Introduction
Sepsis appears as a result of the host's inadequate
and irregular response to an infection, leading to the disruption
of critical organ functions. Sepsis is a life-threatening condition
characterized by a series of pathophysiological symptoms that may
progress to septic shock, and associated with an elevated risk of
death due to extreme cellular, metabolic and circulatory
abnormalities (1). The increasing
occurrence and complex clinical presentation of both sepsis and
septic shock pose significant challenges and burdens for emergency
physicians (2,3). Since the initial consensus definition
in 1991, the prevalence of sepsis and septic shock has continued to
rise, with an estimated 49 million sepsis cases and 11 million
sepsis-related deaths reported worldwide in 2017 (4,5).
Consequently, the World Health Organization has recognized sepsis
as a critical global health concern (3). Several factors contribute to the
occurrence of sepsis, including the higher mean age of patients,
the increasing use of invasive procedures, the administration of
immunosuppressive drugs and chemotherapy, and the emergence of
antibiotic resistance. Despite advances in treatment approaches,
individuals affected by sepsis remain at risk of experiencing fatal
outcomes during their hospital stay (6).
In severe conditions such as sepsis, the organs most
often impacted are the kidneys, liver, lungs and heart, as well as
the central nervous and hematological systems (7). The liver holds a central position in
maintaining metabolic and immunological balance, and can suffer
harm from pathogens, toxins or inflammatory agents, making it a
critical organ requiring prompt treatment for survival (8). During an episode of sepsis, liver
injuries manifest through a series of stages, ranging from impaired
liver cell function to liver damage and eventual liver failure.
Liver failure, the most severe form of damage, is characterized by
the loss of 80–90% of liver cell functionality. Identifying liver
dysfunction and failure directly is a crucial factor for the
successful resolution of a sepsis episode (7,9,10).
Renal dysfunction emerges as another focal point in the progression
of sepsis and is the predominant factor contributing to acute
kidney injury (AKI) in critically ill individuals (11). The risk of in-hospital mortality
among patients experiencing sepsis-related AKI is >60%,
surpassing by far the risks faced by patients with sepsis but
without AKI and those with AKI unrelated to sepsis (12). The precise mechanisms behind AKI
have been partially identified, yet the primary focus is on the
onset of renal hypoperfusion. Current evidence, however, indicates
that local microcirculation and inflammatory signals, including
ischemia-reperfusion injury, oxidative stress and tubular
apoptosis, assume an even more pivotal role (13).
In the development of sepsis, inflammation plays a
vital yet intricate role (14).
The uncontrolled escalation in the release of pro-inflammatory
cytokines by the immune system can result in a dysregulated and
exaggerated cytokine storm that leads to vasodilation, increased
vascular permeability and a decrease in blood pressure, adversely
affecting blood flow to the vital organs (15). Moreover, it disrupts the normal
functioning of the blood clotting system, culminating in
disseminated intravascular coagulation (16). These uncontrolled inflammatory
responses may lead to widespread organ dysfunction and failure,
thereby defining the severity of the sepsis. To date, several
contributing biomarkers, genes and pathways have been investigated
for their role in the escalating cytokine storm that may lead to
sepsis (17). Among others, small
non-coding RNAs such as microRNAs (miRNAs/miRs) have emerged as key
regulators over the years due to their role in inflammatory gene
expression at multiple levels, and their potential as biomarkers
and therapeutic targets in controlling abnormal inflammatory
reactions in the body (18,19).
miR-21 has been found to play a role in inflammation and sepsis.
Studies have shown that miR-21 expression is upregulated in
sepsis-induced acute lung injury (ALI) and acute liver injury
(20,21). In septic mice, miR-21 expression is
enhanced in peritoneal macrophages and neutrophils, and its
deletion in myeloid cells leads to improved survival, decreased
bacterial growth, and reduced systemic inflammation and organ
damage (21,22). The protective effect of miR-21 in
sepsis-induced ALI is mediated by regulating phosphatase and tensin
homolog (PTEN) (23). PTEN
negatively regulates the phosphoinositide 3-kinase (PI3K)/AKT
signaling pathway, which alters inflammatory responses through the
release of inflammatory factors, and the recruitment and activation
of immune cells (24,25).
Boron compounds are essential micronutrients for
plants, and while they are not classified as essential nutrients
for humans, they seem to play various roles in plant and animal
physiology (26). Although they
are present in trace amounts in naturally derived food products and
historically have been used as food preservatives, such as during
the 1910s to prevent spoilage during World War I, their use in
medicine is limited and mostly as adjuvants. Boric acid (BA;
chemical formula: H3BO3), known by various
names such as hydrogen borate, boracic acid or orthoboric acid, is
recognized as a derivative of a weak monobasic acid. Borax (BX;
chemical formula:
Na2B4O7·10H2O), also
referred to as sodium borate, sodium tetraborate decahydrate or
disodium tetraborate, is a naturally occurring mineral. Boron
compounds are commonly employed as adjuvants with antiseptic
properties in ophthalmological and, for limited purposes, in
dermatological products (BA) or as a precursor for various chemical
compounds (27–30). Compounds containing boron, such as
BA or BX, have demonstrated a broad spectrum of biological
activities, including antibacterial, antiviral, antifungal,
anti-carcinogenic, anti-invasive, anti-angiogenic, anti-mutagenic,
anti-inflammatory and antioxidant properties (26,31).
Previous studies suggest that boron induces changes in barrier
function, proliferation and apoptosis in rat intestinal epithelial
cells through the PI3K/AKT signaling pathway (32,33).
Given the complex involvement of inflammation in sepsis, the
present study focused on investigating the in vivo effects
of BA and BX on inflammation biomarkers, such as tumor necrosis
factor-α (TNF-α), interleukin (IL)-6 and IL-10, and on alterations
in miR-21/PTEN/AKT pathway genes within the liver and kidney
tissues of rats experiencing sepsis induced by cecal ligation and
puncture (CLP).
Materials and methods
Animals
Sprague Dawley male rats (n=60), aged between 8 and
10 weeks and weighing 200–250 g, were utilized in this study. The
animals were acquired from the Animal Shelter affiliated with
Ataturk University's Medicinal and Experimental Application and
Research Center (Erzurum, Türkiye). All procedures strictly adhered
to the ethical guidelines sanctioned by the ethical committee, and
the animal research used in the scientific procedures in the study
was conducted under the guidance of Directives 2010/63/EU, which
regulate animal research in the European Union regarding the
protection of animals used for scientific purposes. Approval for
all procedures was granted by the Ethics Committee at Kastamonu
University (Kastamonu, Türkiye; approval no. 28/4). The animals
were comfortably housed in standard polypropylene cages within a
meticulously controlled environment, maintained at a temperature of
22±1°C, with relative humidity ranging between 50 and 60%, and
adhering to a 12-h light/dark photoperiod. The animals were
provided with ample quantities of standard food and tap water
available ad libitum following the procedures outlined by
the Animal Shelter at Ataturk University's Medicinal and
Experimental Application and Research Center, with the animal feed
being commercially supplied by the research center.
Experimental design: CLP-induced
sepsis model
Sepsis was modelled through the CLP method, as
previously described (34). Rats
underwent intraperitoneal injection for anesthesia, receiving a
combination of ketamine (90 mg/kg body weight) and xylazine (10
mg/kg body weight). Upon achieving a profound level of anesthesia,
the abdominal region was depilated and a longitudinal midline
incision (3 to 4 cm) was made to expose the cecum. Following a
meticulous dissection of the cecal mesentery, a ligature was
applied at the predetermined position to achieve the desired
severity grade, effectively occluding the cecal lumen. Using an
18-gauge needle, four holes (two on each side) were created along
the mesentery, extending from the distal end of the cecum to the
ligated point, facilitating the milking of the cecum. The induction
of abdominal sepsis ensued, with the bacterial flora from the stool
emerging through the perforations in the milked cecum.
Subsequently, the cecum was reintroduced into the peritoneal
cavity, and 0.5 ml of normal saline was administered through the
peritoneal cavity to all animals before suturing the incision.
Closure of the abdominal incision occurred in two layers using 3.0
silk sutures. Following the experimental procedures, the rats were
returned to their cages. The animals had unrestricted access to
both food and water. A sepsis model induced by mid-grade CLP using
an 18-gauge needle was established, with significant
sepsis-associated changes observed around the 16th hour
post-procedure, as indicated by Hubbard et al (35).
Experimental design: Boron
administration
Six groups, each consisting of 10 rats, were
established for the study with the following interventions: i)
Control group, which did not receive any intervention or CLP sepsis
induction; ii) CLP group, in which the sepsis model was applied,
but no interventions were made; iii) CLP + BX1 group, which was
treated with 20 mg/kg BX; iv) CLP + BX2 group, which was treated
with 40 mg/kg BX; v) CLP + BA1 group, which was treated with 20
mg/kg BA; and vi) CLB + BA2 group, which was treated with 40 mg/kg
BA. The control group and the treatment groups (CLP + BX1, CLP +
BX2, CLP + BA1 and CLP + BA2) received intraperitoneal normal
saline, with the treatment groups receiving BX (cat. no. 1303-96-4;
MilliporeSigma) or BA (cat. no. 10043-35-3; MilliporeSigma)
dissolved in normal saline 12 h after the induction of sepsis. The
CLP group did not receive any treatment, serving as the untreated
sepsis model.
Sacrifice and sample collection
The animals were humanely euthanized via
intraperitoneal administration of ketamine at a dosage of 300 mg/kg
combined with xylazine at a dosage of 30 mg/kg, and their vital
signs were observed until their heartbeats ceased. Euthanasia was
performed 24 h after sepsis induction, and tissue samples from the
kidney and liver were collected for analysis. The kidney and liver
tissues were fixed in a 10% formalin solution at room temperature
for 24 h to ensure adequate preservation for subsequent
histopathological and molecular analyses.
Gene expression analysis
The extraction of total RNA from paraffin-embedded
kidney and liver tissues was performed using the High Pure FFPET
RNA Isolation Kit (Invitrogen; Thermo Fisher Scientific, Inc.),
following the manufacturer's guidelines. Subsequently, cDNA
synthesis was performed with the Second Strand cDNA Synthesis Kit
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol, utilizing a consistent quantity of RNA
from each sample. All primers (5′-3′) used were purchased as TaqMan
Gene Expression Assays (Table I).
The expression data for β-actin and U6 in each tissue were used as
the endogenous control (Table I).
Gene expression levels were assessed through qPCR using the Light
Cycler® 480 II with Light Cycler 480 Probes Master
(Roche Diagnostic GmbH). Each animal's tissue was examined in
triplicate. The annealing temperature for all gene regions was set
at 60°C, qPCR was performed in triplicate for each sample using the
following conditions: Denaturation at 95°C for 10 min, followed by
45 cycles of amplification at 95°C for 10 sec, 60°C for 30 sec,
72°C for 1 sec and cooling at 40°C for 10 sec. Reaction mixture
including no cDNA used as negative control. and the gene expression
levels were calculated as using the 2-ΔΔCq method.
b-actin and U6 were used as reference genes for the calculation of
relative mRNA and miRNA expression levels, respectively (36,37).
 | Table I.Primer sequences and annealing
temperatures. |
Table I.
