Introduction
Peptidyl-prolyl cis-trans isomerase NIMA-interacting
1 (Pin1) is an enzyme that belongs to the peptidyl-prolyl cis-trans
isomerase (PPIase) family (1).
Pin1 induces conformational changes and functional transitions in
target molecules by catalyzing the phosphorylation of
serine/threonine (Ser/Thr) residues as well as the phosphorylation
of Ser/Thr-Pro motifs (Fig. 1)
(2).
The Ser/Thr-pro motif encompasses kinases such as
CDKs, MAPKs (including P38, JNK and ERK) and GSK-3β, as well as
Ser/Thr phosphodiesterases such as PP2A, Fcp1 and Calcineurin. The
former belongs to the proline-directed kinase family, whereas the
latter belongs to the proline-directed phosphodiesterase family.
ERK2, CDK2 and PP2A all exhibit trans-isomeric properties before
and after phosphorylation. Previous studies have also confirmed the
enhanced stability of CyclinD1, β-Catenin and P53 after cis- or
trans-isomerization (3,4), which is closely related to tumor
development (5).
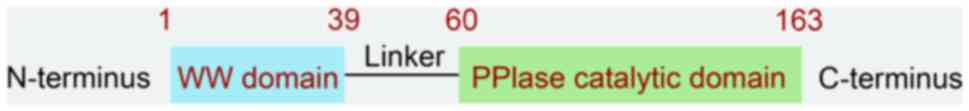
Pin1 consists of 163 amino acids and contains two
major functional domains: The WW structural domain located at the
N-terminal end, which is primarily responsible for recognizing and
binding phosphorylated Ser/Thr-proline motifs; and the PPIase
structural domain located at the C-terminal, which catalyzes the
cis- and trans-isomerization of proline residues (Fig. 1) (6).
Pin1 catalyzes the phosphorylation of the
Ser/Thr-Pro motif through its C-terminal PPIase structural domain,
which induces conversion of the proline residues between the cis
and trans conformation. This cis-trans isomerization results in a
change in protein conformation, which in turn affects protein
function, stability, or interactions with other proteins (Fig. 2) (7). Pin1 can act on numerous
phosphoproteins that regulate cell division (Table I). Previous studies have also shown
that Pin1 is upregulated in several types of cancer where it
promotes cell proliferation and inhibits apoptosis by regulating
the Ser/Thr-Pro motifs of oncogenes and tumor suppressor genes
(8,9). The present review focused on the
mechanisms by which Pin1 acts on certain key proteins in
tumorigenesis.
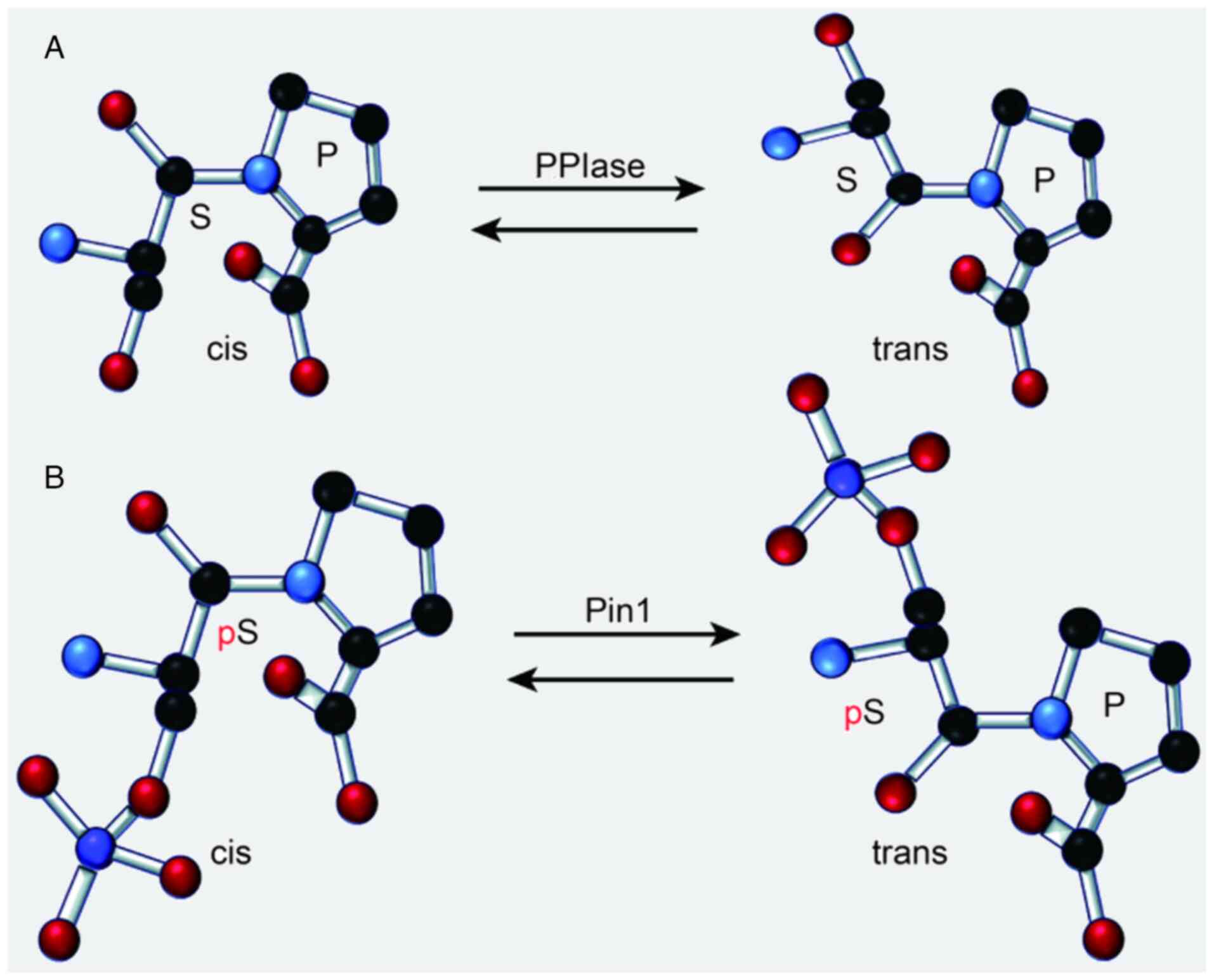 | Figure 2.Functions of (A) PPIase and (B) Pin1.
Adobe Illustrator was used to create this figure, which illustrates
that PPIases are a class of enzymes that catalyze the cis-trans
isomerization of proline residues within proteins. Pin1 is a
specific member of the PPIase family that facilitates the
conversion of phosphorylated Ser/Thr-Pro bonds from the cis to the
trans conformation. This conformational change is crucial for
regulating protein function, as it can induce alterations in
protein structure, thereby affecting cellular signaling pathways,
modulating neuronal activity, or promoting oncogenic processes.
Specifically, Pin1 modulates the activity and interactions of
phosphorylated proteins by altering their conformation, which may
subsequently influence the activity of intracellular signaling
pathways, inhibit neuronal function, or stimulate the proliferation
of cancer cells. PPIase, peptidyl-prolyl cis-trans isomerase; Pin1,
peptidyl-prolyl cis-trans isomerase NIMA-interacting 1. |
 | Table I.Pin1-interacting proteins. |
Table I.
Pin1-interacting proteins.
| Protein | Function |
|---|
| NIMA | Mitotic kinase |
| Cdc25C | Protein phosphatase
for Cdc2 |
| Plk 1 | Mitotic kinase |
| Cdc27 | Anaphase-promoting
complex component |
| Rab4 | GTP-binding
protein |
| p70/S6 kinase | Protein kinase |
| Wee 1 | Mitotic kinase |
| Myt 1 | Mitotic kinase |
| CENP-F | Kinetokore
protein |
| Incenp | Inner centromere
protein |
| Tau |
Microtubule-interacting protein |
| PolII | RNA polymerase
II |
| Sin3-Rpd3 | Histone
deacetylase |
| NHERF-1 |
Na+/H+exchanger
regulatory factor 1 |
| KRMP 1 | Kinesin-related
protein |
| hSpt5 | A DRB
sensitivity-inducing factor component |
| Bcl-2 | Anti-apoptotic
factor |
| c-Jun | Transcription
factor |
| β-catenin | Transcription
activator |
| NFAT | Transcription
factor |
| Cf-2 | Transcriptional
repressor |
| Cyclin D1 | Cell cycle
regulator |
| CK2 | Protein kinase |
| P53 | Transcription
factor |
Mechanism of the Pin1 gene in regulating
tumorigenesis
Pin1 not only exhibits upregulated expression in
several types of cancer, but also plays a crucial role in
tumorigenesis and is a known catalyst of tumorigenesis. Its
overexpression can activate multiple oncogenic pathways (10).
Overactivation of Cdc25C or
upregulated expression of Pin1 abrogates the cell cycle and
promotes tumor growth and proliferation
Cdc25C is a cell cycle protein phosphatase that is
primarily responsible for removing the phosphate group and
activating CDKs, especially CDK1 during the G2/M phase transition,
thus promoting entry into the mitotic phase (11).
