Introduction
Gastric cancer (GC) is the fifth highest cause of
tumor-associated mortalities worldwide (1). At present, the most common treatment
strategies for GC includes chemotherapy, surgery, radiation,
immunotherapy and target treatment such as trastuzumab (2). Despite advancements, the overall
5-year survival rate of patients with advanced GC remains <30%
(3,4), therefore exploring the novel and
effective strategies for the treatment of GC is required.
Recently, traditional Chinese medicines have been
garnering attention as a prospect of tumor treatments due to their
immune-regulation functions, multiple targets and fewer side
effects (5). The traditional
Chinese herb, Long Kui (Solanum nigrum Linn), contains a
number of steroidal alkaloids and has been reported to exert
various bioactive effects, including antitumor and
anti-inflammatory properties (6,7). In
particular, solamargine is an extract from Long Kui that has been
reported to confer antitumor properties in several types of cancer,
including cervical, lung and prostate cancer, in addition to
antiviral and anti-inflammatory properties (7–10).
However, to the best of our knowledge, the molecular mechanism
underlying the antitumor effects of solamargine has not yet been
elucidated.
T cell immunity serves a role in maintaining body
homeostasis by selectively eliminating pathogens and abnormal cells
(10). However, the uncontrolled
hyperactivation of T cells due to immune system disorder can
destroy normal cells (11). A
previous study reported that programmed cell death-1 (PD-1) and
programmed cell death ligand 1 (PD-L1) can maintain the regulation
of T cell activities under normal conditions, thereby preventing
the development of autoimmune reactions (12). Furthermore, STAT3 is a modulator in
cancer and inflammatory responses (13,14).
It has been previously reported that STAT3 activation can promote
the progression of numerous types of cancer, including GC, ovarian
cancer and breast cancer (15,16).
In addition, other studies have demonstrated that STAT3 can promote
tumorigenesis by activating PD-L1 (17,18).
Cancer cells highly expressed PD-L1 and leading to T cell exhaust,
which has been documented to be responsible for cancer immune
escape, which impacts the efficacy of cancer therapy (19). However, to the best of our
knowledge, the possible association between solamargine and
PD-L1/STAT3 signaling remains to be elucidated. Therefore, the
present study aimed to evaluate the mechanism underlying the effect
of solamargine on GC.
Material and methods
Cell culture
GC cell lines, NCI-N87 and HGC-27, and Jurkat T
cells were purchased from the American Type Culture Collection.
Cells were cultured in RPMI 1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 100 µg/ml streptomycin and 100 µg/ml penicillin,
in an incubator with 5% CO2 at 37°C. To evaluate the
effect of solamargine (MedChemExpress) on GC cells, GC cells were
induced with 20 ng/ml IL-6 (MilliporeSigma) for 24 h at 37°C, and
then treated with 10 µM solamargine for 48 h at 37°C.
Cell Counting Kit-8 (CCK-8) assay
For CCK-8 assays, a total of 5×103 HGC-27
or NCI-N87 cells were seeded in culture plates and incubated at
37°C overnight. Following treatment with 5, 10 or 20 µM solamargine
at 37°C for 48 h, cells were then incubated with 10 µl CCK-8
reagent (Beyotime Institute of Biotechnology) at room-temperature
for 4 h. Subsequently, the absorbance of each well was measured at
OD450 using a plate microreader (Thermo Fisher Scientific,
Inc.).
Transwell assay
A total of 5×104 HGC-27 or NCI-N87 cells
were seeded into the upper chamber (serum-free RPMI 1640 medium) of
the Transwell insert (3 µm, cat. no. 3414; Corning, Inc.). For the
invasion assay, the upper chamber was precoated with 50 µl Matrigel
for 3 h at 37°C. As for the migration assay, the upper chamber was
not precoated with Matrigel. Following incubation for 12 h at 37°C,
cells in the upper chamber were removed and those in the lower
chamber (10% FBS RPMI 1640 medium) were fixed with 100% anhydrous
ethanol for 30 min at room temperature, followed by staining with
1% crystal violet for 1 h at room temperature. The cells were then
counted under an inverted light microscope (Leica Microsystems
GmbH).
5-Ethynyl-2′-deoxyuridine (EdU)
assay
Following incubation overnight at 37°C, HGC-27 or
NCI-N87 cells at a density of 4×104 were treated with 50
µM EdU solution (cat. no. C10338; Thermo Fisher Scientific, Inc.)
for 4 h at 37°C. Subsequently, cells were fixed with 4%
formaldehyde for 24 h at room temperature, followed by
permeabilization for 10 min in 0.5% Triton X-100. Cells were then
incubated with 100 µl Apollo reaction cocktail (Guangzhou RiboBio
Co., Ltd) for 30 min at room temperature and DNA was stained with
100 µl/well DAPI (Wuhan Servicebio Technology Co., Ltd.) for 30 min
at room temperature. The stained cells were observed using a
fluorescence microscope (magnification, ×100). A total of three
random fields were selected and the Edu-positive cells were
counted.
Immunofluorescence staining
GC cells were seeded in 24-well plates. After
treatments with 5 or 10 µM solamargine for 48 h at 37°C, cells were
fixed with 4% paraformaldehyde at room temperature for 15 min and
permeabilized with 0.5% Triton X-100 for 2 min at room temperature.