Primer sequences and annealing
temperatures.
| Gene | Forward primer
sequence (5′-3′) | Reverse primer
sequence (5′-3′) | Temperature,
°C | Assay ID |
|---|
| miR-21 |
TAGCTTATCAGACTGATGTTGA |
GCCAGCACAGAATTAATACGAC | 60 | Rn04244285_s1 |
| PTEN |
AGAACAAGATGCTAAAAAGGACAA |
TGTCAGGGTGAGCACAAGAT | 60 | Rn00477208_m1 |
| AKT1 |
GTGGCAAGATGTGTATGAG |
CTGGCTGAGTAGGAGAAC | 60 | Rn00583646_m1 |
| β-actin |
TGGTGGGTATGGGTCAGAAG |
GACAATGCCGTGTTCAATGG | 60 | Hs99999903_m1 |
| U6 |
GCTTCGGCAGCACATATACTAAAAT |
CGCTTCACGAATTTGCGTGTCAT | 60 | 001973 |
Pathological analyses
Histopathology
Liver and kidney tissues obtained from rat
necropsies were preserved in a 10% neutral formalin solution.
Following routine alcohol-xylene processing, the tissues were
embedded in paraffin blocks. Sections (4-µm thick) were placed on
slides and stained with hematoxylin and eosin for 10 min at room
temperature, and a semi-quantitative assessment of microscopic
changes was conducted, with categorization as follows: No changes
(score 0), mild (score 1), moderate (score 2), severe (score 3) and
very severe (score 4).
Immunohistochemistry
For immunohistochemical evaluation, the 4-µm
sections on the slides underwent de-paraffinization in xylene and
alcohol, followed by a 10-min PBS wash and treatment with 3%
H2O2, achieving endogenous peroxidase
inactivation. Antigen retrieval was performed by subjecting the
tissues to a 2×5-min treatment at 500 watts with an antigen
retrieval solution (100X Citrate Buffer; cat. no. ab93678; Abcam).
Subsequently, TNF-α (cat. no. SC-133192; Santa Cruz Biotechnology,
Inc.), IL-6 (cat. no. DF6087; Affinity Biosciences) and IL-10 (cat.
no. SC-8438; Santa Cruz Biotechnology, Inc.) primary antibodies
were applied at a dilution of 1:200 and left to incubate overnight
at 4°C. For the secondary antibody step, the Large Volume Detection
System: Anti-Polyvalent, HRP (cat. no. TP-125-HL; Thermo Fisher
Scientific, Inc.), was used according to the manufacturer's
instructions, typically involving incubation for ~45 min at room
temperature. 3.3′-Diaminobenzidine served as the chromogen. After
counterstaining with Mayer's hematoxylin for 1 min at room
temperature, the slides were covered and examined under a light
microscope. The percentages of immunopositivity detected in six
random distinct fields were analyzed using the Fiji ImageJ program
(License, GPLv3+; http://imagej.net/software/fiji/downloads). Two
different pathologists independently evaluated the samples, scoring
as follows: No reactivity (score 0), mild (score 1), moderate
(score 2), severe (score 3) and very severe (score 4).
Statistical analysis
The disparities in molecular data among distinct
groups were assessed through one-way analysis of variance (ANOVA)
using IBM SPSS 20.0 statistical software (IBM Corp.). Variance
uniformity within groups was confirmed using Levene's test, and
normal distribution within each group was assessed through the
Shapiro-Wilk test. Group differences in gene expression levels were
established using one-way ANOVA, followed by a Tukey's post hoc
test. For the statistical analysis of semiquantitative data
determined histopathologically, intergroup differences were
evaluated using the Kruskal-Wallis test followed by Dunn's post hoc
test, and the group responsible for the variance was determined
through the Mann-Whitney U test. P<0.05 was considered to
indicate a statistically significant difference.
Results
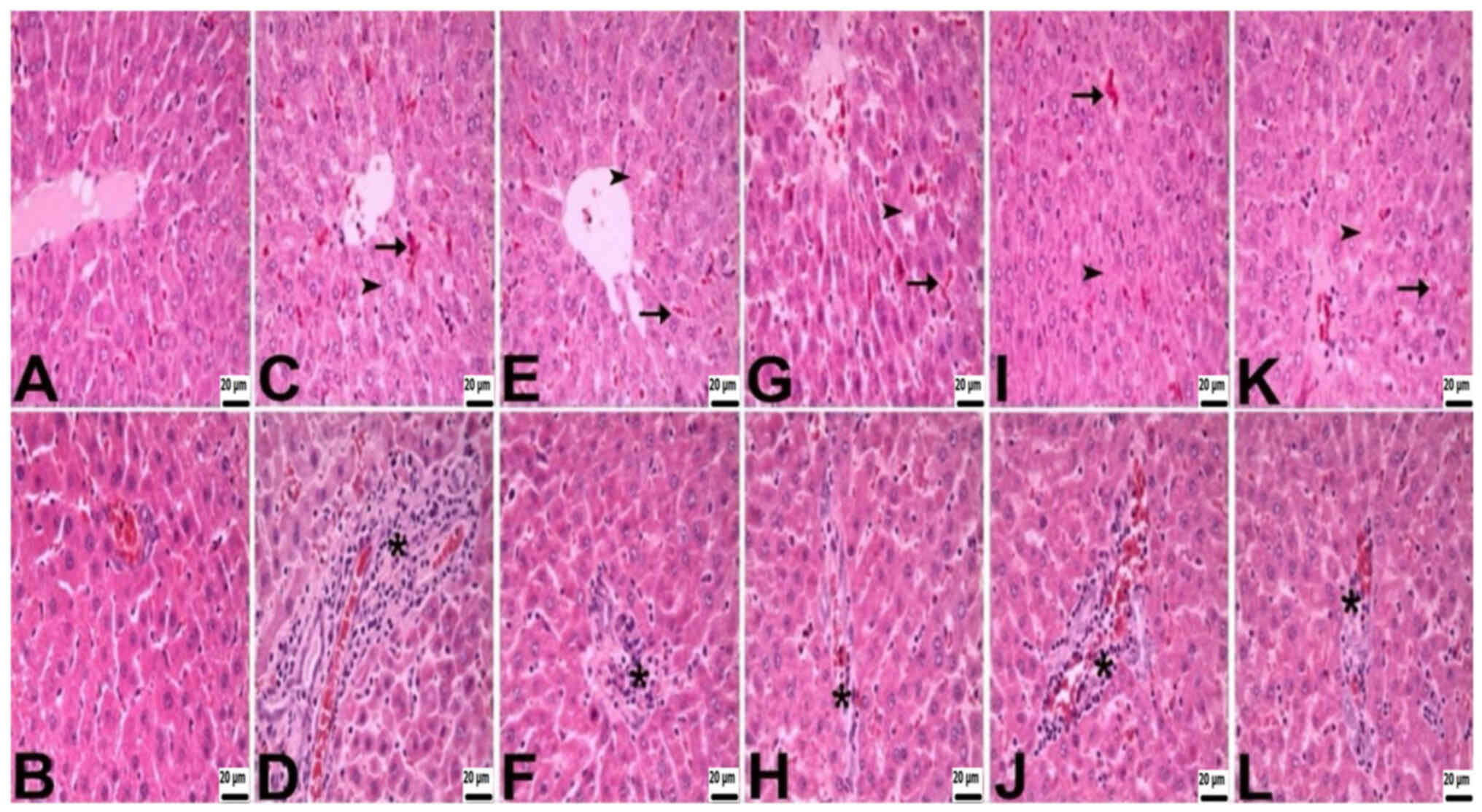
Histopathological findings
During the histopathological assessment of the liver
and kidneys, differences were observed among the groups. Rats in
the control group exhibited a normal histological appearance. By
contrast, rats in the other groups showed histopathological
findings, including necrosis, congestion and mononuclear cell
infiltrations. The severity of necrosis and mononuclear cell
infiltrations varied across groups, with the CLP group experiencing
very severe effects, the CLP + BA1 group showing severe effects,
the CLP + BA2 and CLP + BX1 groups displaying moderate effects, and
the CLP + BX2 group exhibiting mild effects. The degree of
congestion ranged from moderate in the CLP, CLP + BX2, and CLP +
BA1 groups to mild in the CLP + BX1 and CLP + BA2 groups (Table II; Fig. 1).
 | Table II.Statistical evaluation of
histopathological alterations in the liver tissues. |
Table II.
Statistical evaluation of
histopathological alterations in the liver tissues.
