Endometriosis (EM) is a common disease of the female
reproductive system in which endometrial tissue exists outside of
the uterus. Current estimates suggest that the total number of
women diagnosed with endometriosis worldwide is as high as 190
million (1,2). These ectopic endometrial tissues are
usually found in the ovary, ovarian fossa, uterosacral ligament,
and both the anterior and posterior compartments of the pelvis
(3–5). Although EM is recognized as benign
cell proliferation, it has characteristics similar to malignant
tumors, such as progressive and invasive growth, genetic
instability, excessive proliferation, estrogen-dependent growth and
a tendency to metastasize (6).
Studies over the past few decades have shown that there is a
correlation between EM and susceptibility to a variety of
malignancies, including endometrioid carcinoma, clear cell
carcinoma and low-grade serous ovarian cancer (7,8). It
has also been reported that multifocal EM often presents with
clonal growth and an increased mutation load, which are similar
characteristics to cancer (9). The
ectopic epithelial cells of patients with advanced EM even show
signs of atypical hyperplasia. Typical changes of EM, as reported
in the studies by Czernobilsky and Morris (10), and LeGrenade and Silverberg
(11), which are used as
diagnostic criteria in most studies (6), include three features: i) Enlarged
hyperchromatic or morbid nuclei with moderate to considerable
pleomorphism; ii) increased nuclear:cytoplasmic ratio; and iii)
crowding, stratification or tufting of cells. This indicates that
EM may be a transitional form between a benign and malignant
lesion.
The general purpose of EM therapy is to reduce and
eliminate lesions and pain, improve and promote fertility, and
reduce and avoid recurrence. Treatments should strictly follow the
following principles: i) Clinical problem-oriented,
patient-centered, comprehensive long-term management according to
different age stages; ii) empirical drug therapy should be started
as early as possible based on the clinical diagnosis; iii) the
timing of surgery should be standardized and attention should be
paid to the protection of ovarian function and fertility to
maximize the benefits of surgery; iv) after conservative surgery,
long-term drug management and comprehensive treatment should be
used to prevent recurrence; and v) regular review is recommended.
Patients with considerable risk factors for malignant
transformation should receive additional attention to avoid a
missed or delayed diagnosis.
Medical and surgical treatments are both common in
the clinical management of EM (17). Long-term management of EM should
maximize the efficacy of drug therapies by suppressing the activity
and differentiation of lymphocytes, forming an in vivo
hypoestrogenic environment and relieving pain (17,20).
Five main types of drugs are included in the common medical
management of EM: Non-steroidal anti-inflammatory drugs,
progestins, combined oral contraceptives (COCs),
gonadotropin-releasing hormone agonists (GnRH-a) and traditional
Chinese medicine (21–23). Surgical treatment is recommended
for patients who are infertile, who have adnexal cysts with a
diameter >4 cm and who are unresponsive to medical treatment
(24). Different types of surgery
are carried out according to the preoperative evaluation and
personal needs of the patient. Lesion resection (or conservative
surgery), which is mainly conducted laparoscopically, preserves
reproductive function (17,25).
Hysterectomy is suitable for patients with severe symptoms or those
at a high risk of recurrence, who have no reproductive requirements
but wish to preserve their ovarian endocrine function. Hysterectomy
and bilateral adnexectomy are recommended for patients with severe
symptoms, a high risk of recurrence, no reproductive requirements
and who are unresponsive to drug therapies (25). Since EM is prone to relapse and has
a considerable impact on female fertility (26), preserving the reproductive ability
and endocrine function of the ovaries and uterus, and preventing
disease recurrence should be the top priority of EM management.
However, a more thorough understanding and interpretation of the
pathogenesis and etiology of EM is required to make the most of
current medical treatments and to innovate new techniques for
achieving an improved outcome.
The origin and pathogenesis of EM remains unclear.
At present, the most commonly accepted theory is Sampson's
retrograde menstruation theory, in which menstrual debris may be
transferred to the peritoneal cavity through the reverse
peristalsis of the fallopian tube (27,28).
However, it is argued that retrograde menstruation is widespread in
healthy women, and that retrograde menstruation alone does not
necessarily lead to EM (29).
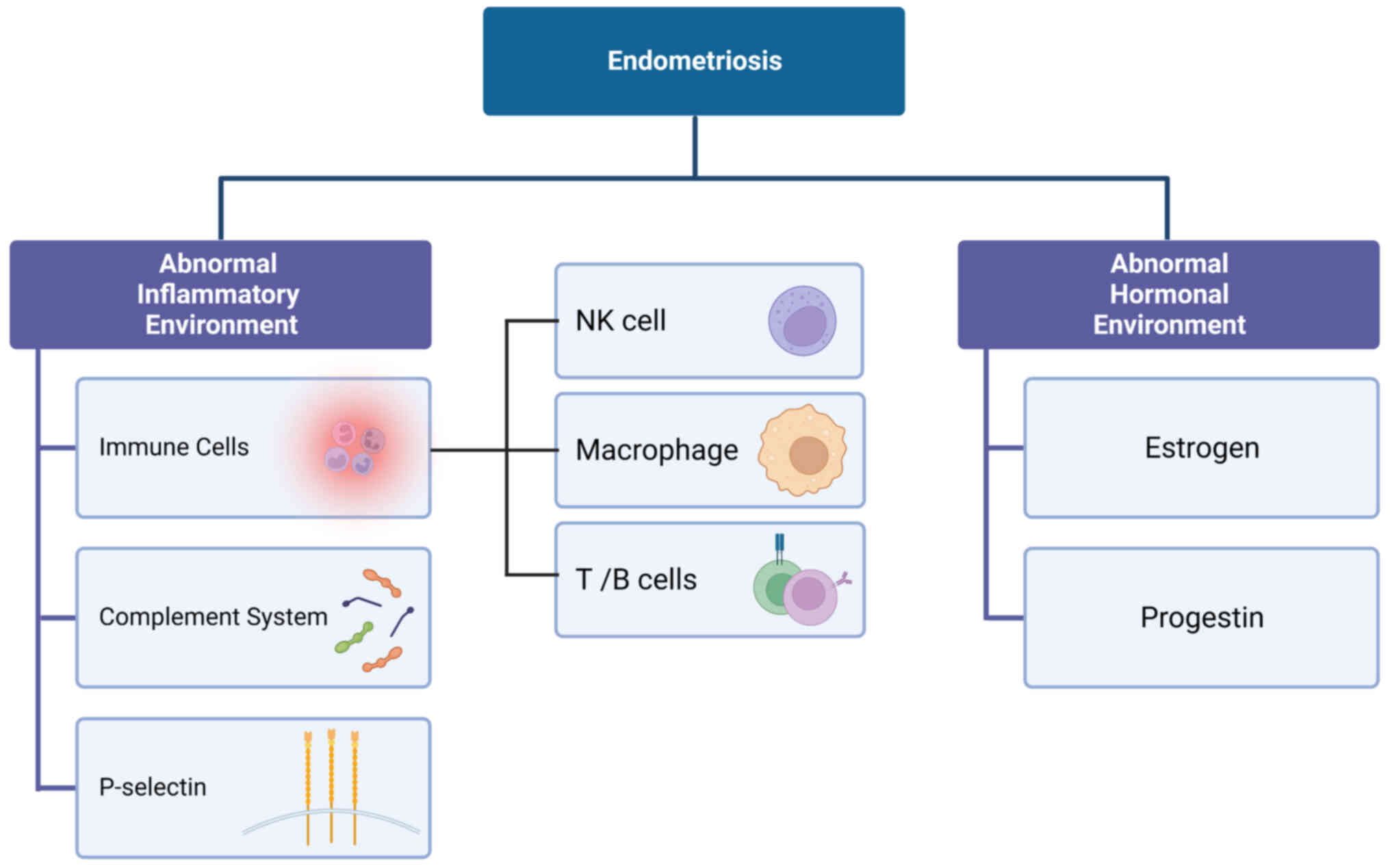
Etiological study of EM shows that it is a
multifactorial disease. Pathological studies have shown that the
immune microenvironment in the ectopic endometrium is considerably
altered. Researchers found that there were considerable
abnormalities in the immune surveillance system of ectopic
endometrial tissue, which permits its implantation into the
peritoneal cavity without clearance by immune tissue (30–32).
Ectopic endometrial tissue not only promotes an oxidative stress
response and chronic inflammation in the ectopic areas, but also
promotes the aggregation and activation of macrophages, thus
inducing the production and release of the growth factors,
angiogenic factors and inflammatory cytokines secreted by
macrophages. This may also be the reason why EM effects fusion of
the spermatocyte and oocyte, embryo implantation and embryo
development, resulting in reproductive disorders (33–35).
The ectopic endometrium also has an abnormal
inflammatory hormone environment that is characterized by local
estrogen levels that are increased several-fold when compared to
that of the peripheral blood. This results in a series of cellular
and cytokine responses that include cell proliferation and the
release of various immune and inflammatory factors, such as tumor
necrosis factor-α (TNF-α), transforming growth factor (TGF)-β1,
interleukin (IL)-1, IL-6, IL-8 and IL-10 (36). Possible subsequent clinical
outcomes include an acute inflammatory response, pain (including
dysmenorrhea, chronic pelvic pain and dyspareunia),
gastrointestinal symptoms (painful bowel movements) and urinary
symptoms (hematuria) that are associated with the menstrual cycle,
as well as EM-associated infertility (35,37–40)
(Fig. 1).
Researchers therefore hypothesize that EM is not
only a gynecological disease, but also a chronic inflammatory
systemic disease that is associated with immunity. Findings that
support this include increases in non-specific inflammatory
markers, such as CA-125 and CRP, and the presence of antinuclear
antibodies in the patient's peripheral blood (40–43).
Immune cells and their products are typically able to detect and
eliminate abnormal cells (44).
Considerable changes have been found in the regulation of various
immune cells in patients with EM, including downregulation of the
cytotoxicity of natural killer (NK) cells, infiltration and
activation of macrophages, infiltration and dysfunction of T and B
lymphocytes, activation of polyclonal B lymphocytes, impaired
apoptosis, dysfunction of Th1 and B cells, and translocation of T
regulatory cells (45–48). This abnormal immune cell regulation
provides various targets for EM therapies. The inhibition of NK
cells and the abnormal activation of macrophages are considered key
factors in the progression of EM, and therefore potential targets
for EM immunotherapy (49–51). In addition to the requirement for a
favorable immune environment for the survival of ectopic
endometrial tissue, hypoxia stress and adhesiveness are two
additional obstacles for the successful implantation of ectopic
endometrial tissue. In a previous study, increased levels of
hypoxia inducible factor-1α (HIF-1α) were observed in ectopic
endometrial tissues compared with those in normal endometrium
(52). HIF-1α is considered the
best biomarker for tissue hypoxia and has an important role in the
hypoxic response to ectopic tissues, including cell adhesion,
angiogenesis and cell proliferation (53). As predicted, the inhibition of
HIF-1α production in a mouse model of EM induced by suturing slices
of uterus to intestinal mesenteric vessels could inhibit EM
progression (54). HIF-1α could
therefore function as the molecular target of EM therapy. Previous
studies have shown that hypoxia promotes the release of angiogenic
factors, such as vascular endothelial growth factor (VEGF-A), and
inflammatory cytokines, such as IL-1β, TNF, TGF-β and IL-8
(55–57). Some responses to hypoxia interact
with and regulate the activity of certain types of immune cells.
For example, hypoxia-induced TGF-β elevation in the peritoneal
fluid of patients with EM was found to be associated with the
suppression of NK cells (58).
Activation of macrophages in the peritoneal fluid in response to
hypoxia was also found to be associated with and possibly
contributed to the reduced cytotoxicity of NK cells in patients
with EM (59). The programmed
death-1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway was also
found to be involved in the immune tolerance that contributes to
the pathogenesis of EM (60).
These results suggest that not only are NK cells potential
therapeutic targets for EM, but that immune checkpoint blocking to
avoid NK cell immunosuppression can be used to investigate
alternative methods for treating EM.
The normal endometrium contains various immune cells
that change in distribution and number throughout the menstrual
cycle (61). The normal periodic
changes in immune cells are dysregulated in EM, considerably
impacting both the composition and function of immune cells.
Lymphocytes and macrophages are the main components in the lesion
microenvironment. Compared with healthy women, women with EM have a
considerably increased proportion of peritoneal macrophages in the
peritoneal fluid, which contributes to the proliferation and
survival of ectopic endometrial cells (62). The levels of the main components of
potential biomarkers in the peritoneal fluid are also increased in
the setting of EM, including phosphatidylcholine, phenylalanine
isoleucine, glycidyl deproteinization, placental protein 14,
midkine, IL-8 and osteoprotegerin (59,60).
Changes in the composition and proportion of certain molecules in
the peritoneal fluid may lead to impaired T cell and NK cell
cytotoxicity (47,63). The aforementioned changes in the
immune environment help to establish an immunosuppressive
microenvironment that is conducive to the proliferation and
invasion of epithelial cells and stromal cells into the ectopic
endometrial tissue, and supports angiogenesis in the ectopic
microenvironment (64,65).
The treatment for EM includes surgical resection and
hormone therapy, both of which have advantages and disadvantages.
Surgical treatment can remove the ectopic cyst and identify the
location of the lesion. However, surgical resection without
long-term medical treatment has a high EM recurrence rate and a
decline in ovarian function. Medical therapy can slow the
progression of EM to a certain extent, delay the need for surgery
and avoid surgical complications. However, it cannot clarify the
nature of the lesion or effectively reduce the lesion size. As
specific targeted immunotherapy is usually not universal and there
is not enough experimental validation, it may be potentially
effective in this domain and deserves more attention (66,67).
NK cells can be divided by their expression level of
CD56 into cd56bright and cd56dim, which have increased and reduced
expression levels of CD56, respectively. Cd56bright NK cells
produce more abundant cytokines, while cd56dim NK cells have
increased cytotoxicity and increased expression levels of FC γ
receptor III (fcgr3), also known as CD16 (70,71).
NK cells spontaneously recognize and eliminate infected, ectopic,
tumorigenic and stress responsive cells so as to automatically
monitor for viral infections, ectopic tissue and malignant cells
(72–74). Repeated exposure to the same target
results in increased accumulation of NK cells and the production of
a specific recall response by NK cells that is characterized by the
enhancement of the functional activity of NK cells against the
target (75–77). The binding of inhibitory killer
cell immunoglobulin-like receptor (KIR) on NK cells with MHC class
1 or human leukocyte antigen (HLA)-1 (KIR/HLA-1) inhibits the
activation of NK cells and allow for further intrinsic
interactions. KIR therefore has an important role in distinguishing
autologous cells from diseased and foreign cells to avoid
non-selective killing of autologous healthy cells (78,79).
The maturation and cytotoxic function of NK cells is based on the
interactions between KIR and autologous MHC molecules, which is
called licensing (80). Once
licensed and functionally mature, NK cells are inhibited by
inhibitory receptors that bind to the autologous MHC. Cells without
MHC class 1 expression will be eliminated by activated NK cells
(81). If NK cells are not
stimulated by interaction with autologous MHC they may lose their
normal function (82). However,
the inhibition of KIRs and MHC is not absolute in mature NK cells
and can also be eliminated or offset by a much stronger active
stimulator. Other MHC receptors are also involved in NK cell
cytotoxicity regulation against target cells, such as leukocyte
immunoglobulin-like receptor subfamily B (LILRB) and natural killer
group protein 2 (NKG2) (83–85).
Among the receptors that activate NK cells, FCGR3,
which is expressed in almost all NK cells, is key for antibody
dependent cytotoxicity. FCGR3 expression levels alone are
sufficient to induce interferon γ and TNF, making it is one of the
most effective activating receptors of NK cells. Other receptors
that can activate NK cell cytotoxicity include NCRs, which are
divided into NCR1, NCR2 and NCR3 (or NKp46, NKp44 and NKp30,
respectively) (86).
In addition to licensing through the interaction
between receptors on the surface of NK cells and the autoantibodies
of the body, exposure to cytokines is also essential to activate
the cytotoxicity of immature NK cells and promote cytokine
secretion (87). IL-2 and IL-15
secretion by macrophages can activate and trigger the maturation of
NK cells and promote their proliferation (88–90).
Simultaneous exposure to IL-12 and IL-18 is not only able to
activate NK cells, but can promote IFN-γ secretion (91,92).
Conversely, increased interferon secretion can also enhance the
cytotoxicity of NK cells, such as the anti-tumor response of NK
cells that is mediated by the cyclic GMP-AMP synthase
(cGAS)-stimulator of interferon genes (STING) pathway activation
(93). Activated NK cells can
induce apoptosis by releasing cytolytic particles against target
cells. They can also by cytotoxic to target cells through a Fas
L-mediated mechanism with the help of the CD95 receptors on the
surface of target cells (94,95).
