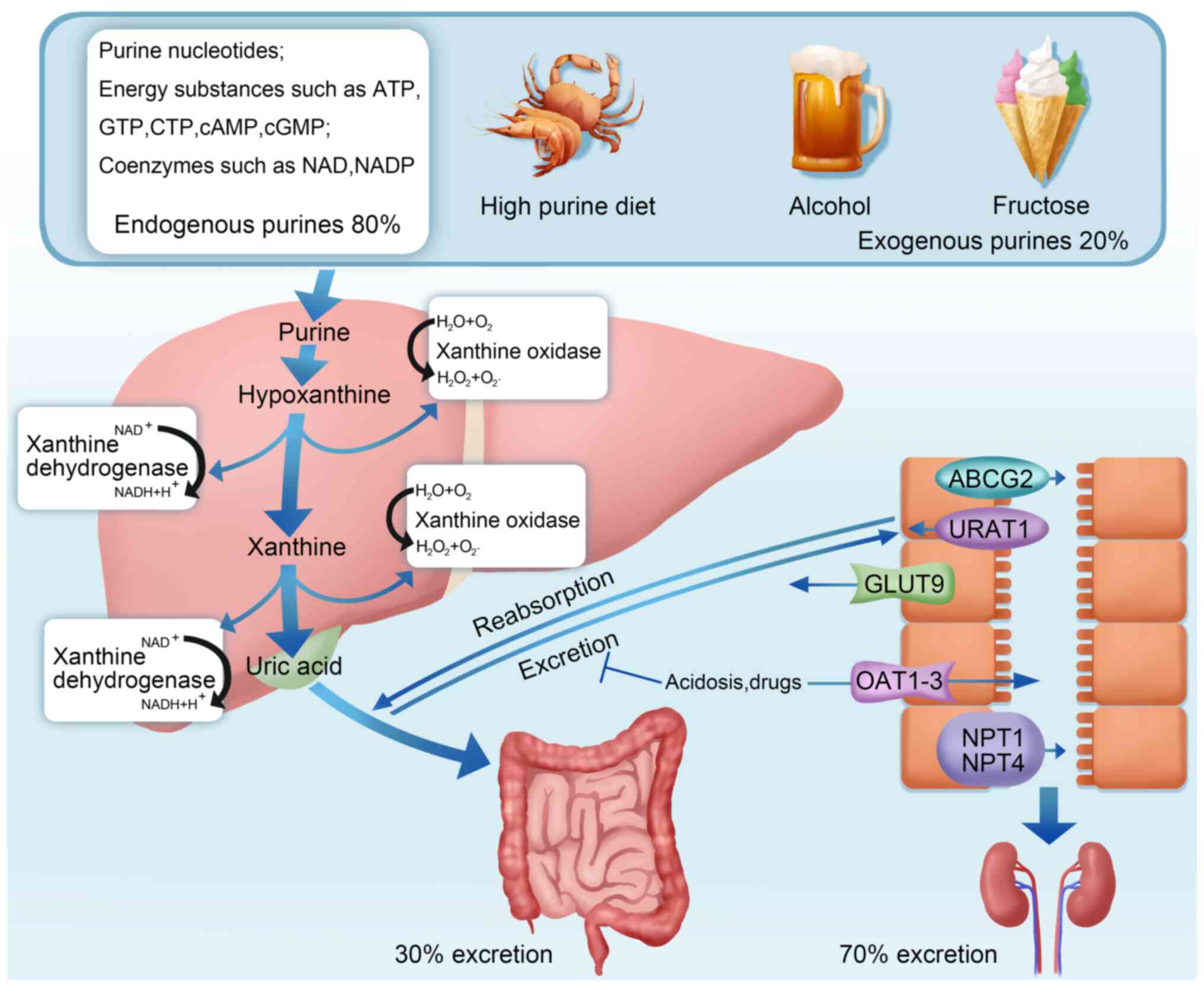
Uric acid (UA), the final product of both exogenous
purines obtained from the diet and endogenous purines released from
damaged, dying and dead cells, is mainly synthesized in the liver,
intestine and vascular endothelium (1,2). Of
the total UA produced daily, ~70% is excreted through the kidneys
and the remaining 30% is excreted from the intestine (3). Hyperuricemia, a condition when the
amount of UA produced exceeds the amount of UA excreted, can occur
due to multiple factors, including acquired factors and rare
genetic factors, such as myeloproliferative diseases, high-purine
diet, alcohol intake, fructose intake, hypoxanthine guanine
phosphoribosyltransferase deficiency and excessive
phosphoribosylpyrophosphate synthase (Fig. 1) (4).
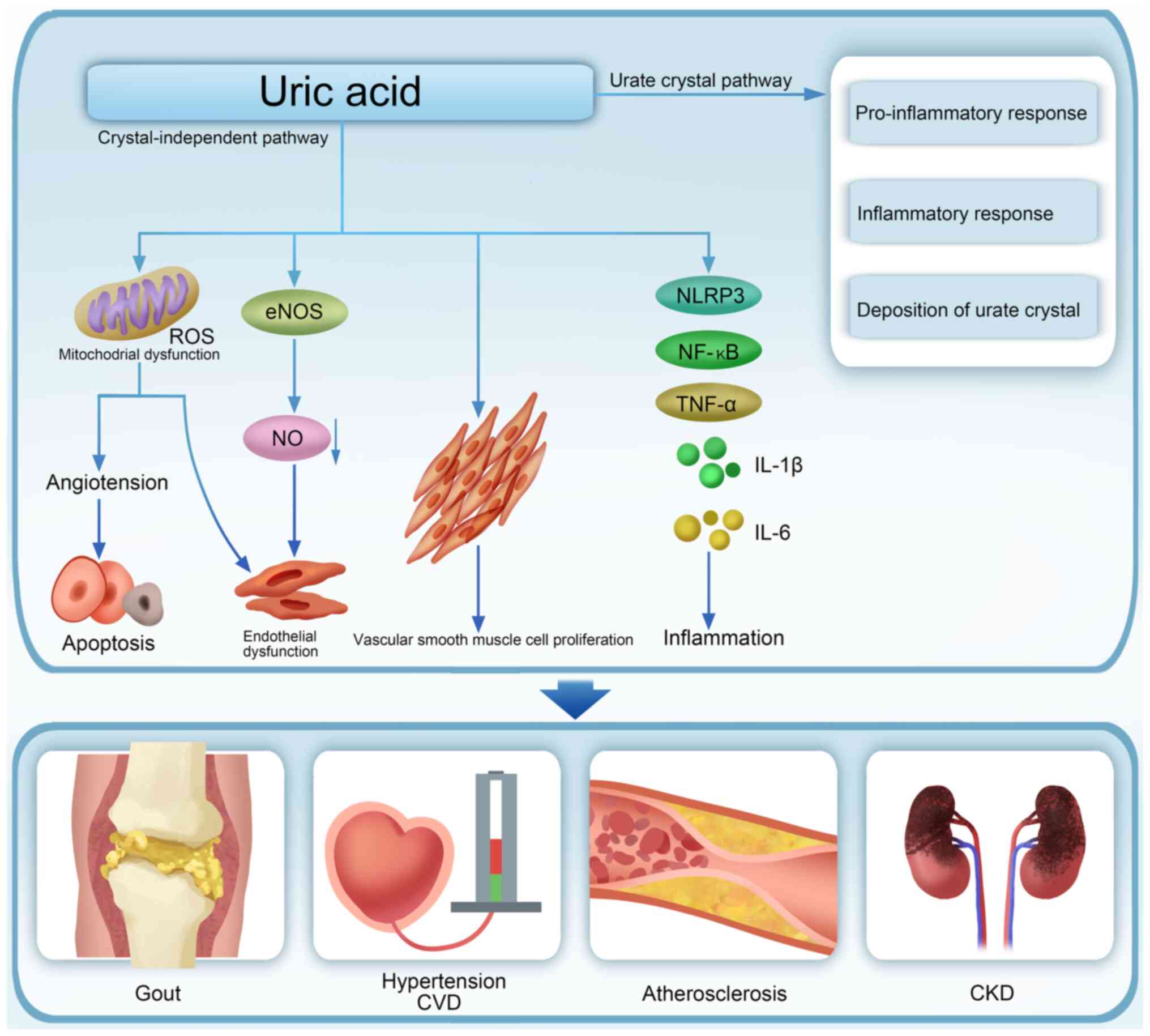
UA has been recognized as a mediator in a number of
pathological processes, including inflammation, apoptosis,
oxidative stress, vascular smooth muscle cell proliferation and
endothelial dysfunction, involved in the development of various
conditions, such as type 2 diabetes mellitus (T2DM), metabolic
syndrome (MS), obesity, hypertension, cardiovascular disease (CVD),
hypertriglyceridemia, metabolic dysfunction-associated steatotic
liver disease (MASLD), acute kidney injury and chronic kidney
disease (CKD; Fig. 2) (5,6). The
incidence and prevalence of hyperuricemia continues to rise,
contributing to increased overall morbidity and mortality rates as
well as a greater economic burden on healthcare. Consequently, it
is now considered a major public health concern.
