Introduction
Allergic asthma, the most common type of asthma, is
an airway inflammatory disease driven by the many kinds of
inflammatory cells (1,2). The primary characteristic of allergic
asthma is the narrowing of airway due to the infiltration of immune
cells, particularly eosinophils, leading to a range of phenomena,
including enhanced mucus production and airway wall remodeling
(3–5). Allergic asthma is characterized by a
Th2 cell response, aberrant accumulation of Th2 cytokines
[interleukin (IL)-4, IL-5, IL-9, and IL-13], and Th2-oriented
cytokines (IL-6 and IL-33), which contribute to goblet cell
dysplasia and accumulation of mucus (6,7).
Therefore, targeting Th2 cytokines is a potential therapeutic
approaches for severe asthma (8–11).
The activation of inflammatory cells in allergic
asthma subsequently leads to the generation of a substantial amount
of reactive oxygen species (ROS) (12). During the normal condition, the
antioxidant and oxidant systems maintain balance; however, with the
excessive accumulation of ROS, the balance was damaged, resulting
in oxidative stress. Various cellular antioxidants can mitigate
oxidative stress, such as superoxide dismutase (SOD), catalase, and
glutathione (GSH) (13,14). Furthermore, the activation of
nuclear factor-kappaB (NF-κB) pathway promotes the production of
pro-inflammatory cytokines and recruits eosinophils, that play
crucial roles in allergic inflammation and oxidative stress
(15–17).
Previous studies suggested that dietary
supplementation of omega-3 fatty acids, enriched with
docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), exerts
beneficial effects in inflammatory diseases, including asthma
(18–20). Notably, it has been observed that
intake of omega-3 fatty acids can elevate the levels of specialized
pro-resolving mediators (SPMs), potentially serving as the primary
mechanism by which omega-3 fatty acids reduce inflammation
(21,22). SPMs act as potent regulators of
cytokines and chemokines production, thereby facilitating a return
to tissue homeostasis (23,24).
Additionally, studies have demonstrated that SPMs exhibit both
anti-inflammatory and pro-resolution properties at more than
thousands times lower doses compared to omega-3 fatty acids
(25–27). Thus, SPMs are promising methods for
asthma. Lipid mediators (LM;17S-monohydroxy docosahexaenoic acid,
resolvin D5, and protectin DX at 3:47:50 ratio), produced from DHA
by soybean lipoxygenase, attenuate atopic dermatitis, which is an
allergic condition involving the inhibition of inflammatory
cytokines and mediators (28).
Thus, we hypothesized that LM could mediate allergic asthma and
evaluated the underlying mechanism of LM on allergic asthma in
OVA-challenged mice.
Materials and methods
Animals
Female BALB/c mice (6 weeks) were obtained from
Orient Bio (Gyeonggi, Korea). The animals were housed at under
controlled conditions of temperature (21–23°C), relative humidity
(60–70%), and a 12-h light/dark cycle. This study was reviewed and
approved by the Institutional Animal Care and Use Committee and
Institutional Animal Ethics Committee of the Korea Research
Institute of Bioscience and Biotechnology (Daejeon, Korea)
(KRIBB-AEC-23236).
Animal model and treatment
Ovalbumin (OVA), the chief globular egg white
protein, has been widely used for allergic models (29,30).
The mice were divided into three groups and the allergic asthma
model was induced by OVA. As depicted in Fig. 1, sensitization of the mice occurred
through intraperitoneal (i.p.) administration of 100 µg of OVA
(Sigma-Aldrich, St. Louis, MO) on days 1, 7, and 14. Subsequently,
150 µg of OVA was intranasally (i.n.) administered to the mice from
days 21 to 24. In the normal control group (NC) and OVA alone
group, saline was orally administered; however, in the OVA + LM
group, LM (10 µg/kg) was orally treated. The mice were sacrificed
for blood, bronchoalveolar lavage fluid (BALF), and lung tissue
collection on Day 25. The dosage selection for LM was based on a
previous study (28,31). The mice were euthanized via 5%
isoflurane in oxygen until breathing ceases.
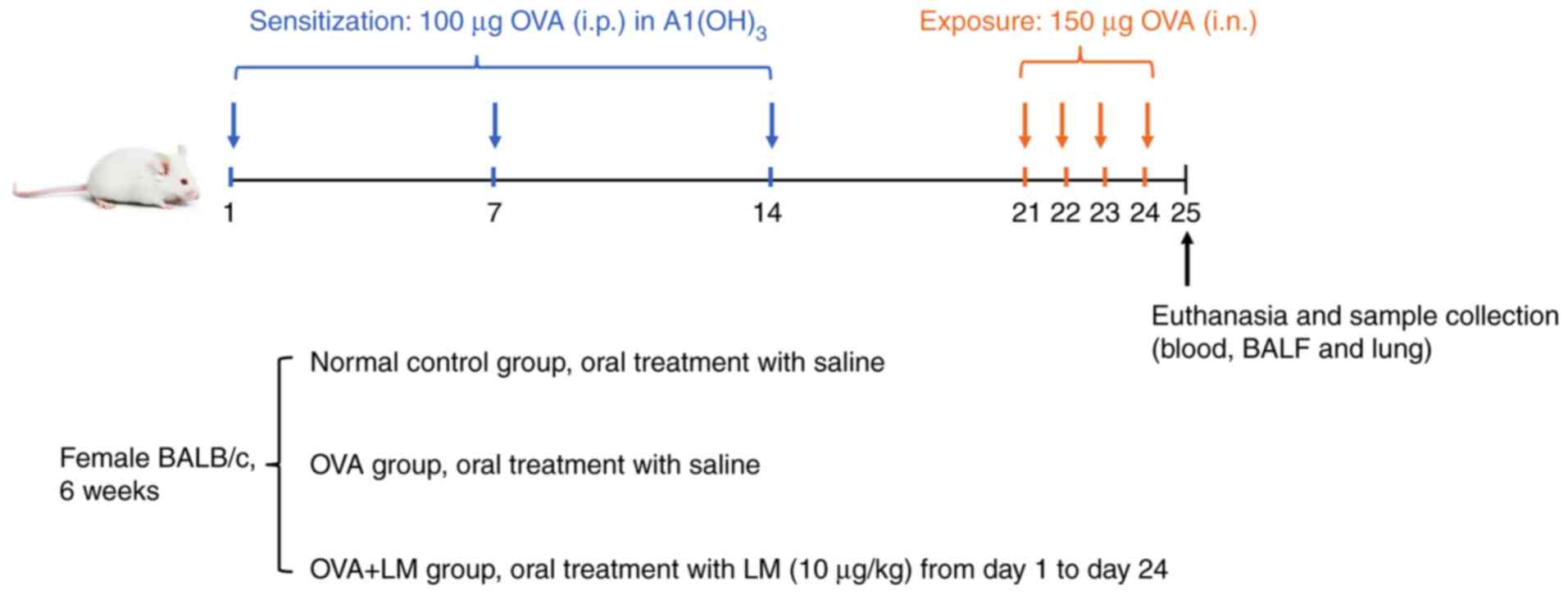 | Figure 1.Schedule of OVA-induced asthma in the
present study. Mice were sensitized via i.p. injection of 100 µg
OVA emulsified in 5 mg hydroxyl aluminum along with 100 µl PBS on
days 1, 7 and 14. Subsequently, the mice underwent i.n. challenge
with 150 µg OVA dissolved in 50 µl PBS on days 21, 22, 23 and 24.
Oral saline was given to the normal control and OVA groups, while
LM (10 µg/kg) was orally administered to the OVA + LM group. The
mice were sacrificed after a period of 24 h following the final
challenge for the collection of blood, BALF and lung tissue. BALF,
bronchoalveolar lavage fluid; i.n., intranasal; i.p.,
intraperitoneal; LM, lipid mediators; OVA, ovalbumin. |
Collection of BALF
The BALF was obtained by lavaging the lungs with
phosphate-buffered saline (PBS), ensuring a slow rinse to protect
the epithelial cells in the bronchioalveolar space. Subsequently,
centrifugation for 10 min was performed to separate the
supernatants for cytokines analysis and resuspend the cell pellets
for cell counting (32).
