Introduction
Viruses are obligate intracellular entities that
exist at the border between the living and non-living world.
Viruses differ from their prokaryotic and eukaryotic counterparts
in that they totally rely on their host and do not have their own
metabolism (1,2). Notably, they do not grow or undergo
any form of division, and they are produced by the assembly of
pre-formed macromolecules.
Viruses do, however, possess genetic material,
either DNA or RNA exclusively. In addition, their structural and
functional traits are inherited, and they are subject to the same
Darwinian evolutionary forces that drive the adaptation and
survival of all living organisms (1). Viruses, especially those with an RNA
genome, exhibit extensive genetic variability, far greater than
that of all the living world, which confers upon them the
evolutionary advantage of continuous adaptation to a multitude of
different organs, systems and hosts (2). Notably, all viruses have been
continuously shaped in both their structure and biological life
cycle by this evolutionary interaction, seeking dynamic equilibrium
with their host that will sustain their fitness and survival, as
they passively follow the stochastic driving forces of natural
selection.
Until a few decades ago, conventional and cell
culture-based systems were relied upon for virus detection and
identification; however, the limited abilities of such systems have
hindered the complete understanding of the presence and role of
viruses in all domains of life. Electron microscopy has provided
the means to distinguish between different viral species, genera
and families purely on the basis of their morphology and size, with
no information about the driving force behind viral fitness and
evolutionary success in the form of adaptation to these diverse
domains of life. The implementation of novel, extremely sensitive,
molecular detection and sequence-based identification methods,
which are applicable to all types of organisms and environmental
samples, has provided an impetus to transform the concept of
viruses (2). A new generation of
sequencing techniques has led to the more precise classification of
viruses and has identified the degree of genetic heterogeneity that
drives viral evolutionary success. Furthermore, this novel
methodology has set the basis for the discovery of novel viruses,
and the rapid detection and characterization of re-emergent viral
strains with novel virulence and epidemic potential. This may
markedly improve the efficiency with which we respond to viral
diseases and outbreaks. Fig. 1
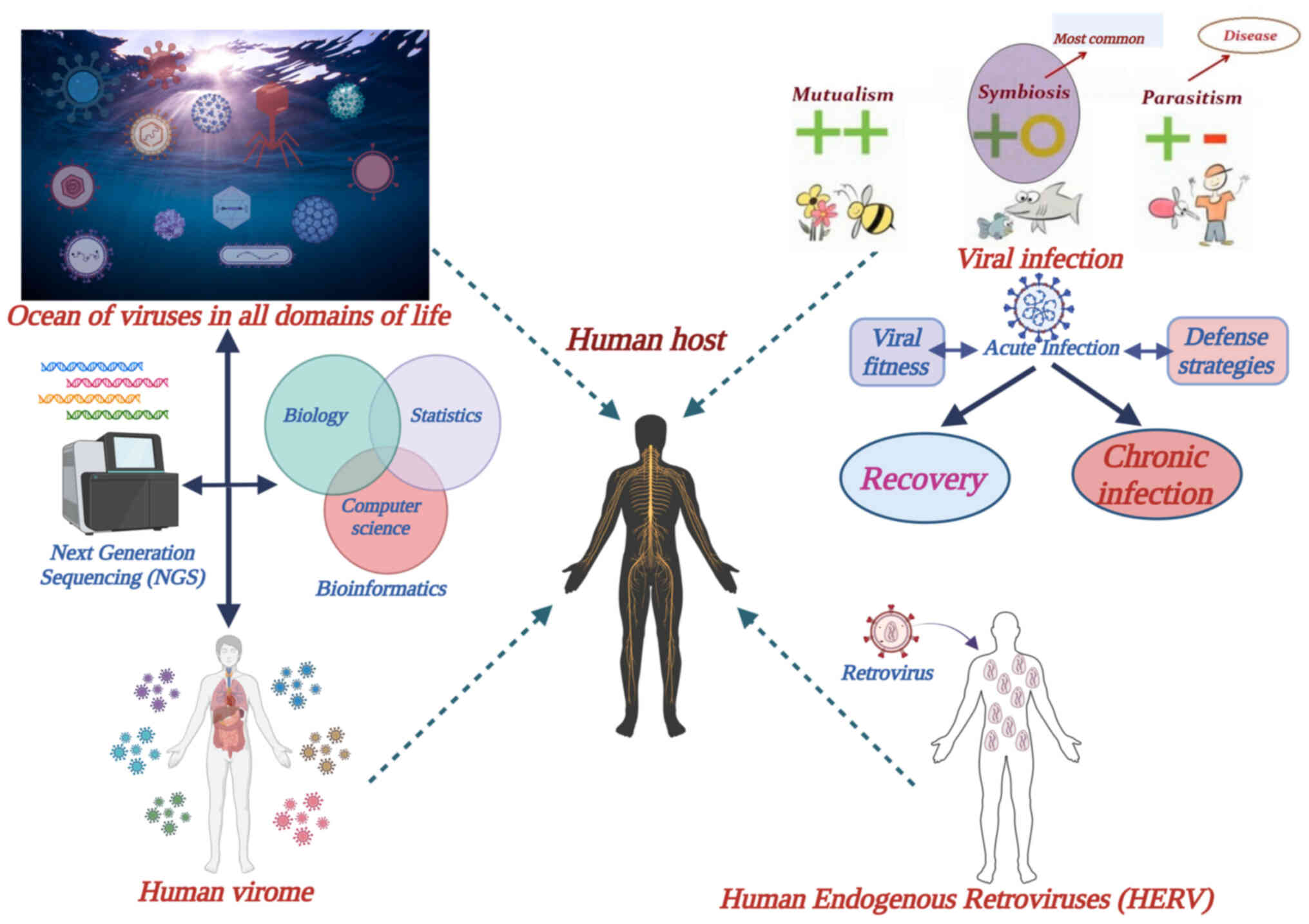
provides information on what is known so far about viruses and
their relationship with their hosts.
First, it is now known that humans live alongside a
considerable number of viruses and continuously interact with them.
Furthermore, viruses are neither confined to a single type of host,
nor a certain ecological niche; they thrive in all domains of life,
affecting both prokaryotic and eukaryotic organisms (1). It is estimated that there are
>1031 viruses on Earth in total (2). For example, >200,000 types of
viruses have been identified in ocean water (3), revealing the universal evolutionary
adaptation of viruses to all diverse ecosystems. A previously
unsuspected multitude and variety of viruses have been discovered
and remain to be discovered (4),
primarily due to continuous advances in genome sequencing and
bioinformatics, the two branches of the scientific discipline
metagenomics.
Despite both the magnitude of virus heterogeneity
and a parasitic life cycle, only a small proportion of viruses can
cause disease (2). Their obligate
reliance on the intracellular environment dictates that their
survival and final evolutionary success depend on the establishment
of a symbiotic relationship with their host, rather than a
parasitic one (1). Notably, the
most successful viruses are those that have reached an equilibrium
with their host by evading the challenges that they face within the
host, such as defensive host mechanisms and the immune system,
which is generally sufficient to eliminate viruses, or to at least
keep viral replication in check.
Nevertheless, there is still a significant number of
viruses that cause disease, either mild or serious, when the
pathogen-host equilibrium cannot be attained. Factors pertinent to
both virus biology and/or human social activity may be responsible
for pathogenicity, especially in the case of epidemics and
pandemics. For example, the emergence of a novel viral variant due
to genetic variation and natural selection in overcrowded societies
with efficient transportation may lead to an epidemic and possibly
to rapid pandemic spread (5,6).
Past pandemics have shaped societies, or even whole human nations
and empires, such as the Spanish flu in 1918 and the recent
COVID-19 pandemic (7). The genetic
novelty of viral variants may not only lead to a lack of pathogen
control by the immune system, but also to new virulence properties
with corresponding, short-term and/or long-term effects on human
physiology and disease. The recent
severeacuterespiratorysyndromecoronavirus-2 (SARS-CoV-2) pandemic
with the subsequent long coronavirus disease (COVID) sequelae is an
example of this (8,9).
Following entry into the host, an acute or a chronic
state of infection will be established, depending on the result of
the interaction between the virus and the host defense mechanisms.
Following an acute infection that is not lethal, the host will
usually recover with final clearance of the virus. However,
long-term sequelae may ensue, either directly by long-term damage
to organs and tissues, and/or indirectly via inflammatory cascades
and other pathophysiological effects irrelevant to organ damage and
dysfunction; this has been demonstrated with SARS-CoV-2 infection
and long COVID syndrome (10).
Chronic viral infections may be the direct result of an acute
infection or may develop months, or even years, after primary entry
of the virus into the host organism. In contrast to acute
infections, chronic infections are characterized by a dynamic and
metastable equilibrium between the host immune system and the virus
(11). Most chronic infections
described thus far are generally benign, where the virus enters a
form of symbiosis with the host; however, there is some evidence
that serious diseases and syndromes can be linked to chronic viral
infections with a continuous inflammatory, or other
pathophysiological, component, such as cancer, immune deficiency
(AIDS), neurodegenerative disorders and cardiovascular disease
(11).
Human endogenous retroviruses (HERVs) provide a
significant example of mutualistic symbiosis between the host and a
virus. These viruses are similar in their genetic constitution to
their infectious counterparts. They actually constitute fossil
retroviruses in the sense that they originate from ancient,
exogenous retroviruses which, during the course of 100 million
years of evolutionary history, integrated cDNA sequences of their
RNA into the genome of human genetic cells through reverse
transcription (12). Gradual
integration and vertical transmission of these sequences led to ~8%
of the human genome being of HERV origin. HERVs do not produce
infectious particles, but partial expression of retroviral genes
has been linked to human physiology and, if aberrantly expressed,
to disease (13). A significant
example of that is the role of the env gene of certain
endogenous retroviruses in human placental morphogenesis and
fetomaternal tolerance (14).
The recent advances in genetic sequence detection
and analysis have resulted in the realization that humans are not
only colonized by diverse prokaryotic and eukaryotic
microorganisms, which constitute the microbiome, but also by a
marked multitude and variety of viruses, collectively termed the
virome. Research has been performed on the composition and dynamics
of the virome, and its role in human health and disease. The virome
consists of viruses that infect human host cells and phages that
infect bacteria of the microbiome (15). Viruses affect the host directly by
interfering with the defense mechanisms, whereas phages may exert
an indirect effect on host physiology and disease by modulation of
the composition and fitness of the bacterial microbiome (16).
The aforementioned information poses a challenge for
the scientific community related to the understanding of the role
of viruses in human health and disease. It is known that the
concept of viruses is more extensive than their exclusive
perception as mere agents of an acute infection, or some chronic
debilitating diseases, such as AIDS or some forms of cancer.
Notably, a causal connection between viruses and disease
pathophysiology may exist; however, there are still a number of
questions that need to be answered. A number of hypotheses have
been put forward regarding viruses and diseases, the etiology of
which was previously unknown, but it remains unclear as to how to
establish a causal connection. In addition, it remains to be
determined how far research has gone regarding the possible role of
viruses in chronic, debilitating diseases such as psychiatric
disorders; and how many, and which, viruses may contribute to the
development of psychiatric disease. Considering the great diversity
of the infectious properties of different viruses, research is also
required regarding how a viral infection could trigger a mental
disorder; whether some viruses have specific virulent properties
that have not been described so far and dictate previously
unrecognized pathophysiological pathways; and whether these new
associations with disease are the result of direct damage and its
sequelae in the long-term, or chronic infection. Furthermore, it
remains to be ascertained as to whether detection is enough, and
how to detect viruses in a chronic state of infection, where viral
load is frequently imperceptible and symptoms are initially
obscure, developing years after viral infection. The role of the
virome and endogenous retroviruses in psychiatric and other
diseases also requires further assessment.
Suggested mechanisms and tentative data
about the possible association of viral infections with psychiatric
disease
Most clinical changes in an individual's cognition
and mental status are heterogeneous and attributed to the
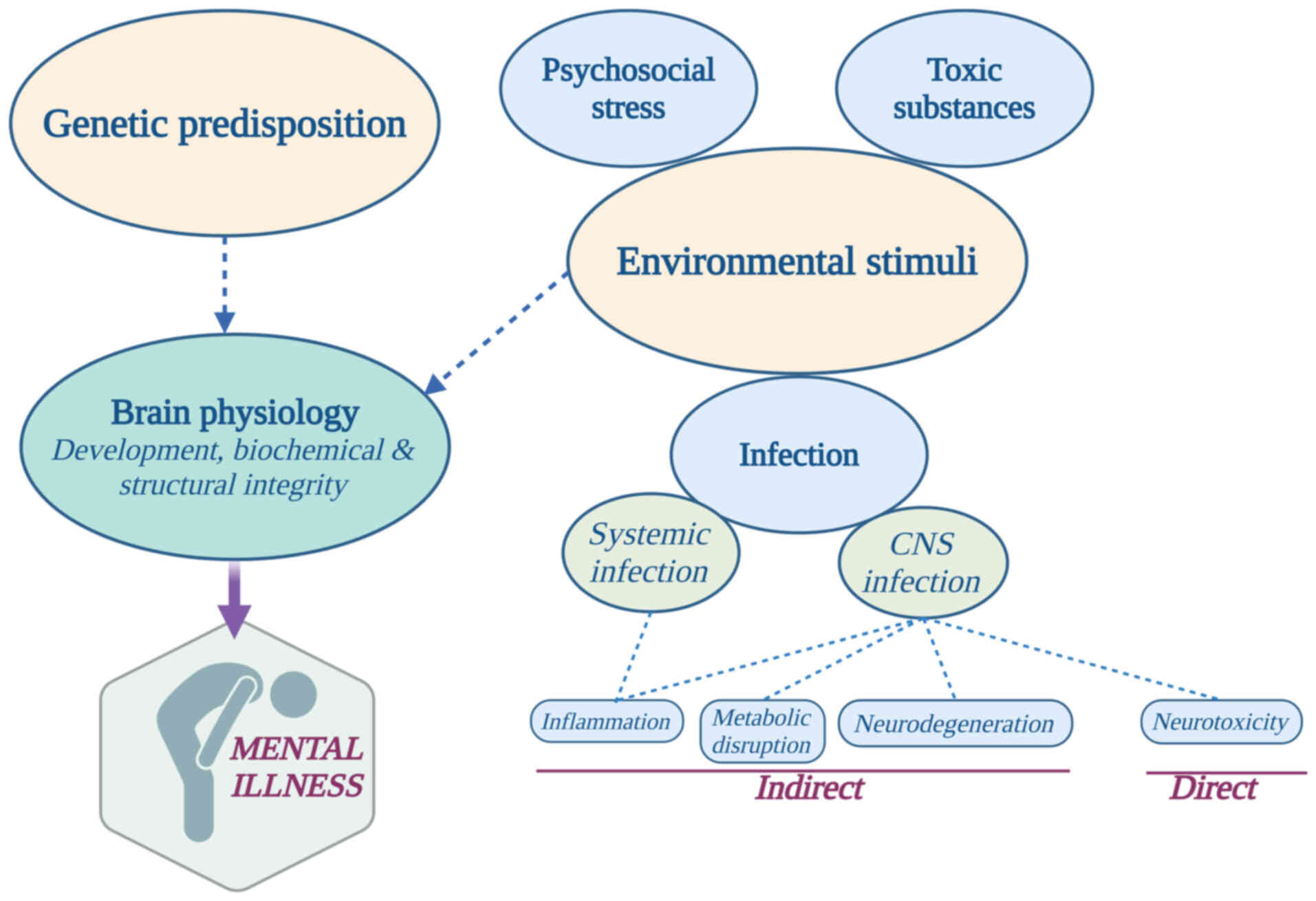
synergistic action of a multitude of different factors. Genetic
predisposition and environmental perturbations, by either stress
from the social surroundings or alterations in the physiological
state of the organism, have been proposed as significant
contributing factors in the development of psychiatric disease
(Fig. 2) (17). Such environmental influences may
include direct insults or indirect sequelae that follow infection
from viruses such as influenza, enteroviruses, arboviruses and
several herpesviruses, or the differential expression of HERVs.
Viruses may contribute to psychiatric diseases such
as schizophrenia, autism, major depression, bipolar disorder and
chronic fatigue syndrome through various mechanisms (18). Thus far, these tentative mechanisms
generally fall into two categories (Fig. 2): Indirect immune, inflammatory,
metabolic and degenerative processes in the central nervous system
(CNS), and/or direct neurotoxic effects following viral invasion
into the CNS (19).
Indirect effects of viral infection on
the physiology of CNS
Virus-induced psychopathology linked to
neurodevelopment
A number of different viruses have been implicated
in a single psychiatric disease, such as schizophrenia, and this
may constitute an immune-mediated disorder, rather than a
pathogen-specific link between viral infection and a psychiatric
disorder (20). The model of
neurodevelopment disruption has been suggested as the most
promising mechanism underlying the onset of schizophrenia (19). Both direct viral infection and
indirect viral effects, via the activation of inflammatory pathways
and the excessive release of cytokines, have been suggested as
possible disrupting mechanisms in normal neurodevelopment (21). Moreover, the timing of disruption
of normal neurological processes that occur at critical stages of
neurodevelopmental processes has been proposed as being of utmost
importance. Specifically, effects incurred by viruses appear to be
stronger when infection occurs during the late 1st and the 2nd
trimesters of gestation (19),
childhood and pre-adolescence (22,23).
Several studies have also shown that childhood viral infection of
the CNS, especially under the age of 3 years or in pre-adolescence,
may be significantly associated with an increased risk for later
psychosis (22,24).
However, virus-induced disruption during both pre-
and postnatal neurodevelopmental processes is not solely
responsible for the possible development of a psychiatric disease.
Psychological trauma during adolescence or early adulthood, in
conjunction with previous maternal or childhood viral infection
during critical stages of neurodevelopment, has been linked with
greater possibilities for the development of schizophrenia
(25,26).
Activation of inflammatory pathways
following infection and their link with psychiatric disease
It has been suggested that systemic or direct CNS
infections may activate neuroinflammatory events mediated by
activated microglia and the release of cytokines in the brain
(27,28). In the case of a systemic viral
infection through, for instance, the circulatory, respiratory and
the gastrointestinal routes, an initial inflammatory event may
ensue that leads to the release of proinflammatory cytokines and
chemokines that can disrupt the integrity of the blood-brain
barrier (BBB), rendering it permeable to viral particles, toxins
and infected immune cells (Fig. 3)
(28). Other neurotropic viruses,
such as α-herpesviruses and rhabdoviruses, invade the CNS directly;
therefore, a correspondingly direct neuroinflammatory event may be
elicited (Fig. 3). In both cases,
oligodendrocytes, astrocytes and microglia constitute the main
mediators of inflammation in the brain (28).
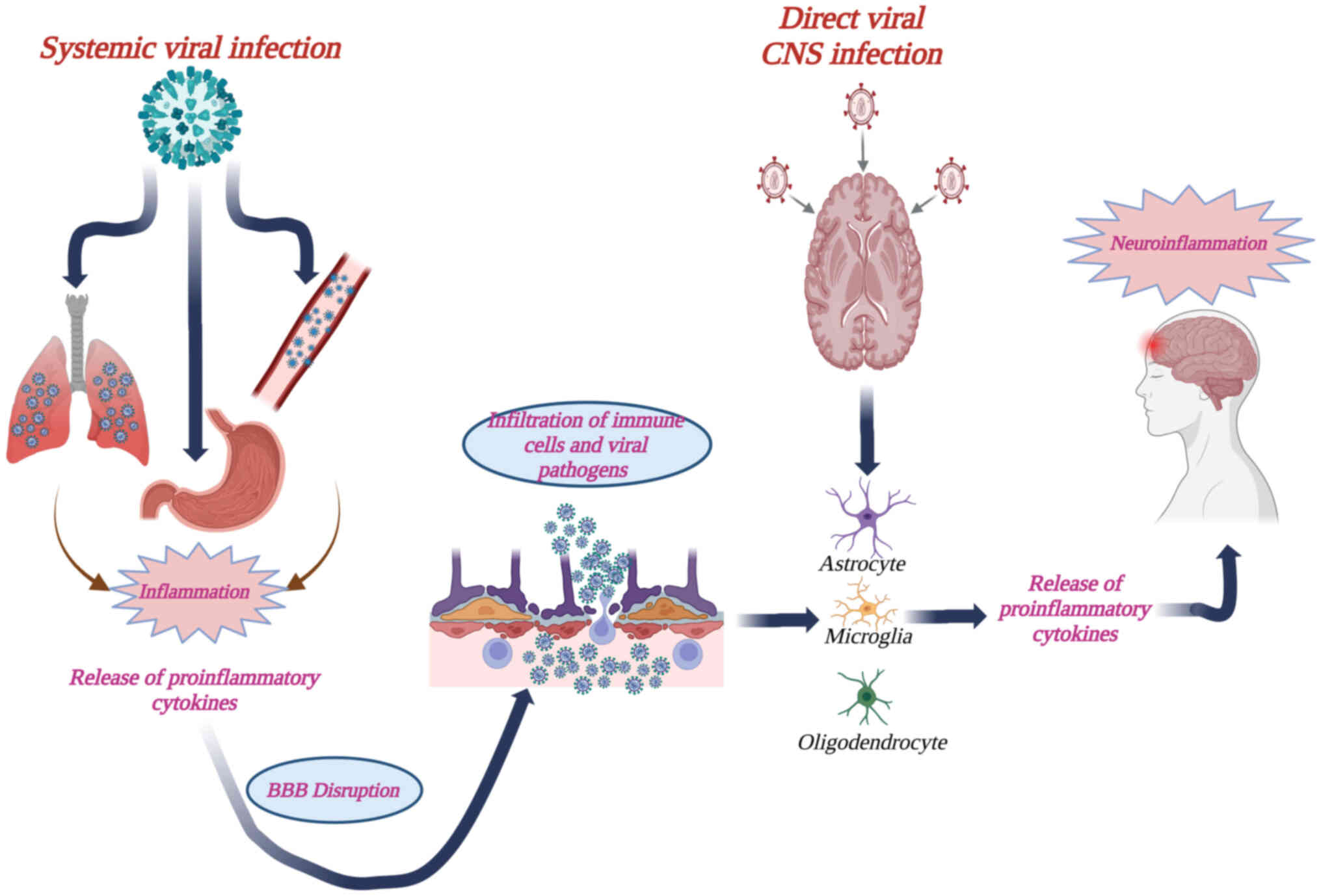 | Figure 3.Diagrammatic presentation of
viral-induced neuroinflammation, following either a systemic, or a
direct CNS infection. Viruses that gain entry to the host through
the circulatory, respiratory and the gastrointestinal routes, can
still elicit neuroinflammation. Initial inflammatory events may
lead to the release of proinflammatory cytokines and chemokines
that can disrupt the integrity of the BBB, thus rendering it
permeable to viral particles, toxins and infected immune cells.
Alternatively, viruses such as α-herpesviruses and rhabdoviruses
invade the CNS directly through spread within neuronal axons. In
both cases, astrocytes and microglia constitute the main immune
mediators within the CNS and their activation via Toll-like
receptors generate further production of proinflammatory cytokines,
leading to neuroinflammation in the brain. Created in https://BioRender.com. BBB, blood-brain barrier; CNS,
central nervous system. |
Extensive data from clinical and experimental models
have shown the induction of proinflammatory cytokines following
infection with several viruses, such as flaviviruses, including
Dengue virus (DENV) and tick-borne encephalitis virus (29,30).
Japanese encephalitis virus (JEV) is another flavivirus that
infects microglial cells, which may serve as a reservoir for the
virus (31). In addition, Zika
virus (ZIKV), as well as directly damaging neuronal cells, may
mediate an exaggerated neuroinflammatory response, especially in
neonates; postnatal microcephaly has been linked with ZIKV-induced
neuroinflammation in both animal models and fatal cases (32,33).
Another example is the neuronal damage caused by the rabies
lyssavirus (RABV), which has also been suggested to be the result
of the high production of inflammatory cytokines in primary
astrocytes and microglia (34).
Regarding the available evidence regarding the role
of these neuroinflammatory events in influencing neurobiological
processes, potentially contributing to the development of
schizophrenia and related psychotic disorders, elevated
proinflammatory cytokines have been found in both the blood and
cerebrospinal fluid (CSF) of patients with cognitive deficits
(35,36). Notably, serum interleukin (IL)-6
and C-reactive protein in both childhood and adolescence have been
shown to be linked to both the presence and severity of disease
during adulthood (37,38). IL-6 is also considered not only as
an important, diagnostic inflammatory marker but as a predictive
one as well, since its increased levels during both childhood and
adolescence have been associated with a high risk for adult
psychotic disorders in a dose-dependent manner (39). IL-8 in the CSF has also been
described as a sensitive marker of inflammation for numerous
neurological and psychiatric diseases, whereas higher IL-12p70
levels have been detected in the serum of patients with
schizophrenia (40).
Complement factors in the brain are also known to
contribute to the process of synaptic pruning, which involves
enhanced activation of proinflammatory cytokine secretion by glial
cells, leading to possible neuronal cell damage and death (41). Moreover, activated complement may
contribute to alterations in the BBB function, which could
subsequently influence the progress of a psychiatric disease
(42). Notably, elevated levels of
complement have been detected in patients following first-episode
psychosis (43,44).
Systemic, or CNS-specific viral infection and the
inflammatory cascades that ensue may not exclusively affect brain
physiology, they may also influence hypothalamic-pituitary-adrenal
(HPA) axis dysregulation, which has been linked to psychiatric
diseases such as schizophrenia (45). HPA axis dysregulation, and brain
neuropathology induced by proinflammatory cascades elicited by
either direct or systemic infections, have a common trait; they
mostly seem to render the organism more vulnerable to a possible
psychiatric disorder but do not lead to the disease on their own.
An environmental, psychosocial stimulus in later life may more
readily affect an individual primed by such inflammatory
complications (46).
Genetic predisposition in human
leukocyte antigen (HLA) genes and immune response to infections
that may prime a psychiatric disease
HLA genes located on chromosome 6 code for cell
surface proteins that serve a fundamental role in immune responses
against foreign antigens. Class I and II HLA molecules represent
two major pathways that act in synergy for host protection
(47). Genome-wide research on the
genetic background of schizophrenia has provided evidence that
genetic polymorphisms within the HLA genes located on chromosome
6are associated with the disease (47). Specifically, polymorphisms
involving HLA-DRB1 alleles of Class II antigens have been
significantly linked with schizophrenia (48). There are polymorphisms, however,
which are negatively associated with schizophrenia (49) or even have a protective effect
(50), at least amongst certain
ethnic populations.
As a consequence of the pivotal role that HLAs have
in foreign antigen presentation and final elimination by the immune
system, an ineffective, polymorphic version of an HLA allele may
lead to more pronounced, adverse effects on the brain and the
persistence of viral antigens, with poor consequences regarding
inflammation and/or autoimmunity with respect to the development of
psychiatric disease. For example, in a recent study, it was
suggested that HLA alleles with a higher affinity for
herpesviruses, leading to a more effective elimination of the
consequences induced by acute infection, may confer protection
against schizophrenia (51).
Viral infection could impair CNS
energy metabolism, which may lead to psychiatric disease
As obligate intracellular entities, viruses rely
exclusively on host cell metabolic machinery to replicate and
sustain their existence. Notably, neurotropic viruses may disrupt
brain physiology by altering neuron and astrocyte metabolism; they
have evolved to alter host cell metabolic activities so that they
can optimally be used for their own benefit, such as glycolysis,
the pentose phosphate pathway, the tricarboxylic acid cycle and
oxidative phosphorylation (52).
For example, there is increasing evidence that ZIKV infection
suppresses neuronal stem cell proliferation and induces premature
differentiation by intervening with glycolysis and oxidative
phosphorylation properties of the host cells, leading to
microcephaly of neonates during pregnancy (53–55).
Moreover, a reduction in glutamine metabolism in astrocytes
following a viral infection may disrupt their cooperation with
neurons to maintain the glutamate-glutamine cycle in the brain,
something that may shape brain activities and lead to
neuropsychiatric disorders (56,57).
For instance, the release of Tat protein by infected cells in
HIV-associated neurocognitive disorders (HAND) has been linked with
disruption of this metabolic cooperation between astrocytes and
neurons (58,59). The same has also been proposed for
SARS-CoV-2, which may also be found in the brain and mainly infects
astrocytes (56,57). Finally, another means for
impairment of neuronal metabolism may be elicited by a viral
infection through alteration of mitophagy and mitochondrial
dynamics, which has been reported for HIV-1 proteins that inhibit
mitophagic flux in human primary neurons, leading to neuronal
damage (60).
Direct impairment of the CNS by
neurotropic virus infection
Evidence and suggestions were previously outlined
above regarding the indirect effects of viral infections on
inflammatory pathways and neurodevelopmental processes that may
lead to severe neuropsychiatric disorders in the long-term and not
immediately after infection. Moreover, these sequelae do not appear
on their own; viral infections and their effects could prepare the
organism to respond more readily to an appropriate environmental
stimulus that would act in synergy to mount the emergence of a
psychiatric disease, later on in the individual's life.
However, a number of viruses have evolved to invade
the CNS directly via numerous pathways, including
leukocyte-mediated transfer through the BBB, neuronal axon
transport from peripheral nerves and invasion of CNS endothelial
cells, or the olfactory nerve (61). Therefore, the possible role of
direct damage inflicted upon the CNS by these neurotropic viruses
in psychiatric disease should be assessed. So far, numerous
neurotropic viruses that belong to a diverse number of families
have generally been linked to psychiatric disease, and these
include herpesviruses, enteroviruses, arboviruses and retroviruses,
as well as respiratory viruses that also exhibit a degree of
neurotropism, such as influenza and coronaviruses, (52,62–64).
Clinical and in vitro studies have shown that these viruses
can be directly neurotoxic via a number of diverse mechanisms,
including manipulation of apoptosis and autophagy, production of
reactive oxygen species (ROS) and disruption of the production of
antioxidants, localized inflammation in the central and peripheral
nervous system (neuroinflammation), and alterations in
neurotransmission (52,65).
Apoptosis and autophagy during viral
infection
Apoptosis and autophagy constitute two important
defense mechanisms against viral spread. It has been reported that
the apoptosis signaling pathway is activated in pediatric patients
with HIV-1 encephalitis (66), as
well as in infections caused by enterovirus A71(EV-A71) (67), RABV (68–72),
West Nile virus (WNV) (73–77)
and JEV (78,79). Some viruses may evade degradation
and the subsequent elimination by the immune system through
inhibition of autophagy, but others modulate autophagy by
exploiting autophagosomes and their secretion pathway as sites for
replication, viral particle maturation and release from the host
cell (80). For example, the VP1
capsid protein of EV-A71 is not only a major modulator of viral
antigenic properties and interaction with the immune system, but it
also contains important neurovirulence properties that induce
autophagy by regulating the mammalian target of rapamycin signaling
pathway to promote viral replication (81–83).
Modulation of autophagy by the HIV-1 proteins Nef and Tat has also
been evaluated and proposed, which may contribute to HAND (84–86).
Oxidative, mitochondrial and
endoplasmic reticulum (ER) stress following viral infection
Infection by neurotropic viruses has been associated
with excessive production of ROS and deficient cellular antioxidant
defenses (52). JEV is a
significant example of such a neurotropic virus that increases ROS
production; it has been suggested as an important contributor to
psychiatric, or other neurologic sequelae, in ~50% of survivors
(87,88). Other neurotropic viruses associated
with excessive oxidative stress include RABV, EV-A71, which is
prevalent in South East Asia, and ZKV (52). Moreover, oxidative stress is not
exclusively seen in viruses causing mainly acute infections, but is
also a characteristic of viruses causing latent, or persistent
infections, such as herpes simplex virus (HSV) and HIV. HSV-1
infection and intracellular ROS generation have been observed in
microglia (89) and neural cells
(90). Glutamate-mediated
excitotoxicity is also an important mechanism of neuronal injury by
viral infection. The effect of HIV-1 proteins on neurotoxicity
induced by glutamatergic system dysregulation has also been
revealed (91).
Several in vitro studies have also shown that
infection with certain viruses may induce mitochondrial and ER
stress (52). For instance, as
well as inducing neuronal damage via neuroinflammation, RABV has
been reported to invoke significant alterations in different
mitochondrial parameters (92).
In vitro studies in human neural stem cells have also shown
that JEV, ZIKV and WNV infection may promote the expression of ER
stress-related proteins (93–95),
whereas the enterovirus EV-A71 may induce oxidative stress both in
the ER (96) and mitochondria
(97), the latter leading to
morphological changes and subsequent functional anomalies in
glioblastoma cells.
Viral infection could interfere with
neurotransmission
Neurotropic viruses may alter the levels of specific
neurotransmitter levels, leading to dysregulation of synaptic nerve
signal transmission and subsequent impairment of specific brain
functions that may trigger psychotic disorders (52). Specifically, viruses affect
pathways that involve dopamine metabolism (98,99)
and glutamate transmission via molecular mimicry at
N-methyl-D-aspartate receptors (100). JEV infection can significantly
increase dopamine production and modulate the rate-limiting enzyme
of dopamine biosynthesis, with an increase in phosphorylated
tyrosine hydroxylase levels at the early stage of infection
(101). JEV may also exploit
dopamine-mediated neuronal communication to increase the
susceptibility of dopamine D2 receptor-expressing cells to JEV
infection, which causes damage to dopaminergic neuron-rich areas
such as the thalamus and midbrain, leading to neuronal loss and
increasing the fatality rate of JEV-infected mice (101).
The quest for establishing an etiological
link between viral infection and psychiatric diseases: Conjectures
and evidence
The present review has thus far provided a summary
of the possible mechanisms with which systemic and/or neurotropic
viral infections may contribute to the development of psychiatric
disease. Nevertheless, determination of the presence of a virus
within the host during an acute, persistent or latent infection
that evolved into an early or a delayed psychiatric abnormality is
essential to establish the possible contribution of viruses to
psychiatric abnormalities. Either seroepidemiological analysis or
the direct detection of viral genetic material in appropriate
clinical samples, such as the CSF, have been employed thus far in
CNS and systemic infections that were subsequently linked to a
psychiatric disease. Significant rates of neuropsychiatric
manifestations (43%) have been found in HSV-2, varicella zoster
virus (VZV) and enterovirus meningitis cases (102–104). Cognitive impairment in adults
shortly after aseptic meningitis and encephalitis caused by HSV,
VZV, enteroviruses, HIV, ZIKV and coronaviruses has also been
reported (105,106).
Epidemiological and serological
evidence about the role of specific viruses in psychiatric
disease
Regarding viral infection and its role in the
disruption of normal neurodevelopment, as long as 4–5 decades ago,
there were initial reports on the greater incidence of patients
with schizophrenia who were born during late winter and spring when
the risk for respiratory tract infections, and most notably
influenza virus infection, is significantly greater (107,108). Numerous studies have subsequently
supported this observation (109,110). A significant risk for
schizophrenia has also been reported for patients whose mothers
were exposed to influenza epidemics during the 2nd trimester of
gestation (111). Prenatal
exposure to rubella is another suggestion for the increased risk of
schizophrenia in the offspring of affected mothers (112). A high frequency of psychiatric
disorders has also been reported for patients with a history of
chronic hepatitis C virus (HCV) infection (113). Additionally, anti-viral treatment
and final HCV clearance have been shown to positively affect the
quality of life in these patients (114). Psychotic diseases, such as major
depressive disorder, anxiety disorder and HAND, have commonly been
reported amongst patients with HIV; however, not much information
is currently available about a possible association between HIV
infection and schizophrenia (46).
Inconsistent results have been reported from such epidemiological
studies and their role in identifying the viral connection in
psychiatric diseases. For instance, in another large-scale study,
no significant association was identified between maternal
infection before, during or after the 1957 influenza epidemic, and
the development of schizophrenia in offspring (115).
Further serological methods have been employed to
decipher an association between prenatal exposure to influenza and
an increased risk of schizophrenia. For example, one study based on
serological investigation of the mothers of psychiatric patients
indicated that the risk of schizophrenia was increased in people
exposed to the influenza virus within the uterus during the first
trimester of pregnancy (112).
Members of the Herpesviridae family are also
very well known for their neurotropic properties and their
persistence in the host, marked by alternating periods of latency
and possible reactivation (116).
Elevated IgG antibody levels against HSV-1 and/or cytomegalovirus
(CMV) have also been associated with a first episode of
schizophrenia, brain morphological changes, increased suicidal
tendencies and cognitive impairment (117,118). In another, large-scale
serological study of CMV IgG detection in plasma samples from
psychiatric patients, high rates of antibody detection (61%) were
associated with a variety of different psychiatric disorders
(119). An aberrant response to
infection by Epstein-Barr virus (EBV) has also been reported in
patients with schizophrenia who have been shown to exhibit elevated
levels of antibodies against the viral capsid antigen of the virus,
but not against the EBV nuclear antigen-1 (120). However, other studies have shown
inconsistent results regarding the potential association of a
previous herpesvirus infection with psychiatric disease. In certain
studies, no significant association between HSV-2, HSV-6 and VZV
infections, and schizophrenia was found (121–123), whereas elevated antibody titers
against Chlamydia trachomatis were more significantly
correlated with schizophrenia than antibodies against other
herpesviruses (124).
Arboviruses have also been implicated as etiological
agents of psychiatric morbidity in patients with clinical and/or
serological evidence of infection. For instance, cognitive
difficulties have previously been reported amongst 49 patients with
clinical and laboratory classification of a WNV infection (64). Persistence of these cognitive
deficits for an extended period of time and their prevalence,
irrespective of whether the infection was neuroinvasive or not, has
been shown to advocate a systemic pattern of neuropsychiatric
disorder manifestation, rather than the result of direct
neurotoxicity of WNV. Chikungunya virus is another arbovirus that
has been linked with depression, anxiety and somatoform disorders
(60), although it has been
suggested that prenatal and postnatal exposure to the virus may not
be associated with impaired neurodevelopment (125). Nevertheless, such studies
regarding the role of these and other arboviruses, such as ZIKV or
DENV, in psychiatric disorders are still scarce and further studies
must be conducted on larger sample sizes and the pathophysiology of
arbovirus infection with psychiatric sequelae.
Direct evidence of the possible
implication of viral infection in psychiatric disorders
Detection of viral genes and their products in
clinical samples of psychiatric patients
Most of the viral infections that have been
recognized so far as contributing factors in the etiology of
psychiatric disease are based on epidemiological and serological
analyses that provide speculative, and not definitive, evidence of
an association between viruses and psychiatric disease. Psychiatric
disorders often develop late, after viral infections during
critical neurodevelopmental stages. This, combined with other
environmental factors, makes it difficult to study the direct
connection if viruses to psychiatric disease during the acute
infection phase. Moreover, viral persistence through latency means
that any viral load, which may be linked with direct neurotoxic, or
indirect inflammatory and neurodevelopmental processes that could
lead to a psychiatric disease, remains at almost imperceptible
levels, posing a significant challenge for their detection, even
when using the most modern, state-of-the-art molecular
methodologies.
More compelling evidence for the role of viruses in
psychiatric diseases comes from studies that detect and analyze
viruses directly in the brain or in CNS-associated clinical
samples, such as the CSF. These studies use highly sensitive
molecular techniques, such as polymerase chain reaction (PCR) and
next-generation sequencing (NGS), which can identify viral genes
and products even at low levels. The potential conferred by these
techniques in accurately detecting and characterizing viral genes
and products, even when minimum viral loads persist during a
dormant part of the biological cycle of the virus, is significant.
A number of viruses, which are not obligate neurotropic viruses,
may also be recognized, such as HCV. The psychopathology behind HCV
infection is complex and poorly understood, involving activation of
glial and local/systemic inflammatory pathways, as well as
metabolic disruptions (126).
However, HCV RNA has been detected in brain autopsy samples of
patients at a 3–5 log lower load than in hepatocytes (127). Moreover, HCV RNA has been found
in astrocytes and microglial cells (128), which may indicate viral spread
within the CNS despite the fact that these cells do not express the
appropriate receptors on their surface for HCV entry. In addition,
HCV RNA has been detected in peripheral blood mononuclear cells
(PBMCs), which may conditionally transport viral particles across
the BBB (129).
Novel sequencing techniques, based on NGS and whole
genome sequencing methodologies, provide a powerful means to delve
into the heterogeneous viral populations of the virome and/or
acutely infecting viruses within a host with psychiatric disorders,
providing a fundamental basis for further analyses and studies of
their possible relationship with the disease. However, few studies
which either report inconsistent, varying results or are largely
incomplete, have been performed to date, and these have reached
controversial conclusions about the connection of viruses with
psychiatric disease. For instance, in one study, whole-genome and
RNA sequencing were employed for the investigation of viral
infection directly in brain tissues and its association with
schizophrenia, bipolar disorder and autism spectrum disorder
(130). Samples from a large
number of patients were analyzed; however, despite detection of
diverse neurotropic DNA and RNA viruses in the brains of patients
with major psychiatric disorders, such as herpesviruses,
polyomaviruses, retroviruses, adenoviruses and others, neither
significant qualitative (types of viruses detected) nor
quantitative (viral load) differences were detected between
patients and controls. Therefore, no association between viral
infection of the brain and major psychiatric disorders could be
inferred.
Another study based on novel sequencing technologies
reported on the development of a sequence-capture method and an
appropriate bioinformatics analysis pipeline that may perform
detailed analysis of the human virome in a variety of clinical
samples, with the aim of investigating its possible association
with psychiatric disease (131).
Detection of HIV-1, Torque teno virus, pegivirus, herpesvirus and
human papillomavirus sequences were reported in PBMC, plasma and
stool samples, but only Torque tenovirus was detected in
psychiatric case samples and not in controls. A limitation of this
previous study, however, was the fact that a limited number of
samples and patients were examined and, notably, only peripheral
samples were analyzed; since viruses present in the CNS may not be
present in peripheral tissues, the study of CSF samples with the
proposed method may enhance identification of viral activity in the
CNS of psychiatric patients (131).
Evidence from research on models using
experimental animals
Several studies on experimental animal models have
been employed during the last two decades in an attempt to support
the viral model of neuropsychiatric abnormalities. For instance, in
such an experiment, transgenic mice that were genetically
manipulated in order to express the phosphoprotein of Borna disease
virus (BDV) in glial cells showed a significant reduction in
synaptic density, and the expression of brain-derived neurotrophic
factor and serotonin receptor, as well as a decrease in synaptic
density (132). These mice then
developed behavioral abnormalities, such as hyperactivity,
increased aggressiveness between males and spatial reference memory
deficit. In summary, the experiment showed that the expression of
BDV phosphoprotein in glial cells may directly induce neuronal
degeneration that could lead to disorders resembling those of
psychiatric patients. In another model of schizophrenia, where mice
were experimentally infected with influenza virus at embryonic days
7, 9, 16 and 18, the mice exhibited significant postnatal brain
structural abnormalities that led to abnormal behavior (133). An experimental study carried out
with conventional mice (adult male and female C57BL/6J, 129X1/SvJ
and nude mice Foxn1nu/Foxn1n, all 8–12 weeks old), infected with
chimeric EcoHIV, as a model to reproduce physiological conditions
for the development of disease in people on antiretroviral therapy
(ART), revealed that ART can prevent AIDS, but not HIV-associated
neuropathogenesis (134). This
study suggested that, although HIV replication is suppressed by
ART, the persistence of integrated, replication-competent HIV in T
cells and macrophages may serve an important role in neurocognitive
and behavioral aberrations.
HERVs and their role in psychiatric
disease
HERVs pose a different challenge from the classical
problem of proving a possible association of viral infections with
psychiatric disease. They are not the mediators of a viral
infection, they are part of the human virome and, as retroviruses,
they have permanently integrated cDNA sequences of their RNA into
the human genome, comprising 8% of its total nucleotide
composition. Therefore, the key point is not to prove any kind of
infection by HERVs, but, since partial expression of retroviral
genes has been linked to either human health or disease, to
determine the association between HERVs and psychiatric disease,
HERV transcripts and/or their products should be detected and
characterized in appropriate clinical samples (135–138).
Aberrant expression of HERVs has recently been
identified in schizophrenia (139). Upregulated transcripts of the
polymerase gene of the HERV-W family have also been detected by
quantitative PCR methods in the CSF samples from a significant
proportion of patients with schizophrenia, or schizoaffective
disorder, as well as in the frontal cortex of brains from
individuals with schizophrenia (135). In addition, elevated levels of
HERV-W transcripts and proteins have been reported in the blood,
CSF and brains of patients with bipolar disorder (136,137). In another study, a more detailed
association between HERVs and psychiatric disease was reported,
where patients with schizophrenia and bipolar disorder could be
differentiated into subgroups with differing inflammatory and
clinical profiles (e.g. earlier disease onset), on the basis of
HERV-W family env protein antigenemia and cytokines (140).
Most studies carried out thus far on the potential
association of HERVs with psychiatric disease have implicated
HERV-W as the most frequently associated family (138). However, insufficient evidence is
available due to the currently limited number of studies and the
small sample sizes that have been employed in these studies.
Further studies are required in order to obtain more unambiguous
data regarding the possible role of HERVs.
The paradigm of SARS-CoV-2: An
interplay of neuroinvasive, inflammatory and environmental
processes
It has been suggested that the neuroinvasive
properties of SARS-CoV-2 in conjunction with hyperactivity of
inflammatory responses may have an impact on the emergence of
neuropsychiatric disorders during both the acute and
post-infectious phases of COVID-19 (141). Specifically, in long COVID
syndrome, it has been proposed that neuropsychiatric sequelae may
be mainly associated with inflammatory, metabolic and degenerative
processes in brain areas where the presence of the virus receptor
ACE2 is significant, extending from the somatosensory cortex to the
rectal/orbital gyrus, the temporal lobe, the thalamus and
hypothalamus, and further, to the brainstem and cerebellar regions
(142). Moreover, the
socioeconomic impact of the pandemic has imposed a significant
strain on human societies and individual lives due to strict
lockdowns, social distancing or isolation measures, incomplete
physical health recovery and a worldwide economic crisis. In
addition, it has been emphasized that individuals with intellectual
and developmental disabilities may be at a greater risk of mental
health deterioration during COVID-19 due to the direct effect of
anxiety caused by the pandemic itself and the media, as well as to
restrictions brought about by lockdown regulations (143–145). These showcase the indirect
effects of SARS-CoV-2 on the increase in the prevalence of
neuropsychiatric disorders, which differ from the purely
biological, neuroinvasive properties of the virus (146–148). Therefore, it is crucial to
broaden the current knowledge about the persistence of psychiatric
disorders in the post-acute phase of infection, in order to
distinguish between the social and the pathophysiological origin of
emergent cases, and to develop an appropriate, targeted approach
for patients.
In a previous study, it was estimated that the
incidence of neurological or psychiatric diagnosis within 6 months
after a SARS-CoV-2 infection was 33.62% in ~250,000 patients
(149). Depression and anxiety
have been reported as the most common psychiatric symptoms in
patients with long COVID (150,151). Notably, post-traumatic stress
disorder (PTSD) has been recorded following recovery from serious
epidemic events in the past and the same applies to the most recent
COVID-19 pandemic (152). Sleep
disturbances, fatigue, memory impairment, concentration,
disorientation, confusion and other serious disorders have been
associated with long COVID with debilitating effects on everyday
life.
Conclusion
The association of viruses with psychiatric diseases
still remains controversial, since we have not crossed the
borderline from hypothesis to proof yet. In most cases of viral
disease and epidemiology, establishing a specific virus as the
causative agent depends fundamentally on confirmation in the
laboratory. However, most evidence available thus far regarding the
possible role of viruses in the development of psychiatric
disorders comes mainly from observational, epidemiological and
serological studies. A number of older diagnostic approaches, such
as serological demonstration of elevated antibody titers against
known viruses, are of limited value. They frequently lack high
specificity, constitute indirect proof of viral infection, and are
ineffective in characterizing novel viruses as potential causes of
psychiatric disease.
Accurate diagnosis is complex with a number of
parameters, including the patient's medical history and immune
status, appropriate sample type and timing of collection, and the
methods used. Despite significant advances in diagnostic laboratory
capabilities due to technological advancements in molecular
methodologies, challenges remain. These advancements have enabled
the high throughput of prompt and more accurate diagnostic results,
reinforcing the liaison between laboratory and clinical staff.
Quantitative results of viral loads and genotypic characterization
of strains relevant to psychiatric or other diseases can now be
obtained, further enhancing the clinical utility of diagnostic
results. Moreover, they have enabled the recognition of emergent,
re-emergent or known viruses that have never been associated with
psychopathology before. However, studies focusing on direct virus
detection in appropriate clinical samples are still limited,
hindering the direct causal connection with disease onset.
Due to notable and inherent difficulties in both
virus detection and the establishment of an etiological connection,
satisfactory results are yet to be obtained. Firstly, detection of
neurotropic viruses is hampered by their ability to establish
chronic infections, either latent or with a late-onset. It is
frequently difficult to determine the insidious persistence of such
neurotropic viruses for months or years within the CNS, something
which is attributed to their very low viral loads, particularly
during dormancy. Moreover, irrespective of whether there is a
direct neuroinvasive effect, or indirect activation of microglial
cells and astrocytes through systemic inflammatory and
neuroinflammatory processes, there is currently a lack of
appropriate methodological approaches to sufficiently understand
the progress of these neurotropic infections and the ensuing
pathophysiology.
Secondly, the neurodevelopmental model of
psychiatric disease suggests that transient viral infections at
critical stages during early childhood or the prenatal period may
lead to psychiatric disease. However, obtaining and preserving
appropriate samples from these critical neurodevelopmental periods
is difficult, especially when such viral infections are
asymptomatic or subclinical.
Thirdly, the complexity of the virome and the
existence of thousands of viral species, a number of which have not
yet been fully described, adds to the challenge. The knowledge of
viral diversity remains incomplete and numerous novel viruses are
yet to be discovered. Additionally, RNA viruses evolve rapidly and
novel strains with potential neuropsychiatric virulence, such
asSARS-CoV-2, may emerge.
Finally, psychiatric diseases are multifactorial
disorders involving a complex mixture of genetic and environmental
influences. Viral agents may prime an organism for the development
of psychiatric disease rather than causing it directly.
Establishing the viral connection is challenging, deviating
significantly from a simplistic, direct connection between
infectious cause and disease, as described by Koch's postulates
(153).
Further experimental research, including larger
population sizes, and modern molecular methodologies accompanied by
appropriate bioinformatics analysis, is essential for elucidating
the complex interactions among genes, pathogens and the immune
system in the etiology of psychiatric disease. The advent and
further development of multi-omics technology, which collectively
includes genomics, transcriptomics, proteomics, metabolomics,
interactomics, epigenomics and pharmacogenomics, may enhance
understanding of virus-host interactions; decipher the
pathophysiological mechanisms that underlie the role of viruses in
various diseases, such as psychiatric disease; identify effective
antiviral treatments and vaccines; and identify reliable biomarkers
of viral infections (154).
Single-cell analysis by multi-omics is particularly promising in
the identification of molecular characteristics of
pathophysiological processes within specific cell types, which, for
example, may be infected by a virus, and how these characteristics
are associated with a specific phenotype, such as a psychiatric
disease. However, there is still the challenge of analyzing the
large amount of biological information collected by single-cell
multi-omics, especially when comparison of data from different cell
types is made, something which greatly relies on the development of
appropriate bioinformatics tools. Moreover, these elaborate
molecular methods need to be supplemented with accurate in
vitro models to obtain a better understanding of the
pathophysiology of viral infections in association with
neuropsychiatric disorders.
Most of the currently available information about
the course of viral pathogenesis in the CNS is limited since it
originates only from lesions analyzed by MRI data and postmortem
histology (155). Despite the
fact that experiments with organoids have been promising for the
evaluation of viral dissemination between neuronal cells and the
activation of astrocytes, their low level of complexity cannot
simulate all cellular factors involved in neuroinvasion and
inflammation (156). Moreover,
the currently available experimental animal models may not fully
replicate the course of neuropathologic conditions in humans for a
very important reason. These experiments are carried out under
carefully controlled laboratory conditions and may not accurately
represent the complexity of those interactions between the human
host, the environment and the viruses that may lead to psychiatric
disease. More elaborate models are needed that will take into
account as many experimental parameters as possible and will more
accurately represent these complex interactions. The continuous
emergence of novel viral variants with unprecedented virulence
potential, as was evident with the recent COVID-19 pandemic, may
also add more complexity to the design of satisfactory experimental
models. Further studies are needed on the study of viral tropism,
and progress before the onset of symptoms and during the course of
infection and, secondly, on the identification of genetic and
epigenetic host factors that may lead to CNS impairment and
subsequent neuropsychiatric disease (91). More extensive, prospective studies
are also needed to follow the sequelae of acute viral infections of
the CNS in conjunction with other environmental stimuli that may
influence the development of psychiatric diseases. By addressing
these challenges, research may move closer to understanding the
role of viruses in psychiatric disorders, potentially developing
targeted interventions that could mitigate their impact.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
NS and CA were major contributors in writing the
manuscript. SP, AT, EA and SK were contributors in writing and
critical revision of the manuscript. DAS was a contributor in
critical revision of the manuscript. ER was a major contributor in
providing the subject of this review manuscript, as well as in
writing and critical revision of the manuscript. Data
authentication is not applicable. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The authors declare that they have no competing interests
References
|
1
|
Nasir A, Forterre P, Kim KM and
Caetano-Anollés G: The distribution and impact of viral lineages in
domains of life. Front Microbiol. 5:1942014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Call L, Nayfach S and Kyrpides NC:
Illuminating the virosphere through global metagenomics. Annu Rev
Biomed Data Sci. 4:369–391. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gregory AC, Zayed AA, Conceição-Neto N,
Temperton B, Bolduc B, Alberti A, Ardyna M, Arkhipova K, Carmichael
M, Cruaud C, et al: Marine DNA viral macro- and microdiversity from
pole to pole. Cell. 177:1109–1123.e14. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neri U, Wolf YI, Roux S, Camargo AP, Lee
B, Kazlauskas D, Chen IM, Ivanova N, Zeigler Allen L, Paez-Espino
D, et al: Expansion of the global RNA virome reveals diverse clades
of bacteriophages. Cell. 185:4023–4037.e18. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohapatra S and Menon NG: Factors
responsible for the emergence of novel viruses: An emphasis on
SARS-CoV-2. Curr Opin Environ Sci Health. 27:1003582022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marie V and Gordon ML: The (Re-)emergence
and spread of viral zoonotic disease: A perfect storm of human
ingenuity and stupidity. Viruses. 15:16382023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Piret J and Boivin G: Pandemics throughout
history. Front Microbiol. 11:6317362021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davis HE, McCorkell L, Vogel JM and Topol
EJ: Long COVID: Major findings, mechanisms and recommendations. Nat
Rev Microbiol. 21:133–146. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boufidou F, Medić S, Lampropoulou V,
Siafakas N, Tsakris A and Anastassopoulou C: SARS-CoV-2
reinfections and long COVID in the post-omicron phase of the
pandemic. Int J Mol Sci. 24:129622023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Castanares-Zapatero D, Chalon P, Kohn L,
Dauvrin M, Detollenaere J, Maertens de Noordhout C, Primus-de Jong
C, Cleemput I and Van den Heede K: Pathophysiology and mechanism of
long COVID: A comprehensive review. Ann Med. 54:1473–1487. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Virgin HW, Wherry EJ and Ahmed R:
Redefining chronic viral infection. Cell. 138:30–50. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grandi N and Tramontano E: HERV envelope
proteins: Physiological role and pathogenic potential in cancer and
autoimmunity. Front Microbiol. 9:4622018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang M, Liang JQ and Zheng S:
Expressional activation and functional roles of human endogenous
retroviruses in cancers. Rev Med Virol. 29:e20252019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luganini A and Gribaudo G: Retroviruses of
the human virobiota: The recycling of viral genes and the resulting
advantages for human hosts during evolution. Front Microbiol.
11:11402020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang G and Bushman FD: The human virome:
Assembly, composition and host interactions. Nat Rev Microbiol.
19:514–527. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seo SU and Kweon MN: Virome-host
interactions in intestinal health and disease. Curr Opin Virol.
37:63–71. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Os J, Rutten BP and Poulton R:
Gene-environment interactions in schizophrenia: Review of
epidemiological findings and future directions. Schizophr Bull.
34:1066–1082. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pearce BD: Viruses and psychiatric
disorders. Siegel A and Zalcman SS: The Neuroimmunological Basis of
Behavior and Mental Disorders. Springer; Boston, MA: pp. 383–410.
2009, View Article : Google Scholar
|
|
19
|
Hobbs JA: The virus connection: How
viruses affect psychiatric pathologies. Psychiatr Times.
33:2016.PubMed/NCBI
|
|
20
|
Müller N and Schwarz MJ: Immune system and
schizophrenia. Curr Immunol Rev. 6:213–220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fatemi SH and Folsom TD: The
neurodevelopmental hypothesis of schizophrenia, revisited.
Schizophr Bull. 35:528–548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blomström Å, Karlsson H, Svensson A,
Frisell T, Lee BK, Dal H, Magnusson C and Dalman C: Hospital
admission with infection during childhood and risk for psychotic
illness-a population-based cohort study. Schizophr Bull.
40:1518–1525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hickie IB, Banati R, Stewart CH, Stewart
CH and Lloyd AR: Are common childhood or adolescent infections risk
factors for schizophrenia and other psychotic disorders? Med J
Aust. 190 (S4):S17–S21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang W and Chikritzhs T: Early childhood
infections and risk of schizophrenia. Psychiatry Res. 200:214–217.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maynard TM, Sikich L, Lieberman JA and
LaMantia AS: Neural development, cell-cell signaling, and the
‘two-hit’ hypothesis of schizophrenia. Schizophr Bull. 27:457–476.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Debost JCPG, Larsen JT, Munk-Olsen T,
Mortensen PB, Meyer U and Petersen L: Joint effects of exposure to
prenatal infection and peripubertal psychological trauma in
schizophrenia. Schizophr Bull. 43:171–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Raison CL, Borisov AS, Majer M, Drake DF,
Pagnoni G, Woolwine BJ, Vogt GJ, Massung B and Miller AH:
Activation of central nervous system inflammatory pathways by
interferon-alpha: Relationship to monoamines and depression. Biol
Psychiatry. 65:296–303. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rocamonde B, Hasan U, Mathieu C and
Dutartre H: Viral-induced neuroinflammation: Different mechanisms
converging to similar exacerbated glial responses. Front Neurosci.
17:11082122023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niranjan R, Muthukumaravel S and
Jambulingam P: The involvement of neuroinfammation in dengue viral
disease: Importance of innate and adaptive immunity.
Neuroimmunomodulation. 26:111–118. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bogovic P and Strle F: Tick-borne
encephalitis: A review of epidemiology, clinical characteristics,
and management. World J Clin Cases. 3:430–441. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lannes N, Neuhaus V, Scolari B,
Kharoubi-Hess S, Walch M, Summerfield A and Filgueira L:
Interactions of human microglia cells with Japanese encephalitis
virus. Virol J. 14:82017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shao Q, Herrlinger S, Yang SL, Lai F,
Moore JM, Brindley MA and Chen JF: Zika virus infection disrupts
neurovascular development and results in postnatal microcephaly
with brain damage. Development. 143:4127–4136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Sousa JR, Azevedo RSS, Martins Filho
AJ, Araujo MTF, Moutinho ERC, Baldez Vasconcelos BC, Cruz ACR,
Oliveira CS, Martins LC, Baldez Vasconcelos BH, et al: Correlation
between apoptosis and in situ immune response in fatal cases of
microcephaly caused by Zika virus. Am J Pathol. 188:2644–2652.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Zhao L, Luo Z, Zhang Y, Lv L, Zhao
J, Sui B, Huang F, Cui M, Fu ZF and Zhou M: Interferon-λ attenuates
rabies virus infection by inducing interferon-stimulated genes and
alleviating neurological inflammation. Viruses. 12:4052020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Marsland AL, Petersen KL, Sathanoori R,
Muldoon MF, Neumann SA, Ryan C, Flory JD and Manuck SB:
Interleukin-6 covaries inversely with cognitive performance among
middle-aged community volunteers. Psychosom Med. 68:895–903. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dickerson SS, Gable SL, Irwin MR, Aziz N
and Kemeny ME: Social-evaluative threat and proinflammatory
cytokine regulation: An experimental laboratory investigation.
Psychol Sci. 20:1237–1244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Metcalf SA, Jones PB, Nordstrom T, Timonen
M, Mäki P, Miettunen J, Jääskeläinen E, Järvelin MR, Stochl J,
Murray GK, et al: Serum C-reactive protein in adolescence and risk
of schizophrenia in adulthood: A prospective birth cohort study.
Brain BehavImmun. 59:253–259. 2017.
|
|
38
|
Müller N: Inflammation in schizophrenia:
Pathogenetic aspects and therapeutic considerations. Schizophr
Bull. 44:973–982. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khandaker GM, Pearson RM, Zammit S, Lewis
G and Jones PB: Association of serum interleukin 6 and C-reactive
protein in childhood with depression and psychosis in young adult
life: A population-based longitudinal study. JAMA Psychiatry.
71:1121–1128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maxeiner HG, Marion Schneider E, Kurfiss
ST, Brettschneider J, Tumani H and Bechter K: Cerebrospinal fluid
and serum cytokine profiling to detect immune control of infectious
and inflammatory neurological and psychiatric diseases. Cytokine.
69:62–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sarma JV and Ward PA: The complement
system. Cell Tissue Res. 343:227–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Keshavan M, Lizano P and Prasad K: The
synaptic pruning hypothesis of schizophrenia: Promises and
challenges. World Psychiatry. 19:110–111. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Druart M and Le Magueresse C: Emerging
roles of complement in psychiatric disorders. Front Psychiatry.
10:5732019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mondelli V, Di Forti M, Morgan BP, Murray
RM, Pariante CM and Dazzan P: Baseline high levels of complement
component 4 predict worse clinical outcome at 1-year follow-up in
first-episode psychosis. Brain BehavImmun. 88:913–915.
2020.PubMed/NCBI
|
|
45
|
Tomonaga K: Virus-induced neurobehavioral
disorders: Mechanisms and implications. Trends Mol Med. 10:71–77.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kotsiri I, Resta P, Spyrantis A,
Panotopoulos C, Chaniotis D, Beloukas A and Magiorkinis E: Viral
infections and schizophrenia: A comprehensive review. Viruses.
15:13452023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sayeh A, Cheikh CB, Mrad M, Lakhal N,
Gritli N, Galelli S, Oumaya A and Fekih-Mrissa N: Association of
HLA-DR/DQ polymorphisms with schizophrenia in Tunisian patients.
Ann Saudi Med. 34:503–507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Prasard S, Semwal P, Deshpande S, Bhatia
T, Nimgaonkar VL and Thelma BK: Molecular genetics of
schizophrenia: Past, present and future. J Biosci. 27 (Suppl
1):S35–S52. 2002. View Article : Google Scholar
|
|
49
|
Wright P, Donaldson PT, Underhill JA,
Choudhuri K, Doherty DG and Murray RM: Genetic association of the
HLA DRB1 gene locus on chromosome 6p21.3 with schizophrenia. Am J
Psychiatry. 153:1530–1533. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nimgaonkar VL, Rudert WA, Zhang X, Trucco
M and Ganguli R: Negative association of schizophrenia with HLA
DQB1*0602: Evidence from a second African-American cohort.
Schizophr Res. 23:81–86. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
James LM, Charonis SA and Georgopoulos AP:
Schizophrenia, human leukocyte antigen (HLA), and herpes viruses:
Immunogenetic associations at the population level. Neurosci
Insights. 18:263310552311664112023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wongchitrat P, Chanmee T and Govitrapong
P: Molecular mechanisms associated with neurodegeneration of
neurotropic viral infection. Mol Neurobiol. 61:2881–2903. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X,
Zhang N, Shi L, Qin CF and Xu Z: Zika virus disrupts neural
progenitor development and leads to microcephaly in mice. Cell Stem
Cell. 19:120–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tiwari SK, Dang JW, Lin N, Qin Y, Wang S
and Rana TM: Zika virus depletes neural stem cells and evades
selective autophagy by suppressing the Fanconi anemia protein
FANCC. EMBO Rep. 21:e491832020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gabriel E, Ramani A, Karow U, Gottardo M,
Natarajan K, Gooi LM, Goranci-Buzhala G, Krut O, Peters F, Nikolic
M, et al: Recent Zika virus isolates induce premature
differentiation of neural progenitors in human brain organoids.
Cell Stem Cell. 20:397–406.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Crunfi F, Carregari VC, Veras FP, Silva
LS, Nogueira MH, Antunes ASLM, Vendramini PH, Valença AGF,
Brandão-Teles C, Zuccoli GDS, et al: Morphological, cellular, and
molecular basis of brain infection in COVID-19 patients. Proc Natl
Acad Sci USA. 119:e22009601192022. View Article : Google Scholar
|
|
57
|
de Oliveira LG, de Souza Angelo Y,
Yamamoto P, Carregari VC, Crunfli F, Reis-de-Oliveira G, Costa L,
Vendramini PH, Duque ÉA, Dos Santos NB, et al: SARS-CoV-2 infection
impacts carbon metabolism and depends on glutamine for replication
in Syrian hamster astrocytes. J Neurochem. 163:113–132. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fan Y and He JJ: HIV-1 Tat induces
unfolded protein response and endoplasmic reticulum stress in
astrocytes and causes neurotoxicity through glial fbrillary acidic
protein (GFAP) activation and aggregation. J Biol Chem.
291:22819–22829. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhou BY, Liu Y, Kim BO, Xiao Y and He JJ:
Astrocyte activation and dysfunction and neuron death by HIV-1 Tat
expression in astrocytes. Mol Cell Neurosci. 27:296–305. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Teodorof-Diedrich C and Spector SA: Human
immunodeficiency virus type 1 gp120 and Tat induce mitochondrial
fragmentation and incomplete mitophagy in human neurons. J Virol.
92:e00993–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
McGavern DB and Kang SS: Illuminating
viral infections in the nervous system. Nat Rev Immunol.
11:318–329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bhatia MS, Gautam P and Jhanjee A:
Psychiatric morbidity in patients with chikungunya Fever: First
report from India. J Clin Diagn Res. 9:VC01–VC03. 2015.PubMed/NCBI
|
|
63
|
Figueiredo T, Dias da Costa M and
Segenreich D: Manic episode after a chikungunya virus infection in
a bipolar patient previously stabilized with valproic acid. J Clin
Psychopharmacol. 38:395–397. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Samaan Z, McDermid Vaz S, Bawor M, Potter
TH, Eskandarian S and Loeb M: Neuropsychological impact of west
nile virus infection: An extensive neuropsychiatric assessment of
49 cases in Canada. PLoS One. 11:e01583642016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Srivastava R, Kalita J, Khan MY and Misra
UK: Free radical generation by neurons in rat model of Japanese
encephalitis. Neurochem Res. 34:2141–2146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
James HJ, Sharer LR, Zhang Q, Wang HG,
Epstein LG, Reed JC and Gelbard HA: Expression of caspase-3 in
brains from paediatric patients with HIV-1 encephalitis.
