Introduction
Stroke is the leading cause of mortality and
disability worldwide (1).
According to the World Health Organization, stroke causes ~5.9
million deaths annually, with ischemic stroke accounting for ~87%
of these cases (2). Mechanical
thrombectomy and early pharmacological thrombolysis using tissue
plasminogen activator are clinically effective interventions for
restoring cerebral blood flow (reperfusion) in ischemic stroke
(3). However, reperfusion can
trigger cerebral ischemia-reperfusion injury (CIRI), a form of
secondary damage that critically affects clinical outcomes
(4–6). CIRI is a complex pathological process
characterized by excitotoxicity, oxidative stress, inflammation and
mitochondrial dysfunction, eventually triggering neuronal apoptosis
(7). As such, there is an urgent
need for effective therapeutic strategies for CIRI.
Mitochondria maintain cellular homeostasis through
the induction of dynamic processes, including fission, fusion,
autophagy and regeneration (8).
Disturbances in these processes, particularly mitochondrial
fission, facilitate neuronal apoptosis (9). Excessive mitochondrial fission is a
prominent early upstream event that drives neuronal apoptosis
during CIRI (10). Dynamin-related
protein 1 (Drp1), a critical regulator of mitochondrial fission, is
primarily activated by the phosphorylation of serine 616, which
promotes its translocation from the cytoplasm to the mitochondrial
membrane, triggering mitochondrial fission (11). Inhibition of Drp1 phosphorylation
by pharmacological inhibition or Drp1 knockdown has been shown to
alleviate CIRI by reducing infarct size and neuronal apoptosis
(12,13).
Drp1-mediated excessive fission not only causes
mitochondrial fragmentation, reactive oxygen species (ROS)
production, reduced mitochondrial membrane potential (MMP) and
increased cytochrome c release, but also leads to the
overactivation of mitophagy (14–17).
Although previous studies have shown that mitophagy exerts
neuroprotective effects and improves the prognosis of CIRI
(18,19), several studies have demonstrated
that type II programmed cell death, induced by overactivated
mitophagy, aggravates brain damage (20–22).
Peroxisome proliferator-activated receptor γ coactivator-1α
(PGC-1α) is a multifunctional co-transcription factor involved in
mitochondrial biosynthesis and function (23), which negatively regulates Drp1
expression by directly binding to the Drp1 promoter, thereby
preventing excessive mitochondrial fission (24). As a direct upstream regulator of
PGC-1α, sirtuin 1 (SIRT1) serves a crucial role in maintaining
mitochondrial function by regulating its expression (25). Previous studies have demonstrated
that activation of the SIRT1/PGC-1α pathway can inhibit excessive
mitochondrial fission and attenuate the development of
diabetes-induced cardiac dysfunction (24,26).
Moreover, the inhibition of Drp1-mediated excessive mitochondrial
fission has been shown to mitigate mitophagy, thereby offering
protection against CIRI (27).
Astragali Radix (Huangqi) is a widely used
traditional Chinese medicine, which exhibits potent pharmacological
effects on cardiovascular diseases and immune regulation (28). Calycosin-7-O-β-D-glucoside (CG), a
major bioactive ingredient of Astragali Radix, has further been
reported to exert neuroprotective effects through
anti-inflammatory, antioxidant and anti-apoptotic mechanisms
(29–31). Although our previous study showed
that CG can alleviate oxygen-glucose deprivation/reperfusion
(OGD/R)-induced HT22 cell damage (32), whether this action is related to
the prevention of excessive mitochondrial fission and mitophagy
overactivation remains unclear. Therefore, the present study
investigated the protective effects of CG against OGD/R-induced
injury by focusing on the inhibition of Drp1-mediated mitochondrial
fission. Additionally, the study investigated whether CG could
inhibit the overactivation of mitophagy.
Materials and methods
Materials
The following reagents were acquired for use in the
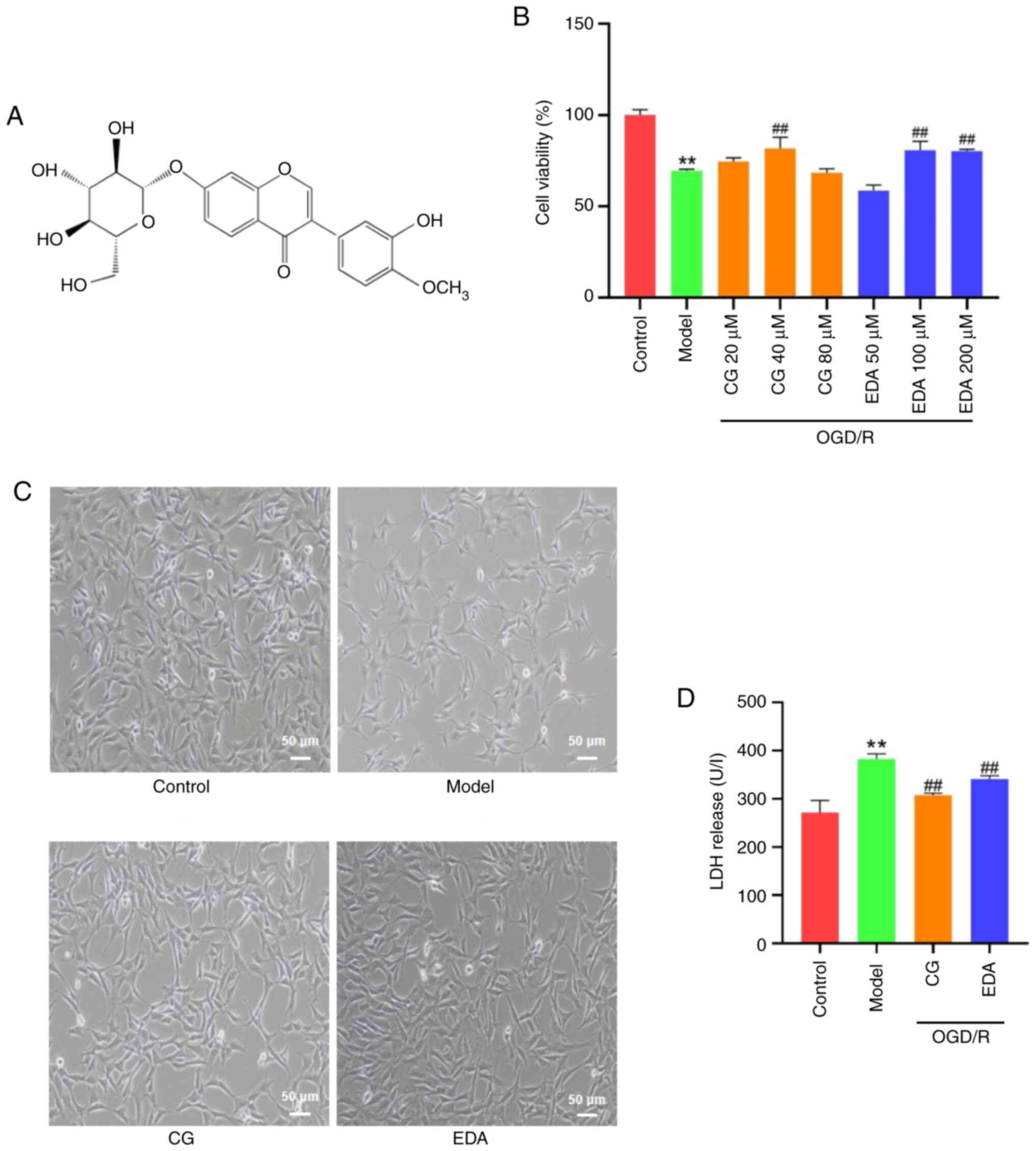
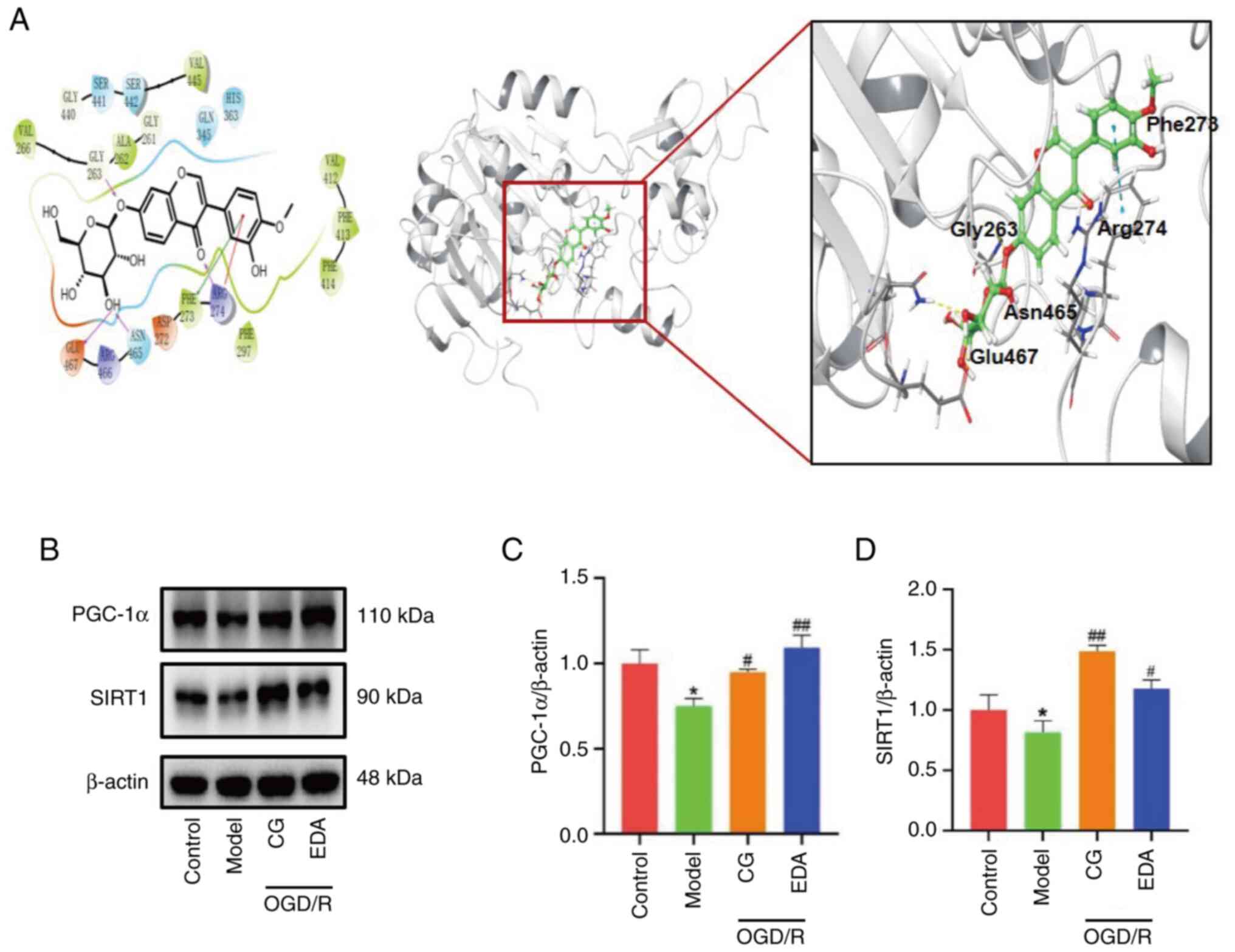
present study: CG (Fig. 1A; purity
>98%; Shanghai Aladdin Biochemical Technology Co., Ltd.);
edaravone (EDA; purity >99%; MilliporeSigma); Cell Counting
Kit-8 (CCK-8; Abbkine Scientific Co., Ltd.); lactate dehydrogenase
(LDH) kit (Nanjing Jiancheng Bioengineering Institute);
MitoTracker™ Orange CMTMRos kit (Thermo Fisher Scientific, Inc.);
MitoSOX™ kit (Thermo Fisher Scientific, Inc.); JC-1, MitoTracker