In different types of cancer, the expression
patterns of Pin1 and Cdc25C show significant differences. For
example, in breast cancer and non-small cell lung cancer,
upregulated expression of Pin1 and Cdc25C is associated with
aggressive tumor behaviors and a poor prognosis (9,12,13).
However, in colorectal cancer, this association is not as clear
(14,15). After phosphorylation by Polo-like
kinase (16), Cdc25C is activated,
but stabilization of its activity requires the catalytic action of
Pin1 to form the trans-isomer. This reversible process involves the
specific action of PP2A, which dephosphorylates trans-Cdc25C,
leading to its inactivation and thus preserving the equilibrium of
Cdc25C activity. The regulatory role of Pin1 is multifaceted in
modulating Cdc25C activity during the G2/M phase. While
insufficient Pin1 activity has been implicated in Cdc25C
dysfunction, the context-dependent nature of Pin1′s effects on
Cdc25C activity suggests a more intricate relationship (11). Overexpression of Cdc25C or
excessive Pin1 activity can lead to cell cycle deregulation,
promoting uncontrolled cell proliferation, a hallmark of cancer
development. This complex interplay is supported by studies showing
both the inhibitory (17–19) and activating effects (20) of Pin1 on Cdc25C, highlighting the
need for a nuanced understanding of the role of Pin1 in
tumorigenesis. Studies have shown that Cdc25 is overexpressed in
several types of cancer (21,22)
and the two isoforms of Cdc25 phosphatase, Cdc25A (23) and Cdc25B (24) may be overexpressed either alone or
in combination in a variety of cancers, including breast, lung,
ovarian, colon, esophageal, gastric, hepatocellular (HCC),
non-small cell lung, non-Hodgkin's lymphoma, pancreatic ductal,
thyroid and head and neck cancer, among others (9,12).
Upregulated expression of Pin1 and Cdc25C are typically associated
with poorer prognostic outcomes, indicating that these proteins may
serve as valuable prognostic indicators for certain types of cancer
(25). Thus, assessing the
expression of Pin1 and Cdc25C in tumors may provide critical
insights into patient prognosis and guide therapeutic
strategies.
Pin1 enhances Cyclin D1 stability and
promotes cancer cell proliferation
In the course of performing the background research
for the present review, >30 target proteins of Pin1 were
identified (Table I), among which
Cyclin D1 has received the most attention. Cyclin D1 is a key
protein in cell cycle regulation and functions primarily in the
G1 phase (26). It
facilitates the cellular transition from G1 to S phase
by binding to CDK4 or CDK6 to form the Cyclin D1-CDK4/6 complex
(27). The stability and
expression levels of Cyclin D1 are critical for cell cycle
progression (Fig. 3).
Regarding the expression pattern of Cyclin D1,
studies have shown that ~50% of patients with breast cancer exhibit
upregulated expression levels of Cyclin D1 and it is also
overexpressed in HCC and squamous cell carcinoma of the head and
neck (26,28). These data suggest that the
co-expression of Pin1 and Cyclin D1 may be a common feature of
several types of cancer, highlighting it as a potential biomarker
(9).
Phosphorylation of Cyclin D1 at the Thr286 site is a
critical step in the regulation of its degradation and function
(29). Pin1 recognizes and binds
phosphorylated Cyclin D1 through its WW structural domain.
Subsequently, Pin1 uses its PPIase structural domain to
cis-trans-isomerize Cyclin D1 and inhibit its binding to CRM1, a
Cyclin D1 efflux factor and thus stabilizes Cyclin D1 (30). In addition to affecting the
stability of Cyclin D1, Pin1 can also indirectly affect the
expression of the Cyclin D1 gene by regulating the activity of
transcription factors (such as c-Jun and AP-1) (31), further highlighting its role in
cell cycle regulation.
Current studies have shown that 50% of patients with
breast cancer exhibit upregulated expression levels of Cyclin D1
(26,27,32).
Additionally, overexpression and gene amplification of Cyclin D1
have been associated with several types of cancer, including head
and neck squamous cell carcinoma, esophageal cancer and HCC
(33). Therefore, Pin1 inhibitors
may pave the way for more precise management of breast cancer
(34).
Pin promotes p53-mediated cell cycle
arrest and apoptosis and suppresses tumorigenesis
P53 is an oncogenic protein that plays a key role in
regulating cell cycle checkpoints, maintaining genomic stability
and promoting apoptosis and is therefore known as the ‘guardian of
the genome’ (35). In various
malignant tumors, >50% of the cases show mutations in the P53
gene (36).
The frequency of P53 mutations varies significantly
in different tumor types. For example, the incidence of P53
mutations is high in breast and colon cancers and relatively low in
certain lymphomas (9). This
difference may affect the role of Pin1 as it promotes the
physiological function of P53, although its function may be
inhibited in the case of mutations.
When DNA is damaged, upstream kinases such as
checkpoint kinase 1/2, ataxia-telangiectasia mutated and ataxia
telangiectasia and Rad3-related protein are activated, which
phosphorylate multiple serine/threonine (Ser/Thr) sites of P53,
including those preceding proline residues, thereby protecting P53
from ubiquitination (37),
ensuring its high level of expression in the cell. This enhances
the tumor suppressor function of P53. Increasing the levels of P53
promotes the expression of p21, leading to cell cycle arrest and
favoring the repair of damaged DNA (38). When DNA damage persists, P53 also
promotes the expression of proteins such as BAX, death receptor
5/killer, Fas and Fas ligand, which in turn triggers apoptosis
(39).
Pin1 protein recognizes and binds phosphorylated P53
through its WW structural domain and this interaction leads to
cis-trans isomerization of P53, further enhancing its stability and
activity as a transcription factor (4). In tumor cells, Pin1 exerts its tumor
suppressor effect by enhancing the function of wild-type P53,
inhibiting tumor cell proliferation and promoting apoptosis
(36). However, in certain types
of cancer, mutations in the P53 gene result in the loss of its
tumor suppressor function, at which point the role of Pin1 may
vary, depending on the nature and location of the mutation
(40).
Pin1 regulates the Ras/AP-1 signaling
pathway and promotes cancer cell proliferation
Ras proteins belong to a family of small GTPases
that serve a crucial role in numerous cell signaling pathways
(41). When Ras binds to GTP, GTP
is activated, which in turn activates a range of downstream
effector molecules. By activating Raf kinase, Ras initiates the
MAPK signaling cascade, which in turn activates MEK (MAPK/ERK
kinase), which activates ERK. Activated ERK translocates to the
nucleus and promotes activation of the AP-1 transcription factor
(42).
In the Ras/AP-1 signaling pathway, Pin1 directly
interacts with members of the AP-1 family (such as c-Fos and c-Jun)
(31) and facilitates cis-trans
isomerization by recognizing their phosphorylated Ser/Thr-Pro
sequences, which enhances the ability of Jun proteins to dimerize,
either in homo- or heterodimeric form and improves their
DNA-binding activity, which in turn enhances transcriptional
activity and stability. Furthermore, the role of Pin1 in the
aberrant activation of the Ras/AP-1 pathway in cancer is critical,
as it may contribute to the sustained proliferation and survival of
cancer cells by maintaining the oncogenic potential of AP-1 family
members under pathological conditions (43,44).
Aberrant activation of the Ras/AP-1 signaling
pathway is closely associated with the development of several types
of cancer (31) and mutations in
the Ras gene typically result in the persistent activation of
downstream signaling pathways to promote uncontrolled cell
proliferation and survival (45),
particularly in pancreatic (46),
colorectal (47) and lung cancer
(48). Overexpression or
overactivation of AP-1 signaling pathway members is also prevalent
in several types of cancer, such as overexpression of c-Jun and
c-Fos, which is associated with aggressiveness and a poor prognosis
in breast cancer, osteosarcoma and other types of cancer (49) and mutations in other components of
the Ras/AP-1 signaling pathway (for example, Raf, MEK and ERK),
also result in sustained activation of the pathway, which enhances
cell viability and the proliferative potential of cancer cells
(50).
The activation pattern of the Ras/AP-1 pathway
varies in different types of cancer. For example, in pancreatic
cancer, Ras gene mutations lead to persistent activation of
downstream signaling pathways, while in certain breast cancer
subtypes, different regulatory mechanisms may be exhibited. This
diversity underscores the importance of Pin1 in regulating the
Ras/AP-1 pathway and its possibility as a potential therapeutic
target (51–53).
Pin1 regulates the Wnt/β-catenin
signaling pathway and promotes cancer cell proliferation
Pin1 recognizes and binds to the phosphorylation of
the Ser/Thr-Pro motif on β-catenin and cis-trans isomerization
β-catenin through its PPIase structural domain, altering its
conformation and enhancing its stability (54). This isomerization protects
β-catenin from ubiquitination and proteasome-mediated degradation,
leading to increased stability and accumulation of β-catenin in the
cytoplasm and facilitating its translocation to the nucleus.
