Introduction
Rotator cuff tears (RCT) are highly prevalent,
especially among individuals over 40 years old and athletes
(1). Beyond tendon tears, the
development of fatty infiltration is a critical factor complicating
RCT (2). Fatty infiltration refers
to the abnormal deposition of fat in the muscle tissue surrounding
the injured tendon, leading to progressive muscular degeneration
(3). This phenomenon is
particularly problematic because it hampers the success of rotator
cuff repair surgeries by increasing the risk of re-tears, reducing
tendon-to-bone healing capacity and impairing shoulder function
(4).
Previous studies have indicated that rotator cuff
injuries often lead to pathological changes, such as fatty
infiltration, severe inflammation, muscular atrophy and fibrosis,
leading to irreversible degeneration and persistent muscle waste
(5). Fatty infiltration is a
primary factor significantly affecting the success of rotator cuff
repair surgery (6). Fatty
infiltration originates from fibro-adipogenic progenitors (FAPs),
which differentiate into adipocytes under certain pathological
conditions (7). When chronic
inflammation and mechanical stress persist after RCT, FAPs
contribute to the accumulation of adipocytes in the muscle,
impairing muscle function (8).
FAPs are multipotent mesenchymal cells residing in
the muscle tissue, where they play a pivotal role in the repair and
regeneration after injury. Under normal conditions, FAPs contribute
to tissue homeostasis and muscle repair by differentiating into
fibroblasts. However, in the context of chronic injuries, such as
RCT, FAPs can aberrantly differentiate into adipocytes, promoting
fatty infiltration (9). This
adipogenic differentiation is driven by signaling pathways, such as
the PI3K/Akt, mTOR and Wnt signaling pathways, which regulate the
fate of FAPs in response to injury (10). The pathological transformation of
FAPs into adipocytes is a major contributor to muscle dysfunction
and a key factor in the failure of rotator cuff repairs (11). Inhibiting pre-adipocyte-related
genes [FABP4 and peroxisome proliferator-activated receptor γ
(PPARγ)] can inhibit fatty infiltration.
RCT significantly modulates fat metabolism,
particularly by dysregulating adipogenesis and lipid accumulation
(12). The injury promotes an
inflammatory response that activates FAPs, driving their
differentiation into adipocytes. This not only disrupts normal
muscle function but also recruits immune cells to the site of
injury (13). Increased
infiltration of immune cells exacerbates muscle degeneration by
sustaining chronic inflammation, further hindering the healing
process (14).
Studies have shown that mTOR, a conserved
serine/threonine kinase, can regulate cell proliferation and
metabolism by integrating various intracellular and extracellular
signals (15). mTOR and its
complexes affect protein translation, lipid synthesis and
metabolism, mitochondrial energy metabolism and autophagy (16). Understanding the role of the mTOR
signaling pathway in fatty infiltration after rotator cuff injuries
may provide new insights into the pathogenesis of rotator cuff
injury.
Through ubiquitination, NEDD4 tags specific
substrate proteins for proteasomal degradation, thereby regulating
their intracellular abundance and activity (17). In the context of RCT, NEDD4
modulates fat metabolism and cell signaling by ubiquitinating key
regulators of adipogenesis and inflammation. This ubiquitination
process modulates the cellular responses to injuries, including the
differentiation of FAPs into adipocytes and the activation of the
mTOR pathway, which is pivotal for tissue repair (18).
Previous studies have highlighted the key roles of
NEDD4 and the mTOR signaling pathway in protein modification and
fat metabolism (19),
respectively. It was hypothesized that NEDD4 regulates the mTOR
pathway through its ubiquitin ligase activity, thereby affecting
adipose infiltration. The present study aimed to investigate the
interaction between NEDD4 and mTOR by constructing NEDD4
overexpression and interference vectors in a rat model of rotator
cuff injury and investigating their mechanisms in regulating fat
infiltration. Understanding these interactions may reveal new
therapeutic strategies, provide a theoretical basis for treating
and preventing fat infiltration after rotator cuff injuries, guide
clinical decision-making, and improve patients' outcomes.
Materials and methods
Vector construction and screening
The overexpression vector pcDNA3.1-NEDD4-3×HA was
purchased from HonorGene (Changsha Abiwei Biotechnology Co., Ltd.).
The plasmid pLVshRNA-EGFP (2A) Puro was used to generate the
knockdown interference vectors. A total of 3 different short
hairpin (sh) RNA sequences were designed to target NEDD4:
sh-NEDD4-1 (5′-GCAGCTCGCAAACCTGTATCT-3′; concentration, 443.6
ng/µl), sh-NEDD4-2 (5′-GGGCTTGTGTAATGAAGATCA-3′; concentration,
560.4 ng/µl) and sh-NEDD4-3 (5′-GCAAACATTCTGGAGGATTCT-3′;
concentration, 682.3 ng/µl). The shRNA negative control (NC)
sequence is shRNA-NC (5-TTCTCCGAACGTGTCACGT; concentration, 519.4
ng/µl). The overexpression plasmid pcDNA3.1-NEDD4-3×HA (687.98
ng/µl) and its NC (754.55 ng/µl) were used for transfection. For
transfection, the PEI transfection reagent (Shanghai Yeasen
Biotechnology Co., Ltd.) was used. Cells were incubated at 37°C for
48 h during transfection. Subsequent experiments were performed 24
h after transfection. The expression levels of NEDD4 were detected
using RT-qPCR, and vectors were screened based on knockdown
efficiency, following the previously described methods (20).
Animal model construction
A total of 18 male SD rats (8-week-old; mean weight,
280±1.03 g) were purchased from SPF (Beijing) Biotechnology Co.,
Ltd [Production license number: SCXK (Jing) 2019–0010]. The rats
had ad libitum access to water and a standard laboratory
diet. Rats were housed in a specific pathogen-free (SPF)
environment at 22±2°C, 40–80% humidity, and a 12/12-h light/dark
cycle. Rats were acclimatized for 5 days before the experiment.
Feed and water were checked daily to ensure they were fresh, clean
and adequate. The body weight of all rats was recorded weekly to
assess their overall health condition. The present study was
approved (approval no. KY20230718-16) by the Experimental Animal
Welfare and Ethics Committee of Beijing Jishuitan Hospital Guizhou
Hospital (Guiyang, China).
The rats undergoing surgery were divided into the
modeling and treatment groups, while the negative control group
received no treatment. A total of three SD rats were used in each
experimental group. The NEDD4 intervention groups were divided into
empty vector groups (sh-NC and OE-NC), overexpression treatment
group (Model + OE-NEDD4) and knockdown treatment group (Model +
sh-NEDD4). The ‘model’ refers to the rotator cuff tear (RCT) injury
model used in the experiment. The experimental design involves five
distinct groups to investigate the role of NEDD4 in RCT. The
negative control group establishes baseline changes without any
intervention. The empty vector groups (sh-NC and OE-NC) serve as
controls to ensure that observed effects are attributable to NEDD4
expression rather than the viral vector itself. The NEDD4
overexpression group is used to assess the impact of increased
NEDD4 levels on tissue repair and fatty infiltration. Conversely,
the NEDD4 knockdown group investigates the effects of reduced NEDD4
expression on disease progression.
The rats were anesthetized using the anesthetic
isoflurane (RWD Life Science Co., Ltd.). Anesthesia was induced
with an isoflurane concentration of 2.0–5.0% in a closed system
until the rats lost their reflex response. During the maintenance
of anesthesia, the isoflurane concentration was reduced to
1.0–2.5%.
Based on previous studies, a method was adopted to
establish the rat model of rotator cuff injury. Before conducting
the surgery to establish the rat model of rotator cuff injury,
animals underwent general anesthesia and received an intramuscular
injection of 100,000 units of penicillin to prevent infection
(21). The surgical procedure
included the following steps: after skin preparation, the surgical
area was disinfected with 2.5% iodine. The shoulder joint was
externally rotated and abducted. Then, an oblique incision was made
along the long axis of the scapula to expose the rotator cuff
insertion site. The supraspinatus muscle (SSP) was dissected at the
bony attachment point, and a 4–0T muscle suture was passed through
the muscle to create a circumferential marker. Thereafter, the
deltoid muscle was closed, and the skin incision was sutured,
followed by a second disinfection of the incision site. For the
first 3 days after the surgery, penicillin was administered at a
dose of 100,000 units/rat via intramuscular injection. Shoulder
cuff repair surgery was conducted after 4 weeks. After opening the
deltoid muscle, the ruptured SSP was identified using the marker
line. The surrounding adhesive tissue was bluntly dissected to
reduce tension, and the SSP was appropriately pulled until the
muscle end was easily relocated to the greater tuberosity. The
synovium and cartilage at the greater tuberosity insertion point
were scraped away until the bone bed was visible. Two parallel bone
tunnels were established using a sterile 0.5-mm drill. Finally, the
SSP tendon was sutured to the insertion point using a 4–0T muscle
suture (Fig. S1).
The treatment started on the second day after
modeling, with all rats in all groups receiving a 200-µl tail vein
injection of NEDD4 lentivirus (concentration: 1×107
TU/ml) once every 2 weeks for 4 weeks.
The humane endpoint criteria were as follows: the
experimental rats becoming debilitated due to immune suppression,
unable to eat or drink, and showing signs of depression accompanied
by hypothermia (body temperature <37°C) without anesthesia or
sedation. None of the experimental animals reached these
criteria.
Euthanasia was carried out using isoflurane (RWD
Life Science Co., Ltd.) anesthesia. At the end of the experiment
(at the conclusion of week 6 post-modeling/eighteen SD rats),
cardiac blood collection was performed under isoflurane anesthesia
following blood sampling.
The rat was anesthetized and positioned supine on a
board. The left index finger was used to palpate the strongest
heartbeat location, typically located at the left 4th or 5th
intercostal space, just below the triangle formed by the front
limbs and xiphoid process. Gentle pressure was applied with the
left thumb and index finger on the right chest area to stabilize
the heart. With the right hand, the needle was inserted vertically
at the point of the strongest heartbeat. Once blood appeared in the
syringe, the left hand was released and the syringe was carefully
supported to slowly draw the blood.