Green kits and Hoechst 33342 (Beyotime Institute of Biotechnology);
BCA kit (Beijing Solarbio Science & Technology Co., Ltd.);
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS)
and penicillin/streptomycin (HyClone; Cytiva); and glucose-free
DMEM (Gibco; Thermo Fisher Scientific, Inc.). The following
antibodies were also purchased: SQSTM1/p62 (cat. no. P0067;
MilliporeSigma); LC3B (cat. no. A19665), Bcl-2 (cat. no. A19693),
Drp1 (cat. no. A21968) and HRP-conjugated secondary antibody (cat.
no. AS014) (all from ABclonal Biotech Co., Ltd.); translocase of
outer mitochondrial membrane 20 (TOM20; cat. no. 42406S),
phosphorylated p-Drp1 (Ser616) (cat. no. 4494S) and caspase-3 (cat.
no. 14220S) (all from Cell Signaling Technology, Inc.); PGC-1α
(cat. no. ab313559), Bax (cat. no. ab32503) and SIRT1 (cat. no.
ab12193) (all from Abcam); β-actin (cat. no. K101527P; Beijing
Solarbio Science & Technology Co., Ltd.).
OGD/R model and drug treatment
HT22 mouse hippocampal neurons were purchased from
Pricella (cat. no. CL-0697), and were cultured in DMEM supplemented
with 10% FBS and antibiotics (penicillin, 100 IU/ml; streptomycin,
100 µg/ml) at 37°C in an incubator under 5% CO2. To
construct the OGD/R model, cells were cultured in glucose-free DMEM
and then transferred to a hypoxia incubator chamber (MIC-101;
Embrient, Inc.) containing 5% CO2 and 95% N2
at 37°C. After 10 h, the cells were transferred to an incubator
with 5% CO2 and 95% air, and the culture medium was
replaced with standard DMEM for 6 h at 37°C (reperfusion). CG (20,
40 and 80 µM) and EDA (50, 100 and 200 µM) were added for 6 h
during the reperfusion phase. The control group was maintained in
standard culture medium without OGD. The concentration of CG was
selected based on a previous study (31).
Cell viability assay
HT22 cells were cultured in 96-well plates at a
density of 5×103 cells/well. After OGD/R modelling and
drug treatment, images were captured under an optical microscope
(Nikon Corporation), after which the medium was discarded, 10 µl
CCK-8 solution was added, and the cells were cultured for 30 min in
a regular incubator. The absorbance was measured at 450 nm using a
microplate reader (BioTek; Agilent Technologies, Inc.).
Measurement of LDH activity
The LDH activity in the supernatant was measured
using an LDH assay kit, according to the manufacturer's
instructions. After OGD/R modelling and drug treatment, the cell
medium was collected and mixed with substrate solution at 37°C for
15 min. The mixture was subsequently incubated with
2,4-dinitrophenylhydrazine for 15 min at 37°C and the absorbance
was measured at 450 nm using a microplate reader (BioTek; Agilent
Technologies, Inc.).
Cell apoptosis assay
Cell apoptosis was determined using an Annexin
V-FITC/PI kit (Beijing Solarbio Science & Technology Co.,
Ltd.), according to the manufacturer's protocol. After treatment,
the cells were harvested by centrifugation at 100 × g for 5 min at
37°C, followed by trypsin digestion. The cells were then
resuspended in binding buffer mixed with 5 µl Annexin V-FITC and
were incubated in the dark for 5 min at 37°C. Subsequently, 1 µl PI
was added and incubated for another 5 min at 37°C. The apoptosis
rate in each group was analyzed using flow cytometry (CytoFLEX
V2-B2-R0; Beckman Coulter, Inc.).
Mitochondrial ROS (mtROS)
detection
According to the manufacturer's instructions,
following treatment, the supernatants were discarded, and the cells
were incubated with MitoSOX™ (3 µM) for 30 min at 37°C, followed by
Hoechst staining for 15 min at 37°C to label the nuclei. The cells
were then washed three times with PBS and observed under an
inverted fluorescence microscope (TS-2; Nikon Corporation).
MMP detection
JC-1 is a widely used probe for monitoring MMP. In
healthy cells with normal MMP levels, JC-1 forms J-aggregates that
emit intense red fluorescence. By contrast, in apoptotic or
unhealthy cells with a low MMP, JC-1 remains in its monomeric form
and emits green fluorescence. Thus, mitochondrial depolarization
can be measured as a decrease in the red/green fluorescence
intensity ratio. According to the manufacturer's protocol,
following treatment, the supernatants were discarded, and cells
were incubated with 1 ml JC-1 solution at 37°C for 50 min. The JC-1
solution was then aspirated and the cells were washed twice with
dye buffer. The fluorescence intensity of the JC-1
aggregate/monomer was subsequently observed under an inverted
fluorescence microscope (TS-2; Nikon Corporation).