| Groups | Necrosis | Congestion | Mononuclear cell
infiltration |
|---|
| Control | 0 (1.00) | 0 (1.00) | 0 (0.00) |
| CLP | 4
(1.00)a | 2
(0.00)a | 4
(0.25)a |
| CLP + BX1 | 2
(1.00)b | 1
(0.25)b | 2
(0.25)b |
| CLP + BX2 | 1
(0.00)c | 2
(0.00)a | 1
(0.00)c |
| CLP + BA1 | 3
(0.00)d | 2
(0.00)a | 3
(0.00)d |
| CLP + BA2 | 2
(0.00)b | 1
(0.00)b | 2
(1.00)b |
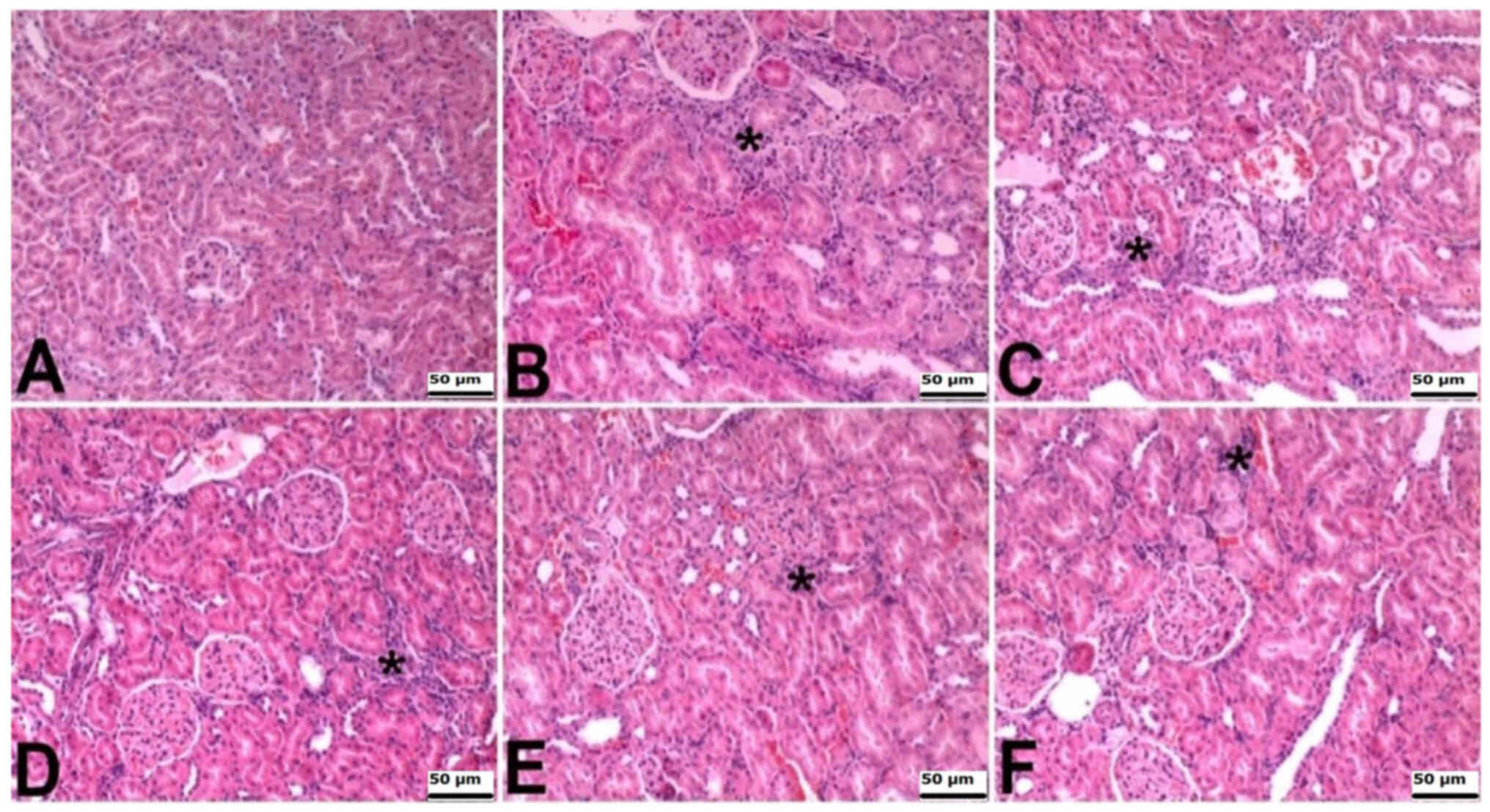
The kidneys of rats in the control group exhibited
normal histological features. Histopathologically, intertubular
areas in the other groups showed signs of interstitial nephritis,
attributed to mononuclear cell infiltrations. This microscopic
finding was severe in the CLP and CLP + BX1 groups, moderate in the
CLP + BX2 group, and mild in the CLP + BA1 and CLP + BA2 groups
(Table III; Fig. 2).
 | Table III.Statistical evaluation of
histopathological alterations in the kidney tissues. |
Table III.
Statistical evaluation of
histopathological alterations in the kidney tissues.
| Groups | Interstitial
nephritis |
|---|
| Control | 0 (0.00) |
| CLP | 3
(0.00)a |
| CLP + BX1 | 3
(0.25)a |
| CLP + BX2 | 2
(0.25)b |
| CLP + BA1 | 1
(0.00)c |
| CLP + BA2 | 1
(0.25)c |
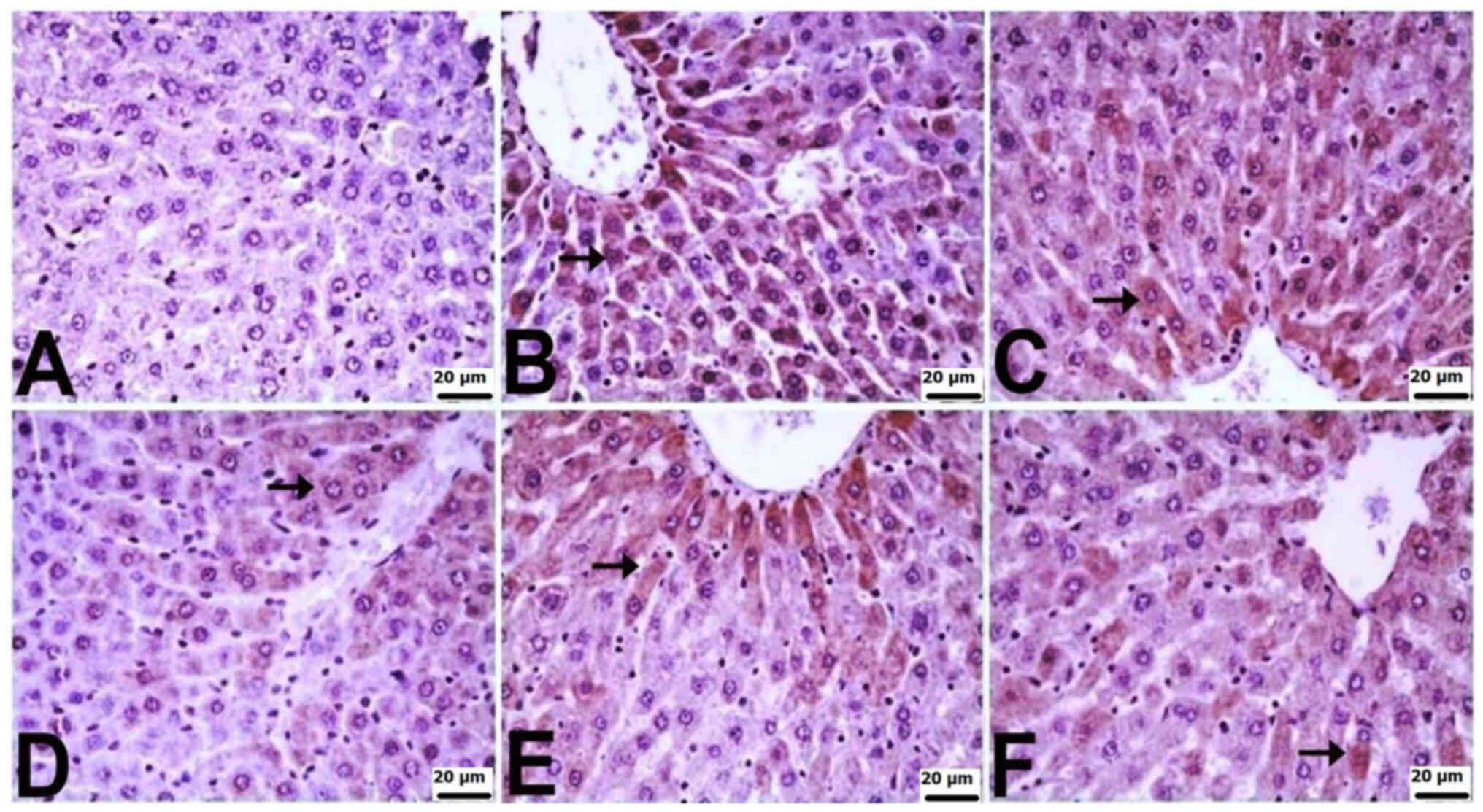
Immunohistochemical findings
Immunohistochemically, significant differences were
observed among the groups in terms of TNF-α, IL-6, and IL-10
staining in the liver and kidneys. In the liver tissues of control
rats, there was no notable immunopositivity in the staining for
TNF-α, IL-6 or IL-10. Varied levels of immunopositivity were
detected in the other treatment groups. For TNF-α staining, the CLP
group exhibited a very severe level, CLP + BX1 and CLP + BA1 groups
showed a severe level, and the CLP + BX2 and CLP + BA2 groups
displayed moderate immunopositivity. IL-6 staining revealed severe
immunopositivity in the CLP group, a mild level in the CLP + BX2
group, and moderate in the other groups. In terms of IL-10
staining, the CLP + BX2 and CLP + BA2 groups exhibited mild
immunopositivity, while the other groups showed moderate
immunopositivity. Positive immunohistochemical findings were
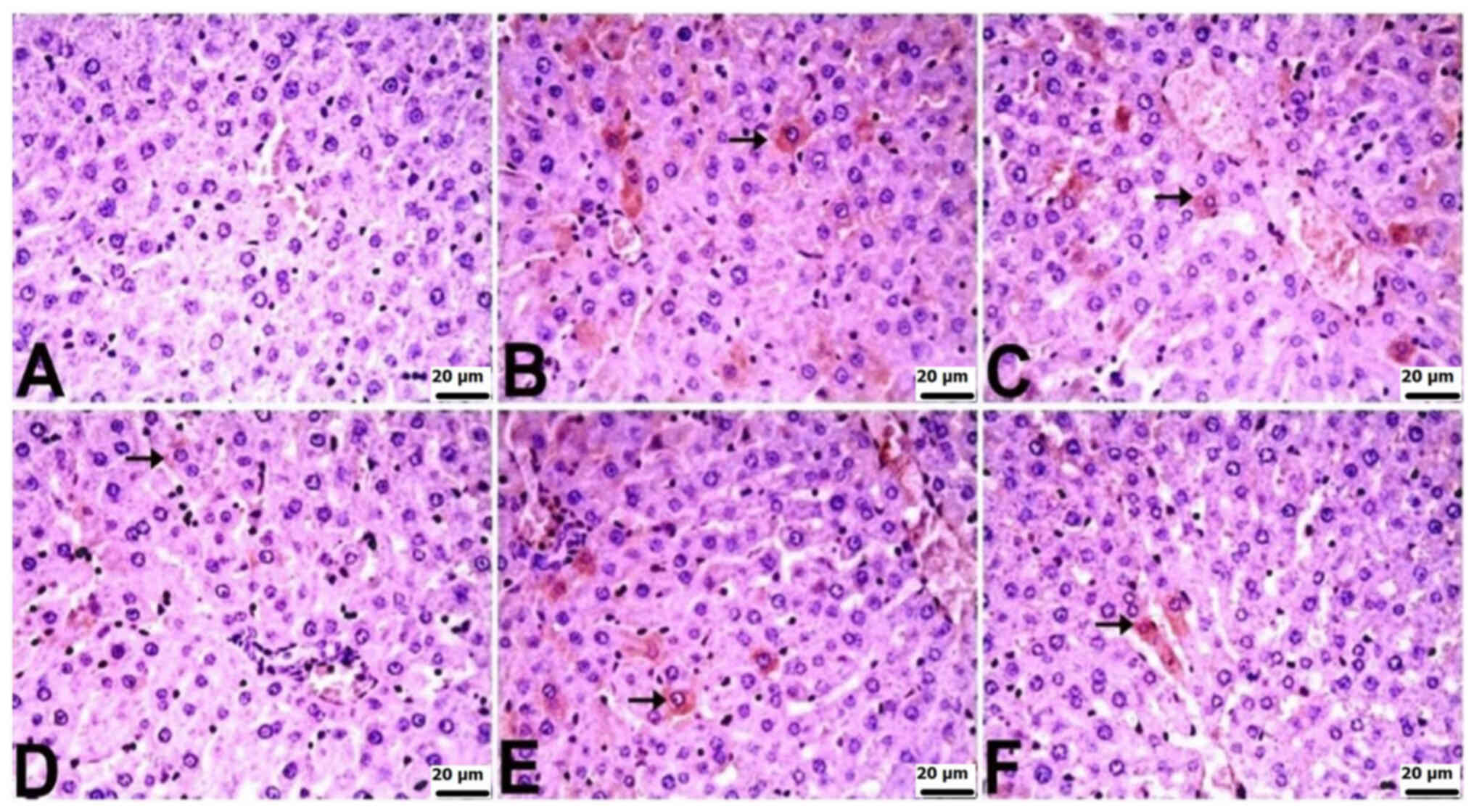
localized intracytoplasmically in hepatocytes (Table IV; Fig. 3, Fig.
