Introduction
Subarachnoid hemorrhage (SAH) is a disease
associated with high rates of mortality and morbidity (1); thus, there is a need to identify
effective treatments to prevent detrimental outcomes. Current
therapeutic approaches are predominantly surgical, including
aneurysm clipping and endovascular coiling (2). However, these surgical methods can
lead to complications, such as delayed cerebral ischemia and
cerebral infarction (3), alongside
various other complications. Additionally, current therapeutic
approaches can result in substantial postoperative cognitive
impairments in patients, underscoring a gap in research (4).
Previous studies have indicated that the global
incidence of SAH is ~9 per 100,000 individuals per year (5–8),
with variations across regions due to economic differences. The
permanent disability and mortality rates associated with SAH remain
high, with a cerebral infarction rate of 54% and a mortality rate
of 13.6% in the United States of America (9), necessitating the need for surgical
interventions (10). Meta-analyses
and clinical trials have suggested that endovascular coiling is a
superior treatment to aneurysm clipping (11); however, neither method effectively
reduces complication rates. For example, the International
Subarachnoid Aneurysm Trial reported that relative to aneurysm
clipping, endovascular coiling resulted in improved neurological
outcomes 1 year after an operation and there was a milder decline
in cognitive function (12).
Despite this, both surgical techniques can result in psychological
complications and adverse outcomes (13–15).
The limited ability of both surgical methods in alleviating
complications has been attributed to variations in clinical and
demographic presentation upon patient admission, such as age,
aneurysm location and treatment method (16,17).
Although aneurysm clipping appears to have an
advantage in managing vasospasms, research supports the use of
endovascular coiling for improved post-surgery cognitive recovery
and independence (10,18). These complications and the
advantages/disadvantages of each approach reflect a need for more
effective treatment modalities since neither surgical method can
fully mitigate the associated complications.
The subsequent complications of SAH, including
vasospasm, neuropsychological sequelae and mortality, are primarily
caused by bleeding and associated secondary brain injuries, rather
than the aneurysm location or surgical method (19,20).
Considering this finding, treatment strategies should target damage
to the brain parenchyma. Specifically, addressing neuronal
apoptosis may potentially relieve the various complications.
Apoptosis, a principal form of cell death, involves key proteins
such as caspase 3, BCL2 and BAX (21,22).
Caspase 3 executes the majority of the apoptotic effects through
intrinsic and extrinsic pathways (23). The intrinsic pathway initiates
mitochondrial cytochrome c release and caspase 9 activation
via apoptotic protease activating pathway-1, while the extrinsic
pathway involves the TNF family of death receptors and caspase 8
activation. Both pathways culminate in the activation of caspase 3
activity, which acts as an apoptosis endpoint (24).
BCL2, located in mitochondrial, nuclear and
endoplasmic reticulum membranes, is an anti-apoptotic protein that
regulates mitochondrial membrane permeability and cytochrome
c release (25).
Conversely, BAX, a pro-apoptotic Bcl-2 family member, promotes
apoptosis (26,27). Research on BAX-knockout mice has
shown resistance to neuronal injury and mortality (28).
Notoginsenoside R1 (NGR1), derived from the
traditional Chinese medicine (TCM) Panax notoginseng, has
numerous clinical benefits, including anti-apoptotic,
anti-inflammatory, anti-osteoporotic, antioxidative, pro-angiogenic
and endothelial protective effects (29,30).
Previous studies have demonstrated the ability of NGR1 to promote
the proliferation of PC12 cells via Akt/cAMP response
element-binding protein and to reduce pathological cardiac
hypertrophy through the PI3K-Akt pathway (31,32).
The present study further investigated the
anti-apoptotic mechanisms of NGR1 in a post-SAH neural model.
Through experiments, the target genes and pathways of NGR1 were
assessed. The PI3K-Akt pathway is known to play a role in some
types of cancer (33,34). PI3K, part of a lipid kinase family,
phosphorylates the inositol ring of phosphoinositide, producing
phosphatidylinositol-3,4,5-triphosphate (35). Activated Akt modulates various
substrates that regulate cell survival, cell cycle progression and
growth. PI3Ks can phosphorylate the 3′-OH group of plasma membrane
phosphatidylinositol, carrying out key roles in inflammation,
metabolism and cell survival (36,37).
Akt, a 57 kDa serine/threonine kinase and cellular homolog of the
viral oncogene v-Akt, is implicated in multiple types of cancer due
to its amplification (38). It has
been indicated that the PI3K-Akt pathway is commonly suppressed in
several cancerous processes, promoting oncogenesis (39).
Based on the analysis of prior studies and their
limitations, the present study was designed to examine potentially
novel and effective SAH treatment strategies. The present study
integrated the understanding of SAH treatment, apoptotic
mechanisms, the anti-apoptotic role of NGR1 and the involvement of
the PI3K-Akt pathway, thereby establishing a comprehensive
foundational body of knowledge for further research.
Materials and methods
In vivo experiments
Male C57BL/6J mice, aged 6–8 weeks, weighing 22–25
g, were obtained from Chengdu Dashuo Laboratory Animal Co., Ltd.
Mice were housed in an incubator with adjustable temperature
(22±3°C) and humidity (35±5%), under a 12-h light/dark cycle
(lights on at 8:00 AM and off at 8:00 PM). They had free access to
food and water, and the breeding environment was closely monitored
and controlled. Body weight and body temperature were measured
daily to serve as sensitive indicators of their health condition.
Neurological deficits were assessed daily using the previously
reported modified Garcia scale (40). All research protocols involving
surgical procedures and animal use were approved by the Southwest
Medical University Experimental Animal Ethics Committee (approval
no. 20211126-005; Luzhou, China) and complied with the American
Veterinary Medical Association Guidelines for the Euthanasia of
Animals: 2020 Edition (41).
A total of 18 mice were divided into the following
six groups for preliminary experiments to optimize drug
concentration: Sham group (n=3), SAH group (n=3), SAH + vehicle
group (n=3), SAH + NGR1 20 mg/kg group (n=3), SAH + NGR1 60 mg/kg
group (n=3) and SAH + NGR1 100 mg/kg group (n=3). A total of 90
mice were divided into the following four groups: Sham (n=24), SAH
(n=24), SAH + vehicle (n=18) and SAH + NGR1 (n=24), with different
numbers of mice undergoing each subsequent experiment, as indicated
in the figure legends. Following model establishment and
pharmacological intervention, short-term neurological assessments
were conducted 24 h post-modeling. Additional analyses included
western blotting, immunofluorescence and measurement of brain water
content. Mice were housed for 1 week before the experiment to allow
for acclimation, and samples were obtained 24 h post-SAH induction.
Mice that displayed clear signs of pain, severe and persistent
distress, or those in a terminal state were humanely euthanized
rather than waiting until the experimental endpoint of 24 h
post-SAH. Mice were excluded if they died from intracranial
herniation or massive bleeding, as confirmed by biopsy. Prior to
planned euthanasia, 14 mice [33.3% in the SAH group (n=8) and 25%
in the SAH + vehicle group (n=6)] died due to complications of
intracranial herniation during the model generation, while they
were still under anesthesia, and 7 mice [12.5% in the SAH group
(n=3) and 16.6% in the SAH + vehicle group (n=4)] exhibited
symptoms requiring humane sacrifice and were subsequently
euthanized.
SAH induction experiment
Male C57BL/6J mice were fed a standard diet (CRF-1;
Chengdu Dashuo Laboratory Animal Co., Ltd.) and underwent a 1-week
acclimation period prior to the establishment of the SAH model. The
SAH model was established using a modified single-clamp puncture
method (40). Mice were initially
anesthetized with 5% isoflurane and then maintained under
anesthesia with 2% isoflurane (42). Mice were positioned supine on the
operating table, and the depth of anesthesia was confirmed,
ensuring no signs of discomfort at the incision site. Following
skin preparation and disinfection, a 1.5-cm incision was made along
the anterior midline of the neck using a scalpel.
The subcutaneous fat, deep fascia and muscle tissue
were sequentially separated with micro tweezers, preserving the
hyoid bone, until the carotid artery was exposed. The blood vessels
and peripheral nerves were then carefully dissected. The right
internal carotid artery was carefully separated and a puncture line
was inserted into the external carotid artery to a depth of ~6 mm
then immediately retracted. The mice were observed for
characteristic changes in respiratory rhythm, indicating successful
indirect induction of SAH. Once there was no active bleeding, the
incision was closed layer by layer, disinfected and the mouse was
placed on a warming pad set at 36.0±0.5°C for recovery. After the
mice regained consciousness, they had free access to water and
food. Subsequently, the mice were individually housed in separate
cages. Mice in the sham operation group underwent a similar
surgical procedure; however, upon encountering resistance at the
bifurcation of the anterior communicating artery and middle
cerebral artery, the puncture needle was promptly withdrawn without
penetrating the blood vessels.
The optimal concentration of NGR1 (cat. no. TTL135;
CAS no. 80418-24-2; Shanghai Ronghe Pharmaceutical Technology
Development Co., Ltd.) was established by injecting mice with 20,
60 and 100 mg/kg of the drug, ultimately determining 100 mg/kg as
the optimum. Mice were subjected to either an intraperitoneal
injection of 100 mg/kg NGR1 (treatment group) or an intraperitoneal
injection of sterile 0.9% sodium chloride (vehicle group)
immediately after SAH induction and again 12 h later.
To euthanize the mice, an intraperitoneal injection
of 100 mg/kg sodium pentobarbital (43) was administered. Mortality was
confirmed by observing cessation of respiration and assessing the
corneal reflex. Brain tissue samples were collected 24 h
post-experimental manipulation and promptly stored in liquid
nitrogen for subsequent analysis.
SAH grading
A blinded SAH scoring assessment was conducted
within 24 h post-SAH occurrence. Briefly, the basal cistern was
partitioned into six segments, each assigned a grade ranging from
0–3. Grades 0, 1, 2 and 3 denoted the absence of significant SAH,
or the presence of mild, moderate and substantial SAH with obscured
circle of Willis respectively. The sum of the score was calculated
from each of the six partitions and mice with a 24-h SAH score of
<8 were excluded from the present study (44), this occurred in five mice. Notably,
control mice were not removed due to having a score of <8.
Short-term neurological function
assessment
Blinded neurobehavioral function assessments were
conducted using the modified Garcia and beam balance tests 24 h
post-SAH, as previously described (44). The modified Garcia test, with a
maximum score of 18, included evaluations of whisker response,
trunk touch, spontaneous activity, limb spontaneous movement,
forelimb extension and climbing ability. During the balance beam
test, the observer placed each mouse on a narrow 60-cm square
wooden beam, which was 1 cm wide and elevated 50 cm above the
ground. The time the mouse remained at the center of the beam was
recorded, up to a maximum of 60 sec, and the observer documented
the average latency to fall for each mouse.
