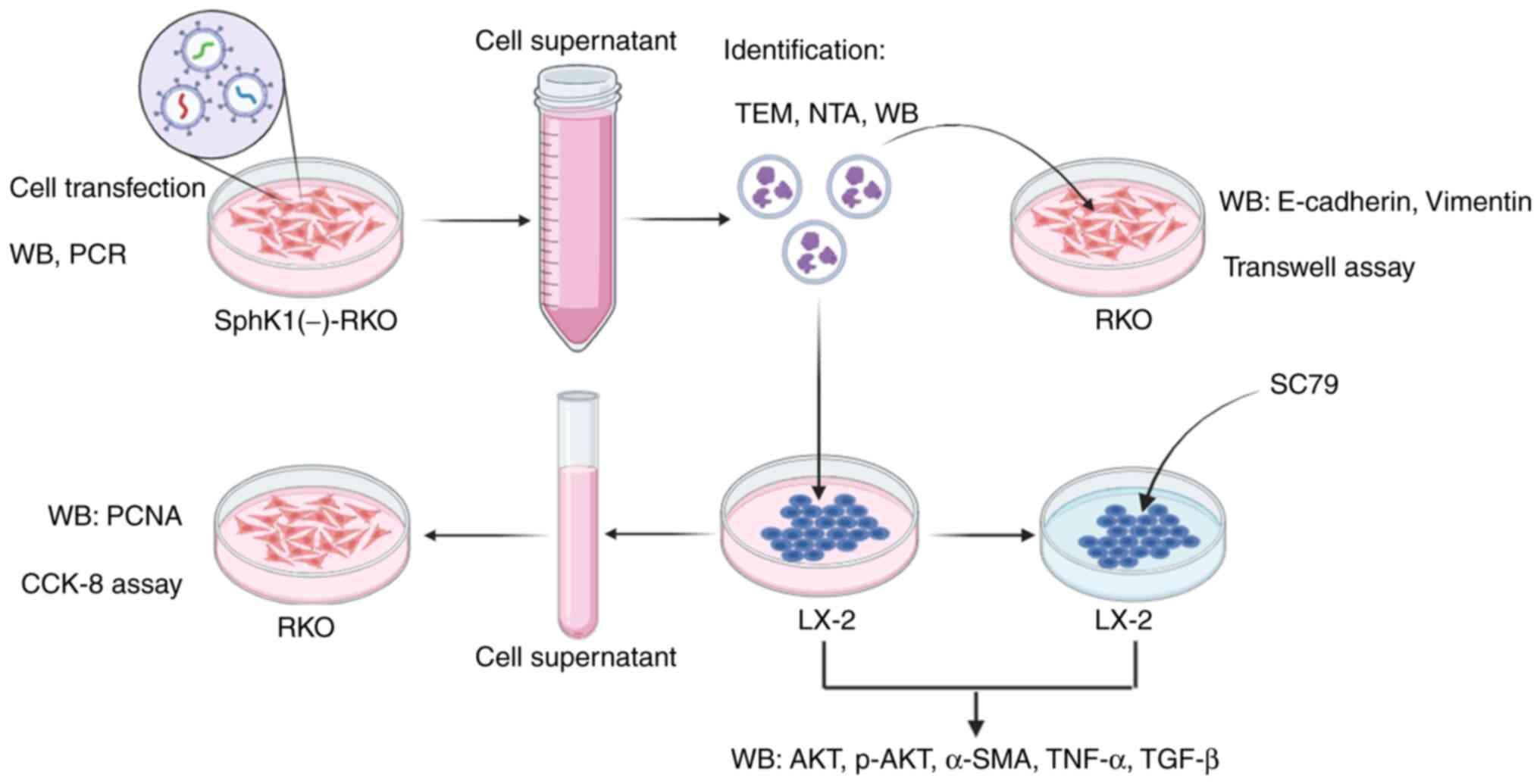
