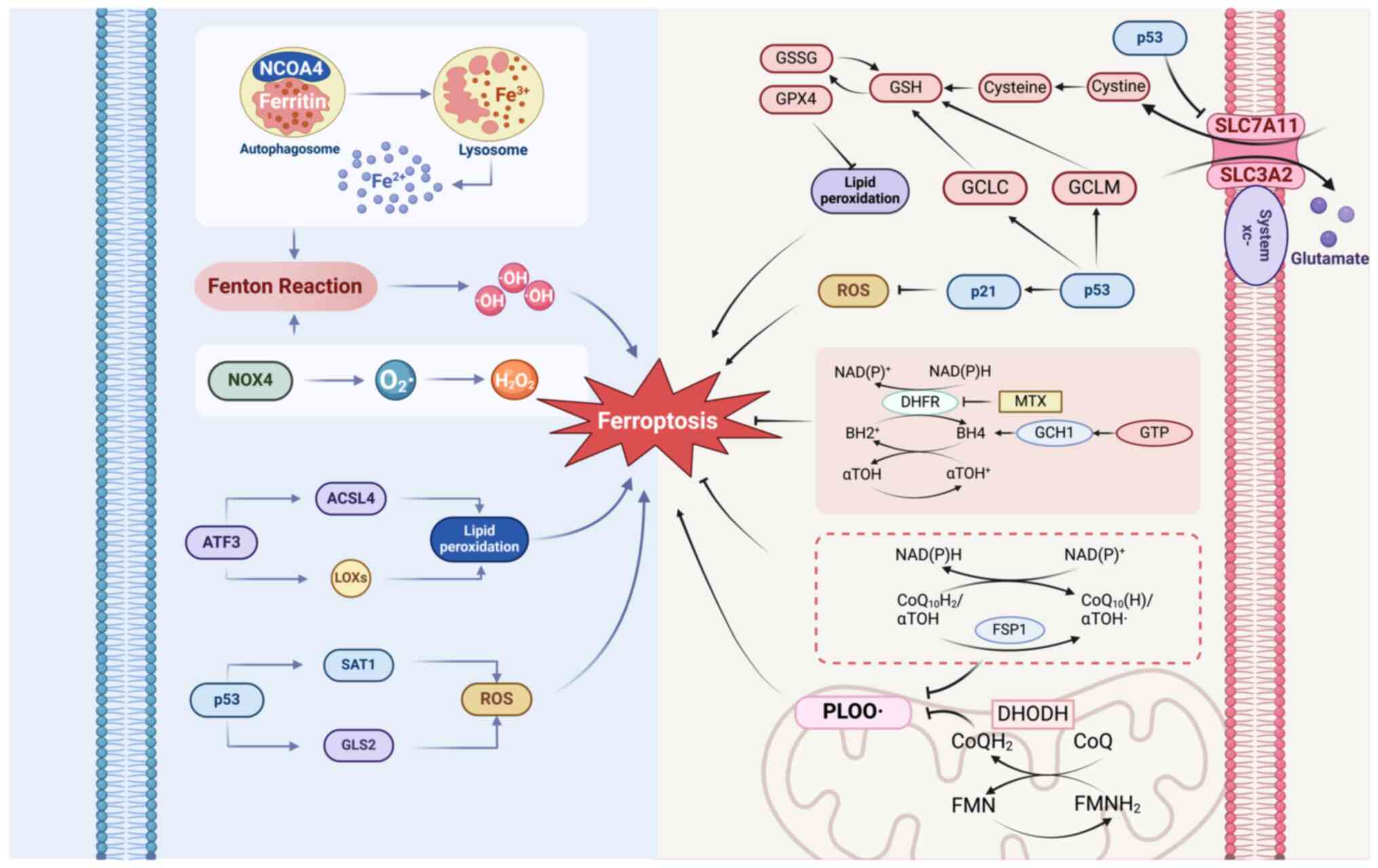
|
1
|
Solmi M, Seitidis G, Mavridis D, Correll
CU, Dragioti E, Guimond S, Tuominen L, Dargél A, Carvalho AF,
Fornaro M, et al: Incidence, prevalence, and global burden of
schizophrenia-data, with critical appraisal, from the global burden
of disease (GBD) 2019. Mol Psychiatry. 28:5319–5327. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schultz SH, North SW and Shields CG:
Schizophrenia: A review. Am Fam Physician. 75:1821–1829.
2007.PubMed/NCBI
|
|
3
|
Bassett AS, Collins EJ, Nuttall SE and
Honer WG: Positive and negative symptoms in families with
schizophrenia. Schizophr Res. 11:9–19. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Azorin JM, Belzeaux R and Adida M:
Negative symptoms in schizophrenia: where we have been and where we
are heading. CNS Neurosci Ther. 20:801–808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marder SR and Umbricht D: Negative
symptoms in schizophrenia: Newly emerging measurements, pathways,
and treatments. Schizophr Res. 258:71–77. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McCutcheon RA, Keefe RSE and McGuire PK:
Cognitive impairment in schizophrenia: Aetiology, pathophysiology,
and treatment. Mol Psychiatry. 28:1902–1918. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bobes J, Arango C, Garcia-Garcia M and
Rejas J; CLAMORS Study Collaborative Group, : Prevalence of
negative symptoms in outpatients with schizophrenia spectrum
disorders treated with antipsychotics in routine clinical practice:
Findings from the CLAMORS study. J Clin Psychiatry. 71:280–286.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Austin SF, Mors O, Budtz-Jørgensen E,
Secher RG, Hjorthøj CR, Bertelsen M, Jeppesen P, Petersen L, Thorup
A and Nordentoft M: Long-term trajectories of positive and negative
symptoms in first episode psychosis: A 10 year follow-up study in
the OPUS cohort. Schizophr Res. 168:84–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pandey A and Kalita KN:
Treatment-resistant schizophrenia: How far have we traveled? Front
Psychiatry. 13:9944252022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ochoa S, Usall J, Cobo J, Labad X and
Kulkarni J: Gender differences in schizophrenia and first-episode
psychosis: A comprehensive literature review. Schizophr Res
Treatment. 2012:9161982012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andriopoulos I, Ellul J, Skokou M and
Beratis S: Suicidality in the ‘prodromal’ phase of schizophrenia.
Compr Psychiatry. 52:479–485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Donnelly L, Rathbone J and Adams CE:
Haloperidol dose for the acute phase of schizophrenia. Cochrane
Database Syst Rev. CD0019512013.PubMed/NCBI
|
|
13
|
Charlson FJ, Ferrari AJ, Santomauro DF,
Diminic S, Stockings E, Scott JG, McGrath JJ and Whiteford HA:
Global epidemiology and burden of schizophrenia: Findings from the
global burden of disease study 2016. Schizophr Bull. 44:1195–1203.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saito Y, Sakurai H, Kane JM, Schooler NR,
Suzuki T, Mimura M and Uchida H: Predicting relapse with residual
symptoms in schizophrenia: A secondary analysis of the PROACTIVE
trial. Schizophr Res. 215:173–180. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Patel KR, Cherian J, Gohil K and Atkinson
D: Schizophrenia: Overview and treatment options. P T. 39:638–645.
