Introduction
Plastics are widely used in modern society; every
year ~320 tons of plastics are manufactured globally (1). Plastics are used in a wide range of
applications, including packaging materials, construction,
automotive parts, electronics, medical devices, textiles and
consumer goods, due to their versatility, durability and
cost-effectiveness. These plastic products are usually resistant to
high temperature, acid, alkali and corrosion and have the benefit
of convenience due to their lightweight nature, ease of
manufacturing, versatility and cost-effectiveness in various
applications. However, there is no efficient and feasible method
for plastic degradation, which results in notable environmental
issues. The most common plastic pollutants in the environment
include polyethylene, polyvinyl chloride, polypropylene,
polyethylene terephthalate and polystyrene (2). In the natural environment, these
plastics are rarely completely degraded by microbiological
activity, radiation and mechanical stress, leading to
disintegration and fragmentation of larger plastic items into
smaller particles; transfer and diffusion are more likely to occur,
resulting in microplastic (MP) pollution of the environment.
Plastic particles are divided into MPs with diameter
<5 mm and nano-plastics (NPs) with diameter <1 µm. MPs are
found in a range of environmental domains, including air, fresh
water, soil and oceans (3). MPs
are released in numerous ways, for example as microfibers from
textiles during washing and from synthetic textiles, personal care
products, synthetic rubber tire erosion and industrial production.
After a series of environmental processes such as decomposition and
migration, they enter animals and plants, and enter the human body
via inhalation, ingestion and skin contact (4). Ingestion of MPs or plastic
derivatives such as chemical additives can cause a variety of
toxicological effects, including growth inhibition, metabolic
disorder, inflammatory response, reproductive problems and
mortality (5,6).
Studies have found MPs and NPs are present in human
blood, placenta and feces (7–9). The
ingestion of MPs is a prevalent route of exposure, with MPs being
detected in food and beverages such as seafood, drinking water and
beer (4). Exposure models in mice
have shown that MPs and NPs accumulate in the stomach, intestine,
liver and other organs (10,11).
Due to the high corrosion resistance of MPs and NPs entering into
the digestive tract, digestive fluid changes the surface roughness
and particle size of MPs and NPs, making them more stable in the
lining of the digestive tract and more prone to adsorption of toxic
substances (12). The barriers
within the tissues do not prevent invasion of MPs and NPs. After
MPs and NPs enter the body, small plastic particles can cross the
epithelial barrier of the digestive system (13–15)
and enter the lymphatic and blood circulation. For example, NPs
with a size of 0.1–10 µm cross the blood-brain barrier and the
placenta (16–18). Ingested MPs and NPs with a particle
size >150 µm pass through the intestinal epithelial cells with
difficulty, resulting in ~90% of MPs being excreted through feces,
with the rest having a localized effect outside the intestinal
epithelial cell membranes. When nanosized plastic particles with
diameter <150 µm come into contact with the villi of the small
intestine, they pass through the small intestinal epithelial cells
(19), enter the lymphatic system
(20) and bloodstream (21), and reach the portal vein through
the capillaries and are spread throughout the body (22–24).
NPs with diameter <150 and >10 µm reach other organs and cell
membranes (17), while those with
a size of <5 µm are absorbed by lymphocytes (19). Smaller nanoparticles diffuse into
the bloodstream via bypass of intercellular tight junctions
(25). Mucus secreted by the
intestinal epithelial cup cells promotes bypass diffusion of the
nanoparticles (19). Larger
nanoparticles (diameter, 50–200 nm) tend to cross intestinal
epithelial cells by endocytosis; 40 nm diameter may be the optimal
size for non-phagocytic uptake (26), while 200 nm may be the optimal size
for crossing the blood-brain barrier (27). In vivo studies have found
that intestinal cells internalize nanoscale particulate matter
using different endocytosis mechanisms; additionally phagocytes can
internalize them through phagocytosis (28), whereas non-phagocytes internalize
smaller nanoparticles with the help of lattice proteins or
cell-membrane-invasion-mediated endocytosis (25), in which actin serves an important
role (29). In addition,
energy-dependent pathways serve a key role in the mechanisms of
endocytosis in intestinal epithelial cells (29). NPs smaller than 3 µm can be
internalized into non-phagocytic cells via non-specific endocytosis
(29), while the maximum particle
size available for endocytosis increases to 5 µm (19), facilitated by the abundant M cells
in the intestinal Peyer's patches (21) and aided by the intestinal mucosal
membranes (30,31). The strong electrostatic interaction
between positively charged particles and the plasma membrane
increases surface tension, which subsequently reduces the
membrane's elasticity (32),
facilitating NPs internalization and their entry into the
bloodstream. In addition to NPs absorbed by the digestive tract,
inhaled MPs and NPs remain in the lungs or enter the circulatory
system through capillaries; particles with a size of <2.5 µm
enter the circulation or penetrate the alveoli (33). NPs that enter the circulation
(diameter, ~100 nm) are surrounded by serum albumin (34), forming a multilayered serum albumin
crown, which may help the NPs evade immune surveillance, increase
their time in the circulatory system and help the particles reach
secondary organs and accumulate in the liver, kidneys and
intestines (34). The binding of
serum albumin to NPs leads to changes in the secondary structure of
the protein (35), which increases
cytotoxicity of the plastic particles.
Although only a small percentage of NPs penetrate
the epithelial barrier of alveolar and gastrointestinal tracts, and
transfer into secondary tissues and organs (9), this low rate of internalization may
have considerable consequences due to long-term exposure of humans
to plastic particles and the potential of accumulation; harmful
effects include oxidative stress, local inflammation, cellular
apoptosis and alteration of intestinal flora (36–39).
An in vivo study showed that following MP ingestion by mice,
interaction between Helicobacter pylori and MPs in the
stomach promoted the rapid colonization of H. pylori in the
epithelial cells of the gastric mucosa (10); proliferation of this pathogenic
bacterium leads to stomach inflammation in the mice. In several
in vivo studies, MPs were found to cause intestinal flora
disorders in mice, with an increase in number of conditionally
pathogenic bacteria, accompanied by intestinal inflammation
(40–42). The effects of NPs on the liver
mainly include disruption of glycolipid metabolism, with an
increase in glucose and diabetes mellitus in NP-exposed mice
(43) and a decrease in hepatic
fat, triglycerides and total cholesterol (44). An in vitro study has found
that NPs enter cells and lead to injury effects (45). Co-culture of human gastric mucosal
epithelial cells with NPs results in decreased proliferation and
increased apoptosis (46). NPs
cause oxidative stress in human intestinal cells (47).
To the best of our knowledge, there are no studies
investigating whether MPs and NPs pass through the food chain to
the human body; however, in vitro and ex vivo studies
reveal the adverse effects of MPs and NPs in the human body
(4,8,9).
Investigating the mechanism of injury effects of MPs and NPs is key
for understanding the impact of MPs and NPs on health, as well as
for prevention and treatment of MP- and NP-induced health
problems.
The present review discusses the mechanisms by which
MPs and NPs damage the human gastrointestinal tract and liver and
limitations of existing research and suggests future research
directions to provide a scientific foundation for investigation of
the effects and mechanisms of MPs and NPs on the human body.
Literature search
A comprehensive online search using PubMed
(https://pubmed.ncbi.nlm.nih.gov/),
Embase (https://www.embase.com/), the Cochrane
Library (https://www.cochranelibrary.com/) and the
International Clinical Trials Registry Platform (https://clinicaltrials.gov/) was performed from their
inception to January 2024 with the following MeSH and EMTREE
keywords: ‘Micro-plastics’, ‘nano-plastics’, ‘gastrointestinal
disease’, ‘liver/hepatic metabolism’, ‘MPs’, ‘NPs’ and ‘digestive
diseases’. All published studies associated with MPs and/or NPs,
gastrointestinal disease or liver metabolism were included. Studies
were excluded if they did not focus on MPs and/or NPs in the
context of gastrointestinal diseases or liver metabolism, or if
they were not published in peer-reviewed journals. Two independent
investigators conducted the literature searches and eligibility
assessment, and discrepancies were resolved by consensus and
consultation with a third reviewer.
