It has been demonstrated that a number of proteins
in the human body undergo post-translational modifications (PTMs)
(1). With the advance of mass
spectrometry techniques, an increasing array of PTMs has been
identified, which can be classified into phosphorylation,
glycosylation, SUMOylation, ubiquitination, acetylation and
methylation, based on the covalent attachment of different small
molecular groups to amino acid residues (2,3).
These modifications induce alterations in the structure and
function of proteins, enhancing the flexibility and diversity of
protein functionalities through different types of PTMs (4). By regulating protein stability,
localization and conformation, PTMs are involved in various
biological processes, including signal transduction and gene
expression regulation (5).
Aberrant levels of PTMs have been closely linked to numerous
diseases, including cancer (6). As
such, PTMs hold considerable potential as biomarkers for disease
and therapeutic targets (7).
Under normal physiological conditions, glucose
within cells undergoes glycolysis and oxidative phosphorylation to
produce substantial amounts of ATP. However, in contrast to normal
cells, tumor cells preferentially generate energy through
glycolysis, even in the presence of oxygen, to adapt to the
hypoxic, acidic and nutrient-deprived tumor microenvironment (TME);
a phenomenon known as the Warburg effect (8). This aerobic glycolysis in tumor cells
results in the accumulation of lactate, which exacerbates the
hypoxic conditions that fuel tumor growth, further intensifying
lactate production and facilitating processes such as angiogenesis,
invasion, metastasis and treatment resistance (9,10).
Traditionally regarded merely as an energy substrate and metabolic
byproduct, lactate is now recognized as a pivotal molecule linking
cellular metabolism to the regulation of cellular activities
(11). Furthermore, lactate serves
as a metabolic substrate that drives PTMs of proteins, with
lactylation, a lactate-induced modification, previously identified
in histones (12). The excessive
production of lactate sustains the hypoxic and acidic TME,
promoting tumor angiogenesis, invasion, metastasis and resistance
to therapies (13). On the other
hand, lactate can influence tumorigenesis through epigenetic
modifications, acting as a bridge between tumor metabolism and
epigenetic regulation (14). This
novel understanding of the role of lactate broadens potential
avenues for cancer therapy.
Cancer-related diseases, as a leading global health
burden, have sparked widespread interest in understanding the
molecular mechanisms underlying tumorigenesis, progression and
treatment (15). Epigenetic
modifications have emerged as critical regulatory factors in cancer
development (16). Lactylation, a
novel form of epigenetic modification, serves a pivotal role in
regulating various biological processes and influencing disease
onset (17). For instance, Kla of
Sox10 leads to macrophage-like vascular smooth muscle cell
accumulation and increases vascular inflammation (18). Recent studies have identified a
growing prevalence of lactylation modifications across multiple
cancer types (19,20), positioning it as a key molecular
mechanism in cancer progression (21–23)
Furthermore, research has shown that protein lactylation is a
dynamic and reversible process (24), suggesting its potential as a
promising therapeutic target for cancer treatment (25).
Lactylation, as an emerging form of modification,
offers a promising therapeutic target for cancer treatment. A
comprehensive understanding of the role lactylation serves in
tumorigenesis and progression will provide profound insights for
the development of novel cancer treatment strategies. This not only
enhances the understanding of lactate metabolism but also paves the
way for future drug identification by offering novel perspectives
and insights into molecular mechanisms. The present review
summarizes the research progress on lactylation modification in
tumor development, examines the underlying mechanisms linking
lactylation with cancer therapy resistance and the TME, and
describes the potential crosstalk between lactylation and gut
microbiota. Furthermore, it explores the therapeutic potential of
targeting lactylation as a novel approach for cancer treatment,
offering valuable insights for the development of innovative
therapeutic strategies.
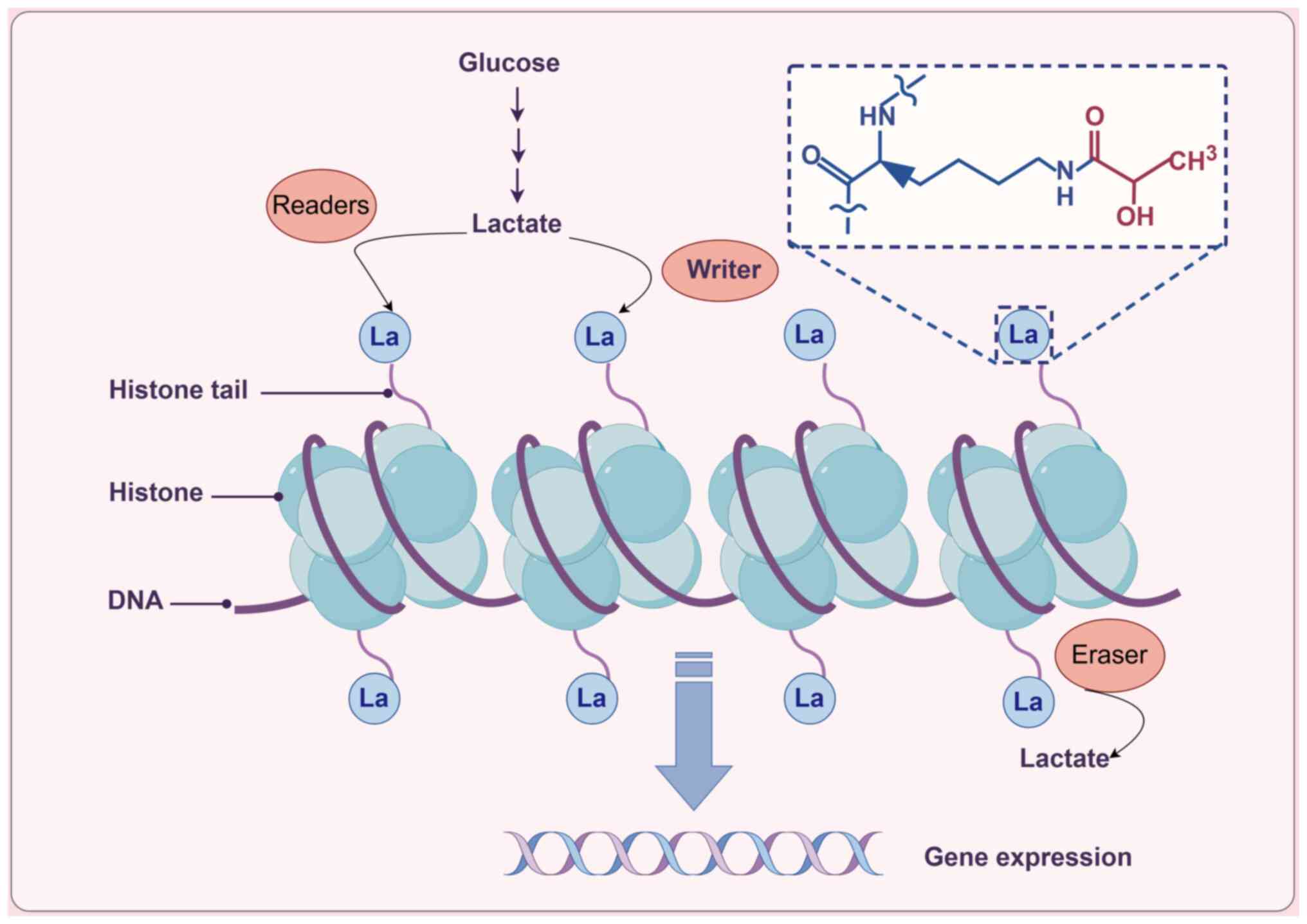
The formation of lactylation is closely linked to
the metabolic processes of tumors. Lactylation primarily arises
from lactate produced during metabolism (26), with endogenous lactate generation
serving as a critical determinant of lactylation levels. Under the
influence of specific enzymes, lactate molecules covalently bind to
lysine residues on proteins. Lactylation can be categorized into
histone lactylation and non-histone lactylation, with histone
lactylation serving a role in regulating gene expression, cellular
signaling and metabolism (27)
(Fig. 1). Currently, lactylation
modifications have been observed in various proteins located in the
nucleus, cytoplasm and mitochondria (28). The identification of lactylation
has broadened the understanding of tumor metabolic reprogramming
and epigenetic modifications.
