Introduction
Lung cancer is the most commonly diagnosed type of
cancer and the leading cause of cancer-related deaths worldwide. At
present, the number of cases of non-small cell lung cancer, the
main pathological subtype of lung cancer, are increasing annually
(1). Despite notable advancements
in chemotherapy, radiotherapy and molecular targeted therapy, the
5-year survival rate of patients with lung cancer remains at
<20%, and patients exhibit high mortality rates (2). Notably, metastasis is a key cause for
treatment failure and poor survival rates of patients with lung
cancer, leading to 90% of lung cancer-related deaths (3,4).
Thus, the development of novel, effective and reliable therapeutic
drugs is required for the clinical treatment of lung cancer.
Research has focused on the potent anticancer
activity of traditional herbal medicines (5). 4-Methoxydalbergione (4-MD) is a
flavonoid extracted from the heartwood of Dalbergia sissoo
Roxb., which has anti-inflammatory and cytoprotective properties
(6,7). The results of previous studies
revealed that 4-MD exhibits anticancer activity in numerous
malignancies, including bladder cancer, liver cancer, esophageal
carcinoma, astroglioma and osteosarcoma; therefore, 4-MD may have
potential as a therapeutic agent against various types of cancer
(8–12). However, the effects of 4-MD on lung
cancer have yet to be fully elucidated.
The induction of tumor cell death is an approach for
cancer therapy. Ferroptosis, an iron-dependent form of regulated
cell death, is characterized by the accumulation of lipid reactive
oxygen species (ROS), altered mitochondrial morphology and
depletion of the endogenous antioxidant glutathione (GSH).
Moreover, ferroptosis is driven by iron-dependent lipid
peroxidation (13,14), and this process is involved in the
regulation of cancer invasion, angiogenesis and metastasis. The
results of a previous study revealed that the induction of
ferroptosis may exhibit potential as a strategy for the inhibition
of cancer cells and the mitigation of cancer metastasis (15).
The present study aimed to characterize the
anticancer effect of 4-MD on lung cancer in vivo and in
vitro, and to elucidate the molecular mechanism of 4-MD against
lung cancer. These findings suggested 4-MD as a promising
therapeutic drug for lung cancer treatment.
Materials and methods
Cell culture and treatment
The lung cancer cell line A549 was obtained from
Procell Life Science & Technology Co., Ltd., and was cultured
in F12K medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.) and 1% penicillin/streptomycin in a humidified incubator with
5% CO2 at 37°C. Cells were treated with 4-MD (purity,
98%; Shanghai Yuanye Biotechnology Co., Ltd.) dissolved in dimethyl
sulfoxide (DMSO) at an initial concentration of 50 mM. A549 cells
were treated with increasing concentrations of 4-MD (3.125, 6.25,
12.5, 25, 50, 100, 200, 400 and 800 µM) for 24 h at 37°C.
Additionally, A549 cells with or without Oe-DNMT1 transfection were
treated with 50 µM 4-MD for 24 h at 37°C.
Cell viability assay
A total of 1×103 cells were seeded into
96-well plates and cultured at 37°C in a humidified incubator with
5% CO2. At 24, 48 and 72 h, 10 µl CCK-8 solution
(Shanghai Yeasen Biotechnology Co., Ltd.) was added to each well,
and the cells were incubated for a further 3 h at 37°C. The
absorbance of each well was measured at 450 nm using a microplate
reader (Omega Bio-Tek, Inc.).
Colony formation assay
A549 cells were seeded into plates (1,000
cells/well) and were cultivated in medium containing 10% FBS for 2
weeks. When cell colonies were visible to the naked eye, A549 cells
were fixed with 4% paraformaldehyde for 5 min and stained with 0.1%
crystal violet 20 min, both at room temperature. The colonies
(>50 cells) were counted using a light microscope (Olympus
Corporation) and ImageJ 1.8.0 software (version 1.8.0; National
Institutes of Health).
5-Ethynyl-2′-deoxyuridine (EDU)
analysis
A549 cells (5×104 cells/well) were seeded
into 96-well plates and cultured at 37°C for 24 h. In total, 100 µl
diluted EDU solution (Thermo Fisher Scientific, Inc.) was added to
each well, and the cells were cultured for a further 2 h at 37°C.
Subsequently, the cells were fixed with 4% paraformaldehyde for 30
min and permeabilized with 5% Triton X-100 for 15 min at room
temperature. Cell nuclei were stained with DAPI staining solution
(Beyotime Institute of Biotechnology) at 37°C for 30 min.
EDU-positive cells were observed under a fluorescence microscope
(Olympus Corporation).
Wound healing assay
Once A549 cells reached 90% confluence, a scratch
was created on the cell monolayer in a 6-well plate using a 200-µl
pipette tip. The cells were then washed with PBS and were cultured
in serum-free medium at 37°C for 24 h. Images of the wound were
captured at 0 and 24 h using a light microscope (Olympus
Corporation), and wound healing was recorded to assess cell
migration.
Transwell invasion assay
A549 cells (5×105 cells/well) were
resuspended in serum-free medium and seeded into the upper chamber
of a 24-well plate (Transwell insert; 8 µm pores; Corning, Inc.).
To assess cell invasion, the membranes were precoated with Matrigel
overnight at 37°C. The lower chamber was filled with complete
medium supplemented with 10% FBS as a chemoattractant. Following
incubation for 24 h at 37°C, invasive cells were fixed with 100%
methanol for 10 min at 37°C and stained with crystal violet for 15
min at 37°C. Subsequently, images of the cells were captured using
a light microscope (Olympus Corporation) and they were counted.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from 5×105 A549
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and the PrimeScript RT reagent kit (Takara Bio,
Inc.) was used to generate cDNA, according to the manufacturers'
recommendations. The ABI 7500 Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was employed to conduct
qPCR with the SYBR-Green PCR Master Mix (Takara Bio, Inc.)
according to the manufacturer's protocol. The following
thermocycling conditions were used: Initial denaturation at 95°C
for 10 min; followed by 35 cycles of denaturation at 95°C for 15
sec, annealing at 60°C for 1 min and extension of 10 min at 65°C.
The relative mRNA expression levels were computed using the
2−ΔΔCq method (16).
GAPDH was used as the housekeeping control. The following primer
sequences were used for qPCR: DNA methyltransferase 1 (DNMT1),
forward 5′-TATCCGAGGAGGGCTACCTG-3′, reverse,
5′-ATGAGCACCGTTCTCCAAGG-3′; GAP DH, forward
5′-AATGGGCAGCCGTTAGGAAA-3′ and reverse
5′-GCGCCCAATACGACCAAATC-3′.
Western blot analysis
Total protein was extracted from A549 cells or mouse
tissues using radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology). Total protein concentration was
quantified using a bicinchoninic acid assay protein assay kit
(Beyotime Institute of Biotechnology), and 30 µg/lane was then
separated by sodium dodecyl-sulfate polyacrylamide gel
electrophoresis on a 12% gel. Separated proteins were subsequently
transferred to polyvinylidene fluoride membranes (MilliporeSigma)
and blocked with non-fat milk at room temperature for 2 h. The
membranes were then incubated with the following primary
antibodies: Anti-E-cadherin (cat. no. 3195T; 1:1,000; Cell
Signaling Technology, Inc.), anti-N-cadherin (cat. no. 22018-1-AP;
1:5,000; Proteintech Group, Inc.), anti-Vimentin (cat. no. ab92547;
1:1,000; Abcam), anti-DNMT1 (cat. no. ab188453; 1:1,000; Abcam),
anti-solute carrier family 7 member 11 [SLC7A11; also known as
cystine-glutamate antiporter; cat. no. ab307601; 1:1,000; Abcam),
anti-GSH peroxidase 4 (GPX4; cat. no. ab125066; 1:1,000; Abcam),
anti-acyl-CoA synthetase long-chain family 4 (ACSL4; cat. no.
ab155282; 1:10,000; Abcam) and anti-GAPDH (cat. no. ab9485;
1:2,500; Abcam) at 4°C overnight, followed by incubation with a
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (cat. no. ab6721; 1:3,000; Abcam) at room temperature for
2 h. Protein bands were visualized using an enhanced
chemiluminescence kit (Beyotime Institute of Biotechnology) and
were semi-quantified using ImageJ software (version 1.8.0).