After blood collection, a high concentration (5%) of
the anesthetic was administered in a closed system to ensure the
animals were euthanized in a state of deep anesthesia. Death was
verified by confirming the absence of both a heartbeat and
respiratory movement. After these signs of death were observed, gas
perfusion was continued for an additional 3 min. The animals were
only removed from the euthanasia chamber after death was confirmed.
If death was not confirmed, alternative euthanasia methods, such as
cervical dislocation, were immediately applied.
This experimental design helped to clarify the
specific role of NEDD4 in the pathogenesis of RCT. By comparing the
results between different groups, the role of NEDD4 in improving or
exacerbating the disease process was investigated.
Histological and pathological
staining
A total of 6 weeks after modeling, all rats were
euthanized. Researchers harvested the distal end of the
supraspinatus tendon along with the connected humeral bone block
from each rat to obtain a complete bone-tendon junction (BTJ)
sample. Some samples were processed for decalcification and stained
using H&E and oil red O staining and terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay following the established protocols for observation
and analysis.
For H&E staining, paraffin-embedded tissue
sections were baked at 64°C for 1 h, followed by deparaffinization
in xylene and rehydration through graded ethanol solutions.
Hematoxylin staining was performed at room temperature for 4 min
and eosin for 6–10 sec.
For Oil Red O staining, fresh frozen tissue sections
were prepared at a 0.5–10 µm thickness, fixed in 10% formaldehyde
for 5–10 min at room temperature, and stained with 0.5% Oil Red O
solution for 10–15 min at room temperature. Nuclei were
counterstained with hematoxylin for 1–2 min at room temperature,
followed by rinsing and sealing with glycerol for observation.
For TUNEL staining, tissue sections were first
equilibrated by adding 50 µl of Equilibration Buffer per sample,
incubating at room temperature for 10 min. TMR-5-dUTP Labeling Mix
and Equilibration Buffer were thawed on ice, and the TdT incubation
buffer was prepared using a ratio of 1 µl Recombinant TdT enzyme: 5
µl TMR-5-dUTP Labeling Mix: 50 µl Equilibration Buffer. This
mixture was adjusted based on slide size. Negative controls were
prepared using ddH2O instead of the enzyme. For
labeling, 56 µl of the TdT incubation buffer was added to each
sample, followed by a 1-h incubation at 37°C in the dark, ensuring
the slides remained moist. Post-incubation, samples were washed
four times with PBS for 5 min each. For nuclear staining, slides
were immersed in a freshly prepared DAPI solution at room
temperature for 8 min in darkness. After washing the samples with
PBS (three times for 5 min each), excess liquid was removed and
anti-fade mounting medium was used for sealing. Fluorescence
microscopy was employed for observation, with immediate analysis
under dark conditions to detect TUNEL-positive cells (red
fluorescence) and DAPI-stained nuclei (blue fluorescence),
examining at least five fields of view per slide.
FAPs' cell culture
The FAPs used were derived from primary isolation
and culture in the laboratory. The FAPs were isolated and cultured
from the primary skeletal muscles of normal rats, not from the rats
used in the animal model construction. Rat FAPs were used in the
study (isolation and culturing of FAPs using a single rat), and
FAPs were cultured following previously described methods (22). Frozen FAPs were thawed from liquid
nitrogen and quickly dissolved in a 37°C water bath. Subsequently,
the cells were transferred to a 15-ml centrifuge tube containing 10
ml of complete culture medium (Shanghai Zhong Qiao Xin Zhou
Biotechnology, Co., Ltd.). After centrifugation (room temperature,
20–25°C) at 200 × g for 5 min, the supernatant was removed, and the
cells were resuspended in fresh complete culture medium before
being transferred to T-25 culture flasks for further cultivation
(37°C with 5% CO2). Euthanasia was performed on the rats
used in the aforementioned experiments.
Lentiviral transfection
Before lentiviral infection, adherent FAPs (primary
isolation and cultivation in the laboratory) were seeded at
3×105 cells per well in a 6-well plate and cultured
until reaching ~70% confluence within 18–24 h. Lentiviral
transduction was performed using lentivirus produced from 293T
cells (iCell Bioscience). The lentiviral vector plasmid (5 µg),
along with packaging plasmid pH1 (3.75 µg) and envelope plasmid pH2
(1.25 µg), was transfected into 293T cells using 20 µl PEI
transfection reagent. Transfection was carried out at 37°C for 24
h. After transfection, lentiviral particles were collected. The
culture medium was replaced with 10 ml fresh medium at 24 h
post-transfection, and supernatants containing lentivirus were
harvested at 48 h. The collected supernatant was centrifuged at 500
× g for 10 min to remove cellular debris. The virus-containing
supernatant was either directly used for infection or stored at
−80°C. The multiplicity of infection for lentiviral transduction
was 300. For lentiviral infection, target cells were exposed to
lentiviral suspension for 24 h at 37°C, followed by medium
replacement. Cells were subsequently selected using 2.5 µg/ml
puromycin for stable expression. Images were captured using a
fluorescence microscope to confirm transduction efficiency.
Experiments were performed 24 h after transduction. The lentivirus
used for transduction was provided in a concentrated format (100X)
and includes a fluorescent tag. Images were captured using a
fluorescence microscope.
Western blotting
Cellular proteins were lysed in a buffer containing
9M urea and a mixture of HALT protease inhibitor (cat. no. 78430;
Thermo Fisher Scientific, Inc.) and HALT phosphatase inhibitor
(cat. no. 78428; Thermo Fisher Scientific, Inc.). Cytoplasmic and
nuclear extracts were prepared using the NE-PER Nuclear and
Cytoplasmic Extraction Kit (cat. no. 78835; Thermo Fisher
Scientific, Inc.) and kept on ice during processing. The lysates
were centrifuged at 16,000 × g for 15 min at 4°C to collect the
supernatant. The protein concentration of the lysates was
determined using the Pierce BCA Protein Assay Kit (cat. no. 23225;
Thermo Fisher Scientific, Inc.). The assay was conducted by
preparing a standard curve with concentrations of 0, 0.25, 0.5, 1,
2, 3 and 5 mg/ml. Each standard and sample (diluted 50×) was
measured in triplicate with 20 µl in a 96-well plate, followed by
the addition of 200 µl BCA working reagent. Plates were incubated
at 37°C for 30 min before measuring absorbance at 562 nm.
Equal amounts of denatured proteins (20 µg per lane)
were dissolved on 4–20% or 12% Mini-PROTEAN TGX Precast Gels
(Bio-Rad Laboratories, Inc.) and transferred onto nitrocellulose
membranes. The membranes were blocked with 5% skimmed milk in TBST
(0.1% Tween-20) for 30 min at room temperature. For phosphoprotein
analysis, 5% BSA in TBST was used instead. The membranes were then
incubated with the following primary antibodies: BACH1 (1:1,000;
cat. no. sc-271211; Santa Cruz Biotechnology, Inc.), HO-1 (1:1,000;
cat. no. MA1-112; Thermo Fisher Scientific, Inc.), HK2 (1:2,000;
cat. no. PA5-29326; Thermo Fisher Scientific, Inc.), β-actin (cat.
no. A228; MilliporeSigma), GAPDH (1:1,000; cat. no. G9295;
MilliporeSigma), Histone 3 (1:5,000; cat. no. ab1791; Abcam), NQ01
(1:2,000; cat. no. HPA007308; MilliporeSigma), KEAP1 (1:1,000; cat.
no. 8047S; Cell Signaling Technology, Inc.) and NRF2 (1:3,000; cat.
no. 12721; Cell Signaling Technology, Inc.). The membranes were
incubated with the primary antibodies overnight at 4°C. The next
day, the membranes were brought to room temperature for 1 h prior
to washing with TBST three times (5 min each).
The membranes were then incubated with secondary
antibodies at room temperature for 1 h. The secondary antibodies
used were HRP-conjugated Goat Anti-Rabbit IgG (H+L) (1:3,000; cat.
no. GB23303; Wuhan Servicebio Technology Co., Ltd.) and
HRP-conjugated Goat Anti-Mouse IgG (H+L) (1:5,000; cat. no.
GB23301; Wuhan Servicebio Technology Co., Ltd.). Detection was
conducted using Clarity Western ECL Substrate (cat. no. 1705061;
Bio-Rad Laboratories, Inc.) and ChemiDoc Touch Imaging System (cat.
no. 1708370; Bio-Rad Laboratories, Inc.). The densitometric
analysis of bands was performed using ImageJ software (version
1.53k; National Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was conducted using the 2^-ΔΔCq method for
relative quantification. RNA was extracted using the RNAsimple
Total RNA Kit (Shanghai Yihui Biological Technology Co., Ltd.).
mRNA was reverse transcribed using the SureScript First-strand cDNA
Synthesis Kit (Guangzhou Saivell Biotechnology Co., Ltd.). Reverse
transcription was conducted under the following conditions: 25°C
for 5 min, 50°C for 15 min, 85°C for 5 sec, and held at 4°C. qPCR
reactions were performed using SYBR Green as the fluorophore (Wuhan
Servicebio Technology Co., Ltd) on a CFX96 Real-Time PCR System
(Bio-Rad Laboratories, Inc.). The thermocycling conditions were as
follows: Initial denaturation: 95°C for 1 min; denaturation: 95°C
for 20 sec; annealing: 55°C for 20 sec; extension: 72°C for 30 sec
(for a total of 40 cycles). The 2−∆∆Cq method was used
for quantification (23). The
primer information used is included in Table SI. β-actin was used as the
reference gene.
Bioinformatics analysis
The procured dataset GSE103266 was obtained from the
Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) of the National
Center for Biotechnology Information. This dataset included data
from bilateral supraspinatus tears and suprascapular nerve
resection surgeries conducted on rodent models. Samples were
collected at 0, 10, 30 and 60 days after injury, with a sample size
of n=4 per group. Untreated specimens were utilized as controls.