Mitochondrial morphology
observation
MitoTracker Green, a live cell mitochondria-specific
fluorescent dye, was applied to distinguish changes in
mitochondrial morphology. Briefly, HT22 cells were incubated with
100 nM MitoTracker Green for 15 min at 37°C. The staining solution
was subsequently removed and the cells were washed twice with HBSS
(Beyotime Institute of Biotechnology). Images were then captured
using a laser confocal microscope (Leica Microsystems GmbH) and the
length of the mitochondria was measured using Image-Pro Plus 6.0
(Media Cybernetics, Inc.).
Immunofluorescence
For immunofluorescence detection, HT22 cells were
first stained with MitoTracker™ Orange CMTMRos (200 nM) at 37°C for
10 min, and then fixed with 4% paraformaldehyde for 15 min at 37°C.
After three washes with PBS, the cells were permeabilized with 0.3%
Triton X-100 in PBS for 15 min at 37°C. The cells were then blocked
with 5% BSA (Beijing Solarbio Science & Technology Co., Ltd.)
for 30 min at 37°C, and incubated overnight at 4°C with primary
antibodies against LC3 (1:500). The next day, after washing three
times with PBS, the samples were incubated with a Alexa Fluor™
488-conjugated goat anti-rabbit IgG secondary antibody (1:500; cat.
no. A11008; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature in the dark, followed by DAPI staining for 30 min at
37°C to label the nuclei. Finally, the fluorescence intensity was
analyzed using an inverted fluorescence microscope (TS-2; Nikon
Corporation).
Molecular docking
The 3D structure of SIRT1 [Protein Data Bank (PDB)
ID: 4KXQ] was downloaded from the PDB (https://www.rcsb.org/), and protein structure was
optimized to ensure it can be used for molecular docking using the
Protein Preparation Wizard module in Schrödinger 12.9 (Schrödinger,
Inc.). The 3D structure of CG was obtained from the PubChem
database (compound CID: 5318267; http://pubchem.ncbi.nlm.nih.gov/compound/5318267).
Molecular docking between CG and SIRT1 was performed using the
Glide module (Schrödinger, Inc.), and the molecular mechanics
generalized Born surface area (MM/GBSA) was calculated. The binding
sites were visualized using PyMOL 3.0 (Schrödinger, Inc.).
Western blotting
Total proteins were extracted from the cells using
RIPA lysis buffer (cat. no. CW2333S; CWBio), whereas protein
concentration was detected using a BCA kit. Proteins (10 µg) were
separated by SDS-PAGE on 10 or 12% gels (the percentage gel used
depended on the molecular weight of the targeted protein), were
transferred to polyvinylidene difluoride membranes and were blocked
for 2 h with 5% skim milk in PBS containing 0.1% Tween-20 (PBST) at
37°C. The membranes were subsequently incubated with primary
antibodies against the following proteins at 4°C overnight: Bax
(1:2,000), Bcl-2 (1:500), caspase-3 (1:4,000), TOM20 (1:4,000), p62
(1:4,000), LC3 (1:1,500), Drp1 (1:1,000), p-Drp1 (1:1,000), PGC-1α
(1:1,000), SIRT1 (1:1,000) and β-actin (1:5,000). After washing
three times with PBST, the membranes were incubated with an
HRP-conjugated secondary antibody for 50 min at room temperature.
The membranes were then scanned using an imaging system (Bio-Rad
Laboratories, Inc.), and Image-Pro Plus 6.0 (Media Cybernetics,
Inc.) was used to analyze the optical density of the bands. The
relative protein expression levels were normalized to β-actin.
Statistical analysis
The data are presented as the mean ± SEM and were
analyzed using SPSS 27.0 (IBM Corporation). Figures were drawn
using GraphPad Prism 8.0 (Dotmatics). One-way ANOVA followed by
Tukey's post hoc test was used for data analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
CG ameliorates damage to HT22 cells
induced by OGD/R challenge
To investigate the protective effects of CG against
OGD/R-induced HT22 cell injury, the viability of HT22 cells was
measured using the CCK-8 assay. The results showed that cell
viability was significantly decreased in the OGD/R group compared
with that in the control group, but it was significantly increased
by CG (40 µM) and EDA (100 and 200 µM) treatment (Fig. 1B); therefore, 40 µM CG and 100 µM
EDA were selected for subsequent experiments. The morphological
characteristics of the OGD/R group included irregular shrinkage,
unclear boundaries and floating (Fig.
1C). Additionally, the release of LDH was markedly increased in
the OGD/R group compared with that in the control group (Fig. 1D). However, these trends were
markedly reversed by CG and EDA.
CG alleviates the OGD/R-induced
apoptosis of HT22 cells
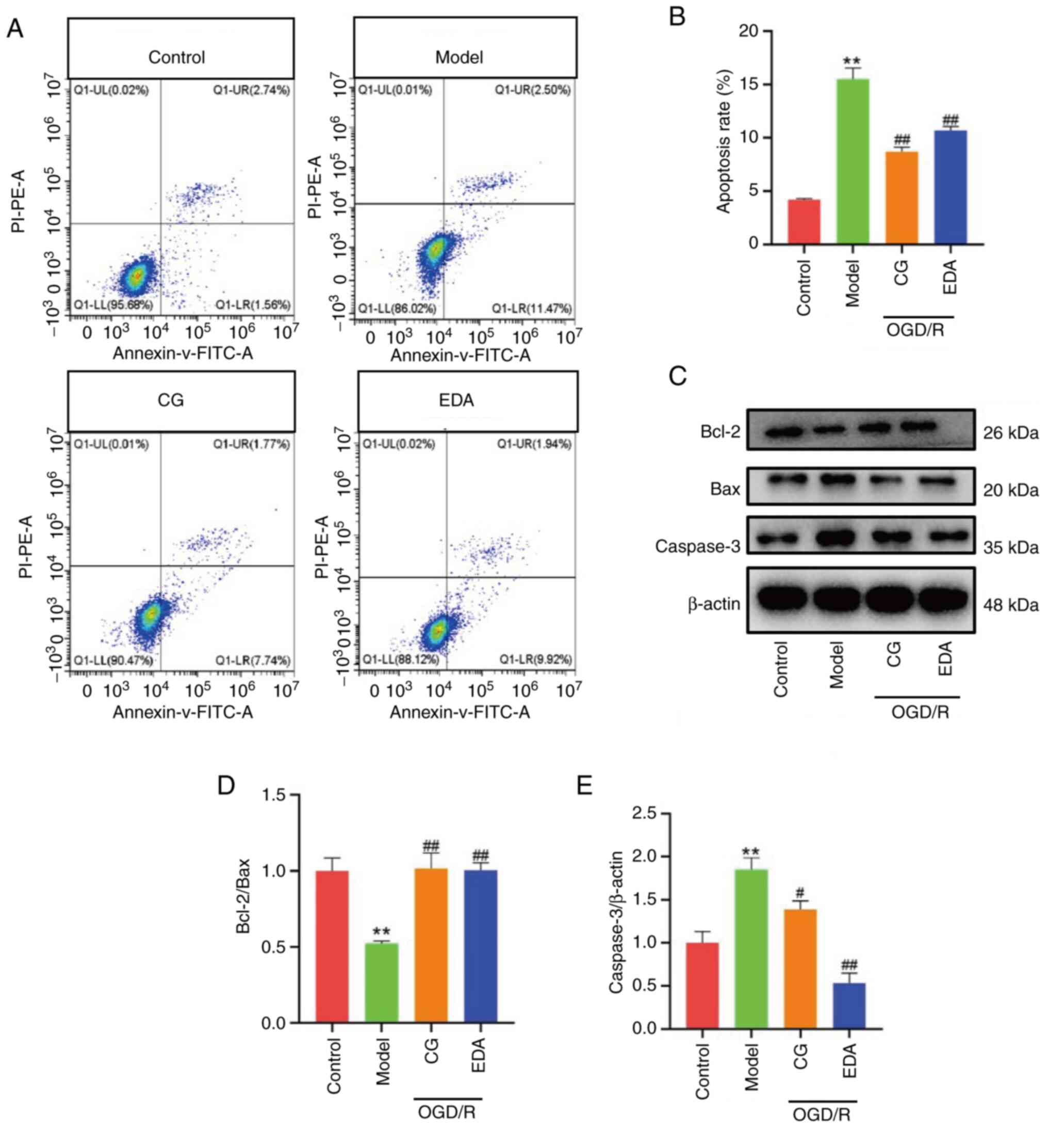
Neuronal apoptosis is the final event of CIRI
(33). To assess the effects of CG
on the apoptosis of HT22 cells, flow cytometry and western blot
analysis were performed. The results revealed a significant
increase in the percentage of apoptotic cells and caspase-3
expression, accompanied by a marked decrease in the Bcl-2/Bax ratio
in the OGD/R group compared with those in the control group
(Fig. 2). However, these changes
were reversed by the CG and EDA treatment. These findings confirmed
the anti-apoptotic effect of CG and indicated that CG ameliorated
OGD/R by reducing neuronal apoptosis.