Mechanism of oxidative stress, inflammation
and apoptosis in the gastrointestinal tract
MPs and NPs enter cells through endocytosis
mechanisms or become adsorbed and accumulate on the surface of
gastrointestinal tissue, causing oxidative stress, inflammation and
apoptosis (Tables I and II). Therefore, it is important to
investigate the mechanism underlying injury effects of MPs and NPs
on the gastrointestinal tract to provide a scientific basis for
prevention and treatment of gastrointestinal diseases caused by MPs
and NPs.
 | Table I.Injury effects and mechanism of
microplastics and nano-plastics in vivo in mice. |
Table I.
Injury effects and mechanism of
microplastics and nano-plastics in vivo in mice.
|
|
| Microplastic |
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Organ | Mouse strain | Type | Size, µm | Exposure route | Dosage | Biomarker | Effect | Mechanism | (Refs.) |
|---|
| Stomach | Balb/c | PE | 10-150 | Intragastric
administration (3 times, 1 week) | 100 µg/ml | IL6, MPO and
TNF-α | Rapid colonization
of Helicobacter pylori in gastric mucosal epithelial cells,
gastric injury and inflammation | Increased
expression of myeloperoxidase, IL-6 and TNF-α | (10) |
| Liver, colon, ileum
and cecum | ICR | PS | 5 | Water consumption
for ~6 weeks | 100, 1,000
µg/l | TG, PYR, TCH, GLU,
HDL-C, LDL-C, CPT1, CPT2 | Disorder of lipid
metabolism, intestinal shielding dysfunction | Changes in
intestinal flora | (11) |
| Colon and
duodenum | C57BL/6 | PE | 10-150 | Consumption of
MP-enriched feed for 5 weeks | 6, 60, 600
µg/day | IL-1α, G-CSF, IL-2,
IL-5, IL-6, IL-9, IP-10 and RANTES | Intestinal
inflammation | Upregulation of
IL-1α, TLR4, AP-1 and IRF5, changes in intestinal
flora, immune imbalance | (40) |
| Colon | C57BL/6 | PS | 5 | Water consumption
for 28 days | 500 µg/day | TNF-α, IL-1β and
IL-6 | Impaired intestinal
barrier and intestinal inflammation, increased intestinal
pathogenic bacteria, interference with intestinal microbial
metabolism | Immune imbalance,
changes in intestinal flora, upregulation of inflammatory factors
(TNF-α, IL-1β and IFN-γ) | (41) |
| Intestines and
cecum | CD-1 | PE | 45-53 | Oral gavage daily
for 30 days | 5.24×104
particles/day | DAO, D-Lac | Increased
intestinal permeability, intestinal inflammation, metabolic
disorder | Changes in
composition of intestinal flora, decreased abundance of bacteria
associated with energy metabolism and immune function;
downregulation of genes associated with oxidative stress, immune
response and lipid metabolism | (42) |
| Liver and
intestine | ICR | PS | 1 | Water consumption
for 1–2 weeks | 55 µg/d | CAT, SOD,
GSH-Px | Insulin resistance,
diabetes mellitus | Metabolic crosstalk
of gut-liver axis | (43) |
| Liver, colon,
ileum | ICR | PS | 5 | Water consumption
for 6 weeks | 100, 1,000
µg/l | TG, TCH, PYR, TBA,
CFTR and NKCC1 | Decreased secretion
of intestinal mucus, damage to intestinal barrier function,
dysbiosis of the gut microbiome and metabolic disorder | NR | (44) |
| Liver and
cecum | ICR | PS | 0.5 and 50 | Water consumption
for 5 weeks | 100, 1,000
µg/l | TG, TCH, GLU,
TBA | Decreased liver
weight, secretion of intestinal mucus, TG and TCH levels and
hepatic lipid disorder | Changes in
intestinal flora, decreased mRNA levels of genes involved in the
synthesis of fats and TG in the liver | (63) |
| Liver | C57BL/6J | PS | 0.5 | Water consumption
for 28 days | 0.5 mg/day | AST, ALB, ALT,
TBIL | Affect the liver
immune microenvironment, hepatic inflammation | Increased immune
infiltration of NK cells and macrophages and decreased immune
infiltration of B cells, increased expression of ALT and aspartate
aminotransferase, activation of the NF-κB signaling pathway | (85) |
| Intestine and
colon | C57/B6 | PS | 5 | Water consumption
for 28 days | 0.2 mg/kg | ZO-1,
claudin-1-3 | Intestinal barrier
dysfunction, increased opportunistic pathogens and decreased tight
junction promoting functional microorganisms | Downregulation of
tight junction protein expression, changes in intestinal flora | (86) |
 | Table II.Injury effects and mechanism of
microplastics and nano-plastics in human cells. |
Table II.
Injury effects and mechanism of
microplastics and nano-plastics in human cells.
|
| Microplastic |
|
|
|
|
|
|
|---|
|
|
| Dosage, Cell | Duration of
Type |
|
|
|
|
|---|
| Size | µg/ml | exposure | Biomarkers | Effects | Mechanism | (Refs.) |
|---|
| Human colon
adenocarcinoma Caco-2, HT29-MTX | PS | 25 and 100 nm | 0-200;
0.01–100 | 4 h | LDH, ROS | Decreased cell
viability, oxidative stress, inflammation, mitochondrial
apoptosis | Upregulation of
HSP70, HO1 and IL- 1β, increased mitochondrial membrane
potential | (15,52) |
| Liver organoids H1
ES | PS | 1 µm | 0.25–25 | 48 h | LDH, ATP, CYP3A4
activity, COL1A1, IL-6, ALT, AST, TG, GSH, GST, SOD | Disrupted function
of metabolic enzymes, increased lipid accumulation, ROS production,
oxidative stress and inflammatory response, hepatotoxicity | Increased release
of ASL and ALT, gene expression associated with disrupt liver
function, expression of HNF4A, CYP2EI, IL-6 and
COL1AI | (39) |
| Human gastric
mucosal epithelial GES-1 | PS | NR | 100 | 24 h | RhoA, Rac1,
F-actin, Rab5, RAB7, LAMP1, LC3B II | Decreased cell
proliferation rate, increased apoptosis | NR | (46) |
| Human colon
adenocarcinoma Caco-2 | PS | 50 nm | 100 | 24 h; 8 weeks | ROS, HO1, GSTP1,
HSP70, SOD2 | Oxidative
stress | HO1 and
SOD 2 transcript levels were significantly increased | (47) |
| Human gastric
adenocarcinoma CRL1739 | PS | 100, | 0.1–100 44 nm | 1 h | c-Myc, ERK-1, Ki67,
CCNE1, CCND1, p38, p53, IL8, IL6, IL-1β, TGF-β1, NF-κB1, HPTR1 | Decreased cell
viability and morphology and inflammation | Upregulation of
IL-6 and IL-8 | (58) |
| Human gastric
carcinoma AGS | PS | 60 and 500 nm | 0.1–100 | 24 h | ROS, apoptotic
protein | Decreased cell
viability; increased apoptosis or necrosis | Disruption of cell
membrane integrity, upregulation of Bax, increased
expression of Caspase-3 and Caspase-8 | (60) |
Reactive oxygen species (ROS) in cells
induce generation of oxidative stress
Cells possess an antioxidant defense system that
maintains intracellular ROS levels and protects biomolecules from
free radical damage (48,49). Increased ROS and oxidative stress
in cells are associated with antioxidant system imbalance and
disease (50). In vitro and
in vivo studies have shown that MPs and NPs increase
intracellular ROS levels (16,31).