RNA sequencing of muscle tissue was conducted to acquire the needed
data. After data acquisition, the DESeq2 package (v1.38.3) of R was
employed for differential analysis. The GSVA package (v1.46.0) was
utilized for immune infiltration and single-sample Gene Set
Enrichment Analysis (ssGSEA), while the ggpmisc package (v0.5.5)
was utilized for correlation analysis. Enrichment analysis was
conducted utilizing the KOBAS (http://bioinfo.org/kobas/).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 8 (Dotmatics). Data are presented as the mean ± standard
error of the mean (SEM). Measurement data were compared between two
groups using unpaired t-tests, and one-way ANOVA was used for
multiple-group comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Rotator cuff injury significantly
activates the PI3K/Akt, mTOR and lipid metabolism-related
pathways
The effect of rotator cuff injury was analyzed using
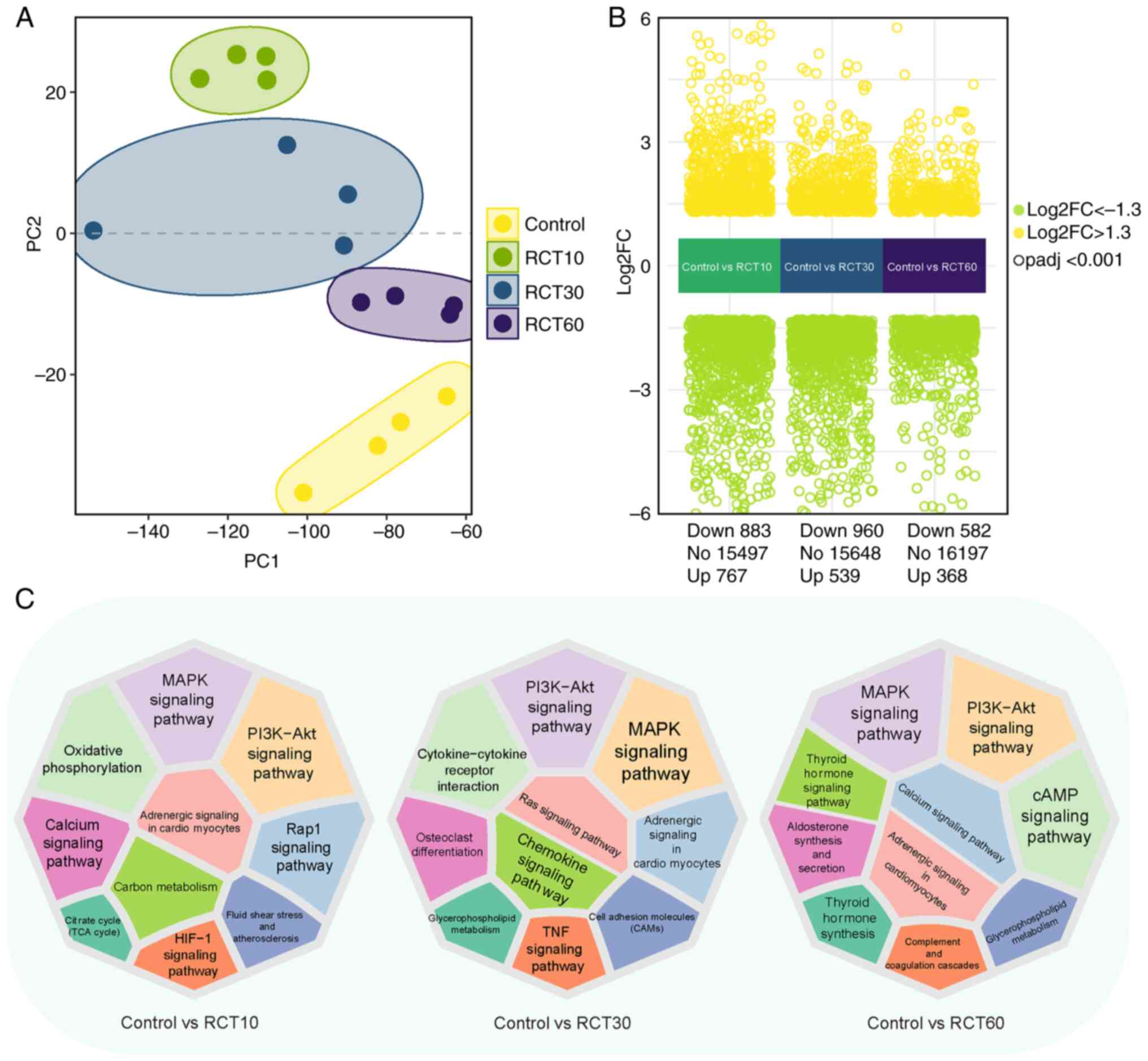
dataset GSE103266. Principal component analysis (PCA) revealed
distinct separation among control and treated groups at 10, 30 and
60 days after injury (Fig. 1A).
Compared with controls, differential gene expression analysis
identified 767, 539 and 368 upregulated and 883, 960 and 582
downregulated genes after 10, 30 and 60 days, respectively
(Fig. 1B). Differential gene
expression in response to RCT revealed significant alterations in
multiple pathways. Specifically, the PI3K-Akt, mTOR and lipid
metabolism pathways showed the most pronounced changes, with
upregulation of adipogenesis-related genes (FABP4 and PPARγ) and
downregulation of lipid degradation pathways. Enrichment analysis
using the KOBAS platform illuminated the significant role of fatty
acid metabolism, oxidative phosphorylation and immune response
pathways, underscoring the complex interplay between muscle
degeneration, inflammation and fat accumulation after RCT (Fig. 1C) (24).
Rotator cuff injury suppresses fat
metabolism and enhances immune activation
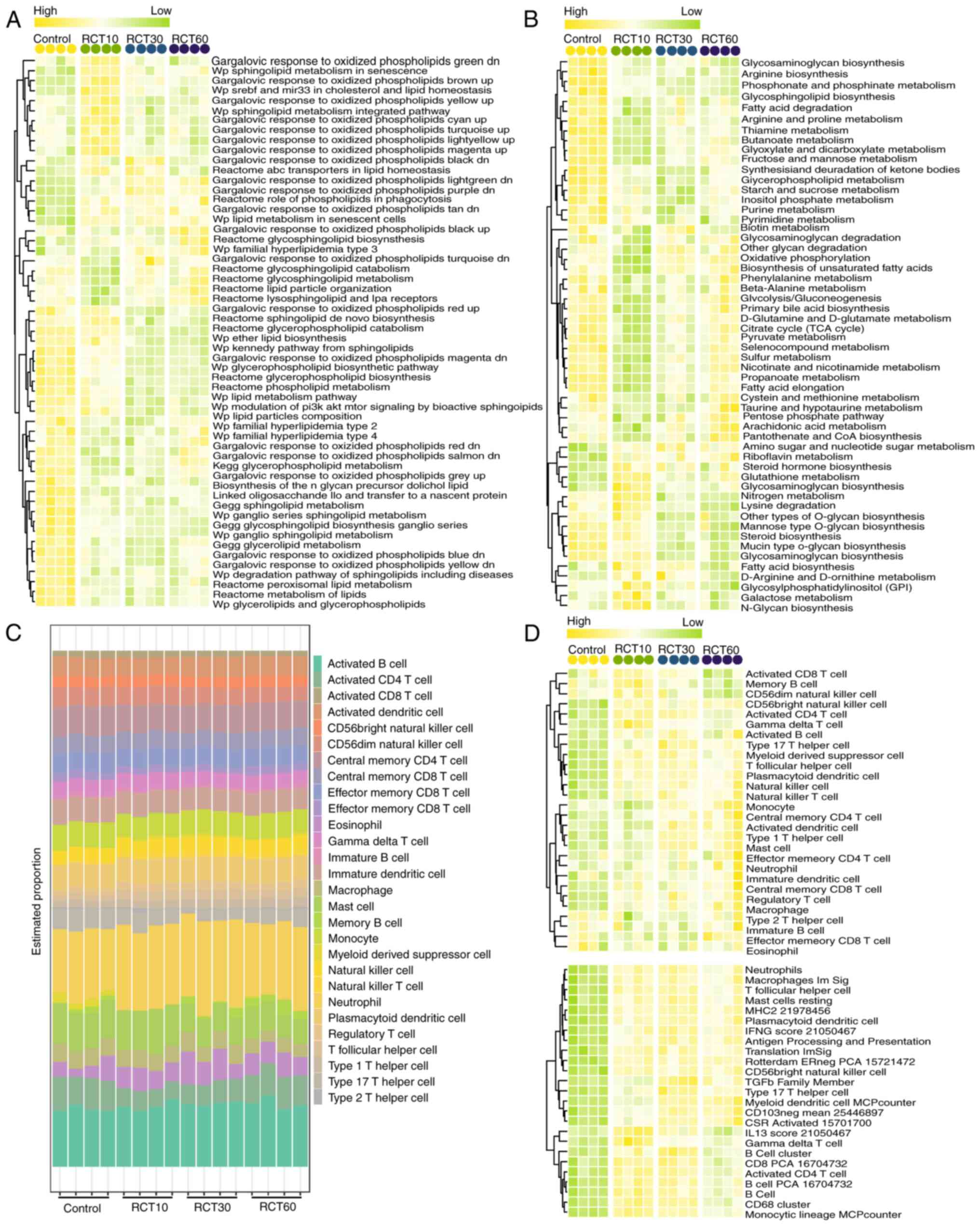
ssGSEA scoring of Kyoto Encyclopedia of Genes and
Genomes (KEGG) pathways associated with fat and other metabolisms
was conducted based on differential gene expression to investigate
the effect of rotator cuff injury on physiological functions and
metabolic pathways. ANOVA revealed significant enrichment of 55 and
56 pathways, respectively (Fig. 2A and
B). Fat metabolism-related pathways, such as Reactome
glycerophospholipid catabolism and Reactome peroxisomal lipid
metabolism, were downregulated, while lipid biosynthesis pathways
were upregulated. Additionally, rotator cuff injury modulated
cellular functions, signaling pathways and disease-related pathways
(Fig. 2A). KEGG enrichment
analysis showed the suppression of metabolic pathways, such as
fatty acid degradation and glycolysis/gluconeogenesis, and only a
few metabolic pathways were upregulated (Fig. 2B). Immune infiltration analysis
identified 28 immune-related cells under different treatments
(Fig. 2C), and scoring analysis
demonstrated the activation of 25 immune-related cells subsequent
to rotator cuff injury (Fig. 2D).