2014.PubMed/NCBI
|
|
16
|
Howes OD, Bukala BR and Beck K:
Schizophrenia: From neurochemistry to circuits, symptoms and
treatments. Nat Rev Neurol. 20:22–35. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Butler AC, Chapman JE, Forman EM and Beck
AT: The empirical status of cognitive-behavioral therapy: A review
of meta-analyses. Clin Psychol Rev. 26:17–31. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SH and Park S: Effectiveness of family
interventions for patients with schizophrenia: A systematic review
and meta-analysis. Int J Ment Health Nurs. 32:1598–1615. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sinclair DJM, Zhao S, Qi F, Nyakyoma K,
Kwong JSW and Adams CE: Electroconvulsive therapy for
treatment-resistant schizophrenia. Schizophr Bull. 45:730–732.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang WS and Stockwell BR: Ferroptosis:
Death by lipid peroxidation. Trends Cell Biol. 26:165–176. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Wan Y, Jiang Y, Zhang L and Cheng
W: GPX4: The hub of lipid oxidation, ferroptosis, disease and
treatment. Biochim Biophys Acta Rev Cancer. 1878:1888902023.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang XD, Liu ZY, Wang MS, Guo YX, Wang
XK, Luo K, Huang S and Li RF: Mechanisms and regulations of
ferroptosis. Front Immunol. 14:12694512023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Doll S, Freitas FP, Shah R, Aldrovandi M,
da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius
E, Scheel CH, et al: FSP1 is a glutathione-independent ferroptosis
suppressor. Nature. 575:693–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen X, Comish PB, Tang D and Kang R:
Characteristics and biomarkers of ferroptosis. Front Cell Dev Biol.
9:6371622021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Friedmann Angeli JP, Schneider M, Proneth
B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch
A, Eggenhofer E, et al: Inactivation of the ferroptosis regulator
Gpx4 triggers acute renal failure in mice. Nat Cell Biol.
16:1180–1191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yagoda N, von Rechenberg M, Zaganjor E,
Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM,
Boniface JJ, et al: RAS-RAF-MEK-dependent oxidative cell death
involving voltage-dependent anion channels. Nature. 447:864–868.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Riegman M, Sagie L, Galed C, Levin T,
Steinberg N, Dixon SJ, Wiesner U, Bradbury MS, Niethammer P,
Zaritsky A and Overholtzer M: Ferroptosis occurs through an osmotic
mechanism and propagates independently of cell rupture. Nat Cell
Biol. 22:1042–1048. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Demuynck R, Efimova I, Naessens F and
Krysko DV: Immunogenic ferroptosis and where to find it? J
Immunother Cancer. 9:e0034302021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang D, Minikes AM and Jiang X:
Ferroptosis at the intersection of lipid metabolism and cellular
signaling. Mol Cell. 82:2215–2227. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang D, Chen X, Kang R and Kroemer G:
Ferroptosis: Molecular mechanisms and health implications. Cell
Res. 31:107–125. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen X, Kang R, Kroemer G and Tang D:
Broadening horizons: The role of ferroptosis in cancer. Nat Rev
Clin Oncol. 18:280–296. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y, Wu S, Li Q, Sun H and Wang H:
Pharmacological inhibition of ferroptosis as a therapeutic target
for neurodegenerative diseases and strokes. Adv Sci (Weinh).
10:e23003252023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bitanihirwe BKY and Woo TUW: Oxidative
stress in schizophrenia: An integrated approach. Neurosci Biobehav
Rev. 35:878–893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lotan A, Luza S, Opazo CM, Ayton S, Lane
DJR, Mancuso S, Pereira A, Sundram S, Weickert CS, Bousman C, et
al: Perturbed iron biology in the prefrontal cortex of people with
schizophrenia. Mol Psychiatry. 28:2058–2070. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Götz ME, Künig G, Riederer P and Youdim
MB: Oxidative stress: Free radical production in neural
degeneration. Pharmacol Ther. 63:37–122. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
David S, Jhelum P, Ryan F, Jeong SY and
Kroner A: Dysregulation of iron homeostasis in the central nervous
system and the role of ferroptosis in neurodegenerative disorders.
Antioxid Redox Signal. 37:150–170. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qin Y, He Y, She Q, Larese-Casanova P, Li
P and Chai Y: Heterogeneity in respiratory electron transfer and
adaptive iron utilization in a bacterial biofilm. Nat Commun.
10:37022019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gomme PT, McCann KB and Bertolini J:
Transferrin: Structure, function and potential therapeutic actions.
Drug Discov Today. 10:267–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kakhlon O and Cabantchik ZI: The labile
iron pool: Characterization, measurement, and participation in
cellular processes(1). Free Radic Biol Med. 33:1037–1046. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Worwood M: Ferritin. Blood Rev. 4:259–269.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
MacKenzie EL, Iwasaki K and Tsuji Y:
Intracellular iron transport and storage: From molecular mechanisms
to health implications. Antioxid Redox Signal. 10:997–1030. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Reinert A, Morawski M, Seeger J, Arendt T
and Reinert T: Iron concentrations in neurons and glial cells with
estimates on ferritin concentrations. BMC Neurosci. 20:252019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sunkara S, Radulović S, Lipovšek S, Birkl
C, Eggenreich S, Birkl-Toeglhofer AM, Schinagl M, Funk D,
Stöger-Pollach M, Haybaeck J, et al: Autolysis affects the iron
cargo of ferritins in neurons and glial cells at different rates in
the human brain. Cell Mol Neurobiol. 43:2909–2923. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hansen TM, Nielsen H, Bernth N and Moos T:
Expression of ferritin protein and subunit mRNAs in normal and iron
deficient rat brain. Brain Res Mol Brain Res. 65:186–197. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang P, Ren Q, Shi M, Liu Y, Bai H and
Chang YZ: Overexpression of mitochondrial ferritin enhances
blood-brain barrier integrity following ischemic stroke in mice by
maintaining iron homeostasis in endothelial cells. Antioxidants
(Basel). 11:12572022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu H, Liu Q, Shan X, Gao W and Chen Q: ATM
orchestrates ferritinophagy and ferroptosis by phosphorylating
NCOA4. Autophagy. 19:2062–2077. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang DL and Rouault TA: How does hepcidin
hinder ferroportin activity? Blood. 131:840–842. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fang X, Ardehali H, Min J and Wang F: The
molecular and metabolic landscape of iron and ferroptosis in
cardiovascular disease. Nat Rev Cardiol. 20:7–23. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Delaby C, Pilard N, Gonçalves AS, Beaumont
C and Canonne-Hergaux F: Presence of the iron exporter ferroportin
at the plasma membrane of macrophages is enhanced by iron loading
and down-regulated by hepcidin. Blood. 106:3979–3984. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ganz T: Hepcidin and its role in
regulating systemic iron metabolism. Hematology Am Soc Hematol Educ
Program. 2006:29–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ramey G, Deschemin JC, Durel B,
Canonne-Hergaux F, Nicolas G and Vaulont S: Hepcidin targets
ferroportin for degradation in hepatocytes. Haematologica.
95:501–504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Qiu B, Zandkarimi F, Bezjian CT, Reznik E,
Soni RK, Gu W, Jiang X and Stockwell BR: Phospholipids with two
polyunsaturated fatty acyl tails promote ferroptosis. Cell.
187:1177–1190.e18. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang WS, Kim KJ, Gaschler MM, Patel M,
Shchepinov MS and Stockwell BR: Peroxidation of polyunsaturated
fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci
USA. 113:E4966–E4975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Talvenmäki H, Lallukka N, Survo S and
Romantschuk M: Fenton's reaction-based chemical oxidation in
suboptimal conditions can lead to mobilization of oil hydrocarbons
but also contribute to the total removal of volatile compounds.
Environ Sci Pollut Res Int. 26:34670–34684. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Henning Y, Blind US, Larafa S, Matschke J
and Fandrey J: Hypoxia aggravates ferroptosis in RPE cells by
promoting the Fenton reaction. Cell Death Dis. 13:6622022.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Abe C and Miyazawa T and Miyazawa T:
Current use of Fenton reaction in drugs and food. Molecules.
27:54512022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Valgimigli L: Lipid peroxidation and
antioxidant protection. Biomolecules. 13:12912023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Minotti G and Aust SD: The role of iron in
oxygen radical mediated lipid peroxidation. Chem Biol Interact.
71:1–19. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gaschler MM and Stockwell BR: Lipid
peroxidation in cell death. Biochem Biophys Res Commun.
