Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic
progressive fibrosing pneumonia of the lung with no known etiology
or pathogenesis. The disease is characterized by a damaged alveolar
network that is gradually replaced with fibrous scars, resulting in
lung deformity and organ failure. It is a fatal disease that kills
patients within 2–5 years of diagnosis. The 5-year survival rate is
20% (1). The most common symptoms
of IPF include dyspnea, persistent cough and fatigue. However,
these symptoms are not specific to IPF and can be found in other
lung conditions, such as chronic obstructive pulmonary disease,
pulmonary tuberculosis and bronchiectasis, making diagnosis
difficult due to lack of definitive diagnostic indicators. Thus,
these challenges necessitate the identification of associated genes
to develop diagnostic models and targeted therapies.
Disrupted RNA methylation and its associated
downstream signaling pathways contribute to development and
progression of various diseases, such as gastric (2) and breast cancer (3). Several types of RNA methylation have
been discovered, including N6-methyladenosine (m6A),
7-methylguanosine and 5-methylcytosine (m5C), the latter being one
of the most common modifications of RNA (4,5).
Using regulatory genes involved in RNA methylation, prognostic
signatures can be created. Studies (6,7) have
been conducted to establish prognostic signatures using RNA
methylation regulatory genes for cancer. Yi et al (6) identified a prognostic signature for
head and neck squamous cell carcinoma based on m6A regulatory
genes. Furthermore, Huang et al (7) developed a m5C-related signature to
predict prognosis in cutaneous melanoma. RNA m5C methylation
modification serves a role in the regulation of several cancers,
including lung, gastric, liver, bladder, prostate and breast
cancer, influencing their development, occurrence and invasive
behavior (8–13). Altered m5C methylation levels can
contribute to tumor progression and a poor prognosis in cancers.
Understanding of the role of RNA methylation in IPF remains
limited. However, research (14–16)
in this area is expanding and yielding promising results about the
potential consequences of altered RNA methylation in diverse
pathologies. Further research is required to confirm and validate
previous findings about this disease. The present study aimed to
identify and validate the function of m5C-associated genes in IPF
diagnosis and typing.
Recent research by Zhou et al (14) using the GSE150910 dataset
investigated the role of m5C regulatory factor in IPF diagnosis;
m5C regulatory factor mediates RNA methylation modification
patterns and immune microenvironment infiltration characteristics,
implying that this genetic factor may be used as an immunotherapy
agent. The present study used data from patients with IPF from the
Gene Expression Omnibus (GEO) to investigate the relationship
between m5C-associated genes and the occurrence of IPF. The present
study created a bleomycin (BLM)-induced IPF mouse model to validate
these findings. The present results may serve as a foundation for
future research into development and progression of IPF, as well as
the search for new and effective therapeutic targets for PF
treatments such as immunotherapy.
Materials and methods
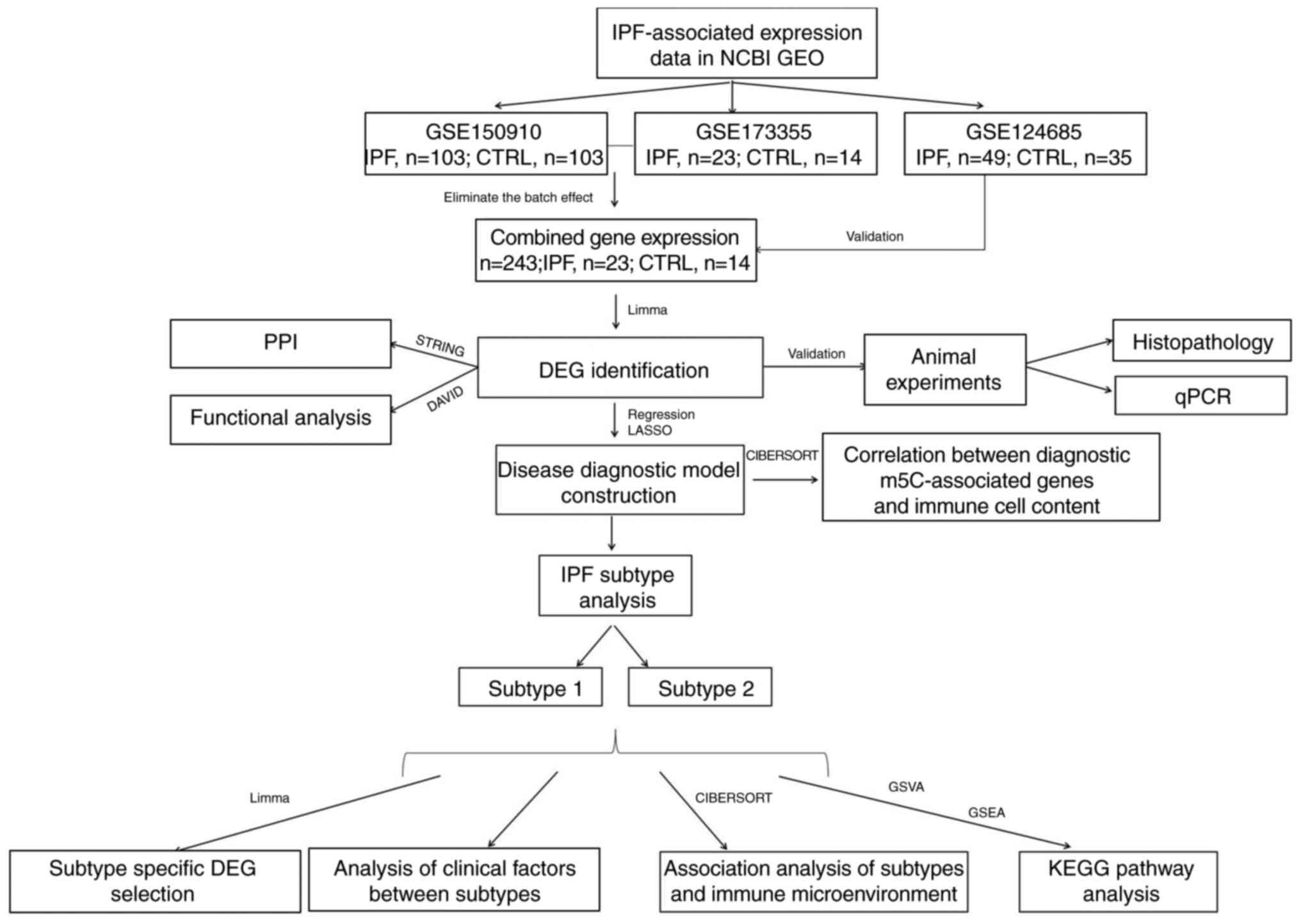
Data collection and processing
A total of three datasets, GSE150910, GSE173355 and
GSE124685 were chosen from the GEO database (hncbi.nlm.nih.gov/geo/). The GSE150910 dataset
included 288 lung tissue samples, with clinical data for 103 IPF
and 103 normal control (CTRL) samples. GSE173355 dataset contained
37 lung tissue samples, including 23 IFP and 14 CTRL samples. Both
datasets had read count data from whole genome detection downloaded
using the detection platform [GPL24676 Illumina NovaSeq 6000
(Homo sapiens)]. Finally, the GSE124685 dataset included 84
lung tissue samples, with clinical data for 49 IPF and 35 CTRL
samples. Fragments per kilobase of exon model per million mapped
fragments data from genome-wide detection was downloaded via the
detection platform [GPL17303 Ion Torrent Proton (H.
sapiens)]. The training datasets were GSE150910 and GSE173355,
while the validation dataset was GSE124685. Because GSE150910 and
GSE173355 contained different batches of gene expression data, sva
package (version 3.38.0) in R3.6.1 (17) was used to eliminate batch effects
and artifacts from the merged data. A total of 243 samples were
collected, including 126 IPF and 117 CTRL samples.
Identification of differentially
expressed genes (DEGs)
The limma package (version 3.34.7) in R3.6.1
(18) was used to investigate
significant differences in m5C gene expression between patients
with IPF and CTRL group. A false discovery rate (FDR) <0.05 was
used to indicate significance. Spearman coefficients between pairs
of DEGs were calculated using the R cor function and results were
visualized with the heatmap package (version 1.0.8) (19,20).
Protein-protein interaction (PPI) and
enrichment of functional pathways
PPI network of DEGs was established using STRING
(version 11.0, string-db.org/) (21) while keeping linkage pairs with
interaction scores ≥0.4, and the interaction network was visualized
using Cytoscape (version 3.9.0) Display (22). The nodes in the network were
annotated using DAVID (version 6.8), based on Gene Ontology (GO)
biological processes and Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway analysis (23,24),
with FDR <0.05 indicating significant enrichment.
