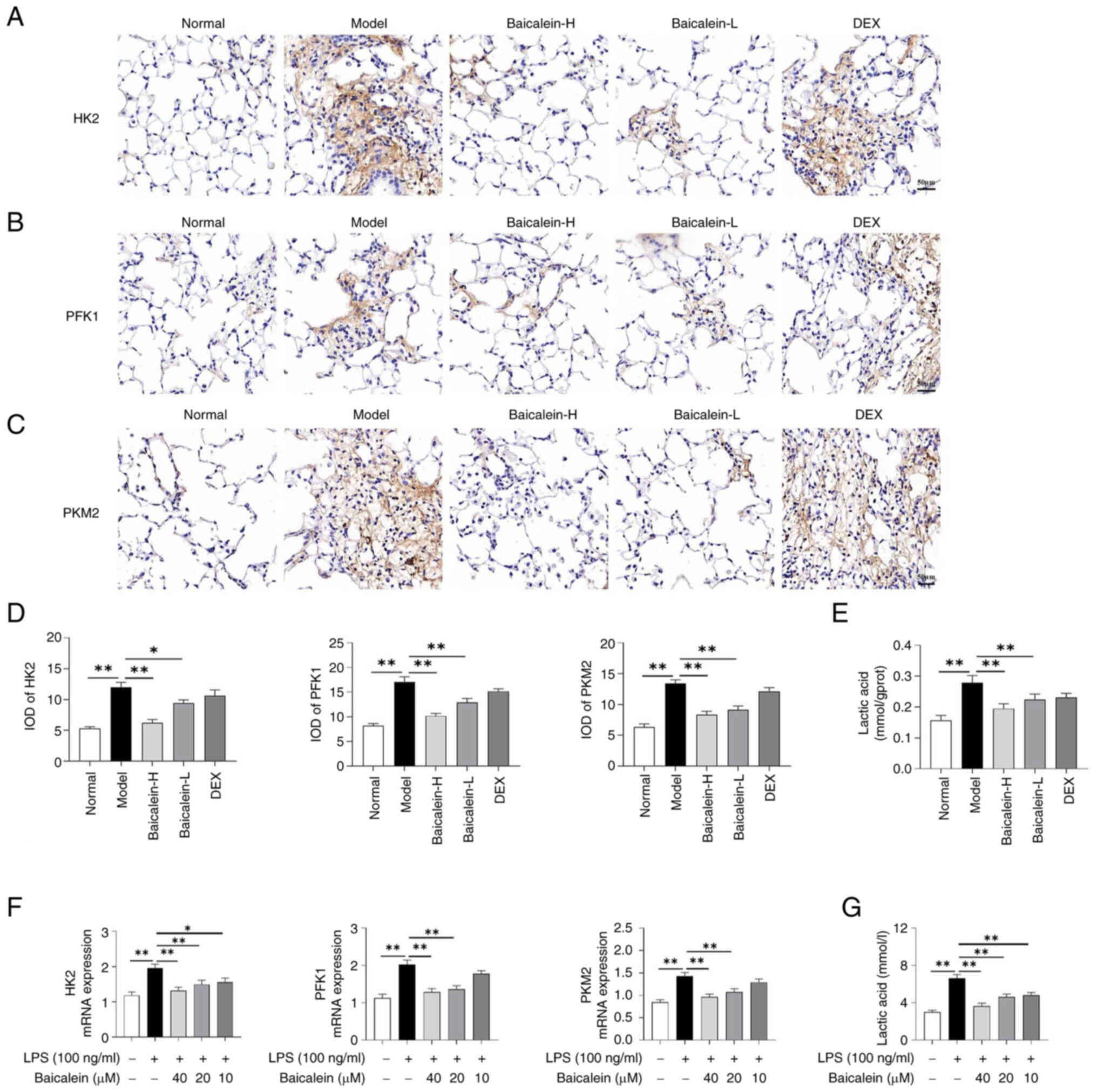 | Figure 4.Baicalein inhibits glycolysis in
LPS-induced macrophages and in the lung tissues of mice with
LPS-induced acute lung injury. Immunohistochemical staining images
of (A) HK2, (B) PFK1 and (C) PKM2 (magnification, ×200) and (D) IOD
values in the lungs. (E) Lactate content in the lung tissues. (F)
mRNA expression levels of HK2, PFK1 and PKM2 in macrophages. (G)
Lactate content in macrophages. Data are presented as the mean ±
SEM. *P<0.05, **P<0.01. DEX, dexamethasone; H, high; HK2,
hexokinase 2; IOD, integrated optical density; L, low; LPS,
lipopolysaccharide; PFK1, phosphofructokinase-1; PKM2, pyruvate
kinase M2. |
Baicalein suppresses HIF-1α signaling
in LPS-induced macrophages and in the lung tissues of mice with
LPS-induced ALI
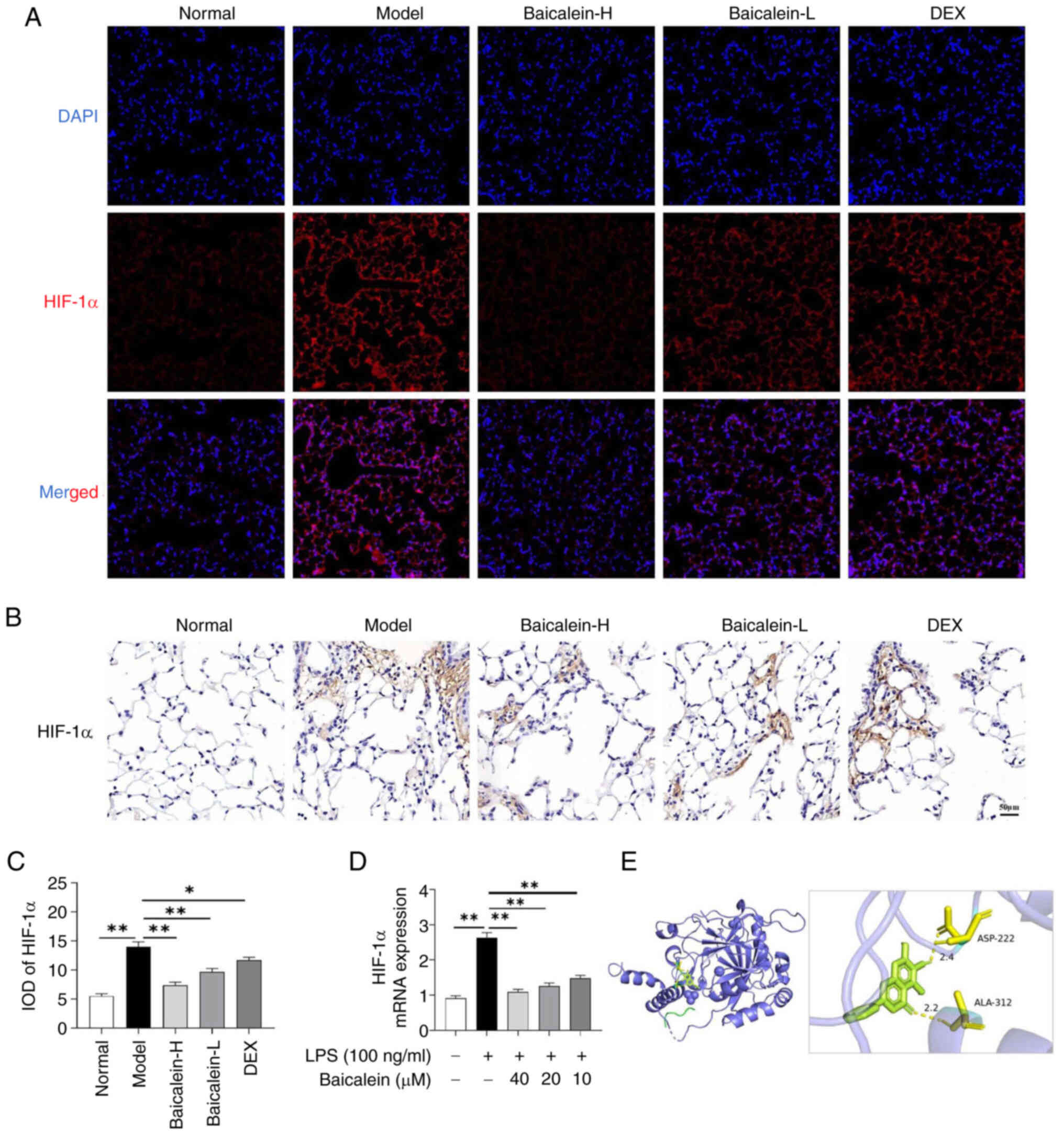
HIF-1α is considered a significant activator of
glycolysis and inflammatory responses. In the present study, the
expression levels of HIF-1α were detected in the lung tissues of
mice with ALI and in LPS-induced macrophages. Notably, baicalein
could significantly inhibit the levels of HIF-1α in the lungs of
LPS-induced ALI mice (Fig. 5A-C)
and in LPS-induced macrophages (Fig.
5D). Furthermore, molecular docking experiments predicted that
baicalein could interact with HIF-1α through the ASP-222 and
ALA-312 residues (Fig. 5E).
Discussion
