Introduction
Epilepsy is a chronic neurological disorder
characterized by recurrent spontaneous seizures. With 65 million
cases worldwide, epilepsy is the third-largest contributor to the
global burden of neurological diseases (1,2).
According to a meta-analysis of international studies, the
incidence of epilepsy is 6.1 per 10,000, with an annual incidence
of 6.78 per 10,000 (3).
Individuals with epilepsy often face numerous other health problems
and co-morbidities are more burdensome than the seizures
themselves. Seizures cause changes in the brain structure and
function that manifest as cognitive and neuropsychological
impairment. Frequent seizures, especially persistent epilepsy,
repeatedly cause oxidative stress, loss of neurons in the
hippocampus or internal olfactory cortex that are closely
associated with cognition, neurogenesis, changes in growth factors
such as brain-derived neurotrophic factor, and inflammation in the
brain (4,5). If seizures are not properly treated
and controlled, permanent cognitive impairment eventually occurs
(6). Approximately 70–80% of
patients with chronic epilepsy have cognitive impairment (7).
Adiponectin (ADPN) was first identified in 1995 and
is one of the most widely studied adipokines to date (8). ADPN receptors (AdipoRs) are expressed
in different parts of the brain, indicating its role in the central
nervous system (CNS). ADPN serves important roles in a number of
physiopathological processes in the CNS, including cognitive
function (9,10). AdipoR1, AdipoR2 and T-cadherin are
three of the known AdipoRs, and both AdipoR1 and AdipoR2 are
expressed in the brain, suggesting that ADPN has physiological
functions outside of peripheral metabolic homeostasis (11). Different neurological diseases have
been linked to AdipoR1 and AdipoR2 signaling (12). When the blood-brain barrier is
compromised due to pathology, ADPN may infiltrate the cerebrospinal
fluid and brain parenchyma (13).
In several CNS illness models, ADPN has been demonstrated to have a
protective effect. For instance, exogenous ADPN supplementation or
lipocalin overexpression lessen ischemic brain injury and enhance
neurological function (14,15).
ADPN may serve a role in the emergence of Alzheimer's disease (AD)
because gene ablation or knockdown of lipocalin or lipocalin
receptors causes severe brain alterations similar to AD, including
memory loss and mood disorders (16,17).
However, the effects and underlying mechanisms of lipocalin on
cognitive impairment in patients with epilepsy remain to be
elucidated.
The present study explored the relationship between
ADPN and cognitive impairment in epilepsy using Spearman's
correlation analysis in patients with epilepsy and healthy
controls. It also investigated the effect of the AdipoR agonist
AdipoRon on cognition in epileptic rats and its underlying
molecular mechanism.
Materials and methods
Clinical sample collection
The present study was conducted in collaboration
with Dr Qian Xue from The First Affiliated Hospital of Hebei North
University (Zhangjiakou, China). Dr Qian Xue participated in the
study design and provided clinical samples collected from The First
Affiliated Hospital of Hebei North University for the current
study. Clinical samples were collected from 20 patients with
epilepsy treated at The First Affiliated Hospital of Hebei North
University between January 2022 and September 2022, as well as 20
healthy volunteers who came to the hospital for physical
examination during the same period. The inclusion criteria for the
epilepsy group were: Patients with epilepsy who met the diagnosis
(18) and had a typical history of
seizures, age >18 years, clear mind, possessing a certain
ability to understand and be able to cooperate to complete the
study, and intracranial magnetic resonance imaging and computed
tomography examination showing no lesions. The patients and their
families consented to the present study and the study was approved
by the Clinical Research Ethics Committee of The First Affiliated
Hospital of Hebei North University (approval no. W2023016;
Zhangjiakou, China). The inclusion criteria for the healthy control
group were: Healthy volunteers were admitted for physical
examinations during the same period and confirmed to be in good
health, and demonstrated the ability to cooperate with examiners.
They possessed sufficient understanding to complete the study and
voluntarily signed the informed consent form.
The exclusion criteria were as follows: Patients
with cognitive impairment due to other reasons such as dementia or
intracranial injury, patients with a history of anxiolytic,
antidepressant, hormonal or immunotherapy therapy within the past 3
months, patients with other pre-existing mental disorders, patients
with cerebral hemorrhage, cerebral infarction or other central
system diseases, patients with hypertension, hyperlipidemia and
diabetes, and patients with infectious diseases or combined liver,
kidney and other organ dysfunction.
Venous blood (3 ml) was collected after >6 h of
fasting. After resting at room temperature for 2 h, serum was
isolated from the blood via centrifugation at 1,000 × g at 4°C for
20 min and kept at −80°C.
ELISA
The levels of ADPN in the serum were quantified
using a human ADPN ELISA kit (cat. no. BMS2032-2; Thermo Fisher
Scientific, Inc.). The blood was centrifuged at 20°C (1,500 × g)
for 10 min. The serum was added to a microplate precoated with
monoclonal antibodies specific to human serum ADPN. After washing
with Tris-buffered saline, the amount of ADPN was determined by
adding streptavidin-conjugated enzyme, substrate and stop solution
in sequence. The optical density was measured at the specified
wavelength to calculate the ADPN concentration.
Assessment of cognition
The following assessment scales were collected for
all subjects: Montreal cognitive assessment (MoCA), Boston naming
test (BNT), Symbol digit modalities test (SDMT) and Rey auditory
verbal learning test (RAVLT) (19–22).
This was performed in a quiet room to avoid external disturbances
and the patients were seizure-free for 24 h before the examination.
The purpose of the test was explained to the subjects before the
test was performed.
Animals and grouping
A total of 45 male specific pathogen-free Sprague
Dawley rats aged 6–8 weeks (200–220 g) were purchased from SPF
Biotechnology Co., Ltd. [cat. no. SCXK(jing) 2019-0010;
qualification certificate no. 110324220104098817]. The rats had
free access to normal sterile food (complete feed included corn,
soybean meal, fish meal, calcium hydrogen phosphate, multiple
vitamins, multiple trace elements and amino acids) and water. The
rats were housed in an SPF animal facility with a temperature range
of 20–25°C, relative humidity range of 40–70% and 12-h light/dark
cycle. The animals were adaptively housed in the animal room
environment for 1 week before the experiment. The animal study was
approved by the Research Ethics Committee of The Second Hospital of
Hebei Medical University (approval no. 2023-AE075; Shijiazhuang,
China).
The 45 rats were randomly divided into two groups:
Epileptic group (n=35) and healthy group (n=10). After modelling,
22 rats with successful modeling from the epileptic group were
selected and randomly divided into the model + AdipoRon group
(n=12) and the model group (n=10).
Induced status epilepticus (SE)
The rat induced SE model was established using
protocols adopted from previous studies (23,24).
A total of 35 rats in the model group were injected
intraperitoneally with 127 mg/kg of lithium chloride (cat. no.
310468; MilliporeSigma), followed by 1 mg/kg of atropine sulphate
(cat. no. PHR1379; MilliporeSigma) 18 h later and 50 mg/kg of
pilocarpine (cat. no. PHR1494; MilliporeSigma) 30 min later. If SE
was not induced after 30 min, pirocarbazine could be given
repeatedly for a maximum of five times. If no seizure occurred
after five times, the model was considered to not have been
successfully established. In 13 rats, the model was not
successfully established and these rats were maintained until their
end-of-life occurred naturally. A total of 22 rats were selected
from the epileptic group and grouped into 10 model rats (model
group) and 12 rats in the drug intervention (model + AdipoRon)
group.