The direct stimulatory effect of exogenous particles increases
intracellular ROS production (51). However, MPs and NPs inhibit
production of antioxidant enzyme transcription factors or decrease
activity of antioxidant enzymes, which in turn inhibits ROS
metabolism and increases mitochondrial membrane potential resulting
in an increase in mitochondrial permeability and ROS production,
thus increasing mitochondrial ROS production; this increases
transfer of ROS produced in mitochondria to the cytoplasm (52). Superoxide dismutase (SOD), catalase
(CAT) and glutathione (GSH) are key biomarkers for measuring the
degree of oxidative stress. Polystyrene NPs can lead to increased
levels of peroxidative biomarkers and markedly decreased SOD, CAT
activity and GSH in the duodenum of mice (53,54);
in vitro experiment using human normal colonic mucosal
epithelial cells has revealed that ROS levels in NP-treated cells
are increased compared with those in untreated cells (53). Therefore, MPs and NPs directly
promote ROS production or indirectly inhibit ROS metabolism by
inhibiting antioxidant enzyme activity and GSH production, leading
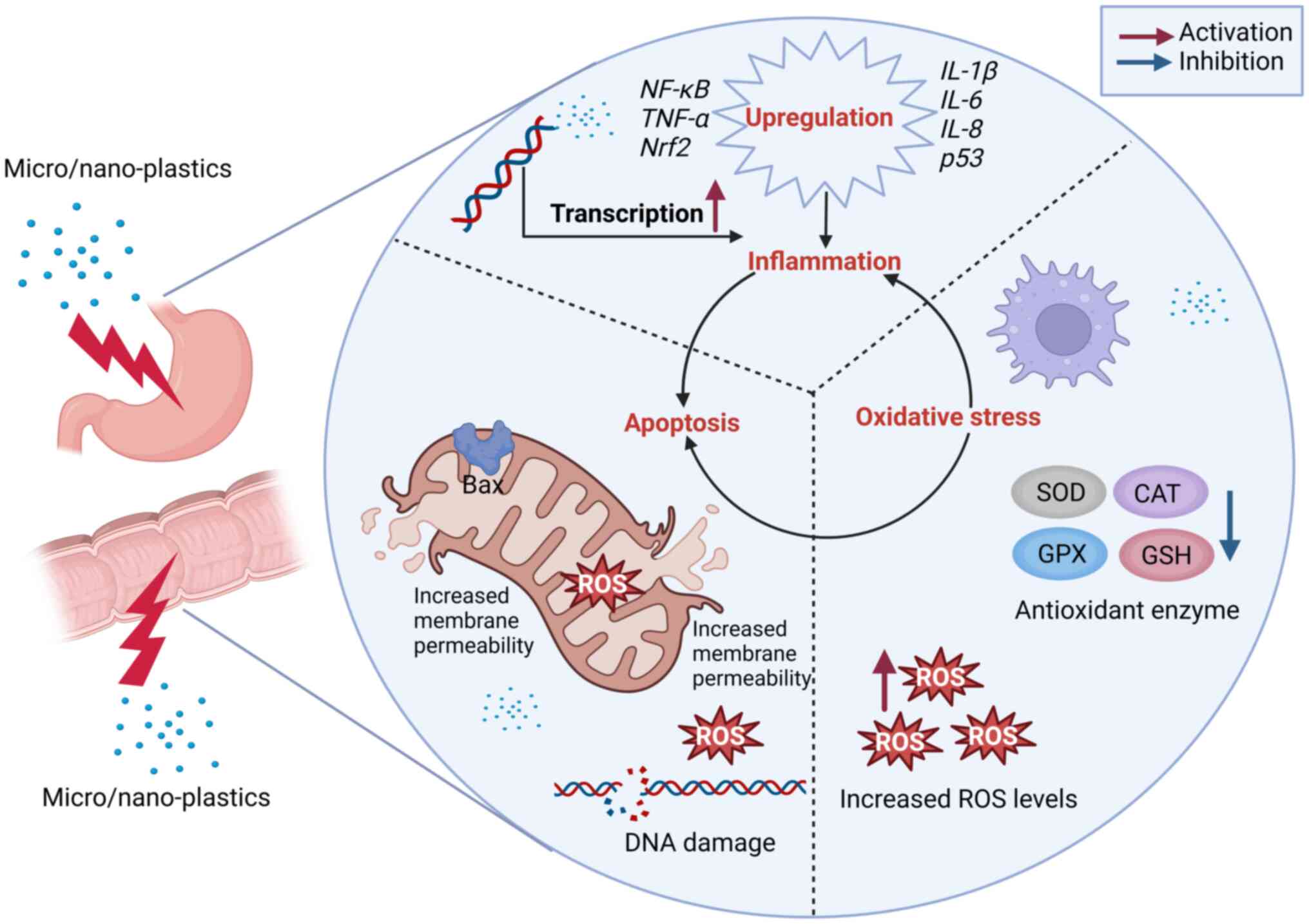
to an increase in ROS (Fig. 1). As
MPs and NPs interact with the cellular microenvironment, increased
ROS settle on the surface of MPs and NPs, leading to oxidative
stress in the cell, which induces localized inflammation in the gut
if MPs and NPs are unable to cross the cellular membrane (55); if the particles are small enough to
cross the intestinal epithelium, ROS toxicity on the surface of
particles is enhanced, mediating a stress response in cells
(56).
Underlying mechanisms of
inflammation
Following ingestion, MPs and NPs accumulate in the
gastrointestinal tract. Mechanical damage or stimulation induced by
MPs and NPs causes inflammation in the gastrointestinal tract
(10,40) due to release of proinflammatory
cytokines (10) or imbalance of
intestinal flora, causing an increase in conditionally pathogenic
bacteria and resulting in immune imbalance and an increase in
lipopolysaccharide content (42,57).
MPs and NPs induce proinflammatory cytokine release
via direct stimulation of proinflammatory cytokine production. An
in vivo study revealed that elevated IL-6 and TNF-α promote
gastric injury and inflammation (10). In vitro studies reveal that
expression of proinflammatory genes such as IL-1β, −6 and −8 is
increased in MP- and NP-treated gastric and small intestinal
epithelial cells, resulting in increased release of proinflammatory
cytokines (15,58). Another mechanism involves oxidative
stress promoting inflammation by activating transcription factors
such as NF-κB, p53, peroxisome proliferator-activated receptor
(PPAR)-γ and nuclear factor erythroid 2-related factor 2 (59), which regulate the expression of
inflammatory cytokines and thus increase release of proinflammatory
cytokines (Fig. 1).
In vivo studies have shown that MPs and NPs
lead to gastrointestinal tract injury in mice (9,15–17).
MPs and NPs promote rapid colonization of H. pylori on the
epithelial cell surface of the gastric mucosa, increase the
efficiency with which NPs enter tissues and promote inflammation
(10). MPs and NPs cause
intestinal dysbiosis, a marked decrease in abundance of immune
function-associated bacteria (42), an increase in the number of
pathogenic bacterial colonies and a decrease in the number of
CD4+ T helper 17 and regulatory T cells, leading to an
immune imbalance, as well as an increase in plasma
lipopolysaccharides (57), which
stimulate intestinal inflammation (41).
Potential mechanisms of apoptosis
Both endogenous and exogenous factors contribute to
DNA damage, and NPs can cross the nuclear membrane, directly
inducing DNA damage (52). In
addition, oxidative stress caused by increased intracellular ROS
levels due to MPs and NPs can lead to DNA damage. If DNA damage is
not repaired rapidly, apoptosis is induced (59). Apoptosis induced by oxidative
stress is observed in in vitro studies (15,60),
accompanied by an increase in mitochondrial membrane potential
(Fig. 1). A study using HaCaT
cells found that under conditions simulating oxidative stress in
vitro, an increase in intracellular expression of inverted
formin-2 leads to ROS overload in mitochondria, which disrupts
cellular redox balance, alters mitochondrial membrane potential,
causes mitochondrial stress and inhibits the hypoxia inducible
factor-1 signaling pathway to mediate apoptosis (61). Bax, a member of the Bcl-2 family,
regulates the release of apoptosis-inducing factors and the
permeability of the outer mitochondrial membrane, with its
overexpression potentially triggering apoptosis (60). Increased expression of Bax
increases permeability of the mitochondrial membrane, leading to
the release of apoptosis-inducing factors from the mitochondria
into the cytoplasm, activating cysteoaspartic enzymes and leading
to apoptosis. N-terminal acetylation of Bax is involved in its
mitochondrial targeting; increase in expression of the Bax
gene leads to an increase in permeability of the mitochondrial
membrane, which results in the release of ROS from the
mitochondria; this leads to ROS accumulation in cells, triggering
apoptosis (Fig. 1). In addition,
the inflammatory response caused by MPs and NPs triggers
apoptosis.