ALI is a respiratory disease associated with a high
mortality rate, which has a notable impact on public health and is
accompanied by acute inflammatory responses (31). Currently, the main interventions
for patients with ALI include protective mechanical ventilation,
anti-inflammatory drugs and corticosteroids (32). The primary pathological features of
ALI commonly include release of inflammatory mediators, pulmonary
edema and the destruction of alveolar structure (33). Therefore, intervening in
inflammatory responses could be beneficial for patients with ALI.
Baicalein possesses various pharmacological effects, such as
scavenging oxygen free radicals (21), antipyretic and analgesic effects,
and anti-inflammatory (22),
anti-angiogenic (23) and
antitumor properties (24). In
addition, it can alleviate intestinal disorders through exerting
regulatory effects on intestinal microorganisms and short-chain
fatty acid production (34).
Therefore, the present study aimed to evaluate the therapeutic
effects of baicalein on ALI. The mechanism underlying the effects
of baicalein intervention on ALI was predicted using network
analysis. Finally, in vitro and in vivo experiments
suggested that baicalein could improve ALI by inhibiting the
inflammatory response through suppressing glycolysis via HIF-1α
signaling.
The mouse model of LPS-induced ALI has been widely
used in basic research on ALI-related diseases due to its maturity,
reliability and high reproducibility (35,36).
After entering the body, LPS binds to cell surface receptors to
stimulate the recruitment and infiltration of inflammatory cells,
and to increase the synthesis and release of inflammatory
mediators, eventually leading to the destruction of lung tissue
structure and functional impairment (37,38).
Therefore, inhibition or alleviation of the inflammatory response
may be an effective intervention measure for ALI. Herein, the
results showed that baicalein could improve LPS-induced ALI in
mice.
Network analysis is commonly used to predict the
targets of active ingredients in treating corresponding diseases
(39,40). In the present study, network
analysis was used to predict the targets of baicalein and genes
associated with ALI. The results showed that the overlapping
baicalein-related targets and ALI-related genes were mainly
associated with the ‘Glycolysis/Gluconeogenesis’ and ‘HIF-1
signaling pathway’. Emerging evidence has suggested that crosstalk
exists between inflammatory processes and metabolic dysregulation
(41). During inflammation, cells
experience metabolic shifts, including a heightened rate of glucose
uptake and an increased production of lactic acid (42). This metabolic change, also known as
glycolysis, induces the recruitment of inflammatory cells to the
site of the inflammation (43).