The drug administration started 3 days after the end
of modelling: Blank control group, intraperitoneal injection of PBS
containing 1% DMSO for 21 days; model group, intraperitoneal
injection of PBS containing 1% DMSO for 21 days; and model +
AdipoRon group, intraperitoneal injection of AdipoRon (5 mg/kg;
cat. no. HY-15848; MedChemExpress) for 21 days.
Morris water maze test
The Morris water maze experiment was conducted at
week 4.
Positioning navigation
Each rat was subjected to a positioning navigation
experiment over a total of 5 days, starting at the same time point
each day, four times a day. In the experiment, the rats faced the
wall of the pool and were placed in the water from each of the four
quadrants. The time from when the rat entered the water to when it
found the underwater platform and stood on it was recorded as the
latency period (sec). After the rat found the platform, it was
allowed to remain on the platform for 15 sec to reinforce its
memory. If the rat failed to find the platform within 120 sec, it
was guided from the water to the platform and remained there for 15
sec, with the latency period recorded as 120 sec. After the
experiment in the water, the rats were kept warm and cleaned.
Spatial exploration
On day 6, the platform hidden under the water
surface was removed and the midpoint of the quadrant opposite the
platform, that is the northeast quadrant, was chosen as the entry
point, and the number of times the rat crossed the platform within
2 min and the total time spent in the platform quadrant were
recorded.
Visible platform
The platform was placed in the opposite quadrant of
the original platform, with 1 cm of the platform above water and a
small blue flag was placed on the platform as a marker. The rats
were placed in the water with their heads facing the platform from
the opposite quadrant of the platform, and the latency to evade and
the average swimming speed were recorded.
Nissl staining
At the endpoint, which was the fifth week after
modeling, rats were sacrificed by CO2 inhalation (60%
volume displacement per min) followed by cervical dislocation,
according to the American Veterinary Medical Association guidelines
for the euthanasia of rodents (25). Tissues samples were then collected
for Nissl staining.
The staining procedure was as follows: Brain tissue
was fixed in 10% paraformaldehyde at 20°C for 8 h, embedded in
paraffin and cut into 4-µm sections. Sections were degreased by
immersion in 70% ethanol solution overnight at 4°C, rinsed in
double-distilled water for 3 min and incubated in 1% methyl violet
(cat. no. 69710; MilliporeSigma) for 5 min at 20–24°C. Color
separation was controlled under the microscope with 0.05% glacial
acetic acid. Tissues were dehydrated in an alcohol gradient,
cleared in xylene and sealed in neutral resin. The number of intact
pyramidal neurons in the dentate gyrus, CA1 and CA3 regions of the
hippocampus in a 1-mm2 area was observed, and images
were captured under an Olympus light microscope (BX51; Olympus
Corporation) with OlyVIA software (version 4.1.1; Olympus
Corporation).
Reverse transcription-quantitative PCR
(RT-qPCR)
Rat brain samples were collected for RNA isolation
according to the manufacturer's instructions. Total RNA was
extracted using TRIzol® reagent (cat. no. 15596026;
Thermo Fisher Scientific, Inc.). cDNA was generated using reverse
transcriptase according to the manufacturer's instructions (cat.
no. K1691; Thermo Fisher Scientific, Inc.) at 42°C for 60 min. The
SYBR Premix Ex Taq kit (cat. no. YB042; Shanghai Yubo Biotechnology
Co., Ltd.) was used for the qPCR assay using the Real-Time PCR
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The qPCR conditions were: Initial denaturation at 94°C for 2
min, followed by denaturation at 94°C for 40 sec, annealing at
60.5°C for 60 sec and elongation at 72°C for 40 sec for a total of
40 cycles, followed by a final elongation at 72°C for 10 min. GAPDH
was used as an endogenous control. The relative expression of genes
was calculated using the 2−ΔΔCq method (26). PCR primers were used as shown in
Table I.
 | Table I.PCR primers. |
Table I.
PCR primers.
| Gene | Primer (5′-3′) |
|---|
| AdipoR1
forward |
AACTGGACTATTCAGGGATTGC |
| AdipoR1
reverse |
ACCATAGAAGTGGACGAAAGC |
| AdipoR2
forward |
CCACCATAGGGCAGATAGG |
| AdipoR2
reverse |
TGAACAAAGGCACCAGCAA |
| GAPDH forward |
AAGCCCATCACCATCTTCCAG |
| GAPDH reverse |
AGAAGACTGTGGATGGCCCCT |
Western blotting
According to the manufacturer's instructions, total
proteins from rat brain tissues were extracted using RIPA buffer
(cat. no. P0013B; Beyotime Institute of Biotechnology). The
Enhanced BCA Protein Assay Kit (cat. no. P0010S; Beyotime Institute
of Biotechnology) was used to measure protein concentrations.
Subsequently, 10% SDS-PAGE was used to separate a total of 8 µg of
protein per lane. The samples were transferred to a polyvinylidene
fluoride membrane. After transfer, the membrane was blocked with 5%
skim milk for 1 h at 22°C to prevent non-specific binding.
Membranes were incubated with primary antibody overnight at 4°C,
followed by incubation with HRP-conjugated secondary antibody
(1:4,000; cat. no. ZB-2301 or ZB-2305; Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.) for 1 h at room temperature, after being
washed four times with 0.05% Tween-20/Tris-buffered saline. The
blots were developed using an enhanced chemiluminescence substrate
kit (cat. no. GS009; Beyotime Institute of Biotechnology) and
visualized using the ChemiDoc system (Bio-Rad Laboratories, Inc.).
The primary antibodies used in the present study were: Anti-GAPDH
(1:5,000; cat. no. AG019; Beyotime Institute of Biotechnology),
anti-ADPN (1:500; cat. no. sc-390251; Santa Cruz Biotechnology,
Inc.), anti-postsynaptic density protein 95 (PSD95; 1:250; cat. no.
sc-71933; Santa Cruz Biotechnology, Inc.), anti-synaptosomal
associated protein 25 (SNAP25; 1:1,000; cat. no. ab41455; Abcam),
anti-synaptophysin (SYP; 1:500; cat. no. 100298-T40; Sino
Biological), anti-AMP-activated protein kinase (AMPK; 1:1,000; cat.
no. 2532; Cell Signaling Technology, Inc.), anti-phosphorylated
(p-)AMPK (1:1,000; cat. no. 2535; Cell Signaling Technology, Inc.),
anti-regulatory-associated protein of mTOR (RAPTOR; 1:1,000; cat.
no. 2280; Cell Signaling Technology, Inc.), anti-p-RAPTOR (1:1,000;
cat. no. 2083; Cell Signaling Technology, Inc.), anti-mTOR
(1:1,000; cat. no. 2983; Cell Signaling Technology, Inc.),
anti-p-mTOR (1:500; cat. no. 2971; Cell Signaling Technology,
Inc.), anti-S6 kinase (S6K; 1:1,000; cat. no. 34475; Cell Signaling
Technology, Inc.) and anti-p-S6K (1:1,000; cat. no. 9234; Cell
Signaling Technology, Inc.). ImageJ software (V1.8.0; National
Institutes of Health) was used for densitometry.
Statistical analysis
SPSS 22.0 statistical software (IBM Corp.) was used
for data analysis and the data are presented as the mean ± SEM.