The levels of protein lactylation are regulated by
various factors, primarily by lactoyltransferases and
de-lactoyltransferases. Lactoyltransferases and
de-lactoyltransferases are responsible for the addition and removal
of lactoyl groups, respectively, thereby influencing lactylation
modifications. For instance, under the action of the
acetyltransferase p300, lactoyl groups are transferred to lysine
residues on histones, leading to covalent binding of lysine
residues with lactate and resulting in lactylation modifications
(29). Lactate transferase
p300/cAMP-response element binding protein (CREB) binding protein
(CBP) includes the cysteine-histidine rich 1, CREB binding
kinase-inducible domain interacting, bromodomain, histone
acetyltransferase, CH3 and steroid receptor coactivator interaction
domains (30). Overexpression or
depletion of the acetyltransferase p300 can increase or decrease
the levels of histone Kla in cells (12).
Alanyl-transfer RNA (tRNA) synthetase (AARS) retains
a conserved prototype structure throughout the entire biological
process, including catalytic, tRNA recognition, editing and C-Ala
domains (31). AARS1 acts as a
lactoyltransferase by recognizing and catalyzing the activation of
lactate molecules, subsequently facilitating the binding of lactate
to lysine residues on target proteins to form lactylation
modifications (32). AARS1 can
also translocate into the nucleus, directly utilizing lactate as a
lactoyl donor and ATP as an energy source to catalyze the
lactylation of histones K90 of yes-associated protein and K108 of
TEA domain transcription factor 1 (33).
As research progresses, more lactoyltransferases
have been identified. For example, lysine acetyltransferase 8
(KAT8) mainly contains an acetyl-CoA binding site and a C2HC zinc
finger motif. Through the KAT8-dependent pathway, it can catalyze
the lactylation of non-histone proteins (34). KAT8 has also been found to serve as
a lactoyltransferase catalyzing the lactylation of non-histone
proteins (35). Researchers have
employed anti-Kla antibodies to confirm the occurrence of lysine
lactylation in Escherichia coli and identified YiaC as a
potential lactoyltransferase (36). YiaC belongs to the GCN5-related
N-acetyltransferase family (37)
and it possesses lysine acetyltransferase activity while also
catalyzing lysine lactylation. Structurally, it has a specific
N-terminal domain, a conserved acetyltransferase domain, conserved
histone acetyltransferase structural domains and a C-terminal
bromodomain at the C-terminus (38). Furthermore, a study has revealed
alternative pathways regulating lactylation modifications, such as
macrophages absorbing extracellular lactate through monocarboxylate
transporters, which promote the lactylation of high mobility group
box 1 via a p300/CBP-dependent mechanism (39).
In the process of lactylation formation,
lactoyltransferases serve a crucial role, while
de-lactoyltransferases can reverse the lactylation modifications.
For instance, histone deacetylases (HDACs) 1–3 have been found to
dynamically and reversibly regulate histone L-lactylation (40). HDAC1-3 contain an N-terminal
catalytic domain (41). A study
has indicated that sirtuin 3 (SIRT3) can de-lactylate the
lactylation at the H4K16la site (42). SIRT3 has a catalytic core region
with a large and structurally homologous Rossmann folding domain
characteristic of NAD-binding proteins and a zinc finger structure
(43). Identifying the key
regulatory enzymes involved in lactylation pathways and their
substrate proteins will lay the groundwork for functional studies
of lactylation modifications and their associated regulatory
pathways.
However, little is currently known regarding how
de-lactoyltransferases exert their reversible effects and
transitions. Furthermore, the precise enzymes and mechanisms
involved in the regulation of lactylation within cells remain
largely unknown, highlighting the need for further research in the
field of de-lactoyltransferases.
It is worth noting that histone acetylation and
lactylation exhibit a high degree of distribution similarity in the
genome (12) and
acetyltransferases p300 and HDAC1-3 may regulate the acetylation
levels of histones while modifying them with lactylation. On the
one hand, it is possible to simultaneously increase the
modification levels of both to regulate gene expression levels
(39). On the other hand,
influenced by the level of lactate metabolism, lactylation
modification may competitively inhibit acetylation modification
(44,45). This crosstalk mechanism between
lactylation and acetylation is not yet fully understood and
requires further in-depth exploration. In addition to
enzyme-catalyzed lactylation, lactoyl glutathione, an intermediate
metabolic product of glyceraldehyde during glycolysis, can directly
modify lysine residues through a non-enzymatic reaction, resulting
in lactylation (46). This
non-enzymatic pathway for lactylation expands the routes through
which lactylation can occur, enriching the understanding of
cellular metabolism and further underscoring the significance of
lactylation modifications in biological processes.
The hallmark features of tumors involve the
dysregulation of critical pathways that maintain the dynamic
balance of normal cells. These pathways include sustaining
proliferative signaling, evading growth suppression, resisting cell
death, achieving replicative immortality, inducing angiogenesis,
and activating invasion and metastasis (47). Lactylation modifications exhibit
differential expression across various tumors and serve a pivotal
role in regulating intracellular signal transduction, thereby
impacting tumor initiation and progression (48).
In non-small cell lung cancer (NSCLC), the
lactylation of SOX9 has been reported to promote stemness,
migration and invasion of NSCLC by enhancing glycolysis (49). Furthermore, a study has
demonstrated that histone H3K18la enhanced immune evasion in NSCLC
cells by activating the POM121 transmembrane
nucleoporin/MYC/programmed death-ligand 1 axis (50). Hypoxia-induced lactylation of the
serine hydroxymethyltransferase 2 protein stimulates glycolysis and
stemness in esophageal cancer cells (51). In colorectal cancer, lactylation of
β-catenin facilitates cancer cell proliferation via the Wnt
signaling pathway (52).
Histone lactylation has also been implicated in
ocular melanoma, where it enhances the recognition of RNA
N6-methyladenosine modifications by YTH N6-methyladenosine RNA
binding protein F2, promoting the degradation of period circadian
regulator 1 and TP53 mRNA, ultimately fostering proliferation and
migration of melanoma cells (53).
In pancreatic cancer, lactylation of transcription factor EB
enhances autophagy and lysosomal activity (54). Elevated lactylation of adenylate
kinase 2 (AK2) in hepatocellular carcinoma has been linked to
metabolic alterations, with lactylation at K28 inhibiting AK2
function, thereby promoting hepatocellular carcinoma cell
proliferation and metastasis (55).