Construction of diagnostic model based
on m5C-related genes
Single-factor logistic regression analysis and
screening of optimal m5C-associated gene combinations
One-way logistic regression analysis in R package
rms (version 6.3–0) (25) was
utilized to screen DEG levels determined in the combined dataset
samples in step with the lars package (version 1.2) (26) using the least absolute shrinkage
and selection operator (LASSO) algorithm for optimization of the
m5C-related genes.
Construction of the diagnostic
model
In the combined training set, the support vector
machine method in R3.6.1 e1071 (version 1.6–8) (27) was used to build the disease
diagnostic classifier based on m5C-associated genes (Core: Sigmoid
Kernel; Cross: 10-fold cross-validation). The receiver operating
characteristic (ROC) curve in R3.6.1 pROC (version 1.12.1)
(28) was used to predict model
accuracy in training and validation cohorts.
KEGG signaling pathway analysis
Genome-wide expression level-based GSVA
quantification of KEGG analysis
KEGG and gene data were obtained from the Gene Set
Enrichment Analysis (GSEA) MSigDB database
(gsea-msigdb.org/gsea/msigdb/index.jsp). The combined sample
genome-wide expression level data were determined for each KEGG
signaling pathway by gene expression levels, again using GSVA
(version 1.36.3) in R3.6.1 language (29). The distribution of quantified KEGG
signaling pathway values in different subtype groupings was
compared with FDR <0.05 set as the threshold for significant
differences.
Subtype grouping-related KEGG
enrichment analysis
GSEA database was used to identify KEGG signaling
pathways that were significantly associated with subtype grouping
based on genome-wide expression levels in combined samples.
Screening DEGs associated with subtype
groupings
In the combined sample expression profiles, limma
(version 3.34.7) package in R3.6.1 (18) was used to identify DEGs between
different subtype subgroups, with |log2FC| >1 and FDR
<0.05 as threshold criteria. KEGG and GO analyses were used to
investigate the biological functions of genes using DAVID (version
6.8) (23,24). FDR<0.05 was used as the
threshold to select DEGs. T test in R3.6.1 language was used to
compare the expression levels of immune checkpoint genes and Human
leukocyte Antigen (HLA) family genes in different subtypes.
BLM-induced PF in mice
A total of 12 male C57BL/6 mice (age, 6–8 weeks;
weight 16–20 g) were obtained from Beijing HFK Bioscience Co., Ltd.
All mice were raised in a pathogen-free environment at 22–25°C,
relative humidity of 50–60% and a 12/12 light-dark cycle. The mice
had unrestricted access to food and water. The animal experiments
were authorized by the Ethics Review Committee of Fujian Medical
University (approval no. IACUC FJMU 2023-0327) and all procedures
followed ARRIVE guidelines (30).
BLM was acquired from Hisun Pfizen Pharmaceuticals Co., Ltd. The
mice were anesthetized by intraperitoneal injection of 1%
pentobarbital sodium (50 mg/kg) to prepare for BLM injection via
the intratracheal route. The skin of the throat was cut lengthwise
along the trachea and the muscles and fascia were bluntly separated
using bending forceps to expose the trachea. A total of 12 mice
were randomly divided into control group (50 µl 0.9% saline,
administered intratracheally once) and BLM group (3.5 mg/kg BLM
dissolved in saline to 50 µl, intratracheally injected once; both
n=6). Overall health and welfare were regularly monitored,
including their weight, respiratory rate, activity levels and food
intake. Humane endpoints were weight loss >20%, respiration rate
>100 breaths/min or abnormal behaviors. However, none of the
mice died or reached the humane endpoints. After 21 days, mice were
euthanized via cervical dislocation following anesthesia with 1%
pentobarbital (50 mg/kg) by intraperitoneal injection and the lung
tissues were harvested and collected for further analysis.
Histopathological analysis
The right lungs were fixed with 10% formalin at room
temperature for 24 h, embedded in paraffin wax and cut into lung
sections with a thickness of 3 µm. The sections were then stained
with hematoxylin for 4 min and eosin for 20 sec and Masson
trichrome (hematoxylin for 5–10 min, Ponceau S acid fuchsin stain
for 5–10 min, and aniline blue for 5 min) at room temperature.
Image acquisition was performed using a light microscope (CX-31,
Olympus Corporation) at ×100 magnification.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from the lung tissue using
the SteadyPure RNA extraction kit (Accurate Biology, China),
following the manufacturer's instructions. qPCR assay was then
carried out using the EVO M-MLV RT kit and SYBR Green (both
Accurate Biology) according to the manufacturer's instructions.
Pre-denaturation was set at 95°C for 30 sec. Additionally,
denaturation, annealing and extension were completed in 40 cycles
at 95°C (5 sec) and 60°C (30 sec) respectively. The relative mRNA
change was normalized using the 2−ΔΔCq method (31). Primer pairs are summarized in
Table SI.
Statistical analysis
The statistical analysis was performed with R
software (version 3.6.1). CIBERSORT
(cibersort.stanford.edu/index.php) (32) was used to calculate relative
abundance of immune cell subtypes in the training cohort and the
proportions of immune cell subtypes in the IPF and CRTL groups were
compared using the Kruskal-Wallis test. Spearman coefficients
between the expression of m5C genes used in the model and immune
cell types with significantly different distributions was
calculated using the cor function in R. ConsensusClusterPlus
(version 1.54.0) (33) in R was
used to analyze samples for subtypes concerning diagnostically
relevant m5C gene levels. The m5C scores of each sample were
calculated using Gene Set Variation Analysis (GSVA) (version
1.36.3) (29). Kruskal-Wallis test
was used to compare distribution of immune cells and m5C scores
across subtypes in the IPF and CTRL groups. R package ESTIMATE was
used to determine the immune, stromal and ESTIMATE scores of IPF
samples and the differences in distribution between the immune cell
subsets and ESTIMATE scores were analyzed using the Kruskal-Wallis
test in R. The association between clinical traits and disease
subtypes was investigated using Fisher's exact test. Additionally,
an unpaired intergroup t test was used to compare gene expression
levels across subtype groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
m5C regulators expressed
differentially between IPF and CTRL samples
Following data processing and quality control, 126
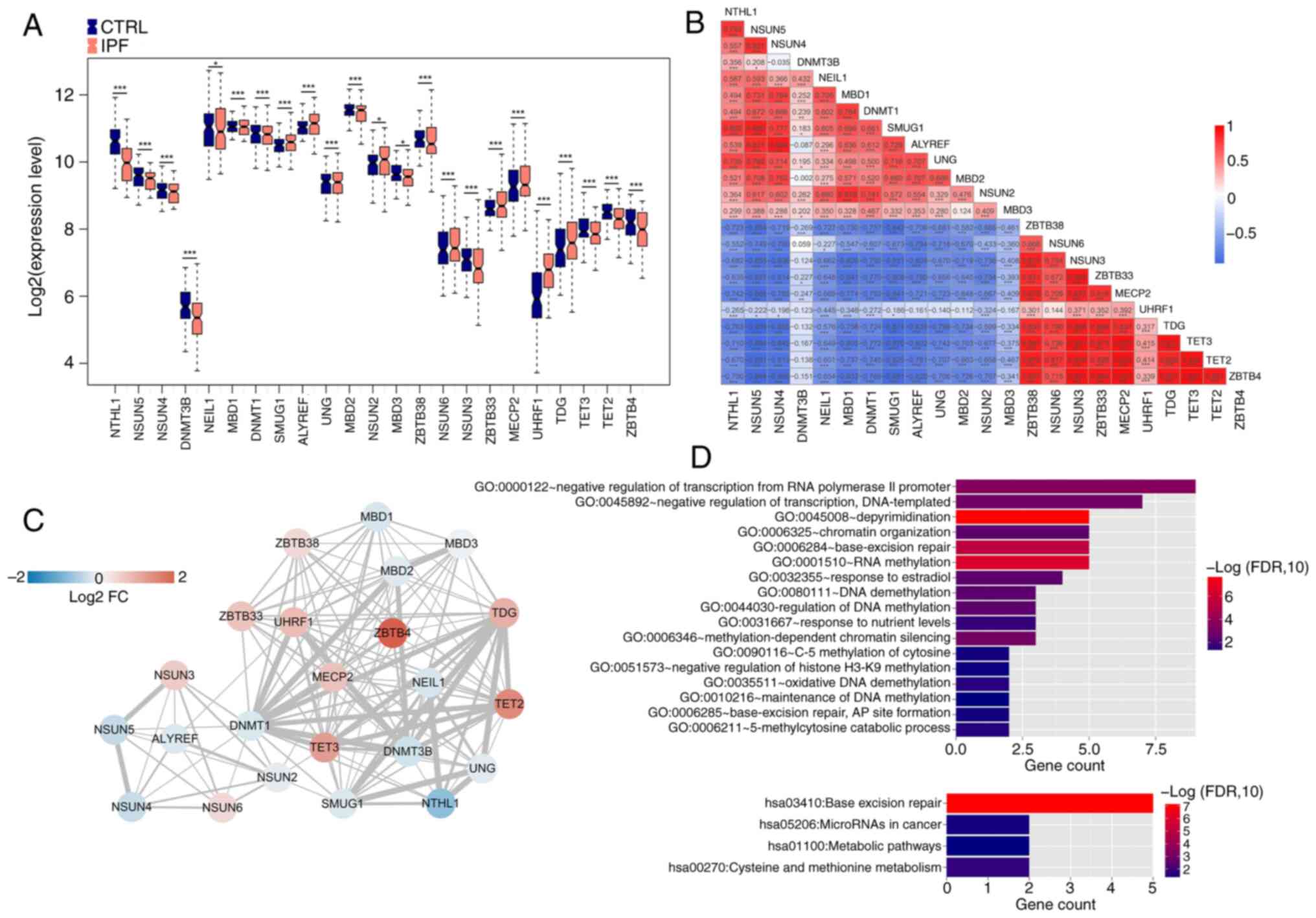
IPF and 117 CTRL samples were examined (Fig. 1). A total of 23 m5C genes were
significantly differentially expressed, of which methyl-CpG binding
domain protein1 (MBD1), Single-strand selective monofunctional
uracil-DNA glycosylase1, Aly/REF export factor (ALYREF), Uracil-DNA
glycosylase (UNG), Zinc finger and BTB domain-containing protein38
(ZBTB38), NSUN6, ZBTB33, Methyl CpG binding protein2 (MECP2), UHRF1
and TDG were significantly upregulated in IPF compared with CTRL
(Fig. 2A). Highest positive
correlations were between TET3 and ZBTB4, TET2 and MECP2, and MECP2
and ZBTB4, while the strongest negative correlations were between
NSUN5 and TDG and TET3 and ZBTB4 (Fig.