Each test was repeated three times. An independent samples unpaired
t-test was used for comparison between groups when the data were
normally distributed. The χ2 test was used for sex
comparisons. Two-way mixed ANOVA was performed for multiple group
comparisons at different timepoints, followed by the Bonferroni
post hoc test. One-way ANOVA was performed for multiple group
comparisons, followed by Tukey's post hoc test. The relationship
between serum ADPN levels and MoCA scores was analyzed by
Spearman's correlation analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Serum ADPN levels are positively
correlated with MoCA in patients with epilepsy
The age, sex and BMI of the two groups did not
differ significantly (P>0.05), while the RAVLT immediate memory
score (P=0.0162), SDMT score (P<0.001), MoCA score (P<0.001)
and BNT score (P=0.0037) were significantly lower in the epilepsy
group than in the healthy control group, indicating a reduction in
cognitive function in the epileptic patients (Table II).
 | Table II.Analysis of demographic and cognitive
functioning assessments. |
Table II.
Analysis of demographic and cognitive
functioning assessments.
| Demographic
feature | Epilepsy group
(n=20) | Healthy group
(n=20) | P-value |
|---|
| Age, years (mean ±
SD) | 40.25±16.51 | 50.20±14.67 | 0.0511 |
| Sex, n
(male/female) | 10/10 | 13/7 | >0.9999 |
| BMI,
kg/m2 | 23.15±1.89 | 22.45±1.94 | 0.2569 |
| Rey auditory verbal
learning test |
|
|
|
|
Immediate | 41.70±10.18 | 48.20±5.46 | 0.0162 |
|
Delay | 6.45±1.76 | 7.50±1.79 | 0.0694 |
| Symbol digit
modalities test | 47.90±5.02 | 57.25±4.97 | <0.001 |
| Montreal cognitive
assessment | 24.00±2.43 | 27.80±1.28 | <0.001 |
| Boston naming
test | 24.65±3.36 | 27.40±2.11 | 0.0037 |
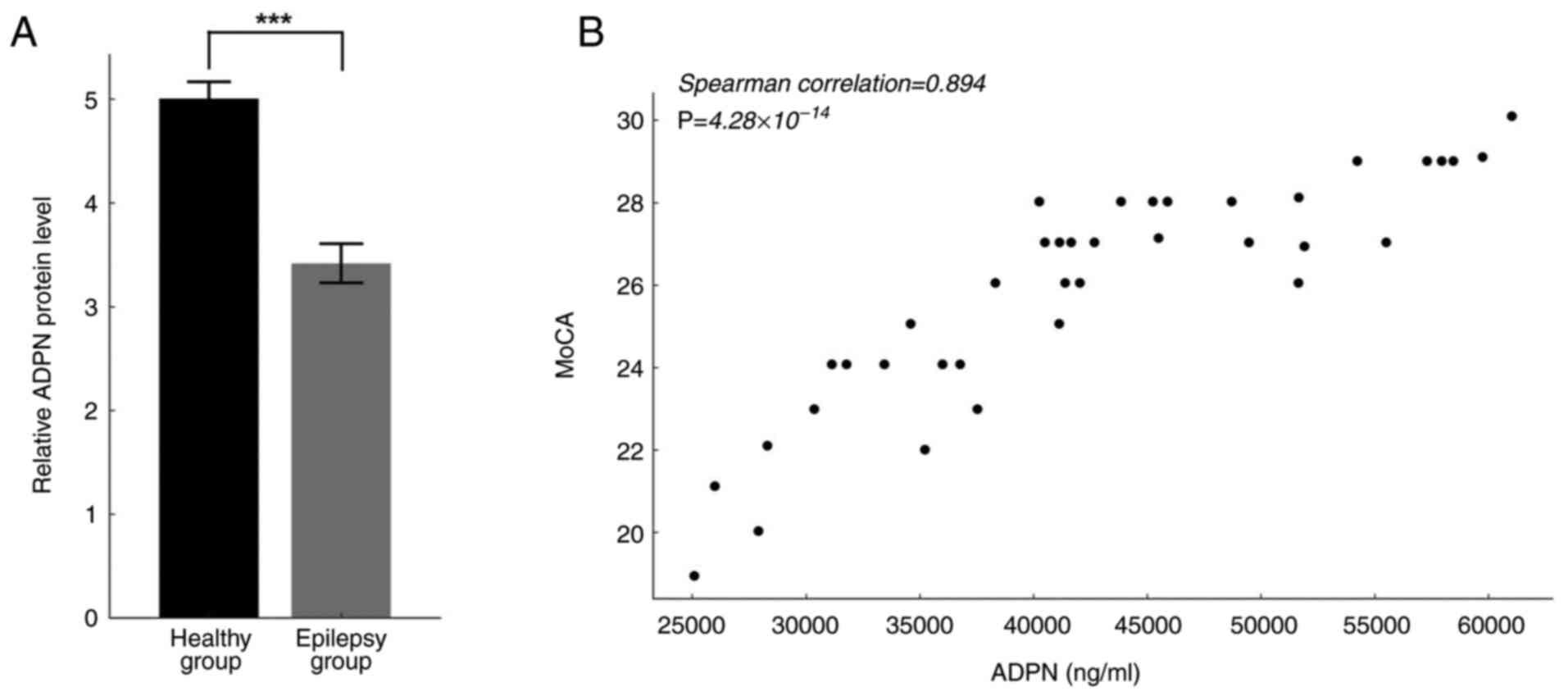
Serum ADPN levels were significantly lower in
patients with epilepsy (Fig. 1A).
Spearman correlation analysis was used to explore the relationship
between serum ADPN levels and MoCA scores in epileptic patients and
healthy volunteers. The results revealed that serum ADPN levels
were positively associated with cognitive function (Spearman
correlation, 0.894; P<0.001; Fig.
1B).