Measurement of cytokines and IgE
level
IL-4, IL-5, IL-13, and IL-33 levels in BALF and
IL-6, TNF-α, and IgE levels in serum were measured using ELISA kits
(Abcam, Cambridge, MA) based on the manufacturer's protocols.
Histological analysis
After dissecting the lungs, tissue sections were
fixed in 4% paraformaldehyde. Subsequently, they underwent
dehydration using ethanol and xylene. The dehydrated tissue was
then embedded in paraffin and stained with H&E (Sigma) to
evaluate the infiltration of inflammatory cells. The inflammatory
score was determined by assigning grades: 0, no inflammation; 1,
minimal inflammation; 2, mild inflammation; 3, moderate
inflammation; and 4, severe inflammation (11). Additionally, the slides were
subjected to periodic acid Schiff (PAS; Abcam) staining for
assessing mucus production.
Oxidative stress assays in lung
tissue
The level of malondialdehyde (MDA) was quantified by
lipid peroxidation colorimetric/fluorometric assay kit (ab118970),
GSH was determined by GSH assay colorimetric kit (ab239727), and
the activity of SOD was analyzed by superoxide dismutase activity
assay kit (ab65354) based on the protocols accompanying the kits
from Abcam.
Western blot analysis
The protein was isolated from homogenized lung
tissue via radio immunoprecipitation assay buffer with protein
inhibitors. Subsequently, the proteins were separated by sodium
dodecyl sulfate-polyacrylamide gel and transferred onto
polyvinylidene fluoride membranes, followed by incubation with
blocking buffer and incubation of primary antibodies against p-p65
(1:1,000, ab76302, Abcam), p65 (1:1,000, ab16502, Abcam), IkB
(1:2,000, ab32518, Abcam), and p-IkB (1:1,000. ab133462, Abcam).
Finally, horseradish peroxidase-conjugated goat anti-rabbit
antibodies (1:20,000, ab205718, Abcam) were applied to the
membranes for a period of 2 h. The relative levels of protein were
calculated using ImageJ software (1.48v; National Institutes of
Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
The lung tissue was subjected to RNA isolation using
a MiniBEST kit (TaKaRa, Tokyo, Japan). Briefly, transcript levels
were quantified using a One-Step AccuPower GreenStar RT-qPCR PreMix
kit (Bioneer Corporation, Daejeon, Korea). RT-qPCR analysis was
performed on the CFX Connect system (Bio-Rad, CA, USA). The
relative mRNA expression of target genes was determined using the
2−ΔΔCq method. Gene-specific primers utilized in this
study are listed in Table I
(33).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Mouse genes | Sequences
(5′-3′) |
|---|
| IL-4 | Forward:
ATCATCGGCATTTTGAACGAGGTC |
|
| Reverse:
ACCTTGGAAGCCCTACAGACGA |
| IL-5 | Forward:
GATGAGGCTTCCTGTCCCTACT |
|
| Reverse:
TGACAGGTTTTGGAATAGCATT |
|
| TCC |
| IL-13 | Forward:
AACGGCAGCATGGTATGGAGTG |
|
| Reverse:
TGGGTCCTGTAGATGGCATTGC |
| IL-6 | Forward:
TACCACTTCACAAGTCGGAGGC |
|
| Reverse:
CTGCAAGTGCATCATCGTTGTTC |
| TNF-α | Forward:
GGTGCCTATGTCTCAGCCTCTT |
|
| Reverse:
GCCATAGAACTGATGAGAGGGAG |
| GAPDH | Forward:
CATCACTGCCACCCAGAAGACTG |
|
| Reverse:
ATGCCAGTGAGCTTCCCGTTCAG |
Statistical analysis
Data were shown as means ± standard deviations
(SDs). Statistical analysis was conducted using GraphPad Prism 9.0
software (GraphPad, San Diego, CA, USA). The Shapiro-Wilk test was
used to test for normality. Comparisons were analyzed by ANOVA,
followed by Dunnett's test as the post hoc test; or by
Kruskal-Wallis test followed by Dunn's test as the post hoc test.
Statistical significance was defined as P<0.05.
Results
LM reduces the level of inflammatory
cells in the BALF in OVA-induced asthma
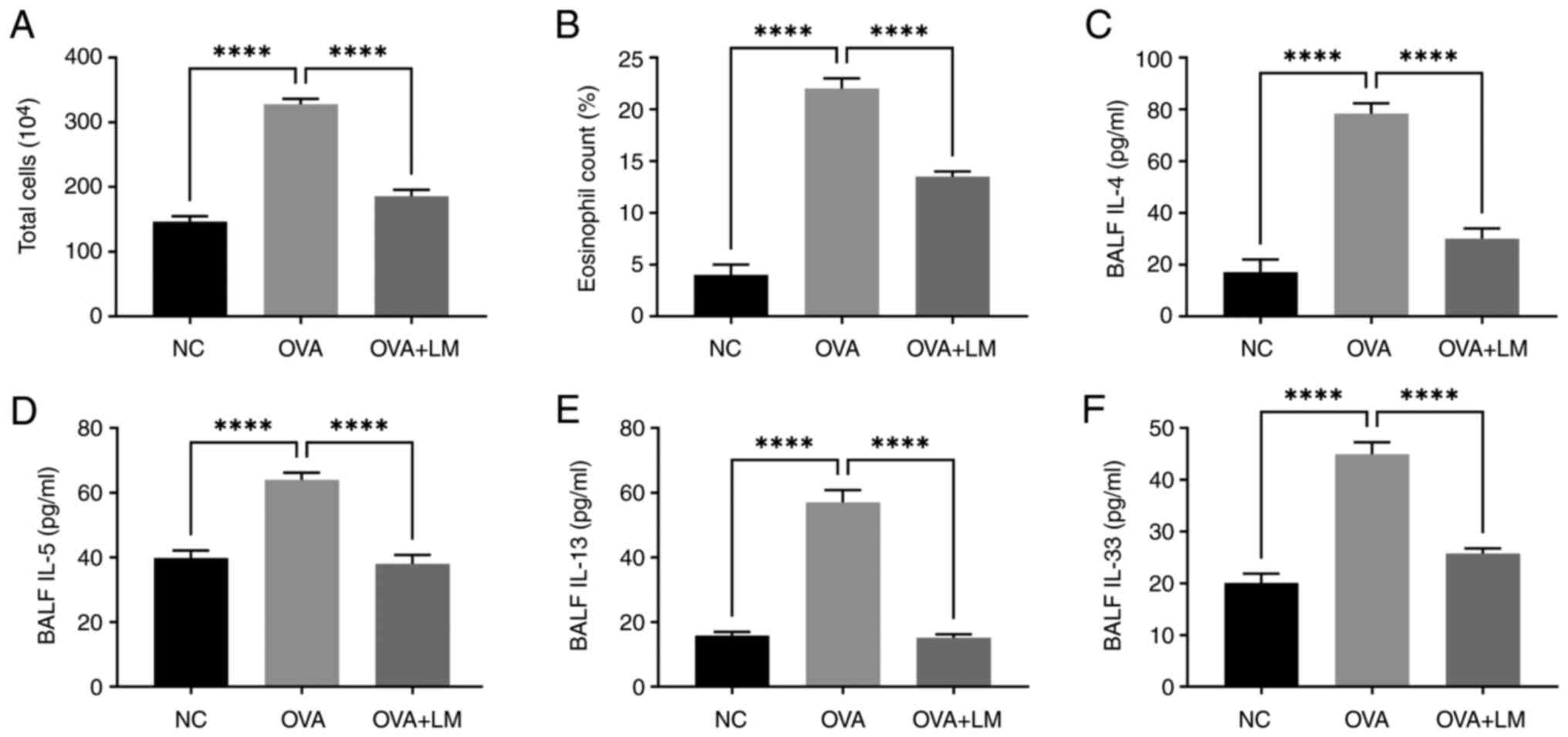
As shown in Fig. 2A and
B, OVA-challenged mice exhibited a higher level in both total
cell number (P<0.0001) and eosinophil percentage (P<0.0001)
compared to the normal condition. However, treatment with LM
effectively attenuated the accumulation of OVA-induced eosinophils
(P<0.0001 vs. OVA group). These findings suggest that LM
possesses the ability to suppress the infiltration of inflammatory
cells, particularly eosinophils, in lung tissue following OVA
exposure.