4, Fig. 5).
 | Table IV.Statistical assessment of
immunopositivity in immunohistochemical staining conducted with
TNF-α, IL-6 and IL-10 in the liver tissues. |
Table IV.
Statistical assessment of
immunopositivity in immunohistochemical staining conducted with
TNF-α, IL-6 and IL-10 in the liver tissues.
| Groups | TNF-α | IL-6 | IL-10 |
|---|
| Control | 0 (1.00) | 0 (0.25) | 0 (0.00) |
| CLP | 4
(1.00)a | 3
(0.25)a | 2
(0.00)a |
| CLP + BX1 | 3
(0.25)b | 2
(0.00)b | 2
(0.00)a |
| CLP + BX2 | 2
(1.00)c | 1
(0.25)c | 1
(0.00)b |
| CLP + BA1 | 3
(1.00)b | 2
(0.00)b | 2
(0.25)a |
| CLP + BA2 | 2
(0.25)c | 2
(0.00)b |
1(0.25)b |
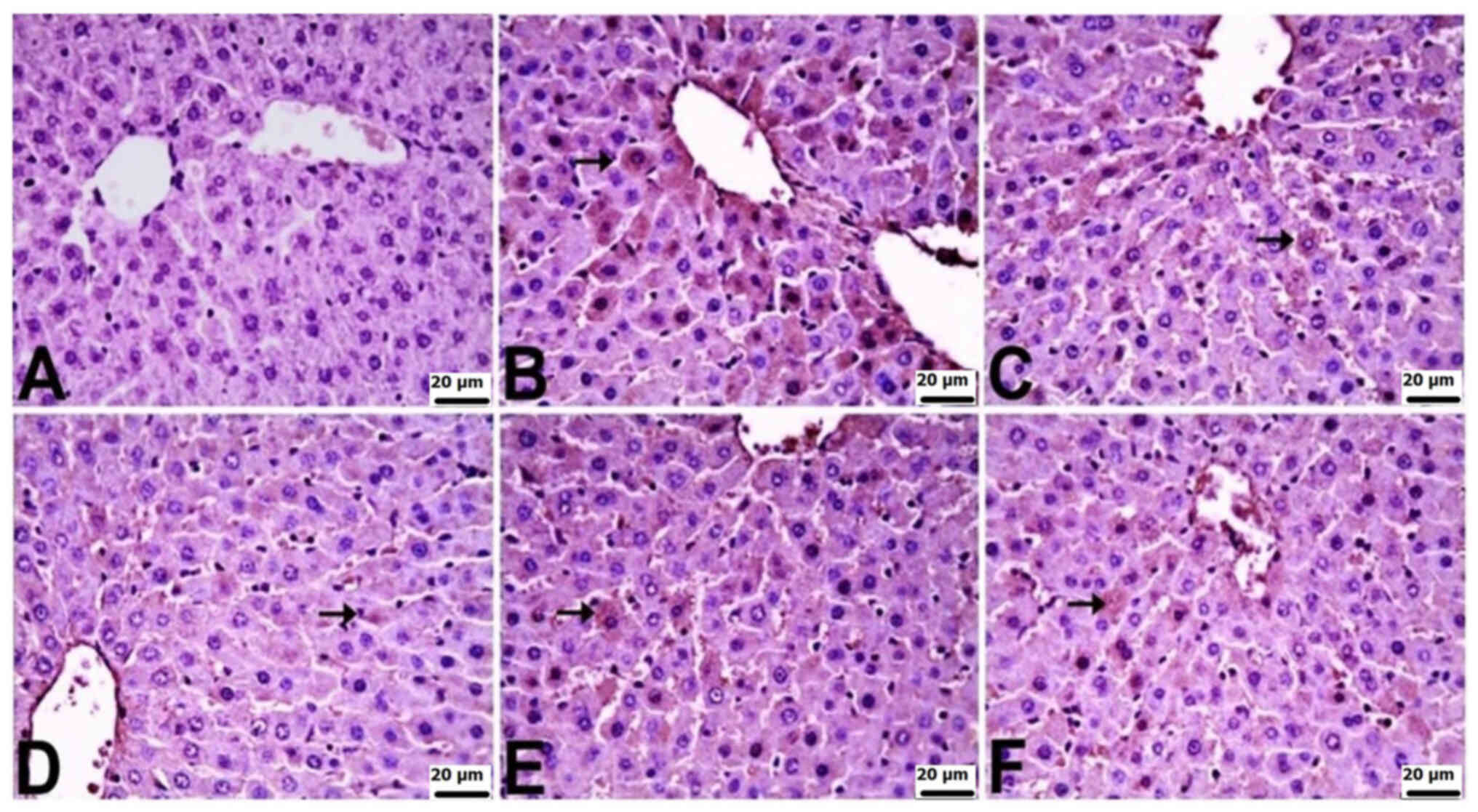
In the kidneys of control rats, there was no
significant immunopositivity in terms of the staining for TNF-α,
IL-6 or IL-10. Varied levels of immunopositivity were observed in
the other groups. TNF-α immunopositivity was severe in the CLP and
CLP + BX1 groups, moderate in the CLP + BX2 group, and mild in the
CLP + BA1 and CLP + BA2 groups. IL-6 immunopositivity was very
severe in the CLP group, severe in the CLP + BX1 and CLP + BX2
groups, and mild in the CLP + BA2 and CLP + BA1 groups. IL-10
immunopositivity was moderate in the CLP group and mild in all the
other groups. TNF-α and IL-10 immunopositivity was observed in