In summary, increased ROS production or decreased
ROS metabolism leads to accumulation of intracellular ROS resulting
in DNA damage and oxidative stress. Immune imbalance caused by
gastrointestinal flora dysbiosis and increased expression of
inflammation-associated cytokines lead to inflammation. Oxidative
stress and inflammation lead to apoptosis. MPs and NPs overexpress
pro-apoptosis-related genes, directly leading to apoptosis
(Fig. 1).
Mechanism of liver glucose and lipid
metabolism disorder
The liver is a key detoxification organ in the human
body. MPs and NPs accumulate on the surface of epithelial cells in
the gastrointestinal tract following ingestion. NPs are absorbed by
epithelial cells and they then enter the lymphatic and blood
circulation, arriving at the liver through the portal vein
(62). Study has also found that
NPs disturb the glucose-lipid metabolism of liver tissues (63), and similar toxic effects have been
detected in an in vitro study of human liver-like organs.
Toxic effects of NPs were also found in in vitro human
liver-like organs (6). Previous
biochemical and transcriptomics studies have investigated the
injury mechanism of NPs causing disruption of glycolipid metabolism
in liver tissue (11,43,57)
and found that NPs affect glycolipid metabolism at both biochemical
and transcriptional levels. NPs cause injury due to effects on
intermediate glycolipid metabolism at the biochemical level and
production of key rate-limiting enzymes in glycolipid metabolism at
the transcriptional level.
Effect of NPs on production of
intermediate metabolites for glycolipid metabolism
NPs affect glycolipid metabolism by influencing the
production of intermediate metabolites. Pyruvate is a key
intermediate metabolite in the glycolytic pathway and creates a
notable association between glucose and lipid metabolism (64). Its increased production may be due
to elevated levels of pyruvate kinase (PK) and phosphoenolpyruvate
carboxykinase (64,65), which may promote the conversion of
glucose to lipid metabolism and lead to increased production of
fatty acids. Elevated levels of glucose and cholesterol in the
liver may increase risk of type II diabetes, hyperlipidemia and
fatty liver disease (64). A study
revealed that biochemical levels of important factors and catalytic
enzymes (Aldh9a1a, Aldh2b, Ehhadh and Echs1) involved in regulation
of glucose metabolism in liver tissues are altered after the
ingestion of NPs (65). The
expression of carbohydrate regulatory element-binding protein
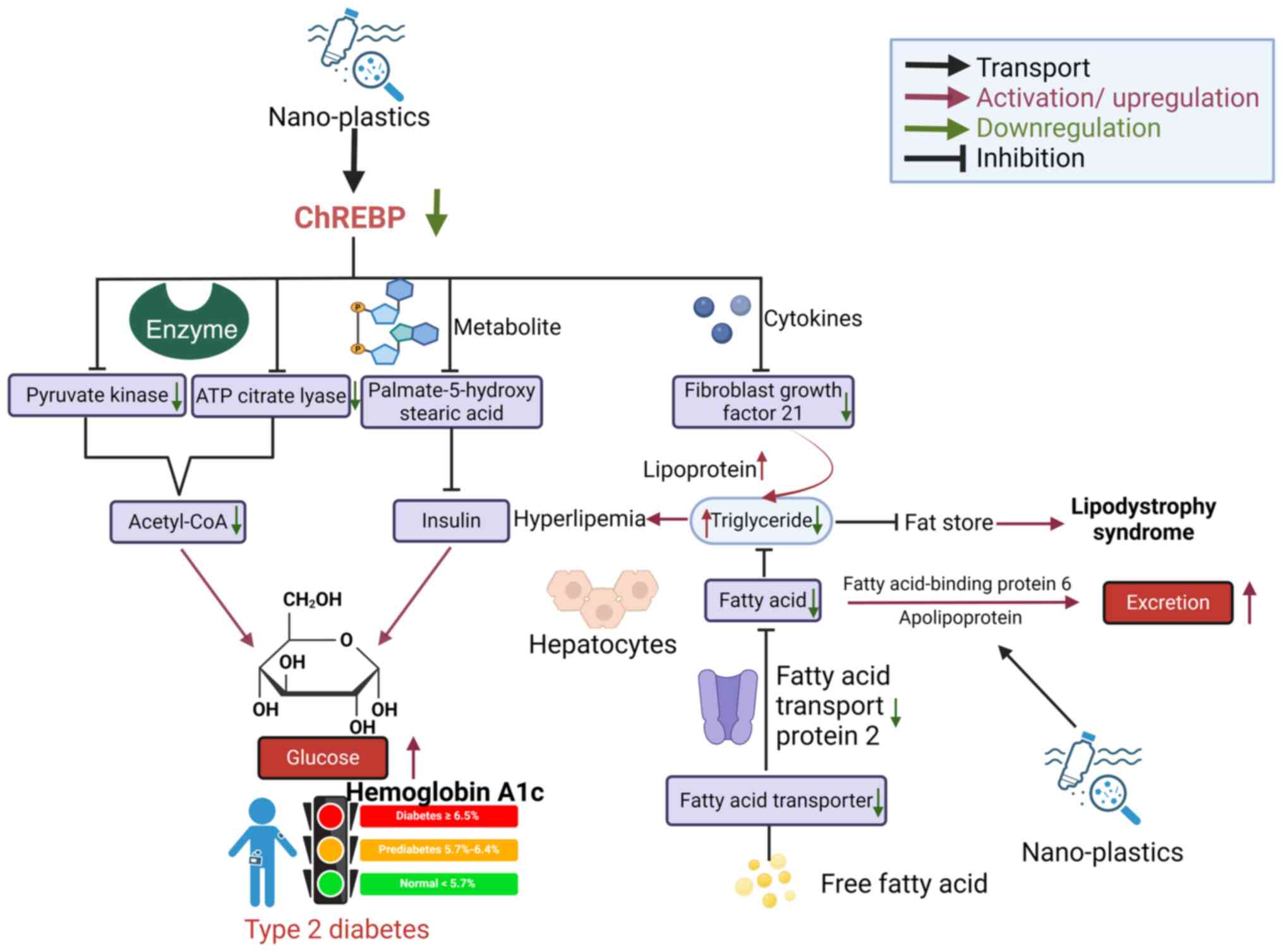
(ChREBP) (63), which prevents the
conversion of glucose to acetyl coenzyme A by inhibiting production
of PK and ATP-citrate lyase (ACL) in the liver cells of mice
following ingestion of NPs, is considerably reduced, resulting in a
marked decrease in glucose metabolism. By inhibiting production of
PK and ACL, ChREBP prevents conversion of glucose to acetyl
coenzyme A, leading to the accumulation of glycogen in the liver
and increasing the risk of type II diabetes (66). In addition, decrease in ChREBP
synthesis also leads to a decrease in the synthesis of
palmitic-5-hydroxystearic acid, which has been shown to increase
insulin sensitivity in adipose tissue (67) and insulin secretion through
activation of G protein-coupled receptor 40 (68); decrease in the expression of ChREBP
as a direct result of NPs indirectly inhibits insulin sensitivity
and secretion. Therefore, NPs indirectly inhibit insulin
sensitivity and secretion and hinder the glycolysis pathway leading
to glucose metabolism disorders (69). NPs can increase the activities of
lactate dehydrogenase and citrate synthase (CS), the key enzymes
participating in glycolysis and gluconeogenesis (69). This leads to glucose metabolism
disorder (Fig. 2), but the
specific mechanism of the influence of NPs on enzyme activities is
unclear (70).