Increased serum UA levels are closely associated
with kidney disease. Kidney disease, particularly when associated
with a decrease in glomerular filtration rate, can lead to
increased serum UA levels due to insufficient UA excretion
resulting from renal failure. Therefore, hyperuricemia may be a
secondary phenomenon in patients with kidney disease (7). However, renal damage may be
associated with increased oxidative stress induced by intracellular
UA (Fig. 1) (8–11).
Hence, studying the interaction between blood UA and the kidneys is
of great significance.
Epidemiological and empirical studies have revealed
an association between hyperuricemia and an increased risk of CKD,
new-onset hypertension, stroke, CVD and coronary heart disease
(CHD) (4,12). These diseases are commonly
accompanied by varying degrees of kidney damage. The present review
summarized the complex correlation between hyperuricemia and renal
injury as well as the pathophysiological factors associated with UA
management. Furthermore, it also assessed the correlation between
UA, CKD, gout, diabetes, obesity, systemic lupus erythematosus
(SLE) and CVD and discussed several existing management strategies
for hyperuricemia.
The regulation of UA levels is complex and involves
multiple factors, including the regulation of UA production in the
liver and its excretion through the kidneys and intestine (13). Of UA, ~80% and 20% is produced from
the endogenous and exogenous purines, respectively. UA from
exogenous sources, primarily from foods rich in purine compounds,
nucleic acids and nucleoproteins such as beans, seafood, animal
viscera, mushrooms, alcohol and meat, endogenous UA is formed by
the conversion, decomposition and metabolism of amino acids,
nucleic acids and phosphoribosyl groups (Fig. 1) (2,14).
Xanthine oxidase, a vital rate-limiting enzyme in
purine metabolism, converts hypoxanthine to xanthine and xanthine
to UA, which are the two essential steps in the UA production from
purines (14). Xanthine oxidase
also converts guanine nucleotides to xanthine, which is further
oxidized to UA by xanthine oxidase (15,16).
Hyperuricemia is primarily caused by excessive intake and decreased
excretion of UA (17). UA
excretion disorders may be caused by various factors originally
involving abnormal expression of urate transporters in the proximal
tubules, such as glucose transporter 9 (GLUT9), uric acid
transporter 1 (URAT1), organic anion transporter 1 (OAT1), OAT3 and
ATP-binding cassette subfamily G member 2 (ABCG2) (18,19).
Notably, regulating the expression of these urate transporters can
improve UA excretion (19,20).
Urate is primarily excreted through three pathways:
glomerular filtration, tubular reabsorption and tubular secretion
(21). Decreased glomerular
filtration, decreased tubular secretion and enhanced tubular
reabsorption can increase UA levels, ultimately causing
hyperuricemia over time. Hyperuricemia is generally associated with
CKD. A recent meta-analysis reported an occurrence of hyperuricemia
in patients with advanced CKD, with a prevalence of 64 and 50% in
patients with stage 3 and 4 or 5 CKD, respectively (22). Therefore, hyperuricemia is a risk
factor for CKD progression (23).
Studies have shown that renal fibrosis, vascular
damage and endothelial dysfunction are the main characteristics of
UA-induced renal injury (4,8,9,24,25).
As a potential mechanism underlying CKD progression due to
hyperuricemia, sodium urate crystals can induce
epithelial-mesenchymal transition (EMT) in renal epithelial cells.
This process is characterized by enhanced α-smooth muscle actin
production, excessive extracellular matrix (ECM) deposition,
activation of NOD-, LRR- and pyrin domain-containing protein 3
(NLRP3) inflammasomes and oxidative stress due to NADPH
oxidase-dependent reactive oxygen species (ROS) and XO-dependent
ROS (8–11,26).
Furthermore, renal interstitial fibrosis is also associated with
the activation of the TGF-β/Smad3 pathway in hyperuricemia
nephropathy (HN) mice (27).
The regulation of the transporters involved in urate
excretion and reabsorption as well as the signaling pathways
involved in urate-induced kidney damage may alleviate
hyperuricemia-induced kidney damage. For example, Fang et al
(18) report that Eucommia
ulmoides cortex ethanol extract significantly reduces serum
uric acid (SUA) levels, which are associated with increased mRNA
expression of OAT1 and OAT3 and significantly
decrease the mRNA levels of GLUT9 and URAT1 in the
kidney.
A clinical trial involving 269,651 patients
demonstrates that UA-lowering therapy is not associated with
beneficial kidney outcomes in patients with kidney function at
least 60 ml/min/1.73 m2 and no albuminuria (28). Moreover, this trial reports a
higher risk of developing CKD and proteinuria in patients with less
severely elevated serum UA concentrations receiving UA-lowering
treatment (28). The 2024 Clinical
Practice Guideline for Chronic Kidney Disease recommends
urate-lowering interventions for symptomatic hyperuricemia, but not
for asymptomatic hyperuricemia, in patients with CKD. The evidence
supporting the use of urate-lowering therapy to delay CKD
progression in patients with asymptomatic hyperuricemia remains
insufficient (29). Consequently,
UA-lowering treatments are not recommended to patients with
asymptomatic hyperuricemia for slowing the progression of kidney
disease. By contrast, a meta-analysis of 17 studies demonstrates
the importance of considering UA-lowering therapy in clinical
strategies for patients with CKD with asymptomatic hyperuricemia
(30). Some studies suggest that
UA lowering is beneficial in preventing CKD development (31,32).
However, the results of these trails may not be conclusive,
considering their quality. Therefore, more clinical trials are
necessary to establish the baseline blood UA concentration
requiring UA-lowering treatment to timely prevent or alleviate
kidney damage associated with elevated UA levels.
Hyperuricemia can induce renal arteriopathy, reduce
renal blood flow, increase urinary albumin excretion and cause gout
nephropathy, also known as HN. It is characterized by renal
interstitial and tubular damage (33). Increased and accumulated UA readily
forms monosodium urate crystals in the kidney following
hyperuricemia. These crystals further damage tubular epithelial
cells by inducing endoplasmic reticulum stress, mitochondrial
disorders, autophagy dysfunction and ultimately apoptosis.
Hyperuricemia leads to both crystal and crystal-independent kidney
injuries (Fig. 2) (34), involving oxidative stress, renal
cell apoptosis, angiotensin system activation and inflammatory
responses (35). The inflammatory
response and the ROS released by injured mitochondria play
essential roles in HN pathogenesis (36,37)
and increased levels of NLRP3 inflammasome, NF-κB and inflammatory
mediators, including IL-1β, IL-6, TNF-α, MCP-1 and ICAM-1, further
accelerate HN progression (19,26,35,37,38).