These findings indicated that rotator cuff injury strongly
modulated fat metabolism, primarily affecting physiological
responses at the site of injury by inhibiting fat metabolism
pathways and promoting immune activation.
NEDD4 is a key regulator of fat
metabolism and immune activation after rotator cuff injury
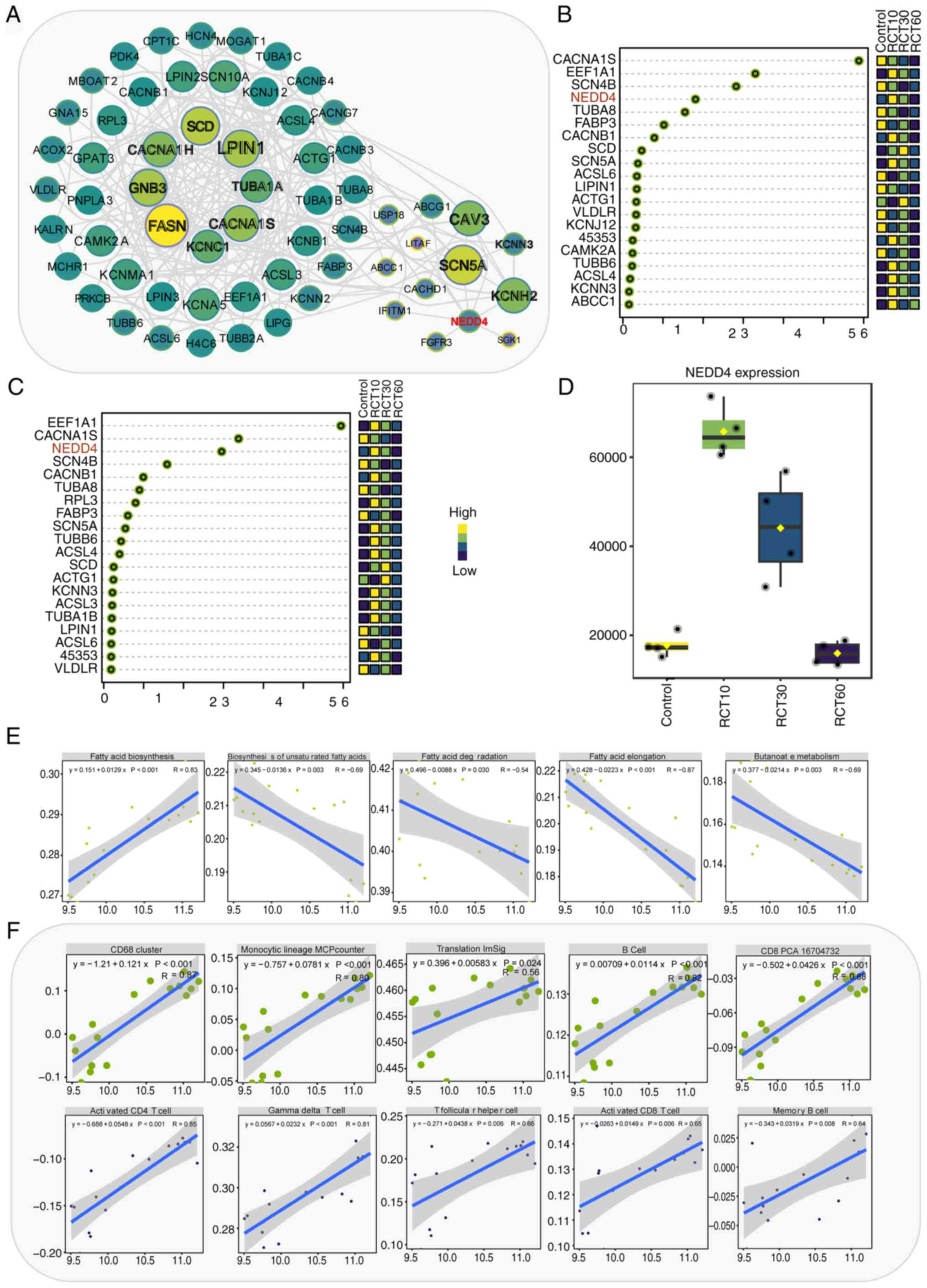
Functional predictions on differential genes across
different groups were performed to elucidate the role of key genes
in abnormal fat metabolism induced by rotator cuff injury. The
results revealed a significant presence of fat metabolism-related
genes in the group treated for 10 days. Gene regulatory network
analysis identified the key roles of genes SCD, CACNA1H, GNB3,
FASN, LPIN1, TUBA1A, CACNA1S, KCNC1, CAV3, SCN5A, KCNH2 and NEDD4
(Fig. 3A). Next, PCA analysis was
conducted on all genes within the network across the four groups.
Thereafter, the top 20 genes that contributed the most to the two
principal components (PC1 and PC2) were selected. By integrating
each gene's contribution to both principal components, CACNA1S,
EEF1A1 and NEDD4 were identified (Fig.
3B and C). NEDD4, an important E3 ubiquitin ligase, exhibited
significantly increased expression after 10 and 30 days of
treatment compared with controls (Fig.
3D). Correlation analysis indicated the close association of
NEDD4 with fat-related signaling pathways, KEGG signaling pathway
and immune infiltration scores (Fig.
3E and F; Fig. S2, Fig. S3, Fig. S4). It was hypothesized that NEDD4
plays a crucial role in rotator cuff injury by regulating fat
metabolism and immune activation.
NEDD4 is a critical regulator of mTOR,
PI3K-Akt and fatty acid metabolic pathways in rotator cuff
injury
To unravel the regulatory mechanism of NEDD4 in
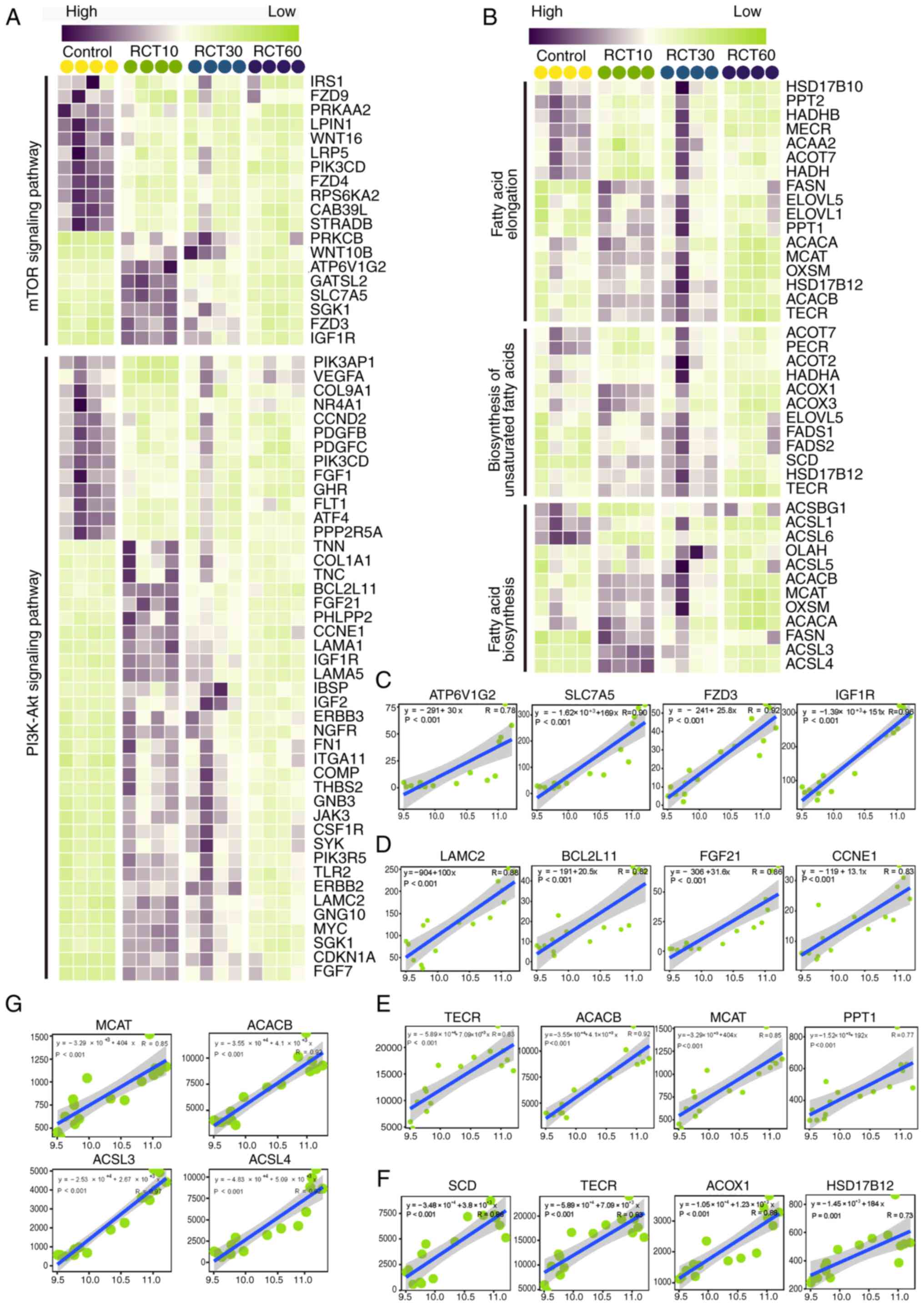
rotator cuff injury, its associations with relevant pathways were
investigated. Gene expression analysis revealed that rotator cuff
injury led to the differential expression of genes related to the
mTOR and PI3K/Akt signaling pathways (Fig. 4A), fatty acid biosynthesis,
unsaturated fatty acid biosynthesis and fatty acid elongation
pathways (Fig. 4B). In the present
study, mTOR activation was assessed as a key component in
accelerating bone-tendon healing in the context of rotator cuff
injury. Correlation analysis showed that NEDD4 was significantly
and positively correlated with genes in the mTOR pathway (ATP6V1G2,
SLC7A5, FZD3 and IGF1R) and the PI3K/Akt pathway (LAMC2, BCL2L11,
FGF21 and CCNE1) (Fig. 4C and D,
respectively). In addition to the listed genes, numerous other
genes were highly correlated with NEDD4 in these pathways (Figs. S5 and S6). Furthermore, NEDD4 was significantly
and positively correlated with genes in fatty acid biosynthesis,
unsaturated fatty acid biosynthesis and fatty acid elongation
(TECR, ACACB, MCAT, PPT1, SCD, ACOX1, HSD17B12, ACSL3 and ACSL4)
(Fig. 4E and G). These findings
suggested that NEDD4 regulated multiple metabolic pathways,
regulating fat metabolism and other physiological functions at the
site of rotator cuff injury in rats.