In terms of lipid metabolism, NPs decrease
expression of ChREBP, leading to a decrease in fibroblast growth
factor 21 (FGF21) synthesis in hepatocytes, which inhibits the role
of FGF21 in decreasing plasma triglycerides by increasing
catabolism of lipoproteins in adipose tissue. Thus, plasma
triglycerides build up, leading to an increased risk of
hyperlipidemia in humans (71,72).
Free fatty acids from the blood enter hepatocytes to synthesize
fatty acids in liver tissues. However, a study revealed that
synthesis of fatty acid transporter (FAT) protein 2 and FAT was
reduced after NP treatment of hepatocytes (63), preventing transport of fatty acids
from the blood to the liver, indirectly impeding synthesis of fatty
acids in the liver. Another study revealed that the synthesis of
ApoE and fatty acid-binding protein 6 was decreased after treatment
of hepatocytes with NPs (73), and
the synthesis of fatty acids in the liver is indirectly impeded.
Therefore, the decreased levels of fatty acids in the liver leads
to insufficient synthesis of triglycerides, which indirectly
affects storage of fat. The lack of fat storage may lead to
lipodystrophy syndrome (73).
Lipodystrophy syndrome is a metabolic disorder that leads to
metabolic complications similar to those observed in obese
patients, such as those with insulin resistance, diabetes mellitus,
hepatic steatosis and dyslipidemia (74).
In summary, in glucose metabolism, NPs inhibit
synthesis of ChREBP, impede the conversion of glucose to
acetyl-coenzyme A and inhibit the sensitivity and secretion of
insulin. Taken together, these lead to the accumulation of glucose
causing disorders of glucose metabolism. In lipid metabolism, NPs
inhibit the production of fatty acids and simultaneously facilitate
the transport of fatty acids out of the cell, indirectly leading to
a decrease in triglyceride content and fat storage (Fig. 2).
NPs affect production of intermediate
metabolites for glycolipid metabolism at the transcription
level
Studies have revealed that NPs can affect key
rate-limiting enzymes involved in glucose metabolism, including
hexokinase 1 (HK1), PK and CS. In a study, zebrafish were given
polystyrene MPs and the liver tissue was extracted for
transcriptome analysis; transcript levels of PK were markedly
decreased in the experimental compared with those in the control
group, while the transcript levels of PK1 markedly increased in the
experimental group (65). HK1 is a
member of the hexokinase family that catalyzes the conversion of
glucose to fructose; increase of the HK1 transcript levels results
in an increase in the synthesis of HK1 protein, which increases
glucose conversion to fructose (74). Decreased levels of PK inhibit the
conversion of fructose to pyruvate, leading to the accumulation of
fructose; the accumulated fructose reaches the intestinal tract
through the blood circulation and accumulates in the intestine,
where it is used by the intestinal flora to produce acetate
(74). The acetate reaches the
liver through the portal vein and is converted into acetyl coenzyme
A, which is used as a substrate for lipogenesis, resulting in an
increase of adipogenesis (75). CS
is a key enzyme in the tricarboxylic acid cycle, converting
oxaloacetic to citric acid. Transcriptome analysis has revealed
that MPs lead to a decrease in the transcription of CS, leading to
a decrease in the synthesis of oleanic and α-ketoglutaric acid
(76), which affects the
tricarboxylic acid cycle and leads to disorders of glucose and
lipid metabolism.
Influencing fatty acid synthesis and β-oxidation at
the transcriptional level is another mechanism by which NPs affect
lipid metabolism. Fatty acid synthesis and β-oxidation are key
components of glycolipid metabolism (76). When studying the effects of NPs on
hepatic lipid metabolism, key enzymes involved in fatty acid
synthesis and β-oxidation, such as SLC27A, ACS and CPT1A, serve as
important biomarkers (77). The
transcript levels of the relevant genes are examined to investigate
the effects of NPs on glycolipid metabolism pathways and the
signaling pathways involved (78,79).
Studies have shown that NPs promote fatty acid synthesis by
promoting transcription of mRNAs for acetyl coenzyme A carboxylase
1, sterol regulatory element-binding protein 1α and fatty acid
synthase (77). NPs inhibit fatty
acid β-oxidation by suppressing the transcription of mRNAs for
acetyl coenzyme A oxidase and cotinine palmitoyl transferase 1
oxidation of fatty acids (78).
PPAR-α and -γ are ligand-activated receptors in the nuclear hormone
receptor family that serve as transcriptional activator proteins to
regulate expression of oxidative enzymes in peroxisomes, which
contain a variety of oxidative enzymes involved in various types of
metabolism, including β-oxidation of fatty acids, bile acids and
cholesterol metabolism (80). NPs
affect the PPAR signaling pathway by increasing the transcript
levels of PPAR-α and -γ. Elevation of the transcript levels of
PPAR-α leads to an increase in the amount of oxidative enzymes in
peroxisomes, which promotes β-oxidation of fatty acids, bile acid
and cholesterol metabolism (77).
A marked increase in size and number of peroxisomes in the liver
can lead to hepatic hypertrophy, hyperplasia and hepatocellular
carcinoma (77). PPAR-γ is
involved in the differentiation and maturation of adipocytes
(81); increases of its
transcriptional level promotes the synthesis of fats, contributing
to disorders of lipid metabolism. Diacylglycerol acyltransferase
(DGAT) is a key enzyme in the synthesis of triglycerides and lipid
droplets in adipocytes; DDGAT serves an important role in the
regulation of lipid metabolism (82). NPs inhibit mRNA transcription of
DGAT, resulting in the reduction of the expression of DGAT,
inhibition of the formation of lipid droplets and fatty acids and
the reduction of lipid storage. DGAT2-deficient mice died soon
after birth due to the severe reduction in energy metabolism
substrates and impaired skin permeability barrier function
(83). Therefore, inhibition of
DGAT mRNA transcription by NPs not only affects lipid metabolism,
but also causes damage to the skin permeability barrier function,
which is harmful to human health.
In summary, after MPs and NPs enter the human body
by ingestion, NPs reach the liver through the circulatory system
and cause disorders in glucose and lipid metabolism of the liver
including enlargement, hyperplasia, type II diabetes,
hyperlipidemia and lipodystrophy syndrome and may contribute to
occurrence of hepatocellular carcinoma.
Discussion
As MPs and NPs are widely located in the biosphere,
their impact on human health is of concern. Studies have
demonstrated that humans are continuously exposed to MPs and NPs by
inhalation or ingestion (4–6).
When MPs and NPs enter the human body, larger particles are
eliminated in feces, while smaller particles are processed by
gastric juices and intestinal mucus, accumulating in the
gastrointestinal tract (84) where
they are absorbed into the cells (16). Current research suggests that a
small proportion of particles cross the lung and intestinal
barriers and accumulate in tissues and organs. Particles with
diameter <150 µm are able to travel from the intestinal lumen to
the lymphatic and circulatory system, accumulating in tissues
throughout the body, including the liver, kidney and brain,
producing various toxic effects (62). MPs and NPs induce oxidative stress
in cells by two methods: Direct stimulation of intracellular ROS
production and inhibition of antioxidant enzymes and GSH synthesis,
resulting in ROS metabolism (50).
The inflammatory response in the gastrointestinal tract is
primarily induced by direct stimulation of phagocytosis to secrete
proinflammatory cytokines and disruption of intestinal bacterial
flora, which leads to an increase in the number of conditionally
pathogenic bacteria (41).