tubular epithelial cells, while IL-6 immunopositivity was observed
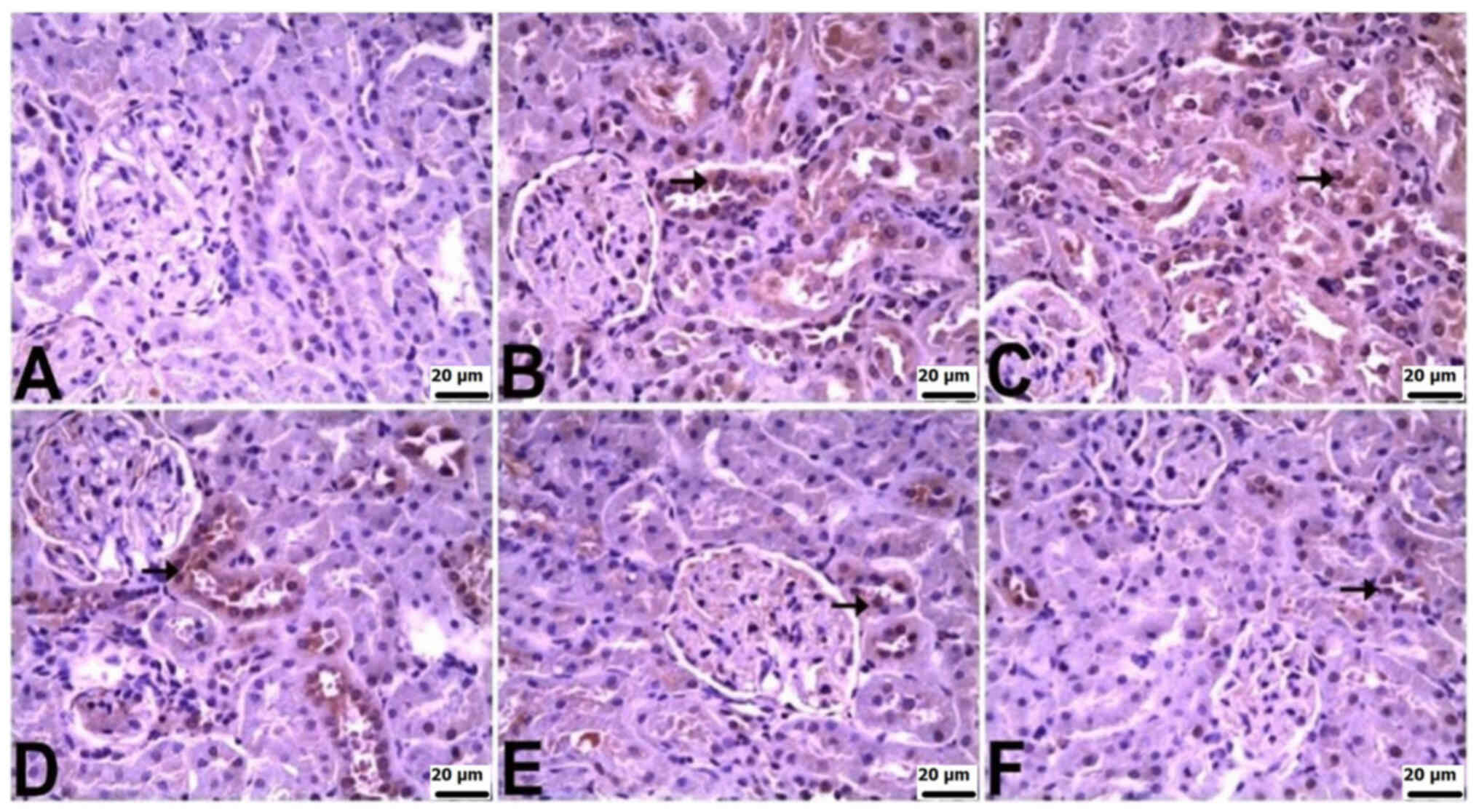
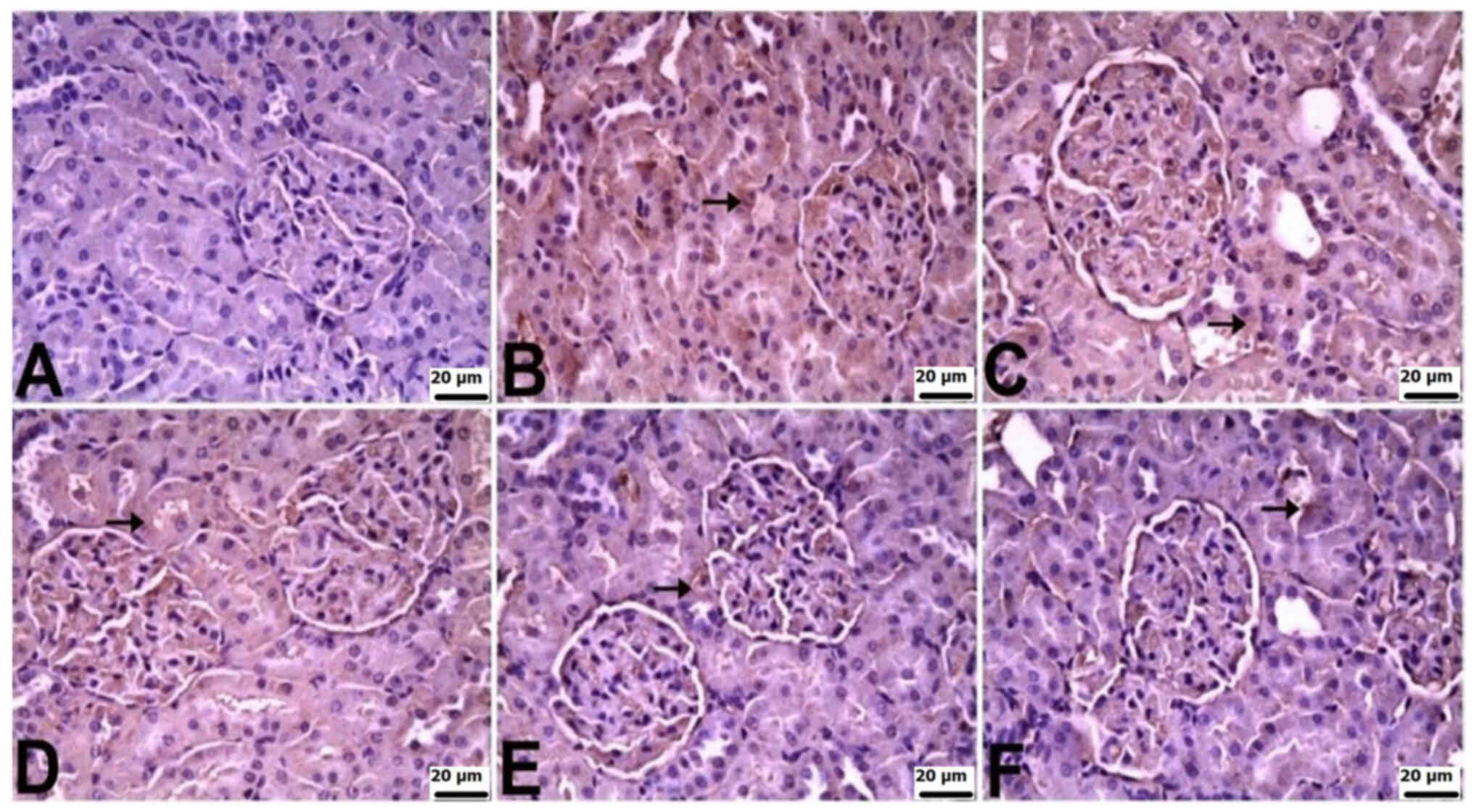
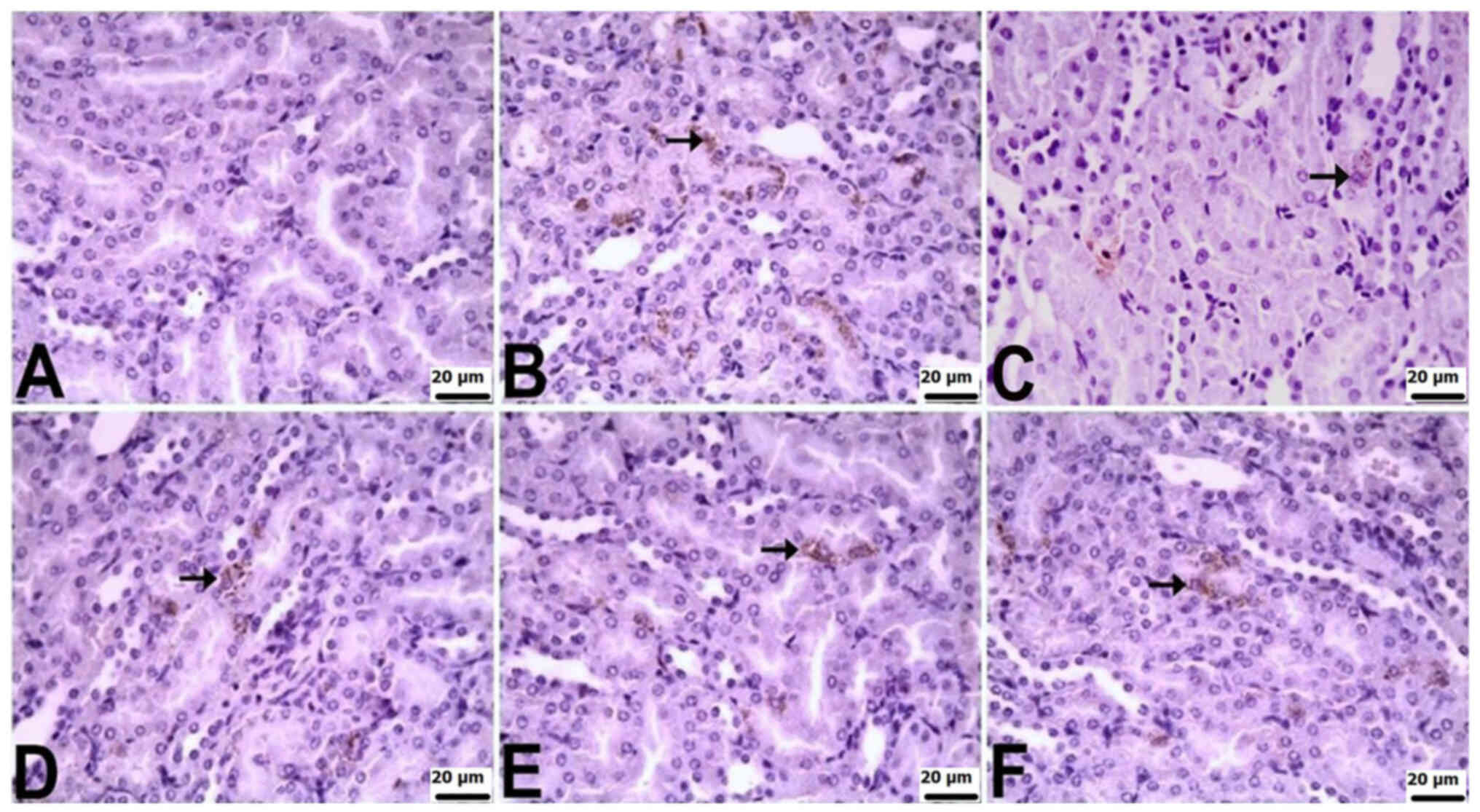
in both tubular epithelial cells and the glomerulus (Table V; Fig.
6, Fig. 7, Fig. 8).
 | Table V.Statistical examination of
immunopositivity in immunohistochemical staining conducted with
TNF-α, IL-6 and IL-10 in the kidney tissues. |
Table V.
Statistical examination of
immunopositivity in immunohistochemical staining conducted with
TNF-α, IL-6 and IL-10 in the kidney tissues.
| Groups | TNF-α | IL-6 | IL-10 |
|---|
| Control | 0 (0.25) | 0 (1.00) | 0 (0.00) |
| CLP | 3
(0.25)a | 4
(0.25)a | 2
(0.00)a |
| CLP + BX1 | 3
(0.25)a | 3
(0.00)b | 1
(0.00)b |
| CLP + BX2 | 2
(0.00)b | 3
(0.00)b | 1
(0.00)b |
| CLP + BA1 | 1
(0.00)c | 1
(0.25)c | 1
(0.00)b |
| CLP + BA2 | 1
(0.00)c | 1
(0.00)c | 1
(0.25)b |
Gene expression analysis
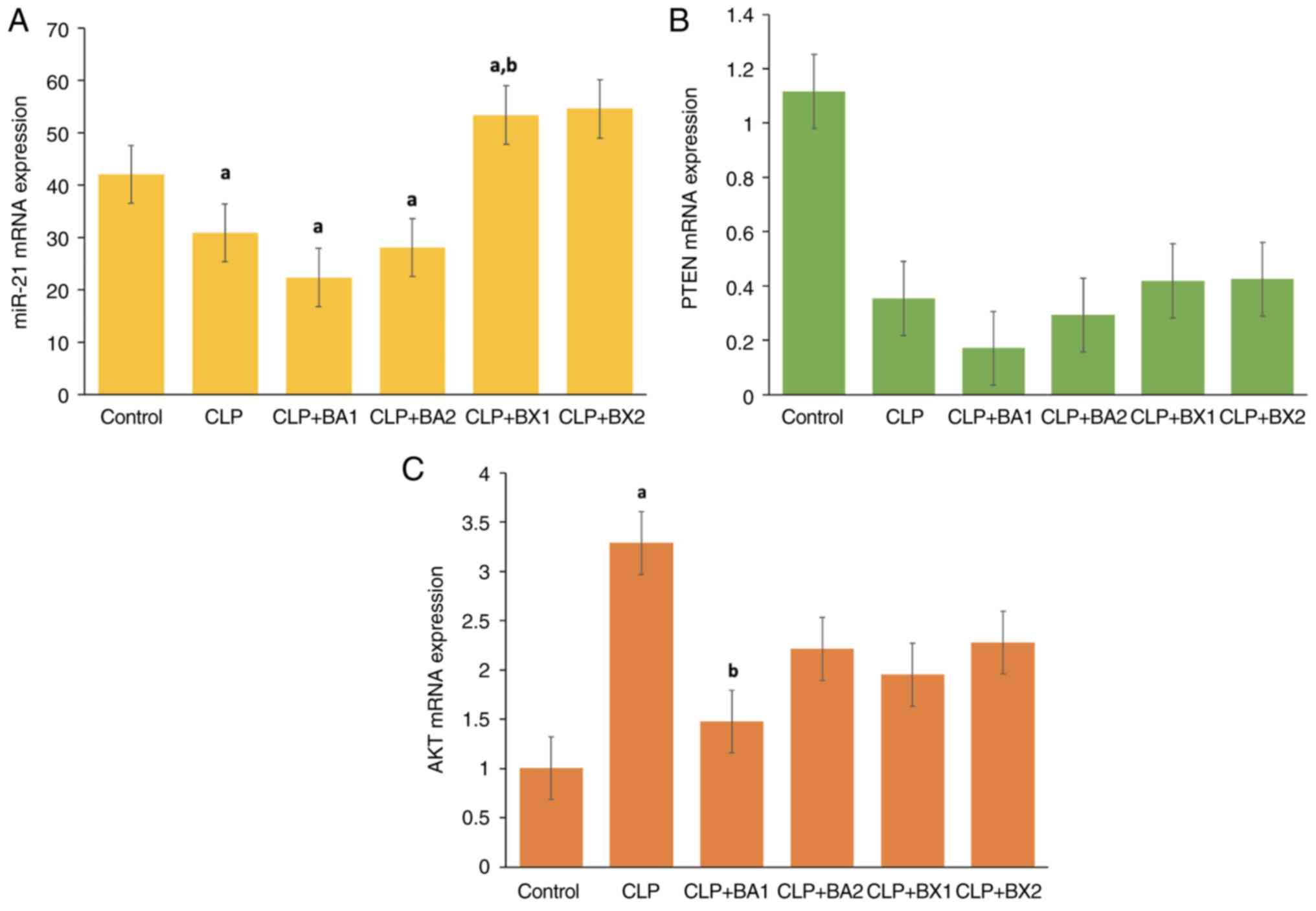
The changes in miR-21 expression levels in the liver
tissue between the control, CLP and various treatment groups are
presented in Fig. 9A. In the
control group, miR-21 expression levels were observed at baseline,
while a significant reduction was detected in the CLP group.