2B).
PPI network and functional pathway
enrichment analysis
A PPI network (Fig.
2C) was created to investigate interactions between proteins
encoded by DEGs. This produced a total of 132 linkage pairs. To
analyze systematic characterization and biological functions of m5C
proteins in the network, GO and KEGG analysis was performed using
DAVID with FDR <0.05 set as the threshold. In total, 17
biological processes and four KEGG signaling pathways were
identified. IPF-associated DEGs were involved in GO biological
processes, including ‘depyrimidination’, ‘RNA methylation’,
‘base-excision repair’, ‘negative regulation of transcription from
RNA polymerase II promoter’ and ‘negative regulation of
transcription, DNA-templated’. KEGG pathway analysis revealed that
IPF-associated DEGs were enriched in ‘base excision repair’,
‘microRNAs in cancer’, ‘metabolic pathways’ and ‘cysteine and
methionine metabolism’ (Fig.
2D).
Construction of a diagnostic model
centered on m5C-related genes
To assess the role of m5C-associated genes in IPF
diagnosis, 23 m5C-related DEGs were screened using univariate Cox
regression. A total of nine genes, including NTHL1, NSUN5, DNMT3B,
MBD3, NSUN6, ZBTB33, UHRF1, TDG and TET2, were identified (Fig. S1). The final optimized m5C-related
genes were then screened using the lars package and the LASSO
algorithm in R (Fig. S2). Based
on feature selection of LASSO algorithm, the ZBTB33 gene was
excluded. In the combined training set, NSUN6, UHRF1, TDG and TET2
were highly expressed in patients with IPF, whereas NTHL1, NSUN5,
DNMT3B and MBD3 were downregulated; similar expression patterns
were also found in the independent validation dataset GSE124685.
Area under the curve was >0.8 in both the training and
validation datasets, indicating the potential effectiveness for IPF
diagnosis (Fig. S3).
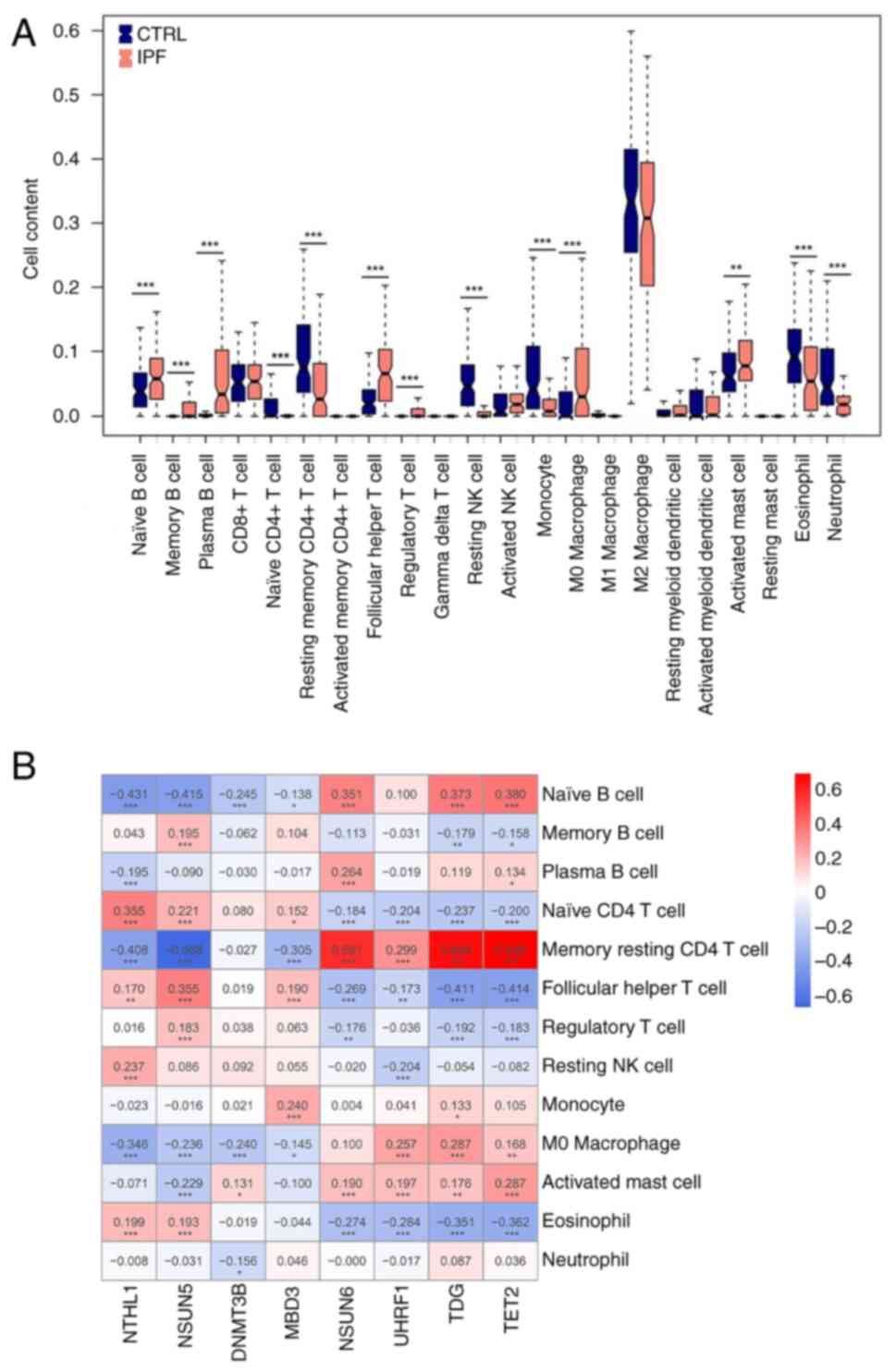
Diagnostic m5C gene and immune
correlation analysis
Studies (34,35)
have demonstrated that there is a fibro-inflammatory response
during formation of PF and immune cells may accumulate around PF
lesions. CIBERSORT was used to calculate immune infiltration in the
training dataset, comparing distribution between IPF and CTRL
groups. The proportion of immune cell infiltration varied among the
13 types (naive, memory, B cell plasma, T cell CD4 naive, T cell
CD4 memory resting, T cell follicular helper, T cell regulatory
Tregs, NK cell resting, Monocyte, Macrophage M0, Mast cell
activated, Eosinophil, and Neutrophil). Resting memory CD4 T cells
were strongly and positively associated with NSUN6, TDG, and TET2
levels and negatively correlated with NSUN5 (Fig. 3).