Inflammation, oxidative stress and DNA damage caused by NPs
entering cells activate the apoptotic signaling pathway, leading to
cell death. At the biochemical level, NPs mainly affect the
production and metabolism of glucose, triglycerides and fatty
acids, while at the transcriptional level, NPs primarily affect
production of rate-limiting enzymes of glycolipid metabolism,
leading to disorders of glycolipid metabolism. The gastrointestinal
toxicity effects of MPs and NPs and effects on hepatic glucose
metabolism increase the risk of gastroenteritis, hyperglycemia,
diabetes mellitus, hepatic hypertrophy, hyperlipidemia and
lipodystrophy, posing a threat to human health (79).
Studies on toxic effects and mechanisms of MPs and
NPs on the gastrointestinal tract and liver are based on human
cells, rodents and aquatic species (43,59).
Although the aforementioned studies have provided evidence of the
possible toxic effects of MPs and NPs on the gastrointestinal tract
and liver in humans, there is lack of knowledge regarding the
absorption, metabolism and excretion of MPs and NPs in the human
body. Additionally, the ability of MPs and NPs to cross the human
tissue barrier is unclear; further studies are needed to
investigate the mechanisms by which MPs and NPs cross the
gastrointestinal barrier and the mechanisms underlying the toxic
effects caused by MPs and NPs. Studies have found that MPs and NPs
impair intestinal barrier function by decreasing intestinal mucus
secretion, inhibiting synthesis of tight junction proteins,
increasing intestinal permeability and causing disruption of
intestinal flora (38,41,47).
To the best of our knowledge, however, there is still a lack of
research on the specific mechanisms by which MPs and NPs impair the
gastrointestinal barrier.
In vitro studies investigating the toxic
effects of MPs and NPs on the gastrointestinal tract and liver use
concentrations of MPs and NPs that are higher than the actual
concentrations humans are exposed to in real life (15,20,30).
Therefore, there is a need to understand the toxic metabolism
kinetics of MPs and NPs within the context of the actual
concentrations to which humans are exposed. Furthermore, there are
differences in the immunological capacity and immune status between
individuals that should be considered when assessing toxicological
effects in humans.
An increasing number of studies have found that MPs
and NPs affect the immune system, as evidenced by the induction of
intestinal flora dysbiosis by MPs and NPs, leading to immune
imbalance and uptake of NPs by lymphocytes (40,57,85).
However, studies of toxic effects of MPs and NPs on immune cells
are limited, and there is lack of studies investigating the toxic
effects of MPs and NPs on the immune system as a whole (59). The gut microbiota, which is not
only an important component of immune and metabolic health but also
affects the central nervous system, has been shown to communicate
through several pathways of the ‘brain-gut axis,’ as identified
using animal models (44,63,86).
Therefore, the toxic effects and mechanisms of MPs and NPs on the
brain-gut axis following gut flora disruption should be further
investigated.
The gastrointestinal tract and liver are key organs
for absorption, metabolism and detoxification. The harmful effects
of MPs and NPs involve the intestinal-hepatic axis, causing
oxidative stress, inflammation, apoptosis and disorders of hepatic
glucose and lipid metabolism in the gastrointestinal tract,
resulting in gastroenteritis, hyperglycemia and hyperlipidemia
(43). MPs and NPs also indirectly
affect the brain-gut axis through the intestinal flora. Therefore,
the toxic effects of MPs and NPs on the gastrointestinal tract and
liver and their mechanisms should be investigated further.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
LZ and YXH conceived the study. LZ and LDR performed
the literature review and data analysis. LZ, YFH and YXH
contributed to the critical revision of the manuscript. LZ, LDR,
YFH and YXH wrote the manuscript and coordinated the revisions.
Data authentication is not applicable. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ragusa A, Svelato A, Santacroce C,
Catalano P, Notarstefano V, Carnevali O, Papa F, Rongioletti MCA,
Baiocco F, Draghi S, et al: Plasticenta: First evidence of
microplastics in human placenta. Environ Int. 146:1062742021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rochman CM, Hoh E, Hentschel BT and Kaye
S: Long-term field measurement of sorption of organic contaminants
to five types of plastic pellets: Implications for plastic marine
debris. Environ Sci Technol. 47:1646–1654. 2013.PubMed/NCBI
|
|
3
|
Wang W, Ge J, Yu X and Li H: Environmental
fate and impacts of microplastics in soil ecosystems: Progress and
perspective. Sci Total Environ. 708:1348412020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prata JC, da Costa JP, Lopes I, Duarte AC
and Rocha-Santos T: Environmental exposure to microplastics: An
overview on possible human health effects. Sci Total Environ.
702:1344552020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pironti C, Ricciardi M, Motta O, Miele Y,
Proto A and Montano L: Microplastics in the environment: intake
through the food web, human exposure and toxicological effects.
Toxics. 9:2242021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thomas PJ, Perono G, Tommasi F, Pagano G,
Oral R, Burić P, Kovačić I, Toscanesi M, Trifuoggi M and Lyons DM:
Resolving the effects of environmental micro- and nanoplastics
exposure in biota: A knowledge gap analysis. Sci Total Environ.
780:1465342021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amereh F, Amjadi N, Mohseni-Bandpei A,
Isazadeh S, Mehrabi Y, Eslami A, Naeiji Z and Rafiee M: Placental
plastics in young women from general population correlate with
reduced foetal growth in IUGR pregnancies. Environ Pollut.
314:1201742022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leslie HA, van Velzen MJM, Brandsma SH,
Vethaak AD, Garcia-Vallejo JJ and Lamoree MH: Discovery and
quantification of plastic particle pollution in human blood.
Environ Int. 163:1071992022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwabl P, Köppel S, Königshofer P,
Bucsics T, Trauner M, Reiberger T and Liebmann B: Detection of
various microplastics in human stool: A prospective case series.
Ann Intern Med. 171:453–457. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tong X, Li B, Li J, Li L, Zhang R, Du Y
and Zhang Y: Polyethylene microplastics cooperate with Helicobacter
pylori to promote gastric injury and inflammation in mice.
Chemosphere. 288((Pt 2)): 1325792022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo T, Wang C, Pan Z, Jin C, Fu Z and Jin
Y: Maternal polystyrene microplastic exposure during gestation and
lactation altered metabolic homeostasis in the dams and their F1
and F2 offspring. Environ Sci Technol. 53:10978–10992. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Wang Y, Xu M, Ma J, Zhang S, Liu
S, Wang K, Tian H and Cui J: Enhanced hepatic cytotoxicity of
chemically transformed polystyrene microplastics by simulated
gastric fluid. J Hazard Mater. 410:1245362021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yee MS, Hii LW, Looi CK, Lim WM, Wong SF,
Kok YY, Tan BK, Wong CY and Leong CO: Impact of microplastics and
nanoplastics on human health. Nanomaterials (Basel). 11:4962021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hesler M, Aengenheister L, Ellinger B,
Drexel R, Straskraba S, Jost C, Wagner S, Meier F, von Briesen H,
Büchel C, et al: Multi-endpoint toxicological assessment of
polystyrene nano- and microparticles in different biological models
in vitro. Toxicol In Vitro. 61:1046102019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cortés C, Domenech J, Salazar M, Pastor S,
Marcos R and Hernández A: Nanoplastics as a potential environmental
health factor: Effects of polystyrene nanoparticles on human
intestinal epithelial Caco-2 cells. Environ Sci Nano. 7:272–285.
2020. View Article : Google Scholar
|
|
16
|
Dong X, Liu X, Hou Q and Wang Z: From
natural environment to animal tissues: A review of
microplastics(nanoplastics) translocation and hazards studies. Sci
Total Environ. 855:1586862023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Campanale C, Massarelli C, Savino I,
Locaputo V and Uricchio VF: A detailed review study on potential
effects of microplastics and additives of concern on human health.
Int J Environ Res Public Health. 17:12122020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barboza LGA, Dick Vethaak A, Lavorante
BRBO, Lundebye AK and Guilhermino L: Marine microplastic debris: An
emerging issue for food security, food safety and human health. Mar
Pollut Bull. 133:336–348. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bouwmeester H, Hollman PC and Peters RJ:
Potential health impact of environmentally released micro- and
nanoplastics in the human food production chain: Experiences from
nanotoxicology. Environ Sci Technol. 49:8932–8947. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Domenech J, Hernández A, Rubio L, Marcos R
and Cortés C: Interactions of polystyrene nanoplastics with in
vitro models of the human intestinal barrier. Arch Toxicol.