AdipoRon improves cognitive impairment
in epileptic rats
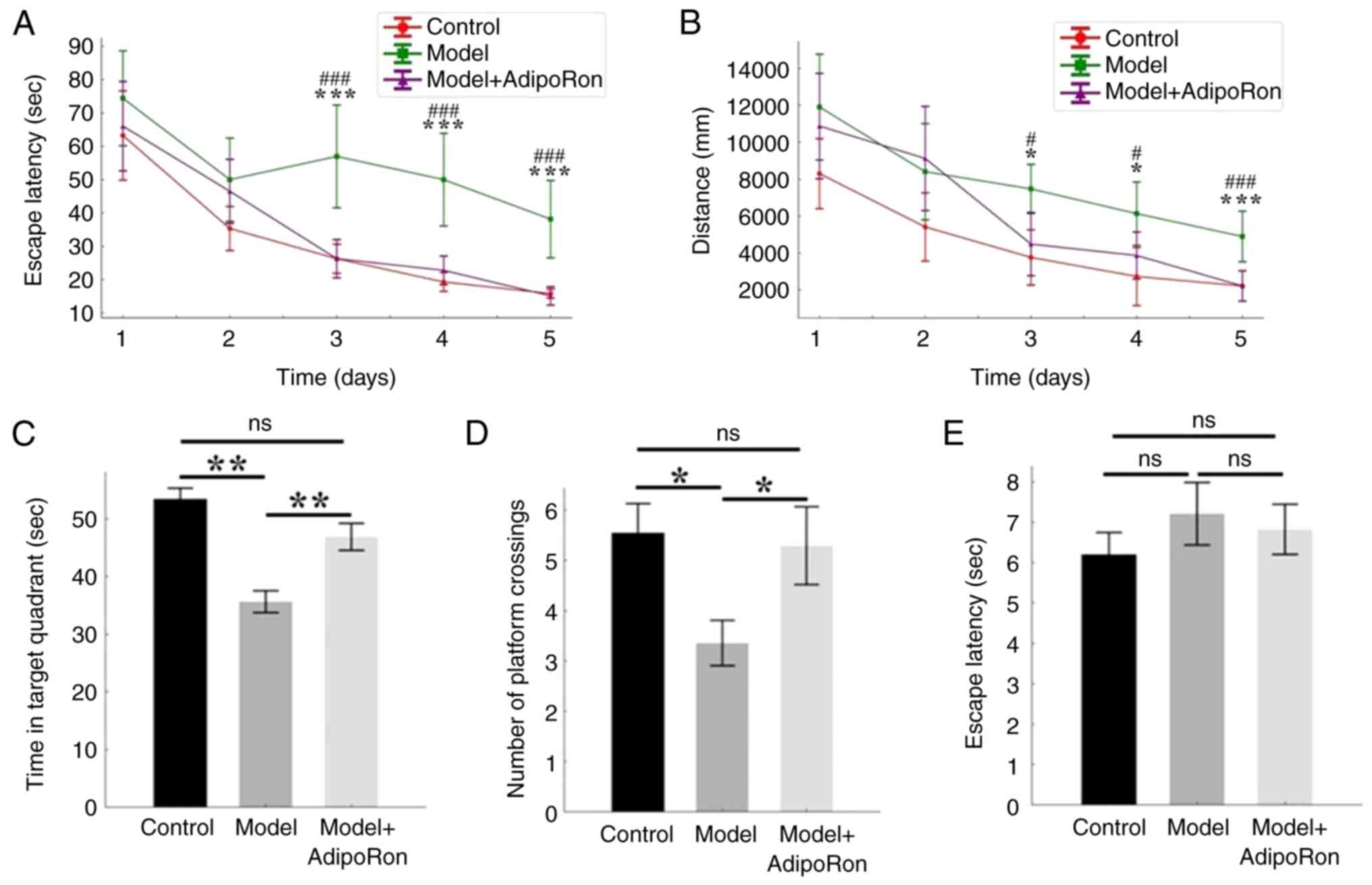
The cognitive differences among the three groups
were explored using the Morris water maze experiment (Fig. 2). The latency to evade and the
distance travelled in the first 5 days of the positioning
navigation experiment are shown in Fig. 2A and B. The latency to evade and
distance traveled decreased with more training time. Two-way mixed
ANOVA with Bonferroni post hoc analysis indicated that both time
(F=38.14; P<0.001) and group (F=10.99; P<0.001) significantly
impacted escape latency. For distance, significant effects were
also observed for both group (F=7.794; P<0.001) and time
(F=36.54; P<0.001). The post hoc test revealed that, starting
from day 3, there was no marked difference between the model +
AdipoRon group and the control group in terms of latency to evade,
whereas there was a significant difference between the model group
and the other two groups (P<0.05) (Fig. 2A and B). The results showed that
there was no difference in the time in target quadrant (Fig. 2C) and number of platform crossings
(Fig. 2D) between the AdipoRon
group and the control group, while there was a significant
difference in the time in target quadrant and number of platform
crossings between the model group and the other two groups
(Fig. 2C and D). These results
showed that AdipoRon treatment did improve the cognitive level of
epileptic rats, including memory and learning ability.
There was no significant difference in the escape
latency among the groups (P>0.05), indicating that the visual
and locomotor abilities of the rats were not affected (Fig. 2E).
AdipoRon effectively protects against
hippocampal neuronal damage caused by epilepsy
The effect of AdipoRon treatment on pathological
changes in the damaged hippocampus of epileptic rats was observed
using Nissl staining. In the control group, a large number of
well-arranged pyramidal cells with normal cell morphology were seen
in the hippocampal region, while in the model group, the number of
neurons was significantly reduced and some neurons in the CA1
region of the hippocampus were lost, with abnormal cell morphology
and reduced volume (Fig. 3A-D).
The difference in the CA3 region was also significant in the model
group compared with the control group (Fig. 3E-H). Following AdipoRon treatment,
hippocampal neuronal tissue staining showed a similar cell
morphology compared with the control group and an increased number
of neurons compared with the model group, with a significant
difference compared with the model group (Fig. 3I-L).
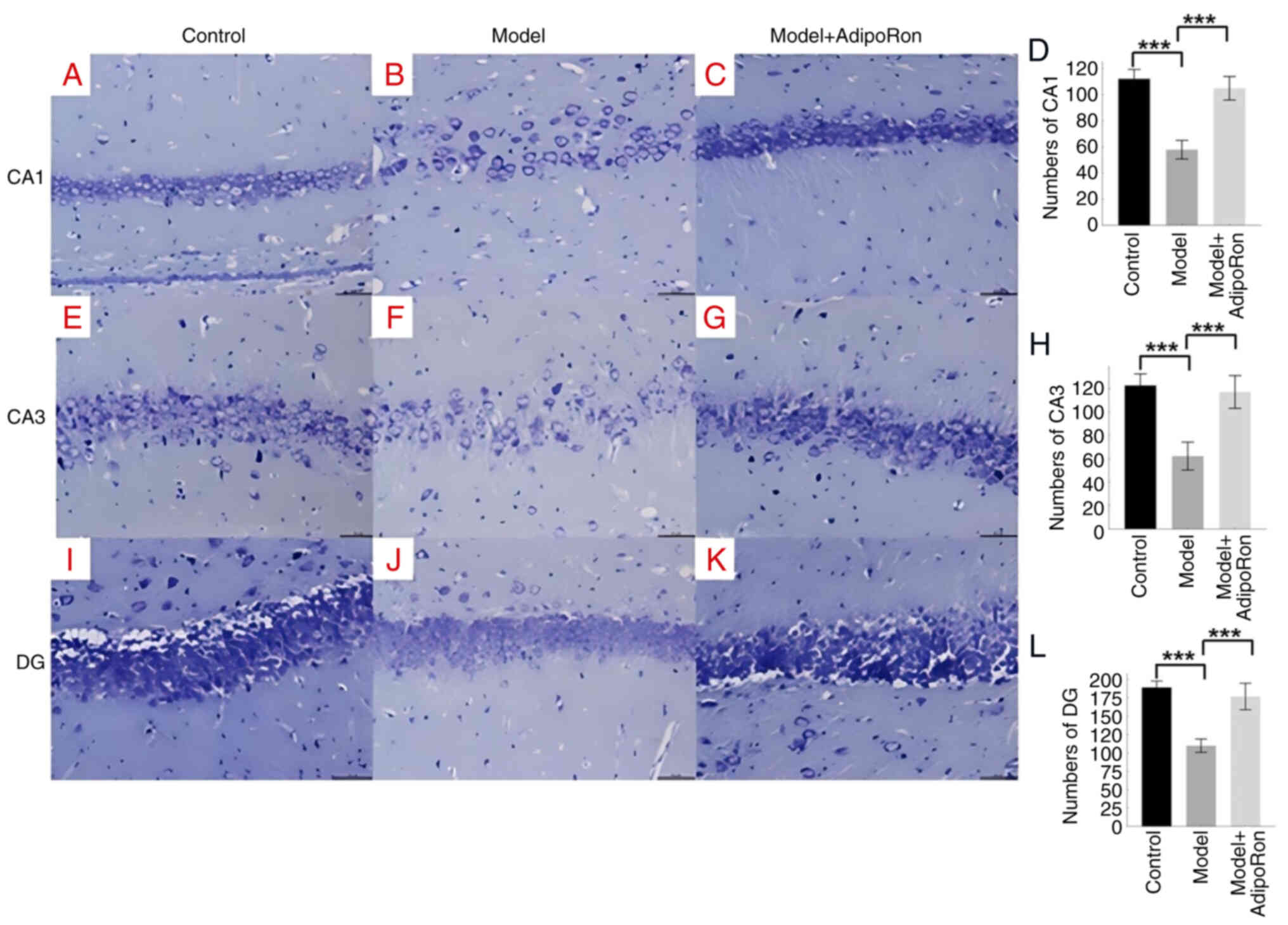 | Figure 3.Nissl staining of rat hippocampus.