482:419–425. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Janero DR: Malondialdehyde and
thiobarbituric acid-reactivity as diagnostic indices of lipid
peroxidation and peroxidative tissue injury. Free Radic Biol Med.
9:515–540. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mas-Bargues C, Escrivá C, Dromant M,
Borrás C and Viña J: Lipid peroxidation as measured by
chromatographic determination of malondialdehyde. Human plasma
reference values in health and disease. Arch Biochem Biophys.
709:1089412021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li Y, Zhao T, Li J, Xia M, Li Y, Wang X,
Liu C, Zheng T, Chen R, Kan D, et al: Oxidative stress and
4-hydroxy-2-nonenal (4-HNE): Implications in the pathogenesis and
treatment of aging-related diseases. J Immunol Res.
2022:22339062022.PubMed/NCBI
|
|
66
|
Dalleau S, Baradat M, Guéraud F and Huc L:
Cell death and diseases related to oxidative stress:
4-Hydroxynonenal (HNE) in the balance. Cell Death Differ.
20:1615–1630. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang X, Hou L, Guo Z, Wang G, Xu J, Zheng
Z, Sun K and Guo F: Lipid peroxidation in osteoarthritis: Focusing
on 4-hydroxynonenal, malondialdehyde, and ferroptosis. Cell Death
Discov. 9:3202023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Butterfield DA: Brain lipid peroxidation
and alzheimer disease: Synergy between the Butterfield and Mattson
laboratories. Ageing Res Rev. 64:1010492020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Villalón-García I, Povea-Cabello S,
Álvarez-Córdoba M, Talaverón-Rey M, Suárez-Rivero JM,
Suárez-Carrillo A, Munuera-Cabeza M, Reche-López D,
Cilleros-Holgado P, Piñero-Pérez R and Sánchez-Alcázar JA: Vicious
cycle of lipid peroxidation and iron accumulation in
neurodegeneration. Neural Regen Res. 18:1196–1202. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shichiri M: The role of lipid peroxidation
in neurological disorders. J Clin Biochem Nutr. 54:151–160. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dietrich-Muszalska A and Kontek B: Lipid
peroxidation in patients with schizophrenia. Psychiatry Clin
Neurosci. 64:469–475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bošković M, Vovk T, Kores Plesničar B and
Grabnar I: Oxidative stress in schizophrenia. Curr Neuropharmacol.
9:301–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen P, Wang D, Xiu M, Chen D, Lackey B,
Wu HE, Wang L and Zhang X: Association of transferrin gene
polymorphism with cognitive deficits and psychiatric symptoms in
patients with chronic schizophrenia. J Clin Med. 11:64142022.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yeh S and Chang C: Cloning and
characterization of a specific coactivator, ARA70, for the androgen
receptor in human prostate cells. Proc Natl Acad Sci USA.
93:5517–5521. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Moore JMR, Galicia SJ, McReynolds AC,
Nguyen NH, Scanlan TS and Guy RK: Quantitative proteomics of the
thyroid hormone receptor-coregulator interactions. J Biol Chem.
279:27584–27590. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kollara A and Brown TJ: Expression and
function of nuclear receptor co-activator 4: Evidence of a
potential role independent of co-activator activity. Cell Mol Life
Sci. 69:3895–3909. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Heinlein CA, Ting HJ, Yeh S and Chang C:
Identification of ARA70 as a ligand-enhanced coactivator for the
peroxisome proliferator-activated receptor gamma. J Biol Chem.
274:16147–16152. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhou ZX, He B, Hall SH, Wilson EM and
French FS: Domain interactions between coregulator ARA(70) and the
androgen receptor (AR). Mol Endocrinol. 16:287–300. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mancias JD, Pontano Vaites L, Nissim S,
Biancur DE, Kim AJ, Wang X, Liu Y, Goessling W, Kimmelman AC and
Harper JW: Ferritinophagy via NCOA4 is required for erythropoiesis
and is regulated by iron dependent HERC2-mediated proteolysis.
Elife. 4:e103082015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Federico G, Carrillo F, Dapporto F,
Chiariello M, Santoro M, Bellelli R and Carlomagno F: NCOA4 links
iron bioavailability to DNA metabolism. Cell Rep. 40:1112072022.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kuno S, Fujita H, Tanaka YK, Ogra Y and
Iwai K: Iron-induced NCOA4 condensation regulates ferritin fate and
iron homeostasis. EMBO Rep. 23:e542782022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Bellelli R, Federico G, Matte' A,
Colecchia D, Iolascon A, Chiariello M, Santoro M, De Franceschi L
and Carlomagno F: NCOA4 deficiency impairs systemic iron
homeostasis. Cell Rep. 14:411–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Arcos M, Liu Z, Villareal LB, Velez PK,
Desai SP, Noureddine A, Zheng H, Martin DR, Brinker J, Zhang D and
Xue X: Myeloid NCOA4 sequesters KEAP1 to reduce ferroptosis for
protection against salmonellosis in mice. Res Sq [Preprint].
rs.3.rs-4278310. 2024.PubMed/NCBI
|
|
84
|
Mancias JD, Wang X, Gygi SP, Harper JW and
Kimmelman AC: Quantitative proteomics identifies NCOA4 as the cargo
receptor mediating ferritinophagy. Nature. 509:105–109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kuno S and Iwai K: Oxygen modulates iron
homeostasis by switching iron sensing of NCOA4. J Biol Chem.
299:1047012023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gryzik M, Srivastava A, Longhi G, Bertuzzi
M, Gianoncelli A, Carmona F, Poli M and Arosio P: Expression and
characterization of the ferritin binding domain of nuclear receptor
coactivator-4 (NCOA4). Biochim Biophys Acta Gen Subj.
1861:2710–2716. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhao H, Lu Y, Zhang J, Sun Z, Cheng C, Liu
Y, Wu L, Zhang M, He W, Hao S and Li K: NCOA4 requires a [3Fe-4S]
to sense and maintain the iron homeostasis. J Biol Chem.
300:1056122024. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li HY, Wei TT, Zhuang M, Tan CY, Xie TH,
Cai J, Yao Y and Zhu L: Iron derived from NCOA4-mediated
ferritinophagy causes cellular senescence via the cGAS-STING
pathway. Cell Death Discov. 9:4192023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hoelzgen F, Nguyen TTP, Klukin E, Boumaiza
M, Srivastava AK, Kim EY, Zalk R, Shahar A, Cohen-Schwartz S,
Meyron-Holtz EG, et al: Structural basis for the intracellular
regulation of ferritin degradation. Nat Commun. 15:38022024.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yuan L, Sun Y, Zhou N, Wu W, Zheng W and
Wang Y: Dihydroquercetin attenuates silica-induced pulmonary
fibrosis by inhibiting ferroptosis signaling pathway. Front
Pharmacol. 13:8456002022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lahiri V, Hawkins WD and Klionsky DJ:
Watch what you (self-) eat: Autophagic mechanisms that modulate
metabolism. Cell Metab. 29:803–826. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Vermot A, Petit-Härtlein I, Smith SME and
Fieschi F: NADPH oxidases (NOX): An overview from discovery,
molecular mechanisms to physiology and pathology. Antioxidants
(Basel). 10:8902021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Nazari B, Jaquet V and Krause KH: NOX
family NADPH oxidases in mammals: Evolutionary conservation and
isoform-defining sequences. Redox Biol. 66:1028512023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lambeth JD: Nox enzymes, ROS, and chronic
disease: An example of antagonistic pleiotropy. Free Radic Biol
Med. 43:332–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chen F, Haigh S, Barman S and Fulton DJR:
From form to function: The role of Nox4 in the cardiovascular
system. Front Physiol. 3:4122012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Altenhöfer S, Radermacher KA, Kleikers
PWM, Wingler K and Schmidt HHHW: Evolution of NADPH oxidase
inhibitors: Selectivity and mechanisms for target engagement.