After blocking with 5% BSA (MilliporeSigma) for 2 h at room
temperature, the cells were then incubated with antibodies against
phosphorylated (p)-STAT3 (1:200; cat. no. ab267373; Abcam), STAT3
(1:200; cat. no. ab68153; Abcam), cleaved caspase 3 (1:200; cat.
no. ab32042; Abcam) or caspase 3 (1:200; cat. no. ab32351; Abcam)
at 4°C overnight. Subsequently, cells were incubated with the goat
anti-rabbit IgG (conjugated to Alexa Fluor® 594)
secondary antibody (1:500; cat. no. ab150080; Abcam) for 1 h at
room temperature. The GC cells were stained with 500 µl DAPI for 10
min at room temperature Finally, images of the stained cells were
captured using a confocal microscope (Carl Zeiss AG).
Western blotting
Total protein was extracted from HGC-27 and NCI-N87
cells using RIPA buffer (Beyotime Institute of Biotechnology) and
protein concentration was quantified using a BCA kit (Beyotime
Institute of Biotechnology). Subsequently, total proteins (20
µg/lane) were separated using SDS-PAGE on a 10% gel and transferred
onto PVDF membranes. Following blocking with 5% non-fat milk at
room temperature for 1 h, the membranes were incubated with primary
antibodies against PD-L1 (1:1,000; Abcam; cat. no. ab228415), c-Myc
(1:1,000; Abcam; cat. no. ab32072) and β-actin (1:1,000; Abcam;
cat. no. ab8227) overnight at 4°C. Subsequently, the membranes were
incubated with the corresponding secondary goat anti-rabbit
antibody (1:5,000; cat. no. ab288151; Abcam) at room temperature
for 1 h. After which, the targeted proteins were visualized using
with ECL kit (Beyotime Institute of Biotechnology). The Odyssey
Imaging System (LI-COR Bio) was used to scan membranes and the data
were analyzed using Odyssey version 2.0 software (LI-COR Bio).
TUNEL staining assay
HGC-27 or NCI-N87 cells were seeded into 24-well
plates. After treatments with 5 or 10 µM solamargine for 48 h at
37°C, cells were fixed with 4% paraformaldehyde at room temperature
for 15 min, before being permeabilized with 0.5% Triton X-100 for 2
min at room temperature. Subsequently, apoptotic cells were stained
for 1 h at 37°C using the One Step TUNEL Apoptosis Assay kit
(Beyotime Institute of Biotechnology). Cell nuclei were stained
with 100 µl/well DAPI (10 µg/ml; Wuhan Servicebio Technology Co.,
Ltd.) for 30 min at room temperature Finally, images of the
positive apoptotic cells in three random fields were captured using
a confocal microscope (Carl Zeiss AG).
Flow cytometry analysis
Jurkat cells were stimulated with anti-CD3/CD28