CG improves the OGD/R-induced
mitochondrial dysfunction of HT22 cells
Mitochondrial dysfunction is an important
characteristic of CIRI (22,34).
To investigate whether CG could restore the mitochondrial
dysfunction caused by OGD/R, mtROS levels were analyzed using
MitoSOX Red. As shown in Fig. 3A and
B, mtROS levels were significantly increased in the OGD/R group
compared with those in the control group, but were notably reduced
following CG and EDA treatment. By contrast, MMP, a hallmark of
mitochondrial integrity, was significantly elevated by CG and EDA
treatment compared with that in the OGD/R group (Fig. 3C and D). Collectively, these
results indicated that CG may protect cells from OGD/R-induced
mitochondrial dysfunction.
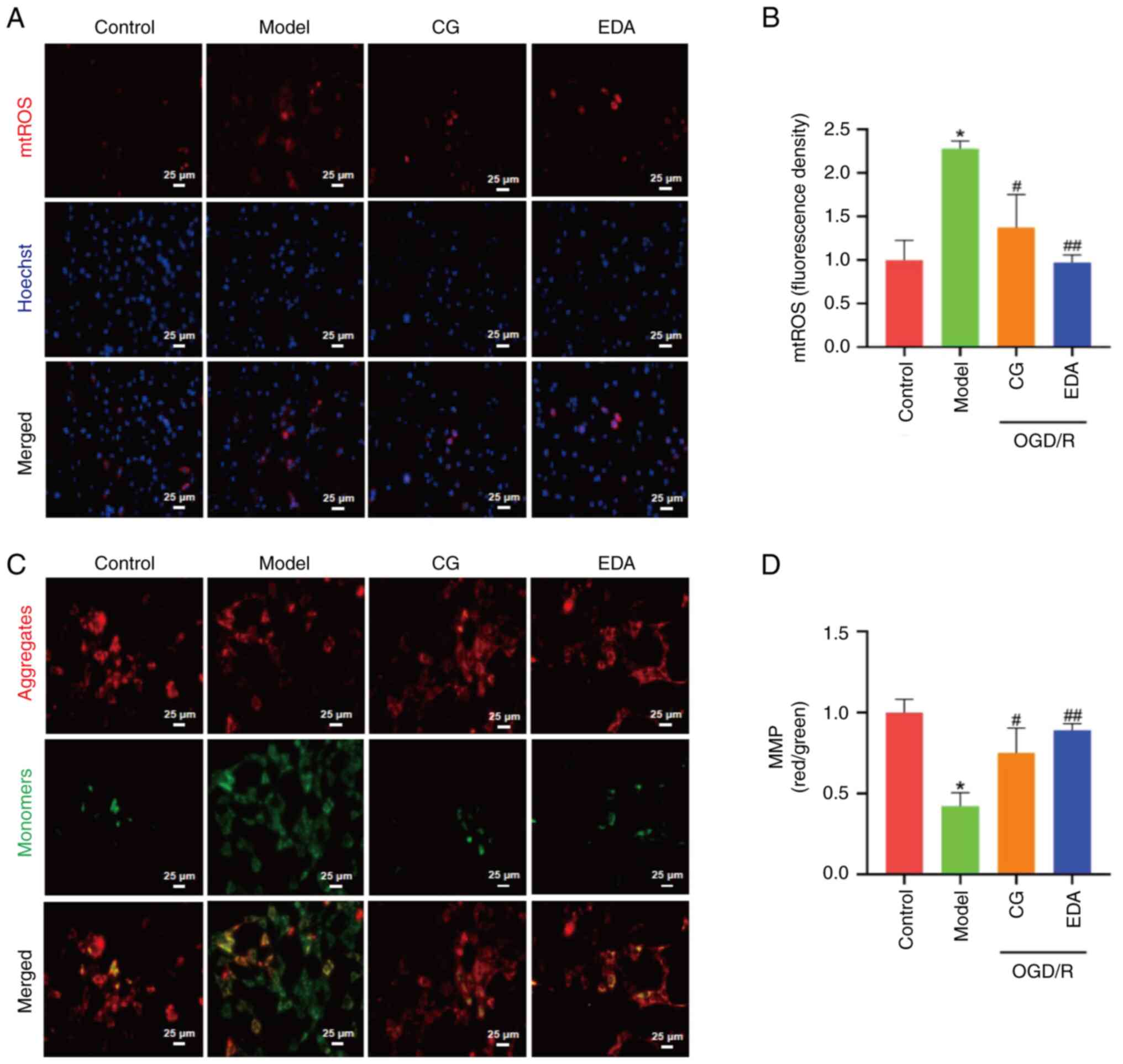 | Figure 3.CG improves OGD/R-induced
mitochondrial dysfunction of HT22 cells. HT22 cells were subjected
to OGD/R, and treated with 40 µM CG or 100 µM EDA. (A) mtROS
detection. Scale bar, 25 µm. (B) Quantification of fluorescence
density of mtROS (four images were randomly selected in each
group). (C) MMP was monitored using the JC-1 probe. Red, JC-1
aggregates; green, JC-1 monomers. Scale bar, 25 µm. (D) Ratio of
aggregates/monomers (four images were randomly selected in each
group). Data are presented as the mean ± SEM, and were analyzed by
one-way ANOVA and Tukey's post hoc test. *P<0.05 vs. control
group; #P<0.05, ##P<0.01 vs. model
group. CG, calycosin-7-O-β-D-glucoside; EDA, edaravone; mtROS,
mitochondrial reactive oxygen species; OGD/R, oxygen-glucose
deprivation/reperfusion. |
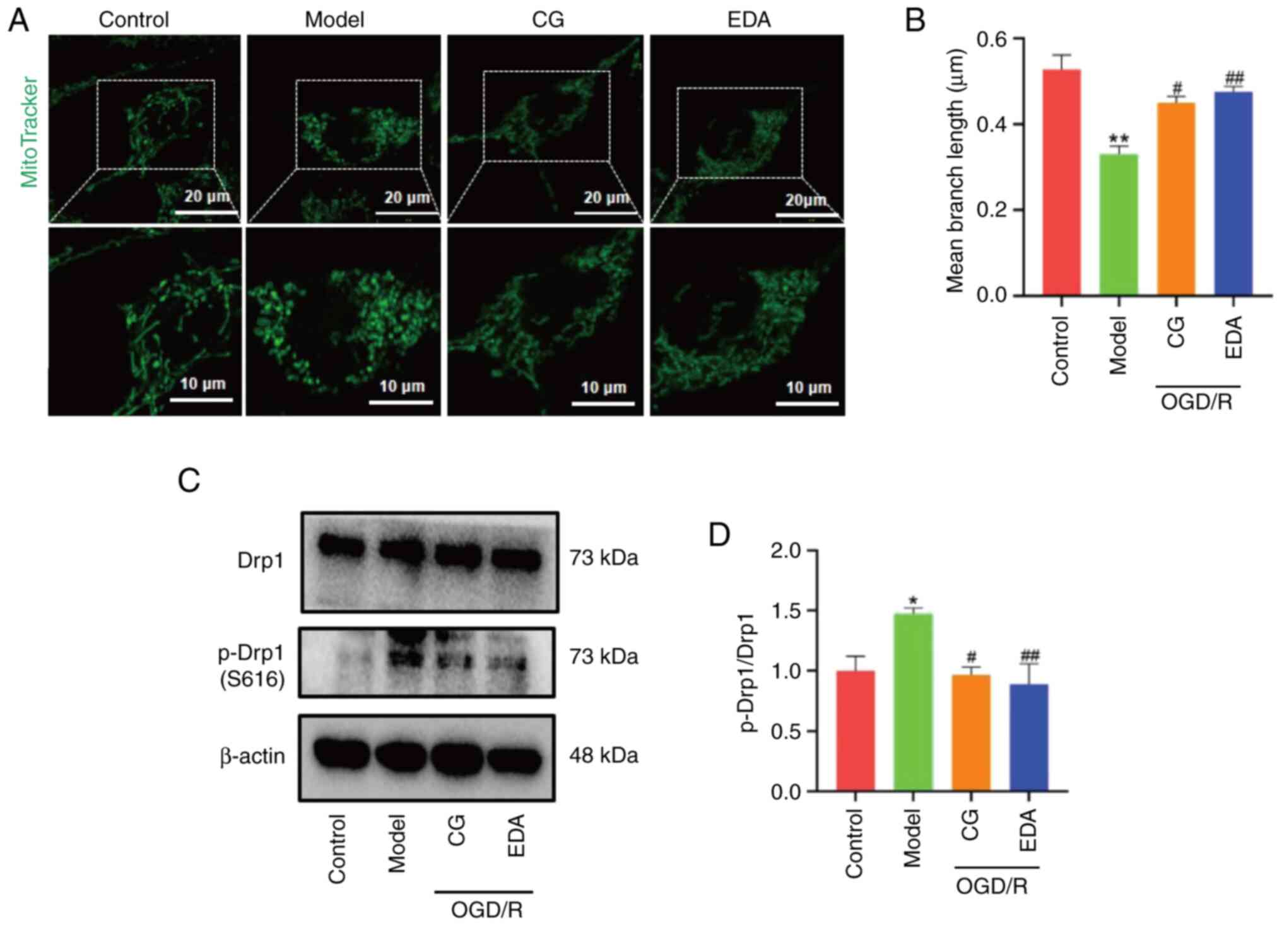
CG prevents OGD/R-induced excessive
mitochondrial fission in HT22 cells
Mitochondria are highly dynamic organelles that
undergo fission and fusion, both of which are processes closely
related to mitochondrial function (8). The MitoTracker Green probe was used
to examine the effects of CG on mitochondrial morphology in
OGD/R-treated HT22 cells. As shown in Fig. 4A and B, the average length of
mitochondria in OGD/R-treated cells was significantly shorter than
that in control cells, indicating a marked decrease in the linear
shape and diffusely interspersed fragmented or dotted mitochondria.
As hypothesized, CG and EDA mitigated mitochondrial fragmentation
in OGD/R-treated HT22 cells. Mitochondrial fission is mediated by
p-Drp1 (Ser616); to confirm whether CG regulated mitochondrial
fission, the expression levels of this marker of mitochondrial
fission were examined. As shown in Fig. 4C and D, an increased p-Drp1/Drp1
ratio was observed in the OGD/R group compared with that in the
control group, which was effectively interrupted by CG and EDA
treatment. These results indicated the protective effect of CG
against OGD/R-induced mitochondrial fission.
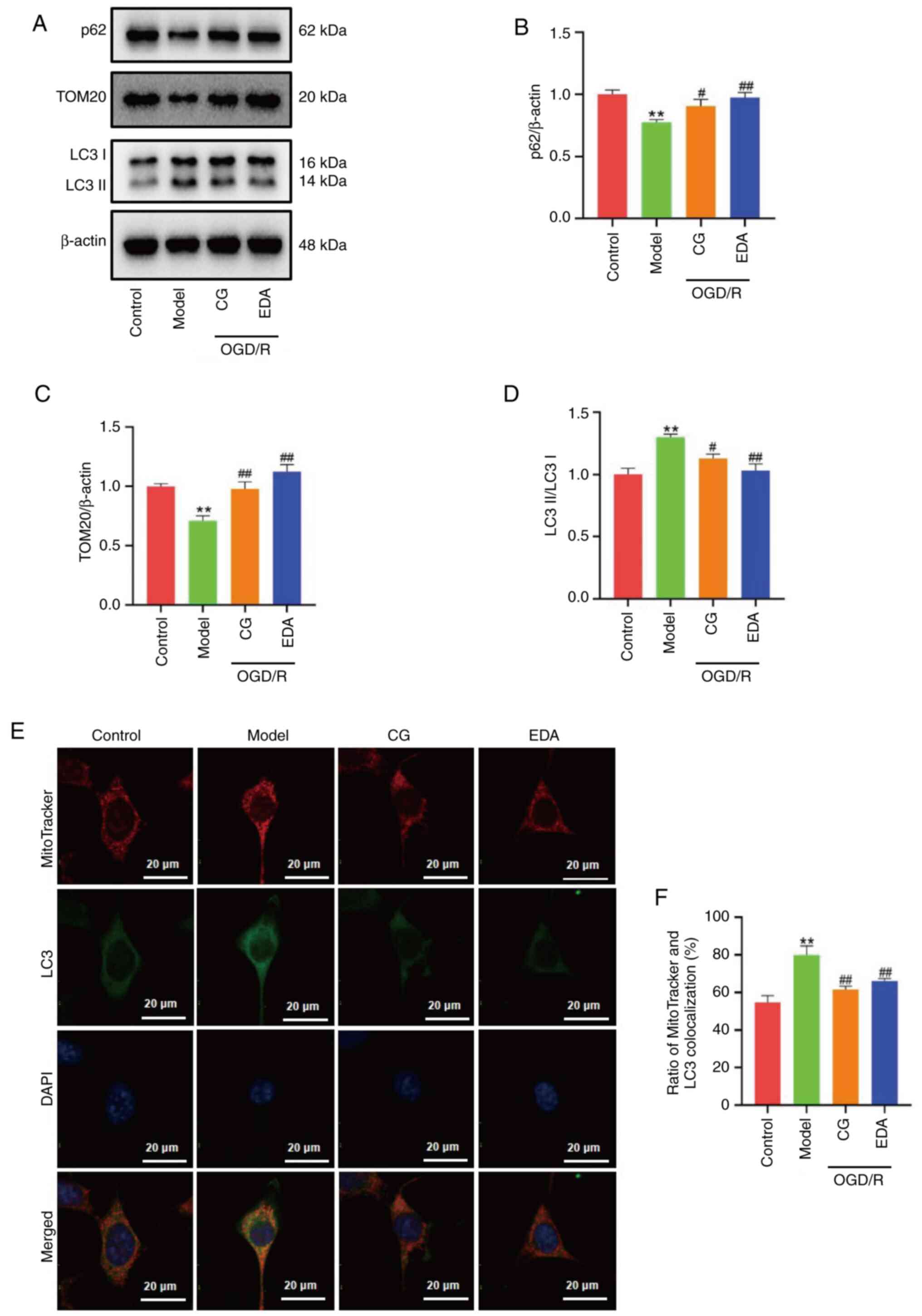
CG downregulates OGD/R-induced
mitophagy overactivation
To investigate whether CG can affect mitophagy, the
expression levels of the mitophagy-related proteins LC3, p62 and
TOM20 were investigated (Fig. 5A).
The results showed an increased LC3II/LC3I ratio in the OGD/R group
compared with that in the control group, and, as expected, this
increase was markedly inhibited by CG treatment (Fig. 5D). By contrast, a significant
increase in the expression levels of p62 and TOM20 were observed
following CG and EDA administration, indicating that CG may reduce
the overactivation of mitophagy induced by OGD/R (Fig. 5B and C). To further observe
overactivated mitophagy, mitochondria and autophagosomes were
colocalized using immunofluorescence staining. As presented in
Fig. 5E and F, the ratio of
colocalization of mitochondria stained with LC3 was greatly
increased in the OGD/R group compared with that in the control
group, and was markedly abrogated by treatment with CG and EDA.