Glycolysis is a metabolic pathway, which provides the energy
necessary for cellular survival, proliferation and differentiation
(44,45). HK2 is considered the key metabolic
enzyme that catalyzes the first step of glycolysis via
phosphorylating glucose to produce glucose-6-phosphate (46). PFK1 is the rate-limiting enzyme in
the glycolytic pathway, which converts fructose-6-phosphate to
fructose-1,6-bisphosphate (47).
The third key enzyme in glycolysis is pyruvate kinase, which
catalyzes the production of pyruvate, thus promoting energy supply
and amino acid synthesis metabolism (48). In addition to providing energy to
cells, the glycolytic pathway has been reported to be associated
with the occurrence of inflammation (49). Under inflammatory stimuli, such as
infection, endotoxins and hypoxia, resting macrophages can polarize
into pro-inflammatory macrophages, glycolysis metabolism becomes
active and large amounts of pro-inflammatory factors, such as TNF-α
and IL-1β, can be released (15).
A previous study suggested that the aforementioned inflammatory
factors could aggravate cell membrane damage, thus leading to LDH
leakage (50), which in turn could
further catalyze the conversion of pyruvate to lactate and
aggravate lung injury. LDH is a marker of cell necrosis, as it is
released into the extracellular space upon cell membrane
disruption. The role of cytokines in inducing cell necrosis and the
subsequent release of LDH have been addressed to connect the
inflammatory processes with cellular damage (51). As a metabolic by-product, lactate
accumulation is influenced by cytokine activity and is associated
with metabolic disorders (52).
Therefore, lactate accumulation could serve as a biomarker
reflecting the interplay between inflammation and metabolic
dysregulation. In the current study, baicalein reduced the levels
of glycolysis-related enzymes and lactate content in the lung
tissues of ALI mice and LPS-induced macrophages.
Glycolysis is regulated by several glycolytic key
enzymes and HIF-1α (53,54). HIF-1α upregulation can directly
induce the excessive expression of glycolysis-related genes, such
as HK and LDH, thus accelerating the glycolytic process (55). Enhanced expression of HIF-1α may
also increase the expression of IL-1β and activate immune cells in
the local environment (55). In
the present study, baicalein downregulated HIF-1α in the lung
tissue of mice with ALI and in LPS-induced macrophages.
In conclusion, the results of the present study
indicated that baicalein could improve ALI by suppressing
inflammatory responses through inhibiting glycolysis via HIF-1α
signaling (Fig. 6). These results
supported the anti-inflammatory effects of baicalein on ALI and
suggested that targeting glycolysis-related catalytic enzymes to
inhibit glycolysis could be a promising therapeutic strategy for
ALI.
Acknowledgements
Not applicable.
Funding
This study was supported by the Top Talent Training Project of
Traditional Chinese Medicine in Henan Province (grant no.
2022ZYBJ30).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZL participated in the study design and manuscript
writing. XZ performed experiments, analyzed data and participated
in manuscript writing. NL and ZW participated in statistical
analysis and manuscript writing. ZL and XZ confirm the authenticity
of all the raw data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Zhumadian Hospital of Traditional Chinese Medicine
(approval no. 2024104001; Zhumadian, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Long ME, Mallampalli RK and Horowitz JC:
Pathogenesis of pneumonia and acute lung injury. Clin Sci (Lond).
136:747–769. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson ER and Matthay MA: Acute lung
injury: Epidemiology, pathogenesis, and treatment. J Aerosol Med
Pulm Drug Deliv. 23:243–252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schmidt GA: Managing Acute Lung Injury.