Cell culture
HT22 cells (cat. no. CL-0697; Procell Life Science
& Technology Co., Ltd.) were maintained according to the
supplier's guidelines in DMEM (cat. no. C11995500BT; Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(cat. no. C04001-500; VivaCell Biosciences), 1% streptomycin (100
µg/ml) and 1% penicillin (100 U/ml) (cat. no. P1400; Beijing
Solarbio Science & Technology Co., Ltd.). Cells were incubated
at 37°C in an incubator (Thermo Fisher Scientific, Inc.) containing
5% CO2 in a humidified atmosphere. To establish the SAH
cell model, cells were exposed to 10 mM oxyhemoglobin (OxyHb) [cat.
no. JP0200; Jinpin Chemical Technology (Shanghai) Co., Ltd. for 24
h at 37°C. The control group was untreated. For the treatment
group, NGR1 was dissolved in DMSO (cat. no. MB5505-L; Dalian Meilun
Biology Technology Co., Ltd.) and added to the culture medium. The
concentrations of NGR1 used were 20, 60 and 100 mM. These
concentrations were used to determine the optimal drug intervention
concentration, which was found to be 100 mM. The cells were then
incubated at 37°C for 24 h. In the OxyHb + vehicle group, cells
were treated with DMSO (10 mM) as the vehicle.
Flow cytometry
For cell surface marker staining and flow cytometric
analysis, adherent HT22 cells were detached using EDTA-free
trypsin, followed by centrifugation at 300 × g and 4°C for 5 min.
The supernatant was removed, and the cells were washed twice with
pre-chilled PBS by centrifugation as previously described.
Subsequently, 100 µl 1X Binding Buffer (Annexin V-FITC/PI Apoptosis
Detection Kit; cat. no. A211-02; Vazyme Biotech Co., Ltd.) was
added and gently mixed to achieve a single-cell suspension. For
cell staining, 5 µl Annexin V-FITC and 5 µl PI Staining Solution
(Annexin V-FITC/PI Apoptosis Detection Kit; cat. no. A211-02;
Vazyme Biotech Co., Ltd.) were added, evenly mixed, and then
incubated in the dark at room temperature (20–25°C) for 10 min.
After staining, the samples were analyzed by flow cytometry within
1 h using a CytoFLEX flow cytometer (CytoFLEX V2-B2-R2; Beckman
Coulter, Inc.). The acquired data were further analyzed using
CytExpert software (Version 2.3; Beckman Coulter, Inc.).
Lactate dehydrogenase (LDH)
release
Following collection, the culture medium was
centrifuged at 4°C and 380 × g for 5 min, and the supernatant was
collected. LDH activity was quantified via ELISA, adhering to the
KeyGEN LDH kit protocol (cat. no. KGA7403-24 Nanjing KeyGen Biotech
Co., Ltd.). The optical density (OD) value of LDH protein was
measured at 450 nm using a microplate reader. A standard curve
showing the association between concentration and OD value was
established. The experiment was conducted in triplicate.
Cell counting kit-8 (CCK-8) assay
Cell viability was assessed using a CCK-8 assay.
Cells were plated at a density of 5×103 cells/well in a
96-well plate and incubated overnight at 37°C. Subsequently, the
cells were exposed to 10 µM OxyHb [cat. no. JP0200; Jinpin Chemical
Technology (Shanghai) Co., Ltd.] for 24 h at room temperature.
After treatment, CCK-8 solution (1:100 dilution; cat. no.
HB-CCK-8-10; Hanbio Biotechnology Co., Ltd.) was added to the cells
and incubated at 37°C for 2 h. The absorbance at 450 nm was then
measured using a microplate reader (cat. no. A51119700DPC; Thermo
Fisher Scientific, Inc.). Each experimental condition had eight
replicate wells. To calculate the rate of cell proliferation
inhibition, the following formula was used: Cell proliferation
inhibition rate (%)=(1-OD value of cells in the experimental
group-blank/OD value of cells in the control group-blank) ×100.
Western blotting
Mouse brain tissues or HT22 cells were homogenized
in RIPA lysis buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology) in an appropriate ratio and then centrifuged (14,000
× g, 15 min, 4°C) to collect the supernatant. The protein
concentration of each sample was determined using the BCA Protein
Quantitative Kit, followed by mixing with loading buffer (100 µl
sample + 400 µl 5X loading buffer). The protein samples were then
boiled at 100°C for 10 min to denature the proteins and stored at
−80°C. Samples (60 µg/lane) were loaded and proteins were separated
by SDS-PAGE on 10% gels, with the parameters set at 80 V for 30 min
for initial resolving and 110 V for 40 min. Following
electrophoresis, the proteins were transferred to PVDF membranes.
Subsequently, the membranes were blocked in 5% skim milk powder
(cat. no. SL1330; Beijing Coolaibo Technology Co., Ltd.) at room
temperature for 2 h. The membranes were then incubated overnight at
4°C with the following primary antibodies: Cleaved-caspase 3
(1:2,000; cat. no. 9664S; Cell Signaling Technology, Inc.), caspase
3 (1:1,000; cat. no. 9662S; Cell Signaling Technology, Inc.), BAX
(1:3,000; cat. no. 50599-2-Ig; ProteinTech Group, Inc.), BCL2
(1:3,000; cat. no. 68103-1-Ig; ProteinTech Group, Inc.), ITGA11
(1:1,000; cat. no. ab198826; Abcam), PI3K (1:1,000; cat. no. 4255S;
Cell Signaling Technology, Inc.), Akt (1:1,000; cat. no. 9272S;
Cell Signaling Technology, Inc.), phosphorylated (p)-Akt (1:1,000;
cat. no. 4051S; Cell Signaling Technology, Inc.) and β-actin
(1:20,000; cat. no. 20536-1-AP; ProteinTech Group, Inc.). The
membrane was then washed three times with PBS-1% Tween-20.
Subsequently, the PVDF membrane was incubated with the horseradish
peroxidase-labeled secondary antibody (1:3,000; cat. nos. SA00001-1
or SA00001-2; ProteinTech Group, Inc.) at room temperature for 2 h.
The bands were visualized using ECL developer (TOUCH IMAGER;
Shanghai Jerome Biotechnology Co., Ltd.) and images were captured
for analysis. The relative expression levels of the proteins were
calculated using ImageJ (version 1.52; National Institutes of
Health), with β-actin serving as the loading control.
Brain tissue immunofluorescence
Immunofluorescence analysis was conducted to assess
the expression levels of cleaved-caspase 3 in the C57BL6J mice.
Brain Tissue sections (5-µm) were fixed in 10% neutral buffered
formalin at 30°C for 24 h and embedded in paraffin following
established procedures (45).
These sections were mounted on glass slides, dewaxed with xylene,
rehydrated in descending alcohol series and antigen retrieval was
conducted using citric acid (10 mM) and heating in a microwave.
Subsequently, the slides were incubated with 5% goat serum (cat.
no. SL038; Beijing Solarbio Science & Technology Co., Ltd.) and
5% BSA (cat. no. A8010-25g; Beijing Solarbio Science &
Technology Co., Ltd.) in a humidified chamber at 26°C in PBS for 1
h to block non-specific binding. Subsequently, the slides were
blocked with 5% BSA at 4°C for 2 h and then incubated overnight at
4°C with an anti-cleaved caspase 3 antibody (1:1,000; cat. no.
9664S; Cell Signaling Technology, Inc.). The following day, the
slides were rinsed with PBS three times for 5 min each and a
secondary antibody conjugated to Alexa Fluor® 488
(1:200; cat. no. ab150077; Abcam.) was applied in the dark at 25°C
for 1 h. Slides were then washed five times with PBS for 5 min
each, and counterstained with DAPI in the dark at room temperature
for 10 min. All images were examined and expression levels were
semi-quantified using NIE confocal microscopy and NIS-Elements
Viewer 4.20 (Nikon Corporation) (16).
HT22 immunofluorescence
Cells were initially seeded in a 24-well plate at a
density of 1×103−2×103 cells/well. After 18
h, a modeling intervention was conducted. Subsequently, 500 µl 4%
paraformaldehyde (PFA) was added to each well and incubated at 4°C
for 25 min to fix the cells. The excess liquid surrounding the
wells was removed using filter paper. Subsequently, the cells were
permeabilized with 0.2% Triton X-100 in PBS at room temperature for
5 min and were blocked in 10% goat serum (cat. no. SL038; Beijing
Solarbio Science & Technology Co., Ltd.) in PBS at room
temperature for 1 h. The cells were then incubated in a humidified
chamber at 37°C for 60 min and subsequently washed with 2 ml PBS
three times for 5 min each, followed by removal of the PBS. The
cells were then were incubated overnight with the anti-cleaved
caspase 3 antibody (1:200 in 10% goat serum; cat. no. 9664S; Cell
Signaling Technology, Inc.) in a humidified chamber at 4°C. The
following day, the wells were washed three times with PBS for 5 min
each, then subjected to incubation with the fluorescent secondary
antibody (1:200; cat. no. ab150077 Abcam.) at room temperature for
1 h. After incubation, the wells were rinsed three times with PBS
for 5 min each. Finally, 5 µl DAPI (cat. no. ab104139; Abcam) was
added to stain the cell nuclei at room temperature for 10 min and a
fluorescence microscope (magnification, ×200; Olympus Corporation)
was used to capture images.
Brain tissue TUNEL staining
Mouse brain tissue sections were fixed in 4% PFA
solution at 4°C for 24 h. TUNEL staining was conducted using TUNEL
BrightRed Apoptosis Detection Kit (cat. no. A113-01; Vazyme Biotech
Co., Ltd.), which was performed according to the manufacturer's
protocol. The mean count of TUNEL-positive cells in specific three
fields of view was determined at a magnification of ×20, three
randomly chosen high-power fields were utilized to verify the cell
count. Cell boundaries were defined by adjusting the brightness of
DAPI staining (2 µg/ml, for 5 min at room temperature in the dark)
to optimize visualization on a slide. Samples were then placed on
slides and images were captured using a fluorescence
microscope.
Neuronal immunostaining
Neuronal staining experiments were performed on
mouse brain tissue sections and HT22 cell samples. TUNEL-positive
cells were evaluated 24 h post-OxyHb treatment. HT22 neurons were
labeled with the neuronal marker NeuN (1:100; cat. no. ab104224;
Abcam) overnight at 4°C, followed by incubation with an Alexa Fluor
488-conjugated secondary antibody (1:200; cat. no. ab150113; Abcam)
at room temperature for 1 h. Cleaved-caspase 3 and NeuN staining
experiments were performed simultaneously, and the TUNEL assay was
conducted using a TUNEL detection kit according to the
manufacturer's protocol (cat. no. C1089; Beyotime Institute of
Biotechnology). For each section, three fields of view were
assessed and the TUNEL-positive neurons near the site of injury
were visualized and counted using a fluorescence microscope
(magnification, ×200; Olympus Corporation). Results are presented
as the apoptotic index and the percentage of TUNEL-positive neurons
relative to the total number of neurons.
Brain water content
Brain water content was determined using the wet/dry
method. The entire brain was harvested 24 h after SAH induction,
and was divided into the left and right hemispheres, and the
cerebellum. The brain specimen was then promptly weighed to obtain
the wet weight, followed by desiccation at 100°C for 72 h, after
which the dry weight was measured. The percentage of brain water
content was calculated as: Water content (%)=[(wet weight-dry
weight)/wet weight] ×100.
