Introduction
Bronchiolitis obliterans (BO) is a destructive
fibrotic lung disease induced by lower respiratory lesions
(1,2). The pathological features of BO
include intraluminal granulation accumulation, progressive airflow
obstruction, inflammation and fibrosis of airway epithelial cells,
collagen and matrix deposition, and epithelial-mesenchymal
transition (EMT) exacerbation (3–7).
Although various modalities have been applied in stopping or
slowing down the progression of BO, no method has yet been proven
to reverse established BO (4,8). The
survival rate of patients with BO is poor; the 5-year survival rate
is ~40% (4,8); therefore, it is of great significance
to identify potential therapeutic targets of BO.
Even though the exact pathogenesis of BO is unclear,
lung transplantation, severe respiratory tract infections and
inhalation exposures to certain chemicals are considered to be
potential causes (9,10). Notably, 2,3-butanedione [also known
as diacetyl (DA)] is a volatile α-diketone, which is added to
popcorn, flavoring and e-cigarettes due to its buttery aroma
(10–12). In 2002, Kreiss et al
(13) reported that inhaling the
volatile butter-flavored ingredient DA was the main cause of BO
occurring in popcorn factory workers. Over the past few decades,
numerous studies have certified that DA is strongly associated with
the development of BO (10–12,14).
Notably, DA is used as a chemical flavoring in a number of fields
and knowledge on how it results in BO remains in its infancy.
Cell proliferation regulating inhibitor of protein
phosphatase 2A (CIP2A) is a dysregulated protein in several types
of cancer, including breast cancer, colorectal cancer, bladder
cancer and hepatocellular carcinoma, which can affect cell
proliferation, cell cycle progression, apoptosis and tumor
formation (15–17). In addition, CIP2A serves a critical
role in nerve diseases, such as inhibiting depression-like
behaviors and promoting the development of Alzheimer's disease
(18–20). The expression of EMT markers,
including Snail, Vimentin and E-cadherin, has been shown to be
regulated by CIP2A (21,22). Furthermore, in a previous study,
the stronger the local inflammation in cancer, the more CIP2A was
detected (23). These findings
indicated that CIP2A may mediate EMT and inflammation. Moreover, in
human primary bronchial epithelial cells (HPBECs) isolated from
patients with chronic obstructive pulmonary disease, CIP2A was
shown to be highly expressed and this enhancement contributed to a
loss of lung function (24).
However, the effect of CIP2A on BO is currently unclear.
The present study aimed to assess the effects of
CIP2A on BO and its underlying mechanism. The findings suggested
the potential of CIPA2 as a new target for BO treatment.
Materials and methods
Animals and groups
Male Sprague Dawley rats (age, 8 weeks; weight, 300
g) were purchased from Changsheng Bio-Technology Co., Ltd. Rats
received 1 week of environmental adaptation (22±1°C; 45–55%
humidity; 12-h light/dark cycle), and had ad libitum access
to water and food. A total of 79 rats were used in the present
study. For experiment 1, 12 rats were included in the control group
and 13 rats in the DA group (one of the 13 rats died naturally
during the modeling process); in experiment 2,12 rats were included
in the control group, 14 rats in the DA group (two of the 14 rats
died naturally during the modeling process), 14 rats in the DA +
vehicle group (one of the 14 rats died naturally during the
modeling process and one rat was used in a preliminary experiment
to determine the antibody concentration required and the detection
conditions of the kit), and 14 rats in the DA + Eth group (one of
the 14 rats died naturally during the modeling process and one rat
was used in a preliminary experiment to determine the antibody
concentration required and the detection conditions of the kit).
Six rats were used to collect bronchoalveolar lavage fluid (BALF)
in each group and another six rats were used for other experiments.
The groups were treated as follows: i) Control group, intratracheal
instillation of aseptic distilled water; ii) DA group,
intratracheal instillation of 125 mg/kg DA (Shanghai Macklin
Biochemical Co., Ltd.); iii) DA + vehicle group, subcutaneous
injection with solvent (10% DMSO + 90% corn oil); iv) DA +
ethoxysanguinarine (Eth; inhibitor of CIP2A) group, subcutaneous
injection with 0.35 mg/kg Eth (Shanghai Macklin Biochemical Co.,
Ltd.) (25). For BO modeling in
vivo, the protocols described in Palmer et al (11) and House et al (26) were performed with minor
modifications; the rats in the last three groups accepted DA
intratracheal instillation once a day for 7 days. Firstly, the rats
were anesthetized using isoflurane (3% to induce anesthesia and 2%
to maintain anesthesia). For intratracheal instillation, the rat
was placed supine on a flat plate at an angle of 30–40° and the
tongue was pulled out. A cannula was inserted into the trachea and
DA from the injector was injected slowly into the trachea via
cannula; the instillation lasted ~5 min. The control rats received
aseptic distilled water. On day 8, rats in the DA + vehicle and DA
+ Eth groups were treated with solvent or Eth once a day for 4
weeks. The rats in the control and DA groups received normal
saline. After 4 weeks, the rats were sacrificed with CO2
(60% replacement rate), and BALF, and left and right lung tissues
with bronchi were collected. Animal death was confirmed by the
absence of toe pinching reflex, breathing and heartbeat. A humane
endpoint was reached when rats lost weight quickly, or experienced
a decline in their physical health, such as a lack or loss of
appetite, lethargy or persistent recumbency. No animal reached
these humane endpoints before the end of the experiment. The
present animal experiments were performed following the Guideline
for the Care and Use of Laboratory Animals (27) and were approved by the Experimental
Animal Ethics Committee of Affiliated Hospital of Shandong
University of Traditional Chinese Medicine [approval number: (2023)
No. 163 application for provincial natural basic experiment; Jinan,
China].
mRNA-sequencing (mRNA-seq)
After isolating mRNA from rat lung tissues with
bronchi using TRIzol® (cat. no. 15596018CN; Invitrogen;
Thermo Fisher Scientific, Inc.), and passing integrity and total
quantity testing, mRNA was used to synthesize cDNA. Amplification
was carried out via PCR, followed by purification using AMPure XP
Beads (Beckman Coulter, Inc.). The type of sequencing, including
nucleotide length and the direction of sequencing, were as follows:
Nucleotide length, 150 bp; direction of sequencing, paired end.
Library quality was detected using an Agilent 2100 system (Agilent
Technologies, Inc.). The loading concentration of the final library
was >2 nM, which was measured by quantitative PCR (qPCR). The
NovaSeq 6000 Illumina high-throughput sequencing platform
(Illumina, Inc.) was used to sequence the library. with the
sequencing kit NovaSeq 6000 S4 Reagent Kit (cat. no. 20028312;
Illumina, Inc.). Differentially expressed genes (DEGs) were defined
as absolute value of log2 fold change (FC) >1.5 and
adjusted-P<0.001. The P-value was adjusted through the
Benjamini-Hochberg method using DESeq2 software. DESeq2 (1.24.0)
(28) was used to analyze the
data. Gene Ontology (GO) analysis was performed using org.Rn.eg.db
Version 3.19.1 (https://bioconductor.org/packages/release/data/annotation/html/org.Rn.eg.db.html)
and clusterProfiler Version 4.12.0 (https://bioconductor.org/packages/release/bioc/html/clusterProfiler.html).
Five gene name lists were downloaded, which were associated with
apoptosis, inflammation, fibrosis, EMT and the epithelium, from
GeneCards (https://www.genecards.org/).
According to the description of the protein on GeneCards, they were
divided into 12 types.
Reverse transcription-qPCR
Total RNA was isolated from tissues using TRIpure
(BioTeke Corporation). RNA then was converted to cDNA using the
All-in-One First-Strand SuperMix (Magen Biotechnology Co., Ltd.)
according to the manufacturer's instructions. qPCR was performed
with 2X Taq PCR MasterMix (Beijing Solarbio Science &
Technology Co., Ltd.) and SYBR Green (Beijing Solarbio Science
& Technology Co., Ltd.). The thermocycling conditions were as
follows: The sample was first incubated at 95°C for 5 min; followed
by 40 cycles at 95°C for 10 sec at 60°C for 10 sec and 72°C for 15
sec. The sample was then incubated at 72°C for 1 min 30 sec, at
40°C for 1 min, and then the melting process was carried out,
gradually increasing the temperature from 60°C to 94°C, with a rate
of 1°C increase every second. Finally, the sample was incubated at
25°C for 1–2 min. β-actin was used as an endogenous control and
data were calculated using the 2−∆∆Cq method (29). The following primers were used for
amplification: CIP2A forward, 5′-TTGTCGGGAGTGGTTTG-3′, reverse,
5′-AGGGCATAGTTAGCTCATCTT-3′; solute carrier family 1 member 6
(SLC1A6) forward, 5′-TCCTGATTGCTGGAAAGA-3′, reverse,
5′-CGGAAAGTGATAGGCAGA-3′; endoplasmic reticulum to nucleus
signaling 2 (ERN2) forward, 5′-TACACCGTGACCTCAAGCC-3′, reverse,
5′-TGCCGGGAATACCAGAAT-3′; ribonuclease A family member 2 (RNASE2)
forward, 5′-GCCATCCAGCACATCTA-3′, reverse,
5′-TGTACTTCTCCCGTCTTTA-3′; tryptase β2 (TPSB2) forward,
5′-ATTGTGGGAGGACGAGA-3′, reverse, 5′-CTGTGGGTGAATGAGGG-3′; and
β-actin forward, 5′-GGAGATTACTGCCCTGGCTCCTAGC-3′ and reverse,
5′-GGCCGGACTCATCGTACTCCTGCTT-3′.
