Introduction
Doxorubicin (DOX), a potent chemotherapeutic agent,
is widely used to treat several types of malignant tumors in
clinical settings, including breast and lung cancer, and others
(1). However, its non-targeted
action limits its therapeutic application (2). DOX-induced cardiomyopathy (DIC)
represents the most severe adverse reaction to DOX (3). It manifests as
concentration-dependent, cumulative and potentially fatal
myocardial injury, resulting in severe cardiomyopathy and heart
failure (4,5).
Ferroptosis serves a pivotal role in the onset and
progression of DIC (6).
Ferroptosis refers to a programmed cell death mode characterized by
excessive Fe2+ production and accumulation of lipid
peroxides. It is characterized by redox system imbalance, leading
to cell membrane destruction and subsequent cell death (7). Glutathione peroxidase 4 (GPX4), a key
antioxidant enzyme, is involved in reducing lipid peroxides to
non-toxic alcohols (8). DOX could
downregulate GPX4, thus triggering ferroptosis by disrupting the
antioxidant system (8).
Transferrin receptor protein 1 (TFR1) serves a key role in
ferroptosis by mediating the entry of Fe3+ into cells to
be converted into Fe2+ (9). In addition, DOX increases TFR1
expression on the cell membrane, allowing the excessive
accumulation of intracellular iron, triggering ferroptosis
(10). Consequently, reducing
ferroptosis during the application of DOX in the treatment of
malignant tumors is of importance (11).
Nuclear factor erythroid 2-related factor 2 (Nrf2)
is involved in regulating ferroptosis in a direct or indirect
manner via several pathways. The pathways include iron regulation,
NADPH regeneration and the antioxidant pathway, and the modulation
of mitochondrial function (12).
Luo et al (13)
demonstrated that astragaloside IV attenuated DOX-induced
myocardial ferroptosis in rats by upregulating Nrf2. Furthermore,
resveratrol alleviates ferroptosis via the p62/Nrf2 axis (14). Li et al (15) demonstrated that fisetin decreased
myocardial ferroptosis by regulating the sirtuin 1/Nrf2 pathway.
Therefore, Nrf2 upregulation could be an effective strategy to
inhibit DOX-induced myocardial ferroptosis.
Ophiopogon japonicus polysaccharide (OJP), a
major bioactive compound within O. japonicus, exhibits
diverse pharmacological activities, including anti-inflammatory,
antioxidant and immunomodulatory effects (16). Previous studies have suggested that
OJP mitigates cardiac injury through several pathways (17,18).
However, to the best of our knowledge, whether OJP attenuates
DOX-induced myocardial ferroptosis has not been elucidated, and its
underlying mechanism remains to be investigated.
The present study aimed to investigate whether OJP
decreases ferroptosis by lowering iron buildup and lipid
peroxidation. In addition, the inhibitory effect of OJP on
ferroptosis by activating Nrf2 to upregulate the downstream GPX4
and decrease the accumulation of Fe2+ in the labile iron
pool was also explored. Overall, the present study could provide a
novel therapeutic strategy for mitigating DOX-induced myocardial
ferroptosis injury.
Materials and methods
Cell culture
The human cardiomyocyte AC16 cell line was obtained
from Ningbo Mingzhou Biotechnology Co., Ltd. (cat. no. MZ-4038).
Cells were cultured in DMEM with high glucose (Shanghai Basal Media
Technologies Co., Ltd.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin solution (Biosharp Life Sciences) at 37°C
in a cell culture incubator with 5% CO2. DOX, ML385 and
ferrostatin-1 (Fer-1) were purchased from MedChemExpress (cat. nos.
HY-15142, HY-100523 and HY-100579, respectively). OJP (purity,
98.50%) was obtained from Shanghai Winherb Medical Technology Co.,
Ltd. (cat. no. TDT017). DOX was utilized at 0.5 µM for 24 h to
establish the myocardial ferroptosis injury model, while OJP was
added (50, 100, 200 µg/ml) for 6 h. In addition, 5 µM Fer-1 or 10
µM ML385 were added for 6 h at 37°C.
Cell viability assay
The viability of AC16 cells was assessed using a
Cell Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology)
assay. Briefly, cells (5,000 cells/well) were inoculated into
96-well culture plates and incubated overnight at 37°C, followed by
treatment with different concentrations of DOX (0.1, 0.5, 1.0, 2
µM) and OJP (50, 100, 200 µg/ml) for 24 h, or pretreated with OJP
or Fer-1 (5 µM) for 6 h, followed by treatment with DOX at 0.5 µM
for 24 h. Cells were washed once with PBS and then 10 µl CCK-8
solution was added, followed by incubation for 1 h at 37°C.
Finally, the absorbance in each well was measured at 450 nm using a
microplate reader (cat. no. PT-3502PC, Beijing Potenov Technology
Co. Ltd.).
Lactate dehydrogenase (LDH)
detection
Following treatment as aforementioned, cell
supernatant was obtained by centrifugation at room temperature 400
g for 5 min, mixed with LDH assay working solution (cat. no. C0016,
Beyotime Institute of Biotechnology) and transferred to a 96-well
plate. Subsequently, the plate was incubated at room temperature in
the dark for an additional 30 min and the absorbance was measured
at 490 nm using a microplate reader.
Creatine phosphokinase-MB (CK-MB) and
cardiac troponin I (cTn-I) assessment
The levels of CK-MB and cTn-I were determined using
the corresponding ELISA kits. Cells were treated as aforementioned.
The supernatant was collected by centrifugation at 1,500 g for 20
min at room temperature. ELISA kits were used according to the
manufacturer's instructions (cat. no. H197-1-1, cat. no. H149-2-1,
Nanjing Jiancheng Bioengineering Institute). Following incubation
for 30 min at room temperature, the plates were washed with
detergent in the kits. After the addition of color developing and
stop solutions, the absorbance was measured at 450 nm with a
microplate reader.
Reactive oxygen species (ROS) level
assessment
Cells (60,000 cells/well) were plated into 6-well
culture plates and treated as aforementioned.
2′-7′-dichlorodihydrofluorescein diacetate (DCFH-DA; Beyotime
Institute of Biotechnology) was diluted in serum-free DMEM and the
appropriate volume (1 ml/well) of diluted DCFH-DA was added to each
well after the culture medium was removed. Subsequently, the cells
were incubated at 37°C for 20 min. Following washing with
serum-free DMEM three times, the cells were observed under a
fluorescence microscope (Olympus Corporation). ImageJ2 (version
1.53, National Institutes of Health) was used in fluorescence
intensity analysis. In addition, cells treated with OJP at 200
µg/ml for 6 h in the presence or absence of 10 µM ML385 were
pretreated for 6 h and then molded at 37°C. Single-cell suspensions
were collected after three washes with PBS and analyzed using flow
cytometry (Agilent NovoCyte Penteon, Agilent Technologies, Inc.).
Analysis of flow cytometry results was performed with NovoExpress
(version 1.4.1, Agilent Technologies, Inc.).
Western blot analysis
Following treatment as aforementioned, cells were
washed three times with PBS and lysed with RIPA lysis buffer (cat.
no. P0013C; Beyotime Institute of Biotechnology) supplemented with
protease inhibitors for 30 min at 4°C. The mixture was centrifuged
at 12,000 × g for 30 min at 4°C and the supernatant was then
collected. Nuclear and Cytoplasmic Protein Extraction Kit (cat. no.
P0027, Beyotime Institute of Biotechnology) was used to isolate
nuclear and cytoplasmic proteins. After the cells were washed with
PBS, cytoplasmic extraction reagent was added, the cells were lysed
at 4°C for 15 min, and then centrifuged at 4°C for 16,000 g for 5
min to obtain the supernatant, which was cytoplasmic protein. The
remaining precipitation was lysed at 4°C for 30 min after adding
nuclear extraction reagent, and the supernatant was obtained after
10 min of centrifugation at 4°C 16,000 g. The protein concentration
was measured using the BCA Protein Quantification kit (cat. no.
P0009, Beyotime Institute of Biotechnology). Proteins (35 µg/lane)
was separated by 10% SDS-PAGE and then transferred to PVDF
membranes. Following blocking with 5% non-fat powdered milk at room
temperature for 1 h, the membranes were incubated with primary
antibodies against TFR1 (1:500, cat. no. sc-393719, Santa Cruz
Biotechnology, Inc.), GPX4 (1:1,000, cat. no. 30388-1-AP), Nrf2
(1:2,000, cat. no. 16396-1-AP), β-actin (1:5,000, cat. no.
66009-1-Ig, all Proteintech Group, Inc.) and histone H3 (1:2,000;
cat. no. 9715S, Cell Signaling Technology, Inc.) overnight at 4°C.
Subsequently, the membranes were incubated with the corresponding
HRP-conjugated secondary antibodies (1:5,000; cat. nos. sc-2357 and
sc-525409, all Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Membranes were treated with Omni-ECL (cat. no. SQ201,
Epizyme Biomedical Technology), the protein bands were visualized
using the Tanon-4600 Chemiluminescence Imaging System (Tanon
Science and Technology Co., Ltd.). The bands were analyzed using
the ImageJ2 software (version 1.53, National Institutes of
Health).
Fe2+ level detection
To measure the relative amount of intracellular
Fe2+ following cell treatment as aforementioned. A
FerroOrange fluorescent probe (cat. no. F374, Dojindo Molecular
Technologies, Inc.) was utilized. Briefly, cells (65,000
cells/well) seeded into 6-well plates were washed three times with
serum-free medium (DMEM, Shanghai Basal Media Technologies Co.,
Ltd.) followed by addition of 1 µmol/l Fe2+ detection
working solution. Subsequently, cells were incubated in a 37°C cell
culture incubator for 30 min prior to examination under a
fluorescence microscope (Olympus Corporation). The fluorescence
intensity analysis was conducted using ImageJ2 (version 1.53,
National Institutes of Health).
Malondialdehyde (MDA) content
measurement
As MDA is the end product of lipid peroxidation
(19), the degree of lipid
peroxidation was evaluated by measuring MDA levels. Following cell
lysis with Western and IP cell lysis buffer (cat. no. P0037,
Beyotime Institute of Biotechnology), the protein concentration was
measured using a BCA kit. The supernatant (12,000 g for 20 min at
4°C) was mixed with MDA working solution (cat. no. S0131, Beyotime
Institute of Biotechnology) and incubated at 100°C for 15 min prior
to cooling to room temperature. Following centrifugation at 1,000 ×
g for 10 min at room temperature, 200 µl supernatant was added into
a 96-well plate. Finally, the absorbance in each well was measured
at a wavelength of 532 nm with the microplate reader.
ATP content assessment
ATP content was detected using the Enhanced ATP
Assay Kit (cat. no. S0027, Beyotime Institute of Biotechnology).
The cell supernatant was obtained following cell lysis with ATP
lysate (Beyotime Institute of Biotechnology). The relative light
unit values were measured with a luminometer (SpectraMax iD5,
Molecular Devices, Inc.) following mixing of 100 µl assay solution
and 20 µl of each sample in a white background 96-well plate.
Mitochondrial membrane potential (MMP)
assessment
An enhanced MMP assay kit with JC-1 (cat. no.
C2003S, Beyotime Institute of Biotechnology) was used to evaluate
the MMP. After the culture medium was removed, cells (60,000
cells/well) in the 6-well plate were supplemented with 1 ml DMEM,
Shanghai Basal Media Technologies Co., Ltd.) and JC-1 staining
solution. Subsequently, cells were incubated for 20 min at 37°C.
Cells were washed twice with JC-1 staining buffer and observed
under a fluorescence microscope (Olympus Corporation). The
fluorescence intensity analysis was performed utilizing ImageJ2
(version 1.53, National Institutes of Health).
Immunofluorescence staining
Cells (50,000 cells/well) were cultured with OJP at
200 µg/ml for 6 h followed by treatment with DOX at 0.5 µM for 24 h
at 37°C in 6-well plates, fixed with 4% paraformaldehyde for 15
min, and permeabilized with 0.1% Triton X-100 for 10 min and
blocked with Immunol staining blocking buffer (cat. no. P0260,
Beyotime Institute of Biotechnology) for 15 min at room
temperature. Cells were incubated with Nrf2 antibody (1:2,000, cat.
no. 16396-1-AP, Proteintech Group, Inc.) at 4°C overnight, followed
by incubation with the corresponding secondary antibody conjugated
to Alexa Fluor® 488 (1:200, cat. no. ab150077, Abcam,
Inc.) for 1 h in room temperature. Following washing with PBS,
cells were stained with DAPI (cat. no. C1002, Beyotime Institute of
Biotechnology) for 5 min and images were captured under a
fluorescence microscope (Leica Microsystem) at room temperature.
Image analyses were processed with the Leica Application Suite X
(version 4.6.0, Leica Microsystem, Inc.).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 8.0 software (Dotmatics). The results are presented
as the mean ± SD of 3 independent experimental repeats. The
differences among multiple groups were compared using one-way ANOVA
followed by Dunnett's or Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
OJP protects against DOX-induced
myocardial injury
DOX was utilized at a concentration of 0.5 µM for
modeling, while OJP was administrated at concentrations of 50–200
µg/ml for pre-treatment. The specific grouping is depicted in
Fig. 1A. To investigate the
potential protective effects of OJP against DOX-induced cardiac
cytotoxicity, a CCK-8 assay was performed. Initially, the optimal
concentration of DOX was determined for model establishment.
Following treatment with 0.5 µM DOX for 24 h, the cell viability
was decreased to ~50% (Fig. 1B).
Consequently, based on previous studies (14,20)
and these preliminary experiments, a concentration of 0.5 µM DOX
was chosen for further experiments. Subsequently, the effect of OJP
on AC16 cell viability was assessed. OJP had no significant effect
on cell viability, suggesting that OJP at 50–200 µg/ml had no
cytotoxic effects on AC16 cells after 24 h of exposure (Fig. 1C). The ferroptosis inhibitor Fer-1
significantly inhibited DOX-mediated cell death, indicating that
ferroptosis was involved in DOX-induced cell death (Fig. 1D). Additionally, OJP relieved
DOX-induced cell death and induced concentration-dependent partial
recovery of cell viability. The effect of OJP with Fer-1 suggested
that OJP could serve a role in reducing ferroptosis. To evaluate
the protective effects of OJP against DIC, the levels of the
myocardial injury-related biomarkers LDH, CK-MB and cTn-I were
measured. DOX-induced myocardial cell damage was demonstrated by
the increased levels of the aforementioned biomarkers. However, OJP
at concentrations ranging from 50 to 200 µg/ml decreased the
secretion levels of LDH and cTn-I, in a dose-dependent manner, with
the most pronounced effect observed at 200 µg/ml (Fig. 1E and G). OJP could alleviate the
increase of CK-MB, with a significant effect observed at 200 µg/ml.
Although the effect at 50 µg/ml was not statistically significant,
it still showed a trend towards alleviation (Fig. 1F). Collectively, the aforementioned
findings suggested that OJP could mitigate DOX-induced myocardial
damage in a concentration-dependent manner, highlighting its
potential as a candidate drug for ameliorating DOX-induced
myocardial injury.
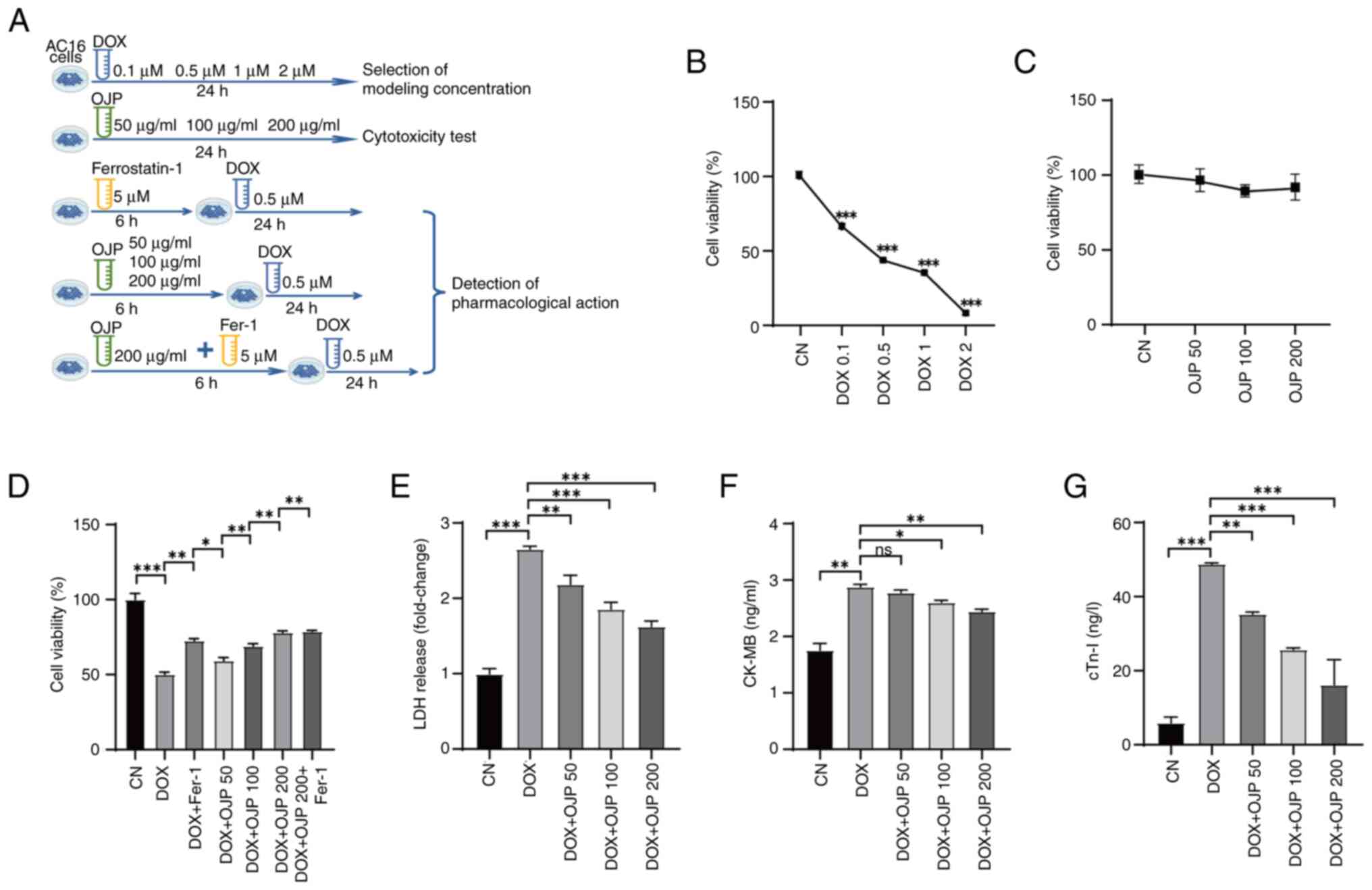 | Figure 1.OJP protects AC16 cells against
DOX-induced injury. (A) Experimental grouping. Effect of (B) DOX
and (C) OJP on cell viability. (D) Effect of OJP on cell viability
in DOX-induced myocardial injury cells. Effect of OJP on (E) LDH
release, (F) CK-MB and (G) cTn-I in DOX-induced myocardial injury
cells. *P<0.05, **P<0.01, ***P<0.001. CK-MB, creatine
phosphokinase-MB; CN, control; cTn-I, cardiac troponin I; DOX,
doxorubicin; Fer-1, ferrostatin-1; LDH, lactate dehydrogenase; ns,
not significant; OJP, Ophiopogon japonicus
polysaccharide. |
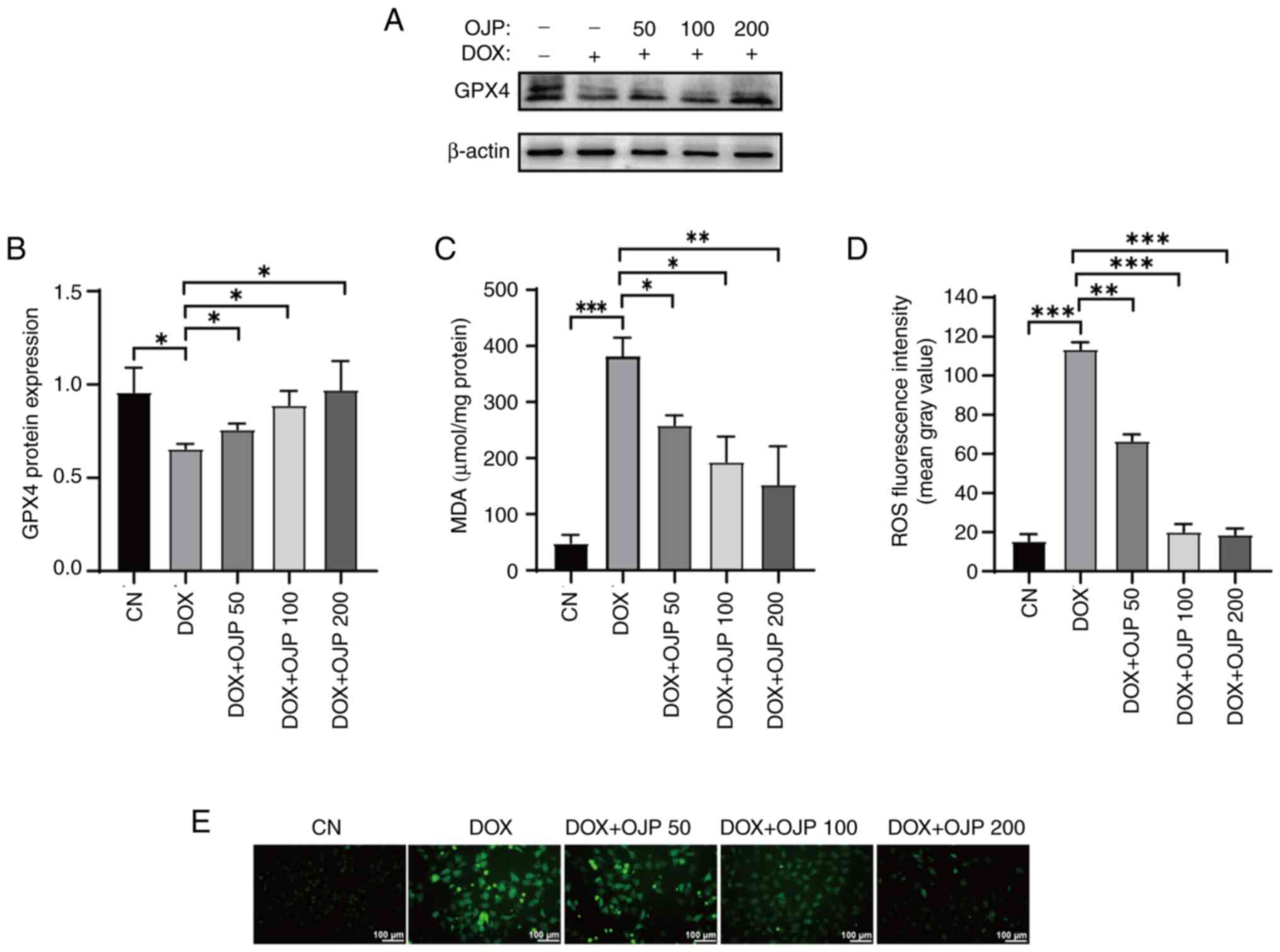
OJP alleviates oxidative damage in
DOX-induced myocardial ferroptosis
To determine whether OJP decreases lipid
peroxidation, changes in the content of GPX4 were assessed. GPX4
serves both as a ferroptosis marker and a crucial endogenous
antioxidant enzyme (21). DOX
notably downregulated GPX4, thus indicating ferroptosis. OJP
partially restored GPX4 expression levels, with the most notable
recovery observed in the 200 µg/ml group (Fig. 2A and B). Subsequently, changes in
MDA levels were assessed. MDA, the ultimate metabolite of lipid
peroxidation, is widely used as a standard marker for assessing the
degree of lipid peroxidation (19,22).
DOX promoted the production of MDA, whereas cell treatment with OJP
significantly reduced MDA content in a concentration-dependent
manner (Fig. 2C). In addition, DOX
induced severe oxidative stress, as evidenced by the notable
increase in green fluorescence, indicating ROS accumulation.
However, OJP gradually attenuated the levels of green fluorescence,
with those in the 200 µg/ml group almost returning to those
observed in the control group (Fig. 2D
and E). These results indicated that OJP could upregulate GPX4
and alleviate lipid peroxidation in myocardial ferroptosis in a
concentration-dependent manner.
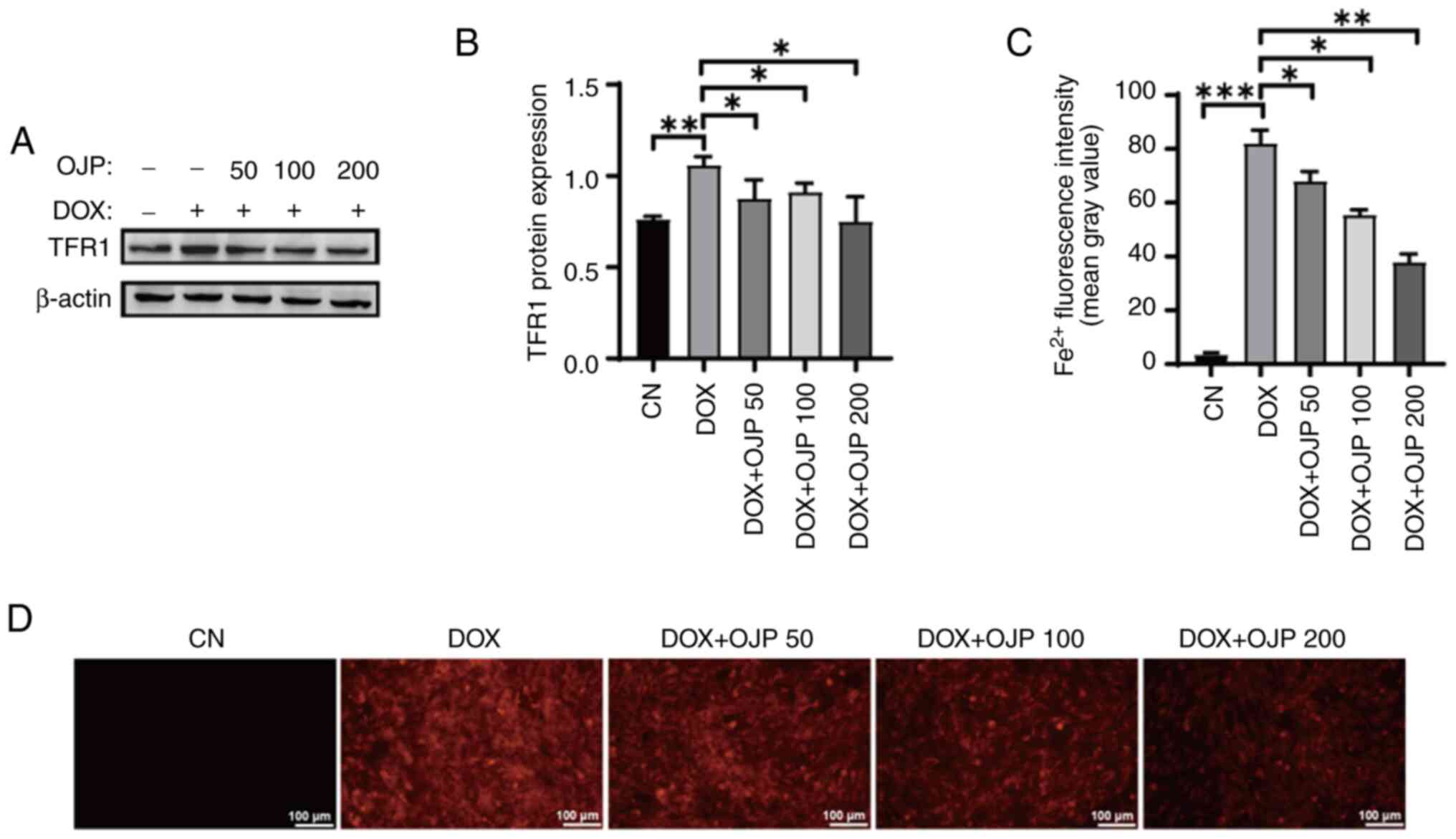
OJP decreases iron accumulation in
DOX-induced myocardial ferroptosis
The effect of OJP on iron accumulation in
DOX-induced myocardial ferroptosis was evaluated by measuring the
expression levels of TFR1 and relative content of Fe2+.
Following cell induction with 0.5 µM DOX for 24 h, the protein
expression levels of TFR1 were increased, and these were then
markedly diminished in OJP-treated cells compared with the DOX
group. The most pronounced decrease was observed in the highest OJP
dose group (Fig. 3A and B).
Fe2+ content serves as an indicator of iron accumulation
(10). In the DOX group, the
FerroOrange probe exhibited bright orange fluorescence upon binding
with Fe2+ (Fig. 3C and
D). Following OJP treatment, the orange fluorescence intensity
was significantly decreased in a concentration-dependent manner,
thus indicating a notable reduction in intracellular
Fe2+ content. Taken together, the aforementioned results
suggested that OJP downregulated TFR1 and alleviated iron
accumulation in DOX-induced myocardial ferroptosis.
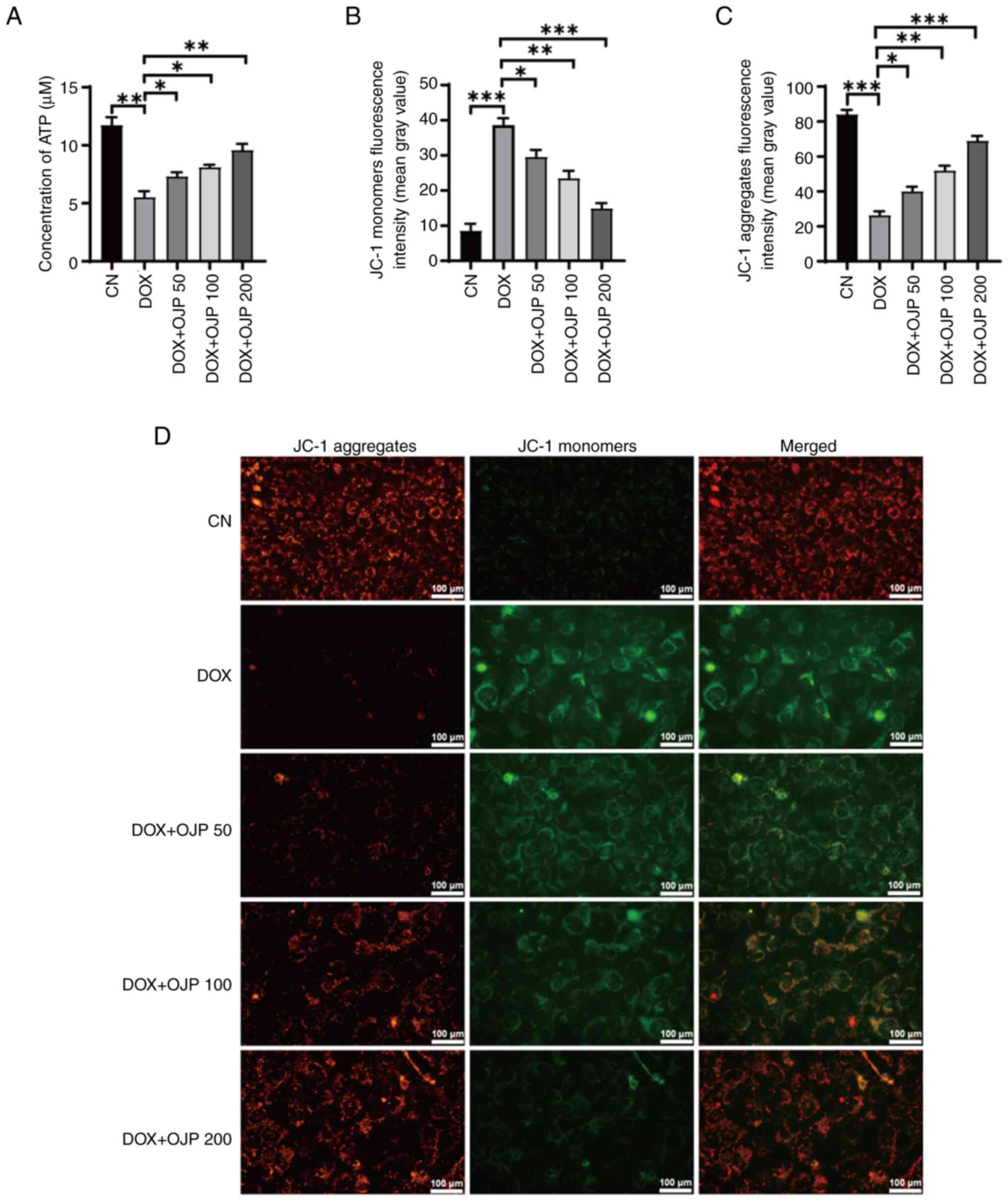
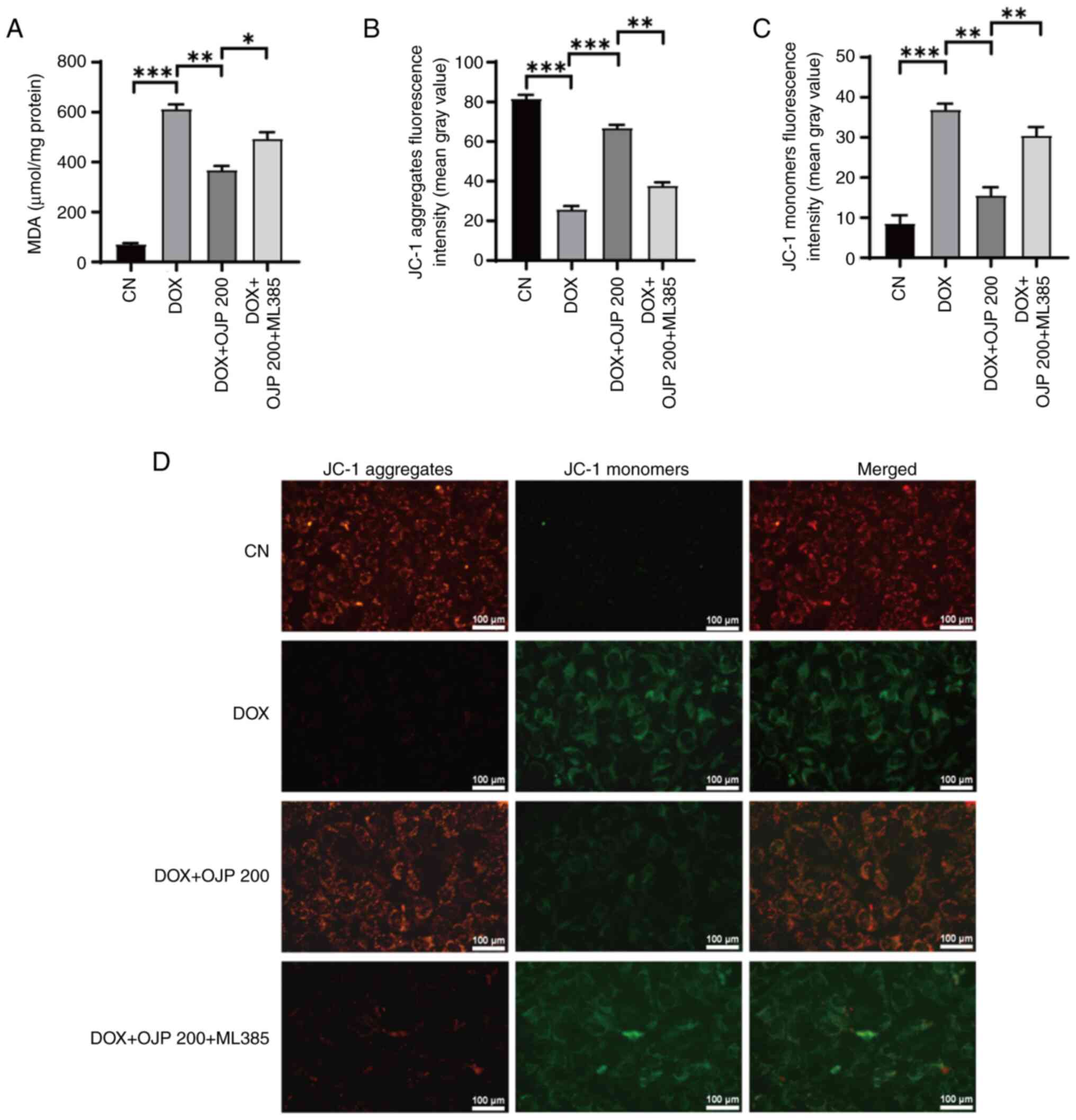
OJP relieves mitochondrial dysfunction
in DOX-induced myocardial ferroptosis
The onset of ferroptosis commonly coincides with
mitochondrial dysfunction, where alterations in ATP generation and
the MMP serve as indicators of mitochondrial performance,
indirectly reflecting the extent of ferroptosis (23). DOX decreased ATP production
compared with the control group, which was restored in the OJP
groups in a concentration-dependent manner (Fig. 4A). In the MMP assessment
experiments using JC-1 staining, changes in the fluorescence color
of the mitochondrial membrane reflected the degree of mitochondrial
injury. The normal control cells exhibited red fluorescence, while
those with an impaired mitochondrial membrane exhibited green
fluorescence. Green, but not red fluorescence, was prominently
observed in cells in the DOX group, thus indicating a decrease in
DOX-induced MMP. By contrast, green fluorescence was gradually
reduced and red fluorescence was gradually restored in the OJP
groups compared with the DOX group, thus indicating an enhanced MMP
(Fig. 4B-D). These results
suggested that OJP alleviated mitochondrial injury during the
progression of myocardial ferroptosis.
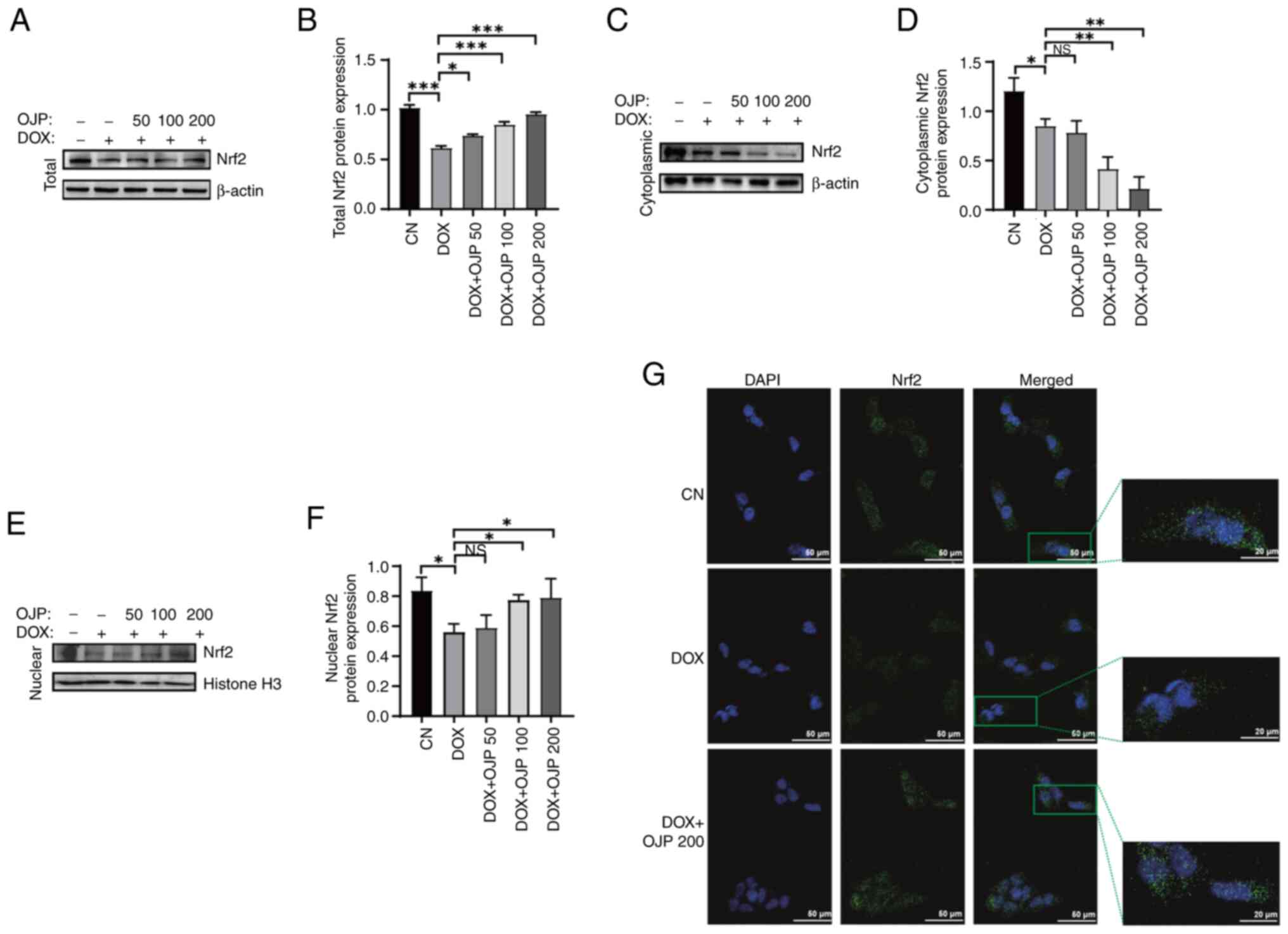
OJP attenuates DOX-induced myocardial
ferroptosis by enhancing the nuclear translocation of Nrf2
It has been reported that Nrf2 serves a pivotal role
in the regulation of ferroptosis (24). To determine the potential mechanism
underlying the effect of OJP on myocardial ferroptosis-induced
injury, the expression levels of Nrf2 were detected. The protein
expression levels of Nrf2 were significantly reduced in the DOX
group compared with the control group. Conversely, cell treatment
with OJP notably upregulated Nrf2 in a concentration-dependent
manner, with 200 µg/ml showing the most pronounced restoring effect
(Fig. 5A and B). Given the key
role of nuclear translocation in the transcriptional activation of
Nrf2 (25), the translocation of
Nrf2 was assessed. OJP treatment led to a dose-dependent decrease
in cytoplasmic Nrf2 content accompanied by an increase in nuclear
Nrf2 accumulation (Fig. 5C-F). The
effect of OJP on the Nrf2 pathway was verified via
immunofluorescence assays. DOX reduced Nrf2 total and nuclear
protein, while OJP could restore Nrf2 expression and promote its
nuclear translocation (Fig. 5G).
These results suggested that OJP could enhance the total protein
levels of Nrf2 and promote its nuclear translocation, thus
supporting its potential as a modulator of myocardial ferroptosis.
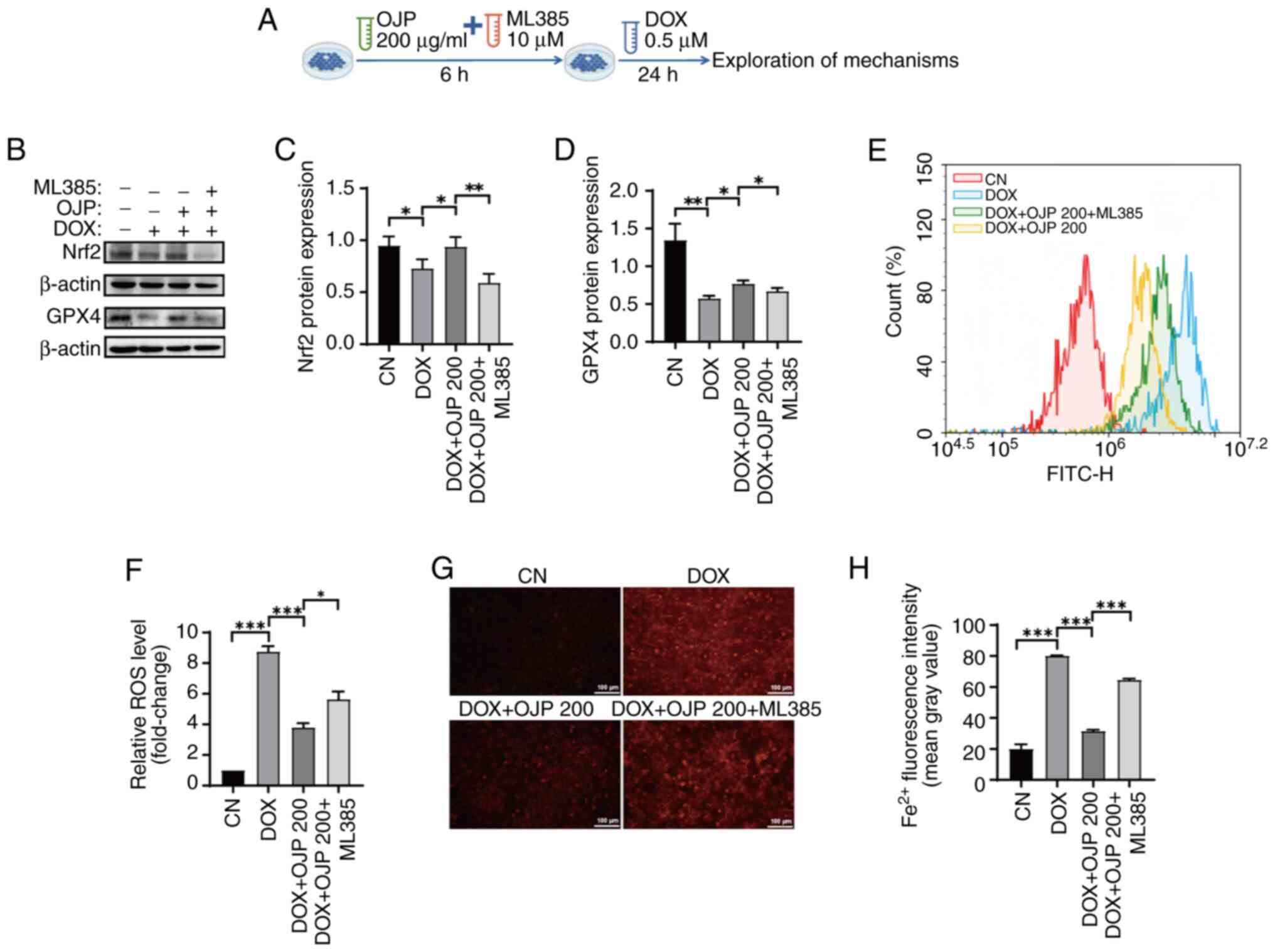
Furthermore, to clarify whether the inhibition of DOX-induced
myocardial ferroptosis by OJP is associated with induction of Nrf2
expression, cells were pretreated with the Nrf2 inhibitor ML385 and
200 µg/ml OJP, followed by DOX administration (Fig. 6A). OJP-induced restoration of Nrf2
expression was partially reversed by ML385. In addition, the
ability of OJP to upregulate GPX4, a downstream signaling molecule
of Nrf2 (26), was inhibited by
ML385 (Fig. 6B-D). Therefore, the
protective effect of OJP against DOX-induced myocardial ferroptosis
was partially mediated via enhancing the Nrf2/GPX4 signaling
pathway. Nrf2 serves as a regulator of ROS and iron homeostasis via
heme metabolism (27). ROS and
Fe2+ levels were measured to assess whether the
OJP-mediated reduction of oxidative stress and iron accumulation in
ferroptosis depended on Nrf2 activation. Consistent with
aforementioned results, OJP significantly attenuated ROS and
Fe2+ levels compared with those in the DOX group. ML385
markedly reversed the ability of OJP to reduce ROS and
Fe2+ levels compared with those in the OJP group
(Fig. 6E-H). MDA and MMP levels
were determined to evaluate whether OJP relieved DOX-induced lipid
peroxidation and mitochondrial injury by enhancing the nuclear
translocation of Nrf2. ML385 significantly reduced the ability of
OJP to alleviate lipid peroxidation and restore the MMP (Fig. 7A-D). These findings suggested that
OJP mitigated DOX-induced ferroptosis in cardiomyocytes by
promoting nuclear translocation of Nrf2. Collectively, the
aforementioned findings suggested the protective effect of OJP
against myocardial ferroptosis was primarily triggered by Nrf2
upregulation.
Discussion
The present study demonstrated that OJP alleviated
myocardial ferroptosis by reducing iron accumulation and ROS
production, reinstating lipid metabolism and maintaining
mitochondrial function. OJP counteracted myocardial ferroptosis via
nuclear translocation of Nrf2. The pharmacological effects of OJP
could ultimately protect cardiomyocytes from DOX-induced myocardial
ferroptosis-triggered injury. The proposed framework of the
protective mechanism of OJP against DOX-induced myocardial
ferroptosis is depicted in Fig.
8.
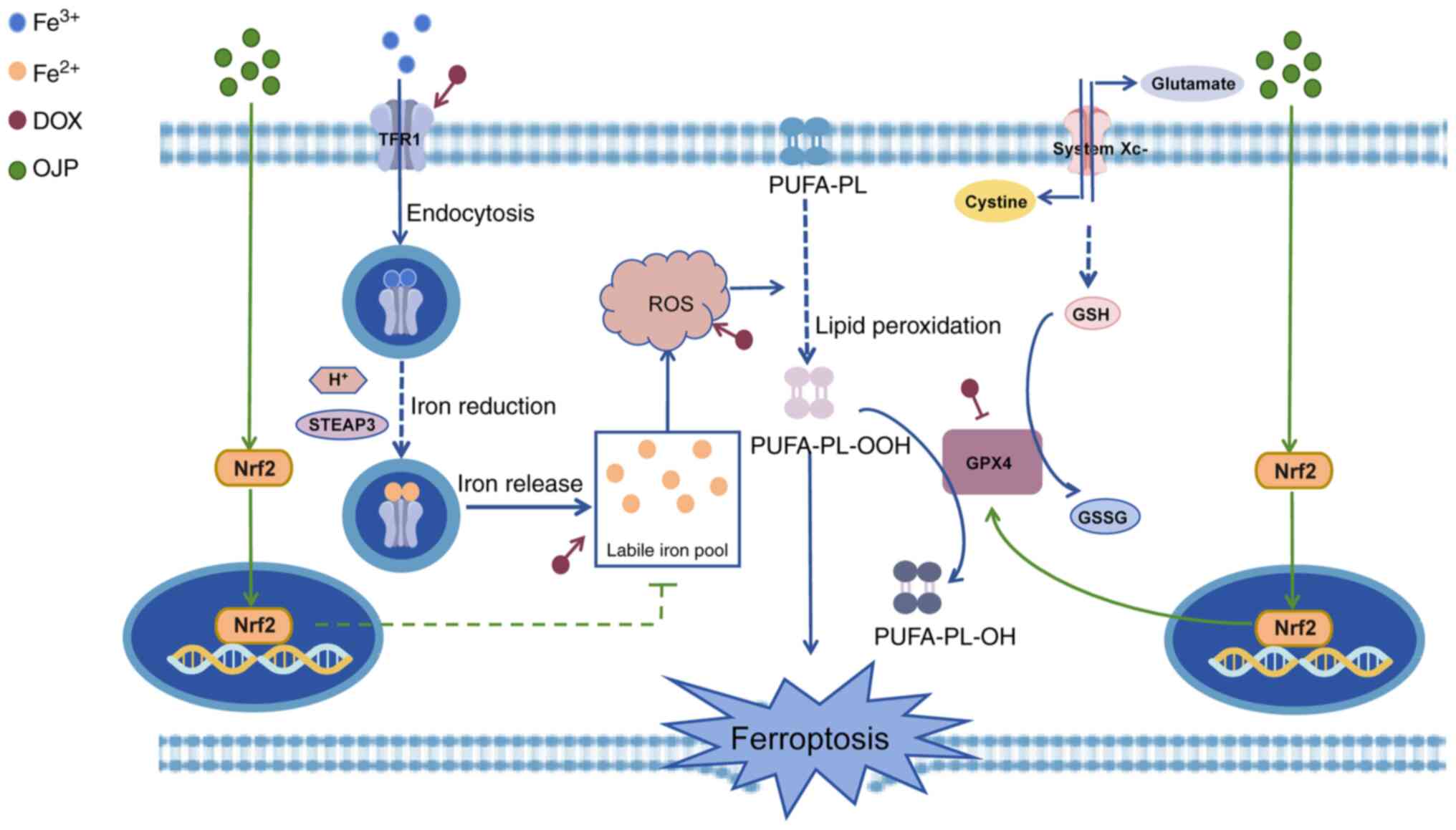 | Figure 8.Pharmacological effects and mechanism
of OJP attenuating DOX-induced myocardial ferroptosis injury. By
promoting the nuclear translocation of Nrf2, OJP reduces the
accumulation of labile iron pools and the production of ROS.
Additionally, it mitigates lipid peroxidation by enhancing the
expression of GPX4, ultimately leading to a decrease in the
occurrence of ferroptosis. DOX, doxorubicin; GPX4, glutathione
peroxidase 4; GSH, glutathione; GSSG, oxidized glutathione; Nrf2,
nuclear factor erythroid 2-related factor 2; OJP, Ophiopogon
japonicus polysaccharide; PUFA-PL, polyunsaturated fatty
acid-phospholipid; ROS, reactive oxygen species; STEAP3,
6-transmembrane epithelial antigen of prostate 3; TFR1, transferrin
receptor protein 1. |
The adverse effects of DOX restrict its clinical
application and lead to severe heart failure (28). Although dexrazoxane is currently
the only drug approved by the US Food and Drug Administration for
prevention of DIC in patients with cancer (29), its clinical application is limited
by its side effects, including vomiting, dermatitis, subcutaneous
necrosis, metabolic abnormality and bone marrow suppression
(29). Therefore, the development
of novel drugs or strategies for preventing DIC is of importance.
Natural compounds are receiving increasing attention due to their
lower incidence of side effects and adverse reactions (30). It has been reported that OJP
displays therapeutic efficacy against myocardial infarction
(31), ischemia-reperfusion injury
(32) and diabetic cardiomyopathy
(18).
Zhang et al (18) found that OJP, when used to treat
myocardial injury associated with diabetes, also decreased blood
glucose levels and hepatic and renal indices in diabetic rats.
Further research has shown that OJP could alleviate lipid
accumulation, hepatic degeneration and inflammation in
non-alcoholic fatty liver disease (33). OJP has also been shown to protect
the integrity of the intestinal epithelial barrier and prevent
inflammation-induced epithelial damage (34). Additionally, ophiopogonin D,
extracted from O. japonicus, has been shown to improve renal
function in diabetic rats (35).
The aforementioned studies suggest that OJP exerts protective
effects across multiple systems in the body without notable toxic
or adverse effects. Traditional Chinese medicine formulations
containing O. japonicus have been applied in clinical
practice. For example, Yang et al (35) collected data from 110 patients with
non-small cell lung cancer and found that, compared with
chemotherapy with gemcitabine and cisplatin alone, the combination
of Shashen Maidong Decoction (SMD, consists of Adenophora stricta,
Polygonatum odoratum, Glycyrrhiza uralensis, Morus alba, O.
japonicus, Lablab purpureus, and Trichosanthes kirilowii) with
chemotherapy led to a greater decrease in inflammation levels.
Additionally, the clinical efficacy was higher, as evidenced by an
improved Karnofsky performance status score, increased body weight
stability and a lower incidence of adverse reactions. Although
there are no clinical reports on the use of O. japonicus
alone (36), the application of
traditional Chinese medicine formulas containing O.
japonicus highlights its advantages both in terms of clinical
efficacy and safety.
The present study also further verified that OJP
could significantly improve DOX-induced cardiomyocyte failure,
evidenced by the enhanced viability of cardiomyocytes and decreased
levels of the myocardial injury-related markers LDH, CK-MB and
cTn-I. Furthermore, OJP did not display cytotoxic effects at the
administered doses. These findings suggest that OJP may serve as a
promising candidate drug with improved safety and fewer side
effects for mitigating DOX-induced cardiomyocyte injury, thereby
delaying progression of myocardial damage.
The involvement of ferroptosis in the pathogenesis
of DIC has been supported by a previous study (37). Therefore, the present study aimed
to explore whether the protective effects of OJP on cardiomyocytes
were mediated by reducing ferroptosis. Ferroptosis is characterized
by iron accumulation and redox system dysregulation (38). Fan et al (39) demonstrated that OJP mitigated
isoproterenol-induced myocardial injury by enhancing the expression
of free radical scavenging enzymes, such as superoxide dismutase
and catalase. Consistently, the present study revealed that OJP
upregulated GPX4 and mitigated DOX-induced oxidative stress and
lipid peroxidation, supporting its potential in alleviating
DOX-induced myocardial ferroptosis by enhancing the antioxidant
system.
Excessive iron accumulation in the body forms the
labile iron pool, eventually promoting ferroptosis (40). However, to the best of our
knowledge, there is limited research on the role of OJP in
modulating iron accumulation (16). To the best of our knowledge, the
present study was the first to demonstrate that cell treatment with
OJP significantly decreased the expression of the iron transport
protein TFR1. Furthermore, intracellular Fe2+ content
was notably decreased in the OJP groups, thus inhibiting
intracellular iron buildup. Ferroptosis disrupts the mitochondrial
balance, thus leading to mitochondrial injury (41). In the present study, OJP
administration partially restored mitochondrial function and
decreased mitochondrial injury. These observations underscore the
potential of OJP as a promising therapeutic agent for managing and
preventing DOX-induced myocardial ferroptosis.
The signaling pathways of ferroptosis are intricate,
affecting cellular antioxidant capacity and resulting in lipid
peroxidation (38). Nrf2 is a key
regulator of antioxidant responses, serving a crucial role in
maintaining cellular redox homeostasis (25). GPX4, a downstream target of Nrf2,
serves a key role in inhibiting ferroptosis by catalyzing the
reduction of lipid peroxides using glutathione (42). The excessive accumulation of
Fe2+ leads to increased lipid peroxidation (43). It has been reported that Nrf2
stabilizes intracellular iron homeostasis by regulating the
expression of heme metabolism-related proteins and the heavy and
light chains of ferritin, thus indirectly regulating
Fe2+ levels in the labile iron pool (44). For example, previous studies have
indicated that dexmedetomidine and naringenin decreased myocardial
ischemia/reperfusion-induced ferroptosis by upregulating Nrf2
(45,46). Similarly, prostaglandin E2 receptor
1, astragaloside IV and fisetin protect cardiomyocytes against
DOX-induced ferroptosis via activation of Nrf2 signaling (15,47,48).
Conversely, Wang et al (49) demonstrated that Nrf2 inhibition
aggravated myocardial injury. OJP has significant cardiovascular
protective effects due to its anti-inflammatory and antioxidant
properties (50), suggesting that
Nrf2 may play a crucial role in these mechanisms. As a key
intracellular antioxidant transcription factor, Nrf2 provides
critical protection against oxidative stress (51). OJP may activate the Nrf2 signaling
pathway, enhancing the activity of antioxidant enzymes and reducing
lipid peroxidation, thereby mitigating ferroptosis-induced
myocardial damage. Furthermore, Nrf2 regulates genes involved in
iron metabolism, aiding in the maintenance of intracellular iron
homeostasis and reducing oxidative stress and cellular injury
caused by iron overload (43). In
the aforementioned study, OJP could increase the protein expression
levels of Nrf2 and promote its nuclear translocation. To ascertain
whether Nrf2 inhibition abrogated the protective effect of OJP,
ML385, an Nrf2 inhibitor, was employed. ML385 not only suppressed
OJP-mediated upregulation of Nrf2 and its downstream target GPX4,
but also reversed the effect of OJP in reducing mitochondrial
injury and iron accumulation. These findings supported the
anti-ferroptosis mechanism of OJP via regulation of the Nrf2/GPX4
signaling pathway and decreasing iron accumulation.
However, the present study has some limitations.
Firstly, only the expression levels of core proteins of the
downstream GPX4 signaling pathway and those involved in regulation
of Fe2+ production were detected. Second, the present
study did not investigate how Nrf2 indirectly regulates TFR1,
leaving the intricate molecular pathways involved for future
exploration. Lastly, the specific molecular mechanisms by which OJP
alleviates mitochondrial damage were not thoroughly examined.
Therefore, the detailed molecular mechanisms underlying the
regulation of Nrf2 signaling during OJP-mediated protection against
ferroptosis should be further investigated. Subsequent studies
should assess the particular activation mechanisms of the Nrf2
signaling pathway by OJP and its effects on downstream molecular
targets.
The present study provided novel insights into the
favorable pharmacological effects of OJP against DOX-induced
myocardial ferroptosis and its underlying mechanisms, thus laying
the groundwork for the future development of novel therapeutic
strategies for the prevention and treatment of DOX-induced
myocardial disease. OJP could be considered as a promising
candidate for potential clinical intervention.
Acknowledgements
Not applicable.
Funding
The present study was supported by Research Project of Health
Commission of Pudong New Area, Shanghai (grant no. PW2021A-69),
Clinical Research Center, Shanghai University of Medicine &
Health Sciences (grant no. 22MC2022002) and Research Grant for
Pudong Health Bureau of Shanghai (grant no. YC-2023-0401).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YC performed experiments, analyzed the data and
wrote the manuscript. LM analyzed data and revised the manuscript.
XW and LC performed experiments and data analysis. ML designed the
study and revised the manuscript. YL and YY analyzed data. All
authors have read and approved the final manuscript. YL and ML
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rawat PS, Jaiswal A, Khurana A, Bhatti JS
and Navik U: Doxorubicin-induced cardiotoxicity: An update on the
molecular mechanism and novel therapeutic strategies for effective
management. Biomed Pharmacother. 139:1117082021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu C, Ma X, Zhuang J, Liu L and Sun C:
Cardiotoxicity of doxorubicin-based cancer treatment: What is the
protective cognition that phytochemicals provide us? Pharmacol Res.
160:1050622020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jones IC and Dass CR: Doxorubicin-induced
cardiotoxicity: Causative factors and possible interventions. Pharm
Pharmacol. 74:1677–1688. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carvalho C, Santos RX, Cardoso S, Correia
S, Oliveira PJ, Santos MS and Moreira PI: Doxorubicin: The good,
the bad and the ugly effect. Curr Med Chem. 16:3267–3285. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Christiansen S and Autschbach R:
Doxorubicin in experimental and clinical heart failure. Eur J
Cardiothorac Surg. 30:611–616. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hardaway BW: Adriamycin-associated
cardiomyopathy: where are we now? updates in pathophysiology, dose
recommendations, prognosis, and outcomes. Curr Opin Cardiol.
34:289–295. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stockwell BR: Ferroptosis turns 10:
Emerging mechanisms, physiological functions, and therapeutic
applications. Cell. 185:2401–2421. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujii J, Homma T and Kobayashi S:
Ferroptosis caused by cysteine insufficiency and oxidative insult.
Free Radic Res. 54:969–980. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kotamraju S, Chitambar CR, Kalivendi SV,
Joseph J and Kalyanaraman B: Transferrin receptor-dependent iron
uptake is responsible for doxorubicin-mediated apoptosis in
endothelial cells: Role of oxidant-induced iron signaling in
apoptosis. J Biol Chem. 277:17179–17187. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang X, Ardehali H, Min J and Wang F: The
molecular and metabolic landscape of iron and ferroptosis in
cardiovascular disease. Nat Rev Cardiol. 20:7–23. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: an iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu J, Zhao Y, Liu M, Lu J and Guan S:
Toward improved human health: Nrf2 plays a critical role in
regulating ferroptosis. Food Funct. 12:9583–9606. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo LF, Guan P, Qin LY, Wang JX, Wang N
and Ji ES: Astragaloside IV inhibits adriamycin-induced cardiac
ferroptosis by enhancing Nrf2 signaling. Mol Cell Biochem.
476:2603–2611. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu W, Chen C, Xu C, Xie D, Wang Q, Liu W,
Zhao H, He F, Chen B, Xi Y, et al: Activation of p62-NRF2 axis
protects against doxorubicin-induced ferroptosis in cardiomyocytes:
A novel role and molecular mechanism of resveratrol. Am J Chin Med.
50:2103–2123. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li D, Liu X, Pi W, Zhang Y, Yu L, Xu C,
Sun Z and Jiang J: Fisetin attenuates doxorubicin-induced
cardiomyopathy in vivo and in vitro by inhibiting ferroptosis
through SIRT1/Nrf2 signaling pathway activation. Front Pharmacol.
12:8084802022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang J, Wang X, Lu M, He X and Yang X:
Recent advances in polysaccharides from Ophiopogon japonicus and
Liriope spicata var. prolifera. Int J Biol Macromol. 114:1257–1266.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen MH, Chen XJ, Wang M, Lin LG and Wang
YT: Ophiopogon japonicus-A phytochemical, ethnomedicinal and
pharmacological review. J Ethnopharmacol. 181:193–213. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Fan S, Mao Y, Ji Y, Jin L, Lu J
and Chen X: Cardiovascular protective effect of polysaccharide from
Ophiopogon japonicus in diabetic rats. Int J Biol Macromol.
82:505–513. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ayala A, Muñoz MF and Argüelles S: Lipid
peroxidation: production, metabolism, and signaling mechanisms of
malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev.
2014:3604382014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao Y, Shen T, Huang X, Lin Y, Chen B,
Pang J, Li G, Wang Q, Zohrabian S, Duan C, et al: Astragalus
polysaccharide restores autophagic flux and improves cardiomyocyte
function in doxorubicin-induced cardiotoxicity. Oncotarget.
8:4837–4848. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie Y, Kang R, Klionsky DJ and Tang D:
GPX4 in cell death, autophagy, and disease. Autophagy.
19:2621–2638. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsikas D: Assessment of lipid peroxidation
by measuring malondialdehyde (MDA) and relatives in biological
samples: Analytical and biological challenges. Anal Biochem.
524:13–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Otasevic V, Vucetic M, Grigorov I,
Martinovic V and Stancic A: Ferroptosis in different pathological
contexts seen through the eyes of mitochondria. Oxid Med Cell
Longev. 2021:55373302021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23:1011072019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He F, Ru X and Wen T: NRF2, a
transcription factor for stress response and beyond. Int J Mol Sci.
21:47772020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang X, Yu M, Wang WK, Zhu LY, Wang X,
Jin HC and Feng LF: The regulation and function of Nrf2 signaling
in ferroptosis-activated cancer therapy. Acta Pharmacol Sin.
45:2229–2240. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fang X, Wang H, Han D, Xie E, Yang X, Wei
J, Gu S, Gao F, Zhu N, Yin X, et al: Ferroptosis as a target for
protection against cardiomyopathy. Proc Natl Acad Sci USA.
116:2672–2680. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prathumsap N, Shinlapawittayatorn K,
Chattipakorn SC and Chattipakorn N: Effects of doxorubicin on the
heart: From molecular mechanisms to intervention strategies. Eur J
Pharmacol. 866:1728182020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cvetković RS and Scott LJ: Dexrazoxane: A
review of its use for cardioprotection during anthracycline
chemotherapy. Drugs. 65:1005–1024. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou D, Zhang H, Xue X, Tao Y, Wang S, Ren
X and Su J: Safety evaluation of natural drugs in chronic skeletal
disorders: A literature review of clinical trials in the past 20
years. Front Pharmacol. 12:8012872022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang S, Lin X, Wang LY, Ruan KF, Feng Y
and Li XY: A polysaccharides MDG-1 augments survival in the
ischemic heart by inducing S1P release and S1P1 expression. Int J
Biol Macromol. 50:734–740. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng Q, Feng Y, Xu DS, Lin X and Chen YZ:
Influence of sulfation on anti-myocardial ischemic activity of
Ophiopogon japonicus polysaccharide. J Asian Nat Prod Res.
11:306–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang L and Wang Y, Wu F, Wang X, Feng Y
and Wang Y: MDG, an Ophiopogon japonicus polysaccharide, inhibits
non-alcoholic fatty liver disease by regulating the abundance of
Akkermansia muciniphila. Int J Biol Macromol. 196:23–34. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin C, Kuo TC, Lin JC, Ho YC and Mi FL:
Delivery of polysaccharides from Ophiopogon japonicus (OJPs) using
OJPs/chitosan/whey protein co-assembled nanoparticles to treat
defective intestinal epithelial tight junction barrier. Int J Biol
Macromol. 160:558–570. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang ZG, Liang X and Zhao YQ: Effect of
shashen maidong tang combined with chemotherapy on immune function
and inflammatory reaction of patients with lung cancer of Qi and
Yin deficiency. Chin J Exp Tradit Med. 23:158–163. 2017.(In
Chinese).
|
|
36
|
Yue L, Xiao L, Zhang X, Niu L, Wen Y, Li
X, Wang Y, Xing G and Li G: Comparative efficacy of Chinese herbal
injections in patients with cardiogenic shock (CS): A systematic
review and Bayesian network meta-analysis of randomized controlled
trials. Front Pharmacol. 15:13483602024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tadokoro T, Ikeda M, Ide T, Deguchi H,
Ikeda S, Okabe K, Ishikita A, Matsushima S, Koumura T, Yamada KI,
et al: Mitochondria-dependent ferroptosis plays a pivotal role in
doxorubicin cardiotoxicity. JCI Insight. 8:e1697562023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Su LJ, Zhang JH, Gomez H, Murugan R, Hong
X, Xu D, Jiang F and Peng ZY: Reactive oxygen species-induced lipid
peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med
Cell Longev. 2019:50808432019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fan S, Zhang J, Xiao Q, Liu P, Zhang Y,
Yao E and Cahen X: Cardioprotective effect of the polysaccharide
from Ophiopogon japonicus on isoproterenol-induced myocardial
ischemia in rats. Int J Biol Macromol. 147:233–240. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dai E, Chen X, Linkermann A, Jiang X, Kang
R, Kagan VE, Bayir H, Yang WS, Garcia-Saez AJ, Ioannou MS, et al: A
guideline on the molecular ecosystem regulating ferroptosis. Nat
Cell Biol. 26:1447–1457. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao M, Yi J, Zhu J, Minikes AM, Monian P,
Thompson CB and Jiang X: Role of mitochondria in ferroptosis. Mol
Cell. 73:354–363. e3. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang M, Tang J, Zhang S, Pang K, Zhao Y,
Liu N, Huang J, Kang J, Dong S, Li H, et al: Exogenous H2S
initiating Nrf2/GPx4/GSH pathway through promoting Syvn1-Keap1
interaction in diabetic hearts. Cell Death Discov. 9:3942023.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kerins MJ and Ooi A: The Roles of NRF2 in
modulating cellular iron homeostasis. Antioxid Redox Signal.
29:1756–1773. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Song X and Long D: Nrf2 and Ferroptosis: A
new research direction for neurodegenerative diseases. Front
Neurosci. 14:2672020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang Z, Yao M, Jiang L, Wang L, Yang Y,
Wang Q, Qian X, Zhao Y and Qian J: Dexmedetomidine attenuates
myocardial ischemia/reperfusion-induced ferroptosis via
AMPK/GSK-3β/Nrf2 axis. Biomed Pharmacother. 154:1135722022.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu S, Wu B, Zhong B, Lin L, Ding Y, Jin X,
Huang Z, Lin M, Wu H and Xu D: Naringenin alleviates myocardial
ischemia/reperfusion injury by regulating the nuclear
factor-erythroid factor 2-related factor 2 (Nrf2)/System
xc-/glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis.
Bioengineered. 12:10924–10934. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang J, Wu C, Gao L, Du G and Qin X:
Astragaloside IV derived from Astragalus membranaceus: A research
review on the pharmacological effects. Adv Pharmacol. 87:89–112.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang B, Jin Y, Liu J, Liu Q, Shen Y, Zuo S
and Yu Y: EP1 activation inhibits doxorubicin-cardiomyocyte
ferroptosis via Nrf2. Redox Biol. 65:1028252023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang Y, Yan S, Liu X, Deng F, Wang P, Yang
L, Hu L, Huang K and He J: PRMT4 promotes ferroptosis to aggravate
doxorubicin-induced cardiomyopathy via inhibition of the Nrf2/GPX4
pathway. Cell Death Differ. 29:1982–1995. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu Q, Lu JJ, Hong HJ, Yang Q, Wang Y and
Chen XJ: Ophiopogon japonicus and its active compounds: A review of
potential anticancer effects and underlying mechanisms.
Phytomedicine. 113:1547182023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen QM and Maltagliati AJ: Nrf2 at the
heart of oxidative stress and cardiac protection. Physiol Genomics.
50:77–97. 2018. View Article : Google Scholar : PubMed/NCBI
|