In clear cell renal cell carcinoma, histone
lactylation stimulates tumor progression by activating the
transcription of platelet-derived growth factor receptor β
(56). In breast cancer,
lactylation of histone lysines induces lactate dehydrogenase A
expression, increasing glycolysis and lactate production, which
drives tumor proliferation, invasion and metastasis (57). Furthermore, non-histone lactylation
of METTL16 at K229 promotes copper ion deposition in gastric cancer
(58). In bladder cancer, histone
H3K18 lactylation serves a pro-oncogenic role by upregulating
lipocalin 2 expression (59).
Additionally, the Warburg effect, mediated by metabolite-driven
histone lactylation, promotes the proliferation of anaplastic
thyroid cancer cells harboring the BRAFV600E mutation (60). Lactylation offers novel insights
for both the diagnosis and treatment of tumors. However, it is
important to note that the role of lactylation may vary across
different types of cancer.
Currently, lactylation sites detected in a number of
tumors are primarily located on histone H3K18 (61,62),
e.g., renal clear cell carcinoma (56) and bladder cancer (59), which may be attributed to the
limitations of existing lactylation detection technologies
(63). To broaden the scope of
lactylation research across various modification sites, it is
imperative to develop more diverse lactylation antibodies or
advanced detection methods. Furthermore, research on non-histone
protein lactylation is still in its infancy, warranting greater
attention to the identification of lactylation sites in non-histone
proteins. Histone and non-histone lactylation are both closely
associated with tumor initiation and progression (Table I).
As scientific advancements continue, it is expected
that more lactylation modification sites will be identified, and
targeting lactylation may provide novel biomarkers and therapeutic
targets for clinical cancer diagnosis and treatment. Further
investigation into lactylation will deepen the understanding of its
role in cancer, offering a promising strategy for cancer
therapy.
Chemotherapy remains one of the primary treatments
for human malignancies, yet chemotherapy resistance in cancer cells
is a factor contributing to poor prognosis in a number of patients
(64). Tumor metabolism,
particularly the production of lactate, can influence chemotherapy
efficacy and DNA repair mechanisms (65). Lactate-induced lactylation
modifications intricately link tumor metabolism with epigenetic
regulation. Investigating the mechanisms by which lactylation
contributes to therapy resistance in tumors will provide deeper
insights into treatment outcomes and prognosis, potentially
offering novel strategies to overcome tumor resistance and improve
therapeutic efficacy.
Current research has identified lactate as serving a
critical role in tumor resistance; however, traditional
lactate-targeting therapies have not achieved the desired clinical
outcomes (72). Consequently, the
development of specific inhibitors targeting histone lactylation
may offer more effective therapeutic strategies. While lactylation
has provided a theoretical basis for understanding tumor
resistance, the knowledge regarding the modification sites,
processes and related proteins remains limited. As technologies for
studying lactylation, such as lactylation proteomics, continue to
advance, it is probable that more lactylation-modified proteins
contributing to tumor resistance will be identified.
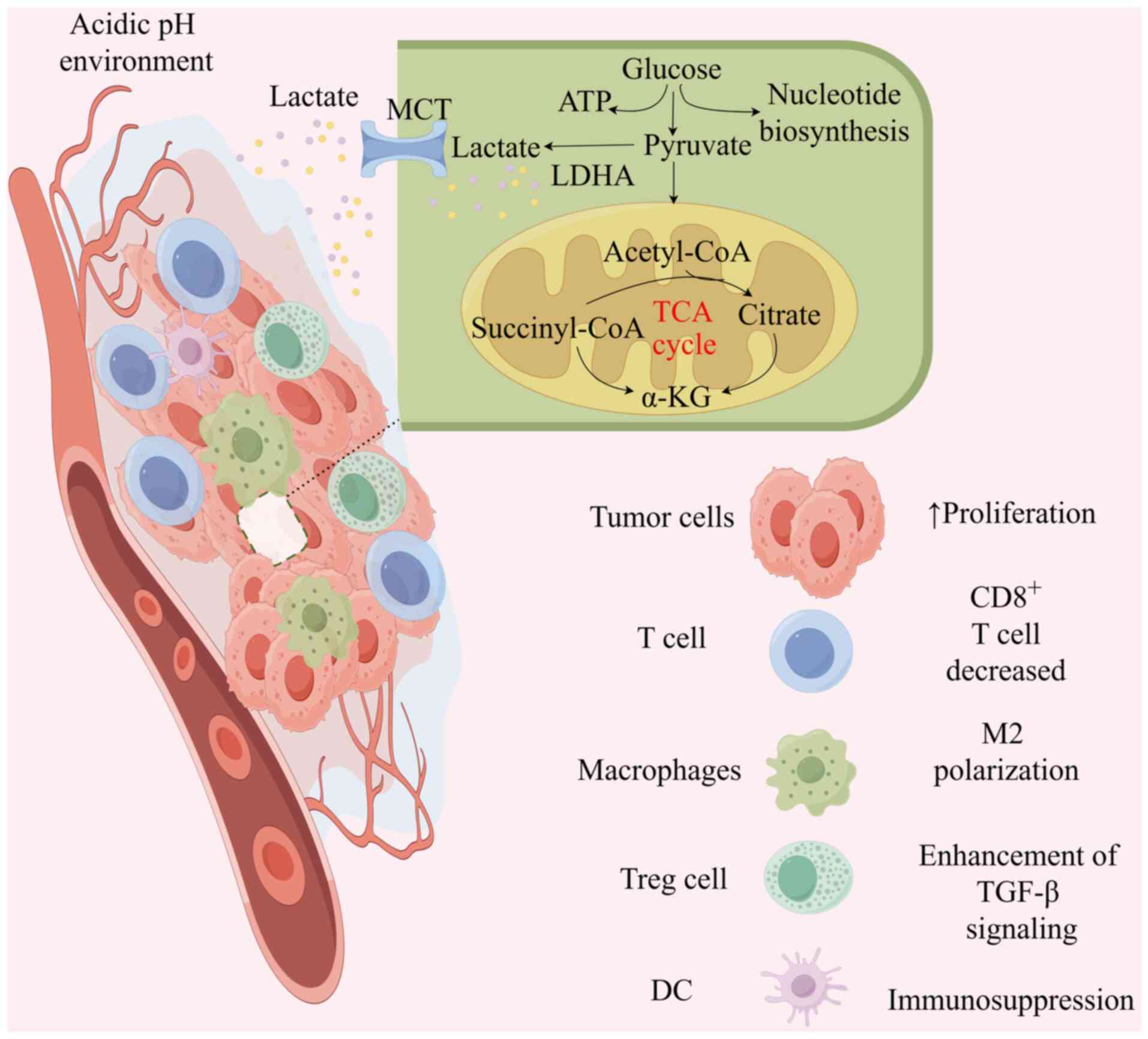
The TME is a highly complex and heterogeneous
ecosystem, comprising tumor cells, extracellular matrix, immune
cells, stromal cells, endothelial cells and an array of signaling
molecules (73). The survival and
growth of tumors are intricately linked to the support provided by
the TME (74). Through the Warburg
effect and glutamine hydrolysis, tumors produce substantial amounts
of lactate, which activates transport receptors on the surface of
tumor cells, facilitating the rapid export of intracellular lactate
to prevent excessive intracellular acidification (75). The accumulation of extracellular
lactate and the resulting acidification of the TME are pivotal
processes that drive tumor progression (76). The extensive buildup of
extracellular lactate increases protein lactylation levels, which
can recruit and regulate cancer-associated fibroblasts,
tumor-infiltrating myeloid cells, including macrophages, dendritic
cells and regulatory T cells (Treg cells), and cancer stem cells.