Transmission electron microscopy
A549 cells were fixed with 2.5% glutaraldehyde for 5
min at 37°C. Following centrifugation (1,000 × g) at room
temperature for 10 min, the cells were fixed with 1% osmic acid for
2 h at room temperature. Cells were washed with phosphate buffer,
dehydrated using alcohol and acetone, embedded with epoxy resin at
room temperature for 2 h and sectioned (70–80 nm) using an
ultramicrotome. The sections were stained with uranium-lead double
staining (2% uranium acetate and 2.6% lead citrate) for 15 min at
room temperature. Cells were then observed under a transmission
electron microscope (JEM-2000EX; JEOL, Ltd.).
Measurements of lipid peroxidation and
iron levels
Lipid peroxidation was assessed using thiobarbituric
acid reactive substance (TBARS) and lipid ROS assays. For the TBARS
assay (cat. no. C10445; Thermo Fisher Scientific, Inc.),
1×106 cells were incubated with 7.5% trichloroacetic
acid and 0.02% butylated hydroxyanisole for 15 min at room
temperature. Following centrifugation (1,000 × g) at room
temperature for 10 min, the supernatants were harvested, incubated
with trichloroacetic acid and boiled for 10 min. After cooling, the
absorbance was measured at 532 nm using a microplate reader (Omega
Bio-Tek, Inc.).
To determine the levels of lipid peroxidation,
1×106 cells were stained with 10 µM BODIPY (581/591) C11
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 30 min in
the dark. Fluorescent signals were detected at 484/510 and 581/610
nm using a laser scanning confocal microscope (Olympus Corporation)
to determine the levels of lipid ROS.
To determine the levels of Fe2+, 5 ×
104 cells were stained with 1 µM FerroOrange
(Fe2+ indicator; Shanghai Maokang Bio) at 37°C for 30
min in the dark. Fluorescent signals were detected at 532/572 nm
using a fluorescence microscope (Olympus Corporation).
Molecular docking
The target gene of 4-MD was predicted by TargetNet
database (http://targetnet.scbdd.com/). The crystal structure of
DNMT1 was acquired from Protein Data Bank (code, 4WXX; http://www.rcsb.org/). The 3-dimensional structure of
4-MD was obtained from PubChem Database (https://pubchem.ncbi.nlm.nih.gov/). Molecular docking
was used to predict the optimal binding site of 4-MD for binding to
DNMT1 (AutoDock 4.2, http://autodock.scripps.edu/). The optimal binding
mode between 4-MD and DNMT1 was determined under the minimum
binding free energy conformation (−5.9 kcal/mol) and visualized
using PyMol (version, 1.8.2.0; DeLano Scientific, LLC).
Cell transfection
The human full-length DNMT1 was cloned into the
pcDNA3.1 vector (Shanghai GenePharma Co., Ltd.) to generate the
DNMT1-overexpression (Oe) vector (Oe-DNMT1). The empty pcDNA3.1
vector was used as the negative control (Oe-NC). A549 cells were
seeded into 6-well plates and cultured at 37°C in a humidified
incubator with 5% CO2. When cells reached 60–70%
confluence, they were transfected with 50 ng/ml Oe-DNMT1 or Oe-NC
using Lipofectamine® 3000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) at room temperature for 48 h according to
the manufacturer's instructions. At 48 h post-transfection, cells
were harvested for subsequent experiments.
Xenograft experiments
In total, 15 male BALB/c-nu mice (age, 4–6 weeks;
weight, 18–20 g) were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd., and were housed in a standard environment (temp,
20–23°C; humidity, 55±5%; 12-h light/dark cycle) with free access
to water and food. All animal experiments were approved by the
Animal Ethics Committee of The First Affiliated Hospital of Nanjing
Medical University (approval no. IACUC-230814; Nanjing, China) and
were carried out in accordance with the ARRIVE guidelines (17). Animals were divided into three
groups (n=5 per group): Control group, 4-MD 10 mg/kg group and 4-MD
30 mg/kg group. In total, 4×106 A549 cells were
subcutaneously injected into the right flank of each mouse. After 7
days, A549 cell-inoculated mice were intraperitoneally injected
with 4-MD (10 or 30 mg/kg in PBS and 0.01% DMSO) once every 3 days
for 3 weeks. Control mice were injected with the same volume of
solvent (PBS and 0.01% DMSO). Tumor size was measured every 3 days
with a caliper, and tumor volume was calculated using the following
equation: Length × width2/2. Mice were sacrificed 28
days after cell injection with an intraperitoneal injection of 200
mg/kg sodium pentobarbital. Death was confirmed following
observations of respiration, the heartbeat and pupils.
Subsequently, tumors were excised and weighed. The health and
behavior of the animals were monitored every day. A maximum tumor
diameter of 20 mm, as well as tumor ulceration, infection or
necrosis were considered the humane endpoints (18).
Immunohistochemistry (IHC) and
Prussian blue staining
Lung tissues were harvested, fixed with 4%
paraformaldehyde at 4°C for 24 h, embedded in paraffin and cut into
4-µm sections. Following deparaffinization and rehydration in a
descending series of ethanol, antigen retrieval was carried out
using 0.1% sodium citrate buffer at 95°C for 20 min, and sections
were incubated with 3% hydrogen peroxide to block endogenous
peroxidase activity and treated with 0.3% Triton X-100 to increases
the permeability for 30 min at 37°C. For Prussian blue staining,
the sections were immersed in 1% potassium ferricyanide for 1 h at
room temperature. For IHC staining, after blocking the sections
with 10% goat serum (Beijing Solarbio Science & Technology Co.,
Ltd.) for 1 h at 37°C, the sections were incubated with anti-DNMT1
(cat. no. ab188453; 1:100; Abcam) and anti-Ki-67 (cat. no. ab16667;
1:200; Abcam) antibody at 4°C overnight, followed by incubation
with a HRP-conjugated goat anti-rabbit secondary antibody (cat. no.
ab6721; 1:1,000; Abcam) at 37°C for 30 min. Sections were
visualized using DAB, counterstained with hematoxylin for 2 min at
room temperature, mounted in neutral gum and observed under an
optical microscope (Olympus Corporation).
Statistical analysis
All data are presented as the mean ± standard
deviation and all experiments were repeated at least three times.