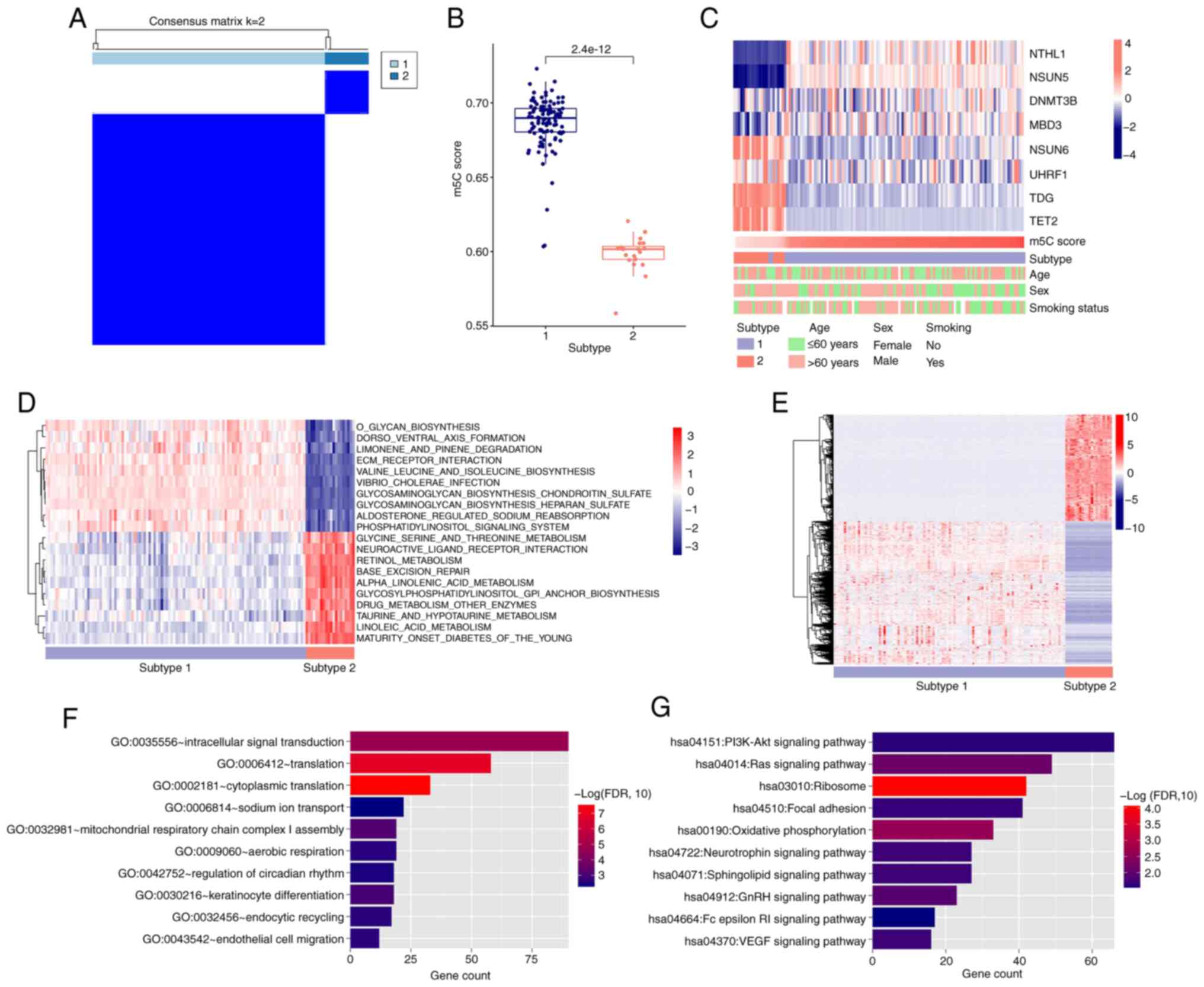
Sample subtype analysis based on
diagnostic m5C genes
All samples were classified into two subtypes based
on the levels of the eight diagnostic m5C genes. Subtype 1 samples
had significantly higher m5C score than subtype 2 (Fig. 4A and B). There was a significant
difference between the subtypes based on sex but age and smoking
had no significant effect (Fig.
4C; Table I). Immune cell
infiltration varied by subtype, with 15 types of immune cell
differently distributed (Fig.
S4A). ESTIMATE scores also differed significantly between
subtypes (Fig. S4B), with subtype
2 showing higher resting memory CD4 T cell infiltration than
subtype 1. Immune checkpoint and HLA family gene analysis revealed
16 HLA family genes with significantly different expression:
Beta-2-Microglobulin, Human Leukocyte Antigen-A (HLA-A), Human
Leukocyte Antigen-C (HLA-C), HLA-DMA, HLA-DOA, HLA-DOB, HLA-DBP1,
HLA-DQA1, HLA-DQA2, HLA-DQB1, HLA-DRA, HLA-E, HLA-F, HLA-G,
Transporter2 ATP-Binding Cassette Sub-Family B (TAP2) and TAP
Binding Protein (TAPBP) (Fig.
S5A). A total of seven immune checkpoint DEGs, CD27, CD274,
CD40, CD70, CD86, Cytotoxic T-lymphocyte-associated protein4
(CTLA4) and Hepatitis A virus cellular receptor2 (HAVCR2), were
also discovered (Fig. S5B).
 | Table I.Clinical information of samples. |
Table I.
Clinical information of samples.
|
| Subtype |
|
|---|
|
|
|
|
|---|
| Characteristic | 1 (n=106) | 2 (n=20) | P-value |
|---|
| Age, years |
|
|
|
|
≤60 | 49 | 6 | 0.2212 |
|
>60 | 56 | 14 |
|
| Sex |
|
|
|
|
Female | 46 | 4 | 0.0479 |
|
Male | 60 | 16 |
|
| Smoking status |
|
|
|
|
Yes | 57 | 14 | 0.3051 |
| No | 40 | 5 |
|
Genome-wide expression-based GSVA
quantification of KEGG analysis
A total of 163 KEGG signaling pathways with
significantly different distribution were screened by comparing
distribution of quantified KEGG signaling pathway values across
subtypes. The logFC values of differences were ordered from
smallest to largest and the top 10 KEGG were identified (Fig. 4D). KEGG signaling pathways of
subtypes 1 and 2 differed significantly: Subtype 1 was primarily
involved in ‘glycan biosynthesis’, ‘dorsoventral axis formation’
and ‘ECM receptor interaction’, whereas subtype 2 was primarily
enriched in metabolic aspects such as ‘glycine, serine and
threonine metabolism’, ‘retinol metabolism’ and ‘alpha-linolenic
acid metabolism’.
Subtype grouping-related KEGG
enrichment analysis
Using GSEA, the KEGG signaling pathways that were
significantly associated with subtype groupings were screened and
12 significantly associated KEGG signaling pathways were identified
(Fig. S6). In general, the higher
the absolute value of NES, the lower the P-value, implying that the
higher the enrichment of the functional gene set, the greater the
confidence in the analysis results.
Screening significantly differentially
expressed genes associated with subtype grouping and enrichment
analysis of GO biological processes and KEGG signaling pathway
A total of 2,870 DEGs were identified (Fig. 4E). DAVID was then used to identify
genes that were significantly differentially expressed for GO
biological processes and KEGG signaling pathways. In total, 55
biological processes and 12 KEGG signaling pathways were screened
(Fig. 4F and G), and the top 10 in
each category were visualized based on FDR value (smallest to
largest). ‘Ribosome’ and ‘oxidative phosphorylation’ showed the
most significant difference.
Normal and fibrotic mouse lung
tissue
Histopathological staining revealed BLM-induced PF
in mice. Mouse lung tissue showed a high concentration of
fibroblasts, severe and extensive interstitial fibrosis, alveolar
structure destruction and irregularly shaped and small alveolar
cavities. In Masson-stained sections, a large blue-stained collagen
deposit could be seen, and the alveolar structure was destroyed,
indicating a more typical form of lung interstitial fibrosis
(Fig. 5A).