These processes collectively remodel the TME and promote tumor
proliferation and progression (77).
In the TME, lactylation of tumor cells, cancer stem
cells and tumor-infiltrating immune cells can actively promote
cancer progression by modulating the expression of downstream genes
(78). Immune cells are critical
components of the TME and their infiltration influences tumor
progression. For instance, in colorectal cancer, METTL3 enhances
its binding affinity to RNA m6A modification targets
through lactylation, mediating the immunosuppressive function of
tumor-infiltrating myeloid cells and facilitating tumor immune
evasion (79). Additionally, the
relationship between T-cell lactylation and tumor progression has
attracted widespread attention. It has been shown that the
lactylation of MOESIN enhances TGF-β signaling, promoting
tumorigenesis by inducing the efficient generation of Treg cells
(80). Treg cells, in turn, impede
the activity of cytotoxic T cells, further driving tumor
progression (81). Additionally,
the lactylation of histone H3 at the K18la site enhances the
activity of pro-tumor macrophages, which suppresses the enrichment
of CD8+ T cells and the proportion of
interferon-γ+CD8+ T cells within the TME,
resulting in an immunosuppressive environment (82). However, inhibiting histone
lactylation in tumor-associated macrophages has been found to
activate macrophage phagocytosis and M2 polarization (12,83).
These studies collectively suggest that lactylation may reshape the
TME and immune evasion, ultimately promoting tumor progression.
Lactic acid produced by tumor metabolism remodels
the TME through lactylation, thereby contributing to tumor
progression (Fig. 2). This marks a
new era in the study of tumor metabolism and the TME, as protein
lactylation not only expands the field of PTMs but also provides
novel directions for research into the role of lactate in tumor
immunity and other areas. By reshaping the TME, lactylation
modulates cancer progression, offering a theoretical foundation for
targeting lactylation as an antitumor strategy. Understanding the
interplay between lactylation and the TME will be key to developing
novel cancer therapies. Furthermore, the current research on the
role and mechanisms of lactylation in relation to other key
cellular components within the TME remains limited and warrants
further investigation.
Epigenetic modifications and metabolic reprogramming
are two fundamental biological processes closely associated with
tumor progression (84). While
current clinical treatments for tumors primarily include targeted
therapies, immunotherapy and surgery, limited research has explored
the therapeutic potential of targeting gut microbiota at the level
of epigenetic and metabolic reprogramming. The gut microbiota
serves a crucial role in regulating the epigenetic modifications of
the host, influencing early development, homeostasis and disease
progression (85). Increasing
evidence suggests that the gut microbiota contributes to the
initiation and progression of malignant tumors (86), influencing tumor development
through various mechanisms (87).
A substantial number of microbial-derived molecules
are absorbed by the host, exerting profound effects on epigenetic
modifications (88). On the one
hand, the gut microbiota synthesizes numerous bioactive compounds,
which serve as substrates, cofactors or regulators of epigenetic
enzymes (89), inducing histone
modifications that result in chromatin alterations (90). On the other hand, the gut
microbiota can suppress aerobic glycolysis in tumors via signaling
molecules, leading to a reduction in lactate production (91), thereby lowering the levels of
lactylation. Furthermore, lactylation can reciprocally affect tumor
metabolic reprogramming, consequently influencing the gut
microbiota composition (92).
The gut microbiota and its metabolites modulate gene
expression through epigenetic regulation, affecting tumor cell
metabolism and thereby affecting lactylation levels. For instance,
a study has shown that lipopolysaccharide induces histone
lactylation, upregulating LINC00152 expression and promoting tumor
invasion, highlighting the potential connection between gut
microbiota and lactylation (93).
The gut microbiota is diverse and different gut microbiota and
their metabolic derivatives have varying effects on lactylation
levels. On one hand, gut microbes can generate lactate through
metabolism (94), which will
increase the lactate-dependent lactylation levels of the host. On
the other hand, the gut microbiota can also reduce the production
of lactate and exert HDAC inhibitory activity by converting lactate
into short-chain fatty acids (95), thereby reducing the overall
lactylation modification levels in the host. Currently, there is
limited research on the effect of lactylation on the gut microbiota
and a study has shown that lactylation modification regulates the
metabolic pathways of the microbiota (96), indirectly affecting the microbial
ecology. Furthermore, lactylation of proteins can enhance the
virulence mediated by pathogen toxins (97). Additionally, lactylation may
co-regulate cellular activities via the crosstalk between tumor
metabolic reprogramming and gut microbiota.
While bioactive metabolites produced by the
microbiota promote epigenetic modifications, the specific
mechanisms by which lactylation and gut microbiota crosstalk
influence tumor progression require further investigation. Future
research on the intricate crosstalk between lactylation and gut
microbiota may pave the way for more targeted and effective
combination therapies for cancer treatment.
The regulation of lactate production and transport
is a crucial strategy for improving tumor prognosis (98). The identification of lactylation
further suggests that targeting lactylation may offer novel options
to inhibit cancer progression and enhance antitumor therapies
(99). Based on the mechanisms of
lactylation and its carcinogenic processes, potential therapeutic
approaches can target lactate metabolism, transport or lactylation
generation (100).
PTMs are an indispensable component in the
regulation of protein functionality, occurring both prior to and
subsequent to protein biosynthesis, thereby enhancing the
functional diversity of the proteome (105). PTMs modulate cellular signaling,
protein localization and the maintenance of cellular functions by
altering protein structure and activity, serving a pivotal role in
cellular development (106).
Lactylation, as a novel type of PTM, is critical for elucidating
the pathological processes associated with tumors.
The present review summarizes the mechanisms
underlying the generation of lactylation and its biological effects
on tumor progression. Additionally, it elucidates the relationship
among lactylation, tumor drug resistance and the remodeling of the
TME. Furthermore, it explores the intricate crosstalk between
lactylation and gut microbiota, and examines the potential of
targeting lactylation as a novel therapeutic strategy for cancer.
By providing a comprehensive overview of the regulatory role of
lactylation in tumor development, the present review aimed to
clarify its scientific significance, highlight critical unresolved
questions and pave the way for novel avenues in targeted cancer
therapy.
It is well established that cancer is a
multifactorial disease arising from a myriad of mechanisms and the
precise mechanisms driving tumorigenesis remain incompletely
understood. Lactylation closely links tumor metabolism with tumor
development (107), providing a
novel perspective on the role of lactate and presenting a promising
potential therapeutic approach for cancer treatment.
However, there is currently a lack of specific
inhibitors targeting lactylation modifications. Furthermore, during
tumorigenesis, lactylation may be activated in tandem with other
oncogenic signals, further complicating targeted therapies.
Additionally, since lactylation shares common writers or erasers
with other PTMs, targeting lactylation may inadvertently affect
other protein modifications. This interdependence poses challenges
to the development of lactylation-specific therapeutic agents
(108).
Lactylation modifications can promote tumor
metabolic reprogramming by regulating gene expression, particularly
through their impact on enzymes involved in glucose metabolism.