Hematoxylin and eosin (H&E)
staining
Tissues were obtained from the rats after
euthanasia, and were fixed with 4% paraformaldehyde at room
temperature for >24 h, dehydrated with different concentrations
of ethanol, permeabilized with xylene for 30 min at room
temperature, embedded in paraffin and cut into slices (5 µm). The
slices were then stained with H&E and their images were
captured using a BX53 fluorescence microscope (Olympus
Corporation). Briefly, slices were incubated with hematoxylin
solution for 5 min and were then differentiated with 1%
hydrochloric acid alcohol, before being soaked in eosin for 3 min
at room temperature. The measurement of severity score was
performed as previously described (30). Briefly, severity was scored as
follows: Normal lung, 0 points; slight fibrous thickening of the
alveolar or bronchial wall, 1 point; moderate thickening of lung
wall without marked damage to lung structure, 2–3 points; worsening
fibrosis with obvious damage to lung architecture and the formation
of fiber bands or small fiber clumps, 4–5 points; serious
structural deformation, a large fiber area including honeycombing,
6–7 points; complete fiber occlusion of the field, 8 points.
Masson's trichrome staining
Masson staining was used to measure fibrosis with
the Masson kit (Leagene; Beijing Regen Biotechnology Co., Ltd.).
Briefly, the aforementioned paraffin-embedded tissue slices were
incubated with Ponceau-Magenta for 10 min. After treating with
phosphomolybdic acid for 2 min, the slices were stained with
aniline blue for 1 min. All steps were carried out at room
temperature. The images were obtained using a BX53 microscope. The
collagenization area was measured based on a previous study
(31).
Giemsa staining
The BALF was collected and part of it was used for
detecting total cell count using a hemocytometer. The remaining
BALF was used for differential counting of inflammatory cells.
Giemsa staining at room temperature for 1 min with Giemsa A and for
7 min with Giemsa B (Giemsa Staining Kit; Nanjing Jiancheng
Bioengineering Institute) was carried out to manually measure the
number of different types of cells under a DP73 fluorescence
microscope (Olympus Corporation), including macrophages,
lymphocytes, neutrophils and eosinophils.
Immunohistochemistry
The aforementioned paraffin-embedded tissue slices
were dewaxed with xylene, rehydrated in a descending alcohol series
and underwent antigen retrieval at 100°C in antigen repair solution
(9 ml citric acid buffer, 41 ml sodium citrate buffer mixed with
450 ml distilled water) for 10 min and were then cooled before
being incubated with 3% H2O2 for 15 min to
eliminate endogenous peroxidase activity at room temperature. After
blocking with 1% BSA (Sangon Biotech Co., Ltd.) for 15 min at 4°C,
the slices were incubated with CIP2A antibody (1:100; cat. no.
bs-5948R; BIOSS) overnight at 4°C and with Goat Anti-Rabbit IgG/HRP
(1:100; cat. no. SE134; Beijing Solarbio Science & Technology
Co., Ltd.) for 45 min. Diaminobenzidine was used as a chromogenic
substrate and the nuclei were counterstained using hematoxylin at
room temperature for 3 min. The images were captured under a BX53
microscope. Relative protein levels were determined as follows:
Relative level=integrated optical density/area. The CIP2A
immunohistochemistry staining was validated using an isotype
control (data not shown).
In vitro model
HPBECs were purchased from iCell Bioscience Inc. and
were cultured in the HPBECs cultivation system (iCell Bioscience
Inc.). The in vitro model of BO was established as
previously described (32). Cells
were exposed to DA (25 mM) for 1 h on days 0, 2 and 4, and were
treated with Eth (GLPBIO Technology LLC) for 24 h on day 6 at 37°C.
For Eth intervention dose in vitro, Jin et al
(25) and Liu et al
(33) were referred to, and the
concentrations of Eth were set to 2, 4 and 6 µM.
Western blotting
Proteins were harvested from tissues and cells using
radio immunoprecipitation assay lysis buffer (Proteintech Group,
Inc.), or the nuclear protein and cytoplasmic protein extraction
kit (Proteintech Group, Inc.). Protein concentrations were measured
using the Bicinchoninic Acid Protein Assay Kit (Proteintech Group,
Inc.). Proteins (15–30 µg/15 µl/lane) were separated by sodium
dodecyl sulfate polyacrylamide gel electrophoresis (5% stacking
gel; 8, 10 or 12% separation gel) and were then transferred to
polyvinylidene fluoride membranes, following by blocking using
Western Blocking Buffer (Proteintech Group, Inc.). The membranes
were then incubated with primary antibodies overnight at 4°C and
with secondary antibodies for 40 min at 37°C. Visualization was
conducted with ECL Western Blotting Substrate (Proteintech Group,
Inc.). The following antibodies (Proteintech Group, Inc.) were used
in the present study: CIP2A monoclonal antibody (cat. no.
67843-1-Ig; 1:10,000), α-smooth muscle actin (α-SMA) polyclonal
antibody (cat. no. 14395-1-AP; 1:1,000), fibronectin polyclonal
antibody (cat. no. 15613-1-AP; 1:10,000), Snail polyclonal antibody
(cat. no. 13099-1-AP; 1:10,000), inducible NO synthase (iNOS)
polyclonal antibody (cat. no. 22226-1-AP; 1:500), NF-κB inhibitor α
(IκBα) polyclonal antibody (cat. no. 10268-1-AP; 1:20,000),
phosphorylated-IκBα (p-IκBα; Ser32/36) recombinant antibody (cat.
no. 82349-1-RR; 1:20,000), p65 polyclonal antibody (cat. no.
10745-1-AP; 1:3,000), HRP-conjugated Affinipure goat anti-rabbit
IgG(H+L) (cat. no. SA00001-2; 1:10,000), HRP-conjugated Affinipure
rabbit anti-goat IgG(H+L) (cat. no. SA00001-4; 1:10,000),
HRP-conjugated Affinipure goat anti-mouse IgG(H+L) (1:10,000),
Histone-H3 polyclonal antibody (cat. no. 17168-1-AP; 1:2,000) and
β-actin monoclonal antibody (cat. no. 66009-1-Ig; 1:20,000).
β-actin was used as an internal control and Histone-H3 was used as
a nuclear loading control.
Immunofluorescence
The aforementioned paraffin-embedded tissue slices
and cell sections were used for immunofluorescence analysis.
Antigen retrieval was performed for 10 min on tissue slices with
antigen repair solution (9 ml citric acid buffer, 41 ml sodium
citrate buffer mixed with 450 ml distilled water) at 100°C and
permeabilization was carried out on cell sections with 0.1% Triton
X-100 for 30 min at room temperature. After blocking with BSA for
15 min at room temperature, the slices and sections were incubated
with primary antibodies and a secondary antibody. The antibodies
used in this analysis included Vimentin (cat. no. AF7013; 1:200)
and E-cadherin (cat. no. AF0131; 1:200) primary antibodies
(Affinity Biosciences), and Cy3-conjugated Goat Anti-Rabbit IgG
(H+L) (1:200; cat. no. SA00009-2; Proteintech Group, Inc.). The PBS
was used as dilution. Subsequently, the nuclei were stained with
DAPI and the images were obtained using a BX53 fluorescence
microscope.
Enzyme-linked immunosorbent assay
(ELISA) kit
The levels of interleukin (IL)-1β, IL-6 and tumor
necrosis factor (TNF)-α were detected using ELISA kits according to
the manufacturer's instructions. All ELISA kits (cat. nos. EK301B,
EK101B, EK306, EK106, EK382 and EK182) were provided by Multi
Sciences (Lianke) Biotech Co., Ltd. The ELX-800 microplate reader
(Bio-Tek Instruments, Inc.) was used to measure absorbance.