Antioxid Redox Signal. 23:406–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Martyn KD, Frederick LM, von Loehneysen K,
Dinauer MC and Knaus UG: Functional analysis of Nox4 reveals unique
characteristics compared to other NADPH oxidases. Cell Signal.
18:69–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Nisimoto Y, Diebold BA, Cosentino-Gomes D
and Lambeth JD: Nox4: A hydrogen peroxide-generating oxygen sensor.
Biochemistry. 53:5111–5120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Serrander L, Cartier L, Bedard K, Banfi B,
Lardy B, Plastre O, Sienkiewicz A, Fórró L, Schlegel W and Krause
KH: NOX4 activity is determined by mRNA levels and reveals a unique
pattern of ROS generation. Biochem J. 406:105–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Di Marzo N, Chisci E and Giovannoni R: The
role of hydrogen peroxide in redox-dependent signaling: Homeostatic
and pathological responses in mammalian cells. Cells. 7:1562018.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Feng S, Tang D, Wang Y, Li X, Bao H, Tang
C, Dong X, Li X, Yang Q, Yan Y, et al: The mechanism of ferroptosis
and its related diseases. Mol Biomed. 4:332023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Rada B and Leto TL: Oxidative innate
immune defenses by Nox/Duox family NADPH oxidases. Contrib
Microbiol. 15:164–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Konno T, Melo EP, Chambers JE and Avezov
E: Intracellular sources of ROS/H2O2 in
health and neurodegeneration: Spotlight on endoplasmic reticulum.
Cells. 10:2332021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Yin H, Xu L and Porter NA: Free radical
lipid peroxidation: Mechanisms and analysis. Chem Rev.
111:5944–5972. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Rochette L, Dogon G, Rigal E, Zeller M,
Cottin Y and Vergely C: Lipid peroxidation and iron metabolism: Two
corner stones in the homeostasis control of ferroptosis. Int J Mol
Sci. 24:4492022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhang H, Wang A, Li G, Zhai Q, Huang Z,
Wang X, Cao Z, Liu L, Liu G, Chen B, et al: Osteoporotic bone loss
from excess iron accumulation is driven by NOX4-triggered
ferroptosis in osteoblasts. Free Radic Biol Med. 198:123–136. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Mancardi D, Mezzanotte M, Arrigo E,
Barinotti A and Roetto A: Iron overload, oxidative stress, and
ferroptosis in the failing heart and liver. Antioxidants (Basel).
10:18642021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Orino K, Lehman L, Tsuji Y, Ayaki H, Torti
SV and Torti FM: Ferritin and the response to oxidative stress.
Biochem J. 357:241–247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhu J, Xiong Y, Zhang Y, Wen J, Cai N,
Cheng K, Liang H and Zhang W: The Molecular mechanisms of
regulating oxidative stress-induced ferroptosis and therapeutic
strategy in tumors. Oxid Med Cell Longev. 2020:88107852020.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Cipriano A, Viviano M, Feoli A, Milite C,
Sarno G, Castellano S and Sbardella G: NADPH oxidases: From
molecular mechanisms to current inhibitors. J Med Chem.
66:11632–11655. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wu J, Feng Z, Chen L, Li Y, Bian H, Geng
J, Zheng ZH, Fu X, Pei Z, Qin Y, et al: TNF antagonist sensitizes
synovial fibroblasts to ferroptotic cell death in collagen-induced
arthritis mouse models. Nat Commun. 13:6762022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wang L, Liu Y, Du T, Yang H, Lei L, Guo M,
Ding HF, Zhang J, Wang H, Chen X and Yan C: ATF3 promotes
erastin-induced ferroptosis by suppressing system Xc. Cell Death
Differ. 27:662–675. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Inaba Y, Hashiuchi E, Watanabe H, Kimura
K, Oshima Y, Tsuchiya K, Murai S, Takahashi C, Matsumoto M,
Kitajima S, et al: The transcription factor ATF3 switches cell
death from apoptosis to necroptosis in hepatic steatosis in male
mice. Nat Commun. 14:1672023. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Liu S, Li Z, Lan S, Hao H, Baz AA, Yan X,
Gao P, Chen S and Chu Y: The dual roles of activating transcription
factor 3 (ATF3) in inflammation, apoptosis, ferroptosis, and
pathogen infection responses. Int J Mol Sci. 25:8242024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wu J, Huang Y, Zhou X, Xiang Z, Yang Z,
Meng D, Wu D, Zhang J and Yang J: ATF3 and its emerging role in
atherosclerosis: A narrative review. Cardiovasc Diagn Ther.
12:926–942. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Shao CJ, Zhou HL, Gao XZ and Xu CF:
Downregulation of miR-221-3p promotes the ferroptosis in gastric
cancer cells via upregulation of ATF3 to mediate the transcription
inhibition of GPX4 and HRD1. Transl Oncol. 32:1016492023.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Feng Z, Cao K, Sun H and Liu X: SEH1L
siliencing induces ferroptosis and suppresses hepatocellular
carcinoma progression via ATF3/HMOX1/GPX4 axis. Apoptosis.
29:1723–1737. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Lu H, Zheng Y and Wang D: ATF3 affects
osteogenic differentiation in inflammatory hPDLSCs by mediating
ferroptosis via regulating the Nrf2/HO-1 signaling pathway. Tissue
Cell. 89:1024472024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Lei P, Bai T and Sun Y: Mechanisms of
ferroptosis and relations with regulated cell death: A review.
Front Physiol. 10:1392019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wu Y, Zhang S, Gong X, Tam S, Xiao D, Liu
S and Tao Y: The epigenetic regulators and metabolic changes in
ferroptosis-associated cancer progression. Mol Cancer. 19:392020.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Wei S, Wang H, Lu C, Malmut S, Zhang J,
Ren S, Yu G, Wang W, Tang DD and Yan C: The activating
transcription factor 3 protein suppresses the oncogenic function of
mutant p53 proteins. J Biol Chem. 289:8947–8959. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Li X, Guo M, Cai L, Du T, Liu Y, Ding HF,
Wang H, Zhang J, Chen X and Yan C: Competitive ubiquitination
activates the tumor suppressor p53. Cell Death Differ.
27:1807–1818. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zhao J, Li X, Guo M, Yu J and Yan C: The
common stress responsive transcription factor ATF3 binds genomic
sites enriched with p300 and H3K27ac for transcriptional
regulation. BMC Genomics. 17:3352016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Akbarpour Arsanjani A, Abuei H,
Behzad-Behbahani A, Bagheri Z, Arabsolghar R and Farhadi A:
Activating transcription factor 3 inhibits NF-κB p65 signaling
pathway and mediates apoptosis and cell cycle arrest in cervical
cancer cells. Infect Agent Cancer. 17:622022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Kwon JW, Kwon HK, Shin HJ, Choi YM, Anwar
MA and Choi S: Activating transcription factor 3 represses
inflammatory responses by binding to the p65 subunit of NF-κB. Sci
Rep. 5:144702015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Liu J, Zhang C, Wang J, Hu W and Feng Z:
The regulation of ferroptosis by tumor suppressor p53 and its
pathway. Int J Mol Sci. 21:83872020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kang R, Kroemer G and Tang D: The tumor
suppressor protein p53 and the ferroptosis network. Free Radic Biol
Med. 133:162–168. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Comporti M: Glutathione depleting agents
and lipid peroxidation. Chem Phys Lipids. 45:143–169. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Ou Y, Wang SJ, Li D, Chu B and Gu W:
Activation of SAT1 engages polyamine metabolism with p53-mediated
ferroptotic responses. Proc Natl Acad Sci USA. 113:E6806–E6812.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Suzuki S, Tanaka T, Poyurovsky MV, Nagano
H, Mayama T, Ohkubo S, Lokshin M, Hosokawa H, Nakayama T, Suzuki Y,
et al: Phosphate-activated glutaminase (GLS2), a p53-inducible
regulator of glutamine metabolism and reactive oxygen species. Proc
Natl Acad Sci USA. 107:7461–7466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Hu W, Zhang C, Wu R, Sun Y, Levine A and
Feng Z: Glutaminase 2, a novel p53 target gene regulating energy
metabolism and antioxidant function. Proc Natl Acad Sci USA.