Statistical analysis was conducted using GraphPad Prism 8 (GraphPad
Software; Dotmatics), and comparisons between groups were
determined using one-way or two-way analysis of variance followed
by Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
4-MD suppresses the proliferation,
migration and invasion of A549 cells
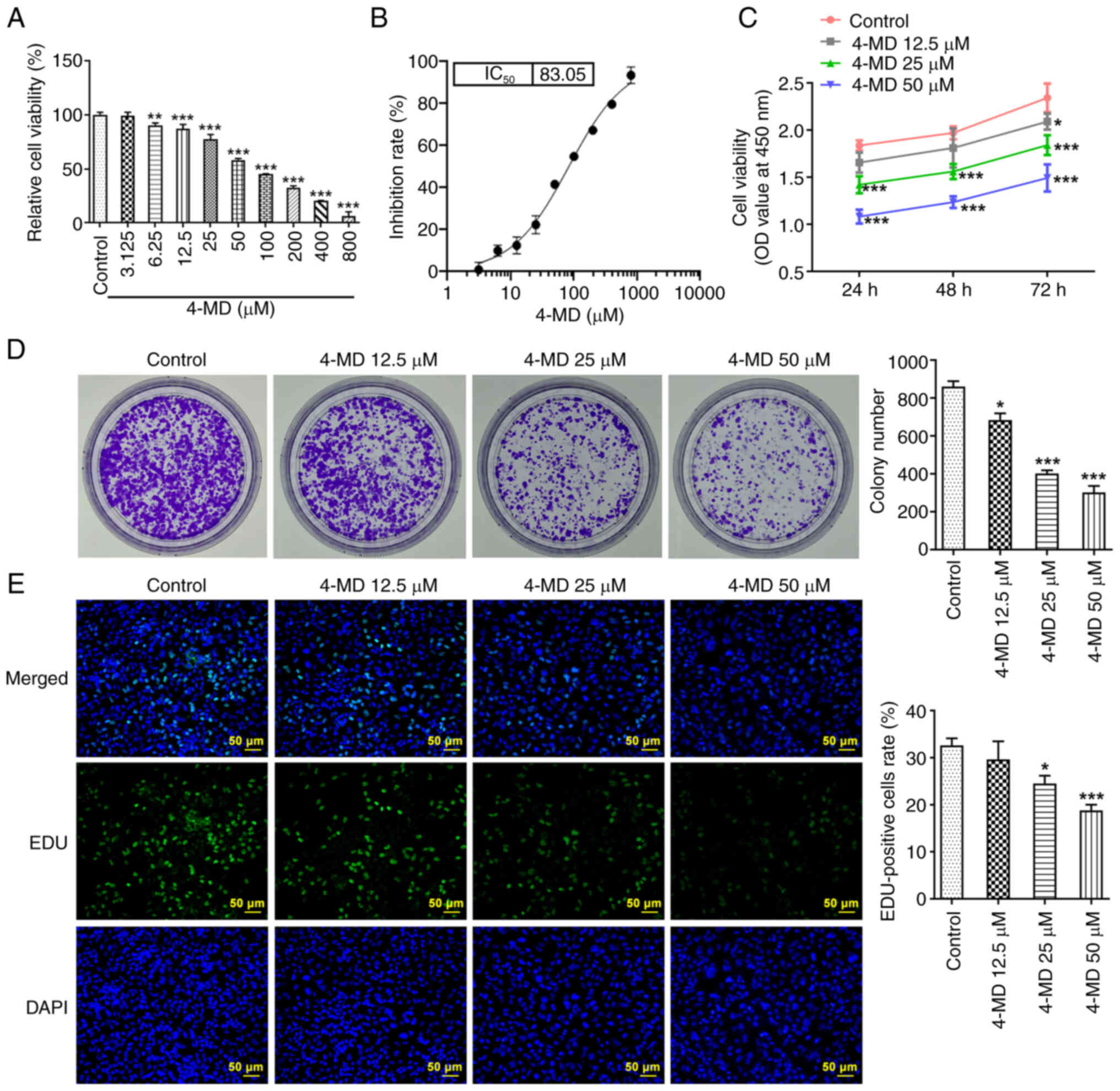
In the present study, A549 cells were treated with
increasing concentrations of 4-MD for 24 h (Fig. 1A). The results demonstrated that
4-MD treatment (≥6.25 µM) significantly reduced cell viability, and
the IC50 of 4-MD was 83.05 µM (Fig. 1B); therefore, 12.5, 25 and 50 µM
4-MD were used in subsequent experiments, since 4-MD concentrations
higher than the IC50 may cause cytotoxicity. The
experiments were performed at concentrations below IC50
to better assess the effect of 4-MD on the cells, which is also the
most common criteria for selecting drug concentrations (19). Collectively, the results of cell
experiments demonstrated that 4-MD reduced cell viability, colony
formation and the number of EDU-positive cells in a
concentration-dependent manner, highlighting the anti-proliferative
activity of 4-MD in A549 cells (Fig.
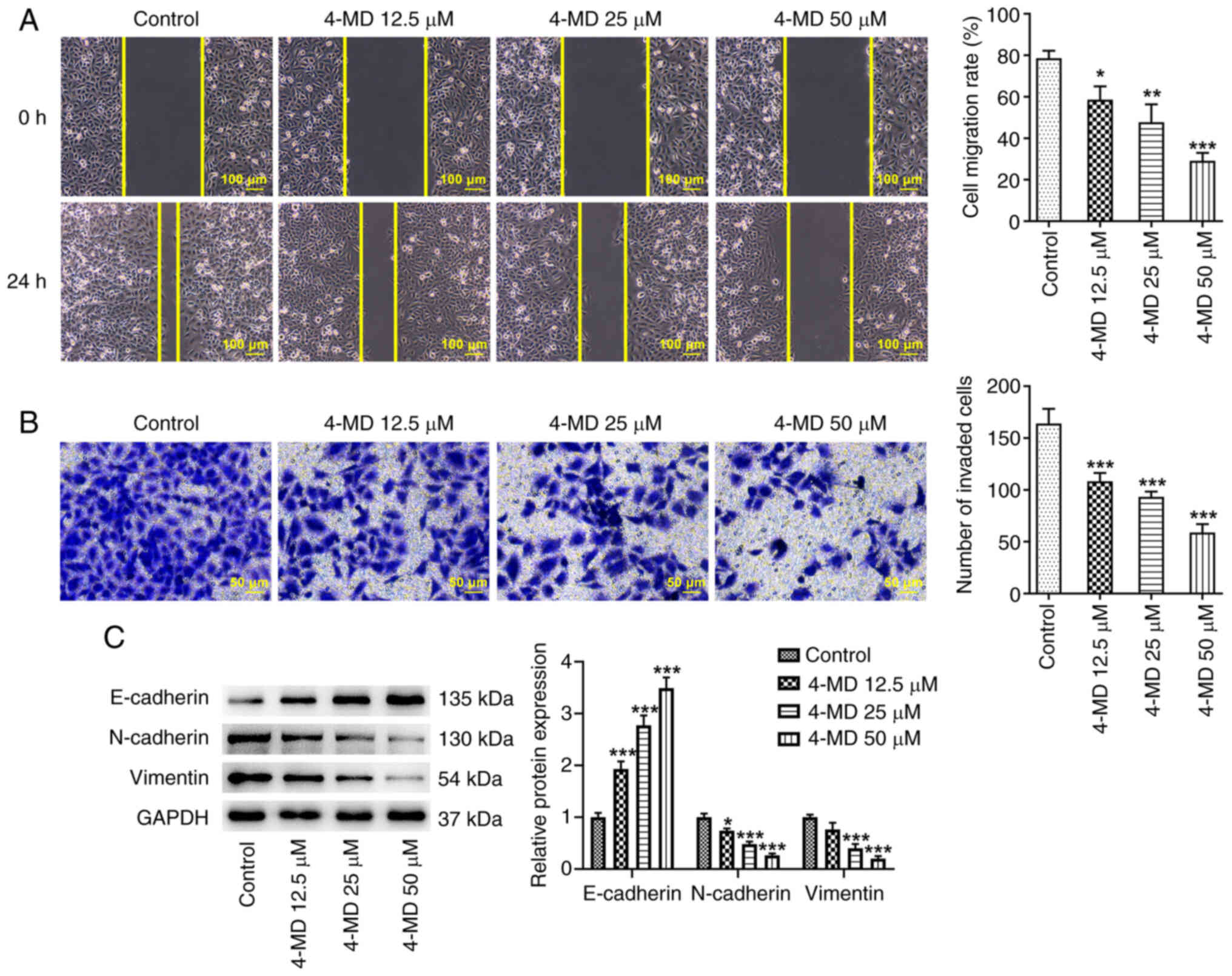
1C-E). In addition, the results of the wound healing and
Transwell assays revealed that 4-MD significantly suppressed cell
migration and invasion in a concentration-dependent manner
(Fig. 2A and B). Furthermore, 4-MD
markedly upregulated the protein expression levels of E-cadherin,
an epithelial cell marker, and downregulated the protein expression
levels of N-cadherin and Vimentin, mesenchymal cell markers
(Fig. 2C). These results suggested
that 4-MD may inhibit epithelial-mesenchymal transition (EMT).