antibodies (1:100; Thermo Fisher Scientific, Inc.; cat. no. 11161D)
for 24 h at 37°C and then co-cultured with NCI-N87 or HGC-27 cells
pretreated with 10 µM solamargine for 48 h at 37°C. To isolate the
Jurkat cells, 5×105 cells were centrifuged for 2 min at
500 × g at 4°C and incubated with APC anti-CD69 (dilution
1:100; Biolegend; cat. no. 985206) for 30 min at room temperature
in the dark, followed washing with PBS for three times and
centrifugation at 500 × g for 2 min at 4°C. The stained
cells (HGC-27 or NCI-N87 cells) were analyzed using flow cytometry
(BD Biosciences). Flow.JoX (version 10.0.7; FlowJo LLC) software
was used for data analysis.
Statistical analysis
All data are expressed as the mean ± standard
deviation. The results were analyzed using GraphPad Prism (version
7.0; Dotmatics). The differences between two groups were assessed
using an unpaired Student's t-test, whilst those among multiple
groups were compared using one-way ANOVA, followed by Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Solamargine reduces the proliferation
ability of GC cells
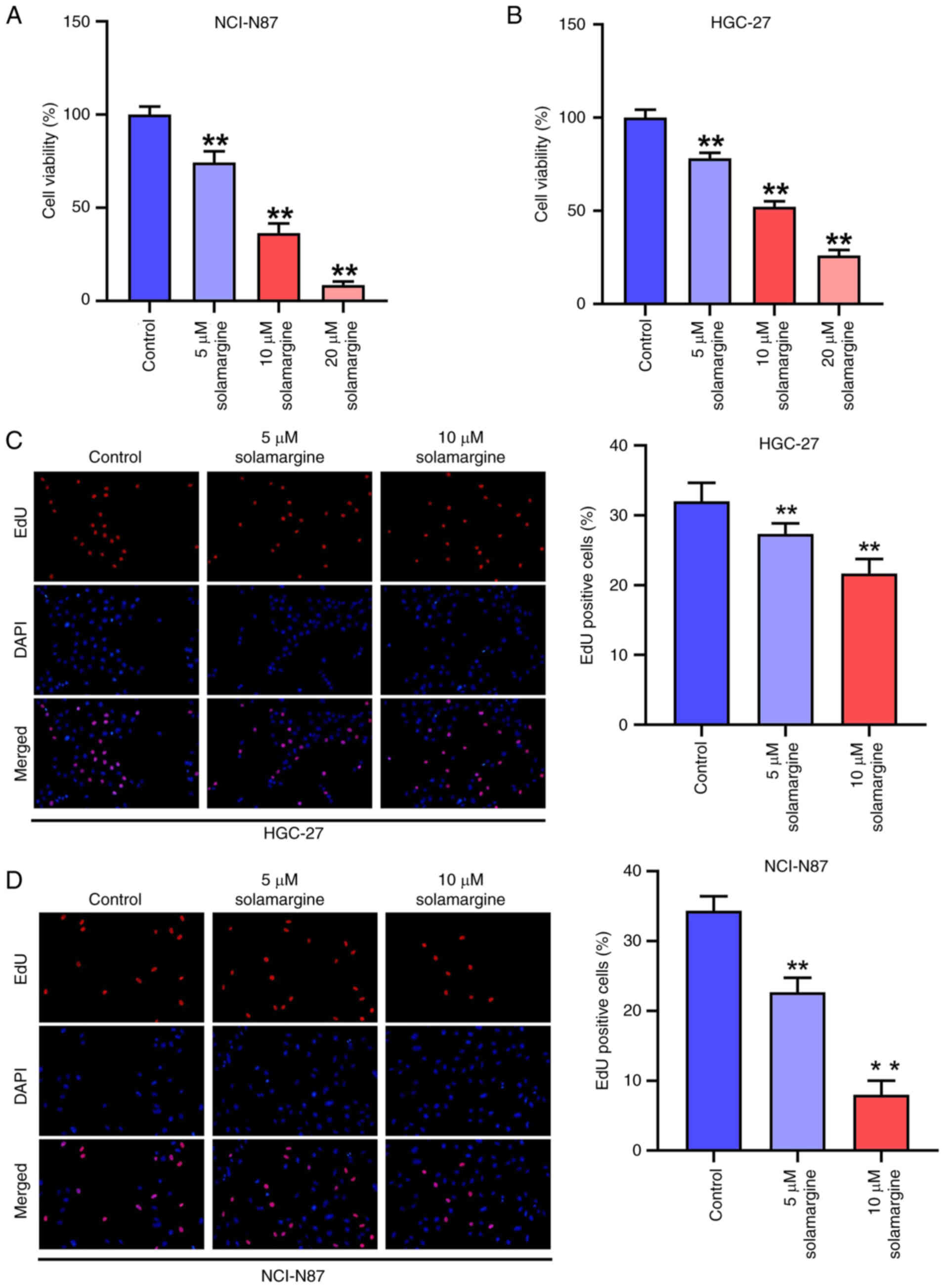
To investigate the effect of solamargine on GC, GC
cells were treated with 5, 10 or 20 µM solamargine for 48 h. It was
demonstrated that solamargine decreased the viability of NCI-N87
(Fig. 1A) and HGC-27 cells
(Fig. 1B), with all concentrations
of solamargine significantly reducing the cell viability compared
with that in the control group. In addition, the percentage of
EdU-positive HGC-27 (Fig. 1C) and
NCI-N87 cells (Fig. 1D) was found
to be significantly decreased after treatment with 5 and 10 µM
solamargine compared with that in the control group. These results
suggest that solamargine can significantly attenuate the viability
and proliferation of GC cells.
Solamargine reduces the migration and
invasion of GC cells
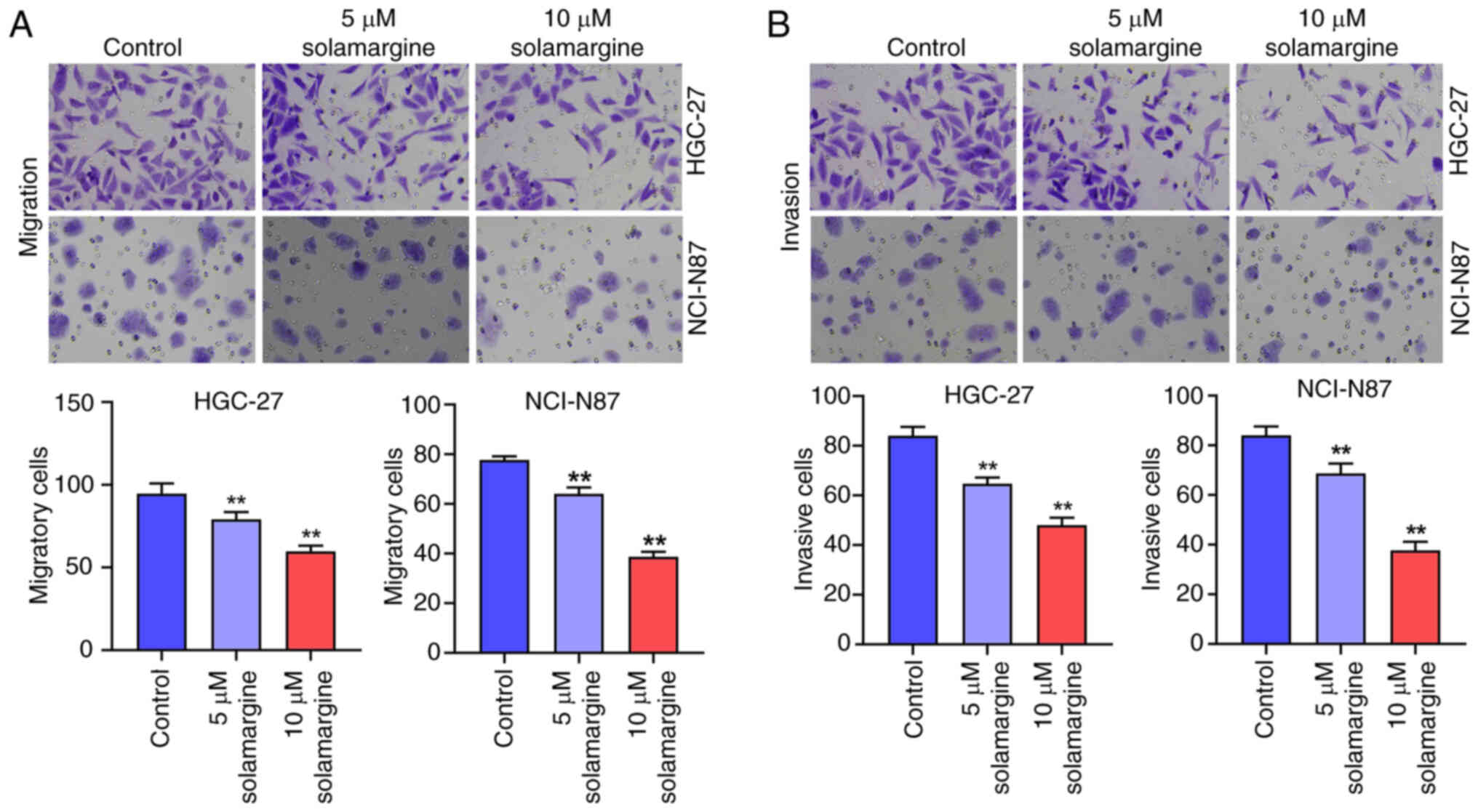
Subsequently, to evaluate the effect of solamargine
on the migration and invasion of GC cells, Transwell assays were
performed. The results indicated that the migration of NCI-N87 and
HGC-27 cells were significantly decreased by 5 and 10 µM
solamargine compared with that in the control group (Fig. 2A). Furthermore, the invasion of
NCI-N87 and HGC-27 cells were also significantly reduced by 5 and
10 µM solamargine compared with that in the control group (Fig. 2B). Taken together, the
aforementioned findings suggest that solamargine can reduce the
migration and invasion of GC cells.