Similarly, in the CLP + BA1 and CLP + BA2 treatment groups, miR-21
expression levels remained low, showing a profile comparable to the
CLP group. By contrast, in the CLP + BX1 treatment group, a
significant increase in miR-21 expression levels was observed
compared with those in both the control and CLP groups The CLP +
BX2 treatment group also showed a tendency to increase miR-21
levels, but this increase was not statistically significant. These
findings indicated that in liver tissue, the CLP model may suppress
miR-21 expression, while BX1 treatment effectively reverses this
suppression. The enhancing effect of BX1 on miR-21 expression
suggests a potential regulatory role in inflammation and related
signaling pathways.
The mRNA expression levels of PTEN in the liver
tissue across the control, CLP and treatment groups are shown in
Fig. 9B. In the control group,
PTEN expression levels were observed to be the highest, indicating
its normal physiological activity under baseline conditions. By
contrast, the CLP group exhibited a marked reduction in PTEN
expression, suggesting a suppression of PTEN activity in response
to the CLP-induced inflammatory state. Notably, treatment with BA1
and BA2 did not significantly restore PTEN expression, as the
levels remained comparable to the CLP group; however, BX1 and BX2
treatments showed a tendency to increase PTEN expression, although
these changes were not statistically significant.
The mRNA expression levels of AKT in the liver
tissue across the control, CLP and treatment groups are shown in
Fig. 9C. In the control group, AKT
expression was observed at baseline levels. In the CLP group, a
significant increase in AKT expression was detected compared with
that in the control group, highlighting the activation of AKT in
response to the CLP-induced inflammatory process. By contrast,
treatment with BA1 resulted in a significant reduction in AKT
expression compared with in the CLP group, suggesting that BA1 may
suppress AKT activation. However, BA2, BX1 and BX2 treatments
showed no significant differences compared with the CLP group, with
AKT expression levels remaining elevated.
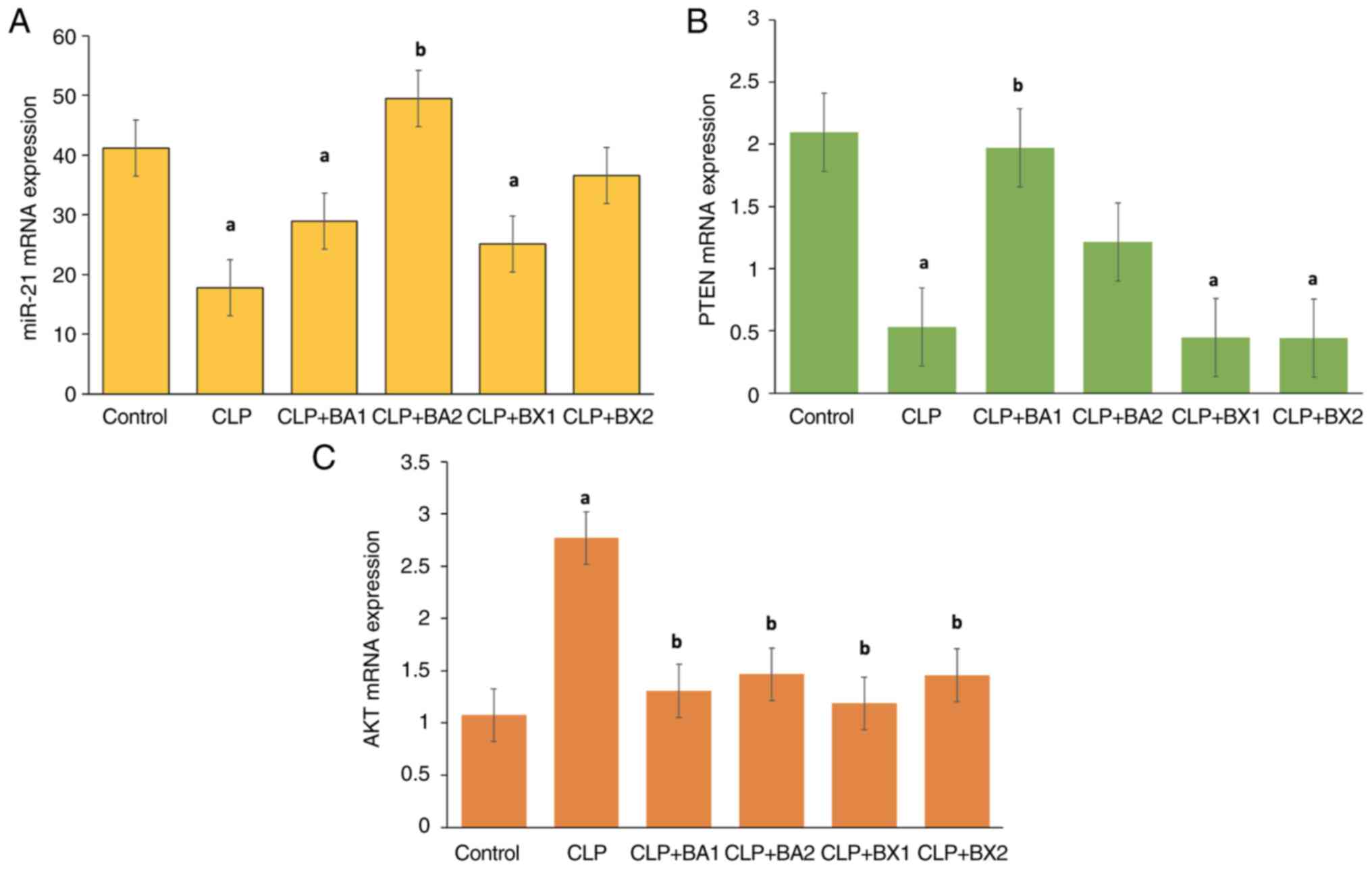
The expression levels of miR-21 in the kidney tissue
across the control, CLP and treatment groups are shown in Fig. 10A. While miR-21 expression levels
were observed at baseline in the control group, a statistically
significant decrease was detected in the CLP group compared with
those in the control group. Similarly, in the CLP + BA1 and CLP +
BX1 treatment groups, miR-21 levels remained lower than those in
the control group, indicating that these treatments failed to
restore miR-21 expression to baseline levels. By contrast, the CLP
+ BA2 group exhibited a statistically significant increase in
miR-21 expression compared with that in the CLP group. The CLP +
BX2 group demonstrated a moderate increase in miR-21 expression
compared with that in the CLP group; however, this change was not
statistically significant.
The mRNA expression levels of PTEN in the kidney
tissue across the control, CLP and treatment groups are shown in
Fig. 10B. In the control group,
PTEN expression levels were observed to be the highest. In the CLP
group, a significant decrease in PTEN expression was observed
compared with that in the control group, suggesting that
CLP-induced inflammation suppressed PTEN expression in kidney
tissue. The CLP + BA1 group exhibited a significant increase in
PTEN expression compared with that in the CLP group, indicating a
potential restorative effect of BA1 treatment. However, in the CLP
+ BX1 and CLP + BX2 groups, PTEN expression levels remained
significantly lower compared with those in the control group,
suggesting that these treatments were unable to restore PTEN
expression to baseline levels.
The mRNA expression levels of AKT in the kidney
tissue across the control, CLP and treatment groups are shown in
Fig. 10C. In the CLP group, a
significant increase in AKT expression was observed compared with
that in the control group; by contrast, the CLP + BA1, CLP + BA2,
CLP + BX1 and CLP + BX2 groups showed a significant reduction in
AKT expression compared with that in the CLP group, indicating that
these treatments effectively suppressed AKT activation.
Discussion
Sepsis is a complex clinical manifestation
characterized by insufficient oxygen delivery to tissues, driven by
processes that induce microcirculatory changes (38). In the early phases,
pro-inflammatory cytokines are released, initiating a robust
inflammatory reaction leading to microcirculatory modifications.
This disturbance affects cellular components, including endothelial
cells, vascular smooth muscle cells, erythrocytes, leukocytes,
platelets and parenchymal cells. Leukocyte activation amplifies
inflammation, triggering platelet activation, the coagulation
cascade and the complement system (39,40).
Cytokines, essential for cellular communication, are
small proteins synthesized by macro-phages and helper T
lymphocytes; they exert their effects not only on the secreting
cells but also through autocrine, paracrine and endocrine actions.
Typically released in a cascading manner, the stimulation of target
cells by one cytokine triggers the production of others in a
sequential cascade (41). In
sepsis, they contribute to immunopathological processes by
increasing the release of key inflammatory cytokines, such as
TNF-α, IL-1β, IL-6 and monocytic chemotactic protein (42). The resulting cytokine storm poses a
potential threat, as it establishes a feedback loop between
cytokines and immune cells. While the immune system combats the
pathogen, cytokines prompt lymphocytes and macrophages to migrate
toward the inflamed site, encouraging the production of effector
cytokines (43). The resulting
cytokine storm establishes a feedback loop between cytokines and
immune cells, potentially spiraling out of control, causing
localized hyperactivation of immune cells and damage to inflamed
organs. Circulating cytokines can also induce damage to distant
organs (44).
TNF-α, primarily produced by activated macrophages
but also synthesized by various cell types, (e.g., lymphocytes,
neutrophils, mast cells and eosinophils), serves as an acute phase
reactant and a signaling protein contributing to systemic
inflammation; its main function involves the regulation of immune
cells. IL-6, classified as a crucial mediator in the inflammatory
response alongside other pro-inflammatory cytokines, such as IL-1
and TNF-α, is predominantly synthesized by fibroblasts, endothelial
cells and monocytes (45).