107:7455–7460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Engeland K: Cell cycle regulation:
p53-p21-RB signaling. Cell Death Differ. 29:946–960. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Engeland K: Cell cycle arrest through
indirect transcriptional repression by p53: I have a DREAM. Cell
Death Differ. 25:114–132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Chen W, Sun Z, Wang XJ, Jiang T, Huang Z,
Fang D and Zhang DD: Direct interaction between Nrf2 and
p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response.
Mol Cell. 34:663–673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Pouremamali F, Pouremamali A, Dadashpour
M, Soozangar N and Jeddi F: An update of Nrf2 activators and
inhibitors in cancer prevention/promotion. Cell Commun Signal.
20:1002022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Villeneuve NF, Sun Z, Chen W and Zhang DD:
Nrf2 and p21 regulate the fine balance between life and death by
controlling ROS levels. Cell Cycle. 8:3255–3256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Franklin CC, Backos DS, Mohar I, White CC,
Forman HJ and Kavanagh TJ: Structure, function, and
post-translational regulation of the catalytic and modifier
subunits of glutamate cysteine ligase. Mol Aspects Med. 30:86–98.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Lu SC: Regulation of glutathione
synthesis. Mol Aspects Med. 30:42–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Dahl EL and Mulcahy RT: Cell-type specific
differences in glutamate cysteine ligase transcriptional regulation
demonstrate independent subunit control. Toxicol Sci. 61:265–272.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Wang X, Shen T, Lian J, Deng K, Qu C, Li
E, Li G, Ren Y, Wang Z, Jiang Z, et al: Resveratrol reduces
ROS-induced ferroptosis by activating SIRT3 and compensating the
GSH/GPX4 pathway. Mol Med. 29:1372023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Weaver K and Skouta R: The selenoprotein
glutathione peroxidase 4: From molecular mechanisms to novel
therapeutic opportunities. Biomedicines. 10:8912022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Xie Y, Kang R, Klionsky DJ and Tang D:
GPX4 in cell death, autophagy, and disease. Autophagy.
19:2621–2638. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Labrecque CL and Fuglestad B:
Electrostatic drivers of GPx4 interactions with membrane, lipids,
and DNA. Biochemistry. 60:2761–2772. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi
AA and Lei P: Ferroptosis: Mechanisms and links with diseases.
Signal Transduct Target Ther. 6:492021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Sugezawa K, Morimoto M, Yamamoto M,
Matsumi Y, Nakayama Y, Hara K, Uejima C, Kihara K, Matsunaga T,
Tokuyasu N, et al: GPX4 regulates tumor cell proliferation via
suppressing ferroptosis and exhibits prognostic significance in
gastric cancer. Anticancer Res. 42:5719–5729. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Li J, Liu J, Zhou Z, Wu R, Chen X, Yu C,
Stockwell B, Kroemer G, Kang R and Tang D: Tumor-specific GPX4
degradation enhances ferroptosis-initiated antitumor immune
response in mouse models of pancreatic cancer. Sci Transl Med.
15:eadg30492023. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Li H, Sun Y, Yao Y, Ke S, Zhang N, Xiong
W, Shi J, He C, Xiao X, Yu H, et al: USP8-governed GPX4 homeostasis
orchestrates ferroptosis and cancer immunotherapy. Proc Natl Acad
Sci USA. 121:e23155411212024. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Shimizu J, Murao A, Nofi C, Wang P and
Aziz M: Extracellular CIRP promotes GPX4-mediated ferroptosis in
sepsis. Front Immunol. 13:9038592022. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Niu B, Liao K, Zhou Y, Wen T, Quan G, Pan
X and Wu C: Application of glutathione depletion in cancer therapy:
Enhanced ROS-based therapy, ferroptosis, and chemotherapy.
Biomaterials. 277:1211102021. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Wu M, Xu LG, Li X, Zhai Z and Shu HB:
AMID, an apoptosis-inducing factor-homologous
mitochondrion-associated protein, induces caspase-independent
apoptosis. J Biol Chem. 277:25617–25623. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Ohiro Y, Garkavtsev I, Kobayashi S,
Sreekumar KR, Nantz R, Higashikubo BT, Duffy SL, Higashikubo R,
Usheva A, Gius D, et al: A novel p53-inducible apoptogenic gene,
PRG3, encodes a homologue of the apoptosis-inducing factor (AIF).
FEBS Lett. 524:163–171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Nakamura T, Mishima E, Yamada N, Mourão
ASD, Trümbach D, Doll S, Wanninger J, Lytton E, Sennhenn P, Nishida
Xavier da Silva T, et al: Integrated chemical and genetic screens
unveil FSP1 mechanisms of ferroptosis regulation. Nat Struct Mol
Biol. 30:1806–1815. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Lv Y, Liang C, Sun Q, Zhu J, Xu H, Li X,
Li YY, Wang Q, Yuan H, Chu B and Zhu D: Structural insights into
FSP1 catalysis and ferroptosis inhibition. Nat Commun. 14:59332023.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Deshwal S, Onishi M, Tatsuta T, Bartsch T,
Cors E, Ried K, Lemke K, Nolte H, Giavalisco P and Langer T:
Mitochondria regulate intracellular coenzyme Q transport and
ferroptotic resistance via STARD7. Nat Cell Biol. 25:246–257.
2023.PubMed/NCBI
|
|
157
|
Frei B, Kim MC and Ames BN: Ubiquinol-10
is an effective lipid-soluble antioxidant at physiological
concentrations. Proc Natl Acad Sci USA. 87:4879–4883. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Ernster L, Forsmark P and Nordenbrand K:
The mode of action of lipid-soluble antioxidants in biological
membranes. Relationship between the effects of ubiquinol and
vitamin E as inhibitors of lipid peroxidation in submitochondrial
particles. J Nutr Sci Vitaminol (Tokyo) Spec No. 548–551. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Sharifi-Rad M, Anil Kumar NV, Zucca P,
Varoni EM, Dini L, Panzarini E, Rajkovic J, Tsouh Fokou PV, Azzini
E, Peluso I, et al: Lifestyle, oxidative stress, and antioxidants:
Back and forth in the pathophysiology of chronic diseases. Front
Physiol. 11:6942020. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Wang Y and Hekimi S: Understanding
ubiquinone. Trends Cell Biol. 26:367–378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Naguib YW, Saha S, Skeie JM, Acri T, Ebeid
K, Abdel-Rahman S, Kesh S, Schmidt GA, Nishimura DY, Banas JA, et
al: Solubilized ubiquinol for preserving corneal function.
Biomaterials. 275:1208422021. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Zameitat E, Freymark G, Dietz CD, Löffler
M and Bölker M: Functional expression of human dihydroorotate
dehydrogenase (DHODH) in pyr4 mutants of ustilago maydis allows
target validation of DHODH inhibitors in vivo. Appl Environ
Microbiol. 73:3371–3379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Reis RAG, Calil FA, Feliciano PR, Pinheiro
MP and Nonato MC: The dihydroorotate dehydrogenases: Past and
present. Arch Biochem Biophys. 632:175–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Evans DR and Guy HI: Mammalian pyrimidine
biosynthesis: Fresh insights into an ancient pathway. J Biol Chem.