Solamargine promotes the apoptosis of
GC cells through the caspase 3 pathway
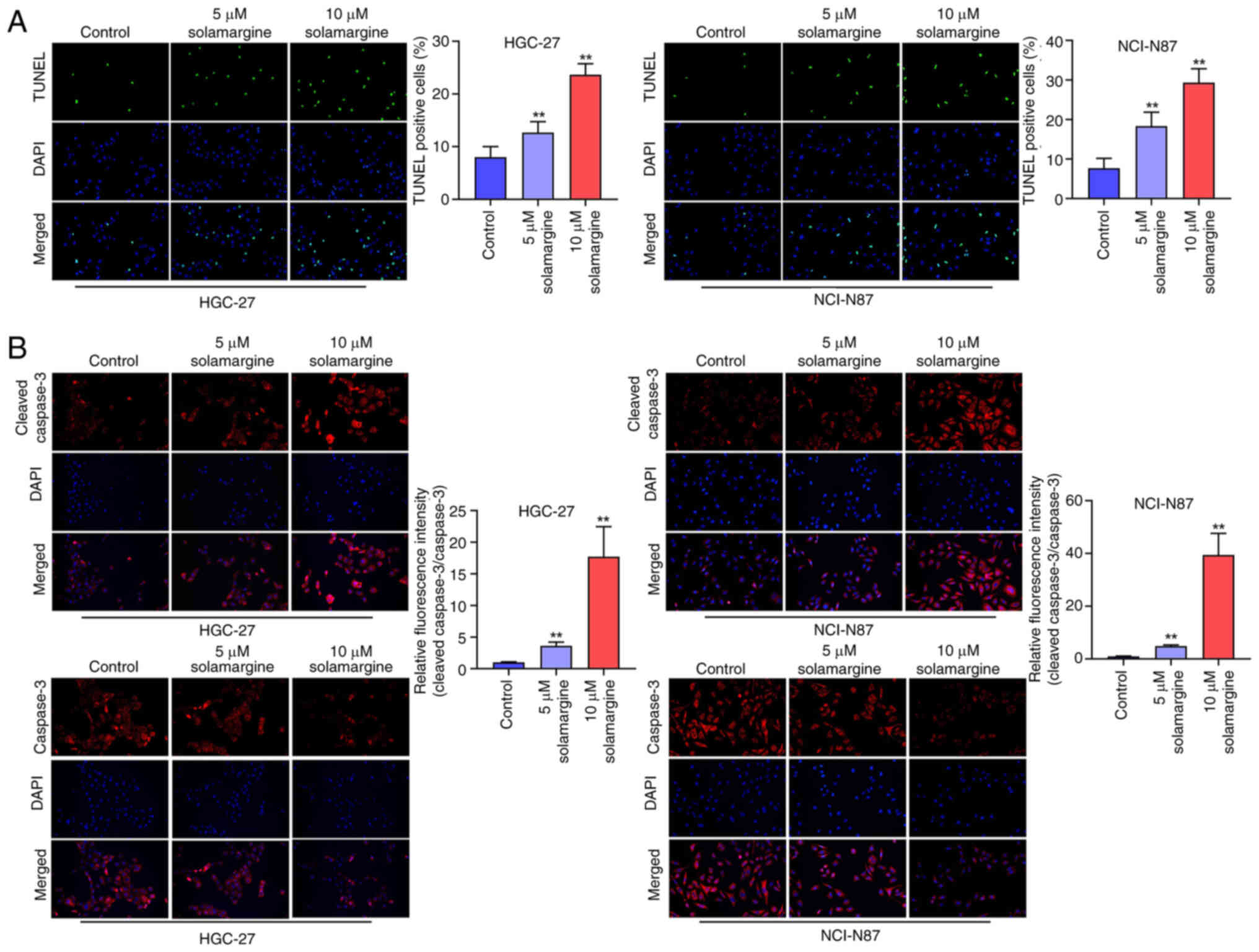
To assess the effect of solamargine on GC cell
apoptosis, a TUNEL staining assay was next performed. Subsequently,
5 and 10 µM solamargine was found to significantly increase the
percentage of TUNEL-positive NCI-N87 and HGC-27 cells compared with
that in the control group (Fig.