Taken together, these results indicated that CG downregulated
OGD/R-induced mitophagy overactivation.
CG upregulates the expression of SIRT1
and PGC-1α after OGD/R
The SIRT1/PGC-1α signaling pathway serves an
essential role in mitochondrial protection (24). Thus, it was hypothesized that the
SIRT1/PGC-1α pathway may be involved in the protective effect of CG
against neuronal apoptosis by inhibiting mitophagy overactivation.
To assess whether SIRT1 is a target of CG, the affinity between CG
and SIRT1 was analyzed using molecular docking. As presented in
Fig. 6A, CG can bind well with
amino acids in SIRT1, including Asn465, Glu467, Gly263, Arg274 and
Phe273, and the docking score of CG and SIRT1 was −8.02 kcal/mol.
Furthermore, the effects of CG on the protein expression levels of
SIRT1 and PGC-1α were examined, with results showing that the
levels of SIRT1 and PGC-1α were markedly reduced in the OGD/R group
compared with those in the control group, while CG and EDA
treatment upregulated the expression of SIRT1 and PGC-1α (Fig. 6B-D). These results indicated that
CG may attenuate mitochondrial dysfunction through the expression
of SIRT1 and PGC-1α.
Discussion
High rates of disability and recurrence are
characteristic of ischemic stroke, a common cerebral disease that
represents a serious global public health concern (35). According to our previous study, CG
may mitigate OGD/R-induced injury by improving mitochondrial
function (32). Notably, abundant
evidence has demonstrated that mitophagy overactivation, triggered
by excessive mitochondrial fission, is central to neuronal
apoptosis following cerebral ischemia/reperfusion (36,37).
However, whether the protective effects of CG against OGD/R-induced
injury are related to the modulation of mitochondrial fission and
mitophagy has not yet been investigated, to the best of our
knowledge.
The results of the present study indicated that CG
notably enhanced cell viability, increased the Bcl-2/Bax ratio, and
decreased caspase-3 expression and LDH release following OGD/R,
thus validating the protective effects of CG against OGD/R-induced
neuronal apoptosis. It has previously been well established that
mitochondrial dysfunction damages neurons and is a major factor in
the etiology of CIRI (22). One of
the main causes of CIRI is the overproduction of mtROS (6). This process contributes to and
permeates all aspects of CIRI, including oxidative stress,
mitochondrial swelling, membrane instability and reduced MMP
(38). Furthermore, cerebral
ischemia-reperfusion causes mitochondrial morphological
fragmentation, which ultimately triggers neurological injury
(39,40). Improving mitochondrial morphology
has been shown to effectively reduce the damage caused by CIRI
(39,41). EDA was first approved for the
treatment of ischemic stroke in Japan in 2001 (42). Notably, ~50% of EDA is present in
the body as the EDA anion, which transfers electrons to remove
different types of radicals, such as H2O2 and
O-2. The produced EDA radical at that time then combines with the
oxygen molecules in the reaction system to form EDA peroxyradical,
and ultimately 2-oxo-(phenylhydrazono)-butanoic acid, a reaction
product unrelated to radicals (43–45).
Thus, in order to assess the effectiveness and mechanism of CG, EDA
was selected as a positive control medication. The present study
verified that CG protected the mitochondria of OGD/R-induced HT22
cells, as demonstrated by decreased mtROS, increased MMP and
improved mitochondrial morphology. The present data further
suggested that CG may reduce OGD/R-induced damage by improving
mitochondrial dysfunction.
Under normal physiological conditions, Drp1 is
typically found in the cytoplasm and is an essential regulator of
mitochondrial fission. Upon stimulation, Drp1 is recruited to the
mitochondrial surface to induce fission (11). Previous studies have shown that
when Drp1 is translocated to the mitochondria, it releases Beclin1
and interacts with LC3 to activate autophagy (16,46).
Excessive mitochondrial fission during cerebral
ischemia-reperfusion triggers mitophagy, a crucial process in
maintaining mitochondrial quality control (47,48).
Although mitophagy is essential for cellular homeostasis, it is a
double-edged sword and its overactivation can lead to apoptosis
(49). Prior research has shown
that preventing mitophagy overactivation caused by excessive
mitochondrial fission can alleviate CIRI (39,40,50).
In the present study, excessive mitochondrial fission and mitophagy
overactivation were detected following OGD/R, as evidenced by the
upregulation of p-Drp1/Drp1 and LC3II/LC3I, and decreased p62 and
TOM20 expression. As expected, treatment with CG reversed the
changes induced by OGD/R.
SIRT1 is the most researched member of the SIRT
family (51), with numerous
studies showing that SIRT1 serves a role in autophagy and apoptosis
following cerebral ischemia (52–54).
PGC-1α, a transcription factor downstream of SIRT1, is crucial for
controlling mitochondria, and may be used as a therapeutic target
for a variety of neurodegenerative illnesses (23). To explore whether the
downregulation of p-Drp1 by CG is related to the expression of
SIRT1 and PGC-1α, the binding ability of CG to SIRT1 was simulated
through molecular docking, and the protein expression levels of
SIRT1 and PGC-1α were detected. The results of these analyses
demonstrated that CG has the ability to directly bind to SIRT1, and
regulate the expression of SIRT1 and PGC-1α. These results
indicated that CG may reduce Drp1 phosphorylation via regulation of
the expression of SIRT1 and PGC-1α, thereby preventing the
overactivation of mitophagy induced by excessive mitochondrial
fission.
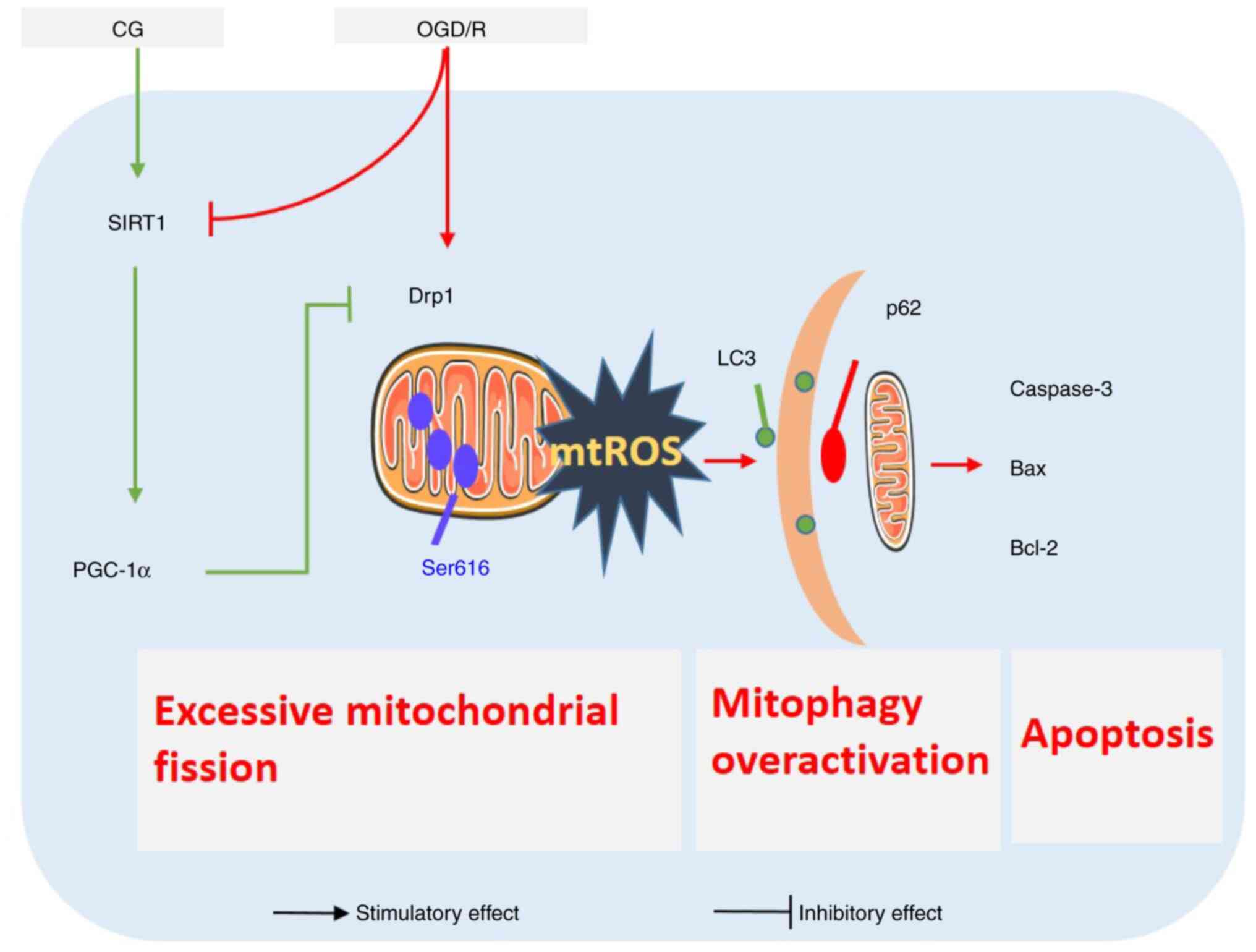
In conclusion, the present study provided compelling
evidence that CG alleviated OGD/R-induced HT22 cell apoptosis by
preventing the overactivation of mitophagy induced by excessive
mitochondrial fission by regulating the expression of SIRT1 and
PGC-1α (Fig. 7). These findings
provide a theoretical basis for the development of clinical
strategies for the use of CG in the management of CIRI. However,
the current study only focused on the protective effects of CG
in vitro; the precise mechanism by which CG protects against
CIRI through the regulation of mitochondrial fission and mitophagy,
and how excessive mitochondrial fission leads to mitophagy
overactivation, still requires further elucidation in vivo.