Clin Chest Med. 37:647–658. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fanelli V and Ranieri VM: Mechanisms and
clinical consequences of acute lung injury. Ann Am Thorac Soc. 12
(Suppl 1):S3–S8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang X, Xiu H, Zhang S and Zhang G: The
Role of Macrophages in the Pathogenesis of ALI/ARDS. Mediators
Inflamm. 2018:12649132018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dzopalic T, Bozic-Nedeljkovic B and
Jurisic V: Function of innate lymphoid cells in the immune-related
disorders. Hum Cell. 32:231–239. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakaguchi M, Marutani E, Shin HS, Chen W,
Hanaoka K, Xian M and Ichinose F: Sodium thiosulfate attenuates
acute lung injury in mice. Anesthesiology. 121:1248–1257. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kosutova P, Kolomaznik M, Calkovska A,
Mokra D and Mikolka P: Nitric-Oxide-releasing dexamethasone
derivative NCX-1005 improves lung function and attenuates
inflammation in experimental lavage-induced ARDS. Pharmaceutics.
13:20922021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xia L, Zhang C, Lv N, Liang Z, Ma T, Cheng
H, Xia Y and Shi L: AdMSC-derived exosomes alleviate acute lung
injury via transferring mitochondrial component to improve
homeostasis of alveolar macrophages. Theranostics. 12:2928–2947.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gopalakrishnan A, Joseph J, Shirey KA,
Keegan AD, Boukhvalova MS, Vogel SN and Blanco JCG: Protection
against influenza-induced Acute Lung Injury (ALI) by enhanced
induction of M2a macrophages: Possible role of PPARγ/RXR ligands in
IL-4-induced M2a macrophage differentiation. Front Immunol.
13:9683362022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu S, Tang W, Liu L, Wei K, Tang Y, Ma J,
Li H and Ao Y: Obesity-induced downregulation of miR-192
exacerbates lipopolysaccharide-induced acute lung injury by
promoting macrophage activation. Cell Mol Biol Lett. 29:362024.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong WJ, Yang HH, Guan XX, Xiong JB, Sun
CC, Zhang CY, Luo XQ, Zhang YF, Zhang J, Duan JX, et al: Inhibition
of glycolysis alleviates lipopolysaccharide-induced acute lung
injury in a mouse model. J Cell Physiol. 234:4641–4654. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Cao Y, Gorshkov B, Zhou Y, Yang Q,
Xu J, Ma Q, Zhang X, Wang J, Mao X, et al: Ablation of endothelial
Pfkfb3 protects mice from acute lung injury in LPS-induced
endotoxemia. Pharmacol Res. 146:1042922019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jurisic V, Radenkovic S and Konjevic G:
The Actual Role of LDH as tumor marker, biochemical and clinical
aspects. Adv Exp Med Biol. 867:115–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soto-Heredero G, Gómez de Las Heras MM,
Gabande-Rodriguez E, Oller J and Mittelbrunn M: Glycolysis-a key
player in the inflammatory response. FEBS J. 287:3350–3369. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jurisic V, Bumbasirevic V, Konjevic G,
Djuricic B and Spuzic I: TNF-alpha induces changes in LDH isotype
profile following triggering of apoptosis in PBL of non-Hodgkin's
lymphomas. Ann Hematol. 83:84–91. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Corcoran SE and O'Neill LA: HIF1α and
metabolic reprogramming in inflammation. J Clin Invest.
126:3699–3707. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dang B, Gao Q, Zhang L, Zhang J, Cai H,
Zhu Y, Zhong Q, Liu J, Niu Y, Mao K, et al: The glycolysis/HIF-1α
axis defines the inflammatory role of IL-4-primed macrophages. Cell
Rep. 42:1124712023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhuang H, Lv Q, Zhong C, Cui Y, He L,
Zhang C and Yu J: Tiliroside ameliorates ulcerative colitis by
restoring the M1/M2 Macrophage Balance via the HIF-1α/glycolysis
Pathway. Front Immunol. 12:6494632021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sowndhararajan K, Deepa P, Kim M, Park SJ
and Kim S: Baicalein as a potent neuroprotective agent: A review.
Biomed. Pharmacother. 95:1021–1032. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu BY, Li L, Liu GL, Ding W, Chang WG, Xu
T, Ji XY, Zheng XX, Zhang J and Wang JX: Baicalein attenuates
cardiac hypertrophy in mice via suppressing oxidative stress and
activating autophagy in cardiomyocytes. Acta Pharmacol Sin.