In the nucleus, Pin1 enhances the binding of
β-catenin to TCF/LEF transcription factors by elevating its
stability and intranuclear translocation to form a transcriptional
activation complex (55). A
β-catenin/TCF complex activates the transcription of Wnt target
genes (such as c-Myc and Cyclin D1 among others) in the nucleus and
regulates the G1/S transition of the cell cycle. The
β-catenin/TCF complex promotes cell cycle progression, which in
turn promotes the proliferation of cancer cells (56). This phenomenon can be seen in
several types of cancer, including colon cancer (57), HCC (58) and breast cancer (59). There are also differences in the
activation patterns of the Wnt/β-catenin signaling in different
types of cancer. For example, in colon cancer, upregulated
expression of β-catenin is closely associated with tumor
progression and metastasis, while it manifests as dysregulation in
certain types of HCC. The role of Pin1 in these different contexts
may determine its specific role in tumor progression.
Role of Pin1 in multiple oncogenic signaling
pathways
The promoter region of the Pin1 gene lacks a TATA
box and a CAAT box, but contains two GC boxes and three E2F binding
sites (60). Therefore, in breast
cancer cells, due to the high expression of E2F (61), it increases the mRNA expression of
Pin1 (62,63). This integration occurs as E2F, when
activated, binds to the GC boxes in the Pin1 promoter, facilitating
the recruitment of transcriptional co-activators that enhance Pin1
transcription. Aberrant upregulation of E2F is a key factor driving
the upregulation of Pin1 in breast cancer cells, a phenomenon that
has also been shown in other types of tumor cells (64,65).
However, Pin1 activity is not only regulated by gene expression
factors, but also by post-translational modifications, such as
phosphorylation and dephosphorylation, which can modulate its
stability and interaction with target proteins (66). For example, when Pin1 is
phosphorylated at specific residues, its conformational change
enhances its ability to bind and isomerize target proteins such as
Jun and β-catenin. This action not only stabilizes these proteins
but also boosts their transcriptional activity, creating a
feed-forward loop that escalates oncogenic signaling.
Previous studies highlight the critical role of the
E2F/Rb pathway in regulating Pin1 expression (67,68),
which is often upregulated in various cancers (69). The dysregulation of this pathway,
particularly its aberrant upregulation, has been associated with
increased Pin1 levels, resulting in a high oncogenicity rate of
>80% (68,70). This suggests that Pin1 acts as an
activator within oncogenic signaling pathways, contributing
significantly to tumorigenesis (9,44,71).
Pin1 does not directly act on the E2F/Rb pathway,
but rather indirectly influences the occurrence and development of
tumors by regulating various proteins and signaling pathways,
including p53, which is related to cell cycle regulation. A study
by Yao et al (72) revealed
the key role of the Rb-E2F bistable switch at the restriction point
of the cell cycle, highlighting the central position of the E2F/Rb
pathway in cell cycle regulation. Dannenberg et al (73) further clarified the relationship
between the retinoblastoma susceptibility gene-encoded nuclear
phosphoprotein and DNA binding activity, which is a key link in the
E2F/Rb pathway. The study by Engeland (38) provided novel insights into the
regulation of RB function by monophosphorylation codes and RB, as a
key component of the E2F/Rb pathway, its functional state is
crucial for the control of the cell cycle. These studies together
provide a solid scientific foundation for understanding the role of
Pin1 in tumor pathways and emphasize the importance of the E2F/Rb
pathway in the Pin1 regulatory network. Specifically, there have
been studies that have specifically examined the association
between the E2F/Rb pathway and Pin1 (74,75).
For instance, it has been shown that Pin1 is a downstream target
gene of E2F and plays an important role in the transformation of
breast cancer cells induced by Neu/Ras (74,75).
This finding highlights the importance of the E2F/Rb pathway in the
Pin1 regulatory network. Additionally, studies have indicated that
Pin1 can directly bind to phosphorylated Rb (67,76)
and this binding is regulated by G1-S phase-specific
Cyclin/CDK complexes (74). These
results suggest that Pin1 indirectly participates in the regulation
of the E2F/Rb pathway through its interaction with Rb (74,75).
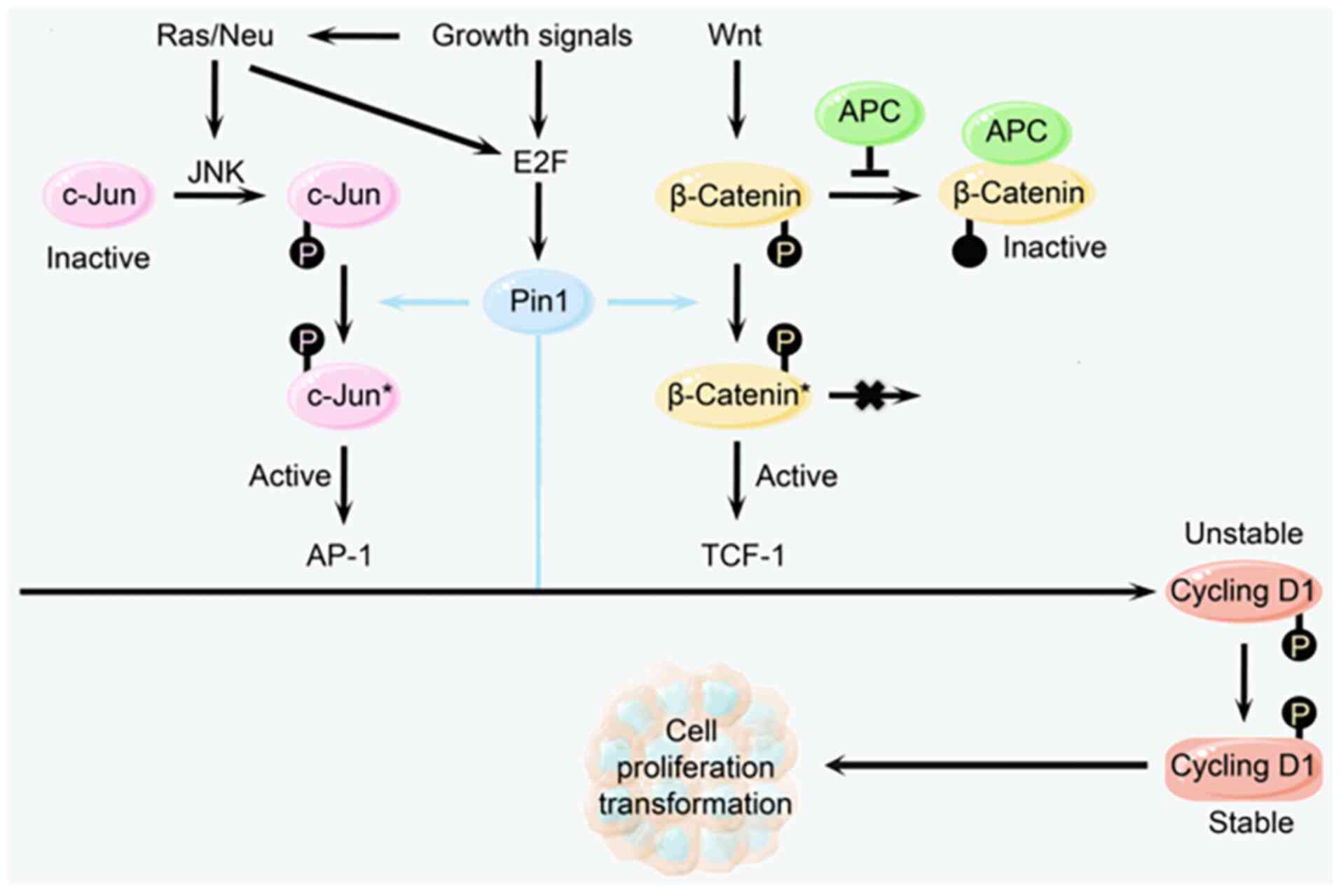
This process involves the following steps that
ultimately lead to tumorigenesis: Growth factors induce expression
of the Pin1 gene via the Ras signaling pathway and simultaneously
contribute to JNK phosphorylation of Jun at the Ser63 and Ser73
sites (77). Then, Pin1
tautomerizes with phosphorylated Jun and enhances its
transcriptional activity (78). At
the same time, Pin1 also tautomerizes with phosphorylated β-Catenin
and prevents it from binding to adenomatous polyposis coli, thereby
increasing the content of β-catenin in the nucleus and thus
promoting Jun gene expression (79). As a result, β-catenin and Jun
jointly promote the expression of CyclinD1; β-catenin also promotes
the expression of Myc (80), which
further induces the expression of CDK4 and E2F (65), which in turn promotes the
expression of Pin1 through a positive feedback mechanism.
Ultimately, these factors work together to promote abnormal cell
proliferation and tumorigenesis (Fig.
4) (81).