3A). Furthermore, the relative fluorescence intensity of
cleaved caspase 3 was significantly increased in 5 and 10 µM
solamargine-treated NCI-N87 and HGC-27 cells (Fig. 3B). This suggests that solamargine
notably induced GC cell apoptosis through the caspase-3 signaling
pathway, which was demonstrated by the relative increase in
fluorescence intensity of cleaved caspase 3.
Solamargine reverses the IL-6-induced
activation of STAT3/PD-L1 signaling
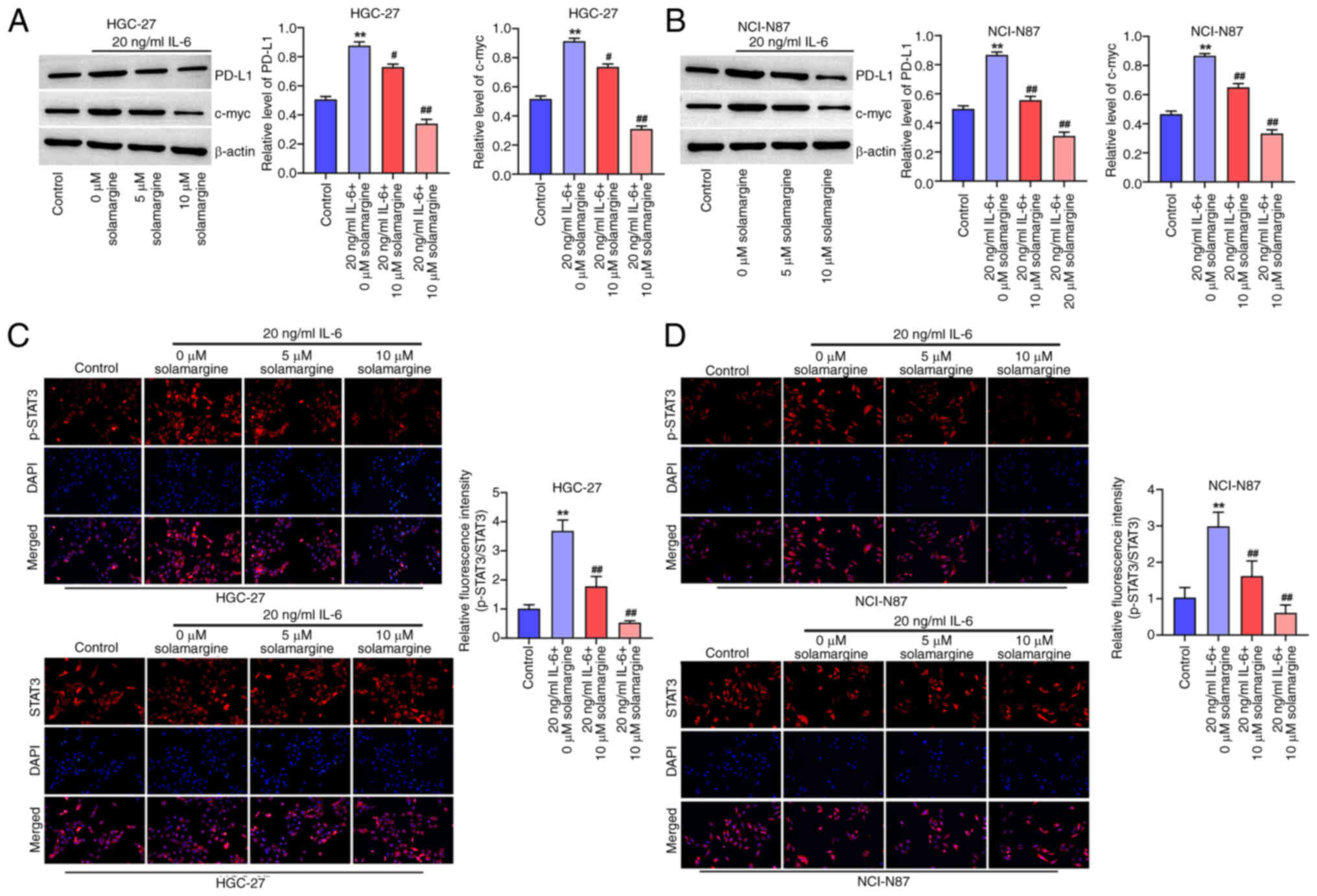
To assess the effect of solamargine on STAT3/PD-L1
signaling, GC cells were treated with IL-6 before the expression
levels of PD-L1 and c-Myc were investigated using western blotting.
It was demonstrated that IL-6 significantly increased the protein
levels of PD-L1 and c-Myc in HGC-27 (Fig. 4A) and NCI-N87 cells (Fig. 4B) compared with that in the control
group. However, treatment with IL-6 and 5 or 10 µM solamargine
significantly reversed this increased protein expression levels of
PD-L1 and c-Myc in HGC-27 (Fig.
4A) and NCI-N87 cells (Fig.
4B) compared with those in the IL-6 treatment alone.
Additionally, the IL-6-induced upregulation of STAT3
phosphorylation in HGC-27 (Fig.
4C) and NCI-N87 cells (Fig.