4-MD promotes lipid peroxidation and
ferroptosis of A549 cells
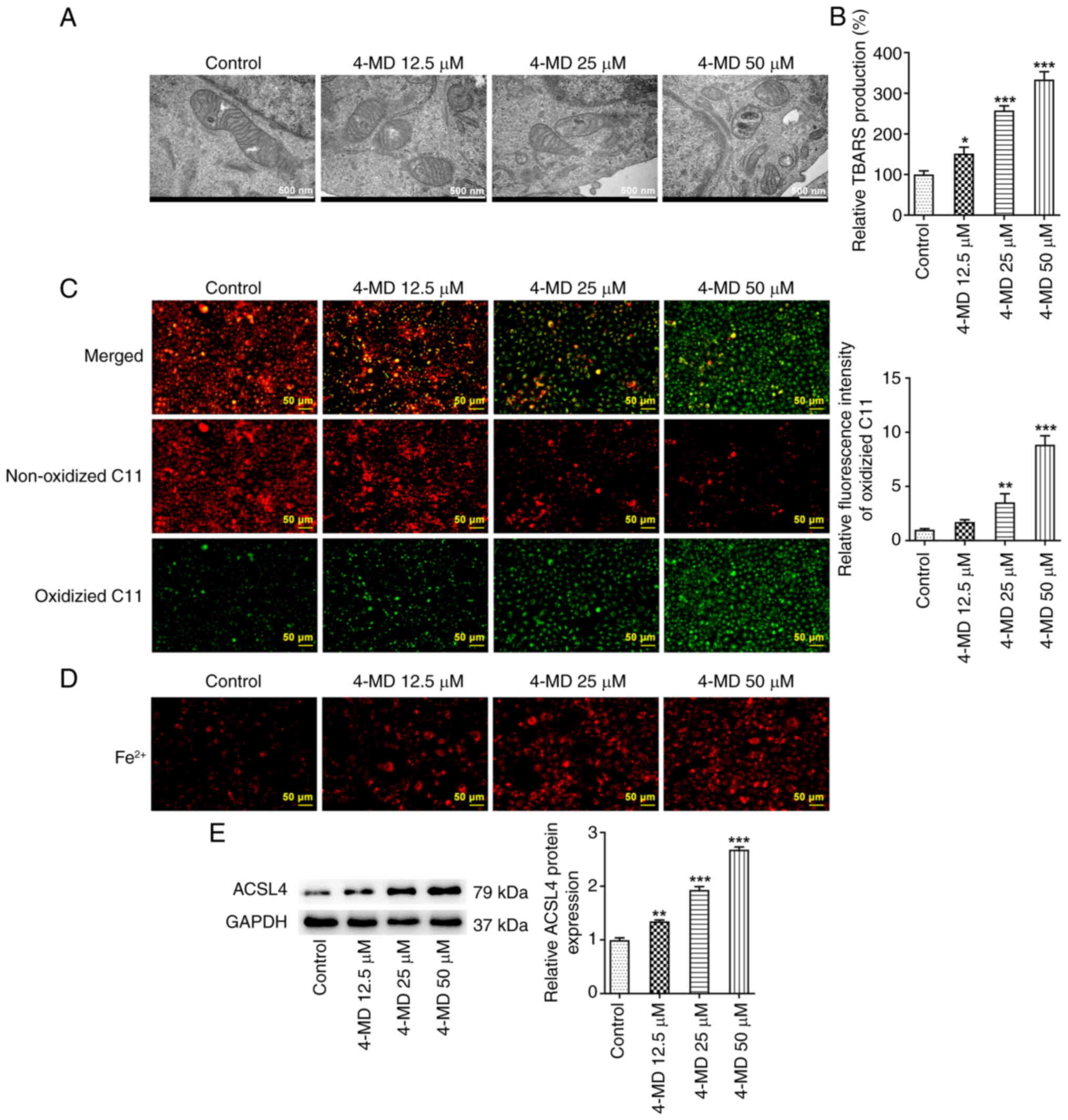
The results obtained using transmission electron
microscopy demonstrated that A549 cells exhibited mitochondria that
were small in size with elevated membrane density following 4-MD
treatment, a typical morphological feature of ferroptosis (Fig. 3A). Moreover, TBARS production and
levels of lipid ROS were increased following treatment with
increasing concentrations of 4-MD, indicating that 4-MD
significantly promoted lipid peroxidation (Fig. 3B and C). It was also revealed that
A549 cells exhibited an excessive deposition of Fe2+
following treatment with 4-MD (Fig.
3D). Notably, ACSL4 is a key step in ferroptosis execution that
promotes lipid peroxidation, which is a critical mediator during
the modulation of ferroptosis. In the present study, treatment with
4-MD significantly upregulated the protein expression levels of
ACSL4 in a concentration-dependent manner (Fig. 3E). Collectively, these results
demonstrated that 4-MD induced ferroptosis in A549 cells.