Zheng et al (82) show the interplay between E2F/Rb
pathway dysregulation and the upregulation of Pin1 expression,
providing further evidence of their correlation in HCC. They
identified that sorafenib, a multikinase inhibitor with proven
efficacy in HCC treatment, downregulated the mRNA and protein
expression levels of the peptidyl-prolyl isomerase Pin1,
potentially through the Rb/E2F pathway (82). The Rb/E2F pathway is pivotal in
cell cycle regulation and proliferation control and sorafenib may
exert its antitumor effects in HCC by modulating this pathway,
thereby inhibiting Pin1 transcription (82). Additionally, all-trans retinoic
acid (ATRA), an anticancer agent, has been shown to suppress and
induce the degradation of active Pin1 in cancer cells, effectively
sensitizing HCC cells to sorafenib-induced cell death in a
caspase-dependent manner. The combined use of ATRA and sorafenib
demonstrated synergistic effects in mouse xenograft models,
improving the inhibition of HCC tumor growth. These findings
underscored the critical role of Pin1 in the antitumor activity of
sorafenib and offered a scientific rationale for the use of Pin1
inhibitors as a novel approach to potentiate the therapeutic
efficacy of sorafenib in HCC treatment (82).
In addition to the aforementioned signaling
pathways, Pin1 plays a pivotal role in other key cancer-related
signaling pathways. For example, the Hippo signaling pathway, which
is critical for organ size regulation and tumor suppression, is
positively regulated by the core components YAP and TAZ proteins
through Pin1 (83). Specifically,
Pin1 modulates the phosphorylation status of YAP and TAZ, which
influences their localization and transcriptional activity in the
nucleus, thus enhancing the expression of tumor-promoting genes.
This modulation may contribute to tumor development and
invasiveness in various types of cancer. Thus, Pin1-mediated
regulation of the Hippo pathway presents another potential
intervention point for cancer therapy.
Pin1 inhibitors
Pin1 knockout (Pin1−/−) significantly
affects cellular response capabilities, primarily in several key
areas: First, Pin1 deficiency reduces the stability and
accumulation of p53, subsequently inhibiting the expression of its
downstream cell cycle regulator, p21. This alteration disrupts the
normal activation of cell cycle checkpoints, allowing cells to
continue dividing despite unresolved DNA damage, thereby increasing
the risk of genetic mutations (84–86).
Additionally, the loss of Pin1 induces morphological changes in
cells and enhances sensitivity to DNA damage, potentially leading
to cell death or transformation. While Pin1 is overexpressed in
various types of cancer and influences the stability of
phosphorylated proteins, its knockout may also suppress tumor
progression (87–90). These findings underscore the
critical role of Pin1 in maintaining genomic stability, regulating
the cell cycle and its involvement in tumorigenesis, highlighting
potential therapeutic targets for cancer treatment (84).
At the cellular level, the functions of Pin1 and its
role in diseases have been extensively studied using a variety of
cellular models (91). For
instance, human embryonic kidney cell line HEK293, human cervical
cancer cell line HeLa, human breast cancer cell line MCF-7 and
human osteosarcoma cell line U2OS have been utilized to investigate
the role of Pin1 in regulating the cell cycle, affecting p53
stability and promoting cancer development (91). Additionally, mouse embryonic
fibroblast cell line NIH 3T3 and MEF cells have been employed to
study the functional changes of Pin1 in knockout and overexpression
models. Human neuroblastoma cell line SH-SY5Y and rat adrenal
pheochromocytoma cell line PC12 have been used to explore the role
of Pin1 in neurodegenerative diseases and neural differentiation,
respectively (91). These in
vitro cell culture models provide important tools for
understanding the mechanisms of Pin1 action in cellular signaling
pathways (91). Studies have shown
that Pin1 interacts with phosphorylated Ser/Thr-Pro motifs through
its PPIase activity and WW domain (66,92),
thereby affecting various cellular signaling pathways, revealing
its significant role in tumorigenesis (93).
In terms of animal models, Pin1 knockout mice
provide an important tool for studying Tau pathology and
Alzheimer's disease (AD). Studies have shown that Pin1 knockout
mice exhibit age-dependent neuropathy characterized by motor and
behavioral deficits, Tau hyperphosphorylation, Tau fiber formation
and neuronal degeneration (94).
These mouse models were used to examine multiple aspects of
neurodegeneration by means of behavioral tests, immunostaining and
immunoblotting (94). In addition,
the Pin1 knockout mouse model also revealed a role for Pin1 in
regulating Tau stability and neurodegenerative phenotypes. For
example, overexpression of mouse Pin1 (mPin1) promotes the
degradation of phosphorylated Tau, whereas mPin1 knockout results
in hyperphosphorylation of Tau, inhibition of Tau degradation and a
neurodegenerative phenotype (91).
For the P301L Tau mutant, the function of mPin1 is reversed and
overexpression of mPin1 increases Tau hyperphosphorylation,
inhibits Tau degradation and induces a neurodegenerative phenotype
(91). Furthermore, Pin1 knockout
mice crossed with Tau transgenic mice show increased cispT231-P Tau
and reduced transpT231-P Tau levels, further supporting the
hypothesis that mPin1 inhibits tau-related neurodegenerative
lesions in mice (91). In addition
to the use of A Pin1 mouse model in Tau pathology studies of AD,
Pin1 knockout mice were also found to affect starch precursor
protein (APP) processing in mice overexpressing APP in the brain
and mPin1 knockdown increased levels of the toxic insoluble Aβ
peptide Aβ42 in an age-dependent manner (91).
Taken together, Pin1 knockout cell and animal models
provide an important experimental basis for understanding the role
of Pin1 in neurodegenerative diseases such as AD and provide
potential targets for future therapeutic strategies.
Pin1 inhibitors inhibit tumor cell growth and
proliferation by blocking Pin1 activity and interfering with its
role in cell cycle regulation, signaling and protein isomerization
(Table II) (95). Therefore, it is particularly
crucial to develop effective Pin1 inhibitors to provide novel
therapeutic options for the management of cancer (96).
 | Table II.Pin1 inhibitors. |
Table II.
Pin1 inhibitors.
| Inhibitor Name | Mechanisms | Research phase |
|---|
| Juglone | Inhibits the
isomerase activity of Pin1 by binding to its PPIase structural
domain. | At the research
stage |
| ATRA | Inhibits the
activity of Pin1 by inducing its degradation. | Preclinical
studies |
| PIB | Directly binds the
PPIase structural domain of Pin1 and interferes with its
interaction with the substrate. | Preclinical
studies |
| GSK3
Inhibitors | Indirectly inhibits
Pin1 function by reducing the phosphorylation state of Pin1. | At the research
stage |
| AL-7 | Inhibits the
isomerization activity of Pin1 by non-competitively binding to its
PPIase structural domain. | At the research
stage |
| Nimbolide | Inhibits the
isomerase activity of Pin1 by binding to its PPIase structural
domain. | Preclinical
studies |
| TAP | Directly binds to
the PPIase structural domain of Pin1 and blocks the isomerization
of Pin1. | Preclinical
studies |
| CBU | Interferes with its
interaction with substrates by binding to Pin1. | At the research
stage |
Depending on how the inhibitor binds to the PPIase
domain, Pin1 inhibitors can be classified into two main categories:
Covalent and non-covalent (97).
Covalent inhibitors bind to the active site of Pin1 by forming a
covalent bond, thereby irreversibly inhibiting its isomerase
activity. This mode of binding usually results in permanent
inactivation of the enzyme activity, as the breaking of the
covalent bond requires specific conditions such as a specific pH or
further modification of the active center of the enzyme. Juglone,
as the first identified covalent Pin1 inhibitor, was initially
extracted from the walnut tree (98). It inhibits the isomerase activity
of Pin1 by covalently binding to its catalytically active site,
thus interfering with Pin1-mediated cis-trans isomerization of
proteins, resulting in an inhibitory effect on tumor cell growth
and also inducing apoptosis (99).
This type of inhibitor has demonstrated potential for the long-term
inhibition of Pin1 activity. However, concurrently, it might also
induce nonspecific responses to other proteins within the cell due
to covalent modification. Thus, the poor stability and high
toxicity of Juglone in vivo restrict its clinical
application (100).
Based on structural optimization, research has
discovered a novel covalent Pin1 inhibitor, KPT-6566, which has
higher specificity and biological activity. KPT-6566 inhibits the
isomerase activity of Pin1 through covalent modification of its
active site, interferes with its intracellular function and
inhibits the proliferation and promotes apoptosis of cancer cells
by inducing the degradation of the Pin1 protein (101). In animal experiments, KPT-6566
demonstrated good tolerability and safety, but its antitumor effect
and safety in humans still needs to be further verified (102).
Notably, Pin1-targeting inhibitors, including
Sulfopin and BJP-06-005-3, have exhibited promising therapeutic
potential in preclinical in vivo studies (97,103). Sulfopin is a covalent inhibitor
that blocks MyC-driven tumors by forming a covalent bond with
Cys113 of Pin1 (103). Sulfopin
is able to reduce the expression of Myc target genes and reduce
tumor progression in N-Myc-driven neuroblastoma and pancreatic
cancer models (103). Although
Sulfopin has a moderate effect on tumor cell activity in
vitro, it shows activity in vivo with very low toxicity
(103), suggesting that higher
doses or in combination with other therapies may be needed in the
future in the treatment of MYC-driven malignancies (103). BJP-06-005-3 is another covalent
Pin1 inhibitor designed to probe Cys113 at the Pin1 active site and
inhibit cell viability in pancreatic ductal adenocarcinoma
(91). Although BJP-06-005-3 is a
promising tool, further chemical optimization may be required to
improve its bioavailability and in vivo performance
(91). These findings highlight
the potential of Sulfopin and BJP-06-005-3 as Pin1 inhibitors in
cancer therapy, especially in enhancing the sensitivity of cancer
cells to radiotherapy and chemotherapy. The development of these
inhibitors not only provides new strategies for cancer treatment,
but also provides an important theoretical basis for future
clinical cancer treatment.