Electron microscopy
Mitochondrial structure in individual cells was
examined using confocal and electron microscopy/tomography. After
confocal imaging (magnification, ×25,000; HITACHI HT-7700; Hitachi,
Ltd.), cells were rapidly fixed by cold incubation (4°C) in a
solution containing 2.5% PFA, 2.5% glutaraldehyde and 0.1 M sodium
calcium carbonate buffer (pH 7.4) for 1 h. Following fixation, the
cells were treated with 1% osmium tetroxide and then incubated in a
mixture of 0.8% potassium ferrocyanide and calcium carbonate in 0.1
M sodium carbonate on ice for 60 min. After washing three times
with distilled water (3 min per wash), the fixed cells were stained
and preserved with 2% uranyl acetate for 30 min on ice, followed by
gradual dehydration in a series of cold ethanol solutions (20, 50,
70 and 90%).
Subsequently, the cells underwent three cycles of
dehydration in 100% ethanol at room temperature, each lasting 3 min
and were then permeabilized with a thoroughly mixed 50% ethanol/50%
Durcuban ACM resin (Fluka) solution for 60 min with agitation at
room temperature. This was followed by two agitation cycles in 100%
Durcuban ACM for 1 h each, after which the samples were polymerized
in an oven at 60–80°C for a minimum of 48 h. The glass coverslip
was removed from the bottom surface using a razor blade and
specific regions were sectioned and affixed to the block (Durcuban
ACM resin) for subsequent slicing. Thin sections of ~80 nm were
prepared, stained with 2% uranyl acetate and Sato lead at 4°C for 2
h, and analyzed using FEI Tecnai 12 transmission electron
microscopy (Thermo Fisher Scientific, Inc.). ImageJ software
(ImageJ 2021 Version 1.53m; National Institutes of Health) was used
to identify and mark normal and damaged mitochondria. The damage
ratio was calculated as follows: Damage ratio (%)=(number of
damaged mitochondria/total number of mitochondria) ×100.
RNA-sequencing (RNA-seq) method
RNA-seq was conducted by Shenzhen Hypros
Biotechnology Co., Ltd. Briefly, total RNA was extracted from HT22
cells in the NGR1 + OxyHb and OxyHb groups using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The quality and purity of RNA were
examined using a NanoDrop™ One/OneC spectrophotometer
(Thermo Fisher Scientific, Inc.) and Qubit RNA Broad-Range Assay
Kit (Invitrogen; Themo Fisher Scientific, Inc.). RNA integrity was
analyzed using the Agilent 4200 TapeStation system (Agilent
Technologies, Inc.). The NEBNext® Poly(A) mRNA Magnetic
Isolation Module (New England Biolabs, Inc.) was used to isolate
mRNA, followed by library construction using the NEBNext
Ultra™ II mRNA Library Prep Kit for Illumina®
(New England Biolabs, Inc.). Purified cDNA libraries were prepared
for cluster generation and paired-end sequencing (150 bp on each
end) on an Illumina NovaSeq 6000 platform using the NovaSeq 6000
Reagent Kit (cat. no. 20028312) (both from Illumina, Inc.)
according to the manufacturer's protocol.
Subsequently, a comprehensive bioinformatics data
analysis was conducted. The process began with quality inspection
using Fastp version 0.20.0 (46).
Next, sequence alignment was performed using HISAT2 version 2.1.0
(47). Gene quantification
followed, employing HTSeq version 0.10.0 (48), as documented in their online
installation guide. Differential expression analysis was then
carried out using DESeq2 version 1.18.1 (49) and edgeR version 3.20.9 (50), both available through Bioconductor.
For functional enrichment annotation, the clusterProfiler (51) tool version 3.6.0 was utilized,
which was also accessed via Bioconductor.
Differential expression analysis and
kyoto encyclopedia of genes and genomes (KEGG) enrichment
analysis
Differential expression analysis to detect
differentially expressed genes (DEGs) between the NGR1 + OxyHb and
OxyHb groups was conducted using the DESeq2 package in R (52,53)
(version 2022.07.2), with the criteria set at an adjusted P<0.01
and absolute log2 fold change >2. Subsequently, the
clusterProfiler (51) (version
4.0.5) package was used for KEGG pathway analysis, with a false
discovery rate of <0.05 considered to indicate significantly
enriched functional pathways.
Statistical analysis
Statistical analysis was conducted using GraphPad
Prism (version 10.0; GraphPad Software, Inc.). Categorical data are
presented as the median (interquartile range) and were analyzed
using the Kruskal-Wallis test and Dunn's post hoc test. Continuous
data are presented as the mean ± standard error of the mean derived
from at least three independent experiments. For comparisons
involving three or more independent groups, one-way analysis of
variance was used, followed by Tukey's Honestly Significant
Difference post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparative analysis of basilar
appearance and neurological scores in mice with surgically-induced
SAH
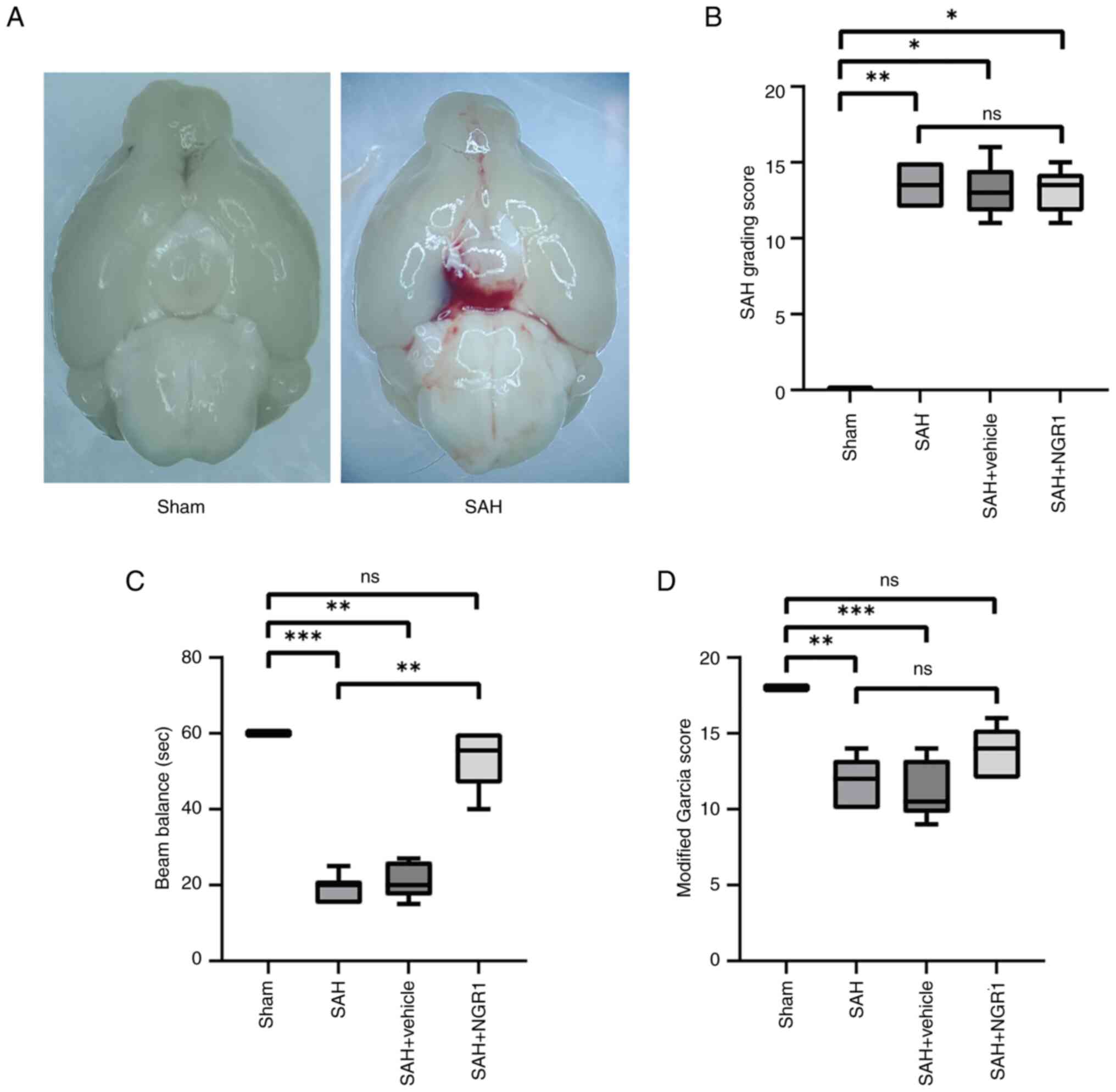
The results of the present study indicated that NGR1
(Fig. S1A) mitigated short-term
brain functional impairment following SAH. SAH was induced in mice
via vascular puncture, with the sham-operated group serving as a
control (Fig. 1A). SAH grading
assessment confirmed that most experimental mice met the criteria
of the present study, with five mice excluded based on a SAH grade
of <8 (Fig. 1B). The outcomes
of the balance beam experiment indicated that the administration of
NGR1 significantly reduced acute neurological impairments in mice
compared with those in the SAH group (Fig. 1C), and improved the modified Garcia
score compared with those in the SAH group (Fig. 1D). The drug concentrations used in
the experiment are shown in Fig. S2A
and C. As shown in Fig. S2A and
C, various concentrations of NGR1 were used, and the
appropriate drug concentration (100 mg/kg NGR1) for in vivo
experiments was selected, where the expression levels of
apoptosis-related proteins (cleaved-caspase 3, BAX, BCL2 and
caspase 3) showed no significant difference compared with the Sham
group. These findings indicated the potential beneficial effects of
NGR1 on short-term neurological function in mice. To further
investigate the mechanism by which NGR1 exerts its effects,
subsequent experiments were conducted to investigate its
anti-apoptotic effects.
Cytotoxicity and cell viability
assessment of NGR1 in vitro
Analysis of the in vitro SAH model results
indicated that NGR1 may possess potential anti-apoptotic effects.
Protein expression levels in cells treated with NGR1 in the various
experimental groups were analyzed by western blotting to determine
the optimal dosage of NGR1 in vitro (100 mM), as
aforementioned (Fig. S2A and C).
As shown in Fig. S1B, a
substantial number of dead cells was observed in the OxyHb group
and the OxyHb + Vehicle group, with a notably reduced number of
surviving cells; this was attributed to the stimulation by OxyHb.
Conversely, the opposite was observed in the Control group and the
OxyHb + NGR1 group, where a higher number of surviving cells was
detected. The optimal anti-apoptotic concentration of NGR1 for
subsequent experiments was based on the expression levels of
apoptosis-related proteins (cleaved-caspase3, BAX, BCL2 and caspase
3) after drug intervention. A dosage of 100 mg/kg NGR1 was selected
for the in vivo experiments (Fig. S2A and C) and 100 mM for the in
vitro experiments (Fig. S2B and
D). Subsequent in vitro experiments demonstrated that
NGR1 significantly reduced LDH release (Fig. S1C) and increased cell viability
(Fig. S1D). In addition, flow
cytometric analysis indicated a reduction in apoptosis following
NGR1 treatment (Fig. S3A and
B).
These findings suggested that NGR1 may alleviate
cytotoxicity and improve cell viability in an in vitro SAH
model. To further confirm these results, in subsequent experiments,
the expression levels of apoptosis-associated proteins were
assessed to directly validate the anti-apoptotic effects of
NGR1.