Statistical analysis
GraphPad (version 9.5.0; Dotmatics) was used for
data analysis. Comparisons among groups were statistically analyzed
using unpaired Student's t-test (two groups) or one-way ANOVA (four
groups) with Tukey's multiple comparison test. The severity score
was analyzed with nonparametric tests, either Mann-Whitney test or
Kruskal-Wallis test with Dunn's multiple comparisons test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
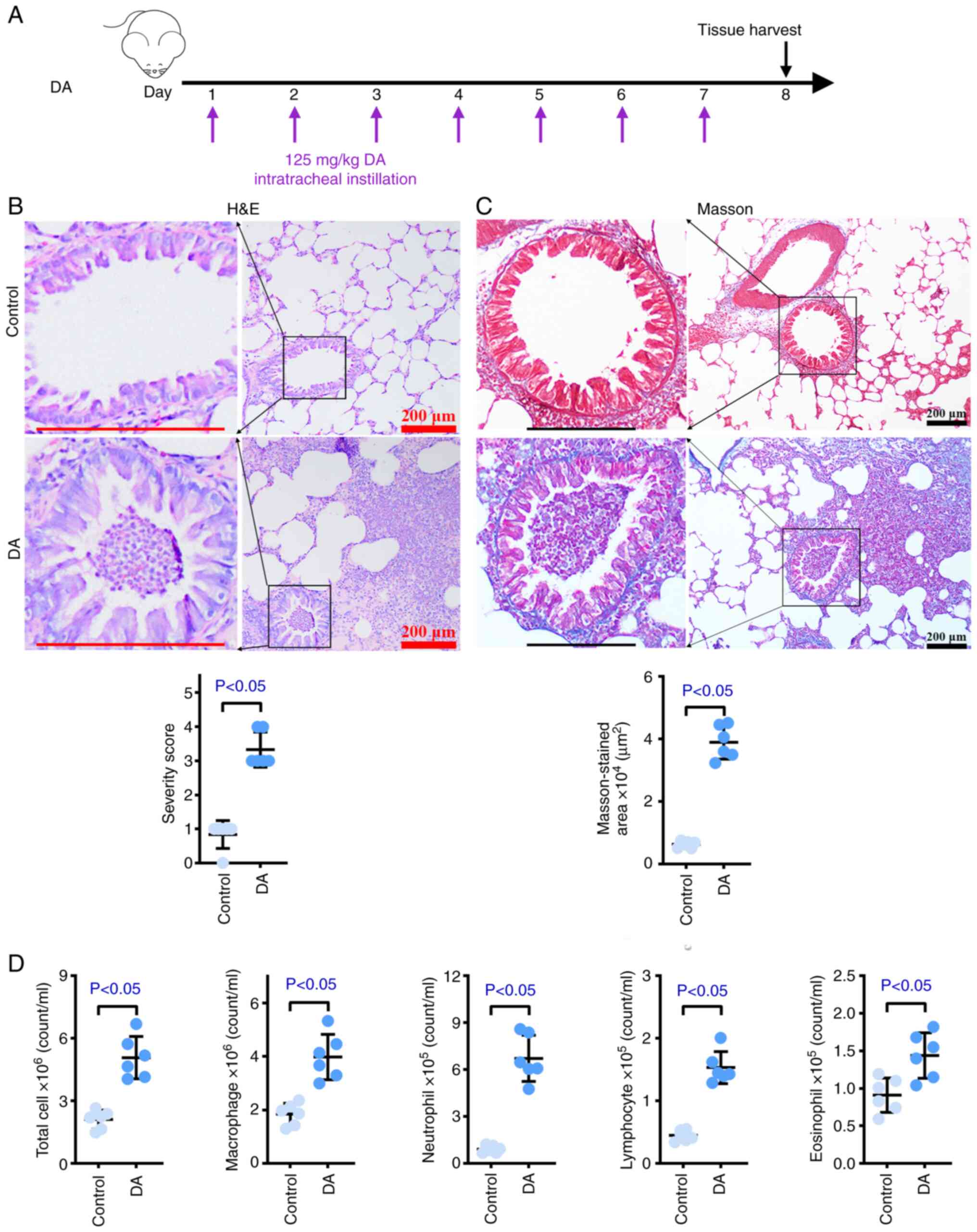
DA induces symptoms of BO in Sprague
Dawley rats
A DA-induced rat model of BO was established to
carry out the following experiments (Fig. 1A). As shown in Fig. 1B, more evident fibrosis accompanied
by more extensive inflammatory infiltration was observed in the
airway of rats that underwent endotracheal instillation with DA
compared with in the control rats. DA also caused partial blockages
in the airways compared with distilled water, and higher severity
scores were observed in the DA group. The Masson's trichrome
staining results of healthy and DA-treated tissues demonstrated
that larger levels of collagenous fiber were present in model
tissues compared with those in healthy tissues (Fig. 1C). Occlusion was also found in the
DA group according to Masson staining, which was in line with the
results of H&E staining. To quantify the effects of DA on
inflammatory infiltration, inflammatory cells were counted in the
BALF (Figs. 1D and S1A). The numbers of macrophages,
neutrophils, lymphocytes, eosinophils and total cells in the BALF
were significantly increased in the BO model rats compared with
those in the control rats. These results indicated that the in
vivo BO model was successfully established.
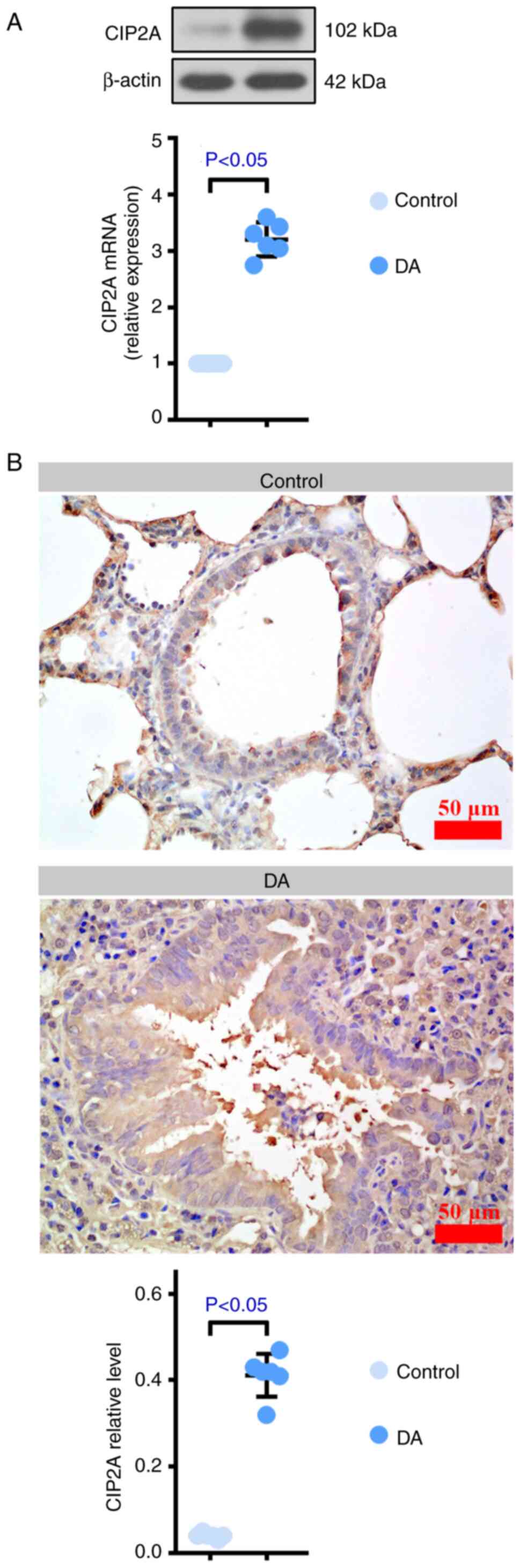
CIP2A expression is increased in
DA-treated rats
In order to explore the related molecular mechanism
of BO, high-throughput analysis was conducted using the lung
tissues of rats. There were 150 upregulated DEGs and 55
downregulated DEGs in tissues from the DA group compared with in
normal tissues (Fig. 2A). RT-qPCR
was carried out to verify the results of mRNA-seq that SLC1A6 and
ERN2 were increased, and RNASE2 and TPSB2 were reduced in samples
from the DA group (Fig. 2B). GO
outcomes revealed that DEGs were enriched in apoptosis,
inflammation, fibrosis, EMT and epithelium-associated items,
including ‘negative regulation of neutrophil apoptotic process’,
‘endothelial cell apoptotic process’, ‘cyclin A2-CDK2 complex’,
‘cyclin B1-CDK1 complex’, ‘cyclin-dependent protein kinase
holoenzyme complex’, ‘DNA replication origin binding’,
‘cyclin-dependent protein serine/threonine kinase regulator
activity’, ‘DNA helicase activity’, ‘regulation of leukotriene
production involved in inflammatory response’, ‘regulation of
inflammatory response’, ‘interleukin-8 receptor activity’, ‘immune
receptor activity’, ‘fibroblast proliferation’, ‘response to
fibroblast growth factor’, ‘elastic fiber’, ‘banded collagen
fibril’, ‘contractile fiber’, ‘regulation of epithelial to
mesenchymal transition’, ‘collagen metabolic process’,
‘collagen-containing extracellular matrix’, ‘fibronectin binding’,
‘epithelium regeneration’ and ‘epithelium migration’ (Fig. 2C), indicating that BO may be
related to them. Therefore, five datasets were downloaded, which
were associated with apoptosis, inflammation, fibrosis, EMT and the
epithelium, from GeneCards, and a Venn analysis was conducted using
these datasets and the identified DEGs. As shown in Fig. 2D, only 47 DEGs simultaneously
existed in the six datasets (the five datasets provided by
GeneCards and the present mRNA-seq results). Proteins encoded by
the 47 DEGs were divided into 12 categories, including functional
protein, constitutive protein, receptor, binding protein, enzyme,
cyclin, glycoprotein, growth factor, proteoglycan, transcription
factor, cytokines and chemokines (Fig.