Furthermore, establishing the pharmacokinetics and ideal dosage of
CG in the treatment of ischemic stroke is essential for its
possible integration into clinical practice.
Acknowledgements
The authors would like to thank Dr Yong Yuan
(Academy of Chinese Medicine of Henan University of Chinese
Medicine) for their technological help with laser confocal
microscopy.
Funding
This study was supported by the National Natural Science
Foundation of China (grant nos. 82304771 and 82274496), the Joint
Fund of Science and Technology Research and Development Project of
Henan Province (grant no. 232301420018) and the Science and
Technology Research Project of Henan Province (grant nos.
232102311193 and 232102310419).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XY designed the experiments. SQ and RG performed the
experiments. XY and SQ wrote the original draft. ZL, MB and BW
analyzed the data. PS analyzed the data and confirmed the version
to be published. EX and YL designed the experiments, drafted the
manuscript and confirm the authenticity of all the raw data. All
authors contributed to the critical revision of the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CIRI
|
cerebral ischemia-reperfusion
injury
|
|
OGD/R
|
oxygen-glucose
deprivation/reperfusion
|
|
CG
|
calycosin-7-O-β-D-glucoside
|
|
EDA
|
edaravone
|
|
mtROS
|
mitochondrial reactive oxygen
species
|
|
MMP
|
mitochondrial membrane potential
|
|
Drp1
|
dynamin-related protein 1
|
|
PGC-1α
|
peroxisome proliferator-activated
receptor γ coactivator-1α
|
|
SIRT1
|
sirtuin 1
|
References
|
1
|
Saini V, Guada L and Yavagal DR: Global
epidemiology of stroke and access to acute ischemic stroke
interventions. Neurology. 97 (20 Suppl 2):S6–S16. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iadecola C, Buckwalter MS and Anrather J:
Immune responses to stroke: Mechanisms, modulation, and therapeutic
potential. J Clin Invest. 130:2777–2788. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jolugbo P and Ariëns RAS: Thrombus
composition and efficacy of thrombolysis and thrombectomy in acute
ischemic stroke. Stroke. 52:1131–1142. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma R, Xie Q, Li Y, Chen Z, Ren M, Chen H,
Li H, Li J and Wang J: Animal models of cerebral ischemia: A
review. Biomed Pharmacother. 131:1106862020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paul S and Candelario-Jalil E: Emerging
neuroprotective strategies for the treatment of ischemic stroke: An
overview of clinical and preclinical studies. Exp Neurol.
335:1135182021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zeng X, Zhang YD, Ma RY, Chen YJ, Xiang
XM, Hou DY, Li XH, Huang H, Li T and Duan CY: Activated Drp1
regulates p62-mediated autophagic flux and aggravates inflammation
in cerebral ischemia-reperfusion via the ROS-RIP1/RIP3-exosome
axis. Mil Med Res. 9:252022.PubMed/NCBI
|
|
7
|
Zhang M, Liu Q, Meng H, Duan H, Liu X, Wu
J, Gao F, Wang S, Tan R and Yuan J: Ischemia-reperfusion injury:
Molecular mechanisms and therapeutic targets. Signal Transduct
Target Ther. 9:122024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song J, Herrnann JM and Becker T: Quality
control of the mitochondrial proteome. Nat Rev Mol Cell Biol.
22:54–70. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hou Y, Fan F, Xie N, Zhang Y, Wang X and
Meng X: Rhodiola crenulata alleviates hypobaric hypoxia-induced
brain injury by maintaining BBB integrity and balancing energy
metabolism dysfunction. Phytomedicine. 128:1555292024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anaell AR, Maizy R, Przyklenk K and
Sanderson TH: Mitochondrial quality control and disease: Insights
into ischemia-reperfusion injury. Mol Neurobiol. 55:2547–2564.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duan CY, Wang L, Zhang J, Xiang X, Wu Y,
Zhang Z, Li Q, Tian K, Xue M, Liu L and Li T: Mdivi-1 attenuates
oxidative stress and exerts vascular protection in ischemic/hypoxic
injury by a mechanism independent of Drp1 GTPase activity. Redox
Biol. 37:1017062020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, Wang Y, Wang G, Ye X, Zhang J, Cao
G, Zhao Y, Gao Z, Zhang Y, Yu B and Kou J: YiQiFuMai powder
injection protects against ischemic stroke via inhibiting neuronal
apoptosis and PKCδ/Drp1-mediated excessive mitochondrial fission.
Oxid Med Cell Longev. 2017:18320932017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu B, Luo H, Zhou X, Cheng CY, Lin L, Liu
BL, Liu K, Li P and Yang H: Succinate-induced neuronal
mitochondrial fission and hexokinase II malfunction in ischemic
stroke: Therapeutical effects of kaempferol. Biochim Biophys Acta
Mol Basis Dis. 1863:2307–2318. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mao K and Klionsky DJ: Mitochondrial
fission facilitates mitophagy in Saccharomyces cerevisiae.
Autophagy. 9:1900–1901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vásquez-Trincado C, García-Carvajal I,
Pennanen C, Parra V, Hill JA, Rothermel BA and Lavandero S:
Mitochondrial dynamics, mitophagy and cardiovascular disease. J
Physiol. 594:509–525. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shirihai OS, Song M and Dorn GW II: How
mitochondrial dynamism orchestrates mitophagy. Circ Res.
116:1835–1849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikeda Y, Shirakabe A, Maejima Y, Zhai P,
Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, et
al: Endogenous Drp1 mediates mitochondrial autophagy and protects
the heart against energy stress. Circ Res. 116:264–278. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai Y, Yang E, Yao X, Zhang X, Wang Q,
Wang Y, Liu J, Fan W, Yi K, Kang C and Wu J: FUNDC1-dependent
mitophagy induced by tPA protects neurons against cerebral
ischemia-reperfusion injury. Redox Biol. 38:1017922021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Q, Liu J, Mao Z, Tian L, Wang N, Wang
G, Wang Y and Seto S: Ligustilide attenuates ischemic stroke injury
by promoting Drp1-mediated mitochondrial fission via activation of
AMPK. Phytomedicine. 95:1538842022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li TH, Sun HW, Song LJ, Yang B, Zhang P,
Yan DM, Liu XZ and Luo YR: Long non-coding RNA MEG3 regulates
autophagy after cerebral ischemia/reperfusion injury. Neural Regen
Res. 17:824–831. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ling J, Cai H, Lin M, Qi S, Du J and Chen
L: RTN1-C mediates cerebral ischemia/reperfusion injury via
modulating autophagy. Acta Biochim Biophys Sin (Shanghai).