Successful establishment of NEDD4
interference and overexpression models in rats to assess the role
of NEDD4 in rotator cuff injury
Interference and overexpression vectors were
constructed to investigate the role of NEDD4 in rat rotator cuff
injury. Interference vectors included sh-NC (empty vector),
sh-NEDD4-1, sh-NEDD4-2 and sh-NEDD4-3, while overexpression vectors
included OE-NC and OE-NEDD4. NEDD4 expression levels were assessed
using RT-qPCR, with the lowest expression observed in rats treated
with sh-NEDD4-1 (Fig. S7A).
Fluorescence staining confirmed successful vector packaging, with
over 90% of vectors exhibiting high fluorescence intensity
(Fig. S7C).
Rats underwent NEDD4 interference and overexpression
treatments to evaluate the effects of vector on rotator cuff
injury. Monitoring body weight over 49 days revealed significant
differences between groups (P<0.05) but there were no overall
differences (Fig. S7B),
suggesting the successful establishment of the rat model. Blood and
tendon attachment tissues were collected from each group for
further analysis.
NEDD4 promotes tissue recovery and
reduces apoptosis in rat rotator cuff injury
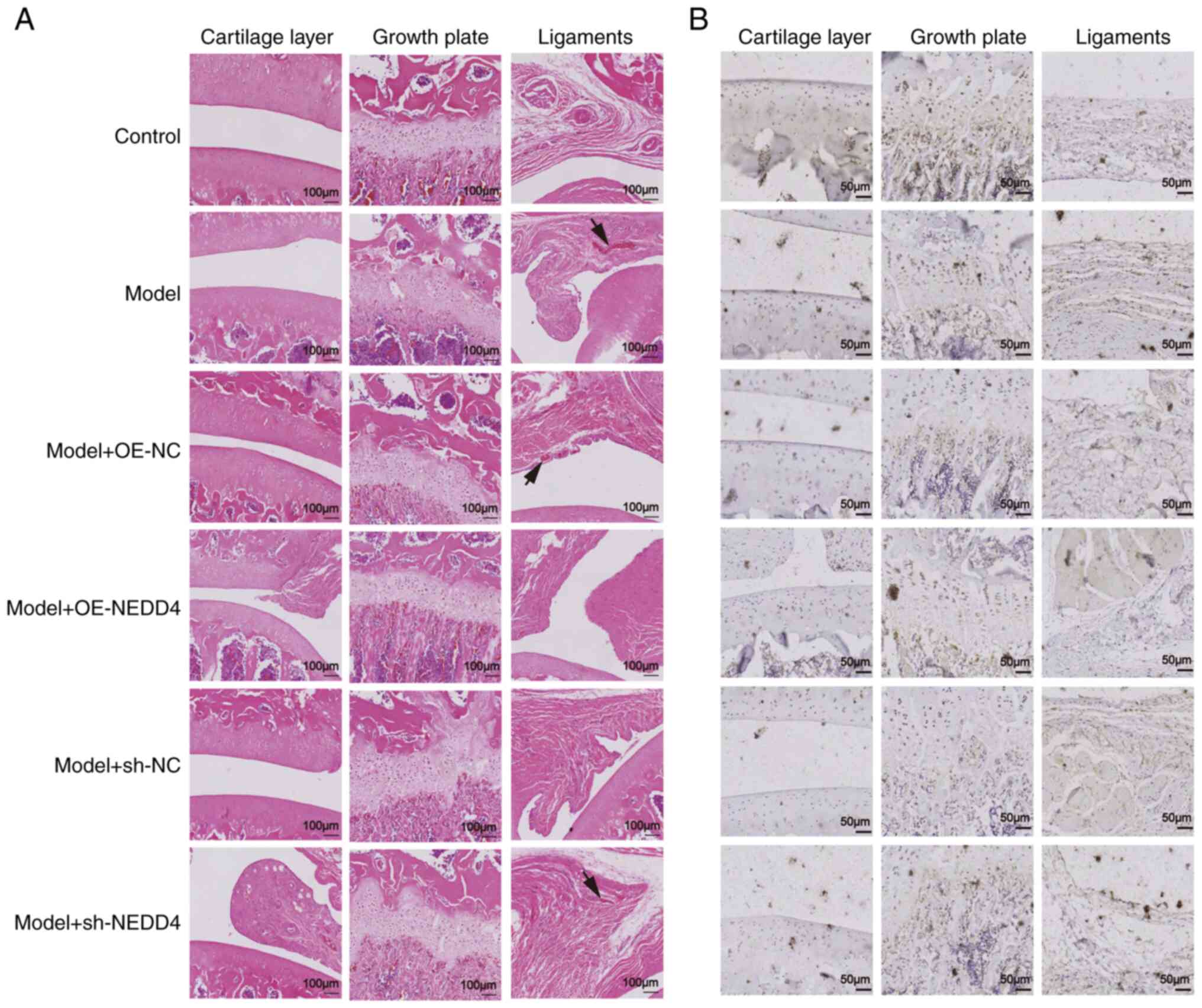
Histological examination of rat rotator cuff
injuries using H&E staining revealed distinct differences among
the treatment groups. In the control group, chondrocytes were
well-organized in the growth plate, and inflammatory bodies were
minimal within the ligament, indicating healthy tissue structure.
In the model group, there was a marked increase in the abundance of
inflammatory bodies in the ligament, accompanied by disorganized
chondrocytes in the growth plate, suggesting significant tissue
damage., Compared with the empty vector group, the NEDD4
overexpression group displayed fewer inflammatory bodies in the
ligament and better-organized chondrocytes, indicating enhanced
tissue recovery. Conversely, the NEDD4 knockdown group showed a
higher number of inflammatory bodies and more disorganized cells,
suggesting severe tissue damage (Fig.
5A).
To further elucidate the role of NEDD4, TUNEL
staining was conducted on rotator cuff cells. The model group
exhibited a significant increase in cell apoptosis compared with
the control group, highlighting the detrimental effect of rotator
cuff injury on cell viability (Fig.
S8). The apoptotic rate was significantly lower in the NEDD4
overexpression group than in the empty vector group, suggesting
that NEDD4 promoted normal cell proliferation and mitigated cell
death (Fig. 5B). On the other
hand, the NEDD4 knockdown group showed a slightly higher rate of
apoptosis compared with the empty vector group, indicating that low
NEDD4 expression impairs normal cell proliferation and exacerbates
tissue damage.
These detailed observations underscore the critical
role of NEDD4 in regulating cell apoptosis and promoting tissue
repair after rotator cuff injuries.
NEDD4 regulates fat metabolism and
reduces lipid accumulation in rotator cuff injury
Oil Red O staining was applied to tissue samples
from each treatment group to investigate the effect of fat
infiltration on rotator cuff injuries. The results revealed an
increase in the abundance of lipid droplets in the model group
compared with the control group, underscoring the significance of
fat deposition in rotator cuff injury (Fig. 6A and B). Notably, NEDD4
overexpression reduced the abundance of lipid droplets, suggesting
its role in mitigating fat deposition. By contrast, NEDD4 knockdown
resulted in increased lipid accumulation compared with the empty
vector control, highlighting the potential of NEDD4 as a
therapeutic target (Fig. 6B).
Further molecular experiments were conducted to elucidate the
relationship between NEDD4 activity and the expression of
adipogenic genes. RT-qPCR revealed that the model group exhibited
elevated levels of adipogenic markers C/ebpα, PPARγ and
Fabp4, with corresponding reductions in PI3k, Akt and mTOR,
suggesting impaired fat metabolism pathways (Fig. 6C). Conversely, NEDD4 overexpression
normalized their expression levels, reducing C/ebpα, PPARγ
and Fabp4 expression levels and enhancing PI3k, Akt and mTOR
expression levels. These findings demonstrated the regulatory
effects of NEDD4 on these pathways. The knockdown group mirrored
the model group's trends, further validating the regulatory role of
NEDD4 in fat metabolism (Fig. 6C).
Western blotting confirmed the transcriptional trends observed in
RT-qPCR, with protein expression patterns aligning closely across
groups. The model group showed increased levels of adipogenic
proteins, which were moderated in the NEDD4 overexpression group,
illustrating the function of these genes in controlling adipocyte
differentiation and fat deposition (Fig. 6D and E). These results collectively
suggested that NEDD4 not only affects adipocyte differentiation but
also plays a crucial role in regulating fat metabolism, potentially
offering a novel intervention for managing rotator cuff injuries by
targeting fat infiltration.