2E). The present study subsequently focused attention on
functional proteins. Among these functional proteins, CIP2A,
located on chromosome 11, was increased in DA-treated lung tissues
(Fig. 2F). The results of RT-qPCR,
western blotting and immunohistochemistry verified the mRNA-seq
results that CIP2A was higher in DA-treated rats than that in
control rats (Fig. 3A-C). Based on
these findings, it may be hypothesized that the development of BO
is related to several factors and CIP2A may serve an important role
during this process.
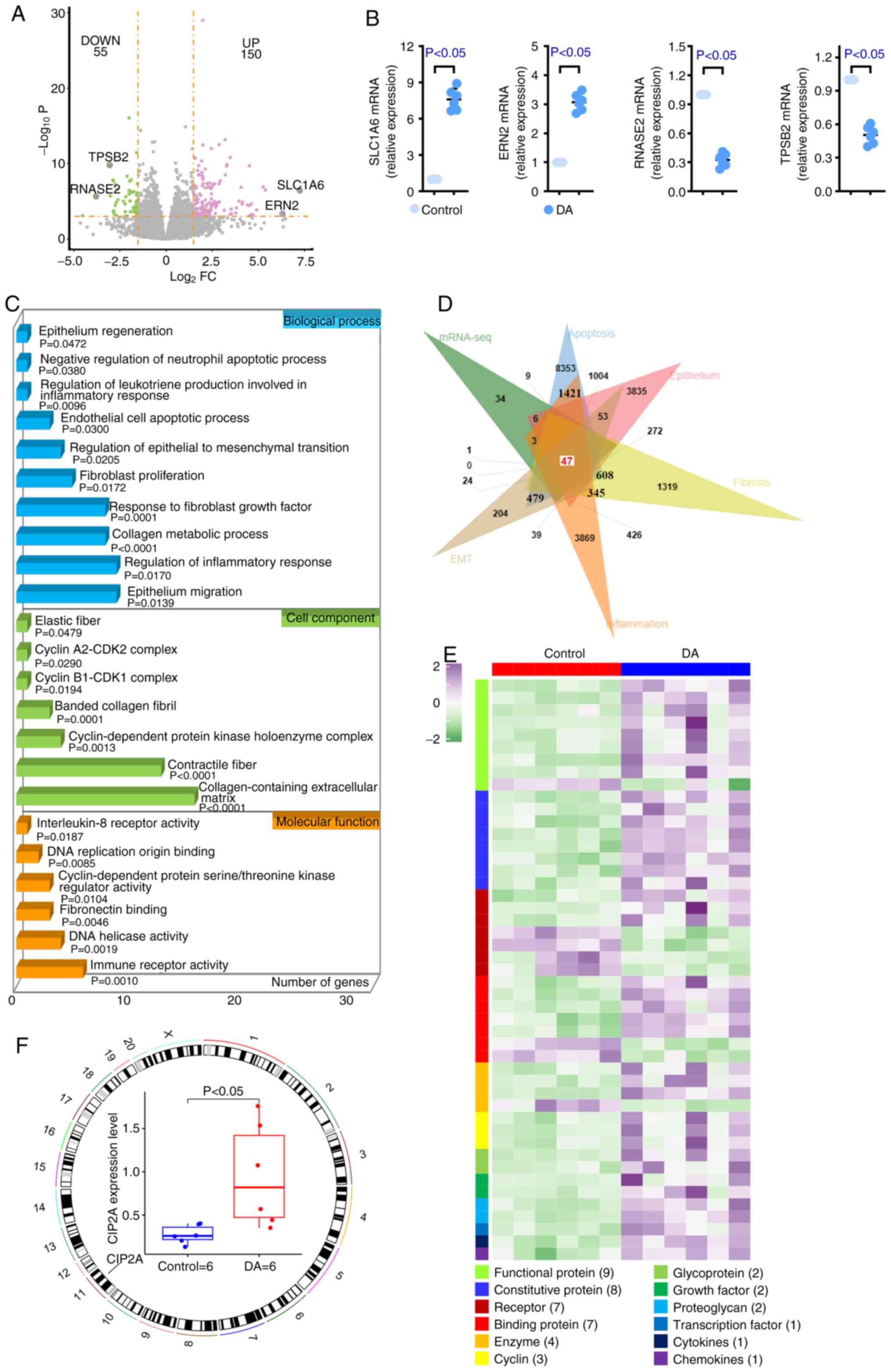 | Figure 2.mRNA-seq of rat tissues. To explore
the related molecular mechanism of bronchiolitis obliterans,
high-throughput analysis was conducted using the lung tissues of
rats. (A) DEGs in DA group rats relative to the control are shown
using a volcano plot. DEGs were defined as an absolute value of
log2FC >1.5 and adjusted-P<0.001. (B) Quantitative PCR was
carried out to verify the results of mRNA-seq. Four DEGs were
randomly selected for verification. (C) Gene Ontology analysis
revealed that apoptosis, inflammation, fibrosis, EMT and
epithelium-associated items were enriched by DEGs. (D) Venn diagram
shows that 47 DEGs commonly existed in the six datasets. (E)
Heatmap of 47 DEGs demonstrated that most genes were functional
proteins. (F) CIP2A was located on chromosome 11 and was increased
in DA-treated rats. n=6. CIP2A, cell proliferation regulating
inhibitor of protein phosphatase 2A; DA, diacetyl; DEGs,
differentially expressed genes; EMT, epithelial-mesenchymal
transition; FC, fold change; mRNA-seq, mRNA-sequencing; SLC1A6,
solute carrier family 1 member 6; ERN2, endoplasmic reticulum to
nucleus signaling 2; RNASE2, ribonuclease A family member 2; TPSB2,
tryptase β2. |
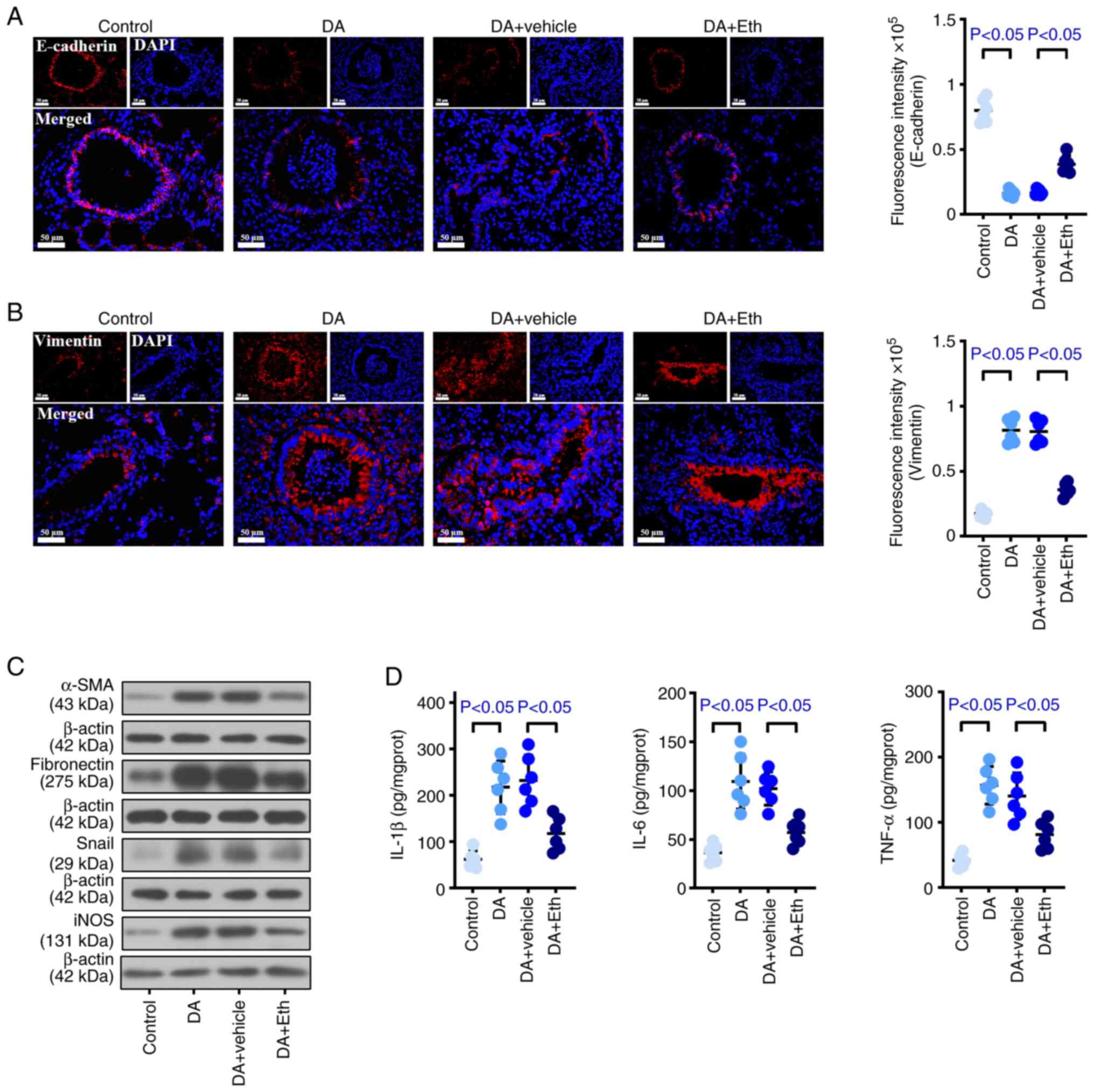
Inhibiting CIP2A attenuates the
injuries induced by DA in the bronchus and lung of rats
To evaluate the effect of CIP2A inhibition on BO,
Eth, a CIP2A inhibitor, was injected subcutaneously into DA-treated
rats (Fig. 4A). DA induced airway
and alveolar injury compared with that in control rats; however,
the fibrosis, inflammatory infiltration and blocking that were