Furthermore, autophagy is also involved in the development of renal
fibrosis, which is associated with the activation of renal
fibroblasts, EMT, mitochondrial fission, cell pyroptosis and
apoptosis (38,39). In a hyperuricemia rat model, the
autophagy inhibitor 3-MA, when administered as a delayed treatment,
effectively reduces the deposition of ECM proteins by blocking EMT,
as well as the phosphorylation of STAT3 and NF-κB. Furthermore, it
inhibits the release of various profibrotic cytokines/chemokines in
damaged kidneys (39). Notably,
the inhibition of NLRP3 inflammasome-mediated pyroptosis due to
autophagy blockade prevents HN progression (38).
Multiple compounds and traditional Chinese medicines
can improve HN by inhibiting inflammation, modulating the
expression of UA transporters and reducing cell apoptosis. A recent
study demonstrates that the SGLT2 inhibitor dapagliflozin
ameliorates UA-induced tubular dysfunction and fibrotic activation
in HN by activating the ERRα-OAT1 axis to enhance UA excretion
(40). The naturally occurring
flavonol fisetin exhibits anti-inflammatory, antioxidant and
antiangiogenic properties (35).
Ren et al (35) demonstrate
that UA levels can be lowered by regulating the expression of
kidney urate transporters, including OAT1, OAT3, URAT1 and ABCG2.
Based on traditional Chinese medicine theory and clinical practice
in kidney disease treatment, a self-designed renal herb formula
protects against HN by inhibiting the apoptosis of resident kidney
cells and inflammatory response by targeting the NF-κB and p53
signaling pathways (34).
Furthermore, an ethanol extract of the bark of Liriodendron
chinense (Hemsl.) Sarg (EELC) can increase urine UA excretion
in HN mice by upregulating OAT1, OAT3 and ABCG2 (41). Additionally, inhibition of
JAK2/STAT3 signaling attenuates HN progression by alleviating renal
inflammation (35,41).
Diabetes, a multisystemic disorder caused by
absolute or relative insulin deficiency or peripheral tissue
resistance to insulin, is one of the most important comorbidities
of MS (42–44). Hyperuricemia is a common
complication of T2DM and serum UA levels are an important risk
factor for T2DM occurrence and development, as well as its
associated complications (Table I)
(45). The magnitudes of insulin
resistance and serum UA concentration are significantly correlated
and insulin resistance, a risk factor for hyperuricemia,
contributes to decreased UA excretion by the kidneys, resulting in
hyperuricemia (46,47). In a previous study, decreased
urinary urate excretion following insulin administration in rats is
associated with increased and decreased expressions of URAT1 (a
major urate reabsorption transporter) and ABCG2 (a major urate
secretory transporter), respectively (48). A study involving patients with
hyperuricemia and T2DM reports reduced albuminuria and serum urate
concentration following intensive urate-lowering therapy with
verinurad combined with the XOI febuxostat (49).
A number of epidemiological studies report a
correlation between serum UA levels and the risk of diabetic kidney
disease (DKD). Jalal et al (50) conducted a prospective observational
study analyzing data from a coronary artery calcification study of
patients with type 1 diabetes (T1D) involving a stepwise logistic
regression model to predict the development of microalbuminuria or
macroalbuminuria after a 6-year follow-up in 324 participants
without evidence of trace or macroalbuminuria at baseline. Baseline
serum UA levels, HbA1c and prealbuminuria are predictive factors
for microalbuminuria or macroalbuminuria. The study revealed that
every 1 mg/dl increase in serum UA baseline levels increased the
risk of developing trace or large amounts of albuminuria at 6 years
by 80% (50). Similarly, a
cross-sectional study involving 20,464 adult patients with T1D from
Italy, with available SUA measurements for 11,162 patients,
reported an association between elevated serum UA levels and low
estimated glomerular filtration rate (eGFR) (51). Previous research also establishes
an association between SUA levels and DKD risk in patients with T2D
(52,53). Additionally, a meta-analysis
involving 25,741 patients with T2DM demonstrates an association
between serum UA levels and an increased risk of DKD in these
patients (54).
Two large randomized clinical trials examining UA
reduction in patients with T1D found that treatment of
hyperuricemia did not improve the progression of preexisting kidney
disease (55,56). In the Preventing Early Renal
Function Loss in T1D study, the reduction of SUA levels with
allopurinol did not provide any benefits for reducing GFR rate or
to other renal outcomes in patients with T1D, early to moderate
diabetic nephropathy, or high normal SUA levels (56). Overall, these results failed to
demonstrate a statistically significant effect of allopurinol on
kidney outcomes in these patients. However, some studies have
demonstrated that febuxostat plus verinurad can improve albuminuria
in patients with T2DM by reducing serum UA levels (49). Therefore, UA-lowering therapy may
exert different effects on kidney damage caused by different types
of diabetes. Nevertheless, more clinical studies are warranted in
the future.
A close relationship has been reported between
obesity and the development of end-stage renal disease in later
years (57,58). Obesity is also associated with CKD
progression (58) and
obesity-related glomerular diseases include proteinuria, glomerular
enlargement, progressive glomerulosclerosis and CKD. UA is an
important risk factor for renal injury in metabolically unhealthy
patients with obesity (59).
Furthermore, obesity is a risk factor for hyperuricemia (60). A retrospective analysis of 8,522
participants showed a positive correlation between SUA levels and
obesity or being overweight (61).
Weight loss effectively lowers UA levels (62,63).
Moreover, bariatric surgery significantly reduced serum UA levels
within 12 and 24 months in patients with and without diabetes
(60).
Bariatric surgery can significantly lower serum UA
levels in patients with severe obesity (66–68).
Furthermore, a meta-analysis by Yeo et al (69) indicates that weight loss achieved
through bariatric surgery reduces serum UA levels and decreases the
frequency of gout attacks (70).
These results suggest a close relationship between obesity and
hyperuricemia. However, further research is needed to determine
whether UA-lowering therapy can improve renal function in patients
with obesity.
A strong inflammatory response in the kidneys can
damage the glomeruli and tubulointerstitium. As UA is produced by
damaged cells and promotes immune inflammatory responses, it is
considered a key molecule in the pathogenesis of diseases such as
hypertension, kidney disease and SLE (71,72).
SLE is an autoimmune disease that affects several organs. Lupus
nephritis (LN) is a serious complication of SLE. LN is closely
associated with hyperuricemia and UA is considered a risk factor
for renal injury in SLE (Table
II) (72,73). Multivariate analysis in a previous
study confirms high UA levels as an independent variable associated
with LN (73). Serum UA levels are
generally considered markers of renal dysfunction. Particularly, UA
is associated with the severity of kidney disease in patients with
LN and is an indicator of poor prognosis in SLE (74,75).
Higher UA levels contribute to the development of new kidney damage
in patients with SLE independent of other known risk factors
(74). A study involving 45
patients reported age, hemoglobin, blood UA, urine protein, IL-17
and IL-34 as independent risk factors for poor prognosis in LN
(76). Serum UA levels of <6.05
mg/dl at 12 months of follow-up indicate good long-term renal
outcomes in LN (75).
UA is constitutively expressed in cells and an
increase in its concentration following cell damage stimulates
dendritic cell maturation, recruiting other immune cells and
leading to the release of inflammatory mediators. UA is considered
a key mediator produced by damaged cells, acting as a danger signal
and promoting inflammatory responses (72). Hyperuricemia may serve as an
adjuvant for the development and progression of renal injury in SLE
(77). UA-lowering therapy may
slow down the deterioration in LN and can effectively delay the
progression of CKD. Notably, analysis of GFR and serum creatinine
levels revealed significant benefits of UA-lowering treatment in
hospitalized patients compared with the patients in the control
group. Additionally, UA-lowering treatment may improve kidney
outcomes. In a previous study, the control group had a higher
number of patients with significantly worse renal function than the
treatment group (78). Therefore,
treating hyperuricemia may reduce kidney damage and slow the
SLE-induced renal function loss.