LM decreases Th2 cytokines in
BALF
OVA challenge resulted in elevated levels of IL-4
(78.21±4.10 pg/ml, P<0.0001 vs. NC group), IL-5 (64.09±2.21
pg/ml, P<0.0001 vs. NC group), and IL-13 (57.02±3.96 pg/ml,
P<0.0001 vs. NC group) in BALF; however, treatment with LM
markedly reduced the expression of these cytokines, as evidenced by
a reduction in IL-4 level to 30.24±3.76 pg/ml (P<0.0001 vs. OVA
group), IL-5 to 37.99±2.78 pg/ml (P<0.0001 vs. OVA group), and
IL-13 to 15.16±1.10 pg/ml (P<0.0001 vs. OVA group) (Fig. 2C-E). Lung epithelial cells produce
IL-33, a type of Th2-oriented cytokine that promotes IL-5
production (34). The IL-33 level
was markedly enhanced almost 2 times in OVA mice (45.83±2.69 pg/ml,
P<0.0001) compared to the normal control group. However, LM
treatment recovered IL-33 to 26.00±1.41 pg/ml (P<0.0001 vs. OVA
group), close to the normal level (Fig. 2F).
LM inhibits pro-inflammatory cytokines
and IgE level in the serum
The levels of IL-6 and TNF-α, which are
representative inflammatory cytokines, were significantly elevated
in the serum of asthmatic animals (167.12±6.25 pg/ml, P<0.0001;
109.17±7.17 pg/ml, P<0.0001, respectively) compared to normal
controls (58.97±6.35 pg/ml; 30.50±3.81 pg/ml, respectively).
However, treatment with LM effectively suppressed the expression of
both cytokines induced by OVA allergen (99.45±6.12 pg/ml,
P<0.001, vs. OVA group; 62.51±4.03 pg/ml, P<0.001 vs. OVA
group) (Fig. 3A and B). The
OVA-induced asthma group exhibited a significant increase in IgE
level, reaching to 90.24±5.98 ng/ml (P<0.0001 vs. NC group),
which was nearly three times of the control group. However,
treatment with LM resulted in a substantial reduction in IgE level
to 56.50±2.70 ng/ml (P<0.001 vs. OVA group) (Fig. 3C).
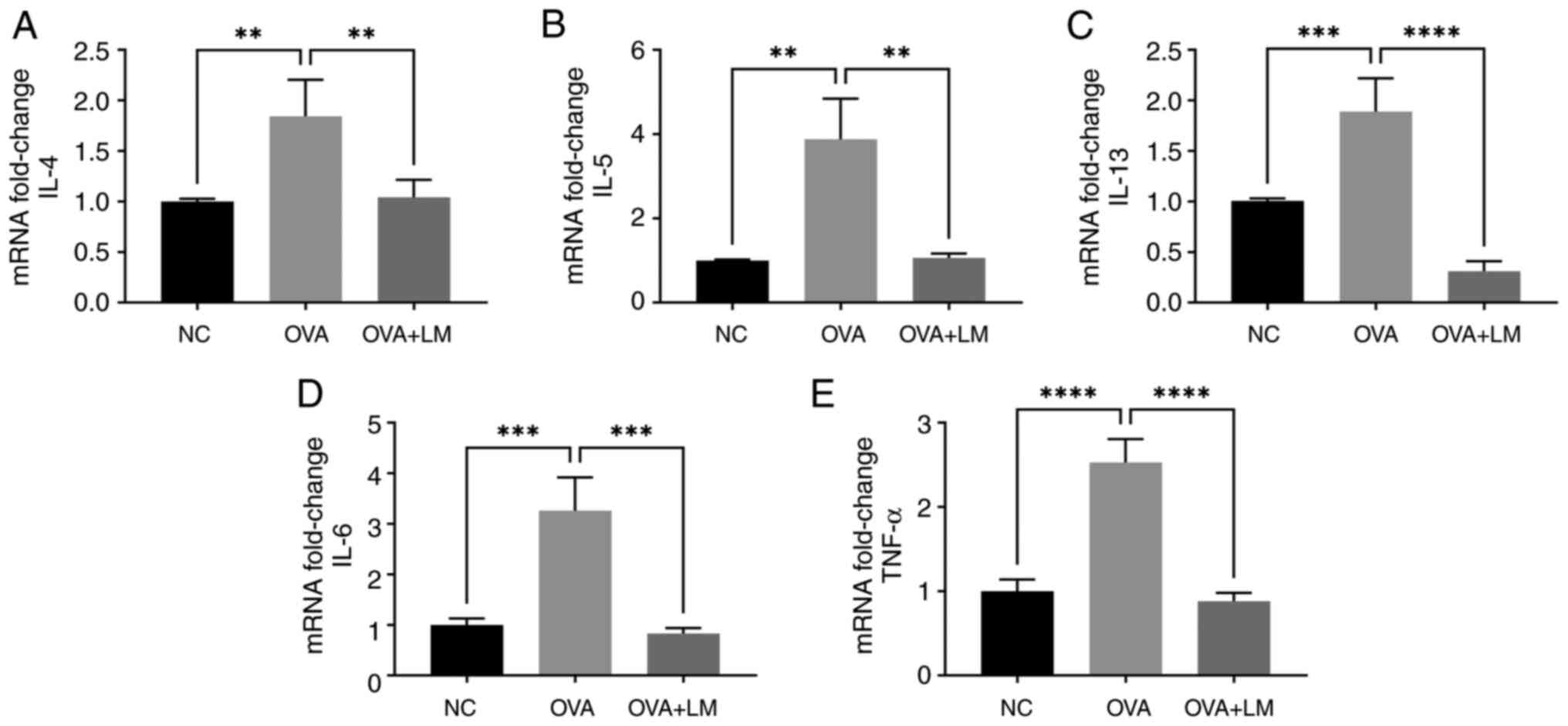
LM inhibits the expression of
inflammatory cytokine in OVA-induced asthma
The expression levels of IL-4, IL-5, and IL-13 were
upregulated in lung tissues following OVA challenge compared to the
normal control group (P<0.01, P<0.01, P<0.001,
respectively). However, treatment with LM effectively suppressed
these cytokines at the gene level in allergic asthma (P<0.01,
P<0.01, P<0.0001 vs. OVA group, respectively) (Fig. 4A-C). Additionally, LM treatment
resulted in downregulation of IL-6 and TNF-α expressions compared
to OVA-induced asthma (P<0.001, P<0.0001, respectively)
(Fig. 4D and E).