Validation of the anti-apoptotic
properties of NGR1 in vivo and in vitro
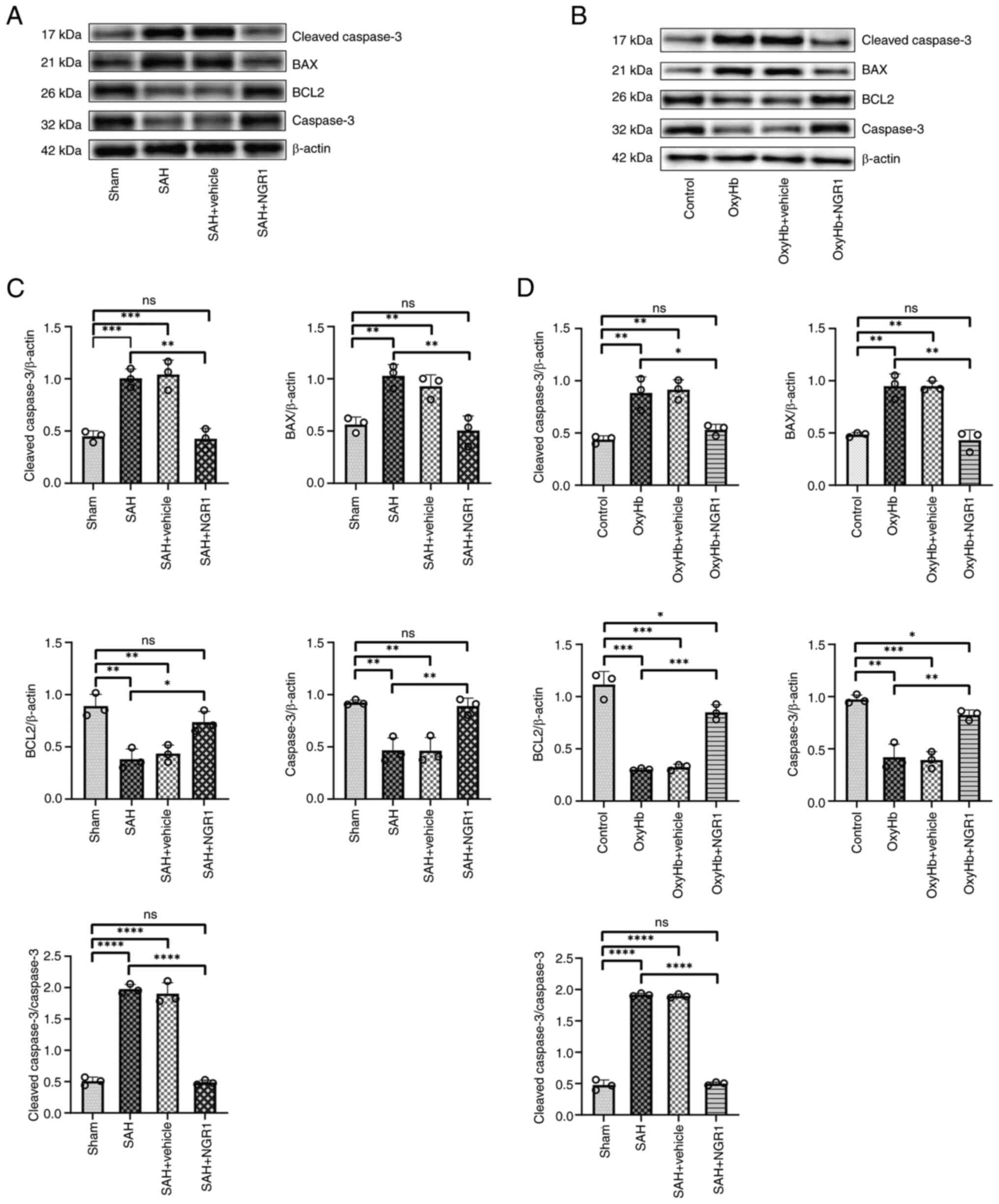
Western blotting demonstrated that NGR1 exhibited
significant anti-apoptotic effects in vivo and in
vitro. In the in vivo model, the expression of
cleaved-caspase 3 in the SAH + NGR1 group was downregulated
compared with that in the SAH group, with no significant difference
observed compared with the control group; notably, there were
significant differences when both the SAH group and the SAH +
vehicle group were compared to the Sham group (Fig. 2A and C). The expression pattern of
Bax exhibited a similar trend. By contrast, the expression trends
of caspase 3 and BCL2 were the opposite. Furthermore, the ratio of
cleaved-caspase 3 to caspase 3 was significantly reduced in the SAH
+ NGR1 group compared with that in the SAH group. A similar trend
was observed in the in vitro model when comparing the OxyHb
group to the OxyHb + NGR1 group (Fig.
2B and D). These findings suggested that NGR1 exerted
anti-apoptotic effects in the brain tissue of mice with SAH, with
similar trends observed in HT22 cells. At the critical 24-h time
point, NGR1 conferred notable anti-apoptotic effects at the protein
level in vivo and in vitro. To further confirm these
findings and to provide insights into the specific anti-apoptotic
mechanism by which NGR1 exerted its effects, caspase 3 activity
assays were conducted. Caspase 3 activity serves as an indirect
indicator of apoptotic pathway activation, aiding in understanding
the initiation of apoptosis within cells. Additionally, TUNEL
staining was carried out, which is a direct method for detecting
DNA fragmentation, which is indicative of apoptosis. Considering
these distinctive characteristics, both methods were performed.
Cleaved-caspase 3 and TUNEL
immunofluorescence staining validate the anti-apoptotic effects of
NGR1
TUNEL and cleaved-caspase 3 immunofluorescence
staining were conducted on mouse brain tissues and HT22 neuronal
cells. Co-staining for neurons and cleaved-caspase 3-positive cells
was conducted to evaluate the impact of NGR1. Notably, the OxyHb
group exhibited significant neuronal apoptosis, which was reduced
in the NGR1 group, as indicated by the decreased number of
cleaved-caspase 3-positive neurons in the OxyHb + NGR1 group
compared with that in the OxyHb group (Fig. S4A and B). The TUNEL assay further
corroborated these observations (Fig.
S4C and D). Additionally, In the SAH group, the significant
increase in cleaved-caspase 3 protein levels and the presence of
TUNEL-positive neurons confirmed the occurrence of neuronal
apoptosis in brain tissues. The results also indicated that NGR1
intervention significantly attenuated apoptosis in the in
vivo model compared with that in the SAH group (Fig. 3A-D). By cross-validating caspase 3
activity assessment and TUNEL staining results, it was confirmed
that NGR1 exhibited anti-neuronal apoptotic effects in both mouse
brain tissues and in vitro neuronal cultures. Given that the
endogenous apoptotic pathway involves the release of cytochrome
c from mitochondria, which activates pro-caspase into active
caspase 3, thereby initiating apoptosis, mitochondrial structural
changes before and after NGR1 treatment were examined using
electron microscopy.
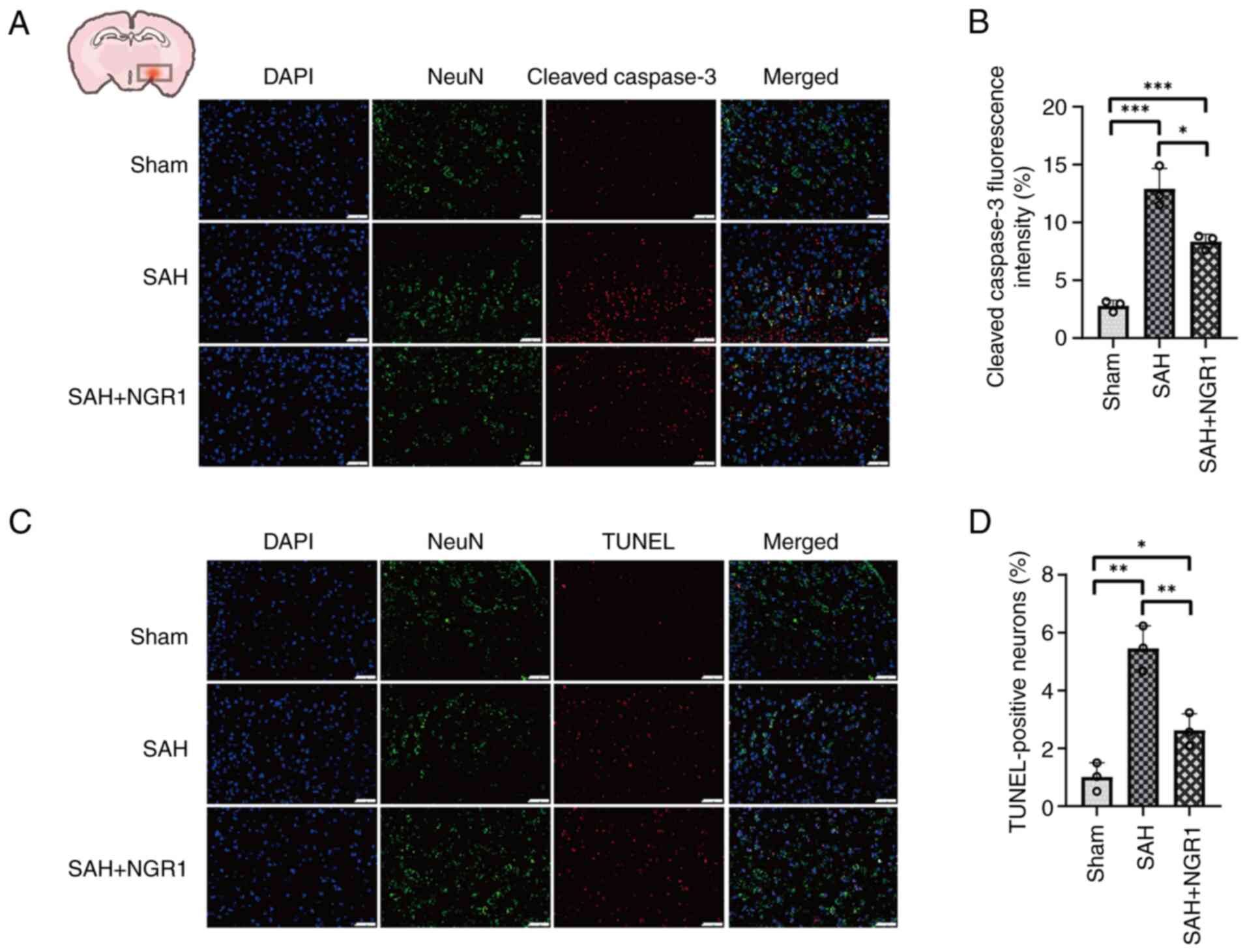 | Figure 3.Verification of the anti-apoptotic
effects of NGR1 using cleaved-caspase 3 and TUNEL assays. (A)
Following exogenous NGR1 administration, a significant reduction in
cleaved-caspase 3-positive neurons was observed. Staining
represents cleaved-caspase 3 (red), neurons (green) and
DAPI-stained nuclei (blue). (B) Statistical analysis of
cleaved-caspase 3 fluorescence intensity. (C) Following exogenous
NGR1 administration, a significant reduction in TUNEL-positive
neurons was observed, indicative of reduced apoptosis. Staining
represents TUNEL (red), neurons (green) and DAPI-stained nuclei
(blue). (D) Statistical analysis of TUNEL fluorescence intensity.