Originally termed a ‘cytokine synthesis inhibitory factor’, IL-10
functions by inhibiting the gene expression and synthesis of
proinflammatory cytokines in macrophages, as well as T cell
cytokines. Additionally, IL-10 suppresses the function of
antigen-presenting cells (46).
Research data suggests that boron compounds (e.g.,
BA) could influence the synthesis of cytokines responsible for
regulating reactive oxygen species (ROS) (47). Furthermore, BA is proposed to
exhibit antioxidant characteristics by neutralizing protons on
oxidative molecules. While BA actively participates in
anti-inflammatory mechanisms, its specific advantages in the
context of sepsis remain to be fully elucidated (48). Boron compounds play a role in
modulating cell membrane functions, influencing transmembrane
signaling and regulating the movement of ions. Additionally, they
may serve as metabolic regulators in certain enzymatic systems. By
elevating the levels of reduced glutathione, boron compounds
inhibit the generation of ROS and apoptosis, thereby mitigating the
impact of oxidative damage in the body (49,50).
Research has demonstrated the hepatoprotective effects of boron
compounds in countering carbon tetrachloride-induced liver
degeneration (51). Another study
underscored the protective capabilities of these compounds against
damage induced by cyclophosphamide (52). In the present study, within the
liver tissue, the CLP + BA2 group exhibited reduced expression
levels of TNF-α and IL-6, classified as pro-inflammatory cytokines,
whereas the level of IL-10, an anti-inflammatory cytokine,
demonstrated a noteworthy increase in comparison with the control
group. The histopathological evaluation of the liver in this
particular group revealed lower score points indicative of necrosis
and mononuclear cell infiltration compared with the other treatment
groups. Similarly for BA administration, but for the kidney
analysis, both the CLP + BA1 and CLP + BA2 groups exhibited a
reduction in TNF-α and IL-6 levels, along with an increase in IL-10
levels. The histopathological assessment of these groups indicated
a lower score for interstitial nephritis compared with that in the
other treatment groups. The synthesis of diverse miRNAs, triggered
by inflammation, governs the expression of molecules responsible
for regulating the inflammatory process. Notably, miR-21, a highly
expressed miRNA in numerous mammalian cells, orchestrates
anti-inflammatory responses (53).
Research indicates that miR-21 plays a crucial role in resolving
inflammation by engaging in the negative feedback mechanism within
inflammatory pathways. It has been proposed that overexpression of
miR-21 in macro-phages leads to a reduction in TNF-α and IL-6
secretion, coupled with an increase in IL-10 production,
underscoring the multifaceted nature of miR-21 in immune modulation
(54). These findings also
highlight the anti-inflammatory activity of miR-21, demonstrating
its impact on the toll-like receptor 4-nuclear factor-κB (NF-κB)
pathway and subsequent reduction in lipopolysaccharide
(LPS)-induced inflammatory responses in macrophages. Due to its
association with inflammation suppression and organ protection,
miR-21 holds potential as a predictive marker for a decreased risk
of sepsis (54). In the present
study, the miR-21 levels were significantly increased in the liver
tissues of the CLP + BX1 and CLP + BX2 groups (not significant),
and in the kidney tissues of the CLP + BA2 group, compared with the
control group. The marked increase of miR-21 levels in the liver
for the CLP + BX1 and CLP + BX2 groups, relative to the CLP group,
was associated with decreased TNF-α and IL-6 levels, along with
increased IL-10 levels. Additionally, the CLP + BA2 group exhibited
similar effects in the kidney tissue.
The PTEN gene, recognized as phosphatase and tensin
homolog deleted on chromosome 10, interferes with diverse cellular
processes, such as cellular growth, proliferation and movement, by
counteracting PI3K. Further comprehensive exploration is required
to understand the means through which PTEN expression is controlled
(55). Research conducted by Kim
et al (56) revealed that
exposing cancer cell lines to TNF-α results in the reduction of
PTEN expression, while TNF-α/NF-κB promotes the conveyance of the
TNF-α signal, leading to the activation of AKT. The downregulation
of PTEN by NF-κB underscores its involvement in regulating
PI3K/AKT, given that TNF-α/TNF receptor also conveys signals
through the activation of the PI3K/AKT pathway (56). Studies have demonstrated heightened
baseline levels of AKT phosphorylation in the tumors of mice
lacking PTEN. The present study findings demonstrated that PTEN
expression was markedly diminished in the liver and kidney tissues
in the CLP group. However, notably, it was observed that the PTEN
expression within the kidney tissue of the CLP + BA1 group was at a
similar level compared with that of the control group.
PI3K, where the primary downstream kinase is AKT,
constitutes a widely distributed family of protein kinases engaged
in signal transduction. This intricate process is mediated through
receptor tyrosine kinases or G protein-coupled receptors, which
include TNF-α receptors (57). In
macrophages, the activation of PI3K responds to LPS, setting off a
series of coordinated events that consecutively activate AKT,
resulting in its phosphorylation. This phosphorylation, in a
cascading fashion, triggers the activation of various downstream
targets, orchestrating diverse cellular functions such as nuclear
factor-κB or PI3K/AKT pathways (58,59).
In the present study, within the kidney tissues, the CLP group
exhibited elevated AKT gene expression levels in comparison with
the control group. However, there was no alteration observed in the
treatment groups. In the liver tissues, both the CLP and treatment
groups displayed a notable rise in AKT levels compared with the
control group. Nevertheless, within the treatment groups, AKT
levels experienced a substantial reduction in comparison with those
in the CLP group. Within these treatment groups, there was a
notable reduction in TNF-α and IL-6 levels compared with the CLP
group, accompanied by an increase in IL-10 levels.
In the present study, the primary emphasis was on
assessing the impact of the natural compounds BA and BX on
pro-inflammatory and anti-inflammatory parameters within the liver
and kidney tissues, recognized as target organs in the CLP-induced
sepsis model. It is worth mentioning that despite the accumulated
evidence as to their mechanisms of action and despite being present
in >400 different products, such as fertilizers, pesticides,
cosmetics, medicines, food supplements, cleaning agents and
personal care items, boron compounds have not undergone widespread
clinical evaluation due to misconceptions about potential serious
toxic effects. Recent research has debunked these concerns,
revealing that boron compounds do not induce toxicity in healthy
cells (28,29,60).
The present results further support the notion that boron compounds
could be further explored with regard to their pharmacological
actions against inflammatory processes.
As our understanding of the anti-inflammatory
properties of these compounds is limited, it becomes essential to
unveil the mechanisms underlying their anti-inflammatory activity.
In the present investigation, in liver tissue, the CLP + BX1 and
CLP + BX2 groups exhibited elevated miR-21 expression; whereas in
kidney tissue, only the CLP + BA2 group exhibited elevated miR-21
expression. Additionally, these groups showed the downregulation of
PTEN expression and the upregulation of AKT expression. The
findings led to the conclusion that, despite a decrease in TNF-α
and IL-6 levels, there was a notable increase in miR-21 levels
within this group when compared with those in the sepsis group.
While these results align with each other, more intricate analyses
are required to elucidate the mechanism in greater detail.
The present study has several limitations that
should be acknowledged. Firstly, while the use of boron compounds,
such as BA and BX has shown promising anti-inflammatory effects in
sepsis-induced liver and kidney damage in a rat model, these
findings are limited to preclinical settings and may not directly
translate to clinical applications. Secondly, the present study
focused on specific pathways and biomarkers, such as
miR-21/PTEN/AKT, which does not encompass the full spectrum of
potential inflammatory and molecular interactions that may occur in
sepsis. Furthermore, the relatively short experimental duration of
24 h post-sepsis induction restricts the evaluation of long-term
effects and potential recovery outcomes. Finally, although efforts
were made to standardize treatment dosages and conditions,
individual variability among the animal models could not be
entirely accounted for. Future research should include a broader
range of experimental conditions, prolonged observation periods,
and clinical trials to validate these findings and explore the
detailed mechanisms of boron compounds in mitigating sepsis-related
organ damage. Despite the limitations, the present investigation
underscores the necessity for further research into the potential
benefits of using a low dose of BA to alleviate kidney damage
caused by sepsis.
Given that the early release of pro-inflammatory
cytokines during sepsis initiates a robust inflammatory response,
leading to microcirculation alterations, the prompt organization
and management of this cytokine storm are imperative. Boron
compounds have recently gained popularity across various fields.
The present study demonstrates the notable anti-inflammatory
effects of boron compounds in rats with induced sepsis. BA, in
particular, showcased promising results in mitigating kidney
damage. Immunohistochemical analyses revealed a significant
reduction in pro-inflammatory markers (TNF-α and IL-6) and an
increase in the anti-inflammatory marker IL-10. These findings
suggest the potential of BA as a potential new agent to alleviate
sepsis-induced inflammatory responses, proposing the need for
further research. The present study underscores the need for
detailed investigations into the underlying mechanisms and supports
exploring the use of low-dose BA and BX for managing sepsis-induced
organ damage. Overall, the results contribute valuable insights to
the understanding of the pharmacological actions of boron compounds
in inflammatory conditions.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CS and ATs conceptualized and designed the study.
MO, MK, SG, ASM and YY engaged in the acquisition, analysis and
interpretation of the data. ATa, CS, MS and SVK contributed to the
interpretation of the data, along with manuscript drafting and
finalization. MS, ATa, EO and DAS were involved in the
interpretation of the data and in critical revisions of the
intellectual content. CS, DAS and ATs confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study complied with the ethical guidelines
approved by the ethical committee, and all procedures involving
animal research were conducted in accordance with Directives
2010/63/EU, which regulate the protection and welfare of animals
used for scientific purposes within the European Union. Approval
for these procedures was granted by the Ethics Committee at
Kastamonu University (approval no. 28/4). The study also complied
with ARRIVE guidelines and the AVMA euthanasia guidelines 2020.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Singer M, Deutschman CS, Seymour C,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rubulotta FM, Ramsay G, Parker MM,
Dellinger RP, Levy MM and Poeze M; Surviving Sepsis Campaign
Steering Committee; European Society of Intensive Care Medicine;
Society of Critical Care Medicine, : An international survey:
Public awareness and perception of sepsis. Crit Care Med.
37:167–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guarino M, Perna B, Cesaro AE, Maritati M,
Spampinato MD, Contini C and De Giorgio R: 2023 Update on sepsis
and septic shock in adult patients: Management in the emergency
department. J Clin Med. 12:31882023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
WHO, . Global report on the epidemiology
and burden of sepsis: Current evidence identifying gaps and future
directions. World Health Organization; Geneva: 2020
|
|
5
|
Chiu C and Legrand M: Epidemiology of
sepsis and septic shock. Curr Opin Anaesthesiol. 34:71–76. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vakkalanka JP, Harland KK, Swanson MB and
Mohr NM: Clinical and epidemiological variability in severe sepsis:
An ecological study. J Epidemiol Community Health. 72:741–745.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caraballo C and Jaimes F: Organ
dysfunction in sepsis: An ominous trajectory from infection to
death. Yale J Biol Med. 92:629–640. 2019.PubMed/NCBI
|
|
8
|
Canabal JM and Kramer DJ: Management of
sepsis in patients with liver failure. Curr Opin Crit Care.