induced by DA were reversed after suppressing CIP2A (Fig. 4B). As shown in Fig. 4C, more collagen deposition was
detected in the BO group than that in the control mice, whereas
collagen deposition was reduced when CIP2A was inhibited. The
number of macrophages, lymphocytes, neutrophils and total cells in
animals with CIP2A reduction was decreased compared with that in
the DA group (Figs. 4D and
S1B), suggesting that CIP2A may
serve a role by recruiting inflammatory cells, especially
lymphocytes and neutrophils. Notably, there was no difference in
the number of eosinophils between the Eth-treated and untreated
DA-induced rats. Western blotting and immunohistochemical analysis
suggested that DA induced an increase in CIP2A, whereas Eth
effectively reduced the protein expression levels of CIP2A
(Fig. 4E and F). In addition, more
occlusion was detected in the DA group, accompanied by an increase
in CIP2A; by contrast, inhibition of CIP2A not only reduced the
CIP2A levels but also decreased the occlusion area (Fig. 4F). Furthermore, stronger Vimentin
and weaker E-cadherin expression were observed in BO model rats
compared with in the control animals, whereas CIP2A inhibition
reversed these results of immunofluorescence staining (Fig. 5A and B). In DA-treated rats,
increased α-SMA, fibronectin, Snail and iNOS expression levels were
detected compared with those in the control rats; by contrast,
CIP2A inhibition reduced the expression levels of α-SMA,
fibronectin, Snail and iNOS, as determined using western blotting
(Fig. 5C). In addition, IL-1β,
IL-6 and TNF-α levels were higher in BO model rats than those in
the normal rats, whereas Eth suppressed these inflammatory factors
(Fig. 5D). These data revealed
that CIP2A reduction may contribute to relieve the fibrosis, EMT
and inflammation caused by DA in rats.
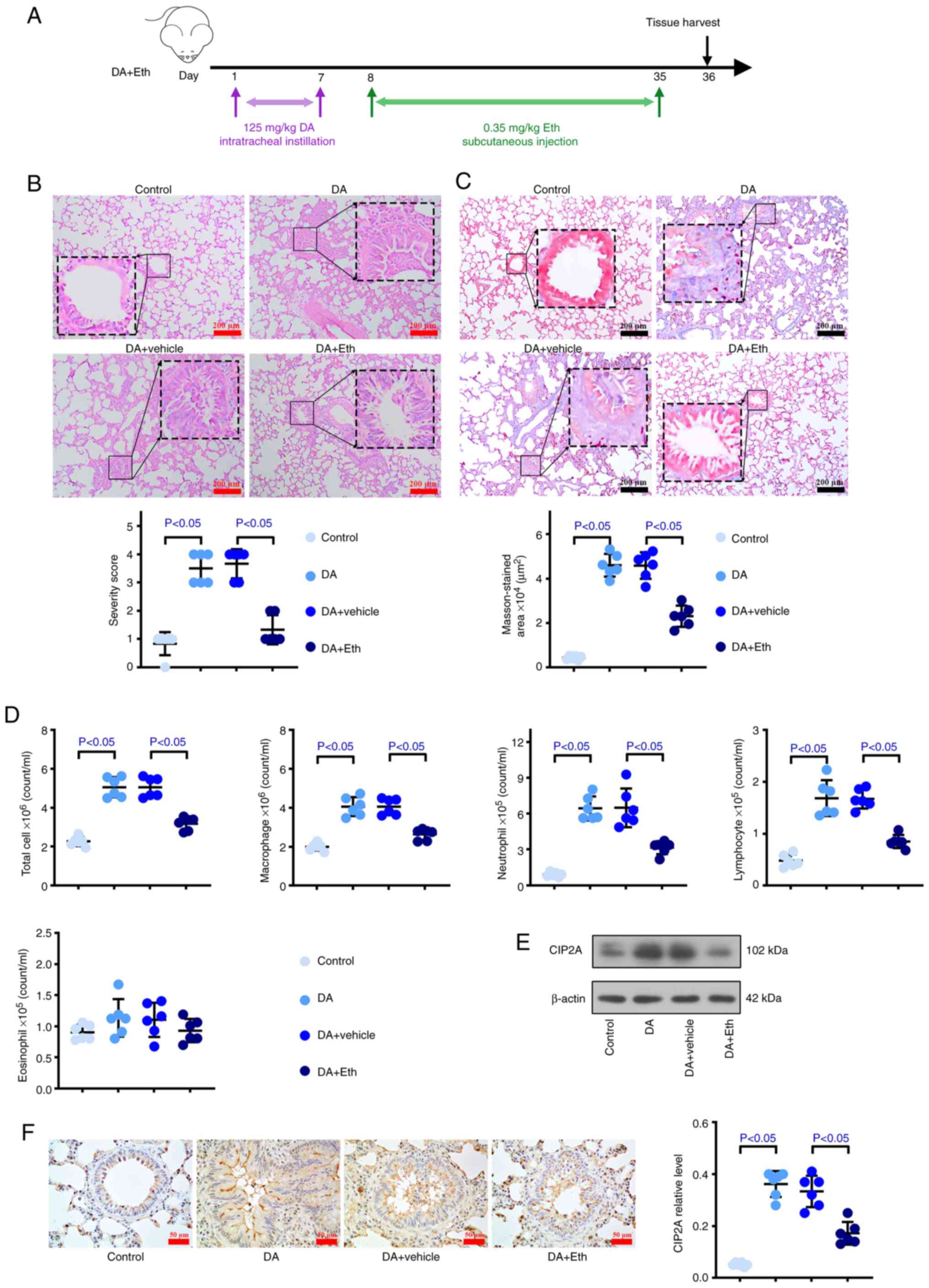 | Figure 4.Inhibiting CIP2A attenuates the
injuries induced by DA in the bronchi and lungs of rats. To
evaluate the effect of CIP2A inhibition on bronchiolitis
obliterans, Eth, a CIP2A inhibitor, was injected subcutaneously
into DA group rats. (A) Animal experimental timeline.