Data are presented as the mean ± SD and were analyzed using a
one-way ANOVA followed by a Tukey's honestly significant difference
post hoc test. *P<0.05, **P<0.01, ***P<0.001. n=3 per
group. Scale bar, 50 µm. SAH, subarachnoid hemorrhage; NGR1,
notoginsenoside R1. |
NGR1 attenuates mitochondrial
structural damage post-SAH
Electron microscopy of HT22 cells revealed that the
control group maintained a largely intact neuronal and
mitochondrial morphology. By contrast, the OxyHb group exhibited
signs of organelle swelling and dissolution, karyolysis and
compromised membrane integrity. In mice administered NGR1, these
phenotypic structural damages were markedly reduced compared with
those in the OxyHb group (Fig. 4A and
B).
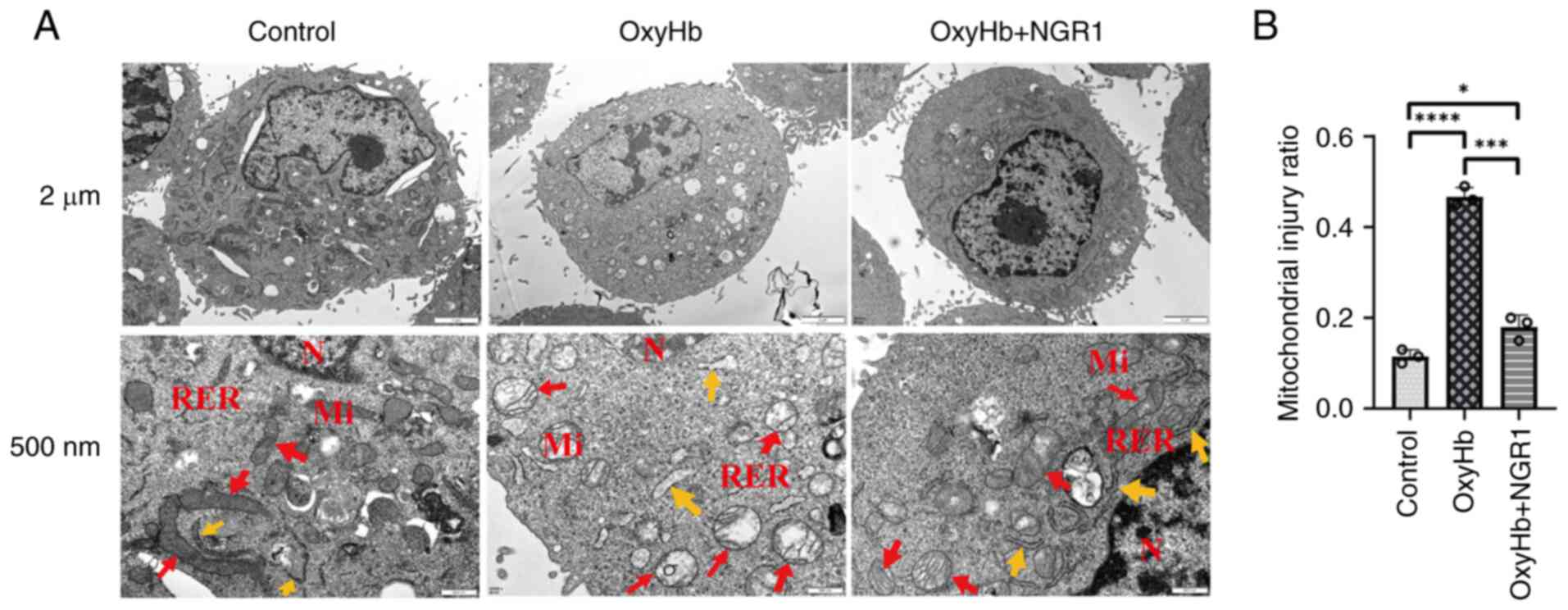 | Figure 4.Effect of NGR1 treatment on
mitochondrial morphology in HT22 neurons treated with OxyHb for 24
h. (A) Control untreated cells: Normal mitochondria (red arrows),
normal RER (yellow arrows). Cells treated with OxyHb: Swollen
mitochondria (red arrows), mildly dilated RER (yellow arrows).
Cells treated with 100 mM NGR1 after OxyHb treatment: Mildly
swollen mitochondria (red arrows), normal RER (yellow arrows);
magnification, ×25,000; scale bar, 2 µm, 500 nm. (B) Statistical
analysis. Data are presented as the mean ± SD and were analyzed
using a one-way ANOVA followed by a Tukey's honestly significant
difference post hoc test. n=3 per group. *P<0.05,
****P<0.0001. NGR1, notoginsenoside R1; N, nucleus; Mi,
mitochondria; RER, rough endoplasmic reticulum; OxyHb,
oxyhemoglobin. |
These experimental results indicated that NGR1
mitigated mitochondrial structural damage following SAH. To further
investigate the target genes associated with the anti-apoptotic
effects of NGR1, sequencing experiments were conducted. Moreover,
NGR1 alleviated brain edema post-SAH compared with in the SAH group
(Fig. S5).
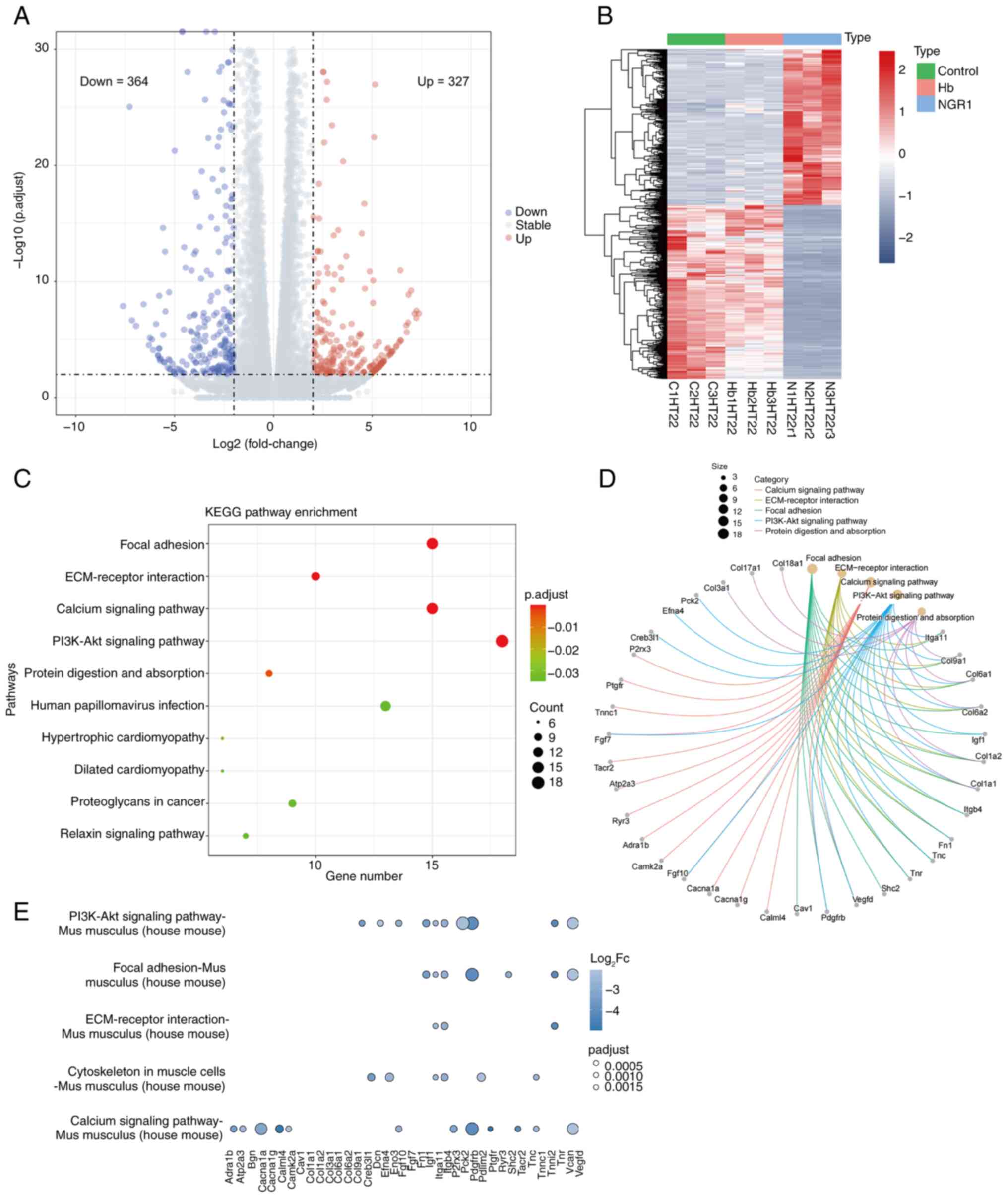
Transcriptome analysis and target gene
identification
To delve deeper into the specific mechanisms of the
anti-apoptotic effects of NGR1 following SAH, drug intervention in
HT22 cells was modeled and conducted, followed by RNA-seq analysis.
ITGA11 was identified as the target gene for subsequent protein
expression level validation using transcriptome analysis. The
analysis revealed 691 DEGs in the NGR1 + OxyHb group vs. the OxyHb
group, including 327 upregulated and 364 downregulated genes
(Fig. 5A; Table SI). A heatmap depicting these
genes highlighted differences in gene expression levels (Fig. 5B). Subsequent KEGG pathway
enrichment analysis (Fig. 5C)
identified the top five affected pathways, which were ‘focal
adhesion’, ‘ECM-receptor interaction’, ‘calcium signaling pathway’,
‘PI3K-Akt signaling pathway’, and ‘protein digestion and
absorption’. The downregulation of these pathways may be associated
with the anti-apoptotic efficacy of NGR1. To precisely locate
potentially relevant genes, a chord diagram (Fig. 5D) was used to visualize all genes
involved in the aforementioned pathways. Based on the degree of
enrichment, the top five enriched genes were selected: ITGA11,
COL9a1, COL6a1, COL6a2 and IGF1 (Fig.
5D). Comparative analysis showed that although ITGA11 gene
expression levels did not significantly change post-OxyHb
stimulation alone, its expression level decreased four-fold with
the addition of NGR1 compared with in the OxyHb stimulation group
(Table SI). Finally, enrichment
of ITGA11 was seen in the ‘PI3K-Akt pathway’ in the KEGG scatter
plot (Fig. 5E), thus, the PI3K-Akt
pathway was chosen for subsequent analysis.
Based on the aforementioned experimental results,
ITGA11 was selected as the target gene; therefore, it was used for
subsequent protein expression level validation. To validate the
function of this gene, in vivo experiments and western
blotting on mouse brain tissues were conducted.