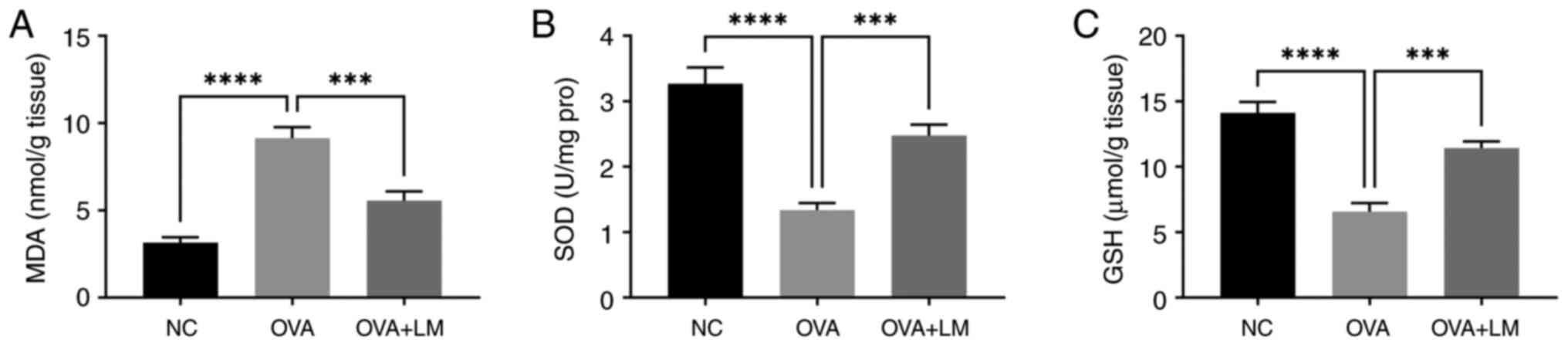
LM modulates antioxidant markers in
OVA-induced asthma
To investigate the role of LM in oxidative stress,
we quantified key antioxidant biochemical markers in lung tissue,
including MDA, SOD, and GSH (35,36).
In OVA-induced mice, MDA level was significantly increased to
9.14±0.62 nmol/g (P<0.0001 vs. NC group) (Fig. 5A), while the crucial antioxidants
SOD and GSH were significantly reduced to 1.34±0.11 U/mg protein
(P<0.0001 vs. NC group) and 6.52±0.66 µmol/g (P<0.0001 vs. NC
group) in lung tissue (Fig. 5B and
C). Notably, administration of LM effectively restored these
mediators to near-normal levels, with MDA decreasing to 5.55±0.53
nmol/g (P<0.001 vs. OVA group), SOD increasing to 2.48±0.17 U/mg
protein (P<0.001 vs. OVA group), and GSH enhancing to 11.42±0.52
µmol/g (P<0.001 vs. OVA group). Overall, LM exhibited the
ability to modulate oxidative stress in an OVA-induced asthma
model.
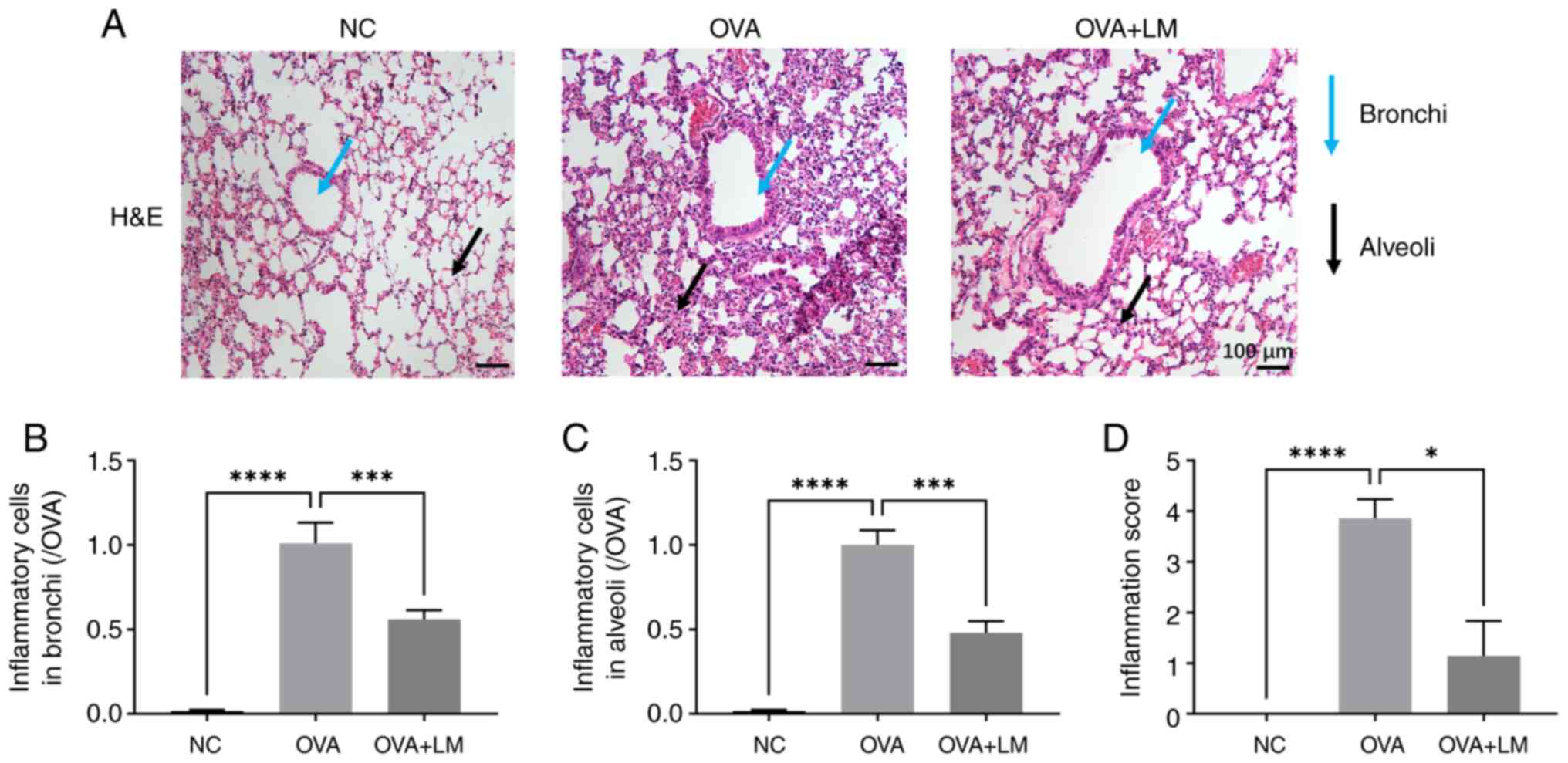
LM ameliorates the histopathological
changes in OVA-induced asthma
As depicted in Fig.
6, OVA stimulation resulted in inflammatory cell infiltration
in both bronchi and alveoli of lung tissues (P<0.0001 vs. NC
group); however, treatment with LM significantly modulated the
inflammatory cell profile within the lung (P<0.001 vs. OVA
group) (Fig. 6A-C). Furthermore,
the inflammatory score was markedly elevated in the OVA group
(P<0.0001 vs. NC group), which was then attenuated by LM
(P<0.05 vs. OVA group) (Fig.