4-MD directly targets DNMT1 to
regulate ferroptosis
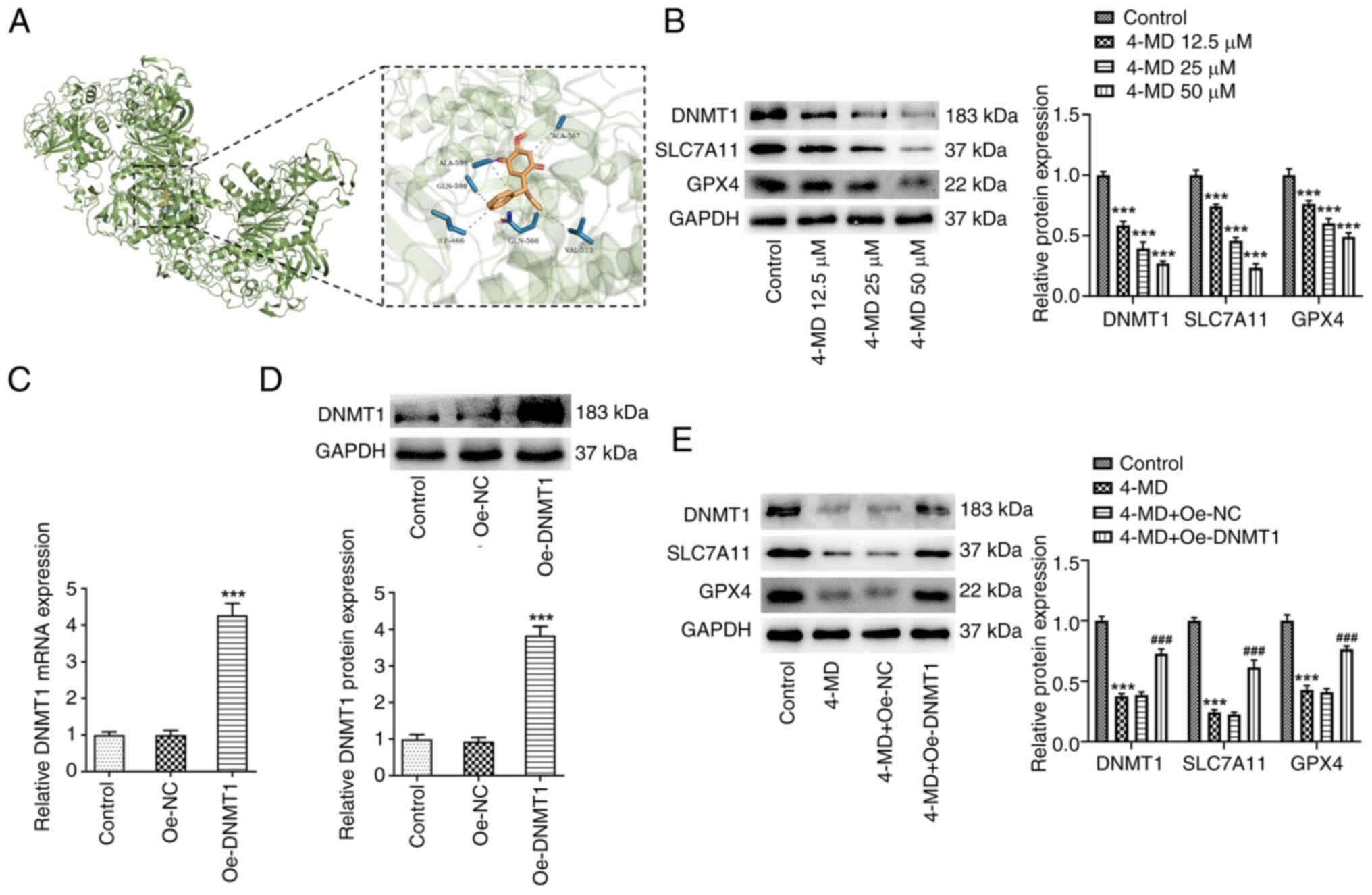
Further investigations were conducted to elucidate
the molecular mechanisms underlying the pro-ferroptosis activity of
4-MD. In the present study, results obtained using TargetNet
(http://targetnet.scbdd.com/) revealed
that DNMT1 exhibited potential as a target of 4-MD, and further
results obtained using molecular docking identified binding sites
between DNMT1 and 4-MD (Fig. 4A).
The results of a previous study demonstrated that 6-thioguanine, a
DNMT1 degrader, inactivated system Xc−, inhibited the
production of GSH, reduced GPX4 expression and increased the levels
of lipid ROS; thus, promoting Fe2+-dependent
ferroptosis. These results suggested that DNMT1 degradation may
possess the potential to induce ferroptosis (20). The results of the present study
revealed that the protein expression levels of DNMT1, SLC7A11 and
GPX4 were significantly reduced in A549 cells following treatment
with 4-MD (Fig. 4B). Thus, it was
hypothesized that 4-MD may directly bind to DNMT1 for subsequent
inhibition, leading to downregulation of SLC7A11 and GPX4, and the
induction of ferroptosis.
In the present study, A549 cells were transfected
with Oe-DNMT1 to induce DNMT1 overexpression (Fig. 4C and D). A549 cells with and
without Oe-DNMT1 transfection were treated with 50 µM 4-MD. It was
revealed that the 4-MD-mediated inhibition of DNMT1, SLC7A11 and
GPX4 protein expression levels was partially abolished following
DNMT1 overexpression (Fig. 4E). In
addition, 4-MD-mediated excessive Fe2+ production was
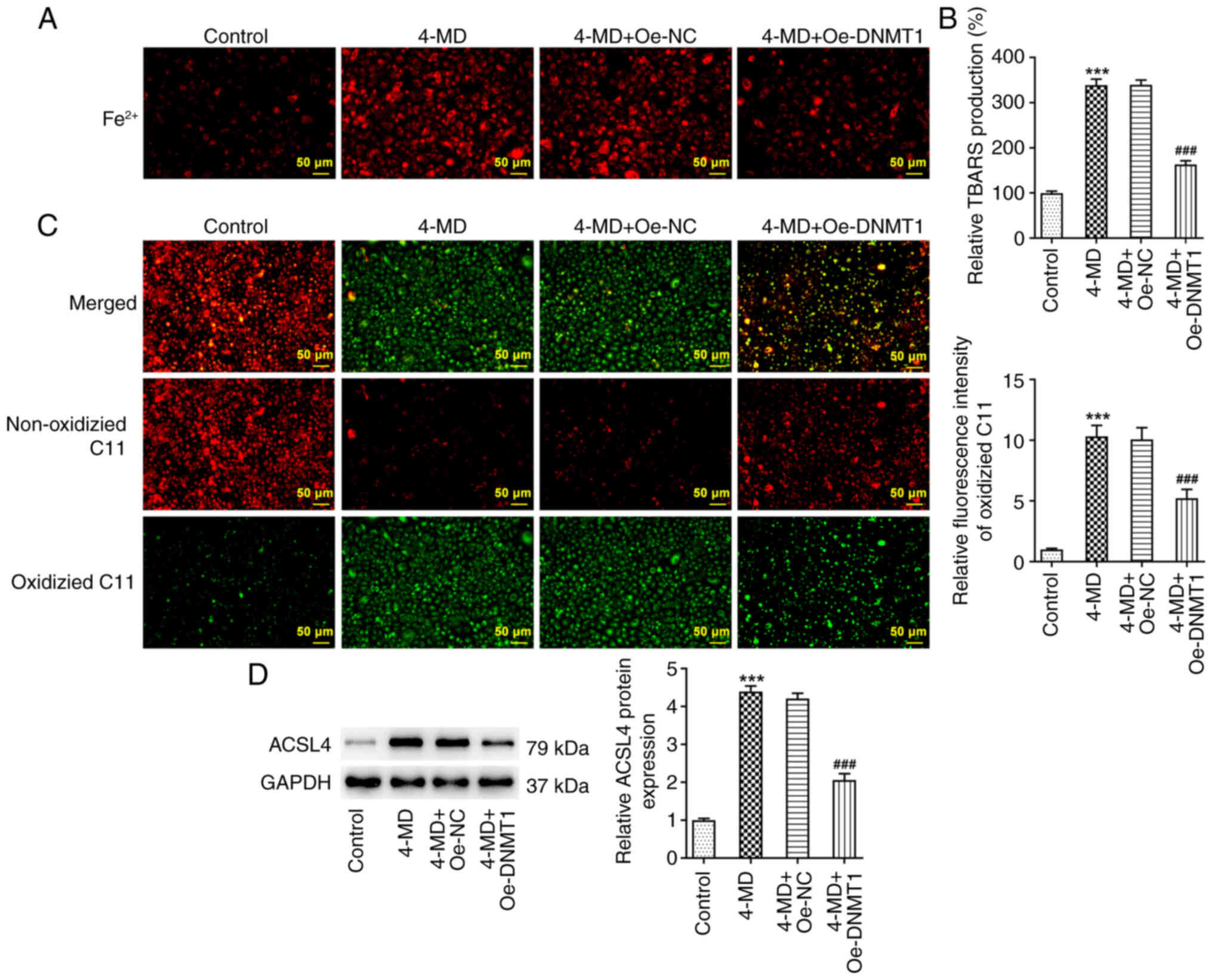
markedly reduced following DNMT1 overexpression (Fig. 5A). The results also demonstrated
that TBARS production and lipid ROS levels were reduced in the 4-MD
+ Oe-DNMT1 group, compared with those in the 4-MD + Oe-NC group,
highlighting that DNMT1 overexpression inhibited 4-MD-mediated
lipid peroxidation in A549 cells (Fig.