The effect of AdipoRon treatment on pathological changes in the
damaged hippocampus of epileptic rats in the control (n=10), model
(n=10) and model + AdipoRon (n=12) groups was evaluated using Nissl
staining. (A-C) Nissl staining of the CA1 region of the hippocampus
(magnification, ×200) in the (A) control, (B) model and (C) model +
AdipoRon groups. (D) Quantification of the CA1 region in the
control, model and model + AdipoRon groups. (E-G) Nissl staining of
the CA3 region of the hippocampus (magnification, ×200) in the (E)
control, (F) model and (G) model + AdipoRon groups. (H)
Quantification of the CA3 region in the control, model and model +
AdipoRon groups. (I-K) Nissl staining of the DG region of the
hippocampus (magnification, ×200) in the (I) control, (J) model and
(K) model + AdipoRon groups. (L) Quantification of the DG region in
the control, model and model + AdipoRon groups. ***P<0.001. DG,
dentate gyrus. |
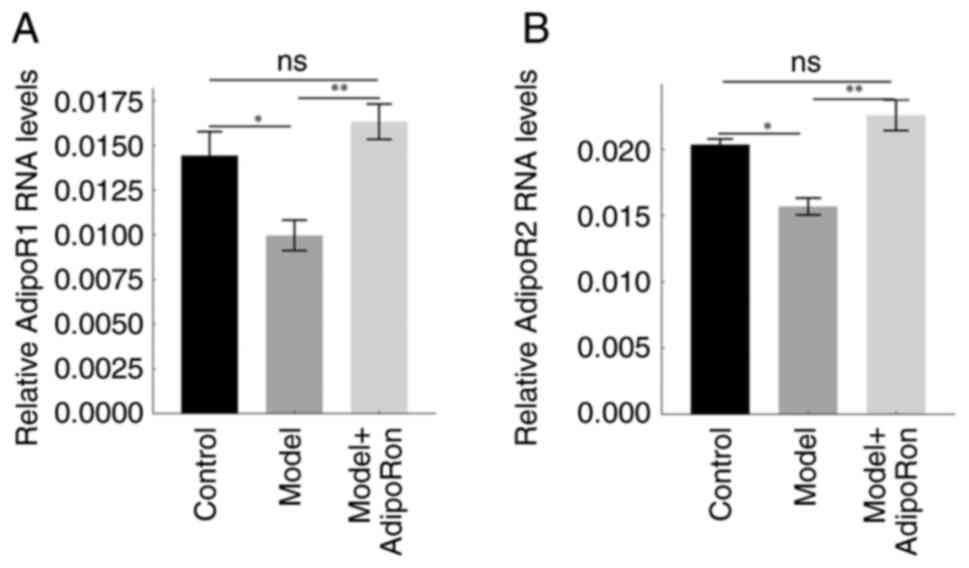
AdipoRon increases AdipoR1 and AdipoR2
expression in the brain
AdipoR1 and AdipoR2 expression in the rat brain of
the model group was considerably lower than that of the control
group and the model + AdipoRon group, whereas no significant
difference was observed between the control and model + AdipoRon
groups (Fig. 4). These results
demonstrated that the expression of AdipoRs was downregulated in
epileptic rats, while treatment with AdipoRon rescued their
expression in the brain.
AdipoRon improves cognition in
epileptic rats by targeting the AMPK/mTOR signaling pathway
The specific mechanisms underlying the cognitive
effects of AdipoRon in epileptic rats were further investigated.
Compared with that in the control group, ADPN expression was
considerably reduced in the epilepsy model animals but AdipoRON
enhanced ADPN expression (Fig. 5A and
B). The expression of the synapse-associated proteins PSD95,
SNAP25 and SYP was significantly reduced in the model group and was
recovered following treatment with AdipoRon (Fig. 5C-E). This suggested that AdipoRon
ameliorated cognitive dysfunction in epileptic rats by modulating
the expression of synapse-associated proteins. In addition, the
levels of p-AMPK and phosphorylation of its target RAPTOR (pRAPTOR)
were lower, while the levels of p-mTOR and phosphorylation of its
downstream target S6K1 (pS6K1) were higher in the model group
compared with the control group (Fig.
5F-I). Increased p-AMPK levels and reduced p-mTOR levels were
observed following AdipoRon treatment, indicating that AdipoRon may
affect the cognitive level of epileptic rats via the AMPK/mTOR
signaling pathway.
 | Figure 5.AdipoRon regulates the activation of
the AMPK/mTOR pathway in the hippocampus of pilocarpine-treated
rats. The expression and phosphorylation of key proteins in the
AMPK/mTOR signaling pathway in the control (n=10), model (n=10) and
model + AdipoRon (n=12) groups were determined by western blotting.
(A) Representative results of western blotting. (B) Relative
expression of ADPN. (C) Relative expression of PSD95. (D) Relative
expression of SNAP25. (E) Relative expression of SYP. (F) Relative
expression of p-AMPK/AMPK. (G) Relative expression of p-mTOR/mTOR.
(H) Relative expression of p-RAPTOR/RAPTOR. (I) Relative expression
of p-S6K/S6K. The expression levels were normalized to GAPDH
expression. Data are presented as the mean ± SEM. *P<0.05,
**P<0.01, ***P<0.001. ADPN, adiponectin; AMPK, AMP-activated
protein kinase; p-, phosphorylated; PSD95, postsynaptic density
protein 95; RAPTOR, anti-regulatory-associated protein of mTOR;
S6K, S6 kinase; SNAP25, synaptosomal associated protein 25; SYP,
synaptophysin. |
Discussion
As one of the most prevalent neurological
conditions, epilepsy affects >65 million individuals worldwide
(27). Daily activities are
limited for individuals with epilepsy and they also have cognitive
impairment, social dysfunction and family problems (28). Verbal memory, language, executive
function and attention are among the cognitive domains where the
impacts of epilepsy are frequently discussed (29–31).
Focal and generalized seizures are both associated with cognitive
deficits (32). Long-term
situational and semantic memory problems, executive dysfunction,
attention issues, language abnormalities and other cognitive
impairments are also present in patients with focal epilepsy
(33,34). In addition to short- and long-term
information processing and retrieval problems, patients with
generalized epilepsy also show cognitive impairments (33,35).
The present study revealed that cognitive impairment in individuals
with epilepsy compared with the non-affected population was
associated with reduced serum ADPN levels. Furthermore,
administration of AdipoRon in epileptic rats alleviated cognitive
dysfunction via the AMPK/mTOR pathway, providing novel insights
regarding the pathogenesis and treatment of epilepsy.
ADPN is the most prevalent adipokine in human plasma
(36). AdipoR1 and AdipoR2 are
critical regulators of inflammation, oxidative stress, glucose and
lipid metabolism in vivo (37,38).
Activation of AdipoR1 with recombinant C1q/TNF-related protein 9, a
homologue of adiponectin, exerts neuroprotective effects through
the APPL1/AMPK/nuclear factor erythroid 2-related factor 2
signaling pathway (39). A
previous study has indicated that ADPN activated the
AdipoR1/APPL1/LKB1/AMPK pathway in newborn rats to reduce the
effects of hypoxia-ischemia-induced neuronal apoptosis (40). In addition, ADPN directly affects
synaptic function via AdipoR2, and lipocalin knockout mice exhibit
cognitive and synaptic deficits (41). Deficiency in ADPN increases fat
storage, poor glucose tolerance, hyperlipidemia, seizure severity
and hippocampus damage in mice with a high-fat diet (42). The findings of the present study
demonstrated that serum levels of ADPN were positively associated
with MoCA scores, and that serum ADPN levels were lower in patients
with epilepsy than in the general population.
AdipoRon, the first reported agonist of AdipoRs
identified by Okada-Iwabu et al (43) in 2013, acts on AdipoR1 and AdipoR2
in liver and skeletal muscle in an experimental mouse model of
diabetes mellitus (44). AdipoRon
reduces brain hemorrhage-induced injury by promoting microglia
polarization to the M2 type and reducing neuronal apoptosis via the
AdipoR1-AMPK signaling pathway (45). AdipoRon also induces AMPK
activation, increases insulin sensitivity, reduces amyloid β plaque
deposition and improves cognitive dysfunction in mice with
Alzheimer's disease (46). Yu
et al (47) revealed that
AdipoRon prevented secondary brain injury following cerebral
hemorrhage by reducing mitochondrial dysfunction through the
AdipoR1-AMPK-peroxisome proliferator-activated receptor-γ
coactivator 1α signaling pathway. In addition, Yan et al
(48) demonstrated that AdipoRon
inhibited deep hypothermic arrest cycle-induced neuroinflammation
by activating the AMPK/NF-κB signaling pathway in the hippocampus.
In the present study, AMPK was activated by AdipoRon in epileptic
rats and it was further demonstrated that the downstream mTOR was
activated.
The synapse-associated protein PSD95 is a
scaffolding protein located primarily in the excitatory
glutamatergic postsynaptic membrane (49). A previous study has shown that
PSD95 is a key protein in promoting synaptic maturation and
maintaining dendritic spine stability (50). The synaptic vesicle-associated
protein SNAP25 is also a synapse-associated protein involved in the
regulation of synaptic vesicle cytokinesis (51). It has been found that a reduction
in postsynaptic SNAP25 leads to a decrease in learning and memory
capacity (52). Furthermore, SYP
expression in the hippocampus is closely associated with learning
memory (53). The SYP levels in
the hippocampus and cortex of mice exhibit age-dependent
alterations, and these changes are associated with cognitive
function (54). A study has shown
that higher SYP expression is linked to improved long-term memory
due to enhanced synaptic plasticity (55). In the present study,
synapse-associated proteins PSD95, SNAP25 and SYP were expressed
from high to low in the normal control group, AdipoRon intervention
group and the epileptic group, respectively. PSD95, SNAP25 and SYP
were all expressed at lower levels in the model group compared with
the normal control and AdipoRon intervention groups, and the
expression differed between the model group and the other two
groups, suggesting that AdipoRon may improve cognitive impairment
in epileptic rats by regulating the expression of
synapse-associated proteins.
AMPK was originally found to act as a regulator of
acetyl-CoA carboxylase and 3-hydroxy-3-methylglutaryl coenzyme A
reductase, which regulate the synthesis of fatty acids and sterols,
respectively (56). It has been
shown that AMPK activated by energy changes inhibited neuronal
developmental growth at multiple stages, including axonal growth,
dendritic growth and bifurcation (57). Mice with knockout of AMPK have
reduced intracerebral lactate, are seizure-prone and develop
cortical lesions as evidenced by thinning of cortical thickness,
neuronal damage and reactive proliferation of glial cells (58,59).
Initially identified as a target of rapamycin, mTOR
serves a key role in neurophysiological processes such as
neurodevelopment and the formation of intracerebral pathways as a
key regulator of protein synthesis and autophagy (60,61).
mTOR signaling dysregulation is involved in the development of
infection and inflammation, and is also part of the complex
mechanism of epilepsy-associated neuroinflammation (62). In epilepsy-associated
neuroinflammation, mTOR hyperactivation leads to disruption of the
blood-brain barrier, which promotes infiltration of peripheral
immune cells (63). In
experimental models, rapamycin and other mTOR inhibitors reduce
seizures and delay the progression of epilepsy (64). Abnormalities in the mTOR signaling
pathway may be a key condition for the onset and progression of
epilepsy. The results of the present study showed that the
p-AMPK/AMPK ratio was reduced and the p-mTOR/mTOR ratio was
increased in the hippocampal tissue of epileptic rats compared with
normal controls, that AdipoRon intervention activated the AMPK/mTOR
pathway in the hippocampal tissue of epileptic rats, and that
AdipoRon intervention treatment improved spatial learning memory
capacity, hippocampal neuronal survival and synapse-associated
protein expression. This suggested that AdipoRon protected
hippocampal neurons by activating the AMPK/mTOR signaling pathway,
increased the expression of synapse-associated proteins and
improved cognitive function in epileptic rats.
The present study revealed that cognitive function
reduction in patients with epilepsy was positively associated with
decreased serum ADPN levels. AdipoRon may protect neurons, regulate
the expression of synapse-associated proteins and improve cognitive
function in epileptic rats by targeting the AMPK/mTOR signaling
pathway.
Acknowledgements
The authors would like to thank Professor Qian Xue
(Department of Neurology, Hebei North University Affiliated First
Hospital, Zhangjiakou, Hebei, China) for their support in the
collection of samples and securing ethical approval for the
study.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YZ was responsible for the study conception and
design, literature research, clinical studies, data analysis,
statistical analysis, manuscript preparation and editing. ZQ was
involved in the study conception, design and drafting the
manuscript, and was responsible for the definition of intellectual
content. ZM was responsible for the experimental studies. HL was
responsible for data acquisition. WW and LJ were involved in the
study conception, design and drafting the manuscript, and were
responsible for the guarantee of integrity of the entire study and
manuscript revision. WW and LJ confirmed the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The participants provided written informed consent.
The human study was approved by the Ethical Committee of The First
Affiliated Hospital of Hebei North University (grant no. W2023016;
Zhangjiakou, China). The animal study was approved by Ethical
Committee of The Second Hospital of Hebei Medical University (grant
no. 2023-AE075; Shijiazhuang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADPN
|
adiponectin
|
|
CNS
|
central nervous system
|
|
MoCA
|
Montreal cognitive assessment
|
|
BNT
|
Boston naming test
|
|
SDMT
|
symbol digit modalities test
|
|
RAVLT
|
Rey auditory verbal learning test
|
|
SE
|
status epilepticus
|
References
|
1
|
GBD 2015 Disease and Injury Incidence and
Prevalence Collaborators, . Global, regional, national incidence,
prevalence, years lived with disability for 310 diseases and
injuries1990-2015: A systematic analysis for the global burden of
disease study 2015. Lancet. 388:1545–1602. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ngugi AK, Bottomley C, Kleinschmidt I,
Sander JW and Newton CR: Estimation of the burden of active and
life-time epilepsy: A meta-analytic approach. Epilepsia.
51:883–890. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fiest KM, Sauro KM, Wiebe S, Patten SB,
Kwon CS, Dykeman J, Pringsheim T, Lorenzetti DL and Jetté N:
Prevalence and incidence of epilepsy: A systematic review and
meta-analysis of international studies. Neurology. 88:296–303.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Rijckevorsel K: Cognitive problems
related to epilepsy syndromes, especially malignant epilepsies.
Seizure. 15:227–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holmes GL: Cognitive impairment in
epilepsy: The role of network abnormalities. Epileptic Disord.
17:101–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Landi S, Petrucco L, Sicca F and Ratto GM:
Transient cognitive impairment in epilepsy. Front Mol Neurosci.
11:4582019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Helmstaedter C and Witt JA: Epilepsy and
cognition-A bidirectional relationship? Seizure. 49:83–89. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Straub LG and Scherer PE: Metabolic
messengers: Adiponectin. Nat Metab. 1:334–339. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Waragai M, Adame A, Trinh I, Sekiyama K,
Takamatsu Y, Une K, Masliah E and Hashimoto M: Possible involvement
of adiponectin, the anti-diabetes molecule, in the pathogenesis of
Alzheimer's disease. J Alzheimers Dis. 52:1453–1459. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jian M, Kwan JSC, Bunting M, Ng RCL and
Chan KH: Adiponectin suppresses amyloid-β oligomer (AβO)-induced
inflammatory response of microglia via AdipoR1-AMPK-NF-κB signaling
pathway. J Neuroinflammation. 16:1102019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guillod-Maximin E, Roy AF, Vacher CM,
Aubourg A, Bailleux V, Lorsignol A, Pénicaud L, Parquet M and
Taouis M: Adiponectin receptors are expressed in hypothalamus and
colocalized with proopiomelanocortin and neuropeptide Y in rodent
arcuate neurons. J Endocrinol. 200:93–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bloemer J, Pinky PD, Govindarajulu M, Hong
H, Judd R, Amin RH, Moore T, Dhanasekaran M, Reed MN and
Suppiramaniam V: Role of adiponectin in central nervous system
disorders. Neural Plast. 2018:45935302018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kusminski CM, Mcternan PG, Schraw T, Kos
K, O'Hare JP, Ahima R, Kumar S and Scherer PE: Adiponectin
complexes in human cerebrospinal fluid: Distinct complex
distribution from serum. Diabetologia. 50:634–642. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishimura M, Izumiya Y, Higuchi A, Shibata
R, Qiu J, Kudo C, Shin HK, Moskowitz MA and Ouchi N: Adiponectin
prevents cerebral ischemic injury through endothelial nitric oxide
synthase dependent mechanisms. Circulation. 117:216–223. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen L, Miao J, Yuan F, Zhao Y, Tang Y,
Wang Y, Zhao Y and Yang GY: Overexpression of adiponectin promotes
focal angiogenesis in the mouse brain following middle cerebral
artery occlusion. Gene Ther. 20:93–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim MW, Abid NB, Jo MH, Jo MG, Yoon GH and
Kim MO: Suppression of adiponectin receptor 1 promotes memory
dysfunction and Alzheimer's disease-like pathologies. Sci Rep.
7:124352017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ng RC, Cheng OY, Jian M, Kwan JS, Ho PW,
Cheng KK, Yeung PK, Zhou LL, Hoo RL, Chung SK, et al: Chronic
adiponectin deficiency leads to Alzheimer's disease-like cognitive
impairments and pathologies through AMPK inactivation and cerebral
insulin resistance in aged mice. Mol Neurodegener. 11:712016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bo X and Luo Z: The Latest Clinical
Guidelines for Diagnosis and Treatment of Epilepsy: Coexistence of
Opportunities and Challenges. Med J Peking Union Med Coll Hosp.
8:122–126. 2017.
|
|
19
|
Li C, Hong Y, Yang X, Zeng X,
Ocepek-Welikson K, Eimicke JP, Kong J, Sano M, Zhu C, Neugroschl J,
et al: The use of subjective cognitive complaints for detecting
mild cognitive impairment in older adults across cultural and
linguistic groups: A comparison of the cognitive function
instrument to the montreal cognitive assessment. Alzheimers Dement.
19:1764–1774. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Madore MR, Scott TM, Fairchild JK and
Yochim BP: Validity of the verbal naming test and boston naming
test in a sample of older veterans. Clin Neuropsychol.
36:1679–1690. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Benedict RH and Smerbeck A: Construct
validity of the symbol-digit modalities test. Mult Scler.
29:483–485. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gottlieb A, Doniger GM, Kimel-Naor S,
Ben-Gal O, Cohen M, Iny H, Beeri MS and Plotnik M: Development and
validation of virtual reality-based rey auditory verbal learning
test. Front Aging Neurosci. 14:9800932022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan J, Shan W, Yang H, Zhu F, Liu X and
Wang Q: Neural activities in multiple rat brain regions in
lithium-pilocarpine-induced status epilepticus model. Front Mol
Neurosci. 12:3232020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Zhao Y, Zhang D, Li J, Yang K,
Yang J and Li B: Neuroprotective effects of quinpirole on lithium
chloride pilocarpine-induced epilepsy in rats and its underlying
mechanisms. Eur J Med Res. 29:1212024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu J, Zhang Y, Pan X, Wang J, Yan G, Zhou
J, Zhu L, Chen X, Li Y and Pang W: A brief interpretation of AVMA
guidelines on euthanasia of animals: 2020 Edition. Lab Anim Comp
Med. 41:195–206. 2021.
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L, Chen S, Liu C, Lin W and Huang H:
Factors for cognitive impairment in adult epileptic patients. Brain
Behav. 10:e014752020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
England MJ, Liverman CT, Schultz AM and
Strawbridge LM: Summary: A reprint from epilepsy across the
spectrum: promoting health and understanding. Epilepsy Curr.
12:245–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rai VK, Shukla G, Afsar M, Poornima S,
Pandey RM, Rai N, Goyal V, Srivastava A, Vibha D and Behari M:
Memory, executive function and language function are similarly
impaired in both temporal and extra temporal refractory epilepsy-A
prospective study. Epilepsy Res. 109:72–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chakravarty K, Shukla G, Poornima S,
Agarwal P, Gupta A, Mohammed A, Ray S, Pandey RM, Goyal V,
Srivastava A and Behari M: Effect of sleep quality on memory,
executive function, and language performance in patients with
refractory focal epilepsy and controlled epilepsy versus healthy
controls-A prospective study. Epilepsy Behav. 92:176–183. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brissart H, Forthoffer N and Maillard L:
Attention disorders in adults with epilepsy. Determinants and
therapeutic strategies. Rev Neurol (Paris). 175:135–140. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khalife MR, Scott RC and Hernan AE:
Mechanisms for cognitive impairment in epilepsy: Moving beyond
seizures. Front Neurol. 13:8789912022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gauffin H, Landtblom AM, Vigren P, Frick
A, Engström M, McAllister A and Karlsson T: Similar profile and
magnitude of cognitive impairments in focal and generalized
epilepsy: A pilot study. Front Neuro. 12:7463812022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bartha-Doering L and Trinka E: The
interictal language profile in adult epilepsy. Epilepsia.