Representative (B) H&E and (C) Masson's trichrome-stained lung
slices (scale bar: 200 µm). Quantitative analysis of severity is
shown. (D) Bronchoalveolar lavage fluid total cell count, and
macrophage, neutrophil, lymphocyte and eosinophil counts in the
control rats, DA-exposed rats and Eth-treated DA-exposed rats. (E)
Western blotting and (F) immunohistochemistry were used to measure
the protein expression levels of CIP2A in lung tissues. n=6. CIP2A,
cell proliferation regulating inhibitor of protein phosphatase 2A;
DA, diacetyl; Eth, ethoxysanguinarine; H&E, hematoxylin and
eosin. |
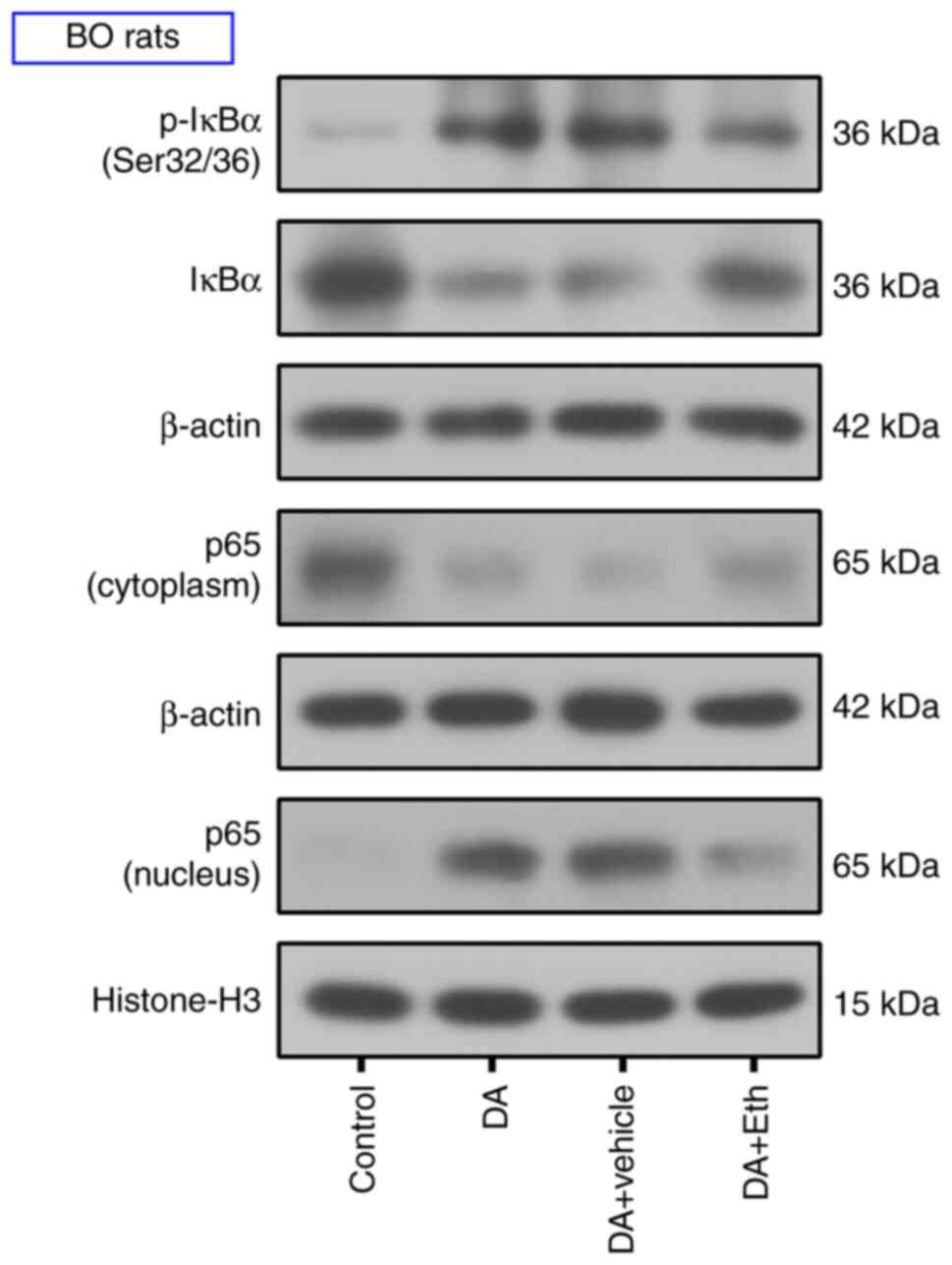
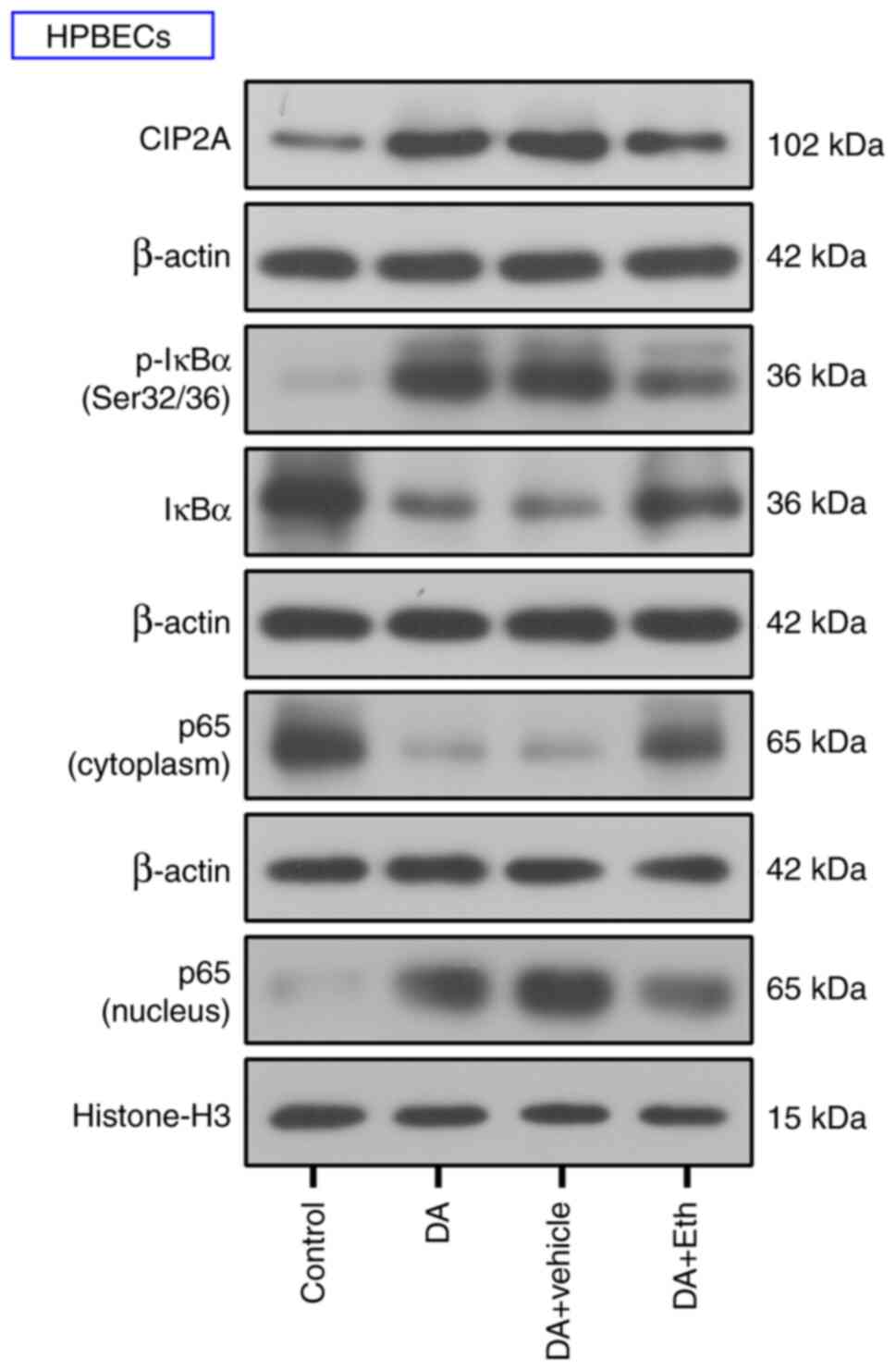
Inhibiting CIP2A suppresses the NF-κB
pathway in BO rats
The present study further investigated the pathway
mediating the effects of CIP2A on BO development. After DA
endotracheal instillation, increased p-IκBα and p65 nuclear
translocation were observed; however, CIP2A inhibition attenuated
the phosphorylation of IKBα and nuclear translocation of p65
(Fig. 6). Western blotting results
suggested that CIP2A inhibition may suppress activation of the
NF-κB pathway.
Inhibiting CIP2A reduces damage caused
by DA in HPBECs
To explore the cellular mechanisms, the present
study employed an in vitro cellular model of BO. The cells
were treated with Eth after DA modeling. As depicted in Fig. 7A, CIP2A expression was reduced in
response to Eth treatment in a dose-dependent manner. A
concentration of 6 µM Eth exhibited the best inhibitory effect;
therefore, this concentration was selected for subsequent
experiments. The expression levels of α-SMA, fibronectin, Snail and
iNOS were higher in DA-treated cells than in control HPBECs. By
contrast, reduced α-SMA, fibronectin, Snail and iNOS levels were
observed in DA + Eth-treated cells (Fig. 7B). As shown in Fig. 7D, the expression of Vimentin was
promoted by DA, whereas E-cadherin exhibited the opposite trend.
Inhibiting CIP2A weakened the expression of Vimentin and enhanced
E-cadherin expression. Furthermore, DA increased the production of
IL-1β, IL-6 and TNF-α, whereas CIP2A reduction deceased this
elevation (Fig. 7C). These data
demonstrated that CIP2A deficiency may inhibit the fibrosis, EMT
and inflammation of DA-treated HPBECs.