279:33035–33038. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Leban J, Kralik M, Mies J, Gassen M,
Tentschert K and Baumgartner R: SAR, species specificity, and
cellular activity of cyclopentene dicarboxylic acid amides as DHODH
inhibitors. Bioorg Med Chem Lett. 15:4854–4857. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Vyas VK and Ghate M: Recent developments
in the medicinal chemistry and therapeutic potential of
dihydroorotate dehydrogenase (DHODH) inhibitors. Mini Rev Med Chem.
11:1039–1055. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Rodriguez JMO, Krupinska E, Wacklin-Knecht
H and Knecht W: Preparation of human dihydroorotate dehydrogenase
for interaction studies with lipid bilayers. Nucleosides
Nucleotides Nucleic Acids. 39:1306–1319. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Peeters MJW, Aehnlich P, Pizzella A,
Mølgaard K, Seremet T, Met Ö, Rasmussen LJ, Thor Straten P and
Desler C: Mitochondrial-linked de novo pyrimidine biosynthesis
dictates human T-cell proliferation but not expression of effector
molecules. Front Immunol. 12:7188632021. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Martínez-Reyes I, Cardona LR, Kong H,
Vasan K, McElroy GS, Werner M, Kihshen H, Reczek CR, Weinberg SE,
Gao P, et al: Mitochondrial ubiquinol oxidation is necessary for
tumour growth. Nature. 585:288–292. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Yang C, Zhao Y, Wang L, Guo Z, Ma L, Yang
R, Wu Y, Li X, Niu J, Chu Q, et al: De novo pyrimidine biosynthetic
complexes support cancer cell proliferation and ferroptosis
defence. Nat Cell Biol. 25:836–847. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee
H, Koppula P, Wu S, Zhuang L, Fang B, et al: DHODH-mediated
ferroptosis defence is a targetable vulnerability in cancer.
Nature. 593:586–590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Ding Q, Tang W, Li X, Ding Y, Chen X, Cao
W, Wang X, Mo W, Su Z, Zhang Q and Guo H: Mitochondrial-targeted
brequinar liposome boosted mitochondrial-related ferroptosis for
promoting checkpoint blockade immunotherapy in bladder cancer. J
Control Release. 363:221–234. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Wang F and Min J: DHODH tangoing with GPX4
on the ferroptotic stage. Signal Transduct Target Ther. 6:2442021.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Diao J, Jia Y, Dai E, Liu J, Kang R, Tang
D, Han L, Zhong Y and Meng L: Ferroptotic therapy in cancer:
Benefits, side effects, and risks. Mol Cancer. 23:892024.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Westphal S and Kalthoff H: Apoptosis:
Targets in pancreatic cancer. Mol Cancer. 2:62003. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Todaro M, Lombardo Y, Francipane MG, Alea
MP, Cammareri P, Iovino F, Di Stefano AB, Di Bernardo C, Agrusa A,
Condorelli G, et al: Apoptosis resistance in epithelial tumors is
mediated by tumor-cell-derived interleukin-4. Cell Death Differ.
15:762–772. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Miao Z, Xu L, Gu W, Ren Y, Li R, Zhang S,
Chen C, Wang H, Ji J and Chen J: A targetable PRR11-DHODH axis
drives ferroptosis- and temozolomide-resistance in glioblastoma.
Redox Biol. 73:1032202024. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Fanet H, Capuron L, Castanon N, Calon F
and Vancassel S: Tetrahydrobioterin (BH4) pathway: From metabolism
to neuropsychiatry. Curr Neuropharmacol. 19:591–609. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Feng Y, Feng Y, Gu L, Liu P, Cao J and
Zhang S: The critical role of tetrahydrobiopterin (BH4) metabolism
in modulating radiosensitivity: BH4/NOS axis as an angel or a
devil. Front Oncol. 11:7206322021. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Burg AW and Brown GM: The biosynthesis of
folic acid. 8. Purification and properties of the enzyme that
catalyzes the production of formate from carbon atom 8 of guanosine
triphosphate. J Biol Chem. 243:2349–2358. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Niederwieser A, Staudenmann W and Wetzel
E: High-performance liquid chromatography with column switching for
the analysis of biogenic amine metabolites and pterins. J
Chromatogr. 290:237–246. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Jiang Y, Zhao J, Li R, Liu Y, Zhou L, Wang
C, Lv C, Gao L and Cui D: CircLRFN5 inhibits the progression of
glioblastoma via PRRX2/GCH1 mediated ferroptosis. J Exp Clin Cancer
Res. 41:3072022. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Liang D, Shu R, Jiang S, Gan Q, Wu S, Zhao
Y, Yang L, Xu M, Gao J and Meng Y: Expression of BmDHFR is
up-regulated to trigger an increase in the BH4/BH2 ratio when the
de novo synthesis of BH4 is blocked in silkworm, Bombyx mori. Int J
Biol Macromol. 225:625–633. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Ebenhoch R, Bauer M, Reinert D, Kersting
A, Huber S, Schmid A, Hinz I, Feiler M, Müller K and Nar H:
Biophysical and structural investigation of the regulation of human
GTP cyclohydrolase I by its regulatory protein GFRP. J Struct Biol.
213:1076912021. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Kraft VAN, Bezjian CT, Pfeiffer S,
Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X,
Anastasov N, Kössl J, et al: GTP cyclohydrolase
1/tetrahydrobiopterin counteract ferroptosis through lipid
remodeling. ACS Cent Sci. 6:41–53. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Wang D, Liang W, Huo D, Wang H, Wang Y,
Cong C, Zhang C, Yan S, Gao M, Su X, et al: SPY1 inhibits neuronal
ferroptosis in amyotrophic lateral sclerosis by reducing lipid
peroxidation through regulation of GCH1 and TFR1. Cell Death
Differ. 30:369–382. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Vasquez-Vivar J, Shi Z and Tan S:
Tetrahydrobiopterin in cell function and death mechanisms. Antioxid
Redox Signal. 37:171–183. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Eichwald T, da Silva LDB, Staats Pires AC,
Niero L, Schnorrenberger E, Filho CC, Espíndola G, Huang WL,
Guillemin GJ, Abdenur JE and Latini A: Tetrahydrobiopterin: Beyond
its traditional role as a cofactor. Antioxidants (Basel).
12:10372023. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Villaume WA: Marginal BH4 deficiencies,
iNOS, and self-perpetuating oxidative stress in post-acute sequelae
of Covid-19. Med Hypotheses. 163:1108422022. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Soula M, Weber RA, Zilka O, Alwaseem H, La
K, Yen F, Molina H, Garcia-Bermudez J, Pratt DA and Birsoy K:
Metabolic determinants of cancer cell sensitivity to canonical
ferroptosis inducers. Nat Chem Biol. 16:1351–1360. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Xiao Y, Yuan Y, Yang Y, Liu B, Ding Z, Luo
J, Chen S and Yu L: GCH1 reduces LPS-induced alveolar macrophage
polarization and inflammation by inhibition of ferroptosis. Inflamm
Res. 72:1941–1955. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Famitafreshi H and Karimian M: Paradoxical
regulation of iron in hippocampus and prefrontal cortex induces
schizophrenic-like symptoms in male rats. Int J Neurosci.
130:384–390. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Kim SW, Stewart R, Park WY, Jhon M, Lee
JY, Kim SY, Kim JM, Amminger P, Chung YC and Yoon JS: Latent iron
deficiency as a marker of negative symptoms in patients with
first-episode schizophrenia spectrum disorder. Nutrients.
10:17072018. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Wei N, Ju M, Su X, Zhang Y, Huang Y, Rao
X, Cui L, Lin Z and Dong Y: Transplantation of gut microbiota
derived from patients with schizophrenia induces schizophrenia-like
behaviors and dysregulated brain transcript response in mice.