94:2997–3012. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hussain N, Jaitley V and Florence AT:
Recent advances in the understanding of uptake of microparticulates
across the gastrointestinal lymphatics. Adv Drug Deliv Rev.
50:107–142. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eldridge JH, Meulbroek JA, Staas JK, Tice
TR and Gilley RM: Vaccine-containing biodegradable microspheres
specifically enter the gut-associated lymphoid tissue following
oral administration and induce a disseminated mucosal immune
response. Adv Exp Med Biol. 251:191–202. 1989.PubMed/NCBI
|
|
23
|
Jani PU, McCarthy DE and Florence AT:
Nanosphere and microsphere uptake via Peyer's patches: Observation
of the rate of uptake in the rat after a single oral dose. Int J
Pharm. 86:239–246. 1992. View Article : Google Scholar
|
|
24
|
Volkheimer G: Hematogenous dissemination
of ingested polyvinyl chloride particles. Ann N Y Acad Sci.
246:164–171. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Banerjee A and Shelver WL: Micro- and
nanoplastic induced cellular toxicity in mammals: A review. Sci
Total Environ. 755((Pt 2)): 1425182021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Varela JA, Bexiga MG, Åberg C, Simpson JC
and Dawson KA: Quantifying size-dependent interactions between
fluorescently labeled polystyrene nanoparticles and mammalian
cells. J Nanobiotechnology. 10:392012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nowak M, Brown TD, Graham A, Helgeson ME
and Mitragotri S: Size, shape, and flexibility influence
nanoparticle transport across brain endothelium under flow. Bioeng
Transl Med. 5:e101532020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Firdessa R, Oelschlaeger TA and Moll H:
Identification of multiple cellular uptake pathways of polystyrene
nanoparticles and factors affecting the uptake: Relevance for drug
delivery systems. Eur J Cell Biol. 93:323–337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gratton SE, Ropp PA, Pohlhaus PD, Luft JC,
Madden VJ, Napier ME and DeSimone JM: The effect of particle design
on cellular internalization pathways. Proc Natl Acad Sci USA.
105:11613–11618. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carr KE, Smyth SH, McCullough MT, Morris
JF and Moyes SM: Morphological aspects of interactions between
microparticles and mammalian cells: Intestinal uptake and onward
movement. Prog Histochem Cytochem. 46:185–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmidt C, Lautenschlaeger C, Collnot EM,
Schumann M, Bojarski C, Schulzke JD, Lehr CM and Stallmach A: Nano-
and microscaled particles for drug targeting to inflamed intestinal
mucosa: A first in vivo study in human patients. J Control Release.
165:139–145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li S and Malmstadt N: Deformation and
poration of lipid bilayer membranes by cationic nanoparticles. Soft
Matter. 9:4969–4976. 2013. View Article : Google Scholar
|
|
33
|
Xie W, You J, Zhi C and Li L: The toxicity
of ambient fine particulate matter (PM2.5) to vascular endothelial
cells. J Appl Toxicol. 41:713–723. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gopinath PM, Saranya V, Vijayakumar S,
Mythili Meera M, Ruprekha S, Kunal R, Pranay A, Thomas J, Mukherjee
A and Chandrasekaran N: Assessment on interactive prospectives of
nanoplastics with plasma proteins and the toxicological impacts of
virgin, coronated and environmentally released-nanoplastics. Sci
Rep. 9:88602019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hollóczki O and Gehrke S: Nanoplastics can
change the secondary structure of proteins. Sci Rep. 9:160132019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goodman KE, Hare JT, Khamis ZI, Hua T and
Sang QA: Exposure of human lung cells to polystyrene microplastics
significantly retards cell proliferation and triggers morphological
changes. Chem Res Toxicol. 34:1069–1081. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu M, Halimu G, Zhang Q, Song Y, Fu X, Li
Y, Li Y and Zhang H: Internalization and toxicity: A preliminary
study of effects of nanoplastic particles on human lung epithelial
cell. Sci Total Environ. 694:1337942019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hirt N and Body-Malapel M: Immunotoxicity
and intestinal effects of nano- and microplastics: A review of the
literature. Part Fibre Toxicol. 17:572020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cheng W, Li X, Zhou Y, Yu H, Xie Y, Guo H,
Wang H, Li Y, Feng Y and Wang Y: Polystyrene microplastics induce
hepatotoxicity and disrupt lipid metabolism in the liver organoids.
Sci Total Environ. 806((Pt 1)): 1503282022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li B, Ding Y, Cheng X, Sheng D, Xu Z, Rong
Q, Wu Y, Zhao H, Ji X and Zhang Y: Polyethylene microplastics
affect the distribution of gut microbiota and inflammation
development in mice. Chemosphere. 244:1254922020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu S, Li H, Wang J, Wu B and Guo X:
Polystyrene microplastics aggravate inflammatory damage in mice
with intestinal immune imbalance. Sci Total Environ.
833:1551982022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Deng Y, Yan Z, Shen R, Wang M, Huang Y,
Ren H, Zhang Y and Lemos B: Microplastics release phthalate esters
and cause aggravated adverse effects in the mouse gut. Environ Int.
143:1059162020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shi C, Han X, Guo W, Wu Q, Yang X, Wang Y,
Tang G, Wang S, Wang Z, Liu Y, et al: Disturbed Gut-liver axis
indicating oral exposure to polystyrene microplastic potentially
increases the risk of insulin resistance. Environ Int.
164:1072732022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jin Y, Lu L, Tu W, Luo T and Fu Z: Impacts
of polystyrene microplastic on the gut barrier, microbiota and
metabolism of mice. Sci Total Environ. 649:308–317. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hwang J, Choi D, Han S, Choi J and Hong J:
An assessment of the toxicity of polypropylene microplastics in
human derived cells. Sci Total Environ. 684:657–669. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ding Y, Zhang R, Li B, Du Y, Li J, Tong X,
Wu Y, Ji X and Zhang Y: Tissue distribution of polystyrene
nanoplastics in mice and their entry, transport, and cytotoxicity
to GES-1 cells. Environ Pollut. 280:1169742021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Domenech J, de Britto M, Velázquez A,
Pastor S, Hernández A, Marcos R and Cortés C: Long-term effects of
polystyrene nanoplastics in human intestinal caco-2 cells.
Biomolecules. 11:14422021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Eleutherio ECA, Silva Magalhães RS, de
Araújo Brasil A, Monteiro Neto JR and de Holanda Paranhos L: SOD1,
more than just an antioxidant. Arch Biochem Biophys.
697:1087012021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Johnson P: Antioxidant enzyme expression
in health and disease: Effects of exercise and hypertension. Comp
Biochem Physiol C Toxicol Pharmacol. 133:493–505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jakubczyk K, Dec K, Kałduńska J, Kawczuga
D, Kochman J and Janda K: Reactive oxygen species-sources,
functions, oxidative damage. Pol Merkur Lekarski. 48:124–127.
2020.PubMed/NCBI
|
|
51
|
Wang X, Zheng H, Zhao J, Luo X, Wang Z and
Xing B: Photodegradation elevated the toxicity of polystyrene
microplastics to grouper (Epinephelus moara) through disrupting
hepatic lipid homeostasis. Environ Sci Technol. 54:6202–6212. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
DeLoid GM, Cao X, Bitounis D, Singh D,
Llopis PM, Buckley B and Demokritou P: Toxicity, uptake, and
nuclear translocation of ingested micro-nanoplastics in an in vitro
model of the small intestinal epithelium. Food Chem Toxicol.