Several reports have shown that NK cells in patients
with EM have reduced abilities both in clearing out of ectopic
endometrium and in participating in local and systemic immunity,
which creates a favorable environment for the survival and growth
of ectopic endometrial tissue (96–98).
NK cytotoxicity decreases not only in ectopic endometrial tissue,
but also in the peripheral blood and peritoneal fluid (99).
Decreased NK cytotoxicity is currently debated, as
to date, studies on this topic have not adequately shown this
cytotoxicity to occur (48,100,101).
It has been speculated that the upregulation of NK cell inhibitory
receptors and the downregulation of stimulatory receptors in
ectopic endometrium may be caused by cytokines such as IL-2, IFNs
and TGF-β. This indicates that cytokine therapy targeting NK cell
inhibitory or stimulatory receptors is feasible (102,103). Most studies show that the
proportion of NK cells decreases in patients with EM. However,
there are also studies showing that the proportion of NK cells in
patients with EM increases when compared with that in healthy
controls (104,105). These results suggest that the
decreased NK cytotoxicity in patients with EM is not the result of
decreased NK cell infiltration, but rather the abnormal expression
level of NK cell activation receptor and/or inhibition receptor.
However, only the upregulation of NK cell inhibitory receptors is
supported by current research, while the regulation of stimulatory
receptors remains unclear due to the lack of studies and
considerable results.
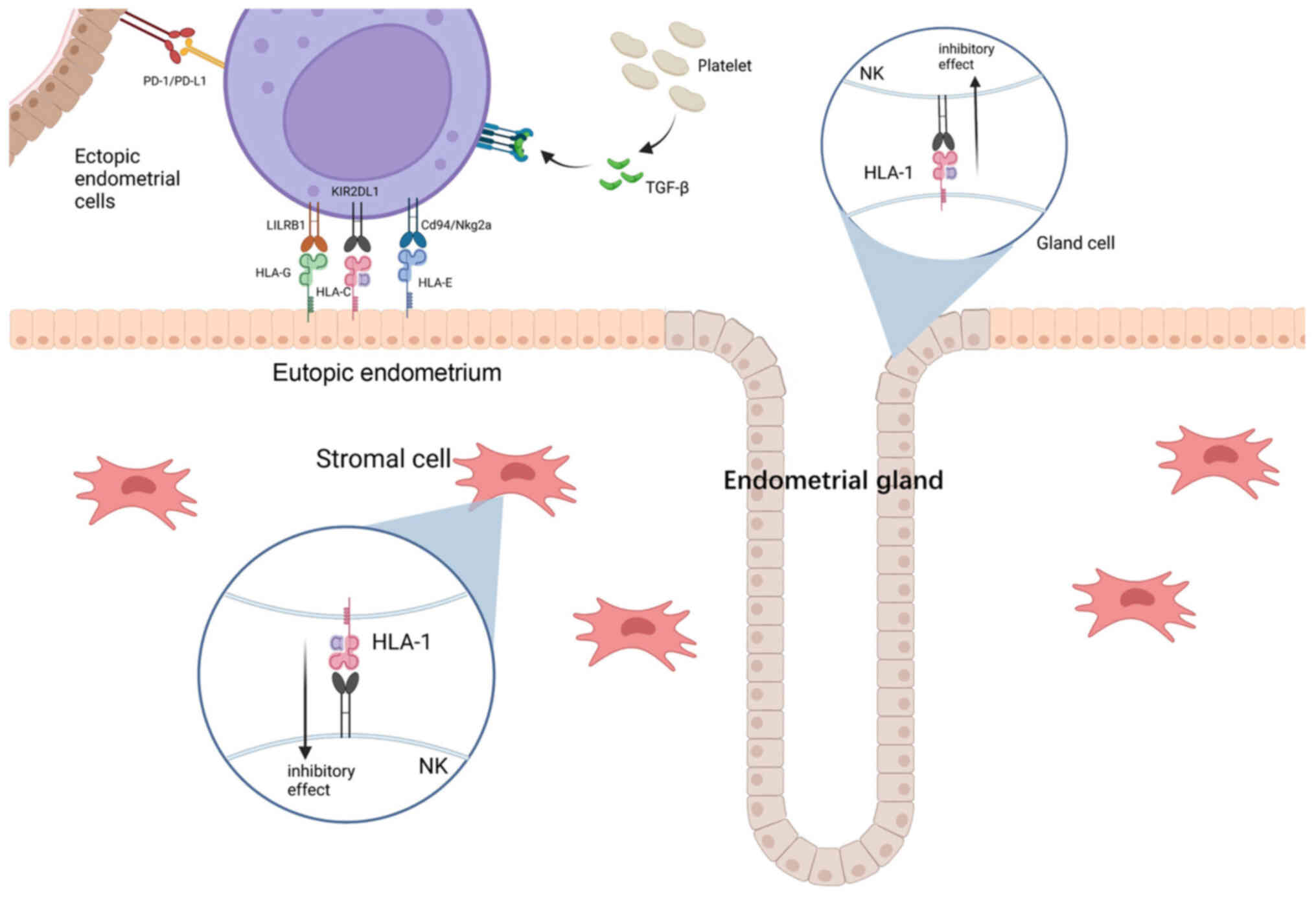
Overexpression of inhibitory receptors is considered
vital for modulating immune evasion and maintaining immune
tolerance in EM. Increased levels of HLA-1 in the glandular and
stromal cells of endometrial tissue was observed in the study by
Vernet-Tomas Mdel et al (106), which may result in increased
resistance to NK cytolysis in patients with EM (106). The case-control study by Wu et
al (107) finds that the
levels of KIR in the peritoneal NK cells of patients with EM also
increased (including NKB1 and EB6), thus further reducing the
cytotoxicity of NK cells (107).
Overexpression of other inhibitory receptors and their ligands were
observed in patients with EM, including the inhibitory receptors
KIR2DL1, CD94/NKG2a and LILRB1 on peritoneal NK cells, and their
separate endogenous ligands HLA-C, HLA-E and HLA-G (108–110). The altered presence and distinct
combined presence of different KIR genes contributes to a unique
genetic background of patients with EM. It should be noted that not
all KIR/HLA binding promotes the development of EM. For example,
receptor KIR2DS5 in combination with its ligand HLA-C C2 has a
protective effect against EM (111).
Decreased expression levels of NKG2D, a stimulatory
receptor of NK cells, was also reported in patients with EM
(112). It is plausible that the
downregulation of NKG2D is the result of increased levels of TGF-β
and ectopic endometrial tissue-derived IL-15 (113,114). However, the ligands for NKG2D,
including MHC class-I chain-associated proteins (MIC)A and B that
were upregulated in the peritoneal fluid of patients with EM
(115), paradoxically exert an
inhibitory effect on NK cells (116,117). The precise mechanism behind how
the regulation of NK cell stimulatory receptors influences the
pathogenesis of EM requires further verification and
examination.
IL-12 can regulate the immune recognition of NK
cells in the endometrium. IL-12 is composed of two heterologous
polypeptide chains, p40 and p35. A study found that the
concentration of IL-12 in patients with EM is similar compared with
that of healthy controls. However, increased levels of free p40 in
the peritoneal fluid of these patients indicated that
overexpression of the p40 subunit alone could reduce the
cytotoxicity induced by IL-12 (118). The ratio of FCGR3-negative NK
cells to FCGR3-positive NK cells in the peritoneal cavity of
patients with EM was increased (119). Based on these findings, the
increased expression levels of inhibitory receptors and ligands has
a key role in the decline of NK cytotoxicity.
Studies on the changes and mechanisms of NK cells in
patients with EM provide a solid experimental basis for the
targeted treatment of EM. The present focus of immunotherapy
targeting NK cells is to restore their cytotoxicity. To reach this
goal, three possible aspects should be considered: i) Blocking
inhibitory receptors; ii) anti-inhibitory or stimulatory cytokine
therapy; and iii) immune checkpoint therapy.
KIR2DL1, LILRB1/2 and CD94/NKG2a are inhibitory
receptors that are overexpressed in the NK cells of women with EM.
The ability to interfere with the binding of the inhibitory
receptors and their ligands to improve the cytotoxicity of NK cells
has been tested by multiple studies (124,125). Disruption of the inhibitory
receptors on NK cells contributing to enhanced cytotoxicity was
found in a human cell model treated with 5-aza-2′-deoxycytidine
reported by Binyamin et al (124) in 2008. The study observed
increased NK cell cytotoxicity when the inhibitor KIR2DL1 was
applied to the NK cells of a healthy woman. It was also observed
that blocking KIR2DL1 can enhance the effects of rituximab (an
anti-CD20 monoclonal antibody known to recruit the immune system to
attack and kill B cells) by increasing the cytotoxicity of NK cells
(124,126). The study by Andre et al
(125) targeted NKG2A on NK cells
with monalizumab combined with cetuximab (an EGFR inhibitor) in
patients with head and neck carcinoma, resulting in increased NK
cell cytotoxicity (125). Whilst
neither of these studies were based on EM, this blocking of
inhibitory receptors therapy may be a promising treatment for EM
and has been shown to be effective against some malignancies
(125,127); however, further studies on their
role in EM are required to make conclusive statements.
Previous studies tried to identify cytokines that
affect the regulation of NK cell activity as new targets for
immunotherapy. Some ILs (such as IL-2 and IL-12) and IFNs are NK
cell stimulative cytokines. Intraperitoneal injection of IL-2 in
surgically implanted EM rat models was found to be capable of
recruiting leukocytes into EM lesions and reducing lesion size
(128,129). However, existing studies on the
therapeutic effects of IL-2 on EM are based in animal models,
therefore, further studies and analysis are needed on the
applicability to human patients. IL-12 is another important NK cell
stimulative cytokine. Researchers pretreated NK cells with an IL-12
heterodimer to reduce the ratio of free p40 to IL-12 and enhance
the cytotoxicity of NK cells in ectopic endometrial tissue,
resulting in suppressed development of ectopic endometrial tissue.
IL-12 is therefore considered a potential specific target for
correcting the increase in free p40 levels in patients with EM
(118). Type I IFNs, which
include IFN-α2b, IFN-β1a and type II IFN (IFN-γ), can activate NK
cells and enhance their cytotoxicity. However, to the best of our
knowledge, no work has studied the feasibility of using the NK cell
activating effects of IFNs to treat EM. The study by Dicitore et
al (130) showed that IFN-β1a
is superior to IFN-α2b at inhibiting the proliferation and
migratory activities of endometrial stromal cells.
TGF-β suppresses the cytotoxicity of NK cells and
the function of other immune cells (132,133). TGF-β secretion is upregulated in
women with EM and is considered important to EM pathogenesis
(134,135). Anti-TGF-β therapies are currently
being evaluated clinically as treatments for malignancies and other
diseases, such as diabetes. However, the results are generally
unsatisfactory, reporting non-responsiveness and potential
systematic side effects (136,137). There are also concerns that
anti-TGF-β therapies would cause systematic suppression and result
in severe systematic side effects due to the important role that
TGF-β has in multiple vital signaling pathways, such as cell
proliferation and differentiation. In vitro and in
vivo studies are needed to further study anti-TGF-β therapy for
EM.
Other immunotherapies may restore NK cell function
in EM, most of which are based on immunotherapy models of other
diseases. In 2004, the study by Clayton et al (138) first proposed the possibility of
using Mycobacterium to restore NK cell activity in EM
(138). However, the hypothesis
was only supported by in vitro studies and further
verification is required.
Immune checkpoint blocks (ICBs) are also new
immunotherapies that researchers are currently evaluating.
PD-1/PD-L1 pathway-associated inhibitors are a type of checkpoint
blocking therapy. Previous studies have reported increased PD-1
expression levels in the peripheral blood cells of patients with
EM, and increased PD-L1 expression levels in both the ectopic and
non-ectopic endometrial tissues of patients with EM (139,140). This indicates that the peripheral
tolerance caused by PD-1/PD-L1-induced T cell suppression may
contribute to the immune abnormalities noted in EM. ICB therapy
using PD-1/PD-L1 inhibitors is a promising treatment for preventing
immune tolerance to EM (141–143). However, studies have reported
that PD-1/PD-L1 inhibitor treatment can lead to adverse reactions
in a variety of tissues and organs throughout the body. The use of
ICBs in the treatment of EM should therefore be carefully selected,
and inhibitors with the strongest specificity for EM should be
utilized (144,145). In addition to ICB therapy, the
use of genetically modified NK cells, such as chimeric antigen
receptor (CAR)-NK cells, in tumor immunotherapy has also attracted
increased attention (146–148).
However, engineering a CAR-NK structure requires a biomarker
specifically expressed on the surface of ectopic endometrial cells,
which, to the best of our knowledge, has not yet been
discovered.
Although the aforementioned targets for the
treatment of EM have been supported in theory by in vitro
and animal experiments, limited clinical trials have been
reported.
The exact mechanism behind how immunosuppression in
ectopic endometrial tissue and its environment damages the
cytotoxicity of NK cells is unclear, which makes it difficult to
identify an appropriate immunotherapy target. Three main specific
inhibitory NK cell receptor families have been identified: KIR,
LILRB and NKG2. To the best of our knowledge, there are no reports
on the inhibition of these receptor/ligand interactions. Cytokine
therapy and the upregulation of associated activated receptors also
requires further research. The possibility of utilizing
immunotherapy in the treatment of EM needs further analysis due to
the lack of tissue/cell specificity, which results in systemic side
effects. It requires investigation on the epigenic differences
between the ectopic and eutopic cells to develop treatments with
increased specificity.
It should be noted that whether enhanced NK cell
cytotoxicity is associated with abortion is debatable (149,150). Further examinations and analyses
are needed before NK cell treatment can enter clinical
research.
Macrophages are a late differentiation cell type of
the mononuclear-phagocyte system, which have an important role in
both the non-specific and specific immune response. Macrophages
were previously considered to be solely derived from blood
monocytes, which are widely distributed and participate in the
innate immunity of the body (151). This notion has changed due to the
discovery of macrophages derived from and residing in specific
tissues without the participation of circulating monocytes
(152). Macrophages can be
polarized into different directions based on the effects of
different microenvironments and stimulating factors. Based on the
surface markers of polarized macrophages and their functions,
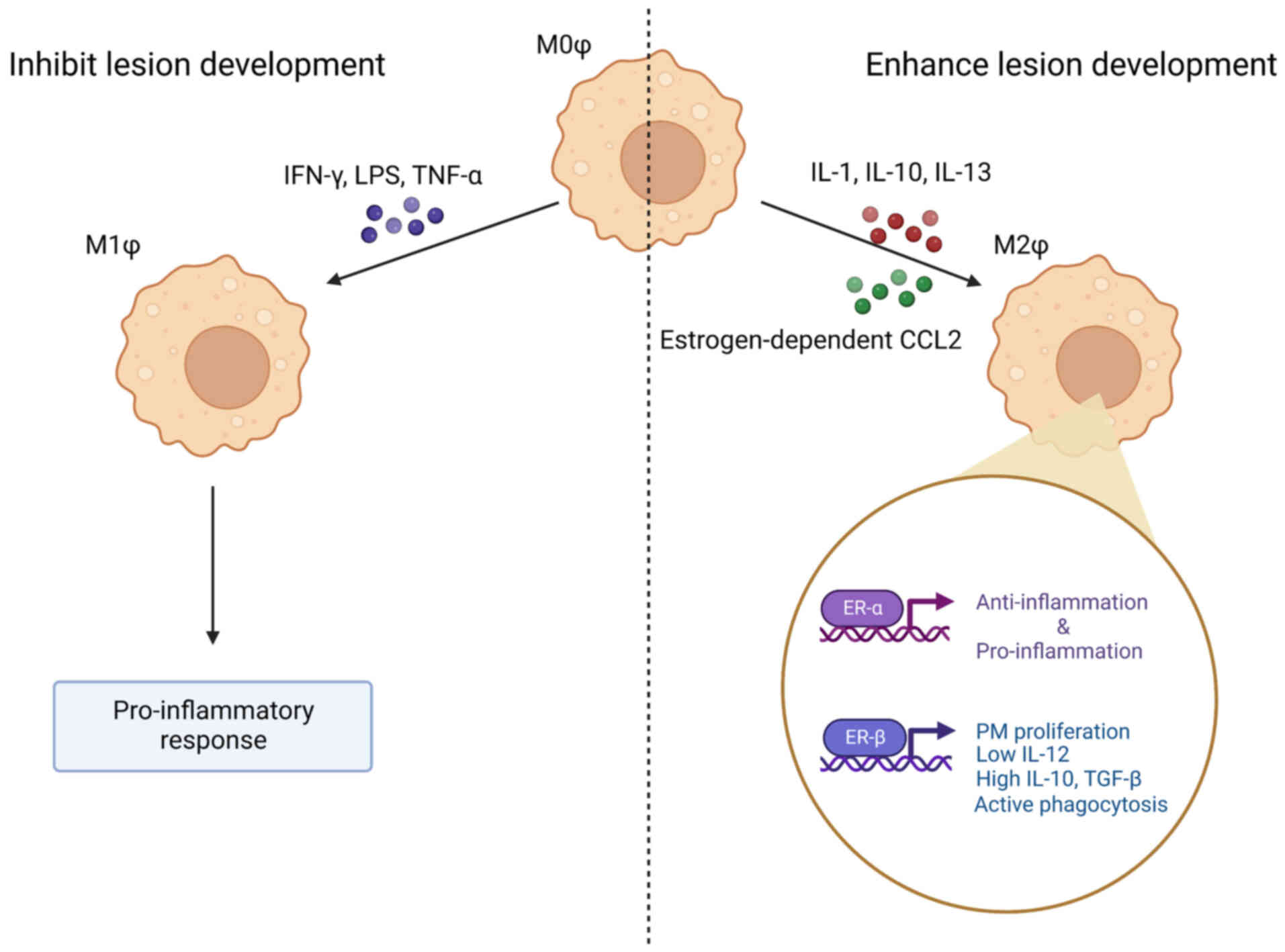
polarized macrophages can be categorized into two types:
Classically activated macrophages (M1) and alternatively activated
macrophages (M2) (153,154). M1 has a pro-inflammatory effect
on the early stages of inflammation, phagocytizes and digests
foreign pathogens, secretes pro-inflammatory factors, activates the
T cell-dependent immune response and promotes the Th1 immune
response. M2 can promote tissue repair and wound healing, regulates
the Th2 immune response and contributes to disease recovery during
the later stage of inflammation, which results in an
anti-inflammatory effect (153,154).