Hypertension is a major cardiovascular risk factor
owing to its high prevalence, correlation with other risk factors
and effect on major cardiovascular events (84). Several clinical trials and animal
studies demonstrate that UA can cause hypertension, kidney disease
and CVD. Hyperuricemia is associated with an increased risk of CVD
but not with stroke or CHD alone in patients with hypertension
(12). Hyperuricemia occurs in
25–40% of individuals with untreated hypertension (85). Mild hyperuricemia is linked with
early signs of renal injury regardless of the eGFR in primary
hypertension (86). Randomized
controlled trials report a substantial decrease in blood pressure
following an uricosuric agent- or XOR inhibitor-induced reduction
in serum UA levels (87–89). Increased oxidative stress
associated with the biochemical processes involved in UA production
could explain the interactions between elevated SUA levels and
hypertension (90). UA may
contribute to hypertension via endothelial dysfunction induced by
both crystal-dependent (extracellular UA) and crystal-independent
(intracellular UA) pathways (87).
Elevated circulating serum UA levels are strongly
associated with the development of hypertension and renal disease
(91) and previous research
demonstrates an increase in the incidence of kidney disease and
hypertension in patients with gout. Furthermore, a correlation is
reported among elevated UA levels, renal artery disease and
hypertension. Moreover, some randomized intervention studies
demonstrate benefits of the treatment of asymptomatic hyperuricemia
in improving blood pressure regulation and renal function (92).
UA induces hypertension through its effect on
endothelial function and impairment of nitric oxide production
(91,93). Hypertension may be the primary
cause of subclinical kidney injury (94,95).
Therefore, UA, urate crystals and XOR (especially XO, which
produces oxidative stress) may contribute to the development of
renal disease, hypertension and CVD through tubular interstitial
disorder, endothelial dysfunction, stimulation of the
renin-angiotensin system and vascular smooth muscle cell
proliferation (90,91,93).
A clinical trial involving 30 adolescents with newly diagnosed
stage 1 essential hypertension and serum UA levels of ≥6.0 mg/dl
shows that treatment with allopurinol markedly decreases UA levels
and significantly reduces casual and ambulatory systolic and
diastolic blood pressure (89).
However, this effect of allopurinol may not be due to UA reduction
but rather by regulating endothelial dysfunction by decreasing UA
and xanthine oxidase-induced oxidants. Nevertheless, more clinical
trials are needed to validate these results and assess their
general applicability to a larger hypertensive population.
Lifestyle management, as the overall principle of
non-pharmacological treatment for hyperuricemia, includes limiting
alcohol consumption, diet control, exercise and weight loss in
individuals with obesity, followed by the management of related
comorbidities and risk factors such as hypertension,
hyperlipidemia, hyperglycemia and smoking. In terms of diet, animal
foods with high purine content (e.g., animal viscera, seafood)
should be restricted. In addition, sweet fruits and soft drinks
containing fructose should be consumed in moderate amounts, as they
can increase blood UA levels.
Individualized therapeutic approaches should be
adopted while selecting UA-lowering drugs. UA-lowering therapy
drugs are categorized based on their mechanisms of action as
follows: i) Inhibitors of UA synthesis, including XOIs such as
allopurinol and febuxostat; ii) enhancers of UA excretion,
including probenecid, benzbromarone and selective UA reabsorption
transporter inhibitors; and iii) promoters of UA dissolution, such
as uricase (96).
Allopurinol acts as a precursor of oxypurinol by
targeting the active site of xanthine oxidase and inhibiting the
final step of purine metabolism, thereby reducing UA production
without disrupting purine nucleoside synthesis. As selective
inhibitors of xanthine oxidase, allopurinol and other XOIs
effectively lower UA levels with an acceptable safety profile,
making them commonly prescribed treatments for hyperuricemia
(97–99). Febuxostat is a non-purine XOI that
exhibits an efficacy similar to that of allopurinol in patients
with hypersensitivity to allopurinol. This medication, similar to
oxypurinol, is well-tolerated in individuals with CKD owing to its
tight binding to the active site of xanthine oxidase, thereby
inhibiting the conversion of purines into UA. Xanthine oxidase is
predominantly localized in the liver, where it exhibits the highest
activity (46,97). Uricosurics, including drugs such as
probenecid, benzbromarone and sulfinpyrazone, can lower UA levels
by increasing renal clearance of urate. The American College of
Rheumatology recommends probenecid as the preferred uricosuric drug
because it prevents urate reabsorption in the proximal tubule,
thereby decreasing serum urate levels (96,100).
Urate-lowering therapies can effectively prevent
kidney damage during the progression of CKD, CVD, gout and obesity
(Table III) (101). Notably, a 2-year clinical trial
demonstrates that allopurinol treatment improves the eGFR and
reduces CV risk (101).
Furthermore, benzbromarone exhibits improved efficacy in rapidly
reducing SUA levels and inhibiting inflammation in patients with
hyperuricemia and gout compared with febuxostat (102). According to the 2020 American
College of Rheumatology Guidelines for the Management of Gout,
urate-lowering therapy is recommended in patients with gout and CKD
(103). Sodium-glucose
cotransporter type 2 inhibitors (SGLT2i) are revolutionary
treatments for patients with T2DM with cardiovascular, kidney and
serum urate-lowering benefits (104). SGLT2i significantly lowers UA
levels and cardiovascular kidney metabolic risk in patients with
gout (105). Furthermore, SGLT2i
dapagliflozin ameliorates UA-induced tubular dysfunction and
fibrotic activation in patients with HN by enhancing UA excretion
(40). Losartan is currently the
only angiotensin II receptor blocker that significantly reduces UA
levels. Clinical guidelines recommend the addition or switching to
losartan as an antihypertensive drug for patients with gout, as it
lowers both blood pressure and UA levels (106). Losartan can ameliorate renal
interstitial fibrosis through different molecular mechanisms in
both clinical and animal experiments (107–110).
A clinical controlled trial involving patients with
stage 3 or 4 CKD, with most patients having hyperuricemia (despite
hyperuricemia not being an inclusion criterion), receiving
allopurinol or placebo treatment for 2 years reports that
allopurinol treatment did not significantly affect the rate of GFR
decline (55). Another clinical
trial involving 530 patients with T1D and early-to-moderate
diabetic nephropathy reports no clinical benefit of reducing serum
urate using allopurinol for renal prognosis (56). These inconsistent results indicate
that response to treatment aimed at reducing UA levels may vary due
to the highly complex clinical features of patients, including
factors such as age, weight, sex and complications. Accordingly,
the selection of appropriate patients and careful clinical trial
design are crucial for determining the efficacy of such treatments.
Furthermore, clinical trials results may guide intervention
strategies and must be seriously analyzed and summarized.
Importantly, high-quality randomized controlled trials are
essential for accurately identifying the indications of UA-lowering
therapy.
UA, urate crystals and XOR-mediated oxidative stress
probably participate in the progression of CKD, hypertension and
CVD through pathological mechanisms such as vascular smooth muscle
cell proliferation, endothelial dysfunction and renal
tubulointerstitial disorders. However, whether urate-lowering
therapy effectively prevents the progression of diabetic
nephropathy, LN, CKD, CVD and obesity in asymptomatic patients with
hyperuricemia remains controversial. Prior to reaching a definitive
conclusion on initiating treatment for hyperuricemia, personalized
treatment for patients with hyperuricemia combined with other
diseases should be considered to effectively reduce SUA levels.
Moreover, high-quality and comprehensive clinical and basic
scientific research on hyperuricemia and purine metabolism, as well
as a definitive assessment of the effects of urate-lowering therapy
on kidney function preservation, is required through larger
clinical studies in the future.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant no. 82100727), promotion plan of basic
and clinical cooperative research in Anhui Medical University
(grant no. 2020×kjT016) and the Open Fund of Inflammation and
Immune Mediated Diseases Laboratory of Anhui Province (grant no.
IMMDL202002).
Not applicable.