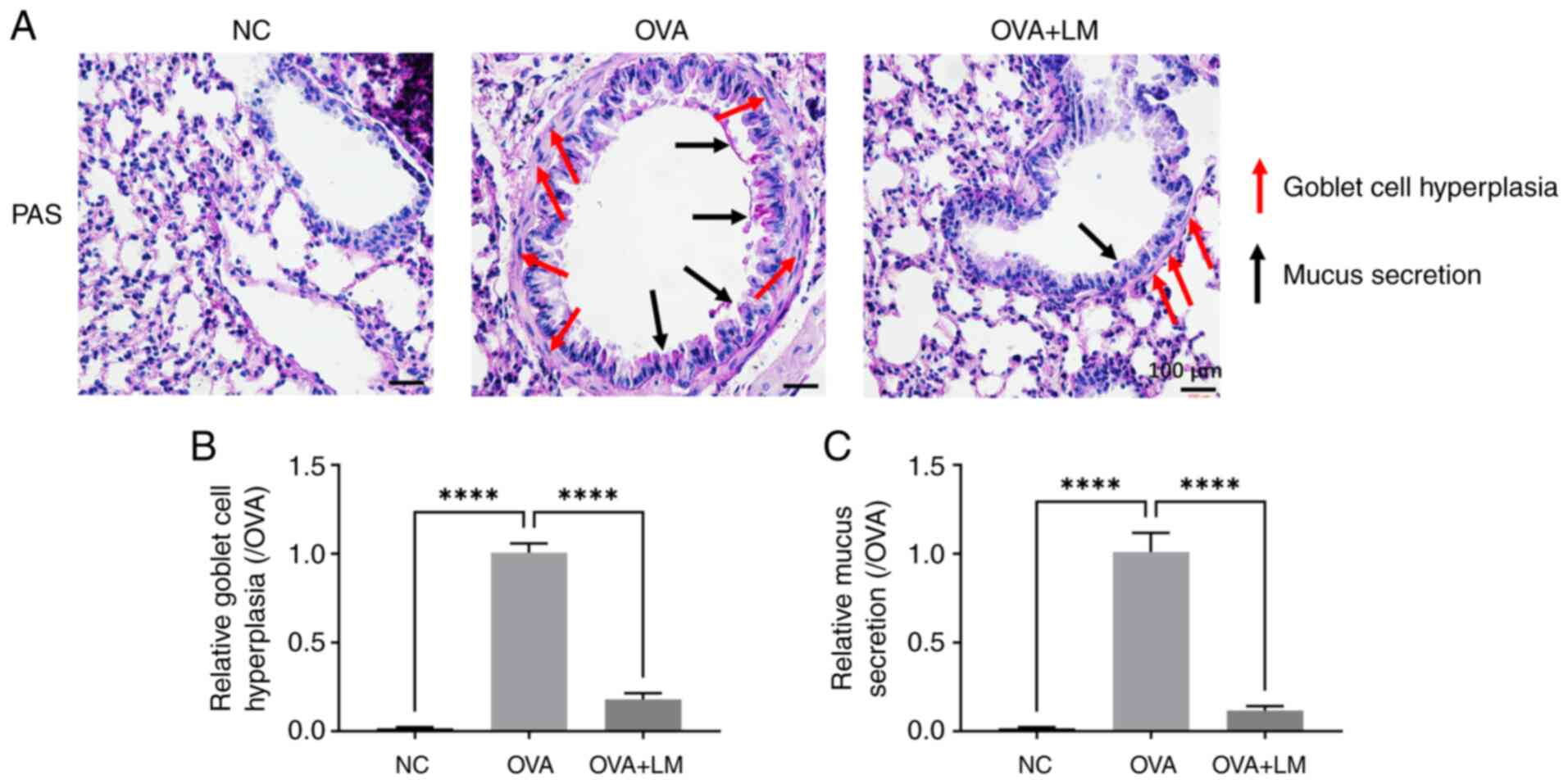
6D). Goblet cell hyperplasia and mucus excessive production are
commonly employed to assess the airway remodeling (37). As shown in Fig. 7, OVA stimulation induced goblet
cell dysplasia in the asthma group (P<0.0001 vs. NC group),
which was mitigated by LM treatment (P<0.0001 vs. OVA group).
Furthermore, LM treatment suppressed OVA-induced mucus secretion in
the bronchi (P<0.0001 vs. OVA group). These findings suggest
that LM modulated the histopathological alterations associated with
OVA-induced asthma.
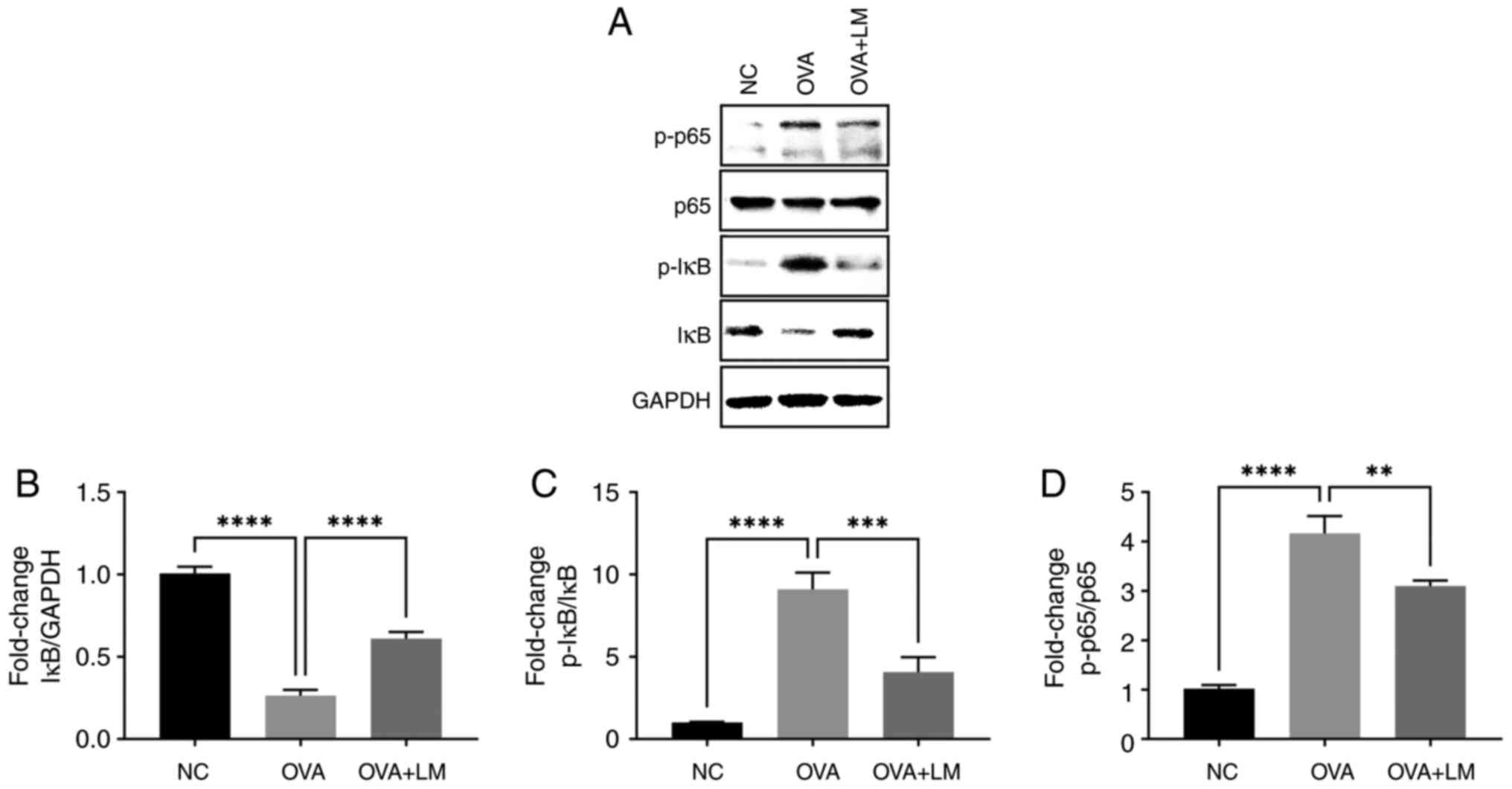
LM suppresses NF-κB signaling pathway
during OVA-induced asthma
In this study, we postulated that LM could
effectively inhibit NF-κB signaling in OVA-induced asthma. As shown
in Fig. 8, OVA challenge
upregulated the phosphorylated IκB and NF-κB (p65) expression, and
increased the degradation of IκB (P<0.0001 vs. NC group,
respectively). However, treatment with LM significantly suppressed
NF-κB activation as evidenced by inhibition of IκB degradation
(P<0.0001 vs. OVA group), and downregulated levels of p-IκB
(P<0.001 vs. OVA group) and p-p65 (P<0.01 vs. OVA group).
These findings demonstrate the anti-inflammatory potential of LM
through inhibition of the NF-κB pathway in OVA-sensitized mice.
Discussion
The main features of allergic asthma include
inflammation, excessive mucus production, and remodeling in the
airway (32). Despite the
availability of a few drugs, it is imperative to explore more
effective approaches for treating asthma. Over the years, there
have been numerous conflicting reports regarding the
supplementation of omega-3 fatty acids in asthma management.
Several studies have demonstrated that intake of omega-3 fatty
acids exerts a protective effect in asthma (38–42).
However, other findings suggest that omega-3 fatty acid
supplementation may either exacerbate pulmonary inflammation or
exhibit no significant reduction in its severity, rendering it
ineffective in human trials (43,44).