Schizophrenia (Heidelb). 10:442024. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Feng S, Chen J, Qu C, Yang L, Wu X, Wang
S, Yang T, Liu H, Fang Y and Sun P: Identification of
ferroptosis-related genes in schizophrenia based on bioinformatic
analysis. Genes (Basel). 13:21682022. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Liu X, Fan L, Lu C, Yin S and Hu H:
Functional role of p53 in the regulation of chemical-induced
oxidative stress. Oxid Med Cell Longev. 2020:60397692020.PubMed/NCBI
|
|
197
|
Byeon SH, Lee SC, Choi SH, Lee HK, Lee JH,
Chu YK and Kwon OW: Vascular endothelial growth factor as an
autocrine survival factor for retinal pigment epithelial cells
under oxidative stress via the VEGF-R2/PI3K/Akt. Invest Ophthalmol
Vis Sci. 51:1190–1197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Martín-Vázquez E, Cobo-Vuilleumier N,
López-Noriega L, Lorenzo PI and Gauthier BR: The
PTGS2/COX2-PGE2 signaling cascade in inflammation: Pro
or anti? A case study with type 1 diabetes mellitus. Int J Biol
Sci. 19:4157–4165. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Lian K, Li Y, Yang W, Ye J, Liu H, Wang T,
Yang G, Cheng Y and Xu X: Hub genes, a diagnostic model, and immune
infiltration based on ferroptosis-linked genes in schizophrenia.
IBRO Neurosci Rep. 16:317–328. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Feng Y and Shen J: Machine learning-based
predictive models and drug prediction for schizophrenia in multiple
programmed cell death patterns. Front Mol Neurosci. 16:11237082023.
View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Zhang D, Wu X, Xue X, Li W, Zhou P, Lv Z,
Zhao K and Zhu F: Ancient dormant virus remnant ERVW-1 drives
ferroptosis via degradation of GPX4 and SLC3A2 in schizophrenia.
Virol Sin. 39:31–43. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Ursini F and Maiorino M: Lipid
peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic
Biol Med. 152:175–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Forcina GC and Dixon SJ: GPX4 at the
crossroads of lipid homeostasis and ferroptosis. Proteomics.
19:e18003112019. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Casanova MF, Crapanzano KA, Mannheim G and
Kruesi M: Sydenham's chorea and schizophrenia: A case report.
Schizophr Res. 16:73–76. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Casanova MF, Comparini SO, Kim RW and
Kleinman JE: Staining intensity of brain iron in patients with
schizophrenia: A postmortem study. J Neuropsychiatry Clin Neurosci.
4:36–41. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Madden DJ and Merenstein JL: Quantitative
susceptibility mapping of brain iron in healthy aging and
cognition. Neuroimage. 282:1204012023. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Ravanfar P, Syeda WT, Jayaram M, Rushmore
RJ, Moffat B, Lin AP, Lyall AE, Merritt AH, Yaghmaie N, Laskaris L,
et al: In vivo 7-tesla MRI investigation of brain iron and its
metabolic correlates in chronic schizophrenia. Schizophrenia
(Heidelb). 8:862022. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Sonnenschein SF, Parr AC, Larsen B,
Calabro FJ, Foran W, Eack SM, Luna B and Sarpal DK: Subcortical
brain iron deposition in individuals with schizophrenia. J
Psychiatr Res. 151:272–278. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Cao B, Yan L, Ma J, Jin M, Park C, Nozari
Y, Kazmierczak OP, Zuckerman H, Lee Y, Pan Z, et al: Comparison of
serum essential trace metals between patients with schizophrenia
and healthy controls. J Trace Elem Med Biol. 51:79–85. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Santa Cruz EC, Madrid KC, Arruda MAZ and
Sussulini A: Association between trace elements in serum from
bipolar disorder and schizophrenia patients considering treatment
effects. J Trace Elem Med Biol. 59:1264672020. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Owiredu WKBA, Brenya PK, Osei Y, Laing EF,
Okrah CO, Obirikorang C, Anto EO, Acheampong E and Donkor S:
Evaluation of serum iron overload, AST:ALT ratio and
log10ferritin:AST ratio among schizophrenia patients in
the Kumasi Metropolis, Ghana: A case-control study. BMC Res Notes.
12:8022019. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Kumar J, Liddle EB, Fernandes CC,
Palaniyappan L, Hall EL, Robson SE, Simmonite M, Fiesal J, Katshu
MZ, Qureshi A, et al: Glutathione and glutamate in schizophrenia: A
7T MRS study. Mol Psychiatry. 25:873–882. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Nucifora LG, Tanaka T, Hayes LN, Kim M,
Lee BJ, Matsuda T, Nucifora FC Jr, Sedlak T, Mojtabai R, Eaton W
and Sawa A: Reduction of plasma glutathione in psychosis associated
with schizophrenia and bipolar disorder in translational
psychiatry. Transl Psychiatry. 7:e12152017. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Tenório MCDS, Graciliano NG, Moura FA, de
Oliveira ACM and Goulart MOF: N-acetylcysteine (NAC): Impacts on
human health. Antioxidants (Basel). 10:9672021. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Raghu G, Berk M, Campochiaro PA, Jaeschke
H, Marenzi G, Richeldi L, Wen FQ, Nicoletti F and Calverley PMA:
The multifaceted therapeutic role of N-acetylcysteine (NAC) in
disorders characterized by oxidative stress. Curr Neuropharmacol.
19:1202–1224. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
das Neves Duarte JM, Kulak A,
Gholam-Razaee MM, Cuenod M, Gruetter R and Do KQ: N-acetylcysteine
normalizes neurochemical changes in the glutathione-deficient
schizophrenia mouse model during development. Biol Psychiatry.
71:1006–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Berk M, Copolov DL, Dean O, Lu K, Jeavons
S, Schapkaitz I, Anderson-Hunt M and Bush AI: N-acetyl cysteine for
depressive symptoms in bipolar disorder-a double-blind randomized
placebo-controlled trial. Biol Psychiatry. 64:468–475. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Conus P, Seidman LJ, Fournier M, Xin L,
Cleusix M, Baumann PS, Ferrari C, Cousins A, Alameda L,
Gholam-Rezaee M, et al: N-acetylcysteine in a double-blind
randomized placebo-controlled trial: Toward biomarker-guided
treatment in early psychosis. Schizophr Bull. 44:317–327. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Rapado-Castro M, Dodd S, Bush AI, Malhi
GS, Skvarc DR, On ZX, Berk M and Dean OM: Cognitive effects of
adjunctive N-acetyl cysteine in psychosis. Psychol Med. 47:866–876.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Sepehrmanesh Z and Heidary M, Akasheh N,
Akbari H and Heidary M: Therapeutic effect of adjunctive N-acetyl
cysteine (NAC) on symptoms of chronic schizophrenia: A
double-blind, randomized clinical trial. Prog Neuropsychopharmacol
Biol Psychiatry. 82:289–296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Farokhnia M, Azarkolah A, Adinehfar F,
Khodaie-Ardakani MR, Hosseini SM, Yekehtaz H, Tabrizi M, Rezaei F,
Salehi B, Sadeghi SM, et al: N-acetylcysteine as an adjunct to
risperidone for treatment of negative symptoms in patients with
chronic schizophrenia: A randomized, double-blind,
placebo-controlled study. Clin Neuropharmacol. 36:185–192. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Breier A, Liffick E, Hummer TA, Vohs JL,
Yang Z, Mehdiyoun NF, Visco AC, Metzler E, Zhang Y and Francis MM:
Effects of 12-month, double-blind N-acetyl cysteine on symptoms,
cognition and brain morphology in early phase schizophrenia
spectrum disorders. Schizophr Res. 199:395–402. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Medvedev ON, Berk M, Dean OM, Brown E,
Sandham MH, Dipnall JF, McNamara RK, Sumich A, Krägeloh CU,
Narayanan A and Siegert RJ: A novel way to quantify schizophrenia
symptoms in clinical trials. Eur J Clin Invest. 51:e133982021.