JJ and XM were responsible for project
administration, conceptualisation and also designed the method for
writing the review. HY and JY wrote and edited the manuscript. TZ
reviewed and made significant revisions to the manuscript. Data
authentication is not applicable. All authors read and approved the
final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Furuhashi M: New insights into purine
metabolism in metabolic diseases: Role of xanthine oxidoreductase
activity. Am J Physiol Endocrinol Metab. 319:E827–E834. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El Ridi R and Tallima H: Physiological
functions and pathogenic potential of uric acid: A review. J Adv
Res. 8:487–493. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lima WG, Martins-Santos MES and Chaves VE:
Uric acid as a modulator of glucose and lipid metabolism.
Biochimie. 116:17–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yanai H, Adachi H, Hakoshima M and
Katsuyama H: Molecular biological and clinical understanding of the
pathophysiology and treatments of hyperuricemia and its association
with metabolic syndrome, cardiovascular diseases and chronic kidney
disease. Int J Mol Sci. 22:92212021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spatola L, Ferraro PM, Gambaro G,
Badalamenti S and Dauriz M: Metabolic syndrome and uric acid
nephrolithiasis: Insulin resistance in focus. Metabolism.
83:225–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakanishi K and Morita H: Uric acid. Int
Heart J. 63:423–425. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ejaz AA, Nakagawa T, Kanbay M, Kuwabara M,
Kumar A, Garcia Arroyo FE, Roncal-Jimenez C, Sasai F, Kang DH,
Jensen T, et al: Hyperuricemia in kidney disease: A Major risk
factor for cardiovascular events, vascular calcification, and renal
damage. Semin Nephrol. 40:574–585. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sánchez-Lozada LG: The pathophysiology of
uric acid on renal diseases. Contrib Nephrol. 192:17–24. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maruhashi T, Hisatome I, Kihara Y and
Higashi Y: Hyperuricemia and endothelial function: From molecular
background to clinical perspectives. Atherosclerosis. 278:226–231.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kushiyama A, Okubo H, Sakoda H, Kikuchi T,
Fujishiro M, Sato H, Kushiyama S, Iwashita M, Nishimura F,
Fukushima T, et al: Xanthine oxidoreductase is involved in
macrophage foam cell formation and atherosclerosis development.
Arterioscler Thromb Vasc Biol. 32:291–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ives A, Nomura J, Martinon F, Roger T,
LeRoy D, Miner JN, Simon G, Busso N and So A: Xanthine
oxidoreductase regulates macrophage IL1β secretion upon NLRP3
inflammasome activation. Nat Commun. 6:65552015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng L, Zhu Y, Ma Y, Zhang H, Zhao H,
Zhang Y, Yang Z and Liu Y: Relationship between hyperuricemia and
the risk of cardiovascular events and chronic kidney disease in
both the general population and hypertensive patients: A systematic
review and meta-analysis. Int J Cardiol. 399:1317792024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chaudhary K, Malhotra K, Sowers J and
Aroor A: Uric acid-key ingredient in the recipe for cardiorenal
metabolic syndrome. Cardiorenal Med. 3:208–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benn CL, Dua P, Gurrell R, Loudon P, Pike
A, Storer RI and Vangjeli C: Physiology of hyperuricemia and
urate-lowering treatments. Front Med (Lausanne). 5:1602018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maiuolo J, Oppedisano F, Gratteri S,
Muscoli C and Mollace V: Regulation of uric acid metabolism and
excretion. Int J Cardiol. 213:8–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gherghina ME, Peride I, Tiglis M, Neagu
TP, Niculae A and Checherita IA: Uric acid and oxidative
stress-relationship with cardiovascular, metabolic, and renal
impairment. Int J Mol Sci. 23:31882022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou Y, Chen M, Zheng J, Shui X, He Y, Luo
H and Lei W: Insights into the relationship between serum uric acid
and pulmonary hypertension (Review). Mol Med Rep. 29:102024.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang C, Chen L, He M, Luo Y, Zhou M, Zhang
N, Yuan J, Wang H and Xie Y: Molecular mechanistic insight into the
anti-hyperuricemic effect of Eucommia ulmoides in mice and
rats. Pharm Biol. 57:112–119. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun HL, Bian HG, Liu XM, Zhang H, Ying J,
Yang H, Zu T, Cui GQ, Liao YF, Xu MF, et al: GRP/GRPR signaling
pathway aggravates hyperuricemia-induced renal inflammation and
fibrosis via ABCG2-dependent mechanisms. Biochem Pharmacol.
218:1159012023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Z, Dong Y, Zhou H, Liu J and Zhao W:
MiR-143-3p directly targets GLUT9 to reduce uric acid reabsorption
and inflammatory response of renal tubular epithelial cells.
Biochem Biophys Res Commun. 517:413–420. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mandal AK and Mount DB: The molecular
physiology of uric acid homeostasis. Annu Rev Physiol. 77:323–345.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roughley MJ, Belcher J, Mallen CD and
Roddy E: Gout and risk of chronic kidney disease and
nephrolithiasis: Meta-analysis of observational studies. Arthritis
Res Ther. 17:902015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagaraju SP, Shenoy SV, Rao I, Prabhu RA,
Rangaswamy D, Bhojaraja MV and Guddattu V: Effect of febuxostat
versus allopurinol on the glomerular filtration rate and
hyperuricemia in patients with chronic kidney disease. Saudi J
Kidney Dis Transpl. 34:279–287. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agnoletti D, Cicero AFG and Borghi C: The
impact of uric acid and hyperuricemia on cardiovascular and renal
systems. Cardiol Clin. 39:365–376. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Balakumar P, Alqahtani A, Khan NA,
Mahadevan N and Dhanaraj SA: Mechanistic insights into
hyperuricemia-associated renal abnormalities with special emphasis
on epithelial-to-mesenchymal transition: Pathologic implications
and putative pharmacologic targets. Pharmacol Res. 161:1052092020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu N, Wang L, Yang T, Xiong C, Xu L, Shi
Y, Bao W, Chin YE, Cheng SB, Yan H, et al: EGF receptor inhibition
alleviates hyperuricemic nephropathy. J Am Soc Nephrol.
26:2716–2729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan J, Shi M, Li L, Liu J, Guo F, Feng Y,
Ma L and Fu P: Pterostilbene, a bioactive component of blueberries,
alleviates renal fibrosis in a severe mouse model of hyperuricemic
nephropathy. Biomed Pharmacother. 109:1802–1808. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hassan W, Shrestha P, Sumida K, Thomas F,
Sweeney PL, Potukuchi PK, Rhee CM, Streja E, Kalantar-Zadeh K and
Kovesdy CP: Association of uric acid-lowering therapy with incident
chronic kidney disease. JAMA Netw Open. 5:e22158782022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kidney Disease: Improving Global Outcomes
(KDIGO) CKD Work Group: KDIGO 2024 clinical practice guideline for
the evaluation and management of chronic kidney disease. Kidney
Int. 105((4S)): S117–S314. 2024.PubMed/NCBI
|
|
30
|
Luo Y, Song Q, Li J, Fu S, Yu W, Shao X,
Li J, Huang Y, Chen J and Tang Y: Effects of uric acid-lowering
therapy (ULT) on renal outcomes in CKD patients with asymptomatic
hyperuricemia: A systematic review and meta-analysis. BMC Nephrol.
25:632024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kanbay M, Huddam B, Azak A, Solak Y,
Kadioglu GK, Kirbas I, Duranay M, Covic A and Johnson RJ: A
randomized study of allopurinol on endothelial function and
estimated glomular filtration rate in asymptomatic hyperuricemic
subjects with normal renal function. Clin J Am Soc Nephrol.
6:1887–1894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanbay M, Ozkara A, Selcoki Y, Isik B,
Turgut F, Bavbek N, Uz E, Akcay A, Yigitoglu R and Covic A: Effect
of treatment of hyperuricemia with allopurinol on blood pressure,
creatinine clearence, and proteinuria in patients with normal renal
functions. Int Urol Nephrol. 39:1227–1233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miake J, Hisatome I, Tomita K, Isoyama T,
Sugihara S, Kuwabara M, Ogino K and Ninomiya H: Impact of hyper-
and hypo-uricemia on kidney function. Biomedicines. 11:12582023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang GY, Li S, Xu Y, Zhang C, Xu XY, Xu L,
Wang N and Feng Y: Renal herb formula protects against
hyperuricemic nephropathy by inhibiting apoptosis and inflammation.
Phytomedicine. 116:1548122023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ren Q, Tao S, Guo F, Wang B, Yang L, Ma L
and Fu P: Natural flavonol fisetin attenuated hyperuricemic
nephropathy via inhibiting IL-6/JAK2/STAT3 and TGF-β/SMAD3
signaling. Phytomedicine. 87:1535522021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo Y, Li H, Liu Z, Li C, Chen Y, Jiang C,
Yu Y and Tian Z: Impaired intestinal barrier function in a mouse
model of hyperuricemia. Mol Med Rep. 20:3292–3300. 2019.PubMed/NCBI
|
|
37
|
Jalal DI, Chonchol M, Chen W and Targher
G: Uric acid as a target of therapy in CKD. Am J Kidney Dis.
61:134–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu Y, Shi Y, Chen H, Tao M, Zhou X, Li J,
Ma X, Wang Y and Liu N: Blockade of autophagy prevents the
progression of hyperuricemic nephropathy through inhibiting NLRP3
inflammasome-mediated pyroptosis. Front Immunol. 13:8584942022.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi Y, Tao M, Ma X, Hu Y, Huang G, Qiu A,
Zhuang S and Liu N: Delayed treatment with an autophagy inhibitor
3-MA alleviates the progression of hyperuricemic nephropathy. Cell
Death Dis. 11:4672020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu H, Li W, Hao Y, Peng Z, Zou Z, Wei J,
Zhou Y, Liang W and Cao Y: The SGLT2 inhibitor dapagliflozin
ameliorates renal fibrosis in hyperuricemic nephropathy. Cell Rep
Med. 5:1016902024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pan J, Zhang C, Shi M, Guo F, Liu J, Li L,
Ren Q, Tao S, Tang M, Ye H, et al: Ethanol extract of
Liriodendron chinense (Hemsl.) Sarg barks attenuates
hyperuricemic nephropathy by inhibiting renal fibrosis and
inflammation in mice. J Ethnopharmacol. 264:1132782021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Asma Sakalli A, Küçükerdem HS and Aygün O:
What is the relationship between serum uric acid level and insulin
resistance?: A case-control study. Medicine (Baltimore).
102:e367322023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Eckel RH, Grundy SM and Zimmet PZ: The
metabolic syndrome. Lancet. 365:1415–1428. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lann D and LeRoith D: Insulin resistance
as the underlying cause for the metabolic syndrome. Med Clin North
Am. 911063–1077. (viii)2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dong M, Chen H, Wen S, Yuan Y, Yang L, Xu
D and Zhou L: The mechanism of sodium-glucose cotransporter-2
inhibitors in reducing uric acid in type 2 diabetes mellitus.
Diabetes Metab Syndr Obes. 16:437–445. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dalbeth N, Gosling AL, Gaffo A and
Abhishek A: Gout. Lancet. 397:1843–1855. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
So A and Thorens B: Uric acid transport
and disease. J Clin Invest. 120:1791–1799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Toyoki D, Shibata S, Kuribayashi-Okuma E,
Xu N, Ishizawa K, Hosoyamada M and Uchida S: Insulin stimulates
uric acid reabsorption via regulating urate transporter 1 and
ATP-binding cassette subfamily G member 2. Am J Physiol Renal
Physiol. 313:F826–F834. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Stack AG, Dronamraju N, Parkinson J,
Johansson S, Johnsson E, Erlandsson F and Terkeltaub R: Effect of
intensive urate lowering with combined verinurad and febuxostat on
albuminuria in patients with type 2 diabetes: A randomized trial.
Am J Kidney Dis. 77:481–489. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jalal DI, Rivard CJ, Johnson RJ, Maahs DM,
McFann K, Rewers M and Snell-Bergeon JK: Serum uric acid levels
predict the development of albuminuria over 6 years in patients
with type 1 diabetes: Findings from the coronary artery
calcification in type 1 diabetes study. Nephrol Dial Transplant.
25:1865–1869. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pacilli A, Viazzi F, Fioretto P, Giorda C,
Ceriello A, Genovese S, Russo G, Guida P, Pontremoli R and De Cosmo
S; AMD-Annals Study Group, : Epidemiology of diabetic kidney
disease in adult patients with type 1 diabetes in Italy: The
AMD-Annals initiative. Diabetes Metab Res Rev. 33:2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tafese R, Genet S and Addisu S:
Association of serum total bilirubin and uric acid with low
glomerular filtration rate diabetic kidney disease in type 2
diabetic patients. Diabetes Metab Syndr Obes. 15:3993–3999. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Han R, Duan L, Zhang Y and Jiang X: Serum
uric acid is a better indicator of kidney impairment than serum
uric acid-to-creatinine ratio and serum uric acid-to-high-density
lipoprotein ratio: A cross-sectional study of type 2 diabetes
mellitus patients. Diabetes Metab Syndr Obes. 16:2695–2703. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ji P, Zhu J, Feng J, Li H, Yu Q, Qin H,
Wei L and Zhang J: Serum uric acid levels and diabetic kidney
disease in patients with type 2 diabetes mellitus: A dose-response
meta-analysis. Prim Care Diabetes. 16:457–465. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Badve SV, Pascoe EM, Tiku A, Boudville N,
Brown FG, Cass A, Clarke P, Dalbeth N, Day RO, de Zoysa JR, et al:
Effects of allopurinol on the progression of chronic kidney
disease. N Engl J Med. 382:2504–2513. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Doria A, Galecki AT, Spino C, Pop-Busui R,
Cherney DZ, Lingvay I, Parsa A, Rossing P, Sigal RJ, Afkarian M, et
al: Serum urate lowering with allopurinol and kidney function in
type 1 diabetes. N Engl J Med. 382:2493–2503. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rhee CM, Ahmadi SF and Kalantar-Zadeh K:
The dual roles of obesity in chronic kidney disease: A review of
the current literature. Curr Opin Nephrol Hypertens. 25:208–216.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Panwar B, Hanks LJ, Tanner RM, Muntner P,
Kramer H, McClellan WM, Warnock DG, Judd SE and Gutiérrez OM:
Obesity, metabolic health, and the risk of end-stage renal disease.
Kidney Int. 87:1216–1222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Di Sessa A, Passaro AP, Colasante AM,
Cioffi S, Guarino S, Umano GR, Papparella A, Miraglia Del Giudice E
and Marzuillo P: Kidney damage predictors in children with
metabolically healthy and metabolically unhealthy obesity
phenotype. Int J Obes (Lond). 47:1247–1255. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mills DW, Woolley DM, Ammori BJ, Chinoy H
and Syed AA: Changes in serum urate levels after bariatric surgery
in patients with obesity: An observational study. Obes Surg.
34:1737–1741. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ye W, Zhou X, Xu Y, Zheng C and Liu P:
Serum uric acid levels among chinese children: Reference values and
association with overweight/obesity. Clin Pediatr (Phila).
63:1684–1690. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nielsen SM, Bartels EM, Henriksen M,
Wæhrens EE, Gudbergsen H, Bliddal H, Astrup A, Knop FK, Carmona L,
Taylor WJ, et al: Weight loss for overweight and obese individuals
with gout: A systematic review of longitudinal studies. Ann Rheum
Dis. 76:1870–1882. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Choi HK and Zhang YQ: Bariatric surgery as
urate-lowering therapy in severe obesity. Ann Rheum Dis.
73:791–793. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Andres-Hernando A, Cicerchi C, Kuwabara M,
Orlicky DJ, Sanchez-Lozada LG, Nakagawa T, Johnson RJ and Lanaspa
MA: Umami-induced obesity and metabolic syndrome is mediated by
nucleotide degradation and uric acid generation. Nat Metab.
3:1189–1201. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Primo D, Izaola O and de Luis D:
Resistin/uric acid index as a marker of metabolic syndrome in
females with obesity. Int J Obes (Lond). 47:393–398. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu W, Zhang H, Han X, Zhang P and Mao Z:
Uric acid level changes after bariatric surgery in obese subjects
with type 2 diabetes mellitus. Ann Transl Med. 7:3322019.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lu J, Bai Z, Chen Y, Li Y, Tang M, Wang N,
Zhu X, Dai H and Zhang W: Effects of bariatric surgery on serum
uric acid in people with obesity with or without hyperuricaemia and
gout: A retrospective analysis. Rheumatology (Oxford).
60:3628–3634. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Qu X, Zheng L, Zu B, Jia B and Lin W:
Prevalence and clinical predictors of hyperuricemia in chinese
bariatric surgery patients. Obes Surg. 32:1508–1515. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yeo C, Kaushal S, Lim B, Syn N, Oo AM, Rao
J, Koura A and Yeo D: Impact of bariatric surgery on serum uric
acid levels and the incidence of gout-A meta-analysis. Obes Rev.
20:1759–1770. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Vafa L, Amini M, Kamran H, Aghakhani L,
Hosseini SV, Mohammadi Z and Haghighat N: The impact of obesity
surgery on serum uric acid in people with severe obesity: A
retrospective study. Clin Nutr Res. 12:21–28. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dos Santos M, Veronese FV and Moresco RN:
Uric acid and kidney damage in systemic lupus erythematosus. Clin
Chim Acta. 508:197–205. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hafez EA, Hassan SAEM, Teama MAM and Badr
FM: Serum uric acid as a predictor for nephritis in Egyptian
patients with systemic lupus erythematosus. Lupus. 30:378–384.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Calich AL, Borba EF, Ugolini-Lopes MR, da
Rocha LF, Bonfá E and Fuller R: Serum uric acid levels are
associated with lupus nephritis in patients with normal renal
function. Clin Rheumatol. 37:1223–1228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Reátegui-Sokolova C, Ugarte-Gil MF,
Gamboa-Cárdenas RV, Zevallos F, Cucho-Venegas JM, Alfaro-Lozano JL,
Medina M, Rodriguez-Bellido Z, Pastor-Asurza CA, Alarcón GS and
Perich-Campos RA: Serum uric acid levels contribute to new renal
damage in systemic lupus erythematosus patients. Clin Rheumatol.
36:845–852. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ugolini-Lopes MR, Gavinier SS, Leon E,
Viana VT, Borba EF and Bonfá E: Is serum uric acid a predictor of
long-term renal outcome in lupus nephritis? Clin Rheumatol.
38:2777–2783. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Cheng Y, Yang X, Zhang X and An Z:
Analysis of expression levels of IL-17 and IL-34 and influencing
factors for prognosis in patients with lupus nephritis. Exp Ther
Med. 17:2279–2283. 2019.PubMed/NCBI
|
|
77
|
Han Y, Lu X, Xiao S, Qin J, Zheng L, Feng
Y, Cai Y, Qiu R, Huang Q and Yang M: Association between serum uric
acid level and systemic lupus erythematosus kidney outcome: An
observational study in Southern Chinese population and a
meta-analysis. Lupus. 32:83–93. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liu X, Zhai T, Ma R, Luo C, Wang H and Liu
L: Effects of uric acid-lowering therapy on the progression of
chronic kidney disease: A systematic review and meta-analysis. Ren
Fail. 40:289–297. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
GBD 2019 Risk Factors Collaborators, .
Global burden of 87 risk factors in 204 countries and territories,
1990–2019: A systematic analysis for the global burden of disease
study 2019. Lancet. 396:1223–1249. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kimura Y, Yanagida T, Onda A, Tsukui D,
Hosoyamada M and Kono H: Soluble uric acid promotes atherosclerosis
via AMPK (AMP-activated protein kinase)-mediated inflammation.
Arterioscler Thromb Vasc Biol. 40:570–582. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang W, Iso H, Murakami Y, Miura K, Nagai
M, Sugiyama D, Ueshima H and Okamura T; EPOCH-JAPAN GROUP, : Serum
uric acid and mortality form cardiovascular disease: EPOCH-JAPAN
study. J Atheroscler Thromb. 23:692–703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kuwabara M, Niwa K, Hisatome I, Nakagawa
T, Roncal-Jimenez CA, Andres-Hernando A, Bjornstad P, Jensen T,
Sato Y, Milagres T, et al: Asymptomatic hyperuricemia without
comorbidities predicts cardiometabolic diseases: Five-year japanese
cohort study. Hypertension. 69:1036–1044. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Perticone M, Maio R, Shehaj E, Gigliotti
S, Caroleo B, Suraci E, Sciacqua A, Andreozzi F and Perticone F:
Sex-related differences for uric acid in the prediction of
cardiovascular events in essential hypertension. A population
prospective study. Cardiovasc Diabetol. 22:2982023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
NCD Risk Factor Collaboration (NCD-RisC),
. Worldwide trends in hypertension prevalence and progress in
treatment and control from 1990 to 2019: A pooled analysis of 1201
population-representative studies with 104 million participants.
Lancet. 398:957–980. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gois PHF and Souza ERM: Pharmacotherapy
for hyperuricemia in hypertensive patients. Cochrane Database Syst
Rev. 4:Cd0086522017.PubMed/NCBI
|
|
86
|
Viazzi F, Leoncini G, Ratto E, Falqui V,
Parodi A, Conti N, Derchi LE, Tomolillo C, Deferrari G and
Pontremoli R: Mild hyperuricemia and subclinical renal damage in
untreated primary hypertension. Am J Hypertens. 20:1276–1282. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lanaspa MA, Andres-Hernando A and Kuwabara
M: Uric acid and hypertension. Hypertens Res. 43:832–834. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Soletsky B and Feig DI: Uric acid
reduction rectifies prehypertension in obese adolescents.
Hypertension. 60:1148–1156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Feig DI, Soletsky B and Johnson RJ: Effect
of allopurinol on blood pressure of adolescents with newly
diagnosed essential hypertension: A randomized trial. JAMA.
300:924–932. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yu MA, Sánchez-Lozada LG, Johnson RJ and
Kang DH: Oxidative stress with an activation of the
renin-angiotensin system in human vascular endothelial cells as a
novel mechanism of uric acid-induced endothelial dysfunction. J
Hypertens. 28:1234–1242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mazzali M, Hughes J, Kim YG, Jefferson JA,
Kang DH, Gordon KL, Lan HY, Kivlighn S and Johnson RJ: Elevated
uric acid increases blood pressure in the rat by a novel
crystal-independent mechanism. Hypertension. 38:1101–1106. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kanbay M, Solak Y, Dogan E, Lanaspa MA and
Covic A: Uric acid in hypertension and renal disease: The chicken
or the egg? Blood Purif. 30:288–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang S, Wang Y, Cheng J, Huangfu N, Zhao
R, Xu Z, Zhang F, Zheng W and Zhang D: Hyperuricemia and
cardiovascular disease. Curr Pharm Des. 25:700–709. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ilatovskaya DV, Behr A, Staruschenko A,
Hall G and Palygin O: Mechanistic insights into redox damage of the
podocyte in hypertension. Hypertension. Nov 13–2024.(Epub ahead of
print). PubMed/NCBI
|
|
95
|
Russo E, Bussalino E, Macciò L, Verzola D,
Saio M, Esposito P, Leoncini G, Pontremoli R and Viazzi F:
Non-haemodynamic mechanisms underlying hypertension-associated
damage in target kidney components. Int J Mol Sci. 24:94222023.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Peng X, Li X, Xie B, Lai Y, Sosnik A,
Boucetta H, Chen Z and He W: Gout therapeutics and drug delivery. J
Control Release. 362:728–754. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Sivera F, Andrés M and Quilis N: Gout:
Diagnosis and treatment. Med Clin (Barc). 148:271–276. 2017.(In
English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Stamp LK and Barclay ML: How to prevent
allopurinol hypersensitivity reactions? Rheumatology (Oxford). 57
(Suppl 1):i35–i41. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Stamp LK, Chapman PT and Palmer SC:
Allopurinol and kidney function: An update. Joint Bone Spine.