However, there is limited research on the role of lactylation in
other fundamental metabolic pathways. Further exploration of the
relationship between lactylation modifications and tumor metabolism
will enhance the comprehensive understanding of tumor epigenetics
and metabolism, laying the groundwork for the development of novel
therapeutic targets in cancer treatment. Additionally, the
potential interplay between lactylation and gut microbiota may
offer novel insights for cancer therapy. Dysbiosis of the gut
microbiota not only promotes tumor progression but also affects the
efficacy of antitumor drugs (109). Combining treatments that target
both the gut microbiota and lactylation may enhance the
effectiveness of cancer therapies (110).
Lactylation, along with a spectrum of other PTMs,
adds a layer of complexity to the epigenetic regulation of tumors,
making the development of sensitive and specific PTM detection
methods a crucial direction for research on tumor modifications.
Furthermore, the interactions between different PTMs are not
mutually independent; rather, their crosstalk influences disease
onset and progression (111). The
interplay between these modifications serves a pivotal role in
tumorigenesis (112). However,
the mechanisms through which lactylation interacts with other PTMs
to affect tumor development require further investigation. In
summary, understanding the crosstalk between lactylation and
various modifications will provide valuable insights into the
intricate relationship between tumor metabolism and epigenetic
processes.
Future research on lactylation could prioritize the
following areas: Investigating the effect of lactylation-related
enzymes on tumor progression; unveiling the complex molecular
mechanisms by which different lactylation modification sites
influence cancer development; and developing specific lactylation
inhibitors for cancer treatment.
In summary, lactylation, as a novel PTM, serves a
role in the pathogenesis of tumors and holds substantial potential
for novel diagnostic and therapeutic approaches in cancer.
Currently, research targeting lactylation remains largely
theoretical, with a number of mechanisms of non-histone lactylation
yet to be elucidated. Future investigations should focus on
exploring the specific mechanisms of lactylation and its intricate
relationships with other PTMs. A deeper understanding of the
regulatory roles of lactylation in various pathological processes
may pave the way for the development of innovative therapeutic
strategies, offering more options and hope in clinical
settings.
Not applicable.
The present study was supported by the Natural Science and
Technology Fund of Guizhou Province [grant no. Qiankehe Basic-ZK
(2022) General 644].
Not applicable.
ZZ, XZ and PZ conceived and designed the review. ZZ
and XZ wrote the manuscript. GX, CC and XK critically revised and
polished the manuscript. Data authentication is not applicable. All
authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Doyle HA and Mamula MJ: Post-translational
protein modifications in antigen recognition and autoimmunity.
Trends Immunol. 22:443–449. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramazi S and Zahiri J: Posttranslational
modifications in proteins: Resources, tools and prediction methods.
Database (Oxford). 2021:baab0122021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang H, Yang L, Liu M and Luo J: Protein
post-translational modifications in the regulation of cancer
hallmarks. Cancer Gene Ther. 30:529–547. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang H and Han W: Protein
post-translational modifications in head and neck cancer. Front
Oncol. 10:5719442020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Visconti A and Qiu H: Recent advances in
serum response factor posttranslational modifications and their
therapeutic potential in cardiovascular and neurological diseases.
Vascul Pharmacol. 156:1074212024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Z, Li M, Jiang H, Luo S, Shao F, Xia
Y, Yang M, Ren X, Liu T, Yan M, et al: Fructose-1,6-bisphosphatase
1 functions as a protein phosphatase to dephosphorylate histone H3
and suppresses PPARα-regulated gene transcription and tumour
growth. Nat Cell Biol. 24:1655–1665. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhong Q, Xiao X, Qiu Y, Xu Z, Chen C,
Chong B, Zhao X, Hai S, Li S, An Z and Dai L: Protein
posttranslational modifications in health and diseases: Functions,
regulatory mechanisms, and therapeutic implications. MedComm
(2020). 4:e2612023. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu T, Wang Y, Fan Y, Fang N, Wang T, Xu T
and Shu Y: CircRNAs in cancer metabolism: A review. J Hematol
Oncol. 12:902019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
San-Millan I, Sparagna GC, Chapman HL,
Warkins VL, Chatfield KC, Shuff SR, Martinez JL and Brooks GA:
Chronic lactate exposure decreases mitochondrial function by
inhibition of fatty acid uptake and cardiolipin alterations in
neonatal rat cardiomyocytes. Front Nutr. 9:8094852022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brooks GA: Lactate as a fulcrum of
metabolism. Redox Biol. 35:1014542020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu W, Guo S, Sun J, Zhao Y and Liu C:
Lactate and lactylation in cardiovascular diseases: Current
progress and future perspectives. Metabolism. 158:1559572024.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang D, Tang Z, Huang H, Zhou G, Cui C,
Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al: Metabolic
regulation of gene expression by histone lactylation. Nature.
574:575–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pérez-Tomás R and Pérez-Guillén I: Lactate
in the tumor microenvironment: An essential molecule in cancer
progression and treatment. Cancers (Basel). 12:32442020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu X, Yang J, Xu J, Pan H, Wang W, Yu X
and Shi S: Histone lactylation: From tumor lactate metabolism to
epigenetic regulation. Int J Biol Sci. 20:1833–1854. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heydari Z, Moeinvaziri F, Mirazimi SMA,
Dashti F, Smirnova O, Shpichka A, Mirzaei H, Timashev P and Vosough
M: Alteration in DNA methylation patterns: Epigenetic signatures in
gastrointestinal cancers. Eur J Pharmacol. 973:1765632024.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rungratanawanich W, Ballway JW, Wang X,
Won KJ, Hardwick JP and Song BJ: Post-translational modifications
of histone and non-histone proteins in epigenetic regulation and
translational applications in alcohol-associated liver disease:
Challenges and research opportunities. Pharmacol Ther.