Conversely, SPMs, derived from DHA or EPA, are demonstrated robust
and favorable effects on inflammatory diseases even at doses
thousands of times lower than DHA or EPA, including asthma
(45–47). Revealing the involvement of SPMs in
inflammatory responses associated with asthma enhances the
comprehension of dysfunctional inflammation resolution mechanisms
and unveils potential therapeutic targets for managing this
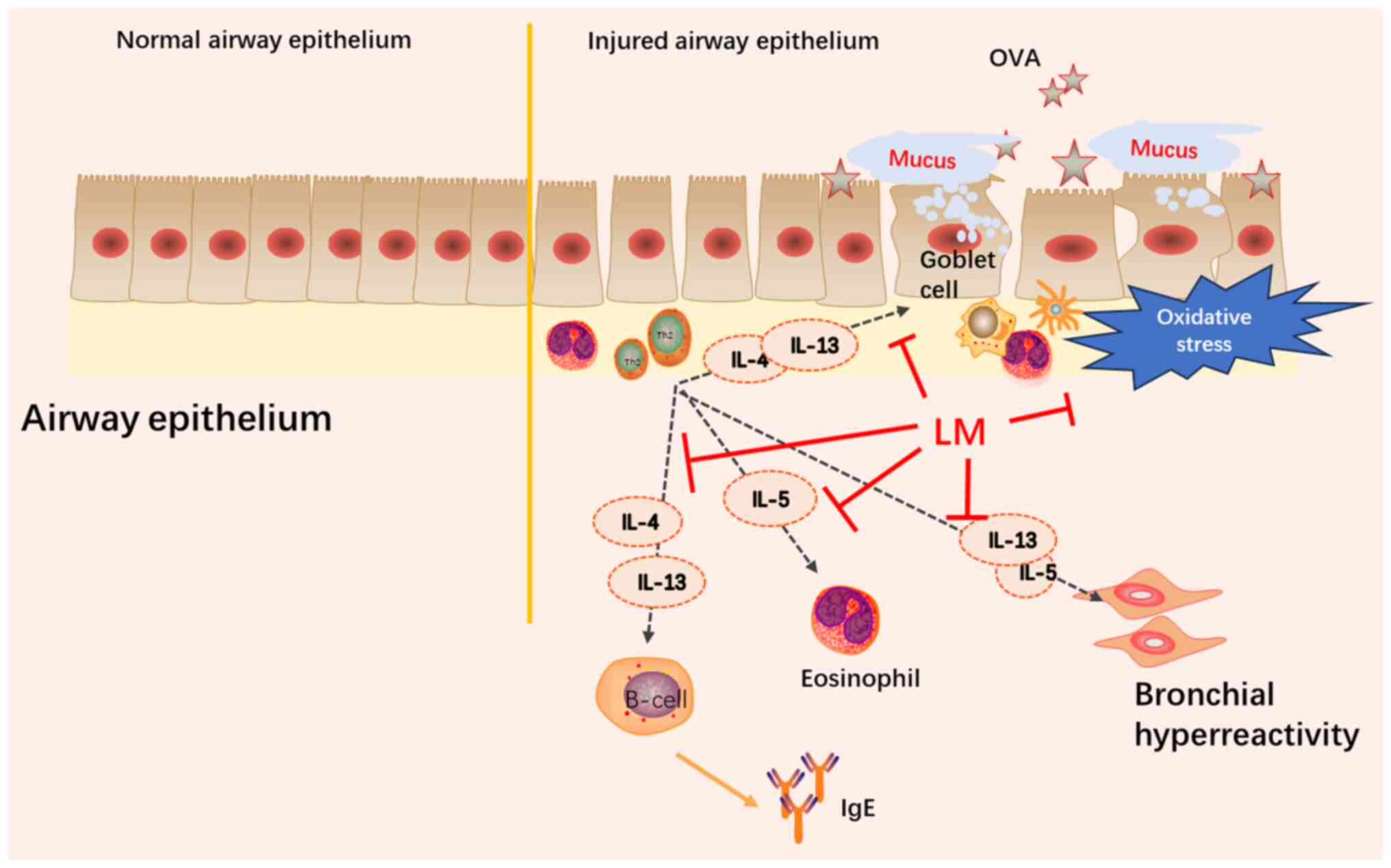
condition. In this study, OVA exposure successfully induced
asthmatic features in mice including elevated eosinophils in BALF
and lung pro-inflammatory symptoms along with goblet cell
hyperplasia, increased mucus production, and oxidative stress.
Furthermore, treatment with LM significantly reduced inflammatory
cell infiltration into the airway and lung while attenuating airway
remodeling and modulating oxidative stress levels. These findings
demonstrate the efficacy of LM in regulating inflammation in asthma
(Fig. 9).
Eosinophils play a pivotal role in allergic
inflammation and the development of airway remodeling during
Th2-type allergic asthma (4). Th2
cytokines, such as IL-4, IL-5, and IL-13, promote the infiltration
of eosinophils into lung tissue (48). IL-5 facilitates the eosinophils to
migrate into the lungs (6). IL-4
and IL-13 stimulate B cells to secrete IgE, which subsequently
activates mast cells and basophils in allergic diseases (49). Additionally, IL-13 influences
smooth muscle activity and airway mucus secretion (50,51).
Therefore, these cytokines represent important targets for
suppressing asthma. In this study, we observed excessive production
of these Th2-related cytokines following OVA induction in mice,
suggesting an allergic-like asthma model. Resolvin D1 and resolvin
E1 markedly decreased airway eosinophilia and mucus metaplasia,
accompanied with decreased Th2 cytokines in mice asthma model
(25–27). Similarly, treatment with LM
significantly reduced levels of Th2-related cytokines, while
concurrently decreasing eosinophil counts in BALF and lung tissues.
Goblet cell hyperplasia is a pathophysiological characteristic of
asthma, significantly augmenting mucus production, thereby leading
to airway obstruction (52,53).
As anticipated, OVA induced goblet cell hyperplasia and resulted in
excessive mucus accumulation; however, oral treatment with LM
substantially mitigated goblet cell dysplasia and suppressed mucus
secretion, indicating the inhibitory role of LM in asthmatic airway
remodeling.
IgE is induced by Th2 cytokines and contributes to
the asthma (10,48). In present study, OVA challenge led
to an elevated level of IgE in the serum, while LM effectively
attenuated the OVA-induced increase in serum IgE. IL-6 and TNF-α
are widely recognized as key markers of inflammation (34). TNF-α has recently emerged as a
crucial factor in refractory asthma and plays multiple roles in
airway pathology during asthmatic conditions (54). Additionally, IL-6 promotes Th2
differentiation and IL-4 production (55). Our findings demonstrate that
stimulation with OVA resulted in upregulated expression of both
IL-6 and TNF-α in lung tissue and serum, which were significantly
suppressed by LM treatment. These results highlight the
anti-inflammatory effects of LM on asthma.
Oxidative stress is crucial in the pathogenesis of
asthma, as it may contribute to the airway inflammation via airway
hyper-responsiveness, mucus secretion, and pro-inflammatory
cytokines (56,57). In our study, we observed elevated
level of MDA along with decreased SOD activity and GSH level during
OVA-induced asthma, indicating oxidative stress in OVA-induced
asthma model. Importantly, LM highly mediated these anti-oxidative
parameters which were associated with the protective role exerted
by LM in mitigating asthma pathology.
NF-κB is a pivotal mediator in the progression of
asthma (58). IκB, which inhibits
NF-κB, is bound to NF-κB in the cytoplasm. Within the inducer, IκB
was phosphorylated and degraded, resulting in the activation of
NF-κB (59). Activation of NF-κB
leads to the expression of inflammatory cytokines and chemokines,
contributing to the Th2 cell differentiation in allergic asthma
(60). In our previous
investigations, we found LM attenuated NF-κB signaling pathway in
RAW264.7 cells and atopic dermatitis (28,31).