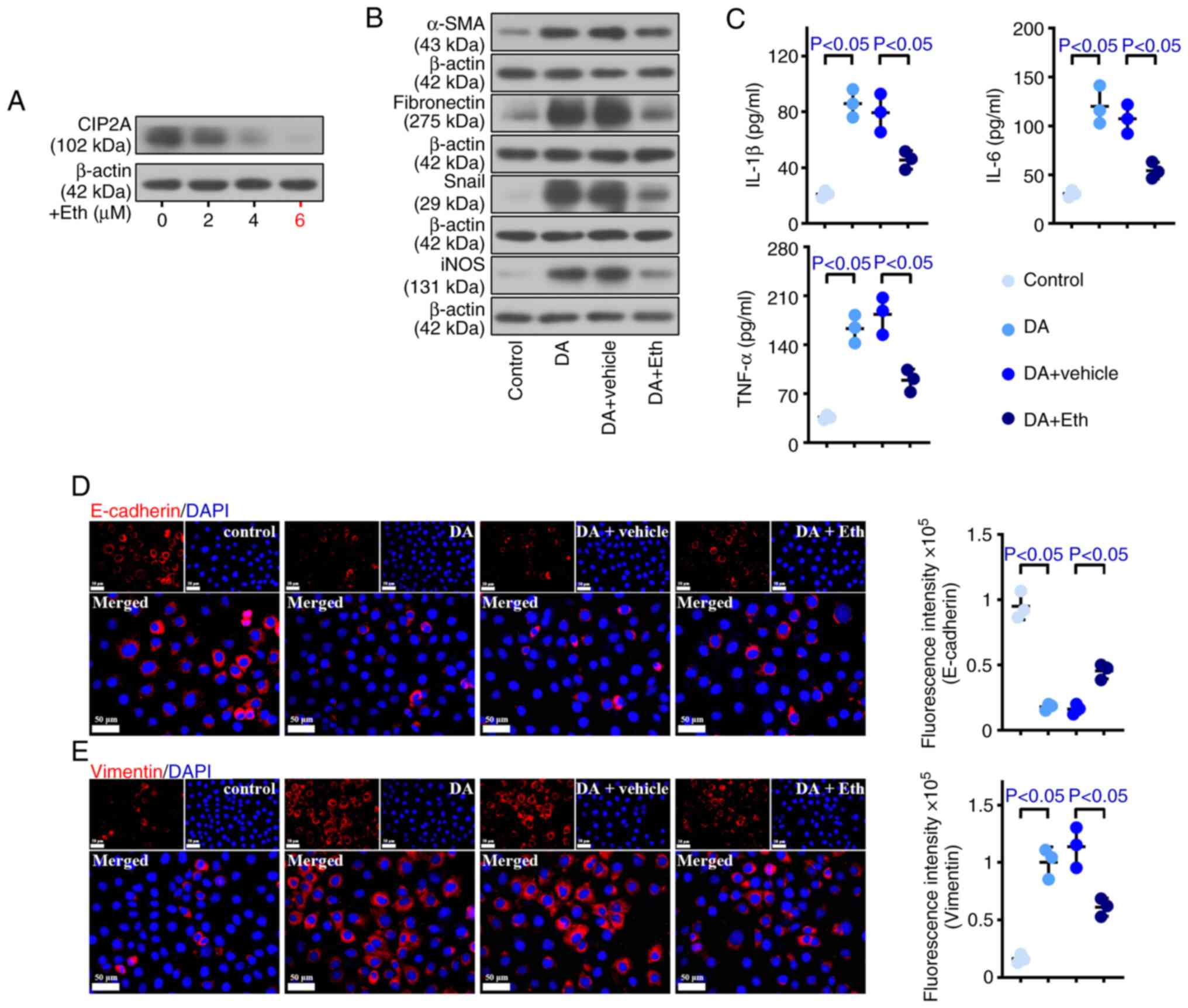 | Figure 7.Inhibiting CIP2A reduces damage
caused by DA in human primary bronchial epithelial cells. To
explore the cellular mechanisms, an in vitro cellular model
of bronchiolitis obliterans was employed. (A) Eth (6 µM) showed the
best inhibitory effect on CIP2A expression. (B) Western blotting
was used to measure the protein expression levels of α-SMA,
fibronectin, Snail and iNOS. (C) Enzyme-linked immunosorbent assay
kits were used to analyze the levels of IL-1β, IL-6 and TNF-α. (D)
E-cadherin and (E) Vimentin levels were detected by
immunofluorescence (scale bar: 50 µm). n=3. α-SMA, α-smooth muscle
actin; CIP2A, cell proliferation regulating inhibitor of protein
phosphatase 2A; DA, diacetyl; Eth, ethoxysanguinarine; IL,
interleukin; iNOS, inducible NO synthase; TNF-α, tumor necrosis
factor-α. |
Inhibiting CIP2A blocks the activation
of NF-κB signaling in HPBECs
The present study further verified the effect of
CIP2A on the NF-κB pathway in vitro. Western blotting
suggested that DA induced an increase in CIP2A and Eth effectively
reduced the protein expression levels of CIP2A in HPBECs (Fig. 8). The expression levels of p-IκBα
and p65 nuclear translocation were increased by DA treatment
compared with that in the control cells; however, CIP2A suppression
decreased the phosphorylation of IκBα and nuclear translocation of
p65 (Fig. 8). These results
indicated that CIP2A may activate the NF-κB signaling pathway.
Discussion
In the present study, candidate genes that possibly
participate in BO pathogenesis were detected by mRNA-seq in the
tissues of rats with BO. Among them, CIP2A was increased and
immunohistochemistry provided further information on the
distribution of CIP2A in the affected lungs with bronchi. Notably,
CIP2A aggravated the symptoms of BO in vivo and in
vitro. These findings supported the association between CIP2A
and BO development, and confirmed the preliminary results.
Due to multiple etiologies, a diverse clinical
spectrum, various pathological appearances and the limited
availability of animal models, effective treatments for BO are
scarce and are usually disappointing (5,11).
Once BO occurs, patients experience irreversible airflow
obstruction, which ultimately develops into respiratory failure
(11). Therefore, clarifying the
mechanisms of BO progression and the identification of new targets
is important. The present study established an animal model of BO
using DA and demonstrated that this model replicated the
pathological features of human patients with BO. Notably, more
serious airway epithelial injury, larger inflammatory infiltration
and fibrosis, more obvious blocking and more collagen deposition
were observed in DA tissues with H&E and Masson's trichrome
staining compared with those in the control tissues. However, the
lack of physiological assessments of lung function is a limitation
of the present study. Palmer et al (11) proposed that the obstruction of
airways may be caused by intraluminal granulation growth or
intramural fibrosis. Considering the absence of knowledge regarding
DA-induced BO, changes in the gene expression of rats treated with
DA were assessed. The expression of the four genes (SLC1A6, ERN2,
RNASE2 and TPSB2) that had the largest absolute value of log2FC,
based on mRNA-seq data, were evaluated to verify the accuracy of
the sequencing. The lack of further validation, such as validating
the expression of the four genes in animal tissue samples by
western blotting or immunohistochemistry, was a limitation of the
current study.
GO analysis of DEGs revealed that DA-induced BO was
associated with apoptosis, inflammation, fibrosis, EMT and the
epithelium. Hence, GeneCards data were used to filter these
relevant DEGs. CIP2A was not only significantly increased in DA
samples but was also related to the aforementioned five aspects.
Since its effect on BO was unknown, the present study assessed it.
In the present study, the results of H&E and Masson's trichrome
staining suggested that CIP2A inhibition ameliorated epithelial
damage, inflammatory infiltration, fibrosis, blocking and collagen
deposition in tissues from DA-treated rats. Fibrosis is defined as
an excessive accumulation of collagen and fibronectin around
damaged tissues (34). EMT is a
process during which epithelial cells lose their original
epithelial characteristics and gain mesenchymal features, which is
characterized by decreased E-cadherin, and increased Vimentin and
α-SMA (35–37). Facilitated EMT has been observed in
patients with BO and animals accompanied by increased inflammation
(7,38). In the present study, CIP2A
suppression markedly affected the expression of EMT and fibrosis
markers, as evidenced by increased E-cadherin, and reduced
Vimentin, α-SMA, fibronectin and Snail expression. In cancer cells,
inhibition of CIP2A has been shown to increase E-cadherin
expression, but to suppress the expression of Snail and Vimentin,
thereby restraining the EMT process (21,39–41).
Furthermore, CIP2A depletion has been reported to suppress
fibronectin-accelerated cell proliferation (15). By contrast, overexpression of CIP2A
may lead to enhanced levels of proinflammatory cytokines, including
IL-1 and TNF-α, in aging-related chronic inflammation (42). In addition, downregulating CIP2A
has been shown to result in devitalized cytokines, such as TNF-α,
and decreased tissue damage and inflammatory cell infiltration,
eventually inhibiting the progression of chronic obstructive
pulmonary disease and lung cancer (43). In the present study, it was
revealed that inhibition of CIP2A suppressed the levels of
inflammatory factors, including IL-1β, IL-6, TNF-α and iNOS, in a
model of BO after Eth treatment.