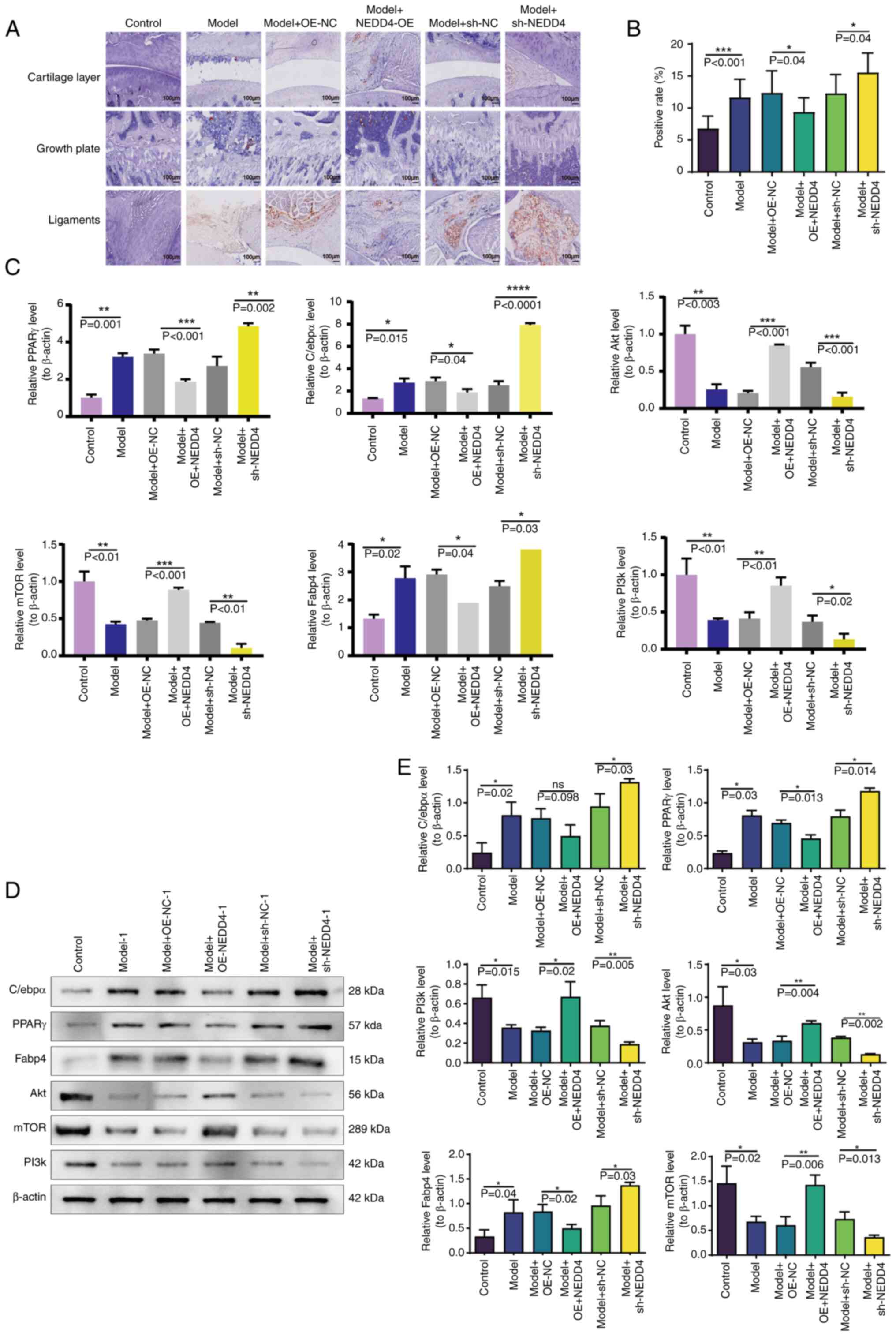 | Figure 6.Research results on NEDD4 and fat
formation and depletion in rotator cuff injuries. (A) Oil Red O
staining results of rotator cuff tissue showing lipid droplets. (B)
Histogram demonstrating lipid droplet positivity in rotator cuff
tissue across different treatment groups. (C) Reverse
transcription-quantitative PCR results for C/ebpα, PPARγ,
Fabp4, PI3k, Akt and mTOR expression levels. (D) Western blotting
results displaying protein levels of C/ebpα, PPARγ, Fabp4,
PI3k, Akt and mTOR. (E) Histogram showing protein levels of
C/ebpα, PPARγ, Fabp4, PI3k, Akt and mTOR across different
treatment groups. *P<0.05, **P<0.01, ***P<0.001 and
****P<0.0001. PPARγ, peroxisome proliferator-activated
receptor γ; OE, overexpression; sh- short hairpin; NC,
negative control. |
NEDD4 inhibits FAP differentiation
into adipocytes
In vitro experiments were performed to
investigate the effect of NEDD4 on the differentiation of FAPs. FAP
cells underwent NEDD4 knockdown and overexpression, and lentiviral
infection status was monitored by fluorescence at 0, 4 and 8 days
after infection (Fig. 7A). These
results suggested that NEDD4 may regulate the differentiation of
FAP cells, with a greater effect after prolonged lentiviral
infection. Fluorescence intensity increased over time in both
groups, indicating that the role of NEDD4 in regulating FAP
differentiation may be greater after longer lentiviral infection.
After knockdown and overexpression interventions, RT-qPCR was
conducted to measure NEDD4 levels in FAP cells to verify vector
effectiveness. NEDD4 knockdown in FAP cells decreased NEDD4 levels
on days 4 and 8, while NEDD4 overexpression increased NEDD4 levels
on those days. Both treatments showed significant differences
compared with the control group. This suggests that the lentiviral
vector affected gene expression, modulated the transcription or
translation of target genes, and altered NEDD4 expression. Over
time, NEDD4 levels significantly increased in the NEDD4
overexpression group (Fig. 7B),
suggesting a stronger regulatory effect on the differentiation of
FAPs with time. Oil Red O staining was performed on FAP cells after
NEDD4 overexpression and knockdown to confirm the role of NEDD4 in
regulating differentiation into adipocytes. Over time, the lipid
droplet rate increased in both control groups and the NEDD4
knockdown group, while decreasing in the NEDD4 overexpression group
(Fig. 7C and D). These findings
suggested that NEDD4 overexpression can inhibit lipid droplet
accumulation, indicating that NEDD4 suppresses FAP cell
differentiation into adipocytes. Therefore, in rotator cuff
injuries with fat infiltration, NEDD4 may be a key factor
inhibiting the differentiation of FAP cells into adipocytes.
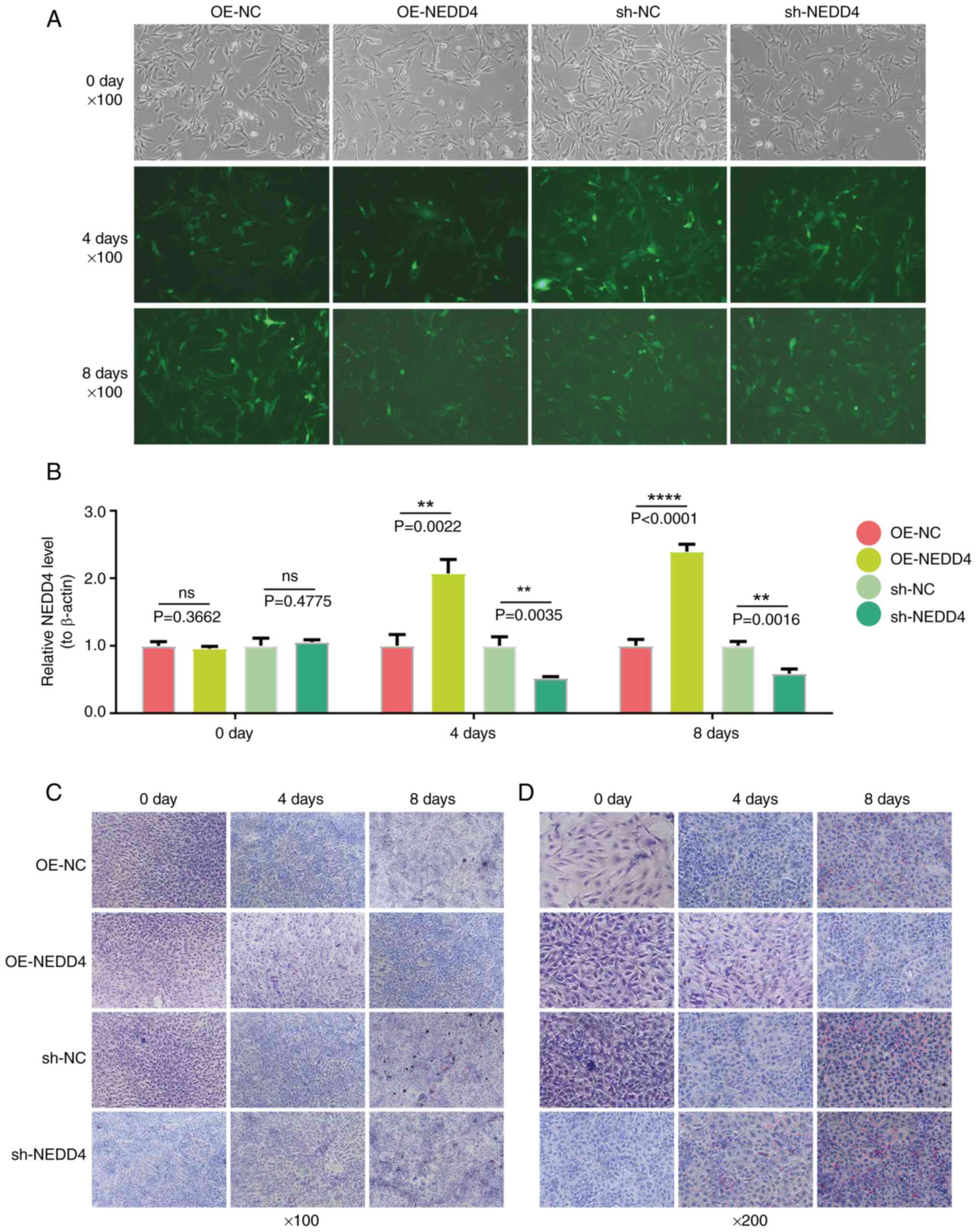 | Figure 7.Analysis of NEDD4′s regulation of
FAPs' cell differentiation. (A) Fluorescence results showing
lentiviral transfection of FAP cells at 0, 4- and 8-days
post-infection for OE-NC, OE-NEDD4, sh-NC and sh-NEDD4. (B) qPCR
detection results of NEDD4 levels in FAP cells at 0, 4 and 8 days
post-infection for 4group. (C) Oil Red O staining results of FAP
cells at 100× magnification showing lipid droplets at 0, 4- and
8-days post-infection for 4 group. (D) Oil Red O staining results
of FAP cells at ×200 magnification showing lipid droplets at 0, 4-
and 8-days post-infection for 4 group. **P<0.01 and
****P<0.0001. FAP, fibro-adipogenic progenitor; OE,
overexpression; sh- short hairpin; NC, negative control; ns, no
significance. |
Discussion
The present study explored the therapeutic
mechanisms of rotator cuff injury using a rat model and in
vitro experiments with FAP cells. The present findings revealed
that NEDD4 protects against fat infiltration and promotes the
recovery of rotator cuff injury by modulating the mTOR signaling
pathway.