4D) was reversed by 5 and 10 µM solamargine treatment compared
with that in the IL-6 treatment alone group. This data suggest that
solamargine can reverse the IL-6-induced activation of STAT3/PD-L1
signaling.
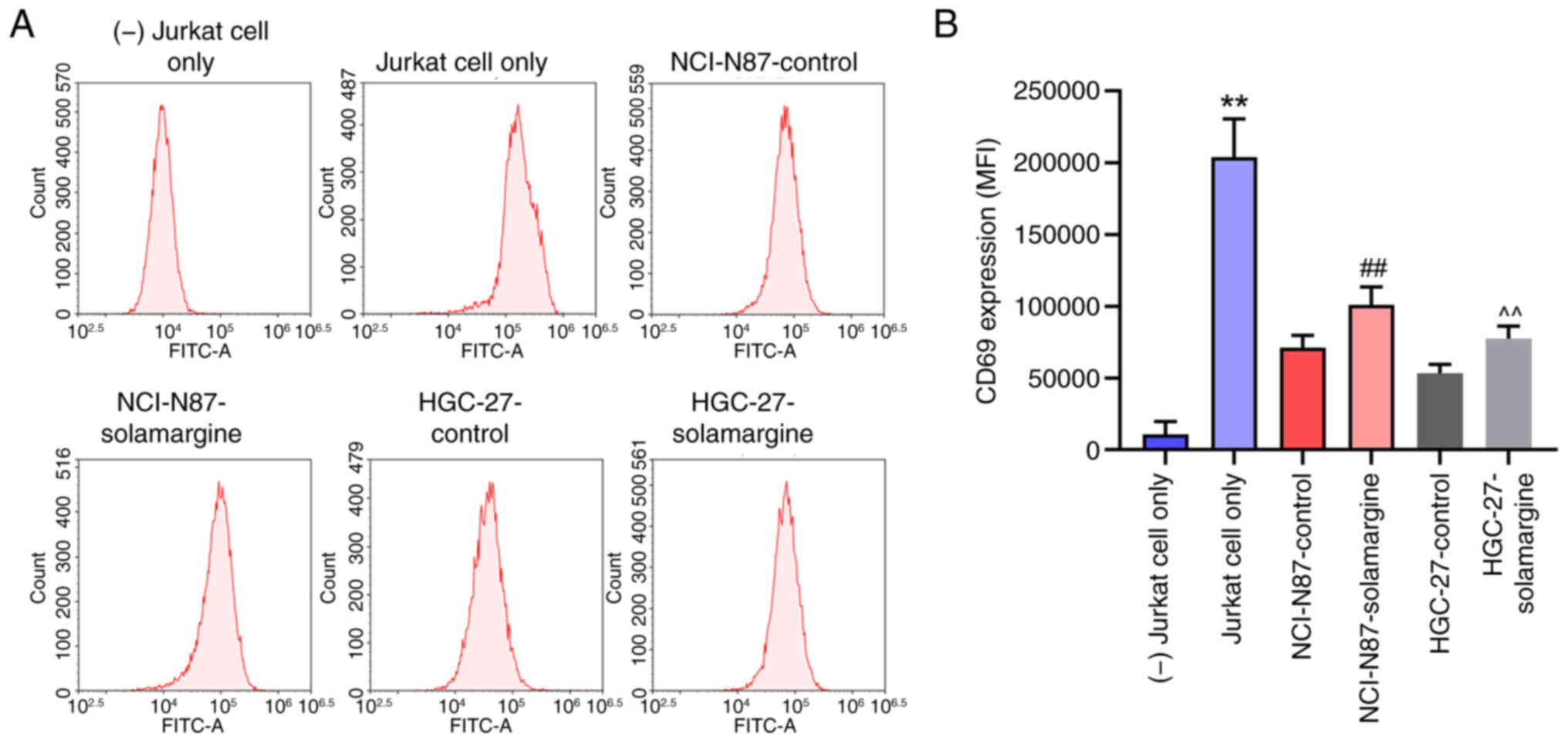
Solamargine activation of T cells
A previous study reported that PD-L1 upregulation
can promote the escape of tumor cells from the attack of T cells
(20). Furthermore, the
aforementioned results of the present study demonstrated that
solamargine can reduce the protein expression levels of PD-L1.
Therefore, to investigate the effect of solamargine on T cells,
Jurkat T cells were stimulated with anti-CD3/CD28 antibodies.
Subsequently, Jurkat T cells were co-cultured with non-treated or
solamargine-treated NCI-N87 or HGC-27 cells. The results
demonstrated that solamargine could increase the expression of CD69
(T cell activation marker) in Jurkat T cells (Fig. 5A and B). In summary, solamargine
may activate T cells.
Discussion
The incidence of GC worldwide had increased by ~75%
in 2023 (21). Therefore, the
pathogenic mechanism of GC should be determined. In the present
study, the results demonstrated that solamargine can reduce the
migration and invasion of GC cells. In addition, solamargine was
found to reverse the IL-6-induced PD-L1 and STAT3 phosphorylation
upregulation in GC cells, in addition to promoted T cell
activation. To the best of our knowledge, the present study was the
first to investigate the association between solamargine and T cell
activation. The results of the present study suggest that
solamargine may serve as a novel therapeutic agent in GC by
activation of T cells.
PD-L1 serves a role in immunotherapy, since PD-L1
downregulation enables T cells to recognize tumor cells (22,23).