Noncovalent inhibitors bind Pin1 through noncovalent
interactions, such as hydrogen bonding, hydrophobic interactions
and van der Waals forces, competitively preventing substrate
binding to Pin1. These inhibitors are often reversible, meaning
that they can dissociate from the enzyme, allowing the enzyme
activity to be restored after the inhibitor is removed. Subsequent
studies reveal that ATRA, a drug used in the treatment of acute
promyelocytic leukemia (104),
also possesses Pin1 inhibitory activity (105). ATRA inhibits Pin1 activity
indirectly by inducing degradation of Pin1 proteins that bind to
the PPIase structural domain, indirectly inhibiting Pin1 activity.
In a variety of cancer cell lines, ATRA shows inhibition of Pin1
expression and activity and can suppress tumor cell proliferation
and induce differentiation (15).
The two types of inhibitors have potential
applications in cancer therapy, but their mechanisms of action and
potential side effects need to be elucidated in further studies.
Covalent inhibitors may provide a more durable therapeutic effect
due to their irreversibility, but they may also cause more
non-specific side effects (106,107). Noncovalent inhibitors may be a
safer treatment option because of their reversibility and
specificity (105).
In the clinical trial NCT00599937, acute
promyelocytic leukemia (APL) was highly curable when treated with a
combination of ATRA and anthracycline-based chemotherapy (CT),
although the long-term outcomes and the optimal timing for the
addition of CT remain unclear (108). In the APL93 study, 576 newly
diagnosed patients were followed for a median of 10 years, with a
10-year survival rate of 77%. Maintenance therapy significantly
reduced the 10-year cumulative relapse rate from 43.2 to 13.4%
through a regimen of intermittent ATRA, continuous 6-mercaptopurine
and methotrexate, demonstrating particular efficacy in patients
with high white blood cell counts (>5×109/l). Early
addition of CT markedly improves the 10-year event-free survival
but had no significant effect on overall survival (108). These findings underscore the
importance of ATRA in conjunction with CT and maintenance therapy,
especially in high-risk patients. However, ATRA has some drawbacks
and limitations, such as the possibility of inducing serious side
effects including Pseudotumor Cerebri syndrome, limiting its
application in the clinic (108,109). Therefore, the development of safe
and efficient Pin1 inhibitors may become an important direction for
future research (110).
After conducting a thorough search on the clinical
trials.gov website, it appears that only one clinical trial has
been identified that is specifically investigating Pin1 inhibitors
at present (108). The academic
community has recognized the potential of Pin1 as a therapeutic
target due to its role in regulating multiple oncogenic pathways
and its overexpression in various cancers, which is associated with
poor clinical prognosis (96).
Despite the identification of several Pin1 inhibitors with
promising anticancer activity in preclinical studies, the
translation of these findings into clinical trials has been slow,
probably due to challenges in drug solubility and the need for
improved drug delivery systems (96). These clinical trials underscore the
active investigation into the role of Pin1 inhibitors in cancer
treatment and the ongoing quest for effective therapeutic
strategies targeting this key regulatory enzyme (96).
Comparative novelty of the present
review
The present review provided an exhaustive
examination of the critical role played by Pin1 in oncogenic
signaling pathways, its regulatory mechanisms and its potential as
a therapeutic target. Distinct from prior reviews (9,44,81,111), the present analysis introduces
several innovative elements that enhance the comprehension of the
complex role of Pin1 in cancer biology and its therapeutic
implications.
The present review offered a comprehensive
discussion on Pin1′s modulation of multiple cancer-related
signaling pathways, thereby elucidating its intricate mechanisms in
tumorigenesis (112). This
synthesis of recent research advancements afforded a novel
perspective on the subject. Additionally, the present review
underscored the potential of Pin1 as a clinical biomarker, with an
in-depth analysis of the correlation between Pin1 expression levels
and patient outcomes across a spectrum of types of cancer,
highlighting its prognostic significance (69).
The development and application of Pin1 inhibitors
in cancer therapy were thoroughly explored, including their effects
on cancer cell growth and proliferation, an area of research with
immediate clinical relevance (82). The present review also introduced
the effect of Pin1 on the tumor immune microenvironment and its
influence on resistance to cancer treatments, a relatively nascent
domain in the field (6).
Furthermore, the present review discussed the
potential for combining Pin1 inhibitors with existing cancer
therapies to surmount treatment resistance, proposing a novel
strategy to augment the efficacy of current therapeutic protocols
(25). By incorporating the latest
research and clinical trial outcomes, the present review presented
a contemporary view on the role of Pin1 in cancer, addressing gaps
that may have been overlooked in previous reviews due to the rapid
evolution of this research area (108).
The present review extended its scope to analyze the
function of Pin1 across various types of cancer, emphasizing the
commonalities and specificities of its role, thereby enriching the
understanding of its oncogenic potential (33). The present review also incorporated
structural insights into the function of Pin1, which are pivotal
for the rational design of more potent inhibitors, a topic that has
not been extensively covered in previous reviews (84–90).
Collectively, the innovation of the present review
is reflected in its holistic and contemporary analysis of the
multifaceted roles of Pin1 in cancer, its potential as a biomarker
and the therapeutic potential of targeting Pin1, including advances
in inhibitor design and overcoming therapeutic resistance (69). These contributions not only
expanded the existing body of scientific knowledge but also chart
new avenues for future research and clinical applications.
Conclusions and future perspectives
Pin1, as a novel regulatory enzyme following its
phosphorylation, plays a crucial role in the regulation of cell
cycle progression, cell proliferation and apoptosis. In particular,
its highly active form is closely associated with tumor development
(71). As a key molecular switch,
Pin1 regulates numerous cancer-related signaling pathways and
cellular processes (112) and is
directly related to tumor aggressiveness and patient prognosis
(7).
The expression level of Pin1 is significantly
increased in a number of types of cancer, such as breast cancer,
lung cancer and hepatocellular carcinoma, and is associated with
poor prognosis of patients (9,12,82).
For example, high Pin1 expression in breast cancer tissues is
associated with tumor aggressiveness and chemotherapy resistance
(9). In HCC, Pin1 overexpression
is correlated with tumor size and lymph node metastasis, suggesting
its important role in tumor progression. In addition, the
expression level of Pin1 is also correlated with the survival time
of patients with multiple cancers, which further confirms its
potential as a prognostic biomarker. Pin1 expression levels vary
not only among different types of cancer, but also among different
subtypes of the same cancer type. This difference in expression
pattern may be related to the biological behavior of the tumor and
the responsiveness of the patient to treatment. For example, in
non-small cell lung cancer, high Pin1 expression is associated with
early tumor recurrence and poor overall survival (12). These findings suggest that the
expression level of Pin1 may serve as a useful indicator for
predicting patient outcome. Future studies are needed to further
validate the clinical utility of Pin1 as a biomarker. The
relationship between Pin1 expression level and patient prognosis
can be more accurately evaluated by large-scale clinical sample
studies. In addition, the combination of other biomarkers and
clinical parameters may improve the accuracy and reliability of
Pin1 as a prognostic assessment tool. In conclusion, Pin1, as an
emerging biomarker, has shown great potential in the diagnosis,
prognostic evaluation and therapeutic response monitoring of
cancer. Future studies are expected to further underscore the
potential of Pin1 as a biomarker for cancer diagnosis and prognosis
assessment. By detecting Pin1 levels in tumor tissues or blood, it
may be possible to achieve early cancer diagnosis, thereby
improving patient survival (69).
In addition, Pin1 inhibitors show a broad potential
for application in the field of cancer therapy (9). Future research directions may include
the development of novel Pin1 inhibitors with improved efficiency
and greater specificity, improvement of the drug properties of
existing inhibitors, exploration of the combined application of
Pin1 inhibitors with other therapeutic means and further in-depth
study of their molecular mechanisms and drug resistance mechanisms
(113). Through systematic and
in-depth research, Pin1 inhibition may emerge as a viable clinical
approach and provide novel treatment options for patients with
cancer, with the aim of improving treatment efficacy and patient
survival.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
YW conceived and designed the present review. SL and
ML wrote the manuscript. All authors revised and edited the
manuscript. Data authentication is not applicable. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Han HJ, Choi BY and Surh YJ: Dual roles of
Pin1 in cancer development and progression. Curr Pharm Des.
23:4422–4425. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jeong J, Usman M, Li Y, Zhou XZ and Lu KP:
Pin1-catalyzed conformation changes regulate protein ubiquitination
and degradation. Cells. 13:7312024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gurung D, Danielson JA, Tasnim A, Zhang
JT, Zou Y and Liu JY: Proline isomerization: From the chemistry and
biology to therapeutic opportunities. Biology (Basel).