5B and C). Moreover, 4-MD-mediated alterations in ACSL4
expression were also reversed following DNMT1 overexpression
(Fig. 5D). These findings
suggested that 4-MD may promote ferroptosis through regulation of
the DNMT1/system Xc−/GPX4 pathway.
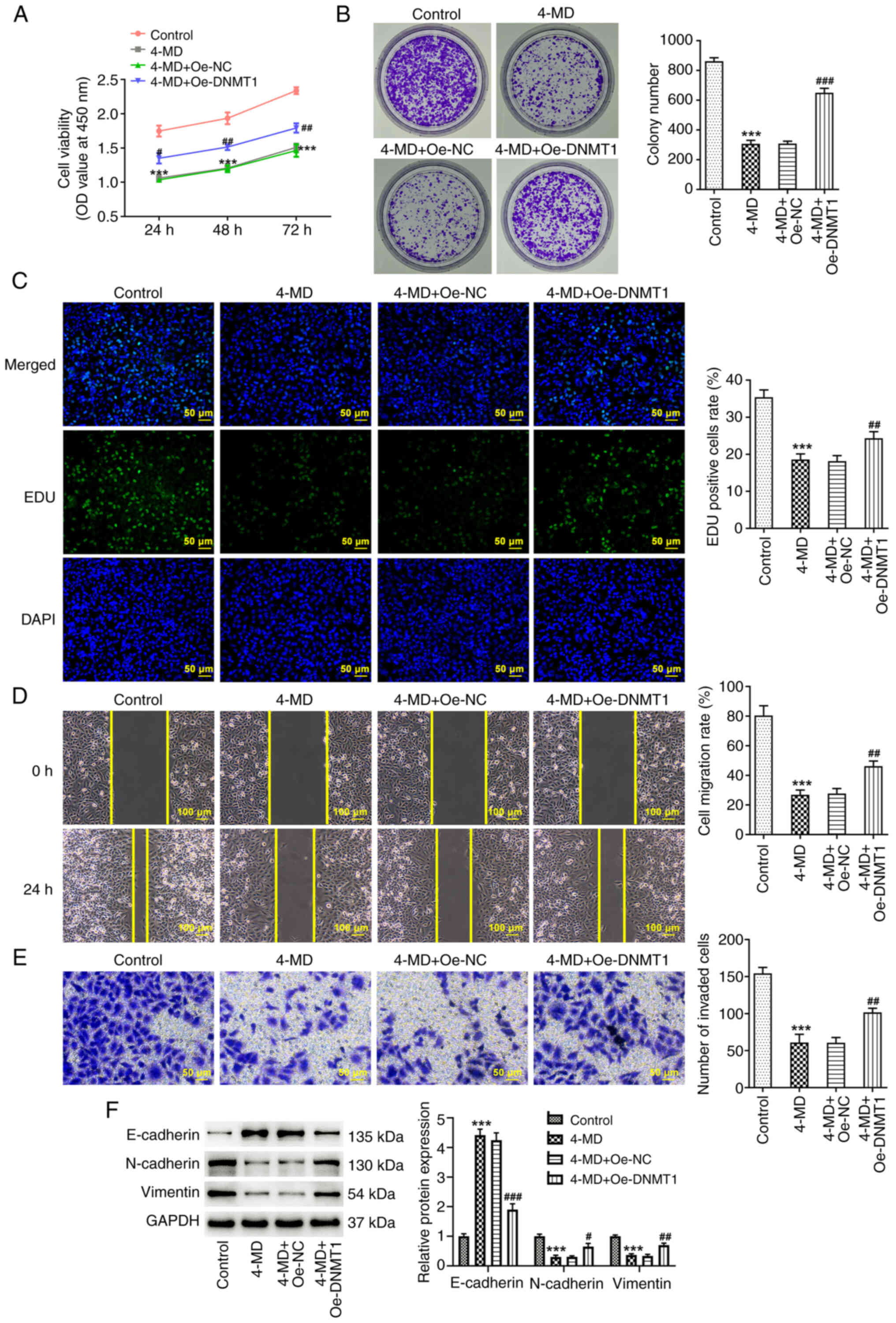
DNMT1 overexpression partially
abolishes the inhibitory effects of 4-MD on A549 cells
To further determine the regulatory role of the
4-MD/DNMT1 axis in lung cancer, the malignant behavior of cells was
assessed using a rescue assay. As shown in Fig. 6A-C, cell viability, colony number
and the number of EDU-positive cells were increased in the 4-MD +
Oe-DNMT1 group, compared with those in the 4-MD + Oe-NC group.
These results indicated that the 4-MD-mediated effects on A549 cell
proliferation were partially abolished following DNMT1
overexpression. Moreover, it was demonstrated that wound healing
and cell invasion were increased in the 4-MD + Oe-DNMT1 group,
compared with those in the 4-MD + Oe-NC group (Fig. 6D and E). These results indicated
that the inhibitory effects of 4-MD on A549 cell migration and
invasion were reduced following DNMT1 overexpression. The results
of the western blot analysis also demonstrated that the
4-MD-mediated alterations in EMT-associated protein expression
levels were partially abolished following DNMT1 overexpression
(Fig. 6F).
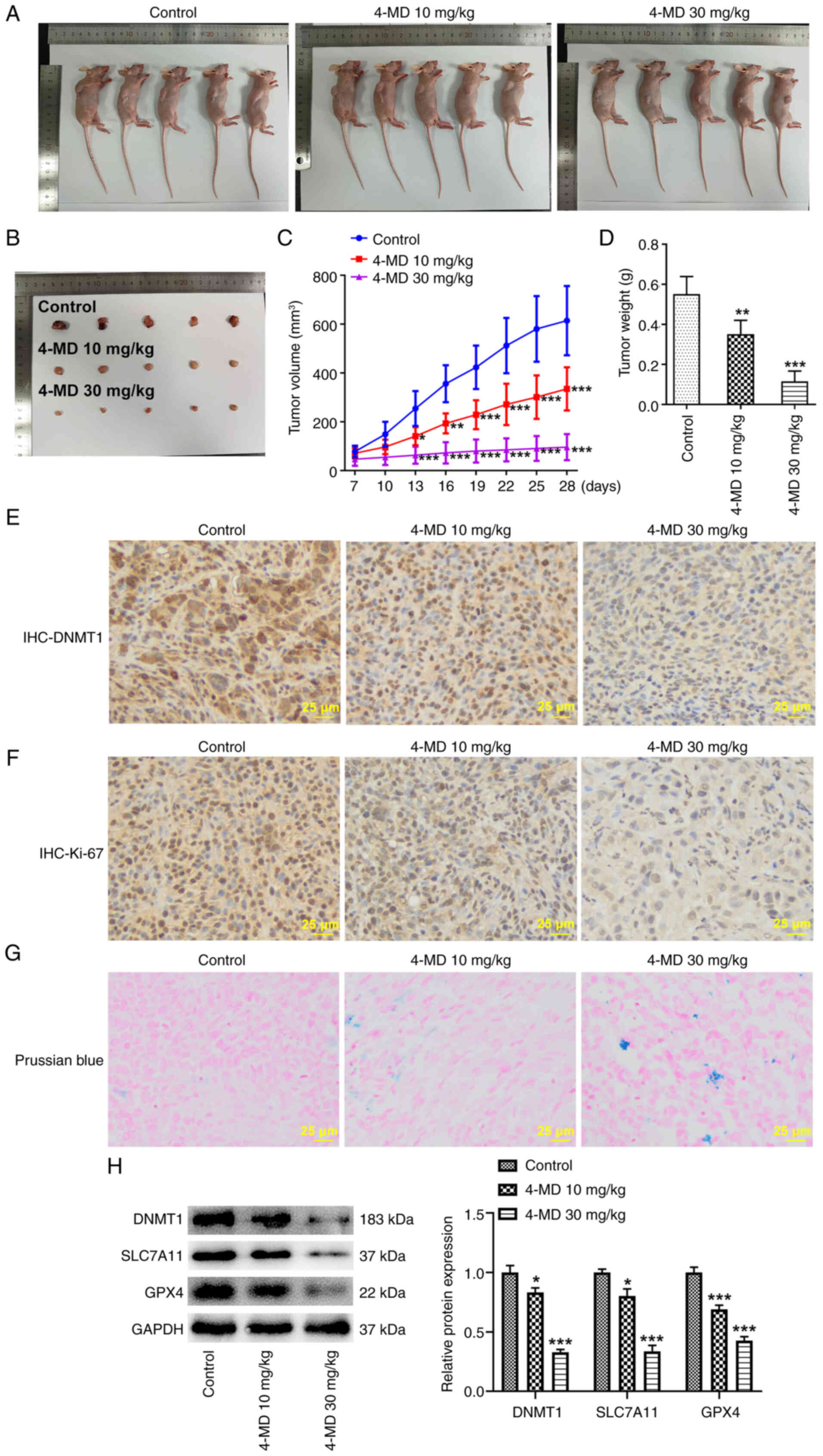
4-MD reduces tumor growth and promotes
ferroptosis in mice
In the present study, animal experiments were
carried out to further verify the antitumor activity of 4-MD in
lung cancer. Notably, 4-MD significantly reduced tumor size and
weight in a concentration-dependent manner, highlighting that 4-MD
may suppress tumor growth (Fig.
7A-D). Tumor ulcerations were not observed in the present
study. The color of the tumors obtained from the first two animals
from the control group in Fig. 7B
may be due to bleeding around the tumor, since blood oxidation
leads to tumor blackening. Alternatively, the bleeding may be due
to local bleeding at the time of A549 cell-inoculation, which was
small enough to not be observed at the time of inoculation.
Subsequent IHC staining of tumor tissues revealed that DNMT1 and
Ki-67 expression levels were reduced following treatment with 4-MD
(Fig. 7E and F). Moreover, the
results of the Prussian blue staining revealed that iron deposition
was present in mice following treatment with 4-MD, and DNMT1,
SLC7A11 and GPX4 protein expression levels were markedly reduced
(Fig. 7G and H). Collectively,
these results demonstrated that 4-MD may promote ferroptosis
through the DNMT1/system Xc−/GPX4 pathway.
Discussion
Lung cancer poses a major threat to human health,
with high rates of incidence and mortality (21). According to GLOBOCAN 2020 cancer
estimates, lung cancer is the second most frequent malignancy, with
an estimated 2.2 million new cancer cases and 1.8 million
mortalities worldwide in 2020 (22). Although advances have been made in
the treatment of lung cancer, the prognosis of patients remains
unsatisfactory, and tumor metastasis and recurrence are key factors
in treatment failure (23). Thus,
the development of novel therapeutic strategies for lung cancer is
required. The results of the present study highlighted that 4-MD
may exhibit potential in the treatment of lung cancer, through
inhibiting cell proliferation, migration and invasion in
vitro, and limiting tumor growth in vivo. Further
exploration of the specific molecular mechanisms revealed that 4-MD
may target DNMT1 directly, subsequently reducing expression and
inducing ferroptosis via the DNMT1/system
Xc−/GPX4 pathway.
Tumor metastasis is a complex biological process
involving multiple influencing factors and molecular mechanisms.
Notably, EMT is crucial in facilitating the motility and invasion
of solid tumor cells, and is the key initial step in cancer cell
metastasis (24–26). During EMT, cells presenting with
epithelium-associated phenotypes and behaviors undergo a
transitional process, leading to the development of mesenchymal
phenotypes, and increased migration and invasion. During
metastasis, the expression of the epithelial adhesion protein,
E-cadherin, is reduced, whereas the expression of mesenchymal
markers, including N-cadherin and Vimentin, is increased (21,27).
The results of a previous study demonstrated that natural compounds
and reagents that exert EMT-suppressive activity may exhibit
potential in the treatment of lung cancer (26). The present study demonstrated that
E-cadherin expression levels were significantly increased in A549
cells, whereas the expression levels of Vimentin and N-cadherin
were markedly decreased following treatment with 4-MD. Thus, 4-MD
may exhibit potential in reducing the proliferation and invasion of
A549 cells.
Active components of Traditional Chinese Medicine
are considered inducers of ferroptosis; thus, exhibiting potential
in the treatment of cancer (28).
The results of a previous study demonstrated that Sanguinarine, a
natural benzophenanthridine alkaloid derived from the root of
Sanguinaria canadensis Linn., inhibited the growth and
metastasis of lung cancer through facilitating GPX4-dependent
ferroptosis (29). Shikonin, the
major active constituent purified from the roots of Lithospermum
erythrorhizon, has been shown to significantly inhibit cell
proliferation, migration and invasion, and reduce tumor growth in
lung cancer through inducing ferroptosis (30). Thus, compounds that target
ferroptosis may exhibit potential in the treatment of lung cancer.
The results of the present study indicated that 4-MD may
significantly induce ferroptosis in lung cancer cells. Notably,
multiple factors are involved in the modulation of ferroptosis,
including GPX4, SLC7A11 and ACSL4. At present, two mechanisms are
considered inducers of ferroptosis; namely, GPX4 inhibition and
system Xc− inhibition (31). The present study revealed that 4-MD
may inhibit GPX4 and SLC7A11 expression. Thus, 4-MD may exhibit
potential in the treatment of cancer through the induction of
ferroptosis.
Drug-target interactions are critical for the
development of novel therapeutic strategies; however,
identification of potential targets is complex. In the present
study, TargetNet (32), an open
website for predicting target binding in molecules, was used to
predict the potential target of 4-MD. The results obtained using
TargetNet and molecular docking demonstrated that 4-MD may directly
bind to DNMT1. DNMT1, a maintenance DNA methyltransferase, is
upregulated in lung cancer. This protein increases the methylation
of tumor suppressor gene promoters to inhibit gene expression, thus
facilitating the development of lung cancer (33,34).
The results of a previous study revealed that DNMT1 can inhibit
ferroptosis through regulating the methylation of
ferroptosis-associated genes (35). Moreover, 6-thioguanine, a DNMT1
degrader, has been reported to inactivate system Xc− and
downregulate the expression of GPX4; thus, inducing ferroptosis via
the inhibition of DNMT1 (20).
These findings suggested that DNMT1 inhibition may exhibit
potential in promoting ferroptosis. In the present study, the
results of the rescue assay revealed that the anticancer activity
and ferroptosis-promoting effects of 4-MD in lung cancer were
partially abolished following DNMT1 overexpression. Thus, 4-MD may
reduce the progression of lung cancer through the inhibition of
DNMT1.
To the best of our knowledge, the present study is
the first to demonstrate the anticancer activity of 4-MD in lung
cancer. The results of the present study revealed that 4-MD
effectively suppressed cell proliferation, migration and invasion,
and reduced EMT in A549 cells, and inhibited tumor growth in
vivo. Mechanistically, 4-MD may directly bind to DNMT1, thus
exerting anticancer effects via the inhibition of DNMT1. Moreover,
4-MD may induce ferroptosis in lung cancer cells via the
DNMT1/system Xc−/GPX4 pathway. Therefore, 4-MD may
exhibit potential as a novel therapeutic agent in the treatment of
lung cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science
Foundation of China (grant no. 81972175).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LC designed the study. JF, HL and JL conducted the
experiments and analyzed the data. JF and HL drafted the
manuscript, and LC critically revised the manuscript. JF and LC
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All animal studies were carried out in compliance
with the ARRIVE guidelines and were approved by the Animal Ethics
Committee of The First Affiliated Hospital of Nanjing Medical
University (approval no. IACUC-230814).