14:189–197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jarrar D, Wang P and Chaudry IH:
Hepatocellular dysfunction-basic considerations. Holzheimer RG and
Mannick JA: Surgical Treatment: Evidence-Based and Problem-Oriented
Munich, Zuckschwerdt: 2001, PubMed/NCBI
|
|
10
|
Yan J and Li S and Li S: The role of the
liver in sepsis. Int Rev Immunol. 33:498–510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bagshaw SM, Uchino S, Bellomo R, Morimatsu
H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, et al:
Septic acute kidney injury in critically ill patients: Clinical
characteristics and outcomes. Clin J Am Soc Nephrol. 2:431–439.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khwaja A: KDIGO clinical practice
guidelines for acute kidney injury. Nephron Clin Pract.
120:c179–c184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma S, Evans RG, Iguchi N, Tare M,
Parkington HC, Bellomo R, May CN and Lankadeva YR: Sepsis-induced
acute kidney injury: A disease of the microcirculation.
Microcirculation. 26:e124832019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nedeva C, Menassa J and Puthalakath H:
Sepsis: Inflammation is a necessary evil. Front Cell Dev Biol.
7:1082019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sprague AH and Khalil RA: Inflammatory
cytokines in vascular dysfunction and vascular disease. Biochem
Pharmacol. 78:539–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Simmons J and Pittet JF: The coagulopathy
of acute sepsis. Curr Opin Anaesthesiol. 28:227–236. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang YY and Ning BT: Signaling pathways
and intervention therapies in sepsis. Signal Transduct Target Ther.
6:4072021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou S, Sun Y, Zhao K, Gao Y, Cui J, Qi L
and Huang L: miR-21/PTEN pathway mediates the cardioprotection of
geniposide against oxidized low-density lipoprotein-induced
endothelial injury via suppressing oxidative stress and
inflammatory response. Int J Mol Med. 45:1305–1316. 2020.PubMed/NCBI
|
|
19
|
Das K and Rao LVM: The role of microRNAs
in inflammation. Int J Mol Sci. 23:154792022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ge J, Yao Y, Jia H, Li P and Sun W:
Inhibition of miR-21 ameliorates LPS-induced acute lung injury
through increasing B cell lymphoma-2 expression. Innate Immun.
26:693–702. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Formosa A, Turgeon P and Dos Santos CC:
Role of miRNA dysregulation in sepsis. Mol Med. 28:992022.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Melo P, Pineros Alvarez AR, Ye X,
Blackman A, Alves-Filho JC, Medeiros AI, Rathmell J, Pua H and
Serezani CH: Macrophage-derived MicroRNA-21 drives overwhelming
glycolytic and inflammatory response during sepsis via repression
of the PGE2/IL-10 axis. J Immunol. 207:902–912. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu D, Dong J, Li P, Tang C, Cheng W, Xu Z,
Zhou W, Ge J, Xia C and Zhang Z: MiRNA-21 has effects to protect
kidney injury induced by sepsis. Biomed Pharmacother. 94:1138–1144.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Acosta-Martinez M and Cabail MZ: The
PI3K/Akt pathway in meta-inflammation. Int J Mol Sci. 23:153302022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vidotto T, Melo CM, Castelli E, Koti M,
Dos Reis RB and Squire JA: Emerging role of PTEN loss in evasion of
the immune response to tumours. Br J Cancer. 122:1732–1743. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Turkez H, Arslan ME, Tatar A and
Mardinoglu A: Promising potential of boron compounds against
Glioblastoma: In vitro antioxidant, anti-inflammatory and
anticancer studies. Neurochem Int. 149:1051372021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yıldız K, Makav M, Adalı Y and Bulut M:
Therapeutic effects of boric acid in a septic arthritis model
induced by escherichia coli in rats. Biol Trace Elem Res.
200:4762–4770. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bolt HM, Başaran N and Duydu Y: Effects of
boron compounds on human reproduction. Arch Toxicol. 94:717–724.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hadrup N, Frederiksen M and Sharma AK:
Toxicity of boric acid, borax and other boron containing compounds:
A review. Regul Toxicol Pharmacol. 121:1048732021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Richold M: Boron exposure from consumer
products. Biol Trace Elem Res. 66:121–129. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nielsen FH and Meacham SL: Growing
evidence for human health benefits of boron. J Evid Based
Complement Altern Med. 16:169–180. 2011. View Article : Google Scholar
|
|
32
|
Chen S, Huang J, Liu T, Zhang F, Zhao C,
Jin E and Li S: PI3K/Akt signaling pathway mediates the effect of
low-dose boron on barrier function, proliferation and apoptosis in
rat intestinal epithelial cells. Sci Rep. 14:3932024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang C, Jin E, Deng J, Pei Y, Ren M, Hu Q,
Gu Y and Li S: GPR30 mediated effects of boron on rat spleen
lymphocyte proliferation, apoptosis, and immune function. Food Chem
Toxicol. 146:1118382020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wen H: Sepsis induced by cecal ligation
and puncture. Methods Mol Biol. 1031:117–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hubbard WJ, Choudhry M, Schwacha MG, Kerby
JD, Rue LW III, Bland KI and Chaudry IH: Cecal ligation and
puncture. Shock. 24 (Suppl 1):S52–S57. 2005. View Article : Google Scholar
|
|
36
|
Solbach P, Potthoff A, Raatschen HJ,
Soudah B, Lehmann U, Schneider A, Gebel MJ, Manns MP and Vogel A:
Testosterone-receptor positive hepatocellular carcinoma in a
29-year old bodybuilder with a history of anabolic androgenic
steroid abuse: A case report. BMC Gastroenterol. 15:602015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sygitowicz G and Sitkiewicz D: Molecular
mechanisms of organ damage in sepsis: An overview. Braz J Infect
Dis. 24:552–560. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ince C: The microcirculation is the motor
of sepsis. Crit Care. 9 (Suppl 4):S13–S19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Legrand M, Klijn E, Payen D and Ince C:
The response of the host microcirculation to bacterial sepsis: Does
the pathogen matter? J Mol Med (Berl). 88:127–133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Blackwell TS and Christman JW: Sepsis and
cytokines: Current status. Br J Anaesth. 77:110–117. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oppenheim JJ: Cytokines: Past, present,
and future. Int J Hematol. 74:3–8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ono S, Tsujimoto H, Hiraki S and Aosasa S:
Mechanisms of sepsis-induced immunosuppression and immunological
modification therapies for sepsis. Ann Gastroenterol Surg.
2:351–358. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Matsuda N and Hattori Y: Systemic
inflammatory response syndrome (SIRS): Molecular pathophysiology
and gene therapy. J Pharmacol Sci. 101:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tekeli H, Ekren Asıcı GS and Bildik A:
Anti-inflammatory effect of boric acid on cytokines in
ovariectomy-induced rats. Cell Mol Biol (Noisy-le-grand).
67:313–320. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Oberholzer A, Oberholzer C and Moldawer
LL: Interleukin-10: A complex role in the pathogenesis of sepsis
syndromes and its potential as an anti-inflammatory drug. Crit Care
Med. 30 (1 Suppl):S58–S63. 2002. View Article : Google Scholar
|
|
47
|
Baker SJ, Ding CZ, Akama T, Zhang YK,
Hernandez V and Xia Y: Therapeutic potential of boron-containing
compounds. Future Med Chem. 1:1275–1288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Romero-Aguilar KS, Arciniega-Martínez IM,
Farfán-García ED, Campos-Rodríguez R, Reséndiz-Albor AA and
Soriano-Ursúa MA: Effects of boron-containing compounds on immune
responses: Review and patenting trends. Expert Opin Ther Pat.
29:339–351. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bolat I, Kapakin KAT, Kirman EM, Gundogdu
G, Gundogdu K, Miloglu FD and Tasci SY: Investigation of the
effects of boric acid used in the treatment of rat models with knee
osteoarthritis induced by monosodium iodoacetate on liver tissue.
Harran Üniv Vet Fak Derg. 12:202–208. 2023.
|
|
50
|
Soriano-Ursúa MA, Das BC and
Trujillo-Ferrara JG: Boron-containing compounds: Chemico-biological
properties and expanding medicinal potential in prevention,
diagnosis and therapy. Expert Opin Ther Pat. 24:485–500. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ince S, Keles H, Erdogan M, Hazman O and
Kucukkurt I: Protective effect of boric acid against carbon
tetrachloride-induced hepatotoxicity in mice. Drug Chem Toxicol.
35:285–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ince S, Kucukkurt I, Demirel HH, Acaroz
DA, Akbel E and Cigerci IH: Protective effects of boron on
cyclophosphamide induced lipid peroxidation and genotoxicity in
rats. Chemosphere. 108:197–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nara K, Kawashima N, Noda S, Fujii M,
Hashimoto K, Tazawa K and Okiji T: Anti-inflammatory roles of
microRNA 21 in lipopolysaccharide-stimulated human dental pulp
cells. J Cell Physiol. 234:21331–21341. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Feng J, Li A, Deng J, Yang Y, Dang L, Ye
Y, Li Y and Zhang W: miR-21 attenuates lipopolysaccharide-induced
lipid accumulation and inflammatory response: Potential role in
cerebrovascular disease. Lipids Health Dis. 13:272014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Guo D and Donner DB: Tumor necrosis factor
promotes phosphorylation and binding of insulin receptor substrate
1 to phosphatidylinositol 3-kinase in 3T3-L1 adipocytes. J Biol
Chem. 271:615–618. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kim S, Domon-Dell C, Kang J, Chung DH,
Freund JN and Evers BM: Down-regulation of the tumor suppressor
PTEN by the tumor necrosis factor-alpha/nuclear factor-kappaB
(NF-kappaB)-inducing kinase/NF-kappaB pathway is linked to a
default IkappaB-alpha autoregulatory loop. J Biol Chem.
279:4285–4291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Puri KD, Doggett TA, Huang CY, Douangpanya
J, Hayflick JS, Turner M, Penninger J and Diacovo TG: The role of
endothelial PI3Kgamma activity in neutrophil trafficking. Blood.
106:150–157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yasuda T: Hyaluronan inhibits Akt, leading
to nuclear factor-κB down-regulation in
lipopolysaccharide-stimulated U937 macrophages. J Pharmacol Sci.
115:509–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Huang JB, Ding Y, Huang DS, Liang AJ, Zeng
WK, Zeng ZP, Qin CQ and Barden B: Inhibition of the PI3K/AKT
pathway reduces tumor necrosis factor-alpha production in the
cellular response to wear particles in vitro. Artif Organs.
37:298–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Scientific Committee on Consumer Safety
(SCCS), . Opinion of the boron compounds. SCCS, 2010. https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_027.pdf
|