12:10082023.PubMed/NCBI
|
|
4
|
Lanni C, Masi M, Racchi M and Govoni S:
Cancer and Alzheimer's disease inverse relationship: an
age-associated diverging derailment of shared pathways. Mol
Psychiatry. 26:280–295. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marcolino TF, Pimenta CAM, Artigiani Neto
R, Castelo P, Silva MS, Forones NM and Oshima CTF: p53, Cyclin-D1,
β-catenin, APC and c-myc in tumor tissue from colorectal and
gastric cancer patients with suspected lynch syndrome by the
bethesda criteria. Asian Pac J Cancer Prev. 21:343–348. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Born A, Henen MA and Vogeli B: Activity
and affinity of Pin1 variants. Molecules. 25:362019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stewart R, Sharma S, Wu T, Okuda S, Xie G,
Zhou XZ, Shilton B and Lu KP: The role of the master cancer
regulator Pin1 in the development and treatment of cancer. Front
Cell Dev Biol. 12:13439382024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu C, Dan L, Li Q, Bajinka O and Yuan X:
The mechanisms of Pin1 as targets for cancer therapy. Front
Immunol. 15:14820882024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu JH, Im CY and Min SH: Function of PIN1
in Cancer development and its inhibitors as cancer therapeutics.
Front Cell Dev Biol. 8:1202020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma JQ, Yang Y, Juan J, Guo CF, Tuerxun M,
Ting W and Hasim A: Over-expression of prolyl isomerase Pin1
promotes cervical tumorigenesis and metastasis. Int J Clin Exp
Pathol. 11:664–674. 2018.PubMed/NCBI
|
|
11
|
Liu K, Zheng M, Lu R, Du J, Zhao Q, Li Z,
Li Y and Zhang S: The role of CDC25C in cell cycle regulation and
clinical cancer therapy: A systematic review. Cancer Cell Int.
20:2132020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang JN, Zhang ZR, Che Y, Yuan ZY, Lu ZL,
Li Y, Li N, Wan J, Sun HD, Sun N, et al: Acetyl-macrocalin B, an
ent-kaurane diterpenoid, initiates apoptosis through the
ROS-p38-caspase 9-dependent pathway and induces G2/M phase arrest
via the Chk1/2-Cdc25C-Cdc2/cyclin B axis in non-small cell lung
cancer. Cancer Biol Ther. 19:609–621. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia Z, Ou-Yang W, Hu T and Du K:
Prognostic significance of CDC25C in lung adenocarcinoma: An
analysis of TCGA data. Cancer Genet. 233–234. 67–74.
2019.PubMed/NCBI
|
|
14
|
Wu C, Lyu J, Yang EJ, Liu Y, Zhang B and
Shim JS: Targeting AURKA-CDC25C axis to induce synthetic lethality
in ARID1A-deficient colorectal cancer cells. Nat Commun.
9:32122018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Yuan Z, Wang L, Yang J, Pu P, Le Y,
Chen X, Wang C, Gao Y, Liu Y, et al: Prolyl isomerase Pin1 sculpts
the immune microenvironment of colorectal cancer. Cell Signal.
115:1110412024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maegawa S and Gopalakrishnan V: PLK
inhibitors come of age in pediatric brain tumors. Neuro Oncol.
24:427–428. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Crenshaw DG, Yang J, Means AR and
Kornbluth S: The mitotic peptidyl-prolyl isomerase, Pin1, interacts
with Cdc25 and Plx1. EMBO J. 17:1315–1327. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen M, Stukenberg PT, Kirschner MW and Lu
KP: The essential mitotic peptidyl-prolyl isomerase Pin1 binds and
regulates mitosis-specific phosphoproteins. Genes Dev. 12:706–720.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou XZ, Kops O, Werner A, Lu PJ, Shen M,
Stoller G, Küllertz G, Stark M, Fischer G and Lu KP: Pin1-dependent
prolyl isomerization regulates dephosphorylation of Cdc25C and tau
proteins. Mol Cell. 6:873–883. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stukenberg PT and Kirschner MW: Pin1 acts
catalytically to promote a conformational change in Cdc25. Mol
Cell. 7:1071–1083. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fei F, Qu J, Liu K, Li C, Wang X, Li Y and
Zhang S: The subcellular location of cyclin B1 and CDC25 associated
with the formation of polyploid giant cancer cells and their
clinicopathological significance. Lab Invest. 99:483–498. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dakilah I, Harb A, Abu-Gharbieh E,
El-Huneidi W, Taneera J, Hamoudi R, Semreen MH and Bustanji Y:
Potential of CDC25 phosphatases in cancer research and treatment:
Key to precision medicine. Front Pharmacol. 15:13240012024.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang C, Zeng J, Li LJ, Xue M and He SL:
Cdc25A inhibits autophagy-mediated ferroptosis by upregulating
ErbB2 through PKM2 dephosphorylation in cervical cancer cells. Cell
Death Dis. 12:10552021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen YC, Hsieh HH, Chang HC, Wang HC, Lin
WJ and Lin JJ: CDC25B induces cellular senescence and correlates
with tumor suppression in a p53-dependent manner. J Biol Chem.
296:1005642021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khoei SG, Mohammadi C, Mohammadi Y, Sameri
S and Najafi R: Prognostic value of peptidyl-prolyl cis-trans
isomerase 1 (PIN1) in human malignant tumors. Clin Transl Oncol.
22:1067–1077. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Montalto FI and De Amicis F: Cyclin D1 in
cancer: A molecular connection for cell cycle control, adhesion and
invasion in tumor and stroma. Cells. 9:26482020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cai Z, Wang J, Li Y, Shi Q, Jin L, Li S,
Zhu M, Wang Q, Wong LL, Yang W, et al: Overexpressed Cyclin D1 and
CDK4 proteins are responsible for the resistance to CDK4/6
inhibitor in breast cancer that can be reversed by PI3K/mTOR
inhibitors. Sci China Life Sci. 66:94–109. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lundberg A, Lindstrom LS, Li J, Harrell
JC, Darai-Ramqvist E, Sifakis EG, Foukakis T, Perou CM, Czene K,
Bergh J and Tobin NP: The long-term prognostic and predictive
capacity of cyclin D1 gene amplification in 2305 breast tumours.
Breast Cancer Res. 21:342019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi Q, Li Y, Li S, Jin L, Lai H, Wu Y, Cai
Z, Zhu M, Li Q, Li Y, et al: LncRNA DILA1 inhibits Cyclin D1
degradation and contributes to tamoxifen resistance in breast
cancer. Nat Commun. 11:55132020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tchakarska G and Sola B: The double
dealing of cyclin D1. Cell Cycle. 19:163–178. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song D, Lian Y and Zhang L: The potential
of activator protein 1 (AP-1) in cancer targeted therapy. Front
Immunol. 14:12248922023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mst Nazneen Nahar Rina D, Dr. Naba Kumar
Saha P and Mostafa Kamal D: Significance of cyclin D1
immunoexpression in breast carcinoma. J Cancer Sci Clin Ther.
8:321–326. 2024. View Article : Google Scholar
|
|
33
|
Fang M, Wu HK, Pei Y, Zhang Y, Gao X, He
Y, Chen G, Lv F, Jiang P, Li Y, et al: E3 ligase MG53 suppresses
tumor growth by degrading cyclin D1. Signal Transduct Target Ther.
8:2632023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nashaat S, Henen MA, El-Messery SM and
Eisa H: Synthesis, state-of-the-art NMR-binding and molecular
modeling study of new benzimidazole core derivatives as Pin1
inhibitors: Targeting breast cancer. Bioorg Med Chem.
28:1154952020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang C, Liu J, Xu D, Zhang T, Hu W and
Feng Z: Gain-of-function mutant p53 in cancer progression and
therapy. J Mol Cell Biol. 12:674–687. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu J, Cao J, Topatana W, Juengpanich S, Li
S, Zhang B, Shen J, Cai L, Cai X and Chen M: Targeting mutant p53
for cancer therapy: Direct and indirect strategies. J Hematol
Oncol. 14:1572021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deng L, Meng T, Chen L, Wei W and Wang P:
The role of ubiquitination in tumorigenesis and targeted drug
discovery. Signal Transduct Target Ther. 5:112020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Engeland K: Cell cycle regulation:
p53-p21-RB signaling. Cell Death Differ. 29:946–960. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vaddavalli PL and Schumacher B: The p53
network: cellular and systemic DNA damage responses in cancer and
aging. Trends Genet. 38:598–612. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marei HE, Althani A, Afifi N, Hasan A,
Caceci T, Pozzoli G, Morrione A, Giordano A and Cenciarelli C: p53
signaling in cancer progression and therapy. Cancer Cell Int.
21:7032021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tomazini A and Shifman JM: Targeting Ras
with protein engineering. Oncotarget. 14:672–687. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ullah R, Yin Q, Snell AH and Wan L:
RAF-MEK-ERK pathway in cancer evolution and treatment. Semin Cancer
Biol. 85:123–154. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheng CW and Tse E: PIN1 in cell cycle
control and cancer. Front Pharmacol. 9:13672018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen Y, Wu YR, Yang HY, Li XZ, Jie MM, Hu
CJ, Wu YY, Yang SM and Yang YB: Prolyl isomerase Pin1: A promoter
of cancer and a target for therapy. Cell Death Dis. 9:8832018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sciacchitano S, Sacconi A, De Vitis C,
Blandino G, Piaggio G, Salvati V, Napoli C, Marchetti P, Taurelli
BS, Coluzzi F, et al: H-Ras gene takes part to the host immune
response to COVID-19. Cell Death Discov. 7:1582021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Luo J: KRAS mutation in pancreatic cancer.