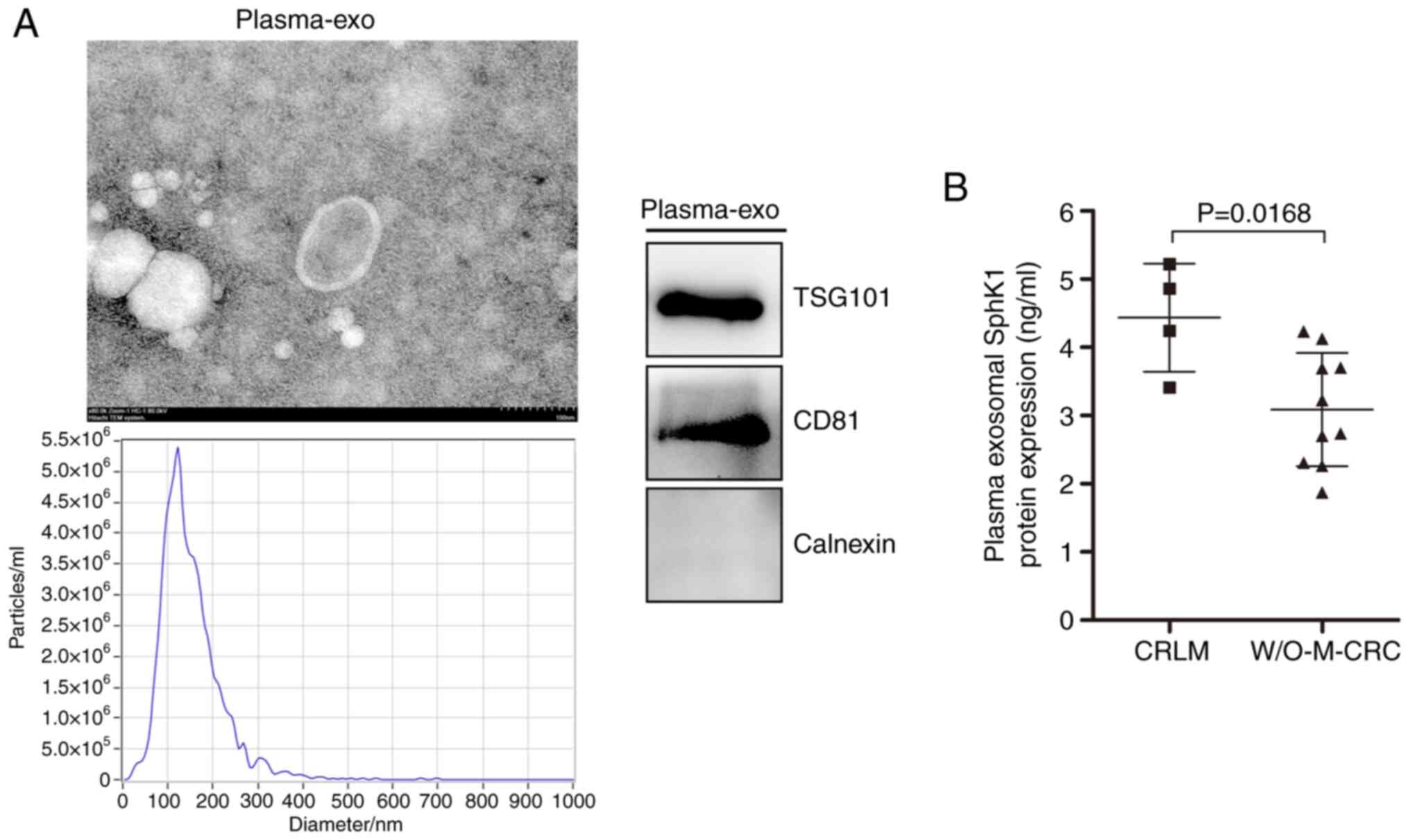
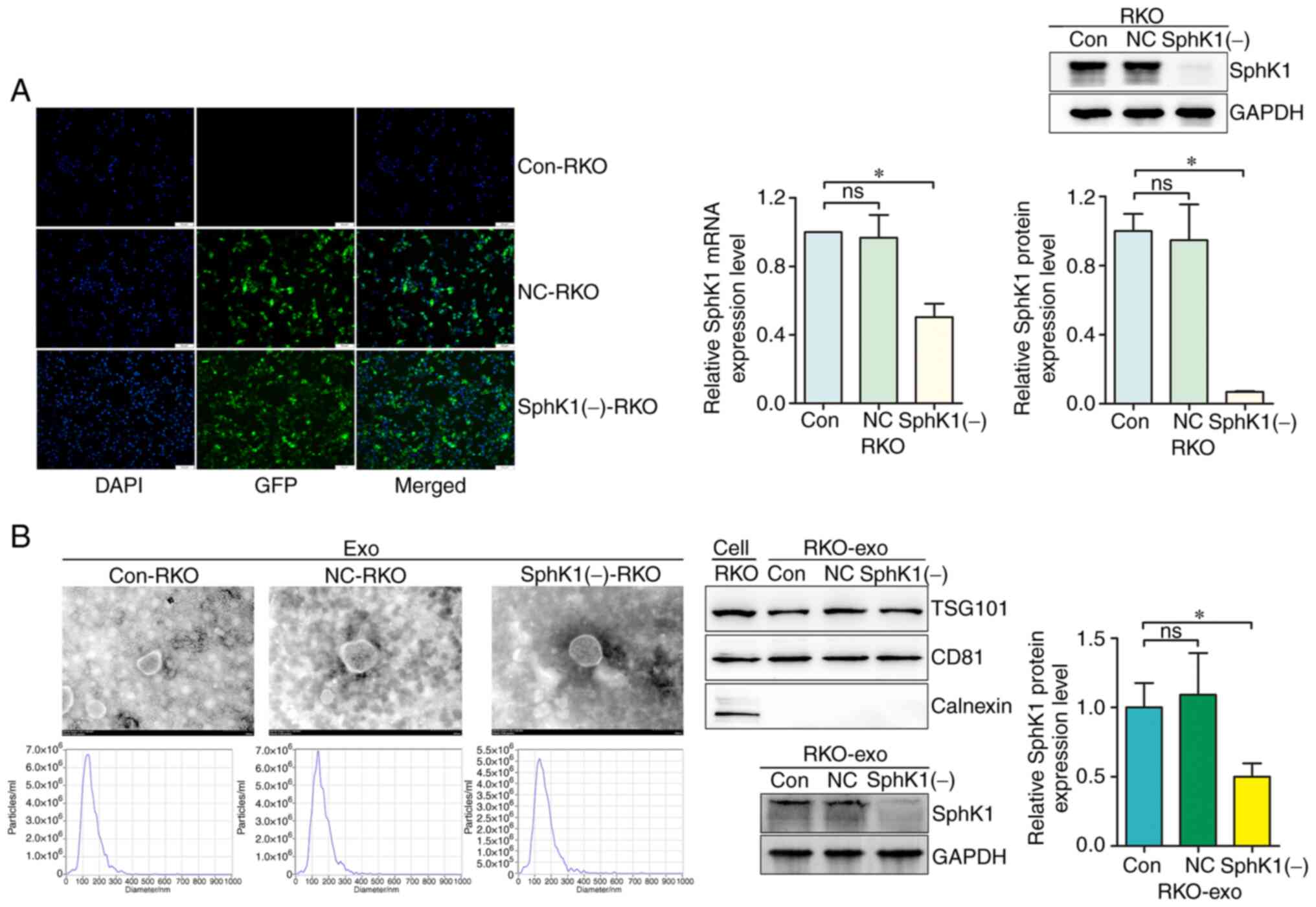
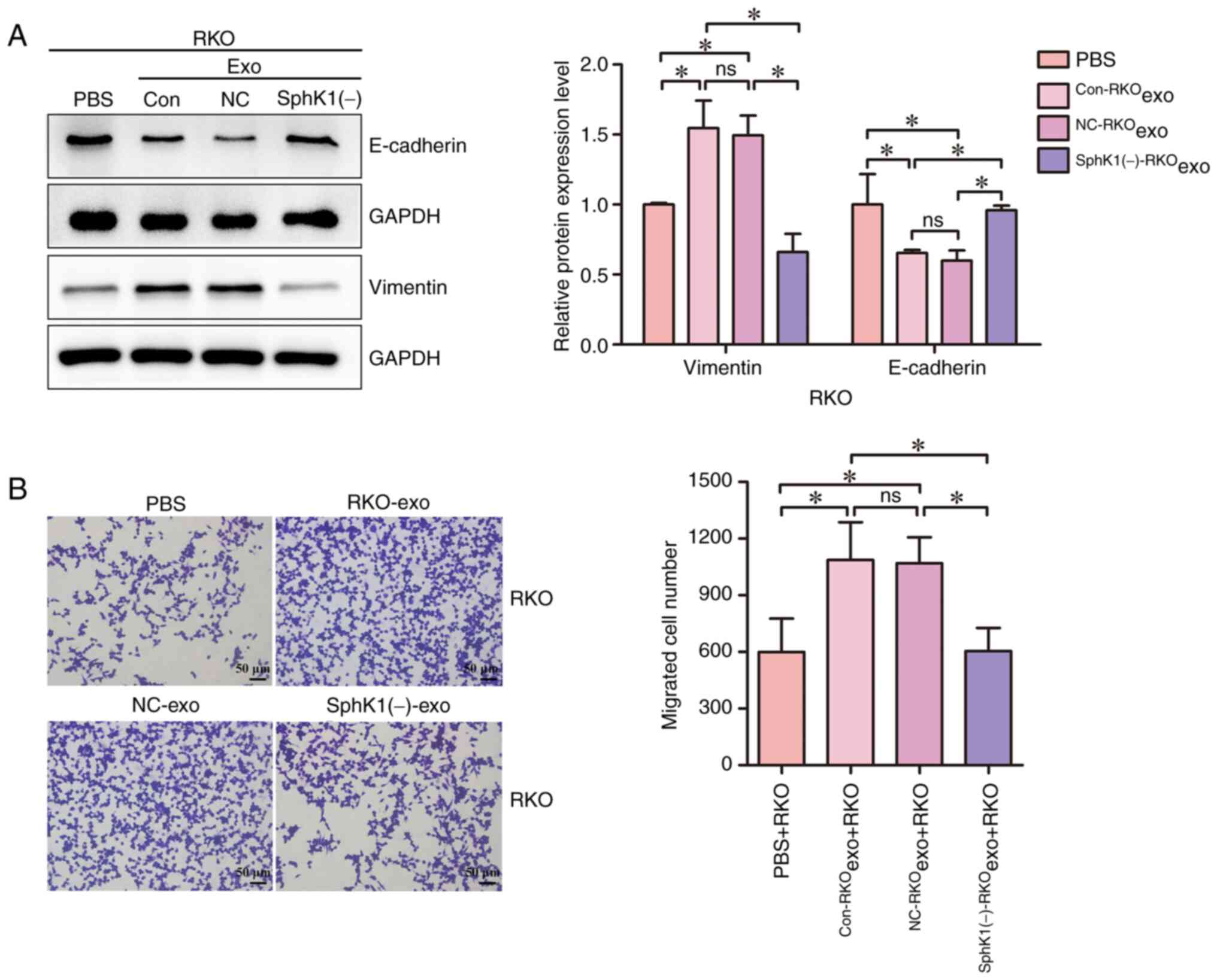
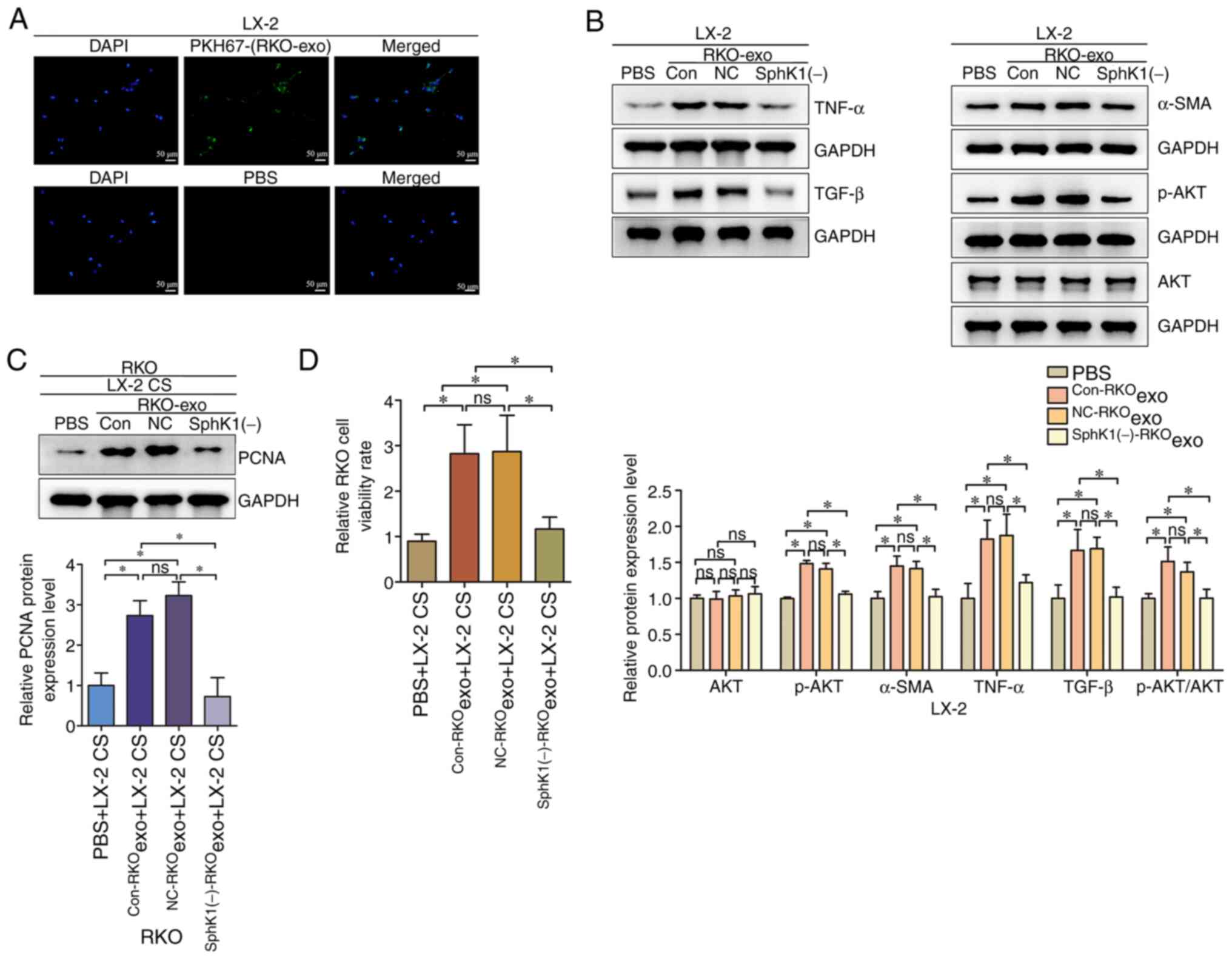
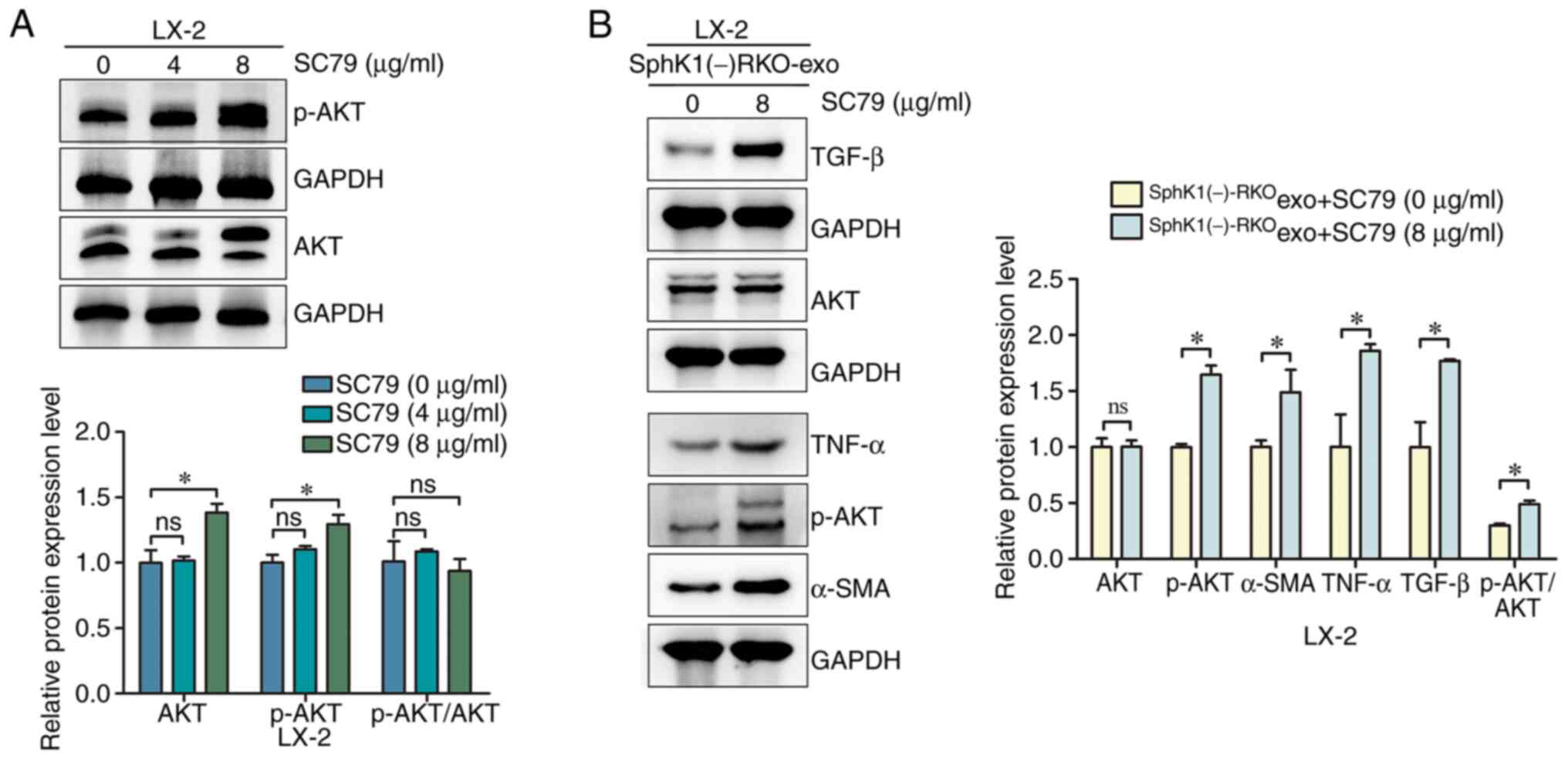
|
1
|
Viale PH: The American cancer society's
facts & figures: 2020 edition. J Adv Pract Oncol. 11:135–136.
2022.PubMed/NCBI
|
|
2
|
Pallares-Rusiñol A, Bernuz M, Moura SL,
Fernández-Senac C, Rossi R, Martí M and Pividori MI: Advances in
exosome analysis. Adv Clin Chem. 112:69–117. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rahnama M, Heidari M, Poursalehi Z and
Golchin A: Global trends of exosomes application in clinical
trials: A scoping review. Stem Cell Rev Rep. 20:2165–2193. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yáñez-Mó M, Siljander PR, Andreu Z, Zavec
AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J,
et al: Biological properties of extracellular vesicles and their
physiological functions. J Extracell Vesicles. 4:270662015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang C, Li J, Xie Z, Hu X and Huang Y:
Relationship between exosomes and cancer: Formation, diagnosis, and
treatment. Int J Biol Sci. 21:40–62. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye Z, Yi J, Jiang X, Shi W, Xu H, Cao H,
Qin L, Liu L, Wang T, Ma Z and Jiao Z: Gastric cancer-derived
exosomal let-7 g-5p mediated by SERPINE1 promotes macrophage M2
polarization and gastric cancer progression. J Exp Clin Cancer Res.
44:22025. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie H, Wu Y, Huang J, Shen Q, Li X, Wang
L, Lin J, Chi Z, Ke K, Lin X, et al: NK cell exosomes alleviate
PD-l1 expression and facilitate tumor immunity by repressing
PI3K-AKT-mTOR signaling. Immunol Invest. 2:1–14. 2025. View Article : Google Scholar
|
|
8
|
Yin J, Zhu W, Feng S, Yan P and Qin S: The
role of cancer-associated fibroblasts in the invasion and
metastasis of colorectal cancer. Front Cell Dev Biol.
12:13755432024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rom AD, Dragicevic S, Jankovic R, Skodric
SR, Sabljak P, Markovic V, Stojkovic JR, Barisic G and Nikolic A:
Markers of epithelial-mesenchymal transition and mucinous histology
are significant predictors of disease severity and tumor
characteristics in early-onset colorectal cancer. Diagnostics
(Basel). 14:15122024. View Article : Google Scholar
|
|
10
|
Ramos R, Vinyals A, Campos-Martin R, Cabré
E, Bech JJ, Vaquero J, Gonzalez-Sanchez E, Bertran E, Ferreres JR,
Lorenzo D, et al: New insights into the exosome-induced migration
of uveal melanoma cells and the pre-metastatic niche formation in
the liver. Cancers (Basel). 16:29772024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alkafaas SS, Elsalahaty MI, Ismail DF,
Radwan MA, Elkafas SS, Loutfy SA, Elshazli RM, Baazaoui N, Ahmed
AE, Hafez W, et al: The emerging roles of sphingosine 1-phosphate
and SphK1 in cancer resistance: A promising therapeutic target.
Cancer Cell Int. 24:892024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu SQ, Xu CY, Wu WH, Fu ZH, He SW, Qin MB
and Huang JA: Sphingosine kinase 1 promotes the metastasis of
colorectal cancer by inducing the epithelial-mesenchymal transition
mediated by the FAK/AKT/MMPs axis. Int J Oncol. 54:41–52.
2019.PubMed/NCBI
|
|
13
|
Xu CY, Liu SQ, Qin MB, Zhuge CF, Qin L,
Qin N, Lai MY and Huang JA: SphK1 modulates cell migration and
EMT-related marker expression by regulating the expression of p-FAK
in colorectal cancer cells. Int J Mol Med. 39:1277–1284. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao S, Mi Y, Zheng B, Wei P, Gu Y, Zhang
Z, Xu Y, Cai S, Li X and Li D: Highly-metastatic colorectal cancer
cell released miR-181a-5p-rich extracellular vesicles promote liver
metastasis by activating hepatic stellate cells and remodelling the
tumour microenvironment. J Extracell Vesicles. 1:e121862022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang W, Gao K, Bai Y, Xu D, Zhao M, Tao X
and Wang J: Wedelolactone attenuates liver fibrosis and hepatic
stellate cell activation by suppressing the hippo pathway.