Patient consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim N, Kim HK, Lee K, Hong Y, Cho JH, Choi
JW, Lee JI, Suh YL, Ku BM, Eum HH, et al: Single-cell RNA
sequencing demonstrates the molecular and cellular reprogramming of
metastatic lung adenocarcinoma. Nat Commun. 11:22852020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lambert AW, Pattabiraman DR and Weinberg
RA: Emerging biological principles of metastasis. Cell.
168:670–691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo H, Vong CT, Chen H, Gao Y, Lyu P, Qiu
L, Zhao M, Liu Q, Cheng Z, Zou J, et al: Naturally occurring
anti-cancer compounds: Shining from Chinese herbal medicine. Chin
Med. 14:482019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan SC, Chang YS, Wang JP, Chen SC and
Kuo SC: Three new flavonoids and antiallergic, anti-inflammatory
constituents from the heartwood of Dalbergia odorifera. Planta Med.
64:153–158. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim DC, Lee DS, Ko W, Kim KW, Kim HJ, Yoon
CS, Oh H and Kim YC: Heme Oxygenase-1-Inducing Activity of
4-Methoxydalbergione and 4′-Hydroxy-4-methoxydalbergione from
Dalbergia odorifera and Their Anti-inflammatory and Cytoprotective
Effects in Murine Hippocampal and BV2 microglial cell line and
primary rat microglial cells. Neurotox Res. 33:337–352. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du H, Tao T, Xu S, Xu C, Li S, Su Q, Yan
J, Liu B and Li R: 4-Methoxydalbergione Inhibits Bladder Cancer
Cell Growth via Inducing Autophagy and Inhibiting Akt/ERK Signaling
Pathway. Front Mol Biosci. 8:7896582022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeng L, Qin Y, Lu X, Fang X, Huang J, Yu C
and Feng ZB: 4-Methoxydalbergione Elicits Anticancer Effects by
Upregulation of GADD45G in Human Liver Cancer Cells. J Healthc Eng.
2023:67108802023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li M, Xiao Y, Liu P, Wei L, Zhang T, Xiang
Z, Liu X, Zhang K, Zhong Q and Chen F: 4-Methoxydalbergione
inhibits esophageal carcinoma cell proliferation and migration by
inactivating NF-ĸB. Oncol Rep. 49:422023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li R, Xu CQ, Shen JX, Ren QY, Chen DL, Lin
MJ, Huang RN, Li CH, Zhong RT, Luo ZH, et al: 4-Methoxydalbergione
is a potent inhibitor of human astroglioma U87 cells in vitro and
in vivo. Acta Pharmacol Sin. 42:1507–1515. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park KR, Yun HM, Quang TH, Oh H, Lee DS,
Auh QS and Kim EC: 4-Methoxydalbergione suppresses growth and
induces apoptosis in human osteosarcoma cells in vitro and in vivo
xenograft model through down-regulation of the JAK2/STAT3 pathway.
Oncotarget. 7:6960–6971. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi
AA and Lei P: Ferroptosis: Mechanisms and links with diseases.
Signal Transduct Target Ther. 6:492021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hassannia B, Vandenabeele P and Vanden
Berghe T: Targeting ferroptosis to iron out cancer. Cancer Cell.
35:830–849. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang C, Zhang X, Yang M and Dong X:
Recent progress in ferroptosis inducers for cancer therapy. Adv
Mater. 31:e19041972019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Percie du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: Updated guidelines for
reporting animal research. PLoS Biol. 18:e30004102020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pritt SL and Smith TM: Institutional
animal care and use committee postapproval monitoring programs: A
proposed comprehensive classification scheme. J Am Assoc Lab Anim
Sci. 59:127–131. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Wang R, Piotrowski M, Zhang H and
Leach KL: Intracellular concentrations determine the cytotoxicity
of adefovir, cidofovir and tenofovir. Toxicol In Vitro. 29:251–258.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Gao M, Niu Y and Sun J: From
DNMT1 degrader to ferroptosis promoter: Drug repositioning of
6-Thioguanine as a ferroptosis inducer in gastric cancer. Biochem
Biophys Res Commun. 603:75–81. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng J, Li X, Liang L, Duan H, Xie S and
Wang C: Phosphorylation of CAP1 regulates lung cancer
proliferation, migration, and invasion. J Cancer Res Clin Oncol.
148:137–153. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muthusamy B, Patil PD and Pennell NA:
Perioperative systemic therapy for resectable non-small cell lung
cancer. J Natl Compr Canc Netw. 20:953–961. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie S, Wu Z, Qi Y, Wu B and Zhu X: The
metastasizing mechanisms of lung cancer: Recent advances and
therapeutic challenges. Biomed Pharmacother. 138:1114502021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bakir B, Chiarella AM, Pitarresi JR and
Rustgi AK: EMT, MET, plasticity, and tumor metastasis. Trends Cell
Biol. 30:764–776. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chanvorachote P, Petsri K and Thongsom S:
Epithelial to mesenchymal transition in lung cancer: Potential
EMT-Targeting natural product-derived compounds. Anticancer Res.
42:4237–4246. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Menju T and Date H: Lung cancer and
epithelial-mesenchymal transition. Gen Thorac Cardiovasc Surg.
69:781–789. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ye J, Zhang R, Wu F, Zhai L, Wang K, Xiao
M, Xie T and Sui X: Non-apoptotic cell death in malignant tumor
cells and natural compounds. Cancer Lett. 420:210–227. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu R, Wu J, Luo Y, Wang Y, Tian J, Teng W,
Zhang B, Fang Z and Li Y: Sanguinarine represses the growth and
metastasis of non-small cell lung cancer by facilitating
ferroptosis. Curr Pharm Des. 28:760–768. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qian X, Zhu L, Xu M, Liu H, Yu X, Shao Q
and Qin J: Shikonin suppresses small cell lung cancer growth via
inducing ATF3-mediated ferroptosis to promote ROS accumulation.
Chem Biol Interact. 382:1105882023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang WS and Stockwell BR: Ferroptosis:
Death by lipid peroxidation. Trends Cell Biol. 26:165–176. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yao ZJ, Dong J, Che YJ, Zhu MF, Wen M,
Wang NN, Wang S, Lu AP and Cao DS: TargetNet: A web service for
predicting potential drug-target interaction profiling via
multi-target SAR models. J Comput Aided Mol Des. 30:413–424. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu XY, Chen HC, Li WW, Yan JD and Lv RY:
DNMT1 promotes cell proliferation via methylating hMLH1 and hMSH2
promoters in EGFR-mutated non-small cell lung cancer. J Biochem.
168:151–157. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma F, Lei YY, Ding MG, Luo LH, Xie YC and
Liu XL: LncRNA NEAT1 Interacted With DNMT1 to regulate malignant
phenotype of cancer cell and cytotoxic T Cell Infiltration via
Epigenetic Inhibition of p53, cGAS, and STING in Lung Cancer. Front
Genet. 11:2502020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zou P, Chen Z, He Q and Zhuo Y:
Polyphyllin I induces ferroptosis in castration-resistant prostate
cancer cells through the ERK/DNMT1/ACSL4 axis. Prostate. 84:64–73.
2024. View Article : Google Scholar : PubMed/NCBI
|