Consistent with previous findings, H&E staining
showed that NEDD4 overexpression positively affects tissue recovery
(25). Furthermore, TUNEL staining
indicated that NEDD4 negatively regulates apoptosis, thereby
decreasing the severity of rotator cuff injuries, promoting cell
proliferation, and facilitating tissue repair (26). As a member of the HECT E3 ubiquitin
ligase family, NEDD4 plays a key role in the ubiquitination
process. It tags specific substrate proteins for proteasomal
degradation, thus regulating their abundance and activity (27). In the context of rotator cuff
injuries, NEDD4 regulates fat metabolism and cell signaling by
ubiquitinating key regulators of adipogenesis and inflammation.
This process affects the cellular response to harmful stimuli,
including the differentiation of FAP cells into adipocytes and the
activation of the mTOR pathway, both of which are critical for
tissue repair (28,29).
Rotator cuff injuries involve complex molecular
signaling, in which protein ubiquitination is essential for
regulating protein function, stability and cellular localization
(30,31). This process can affect muscle
inflammation, apoptosis and autophagy, thereby controlling the
viability and function of myocytes (32,33).
Therefore, NEDD4 may play a pivotal role in modulating fat
metabolism and tissue repair by stabilizing key proteins in
critical signaling pathways (34).
By ubiquitinating specific proteins, NEDD4 ensures their
degradation in the proteasome, thereby precisely controlling their
abundance and activity (35). In
the context of rotator cuff injuries, this mechanism is crucial for
regulating fat infiltration and promoting tissue repair (36,37).
The present study underscored the importance of
NEDD4 in regulating fat deposition after rotator cuff injury.
Specifically, a significant and positive correlation was found
between NEDD4 and several lipid metabolism-related genes, such as
ATP6V1G2, SLC7A5 and FZD3 (38).
IGF1R and SLC7A5 are pivotal for adipocyte growth and nutrient
uptake (39), while FGF21, ACOX1
and ACSL3/4 play central roles in fatty acid oxidation and lipid
catabolism (40–43). NEDD4 may exert its effects by
ubiquitinating these targets, thus regulating lipid synthesis,
fatty acid oxidation and energy homeostasis (44). This correlation highlights the
potential role of NEDD4 in metabolic disorders, such as obesity and
metabolic syndrome whose hallmark is dysregulated lipid metabolism
(45).
Moreover, the present study demonstrated that NEDD4
overexpression significantly reduced fat deposition in rotator cuff
tissues, evidenced by decreased expression of adipogenic markers,
such as C/EBPα, PPARγ and Fabp4. This suggests that NEDD4 inhibits
adipocyte differentiation and fat accumulation, providing a more
favorable environment for tissue repair. Conversely, NEDD4
knockdown leads to increased fat deposition, which is associated
with impaired tissue repair. These findings indicate that NEDD4 may
mitigate fat infiltration by regulating the expression of genes
involved in adipogenesis and lipid metabolism, thereby promoting
tissue repair.
Consistent with previous studies, Oil Red O staining
also confirmed the pathological role of fat deposition in rotator
cuff injury (2). Fat cell
infiltration may hinder skeletal muscle function and contribute to
the development of injuries (46,47).
The current experimental results revealed that NEDD4 overexpression
reduces fat deposition in rotator cuff injuries, while its
inhibition exacerbates this condition. Thus, NEDD4 may promote
tissue recovery by preventing fat deposition. In vitro
experiments showed that NEDD4 significantly inhibited the
differentiation of FAP cells into adipocytes. NEDD4 overexpression
prevented lipid accumulation in FAPs, whereas NEDD4 knockdown
exhibited the opposite effect. These results suggest that NEDD4 is
a potential therapeutic target for preventing fat infiltration in
rotator cuff injuries (48).
Mechanistically, the effects of NEDD4 may be
mediated through the ubiquitination and degradation of key
adipogenic regulators, such as PPARγ and C/EBPα, which promote
adipogenesis (49,50). By facilitating the ubiquitination
of these factors, NEDD4 reduces their stability, thereby inhibiting
adipogenesis. The direct regulatory effects of adipogenesis-related
transcription factors further underscore the potential of NEDD4 in
controlling fat infiltration in pathological conditions, where
abnormal FAP differentiation leads to muscular dysfunction and
impaired tendon healing (51,52).
Additionally, NEDD4 regulates the PI3K/Akt/mTOR
axis, affecting lipid metabolism and cell viability (53). NEDD4 overexpression activates the
mTOR pathway, which is associated with the inhibition of
adipogenesis, enhanced myocyte regeneration and accelerated tissue
repair (54). By contrast, NEDD4
knockdown suppresses the mTOR pathway, leading to the
differentiation of FAP into adipocytes, thereby exacerbating fat
deposition in injured rotator cuff tissues. This mechanism explains
the correlation between fat infiltration and impaired healing in
rotator cuff injuries.
Overall, the present study elucidated the critical
role of NEDD4 in the recovery of rotator cuff injury, by regulating
protein ubiquitination and fat metabolism. Through protein
ubiquitination, NEDD4 exerts a dual role in lipid metabolism. On
one hand, it inhibits adipocyte differentiation and reduces fat
deposition by regulating adipogenesis-related genes. On the other
hand, it affects lipid metabolism and cell viability through key
signaling pathways, such as the PI3K/Akt/mTOR axis, thereby
promoting tissue repair. The mechanism of NEDD4 provides a deeper
insight into the relationship between fat infiltration and impaired
healing of rotator cuff injuries.
Although NEDD4 overexpression shows beneficial
effects in rotator cuff injuries, further studies are needed to
uncover additional mechanisms (35). Moreover, NEDD4 may interact with
other proteins to form complexes that indirectly regulate lipid
synthesis by affecting the function or stability of these proteins
(55). NEDD4 may also regulate
autophagy in adipocytes by promoting fatty acid degradation and
enhancing energy consumption, thus reducing fat deposition
(45,56). Additionally, NEDD4 may modulate the
insulin signaling pathway, weakening the effect of insulin on lipid
synthesis and deposition (56,57).
In conclusion, NEDD4 reduced fat infiltration in
rotator cuff injury by targeting the mTOR signaling pathway and
regulating protein ubiquitination, thereby enhancing protein
stability in key signaling pathways involved in fat metabolism and
tissue repair. The present study highlighted the significance of
NEDD4 in modulating the mTOR pathway, reducing fat infiltration,
and promoting tissue repair in rotator cuff injuries, suggesting
its potential as a therapeutic target for improving the outcomes of
rotator cuff injury.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Guizhou Provincial
Natural Science Foundation [grant no. Qiankehebasis-ZK (2024)
genera 241], the Start-up Fund for Doctoral Research at the
Affiliated Hospital of Guizhou Medical University (grant no.
gyfybsky-2022-38) and the National Natural Science Foundation of
China Cultivation Program of Affiliated Hospital of Guizhou Medical
University [grant no. gyfynsfc (2023)-62].
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JL conceptualized the study, developed methodology,
validated and curated data, wrote the original draft, and wrote,
reviewed and edited the manuscript. YP performed validation and
formal analysis. DZ conducted software analysis. CG conceptualized
the study, performed formal analysis, data curation and project
administration and acquired funding. WP conceptualized the study,
developed methodology curated data, and performed project
administration. JL and CG confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
KY20230718-16) by the Experimental Animal Welfare and Ethics
Committee of Beijing Jishuitan Hospital Guizhou Hospital (Guiyang,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Harada Y, Yokoya S, Sumimoto Y, Iwahori Y,
Kajita Y, Deie M and Adachi N: Prevalence of rotator cuff tears
among older tennis players and its impact on clinical findings and
shoulder function. J Sport Rehabil. 31:849–855. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Merriman MA Jr, Chapman JH, Whitfield T,
Hosseini F, Ghosh D and Laurencin CT: Fat expansion not fat
infiltration of muscle post rotator cuff tendon tears of the
shoulder: Regenerative engineering implications. Regen Eng Transl
Med. 1–14. 2023.
|
|
3
|
Zhu Y, Hu Y, Pan Y, Li M, Niu Y, Zhang T,
Sun H, Zhou S, Liu M, Zhang Y, et al: Fatty infiltration in the
musculoskeletal system: Pathological mechanisms and clinical
implications. Front Endocrinol. 15:14060462024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alenabi ST: Modifications in early
rehabilitation protocol after rotator cuff repair: EMG Studies
(unpublished PhD thesis). University of Montreal; 2016
|
|
5
|
Fu C, Huang AH, Galatz LM and Han WM:
Cellular and molecular modulation of rotator cuff muscle
pathophysiology. J Orthop Res. 39:2310–2322. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee S, Park I, Lee HA and Shin SJ: Factors
related to symptomatic failed rotator cuff repair leading to
revision surgeries after primary arthroscopic surgery. Arthroscopy.
36:2080–2088. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giuliani G, Rosina M and Reggio A:
Signaling pathways regulating the fate of fibro/adipogenic
progenitors (FAPs) in skeletal muscle regeneration and disease.