251:1085472023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Wang Z, Wang Q, Li X and Guo Y:
Ubiquitous protein lactylation in health and diseases. Cell Mol
Biol Lett. 29:232024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu X, Zhang DD, Kong P, Gao YK, Huang XF,
Song Y, Zhang WD, Guo RJ, Li CL, Chen BW, et al: Sox10 escalates
vascular inflammation by mediating vascular smooth muscle cell
transdifferentiation and pyroptosis in neointimal hyperplasia. Cell
Rep. 42:1128692023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang D, Yin J, Shan L, Yi X, Zhang W and
Ding Y: Identification of lysine-lactylated substrates in gastric
cancer cells. iScience. 25:1046302022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen M, Cen K, Song Y, Zhang X, Liou YC,
Liu P, Huang J, Ruan J, He J, Ye W, et al:
NUSAP1-LDHA-glycolysis-lactate feedforward loop promotes Warburg
effect and metastasis in pancreatic ductal adenocarcinoma. Cancer
Lett. 567:2162852023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng Q, Sun H, Zhang Y, Yang X, Hao S, Liu
B, Zhou H, Xu ZX and Wang Y: Lactylation stabilizes DCBLD1
activating the pentose phosphate pathway to promote cervical cancer
progression. J Exp Clin Cancer Res. 43:362024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chu YD, Cheng LC, Lim SN, Lai MW, Yeh CT
and Lin WR: Aldolase B-driven lactagenesis and CEACAM6 activation
promote cell renewal and chemoresistance in colorectal cancer
through the Warburg effect. Cell Death Dis. 14:6602023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gu X, Zhuang A, Yu J, Yang L, Ge S, Ruan
J, Jia R, Fan X and Chai P: Histone lactylation-boosted ALKBH3
potentiates tumor progression and diminished promyelocytic leukemia
protein nuclear condensates by m1A demethylation of SP100A. Nucleic
Acids Res. 52:2273–2289. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu R, Wu J, Guo H, Yao W, Li S, Lu Y, Jia
Y, Liang X, Tang J and Zhang H: Post-translational modifications of
histones: Mechanisms, biological functions, and therapeutic
targets. MedComm (2020). 4:e2922023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan RY, He L, Zhang J, Liu X, Liao Y, Gao
J, Liao Y, Yan Y, Li Q, Zhou X, et al: Positive feedback regulation
of microglial glucose metabolism by histone H4 lysine 12
lactylation in Alzheimer's disease. Cell Metab. 34:634–648.e6.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Levine AJ and Puzio-Kuter AM: The control
of the metabolic switch in cancers by oncogenes and tumor
suppressor genes. Science. 330:1340–1344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Song H, Li M and Lu P: Histone
lactylation bridges metabolic reprogramming and epigenetic rewiring
in driving carcinogenesis: Oncometabolite fuels oncogenic
transcription. Clin Transl Med. 14:e16142024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu Y, He Z, Li Z, Wang Y, Wu N, Sun H,
Zhou Z, Hu Q and Cong X: Lactylation: The novel histone
modification influence on gene expression, protein function, and
disease. Clin Epigenetics. 16:722024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu D, Spencer CB, Ortoga L, Zhang H and
Miao C: Histone lactylation-regulated METTL3 promotes ferroptosis
via m6A-modification on ACSL4 in sepsis-associated lung injury.
Redox Biol. 74:1031942024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu F, Hua Y, Kaochar S, Nie S, Lin YL, Yao
Y, Wu J, Wu X, Fu X, Schiff R, et al: Discovery, structure-activity
relationship, and biological activity of histone-competitive
inhibitors of histone acetyltransferases P300/CBP. J Med Chem.
63:4716–4731. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Antika TR, Chrestella DJ, Tseng YK, Yeh
YH, Hsiao CD and Wang CC: A naturally occurring mini-alanyl-tRNA
synthetase. Commun Biol. 6:3142023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zong Z, Xie F, Wang S, Wu X, Zhang Z, Yang
B and Zhou F: Alanyl-tRNA synthetase, AARS1, is a lactate sensor
and lactyltransferase that lactylates p53 and contributes to
tumorigenesis. Cell. 187:2375–2392.e33. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ju J, Zhang H, Lin M, Yan Z, An L, Cao Z,
Geng D, Yue J, Tang Y, Tian L, et al: The alanyl-tRNA synthetase
AARS1 moonlights as a lactyltransferase to promote YAP signaling in
gastric cancer. J Clin Invest. 134:e1745872024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoo L, Mendoza D, Richard AJ and Stephens
JM: KAT8 beyond acetylation: A survey of its epigenetic regulation,
genetic variability, and implications for human health. Genes
(Basel). 15:6392024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie B, Zhang M, Li J, Cui J, Zhang P, Liu
F, Wu Y, Deng W, Ma J, Li X, et al: KAT8-catalyzed lactylation
promotes eEF1A2-mediated protein synthesis and colorectal
carcinogenesis. Proc Natl Acad Sci USA. 121:e23141281212024.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dong H, Zhang J, Zhang H, Han Y, Lu C,
Chen C, Tan X, Wang S, Bai X, Zhai G, et al: YiaC and CobB regulate
lysine lactylation in Escherichia coli. Nat Commun.
13:66282022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Parks AR and Escalante-Semerena JC:
Modulation of the bacterial CobB sirtuin deacylase activity by
N-terminal acetylation. Proc Natl Acad Sci USA. 117:15895–15901.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mutlu B and Puigserver P: GCN5
acetyltransferase in cellular energetic and metabolic processes.
Biochim Biophys Acta Gene Regul Mech. 1864:1946262021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang K, Fan M, Wang X, Xu J, Wang Y, Tu F,
Gill PS, Ha T, Liu L, Williams DL and Li C: Lactate promotes
macrophage HMGB1 lactylation, acetylation, and exosomal release in
polymicrobial sepsis. Cell Death Differ. 29:133–146. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moreno-Yruela C, Zhang D, Wei W, Bæk M,
Liu W, Gao J, Danková D, Nielsen AL, Bolding JE, Yang L, et al:
Class I histone deacetylases (HDAC1-3) are histone lysine
delactylases. Sci Adv. 8:eabi66962022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Micelli C and Rastelli G: Histone
deacetylases: Structural determinants of inhibitor selectivity.
Drug Discov Today. 20:718–735. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fan Z, Liu Z, Zhang N, Wei W, Cheng K, Sun
H and Hao Q: Identification of SIRT3 as an eraser of H4K16la.
iScience. 26:1077572023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tao Z, Jin Z, Wu J, Cai G and Yu X:
Sirtuin family in autoimmune diseases. Front Immunol.
14:11862312023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hagihara H, Shoji H, Otabi H, Toyoda A,
Katoh K, Namihira M and Miyakawa T: Protein lactylation induced by
neural excitation. Cell Rep. 37:1098202021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rho H, Terry AR, Chronis C and Hay N:
Hexokinase 2-mediated gene expression via histone lactylation is
required for hepatic stellate cell activation and liver fibrosis.
Cell Metab. 35:1406–1423.e8. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gaffney DO, Jennings EQ, Anderson CC,
Marentette JO, Shi T, Schou Oxvig AM, Streeter MD, Johannsen M,
Spiegel DA, Chapman E, et al: Non-enzymatic lysine lactoylation of
glycolytic enzymes. Cell Chem Biol. 27:206–213.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kiri S and Ryba T: Cancer, metastasis, and
the epigenome. Mol Cancer. 23:1542024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lv X, Lv Y and Dai X: Lactate, histone
lactylation and cancer hallmarks. Expert Rev Mol. 25:e72023.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yan F, Teng Y, Li X, Zhong Y, Li C, Yan F
and He X: Hypoxia promotes non-small cell lung cancer cell
stemness, migration, and invasion via promoting glycolysis by
lactylation of SOX9. Cancer Biol Ther. 25:23041612024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang C, Zhou L, Zhang M, Du Y, Li C, Ren
H and Zheng L: H3K18 lactylation potentiates immune escape of
non-small cell lung cancer. Cancer Res. 84:3589–3601. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Qiao Z, Li Y, Li S, Liu S and Cheng Y:
Hypoxia-induced SHMT2 protein lactylation facilitates glycolysis
and stemness of esophageal cancer cells. Mol Cell Biochem.
479:3063–3076. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Miao Z, Zhao X and Liu X: Hypoxia induced
β-catenin lactylation promotes the cell proliferation and stemness
of colorectal cancer through the wnt signaling pathway. Exp Cell
Res. 422:1134392023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yu J, Chai P, Xie M, Ge S, Ruan J, Fan X
and Jia R: Histone lactylation drives oncogenesis by facilitating
m6A reader protein YTHDF2 expression in ocular melanoma.