53:170–178. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan ZL, Mo YZ, Li DL, Xie L and Chen MH:
Inhibition of ERK downregulates autophagy via mitigating
mitochondrial fragmentation to protect SH-SY5Y cells from OGD/R
injury. Cell Commun Signal. 21:2042023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Xu E, Musich PR and Lin F:
Mitochondrial dysfunction in neurodegenerative diseases and the
potential countermeasure. CNS Neurosci Ther. 25:816–824. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ding M, Feng N, Tang D, Feng J, Li Z, Jia
M, Liu Z, Gu X, Wang Y, Fu F and Pei J: Melatonin prevents
Drp1-mediated mitochondrial fission in diabetic hearts through
SIRT1-PGC1α pathway. J Pineal Res. 65:1–16. 2018. View Article : Google Scholar
|
|
25
|
Lei MY, Cong L, Liu ZQ, Liu ZF, Ma Z, Liu
K, Li J, Deng Y, Liu W and Xu B: Resveratrol reduces DRP1-mediated
mitochondrial dysfunction via the SIRT1-PGC1α signaling pathway in
manganese-induced nerve damage in mice. Environ Toxicol.
37:282–298. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rodgers JT, Lerin C, Haas W, Gygi SP,
Spiegelman BM and Puigserver P: Nutrient control of glucose
homeostasis through a complex of PGC-1alpha and SIRT1. Nature.
434:113–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang J, Hu Z, Tan J, Yang S and Zeng L:
Parkin protects against oxygen-glucose deprivation/reperfusion
insult by promoting Drp1 degradation. Oxid Med Cell Longev.
2016:84743032016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li M, Han B, Zhao H, Xu C, Xu D,
Sieniawska E, Lin X and Kai G: Biological active ingredients of
Astragali Radix and its mechanisms in treating cardiovascular and
cerebrovascular diseases. Phytomedicine. 98:1539182022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu W, Zhou F, Zhu Q, Bai M, Luo T, Zhou L
and Deng R: Calycosin-7-O-β-D-glucoside attenuates
palmitate-induced lipid accumulation in hepatocytes through AMPK
activation. Eur J Pharmacol. 925:1749882022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Y, Che G, Di Z, Sun W, Tian J and Ren
M: Calycosin-7-O-β-D-glucoside attenuates myocardial
ischemia-reperfusion injury by activating JAK2/STAT3 signaling
pathway via the regulation of IL-10 secretion in mice. Mol Cell
Biochem. 463:175–187. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park KR, Park JE, Kim B, Kwon IK, Hong JT
and Yun HM: Calycosin-7-O-β-glucoside isolated from astragalus
membranaceus promotes osteogenesis and mineralization in human
mesenchymal stem cells. Int J Mol Sci. 22:113622021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan X, Yu A, Zheng H, Wang S, He Y and
Wang L: Calycosin-7-O-β-D-glucoside attenuates OGD/R-induced damage
by preventing oxidative stress and neuronal apoptosis via the
SIRT1/FOXO1/PGC-1α pathway in HT22 cells. Neural Plast.
2019:87980692019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu S, Huang P, Yang J, Du H, Wan H and He
Y: Calycosin alleviates cerebral ischemia/reperfusion injury by
repressing autophagy via STAT3/FOXO3a signaling pathway.
Phytomedicine. 115:1548452023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mao Z, Tia L, Liu J, Wu Q, Wang N, Wang G,
Wang Y and Seto S: Ligustilide ameliorates hippocampal neuronal
injury after cerebral ischemia reperfusion through activating
PINK1/Parkin-dependent mitophagy. Phytomedicine. 101:1541112022.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feske SK: Ischemic stroke. Am J Med.
134:1457–1464. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Quintana DD, Garcia JA, Sarkar SN, Jun S,
Engler-Chiurazzi EB, Russell AE, Cavendish JZ and Simpkins JW:
Hypoxia-reoxygenation of primary astrocytes results in a
redistribution of mitochondrial size and mitophagy. Mitochondrion.
47:244–255. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sanderson TH, Raghunayakula S and Kumar R:
Neuronal hypoxia disrupts mitochondrial fusion. Neuroscience.
301:71–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu L, Gao Y, Hu M, Dong Y, Xu J, Zhang J
and Lv P: Edaravone dexborneol protects cerebral ischemia
reperfusion injury through activating Nrf2/HO-1 signaling pathway
in mice. Fundam Clin Pharmacol. 36:790–800. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Grohm J, Kin SW, Mamrak U, Tobaben S,
Cassidy-Stone A, Nunnari J, Plesnila N and Culmsee C: Inhibition of
Drp1 provides neuroprotection in vitro and in vivo. Cell Death
Differ. 19:1446–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao YX, Cui M, Chen SF, Dong Q and Liu
XY: Amelioration of ischemic mitochondrial injury and Bax-dependent
outer membrane permeabilization by Mdivi-1. CNS Neurosci Ther.
20:528–538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo X, Sesaki H and Qi X: Drp1 stabilizes
p53 on the mitochondria to trigger necrosis under oxidative stress
conditions in vitro and in vivo. Biochem J. 461:137–146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Higashi Y, Jitsuiki D, Chayama K and
Yoshizumi M: Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a
novel free radical scavenger, for treatment of cardiovascular
diseases. Recent Pat Cardiovasc Drug Discov. 1:85–93. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang DX, Li Y, Yu D, Guan B, Ming Q, Li Y
and Chen LQ: Human urinary kallidinogenase combined with edaravone
in treating acute ischemic stroke patients: A meta-analysis. Brain
Behav. 11:e24312021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Watanabe T, Tanaka M, Watanabe K,
Takamatsu Y and Tobe A: Research and development of the free
radical scavenger edaravone as a neuroprotectant. Yakugaku Zasshi.
124:99–111. 2004.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Matsumoto S, Murozono M, Kanazawa M, Nara
T, Ozawa T and Watanabe Y: Edaravone and cyclosporine A as
neuroprotective agents for acute ischemic stroke. Acute Med Surg.
5:213–221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu H, Li G, Chen W, Luo W, Yang Z, You Z
and Zou Y: Drp1 knockdown represses apoptosis of rat retinal
endothelial cells by inhibiting mitophagy. Acta Histochem.
124:1518372022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Feng J, Chen X, Guan B, Li C, Qiu J and
Shen J: Inhibition of peroxynitrite-induced mitophagy activation
attenuates cerebral ischemia-reperfusion injury. Mol Neurobiol.
55:6369–6386. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Feng J, Chen X, Lu S, Li W, Yang D, Su W,
Wang X and Shen J: Naringin attenuates cerebral
ischemia-reperfusion injury through inhibiting
peroxynitrite-mediated mitophagy activation. Mol Neurobiol.
55:9029–9042. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li L, Yang S, Lu X, Zhang Y and Li T:
Research progress on the mechanism of mitochondrial autophagy in
cerebral stroke. Front Aging Neurosci. 13:6986012021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yuan Y, Zhang X, Zheng Y and Chen Z:
Regulation of mitophagy in ischemic brain injury. Neurosci Bull.
31:395–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wu J, Zhang D, Chen L, Li J, Wang J, Ning
C, Yu N, Zhao F, Chen D, Chen X, et al: Discovery and mechanism
study of SIRT1 activators that promote the deacetylation of
fluorophore-labeled substrate. J Med Chem. 56:761–780. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tang H, Wen J, Qin T, Chen Y, Huang J and
Yang Q, Jiang P, Wang L, Zhao Y and Yang Q: New insights into
Sirt1: Potential therapeutic targets for the treatment of cerebral
ischemic stroke. Front Cell Neurosci. 17:12287612023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Huang S, Hong Z, Zhang L, Guo J, Li Y and
Li K: CERKL alleviates ischemia reperfusion-induced nervous system
injury through modulating the SIRT1/PINK1/Parkin pathway and
mitophagy induction. Biol Chem. 403:691–701. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li M, Li SC, Dou BK, Zou YX, Han HZ, Liu
DX, Ke ZJ and Wang ZF: Cycloastragenol upregulates SIRT1
expression, attenuates apoptosis and suppresses neuroinflammation
after brain ischemia. Acta Pharmacol Sin. 41:1025–1032. 2020.
View Article : Google Scholar : PubMed/NCBI
|