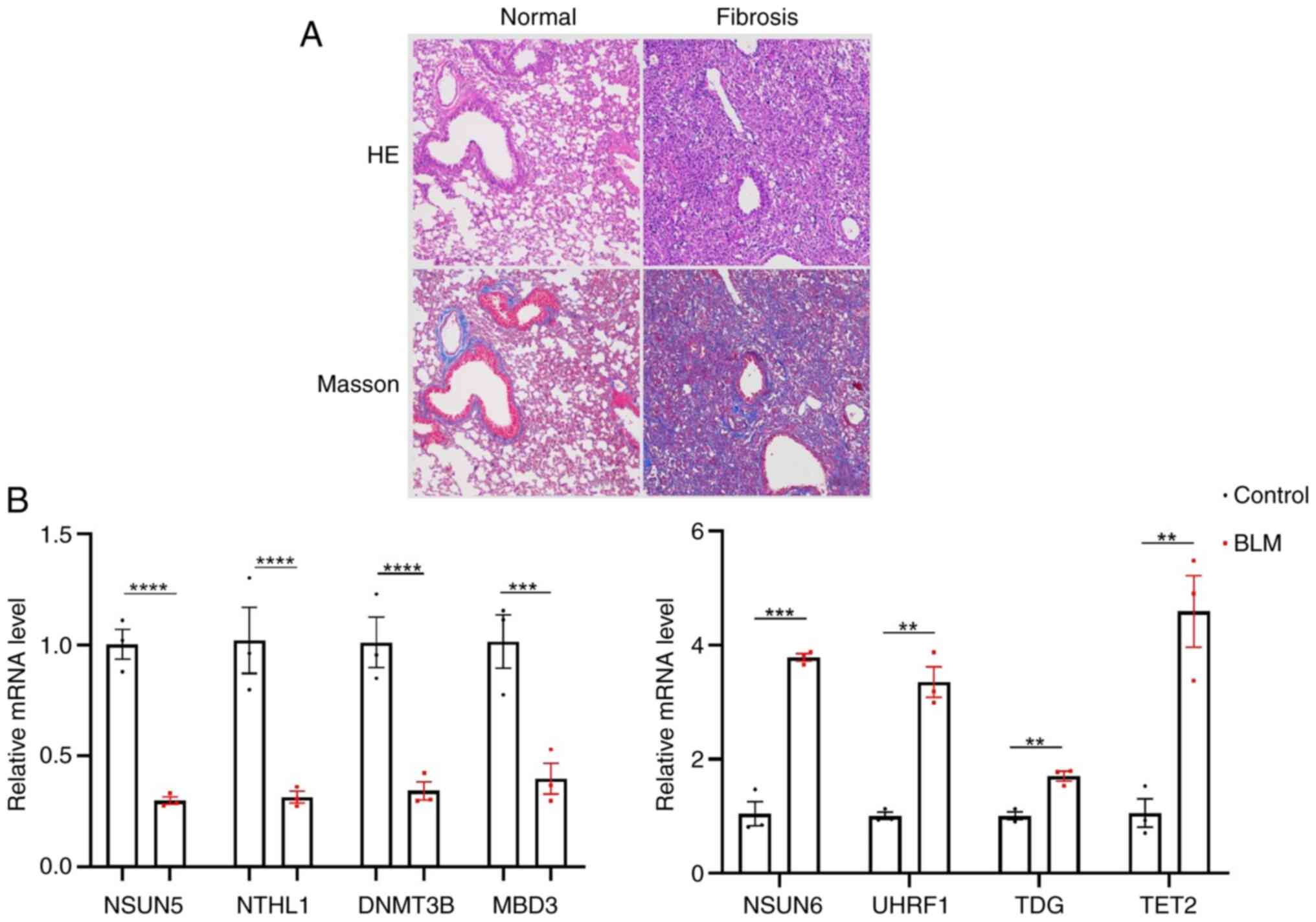 | Figure 5.Lung fibrosis tissue in mice. (A)
Normal and fibrotic lung tissue. Magnification, ×100. (B)
Expression of eight diagnostic 5-methylcytosine -related genes in
mouse lung tissue. Control vs. BLM. **P<0.01, ***P<0.001,
****P<0.0001. HE, hematoxylin and eosin; NSUN, nucleolar RNA
methyltransferase; NTHL, nth-like; DNMT3B, DNA methyltransferase3
beta; MBD, methyl-CpG binding domain; UHRF, ubiquitin-like with
plant homeodomain and ring finger; TDG, thymine DNA glycosylase;
TET2, ten-eleven translocation2; BLM, bleomycin |
Expression of m5C methylation-related
genes in lung fibrosis tissue samples
NSUN6, UHRF1, TDG and TET2 were significantly
upregulated, while the expression levels of NSUN5, NTHL1, DNMT3B
and MBD3 were downregulated in fibrotic compared with normal lung
tissue (Fig. 5B).
Discussion
IPF is a progressive fatal lung disease whose
symptoms frequently overlap with other conditions, making diagnosis
difficult. Identifying factors involved in its development and
progression can facilitate development of reliable markers capable
of accurately predicting the prognosis of patients with IPF
(4). RNA modifications such as
m6A, m5C and m1A are increasingly recognized to serve critical
roles in diverse biological processes, and their dysregulation has
been linked to development and progression of several diseases,
particularly cancer, by influencing gene expression (5,13,36,37).
There has been a great deal of research on m6A regulators and their
role in the etiology of IPF (15,16),
but to the best of our knowledge, there have been no studies on
m5C. The present study aimed to develop a reliable and effective
diagnostic model for IPF using m5C-associated regulatory genes. The
differential expression and interactions of 23 IPF-associated m5C
regulatory genes were investigated using GEO database, resulting in
the development of a diagnostic model. The model identified two
subtypes of patients with IPF. Furthermore, CIBERSORT was used to
compare differences in immune cell distribution and immune
checkpoint and HLA family gene expression.
The present study identified eight differentially
expressed IPF-associated m5C regulatory genes. These included
NTHL1, DNMT3B, MBD3, UHRF1, TDG, NSUN5 and 6 and TET2. The model
efficacy was assessed in training and validation datasets, and each
of the genes was further validated using a PF mouse model. The
results were consistent with the information obtained from GEO:
NSUN6, UHRF1 and TDG were significantly upregulated, while the
expression levels of NSUN5, NTHL1, DNMT3B and MBD3 were
downregulated in fibrotic compared with normal lung tissue. TET2
demethylates both DNA and RNA (38). It preserves the stemness of
trophoblast stem cells by converting 5-methylcytosine to
5-hydroxymethylcytosine and its inhibition slows trophoblast stem
cell proliferation and promotes epithelial-mesenchymal transition
(EMT) (39). EMT process involves
transformation of epithelial cells into mesenchymal-like cells. In
~33.3% of cases of PF, myofibroblasts undergo EMT (40). Furthermore, TET3 has been shown to
be upregulated in IPF fibroblasts, which contradicts the present
findings (41). Macrophage
polarization in the alveoli has also been found in PF caused by low
levels of DNMT3B, IL-4 and transforming growth factor β1 (TGF-β1)
(42). This suggests DNMT3B may
play a protective role against fibrosis. This is consistent with
the present findings, which showed that DNMT3B was downregulated in
the IPF samples compared with CTRL. Furthermore, development of
fibrosis has been linked to DNMT1, which regulates DNA methylation
in fibroblasts and alveolar epithelial cells (43). NTHL1 encodes a DNA repair enzyme
that primarily removes oxidatively damaged bases from DNA
molecules. Inflammation in the lungs can cause oxidative DNA damage
(44), which NTHL1 can help to
mitigate. However, aberrations in the NTHL1 repair system cause
pathological oxidative damage in the lung, eventually leading to PF
pathogenesis (45,46). One study found UHRF1 is a mediator
of KRAS-driven oncogenesis in lung adenocarcinoma (47). The protein encoded by TDG is a key
DNA repair enzyme that is primarily responsible for the removal of
modified pyrimidine bases, specifically demethylated thymine. It is
key for maintaining genomic stability and regulating the DNA damage
response. A study discovered a link between DNA damage and
development of PF, implying that TDG could play an important role
in repairing damage (48). NSUN5
and NSUN6 are members of the NSUN family, encoding RNA
methyltransferase proteins (49).
Previous studies (50,51) on NSUN5 have concentrated on its
role in neurological disorder. NSUN6 is associated with development
of pancreatic (52) and colorectal
cancer (53). However, no link has
been found between NSUN5, NSUN6 and PF to date. Compared with mice
without macrophage MBD2 deficiency, mice with MBD2 deficiency in
macrophages are protected from BLM-induced pulmonary fibrosis and
exhibit significantly lower levels of TGF-β1 and M2 macrophages
(54). MECP2 is associated with
pathological fibrosis in the heart by promoting fibroblast
proliferation and fibrosis via Dual Specificity Phosphatase5
downregulation (55). Here, MECP2
was highly expressed in IPF samples compared with CTRL, implying
that the gene may also regulate lung fibrosis, as confirmed by
Cheng et al (56). The
present findings indicated that ZBTB4 may regulate development of
PF via m5C methylation, as it was significantly differentially
expressed in the IPF and CTRL groups. This demonstrated that the
model based on these m5C-related genes, combined with disease
subtype classification, may be useful for diagnosing IPF.
IPF is characterized by increased infiltration of
inflammatory cells; whether this is a primary cause of IPF or an
epiphenomenon remains unknown (34). Furthermore, immune dysregulation is
hypothesized to contribute to IPF development (35). In the present study, the proportion
of inflammatory cells such as T and B cells, macrophages,
monocytes, natural killer (NK) cells, neutrophils and eosinophils
was significantly different between IPF and CTRL groups.
Macrophages are abundant in healthy lungs (57). Alveolar macrophages (AMs) maintain
homeostasis by removing cell debris, including apoptotic cells,
regulating wound healing and initiating anti-pathogen immune
responses. Monocytes are recruited and stimulated to differentiate
into macrophages in response to lung injury (58). However, M2 macrophages predominate
in the lung of patients with IPF/Usual Interstitial Pneumonia and
animal studies indicate that M2 macrophages may be a useful target
for treating and preventing PF (59,60).
These cells produce a high-affinity IL-13 receptor, IL-13Rα2, which
interacts with IL-13 and increases production of TGF-β1, promoting
fibrosis (61). Although patients
with IPF had significantly higher levels of M0 macrophages, the
present study found no difference in number of M2 macrophages
between the IPF and CTRL groups. Little is understood about how NK
cells and M0 macrophages contribute to PF. The present findings may
provide novel insight into how immunological infiltration occurs in
PF. Resting memory CD4+ T cells are hypothesized to serve as
reservoirs for viruses such as HIV and, if activated, they can
promote HIV infection (62).
Resting memory CD4+ T cell levels were significantly lower in
patients with IPF and they showed strong positive correlations with
TET2 and TDG, as well as a negative correlation with NSUN5. TET2 is
involved in development of IPF (63). Thus, combination of resting memory
CD4+ T cells and TET2 may offer a novel approach to IPF
diagnosis.
Based on expression of m5C-associated genes, samples
were classified into two subtypes. Subtype 1 had significantly
higher m5C scores compared with subtype 2 samples. Sex was the only
clinical characteristic that distinguished subtypes. The
distribution of immune cells revealed that subtype 1 contained
significantly more M2 macrophages than subtype 2. By contrast,
resting memory CD4+ T cells, which have not previously been linked
to IPF tissues, were highly expressed in subtype 2. However,
currently, IPF is not clinically classified into distinct subtypes.
The present study found differences in the expression of
m5C-related genes across subtypes. Future studies should enroll
patients and measure the expression levels of these m5C genes and
assess correlation with the clinical characteristics associated
with each subtype, resulting in a more complete understanding of
potential stratification in IPF.
Subtype 1 had significantly lower levels of CD27 and
CD70 expression than subtype 2. CD27, a member of the TNF receptor
family, is found almost exclusively on naive CD4 T cells (64). CD27- and CD28-expressing T cells
are associated with various inflammatory lung conditions, including
IPF, and CD27 levels are associated with lung function parameters
in patients with IPF (65). A key
aspect of the pathophysiology of fibrosis is the secretion of
extracellular matrix proteins, which is reduced when CD70 binds to
fibroblasts (58). CD70 is a type
II transmembrane member of the TNF family found in both fibroblasts
and lung tissue. CD70 and CD27 work together as a ligand-receptor
system to regulate T cell co-stimulation and interaction with
fibroblasts (65). Thus, CD27-CD70
interaction may be a promising target for fibrosis treatment. CD40
regulates a range of processes, including innate and adaptive
immune responses. Several inflammatory pulmonary conditions,
including acute lung injury, bronchial asthma interstitial
pneumonia and acute respiratory distress syndrome, are associated
with CD40-CD40L interactions (66). CD40 also decreases inflammation in
the early stages of IPF, making it a potential target for slowing
progression of PF. Furthermore, CD274, CD86, CTLA4 and HAVCR2
expression were significantly higher in subtype 2 compared with
subtype 1. Programmed cell death 1 ligand 1 (PD-L1), also called
CD274 or B7-H1, is a member of the B7 family of immune regulatory
molecules. PD-L1 is highly expressed in numerous types of tissues,
including the heart and lungs. Wang et al (67) found that PD-L1 knockout in septic
mice decreases plasma levels of TNF-α and IL-6 and alveolar edema.
This suggests that PD-L1 serves a protective role against lung
inflammation. Immune checkpoint inhibitor-associated pneumonia
(CIP) frequently develops in patients receiving PD-L1 inhibitors in
clinical practice (68–70). CIP frequently presents with
interstitial lung changes, which can progress to PF in severe cases
(70). The present findings
support the protective effects of PD-L1, which may be applicable in
IPF. The immunoglobulin superfamily includes B
lymphocyte-activating antigen B7-2, also known as CD86, which is
expressed as a type I membrane protein in dendritic and Langerhans
cells and binds to T cell surfaces, activating CD28 and CTLA-4
(71). CD86 negatively regulates T
cell activation and decreases immune responses when bound to CTLA4
(72,73). HAVCR2, also known as TIM3, is an
immune checkpoint receptor protein that inhibits antitumor immune
responses. Its overexpression by AMs exacerbates PF (68). HLA-G expression on mast cells helps
to counteract fibrosis (74).
Certain morphological features, such as male sex, lower baseline
forced vital capacity and diffusing capacity of the lung for carbon
monoxide (DLCO) and older age, have been identified in
retrospective studies as risk factors for progressive fibrosing
interstitial lung disease progression and mortality (75–77).
Patients with IPF are more likely to be male and the present study
found a significant difference in the sex distribution of subtypes,
with subtype 2 having a higher proportion of male patients than
subtype 1. Subtype 2 is hypothesized to have a worse prognosis than
subtype 1 because it expresses lower levels of CD27 and CD70 and
has higher levels of CD274, CD86, CTLA4 and HAVCR2 compared with
subtype 1. This requires additional clinical and experimental
verification. The association between m5C methylation factors and
immune checkpoints also warrants further investigation.
The present study had limitations. First, due to
individual differences and other confounding factors, findings
based on existing databases may not be accurate. The present
results should be verified with a well-designed multicenter
clinical study based on patient clinical data. The small sample
sizes in GSE150910 and GSE173355 datasets are a second limitation.
Finally, because IPF is rare, insufficient specimens have been
collected to confirm the predicted DEGs and the disease diagnosis
model; this should be validated in future studies.
To the best of our knowledge, the present study is
the first to develop an effective prognostic signature for IPF and
the findings highlighted the significance of m5C-related genes in
diagnosing and typing IPF, as well as expression of immune cells
and immune checkpoint-associated genes in patients with IPF and
different disease subtypes.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82171566), Joint Funds for the
Innovation of Science and Technology, Fujian Province (grant no.
2020Y9086) and Foundation of Fujian Provincial Department of
Finance (grant no. 2021XH005).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LC designed and supervised the study. LT analyzed
data. LT and WS wrote the manuscript. WS, JW and YL performed
bioinformatics analysis. LT and LC confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Ethics
Review Committee of Fujian Medical University (approval no. IACUC
FJMU 2023-0327) and all steps were carried out in accordance with
ARRIVE guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sharif R: Overview of idiopathic pulmonary
fibrosis (IPF) and evidence-based guidelines. Am J Manag Care. 23
(11 Suppl):S176–S182. 2017.PubMed/NCBI
|
|
2
|
Zhang C, Zhang M, Ge S, Huang W, Lin X,
Gao J, Gong J and Shen L: Reduced m6A modification predicts
malignant phenotypes and augmented Wnt/PI3K-Akt signaling in
gastric cancer. Cancer Med. 8:4766–4781. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang C, Samanta D, Lu H, Bullen JW, Zhang
H, Chen I, He X and Semenza GL: Hypoxia induces the breast cancer
stem cell phenotype by HIF-dependent and ALKBH5-mediated
m6A-demethylation of NANOG mRNA. Proc Natl Acad Sci USA.
113:E2047–E2056. 2016.PubMed/NCBI
|
|
4
|
Huang T and Zhou HF: A novel
5-methylcytosine- and immune-related prognostic signature is a
potential marker of idiopathic pulmonary fibrosis. Comput Math
Methods Med. 2022:16853842022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han X, Wang M, Zhao YL, Yang Y and Yang
YG: RNA methylations in human cancers. Semin Cancer Biol.
75:97–115. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yi L, Wu G, Guo L, Zou X and Huang P:
Comprehensive analysis of the PD-L1 and immune infiltrates of
m6A RNA methylation regulators in head and neck squamous
cell carcinoma. Mol Ther Nucleic Acids. 21:299–314. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang M, Zhang Y, Ou X, Wang C, Wang X,
Qin B, Zhang Q and Yu J, Zhang J and Yu J: m5C-related signatures
for predicting prognosis in cutaneous melanoma with machine
learning. J Oncol. 2021:61732062021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan J, Huang Z and Xu Y: m5C RNA
methylation regulators predict prognosis and regulate the immune
microenvironment in lung squamous cell carcinoma. Front Oncol.
11:6574662021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mei L, Shen C, Miao R, Wang JZ, Cao MD,
Zhang YS, Shi LH, Zhao GH, Wang MH, Wu LS and Wei JF: RNA
methyltransferase NSUN2 promotes gastric cancer cell proliferation
by repressing p57Kip2 by an m5C-dependent
manner. Cell Death Dis. 11:2702020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Z, Xue S, Zhang M, Xu H, Hu X, Chen S,
Liu Y, Guo M and Cui H: Aberrant NSUN2-mediated m5C
modification of H19 lncRNA is associated with poor differentiation
of hepatocellular carcinoma. Oncogene. 39:6906–6919. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Li A, Sun BF, Yang Y, Han YN, Yuan
X, Chen RX, Wei WS, Liu Y, Gao CC, et al: 5-Methylcytosine promotes
pathogenesis of bladder cancer through stabilizing mRNAs. Nat Cell
Biol. 21:978–990. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun G, Ma S, Zheng Z, Wang X, Chen S,
Chang T, Liang Z, Jiang Y, Xu S and Liu R: Multi-omics analysis of
expression and prognostic value of NSUN members in prostate cancer.
Front Oncol. 12:9655712022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang Z, Pan J, Wang H, Du X, Xu Y, Wang Z
and Chen D: Prognostic significance and tumor immune
microenvironment heterogenicity of m5C RNA methylation regulators
in triple-negative breast cancer. Front Cell Dev Biol.
9:6575472021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Y, Hu Z, Sun Q and Dong Y:
5-Methyladenosine regulators play a crucial role in development of
chronic hypersensitivity pneumonitis and idiopathic pulmonary
fibrosis. Sci Rep. 13:59412023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Y, Fang C, Sun Q and Dong Y:
Relevance of RNA N6-methyladenosine regulators for pulmonary
fibrosis: Implications for chronic hypersensitivity pneumonitis and
idiopathic pulmonary fibrosis. Front Genet. 13:9391752022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang JX, Huang PJ, Wang DP, Yang WY, Lu
J, Zhu Y, Meng XX, Wu X, Lin QH, Lv H, et al: m6A
modification regulates lung fibroblast-to-myofibroblast transition
through modulating KCNH6 mRNA translation. Mol Ther. 29:3436–3448.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leek JT, Johnson WE, Parker HS, Jaffe AE
and Storey JD: The sva package for removing batch effects and other
unwanted variation in high-throughput experiments. Bioinformatics.
28:882–883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang L, Cao C, Ma Q, Zeng Q, Wang H, Cheng
Z, Zhu G, Qi J, Ma H, Nian H and Wang Y: RNA-seq analyses of
multiple meristems of soybean: Novel and alternative transcripts,
evolutionary and functional implications. BMC Plant Biol.
14:1692014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eisen MB, Spellman PT, Brown PO and
Botstein D: Cluster analysis and display of genome-wide expression
patterns. Proc Natl Acad Sci USA. 95:14863–14868. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szklarczyk D, Gable AL, Nastou KC, Lyon D,
Kirsch R, Pyysalo S, Doncheva NT, Legeay M, Fang T, Bork P, et al:
Correction to ‘The STRING database in 2021: Customizable
protein-protein networks, and functional characterization of
user-uploaded gene/measurement sets’. Nucleic Acids Res.
49:108002021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang DW, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang DW, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan X, Jin X, Wang J, Hu Q and Dai B:
Placenta inflammation is closely associated with gestational
diabetes mellitus. Am J Transl Res. 13:4068–4079. 2021.PubMed/NCBI
|
|
26
|
Goeman JJ: L1 penalized estimation in the
Cox proportional hazards model. Biom J. 52:70–84. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Q and Liu X: Screening of feature
genes in distinguishing different types of breast cancer using
support vector machine. Onco Targets Ther. 8:2311–2317.
2015.PubMed/NCBI
|
|
28
|
Robin X, Turck N, Hainard A, Tiberti N,
Lisacek F, Sanchez JC and Müller M: pROC: An open-source package
for R and S+ to analyze and compare ROC curves. BMC Bioinformatics.
12:772011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye L, Zhang T, Kang Z, Guo G, Sun Y, Lin
K, Huang Q, Shi X, Ni Z, Ding N, et al: Tumor-infiltrating immune
cells Act as a marker for prognosis in colorectal cancer. Front
Immunol. 10:23682019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Percie du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: Updated guidelines for
reporting animal research. PLoS Biol. 18:e30004102020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen B, Khodadoust MS, Liu CL, Newman AM
and Alizadeh AA: Profiling tumor infiltrating immune cells with
CIBERSORT. Methods Mol Biol. 1711:243–259. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang X, Ren L, Yan X, Shan Y, Liu L, Zhou
J, Kuang Q, Li M, Long H and Lai W: Identification of
immune-related lncRNAs in periodontitis reveals regulation network
of gene-lncRNA-pathway-immunocyte. Int Immunopharmacol.
84:1066002020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jee AS, Sahhar J, Youssef P, Bleasel J,
Adelstein S, Nguyen M and Corte TJ: Review: Serum biomarkers in
idiopathic pulmonary fibrosis and systemic sclerosis associated
interstitial lung disease-frontiers and horizons. Pharmacol Ther.
202:40–52. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Harrell CR, Sadikot R, Pascual J,
Fellabaum C, Jankovic MG, Jovicic N, Djonov V, Arsenijevic N and
Volarevic V: Mesenchymal stem cell-based therapy of inflammatory
lung diseases: Current understanding and future perspectives. Stem
Cells Int. 2019:42369732019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han M, Sun H, Zhou Q, Liu J, Hu J, Yuan W
and Sun Z: Effects of RNA methylation on Tumor angiogenesis and
cancer progression. Mol Cancer. 22:1982023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li D, Li K, Zhang W, Yang KW, Mu DA, Jiang
GJ, Shi RS and Ke D: The m6A/m5C/m1A regulated gene signature
predicts the prognosis and correlates with the immune status of
hepatocellular carcinoma. Front Immunol. 13:9181402022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Y, Xue M, Deng X, Dong L, Nguyen LXT,
Ren L, Han L, Li C, Xue J, Zhao Z, et al: TET2-mediated mRNA
demethylation regulates leukemia stem cell homing and self-renewal.
Cell Stem Cell. 30:1072–1090.e10. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chrysanthou S, Senner CE, Woods L,
Fineberg E, Okkenhaug H, Burge S, Perez-Garcia V and Hemberger M: A
critical role of TET1/2 proteins in cell-cycle progression of
trophoblast stem cells. Stem Cell Reports. 10:1355–1368. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tanjore H, Xu XC, Polosukhin VV, Degryse
AL, Li B, Han W, Sherrill TP, Plieth D, Neilson EG, Blackwell TS
and Lawson WE: Contribution of epithelial-derived fibroblasts to
bleomycin-induced lung fibrosis. Am J Respir Crit Care Med.
180:657–665. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Negreros M, Hagood JS, Espinoza CR,
Balderas-Martínez YI, Selman M and Pardo A: Transforming growth
factor beta 1 induces methylation changes in lung fibroblasts. PLoS
One. 14:e02235122019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qin W, Spek CA, Scicluna BP, van der Poll
T and Duitman J: Myeloid DNA methyltransferase3b deficiency
aggravates pulmonary fibrosis by enhancing profibrotic macrophage
activation. Respir Res. 23:1622022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wei A, Gao Q, Chen F, Zhu X, Chen X, Zhang
L, Su X, Dai J, Shi Y and Cao W: Inhibition of DNA methylation
de-represses peroxisome proliferator-activated receptor-γ and
attenuates pulmonary fibrosis. Br J Pharmacol. 179:1304–1318. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu J, Liu L, Ma X, Cao X, Chen Y, Qu X,
Ji M, Liu H, Liu C, Qin X and Xiang Y: The role of DNA damage and
repair in idiopathic pulmonary fibrosis. Antioxidants (Basel).
11:22922022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Magrin L, Fanale D, Brando C, Fiorino A,
Corsini LR, Sciacchitano R, Filorizzo C, Dimino A, Russo A and
Bazan V: POLE, POLD1, and NTHL1: the last but not the least
hereditary cancer-predisposing genes. Oncogene. 40:5893–5901. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu Q, Qin Y, Ji S, Xu W, Liu W, Sun Q,
Zhang Z, Liu M, Ni Q, Yu X and Xu X: UHRF1 promotes aerobic
glycolysis and proliferation via suppression of SIRT4 in pancreatic
cancer. Cancer Lett. 452:226–236. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kostyrko K, Román M, Lee AG, Simpson DR,
Dinh PT, Leung SG, Marini KD, Kelly MR, Broyde J, Califano A, et
al: UHRF1 is a mediator of KRAS driven oncogenesis in lung
adenocarcinoma. Nat Commun. 14:39662023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tanner L, Single AB, Bhongir RKV, Heusel
M, Mohanty T, Karlsson CAQ, Pan L, Clausson CM, Bergwik J, Wang K,
et al: Small-molecule-mediated OGG1 inhibition attenuates pulmonary
inflammation and lung fibrosis in a murine lung fibrosis model. Nat
Commun. 14:6432023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Heissenberger C, Liendl L, Nagelreiter F,
Gonskikh Y, Yang G, Stelzer EM, Krammer TL, Micutkova L, Vogt S,
Kreil DP, et al: Loss of the ribosomal RNA methyltransferase NSUN5
impairs global protein synthesis and normal growth. Nucleic Acids
Res. 47:11807–11825. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang XW, Wu LY, Liu HR, Huang Y, Qi Q,
Zhong R, Zhu L, Gao CF, Zhou L, Yu J and Wu HG: NSUN5 promotes
progression and predicts poor prognosis in hepatocellular
carcinoma. Oncol Lett. 24:4392022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou J, Kong YS, Vincent KM,
Dieters-Castator D, Bukhari AB, Glubrecht D, Liu RZ, Quilty D,
Findlay SD, Huang X, et al: RNA cytosine methyltransferase NSUN5
promotes protein synthesis and tumorigenic phenotypes in
glioblastoma. Mol Oncol. 17:1763–1783. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang R, Liang X, Wang H, Guo M, Shen H,
Shi Y, Liu Q, Sun Y, Yang L and Zhan M: The RNA methyltransferase
NSUN6 suppresses pancreatic cancer development by regulating cell
proliferation. EBioMedicine. 63:1031952021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cui Y, Lv P and Zhang C: NSUN6 mediates
5-methylcytosine modification of METTL3 and promotes colon
adenocarcinoma progression. J Biochem Mol Toxicol. 38:e237492024.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang Y, Zhang L, Wu GR, Zhou Q, Yue H, Rao
LZ, Yuan T, Mo B, Wang FX, Chen LM, et al: MBD2 serves as a viable
target against pulmonary fibrosis by inhibiting macrophage M2
program. Sci Adv. 7:eabb60752021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tao H, Yang JJ, Hu W, Shi KH, Deng ZY and
Li J: MeCP2 regulation of cardiac fibroblast proliferation and
fibrosis by down-regulation of DUSP5. Int J Biol Macromol.
82:68–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cheng C, Wu Y, Xiao T, Xue J, Sun J, Xia
H, Ma H, Lu L, Li J, Shi A, et al: METTL3-mediated m6A
modification of ZBTB4 mRNA is involved in the smoking-induced EMT
in cancer of the lung. Mol Ther Nucleic Acids. 23:487–500. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Desch AN, Gibbings SL, Goyal R, Kolde R,
Bednarek J, Bruno T, Slansky JE, Jacobelli J, Mason R, Ito Y, et
al: Flow cytometric analysis of mononuclear phagocytes in
nondiseased human lung and lung-draining lymph nodes. Am J Respir
Crit Care Med. 193:614–626. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shenderov K, Collins SL, Powell JD and
Horton MR: Immune dysregulation as a driver of idiopathic pulmonary
fibrosis. J Clin Invest. 131:e1432262021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hancock A, Armstrong L, Gama R and Millar
A: Production of interleukin 13 by alveolar macrophages from normal
and fibrotic lung. Am J Respir Cell Mol Biol. 18:60–65. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rao LZ, Wang Y, Zhang L, Wu G, Zhang L,
Wang FX, Chen LM, Sun F, Jia S, Zhang S, et al: IL-24 deficiency
protects mice against bleomycin-induced pulmonary fibrosis by
repressing IL-4-induced M2 program in macrophages. Cell Death
Differ. 28:1270–1283. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Fichtner-Feigl S, Strober W, Kawakami K,
Puri RK and Kitani A: IL-13 signaling through the IL-13alpha2
receptor is involved in induction of TGF-beta1 production and
fibrosis. Nat Med. 12:99–106. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang Z, Yin X, Ma M, Ge H, Lang B, Sun H,
He S, Fu Y, Sun Y, Yu X, et al: IP-10 Promotes latent HIV infection
in resting memory CD4+ T cells via LIMK-cofilin pathway.
Front Immunol. 12:6566632021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ren L, Chang YF, Jiang SH, Li XH and Cheng
HP: DNA methylation modification in Idiopathic pulmonary fibrosis.
Front Cell Dev Biol. 12:14163252024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wajant H: Therapeutic targeting of CD70
and CD27. Expert Opin Ther Targets. 20:959–973. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tran-Nguyen TK, Xue J, Feghali-Bostwick C,
Sciurba FC, Kass DJ and Duncan SR: CD70 activation decreases
pulmonary fibroblast production of extracellular matrix proteins.
Am J Respir Cell Mol Biol. 63:255–265. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kawabe T, Matsushima M, Hashimoto N,
Imaizumi K and Hasegawa Y: CD40/CD40 ligand interactions in immune
responses and pulmonary immunity. Nagoya J Med Sci. 73:69–78.
2011.PubMed/NCBI
|
|
67
|
Wang JF, Wang YP, Xie J, Zhao ZZ, Gupta S,
Guo Y, Jia SH, Parodo J, Marshall JC and Deng XM: Upregulated PD-L1
delays human neutrophil apoptosis and promotes lung injury in an
experimental mouse model of sepsis. Blood. 138:806–810. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang Y, Kuai Q, Gao F, Wang Y, He M, Zhou
H, Han G, Jiang X, Ren S and Yu Q: Overexpression of TIM-3 in
macrophages aggravates pathogenesis of pulmonary fibrosis in mice.
Am J Respir Cell Mol Biol. 61:727–736. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cui P, Liu Z, Wang G, Ma J, Qian Y, Zhang
F, Han C, Long Y, Li Y, Zheng X, et al: Risk factors for
pneumonitis in patients treated with anti-programmed death-1
therapy: A case-control study. Cancer Med. 7:4115–4120. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang Q, Tang L, Zhou Y, He W and Li W:
Immune checkpoint inhibitor-associated pneumonitis in non-small
cell lung cancer: Current understanding in characteristics,
diagnosis, and management. Front Immunol. 12:6639862021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Esensten JH, Helou YA, Chopra G, Weiss A
and Bluestone JA: CD28 costimulation: From mechanism to therapy.
Immunity. 44:973–988. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kennedy A, Waters E, Rowshanravan B, Hinze
C, Williams C, Janman D, Fox TA, Booth C, Pesenacker AM, Halliday
N, et al: Differences in CD80 and CD86 transendocytosis reveal CD86
as a key target for CTLA-4 immune regulation. Nat Immunol.
23:1365–1378. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Engelhardt JJ, Sullivan TJ and Allison JP:
CTLA-4 overexpression inhibits T cell responses through a
CD28-B7-dependent mechanism. J Immunol. 177:1052–1061. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mouchet N, Vu N, Turlin B, Rioux-Leclercq
N, Jouneau S, Samson M and Amiot L: HLA-G is widely expressed by
mast cells in regions of organ fibrosis in the liver, lung and
kidney. Int J Mol Sci. 22:124902021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zamora-Legoff JA, Krause ML, Crowson CS,
Ryu JH and Matteson EL: Progressive decline of lung function in
rheumatoid arthritis-associated interstitial lung disease.
Arthritis Rheumatol. 69:542–549. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gimenez A, Storrer K, Kuranishi L, Soares
MR, Ferreira RG and Pereira CAC: Change in FVC and survival in
chronic fibrotic hypersensitivity pneumonitis. Thorax. 73:391–392.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wong AW, Ryerson CJ and Guler SA:
Progression of fibrosing interstitial lung disease. Respir Res.
21:322020. View Article : Google Scholar : PubMed/NCBI
|