42:701–714. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ren M, Zhao Y, He Z, Lin J, Xu C, Liu F,
Hu R, Deng H and Wang Y: Baicalein inhibits inflammatory response
and promotes osteogenic activity in periodontal ligament cells
challenged with lipopolysaccharides. BMC Complement Med Ther.
21:432021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li B, Chen K, Qian N, Huang P, Hu F, Ding
T, Xu X, Zhou Q, Chen B, Deng L, et al: Baicalein alleviates
osteoarthritis by protecting subchondral bone, inhibiting
angiogenesis and synovial proliferation. J Cell Mol Med.
25:5283–5294. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang R, Wang C, Lu L, Yuan F and He F:
Baicalin and baicalein in modulating tumor microenvironment for
cancer treatment: A comprehensive review with future perspectives.
Pharmacol. Res. 199:1070322024.PubMed/NCBI
|
|
25
|
Ning B, Shen J, Liu F, Zhang H and Jiang
X: Baicalein Suppresses NLRP3 and AIM2 Inflammasome-Mediated
Pyroptosis in Macrophages Infected by Mycobacterium tuberculosis
via Induced Autophagy. Microbiol Spectr. 11:e4711222023. View Article : Google Scholar
|
|
26
|
Szapiel SV, Elson NA, Fulmer JD,
Hunninghake GW and Crystal RG: Bleomycin-induced interstitial
pulmonary disease in the nude, athymic mouse. Am Rev Respir.
120:893–899. 1979.PubMed/NCBI
|
|
27
|
Li Q, Peng J, Luo Y, Zhou J, Li T, Cao L,
Peng S, Zuo Z and Wang Z: Far infrared light irradiation enhances
Aβ clearance via increased exocytotic microglial ATP and
ameliorates cognitive deficit in Alzheimer's disease-like mice. J
Neuroinflammation. 19:1452022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jurisic V: Multiomic analysis of cytokines
in immuno-oncology. Expert Rev Proteomics. 17:663–674. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT and
Lin CY: cytoHubba: Identifying hub objects and sub-networks from
complex interactome. BMC Syst Biol. 8 (Suppl 4):S112014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie WM, Su W, Liu XY, Zhou J, Wang M, Wang
Y, Wang W, Bai X, Li Z and Li T: FTO Deficiency Alleviate
LPS-induced ALI by TXNIP/NLPR3-mediated alveolar epithelial cell
pyroptosis. Am J Respir Cell Mol Biol. 70:351–363. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kligerman S: Pathogenesis, imaging, and
evolution of acute lung injury. Radiol Clin North Am. 60:925–939.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rubenfeld GD and Herridge MS: Epidemiology
and outcomes of acute lung injury. Chest. 131:554–562. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu Q, Hou S, Xiong B, Wen Y, Wang J, Zeng
J, Ma X and Wang F: Therapeutic effects of baicalin on diseases
related to gut-brain axis dysfunctions. Molecules. 28:65012023.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li J, Deng SH, Li J, Li L, Zhang F, Zou Y,
Wu DM and Xu Y: Obacunone alleviates ferroptosis during
lipopolysaccharide-induced acute lung injury by upregulating
Nrf2-dependent antioxidant responses. Cell Mol Biol Lett.
27:292022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li J, Lu K, Sun F, Tan S, Zhang X, Sheng
W, Hao W, Liu M, Lv W and Han W: Panaxydol attenuates ferroptosis
against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1
pathway. J Transl Med. 19:962021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fukatsu M, Ohkawara H, Wang X, Alkebsi L,
Furukawa M, Mori H, Fukami M, Fukami SI, Sano T, Takahashi H, et
al: The suppressive effects of Mer inhibition on inflammatory
responses in the pathogenesis of LPS-induced ALI/ARDS. Sci Signal.
15:d25332022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luo L, Huang F, Zhong S, Ding R, Su J and
Li X: Astaxanthin attenuates ferroptosis via Keap1-Nrf2/HO-1
signaling pathways in LPS-induced acute lung injury. Life Sci.
311((Pt A)): 1210912022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang P, Zhang D, Zhou W, Wang L, Wang B,
Zhang T and Li S: Network pharmacology: Towards the artificial
intelligence-based precision traditional Chinese medicine. Brief
Bioinform. 25:bbad5182023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nogales C, Mamdouh ZM, List M, Kiel C,
Casas AI and Schmidt HHHW: Network pharmacology: Curing causal
mechanisms instead of treating symptoms. Trends Pharmacol Sci.
43:136–150. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Palomer X, Salvado L, Barroso E and
Vazquez-Carrera M: An overview of the crosstalk between
inflammatory processes and metabolic dysregulation during diabetic
cardiomyopathy. Int J Cardiol. 168:3160–3172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
O'Neill LA and Hardie DG: Metabolism of
inflammation limited by AMPK and pseudo-starvation. Nature.
493:346–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jha MK, Song GJ, Lee MG, Jeoung NH, Go Y,
Harris RA, Park DH, Kook H, Lee IK and Suk K: Metabolic connection
of inflammatory pain: Pivotal role of a pyruvate dehydrogenase
kinase-pyruvate dehydrogenase-lactic acid axis. J Neurosci.
35:14353–14369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen Z, Liu M, Li L and Chen L:
Involvement of the Warburg effect in non-tumor diseases processes.
J Cell Physiol. 233:2839–2849. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gan P, Zhang S and Fred Wong WS: Targeting
reprogrammed metabolism as a therapeutic approach for respiratory
diseases. Biochem. Pharmacol. 228:1161872024.PubMed/NCBI
|
|
46
|
Chen J, Li G, Sun D, Li H and Chen L:
Research progress of hexokinase 2 in inflammatory-related diseases
and its inhibitors. Eur J Med Chem. 264:1159862024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Webb BA, Dosey AM, Wittmann T, Kollman JM
and Barber DL: The glycolytic enzyme phosphofructokinase-1
assembles into filaments. J Cell Biol. 216:2305–2313. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Israelsen WJ and Vander Heiden MG:
Pyruvate kinase: Function, regulation and role in cancer. Semin
Cell Dev Biol. 43:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Puleston DJ, Villa M and Pearce EL:
Ancillary Activity: Beyond core metabolism in immune cells. Cell
Metab. 26:131–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang C, Wang Y, Song D, Su J and Zhang F:
Therapeutic Effects of Modified Si-Miao-Yong-An Decoction in the
Treatment of Rat Myocardial Ischemia/Reperfusion Injury. Evid Based
Complement Alternat Med. 2022:14424052022.PubMed/NCBI
|
|
51
|
Gomez A, Serrano A, Salero E, Tovar A,
Amescua G, Galor A, Keane RW, de Rivero Vaccari JP and Sabater AL:
Tumor necrosis factor-alpha and interferon-gamma induce
inflammasome-mediated corneal endothelial cell death. Exp Eye Res.
207:1085742021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fang Y, Li Z, Yang L, Li W, Wang Y, Kong
Z, Miao J, Chen Y, Bian Y and Zeng L: Emerging roles of lactate in
acute and chronic inflammation. Cell Commun Signal. 22:2762024.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Qiu B, Yuan P, Du X, Jin H, Du J and Huang
Y: Hypoxia inducible factor-1α is an important regulator of
macrophage biology. Heliyon. 9:e171672023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Azzam HN, El-Derany MO, Wahdan SA, Faheim
RM, Helal GK and El-Demerdash E: The role of
mitochondrial/metabolic axis in development of tamoxifen resistance
in breast cancer. Hum Cell. 36:1877–1886. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kierans SJ and Taylor CT: Regulation of
glycolysis by the hypoxia-inducible factor (HIF): Implications for
cellular physiology. J Physiol. 599:23–37. 2021. View Article : Google Scholar : PubMed/NCBI
|