NF-κB is universally acknowledged to have an
important role in inflammatory responses, which promotes downstream
target gene expression and inflammatory mediator secretion when it
is activated by stimuli (44,45).
In addition, there are intricate connections between inflammation
and fibrosis. TNF-α has been shown to accentuate transforming
growth factor β1 (TGF-β1)-driven EMT, and TNF-α and TGF-β1 jointly
activate TGF-β-activated kinase 1 and subsequently activate the
NF-κB pathway. After a series of cascading reactions, EMT can be
initiated, and inflammation and fibrosis are aggravated (46). In patients with BO, western blot
analysis of NF-κB has been reported to be positive (47). Furthermore, activated NF-κB has
been observed in samples from humans and animals with BO, and this
activation may be associated with BO development (48–50).
Notably, the current study observed that the NF-κB pathway was
activated in DA-treated cells, accompanied by enhanced CIP2A
levels, and that inhibition of CIP2A suppressed this activation, as
evidenced by the restrained phosphorylation of IκBα and nuclear
translocation of p65, suggesting that CIP2A may promote BO
progression via the NF-κB pathway. In cancer, CIP2A has been
reported to promote malignant biological behaviors of cancer cells
through the AKT-mTOR (51,52), JNK (53) and GSK-3β/β-catenin (54) signaling pathways. CIP2A is also
vital in osteoblast differentiation by causing ERK phosphorylation
(55). However, whether CIP2A
serves a role in BO through these pathways remains to be
explored.
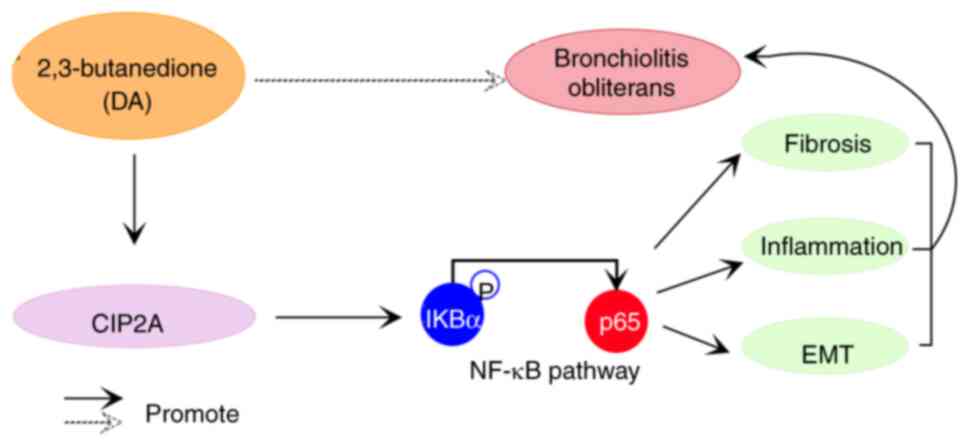
In conclusion, the present study demonstrated that
BO may be caused by DA and mediated by CIP2A. Furthermore, CIP2A
deficiency was shown to inhibit the fibrosis, inflammation and EMT
of BO models through suppressing the NF-κB pathway (Fig. 9).
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant no. 82104933).
Availability of data and materials
The mRNA-sequencing data generated in the present
study may be found in the Gene Expression Omnibus under accession
number GSE279398 or at the following URL: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE279398.
The other data generated in the present study may be requested from
the corresponding author.
Authors' contributions
XuZ, XiZ, YL and BZ conceived the study. XuZ and YL
designed the methodology. XuZ, XiZ and YL performed experiments and
analyzed data. XuZ and BZ wrote and revised the manuscript. XuZ,
XiZ and YL confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present animal experiments were performed
following the Guideline for the Care and Use of Laboratory Animals
and were approved by the Experimental Animal Ethics Committee of
Affiliated Hospital of Shandong University of Traditional Chinese
Medicine [approval number: (2023) No. 163 application for
provincial natural basic experiment]. Ethics approval for the use
of the cells in the present study was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Corresponding author: Dr Xu Zhou ORCID:
0009-0002-8712-6732.
References
|
1
|
Chu CY, Kim SY, Pryhuber GS, Mariani TJ
and McGraw MD: Single-cell resolution of human airway epithelial
cells exposed to bronchiolitis obliterans-associated chemicals. Am
J Physiol Lung Cell Mol Physiol. 326:L135–Ll148. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colom AJ and Teper AM: Post-infectious
bronchiolitis obliterans. Pediatr Pulmonol. 54:212–219. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Flake GP and Morgan DL: Pathology of
diacetyl and 2,3-pentanedione airway lesions in a rat model of
obliterative bronchiolitis. Toxicology. 388:40–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boehler A and Estenne M: Post-transplant
bronchiolitis obliterans. Eur Respir J. 22:1007–1018. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Laohaburanakit P, Chan A and Allen RP:
Bronchiolitis obliterans. Clin Rev Allergy Immunol. 25:259–274.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
D'Amico R, Fusco R, Cordaro M, Siracusa R,
Peritore AF, Gugliandolo E, Crupi R, Scuto M, Cuzzocrea S, Di Paola
R and Impellizzeri D: Modulation of NLRP3 inflammasome through
formyl peptide receptor 1 (Fpr-1) pathway as a new therapeutic
target in bronchiolitis obliterans syndrome. Int J Mol Sci.
21:21442020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang C, Niu Y, Yu L, Lv W, Xu H,
Abuduwufuer A, Cao J and Hu J: The role of epithelial-mesenchymal
transition in the post-lung transplantation bronchiolitis
obliterans. J Cardiothorac Surg. 12:1192017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hakim A, Cooke KR, Pavletic SZ, Khalid M,
Williams KM and Hashmi SK: Diagnosis and treatment of bronchiolitis
obliterans syndrome accessible universally. Bone Marrow Transplant.
54:383–392. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hodge S, Holmes M, Banerjee B, Musk M,
Kicic A, Waterer G, Reynolds PN, Hodge G and Chambers DC:
Posttransplant bronchiolitis obliterans syndrome is associated with
bronchial epithelial to mesenchymal transition. Am J Transplant.
9:727–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Kim SY, House E, Olson HM,
Johnston CJ, Chalupa D, Hernady E, Mariani TJ, Clair G, Ansong C,
et al: Repetitive diacetyl vapor exposure promotes ubiquitin
proteasome stress and precedes bronchiolitis obliterans pathology.
Arch Toxicol. 95:2469–2483. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palmer SM, Flake GP, Kelly FL, Zhang HL,
Nugent JL, Kirby PJ, Foley JF, Gwinn WM and Morgan DL: Severe
airway epithelial injury, aberrant repair and bronchiolitis
obliterans develops after diacetyl instillation in rats. PLoS One.
6:e176442011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kelly FL, Sun J, Fischer BM, Voynow JA,
Kummarapurugu AB, Zhang HL, Nugent JL, Beasley RF, Martinu T and
Gwinn WM: Diacetyl induces amphiregulin shedding in pulmonary
epithelial cells and in experimental bronchiolitis obliterans. Am J
Respir Cell Mol Biol. 51:568–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kreiss K, Gomaa A, Kullman G, Fedan K,
Simoes EJ and Enright PL: Clinical bronchiolitis obliterans in
workers at a microwave-popcorn plant. N Engl J Med. 347:330–338.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Rooy FGBGJ, Rooyackers JM, Prokop M,
Houba R, Smit LAM and Heederik DJJ: Bronchiolitis obliterans
syndrome in chemical workers producing diacetyl for food
flavorings. Am J Respir Crit Care Med. 176:498–504. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao F, Xu T, Wang X, Zhong S, Chen S,
Zhang M, Zhang X, Shen Y, Wang X, Xu C and Shen Z: CIP2A mediates
fibronectin-induced bladder cancer cell proliferation by
stabilizing β-catenin. J Exp Clin Cancer Res. 36:702017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen W, Liang JL, Zhou K, Zeng QL, Ye JW
and Huang MJ: Effect of CIP2A and its mechanism of action in the
malignant biological behavior of colorectal cancer. Cell Commun
Signal. 18:672020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laine A, Nagelli SG, Farrington C, Butt U,
Cvrljevic AN, Vainonen JP, Feringa FM, Grönroos TJ, Gautam P, Khan
S, et al: CIP2A interacts with TopBP1 and drives Basal-like breast
cancer tumorigenesis. Cancer Res. 81:4319–4331. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu WT, Liuyang ZY, Tian Y, Liang JW, Zhang
XL, Zhang HL, Wang G, Huo Y, Shentu YP, Wang JZ, et al: CIP2A
deficiency promotes depression-like behaviors in mice through
inhibition of dendritic arborization. EMBO Rep. 23:e549112022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Y, Yang D, Chen H, Zheng C, Jiang H,
Liu X, Huang X, