Introduction
Ischemic stroke is a pathological condition
characterized by impaired blood supply to the brain, leading to
local ischemia and hypoxia in the brain tissue, resulting in
neurological deficits (1). Despite
significant advancements in research, treatment of ischemic stroke
remains a global challenge. Over 13 million individuals worldwide
suffer from a new stroke and 5.5 million succumb to stroke each
year. Stroke is the second leading cause of death and disability
globally, with an associated economic burden exceeding $891 billion
and continuing to increase annually (2,3).
Ischemic stroke represents the most prevalent form of stroke,
accounting for 65.3% of new stroke cases globally; it is associated
with a significant economic burden and social impact on a global
scale (4). The primary treatment
for ischemic stroke is intravenous or mechanical thrombolysis,
which rapidly restores blood flow to the affected brain area and
reduces disability. However, numerous patients miss the therapeutic
window, and some who receive treatment still experience infarcts
(5). Therefore, new therapeutic
approaches are needed in the current situation. Pathophysiological
changes following ischemic stroke involve inflammation, abnormal
activation of immune cells, ionic imbalance and blood-brain barrier
(BBB) dysfunction. Although extensive researches have been
conducted on neurological damage after ischemic stroke, the exact
mechanism remains unclear (6,7).
Atherosclerosis is a major etiological factor in the
pathogenesis of ischemic stroke, and aortic atherosclerotic
cerebral infarction is the most prevalent form of this condition.
It is characterized by the formation of plaques within the arterial
wall, which can lead to narrowing or complete obstruction of the
blood vessels, precipitating an ischemic event (8). Following the onset of cerebral
ischemia, the inflammatory response can be initiated and amplified
rapidly, resulting in a substantial elevation of inflammatory
cytokines and exacerbation of brain tissue damage, which may lead
to various complications. The BBB is instrumental in sustaining
homeostasis of the central nervous system (CNS). The robust
inflammatory response that follows a stroke impairs the integrity
of the BBB, worsens the clinical course and negatively affects
prognosis (9).
Annexin-A1 (ANXA1) is an important member of the
annexin superfamily, consisting of 346 amino acids. It is widely
distributed and expressed in eosinophils, neutrophils, monocytes,
lymphocytes and endothelial cells as well as in the heart, brain,
kidney, lung, vascular tissues and other cells (10,11).
ANXA1 exerts a wide range of effects, including inhibition of
cytokine release, blocking leukocyte recruitment, stimulation of
phagocytosis, promotion of apoptosis and reduction of vascular
permeability (12). ANXA1 has
gained increasing attention in recent years owing to its
significant role in various diseases, including ischemic stroke
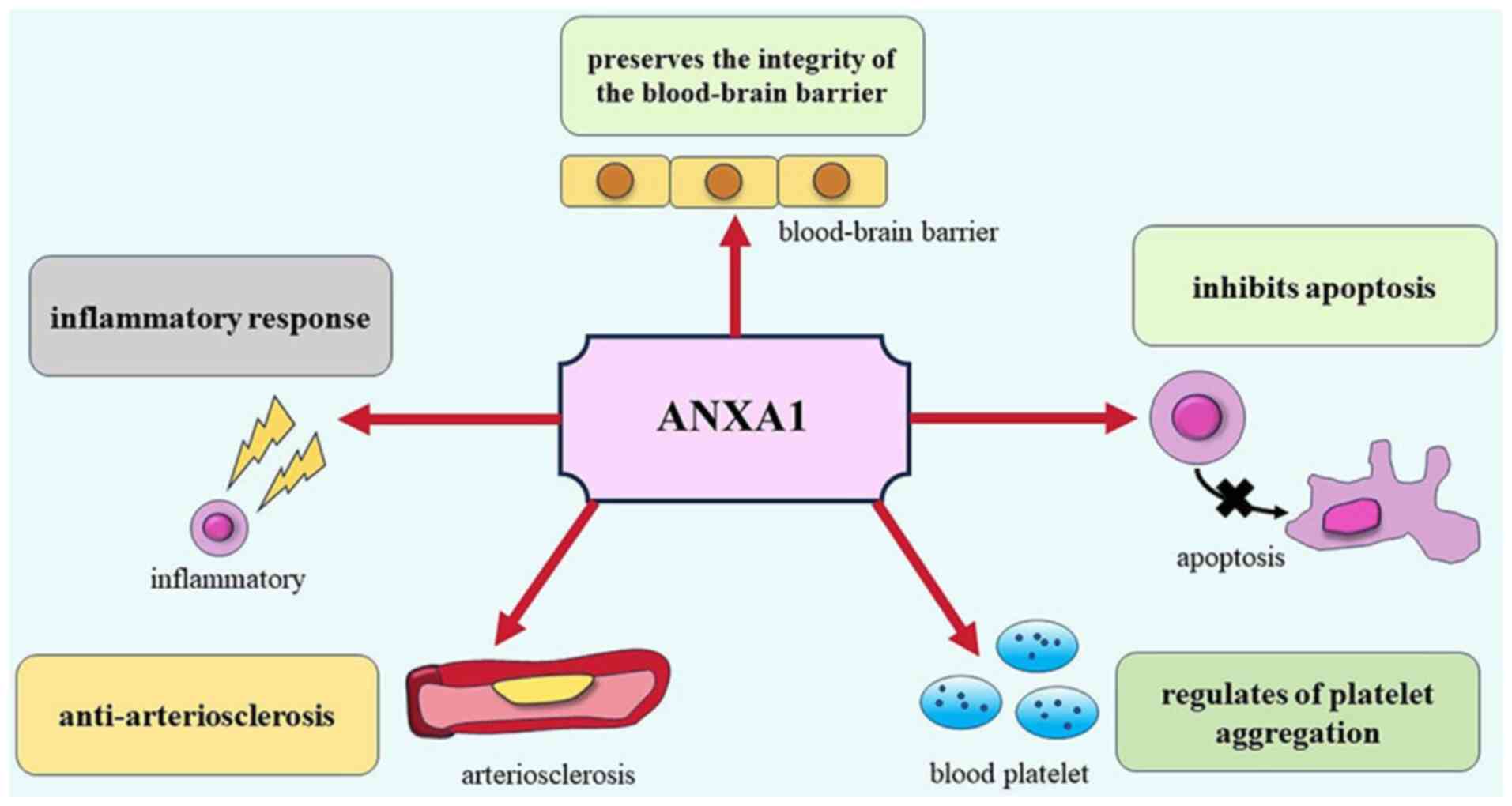
(Fig. 1). The present review aimed
to summarize the roles of ANXA1 in various aspects of ischemic
stroke, provide new insights into the mechanism of nerve injury
following ischemic stroke, and explore the potential of ANXA1 as a
novel therapeutic target for ischemic stroke.
The structure and biological function
of Annexins
When members of the annexin family were first
discovered in the late 1970s and the early 1980s, they were
assigned a multitude of disparate nomenclature, each reflecting the
individual biochemical properties of the respective proteins.
However, as techniques for protein sequence analysis, cDNA
sequencing, and gene cloning have advanced, researchers have
recognized that these proteins share key biochemical properties, as
well as gene structure and sequence features. To unify the
terminology and resolve the confusion surrounding the naming of
these proteins, the term ‘annexin’ was introduced (13). The term ‘annexin’ H was derived
from the Greek word ‘annex’, meaning ‘union’ or ‘binding’, this
nomenclature encapsulates the fundamental attribute of the Annexin
family, namely their universal capacity to interact with biological
membranes. Moreover, this designation reflects the collective
objective of pioneering researchers who independently investigated
these proteins, seeking a scaffolding protein capable of acting as
a conduit between cellular structures.
Annexins are calcium-dependent phospholipid-binding
proteins found in a wide variety of tissues and cells in
eukaryotes. It is a large family comprising of 13 proteins with
similar structural features (14,15).
They are characterized by one or two homologous carboxyl-terminal
‘cores’, each consisting of four sequence repeats involved in
membrane binding. Consequently, annexins are mainly differentiated
by their relatively short non-homologous amino-terminal sequence
(16). Members of the annexin
superfamily are involved in a wide range of cellular activities,
including cell division, apoptosis, vesicular transport, calcium
signaling and growth regulation, contributing to overall cell
functioning (13).
Annexins can be classified into five categories A-E,
based on their molecular structure, evolutionary relationships and
chromosomal localization. Annexin A is expressed in vertebrate
cells, annexin B in invertebrate cells, annexin C in mononuclear
eukaryotes and fungi, annexin D in plants, and annexin E in
prokaryotic cells (17). The
annexin A subfamily comprises 12 members, specifically annexins
A1-A11 and A13 (18). Notably,
although typically numbered sequentially, annexin A12 is absent
from the annexin A family, potentially due to gene loss or other
evolutionary processes. Such discrepancies in gene nomenclature are
not uncommon in biological classifications.
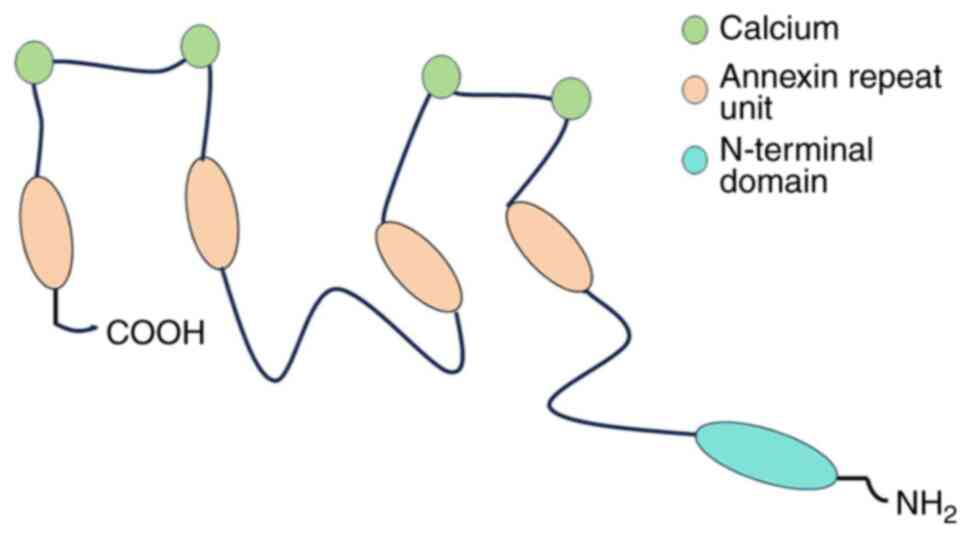
From a molecular perspective, members of the annexin
A family consist of two main structural domains; a highly conserved
C-terminal domain and a relatively variable N-terminal domain. The
C-terminal domain, which serves as the central backbone of the
proteins, contains four annexin repeat units (eight in ANXA6) that
are tightly stacked through hydrophobic interactions, forming the
characteristic ‘type 2’ calcium binding site that stabilize the
overall protein structure (19).
By contrast, the N-terminal domain, also known as the tail
structural domain, exhibits significant variability within the
annexin A family of proteins. This domain contains several
post-translational modification sites unique to each family member,
conferring a high degree of specificity in protein-protein
interactions and binding to a wide range of ligands (20) (Fig.
2). As a result, the N-terminal domains of each member not only
differ in spatial location but also exhibit specificity in
biological function, enabling members of the annexin A family to
play key roles in a wide variety of biological processes.
Evidence suggests that Annexins are strongly
associated with various human diseases, including cardiovascular
diseases, cerebrovascular diseases and cancer (21). ANXA1, a pivotal member of the
annexin family of proteins, has been the focus of extensive
researches and has been shown to exert regulatory effects on
numerous biological processes, particularly those related to
cerebral ischemia-reperfusion events (22). Formyl peptide receptors (FPRs), a
family of G-protein coupled receptors including FPR1, FPR2, and
FPR3, are the primary pathways through which ANXA1 exerts its
biological effects (23). ANXA1
has been shown to exert reparative and regenerative effects on the
nervous system via FPR2. Although the evidence is still limited,
ANXA1 also displays a protective function in nerve conduction
structures under both physiological and pathological conditions
(24).
ANXA1 and ischemic stroke
ANXA1 exerts anti-arteriosclerosis
effects
Atherosclerosis is a chronic inflammatory disease
that occurs within blood vessel walls and progresses through
various pathological stages. It is mainly characterized by the
formation of fatty plaques on the walls of large and medium-sized
arteries, particularly in regions with disturbed blood flow
(25). At the site of the lesion,
monocytes differentiate into macrophages when they phagocytose
lipoproteins, transforming into foam cells and forming ‘fatty
streaks’. In advanced atherosclerosis, defective clearance of
apoptotic cells, excessive lipid loading, and accumulation of
cholesterol crystals contribute to macrophage apoptosis and
formation of a necrotic core. Infiltrating macrophages trigger an
inflammatory response, leading to apoptosis of vascular smooth
muscle cells, thinning of the fibrous cap, and degradation of the
extracellular matrix. These processes ultimately destabilize the
plaque and increase the risk of acute cerebrovascular events
(26).
It has been revealed that ANXA1 expression is
elevated in atherosclerotic plaques, which contain numerous
apoptotic cells. Studies have shown that impaired clearance of
apoptotic cells exacerbates the development of atherosclerosis, in
which ANXA1 secreted by apoptotic cells binds to the FRP1 receptor
on antigen-presenting cells (APCs) and helps APCs to invade dead
cells, thereby promoting apoptotic cell clearance. ANXA1 may play a
role in promoting apoptosis of inflammatory cells and influencing
macrophage burial (27).
Furthermore, a study analyzing 34 patients with carotid
atherosclerosis found that ANXA1 is expressed in all plaques.
Notably, ANXA1 expression in carotid plaques is significantly
higher in asymptomatic patients than in those with neurological
symptoms, suggesting that elevated ANXA1 expression levels may have
a stabilizing effect on asymptomatic carotid plaques (28). In order to investigate the
pharmacological effects of human recombinant ANXA1 (hrANXA1) on the
formation and progression of atherosclerosis, Kusters et al
(29) used a low-density
lipoprotein gene-deficient (LDLR-/-) mouse model and fed the mice a
Western Type Diet to induce atherosclerosis. The observation group
was administered intraperitoneal injections of hrANXA1 protein at a
dose of 1 mg/kg three times per week for six weeks. The control
group was administered an equal volume of PBS. Subsequently, the
total plaque area was quantified in the aortic arch and major
arterial branches. The progression of arterial plaques was
evaluated by immunohistochemistry, and the results revealed that in
mice with pre-existing plaques, the observation group exhibited a
reduction in plaque area of ~50% and a reduction in necrotic core
volume of 76% in the observation group. The study demonstrated that
hrANXA1 had a similar plaque-stabilizing effect, significantly
reducing the progression of lesions to unstable plaques.
Additionally, hrANXA1 can act as a bridging molecule between
apoptotic cells and macrophages, contributing to increased
phagocytosis by macrophages and a reduction in inflammatory factors
(29). Macrophages play a
multifaceted role in the inhibition of inflammation and clearance
of cellular debris and apoptotic cells, and are instrumental in the
onset, progression and regression of atherosclerosis (30). The aforementioned studies also
indicated that the plaque-stabilizing effect of ANXA1 may be linked
to macrophages.
Altered lipid metabolism is a key risk factor and
characteristic manifestation of atherosclerosis. Several studies
have indicated a correlation between ANXA1 and lipid metabolism
(31–33). The aim of this study was to
investigate the effects of ANXA1 deficiency on obesity and
metabolism by establishing a diet-induced mouse obesity model by
feeding a high-fat diet to ANXA1 knockout mice and wild-type mice
(31). This was performed to
investigate the effects of ANXA1 deficiency on obesity and
metabolism. It was observed that the expression of key enzymes and
proteins associated with adipose tissue lipolysis, including
adipose triglyceride lipase, hormone-sensitive lipase and
galectin-12, was significantly upregulated in wild-type mice.
However, this was not observed in ANXA1-knockout mice, indicating
that ANXA1 deficiency may inhibit lipolytic processes and
contribute to the development of obesity. Furthermore, plasma
corticosterone levels were found to be significantly elevated in
ANXA1 knockout mice, suggesting that ANXA1 deficiency may also
influence the hypothalamic-pituitary-adrenal axis, thereby
promoting the development of obesity (31). Another study also demonstrated an
increase in ANXA1 expression in the subcutaneous fat of both young
and elderly overweight patients (32). Similarly, a study conducted in
Spain found higher levels of ANXA1 expression in obese children
than in those of normal weight. Interestingly, despite increased
expression, plasma levels of ANXA1 were negatively correlated with
adipose markers and positively correlated with HDL cholesterol
levels (33).
The relationship between diabetes and
atherosclerosis is similarly robust, with multiple pathological
pathways interlinking these two conditions. Evidence has
demonstrated that patients with diabetes have a markedly elevated
risk of atherosclerosis (34).
ANXA1 is inextricably linked to blood glucose regulation.
Additionally, Purvis et al (35) constructed an obese and
insulin-resistant mouse model. They observed that ANXA1-knockout
mice exhibited elevated blood glucose levels, impaired glucose
tolerance and more pronounced insulin resistance. Following the
administration of hrANXA1 via intraperitoneal injection for a
period of six weeks, a notable reduction in both blood glucose
levels and the rate of weight gain was observed. And the results
showed the administration of hrANXA1 reduced blood glucose levels
which may be related to the attenuation of the phosphorylation
level at the Ser307 site on insulin receptor substrate-1. These
findings indicate that ANXA1 has the potential to significantly
reduce insulin resistance (35).
Indeed, a considerable number of pharmaceutical agents employed in
the treatment of diabetes are known to exert their effects by
inhibiting of the Ras homolog gene family member A (GTPase RhoA).
This includes metformin. Furthermore, evidence indicates that
fasudil influences glucose metabolism by inhibiting the GTPase RhoA
(36–38). It is of particular interest to note
that RhoA activation is inhibited by ANXA1 (35), which provides further evidence of a
close link between ANXA1 and glucose regulation.
Moreover, ANXA1 has significant effect on
atherosclerosis. Activation of the lipoxin A4 receptor (ALX) serves
as an endogenous anti-inflammatory effector, and FPR2 is a key
signaling molecule that controls the cellular inflammatory
response. Glucocorticoids have been shown to induce the release of
ANXA1 in macrophages and neutrophils, activating ALX/FPR2 to exert
anti-inflammatory effects (39).
There is a robust correlation between the levels of cellular
inflammatory factors and severity of atherosclerosis (40,41).
This suggests that ANXA1 may positively affect atherosclerosis
through its anti-inflammatory action. Additionally, Al-Kuraishy
et al (42) discovered that
ANXA1 inhibits integrin activation and myeloid cell accumulation in
the arterial wall, reduces necrotic areas in the center of plaques,
induces neutrophil apoptosis, reduces the risk of plaque exposure
to deleterious neutrophilic intracellular contents, reduces the
expression of endothelial cell adhesion molecules, and attenuates
inflammatory responses, thereby reducing the incidence of
atherosclerosis. The inflammatory response to arterial injury
accelerates the growth of neointima and can lead to restenosis of
blood vessels. In the ANXA1-knockout mouse model, the accumulation
of proliferating macrophages in the injured tissue exacerbates the
growth of neointima, and then promotes the occurrence of vascular
stenosis. de Jong et al (43) found that the levels of ANXA1 in
plasma and at the lesion were negatively correlated with the size
of neointima. Additionally, ANXA1 can inhibit the proliferation of
macrophages by suppressing the production and release of macrophage
colony-stimulating factor, thereby preventing the occurrence of
arterial restenosis (43).
AnxA1Ac2-26, the mimetic peptide of ANXA1, significantly
improves vascular remodeling parameters including reducing the
thickness of the arterial intima and media, decreasing collagen
deposition, and repairing elastic fiber breaks in ANXA1-deficient
mouse models. ANXA1 notably slowed down the aging process of
endothelial cells by inhibiting the expression of pro-senescence
related molecules such as p53 and p21, and senescence-associated
secretory phenotype factors tumour necrosis factor α (TNF-α) and
interleukin-6 (IL-6). At the same time, it restores the
proliferative and migratory abilities of endothelial cells by
reducing DNA damage markers such as γ-H2AX, thereby promoting
endothelial repair (44). The
evidence indicates that ANXA1 may play a protective role in the
repair of arterial injury and the prevention of restenosis.
ANXA1 plays a role in the inflammatory
response
After the onset of ischemic stroke, the primary
treatment goal is to restore blood flow to restore glucose and
oxygen delivery to the ischemic brain tissue as quickly as
possible. However, reperfusion exposes the infarct area to
peripheral immune cells, which leads to immune activation and
subsequent inflammatory damage. In addition, post-infarction, dead
and dying cells stimulate the production of various inflammatory
factors and chemokines, including CCR2, CCR5, CCR6, CXCL8 and
CXCR2, further exacerbating the inflammatory response (45). Elevated levels of these
inflammatory factors increase cerebral infarct areas, raise early
mortality rates, and worsen patient prognosis (46).
Liu et al (47) investigated the effects of chloral
hydrate on stroke in mice. They observed a significant increase in
ANXA1 expression in chloral hydrate-treated mice during the acute
phase of ischemic stroke, which was accompanied by a reduction in
inflammatory factors such as TNF-α, IL-6 and interleukin-1β
(IL-1β), effectively attenuating edema and damage to brain tissue
following ischemia. These results were further verified using an
ANXA1 inhibitor, confirming that ANXA1 plays a neuroprotective role
by reducing the expression of inflammatory factors (47). The aforementioned finding is also
consistent with another study suggesting that ANXA1 may play a
pivotal role in the post-stroke inflammatory response (48).
Microglia, key regulators of the inflammatory
response within the CNS, are activated by environmental stimuli
following ischemic stroke. This activation leads to microglial
polarization and shifts to different phenotypes, typically
classified as pro-inflammatory or anti-inflammatory microglia
(49,50). Proinflammatory microglia secrete a
variety of inflammatory factors, including IL-1β, IL-6, TNF-α and
inducible nitric oxide synthase, which exacerbate the inflammatory
response and accelerate the pathological progression of cerebral
ischemia. By contrast, anti-inflammatory microglia help to reverse
the damage caused by cerebral ischemia by secreting relevant
anti-inflammatory mediators (51).
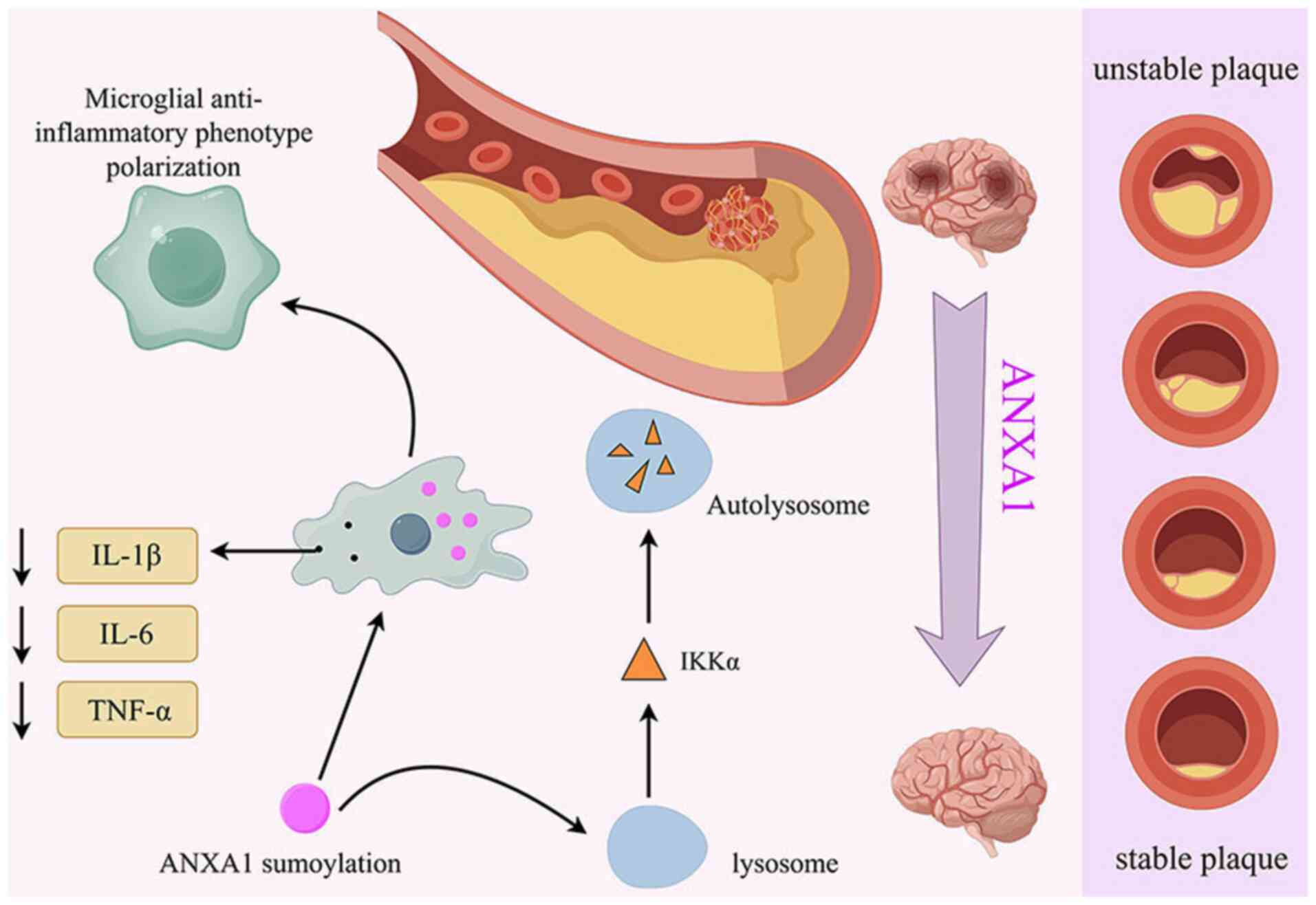
ANXA1 is abundant in microglia and its biological
function is tightly regulated by post-translational modifications
(52), including SUMOylation a
modification involving small ubiquitin-like modifier (SUMO)
proteins. SUMOylated ANXA1 has been shown to effectively induce
oxygen-glucose deprivation and reoxygenation-injured microglia to
polarize towards an anti-inflammatory phenotype, thereby
attenuating inflammatory stimuli and exerting neuroprotective
effects (51,53). One research team discovered that
Tat-Nuclear Translocation Signal (Tat-NTS), a peptide that
increases ANXA1 SUMOylation, promotes microglia to adopt an
anti-inflammatory phenotype. This process also selectively degrades
IκB kinase α (IKKα) through autophagy, blocking the activation of
the NF-κB pathway triggered by cerebral ischemia-reperfusion
injury. As a result, apoptosis of ischemic neurons was reduced, and
neurological function was improved in experimental mice (54) (Fig.
3). Furthermore, another experimental study indicated that
ANXA1 promotes the conversion of microglia to an anti-inflammatory
phenotype and induces microglial migration to protect neurons from
ischemic injury. This process is closely linked to the release of
ATP and glutamate from injured neurons (55). Previous evidence also demonstrated
a significant protective effect of ANXA1 against cellular edema and
glutamate overload in the ischemic environment (56).
Leukocytosis is an important marker of the
inflammatory response after stroke, in which neutrophils undergo
conformational changes and migrate through the endothelium of the
vessel wall. They are then attracted to ischemic tissue by
chemokines, releasing pro-inflammatory factors, matrix
metalloproteinases (MMPs), reactive oxygen species, and other
signals that cause secondary damage, leading to altered BBB
permeability and post-ischemic edema (57,58).
ANXA1 can limit neutrophil recruitment and migration, inhibit the
production of proinflammatory factors, and promote the clearance of
apoptotic neutrophils, thereby reducing the inflammatory response
(59). In an experiment,
researchers observed cerebral microcirculation in mice with
cerebral infarction and found that ANXA1-deficient mice had more
leukocyte adherence in the small veins of the brain, larger
infarcts, and worse neurological scores than the control group. The
inflammatory indices significantly decreased after administration
of an ANXA1 peptidomimetic, confirming that the anti-inflammatory
circuits centered on ANXA1 have neuroprotective effects (60).
In addition to neutrophils, platelets also play a
crucial role in the inflammatory response after stroke. Platelets
are involved in several processes, including thrombosis and
inflammation. The inflammatory response after ischemia-reperfusion
leads to microvascular dysfunction and platelet adhesion to blood
vessels, thereby increasing the risk of recurrent cerebrovascular
events (61). Administration of
ANXA1 via intravenous injection significantly increased blood flow
in the cerebral arteries and veins, altered the
thrombotic-inflammatory environment, and prevented thrombotic
events after cerebral ischemia-reperfusion (62). Further findings revealed that ANXA1
inhibits thrombin-induced activation of signaling events and
integrins, thereby reducing platelet aggregation and preventing
thrombosis (62). It has been
reported that ANXA1 is associated with cellular senescence and
multiple inflammatory pathways, including the chemokine, NF-κB and
TNF signaling pathways. ANXA1 may prevent vascular aging by
inhibiting inflammatory responses (44).
To date, substantial evidence has shown that ANXA1
can exert anti-inflammatory effects. However, it is noteworthy that
ANXA1 has pro-inflammatory functions. The N-terminal structural
domain of ANXA1 mediates anti-inflammatory effects, including the
inhibition of leukocyte migration, whereas the core region of ANXA1
has been shown to promote endothelial cell aggregation and
migration, resulting in pro-inflammatory effects. This suggests
that ANXA1 contains two oppositely acting fragments (11). However, research on its
pro-inflammatory effects in ischemic stroke is limited. Further
studies are needed to explore its potential clinical applications,
as the anti-inflammatory capacity and neuroprotective effects of
ANXA1 could provide an effective therapeutic strategy for
stroke.
ANXA1 preserves the integrity of
BBB
The BBB is a dynamic component of cerebral blood
vessels that plays a crucial role in regulating the permeation of
solutes into the brain tissue and maintaining brain homeostasis
(63). The cerebrovascular
structure is unique in that there are very dense tight junctions
between adjacent endothelial cells, and the integrity of this
structure is essential for maintaining the BBB function (64). In the pathological processes of
numerous neurological diseases, destruction of the BBB leads to
disease progression (65). After
the onset of cerebral ischemia, BBB disruption occurs, leading to
increased vascular permeability and leukocyte infiltration,
resulting in cerebral edema (66).
Simultaneously, BBB disruption promotes the occurrence of secondary
brain injury and aggravates the incidence of post-stroke
hemorrhagic transformation, which seriously affects the prognosis
of patients (67).
ANXA1 functions as an anti-inflammatory messenger
for glucocorticoids and is expressed in a wide range of cells
within the brain, particularly in the endothelial cells of the
cerebral microvascular system and at tight junctions in areas of
intercellular contact (68).
Glucocorticoids have been shown to upregulate ANXA1 expression and
increase BBB tightness in the brain (69,70).
Thus, ANXA1 may play a vital role in regulating BBB permeability.
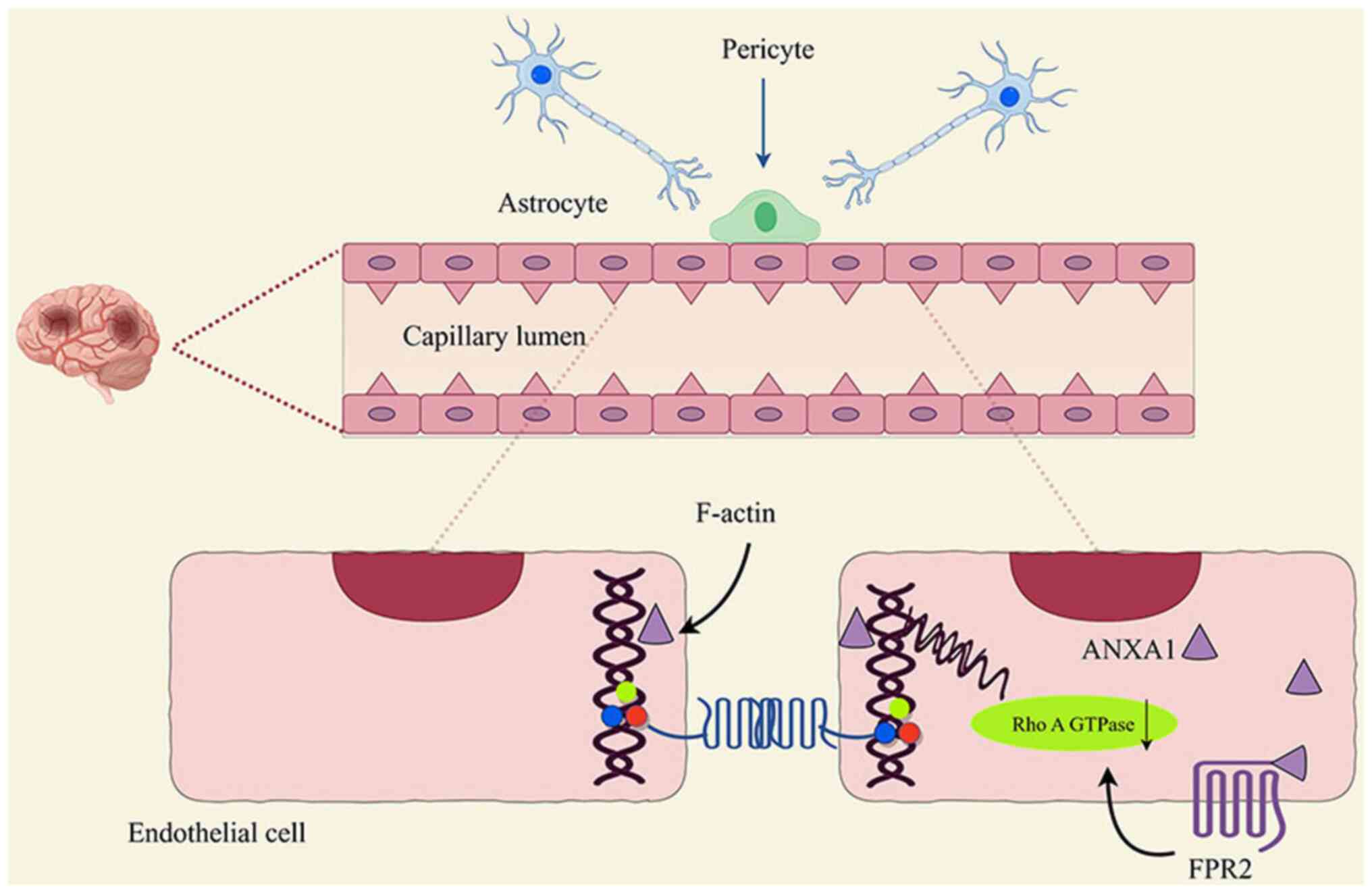
Cristante et al (71) found
that ANXA1 co-regulates paracellular permeability of the BBB in two
ways through interactions with the actin cytoskeleton and paracrine
downregulation of RhoA GTPase activity by FPR2 (71) (Fig.
4), which is tightly correlated with maintaining the integrity
and normal function of the BBB. Another study has found that ANXA1
maintains tight junctions between endothelial cells in BBB,
prevents lipopolysaccharide (LPS) permeation through the BBB, and
limits peripheral effects on the brain in pathological conditions
(72). Further evidence has
emerged to substantiate the protective effects of ANXA1 against
ischemic stroke and other neurovascular diseases. While numerous
studies have focused on the regulation of inflammation, there is
evidence indicating its role in the cerebral vasculature (71). Other researchers administered
hrANXA1 to mice with brain injuries via intravenous injection and
evaluated the extent of brain edema, presence of neurological
deficits and integrity of the BBB. The results demonstrated that in
mice treated with hrANXA1, Evans blue staining extravasation and
immunoglobulin G extravasation in the damaged cerebral hemispheres
were markedly diminished, neutrophil infiltration and inflammatory
factor levels were significantly attenuated, and cerebral edema and
BBB disruption were significantly reduced (73). In animals lacking ANXA1 receptors,
more severe BBB leakage has been observed after the onset of
ischemia (60). One study found
that hrANXA1 administration restored BBB function, which was
associated with increased expression of tight junction proteins
including occludin and claudin-5, decreased activity of MMPs,
increased levels of MMP inhibitors and stabilization of F-actin
(74). There is a clear sex
difference in the incidence of neurovascular diseases including
stroke, with women tending to have a lower incidence than men
(75). Although numerous factors
contribute to neuroprotection, estrogen plays a dominant role
(76). Estrogen appears to play a
role in protecting against oxidative stress. There is evidence that
estrogen exerts specific protective effects on the BBB by
regulating the expression of inter-endothelial tight and adhesion
junction proteins. Furthermore, estrogen reduces the expression of
adhesion molecules on the luminal surface of the endothelium and
prevents leukocyte adhesion and migration during inflammatory
response. Of particular interest is the fact that both the
important effects of estrogen appear to be mediated by ANXA1
(77).
ANXA1 regulates of platelet
aggregation
Platelets prevent bleeding after vascular injury and
play a central role in maintaining hemostasis. However, abnormal
platelet activation can lead to intravascular thrombosis, resulting
in obstruction of blood flow and then triggering cardiovascular and
cerebrovascular events in various pathological conditions, such as
atherosclerotic plaque rupture (78). A previous study investigated the
effects of AnxA1Ac2-26, a mimetic peptide of ANXA1, on
ischemia and reperfusion in mice. The results demonstrated that the
adhesion of platelets to the endothelium increased in the ANXA1
knockout group at specific time points, namely 4 and 24 h after the
onset of ischemia and reperfusion (79). In addition, the study also revealed
that platelet aggregation was markedly attenuated in mice treated
with ANXA1 via intravenous injection, accompanied by a significant
increase in blood flow within the small cerebral arteries and
veins. These findings indicate that AnxA1Ac2-26 reduces
the bleeding time, regulates platelet aggregation, and mitigates
the risk of thrombosis. Furthermore, the study found that the
mechanism of thrombosis inhibition by AnxA1Ac2-26 was
closely related to the regulation of the glycoprotein VI (GPVI)
signaling pathway, reduction of αIIbβ3 activation and expression of
P-selectin (79). Arachidonic acid
(AA) and eicosanoids promote platelet aggregation and
vasoconstriction. Macrophages deficient in ANXA1 have been shown to
increase the production of AA and eicosanoids. Additionally, a
deficiency in ANXA1 results in a hypersensitive cellular response
to LPS (endotoxin), which in turn promotes the activation of
inflammatory vesicles, NLRP3, and increases the risk of thrombosis
(80). These novel findings
demonstrate that ANXA1 may improve cerebral ischemia-reperfusion
injury by regulating platelet function suggesting that ANXA1 has
great potential as a therapeutic agent for thrombophilia, opening a
new avenue for the treatment of cerebral infarction.
ANXA1 inhibits apoptosis
Apoptosis is one of the major pathways that leads to
cell death and plays a key role in ischemic brain injury. The high
metabolic rate of neurons makes them vulnerable to injury. After
the onset of cerebral ischemia-reperfusion, endogenous or exogenous
apoptotic pathways are triggered, leading to neurons undergoing
apoptosis in the ischemic penumbra or peri-infarct zone within
hours or days (81). It can be
reasonably deduced that targeted inhibition of pro-apoptotic
factors would be an efficacious therapeutic strategy for ischemic
stroke. Indeed, some researchers have already identified that acute
ischemic stroke (AIS) promotes the translocation of ANXA1 from the
neuronal cytoplasm to the nucleus, which in turn activates the
neuronal apoptotic pathway and ultimately leads to cell death
(82,83). This indicates that nuclear
translocation of ANXA1 participate in neuronal apoptosis following
AIS, which is of particular importance in the context of
neurological injuries, and it has been shown that S100A11 can bind
to ANXA1, inhibit nuclear translocation of ANXA1 and reduce
apoptosis after ischemic stroke (84). Furthermore, evidence indicates that
Sentrin/SUMO-specific protease 6 (SENP6) is tightened by the
nuclear translocation of ANXA1 and the activation of p53-dependent
apoptotic pathways, including increased BH3 interacting domain
death agonist expression (Bid) and the activated caspase-3 pathway.
Silencing SENP6 expression resulted in the inhibition of ANXA1
nuclear translocation, reduction in neuronal apoptosis, and
improvement in neurological function following cerebral
ischemia/reperfusion in mice (85). Another study established a cerebral
ischemia-reperfusion injury induced by 60 min of middle cerebral
artery occlusion in mice. The animals were then injected with a
cell-penetrating peptide, fluorescein isothiocyanate
(FITC)-Tat-NTS, labelled with FITC, into the unilateral lateral
ventricle. The data showed that administration of Tat-NTS resulted
in a significant reduction in ANXA1 levels within the nucleus
accumbens, accompanied by inhibition of ANXA1 nuclear translocation
(86). Further studies have
demonstrated that the level of the pro-apoptotic protein Bid was
significantly diminished in mice treated with the Tat-NTS peptide
24 h after reperfusion (86).
Another study demonstrated that ANXA1 in mouse microglia was
localized in the cytoplasm and exhibited a uniform distribution in
the physiological state (54).
Furthermore, ANXA1 expression was increased and shifted to the
nucleus after MCAO. After Tat-NTS treatment, the nuclear
translocation of ANXA1 was downregulated and promoted the
conversion of microglia to an anti-inflammatory phenotype, thereby
reducing the area of cerebral infarction areas in mice (54). These findings suggest that the
Tat-NTS peptide may exert a profound neuroprotective effect by
inhibiting ANXA1 nuclear translocation and reducing neuronal
apoptosis.
Advances in the development of activators
and molecular drugs
The development of ANXA1 activators and
small-molecule drugs has made significant progress in recent years
and has demonstrated considerable clinical potential, particularly
in the fields of anti-inflammatory and neuroprotective therapies.
Researchers have concentrated their efforts on the creation of
peptide and non-peptide small-molecule drugs that imitate the
biological activity of ANXA1, with the objective of treating
cerebral ischemia, including Tat-NTS peptide,
AnxA1Ac2-26 and hrANXA1, among others (73,79).
AnxA1Ac2-26
AnxA1Ac2-26, as an ANXA1 mimetic peptide,
was medicated by the key resolution receptor, FPR 2/ALX. It has
been demonstrated that AnxA1Ac2-26 administration could
inhibit inflammation-induced microvascular thrombosis, and decrease
platelet stimulation and aggregation by regulating αIIbβ3 and
P-selectin in the cerebral microvasculature (79). Moreover, AnxA1Ac2-26
treatment reduced leukocyte adhesion and leukocyte-endothelial
interactions in mice subjected to bilateral common carotid artery
occlusion (87). Furthermore, a
recent study has showed that AnxA1Ac2-26 administration
downregulates cerebral thrombotic responses and mediates protein
kinase B (Akt) and extracellular signal-regulated kinases (ERK1/2)
to activate sickle cell disease neutrophils and enable resolution
(88). At the start of
reperfusion, Ac2-26 administration shifts microglia/macrophage
polarization toward anti-inflammatory M2 phenotype, and improved
cerebral ischemia-reperfusion injury in the in vivo and
in vitro experiments through activating the AMPK-mTOR
pathway by binding the FPR2/ALX (89).
Tat-NTS peptide
Tat-NTS peptide has previously been reported as a
novel cell-penetrating peptide developed to prevent nuclear
translocation of ANXA1. Tat-NTS peptide improves neuronal survival
by reducing the transcriptional activity of p53 and activation of
caspase-3 apoptosis pathway, downregulating expression of Bid
following oxygen-glucose deprivation and reperfusion. In addition,
Tat-NTS peptide administration significantly reduces infarct volume
and improves neurological function after local brain ischemia
(86). Interestingly, the latest
study has demonstrated Tat-NTS regulates microglia ANXA1 function,
and exerts neuroprotective effects following cerebral ischemia
(54).
hrANXA1
Up to now, the studies on rANXA1 have mainly focused
on traumatic brain injury and inflammatory brain diseases, but not
been applied in cerebral ischemia related studies. The current
studies have found that rANXA1 treatment notably protects neurons
against brain damages induced by controlled cortical impact by
alleviating BBB protection, reducing degradation of endothelial
junction proteins and inhibiting inflammatory response through RhoA
inhibition (73). Moreover, other
evidence has supported that rANXA1 reduces neuro-inflammation by
inhibiting the action of LPS-induced translocator protein-18KDa,
which may be tightly related with the mechanisms of ANXA1 on
inflammatory brain diseases (90).
The aforementioned findings focus on clinical prospects, safety and
efficacy of ANXA1, and provide conceptual evidence that targeting
ANXA1 may be a new therapeutic strategy for cerebral ischemia.
Conclusions and clinical prospects
Researches on Annexin are gradually increasing, and
available evidence indicates that ANXA1 plays a multifaceted role
in the development of ischemic stroke. This role encompasses
anti-atherosclerotic effects, participation in inflammation,
protection of the BBB, regulation of platelet aggregation and
resistance to apoptosis.
So far, no relevant studies on ANXA1 in the
treatment of cerebral ischemia have been found. However,
ANXA1-related studies in stroke patients can be retrieved, which
has great implications for clinical research and treatment. One
study detects the protein and gene changes of the infarct core
region of human brain and the corresponding contralateral brain
region from the patients with ischemic stroke. The results
indicated that ANXA1s, as one of the most influential molecules,
are significantly dysregulated after stroke (91). Another clinical study revealed that
significantly lower plasma levels of ANXA1 was detected in the
patients with AIS during the onset stage compared with that in
healthy controls (89). Decreased
ANXA1 levels are recovered on 2–3 days post successful
recanalization by endovascular thrombectomy. Furthermore, clinical
evidence has demonstrated a negative correlation between ANXA1
levels and the Modified Rankin Scale score in patients three months
postoperatively. These results showed that ANXA1 levels are
positively correlated with clinical outcomes, indicating that
plasma ANXA1 may be a promising biomarker to predict favorable
prognosis of patients with AIS (89). Despite extensive studies on ANXA1,
it is regrettable that its exploration has mainly focused on basic
experiments. To date, there is still a lack of large-scale
case-control trials for clinical application. In the field of
ischemic stroke, studies related to ANXA1 have remained at the
laboratory stage, and only a small number have been applied in
clinical practice. Further studies are required to ascertain the
safety of the clinical application of ANXA1 and its effect on
patient prognosis. The present review summarizes the main
mechanisms of ANXA1 in the treatment of ischemic stroke and
highlights its potential as a novel therapeutic target and
biomarker for stroke in the future. As research progresses,
promising therapeutic strategies for ANXA1 may become available in
the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81973618 and 81503422), the Henan
science and technology research and development plan joint fund
(grant no. 242301420094), the Key Scientific Research Project of
Higher Education of Henan (grant no. 25A360005) and the Natural
Science Foundation of Henan (grant no. 202300410399).
Availability of data and materials
Not applicable.
Authors' contributions
CT, RL and ZZ conceived the subject of the review,
wrote and edited the original draft. DM reviewed and polished the
manuscript. MZ reviewed the manuscript and made revisions regarding
the intellectual content. YZ and HL wrote the original draft. SL
and JY conducted a formal literature search and analyses. BL and HY
completed the production of illustrations and made revisions to the
manuscript. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gulati A, Agrawal N, Vibha D, Misra UK,
Paul B, Jain D, Pandian J and Borgohain R: Safety and efficacy of
sovateltide (IRL-1620) in a multicenter randomized controlled
clinical trial in patients with acute cerebral ischemic stroke. CNS
Drugs. 35:85–104. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lindsay MP, Norrving B, Sacco RL, Brainin
M, Hacke W, Martins S, Pandian J and Feigin V: World stroke
organization (WSO): Global stroke fact sheet 2019. Int J Stroke.
14:806–817. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feigin VL, Brainin M, Norrving B, Martins
S, Sacco RL, Hacke W, Fisher M, Pandian J and Lindsay P: World
stroke organization (WSO): Global stroke fact sheet 2022. Int J
Stroke. 17:18–29. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
GBD 2019 Stroke Collaborators: Global,
regional, and national burden of stroke and its risk factors,
1990–2019: A systematic analysis for the global burden of disease
study 2019. Lancet Neurol. 20:795–820. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campbell BCV, De Silva DA, Macleod MR,
Coutts SB, Schwamm LH, Davis SM and Donnan GA: Ischaemic stroke.
Nat Rev Dis Primers. 5:702019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang X, Andjelkovic AV, Zhu L, Yang T,
Bennett MVL, Chen J, Keep RF and Shi Y: Blood-brain barrier
dysfunction and recovery after ischemic stroke. Prog Neurobiol.
163–164. 144–171. 2018.
|
|
7
|
Zhu H, Hu S, Li Y, Sun Y, Xiong X, Hu X,
Chen J and Qiu S: Interleukins and ischemic stroke. Front Immunol.
13:8284472022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang L, Jia H, Huang B, Lu S, Chen Z, Shen
J, Zou Y, Wang C and Sun Y: Identification of differently expressed
mrnas in atherosclerosis reveals CDK6 is regulated by
circHIPK3/miR-637 axis and promotes cell growth in human vascular
smooth muscle cells. Front Genet. 12:5961692021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Candelario-Jalil E, Dijkhuizen RM and
Magnus T: Neuroinflammation, stroke, blood-brain barrier
dysfunction, and imaging modalities. Stroke. 53:1473–1486. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spurr L, Nadkarni S, Pederzoli-Ribeil M,
Goulding NJ, Perretti M and D'Acquisto F: Comparative analysis of
annexin A1-formyl peptide receptor 2/ALX expression in human
leukocyte subsets. Int Immunopharmacol. 11:55–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kelly L, McGrath S, Rodgers L, McCall K,
Tulunay Virlan A, Dempsey F, Crichton S and Goodyear CS:
Annexin-A1: The culprit or the solution? Immunology. 166:2–16.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vital SA, Becker F, Holloway PM, Russell
J, Perretti M, Granger DN and Gavins FNE: FormylFormyl-peptide
receptor 2/3/Lipoxin A4 receptor regulates neutrophil-platelet
aggregation and attenuates cerebral inflammation: Impact for
therapy in cardiovascular disease. Circulation. 133:2169–2179.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gerke V and Moss SE: Annexins: From
structure to function. Physiol Rev. 82:331–371. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benz J and Hofmann A: Annexins: From
structure to function. Biol Chem. 378:177–183. 1997.PubMed/NCBI
|
|
15
|
Camors E, Monceau V and Charlemagne D:
Annexins and Ca2+ handling in the heart. Cardiovasc Res.
65:793–802. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Souza Ferreira LP, da Silva RA, Gil CD
and Geisow MJ: Annexin A1, A2, A5, and A6 involvement in human
pathologies. Proteins. 91:1191–1204. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xi Y, Ju R and Wang Y: Roles of annexin A
protein family in autophagy regulation and therapy. Biomed
Pharmacother. 130:1105912020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei B, Guo C, Liu S and Sun MZ: Annexin A4
and cancer. Clin Chim Acta. 447:72–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grewal T, Hoque M, Conway JRW, Reverter M,
Wahba M, Beevi SS, Timpson P, Enrich C and Rentero C: Annexin A6-A
multifunctional scaffold in cell motility. Cell Adh Migr.
11:288–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu J, Chen L, Ruan J and Chen X: The role
of the annexin A protein family at the maternal-fetal interface.
Front Endocrinol (Lausanne). 15:13142142024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hayes MJ and Moss SE: Annexins and
disease. Biochem Biophys Res Commun. 322:1166–1170. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gavins FNE and Hickey MJ: Annexin A1 and
the regulation of innate and adaptive immunity. Front Immunol.
3:3542012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Caso VM, Manzo V, Pecchillo Cimmino T,
Conti V, Caso P, Esposito G, Russo V, Filippelli A, Ammendola R and
Cattaneo F: Regulation of inflammation and oxidative stress by
formyl peptide receptors in cardiovascular disease progression.
Life (Basel). 11:2432021.PubMed/NCBI
|
|
24
|
Wang A, Zhang H, Li X and Zhao Y: Annexin
A1 in the nervous and ocular systems. Neural Regen Res. 19:591–597.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saigusa R, Winkels H and Ley K: T cell
subsets and functions in atherosclerosis. Nat Rev Cardiol.
17:387–401. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Silvestre-Roig C, de Winther MP, Weber C,
Daemen MJ, Lutgens E and Soehnlein O: Atherosclerotic plaque
destabilization: Mechanisms, models, and therapeutic strategies.
Circ Res. 114:214–226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen X, Zhang S, Guo Z, Xing D and Chen W:
The crosstalk of ABCA1 and ANXA1: A potential mechanism for
protection against atherosclerosis. Mol Med. 26:842020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheuk BLY and Cheng SWK: Annexin A1
expression in atherosclerotic carotid plaques and its relationship
with plaque characteristics. Eur J Vasc Endovasc Surg. 41:364–371.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kusters DHM, Chatrou ML, Willems BAG, De
Saint-Hubert M, Bauwens M, van der Vorst E, Bena S, Biessen EA,
Perretti M, Schurgers LJ and Reutelingsperger CPM: Pharmacological
treatment with annexin A1 reduces atherosclerotic plaque burden in
LDLR-/- mice on western type diet. PLoS One. 10:e01304842015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Pang S, Jiang Y, Wang L and Liu Y:
The role of macrophages in atherosclerosis: Participants and
therapists. Cardiovasc Drugs Ther. October 21–2023.(Epub ahead of
print). View Article : Google Scholar
|
|
31
|
Akasheh RT, Pini M, Pang J and Fantuzzi G:
Increased adiposity in annexin A1-deficient mice. PLoS One.
8:e826082013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alfadda AA, Benabdelkamel H, Masood A,
Moustafa A, Sallam R, Bassas A and Duncan M: Proteomic analysis of
mature adipocytes from obese patients in relation to aging. Exp
Gerontol. 48:1196–1203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aguilera CM, Gomez-Llorente C, Tofe I,
Gil-Campos M, Cañete R and Gil Á: Genome-wide expression in
visceral adipose tissue from obese prepubertal children. Int J Mol
Sci. 16:7723–7737. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Poznyak A, Grechko AV, Poggio P,
Myasoedova VA, Alfieri V and Orekhov AN: The diabetes
mellitus-atherosclerosis connection: The role of lipid and glucose
metabolism and chronic inflammation. Int J Mol Sci. 21:18352020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Purvis GSD, Collino M, Loiola RA,
Baragetti A, Chiazza F, Brovelli M, Sheikh MH, Collotta D, Cento A,
Mastrocola R, et al: Identification of annexinA1 as an endogenous
regulator of RhoA, and its role in the pathophysiology and
experimental therapy of type-2 diabetes. Front Immunol. 10:5712019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kanda T, Wakino S, Homma K, Yoshioka K,
Tatematsu S, Hasegawa K, Takamatsu I, Sugano N, Hayashi K and
Saruta T: Rho-kinase as a molecular target for insulin resistance
and hypertension. FASEB J. 20:169–171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kikuchi Y, Yamada M, Imakiire T, Kushiyama
T, Higashi K, Hyodo N, Yamamoto K, Oda T, Suzuki S and Miura S: A
Rho-kinase inhibitor, fasudil, prevents development of diabetes and
nephropathy in insulin-resistant diabetic rats. J Endocrinol.
192:595–603. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Agard C, Rolli-Derkinderen M,
Dumas-de-La-Roque E, Rio M, Sagan C, Savineau JP, Loirand G and
Pacaud P: Protective role of the antidiabetic drug metformin
against chronic experimental pulmonary hypertension. Br J
Pharmacol. 158:1285–1294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li YZ, Wang YY, Huang L, Zhao YY, Chen LH
and Zhang C: Annexin A protein family in atherosclerosis. Clin Chim
Acta. 531:406–417. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Koushki K, Shahbaz SK, Mashayekhi K,
Sadeghi M, Zayeri ZD, Taba MY, Banach M, Al-Rasadi K, Johnston TP
and Sahebkar A: Anti-inflammatory action of statins in
cardiovascular disease: The role of inflammasome and toll-like
receptor pathways. Clin Rev Allergy Immunol. 60:175–199. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kurilenko N, Fatkhullina AR, Mazitova A
and Koltsova EK: Act locally, Act globally-microbiota, barriers,
and cytokines in atherosclerosis. Cells. 10:3482021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Al-Kuraishy HM, Al-Gareeb AI and Samy OM:
Statin therapy improves serum annexin A1 levels in patients with
acute coronary syndrome: A case-controlled study. Int J Crit Illn
Inj Sci. 11:4–8. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
de Jong RJ, Paulin N, Lemnitzer P, Viola
JR, Winter C, Ferraro B, Grommes J, Weber C, Reutelingsperger C,
Drechsler M and Soehnlein O: Protective aptitude of annexin A1 in
arterial neointima formation in atherosclerosis-prone mice-brief
report. Arterioscler Thromb Vasc Biol. 37:312–315. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
You Q, Ke Y, Chen X, Yan W, Li D, Chen L,
Wang R, Yu J and Hong H: Loss of endothelial annexin A1 aggravates
inflammation-induched vascular aging. Adv Sci (Weinh).
11:e23070402024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
DeLong JH, Ohashi SN, O'Connor KC and
Sansing LH: Inflammatory responses after ischemic stroke. Semin
Immunopathol. 44:625–648. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vila N, Castillo J, Dávalos A and Chamorro
A: Proinflammatory cytokines and early neurological worsening in
ischemic stroke. Stroke. 31:2325–2329. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu JH, Feng D, Zhang YF, Shang Y, Wu Y,
Li XF and Pei L: Chloral hydrate preconditioning protects against
ischemic stroke via upregulating annexin A1. CNS Neurosci Ther.
21:718–726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nie QQ, Zheng ZQ, Liao J, Li YC, Chen YT,
Wang TY, Yuan GQ, Wang Z and Xue Q: SPP1/AnxA1/TIMP1 as essential
genes regulate the inflammatory response in the acute phase of
cerebral ischemia-reperfusion in rats. J Inflamm Res. 15:4873–4890.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lan X, Han X, Li Q, Yang QW and Wang J:
Modulators of microglial activation and polarization after
intracerebral haemorrhage. Nat Rev Neurol. 13:420–433. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xue Y, Nie D, Wang LJ, Qiu HC, Ma L, Dong
MX, Tu WJ and Zhao J: Microglial polarization: Novel therapeutic
strategy against ischemic stroke. Aging Dis. 12:466–479. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li X, Xia Q, Mao M, Zhou H, Zheng L, Wang
Y, Zeng Z, Yan L, Zhao Y and Shi J: Annexin-A1 SUMOylation
regulates microglial polarization after cerebral ischemia by
modulating IKKα stability via selective autophagy. Sci Adv.
7:eabc55392021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
D'Acunto CW, Gbelcova H, Festa M and Ruml
T: The complex understanding of annexin A1 phosphorylation. Cell
Signal. 26:173–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu S, Gao Y, Yu X, Zhao B, Liu L, Zhao Y,
Luo Z and Shi J: Annexin-1 mediates microglial activation and
migration via the CK2 pathway during oxygen-glucose
deprivation/reperfusion. Int J Mol Sci. 17:17702016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhou H, Yan L, Huang H, Li X, Xia Q, Zheng
L, Shao B, Gao Q, Sun N and Shi J: Tat-NTS peptide protects neurons
against cerebral ischemia-reperfusion injury via ANXA1 SUMOylation
in microglia. Theranostics. 13:5561–5583. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Luo ZZ, Gao Y, Sun N, Zhao Y, Wang J, Tian
B and Shi J: Enhancing the interaction between annexin-1 and formyl
peptide receptors regulates microglial activation to protect
neurons from ischemia-like injury. J Neuroimmunol. 276:24–36. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shijo M, Hamasaki H, Honda H, Suzuki SO,
Tachibana M, Ago T, Kitazono T, Iihara K and Iwaki T: Upregulation
of annexin A1 in reactive astrocytes and its subtle induction in
microglia at the boundaries of human brain infarcts. J Neuropathol
Exp Neurol. 78:961–970. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jickling GC, Liu D, Ander BP, Stamova B,
Zhan X and Sharp FR: Targeting neutrophils in ischemic stroke:
Translational insights from experimental studies. J Cereb Blood
Flow Metab. 35:888–901. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Martynov MY and Gusev EI: Current
knowledge on the neuroprotective and neuroregenerative properties
of citicoline in acute ischemic stroke. J Exp Pharmacol. 7:17–28.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sugimoto MA, Vago JP, Teixeira MM and
Sousa LP: Annexin A1 and the resolution of inflammation: Modulation
of neutrophil recruitment, apoptosis, and clearance. J Immunol Res.
2016:82392582016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gavins FNE, Dalli J, Flower RJ, Granger DN
and Perretti M: Activation of the annexin 1 counter-regulatory
circuit affords protection in the mouse brain microcirculation.
FASEB J. 21:1751–1758. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Iadecola C and Anrather J: The immunology
of stroke: From mechanisms to translation. Nat Med. 17:796–808.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Senchenkova EY, Ansari J, Becker F, Vital
SA, Al-Yafeai Z, Sparkenbaugh EM, Pawlinski R, Stokes KY, Carroll
JL, Dragoi AM, et al: Novel role for the AnxA1-Fpr2/ALX signaling
axis as a key regulator of platelet function to promote resolution
of inflammation. Circulation. 140:319–335. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ronaldson PT and Davis TP: Regulation of
blood-brain barrier integrity by microglia in health and disease: A
therapeutic opportunity. J Cereb Blood Flow Metab. 40 (1
Suppl):S6–S24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Abbott NJ, Patabendige AA, Dolman DE,
Yusof SR and Begley DJ: Structure and function of the blood-brain
barrier. Neurobiol Dis. 37:13–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Engelhardt B and Sorokin L: The
blood-brain and the blood-cerebrospinal fluid barriers: Function
and dysfunction. Semin Immunopathol. 31:497–511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fan W, Chen H, Li M, Fan X, Jiang F, Xu C,
Wang Y, Wei W, Song J, Zhong D and Li G: NRF2 activation
ameliorates blood-brain barrier injury after cerebral ischemic
stroke by regulating ferroptosis and inflammation. Sci Rep.
14:53002024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fang W, Sha L, Kodithuwakku ND, Wei J,
Zhang R, Han D, Mao L and Li Y: Attenuated blood-brain barrier
dysfunction by XQ-1H following ischemic stroke in hyperlipidemic
rats. Mol Neurobiol. 52:162–175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Purvis GSD, Solito E and Thiemermann C:
Annexin-A1: Therapeutic potential in microvascular disease. Front
Immunol. 10:9382019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Go KG, Zuiderveen F, De Ley L, Ter Haar
JG, Parente L, Solito E and Molenaar WM: Effect of steroids on
brain lipocortin immunoreactivity. Acta Neurochir Suppl (Wien).
60:101–103. 1994.PubMed/NCBI
|
|
70
|
de Boer AG and Gaillard PJ: Blood-brain
barrier dysfunction and recovery. J Neural Transm (Vienna).
113:455–462. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cristante E, McArthur S, Mauro C, Maggioli
E, Romero IA, Wylezinska-Arridge M, Couraud PO, Lopez-Tremoleda J,
Christian HC, Weksler BB, et al: Identification of an essential
endogenous regulator of blood-brain barrier integrity, and its
pathological and therapeutic implications. Proc Natl Acad Sci USA.
110:832–841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Maggioli E, McArthur S, Mauro C, Kieswich
J, Kusters DHM, Reutelingsperger CPM, Yaqoob M and Solito E:
Estrogen protects the blood-brain barrier from inflammation-induced
disruption and increased lymphocyte trafficking. Brain Behav Immun.
51:212–222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu H, He J, Wu Y, Du Y, Jiang Y, Chen C,
Yu Z, Zhong J, Wang Z, Cheng C, et al: Endothelial regulation by
exogenous annexin A1 in inflammatory response and BBB integrity
following traumatic brain injury. Front Neurosci. 15:6271102021.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sheikh MH, Errede M, d'Amati A, Khan NQ,
Fanti S, Loiola RA, McArthur S, Purvis GSD, O'Riordan CE, Ferorelli
D, et al: Impact of metabolic disorders on the structural,
functional, and immunological integrity of the blood-brain barrier:
Therapeutic avenues. FASEB J. 36:e221072022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gillies GE and McArthur S: Estrogen
actions in the brain and the basis for differential action in men
and women: A case for sex-specific medicines. Pharmacol Rev.
62:155–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Suzuki S, Brown CM and Wise PM:
Neuroprotective effects of estrogens following ischemic stroke.
Front Neuroendocrinol. 30:201–211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
McArthur S, Loiola RA, Maggioli E, Errede
M, Virgintino D and Solito E: The restorative role of annexin A1 at
the blood-brain barrier. Fluids Barriers CNS. 13:172016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zharkova O, Salamah MF, Babak MV, Rajan E,
Lim LHK, Andrade F, Gil CD, Oliani SM, Moraes LA and Vaiyapuri S:
Deletion of annexin A1 in mice upregulates the expression of its
receptor, Fpr2/3, and reactivity to the AnxA1 mimetic peptide in
platelets. Int J Mol Sci. 24:34242023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Vital SA, Senchenkova EY, Ansari J and
Gavins FNE: Targeting AnxA1/Formyl peptide receptor 2 pathway
affords protection against pathological thrombo-inflammation.
Cells. 9:24732020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sanches JM, Branco LM, Duarte GHB, Oliani
SM, Bortoluci KR, Moreira V and Gil CD: Annexin A1 regulates NLRP3
inflammasome activation and modifies lipid release profile in
isolated peritoneal macrophages. Cells. 9:9262020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Broughton BRS, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–e339.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhao Y, Wang J, Jiang H, Yu Z, Li X and
Shi J: Following OGD/R, annexin 1 nuclear translocation and
subsequent induction of apoptosis in neurons are assisted by myosin
IIA in a TRPM7 kinase-dependent manner. Mol Neurobiol. 51:729–742.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li X, Zhao Y, Xia Q, Zheng L, Liu L, Zhao
B and Shi J: Nuclear translocation of annexin 1 following
oxygen-glucose deprivation-reperfusion induces apoptosis by
regulating Bid expression via p53 binding. Cell Death Dis.
7:e23562016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xia Q, Li X, Zhou H, Zheng L and Shi J:
S100A11 protects against neuronal cell apoptosis induced by
cerebral ischemia via inhibiting the nuclear translocation of
annexin A1. Cell Death Dis. 9:6572018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Xia Q, Mao M, Zeng Z, Luo Z, Zhao Y, Shi J
and Li X: Inhibition of SENP6 restrains cerebral
ischemia-reperfusion injury by regulating annexin-A1 nuclear
translocation-associated neuronal apoptosis. Theranostics.
11:7450–7470. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li X, Zheng L, Xia Q, Liu L, Mao M, Zhou
H, Zhao Y and Shi J: A novel cell-penetrating peptide protects
against neuron apoptosis after cerebral ischemia by inhibiting the
nuclear translocation of annexin A1. Cell Death Differ. 26:260–275.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Smith HK, Gil CD, Oliani SM and Gavins
FNE: Targeting formyl peptide receptor 2 reduces
leukocyte-endothelial interactions in a murine model of stroke.
FASEB J. 29:2161–2171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ansari J, Senchenkova EY, Vital SA,
Al-Yafeai Z, Kaur G, Sparkenbaugh EM, Orr AW, Pawlinski R, Hebbel
RP, Granger DN, et al: Targeting the AnxA1/Fpr2/ALX pathway
regulates neutrophil function, promoting thromboinflammation
resolution in sickle cell disease. Blood. 137:1538–1549. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Xu X, Gao W, Li L, Hao J, Yang B, Wang T,
Li L, Bai X, Li F, Ren H, et al: Annexin A1 protects against
cerebral ischemia-reperfusion injury by modulating
microglia/macrophage polarization via FPR2/ALX-dependent AMPK-mTOR
pathway. J Neuroinflammation. 18:1192021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Pantaleão L, Rocha GHO, Reutelingsperger
C, Tiago M, Maria-Engler SS, Solito E and Farsky SP: Connections of
annexin A1 and translocator protein-18 kDa on toll like receptor
stimulated BV-2 cells. Exp Cell Res. 367:282–290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ramiro L, García-Berrocoso T, Briansó F,
Goicoechea L, Simats A, Llombart V, Gonzalo R, Hainard A,
Martínez-Saez E, Canals F, et al: Integrative multi-omics analysis
to characterize human brain ischemia. Mol Neurobiol. 58:4107–4121.
2021. View Article : Google Scholar : PubMed/NCBI
|