In the present study, OVA exposure increased the expression of
p-IκB and p-p65, leading to the activation of the NF-κB signaling
pathway. Treatment with LM drastically inhibited the degradation
and phosphorylation of IκB. Furthermore, LM inhibited the
expression of p-p65. Taken together, the results suggest that LM
ameliorates OVA-induced allergic asthma by regulating the NF-κB
activation.
In conclusion, this study demonstrates that LM shows
promise as a viable option for both alternative and adjunctive
therapy in the management of asthma. Firstly, LM is endogenous to
the human body, ensuring its safety; Secondly, LM exhibits
significant improvements at significantly lower doses compared to
omega-3 fatty acids, facilitating ease of intake. However, more
research is required to explore optimal therapeutic strategies
including dose-dependency, administration methods, and clinical
testing.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Microbial Biotechnology
Research Center, Korea Research Institute of Bioscience and
Biotechnology from the Ministry of Science and ICT (grant no.
KGM5482423) and the National Research Foundation of Korea grant
funded by the Korean government (grant no.
NRF2021R1A2C2013498).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YS conceptualized and designed the study, performed
experiments, analyzed data, and wrote and revised manuscript. HSC
analyzed data, contributed to critical revisions and contributed to
the final manuscript. SKK and YH performed experiments and analyzed
data. SCC and JHS investigated the literature, supplied the
materials and analyzed data. YSJ contributed to data analysis and
critical revisions of the intellectual content. JHC contributed to
data analysis, and the draft and final manuscript. JWS
conceptualized the study, and contributed to the draft and final
manuscript. YS and JWS confirmed the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Institutional Animal Care and Use Committee and Institutional
Animal Ethics Committee of the Korea Research Institute of
Bioscience and Biotechnology (Daejeon, South Korea; approval no.
KRIBB-AEC-23236).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pawankar R: Allergic diseases and asthma:
A global public health concern and a call to action. World Allergy
Organ J. 7:122014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Agache I, Eguiluz-Gracia I, Cojanu C,
Laculiceanu A, Del Giacco S, Zemelka-Wiacek M, Kosowska A, Akdis CA
and Jutel M: Advances and highlights in asthma in 2021. Allergy.
76:3390–3407. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lambrecht BN and Hammad H: The immunology
of asthma. Nat Immunol. 16:45–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Possa SS, Leick EA, Prado CM, Martins MA
and Tibério IFLC: Eosinophilic inflammation in allergic asthma.
Front Pharmacol. 4:462013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hammad H and Lambrecht BN: The basic
immunology of asthma. Cell. 184:1469–1485. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stokes JR and Casale TB: Characterization
of asthma endotypes: Implications for therapy. Ann Allergy Asthma
Immunol. 117:121–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abdelaziz MH, Abdelwahab SF, Wan J, Cai W,
Huixuan W, Jianjun C, Kumar KD, Vasudevan A, Sadek A, Su Z, et al:
Alternatively activated macrophages; a double-edged sword in
allergic asthma. J Transl Med. 18:582020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma Y, Ge A, Zhu W, Liu YN, Ji NF, Zha WJ,
Zhang JX, Zeng XN and Huang M: Morin attenuates ovalbumin-induced
airway inflammation by modulating oxidative stress-responsive MAPK
signaling. Oxid Med Cell Longev. 2016:58436722016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saeedavi M, Goudarzi M, Mehrzadi S, Basir
Z, Hasanvand A and Hosseinzadeh A: Sinapic acid ameliorates airway
inflammation in murine ovalbumin-induced allergic asthma by
reducing Th2 cytokine production. Life Sci. 307:1208582022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ezz-Eldin YM, Aboseif AA and Khalaf MM:
Potential anti-inflammatory and immunomodulatory effects of
carvacrol against ovalbumin-induced asthma in rats. Life Sci.
242:1172222020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zafar MS, Shahid K, Gobe GC, Yasmin R,
Naseem N and Shahzad M: Suppression of cytokine storm and
associated inflammatory mediators by salicylaldehyde derivative of
pregabalin: An innovative perspective for alleviating airway
inflammation and lung remodeling. J King Saud Univ Sci.
34:1018772022. View Article : Google Scholar
|
|
12
|
Sahiner UM, Birben E, Erzurum S, Sackesen
C and Kalayci O: Oxidative stress in asthma. World Allergy Organ J.
4:151–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bowler RP and Crapo JD: Oxidative stress
in airways: Is there a role for extracellular superoxide dismutase?
Am J Respir Crit Care Med. 166:S38–S43. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Michaeloudes C, Abubakar-Waziri H, Lakhdar
R, Raby K, Dixey P, Adcock IM, Mumby S, Bhavsar PK and Chung KF:
Molecular mechanisms of oxidative stress in asthma. Mol Aspects
Med. 85:1010262022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee KS, Lee HK, Hayflick JS, Lee YC and
Puri KD: Inhibition of phosphoinositide 3-kinase delta attenuates
allergic airway inflammation and hyperresponsiveness in murine
asthma model. FASEB J. 20:455–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ganesh Yerra V, Negi G, Sharma SS and
Kumar A: Potential therapeutic effects of the simultaneous
targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy.
Redox Biol. 1:394–397. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
El-Hashim AZ, Renno WM, Abduo HT, Jaffal
SM, Akhtar S and Benter IF: Effect of inhibition of the
ubiquitin-proteasome-system and IκB kinase on airway inflammation
and hyperresponsiveness in a murine model of asthma. Int J
Immunopathol Pharmacol. 24:33–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Xun P, Zamora D, Sood A, Liu K,
Daviglus M, Iribarren C, Jacobs D Jr, Shikany JM and He K: Intakes
of long-chain omega-3 (n-3) PUFAs and fish in relation to incidence
of asthma among American young adults: The CARDIA study. Am J Clin
Nutr. 97:173–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wendell SG, Baffi C and Holguin F: Fatty
acids, inflammation, and asthma. J Allergy Clin Immunol.
133:1255–1264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kitz R, Rose MA, Schubert R, Beermann C,
Kaufmann A, Böhles HJ, Schulze J and Zielen S: Omega-3
polyunsaturated fatty acids and bronchial inflammation in grass
pollen allergy after allergen challenge. Respir Med. 104:1793–1798.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siddiquee A, Patel M, Rajalingam S, Narke
D, Kurade M and Ponnoth DS: Effect of omega-3 fatty acid
supplementation on resolvin (RvE1)-mediated suppression of
inflammation in a mouse model of asthma. Immunopharmacol
Immunotoxicol. 41:250–257. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyata J and Arita M: Role of omega-3
fatty acids and their metabolites in asthma and allergic diseases.
Allergol Int. 64:27–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duvall MG and Levy BD: DHA- and
EPA-derived resolvins, protectins, and maresins in airway
inflammation. Eur J Pharmacol. 785:144–155. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koltsida O, Karamnov S, Pyrillou K,
Vickery T, Chairakaki AD, Tamvakopoulos C, Sideras P, Serhan CN and
Andreakos E: Toll-like receptor 7 stimulates production of
specialized pro-resolving lipid mediators and promotes resolution
of airway inflammation. EMBO Mol Med. 5:762–775. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Levy BD: Resolvin D1 and Resolvin E1
promote the resolution of allergic airway inflammation via shared
and distinct molecular counter-regulatory pathways. Front Immunol.
3:3902012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rogerio AP, Haworth O, Croze R, Oh SF,
Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN and Levy BD:
Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of
allergic airways responses. J Immunol. 189:1983–1991. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aoki H, Hisada T, Ishizuka T, Utsugi M,
Kawata T, Shimizu Y, Okajima F, Dobashi K and Mori M: Resolvin E1
dampens airway inflammation and hyperresponsiveness in a murine
model of asthma. Biochem Biophys Res Commun. 367:509–515. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Su Y, Han Y, Choi HS, Lee GY, Cho HW, Choi
H, Jang YS, Choi JH and Seo JW: Lipid mediators derived from DHA
alleviate DNCB-induced atopic dermatitis and improve the gut
microbiome in BALB/c mice. Int Immunopharmacol. 124:1109002023.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou R, Shi X, Gao Y, Cai N, Jiang Z and
Xu X: Anti-inflammatory activity of guluronate oligosaccharides
obtained by oxidative degradation from alginate in
lipopolysaccharide-activated murine macrophage RAW 264.7 cells. J
Agric Food Chem. 63:160–168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun N, Teng A, Zhao Y, Liu H, Tu J, Jia Q
and Wang Q: Immunological and anticancer activities of
seleno-ovalbumin (Se-OVA) on H22-bearing mice. Int J Biol Macromol.