The results of the present study demonstrated that solamargine
reduced the proliferation of GC cells and reduced the protein
expression levels of PD-L1. Traditional Chinese medicine monomers
have been previously documented to complement the efficacy of
immunotherapy for the treatment of malignant tumors. A previous
study by Yu et al (24)
demonstrated that the traditional Chinese medicine monomer
ailanthone can improve the therapeutic efficacy of anti-PD-L1 mAb
(Bio X Cell) in melanoma cells by inhibiting the c-Jun signaling
pathway. Additionally, another study by Liu et al (25) previously revealed that berberine
can reduce PD-L1 expression levels in non-small cell lung cancer
cells and promote antitumor immunity, thus inhibiting the
deubiquitination activity of CSN5 and served as an immune
checkpoint inhibitor. Therefore, the anti-PD-L1 potential of
monomers in GC cancer treatment should be further investigated.
The results of the present study also suggested that
solamargine reduced the levels of STAT3 in GC cells. Emerging
evidence suggests that STAT3 activation can promote tumorigenesis
in several types of cancer, including GC (26–28).
Therefore, it was hypothesized that solamargine can inhibit the
tumorigenesis of GC by inactivating the STAT3 signaling pathway.
Furthermore, PD-L1 has been previously shown to be positively
regulated by STAT3 during cancer progression. Jiang et al
(29) previously found that
tripartite motif-containing 29 can induce antitumor immunity by
downregulating STAT3 to inhibit the expression levels of PD-L1 in
GC. Additionally, Wang et al (30) revealed that pumilio1 can increase
the NPM3/NPM1 axis to promote PD-L1-mediated immune escape in GC.
These aforementioned findings suggest that solamargine may increase
the antitumor immunity during the progression of GC by inhibiting
STAT3/PD-L1 signaling.
However, the present study has a number of
limitations. The mechanisms underlying the effect of solamargine on
GC requires further investigation. Additionally, in vivo
studies are required to verify the results of the present study. In
addition, solamargine in combination with other therapies including
chemotherapy or targeted therapy should be investigated in
future.
In summary, the present study indicated that
solamargine can inhibit GC cell proliferation and invasion by
inactivating the STAT3 and PD-L1 signaling pathways. This finding
may provide a novel theoretical basis for drug discovery for the
treatment of GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Xiangtan Science and
Technology Bureau Project (Clinical study of endoscopic submucosal
dissection in the treatment of early carcinoma and precancerous
lesions of the digestive tract; grant no. SF-YB20171007).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XL and LS designed the study. WL, BL and LL
performed the experiments. XL and YS analyzed the data. WL and YS
prepared the manuscript. All authors read and approved the final
version of the manuscript and XL and LS confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jeon M, Jang H, Jeon H, Park CG and Kim S:
Long-term late effects in older gastric cancer survivors: Survival
analysis using Cox hazard regression model by retrospective
electronic health records. Support Care Cancer. 32:292023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun Z, Liu Y, Deng H, Wang S, Zhang J,
Xing C and Xu C: Modified Chaishao Liujunzi Decoction inhibits bile
acid-induced gastric intestinal metaplasia: From network prediction
to experimental verification. Aging (Albany NY). 15:13998–14018.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fang S, Liu Z, Qiu Q, Tang Z, Yang Y,
Kuang Z, Du X, Xiao S, Liu Y, Luo Y, et al: Diagnosing and grading
gastric atrophy and intestinal metaplasia using semi-supervised
deep learning on pathological images: Development and validation
study. Gastric Cancer. 27:343–354. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu Y, Huang C, Zhang C, Zhou Y, Zhao E,
Zhang Y, Pan X, Huang H, Liao W and Wang X: LncRNA MIR200CHG
inhibits EMT in gastric cancer by stabilizing miR-200c from
target-directed miRNA degradation. Nat Commun. 14:81412023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pei H, Yang J, Li W, Luo X, Xu Y, Sun X,
Chen Q, Zhao Q, Hou L, Tan G and Ji D: Solanum nigrum Linn:
Advances in anti-cancer activity and mechanism in digestive system
tumors. Med Oncol. 40:3112023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Long K, Zhou H, Li Y, Liu L and Cai J: The
value of chest computed tomography in evaluating lung cancer in a
lobe affected by stable pulmonary tuberculosis in middle-aged and
elderly patients: A preliminary study. Front Oncol. 12:8681072022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han Y, Shi J, Xu Z, Zhang Y, Cao X, Yu J,
Li J and Xu S: Identification of solamargine as a cisplatin
sensitizer through phenotypical screening in cisplatin-resistant
NSCLC organoids. Front Pharmacol. 13:8021682022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qu X, Xie J, Zhang Y and Wang Z:
Solamargine alleviates proliferation and metastasis of cervical
cancer cells by blocking the CXCL3-Mediated Erk signaling pathway.
Evid Based Complement Alternat Med. 2022:76347542022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ge J, Wang P, Ma H and Zhang J:
Solamargine inhibits prostate cancer cell growth and enhances the
therapeutic efficacy of docetaxel via Akt Signaling. J Oncol.
2022:90559542022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang S, Sun M, Ren Y, Luo T, Wang X, Weng
G and Cen D: Solamargine induces apoptosis of human renal carcinoma
cells via downregulating phosphorylated STAT3 expression. Oncol
Lett. 26:4932023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cha JH, Chan LC, Li CW, Hsu JL and Hung
MC: Mechanisms Controlling PD-L1 expression in cancer. Mol Cell.
76:359–370. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ai L, Xu A and Xu J: Roles of PD-1/PD-L1
Pathway: Signaling, cancer, and beyond. Adv Exp Med Biol.