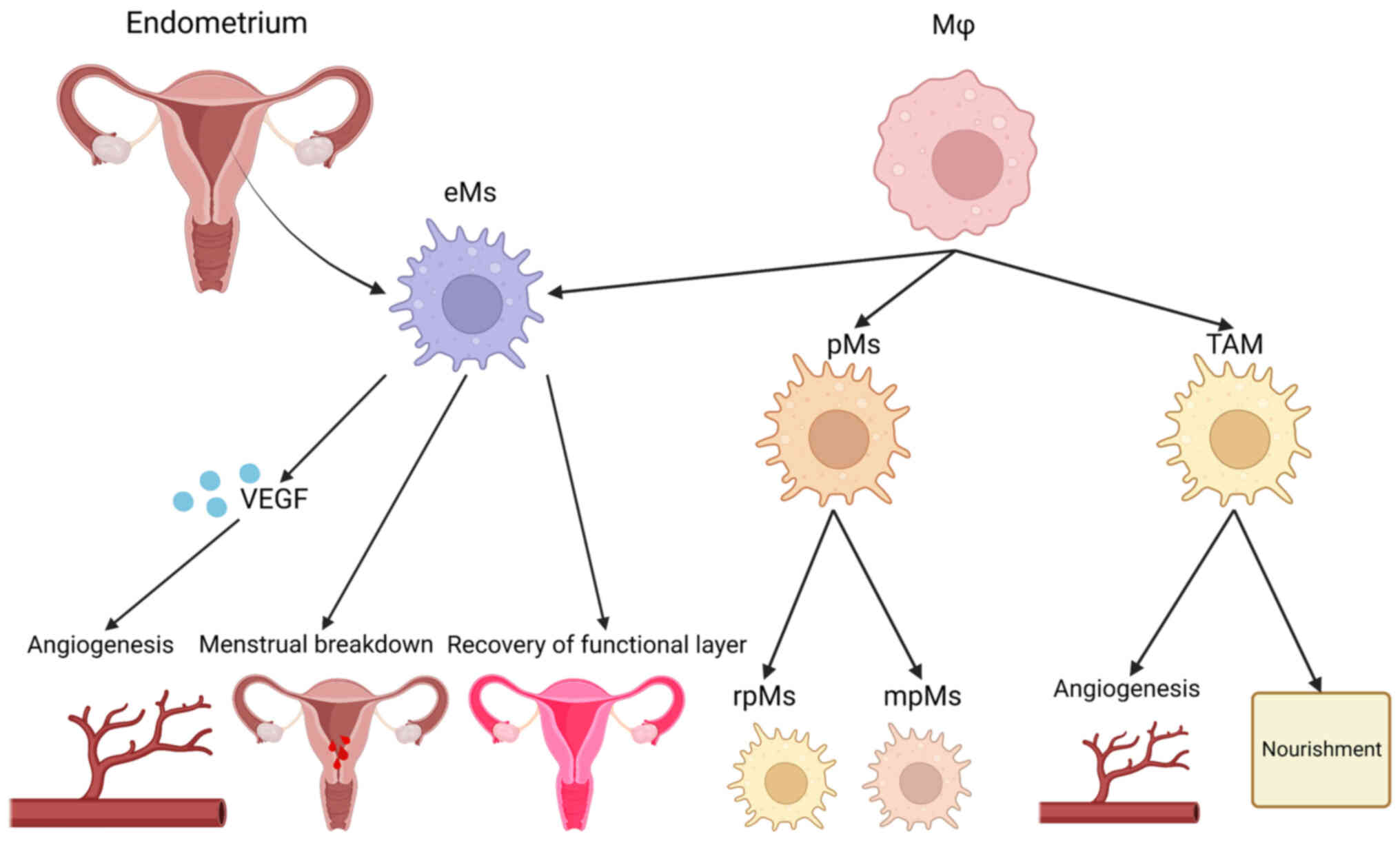
There are two types of macrophages in the female
pelvis: Endometrial and peritoneal. Endometrial macrophages (eMs)
are involved in triggering and regulating the process of
endometrial breakdown, and the subsequent repair of the endometrial
functional layer by facilitating cell proliferation and
angiogenesis (155,156). eMs function in the following
three ways: i) Production and release of VEGF to promote
angiogenesis; ii) participation in triggering and controlling the
shedding process; and iii) facilitating and rebuilding the
functional layer (157).
Peritoneal macrophages (pMs) are distributed in ectopic endometrial
tissues outside of the reproductive tract. The increased
macrophages in patients with EM are mainly pMs. pMs have an immune
monitoring role on the peritoneal surface. pMs can be classified
into resident pMs and monocyte-derived pMs of bone marrow origin
(158,159).
Based on the differences in MHCII and F4/80
expression levels, pMs can be divided into two phenotypes: Big,
tissue-resident pMs and small, monocyte-derived pMs. Both types of
macrophages can be either polarized into M1, which is
pro-inflammatory, or M2, which is anti-inflammatory, depending on
the stimulation of pathogen-associated molecular patterns (51). The pro-inflammatory M1 phenotype of
pMs, similar to the classification of helper T cells, is activated
mainly through the activation of IFN-γ, LPS, TNF-α or a combination
of the three. The anti-inflammatory M2 phenotype of pM is mainly
activated by IL-1, IL-10 and IL-13. The polarization of pMs
produces corresponding molecular markers, which allows researchers
to detect the regulation of macrophages (160–162). Another type of macrophage,
tumor-associated macrophage, has a role in the nutrition and
angiogenesis of patients with EM and endometrial cancer (Fig. 3) (64).
Although the number and activation of pMs in
patients with EM are increased, the phagocytic capacity of these
pMs is still unable to remove the ectopic endometrial tissue
debris. pMs obtained from women with EM show a reduced capacity for
phagocytosis due to the decreased expression level and activity of
matrix metalloproteinase-9, which is regulated by prostaglandin E2
(PGE2) and is the enzyme that is necessary in the degradation of
the extracellular matrix (163–165).
Ectopic endometrial tissue is in a hormonal
environment that contains abnormal concentrations of estrogen and
androgens. The secretion of C-C motif chemokine ligand 2 (CCL2) by
endometrial stromal cells is upregulated in the ectopic milieu,
which has been confirmed to be mediated by estrogen (166). CCL2 mediates the polarization of
macrophages to M2 instead of M1 (167). The abnormal EM environment also
promotes the elevation of distinct anti-inflammatory phenotypes of
macrophages, forming an immunosuppressive microenvironment by
stimulating the proliferation of epithelial and stromal cells in
endometriotic foci, and promoting angiogenesis (168). However, chronic inflammation is
still observed in the lesion microenvironment. It could be possible
that the upregulation of M2 is compensatory, induced by persistent
inflammation and tissue repair. According to the macrophage
depletion study by Bacci et al (169), anti-inflammatory M2 induced by
macrophage colony-stimulating factor and IL-10 is considered to be
of importance to the growth and development of ectopic EM tissue,
while pro-inflammatory M1 induced by IFN-γ is capable of
eliminating the ectopic tissue (169). The phenotypic plasticity of pMs
makes it possible to investigate potential therapeutic targets for
EM based on the suppression of the M2 phenotype in pMs or the
activation of the M1 phenotype. The suppression of M2 polarization
has already been proposed as chemical therapy for colon tumors,
such as by using ovatodiolide to prevent the polarization of M2
tumor-associated macrophages (170).
Estrogen receptors on macrophages can be classified
into surface receptors and nuclear receptors. Estrogen nuclear
receptors can be divided into ER-α (ER1) and ER-β (ER2). ER2
promotes inflammation and disease progression by increasing the
production of inflammatory cytokines, including IL-1β and IL-6
(171,172). IL-6 mediates the recruitment of
monocytes and their differentiation into macrophages, which
contributes to the increased macrophage infiltration into the EM
lesions. ER2 also inhibits apoptosis by interacting with the NLRP3
sensor, caspase 1 and apoptosis signal regulated kinase-1 (173). However, chloroindazole, an ER2
ligand developed in 2015, can suppress inflammation and
angiogenesis within the EM lesion, thereby suppressing EM
progression (174). These data
indicate that the activation of ER2 can also be anti-inflammatory
and can serve as a possible target for EM treatment.
ER1 has two main roles. Firstly, ER1 promotes the
secretion of pro-inflammatory cytokines, such as IFN-1,
contributing to the inflammatory response (175,176). Secondly, ER1 activation also
inhibits the NF-κB pathway, which limits the extent of the
inflammation (177,178).
Upregulation of ER2 expression levels and
downregulation of ER1 expression levels in macrophages and
endometrial stromal cells will result in an extremely low ratio of
ER1:ER2. There are controversies on whether the influence the ER1
deficiency and ER2 overexpression have an inflammatory or
anti-inflammatory effect on the EM environment due to the opposing
findings of previous studies (172,179). Despite these controversies, the
consensus is that the dysregulation of estrogen and ERs contributes
to inflammation in EM. The regulation of inflammatory pathways and
immune cells by ERs and estrogen in endometriotic stromal cells
will be discussed in further detail below.
Estrogen receptors on the cell surface are G
protein-coupled ERs (GPERs), which are expressed on the surface of
macrophages in this hormonal environment (180). GPERs are seven
transmembrane-spanning receptors that bind to estrogen and mediate
rapid non-genomic signaling pathways, such as the mitogen-activated
protein kinases (MAPK) pathway and the
phosphatidylinositide-3-kinases/Akt (PI3K/Akt) pathways, which can
be initiated within seconds and rapidly induce a physiological
response in target cells (181).
GPER expression levels in macrophages within ectopic endometrium
are increased, suggesting that this abnormality may be important to
the regulation of the macrophage immune response (182). The roles of GPERs in EM have been
gradually discovered and reported, making them a promising target
for EM therapy. Activation of GPERs by their agonist G-1 can
inhibit the secretion of TNF-α and IL-6 that was induced by LPS,
resulting in an anti-inflammatory effect on GPER-expressing
macrophages (183).
Continuously elevated estrogen levels also lead to
the synthesis and secretion of inflammatory cytokines by
macrophages, such as IL-1, IL-6 and TNF-α, which trigger a series
of pro-inflammatory responses (173,184). Macrophages have a two-way
response to estrogen: Upregulation of pro-inflammatory cytokines is
induced by comparatively low concentrations of estrogen and
inhibited by increased concentrations of estrogen (185). It has been hypothesized that the
function of macrophages in ectopic endometrial tissue may be
estrogen-dependent, and that estrogen may regulate the immune
response through the GPERs and ERs of macrophages in ectopic
endometrial tissue (182,186). These observations indicate that
whether these estrogen-dependent events have a pro- or
anti-inflammatory role on macrophages in EM depends on the types of
ERs and the local concentration of estrogen within the lesion.
The chronic inflammatory environment in ectopic
endometrial tissue triggers the secretion of inflammatory
cytokines, such as IL-1β, IL-17A and TNF-α (187). Increased inflammatory cytokines
can lead to the abnormal activation of the mTOR/PI3K signaling
pathway, and the abnormal activation and proliferation of
keratinocytes (188). Whether the
same inference can be made on macrophages in EM requires further
study. In addition, inflammatory cytokine IL-6 in inflamed tissue
acts as a superior coordinator of protein synthesis capacity and
cell growth rate by stimulating the translation of c-Myc mRNA, an
oncogenic transcription factor that activates transcription via all
three nuclear RNA polymerases. RNA polymerase I-associated
transcription factors are recruited to rDNA by IL-6 when quiescent
cells are stimulated to re-enter the cell cycle. Stimulated c-Myc
mRNA will therefore eventually lead to an upregulation of rRNA
transcription and enhanced proliferation of macrophages (189).
Macrophages are also involved in the induction of
the immunosuppressive peritoneal environment in EM. IL-8 secreted
by macrophages increases the expression level of Fas ligand (FasL)
in endometrial cells, and the binding of FasL to Fas on T cells
triggers apoptosis (190). It has
been reported that the mRNA level of IL-8 in the peripheral blood
and peritoneal fluid of patients with EM is considerably increased.
The expression level of FasL on endometrial cells is also
increased, which contributes to the formation of the
immunosuppressed and immune tolerant microenvironment that is
conducive to the adhesion of ectopic cells (191). Serum IL-8 levels have the
potential to be an early indicator of EM (192). However, there seems to be little
research assessing relevant immunotherapeutic targeting of Fas/FasL
for EM. Other immunosuppressive cytokines are also secreted by
macrophages in ectopic endometrial tissues, including IL-10 and
TGF-β, leading to the inhibition of NK cells in the peritoneal
cavity (Fig. 4) (59).
Similar to malignant tumors, the expression level of
oncogenes and tumor suppressor genes in EM is considerably altered.
Studies have shown that c-Myc, a recognized oncogene, is
overexpressed in most patients with EM. It has been proposed that
c-Myc also participates in the pathogenesis of EM (189,193).
In addition to the aforementioned inflammatory
cytokines that have important roles in the pathogenesis of EM,
Tie2-expressing macrophages in ectopic endometrial tissue inhibit
endothelial cell apoptosis by preventing the caspase-3 activation
of neovascular endothelial cells. This may also be used as a
potential therapeutic target for EM (194).
EM is characterized by considerably increased levels
of macrophage migration inhibitory factor (MIF), a multipotent
protein that has a range of immune regulatory functions and is a
key upstream regulator of both the non-specific and specific immune
responses. During the onset of premenstrual syndrome, patients with
EM have elevated MIF levels in the normal endometrium, early
ectopic endometrium, peritoneal fluid and systemic circulation
(195–198). Studies have shown that MIF and
its specific inhibitors can be used not only to improve the
accuracy of EM diagnosis, but also to develop new therapeutic
strategies against EM. The study by Seeber et al (199) found that the combined use of MIF
and factors such as CA-125, monocyte chemoattractant protein 1 and
leptin, can improve the accuracy of EM diagnosis to 93% (199). The concentration of macrophages
in the normal endometrium of patients with EM is considerably
reduced when compared with that of healthy controls, which
predisposes patients to a poor prognosis (200).
It has been suggested that abnormal macrophage
regulation may also be associated with the various clinical
features of EM. For example, the increased concentration of pMs may
disturb normal fertilization and lead to infertility in women with
EM (201). Decreased insulin-like
growth factor-1 (IGF-1) production by macrophages may also be
associated with pelvic pain associated with EM (202).
In conclusion, patients with EM have increased
macrophage levels and activation mediated by the mTOR/PI3K
signaling pathway, c-Myc oncogene expression levels, and its
resulting ribosome biogenesis in EM lesions and peritoneal fluid.