83:19–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Punzi L, Galozzi P, Luisetto R, Scanu A,
Ramonda R and Oliviero F: Gout: One year in review 2023. Clin Exp
Rheumatol. 42:1–9. 2024.PubMed/NCBI
|
|
101
|
Goicoechea M, Garcia de Vinuesa S,
Verdalles U, Verde E, Macias N, Santos A, Pérez de Jose A, Cedeño
S, Linares T and Luño J: Allopurinol and progression of CKD and
cardiovascular events: Long-term follow-up of a randomized clinical
trial. Am J Kidney Dis. 65:543–549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wu F, Chen L and Du Y: Comparison of the
efficacy and safety of benzbromarone and febuxostat in gout and
hyperuricemia: A systematic review and meta-analysis. Clin
Rheumatol. 43:1745–1754. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
FitzGerald JD, Dalbeth N, Mikuls T,
Brignardello-Petersen R, Guyatt G, Abeles AM, Gelber AC, Harrold
LR, Khanna D, King C, et al: 2020 American college of rheumatology
guideline for the management of gout. Arthritis Rheumatol.
72:879–895. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
McCormick N, Yokose C, Lu N, Wexler DJ,
Aviña-Zubieta JA, De Vera MA, McCoy RG and Choi HK: Sodium-glucose
cotransporter-2 inhibitors vs sulfonylureas for gout prevention
among patients with type 2 diabetes receiving metformin. JAMA
Intern Med. 184:650–660. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yokose C, Challener G, Jiang B, Zhou B,
McCormick N, Tanikella S, Panchot KMQ, Kohler MJ, Yinh J, Zhang Y,
et al: Serum urate change among gout patients treated with
sodium-glucose cotransporter type 2 inhibitors vs sulfonylurea: A
comparative effectiveness analysis. Semin Arthritis Rheum.
66:1524412024. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Saad M: Hyperuricemia and gout: The role
of losartan. Sr Care Pharm. 38:359–360. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Costantino VV, Gil Lorenzo AF, Bocanegra V
and Vallés PG: Molecular mechanisms of hypertensive nephropathy:
Renoprotective effect of losartan through Hsp70. Cells.
10:31462021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
He YM, Feng L, Huo DM, Yang ZH and Liao
YH: Enalapril versus losartan for adults with chronic kidney
disease: A systematic review and meta-analysis. Nephrology
(Carlton). 18:605–614. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Khazaeli M, Nunes ACF, Zhao Y, Khazaali M,
Prudente J, Vaziri ND, Singh B and Lau WL: Tetrahydrocurcumin
Add-On therapy to losartan in a rat model of diabetic nephropathy
decreases blood pressure and markers of kidney injury. Pharmacol
Res Perspect. 11:e010792023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zou J, Zhou X, Ma Y and Yu R: Losartan
ameliorates renal interstitial fibrosis through metabolic pathway
and Smurfs-TGF-β/Smad. Biomed Pharmacother. 149:1129312022.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zhou Q, Ke S, Yan Y, Guo Y and Liu Q:
Serum uric acid is associated with chronic kidney disease in
elderly Chinese patients with diabetes. Ren Fail. 45:22388252023.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Aktas G, Yilmaz S, Kantarci DB, Duman TT,
Bilgin S, Balci SB and Atak Tel BM: Is serum uric acid-to-HDL
cholesterol ratio elevation associated with diabetic kidney injury?
Postgrad Med. 135:519–523. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Pilemann-Lyberg S, Lindhardt M, Persson F,
Andersen S and Rossing P: Serum uric acid and progression of
diabetic nephropathy in type 1 diabetes. J Diabetes Complications.
32:470–473. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
D'Elia L, Masulli M, Cirillo P, Virdis A,
Casiglia E, Tikhonoff V, Angeli F, Barbagallo CM, Bombelli M,
Cappelli F, et al: Serum uric acid/serum creatinine ratio and
cardiovascular mortality in diabetic individuals-the uric acid
right for heart health (URRAH) project. Metabolites. 14:1642024.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ahola AJ, Sandholm N, Forsblom C,
Harjutsalo V, Dahlström E and Groop PH; FinnDiane Study Group, :
The serum uric acid concentration is not causally linked to
diabetic nephropathy in type 1 diabetes. Kidney Int. 91:1178–1185.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Oh TR, Choi HS, Kim CS, Ryu DR, Park SH,
Ahn SY, Kim SW, Bae EH and Ma SK: Serum uric acid is associated
with renal prognosis of lupus nephritis in women but not in men. J
Clin Med. 9:7732020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Elnady B, Almalki A, Abdel-Fattah MM,
Desouky DES and Attar M: Serum uric acid as a sensitive concordant
marker with lupus nephritis and new onset of renal damage: A
prospective cohort study. Clin Rheumatol. 40:1827–1834. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wang H, Qiu F, Liu J, Luo C and Liu X:
Elevated serum uric acid is associated with renal arteriolopathy
and predict poor outcome in patients with lupus nephritis. Clin Exp
Rheumatol. 42:30–38. 2024.PubMed/NCBI
|
|
119
|
Wen Q, Tang X, Zhou Q, Chen W and Yu X:
Clinicopathological patterns and outcomes in patients with lupus
nephritis and hyperuricemia. J Clin Med. 11:30752022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Goicoechea M, de Vinuesa SG, Verdalles U,
Ruiz-Caro C, Ampuero J, Rincón A, Arroyo D and Luño J: Effect of
allopurinol in chronic kidney disease progression and
cardiovascular risk. Clin J Am Soc Nephrol. 5:1388–1393. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kwak CH, Sohn M, Han N, Cho YS, Kim YS and
Oh JM: Effectiveness of febuxostat in patients with
allopurinol-refractory hyperuricemic chronic kidney disease. Int J
Clin Pharmacol Ther. 56:321–327. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Kao MP, Ang DS, Gandy SJ, Nadir MA,
Houston JG, Lang CC and Struthers AD: Allopurinol benefits left
ventricular mass and endothelial dysfunction in chronic kidney
disease. J Am Soc Nephrol. 22:1382–1389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Smink PA, Bakker SJL, Laverman GD, Berl T,
Cooper ME, de Zeeuw D and Lambers Heerspink HJ: An initial
reduction in serum uric acid during angiotensin receptor blocker
treatment is associated with cardiovascular protection: A post-hoc
analysis of the RENAAL and IDNT trials. J Hypertens. 30:1022–1028.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Castilla-Ojo N, Turkson-Ocran RA, Conlin
PR, Appel LJ, Miller ER III and Juraschek SP: Effects of the DASH
diet and losartan on serum urate among adults with hypertension:
Results of a randomized trial. J Clin Hypertens (Greenwich).
25:915–922. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Heerspink HJL, Stack AG, Terkeltaub R,
Jongs N, Inker LA, Bjursell M, Maklad N, Perl S, Eklund O, Rikte T,
et al: Combination treatment with verinurad and allopurinol in CKD:
A randomized placebo and active controlled trial. J Am Soc Nephrol.
35:594–606. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Xin W, Mi S and Lin Z: Allopurinol therapy
improves vascular endothelial function in subjects at risk for
cardiovascular diseases: A meta-analysis of randomized controlled
trials. Cardiovasc Ther. 34:441–449. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Konishi M, Kojima S, Uchiyama K, Yokota N,
Tokutake E, Wakasa Y, Hiramitsu S, Waki M, Jinnouchi H, Kakuda H,
et al: Effect of febuxostat on clinical outcomes in patients with
hyperuricemia and cardiovascular disease. Int J Cardiol.
349:127–133. 2022. View Article : Google Scholar : PubMed/NCBI
|