1248:33–59. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Civriz AH, Teke K, Akdas EM, Dillioglugil
O, Vural C and Yaprak Bayrak B: The prognostic value of expressions
of STAT3, PD-L1, and PD-L2 in Ta/T1 urothelial carcinoma before and
after BCG treatment. Ural Oncol. 41:486.e1–486.e13. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wen J, Peng H, Wang D, Wen ZM, Liu YT, Qu
J, Cui HX, Wang YY, DU YL, Wang T, et al: Lipopolysaccharides
protect mesenchymal stem cell against cardiac ischemia-reperfusion
injury by HMGB1/STAT3 signaling. J Geriatr Cardiol. 20:801–812.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Erlichman N, Meshel T, Baram T, Abu Raiya
A, Horvitz T, Ben-Yaakov H and Ben-Baruch A: The Cell-Autonomous
Pro-Metastatic Activities of PD-L1 in Breast Cancer Are Regulated
by N-Linked Glycosylation-Dependent Activation of STAT3 and STAT1.
Cells. 12:23382023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang R, Ye H, Yang B, Ao M, Yu X, Wu Y, Xi
M and Hou M: m6A-modified circNFIX promotes ovarian cancer
progression and immune escape via activating IL-6R/JAK1/STAT3
signaling by sponging miR-647. Int Immunopharmacol. 124((Pt A)):
1108792023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye H, Yu W, Li Y, Bao X, Ni Y, Chen X, Sun
Y, Chen A, Zhou W and Li J: AIM2 fosters lung adenocarcinoma immune
escape by modulating PD-L1 expression in tumor-associated
macrophages via JAK/STAT3. Hum Vaccin Immunother. 19:22697902023.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Carvalho T, Lara P, Jorquera-Cordero C,
Aragão CFS, de Santana Oliveira A, Garcia VB, de Paiva Souza SV,
Schomann T, Soares LAL, da Matta Guedes PM and de Araújo Júnior RF:
Inhibition of murine colorectal cancer metastasis by targeting
M2-TAM through STAT3/NF-kB/AKT signaling using macrophage 1-derived
extracellular vesicles loaded with oxaliplatin, retinoic acid, and
Libidibia ferrea. Biomed Pharmacother. 168:1156632023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han Y, Liu D and Li L: PD-1/PD-L1 pathway:
Current researches in cancer. Am J Cancer Res. 10:727–742.
2020.PubMed/NCBI
|
|
20
|
Dermani FK, Samadi P, Rahmani G, Kohlan AK
and Najafi R: PD-1/PD-L1 immune checkpoint: Potential target for
cancer therapy. J Cell Physiol. 234:1313–1325. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan J, Fan F, He J and Sun Y: Agaricus
blazei Murill extract FA-2-b-β inhibits gastric cancer cell
proliferation by apoptosis by the mitochondrial pathway and
blocking the cell cycle. Asian J Surg. 47:1560–1561. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han EK, Woo JW, Suh KJ, Kim SH, Kim JH and
Park SY: PD-L1 (SP142) expression in primary and
recurrent/metastatic triple-negative breast cancers and its
clinicopathological significance. Cancer Res Treat. 56:557–566.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Anderson HG, Takacs GP, Harris DC, Kuang
Y, Harrison JK and Stepien TL: Global stability and parameter
analysis reinforce therapeutic targets of PD-L1-PD-1 and MDSCs for
glioblastoma. J Math Biol. 88:102023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu P, Wei H, Li K, Zhu S, Li J, Chen C,
Zhang D, Li Y, Zhu L, Yi X, et al: The traditional chinese medicine
monomer Ailanthone improves the therapeutic efficacy of anti-PD-L1
in melanoma cells by targeting c-Jun. J Exp Clin Cancer Res.
41:3462022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Liu X, Zhang N, Yin M, Dong J, Zeng
Q, Mao G, Song D, Liu L and Deng H: Berberine diminishes cancer
cell PD-L1 expression and facilitates antitumor immunity inhibiting
the deubiquitination activity of CSN5. Acta Pharm Sin B.
10:2299–2312. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, He X, Jia Z, Yan A, Xiao K, Liu S,
Hou M, Long Y and Ding X: Shenqi Fuzheng injection restores the
sensitivity to gefitinib in non-small cell lung cancer by
inhibiting the IL-22/STAT3/AKT pathway. Pharm Biol. 62:33–41. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li XH, Huang GZ, Xu ZL, Zhao CY, Dong XY,
Cui BK and Lin XJ: IL20RB signaling enhances stemness and
chemotherapy resistance in pancreatic cancer. J Transl Med.
21:9112023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu C, Shen A, Song J, Cheng L, Zhang M,
Wang Y and Liu X: LncRNA-CCAT5-mediated crosstalk between
Wnt/β-Catenin and STAT3 signaling suggests novel therapeutic
approaches for metastatic gastric cancer with high Wnt activity.
Cancer Commun (Lond). 44:76–100. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang T, Xia Y, Li Y, Lu C, Lin J, Shen Y,
Lv J, Xie L, Gu C, Xu Z and Wang L: TRIM29 promotes antitumor
immunity through enhancing IGF2BP1 ubiquitination and subsequent
PD-L1 downregulation in gastric cancer. Cancer Lett.
581:2165102024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang H, Zhou Z, Zhang J, Hao T, Wang P, Wu
P, Su R, Yang H, Deng G, Chen S, et al: Pumilio1 regulates
NPM3/NPM1 axis to promote PD-L1-mediated immune escape in gastric
cancer. Cancer Lett. 581:2164982024. View Article : Google Scholar : PubMed/NCBI
|