Macrophages that co-exist with ectopic EM cells in vitro are
immunosuppressive and macrophages in the peritoneal fluid of women
with EM exist as a mix of both pro-inflammatory and
anti-inflammatory cells. Anti-inflammatory macrophages in
peritoneal fluid, which are M2, promote the development of EM
lesions, while pro-inflammatory macrophages, which are M1, are
antagonistic. Increased concentrations of estrogen in the ectopic
EM microenvironment promote re-polarization from M1 to M2, which
further contributes to the growth of the lesion.
Several theories for immune-associated therapies
targeting the upregulation of pMs in EM have been proposed. The
main hypotheses regarding the upregulation of pMs in the peritoneal
fluid of patients with EM include the estrogen dependency theory,
the mTOR/PI3K signaling pathway theory, overexpression of the
oncogene c-Myc, ribosome biogenesis and the overexpression of MIF.
Several potential medication therapies for managing these
etiologies have been proposed.
Regarding the estrogen dependency theory, estrogen
replacement and other associated therapies are being investigated
and introduced into clinical management. The estrogen replacement
therapy is currently the most commonly used method for treating EM
that can achieve complete ovarian suppression, and has been in use
since it was first reported in 1948 (203). Low-doses of combined estrogen and
progestin or progestin alone can effectively relieve the clinical
symptoms of pelvic pain caused by EM, and can also reduce the
adverse effects of low estrogen that are induced by GnRH agonists
(204). However, GnRH therapy can
lead to several clinical side effects, including increased
follicular development (205).
In addition to the estrogen-dependent theory, the
mTOR/PI3K signaling pathway theory is also important, where
mTOR/PI3K functions as the upstream regulator of ribosome
biogenesis and plays a key role in protein synthesis (206). The mTOR/PI3K inhibitor GSK2126458
and the RNA polymerase 1 inhibitors CX5461 and BMH21 have been
developed, all of which have shown very good therapeutic effects in
a mouse model of human EM (206).
The complement system is an indispensable part of
the innate immune response and is involved in the identification
and elimination of pathogens and abnormal cells, such as apoptotic
and necrotic cells (208–210). The complement system recognizes
and tags pathogens and altered or transformed self-cells, thereby
activating the inflammatory response and modulating the adaptive
immune response, and ultimately leading to the lysis of target
cells or pathogens (211). The
complement system is a functionally complex system that can trigger
a severe immune response or inflammatory process (211). This system may be harmful to the
body when it is excessively or abnormally activated in conditions
such as inflammation or tissue damage (212–215). The complement system has a
considerable role in peritoneal inflammation, which is associated
with the early stages of EM (216).
The complement system is formed from a group of
small proteins that demonstrate enzymatic activity after activation
and exist in the serum and tissue fluid of healthy individuals and
animals. The components of the complement system are extremely
complex and variable. The study by Aslan et al (217) reported that 23 out of 84 immune
response genes were upregulated in patients with EM, two of them
considerably so. Some of these differentiating molecules were later
confirmed to be members of the complement system (217).
In a previous study, most components of the
complement system that are associated with EM were found to be
upregulated in patients with EM (218). To the best of our knowledge, a
limited number of complement components were found to be decreased.
Such decreased components included mannose/mannan binding
lectin-associated serine protease-1 (MASP-1), and several remain
controversial (219).
In a previous study, the quantities of C1q and
C1INH in the peritoneal fluid of patients with EM at various stages
were considerably increased compared with those in normal controls
(220). Moreover, increased
levels of C1q and C1INH were found in the peritoneal fluid of early
stage EM (220). These results
suggest that immunoglobulins participate in the initiation of the
classical pathway in ectopic endometrial tissue, especially during
early EM (220). Furthermore,
C1-associated genes (including C1QA, C1QB, C1R and C1S) and C2
genes were increased in ectopic endometrial tissues compared with
those in healthy controls (221).
C3 is usually expressed in the ectopic tissue of patients with EM
despite its regular expression levels in the glandular epithelium
of normal endometrium (222,223). The overall C3 levels in the
peritoneal fluid and peripheral blood of patients with EM were
increased compared with those of normal controls, especially C3c
and C3b (219,224–227).
A growing body of evidence has reported that the C3
levels in the serum of patients with EM were considerably
upregulated, in particular among patients with mild EM, when
compared with that in healthy subjects and patients with severe EM
(228–231). Other studies found that the C3
levels in the eutopic endometrium of patients with EM were also
considerably increased under the influence of ectopic endometrial
tissue (228,232). Elevated iC3b in the peritoneal
fluid of patients with EM may negatively regulate NK cell activity
via the iC3b/CR3 signaling pathway, thereby downregulating NK cell
cytotoxicity (219,233).
The levels of other members of the complement
system, such as C5, C6, C7 and C8A, were also upregulated in the
ectopic endometrial tissue of patients with EM (217,221). C6 levels were considerably
increased in patients with early stage EM compared with those in
their healthy counterparts (234). C7 was also upregulated in ectopic
endometrial tissue (221).
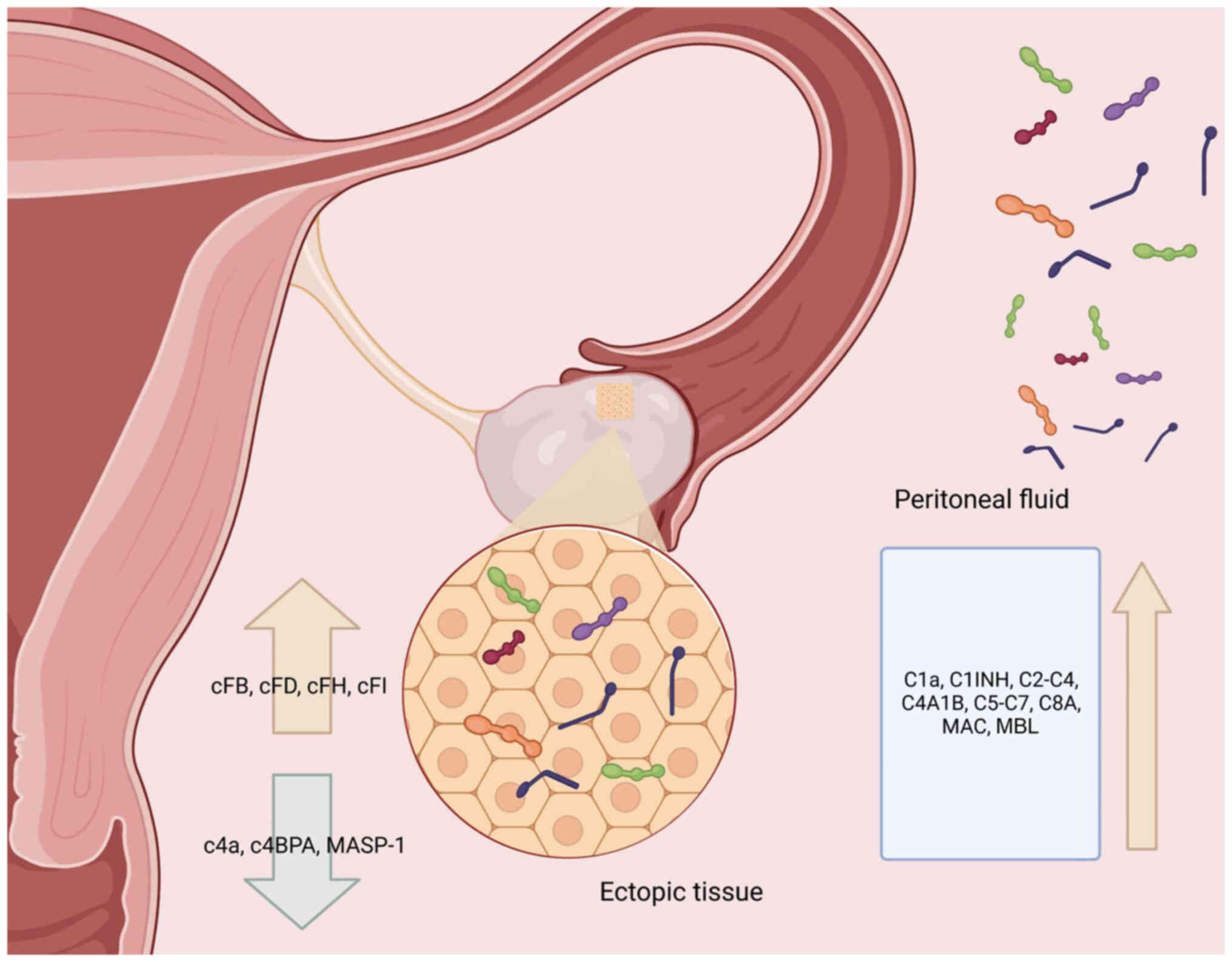
The expression levels of complement factor (CF)B,
CFD, CFH and CFI in the complement system are upregulated in
ectopic EM tissue, while the expression level of MASP-1 is
downregulated (Fig. 5).
There are controversial theories about the
relationship between C4 and EM. Studies have shown that the
concentration of C4 in the peripheral blood and peritoneal
secretions of women with EM are increased compared with those of
normal controls (219,224,225). C4a was considerably decreased in
the peritoneal fluid of patients with peritoneal and ovarian EM
(218,235). The C4A/B gene expression level
was upregulated in ectopic endometrial tissues, while complement
component 4 binding protein α (C4BPA) expression level was reduced
(221).
Two studies have examined the membrane attack
complex (MAC; also known as SC5b-9) in patients with EM. One study
found that the MAC levels in the peritoneal fluid and peripheral
blood of patients with EM were increased. The concentrations of
terminal complex were also increased in patients with advanced EM
(219). However, another study
found no considerable difference in the MAC level in the peritoneal
fluid in patients with EM compared with that in normal controls
(236,237).
The relationship between mannose-binding lectin
(MBL) and EM is also controversial. Some studies have shown that
equivalent MBL levels exist between normal controls and patients
with EM (238,239), while the study by Sikora et
al (220) observed an
increased level of MBL in the peritoneal fluid of patients with EM.
Furthermore, the concentration of MBL in patients with early EM was
increased compared with that in patients with late EM (218,220,236,237,240).
Complement C3 inhibitors can interrupt the
inflammatory cascade at its earliest stage and reduce the
production of iC3b, which weakens NK cytotoxicity. The blockade of
C5a and C3a to induce macrophage activation, C1q inducing the
transformation of macrophages to the M2 phenotype and angiogenesis
in EM lesions via complement immune therapy could all be promising
targets for EM treatment (241,220).
Estrogen and progesterone are two key sex hormones
that are closely associated with the occurrence and progression of
EM. As previously discussed, estrogen mainly exerts its functions
by interacting with ER and inducing an inflammatory environment.
ER2 is associated with the inhibition of the inflammatory response.
Increased ER2 activity can promote cell survival by inhibiting
TNF1-mediated apoptosis, participating in growth factor signaling
and promoting epithelial mesenchymal transition (242).
The close relationship between sex hormones such as
estrogen and progesterone and the immune system has been frequently
demonstrated. Estrogen can induce the activation of the immune
response and immune cells through nuclear receptors. The
dysregulation of estrogen and progesterone signaling in EM are
termed estrogen dominance and progesterone resistance (243,244).
The binding of progesterone to progesterone
receptor (PR) in epithelial and stromal cells inhibits epithelial
cell proliferation and promotes decidualization (245). These effects of progesterone are
achieved by the integration of the response through two
functionally different subtypes of PR: PR-A and PR-B. These two
subtypes share the same gene but have separate promoters, which
makes their structure and function distinct from one another
(246). PR-A is recognized as the
initial driver of uterine PR function, while PR-B is key to
progesterone-induced morphogenesis during pregnancy, and mainly
improves progesterone reactivity by maintaining an appropriate
ratio to PR-A (247).
Estrogen and its two nuclear receptor subtypes have
already been briefly introduced in the preceding sections. Estrogen
promotion of epithelial cell proliferation and endometrial stromal
decidualization is also mediated by the binding of estrogen and its
receptors. The two receptor subtypes, ER1 and ER2, are transcribed
by different genes (248).
ER1 is expressed in most cells of the immune
system, while ER2 is limited to certain cell types of some immune
organs, such as lymphocytes in human lymph nodes, bone marrow and
thymus. Therefore, ER1 has a stronger impact on the immune system
than ER2 (220). Both ER subtypes
are expressed in the endometrium, with the expression levels of ER1
outnumbering those of ER2 (249).
ER1 also has a more important role in promoting the proliferation
of endometrial epithelial cells, implantation and fertilization
than ER2 (221). The response
induced by estrogen binding to ER1 may be mediated by slower
genomic signaling pathways such as the IGF-1-PI3K/AKT pathway
(250). As aforementioned, ER2 is
associated with the inhibition of the inflammatory response.
Increased ER2 activity can promote cell survival by inhibiting
TNF1-mediated apoptosis, participating in growth factor signaling
and promoting epithelial mesenchymal transition (242).
In addition to the two aforementioned types of sex
steroid hormones, the abnormal elevation of prostaglandins (another
hormone that induces an inflammatory response) is also involved in
the pathophysiological changes of EM. Increased PGE2 is detected in
both the eutopic and ectopic endometrial tissues of patients with
EM. PGE2 participates in the direct and indirect induction of pain
through positive feedback with estradiol (E2). In this positive
feedback loop, E2 activates cyclooxygenase II (COX2) to promote the
production of PGE2, and the upregulation of PGE2 level in turn
promotes the expression of steroidogenesis-associated genes and
aromatase, thereby increasing E2 production (251). Sensitivity to IL-1β is important
in the regulation of COX2, which contributes to the maintenance of
sex hormone-associated inflammation in EM lesions (165).
Abnormal regulation of sex hormone nuclear
receptors in patients with EM has been reported (252). The main dysregulations of sex
steroid hormones in EM can be classified into two types: Estrogen
dominance and progesterone resistance.
Estrogen dominance refers to estrogen-induced cell
proliferation and inflammation. The estrogen response is primarily
triggered by ER1 and ER2. These two receptors have different
behaviors, and thus the expression level and ratio of these two
receptors are important to determine effects of estrogen on EM. The
ratio of ER1:ER2 is decreased in ectopic endometrial tissue of the
ovary. This decreased ratio is caused by the upregulation of ER2
and the downregulation of ER1 due to changes in the methylation
level of their promoters (249).
Decreased methylation of the ER2 promoter leads to increased
expression levels of ER2, while increased methylation of the ER1
promoter leads to decreased ER1 expression levels (251,253). In addition to epigenetic changes,
crosstalk between ER1, ER2 and PRs has also been found to be
important. ER2 directly downregulates the expression of ER1 by
binding to the promotor region of ER1 (254). The downregulation of ER1
contributes to the reduction of PR and further promotes the
development of EM and infertility (255).
ER2 upregulation in EM can activate a variety of
proliferation- and inflammation-associated signaling pathways, such
as the COX2-PGE2 feedback loop, which may be the main reason for
increased lesion survival, cell proliferation and inflammation
(249). Other research has found
that ER2 can interact with inflammatory factors to regulate
apoptosis and the inflammatory response, which is also associated
with the pathogenesis of EM (172).
The other sex steroid hormone dysregulation in EM
is progesterone resistance, in which normal and ectopic endometrial
tissues in patients with EM do not respond to progesterone
(256). Little is known about the
mechanism behind progesterone resistance. Several studies have
suggested that the downregulation of progesterone receptors may be
a potential contributor to progesterone resistance, but this
remains controversial (257,258).
The inflammatory and coagulation systems are the
two main host defense systems. The coagulation system can be
triggered by the inflammatory system (267,268). Inflammation is regulated by
coagulation. P-selectin is a platelet adhesion molecule, whose
expression levels are regulated by protein kinase C. Studies have
found that its expression level is abnormal in patients with EM
(269–271). The study by Guo et al
(272) reported that platelet
aggregation was induced by P-selectin in ectopic endometrium, which
promoted the proliferation and progression of the cell cycle for
endometriotic stromal cells (272). Studies have found that P-selectin
is also involved in leukocyte adhesion and inflammation (273,274). P-selectin is therefore considered
a potential immune-associated therapeutic target. P-selectin can be
targeted and blocked in several ways. For example, inclucumab is a
highly specific recombinant human monoclonal antibody against
P-selectin, and has been in clinical trials for the treatment of
myocardial injury (275). The Fc
fragment of recombinant P-selectin has also been tested in a mouse
model of human EM, where it was effective without signs of bleeding
complications (272). However, to
the best of our knowledge, there are no reports of the clinical use
of P-selectin antagonists or antibodies to treat EM.