Validation of ITGA11 protein
expression levels
The expression levels of ITGA11 were downregulated
in the SAH + NGR1 group compared with those in the SAH group
(Fig. 6A and B). Notably, no
statistically significant differences were observed in the
expression levels of PI3K and p-Akt when comparing the SAH + NGR1
group with the Sham group; however, when comparing the SAH group
and the SAH + vehicle group with the Sham group, the expression
levels of both PI3K and p-Akt were reduced. Additionally, following
NGR1 intervention, the expression levels of BCL2 and BAX aligned
with the anti-apoptotic effects of NGR1, whereas there was no
significant change in Akt protein expression among all of the
groups.
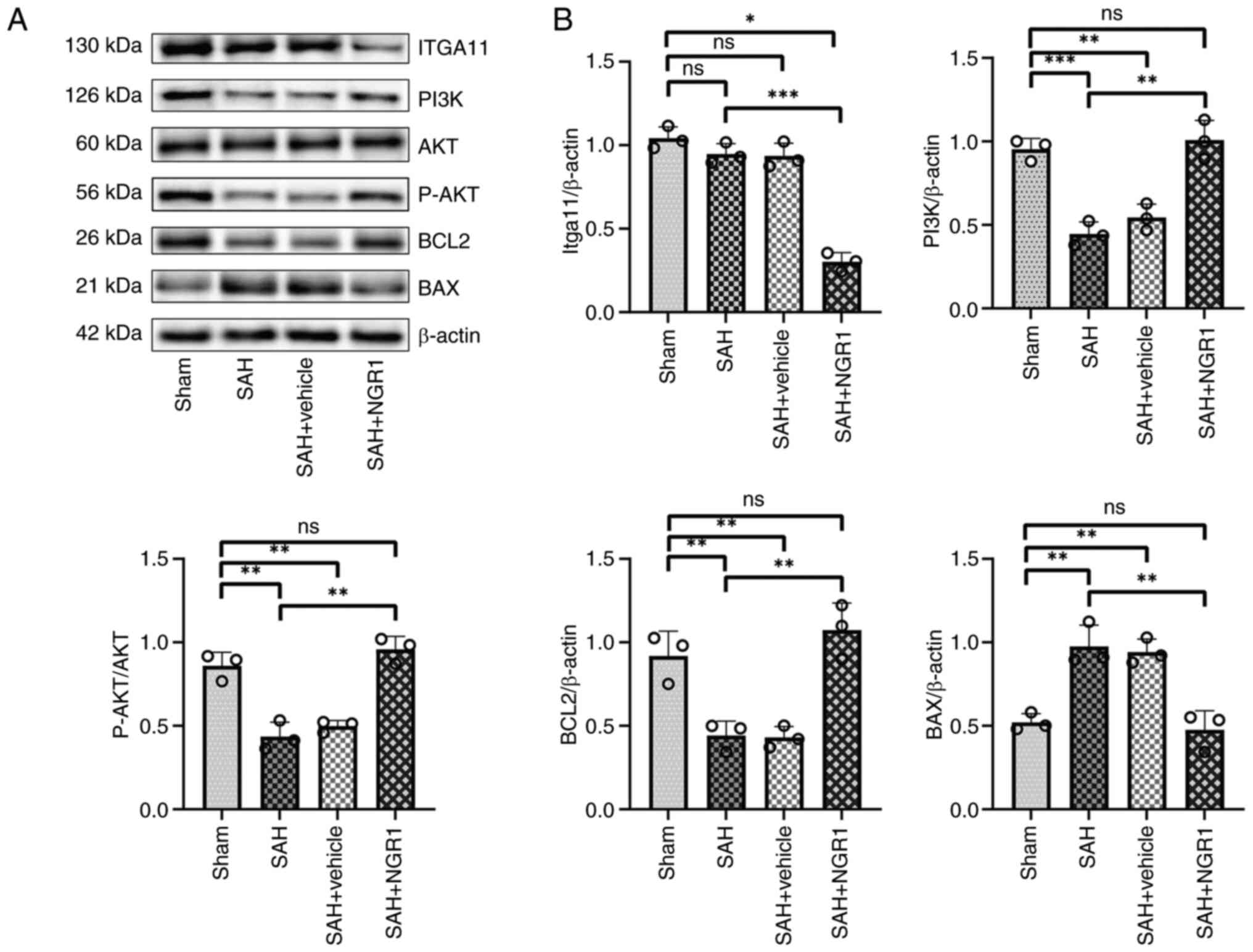 | Figure 6.Western blot analysis validation of
the bioinformatics results. (A) Protein expression levels of
ITGA11, PI3K, Akt, p-Akt, BCL2 and BAX. (B) Densitometric analysis
of the protein expression levels. Data are presented as the mean ±
SD and were analyzed using a one-way ANOVA followed by a Tukey's
honestly significant difference post hoc test. *P<0.05,
**P<0.01, ***P<0.001. ns, not significant; P-,
phosphorylated; SAH, subarachnoid hemorrhage; ITGA11, integrin
subunit α11. |
The aforementioned experimental results suggested
that NGR1 may regulate neuronal apoptosis by inhibiting the
expression levels of ITGA11. These results highlight a novel
pathway, PI3K-Akt-ITGA11, which may be targeted for the treatment
of SAH.
Discussion
The findings of the present study indicated that
NGR1 administration significantly reduced neurological deficits in
experimental SAH models. NGR1 demonstrated neuroprotective effects
by reducing neuronal apoptosis and cerebral edema, potentially due
to its ability to preserve mitochondrial structural integrity. The
findings of the present study also suggested that the regulatory
effects of NGR1 on neuronal apoptosis were mediated, at least
partially, through the ITGA11 pathway, providing novel insights
into its mechanisms of action.
Limitations of the present study were identified.
First, the use of only male mice excluded potential influences from
female physiology, such as hormonal fluctuations, pregnancy and
menstrual cycles (54). Future
studies should incorporate female mice to enhance the
generalizability of the findings.
Another limitation involved discrepancies between
the in vivo and in vitro models. The present study
observed variations in the effects of NGR1 between mouse brain
tissues and HT22 cells, In the in vivo results, there was no
significant difference in the expression levels of BCL2 and
cleaved-caspase 3 after treatment with NGR1 compared with those in
the control group. Furthermore, the downregulatory effect of NGR1
on cleaved-caspase 3 expression was more pronounced in cells than
in brain tissues when compared with the respective control groups,
which may be due to differences in drug solubility, absorption and
permeability, as well as other physiological factors such as
feeding states and drug interactions (55,56).
Additionally, environmental differences between in vivo and
in vitro conditions, such as pH and solubility, may have
influenced the efficacy of the drug, highlighting the need for
further refinement of these models to enhance the reliability of
the experimental results.
The HT22 cell line is commonly used as a model in
SAH research, with numerous studies using this cell line due to its
advantageous characteristics (57–59).
HT22 cells are derived from primary neuronal cells, they can
proliferate indefinitely and are commercially available, which
makes them highly accessible. This cell line is particularly suited
for drug screening as it provides a large, homogeneous population
of cells, ensuring experimental reproducibility. However, the use
of HT22 cells also presents certain limitations (60). Over prolonged culture periods, cell
lines can accumulate genetic mutations and lose important
biological characteristics, leading to deviations from the
properties of primary cells or neurons found in the mouse brain. To
minimize these effects, cells from the 4 to 5th passage were used
in the present study.
Additionally, analysis of the literature revealed
that several studies preferentially use primary cells as in
vitro models (61–63). Primary cells, isolated from mouse
brain tissues offer greater biological specificity and
authenticity. They retain key morphological, functional and
metabolic characteristics of brain tissues, providing a more
accurate representation of in vivo conditions. However, the
isolation and maintenance of primary cells is labor-intensive and
their limited lifespan poses challenges for long-term experiments.
The choice of cell types for in vitro models should be
determined by the specific needs of each experiment. Given the
lower biological variability and reduced risk of genetic mutations,
the use of primary cells will be considered in future experiments
to enhance the reliability and biological relevance of the
findings.
A further limitation of the present study is the
focus on short-term outcomes, without assessing the long-term
effects of NGR1 on neurological recovery and overall survival
(64). While the present study
demonstrated the short-term efficacy of NGR1, long-term studies are
essential to fully understand its therapeutic potential in SAH
treatment and to determine the longevity of its neuroprotective
effects.
Secondly, the behavioral experiments used to
evaluate the efficacy of the drug in mice in the present study
included only the balance beam test and the Garcia score. These
were limited behavioral assessments used to evaluate the efficacy
of NGR1, which primarily demonstrated the anti-apoptotic effects of
NGR1. In future experiments, to improve understanding of the
neurological recovery of the mice, the Bederson score to assess
neurological deficits, the Novel Object Recognition Test to
evaluate long-term memory and recognition abilities, and the Morris
water maze test to measure spatial learning and memory capabilities
will be implemented.
The absence of clinical data represents another
limitation (65). The experiments
were confined to mouse models and cell lines, without validation in
human patients. Although animal models provide valuable preliminary
insights, clinical trials are necessary to confirm the safety and
efficacy of NGR1 in patients with SAH. These trials will be key in
translating the current preclinical findings into potential
therapeutic applications.
While the present study demonstrated the regulatory
role of NGR1 on ITGA11 and the PI3K-Akt signaling pathway, the
precise molecular mechanisms remain unresolved. Further research
should incorporate various molecular biology techniques, such as
protein-protein interaction experiments, gene knockout studies and
pathway inhibition experiments to further investigate the mechanism
of NGR1 action. Notably, it is likely that NGR1 primarily functions
through the ITGA11 and PI3K-Akt pathways, which provides key
insights into its anti-apoptotic effects, and the incomplete
elucidation of its mechanism does not undermine the observed
efficacy of NGR1.
The present study did not comprehensively
investigate the pharmacokinetics of NGR1 or the metabolic pathways,
which can influence optimal administration and therapeutic
efficacy. Further pharmacokinetic and pharmacodynamic studies are
required to ensure that NGR1 reaches effective concentrations in
the brain and remains active for sufficient durations to exert its
neuroprotective effects (66).
Despite these limitations, the multi-methodological
approach used in the present study provides a demonstration of the
efficacy of NGR1 in reducing neuronal apoptosis and cerebral edema
in SAH models in vivo and in vitro. By addressing the
limitations and proposing areas for future research, the present
study lays the groundwork for the further exploration of NGR1 as a
potential therapeutic agent in SAH and provides valuable insights
into the underlying mechanisms involved in its neuroprotective
effects.
Strengths of the present study include a
comprehensive, multi-layered design, using both C57BL/6J mice to
model SAH and HT22 mouse hippocampal neuron cells for in
vitro experiments (67). This
combination of animal and cell models facilitated a thorough
understanding of the mechanism of NGR1. The experimental procedures
were well-controlled, with strict oversight of conditions such as
mouse housing, anesthesia and SAH induction, ensuring the
reliability of the results. Various detection methods were used,
including behavioral tests, biochemical analyses, flow cytometry,
immunofluorescence staining, western blotting and electron
microscopy, contributing to a detailed examination of the effects
of NGR1 (68).