FEBS J. 289:6484–6517. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Theret M, Rossi FMV and Contreras O:
Evolving roles of muscle-resident fibro-adipogenic progenitors in
health, regeneration, neuromuscular disorders, and aging. Front
Physiol. 12:6734042021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Subhash AK, Davies M, Gatto A, Bogdanov
JM, Lan R, Jensen A, Feeley BT and Petrigliano FA:
Fibro-adipogenesis in injured rotator cuff muscle. Curr Tissue
Microenviron Rep. 3:1–9. 2022. View Article : Google Scholar
|
|
10
|
Parker EG: The Role of Fibro-Adipogenic
Progenitor Cells (FAPs) in Muscle Disuse Atrophy. Augusta
University; 2022
|
|
11
|
Bogdanov J, Lan R, Chu TN, Bolia IK, Weber
AE and Petrigliano FA: Fatty degeneration of the rotator cuff:
Pathogenesis, clinical implications, and future treatment. JSES Rev
Rep Tech. 1:301–308. 2021.PubMed/NCBI
|
|
12
|
Wang X, Xu M and Li Y: Adipose tissue
aging and metabolic disorder, and the impact of nutritional
interventions. Nutrients. 14:31342022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen W, You W, Valencak TG and Shan T:
Bidirectional roles of skeletal muscle fibro-adipogenic progenitors
in homeostasis and disease. Ageing Res Rev. 80:1016822022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caballero-Sánchez N, Alonso-Alonso S and
Nagy L: Regenerative inflammation: When immune cells help to
re-build tissues. FEBS J. 291:1597–1614. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu GY and Sabatini DM: mTOR at the nexus
of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol.
21:183–203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Querfurth H and Lee HK:
Mammalian/mechanistic target of rapamycin (mTOR) complexes in
neurodegeneration. Mol Neurodegener. 16:442021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Conway JA, Kinsman G and Kramer ER: The
role of NEDD4 E3 ubiquitin-protein ligases in Parkinson's disease.
Genes. 13:5132022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fitzgerald G: Fibro-adipogenic progenitor
heterogeneity in healthy and diseased skeletal muscle. ETH Zurich.
2022.
|
|
19
|
Szwed A, Kim E and Jacinto E: Regulation
and metabolic functions of mTORC1 and mTORC2. Physiological
reviews. 101:1371–1426. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yue M, Yun Z, Li S, Yan G and Kang Z:
NEDD4 triggers FOXA1 ubiquitination and promotes colon cancer
progression under microRNA-340-5p suppression and ATF1
upregulation. RNA Biology. 18:1981–1995. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Degen RM, Carbone A, Carballo C, Zong J,
Chen T, Lebaschi A, Ying L, Deng XH and Rodeo SA: The effect of
purified human bone marrow-derived mesenchymal stem cells on
rotator cuff tendon healing in an athymic rat. ARTHROSCOPY.
32:2435–2443. 1016. View Article : Google Scholar
|
|
22
|
Sastourné-Arrey Q, Mathieu M, Contreras X,
Monferran S, Bourlier V, Gil–Ortega M, Murphy E, Laurens C, Varin
A, Guissard C, et al: Adipose tissue is a source of regenerative
cells that augment the repair of skeletal muscle after injury. Nat
Commun. 14:802023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Silva-Vignato B, Coutinho LL, Poleti MD,
Cesar ASM, Moncau CT, Regitano LCA and Balieiro JCC: Gene
co-expression networks associated with carcass traits reveal new
pathways for muscle and fat deposition in Nelore cattle. BMC
Genomics. 20:322019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Qin Y, Wu G, Wang J, Cao J, Wang
Y, Wu D, Yang K, Zhao Z, He L, et al: PRRG4 promotes breast cancer
metastasis through the recruitment of NEDD4 and downregulation of
Robo1. Oncogene. 39:7196–7208. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
King SJ: The role of the energy-sensing
AMP-activated protein kinase in intestinal epithelial physiology
and pathophysiology. University of California; Riverside: 2019
|
|
27
|
Ingham RJ, Gish G and Pawson T: The Nedd4
family of E3 ubiquitin ligases: Functional diversity within a
common modular architecture. Oncogene. 23:1972–1984. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Joe AW, Yi L, Natarajan A, Grand FL, So L,
Wang J, Rudnicki MA and Rossi FMV: Muscle injury activates resident
fibro/adipogenic progenitors that facilitate myogenesis. Nature
Cell Biol. 12:153–163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Molina T, Fabre P and Dumont NA:
Fibro-adipogenic progenitors in skeletal muscle homeostasis,
regeneration and diseases. Open Biol. 11:2101102021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang N, Wang H, Shen L, Liu X, Ma Y and
Wang C: Aging-related rotator cuff tears: Molecular mechanisms and
implications for clinical management. Adv Biol. 8:e23003312024.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Frich LH, Fernandes LR, Schrøder HD,
Hejbøl EK, Nielsen PV, Jørgensen PH, Stensballe A and Lambertsen
KL: The inflammatory response of the supraspinatus muscle in
rotator cuff tear conditions. J Shoulder Elbow Surg. 30:e261–e275.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen W, Chen Y, Liu Y and Wang X:
Autophagy in muscle regeneration: Potential therapies for
myopathies. J Cachexia Sarcopenia Muscle. 13:1673–1685. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang XX, Zhang B, Xia R and Jia QY:
Inflammation, apoptosis and autophagy as critical players in
vascular dementia. Eur Rev Med Pharmacol Sci. 24:9601–9614.
2020.PubMed/NCBI
|
|
34
|
Boase NA and Kumar S: NEDD4: The founding
member of a family of ubiquitin-protein ligases. Gene. 557:113–122.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li J: Effect of Nedd4 haploinsufficiency
on insulin sensitivity, adiposity and neuronal behaviors. The
University of Tennessee Health Science Center. 2014.
|
|
36
|
Baloghova N, Lidak T and Cermak L:
Ubiquitin ligases involved in the regulation of Wnt, TGF-β, and
notch signaling pathways and their roles in mouse development and
homeostasis. Genes (Basel). 10:8152019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sopariwala DH, Yadav V, Badin PM, Likhite
N, Sheth M, Lorca S, Vila IK, Kim ER, Tong Q, Song MS, et al:
Long-term PGC1β overexpression leads to apoptosis, autophagy and
muscle wasting. Sci Rep. 7:102372017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Barthelemy C and André B: Ubiquitylation
and endocytosis of the human LAT1/SLC7A5 amino acid transporter.
Sci Rep. 9:167602019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nematbakhsh S, Pei Pei C, Selamat J,
Nordin N, Idris LH and Abdull Razis AF: Molecular regulation of
lipogenesis, adipogenesis and fat deposition in chicken. Genes
(Basel). 12:4142021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Salminen A, Kauppinen A and Kaarniranta K:
FGF21 activates AMPK signaling: Impact on metabolic regulation and
the aging process. J Mol Med. 95:123–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Babaknejad N, Nayeri H, Hemmati R, Bahrami
S and Esmaillzadeh A: An overview of FGF19 and FGF21: The
therapeutic role in the treatment of the metabolic disorders and
obesity. Horm Metab Res. 50:441–452. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang M, Zhou W, Cao Y, Kou L, Liu C, Li
X, Zhang B, Guo W, Xu B and Li S: O-GlcNAcylation regulates
long-chain fatty acid metabolism by inhibiting ACOX1
ubiquitination-dependent degradation. Int J Biol Macromol.
266:1311512024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ndiaye H, Liu JY, Hall A, Minogue S,
Morgan MY and Waugh MG: Immunohistochemical staining reveals
differential expression of ACSL3 and ACSL4 in hepatocellular
carcinoma and hepatic gastrointestinal metastases. Biosci Rep.
40:BSR202002192020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Song F, Li JZ, Wu Y, Wu WY, Wang Y and Li
G: Ubiquitinated ligation protein NEDD4L participates in MiR-30a-5p
attenuated atherosclerosis by regulating macrophage polarization
and lipid metabolism. Mol Ther Nucleic Acids. 26:1303–1317. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Loix M, Zelcer N, Bogie JF and Hendriks
JJ: The ubiquitous role of ubiquitination in lipid metabolism.
Trends Cell Biol. 34:416–429. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sciorati C, Clementi E, Manfredi AA and
Rovere-Querini P: Fat deposition and accumulation in the damaged
and inflamed skeletal muscle: Cellular and molecular players. Cell
Mol Life Sci. 72:2135–2156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hamrick MW, McGee-Lawrence ME and
Frechette DM: Fatty infiltration of skeletal muscle: Mechanisms and
comparisons with bone marrow adiposity. Front Endocrinol
(Lausanne). 7:692016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gumucio JP, Qasawa AH, Ferrara PJ, Malik
AN, Funai K, McDonagh B and Mendias CL: Reduced mitochondrial lipid
oxidation leads to fat accumulation in myosteatosis. FASEB J.
33:7863–7881. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li JJ, Wang R, Lama R, Wang X, Floyd ZE,
Park EA and Liao FF: Ubiquitin ligase NEDD4 regulates PPARγ
stability and adipocyte differentiation in 3T3-L1 cells. Sci Rep.
6:385502016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chowdhury S, Singh AK, Srivastava S,
Upadhyay V, Sethi A, Siddiqui S and Trivedi AK: AIP4 regulates
adipocyte differentiation by targeting C/EBPα for
ubiquitin-mediated proteasomal degradation. J Cell Biochem.
124:961–973. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Te LJ: Enabling skeletal muscle repair and
functional recovery following denervation-induced injury using
ultrasound mediated gene delivery (UMGD). University of Toronto
(Canada); 2021
|
|
52
|
Parra O and Linos K: Molecular
pathogenesis of soft tissue and bone tumors. Diagn Mol Pathol.
485–551. 2024. View Article : Google Scholar
|
|
53
|
Wang K, Liu J, Li YL, Li JP and Zhang R:
Ubiquitination/de-ubiquitination: A promising therapeutic target
for PTEN reactivation in cancer. Biochim Biophys Acta Rev Cancer.
1877:1887232022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
D'Cruz R: Identification of PDLIM7 as a
Nedd4-1 substrate in the regulation of skeletal muscle mass.
University of Toronto (Canada); 2016
|
|
55
|
Huang X, Chen J, Cao W, Yang L, Chen Q, He
J, Yi Q, Huang H, Zhang E and Cai Z: The many substrates and
functions of NEDD4-1. Cell Death Dis. 10:9042019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang H: Protein degradation pathways in
hepatic ER stress and insulin resistance. RMIT University; 2015
|
|
57
|
Yang B and Kumar S: Nedd4 and Nedd4-2:
Closely related ubiquitin-protein ligases with distinct
physiological functions. Cell Death Differ. 17:68–77. 2010.
View Article : Google Scholar : PubMed/NCBI
|