The treatment of EM still primarily uses
hormone-regulating drugs, such as progesterone-based therapy, GnRH
agonists, aromatase and COX inhibitors, and COC. Although clinical
trials have shown the effectiveness of hormone therapy, patients
still focus on its moderate or severe adverse effects, such as
osteoporosis and sexual function inhibition. Studies have tested
the effects of various compounds on EM, hoping that these compounds
have therapeutic effects on EM without side effects. In the present
review, several compounds are discussed that have been shown to be
capable of improving symptoms of EM in animal experiments, clinical
trials or both. Dienogest is a derivative of 19-nortestosterone,
which serves the dual roles of anti-ovulation and
anti-proliferation against endometrial cells, which can effectively
relieve EM symptoms without the side effects of estrogen and
androgens (276). Clinical
randomized controlled trials at different stages have been carried
out in Europe and Japan, whose results show that Dienogest is
superior to other progesterone drugs in terms of efficacy, safety,
receptor selectivity and tolerance in the treatment of EM (277,278). Although Dienogest has numerous
advantages over other hormone drugs, severe bleeding seems to be a
potential serious side effect.
Beyond hormone-associated therapy, researchers are
also committed to studying and developing drugs targeting other
immune-associated factors to treat of EM. Since the first
anti-complement drug eculizumab (anti-C5 antibody) was approved by
the US Food and Drug Administration for the treatment of paroxysmal
nocturnal hemoglobinuria in 2007, more complement pathway blocking
drugs have been fully developed (279).
In addition to studying drugs targeting immune
system factors, researchers are also studying the utility of
ribosome biosynthesis (associated with macrophage proliferation) in
the treatment of EM. Chang et al (206) tested the potential of using
ribosome biogenesis inhibitors targeting mTOR/PI3K and RNA
polymerase I as an alternative to the treatment of EM in an animal
study in 2022. The results showed that the ribosome biogenesis
inhibitor could inhibit inflammation, reduce neutrophils in the
peritoneal fluid and relieve pain in the treatment of an EM mouse
model, which confirmed its therapeutic potential (206).
In previous decades, there has been a push towards
efforts to find other potential targets for the treatment of EM. It
has become a consensus that local and systemic changes to immune
cells and immune-associated factors are important to the
pathogenesis and development of EM. More attention should be paid
to the development of drugs that target the components of the
immune system. To the best of our knowledge, numerous side effects
can be avoided by immunotherapy, which should be the direction of
future research on EM treatment. Immunotherapy targeting NK cells
and macrophages is in the preclinical trial stage, which may
inspire other researchers to seek improved immune-associated
solutions.
Ectopic endometrial tissue in patients with EM is a
clone of ectopic proliferating endometrial cells in the immune
microenvironment of the inflammatory response, which is
characterized by increased estrogen pro-inflammatory cytokine
levels and alterations to the immune cell infiltration spectrum.
The pathogenesis of EM remains unclear, and it is relatively
difficult to find and select a satisfactory treatment for this
disease. To the best of our knowledge, there is no treatment plan
that can completely cure EM. At present, the treatment of EM is
mainly symptomatic, and includes reducing pain, avoiding
infertility and delaying recurrence as much as possible. Compared
with the inefficiency of medical symptomatic treatment,
laparoscopic surgery is still the first choice for patients with EM
of childbearing age due to its high postoperative pregnancy rate.
However, as with all surgeries, conservative surgery in patients
with EM may only require partial ovariectomy. Hysterectomy is
occasionally required, but there is a risk of over-operation or
premature ovarian failure. Hormone therapy for EM is not ideal and
is usually accompanied by side effects. Thus, although there are
limited studies on the clinical application and evaluation of
immune targeted therapy and personalized therapy for EM, it is
still necessary to further investigate this area.
The present review discussed the role of five major
factors (NK cells, macrophages, the complement system, sex steroid
hormones and P-selectin), and summarized their functions,
regulation and association with EM. Several potential therapeutic
targets for EM have also been summarized, whether they are in the
hypothetical stage or established by animal experiments. It is
hoped that through the present review, more attention can be given
to EM and its potential therapeutic targets, further advancing EM
treatment methods.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant no. 81672861) and the Science and
Technology Development Plan of Shandong Province (grant no.
2017GSF218029).
Not applicable.
WZ, KL and XZ designed the research, WZ and KL
collected and analyzed the reference articles, and wrote the draft.
GZ and AJ designed the figures, XZ and GZ critically revised the
manuscript. All authors contributed to manuscript revision, and
read and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Zondervan KT, Becker CM and Missmer SA:
Endometriosis. N Engl J Med. 382:1244–1256. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taylor HS, Kotlyar AM and Flores VA:
Endometriosis is a chronic systemic disease: Clinical challenges
and novel innovations. Lancet. 397:839–582. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Williams EA: Endometriosis in
Clinical-practice-surgical aspects. Irish J Med Sci. 152:14–17.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Gorp T, Amant F, Neven P, Vergote I
and Moerman P: Endometriosis and the development of malignant
tumours of the pelvis. A review of literature. Best Pract Res Clin
Obstet Gynaecol. 18:349–371. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Indrielle-Kelly T, Fruhauf F, Burgetova A,
Fanta M and Fischerova D: Diagnosis of endometriosis 3rd
Part-ultrasound diagnosis of deep endometriosis. Ceska Gynekol.
84:269–275. 2019.PubMed/NCBI
|
|
6
|
Fukunaga M, Nomura K, Ishikawa E and
Ushigome S: Ovarian atypical endometriosis: Its close association
with malignant epithelial tumours. Histopathology. 30:249–255.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pearce CL, Templeman C, Rossing MA, Lee A,
Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund
KG, et al: Association between endometriosis and risk of
histological subtypes of ovarian cancer: A pooled analysis of
case-control studies. Lancet Oncol. 13:385–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He Y, Cao B and Huang Y: Effect of
endometriosis on prognosis of ovarian clear cell carcinoma: A
10-year retrospective study. Front Oncol. 14:14383092024.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anglesio MS, Bashashati A, Wang YK, Senz
J, Ha G, Yang W, Aniba MR, Prentice LM, Farahani H, Li Chang H, et
al: Multifocal endometriotic lesions associated with cancer are
clonal and carry a high mutation burden. J Pathol. 236:201–209.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Czernobilsky B and Morris WJ: A histologic
study of ovarian endometriosis with emphasis on hyperplastic and
atypical changes. Obstet Gynecol. 53:318–323. 1979.PubMed/NCBI
|
|
11
|
LaGrenade A and Silverberg SG: Ovarian
tumors associated with atypical endometriosis. Hum Pathol.
19:1080–1084. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nouri B, Hashemi SH, J Ghadimi D,
Roshandel S and Akhlaghdoust M: Machine Learning-based detection of
endometriosis: A retrospective study in a population of iranian
female patients. Int J Fertil Steril. 18:362–366. 2024.PubMed/NCBI
|
|
13
|
Moradi Y, Shams-Beyranvand M, Khateri S,
Gharahjeh S, Tehrani S, Varse F, Tiyuri A and Najmi Z: A systematic
review on the prevalence of endometriosis in women. Indian J Med
Res. 154:446–454. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mafra F, Catto M, Bianco B, Barbosa CP and
Christofolini D: Association of Wnt4 polymorphisms with
endometriosis in infertile patients. J Assist Reprod Genet.
32:1359–1364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Santulli P, Bourdon M, Presse M, Gayet V,
Marcellin L, Prunet C, de Ziegler D and Chapron C:
Endometriosis-Related infertility: Assisted reproductive technology
has no adverse impact on pain or quality-of-life scores. Fertil
Steril. 105:978–987.e4. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferrero S, Stabilini C, Barra F, Clarizia
R, Roviglione G and Ceccaroni M: Bowel resection for intestinal
endometriosis. Best Pract Res Clin Obstet Gynaecol. 71:114–128.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chapron C, Marcellin L, Borghese B and
Santulli P: Rethinking mechanisms, diagnosis and management of
endometriosis. Nat Rev Endocrinol. 15:666–682. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferrero S, Barra F, Scala C and Condous G:
Ultrasonography for bowel endometriosis. Best Pract Res Clin Obstet
Gynaecol. 71:38–50. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Geng JH and Lee YC: Bladder endometriosis.
N Engl J Med. 381:e432019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Santulli P, Marcellin L, Tosti C,
Chouzenoux S, Cerles O, Borghese B, Batteux F and Chapron C: MAP
kinases and the inflammatory signaling cascade as targets for the
treatment of endometriosis? Expert Opin Ther Targets. 19:1465–1483.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brown J, Crawford TJ, Allen C, Hopewell S
and Prentice A: Nonsteroidal anti-inflammatory drugs for pain in
women with endometriosis. Cochrane Database Syst Rev.
1:CD0047532017.PubMed/NCBI
|
|
22
|
Streuli I, de Ziegler D, Santulli P,
Marcellin L, Borghese B, Batteux F and Chapron C: An update on the
pharmacological management of endometriosis. Expert Opin
Pharmacother. 14:291–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Y, Liu Y, Jia H, Luo C and Chen H:
Treatment of endometriosis with dienogest in combination with
traditional Chinese medicine: A systematic review and
meta-analysis. Front Surg. 9:9924902022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chinese Medical Association Obstetrics,
Gynecology Branch, Endometriosis Professional Committee and Chinese
Medical Association Obstetrics and Gynecology Branch Endometriosis
Collaborative Group, . Long-term management of endometriosis:
Chinese expert consensus. Chin J Obstet Gynecol. 53:836–841.
2018.(In Chinese).
|
|
25
|
Endometriosis Collaborative Group of the
Obstetrics and Gynecology Branch of the Chinese Medical
Association, . Guidelines for the diagnosis and treatment of
endometriosis. Chin J Obstet Gynecol. 3:161–169. 2015.
|
|
26
|
Vercellini P, Viganò P, Somigliana E and
Fedele L: Endometriosis: Pathogenesis and treatment. Nat Rev
Endocrinol. 10:261–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barbieri RL: Stenosis of the external
cervical os: An association with endometriosis in women with
chronic pelvic pain. Fertil Steril. 70:571–573. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sanfilippo JS, Wakim NG, Schikler KN and
Yussman MA: Endometriosis in Association with Uterine Anomaly. Am J
Obstet Gynecol. 154:39–43. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Halme J, Hammond MG, Hulka JF, Raj SG and
Talbert LM: Retrograde menstruation in healthy women and in
patients with endometriosis. Obstet Gynecol. 64:151–154.
1984.PubMed/NCBI
|
|
30
|
Herington JL, Bruner-Tran KL, Lucas JA and
Osteen KG: Immune interactions in endometriosis. Expert Rev Clin
Immunol. 7:611–626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kyama CM, Debrock S, Mwenda JM and
D'Hooghe TM: Potential involvement of the immune system in the
development of endometriosis. Reprod Biol Endocrinol. 1:1232003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Christodoulakos G, Augoulea A,
Lambrinoudaki I, Sioulas V and Creatsas G: Pathogenesis of
endometriosis: The role of defective ‘immunosurveillance’. Eur J
Contracept Reprod Health Care. 12:194–202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koninckx PR, Ussia A, Adamyan L, Wattiez
A, Gomel V and Martin DC: Pathogenesis of endometriosis: The
Genetic/Epigenetic theory. Fertil Steril. 111:327–340. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Foster WG: Hypoxia-induced autophagy,
epithelial to mesenchymal transition, and invasion in the
pathophysiology of endometriosis: A perspective. Biol Reprod.
99:905–906. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanbo T and Fedorcsak P:
Endometriosis-associated infertility: Aspects of pathophysiological
mechanisms and treatment options. Acta Obstet Gynecol Scand.
96:659–667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gibson DA, Simitsidellis I, Collins F and
Saunders PTK: Androgens, oestrogens and endometrium: A fine balance
between perfection and pathology. J Endocrinol. 246:R75–R93. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mohammed Rasheed HA and Hamid P:
Inflammation to infertility: Panoramic view on endometriosis.
Cureus. 12:e115162020.PubMed/NCBI
|
|
38
|
Halis G and Arici A: Endometriosis and
Inflammation in Infertility. Ann N Y Acad Sci. 1034:300–315. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kang YJ, Jeung IC, Park A, Park YJ, Jung
H, Kim TD, Lee HG, Choi I and Yoon SR: An increased level of IL-6
suppresses NK cell activity in peritoneal fluid of patients with
endometriosis via regulation of SHP-2 expression. Hum Reprod.
29:2176–2189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bedaiwy MA, Falcone T, Sharma RK, Goldberg
JM, Attaran M, Nelson DR and Agarwal A: Prediction of endometriosis
with serum and peritoneal fluid markers: A prospective controlled
trial. Hum Reprod. 17:426–431. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vilas Boas L, Bezerra Sobrinho C, Rahal D,
Augusto Capellari C, Skare T and Nisihara R: Antinuclear antibodies
in patients with endometriosis: A cross-sectional study in 94
patients. Hum Immunol. 83:70–73. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dias JA Jr, de Oliveira RM and Abrao MS:
Antinuclear antibodies and endometriosis. Int J Gynaecol Obstet.
93:262–263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Malinowski A, Szpakowski M, Wilczynski J,
Banasik M and Puchala B: Occurrence of antinuclear antibodies in
women with endometriosis and unexplained infertility. Ginekol Pol.
66:420–424. 1995.(In Polish). PubMed/NCBI
|
|
44
|
Swann JB and Smyth MJ: Immune surveillance
of tumors. J Clin Invest. 117:1137–1146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vallve-Juanico J, Houshdaran S and Giudice
LC: The endometrial immune environment of women with endometriosis.
Hum Reprod Update. 25:564–591. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Podgaec S, Abrao MS, Dias JA Jr, Rizzo LV,
de Oliveira RM and Baracat EC: Endometriosis: An inflammatory
disease with a Th2 immune response component. Hum Reprod.
22:1373–1379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Olkowska-Truchanowicz J, Bocian K, Maksym
RB, Bialoszewska A, Wlodarczyk D, Baranowski W, Ząbek J,
Korczak-Kowalska G and Malejczyk J: CD4+
CD25+ FOXP3+ regulatory T cells in peripheral
blood and peritoneal fluid of patients with endometriosis. Hum
Reprod. 28:119–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tanaka E, Sendo F, Kawagoe S and Hiroi M:
Decreased natural killer cell activity in women with endometriosis.
Gynecol Obstet Invest. 34:27–30. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Thiruchelvam U, Wingfield M and O'Farrelly
C: Natural killer cells: Key players in endometriosis. Am J Reprod
Immunol. 74:291–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sciezynska A, Komorowski M, Soszynska M
and Malejczyk J: Nk cells as potential targets for immunotherapy in
endometriosis. J Clin Med. 8:172019. View Article : Google Scholar
|
|
51
|
Artemova D, Vishnyakova P, Khashchenko E,
Elchaninov A, Sukhikh G and Fatkhudinov T: Endometriosis and
cancer: Exploring the role of macrophages. Int J Mol Sci.
22:51962021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu MH, Chen KF, Lin SC, Lgu CW and Tsai
SJ: Aberrant expression of leptin in human endometriotic stromal
cells is induced by elevated levels of hypoxia inducible
factor-1alpha. Am J Pathol. 170:590–598. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Semenza GL: Hif-1 and mechanisms of
hypoxia sensing. Curr Opin Cell Biol. 13:167–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Becker CM, Rohwer N, Funakoshi T, Cramer
T, Bernhardt W, Birsner A, Folkman J and D'Amato RJ:
2-Methoxyestradiol inhibits hypoxia-inducible factor-1{alpha} and
suppresses growth of lesions in a mouse model of endometriosis. Am
J Pathol. 172:534–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hsiao KY, Chang N, Lin SC, Li YH and Wu
MH: Inhibition of dual specificity Phosphatase-2 by hypoxia
promotes interleukin-8-mediated angiogenesis in endometriosis. Hum
Reprod. 29:2747–2755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hsiao KY, Chang N, Tsai JL, Lin SC, Tsai
SJ and Wu MH: Hypoxia-inhibited DUSP2 expression promotes
IL-6/STAT3 signaling in endometriosis. Am J Reprod Immunol.
78:2017.doi: 10.1111/aji.12690. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sharkey AM, Day K, McPherson A, Malik S,
Licence D, Smith SK and Charnock-Jones DS: Vascular endothelial
growth factor expression in human endometrium is regulated by
hypoxia. J Clin Endocrinol Metab. 85:402–409. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kupker W, Schultze-Mosgau A and Diedrich
K: Paracrine changes in the peritoneal environment of women with
endometriosis. Hum Reprod Update. 4:719–723. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yang HL, Zhou WJ, Chang KK, Mei J, Huang
LQ, Wang MY, Meng Y, Ha SY, Li DJ and Li MQ: The crosstalk between
endometrial stromal cells and macrophages impairs cytotoxicity of
NK cells in endometriosis by secreting IL-10 and TGF-β.