The present study also investigated the molecular
mechanisms of NGR1, as RNA-seq transcriptomic analysis identified
significant changes in apoptosis-associated genes and pathways,
particularly the downregulation of ITGA11 and PI3K-Akt signaling
under NGR1 treatment. These findings were validated at the protein
level through western blotting, reinforcing the credibility of the
molecular analyses. Furthermore, the potential for clinical
translation was emphasized, as the anti-apoptotic effects of NGR1,
derived from Panax notoginseng, suggest its potential
application in SAH treatment. The experimental design, including
control and drug intervention groups, further clarified the
specific effects of NGR1 and established a dose gradient for
optimal concentration in in vitro tests.
In summary, the experimental design of the present
study, varied detection methods and comprehensive molecular
analyses demonstrated the role of NGR1 in reducing neuronal
apoptosis and cerebral edema in SAH. These findings offer a novel
perspective on integrating TCM in modern medical research.
In relation to existing research, the findings of
the present study are in agreement with similar studies, which
showed that NGR1 exerts a neuroprotective effect (69–72).
The choice of NGR1 as the therapeutic agent is consistent with
existing studies that have demonstrated its neuroprotective
properties (70,73,74),
such as its role in inhibiting neuroinflammation and promoting
neuronal survival (75). The
present study extends on previous research by applying NGR1
specifically in a model of SAH. Furthermore, it corroborates the
established role of the PI3K-Akt signaling pathway in
neuroprotection (72), reinforcing
the relevance of the effects of NGR1 through this pathway. It also
deepens the understanding of the mechanisms of NGR1 by
investigating the ITGA11 and PI3K-Akt pathways in detail. The use
of multiple evaluation indicators, such as assessment of neuronal
apoptosis and cerebral edema, adds further depth to the
results.
The opportunities for future research are extensive.
The present study highlights novel research directions for
developing SAH-specific therapeutic strategies, with a particular
focus on drug development exploiting the neuroprotective effect of
NGR1. The identification of key molecular pathways, such as ITGA11
and PI3K-Akt, provides a foundation for future drug development
targeting these pathways. The potential for combined treatments
with other pathway regulators is another promising avenue for
exploration. Moreover, the present study lays the foundation for
the translation of these preclinical findings into clinical
research, providing valuable insights into the dose, safety and
efficacy of NGR1.
The experimental design and methods used in the
present study offer guidance for future research, to validate the
reliability and reproducibility of the results. Additionally, the
interdisciplinary nature of the present study, combining
neuroscience and pharmacology, fosters innovation in both basic and
clinical applications. The identification of novel mechanisms for
NGR1 in SAH treatment may promote further basic research into the
broader biological effects of this compound. Importantly, the
findings of the present study suggest the potential for clinical
trials to evaluate the therapeutic potential and safety of NGR1 in
humans.
In conclusion, the present study provides important
insights into the mechanisms and therapeutic potential of NGR1 in
treating SAH. The identification of key pathways, such as ITGA11
and PI3K-Akt, not only advances the understanding of the effects of
NGR1, but also lays the groundwork for future drug development and
combinatorial therapeutic strategies. The methodology and
comprehensive evaluation of the present study offer valuable
guidance for future experimental designs, with the aim of
identifying the value of NGR1 for the treatment of SAH. It is
anticipated that this work will lead to promoting further
advancements in SAH treatment.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the Sichuan Science and
Technology Program (grant nos. 2023YFH0069, 2023NSFSC0028 and
2022YFS0615) and the Luzhou Government-Southwest Medical University
Strategic Cooperation Project of Southwest Medical University
Project (grant nos. 2021LZXNYD-P01 and 2021ZKZD013).
Availability of data and materials
The RNA-seq data generated in the present study may
be found in the Gene Expression Omnibus database under accession
number GSE240154 or at the following URL: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE240154.
The other data generated in the present study may be requested from
the corresponding author.
Authors' contributions
YH designed the present study, prepared the
manuscript and conducted experiments. LZ conducted the
bioinformatics analyses. WM and YJ made substantial contributions
to the analysis and interpretation of the data for the present
study. WM and YJ confirm the authenticity of all the raw data. All
authors have read and approved the final version of the
manuscript.
Ethical approval and consent to
participate
All experimental animal procedures conducted in the
present study were approved by the China Committee for the
Protection and Use of Experimental Animals and complied with the
regulatory requirements of the AVMA Guidelines for the Euthanasia
of Animals: 2020 Edition (32).
All research protocols involving surgical procedures and animal use
were approved by the Laboratory Animal Welfare Ethics Committee
(approval no. 20211126-005).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thilak S, Brown P, Whitehouse T, Gautam N,
Lawrence E, Ahmed Z and Veenith T: Diagnosis and management of
subarachnoid haemorrhage. Nat Commun. 15:18502024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peng L, Qin H, Liu J, Wu N, Wang X, Han L
and Ding X: Neurosurgical clipping versus endovascular coiling for
patients with ruptured anterior circulation aneurysms: A systematic
review and meta-analysis. Neurosurg Rev. 47:682024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chalet FX, Briasoulis O, Manalastas EJ,
Talbot DA, Thompson JC and Macdonald RL: Clinical burden of
angiographic vasospasm and its complications after aneurysmal
subarachnoid hemorrhage: A systematic review. Neurol Ther.
12:371–390. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cahill J and Zhang JH: Subarachnoid
hemorrhage: Is it time for a new direction? Stroke. 40 (3
Suppl):S86–S87. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schupper AJ, Hardigan TA, Mehta A, Yim B,
Yaeger KA, De Leacy R, Fifi JT, Mocco J and Majidi S: Sex and
racial disparity in outcome of aneurysmal subarachnoid hemorrhage
in the United States: A 20-year analysis. Stroke. 54:1347–1356.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lauzier DC, Jayaraman K, Yuan JY, Diwan D,
Vellimana AK, Osbun JW, Chatterjee AR, Athiraman U, Dhar R and
Zipfel GJ: Early brain injury after subarachnoid hemorrhage:
Incidence and mechanisms. Stroke. 54:1426–1440. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang H and Lai LT: Incidence and
case-fatality of aneurysmal subarachnoid hemorrhage in Australia,
2008–2018. World Neurosurg. 144:e438–e446. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xia C, Hoffman H, Anikpezie N, Philip K,
Wee C, Choudhry R, Albright KC, Masoud H, Beutler T, Schmidt E, et
al: Trends in the incidence of spontaneous subarachnoid hemorrhages
in the United States, 2007–2017. Neurology. 100:e123–e132. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qureshi AI, Bhatti IA, Gillani SA, Beall
J, Cassarly CN, Gajewski B, Martin RH, Suarez JI and Kwok CS:
Prevalence, trends, and outcomes of cerebral infarction in patients
with aneurysmal subarachnoid hemorrhage in the USA. J Neuroimaging.
34:790–798. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vasconcellos de Oliveira Souza N, Rouanet
C, Fontoura Solla DJ, Barroso de Lima CV, Trevizo J, Rezende F,
Alves MM, de Oliveira Manuel AL, Righy C, Chaddad Neto F, et al:
Impact of medical and neurologic complications on the outcome of
patients with aneurysmal subarachnoid hemorrhage in a middle-income
country. World Neurosurg. 183:e250–e260. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu W, Ling X, Petersen JD, Liu J, Xiao A
and Huang J: Clipping versus coiling for aneurysmal subarachnoid
hemorrhage: A systematic review and meta-analysis of prospective
studies. Neurosurg Rev. 45:1291–1302. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Liyis BG, Surya SC and Tini K:
Effectivity and safety of endovascular coiling versus microsurgical
clipping for aneurysmal subarachnoid hemorrhage: A systematic
review and meta-analysis. Clin Neurol Neurosurg. 236:1080582024.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Le VT, Nguyen AM and Nguyen PL: Risk
factors for in-hospital seizure and new-onset epilepsy in coiling
and clipping treatment of aneurysmal subarachnoid hemorrhage. World
Neurosurg. 184:e460–e467. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Früh A, Wolf S, Wasilewski D, Vajkoczy P
and Truckenmueller P; EARLYDRAIN study group, : Early complications
and outcome after treatment of ruptured aneurysms in patients with
subarachnoid hemorrhage-A post hoc analysis of the EARLYDRAIN
trial. World Neurosurg. 184:e720–e730. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li M, Tian Z, Ru X, Shen J, Chen G, Duan Z
and Cui J: Comparison of endovascular interventional embolization
and microsurgical clipping for ruptured cerebral aneurysms: Impact
on patient outcomes. Int J Neurosci. 1–8. 2024. View Article : Google Scholar
|
|
16
|
Hoh BL, Topcuoglu MA, Singhal AB, Pryor
JC, Rabinov JD, Rordorf GA, Carter BS and Ogilvy CS: Effect of
clipping, craniotomy, or intravascular coiling on cerebral
vasospasm and patient outcome after aneurysmal subarachnoid
hemorrhage. Neurosurgery. 55:779–789. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tawakul A, Alluqmani MM, Badawi AS, Alawfi
AK, Alharbi EK, Aljohani SA, Mogharbel GH, Alahmadi HA and Khawaji
ZY: Risk factors for cerebral vasospasm after subarachnoid
hemorrhage: A systematic review of observational studies. Neurocrit
Care. 41:1081–1099. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lele AV, Fong CT, Walters AM and Souter
MJ: External ventricular drain placement, critical care
utilization, complications, and clinical outcomes after spontaneous
subarachnoid hemorrhage: A single-center retrospective cohort
study. J Clin Med. 13:10322024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kamp MA, Lieshout JHV, Dibué-Adjei M,
Weber JK, Schneider T, Restin T, Fischer I and Steiger HJ: A
systematic and meta-analysis of mortality in experimental mouse
models analyzing delayed cerebral ischemia after subarachnoid
hemorrhage. Transl Stroke Res. 8:206–219. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dayyani M, Sadeghirad B, Grotta JC,
Zabihyan S, Ahmadvand S, Wang Y, Guyatt GH and Amin-Hanjani S:
Prophylactic therapies for morbidity and mortality after aneurysmal
subarachnoid hemorrhage: A systematic review and network
meta-analysis of randomized trials. Stroke. 53:1993–2005. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun G: Death and survival from executioner
caspase activation. Semin Cell Dev Biol. 156:66–73. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tan W, Li Y, Ma L, Fu X, Long Q, Yan F, Li
W, Liu X, Ding H, Wang Y and Zhang W: Exosomes of endothelial
progenitor cells repair injured vascular endothelial cells through
the Bcl2/Bax/caspase-3 pathway. Sci Rep. 14:44652024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu C, Fan F, Li CY, Xiong Y and Liu X:
Caspase-3 promotes oncogene-induced malignant transformation via
EndoG-dependent Src-STAT3 phosphorylation. Cell Death Dis.
15:4862024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hongmei Z: Extrinsic and intrinsic
apoptosis signal pathway review. Ntuli T: Apoptosis and Medicine.
IntechOpen; London, UK: 2012, View
Article : Google Scholar
|
|
25
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update.