163:657–665. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su Y, Han Y, Choi HS, Lee GY, Cho HW, Choi
H, Choi JH, Jang YS and Seo JW: Lipid mediators obtained from
docosahexaenoic acid by soybean lipoxygenase attenuate
RANKL-induced osteoclast differentiation and rheumatoid arthritis.
Biomed Pharmacother. 171:1161532024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu D, Li S, Liu X, Xu J, Jiang A, Zhang Y,
Liu Z, Wang J, Zhou E, Wei Z, et al: Alpinetin prevents
inflammatory responses in OVA-induced allergic asthma through
modulating PI3K/AKT/NF-κB and HO-1 signaling pathways in mice. Int
Immunopharmacol. 89:1070732020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lambrecht BN, Hammad H and Fahy JV: The
cytokines of asthma. Immunity. 50:975–991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karadogan B, Beyaz S, Gelincik A,
Buyukozturk S and Arda N: Evaluation of oxidative stress biomarkers
and antioxidant parameters in allergic asthma patients with
different level of asthma control. J Asthma. 59:663–672. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cho YS and Moon H-B: The role of oxidative
stress in the pathogenesis of asthma. Allergy Asthma Immunol Res.
2:183–187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang WC, Fang LW and Liou CJ: Phloretin
attenuates allergic airway inflammation and oxidative stress in
asthmatic mice. Front Immunol. 8:1342017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schubert R, Kitz R, Beermann C, Rose MA,
Lieb A, Sommerer PC, Moskovits J, Alberternst H, Böhles HJ, Schulze
J and Zielen S: Effect of n-3 polyunsaturated fatty acids in asthma
after low-dose allergen challenge. Int Arch Allergy Immunol.
148:321–329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mickleborough TD, Lindley MR, Ionescu AA
and Fly AD: Protective effect of fish oil supplementation on
exercise-induced bronchoconstriction in asthma. Chest. 129:39–49.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yokoyama A, Hamazaki T, Ohshita A, Kohno
N, Sakai K, Zhao GD, Katayama H and Hiwada K: Effect of aerosolized
docosahexaenoic acid in a mouse model of atopic asthma. Int Arch
Allergy Immunol. 123:327–332. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Morin C, Fortin S, Cantin AM and Rousseau
É: MAG-EPA resolves lung inflammation in an allergic model of
asthma. Clin Exp Allergy. 43:1071–1082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang T, Li P, Zhao J, Dai L, Sun D, Liu
M, An L, Jia L, Jing X, Wang H, et al: Long-chain polyunsaturated
fatty acids improve airway pathological features and gut microbial
imbalances in BALB/c mice with ovalbumin-induced asthma. J Funct
Foods. 81:1044652021. View Article : Google Scholar
|
|
43
|
Yin H, Liu W, Goleniewska K, Porter NA,
Morrow JD and Peebles RS Jr: Dietary supplementation of omega-3
fatty acid-containing fish oil suppresses F2-isoprostanes but
enhances inflammatory cytokine response in a mouse model of
ovalbumin-induced allergic lung inflammation. Free Radic Biol Med.
47:622–628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schuster GU, Bratt JM, Jiang X, Pedersen
TL, Grapov D, Adkins Y, Kelley DS, Newman JW, Kenyon NJ and
Stephensen CB: Dietary long-chain omega-3 fatty acids do not
diminish eosinophilic pulmonary inflammation in mice. Am J Respir
Cell Mol Biol. 50:626–636. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Miyata J, Fukunaga K, Iwamoto R, Isobe Y,
Niimi K, Takamiya R, Takihara T, Tomomatsu K, Suzuki Y, Oguma T, et
al: Dysregulated synthesis of protectin D1 in eosinophils from
patients with severe asthma. J Allergy Clin Immunol.
131:353–360.e2-e2. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zambalde ÉP, Teixeira MM, Favarin DC, de
Oliveira JR, Magalhães ML, Cunha MM, Silva WC Jr, Okuma CH,
Rodrigues V Jr, Levy BD and Rogerio AP: The anti-inflammatory and
pro-resolution effects of aspirin-triggered RvD1 (AT-RvD1) on
peripheral blood mononuclear cells from patients with severe
asthma. Int Immunopharmacol. 35:142–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Peh HY, Bruggemann TR, Ho WE, Cheng C, Tan
WD, Wong WF and Levy BD: Resolvin D2 promotes the
resolution of allergen-induced lung inflammation. J Immunol. 204 (1
Suppl):S147.192020. View Article : Google Scholar
|
|
48
|
Leigh R, Ellis R, Wattie JN, Hirota JA,
Matthaei KI, Foster PS, O'Byrne PM and Inman MD: Type 2 cytokines
in the pathogenesis of sustained airway dysfunction and airway
remodeling in mice. Am J Respir Crit Care Med. 169:860–867. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Platts-Mills TAE, Schuyler AJ, Erwin EA,
Commins SP and Woodfolk JA: IgE in the diagnosis and treatment of
allergic disease. J Allergy Clin Immunol. 137:1662–1670. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ingram JL and Kraft M: IL-13 in asthma and
allergic disease: Asthma phenotypes and targeted therapies. J
Allergy Clin Immunol. 130:829–844. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Alasandagutti ML, Ansari MSS, Sagurthi SR,
Valluri V and Gaddam S: Role of IL-13 genetic variants in
signalling of asthma. Inflammation. 40:566–577. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Curran DR and Cohn L: Advances in mucous
cell metaplasia: A plug for mucus as a therapeutic focus in chronic
airway disease. Am J Respir Cell Mol Biol. 42:268–275. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lai H and Rogers DF: New pharmacotherapy
for airway mucus hypersecretion in asthma and COPD: Targeting
intracellular signaling pathways. J Aerosol Med Pulm Drug Deliv.
23:219–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Adner M, Rose AC, Zhang Y, Swärd K, Benson
M, Uddman R, Shankley NP and Cardell LO: An assay to evaluate the
long-term effects of inflammatory mediators on murine airway smooth
muscle: Evidence that TNFalpha up-regulates 5-HT(2A)-mediated
contraction contraction. Br J Pharmacol. 137:971–982. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dienz O and Rincon M: The effects of IL-6
on CD4 T cell responses. Clin Immunol. 130:27–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Henderson WR Jr, Chi EY, Teo JL, Nguyen C
and Kahn M: A small molecule inhibitor of redox-regulated NF-kappa
B and activator protein-1 transcription blocks allergic airway
inflammation in a mouse asthma model. J Immunol. 169:5294–5299.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Poynter ME: Airway epithelial regulation
of allergic sensitization in asthma. Pulm Pharmacol Ther.
25:438–446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ma B, Athari SS, Mehrabi Nasab E and Zhao
L: PI3K/AKT/mTOR and TLR4/MyD88/NF-κB signaling inhibitors
attenuate pathological mechanisms of allergic asthma. Inflammation.
44:1895–1907. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bao Z, Zhang P, Yao Y, Lu G, Tong Z, Yan
B, Tu L, Yang G and Zhou J: Deguelin attenuates allergic airway
inflammation via inhibition of NF-κb pathway in mice. Int J Biol
Sci. 13:492–504. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Das J, Chen CH, Yang L, Cohn L, Ray P and
Ray A: A critical role for NF-kappa B in GATA3 expression and TH2
differentiation in allergic airway inflammation. Nat Immunol.
2:45–50. 2001. View
Article : Google Scholar : PubMed/NCBI
|