Reproduction. 154:815–825. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
El Hafny-Rahbi B, Brodaczewska K, Collet
G, Majewska A, Klimkiewicz K, Delalande A, Grillon C and Kieda C:
Tumour angiogenesis normalized by Myo-inositol trispyrophosphate
alleviates hypoxia in the microenvironment and promotes antitumor
immune response. J Cell Mol Med. 25:3284–3299. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Thiruchelvam U, Dransfield I, Saunders PT
and Critchley HO: The importance of the macrophage within the human
endometrium. J Leukoc Biol. 93:217–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mehedintu C, Plotogea MN, Ionescu S and
Antonovici M: Endometriosis still a challenge. J Med Life.
7:349–357. 2014.PubMed/NCBI
|
|
63
|
Matarese G, De Placido G, Nikas Y and
Alviggi C: Pathogenesis of endometriosis: Natural immunity
dysfunction or autoimmune disease? Trends Mol Med. 9:223–228. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hogg C, Horne AW and Greaves E:
Endometriosis-associated macrophages: Origin, phenotype, and
function. Front Endocrinol (Lausanne). 11:72020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Capobianco A and Rovere-Querini P:
Endometriosis, a disease of the macrophage. Front Immunol. 4:92013.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Colette S and Donnez J: Are aromatase
inhibitors effective in endometriosis treatment? Expert Opin
Investig Drugs. 20:917–931. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Check JH: Chronic pelvic pain
syndromes-Traditional and novel therapies: Part I surgical therapy.
Clin Exp Obstet Gynecol. 38:10–13. 2011.PubMed/NCBI
|
|
68
|
Sivori S, Vitale M, Morelli L, Sanseverino
L, Augugliaro R, Bottino C, Moretta L and Moretta A: P46, a novel
natural killer cell-specific surface molecule that mediates cell
activation. J Exp Med. 186:1129–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Freud AG, Zhao S, Wei S, Gitana GM,
Molina-Kirsch HF, Atwater SK and Natkunam Y: Expression of the
activating receptor, NKp46 (CD335), in human natural killer and
T-cell neoplasia. Am J Clin Pathol. 140:853–866. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cooper MA, Fehniger TA and Caligiuri MA:
The biology of human natural killer-cell subsets. Trends Immunol.
22:633–640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cooper MA, Fehniger TA, Turner SC, Chen
KS, Ghaheri BA, Ghayur T, Carson WE and Caligiuri MA: Human natural
killer cells: A unique innate immunoregulatory role for the
CD56(bright) subset. Blood. 97:3146–3151. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hamerman JA, Ogasawara K and Lanier LL: NK
cells in innate immunity. Curr Opin Immunol. 17:29–35. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Vivier E, Tomasello E, Baratin M, Walzer T
and Ugolini S: Functions of natural killer cells. Nat Immunol.
9:503–510. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
74
|
Freud AG, Mundy-Bosse BL, Yu J and
Caligiuri MA: The broad spectrum of human natural killer cell
diversity. Immunity. 47:820–833. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Reeves RK, Li H, Jost S, Blass E, Li H,
Schafer JL, Varner V, Manickam C, Eslamizar L, Altfeld M, et al:
Antigen-specific NK cell memory in rhesus macaques. Nat Immunol.
16:927–932. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hammer Q, Ruckert T and Romagnani C:
Natural killer cell specificity for viral infections. Nat Immunol.
19:800–808. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Nikzad R, Angelo LS, Aviles-Padilla K, Le
DT, Singh VK, Bimler L, Vukmanovic-Stejic M, Vendrame E, Ranganath
T, Simpson L, et al: Human natural killer cells mediate adaptive
immunity to viral antigens. Sci Immunol. 4:eaat81162019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Morvan MG and Lanier LL: Nk cells and
cancer: You can teach innate cells new tricks. Nat Rev Cancer.
16:7–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chiossone L, Dumas PY, Vienne M and Vivier
E: Natural killer cells and other innate lymphoid cells in cancer.
Nat Rev Immunol. 18:671–688. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Raulet DH and Vance RE: Self-tolerance of
natural killer cells. Nat Rev Immunol. 6:520–531. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Karre K, Ljunggren HG, Piontek G and
Kiessling R: Selective rejection of H-2-deficient lymphoma variants
suggests alternative immune defence strategy. Nature. 319:675–678.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Viant C, Fenis A, Chicanne G, Payrastre B,
Ugolini S and Vivier E: SHP-1-mediated inhibitory signals promote
responsiveness and anti-Tumour functions of natural killer cells.
Nat Commun. 5:51082014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
van der Touw W, Chen HM, Pan PY and Chen
SH: LILRB Receptor-mediated regulation of myeloid cell maturation
and function. Cancer Immunol Immunother. 66:1079–1087. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Stojanovic A, Correia MP and Cerwenka A:
The NKG2D/NKG2DL axis in the crosstalk between lymphoid and myeloid
cells in health and disease. Front Immunol. 9:8272018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zingoni A, Molfetta R, Fionda C, Soriani
A, Paolini R, Cippitelli M, Cerboni C and Santoni A: NKG2D and its
ligands: ‘One for all, all for one’. Front Immunol. 9:4762018.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ferlazzo G, Thomas D, Lin SL, Goodman K,
Morandi B, Muller WA, Moretta A and Münz C: The abundant NK cells
in human secondary lymphoid tissues require activation to express
killer cell Ig-like receptors and become cytolytic. J Immunol.
172:1455–1462. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bryceson YT, March ME, Ljunggren HG and
Long EO: Synergy among receptors on resting NK cells for the
activation of natural cytotoxicity and cytokine secretion. Blood.
107:159–166. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Masztalerz A, Van Rooijen N, Den Otter W
and Everse LA: Mechanisms of Macrophage cytotoxicity in IL-2 and
IL-12 mediated tumour regression. Cancer Immunol Immunother.
52:235–242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mukherjee S, Jensen H, Stewart W, Stewart
D, Ray WC, Chen SY, Nolan GP, Lanier LL and Das J: In silico
modeling identifies CD45 as a regulator of IL-2 synergy in the
NKG2D-mediated activation of immature human NK cells. Sci Signal.
10:eaai90622017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Fehniger TA and Caligiuri MA: Interleukin
15: Biology and relevance to human disease. Blood. 97:14–32. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kasaian MT, Whitters MJ, Carter LL, Lowe
LD, Jussif JM, Deng B, Johnson KA, Witek JS, Senices M, Konz RF, et
al: Il-21 limits NK cell responses and promotes Antigen-specific T
cell activation: A mediator of the transition from innate to
adaptive immunity. Immunity. 16:559–569. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Tarannum M and Romee R: Cytokine-induced
memory-like natural killer cells for cancer immunotherapy. Stem
Cell Res Ther. 12:5922021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Marcus A, Mao AJ, Lensink-Vasan M, Wang L,
Vance RE and Raulet DH: Tumor-derived cGAMP triggers a
STING-mediated interferon response in non-tumor cells to activate
the NK cell response. Immunity. 49:754–763.e4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lettau M, Paulsen M, Schmidt H and Janssen
O: Insights into the molecular regulation of fasl (CD178) biology.
Eur J Cell Biol. 90:456–466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Prager I and Watzl C: Mechanisms of
natural killer Cell-mediated cellular cytotoxicity. J Leukoc Biol.
105:1319–1329. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Gazvani R and Templeton A: Peritoneal
environment, cytokines and angiogenesis in the pathophysiology of
endometriosis. Reproduction. 123:217–226. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ulukus M and Arici A: Immunology of
endometriosis. Minerva Ginecol. 57:237–248. 2005.PubMed/NCBI
|
|
98
|
Symons LK, Miller JE, Kay VR, Marks RM,
Liblik K, Koti M and Tayade C: The immunopathophysiology of
endometriosis. Trends Mol Med. 24:748–762. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Oosterlynck DJ, Meuleman C, Waer M,
Vandeputte M and Koninckx PR: The natural killer activity of
peritoneal fluid lymphocytes is decreased in women with
endometriosis. Fertil Steril. 58:290–295. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Maeda N, Izumiya C, Kusum T, Masumoto T,
Yamashita C, Yamamoto Y, Oguri H and Fukaya T: Killer inhibitory
receptor CD158a overexpression among natural killer cells in women
with endometriosis is undiminished by laparoscopic surgery and
gonadotropin releasing hormone agonist treatment. Am J Reprod
Immunol. 51:364–372. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Vigano P, Vercellini P, Di Blasio AM,
Colombo A, Candiani GB and Vignali M: Deficient antiendometrium
Lymphocyte-mediated cytotoxicity in patients with endometriosis.
Fertil Steril. 56:894–899. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lotze MT and Rosenberg SA: Results of
clinical trials with the administration of interleukin 2 and
adoptive immunotherapy with activated cells in patients with
cancer. Immunobiology. 172:420–437. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sikora J, Smycz-Kubanska M,
Mielczarek-Palacz A, Bednarek I and Kondera-Anasz Z: The
involvement of multifunctional TGF-β and related cytokines in
pathogenesis of endometriosis. Immunol Lett. 201:31–37. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Dias JA Jr, Podgaec S, de Oliveira RM,
Carnevale Marin ML, Baracat EC and Abrao MS: Patients with
endometriosis of the rectosigmoid have a higher percentage of
natural killer cells in peripheral blood. J Minim Invasive Gynecol.
19:317–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Oosterlynck DJ, Meuleman C, Lacquet FA,
Waer M and Koninckx PR: Flow cytometry analysis of lymphocyte
subpopulations in peritoneal fluid of women with endometriosis. Am
J Reprod Immunol. 31:25–31. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Vernet-Tomas Mdel M, Perez-Ares CT, Verdu
N, Molinero JL, Fernandez-Figueras MT and Carreras R: The
endometria of patients with endometriosis show higher expression of
Class I human leukocyte antigen than the endometria of healthy
women. Fertil Steril. 85:78–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wu MY, Yang JH, Chao KH, Hwang JL, Yang YS
and Ho HN: Increase in the expression of killer cell inhibitory
receptors on peritoneal natural killer cells in women with
endometriosis. Fertil Steril. 74:1187–1191. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Matsuoka S, Maeda N, Izumiya C, Yamashita
C, Nishimori Y and Fukaya T: Expression of inhibitory-motif killer
immunoglobulin-like receptor, KIR2DL1, is increased in natural
killer cells from women with pelvic endometriosis. Am J Reprod
Immunol. 53:249–254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Maeda N, Izumiya C, Oguri H, Kusume T,
Yamamoto Y and Fukaya T: Aberrant expression of intercellular
adhesion Molecule-1 and killer inhibitory receptors induces immune
tolerance in women with pelvic endometriosis. Fertil Steril.
77:679–683. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Galandrini R, Porpora MG, Stoppacciaro A,
Micucci F, Capuano C, Tassi I, Di Felice A, Benedetti-Panici P and
Santoni A: Increased frequency of human leukocyte Antigen-E
inhibitory receptor Cd94/Nkg2a-Expressing peritoneal natural killer
cells in patients with endometriosis. Fertil Steril. 89 (5
Suppl):S1490–S1496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Nowak I, Ploski R, Barcz E, Dziunycz P,
Kaminski P, Kostrzewa G, Milewski Ł, Roszkowski PI, Senitzer D,
Malejczyk J and Kuśnierczyk P: KIR2DS5 in the presence of HLA-C C2
protects against endometriosis. Immunogenetics. 67:203–209. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Guo SW, Du Y and Liu X: Platelet-derived
Tgf-Beta1 mediates the Down-modulation of Nkg2d expression and may
be responsible for impaired natural Killer (Nk) cytotoxicity in
women with endometriosis. Hum Reprod. 31:1462–1474. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Bellelis P, Frediani Barbeiro D,
Gueuvoghlanian-Silva BY, Kalil J, Abrao MS and Podgaec S:
Interleukin-15 and Interleukin-7 Are the major cytokines to
maintain endometriosis. Gynecol Obstet Invest. 84:435–444. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yu JJ, Sun HT, Zhang ZF, Shi RX, Liu LB,
Shang WQ, Wei CY, Chang KK, Shao J, Wang MY and Li MQ: Il15
promotes growth and invasion of endometrial stromal cells and
inhibits killing activity of NK cells in endometriosis.
Reproduction. 152:151–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Gonzalez-Foruria I, Santulli P, Chouzenoux
S, Carmona F, Batteux F and Chapron C: Soluble ligands for the
NKG2D receptor are released during endometriosis and correlate with
disease Severity. PLoS One. 10:e01199612015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Salih HR, Rammensee HG and Steinle A:
Cutting edge: Down-regulation of mica on human tumors by
proteolytic shedding. J Immunol. 169:4098–4102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Salih HR, Goehlsdorf D and Steinle A:
Release of micb molecules by tumor cells: Mechanism and soluble
micb in sera of cancer patients. Hum Immunol. 67:188–195. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Mazzeo D, Vigano P, Di Blasio AM,
Sinigaglia F, Vignali M and Panina-Bordignon P: Interleukin-12 and
Its Free P40 subunit regulate immune recognition of endometrial
cells: Potential role in endometriosis. J Clin Endocrinol Metab.
83:911–916. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Mei J, Zhou WJ, Zhu XY, Lu H, Wu K, Yang
HL, Fu Q, Wei CY, Chang KK, Jin LP, et al: Suppression of Autophagy
and HCK Signaling Promotes PTGS2high FCGR3−
NK cell differentiation triggered by ectopic endometrial stromal
cells. Autophagy. 14:1376–1397. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Palumbo JS, Talmage KE, Massari JV, La
Jeunesse CM, Flick MJ, Kombrinck KW, Hu Z, Barney KA and Degen JL:
Tumor cell-associated tissue factor and circulating hemostatic
factors cooperate to increase metastatic potential through natural
killer cell-dependent and-independent mechanisms. Blood.
110:133–141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Nieswandt B, Hafner M, Echtenacher B and
Mannel DN: Lysis of tumor cells by natural killer cells in mice is
impeded by platelets. Cancer Res. 59:1295–1300. 1999.PubMed/NCBI
|
|
122
|
Kopp HG, Placke T and Salih HR:
Platelet-derived transforming growth factor-beta down-regulates
NKG2D thereby inhibiting natural killer cell antitumor reactivity.
Cancer Res. 69:7775–7783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhang Q, Ding D, Liu X and Guo SW:
Activated platelets induce estrogen receptor beta expression in
endometriotic stromal cells. Gynecol Obstet Invest. 80:187–192.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Binyamin L, Alpaugh RK, Hughes TL, Lutz
CT, Campbell KS and Weiner LM: Blocking NK cell inhibitory
self-recognition promotes antibody-dependent cellular cytotoxicity
in a model of anti-lymphoma therapy. J Immunol. 180:6392–6401.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Andre P, Denis C, Soulas C,
Bourbon-Caillet C, Lopez J, Arnoux T, Bléry M, Bonnafous C,
Gauthier L, Morel A, et al: Anti-NKG2A mAb is a checkpoint
inhibitor that promotes anti-tumor immunity by unleashing both T
and NK cells. Cell. 175:1731–1743.e13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Chan EY, Yap DY, Colucci M, Ma AL, Parekh
RS and Tullus K: Use of rituximab in childhood idiopathic nephrotic
syndrome. Clin J Am Soc Nephrol. 18:533–548. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Sivori S, Della Chiesa M, Carlomagno S,
Quatrini L, Munari E, Vacca P, Tumino N, Mariotti FR, Mingari MC,
Pende D and Moretta L: Inhibitory receptors and checkpoints in
human NK cells, implications for the immunotherapy of cancer. Front
Immunol. 11:21562020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Velasco I, Quereda F, Bermejo R, Campos A
and Acien P: Intraperitoneal recombinant Interleukin-2 activates
leukocytes in rat endometriosis. J Reprod Immunol. 74:124–132.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Quereda F, Bermejo R, Velasco I, Campos A
and Acien P: The effect of intraperitoneal Interleukin-2 on
surgically induced endometriosis in rats. Eur J Obstet Gynecol
Reprod Biol. 136:243–248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Dicitore A, Castiglioni S, Saronni D,
Gentilini D, Borghi MO, Stabile S, Vignali M, Di Blasio AM, Persani
L and Vitale G: Effects of human recombinant type I IFNs (IFN-α2b
and IFN-β1a) on growth and migration of primary endometrial stromal
cells from women with deeply infiltrating endometriosis: A
preliminary study. Eur J Obstet Gynecol Reprod Biol. 230:192–198.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wu MY, Chao KH, Chen SU, Chen HF, Yang YS,
Huang SC and Ho HN: The suppression of peritoneal cellular immunity
in women with endometriosis could be restored after gonadotropin
releasing hormone agonist treatment. Am J Reprod Immunol.