Genome Biol. 22:852021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Huang Y, Luo G, Peng K, Song Y, Wang Y,
Zhang H, Li J, Qiu X, Pu M, Liu X, et al: Lactylation stabilizes
TFEB to elevate autophagy and lysosomal activity. J Cell Biol.
223:e2023080992024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang Z, Yan C, Ma J, Peng P, Ren X, Cai S,
Shen X, Wu Y, Zhang S, Wang X, et al: Lactylome analysis suggests
lactylation-dependent mechanisms of metabolic adaptation in
hepatocellular carcinoma. Nat Metab. 5:61–79. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang J, Luo L, Zhao C, Li X, Wang Z, Zeng
Z, Yang X, Zheng X, Jie H, Kang L, et al: A positive feedback loop
between inactive VHL-triggered histone lactylation and PDGFRβ
signaling drives clear cell renal cell carcinoma progression. Int J
Biol Sci. 18:3470–3483. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hou X, Ouyang J, Tang L, Wu P, Deng X, Yan
Q, Shi L, Fan S, Fan C, Guo C, et al: KCNK1 promotes proliferation
and metastasis of breast cancer cells by activating lactate
dehydrogenase A (LDHA) and up-regulating H3K18 lactylation. PLoS
Biol. 22:e30026662024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sun L, Zhang Y, Yang B, Sun S, Zhang P,
Luo Z, Feng T, Cui Z, Zhu T, Li Y, et al: Lactylation of METTL16
promotes cuproptosis via m6A-modification on FDX1 mRNA
in gastric cancer. Nat Commun. 14:65232023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xie B, Lin J, Chen X, Zhou X, Zhang Y, Fan
M, Xiang J, He N, Hu Z and Wang F: CircXRN2 suppresses tumor
progression driven by histone lactylation through activating the
Hippo pathway in human bladder cancer. Mol Cancer. 22:1512023.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang X, Ying T, Yuan J, Wang Y, Su X, Chen
S, Zhao Y, Zhao Y, Sheng J, Teng L, et al: BRAFV600E restructures
cellular lactylation to promote anaplastic thyroid cancer
proliferation. Endocr Relat Cancer. 30:e2203442023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dai E, Wang W and Li Y, Ye D and Li Y:
Lactate and lactylation: Behind the development of tumors. Cancer
Lett. 591:2168962024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li F, Si W, Xia L, Yin D, Wei T, Tao M,
Cui X, Yang J, Hong T and Wei R: Positive feedback regulation
between glycolysis and histone lactylation drives oncogenesis in
pancreatic ductal adenocarcinoma. Mol Cancer. 23:902024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jing F, Zhang J, Zhang H and Li T:
Unlocking the multifaceted molecular functions and diverse disease
implications of lactylation. Biol Rev Camb Philos Soc. 100:172–189.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xia Y, Sun M, Huang H and Jin WL: Drug
repurposing for cancer therapy. Signal Transduct Target Ther.
9:922024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen H, Li Y, Li H, Chen X, Fu H, Mao D,
Chen W, Lan L, Wang C, Hu K, et al: NBS1 lactylation is required
for efficient DNA repair and chemotherapy resistance. Nature.
631:663–669. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chen Y, Wu J, Zhai L, Zhang T, Yin H, Gao
H, Zhao F, Wang Z, Yang X, Jin M, et al: Metabolic regulation of
homologous recombination repair by MRE11 lactylation. Cell.
187:294–311.e21. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li G, Wang D, Zhai Y, Pan C, Zhang J, Wang
C, Huang R, Yu M, Li Y, Liu X, et al: Glycometabolic
reprogramming-induced XRCC1 lactylation confers therapeutic
resistance in ALDH1A3-overexpressing glioblastoma. Cell Metab.
36:1696–1710.e10. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yue Q, Wang Z, Shen Y, Lan Y, Zhong X, Luo
X, Yang T, Zhang M, Zuo B, Zeng T, et al: Histone H3K9 lactylation
confers temozolomide resistance in glioblastoma via LUC7L2-mediated
MLH1 intron retention. Adv Sci (Weinh). 11:e23092902024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li F, Zhang H, Huang Y, Li D, Zheng Z, Xie
K, Cao C, Wang Q, Zhao X, Huang Z, et al: Single-cell transcriptome
analysis reveals the association between histone lactylation and
cisplatin resistance in bladder cancer. Drug Resist Updat.
73:1010592024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li W, Zhou C, Yu L, Hou Z, Liu H, Kong L,
Xu Y, He J, Lan J, Ou Q, et al: Tumor-derived lactate promotes
resistance to bevacizumab treatment by facilitating autophagy
enhancer protein RUBCNL expression through histone H3 lysine 18
lactylation (H3K18la) in colorectal cancer. Autophagy. 20:114–130.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Komedchikova EN, Kolesnikova OA, Syuy AV,
Volkov VS, Deyev SM, Nikitin MP and Shipunova VO: Targosomes:
Anti-HER2 PLGA nanocarriers for bioimaging, chemotherapy and local
photothermal treatment of tumors and remote metastases. J Control
Release. 365:317–330. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chen S, Xu Y, Zhuo W and Zhang L: The
emerging role of lactate in tumor microenvironment and its clinical
relevance. Cancer Lett. 590:2168372024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang S, Qi X, Liu D, Xie D, Jiang B, Wang
J, Wang X and Wu G: The implications for urological malignancies of
non-coding RNAs in the the tumor microenvironment. Comput Struct
Biotechnol J. 23:491–505. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li Y, Cao Q, Hu Y, He B, Cao T, Tang Y,
Zhou XP, Lan XP and Liu SQ: Advances in the interaction of
glycolytic reprogramming with lactylation. Biomed Pharmacother.
177:1169822024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li X, Yang Y, Zhang B, Lin X, Fu X, An Y,
Zou Y, Wang JX, Wang Z and Yu T: Lactate metabolism in human health
and disease. Signal Transduct Target Ther. 7:3052022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang JX, Choi SYC, Niu X, Kang N, Xue H,
Killam J and Wang Y: Lactic acid and an acidic tumor
microenvironment suppress anticancer immunity. Int J Mol Sci.
21:83632020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Huber V, Camisaschi C, Berzi A, Ferro S,
Lugini L, Triulzi T, Tuccitto A, Tagliabue E, Castelli C and
Rivoltini L: Cancer acidity: An ultimate frontier of tumor immune
escape and a novel target of immunomodulation. Semin Cancer Biol.
43:74–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Qu J, Li P and Sun Z: Histone lactylation
regulates cancer progression by reshaping the tumor
microenvironment. Front Immunol. 14:12843442023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Xiong J, He J, Zhu J, Pan J, Liao W, Ye H,
Wang H, Song Y, Du Y, Cui B, et al: Lactylation-driven
METTL3-mediated RNA m6A modification promotes
immunosuppression of tumor-infiltrating myeloid cells. Mol Cell.
82:1660–1677.e10. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Gu J, Zhou J, Chen Q, Xu X, Gao J, Li X,
Shao Q, Zhou B, Zhou H, Wei S, et al: Tumor metabolite lactate
promotes tumorigenesis by modulating MOESIN lactylation and
enhancing TGF-β signaling in regulatory T cells. Cell Rep.
40:1111222022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chevrier S, Levine JH, Zanotelli VRT,
Silina K, Schulz D, Bacac M, Ries CH, Ailles L, Jewett MAS, Moch H,
et al: An immune atlas of clear cell renal cell carcinoma. Cell.