Rejuvenation Res. 27:207–219. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Landskron G, De la Fuente M, Thuwajit P,
Thuwajit C and Hermoso MA: Chronic inflammation and cytokines in
the tumor microenvironment. J Immunol Res. 2014:1491852014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paschos KA and Bird NC: Liver regeneration
and its impact on post-hepatectomy metastatic tumour recurrence.
Anticancer Res. 30:2161–2170. 2010.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zou F, Wang S, Xu M, Wu Z and Deng F: The
role of sphingosine-1-phosphate in the gut mucosal microenvironment
and inflammatory bowel diseases. Front Physiol. 14:12356562023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kao WH, Liao LZ, Chen YA, Lo UG, Pong RC,
Hernandez E, Chen MC, Teng CJ, Wang HY, Tsai SC, et al: SPHK1
promotes bladder cancer metastasis via PD-L2/c-Src/FAK signaling
cascade. Cell Death Dis. 15:6782024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu M, Wang S, Zeng Y, Liu P and Li H:
SPHK1 promotes pancreatic cancer lymphangiogenesis through the
activation of ERK in LECs. Mol Biotechnol.
11:10.1007/s12033–024-01192-9. 2024.
|
|
22
|
Zhang C, Zhou C, Xu J and Xue S: Increased
sphingosine kinase 1 expression is associated with poor prognosis
in human solid tumors: A meta-analysis. Dis Markers.
2022:84439322022.PubMed/NCBI
|
|
23
|
Xu C, Zhang W, Liu S, Wu W, Qin M and
Huang J: Activation of the SphK1/ERK/p-ERK pathway promotes
autophagy in colon cancer cells. Oncol Lett. 15:9719–9724.
2018.PubMed/NCBI
|
|
24
|
Gupta P, Kadamberi IP, Mittal S, Tsaih SW,
George J, Kumar S, Vijayan DK, Geethadevi A, Parashar D, Topchyan
P, et al: Tumor derived extracellular vesicles drive T cell
exhaustion in tumor microenvironment through sphingosine mediated
signaling and impacting immunotherapy outcomes in ovarian cancer.
Adv Sci (Weinh). 9:e21044522022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Niu Y, Yang W, Qian H and Sun Y:
Intracellular and extracellular factors of colorectal cancer liver
metastasis: A pivotal perplex to be fully elucidated. Cancer Cell
Int. 22:3412022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu B, Wu T, Lin B, Liu X, Liu Y, Song G,
Fan C and Ouyang G: Periostin-TGF-β feedforward loop contributes to
tumour-stroma crosstalk in liver metastatic outgrowth of colorectal
cancer. Br J Cancer. 130:358–368. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang WH, Zhou MW, Zhu YF, Xiang JB, Li
ZY, Wang ZH, Zhou YM, Yang Y, Chen ZY and Gu XD: The role of
hepatic stellate cells in promoting liver metastasis of colorectal
carcinoma. Onco Targets Ther. 12:7573–7580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Stoess C, Holzmann G, Mogler C,
Stupakov P, Altmayr F, Schulze S, Wang B, Steffani M, Friess H, et
al: Signalling of the neuropeptide calcitonin gene-related peptide
(CGRP) through RAMP1 promotes liver fibrosis via TGFβ1/Smad2 and
YAP pathways. Exp Cell Res. 442:1141932024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watakabe K, Miyoshi M, Kakinuma S, Sato A,
Tsuchiya J, Shimizu T, Mochida T, Inada K, Kaneko S, Kawai-Kitahata
F, et al: A20 in hepatic stellate cells suppresses chronic
hepatitis by inhibiting DCLK1-JNK pathway-dependent chemokines.
FASEB J. 38:e237572024. View Article : Google Scholar : PubMed/NCBI
|