35:510–516. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Regis S, Dondero A, Caliendo F, Bottino C
and Castriconi R: NK cell function regulation by TGF-β-induced
epigenetic mechanisms. Front Immunol. 11:3112020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Yoshimura A, Wakabayashi Y and Mori T:
Cellular and molecular basis for the regulation of inflammation by
TGF-beta. J Biochem. 147:781–792. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Young VJ, Brown JK, Saunders PT, Duncan WC
and Horne AW: The peritoneum is both a source and target of TGF-β
in women with endometriosis. PLoS One. 9:e1067732014. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhang M, Xu T, Tong D, Li S, Yu X, Liu B,
Jiang L and Liu K: Research advances in endometriosis-related
signaling pathways: A review. Biomed Pharmacother. 164:1149092023.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Hawinkels LJ and Ten Dijke P: Exploring
Anti-TGF-β therapies in cancer and fibrosis. Growth Factors.
29:140–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Voelker J, Berg PH, Sheetz M, Duffin K,
Shen T, Moser B, Greene T, Blumenthal SS, Rychlik I, Yagil Y, et
al: Anti-TGF-β1 antibody therapy in patients with diabetic
nephropathy. J Am Soc Nephrol. 28:953–962. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Clayton RD, Duffy SR, Wilkinson N, Garry R
and Jackson AM: Increase in peripheral blood mononuclear cell
(PBMC)- and CD56+ cell-mediated killing of endometrial stromal
cells by mycobacteria; a possible role in endometriosis
immunotherapy? Hum Reprod. 19:1886–1893. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Oksasoglu B, Hepokur C, Misir S, Yildiz C,
Sonmez G and Yanik A: Determination of PD-1 expression in
peripheral blood cells in patients with endometriosis. Gynecol
Endocrinol. 37:157–161. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Wu L, Lv C, Su Y, Li C, Zhang H, Zhao X
and Li M: Expression of programmed death-1 (PD-1) and Its Ligand
PD-L1 is upregulated in endometriosis and promoted by
17beta-estradiol. Gynecol Endocrinol. 35:251–256. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Chen Z, Yang Y, Liu LL and Lundqvist A:
Strategies to augment natural killer (NK) cell activity against
solid tumors. Cancers (Basel). 11:10402019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Memon H and Patel BM: Immune checkpoint
inhibitors in non-small cell lung cancer: A Bird's eye view. Life
Sci. 233:1167132019. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Giannopoulos K: Targeting immune signaling
checkpoints in acute myeloid leukemia. J Clin Med. 8:2362019.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Hofmann L, Forschner A, Loquai C,
Goldinger SM, Zimmer L, Ugurel S, Schmidgen MI, Gutzmer R, Utikal
JS, Göppner D, et al: Cutaneous, gastrointestinal, hepatic,
endocrine, and renal side-effects of Anti-PD-1 therapy. Eur J
Cancer. 60:190–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Sosa A, Lopez Cadena E, Simon Olive C,
Karachaliou N and Rosell R: Clinical assessment of immune-related
adverse events. Ther Adv Med Oncol. 10:17588359187646282018.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Afolabi LO, Adeshakin AO, Sani MM, Bi J
and Wan X: Genetic reprogramming for NK cell cancer immunotherapy
with CRISPR/Cas9. Immunology. 158:63–69. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Wang L, Dou M, Ma Q, Yao R and Liu J:
Chimeric antigen receptor (CAR)-modified NK cells against cancer:
Opportunities and challenges. Int Immunopharmacol. 74:1056952019.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Kloess S, Kretschmer A, Stahl L, Fricke S
and Koehl U: CAR-expressing natural killer cells for cancer
retargeting. Transfus Med Hemother. 46:4–13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Seshadri S and Sunkara SK: Natural killer
cells in female infertility and recurrent miscarriage: A systematic
review and meta-analysis. Hum Reprod Update. 20:429–438. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Hadinedoushan H, Mirahmadian M and
Aflatounian A: Increased natural killer cell cytotoxicity and Il-2
production in recurrent spontaneous abortion. Am J Reprod Immunol.
58:409–414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Hashimoto D, Chow A, Noizat C, Teo P,
Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, et al:
Tissue-resident macrophages self-maintain locally throughout adult
life with minimal contribution from circulating monocytes.
Immunity. 38:792–804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Gomez Perdiguero E, Klapproth K, Schulz C,
Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF,
Geissmann F and Rodewald HR: Tissue-resident macrophages originate
from yolk-sac-derived erythro-myeloid progenitors. Nature.
518:547–551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Gordon S and Martinez FO: Alternative
activation of macrophages: Mechanism and functions. Immunity.
32:593–604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Verreck FA, de Boer T, Langenberg DM,
Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de
Waal-Malefyt R and Ottenhoff TH: Human IL-23-producing type 1
macrophages promote but IL-10-producing type 2 macrophages subvert
immunity to (myco)bacteria. Proc Natl Acad Sci USA. 101:4560–4565.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Bonatz G, Hansmann ML, Buchholz F, Mettler
L, Radzun HJ and Semm K: Macrophage- and lymphocyte-subtypes in the
endometrium during different phases of the ovarian cycle. Int J
Gynaecol Obstet. 37:29–36. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Vallve-Juanico J, Santamaria X, Vo KC,
Houshdaran S and Giudice LC: Macrophages display proinflammatory
phenotypes in the eutopic endometrium of women with endometriosis
with relevance to an infectious etiology of the disease. Fertil
Steril. 112:1118–1128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Maybin JA and Critchley HO: Menstrual
physiology: Implications for endometrial pathology and beyond. Hum
Reprod Update. 21:748–761. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Bain CC and Jenkins SJ: The biology of
serous cavity macrophages. Cell Immunol. 330:126–135. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Hudson QJ, Ashjaei K, Perricos A, Kuessel
L, Husslein H, Wenzl R and Yotova I: Endometriosis patients show an
increased M2 response in the peritoneal CD14+low/CD68+low
macrophage subpopulation coupled with an increase in the T-helper 2
and T-regulatory cells. Reprod Sci. 27:1920–1931. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Roszer T: Understanding the mysterious M2
macrophage through activation markers and effector mechanisms.
Mediators Inflamm. 2015:8164602015. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Mantovani A, Sica A, Sozzani S, Allavena
P, Vecchi A and Locati M: The Chemokine system in diverse forms of
macrophage activation and polarization. Trends Immunol. 25:677–686.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Bardi GT, Smith MA and Hood JL: Melanoma
exosomes promote mixed M1 and M2 macrophage polarization. Cytokine.
105:63–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Simpson JL, Elias S, Malinak LR and
Buttram VC Jr: Heritable aspects of Endometriosis. I. Genetic
studies. Am J Obstet Gynecol. 137:327–331. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Dmowski WP, Gebel H and Braun DP:
Decreased apoptosis and sensitivity to macrophage mediated
cytolysis of endometrial cells in endometriosis. Hum Reprod Update.
4:696–701. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Wu MH, Shoji Y, Wu MC, Chuang PC, Lin CC,
Huang MF and Tsai SJ: Suppression of matrix metalloproteinase-9 by
prostaglandin E(2) in peritoneal macrophage is associated with
severity of endometriosis. Am J Pathol. 167:1061–1069. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Gou Y, Li X, Li P, Zhang H, Xu T, Wang H,
Wang B, Ma X, Jiang X and Zhang Z: Estrogen receptor β upregulates
CCL2 via NF-κB signaling in endometriotic stromal cells and
recruits macrophages to promote the pathogenesis of endometriosis.
Hum Reprod. 34:646–658. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Kwon MJ, Shin HY, Cui Y, Kim H, Thi AH,
Choi JY, Kim EY, Hwang DH and Kim BG: CCL2 mediates
neuron-macrophage interactions to drive proregenerative macrophage
activation following preconditioning injury. J Neurosci.
35:15934–15947. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Johan MZ, Ingman WV, Robertson SA and Hull
ML: Macrophages infiltrating endometriosis-like lesions exhibit
progressive phenotype Changes in a heterologous mouse model. J
Reprod Immunol. 132:1–8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Bacci M, Capobianco A, Monno A, Cottone L,
Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S,
et al: Macrophages are alternatively activated in patients with
endometriosis and required for growth and vascularization of
lesions in a mouse model of disease. Am J Pathol. 175:547–556.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Huang YJ, Yang CK, Wei PL, Huynh TT,
Whang-Peng J, Meng TC, Hsiao M, Tzeng YM, Wu AT and Yen Y:
Ovatodiolide suppresses colon tumorigenesis and prevents
polarization of M2 Tumor-associated macrophages through YAP
oncogenic pathways. J Hematol Oncol. 10:602017. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Monsivais D, Dyson MT, Yin P, Coon JS,
Navarro A, Feng G, Malpani SS, Ono M, Ercan CM, Wei JJ, et al: ERβ-
and prostaglandin E2-regulated pathways integrate cell
proliferation via Ras-like and estrogen-regulated growth inhibitor
in endometriosis. Mol Endocrinol. 28:1304–1315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Han SJ, Jung SY, Wu SP, Hawkins SM, Park
MJ, Kyo S, Qin J, Lydon JP, Tsai SY, Tsai MJ, et al: Estrogen
receptor β modulates apoptosis complexes and the inflammasome to
drive the pathogenesis of endometriosis. Cell. 163:960–974. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Stanojevic S, Curuvija I, Blagojevic V,
Petrovic R, Prijic I and Vujic V: The involvement of estrogen
receptors α and β in the in vitro effects of 17β-estradiol on
secretory profile of peritoneal macrophages from naturally
menopausal female and middle-aged male rats. Exp Gerontol.
113:86–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Zhao Y, Gong P, Chen Y, Nwachukwu JC,
Srinivasan S, Ko C, Bagchi MK, Taylor RN, Korach KS, Nettles KW, et
al: Dual suppression of estrogenic and inflammatory activities for
targeting of endometriosis. Sci Transl Med. 7:271ra92015.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Panchanathan R, Shen H, Zhang X, Ho SM and
Choubey D: Mutually positive regulatory feedback loop between
interferons and estrogen receptor-alpha in mice: Implications for
sex bias in autoimmunity. PLoS One. 5:e108682010. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Smith S, Ni Gabhann J, McCarthy E, Coffey
B, Mahony R, Byrne JC, Stacey K, Ball E, Bell A, Cunnane G, et al:
Estrogen receptor α regulates tripartite motif-containing protein
21 expression, contributing to dysregulated cytokine production in
systemic lupus erythematosus. Arthritis Rheumatol. 66:163–172.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Feldman I, Feldman GM, Mobarak C,
Dunkelberg JC and Leslie KK: Identification of proteins within the
nuclear factor-kappa B transcriptional complex including estrogen
receptor-alpha. Am J Obstet Gynecol. 196:394.e1–13. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Kalaitzidis D and Gilmore TD:
Transcription factor cross-talk: The estrogen receptor and
NF-kappaB. Trends Endocrinol Metab. 16:46–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Greaves E, Temp J, Esnal-Zufiurre A,
Mechsner S, Horne AW and Saunders PT: Estradiol is a critical
mediator of macrophage-nerve cross talk in peritoneal
endometriosis. Am J Pathol. 185:2286–2297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Gaudet HM, Cheng SB, Christensen EM and
Filardo EJ: The G-protein coupled estrogen receptor, gper: The
inside and inside-out story. Mol Cell Endocrinol. 418:207–219.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Revankar CM, Cimino DF, Sklar LA,
Arterburn JB and Prossnitz ER: A Transmembrane intracellular
estrogen receptor mediates rapid cell signaling. Science.
307:1625–1630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Heublein S, Lenhard M, Vrekoussis T,
Schoepfer J, Kuhn C, Friese K, Makrigiannakis A, Mayr D and Jeschke
U: The G-protein-coupled estrogen receptor (GPER) is expressed in
normal human ovaries and is upregulated in ovarian endometriosis
and pelvic inflammatory disease involving the ovary. Reprod Sci.
19:1197–1204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Okamoto M, Suzuki T, Mizukami Y and Ikeda
T: The Membrane-type estrogen receptor G-protein-coupled estrogen
receptor suppresses lipopolysaccharide-induced interleukin 6 via
inhibition of nuclear factor-kappa B pathway in murine macrophage
cells. Anim Sci J. 88:1870–1879. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Calippe B, Douin-Echinard V, Delpy L,
Laffargue M, Lelu K, Krust A, Pipy B, Bayard F, Arnal JF, Guéry JC
and Gourdy P: 17Beta-estradiol promotes TLR4-triggered
proinflammatory mediator production through direct estrogen
receptor alpha signaling in macrophages in vivo. J Immunol.
185:1169–1176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Zhang YH, He M, Wang Y and Liao AH:
Modulators of the balance between M1 and M2 macrophages during
pregnancy. Front Immunol. 8:1202017.PubMed/NCBI
|
|
186
|
Chen S, Liu Y, Zhong Z, Wei C, Liu Y and
Zhu X: Peritoneal immune microenvironment of endometriosis: Role
and therapeutic perspectives. Front Immunol. 14:11346632023.
View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Hirata T, Osuga Y, Takamura M, Kodama A,
Hirota Y, Koga K, Yoshino O, Harada M, Takemura Y, Yano T and
Taketani Y: Recruitment of CCR6-expressing Th17 cells by CCL 20
secreted from IL-1 beta-, TNF-alpha-, and IL-17A-stimulated
endometriotic stromal cells. Endocrinology. 151:5468–5476. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Buerger C, Shirsath N, Lang V, Berard A,
Diehl S, Kaufmann R, Boehncke WH and Wolf P: Inflammation dependent
mTORC1 signaling interferes with the switch from keratinocyte
proliferation to differentiation. PLoS One. 12:e01808532017.
View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Schenken RS, Johnson JV and Riehl RM:
C-myc protooncogene polypeptide expression in endometriosis. Am J
Obstet Gynecol. 164:1031–1037. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Selam B, Kayisli UA, Garcia-Velasco JA,
Akbas GE and Arici A: Regulation of Fas ligand expression by IL-8
in human endometrium. J Clin Endocrinol Metab. 87:3921–3927. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Gazvani MR, Christmas S, Quenby S, Kirwan
J, Johnson PM and Kingsland CR: Peritoneal fluid concentrations of
interleukin-8 in women with endometriosis: Relationship to stage of
disease. Human Reproduction. 13:1957–1961. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Borrelli GM, Abrao MS and Mechsner S: Can
chemokines be used as biomarkers for endometriosis? A systematic
review. Hum Reprod. 29:253–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Schneider J, Jimenez E, Rodriguez F and
del Tanago JG: c-myc, c-erb-B2, nm23 and p53 expression in human
endometriosis. Oncol Rep. 5:49–52. 1998.PubMed/NCBI
|
|
194
|
Capobianco A, Monno A, Cottone L, Venneri
MA, Biziato D, Di Puppo F, Ferrari S, De Palma M, Manfredi AA and
Rovere-Querini P: Proangiogenic Tie2(+) macrophages infiltrate
human and murine endometriotic lesions and dictate their growth in
a mouse model of the disease. Am J Pathol. 179:2651–2659. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Kats R, Collette T, Metz CN and Akoum A:
Marked elevation of macrophage migration inhibitory factor in the
peritoneal fluid of women with endometriosis. Fertil Steril.
78:69–76. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Akoum A, Metz CN, Al-Akoum M and Kats R:
Macrophage migration inhibitory factor expression in the
intrauterine endometrium of women with endometriosis varies with
disease stage, infertility status, and pelvic pain. Fertil Steril.
85:1379–1385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Morin M, Bellehumeur C, Therriault MJ,
Metz C, Maheux R and Akoum A: Elevated levels of macrophage
migration inhibitory factor in the peripheral blood of women with
endometriosis. Fertil Steril. 83:865–872. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Kats R, Metz CN and Akoum A: Macrophage
migration inhibitory factor is markedly expressed in active and
early-stage endometriotic lesions. J Clin Endocrinol Metab.
87:883–889. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Seeber B, Sammel MD, Fan X, Gerton GL,
Shaunik A, Chittams J and Barnhart KT: Panel of markers can
accurately predict endometriosis in a subset of patients. Fertil
Steril. 89:1073–1081. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Zinovkin DA, Pranjol MZI, Bilsky IA and
Zmushko VA: Tumor-associated T-lymphocytes and macrophages are
decreased in endometrioid endometrial carcinoma with melf-pattern
stromal changes. Cancer Microenviron. 11:107–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Jha P, Farooq A, Agarwal N and Buckshee K:
In vitro sperm phagocytosis by human peritoneal macrophages in
endometriosis-associated infertility. Am J Reprod Immunol.