Semin Oncol. 48:10–18. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhu G, Pei L, Xia H, Tang Q and Bi F: Role
of oncogenic KRAS in the prognosis, diagnosis and treatment of
colorectal cancer. Mol Cancer. 20:1432021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shalom B, Farago M, Salaymeh Y, Sebban S,
Risling M, Pikarsky E and Katzav S: Vav1 accelerates Ras-driven
lung cancer and modulates its tumor microenvironment. Cell Signal.
97:1103952022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
He Y, Ling Y, Zhang Z, Mertens RT, Cao Q,
Xu X, Guo K, Shi Q, Zhang X, Huo L, et al: Butyrate reverses
ferroptosis resistance in colorectal cancer by inducing
c-Fos-dependent xCT suppression. Redox Biol. 65:1028222023.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Degirmenci U, Wang M and Hu J: Targeting
aberrant RAS/RAF/MEK/ERK signaling for cancer therapy. Cells.
9:1982020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu J, Wang Y, Mu C, Li M, Li K, Li S, Wu
W, Du L, Zhang X, Li C, et al: Pancreatic tumor eradication via
selective Pin1 inhibition in cancer-associated fibroblasts and T
lymphocytes engagement. Nat Commun. 13:43082022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lu KP and Zhou XZ: The prolyl isomerase
PIN1: A pivotal new twist in phosphorylation signalling and
disease. Nat Rev Mol Cell Biol. 8:904–916. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Luo ML, Gong C, Chen CH, Lee DY, Hu H,
Huang P, Yao Y, Guo W, Reinhardt F, Wulf G, et al: Prolyl isomerase
Pin1 acts downstream of miR200c to promote cancer stem-like cell
traits in breast cancer. Cancer Res. 74:3603–3616. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang
X, Zhou Z, Shu G and Yin G: Wnt/beta-catenin signalling: Function,
biological mechanisms, and therapeutic opportunities. Signal
Transduct Target Ther. 7:32022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Koelman EMR, Yeste-Vazquez A and Grossmann
TN: Targeting the interaction of β-catenin and TCF/LEF
transcription factors to inhibit oncogenic Wnt signaling. Bioorg
Med Chem. 70:1169202022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Delgado-Bellido D, Zamudio-Martinez E,
Fernandez-Cortes M, Herrera-Campos AB, Olmedo-Pelayo J, Perez CJ,
Expósito J, de Álava E, Amaral AT, Valle FO, et al: VE-Cadherin
modulates β-catenin/TCF-4 to enhance vasculogenic mimicry. Cell
Death Dis. 14:1352023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ji Y, Liu Y, Sun C, Yu L, Wang Z, Du X,
Yang W, Zhang C, Tao C, Wang J, et al: ADCK1 activates the
β-catenin/TCF signaling pathway to promote the growth and migration
of colon cancer cells. Cell Death Dis. 12:3542021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang WJ, Tian XP, Bi SX, Zhang SR, He TS,
Song LY, Yun JP, Zhou ZG, Yu RM and Li M: The
β-catenin/TCF-4-LINC01278-miR-1258-Smad2/3 axis promotes
hepatocellular carcinoma metastasis. Oncogene. 39:4538–4550. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhu L, Tian Q, Gao H, Wu K, Wang B, Ge G,
Jiang S, Wang K, Zhou C, He J, et al: PROX1 promotes breast cancer
invasion and metastasis through WNT/β-catenin pathway via
interacting with hnRNPK. Int J Biol Sci. 18:2032–2046. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang JZ, Du WT, Bai J, Cheng SZ and Zhang
YH: The association of rs2233679 in the PIN1 gene promoter with the
risk of Coronary Artery Disease in Chinese female individuals. J
Stroke Cerebrovasc Dis. 29:1049352020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yu S, Wang Y, Gong X, Fan Z, Wang Z, Liang
Z, Wu R, Cao B, Wang N, Bi C, et al: LncRNA AGPG confers endocrine
resistance in breast cancer by promoting E2F1 activity. Cancer Res.
83:3220–3236. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Krishnan N, Titus MA and Thapar R: The
prolyl isomerase pin1 regulates mRNA levels of genes with short
half-lives by targeting specific RNA binding proteins. PLoS One.
9:e854272014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Choi YJ, Kim I, Lee JE and Park JW: PIN1
transcript variant 2 acts as a long non-coding RNA that controls
the HIF-1-driven hypoxic response. Sci Rep. 9:105992019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kassab A, Gupta I and Moustafa AA: Role of
E2F transcription factor in oral cancer: Recent insight and
advancements. Semin Cancer Biol. 92:28–41. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kim S, Armand J, Safonov A, Zhang M, Soni
RK, Schwartz G, McGuinness JE, Hibshoosh H, Razavi P, Kim M, et al:
Sequential activation of E2F via Rb degradation and c-Myc drives
resistance to CDK4/6 inhibitors in breast cancer. Cell Rep.
42:1131982023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chen D, Wang L and Lee TH:
Post-translational modifications of the peptidyl-prolyl isomerase
Pin1. Front Cell Dev Biol. 8:1292020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Maggio J, Armando R, Balcone L, Vilarullo
RN, Casco MDP, Gomez DLM and Gomez DE: Role of PIN1 in human
pathology: Cellular regulation, pathogenesis and therapeutic
implications (Review). World Acad Sci J. 6:52023. View Article : Google Scholar
|
|
68
|
Zhou Y, Nakajima R, Shirasawa M,
Fikriyanti M, Zhao L, Iwanaga R, Bradford AP, Kurayoshi K, Araki K
and Ohtani K: Expanding roles of the E2F-RB-p53 pathway in tumor
suppression. Biology (Basel). 12:15112023.PubMed/NCBI
|
|
69
|
Chuang HH, Zhen YY, Tsai YC, Chuang CH,
Huang MS, Hsiao M and Yang CJ: Targeting Pin1 for modulation of
cell motility and cancer therapy. Biomedicines. 9:3592021.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Bao L, Kimzey A, Sauter G, Sowadski JM, Lu
KP and Wang DG: Prevalent overexpression of prolyl isomerase Pin1
in human cancers. Am J Pathol. 164:1727–1737. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Caligiuri I, Vincenzo C, Asano T, Kumar V
and Rizzolio F: The metabolic crosstalk between PIN1 and the tumour
microenvironment. Semin Cancer Biol. 91:143–157. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yao G, Lee TJ, Mori S, Nevins JR and You
L: A bistable Rb-E2F switch underlies the restriction point. Nat
Cell Biol. 10:476–482. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Dannenberg JH, van Rossum A, Schuijff L
and te Riele H: Ablation of the retinoblastoma gene family
deregulates G(1) control causing immortalization and increased cell
turnover under growth-restricting conditions. Genes Dev.
14:3051–3064. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tong Y, Ying H, Liu R, Li L, Bergholz J
and Xiao ZX: Pin1 inhibits PP2A-mediated Rb dephosphorylation in
regulation of cell cycle and S-phase DNA damage. Cell Death Dis.
6:e16402015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wulf G, Garg P, Liou YC, Iglehart D and Lu
KP: Modeling breast cancer in vivo and ex vivo reveals an essential
role of Pin1 in tumorigenesis. EMBO J. 23:3397–3407. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zannini A, Rustighi A, Campaner E and Del
Sal G: Oncogenic hijacking of the PIN1 signaling network. Front
Oncol. 9:942019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lepore A, Choy PM, Lee NCW, Carella MA,
Favicchio R, Briones-Orta MA, Glaser SS, Alpini G, D'Santos C,
Tooze RM, et al: Phosphorylation and stabilization of PIN1 by JNK
promote intrahepatic cholangiocarcinoma growth. Hepatology.
74:2561–2579. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Poudel M, Bhattarai PY, Shrestha P and
Choi HS: Regulation of Interleukin-36ү/IL-36R Signaling Axis by
PIN1 in epithelial cell transformation and breast tumorigenesis.
Cancers (Basel). 14:36542022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Jawanjal P, Salhan S, Dhawan I, Tripathi R
and Rath G: Peptidyl-prolyl isomerase Pin1-mediated abrogation of
APC-β-catenin interaction in squamous cell carcinoma of cervix. Rom
J Morphol Embryol. 55:83–90. 2014.PubMed/NCBI
|
|
80
|
Ma ZQ, Feng YT, Guo K, Liu D, Shao CJ, Pan
MH, Zhang YM, Zhang YX, Lu D, Huang D, et al: Melatonin inhibits
ESCC tumor growth by mitigating the HDAC7/beta-catenin/c-Myc
positive feedback loop and suppressing the USP10-maintained HDAC7
protein stability. Mil Med Res. 9:542022.PubMed/NCBI
|
|
81
|
Pu W, Zheng Y and Peng Y: Prolyl isomerase
Pin1 in human cancer: Function, mechanism, and significance. Front
Cell Dev Biol. 8:1682020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zheng M, Xu H, Liao XH, Chen CP, Zhang AL,
Lu W, Wang L, Yang D, Wang J, Liu H, et al: Inhibition of the
prolyl isomerase Pin1 enhances the ability of sorafenib to induce
cell death and inhibit tumor growth in hepatocellular carcinoma.