55:1512–1525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Simani L, Roozbeh M, Rostami M, Pakdaman
H, Ramezani M and Asadollahi M: Attention and inhibitory control
deficits in patients with genetic generalized epilepsy and
psychogenic nonepileptic seizure. Epilepsy Behav. 102:1066722020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Turer AT and Scherer PE: Adiponectin:
Mechanistic insights and clinical implications. Diabetologia.
55:2319–2326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS
and Lodish HF: T-cadherin is a receptor for hexameric and
high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad
Sci USA. 101:10308–10313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu B, Liu J, Wang J, Sun F, Jiang S, Hu
F, Wang D, Liu D, Liu C and Yan H: Adiponectin protects against
cerebral ischemic injury through AdipoR1/AMPK pathways. Front
Pharmacol. 10:5972019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao W, Kong F, Gong X, Guo Z, Zhao L and
Wang S: Activation of AdipoR1 with rCTRP9 preserves BBB integrity
through the APPL1/AMPK/Nrf2 signaling pathway in ICH mice. Oxid Med
Cell Longev. 2021:28012632021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu B, Liu J, Wang JG, Liu CL and Yan HJ:
AdipoRon improves cognitive dysfunction of Alzheimer's disease and
rescues impaired neural stem cell proliferation through
AdipoR1/AMPK pathway. Exp Neurol. 327:1132492020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bloemer J, Pinky PD, Smith WD,
Bhattacharya D, Chauhan A, Govindarajulu M, Hong H, Dhanasekaran M,
Judd R, Amin RH, et al: Adiponectin knockout mice display cognitive
and synaptic deficits. Front Endocrinol (Lausanne). 10:8192019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee EB, Warmann G, Dhir R and Ahima RS:
Metabolic dysfunction associated with adiponectin deficiency
enhances kainic acid-induced seizure severity. J Neurosci.
31:14361–14366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Okada-Iwabu M, Yamauchi T, Iwabu M, Honma
T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T,
Shirouzu M, et al: A small-molecule AdipoR agonist for type 2
diabetes and short life in obesity. Nature. 503:493–499. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee S and Kwak HB: Role of adiponectin in
metabolic and cardiovascular disease. J Exerc Rehabil. 10:54–59.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zheng J, Sun Z, Liang F, Xu W, Lu J, Shi
L, Shao A, Yu J and Zhang J: AdipoRon attenuates neuroinflammation
after intracerebral hemorrhage through AdipoR1-AMPK pathway.
Neuroscience. 412:116–130. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Khandelwal M, Manglani K, Upadhyay P, Azad
M and Gupta S: AdipoRon induces AMPK activation and ameliorates
Alzheimer's like pathologies and associated cognitive impairment in
APP/PS1 mice. Neurobiol Dis. 174:1058762022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yu J, Zheng J, Lu J, Sun Z, Wang Z and
Zhang J: AdipoRon protects against secondary brain injury after
intracerebral hemorrhage via alleviating mitochondrial dysfunction:
Possible involvement of AdipoR1-AMPK-PGC1α pathway. Neurochem Res.
44:1678–1689. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yan W, Gao S, Zhang Q, Qi J, Liu G, Teng
Y, Wang J, Yan S and Ji B: AdipoRon inhibits neuroinflammation
induced by deep hypothermic circulatory arrest involving the
AMPK/NF-κB pathway in rats. Pharmaceutics. 14:24672022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Delgado JY, Nall D and Selvin PR: Pin1
binding to phosphorylated PSD-95 regulates the number of functional
excitatory synapses. Front Mol Neurosci. 13:102020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ampuero E, Jury N, Härtel S, Marzolo MP
and van Zundert B: Interfering of the Reelin/ApoER2/PSD95 signaling
axis reactivates dendritogenesis of mature hippocampal neurons. J
Cell Physiol. 232:1187–1199. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Choi UB, Strop P, Vrljic M, Chu S, Brunger
AT and Weninger KR: Single-molecule FRET-derived model of the
synaptotagmin 1-SNARE fusion complex. Nat Struct Mol Biol.
17:318–324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fossati G, Morini R, Corradini I,
Antonucci F, Trepte P, Edry E, Sharma V, Papale A, Pozzi D,
Defilippi P, et al: Reduced SNAP-25 increases PSD-95 mobility and
impairs spine morphogenesis. Cell Death Differ. 22:1425–1436. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ma Q, Geng Y, Wang HL, Han B, Wang YY, Li
XL, Wang L and Wang MW: High frequency repetitive transcranial
magnetic stimulation alleviates cognitive impairment and modulates
hippocampal synaptic structural plasticity in aged mice. Front
Aging Neurosci. 11:2352019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Haley GE, Kohama SG, Urbanski HF and Raber
J: Age-related decreases in SYN levels associated with increases in
MAP-2, apoE, and GFAP levels in the rhesus macaque prefrontal
cortex and hippocampus. Age (Dordr). 32:283–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wi S, Yu JH, Kim M and Cho SR: In vivo
expression of reprogramming factors increases hippocampal
neurogenesis and synaptic plasticity in chronic hypoxic-ischemic
brain injury. Neural Plast. 2016:25808372016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ferrer A, Caelles C, Massot N and Hegardt
FG: Activation of rat liver cytosolic 3-hydroxy-3-methylglutaryl
coenzyme A reductase kinase by adenosine 5′-monophosphate. Biochem
Biophys Res Commun. 132:497–504. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ramamurthy S, Chang E, Cao Y, Zhu J and
Ronnett GV: AMPK activation regulates neuronal structure in
developing hippocampal neurons. Neuroscience. 259:13–24. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Muraleedharan R, Gawali MV, Tiwari D,
Sukumaran A, Oatman N, Anderson J, Nardini D, Bhuiyan MAN, Tkáč I,
Ward AL, et al: AMPK-regulated astrocytic lactate shuttle plays a
non-cell-autonomous role in neuronal survival. Cell Rep.
32:1080922020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Muraleedharan R, Nardini D, Waclaw RR and
Dasgupta B: Analysis of reactive astrogliosis in mouse brain using
in situ hybridization combined with immunohistochemistry. STAR
Protoc. 2:1003752021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lipton JO and Sahin M: The neurology of
mTOR. Neuron. 84:275–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nguyen LH, Xu Y, Mahadeo T, Zhang L, Lin
TV, Born HA, Anderson AE and Bordey A: Expression of 4E-BP1 in
juvenile mice alleviates mTOR-induced neuronal dysfunction and
epilepsy. Brain. 145:1310–1325. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Van Skike CE, Jahrling JB, Olson AB, Sayre
NL, Hussong SA, Ungvari Z, Lechleiter JD and Galvan V: Inhibition
of mTOR protects the blood-brain barrier in models of Alzheimer's
disease and vascular cognitive impairment. Am J Physiol Heart Circ
Physiol. 314:H693–H703. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mcdaniel SS and Wong M: Therapeutic role
of mammalian target of rapamycin (mTOR) inhibition in preventing
epileptogenesis. Neurosci Lett. 497:231–239. 2011. View Article : Google Scholar : PubMed/NCBI
|