36:235–237. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Forster R, Sarginson A, Velichkova A, Hogg
C, Dorning A, Horne AW, Saunders PTK and Greaves E:
Macrophage-derived insulin-like growth Factor-1 is a key
neurotrophic and nerve-sensitizing factor in pain associated with
endometriosis. FASEB J. 33:11210–11222. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Bushnell LF: Endometriosis and estrogen
therapy. Am J Obstet Gynecol. 55:9151948. View Article : Google Scholar
|
|
204
|
Barbieri RL: Endometriosis and the
estrogen threshold theory. Relation to surgical and medical
treatment. J Reprod Med. 43 (3 Suppl):S287–S292. 1998.
|
|
205
|
Howell R, Dowsett M, King N and Edmonds
DK: Endocrine effects of gnrh analogue with low-dose hormone
replacement therapy in women with endometriosis. Clin Endocrinol
(Oxf). 43:609–615. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Chang CY, Chiang AJ, Yan MJ, Lai MT, Su
YY, Huang HY, Chang CY, Li YH, Li PF, Chen CM, et al: Ribosome
biogenesis serves as a therapeutic target for treating
endometriosis and the associated complications. Biomedicines.
10:1852022. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Khoufache K, Bazin S, Girard K,
Guillemette J, Roy MC, Verreault JP, Al-Abed Y, Foster W and Akoum
A: Macrophage migration inhibitory factor antagonist blocks the
development of endometriosis in vivo. PLoS One. 7:e372642012.
View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Daha MR: Role of complement in innate
immunity and infections. Crit Rev Immunol. 30:47–52. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Conigliaro P, Triggianese P, Ballanti E,
Perricone C, Perricone R and Chimenti MS: Complement, infection,
and autoimmunity. Curr Opin Rheumatol. 31:532–541. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Trouw LA, Blom AM and Gasque P: Role of
complement and complement regulators in the removal of apoptotic
cells. Mol Immunol. 45:1199–1207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Hajishengallis G, Reis ES, Mastellos DC,
Ricklin D and Lambris JD: Novel mechanisms and functions of
complement. Nat Immunol. 18:1288–1298. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Lubbers R, van Essen MF, van Kooten C and
Trouw LA: Production of complement components by cells of the
immune system. Clin Exp Immunol. 188:183–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Merle NS, Church SE, Fremeaux-Bacchi V and
Roumenina LT: Complement system part I-molecular mechanisms of
activation and regulation. Front Immunol. 6:2622015. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Merle NS, Noe R, Halbwachs-Mecarelli L,
Fremeaux-Bacchi V and Roumenina LT: Complement system part II: Role
in immunity. Front Immunol. 6:2572015. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Ricklin D, Reis ES and Lambris JD:
Complement in disease: A defence system turning offensive. Nat Rev
Nephrol. 12:383–401. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Zhang T, De Carolis C, Man GCW and Wang
CC: The Link between Immunity, autoimmunity and endometriosis: A
literature update. Autoimmunity Revi. 17:945–955. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Aslan C, Ak H, Askar N, Ozkaya AB,
Ergenoglu AM, Yeniel AO, Akdemir A and Aydin HH: Overexpression of
complement C5 in endometriosis. Clin Biochem. 47:496–498. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Rahal D, Andrade F and Nisihara R:
Insights into the role of complement system in the pathophysiology
of endometriosis. Immunol Lett. 231:43–48. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Kabut J, Kondera-Anasz Z, Sikora J and
Mielczarek-Palacz A: Levels of complement components iC3b, C3c, C4,
and SC5b-9 in peritoneal fluid and serum of infertile women with
endometriosis. Fertil Steril. 88:1298–1303. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Sikora J, Wroblewska-Czech A,
Smycz-Kubanska M, Mielczarek-Palacz A, Cygal A, Witek A and
Kondera-Anasz Z: The role of complement components C1q, MBL and C1
inhibitor in pathogenesis of endometriosis. Arch Gynecol Obstet.
297:1495–1501. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Ahn SH, Khalaj K, Young SL, Lessey BA,
Koti M and Tayade C: Immune-inflammation gene signatures in
endometriosis patients. Fertil Steril. 106:1420–1431.e7. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Weed JC and Arquembourg PC: Endometriosis:
Can it produce an autoimmune response resulting in infertility?
Clin Obstet Gynecol. 23:885–893. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Isaacson KB, Coutifaris C, Garcia CR and
Lyttle CR: Production and secretion of complement component-3 by
endometriotic tissue. J Clin Endocrinol Metab. 69:1003–1009. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Badawy SZA, Cuenca V, Stitzel A, Jacobs
RDB and Tomar RH: Autoimmune phenomena in infertile patients with
endometriosis. Obstet Gynecol. 63:271–275. 1984.PubMed/NCBI
|
|
225
|
Badawy SZA, Cuenca V, Marshall L,
Munchback R, Rinas AC and Coble DA: Cellular-components in
peritoneal-fluid in infertile patients with and without
endometriosis. Fertil Steril. 42:704–708. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Tao XJ, Sayegh RA and Isaacson KB:
Increased expression of complement component 3 in human ectopic
endometrium compared with the matched eutopic endometrium. Fertil
Steril. 68:460–467. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Sayegh RA, Tao XJ, Awwad JT and Isaacson
KB: Localization of the expression of complement component 3 in the
human endometrium by in situ hybridization. J Clin Endocrinol
Metab. 81:1641–1649. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Steele RW, Dmowski WP and Marmer DJ:
Immunological aspects of human endometriosis. Am J Reprod Immunol
(1980). 6:33–36. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Meek SC, Hodge DD and Musich JR:
Autoimmunity in infertile patients with endometriosis. Am J Obstet
Gynecol. 158:1365–1373. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Isaacson KB, Galman M, Coutifaris C and
Lyttle CR: Endometrial synthesis and secretion of complement
component-3 by patients with and without endometriosis. Fertil
Steril. 53:836–841. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Hasan A, Rahim A, Afzal M, Naveed AK, Ayub
S and Jahan S: Serum albumin and C3 complement levels in
endometriosis. J Coll Physicians Surg Pak. 29:702–705. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Bischof P, Planasbasset D, Campana A and
Meisser A: Investigations on the cell type responsible for the
endometrial secretion of complement component-3 (C3). Human Reprod.
9:1652–1659. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Liu CF, Min XY, Wang N, Wang JX, Ma N,
Dong X, Zhang B, Wu W, Li ZF, Zhou W and Li K: Complement receptor
3 Has negative impact on tumor surveillance through suppression of
natural killer cell function. Front Immunol. 8:16022017. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Suryawanshi S, Huang X, Elishaev E, Budiu
RA, Zhang L, Kim S, Donnellan N, Mantia-Smaldone G, Ma T, Tseng G,
et al: Complement pathway is frequently altered in endometriosis
and endometriosis-associated ovarian cancer. Clin Cancer Res.
20:6163–6174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Woelfler MM, Meinhold-Heerlein IM,
Soehngen L, Rath W, Knuechel R, Neulen J, Maass N and Henkel C:
Two-dimensional gel electrophoresis in peritoneal fluid samples
identifies differential protein regulation in patients suffering
from peritoneal or ovarian endometriosis. Fertil Steril.
95:2764–2768. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Riley CF, Moen MH and Videm V:
Inflammatory markers in endometriosis: Reduced peritoneal
neutrophil response in minimal endometriosis. Acta Obstet Gynecol
Scand. 86:877–881. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Kruse C, Steffensen R, Nielsen HJ and
Jensenius JC: Mannan-binding lectin polymorphisms and serum levels
in patients with endometriosis. Eur J Obstet Gynecol Reprod Biol.
181:256–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Kruse C, Steffensen R, Nielsen HJ and
Jensenius JC: Mannan-binding lectin polymorphisms and serum levels
in patients with endometriosis. Eur J Obstet Gynecol Reprod Biol.
181:256–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Kavoussi SK, Mueller MD and Lebovic DI:
Expression of mannose-binding lectin in the peritoneal fluid of
women with and without endometriosis. Fertil Steril. 85:1526–1528.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Ozerkan K, Oral B and Uncu G:
Mannose-binding lectin levels in endometriosis. Fertil Steril.
94:775–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Karadadas E, Hortu I, Ak H, Ergenoglu AM,
Karadadas N and Aydin HH: Evaluation of complement system proteins
C3a, C5a and C6 in patients of endometriosis. Clin Biochem.
81:15–19. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Stefkovich ML, Arao Y, Hamilton KJ and
Korach KS: Experimental models for evaluating non-genomic estrogen
signaling. Steroids. 133:34–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Al-Sabbagh M, Lam EW and Brosens JJ:
Mechanisms of endometrial progesterone resistance. Mol Cell
Endocrinol. 358:208–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Han SJ and O'Malley BW: The dynamics of
nuclear receptors and nuclear receptor coregulators in the
pathogenesis of endometriosis. Hum Reprod Update. 20:467–484. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Patel BG, Rudnicki M, Yu J, Shu Y and
Taylor RN: Progesterone resistance in endometriosis: Origins,
consequences and interventions. Acta Obstet Gynecol Scand.
96:623–632. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Kastner P, Krust A, Turcotte B, Stropp U,
Tora L, Gronemeyer H and Chambon P: Two distinct estrogen-regulated
promoters generate transcripts encoding the two functionally
different human progesterone receptor forms A and B. EMBO J.
9:1603–1614. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Patel B, Elguero S, Thakore S, Dahoud W,
Bedaiwy M and Mesiano S: Role of Nuclear progesterone receptor
isoforms in uterine pathophysiology. Hum Reprod Update. 21:155–173.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Vasquez YM and DeMayo FJ: Role of nuclear
receptors in blastocyst implantation. Semin Cell Dev Biol.
24:724–735. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Chantalat E, Valera MC, Vaysse C, Noirrit
E, Rusidze M, Weyl A, Vergriete K, Buscail E, Lluel P, Fontaine C,
et al: Estrogen receptors and endometriosis. Int J Mol Sci.
21:28152020. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Wang Y, Zhu L, Kuokkanen S and Pollard JW:
Activation of protein synthesis in mouse uterine epithelial cells
by estradiol-17β is mediated by a PKC-ERK1/2-mTOR signaling
pathway. Proc Natl Acad Sci USA. 112:E1382–E1391. 2015.PubMed/NCBI
|
|
251
|
Reis FM, Petraglia F and Taylor RN:
Endometriosis: Hormone regulation and clinical consequences of
chemotaxis and apoptosis. Hum Reprod Update. 19:406–418. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Yilmaz BD and Bulun SE: Endometriosis and
nuclear receptors. Hum Reprod Update. 25:473–485. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E,
Yin P, Milad MP, Confino E, Reierstad S, Innes J and Bulun SE:
Promoter methylation regulates estrogen receptor 2 in human
endometrium and endometriosis. Biol Reprod. 77:681–687. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Trukhacheva E, Lin Z, Reierstad S, Cheng
YH, Milad M and Bulun SE: Estrogen receptor (ER) beta regulates
eralpha expression in stromal cells derived from ovarian
endometriosis. J Clin Endocrinol Metab. 94:615–622. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Bulun SE, Yilmaz BD, Sison C, Miyazaki K,
Bernardi L, Liu S, Kohlmeier A, Yin P, Milad M and Wei J:
Endometriosis. Endocr Rev. 40:1048–1079. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Burney RO, Talbi S, Hamilton AE, Vo KC,
Nyegaard M, Nezhat CR, Lessey BA and Giudice LC: Gene expression
analysis of endometrium reveals progesterone resistance and
candidate susceptibility genes in women with endometriosis.
Endocrinology. 148:3814–3826. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Flores VA, Vanhie A, Dang T and Taylor HS:
Progesterone receptor status predicts response to progestin therapy
in endometriosis. J Clin Endocrinol Metab. 103:4561–4568. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Broi MGD, Rocha CVJ, Meola J, Martins WP,
Carvalho FM, Ferriani RA and Navarro PA: Expression of PGR, HBEGF,
ITGAV, ITGB3 and SPP1 genes in eutopic endometrium of infertile
women with endometriosis during the implantation window: A pilot
study. JBRA Assist Reprod. 21:196–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Strowitzki T, Marr J, Gerlinger C,
Faustmann T and Seitz C: Dienogest is as effective as leuprolide
acetate in treating the painful symptoms of endometriosis: A
24-Week, randomized, multicentre, open-label trial. Hum Reprod.
25:633–641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Saunders PTK and Horne AW: Endometriosis:
Etiology, pathobiology, and therapeutic prospects. Cell.
184:2807–2824. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Ferrero S, Evangelisti G and Barra F:
Current and emerging treatment options for endometriosis. Expert
Opin Pharmacother. 19:1109–1125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Capezzuoli T, Rossi M, La Torre F,
Vannuccini S and Petraglia F: Hormonal drugs for the treatment of
endometriosis. Curr Opin Pharmacol. 67:1023112022. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Dunselman GA, Vermeulen N, Becker C,
Calhaz-Jorge C, D'Hooghe T, De Bie B, Heikinheimo O, Horne AW,
Kiesel L, Nap A, et al: ESHRE guideline: Management of women with
endometriosis. Hum Reprod. 29:400–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Crosignani PG, Luciano A, Ray A and
Bergqvist A: Subcutaneous depot medroxyprogesterone acetate versus
leuprolide acetate in the treatment of endometriosis-associated
pain. Hum Reprod. 21:248–256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Selak V, Farquhar C, Prentice A and Singla
A: Danazol for pelvic pain associated with endometriosis. Cochrane
Database Syst Rev. CD0000682007.PubMed/NCBI
|
|
266
|
Strowitzki T, Faustmann T, Gerlinger C and
Seitz C: Dienogest in the treatment of endometriosis-associated
pelvic pain: A 12-week, randomized, double-blind,
placebo-controlled study. Eur J Obstet Gynecol Reprod Biol.
151:193–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Petaja J: Inflammation and coagulation. An
overview. Thromb Res. 127 (Suppl 2):S34–S37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Demetz G and Ott I: The interface between
inflammation and coagulation in cardiovascular disease. Int J
Inflam. 2012:8603012012.PubMed/NCBI
|
|
269
|
Lessey BA, Castelbaum AJ, Sawin SW, Buck
CA, Schinnar R, Bilker W and Strom BL: Aberrant integrin expression
in the endometrium of women with endometriosis. J Clin Endocrinol
Metab. 79:643–649. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Regidor PA, Vogel C, Regidor M, Schindler
AE and Winterhager E: Expression pattern of integrin adhesion
molecules in endometriosis and human endometrium. Hum Reprod
Update. 4:710–718. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
271
|
Libersan D and Merhi Y: Platelet
P-selectin expression: Requirement for protein kinase C, but not
protein tyrosine kinase or phosphoinositide 3-kinase. Thromb
Haemost. 89:1016–1023. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
272
|
Guo SW, Ding D, Geng JG, Wang L and Liu X:
P-selectin as a potential therapeutic target for endometriosis.
Fertil Steril. 103:990–1000.e8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
273
|
Lorant DE, Patel KD, McIntyre TM, McEver
RP, Prescott SM and Zimmerman GA: Coexpression of Gmp-140 and paf
by endothelium stimulated by histamine or thrombin: A juxtacrine
system for adhesion and activation of neutrophils. J Cell Biol.
115:223–234. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Lefer DJ: Pharmacology of selectin
inhibitors in ischemia/reperfusion states. Annu Rev Pharmacol
Toxicol. 40:283–294. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Kling D, Stucki C, Kronenberg S, Tuerck D,
Rheaume E, Tardif JC, Gaudreault J and Schmitt C: Pharmacological
control of platelet-leukocyte interactions by the human
anti-P-selectin antibody inclacumab-preclinical and clinical
studies. Thromb Res. 131:401–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
276
|
Harada T and Taniguchi F: Dienogest: A new
therapeutic agent for the treatment of endometriosis. Womens Health
(Lond). 6:27–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Kaminski K, Fiegler P, Marr J and Moore C:
Treatment of endometriosis with dienogest: Preliminary report.
Ginekol Pol. 72:299–304. 2001.(In Polish). PubMed/NCBI
|
|
278
|
Harada T, Momoeda M, Taketani Y, Aso T,
Fukunaga M, Hagino H and Terakawa N: Dienogest is as effective as
intranasal buserelin acetate for the relief of pain symptoms
associated with endometriosis-a randomized, double-blind,
multicenter, controlled trial. Fertil Steril. 91:675–681. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
279
|
Rother RP, Rollins SA, Mojcik CF, Brodsky
RA and Bell L: Discovery and development of the complement
inhibitor eculizumab for the treatment of paroxysmal nocturnal
hemoglobinuria. Nat Biotechnol. 25:1256–1264. 2007. View Article : Google Scholar : PubMed/NCBI
|