Oncotarget. 8:29771–29784. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Khanal P, Yeung B, Zhao Y and Yang X:
Identification of Prolyl isomerase Pin1 as a novel positive
regulator of YAP/TAZ in breast cancer cells. Sci Rep. 9:63942019.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wulf GM, Liou YC, Ryo A, Lee SW and Lu KP:
Role of Pin1 in the regulation of p53 stability and p21
transactivation, and cell cycle checkpoints in response to DNA
damage. J Biol Chem. 277:47976–47979. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zheng H, You H, Zhou XZ, Murray SA, Uchida
T, Wulf G, Gu L, Tang X, Lu KP and Xiao ZX: The prolyl isomerase
Pin1 is a regulator of p53 in genotoxic response. Nature.
419:849–853. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zacchi P, Gostissa M, Uchida T, Salvagno
C, Avolio F, Volinia S, Ronai Z, Blandino G, Schneider C and Del
Sal G: The prolyl isomerase Pin1 reveals a mechanism to control p53
functions after genotoxic insults. Nature. 419:853–857. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ryo A, Uemura H, Ishiguro H, Saitoh T,
Yamaguchi A, Perrem K, Kubota Y, Lu KP and Aoki I: Stable
suppression of tumorigenicity by Pin1-targeted RNA interference in
prostate cancer. Clin Cancer Res. 11:7523–7531. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kim JA, Kim MR, Kim O, Phuong NT, Yun J,
Oh WK, Bae K and Kang KW: Amurensin G inhibits angiogenesis and
tumor growth of tamoxifen-resistant breast cancer via Pin1
inhibition. Food Chem Toxicol. 50:3625–3634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Polonio-Vallon T, Krüger D and Hofmann TG:
ShaPINg cell fate upon DNA damage: Role of Pin1 isomerase in DNA
damage-induced cell death and repair. Front Oncol. 4:1482014.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Steger M, Murina O, Hühn D, Ferretti LP,
Walser R, Hänggi K, Lafranchi L, Neugebauer C, Paliwal S, Janscak
P, et al: Prolyl isomerase PIN1 regulates DNA double-strand break
repair by counteracting DNA end resection. Mol Cell. 50:333–343.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lee YM, Teoh DE, Yeung K and Liou YC: The
kingdom of the prolyl-isomerase Pin1: The structural and functional
convergence and divergence of Pin1. Front Cell Dev Biol.
10:9560712022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Jirawatnotai S, Dalton S and
Wattanapanitch M: Role of cyclins and cyclin-dependent kinases in
pluripotent stem cells and their potential as a therapeutic target.
Semin Cell Dev Biol. 107:63–71. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang N, Chai T, Wang XR, Zheng YD, Sang CY
and Yang JL: Pin1: Advances in pancreatic cancer therapeutic
potential and inhibitors research. Bioorg Chem. 153:1078692024.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kondo A, Albayram O, Zhou XZ and Lu KP:
Pin1 knockout mice: A model for the study of tau pathology in
Alzheimer's disease. Methods Mol Biol. 1523:415–425. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Nakatsu Y, Matsunaga Y, Ueda K, Yamamotoya
T, Inoue Y, Inoue MK, Mizuno Y, Kushiyama A, Ono H, Fujishiro M, et
al: Development of Pin1 inhibitors and their potential as
therapeutic agents. Curr Med Chem. 27:3314–3329. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
He S, Li L, Jin R and Lu X: Biological
function of Pin1 in vivo and its inhibitors for preclinical study:
Early development, current strategies, and future directions. J Med
Chem. 66:9251–9277. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Pinch BJ, Doctor ZM, Nabet B, Browne CM,
Seo HS, Mohardt ML, Kozono S, Lian X, Manz TD, Chun Y, et al:
Identification of a potent and selective covalent Pin1 inhibitor.
Nat Chem Biol. 16:979–987. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Dos S, Moreira C, Santos TB, Freitas RHCN,
Pacheco PAF and da Rocha DR: Juglone: A versatile natural platform
for obtaining new bioactive compounds. Curr Top Med Chem.
21:2018–2045. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Cai Y, Zou G, Xi M, Hou Y, Shen H, Ao J,
Li M, Wang J and Luo A: Juglone inhibits listeria monocytogenes
ATCC 19115 by targeting cell membrane and protein. Foods.
11:25582022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Li F, Li Y, Deng ZP, Zhu XJ, Zhang ZG,
Zhang XD, Tian JL, Li W and Zhao P: Traditional uses,
phytochemistry, pharmacology and clinical applications of Cortex
Juglandis Mandshuricae: A comprehensive review. J Ethnopharmacol.
285:1148872022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang Z, Hu Q, Ye S and Xiang L:
Inhibition of the PIN1-NRF2/GPX4 axis imparts sensitivity to
cisplatin in cervical cancer cells. Acta Biochim Biophys Sin
(Shanghai). 54:1325–1335. 2022.PubMed/NCBI
|
|
102
|
Guo YT, Lu Y, Jia YY, Qu HN, Qi D, Wang
XQ, Song PY, Jin XS, Xu WH, Dong Y, Liang YY and Quan CS:
Predictive Value of Pin1 in cervical low-grade squamous
intraepithelial lesions and inhibition of Pin1 exerts potent
anticancer activity against human cervical cancer. Aging Dis.
11:44–59. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Dubiella C, Pinch BJ, Koikawa K, Zaidman
D, Poon E, Manz TD, Nabet B, He S, Resnick E, Rogel A, et al:
Sulfopin is a covalent inhibitor of Pin1 that blocks Myc-driven
tumors in vivo. Nat Chem Biol. 17:954–963. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Liang C, Qiao G, Liu Y, Tian L, Hui N, Li
J, Ma Y, Li H, Zhao Q, Cao W, et al: Overview of all-trans-retinoic
acid (ATRA) and its analogues: Structures, activities, and
mechanisms in acute promyelocytic leukaemia. Eur J Med Chem.
220:1134512021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Giuli MV, Hanieh PN, Forte J, Fabiano MG,
Mancusi A, Natiello B, Rinaldi F, Del Favero E, Ammendolia MG,
Marianecci C, et al: pH-sensitive niosomes for ATRA delivery: A
promising approach to inhibit Pin1 in high-grade serous ovarian
cancer. Int J Pharm. 649:1236722024. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Schaefer D and Cheng X: Recent advances in
covalent drug discovery. Pharmaceuticals (Basel). 16:6632023.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Bjij I, Olotu FA, Agoni C, Adeniji E, Khan
S, El Rashedy A, Cherqaoui D and Soliman MES: Covalent inhibition
in drug discovery: Filling the void in literature. Curr Top Med
Chem. 18:1135–1145. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Adès L, Guerci A, Raffoux E, Sanz M,
Chevallier P, Lapusan S, Recher C, Thomas X, Rayon C, Castaigne S,
et al: Very long-term outcome of acute promyelocytic leukemia after
treatment with all-trans retinoic acid and chemotherapy: The
European APL Group experience. Blood. 115:1690–1696. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Fenaux P, Chastang C, Chevret S, Sanz M,
Dombret H, Archimbaud E, Fey M, Rayon C, Huguet F, Sotto JJ, et al:
A randomized comparison of all transretinoic acid (ATRA) followed
by chemotherapy and ATRA plus chemotherapy and the role of
maintenance therapy in newly diagnosed acute promyelocytic
leukemia. The European APL Group. Blood. 94:1192–1200. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhang F, Zhang A, Xie Y, Wen H, Kankala
RK, Huang J, Zhang A, Wang Q, Chen B, Dong H, et al: Nanocarrier of
Pin1 inhibitor based on supercritical fluid technology inhibits
cancer metastasis by blocking multiple signaling pathways. Regen
Biomater. 10:rbad0142023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wu W, Xue X, Chen Y, Zheng N and Wang J:
Targeting prolyl isomerase Pin1 as a promising strategy to overcome
resistance to cancer therapies. Pharmacol Res. 184:1064562022.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Fagiani F, Vlachou M, Di Marino D,
Canobbio I, Romagnoli A, Racchi M, Govoni S and Lanni C: Pin1 as
molecular switch in vascular endothelium: Notes on its putative
role in age-associated vascular diseases. Cells. 10:32872021.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Poli G, Di Stefano M, Estevez JA, Minutolo
F, Granchi C, Giordano A, Parisi S, Mauceri M, Canzonieri V,
Macchia M, et al: New PIN1 inhibitors identified through a
pharmacophore-driven, hierarchical consensus docking strategy. J
Enzyme Inhib Med Chem. 37:145–150. 2022. View Article : Google Scholar : PubMed/NCBI
|