158:1126092021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
He Y, Li Z, Xu T, Luo D, Chi Q, Zhang Y
and Li S: Polystyrene nanoplastics deteriorate LPS-modulated
duodenal permeability and inflammation in mice via ROS
drived-NF-κB/NLRP3 pathway. Chemosphere. 307((Pt 1)): 1356622022.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Deng Y, Zhang Y, Lemos B and Ren H: Tissue
accumulation of microplastics in mice and biomarker responses
suggest widespread health risks of exposure. Sci Rep. 7:466872017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rubio L, Marcos R and Hernández A:
Potential adverse health effects of ingested micro- and
nanoplastics on humans. Lessons learned from in vivo and in vitro
mammalian models. J Toxicol Environ Health B Crit Rev. 23:51–68.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Powell JJ, Thoree V and Pele LC: Dietary
microparticles and their impact on tolerance and immune
responsiveness of the gastrointestinal tract. Br J Nutr. 98 (Suppl
1):S59–S63. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Huang D, Zhang Y, Long J, Yang X, Bao L,
Yang Z, Wu B, Si R, Zhao W, Peng C, et al: Polystyrene microplastic
exposure induces insulin resistance in mice via dysbacteriosis and
pro-inflammation. Sci Total Environ. 838((Pt 1)): 1559372022.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Forte M, Iachetta G, Tussellino M,
Carotenuto R, Prisco M, De Falco M, Laforgia V and Valiante S:
Polystyrene nanoparticles internalization in human gastric
adenocarcinoma cells. Toxicol In Vitro. 31:126–136. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yan X, Zhang Y, Lu Y, He L, Qu J, Zhou C,
Hong P, Sun S, Zhao H, Liang Y, et al: The complex toxicity of
tetracycline with polystyrene spheres on gastric cancer cells. Int
J Environ Res Public Health. 17:28082020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen Z, Wang C, Yu N, Si L, Zhu L, Zeng A,
Liu Z and Wang X: INF2 regulates oxidative stress-induced apoptosis
in epidermal HaCaT cells by modulating the HIF1 signaling pathway.
Biomed Pharmacother. 111:151–161. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Vethaak AD and Legler J: Microplastics and
human health. Science. 371:672–674. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lu L, Wan Z, Luo T, Fu Z and Jin Y:
Polystyrene microplastics induce gut microbiota dysbiosis and
hepatic lipid metabolism disorder in mice. Sci Total Environ.
631-632:449–458. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wan Z, Wang C, Zhou J, Shen M, Wang X, Fu
Z and Jin Y: Effects of polystyrene microplastics on the
composition of the microbiome and metabolism in larval zebrafish.
Chemosphere. 217:646–658. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhao Y, Bao Z, Wan Z, Fu Z and Jin Y:
Polystyrene microplastic exposure disturbs hepatic glycolipid
metabolism at the physiological, biochemical, and transcriptomic
levels in adult zebrafish. Sci Total Environ. 710:1362792020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shi L and Tu BP: Acetyl-CoA and the
regulation of metabolism: mechanisms and consequences. Curr Opin
Cell Biol. 33:125–131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhou P, Santoro A, Peroni OD, Nelson AT,
Saghatelian A, Siegel D and Kahn BB: PAHSAs enhance hepatic and
systemic insulin sensitivity through direct and indirect
mechanisms. J Clin Invest. 129:4138–4150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Syed I, Lee J, Moraes-Vieira PM, Donaldson
CJ, Sontheimer A, Aryal P, Wellenstein K, Kolar MJ, Nelson AT,
Siegel D, et al: Palmitic acid hydroxystearic acids activate GPR40,
which is involved in their beneficial effects on glucose
homeostasis. Cell Metab. 27:419–427.e4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Vijayakumar A, Aryal P, Wen J, Syed I,
Vazirani RP, Moraes-Vieira PM, Camporez JP, Gallop MR, Perry RJ,
Peroni OD, et al: Absence of carbohydrate response element binding
protein in adipocytes causes systemic insulin resistance and
impairs glucose transport. Cell Rep. 21:1021–1035. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wen B, Zhang N, Jin SR, Chen ZZ, Gao JZ,
Liu Y, Liu HP and Xu Z: Microplastics have a more profound impact
than elevated temperatures on the predatory performance, digestion
and energy metabolism of an Amazonian cichlid. Aquat Toxicol.
195:67–76. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Schlein C, Talukdar S, Heine M, Fischer
AW, Krott LM, Nilsson SK, Brenner MB, Heeren J and Scheja L: FGF21
lowers plasma triglycerides by accelerating lipoprotein catabolism
in white and brown adipose tissues. Cell Metab. 23:441–453. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Iizuka K, Takeda J and Horikawa Y: Glucose
induces FGF21 mRNA expression through ChREBP activation in rat
hepatocytes. FEBS Lett. 583:2882–2886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bi Y, Chang Y, Liu Q, Mao Y, Zhai K, Zhou
Y, Jiao R and Ji G: ERp44/CG9911 promotes fat storage in Drosophila
adipocytes by regulating ER Ca(2+) homeostasis. Aging (Albany NY).
13:15013–15031. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Simha V and Garg A: Lipodystrophy: Lessons
in lipid and energy metabolism. Curr Opin Lipidol. 17:162–169.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Iizuka K, Takao K and Yabe D:
ChREBP-mediated regulation of lipid metabolism: Involvement of the
gut microbiota, liver, and adipose tissue. Front Endocrinol
(Lausanne). 11:5871892020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Nunes-Nesi A, Araújo WL, Obata T and
Fernie AR: Regulation of the mitochondrial tricarboxylic acid
cycle. Curr Opin Plant Biol. 16:335–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bougarne N, Weyers B, Desmet SJ, Deckers
J, Ray DW, Staels B and De Bosscher K: Molecular actions of
PPARalpha in lipid metabolism and inflammation. Endocr Rev.
39:760–802. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wang Q, Wu Y, Zhang W, Shen T, Li H, Wu J,
Zhang L, Qin L, Chen R, Gu W, et al: Lipidomics and transcriptomics
insight into impacts of microplastics exposure on hepatic lipid
metabolism in mice. Chemosphere. 308((Pt 3)): 1365912022.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Fan X, Wei X, Hu H, Zhang B, Yang D, Du H,
Zhu R, Sun X, Oh Y and Gu N: Effects of oral administration of
polystyrene nanoplastics on plasma glucose metabolism in mice.
Chemosphere. 288((Pt 3)): 1326072022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Islinger M, Cardoso MJ and Schrader M: Be
different-the diversity of peroxisomes in the animal kingdom.
Biochim Biophys Acta. 1803:881–897. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Marion-Letellier R, Savoye G and Ghosh S:
Fatty acids, eicosanoids and PPAR gamma. Eur J Pharmacol.
785:44–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Bhatt-Wessel B, Jordan TW, Miller JH and
Peng L: Role of DGAT enzymes in triacylglycerol metabolism. Arch
Biochem Biophys. 655:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Stone SJ, Myers HM, Watkins SM, Brown BE,
Feingold KR, Elias PM and Farese RV Jr: Lipopenia and skin barrier
abnormalities in DGAT2-deficient mice. J Biol Chem.
279:11767–11776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Stock V, Fahrenson C, Thuenemann A, Dönmez
MH, Voss L, Böhmert L, Braeuning A, Lampen A and Sieg H: Impact of
artificial digestion on the sizes and shapes of microplastic
particles. Food Chem Toxicol. 135:1110102020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhao L, Shi W, Hu F, Song X, Cheng Z and
Zhou J: Prolonged oral ingestion of microplastics induced
inflammation in the liver tissues of C57BL/6J mice through
polarization of macrophages and increased infiltration of natural
killer cells. Ecotoxicol Environ Saf. 227:1128822021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Qiao J, Chen R, Wang M, Bai R, Cui X, Liu
Y, Wu C and Chen C: Perturbation of gut microbiota plays an
important role in micro/nanoplastics-induced gut barrier
dysfunction. Nanoscale. 13:8806–8816. 2021. View Article : Google Scholar : PubMed/NCBI
|