169:736–749.e18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cai J, Song L, Zhang F, Wu S, Zhu G, Zhang
P, Chen S, Du J, Wang B, Cai Y, et al: Targeting SRSF10 might
inhibit M2 macrophage polarization and potentiate anti-PD-1 therapy
in hepatocellular carcinoma. Cancer Commun (Lond). 44:1231–1260.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chaudagar K, Hieromnimon HM, Khurana R,
Labadie B, Hirz T, Mei S, Hasan R, Shafran J, Kelley A, Apostolov
E, et al: Reversal of lactate and PD-1-mediated macrophage
immunosuppression controls growth of PTEN/p53-deficient prostate
cancer. Clin Cancer Res. 29:1952–1968. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sun L, Zhang H and Gao P: Metabolic
reprogramming and epigenetic modifications on the path to cancer.
Protein Cell. 13:877–919. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Jiang SS, Kang ZR, Chen YX and Fang JY:
The gut microbiome modulate response to immunotherapy in cancer.
Sci China Life Sci. 68:381–396. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Xie Y, Xie F, Zhou X, Zhang L, Yang B,
Huang J, Wang F, Yan H, Zeng L, Zhang L and Zhou F: Microbiota in
tumors: From understanding to application. Adv Sci (Weinh).
9:e22004702022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Sepich-Poore GD, Zitvogel L, Straussman R,
Hasty J, Wargo JA and Knight R: The microbiome and human cancer.
Science. 371:eabc45522021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Mischke M and Plösch T: The gut microbiota
and their metabolites: Potential implications for the host
epigenome. Adv Exp Med Biol. 902:33–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Woo V and Alenghat T: Epigenetic
regulation by gut microbiota. Gut Microbes. 14:20224072022.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Shock T, Badang L, Ferguson B and
Martinez-Guryn K: The interplay between diet, gut microbes, and
host epigenetics in health and disease. J Nutr Biochem.
95:1086312021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang Z, Chen Y, Zheng Y, Wang L, Shen S,
Yang G, Yang Y and Wang T: Quxie capsule alleviates
colitis-associated colorectal cancer through modulating the gut
microbiota and suppressing A. fumigatus-induced aerobic glycolysis.
Integr Cancer Ther. 21:153473542211385342022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Sun S, Xu X, Liang L, Wang X, Bai X, Zhu
L, He Q, Liang H, Xin X, Wang L, et al: Lactic acid-producing
probiotic saccharomyces cerevisiae attenuates ulcerative colitis
via suppressing macrophage pyroptosis and modulating gut
microbiota. Front Immunol. 12:7776652021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang J, Liu Z, Xu Y, Wang Y, Wang F, Zhang
Q, Ni C, Zhen Y, Xu R, Liu Q, et al: Enterobacterial LPS-inducible
LINC00152 is regulated by histone lactylation and promotes cancer
cells invasion and migration. Front Cell Infect Microbiol.
12:9138152022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wang SP, Rubio LA, Duncan SH, Donachie GE,
Holtrop G, Lo G, Farquharson FM, Wagner J, Parkhill J, Louis P, et
al: Pivotal roles for pH, lactate, and lactate-utilizing bacteria
in the stability of a human colonic microbial ecosystem. mSystems.
5:e00645–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Koh A, De Vadder F, Kovatcheva-Datchary P
and Bäckhed F: From dietary fiber to host physiology: Short-chain
fatty acids as key bacterial metabolites. Cell. 165:1332–1345.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Li Z, Gong T, Wu Q, Zhang Y, Zheng X, Li
Y, Ren B, Peng X and Zhou X: Lysine lactylation regulates metabolic
pathways and biofilm formation in streptococcus mutans. Sci Signal.
16:eadg18492023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wang Y, Liu Y, Xiang G, Jian Y, Yang Z,
Chen T, Ma X, Zhao N, Dai Y, Lv Y, et al: Post-translational toxin
modification by lactate controls staphylococcus aureus virulence.
Nat Commun. 15:98352024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lin J, Liu G, Chen L, Kwok HF and Lin Y:
Targeting lactate-related cell cycle activities for cancer therapy.
Semin Cancer Biol. 86:1231–1243. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Fan H, Yang F, Xiao Z, Luo H, Chen H, Chen
Z, Liu Q and Xiao Y: Lactylation: Novel epigenetic regulatory and
therapeutic opportunities. Am J Physiol Endocrinol Metab.
324:E330–E338. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhang Q, Cao L and Xu K: Role and
mechanism of lactylation in cancer. Zhongguo Fei Ai Za Zhi.
27:471–479. 2024.(In Chinese). PubMed/NCBI
|
|
101
|
De Cesare M, Pratesi G, Giusti A, Polizzi
D and Zunino F: Stimulation of the apoptotic response as a basis
for the therapeutic synergism of lonidamine and cisplatin in
combination in human tumour xenografts. Br J Cancer. 77:434–439.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Shu Y, Yue J, Li Y, Yin Y, Wang J, Li T,
He X, Liang S, Zhang G, Liu Z and Wang Y: Development of human
lactate dehydrogenase a inhibitors: High-throughput screening,
molecular dynamics simulation and enzyme activity assay. J Comput
Aided Mol Des. 38:282024. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Pan L, Feng F, Wu J, Fan S, Han J, Wang S,
Yang L, Liu W, Wang C and Xu K: Demethylzeylasteral targets lactate
by inhibiting histone lactylation to suppress the tumorigenicity of
liver cancer stem cells. Pharmacol Res. 181:1062702022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Su J, Zheng Z, Bian C, Chang S, Bao J, Yu
H, Xin Y and Jiang X: Functions and mechanisms of lactylation in
carcinogenesis and immunosuppression. Front Immunol.
14:12530642023. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Smith LE and Rogowska-Wrzesinska A: The
challenge of detecting modifications on proteins. Essays Biochem.
64:135–153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hao Y, Gu C, Luo W, Shen J, Xie F, Zhao Y,
Song X, Han Z and He J: The role of protein post-translational
modifications in prostate cancer. PeerJ. 12:e177682024. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Xin Q, Wang H, Li Q, Liu S, Qu K, Liu C
and Zhang J: Lactylation: A passing fad or the future of
posttranslational modification. Inflammation. 45:1419–1429. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Gao X, Pang C, Fan Z, Wang Y, Duan Y and
Zhan H: Regulation of newly identified lysine lactylation in
cancer. Cancer Lett. 587:2166802024. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Vétizou M, Pitt JM, Daillère R, Lepage P,
Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong
CPM, et al: Anticancer immunotherapy by CTLA-4 blockade relies on
the gut microbiota. Science. 350:1079–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Gori S, Inno A, Belluomini L, Bocus P,
Bisoffi Z, Russo A and Arcaro G: Gut microbiota and cancer: How gut
microbiota modulates activity, efficacy and toxicity of antitumoral
therapy. Crit Rev Oncol Hematol. 143:139–147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wu Z, Huang R and Yuan L: Crosstalk of
intracellular post-translational modifications in cancer. Arch
Biochem Biophys. 676:1081382019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Tomasi ML and Ramani K: SUMOylation and
phosphorylation cross-talk in hepatocellular carcinoma. Transl
Gastroenterol Hepatol. 3:202018. View Article : Google Scholar : PubMed/NCBI
|