Neuropathol Appl Neurobiol. 25:380–386. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Du X, Wang H, Xu F, Huang Y, Liu Z and Liu
T: Enterovirus 71 induces apoptosis of SH-SY5Y human neuroblastoma
cells through stimulation of endogenous microRNA let-7b expression.
Mol Med Rep. 12:953–959. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Jackson AC and Rossiter JP: Apoptosis
plays an important role in experimental rabies virus infection. J
Virol. 71:5603–5607. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Rutherford M and Jackson AC: Neuronal
apoptosis in immunodeficient mice infected with the challenge virus
standard strain of rabies virus by intracerebral inoculation. J
Neurovirol. 10:409–413. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jackson AC: Apoptosis in experimental
rabies in bax-deficient mice. Acta Neuropathol. 98:288–294. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Fu ZF and Jackson AC: Neuronal dysfunction
and death in rabies virus infection. J Neurovirol. 11:101–106.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kojima D, Park CH, Tsujikawa S, Kohara K,
Hatai H, Oyamad T, Noguchi A and Inoue S: Lesions of the central
nervous system induced by intracerebral inoculation of BALB/c mice
with rabies virus (CVS-11). J Vet Med Sci. 72:1011–1016. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Parquet MC, Kumatori A, Hasebe F, Morita K
and Igarashi A: West Nile virus-induced bax-dependent apoptosis.
FEBS Lett. 500:17–24. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kleinschmidt MC, Michaelis M, Ogbomo H,
Doerr HW and Cinatl J Jr: Inhibition of apoptosis prevents West
Nile virus induced cell death. BMC Microbiol. 7:492007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
van Marle G, Antony J, Ostermann H, Dunham
C, Hunt T, Halliday W, Maingat F, Urbanowski MD, Hobman T, Peeling
J and Power C: West Nile virus-induced neuroinflammation: Glial
infection and capsid protein-mediated neurovirulence. J Virol.
81:10933–10949. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yang JS, Ramanathan MP, Muthumani K, Choo
AY, Jin SH, Yu QC, Hwang DS, Choo DK, Lee MD, Dang K, et al:
Induction of inflammation by West Nile virus capsid through the
caspase-9 apoptotic pathway. Emerg Infect Dis. 8:1379–1384. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ramanathan MP, Chambers JA, Pankhong P,
Chattergoon M, Attatippaholkun W, Dang K, Shah N and Weiner DB:
Host cell killing by the West Nile virus NS2B-NS3 proteolytic
complex: NS3 alone is sufficient to recruit caspase-8-based
apoptotic pathway. Virology. 345:56–72. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Swarup V, Das S, Ghosh S and Basu A: Tumor
necrosis factor receptor-1-induced neuronal death by TRADD
contributes to the pathogenesis of Japanese encephalitis. J
Neurochem. 103:771–783. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mishra MK and Basu A: Minocycline
neuroprotects, reduces microglial activation, inhibits caspase 3
induction, and viral replication following Japanese encephalitis. J
Neurochem. 105:1582–1595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chen T, Tu S, Ding L, Jin M, Chen H and
Zhou H: The role of autophagy in viral infections. J Biomed Sci.
30:52023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Huang SC, Chang CL, Wang PS, Tsai Y and
Liu HS: Enterovirus 71-induced autophagy detected in vitro and in
vivo promotes viral replication. J Med Virol. 81:1241–1252. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liu ZW, Zhuang ZC, Chen R, Wang XR, Zhang
HL, Li SH, Wang ZY and Wen HL: Enterovirus 71 VP1 protein regulates
viral replication in SH-SY5Y cells via the mTOR autophagy signaling
pathway. Viruses. 12:112019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Too IH, Yeo H, Sessions OM, Yan B, Libau
EA, Howe JLC, Lim ZQ, Suku-Maran S, Ong WY, Chua KB, et al:
Enterovirus 71 infection of motor neuron-like NSC-34 cells
undergoes a non-lytic exit pathway. Sci Rep. 6:369832016.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Fields JA, Metcalf J, Overk C, Adame A,
Spencer B, Wrasidlo W, Florio J, Rockenstein E, He JJ and Masliah
E: The anticancer drug sunitinib promotes autophagyand protects
from neurotoxicity in an HIV-1 Tat model of neurodegeneration. J
Neurovirol. 23:290–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cheney L, Guzik H, Macaluso FP, Macian F,
Cuervo AM and Berman JW: HIV Nef and antiretroviral therapy have an
inhibitory effect on autophagy in human astrocytes that may
contribute to HIV-associated neurocognitive disorders. Cells.
9:14262020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fields J, Dumaop W, Elueteri S, Campos S,
Serger E, Trejo M, Kosberg K, Adame A, Spencer B, Rockenstein E, et
al: HIV-1 Tat alters neuronal autophagy by modulating autophagosome
fusion to the lysosome: Implications for HIV-associated
neurocognitive disorders. J Neurosci. 35:1921–1938. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Fischer M, Lindsey N, Staples JE and Hills
S; Centers for Disease Control and Prevention (CDC), : Japanese
encephalitis vaccines: Recommendations of the advisory committee on
immunization practices (ACIP). MMWR Recomm Rep. 59:1–27. 2010.
|
|
88
|
Unni SK, Růžek D, Chhatbar C, Mishra R,
Johri MK and Singh SK: Japanese encephalitis virus: From genome to
infectome. Microbes Infect. 13:312–321. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hu S, Sheng WS, Schachtele SJ and
Lokensgard JR: Reactive oxygen species drive herpes simplex virus
(HSV)-1-induced proinflammatory cytokine production by murine
microglia. J Neuroinflammation. 8:1232011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kavouras JH, Prandovszky E, Valyi-Nagy K,
Kovacs SK, Tiwari V, Kovacs M, Shukla D and Valyi-Nagy T: Herpes
simplex virus type 1 infection induces oxidative stress and the
release of bioactive lipid peroxidation by-products in mouse P19N
neural cell cultures. J Neurovirol. 13:416–425. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gorska AM and Eugenin EA: The glutamate
system as a crucial regulator of CNS toxicity and survival of HIV
reservoirs. Front Cell Infect Microbiol. 10:2612020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Alandijany T, Kammouni W, Roy Chowdhury
SK, Fernyhough P and Jackson AC: Mitochondrial dysfunction in
rabies virus infection of neurons. J Neurovirol. 19:537–549. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ghosh Roy S, Sadigh B, Datan E, Lockshin
RA and Zakeri Z: Regulation of cell survival and death during
flavivirus infections. World J Biol Chem. 5:93–105. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Mukherjee S, Singh N, Sengupta N, Fatima
M, Seth P, Mahadevan A, Shankar SK, Bhattacharyya A and Basu A:
Japanese encephalitis virus induces human neural stem/progenitor
cell death by elevating GRP78, PHB and hnRNPC through ER stress.
Cell Death Dis. 8:e25562017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Tan Z, Zhang W, Sun J, Fu Z, Ke X, Zheng
C, Zhang Y, Li P, Liu Y, Hu Q, et al: ZIKV infection activates the
IRE1-XBP1 and ATF6 pathways of unfolded protein response in neural
cells. J Neuroinflammation. 15:2752018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hu DD, Mai JN, He LY, Li PQ, Chen WX, Yan
JJ, Zhu WD, Deng L, Wei D, Liu DH, et al: Glucocorticoids prevent
enterovirus 71 capsid protein VP1 induced calreticulin surface
exposure by alleviating neuronal ER stress. Neurotox Res.
31:204–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Cheng ML, Weng SF, Kuo CH and Ho HY:
Enterovirus 71 induces mitochondrial reactive oxygen species
generation that is required for efficient replication. PLoS One.
9:e1132342014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Guo L, Xing Y, Pan R, Jiang M, Gong Z, Lin
L, Wang J, Xiong G and Dong J: Curcumin protects microglia and
primary rat cortical neurons against HIV-1 gp120-mediated
inflammation and apoptosis. PLoS One. 8:e705652013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Shah A, Kumar S, Simon SD, Singh DP and
Kumar A: HIV gp120- and methamphetamine-mediated oxidative stress
induces astrocyte apoptosis via cytochrome P450 2E1. Cell Death
Dis. 4:e8502013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ivanov AV, Valuev-Elliston VT, Ivanova ON,
Kochetkov SN, Starodubova ES, Bartosch B and Isaguliants MG:
Oxidative stress during HIV infection: Mechanisms and consequences.
Oxid Med Cell Longev. 2016:89103962016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Simanjuntak Y, Liang JJ, Lee YL and Lin
YL: Japanese encephalitis virus exploits dopamine D2
receptor-phospholipase C to target dopaminergic human neuronal
cells. Front Microbiol. 8:6512017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Damsgaard J, Hjerrild S, Andersen H and
Leutscher PDC: Long-term neuropsychiatric consequences of aseptic
meningitis in adult patients. Infect Dis (Lond). 47:357–363. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Omland LH, Vestergaard BF and Wandall JH:
Herpes simplex virus type 2 infections of the central nervous
system: A retrospective study of 49 patients. Scand J Infect Dis.
40:59–62. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Persson A, Bergström T, Lindh M, Namvar L
and Studahl M: Varicella-zoster virus CNS disease-viral load,
clinical manifestations and sequels. J Clin Virol. 46:249–253.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Sittinger H, Müller M, Schweizer I and
Merkelbach S: Mild cognitive impairment after viral meningitis in
adults. J Neurol. 249:554–560. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Du C, Li G and Han G: Biosafety and mental
health: Virus induced cognitive decline. Biosaf Health. 5:159–167.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Hare EH, Price JS and Slater E:
Schizophrenia and season of birth. Br J Psychiatry. 120:124–125.
1972. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Machón RA, Mednick SA and Schulsinger F:
The interaction of seasonality, place of birth, genetic risk and
subsequent schizophrenia in a high risk sample. Br J Psychiatry.
143:383–388. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Pallast EG, Jongbloet PH, Straatman HM and
Zielhuis GA: Excess seasonality of births among patients with
schizophrenia and seasonal ovopathy. Schizophr Bull. 20:269–276.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Susser ES, Brown AS and Gorman JM:
Prenatal exposures in schizophrenia. American Psychiatric
Association; 1999
|
|
111
|
Mednick SA, Machon RA, Huttunen MO and
Bonett D: Adult schizophrenia following prenatal exposure to an
influenza epidemic. Arch Gen Psychiatry. 45:189–192. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Brown AS, Begg MD, Gravenstein S, Schaefer
CA, Wyatt RJ, Bresnahan M, Babulas VP and Susser ES: Serologic
evidence of prenatal influenza in the etiology of schizophrenia.
Arch Gen Psychiatry. 61:774–780. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Bonkovsky HL, Snow KK, Malet PF,
Back-Madruga C, Fontana RJ, Sterling RK, Kulig CC, Di Bisceglie AM,
Morgan TR, Dienstag JL, et al: Health-related quality of life in
patients with chronic hepatitis C and advanced fibrosis. J Hepatol.
46:420–431. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Gragnani L, Cerretelli G, Lorini S, Steidi
C, Giovannelli A, Monti M, Petraccia L, Sadalla S, Urraro T, Caini
P, et al: Interferon-free therapy in hepatitis C virus mixed
cryoglobulinaemia: A prospective, controlled, clinical and quality
of live analysis. Aliment Pharmacol Ther. 48:440–450. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Torrey EF, Bowler AE and Rawlings R: An
influenza epidemic and the seasonality of schizophrenic births.
Kurstak: Psychiatry and Biological Factors. Springer; Boston, MA:
pp. 109–116. 1991, View Article : Google Scholar
|
|
116
|
Nicoll MP, Proença JT and Efstathiou S:
The molecular basis of herpes simplex virus latency. FEMS Microbiol
Rev. 36:684–705. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Torrey EF, Leweke MF, Schwarz MJ, Mueller
N, Bachmann S, Schroeder J, Dickerson F and Yolken RH:
Cytomegalovirus and schizophrenia. CNS Drugs. 20:879–885. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Prasad KMR, Shirts BH, Yolken RH, Keshavan
MS and Nimgaonkar VL: Brain morphological changes associated with
exposure to HSV1 in first-episode schizophrenia. Mol Psychiatry.
12:105–113. 12007. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Burgdorf KS, Trabjerg BB, Pedersen MG,
Nissen J, Banasik K, Pedersen OB, Sørensen E, Nielsen KR, Larsen
MH, Erikstrup C, et al: Large-scale study of toxoplasma and
cytomegalovirus shows an association between infection and serious
psychiatric disorders. Brain BehavImmun. 79:152–158. 2019.
|
|
120
|
Dickerson F, Jones-Brando L, Ford G,
Genovese G, Stallings C, Origoni A, O'Dushlaine C, Katsafanas E,
Sweeney K, Khushalani S and Yolken R: Schizophrenia is associated
with an aberrant immune response to epstein-barr virus. Schizophr
Bull. 45:1112–1119. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Dickerson FB, Boronow JJ, Stallings C,
Origoni AE, Ruslanova I and Yolken RH: Association of serum
antibodies to herpes simplex virus 1 with cognitive deficits in
individuals with schizophrenia. Arch Gen Psychiatry. 60:466–472.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Thomas P, Bhatia T, Gauba D, Wood J, Long
C, Prasad K, Dickerson FB, Gur RE, Gur RC, Yolken RH, et al:
Exposure to herpes simplex virus, type 1 and reduced cognitive
function. J Psychiatr Res. 47:1680–1685. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yolken RH, Torrey EF, Lieberman JA, Yang S
and Dickerson FB: Serological evidence of exposure to herpes
simplex virus type 1 is associated with cognitive deficits in the
CATIE schizophrenia sample. Schizophr Res. 128:61–65. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Krause D, Matz J, Weidinger E, Wagner J,
Wildenauer A, Obermeier M, Riedel M and Müller N: The association
of infectious agents and schizophrenia. World J Biol Psychiatry.
11:739–743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Waechter R, Ingraham E, Evans R, Cudjoe N,
Krystosik A, Isaac R, Watts A, Noël T, Landon B, Fernandes M, et
al: Pre and postnatal exposure to chikungunya virus does not affect
child neurodevelopmental outcomes at two years of age. PLoS Negl
Trop Dis. 14:e00085462020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Mazzaro C, Quartuccio L, Adinolfi LE,
Roccatello D, Pozzato G, Nevola R, Tonizzo M, Gitto S, Andreone P
and Gattei V: A review on extrahepatic manifestations of chronic
hepatitis C virus infection and the impact of direct-acting
antiviral therapy. Viruses. 13:22492021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Fishman SL, Murray JM, Eng FJ, Walewski
JL, Morgello S and Branch AD: Molecular and bioinformatic evidence
of hepatitis C virus evolution in brain. J Infect Dis. 197:597–607.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wilkinson J, Radkowski M and Laskus T:
Hepatitis C virus neuroinvasion: Identification of infected cells.
J Virol. 83:1312–1319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Laskus T, Radkowski M, Bednarska A,
Wilkinson J, Adair D, Nowicki M, Nikolopoulou GB, Vargas HE and
Rakela J: Detection and analysis of hepatitis C virus sequences in
cerebrospinal fluid. J Virol. 76:10064–10068. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Min S, Gandal MJ, Kopp RF, Liu C and Chen
C: No increased detection of nucleic acids of CNS-related viruses
in the brains of patients with schizophrenia, bipolar disorder, and
autism spectrum disorder. Schizophr Bull. 49:551–558. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Yolken RH, Kinnunen PM, Vapalahti O,
Dickerson F, Suvisaari J, Chen O and Sabunciyan S: Studying the
virome in psychiatric disease. Schizophr Res. 234:78–86. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Kneeland RE and Fatemi SH: Viral
infection, inflammation and schizophrenia. Prog
Neuropsychopharmacol Biol Psychiatry. 42:35–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Gu CJ, Borjabad A, Hadas E, Kelschenbach
J, Kim BH, Chao W, Arancio O, Suh J, Polsky B, McMillan J, et al:
EcoHIV infection of mice establishes latent viral reservoirs in T
cells and active viral reservoirs in macrophages that are
sufficient for induction of neurocognitive impairment. PLoS Pathog.
14:e10070612018. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Gruchot J, Herrero F, Weber-Stadlbauer U,
Meyer U and Küry P: Interplay between activation of endogenous
retroviruses and inflammation as common pathogenic mechanism in
neurological and psychiatric disorders. Brain BehavImmun.
107:242–252. 2023.
|
|
135
|
Kamitani W, Ono E, Yoshino S, Kobayashi T,
Taharaguchi S, Lee BJ, Yamashita M, Kobayashi T, Okamoto M,
Taniyama H, et al: Glial expression of Borna disease virus
phosphoprotein induces behavioral and neurological abnormalities in
transgenic mice. Proc Natl Acad Sci USA. 100:8969–8974. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Li F, Sabunciyan S, Yolken RH, Lee D, Kim
S and Karlsson H: Transcription of human endogenous retroviruses in
human brain by RNA-seq analysis. PLoS One. 14:e02073532019.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Karlsson H, Bachmann S, Schröder J,
McArthur J, Torrey EF and Yolken RH: Retroviral RNA identified in
the cerebrospinal fluids and brains of individuals with
schizophrenia. Proc Natl Acad Sci USA. 98:4634–4639. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Perron H, Hamdani N, Faucard R, Lajnef M,
Jamain S, Daban-Huard C, Sarrazin S, LeGuen E, Houenou J, Delavest
M, et al: Molecular characteristics of human endogenous retrovirus
type-W in schizophrenia and bipolar disorder. Transl Psychiatry.
2:e2012012. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Tamouza R, Meyer U, Foiselle M, Richard
JR, Wu CL, Boukouaci W, Le Corvoisier P, Barrau C, Lucas A, Perron
H and Leboyer M: Identification of inflammatory subgroups of
schizophrenia and bipolar disorder patients with HERV-W ENV
antigenemia by unsupervised cluster analysis. Transl Psychiatry.
11:3772021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Slokar G and Hasler G: Human endogenous
retroviruses as pathogenic factors in the development of
schizophrenia. Front Psychiatry. 6:1832016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Jesuthasan A, Massey F, Manji H, Zandi MS
and Wiethoff S: Emerging potential mechanisms and predispositions
to the neurological manifestations of COVID-19. J Neurol Sci.
428:1176082021. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Guedj E, Campion JY, Dudouet P, Kaphan E,
Bregeon F, Tissot-Dupont H, Guis S, Barthelemy F, Habert P,
Ceccaldi M, et al: 18F-FDG brain PET hypometabolism in
patients with long COVID. Eur J Nucl Med Mol Imaging. 48:2823–2833.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Tromans S, Kinney M, Chester V, Alexander
R, Roy A, Sander JW, Dudson H and Shankar R: Priority concerns for
people with intellectual and developmental disabilities during the
COVID-19 pandemic. BJPsych Open. 6:e1282020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Holmes EA, O'Connor RC, Perry VH, Tracey
I, Wessely S, Arsenault L, Ballard C, Christensen H, Cohen Silver
R, Everall I, et al: Multidisciplinary research priorities for the
COVID-19 pandemic: A call for action for mental health science.
Lancet Psychiatry. 7:547–560. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Ho CS, Chee CY and Ho RC: Mental health
strategies to combat the psychological impact of coronavirus
disease 2019 (COVID-19) beyond paranoia and panic. Ann Acad Med
Singap. 49:155–160. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Tsamakis K, Tsiptsios D, Ouranidis A,
Mueller C, Schizas D, Terniotis C, Nikolakakis N, Tyros G,
Kympouropoulos S, Lazaris A, et al: COVID-19 and its consequences
on mental health (Review). Exp Ther Med. 21:2442021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Efstathiou V, Stefanou MI, Demetriou M,
Siafakas N, Makris M, Tsivgoulis G, Zoumpourlis V, Kympouropoulos
SP, Tsoporis JN, Spandidos DA, et al: Long COVID and
neuropsychiatric manifestations (Review). Exp Ther Med. 23:3632022.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Efstathiou V, Stefanou MI, Demetriou M,
Siafakas N, Katsantoni E, Makris M, Tsivgoulis G, Zoumpourlis V,
Kympouropoulos SP, Tsoporis JN, et al: New-onset neuropsychiatric
sequelae and ‘long-COVID’ syndrome (Review). Exp Ther Med.
24:7052022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Yuan K, Gong YM, Liu L, Sun YK, Tian SS,
Wang YJ, Zhong Y, Zhang AY, Su SZ, Liu XX, et al: Prevalence of
posttraumatic stress disorder after infectious disease pandemics in
the twenty-first century, including COVID-19: A meta-analysis and
systematic review. Mol Psychiatry. 26:4982–4998. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Antonelli G and Cutler S: Evolution of the
Koch postulates: Towards a 21st-century understanding of microbial
infection. Clin Microbiol Infect. 22:583–584. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Taquet M, Geddes JR, Husain M, Luciano S
and Harrison PJ: 6-Month neurological and psychiatric outcomes in
236 379 survivors of COVID-19: A retrospective cohort study using
electronic health records. Lancet Psychiatry. 8:416–427. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Schou TM, Joca S, Wegener G and
Bay-Richter C: Psychiatric and neuropsychiatric sequelae of
COVID-19-A systematic review. Brain Behav Immun. 97:328–348. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Shanbehzadeh S, Tavahomi M, Zanjari N,
Ebrahimi-Takamjani I and Amiri-Arimi S: Physical and mental health
complications post-COVID-19: Scoping review. J Psychosom Res.
147:1105252021. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Chen C, Wang J, Pan D, Wang X, Xu Y, Yan
J, Wang L, Yang X, Yang M and Liu GP: Applications of multi-omics
analysis in human diseases. MedComm (2020). 4:e3152023. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Ong K, Ng K, Ng C, Tan S, Teo W, Karim N,
Kumar S and Wong KT: Neuronal infection is a major pathogenetic
mechanism and cause of fatalities in human acute nipah virus
encephalitis. Neuropathol Appl Neurobiol. 48:e128282022. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Mathieu C, Bovier FT, Ferren M, Lieberman
NAP, Predella C, Lalande A, Peddu V, Lin MJ, Addetia A, Patel A, et
al: Molecular features of the measles virus viral fusion complex
that favor infection and spread in the brain. mBio.
12:e00799212021. View Article : Google Scholar : PubMed/NCBI
|