Arch Toxicol. 89:289–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pisani C, Ramella M, Boldorini R, Loi G,
Billia M, Boccafoschi F, Volpe A and Krengli M: Apoptotic and
predictive factors by bax, caspases 3/9, Bcl-2, p53 and Ki-67 in
prostate cancer after 12 Gy single-dose. Sci Rep. 10:70502020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qian S, Wei Z, Yang W, Huang J, Yang Y and
Wang J: The role of BCL-2 family proteins in regulating apoptosis
and cancer therapy. Front Oncol. 12:9853632022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miller TM, Moulder KL, Knudson CM, Creedon
DJ, Deshmukh M, Korsmeyer SJ and Johnson EM Jr: Bax deletion
further orders the cell death pathway in cerebellar granule cells
and suggests a caspase-independent pathway to cell death. J Cell
Biol. 139:205–217. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Q, Huang Z, Chen J, Tian X, Zhang R,
Liang Q, Liu Z and Cheng Y: Notoginsenoside R1 attenuates ischemic
heart failure by modulating MDM2/β arrestin2-mediated β2-adrenergic
receptor ubiquitination. Biomed Pharmacother. 177:1170042024.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang S, Chen Q, Jin M, Ren J and Sun X,
Zhang Z, Luo Y and Sun X: Notoginsenoside R1 alleviates cerebral
ischemia/reperfusion injury by inhibiting the TLR4/MyD88/NF-κB
signaling pathway through microbiota-gut-brain axis. Phytomedicine.
128:1555302024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeng M, Zhang R, Yang Q, Guo L, Zhang X,
Yu B, Gan J, Yang Z, Li H, Wang Y, et al: Pharmacological therapy
to cerebral ischemia-reperfusion injury: Focus on saponins. Biomed
Pharmacother. 155:1136962022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meng X, Sun G, Ye J, Xu H, Wang H and Sun
X: Notoginsenoside R1-mediated neuroprotection involves estrogen
receptor-dependent crosstalk between Akt and ERK1/2 pathways: A
novel mechanism of Nrf2/ARE signaling activation. Free Radic Res.
48:445–460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang N, Dai Q, Su X, Fu J, Feng X and
Peng J: Role of PI3K/AKT pathway in cancer: The framework of
malignant behavior. Mol Biol Rep. 47:4587–4629. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu R, Chen Y, Liu G, Li C, Song Y, Cao Z,
Li W, Hu J, Lu C and Liu Y: PI3K/AKT pathway as a key link
modulates the multidrug resistance of cancers. Cell Death Dis.
11:7972020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vasan N and Cantley LC: At a crossroads:
How to translate the roles of PI3K in oncogenic and metabolic
signalling into improvements in cancer therapy. Nat Rev Clin Oncol.
19:471–485. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barzegar Behrooz A, Talaie Z, Jusheghani
F, Łos MJ, Klonisch T and Ghavami S: Wnt and PI3K/Akt/mTOR survival
pathways as therapeutic targets in glioblastoma. Int J Mol Sci.
23:13532022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He Y, Sun MM, Zhang GG, Yang J, Chen KS,
Xu WW and Li B: Targeting PI3K/Akt signal transduction for cancer
therapy. Signal Transduct Target Ther. 6:4252021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Peng J, Wu Y, Pang J, Sun X, Chen L, Chen
Y, Tang J, Zhang JH and Yong J: Single clip: An improvement of the
filament-perforation mouse subarachnoid haemorrhage model. Brain
Inj. 33:701–711. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Leary S, Underwood W, Anthony R, Cartner
S, Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin MA, Meyer R,
Miller D, et al: AVMA guidelines for the euthanasia of animals:
2020 Edition*. American Veterinary Medical Association 1931; N.
Meacham Road Schaumburg IL 60173: 2020
|
|
42
|
Huang T, Xiao Y, Zhang Y, Wang C, Chen X,
Li Y, Ge Y and Gao J: miR-223 ameliorates thalamus
hemorrhage-induced central poststroke pain via targeting NLRP3 in a
mouse model. Exp Ther Med. 23:3532022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Feng X, Ma W, Zhu J, Jiao W and Wang Y:
Dexmedetomidine alleviates early brain injury following traumatic
brain injury by inhibiting autophagy and neuroinflammation through
the ROS/Nrf2 signaling pathway. Mol Med Rep. 24:6612021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Peng J, Pang J, Huang L, Enkhjargal B,
Zhang T, Mo J, Wu P, Xu W, Zuo Y, Peng J, et al: LRP1 activation
attenuates white matter injury by modulating microglial
polarization through Shc1/PI3K/Akt pathway after subarachnoid
hemorrhage in rats. Redox Biol. 21:1011212019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang CS, Han Q, Song ZW, Jia HY, Shao TP
and Chen YP: Hydrogen gas post-conditioning attenuates early
neuronal pyroptosis in a rat model of subarachnoid hemorrhage
through the mitoKATP signaling pathway. Exp Ther Med.
22:8362021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen S, Zhou Y, Chen Y and Gu J: fastp: An
ultra-fast all-in-one FASTQ preprocessor. Bioinformatics.
34:i884–i890. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim D, Langmead B and Salzberg SL: HISAT:
A fast spliced aligner with low memory requirements. Nat Methods.
12:357–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Anders S, Pyl PT and Huber W: HTSeq-a
python framework to work with high-throughput sequencing data.
Bioinformatics. 31:166–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Team R, . RStudio: Integrated development
for R. Boston, MA: RStudio. Inc.; pp. 700pp. pp8792015
|
|
53
|
Null RCTR, Team R, Null RCT, Core Writing
T, Null R, Team R, Null RDCT, Core R, Team R and Team RDC: R: A
language and environment for statistical computing. Computing.
1:12–21. 2011.
|
|
54
|
Dinh DD, Wan H, Lidington D and Bolz SS:
Female mice display sex-specific differences in cerebrovascular
function and subarachnoid haemorrhage-induced injury. EBioMedicine.
102:1050582024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kitaeva KV, Rutland CS, Rizvanov AA and
Solovyeva VV: Cell culture based in vitro test systems for
anticancer drug screening. Front Bioeng Biotechnol. 8:3222020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pinto B, Henriques AC, Silva PMA and
Bousbaa H: Three-dimensional spheroids as in vitro preclinical
models for cancer research. Pharmaceutics. 12:11862020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wu Y, Xu Y, Sun JS, Dai K, Wang Z and
Zhang J: Inhibiting RIPK1-driven neuroinflammation and neuronal
apoptosis mitigates brain injury following experimental
subarachnoid hemorrhage. Exp Neurol. 374:1147052024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yuan B, Zhao XD, Shen JD, Chen SJ, Huang
HY, Zhou XM, Han YL, Zhou LJ, Lu XJ and Wu Q: Activation of SIRT1
alleviates ferroptosis in the early brain injury after subarachnoid
hemorrhage. Oxid Med Cell Longev. 2022:90698252022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tang J, Chen R, Wang L, Yu L, Zuo D, Cui G
and Gong X: Melatonin attenuates thrombin-induced inflammation in
BV2 cells and then protects HT22 cells from apoptosis.
Inflammation. 43:1959–1970. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Richter M, Piwocka O, Musielak M,
Piotrowski I, Suchorska WM and Trzeciak T: From donor to the lab: A
fascinating journey of primary cell lines. Front Cell Dev Biol.
9:7113812021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Helms HC, Abbott NJ, Burek M, Cecchelli R,
Couraud PO, Deli MA, Förster C, Galla HJ, Romero IA, Shusta EV, et
al: In vitro models of the blood-brain barrier: An overview of
commonly used brain endothelial cell culture models and guidelines
for their use. J Cereb Blood Flow Metab. 36:862–890. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang J, Yang H, Wu J, Zhang D, Wang Y and
Zhai J: Recent progresses in novel in vitro models of primary
neurons: A biomaterial perspective. Front Bioeng Biotechnol.
10:9530312022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Swartzlander DB, Propson NE, Roy ER, Saito
T, Saido T, Wang B and Zheng H: Concurrent cell type-specific
isolation and profiling of mouse brains in inflammation and
Alzheimer's disease. JCI Insight. 3:e1211092018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
El Amki M, Dubois M, Lefevre-Scelles A,
Magne N, Roussel M, Clavier T, Guichet PO, Gérardin E, Compère V
and Castel H: Long-lasting cerebral vasospasm, microthrombosis,
apoptosis and paravascular alterations associated with neurological
deficits in a mouse model of subarachnoid hemorrhage. Mol
Neurobiol. 55:2763–2779. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fluri F, Schuhmann MK and Kleinschnitz C:
Animal models of ischemic stroke and their application in clinical
research. Drug Des Devel Ther. 9:3445–3454. 2015.PubMed/NCBI
|
|
66
|
Su C, Liu Y, Li R, Wu W, Fawcett JP and Gu
J: Absorption, distribution, metabolism and excretion of the
biomaterials used in Nanocarrier drug delivery systems. Adv Drug
Deliv Rev. 143:97–114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang R, Khan D and Muhammad S:
Establishment of a novel protocol for assessing the severity of
subarachnoid hemorrhage in circle Willis perforation mouse model.
Sci Rep. 14:101472024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Marbacher S, Grüter B, Schöpf S, Croci D,
Nevzati E, D'Alonzo D, Lattmann J, Roth T, Bircher B, Wolfert C, et
al: Systematic review of in vivo animal models of subarachnoid
hemorrhage: Species, standard parameters, and outcomes. Transl
Stroke Res. September 12–2018.(Epub ahead of print). PubMed/NCBI
|
|
69
|
Shi X, Yu W, Yang T, Liu W, Zhao Y, Sun Y,
Chai L, Gao Y, Dong B and Zhu L: Panax notoginseng saponins
provide neuroprotection by regulating NgR1/RhoA/ROCK2 pathway
expression, in vitro and in vivo. J Ethnopharmacol. 190:301–312.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Pei X, Zhang L, Liu D, Wu Y, Li X, Cao Y
and Du X: Notoginsenoside R1 attenuates brain injury in rats with
traumatic brain injury: Possible mediation of apoptosis via ERK1/2
signaling pathway. PLoS One. 18:e02959032023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang D, Gao B, Yang T, Sun H, Ran X and
Lin W: Protective effect of NGR1 against glutamate-induced
cytotoxicity in HT22 hippocampal neuronal cells by upregulating the
SIRT1/Wnt/β-catenin pathway. Evid Based Complement Alternat Med.
2021:43581632021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tu L, Wang Y, Chen D, Xiang P, Shen J, Li
Y and Wang S: Protective effects of notoginsenoside r1 via
regulation of the PI3K-Akt-mTOR/JNK pathway in neonatal cerebral
hypoxic-ischemic brain injury. Neurochem Res. 43:1210–1226. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhai Y, Meng X, Luo Y, Wu Y, Ye T, Zhou P,
Ding S, Wang M, Lu SB, Zhu L, et al: Notoginsenoside R1 ameliorates
diabetic encephalopathy by activating the Nrf2 pathway and
inhibiting NLRP3 inflammasome activation. Oncotarget. 9:9344–9363.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhu T and Wan Q: Pharmacological
properties and mechanisms of Notoginsenoside R1 in
ischemia-reperfusion injury. Chin J Traumatol. 26:20–26. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhang HB, Tu XK, Chen Q and Shi SS:
Propofol reduces inflammatory brain injury after subarachnoid
hemorrhage: Involvement of PI3K/Akt pathway. J Stroke Cerebrovasc
Dis. 28:1043752019. View Article : Google Scholar : PubMed/NCBI
|