View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Rossell SL, Francis PS, Galletly C, Harris
A, Siskind D, Berk M, Bozaoglu K, Dark F, Dean O, Liu D, et al:
N-acetylcysteine (NAC) in schizophrenia resistant to clozapine: A
double blind randomised placebo controlled trial targeting negative
symptoms. BMC Psychiatry. 16:3202016. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Lavoie S, Murray MM, Deppen P, Knyazeva
MG, Berk M, Boulat O, Bovet P, Bush AI, Conus P, Copolov D, et al:
Glutathione precursor, N-acetyl-cysteine, improves mismatch
negativity in schizophrenia patients. Neuropsychopharmacology.
33:2187–2199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Carmeli C, Knyazeva MG, Cuénod M and Do
KQ: Glutathione precursor N-acetyl-cysteine modulates EEG
synchronization in schizophrenia patients: A double-blind,
randomized, placebo-controlled trial. PLoS One. 7:e293412012.
View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Naguy A and Naguy C: N-acetyl-cysteine in
schizophrenia-there is more than meets the eyes! CNS Spectr.
26:446–447. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Deepmala Slattery J, Kumar N, Delhey L,
Berk M, Dean O, Spielholz C and Frye R: Clinical trials of
N-acetylcysteine in psychiatry and neurology: A systematic review.
Neurosci Biobehav Rev. 55:294–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Dean O, Giorlando F and Berk M:
N-acetylcysteine in psychiatry: Current therapeutic evidence and
potential mechanisms of action. J Psychiatry Neurosci. 36:78–86.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Klauser P, Xin L, Fournier M, Griffa A,
Cleusix M, Jenni R, Cuenod M, Gruetter R, Hagmann P, Conus P, et
al: N-acetylcysteine add-on treatment leads to an improvement of
fornix white matter integrity in early psychosis: A double-blind
randomized placebo-controlled trial. Transl Psychiatry. 8:2202018.
View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Tharoor H, Mara S and Gopal S: Role of
novel dietary supplement N-acetyl cysteine in treating negative
symptoms in schizophrenia: A 6-month follow-up study. Indian J
Psychol Med. 40:139–142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Tiihonen J, Tanskanen A and Taipale H:
20-Year nationwide follow-up study on discontinuation of
antipsychotic treatment in first-episode schizophrenia. Am J
Psychiatry. 175:765–773. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Bjørklund G, Shanaida M, Lysiuk R,
Antonyak H, Klishch I, Shanaida V and Peana M: Selenium: An
antioxidant with a critical role in anti-aging. Molecules.
27:66132022. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Brown JS Jr: Role of selenium and other
trace elements in the geography of schizophrenia. Schizophr Bull.
20:387–398. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Deng MG, Cui HT, Nie JQ, Liang Y and Chai
C: Genetic association between circulating selenium level and the
risk of schizophrenia in the European population: A two-sample
Mendelian randomization study. Front Nutr. 9:9698872022. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Cai L, Chen T, Yang J, Zhou K, Yan X, Chen
W, Sun L, Li L, Qin S, Wang P, et al: Serum trace element
differences between schizophrenia patients and controls in the Han
Chinese population. Sci Rep. 5:150132015. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Mattmiller SA, Carlson BA and Sordillo LM:
Regulation of inflammation by selenium and selenoproteins: Impact
on eicosanoid biosynthesis. J Nutr Sci. 2:e282013. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Soares de Oliveira AR, Jayanne Clímaco
Cruz K, Beatriz Silva Morais J, Rocha Dos Santos L, Rodrigues de
Sousa Melo S, Fontenelle LC, Santos de Sousa G, Costa Maia CS,
Oliveira Duarte de Araújo C, Leal Mendes I, et al: Selenium status
and oxidative stress in obese: Influence of adiposity. Eur J Clin
Invest. 51:e135382021. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Rayman MP: The importance of selenium to
human health. Lancet. 356:233–241. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Li Z, Liu Y, Li X, Ju W, Wu G, Yang X, Fu
X and Gao X: Association of elements with schizophrenia and
intervention of selenium supplements. Biol Trace Elem Res.
183:16–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Jamilian H and Ghaderi A: The effects of
probiotic and selenium co-supplementation on clinical and metabolic
scales in chronic schizophrenia: A randomized, double-blind,
placebo-controlled trial. Biol Trace Elem Res. 199:4430–4438. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Calder PC: Omega-3 fatty acids and
inflammatory processes. Nutrients. 2:355–374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Djuricic I and Calder PC: Beneficial
outcomes of omega-6 and omega-3 polyunsaturated fatty acids on
human health: An update for 2021. Nutrients. 13:24212021.
View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Tang W, Wang Y, Xu F, Fan W, Zhang Y, Fan
K, Wang W, Zhang Y and Zhang C: Omega-3 fatty acids ameliorate
cognitive dysfunction in schizophrenia patients with metabolic
syndrome. Brain Behav Immun. 88:529–534. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Hsu MC, Huang YS and Ouyang WC: Beneficial
effects of omega-3 fatty acid supplementation in schizophrenia:
Possible mechanisms. Lipids Health Dis. 19:1592020. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Hsu MC and Ouyang WC: A systematic review
of effectiveness of omega-3 fatty acid supplementation on symptoms,
social functions, and neurobiological variables in schizophrenia.
Biol Res Nurs. 23:723–737. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Goh KK, Chen CYA, Chen CH and Lu ML:
Effects of omega-3 polyunsaturated fatty acids supplements on
psychopathology and metabolic parameters in schizophrenia: A
meta-analysis of randomized controlled trials. J Psychopharmacol.
35:221–235. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Surette ME: The science behind dietary
omega-3 fatty acids. CMAJ. 178:177–180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Fuentes NR, Kim E, Fan YY and Chapkin RS:
Omega-3 fatty acids, membrane remodeling and cancer prevention. Mol
Aspects Med. 64:79–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Joffre C, Rey C and Layé S: N-3
polyunsaturated fatty acids and the resolution of
neuroinflammation. Front Pharmacol. 10:10222019. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Chen Y, Fang ZM, Yi X, Wei X and Jiang DS:
The interaction between ferroptosis and inflammatory signaling
pathways. Cell Death Dis. 14:2052023. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Simopoulos AP: Omega-3 fatty acids in
inflammation and autoimmune diseases. J Am Coll Nutr. 21:495–505.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Gutiérrez S, Svahn SL and Johansson ME:
Effects of omega-3 fatty acids on immune cells. Int J Mol Sci.
20:50282019. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Nuñez MT and Chana-Cuevas P: New
perspectives in iron chelation therapy for the treatment of
neurodegenerative diseases. Pharmaceuticals (Basel). 11:1092018.
View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Devos D, Labreuche J, Rascol O, Corvol JC,
Duhamel A, Guyon Delannoy P, Poewe W, Compta Y, Pavese N, Růžička
E, et al: Trial of deferiprone in Parkinson's disease. N Engl J
Med. 387:2045–2055. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Urrutia PJ, Bórquez DA and Núñez MT:
Inflaming the brain with Iron. Antioxidants (Basel). 10:612021.
View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Costa I, Barbosa DJ, Benfeito S, Silva V,
Chavarria D, Borges F, Remião F and Silva R: Molecular mechanisms
of ferroptosis and their involvement in brain diseases. Pharmacol
Ther. 244:1083732023. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Ramadhan MIA, Sitanaya SN, Hakim AHW and
Ramli Y: The role of iron-chelating therapy in improving
neurological outcome in patients with intracerebral hemorrhage:
Evidence-based case report. Medicina (Kaunas). 59:4532023.
View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Dusek P, Schneider SA and Aaseth J: Iron
chelation in the treatment of neurodegenerative diseases. J Trace
Elem Med Biol. 38:81–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Cutler P: Iron overload and psychiatric
illness. Can J Psychiatry. 39:8–11. 1994. View Article : Google Scholar : PubMed/NCBI
|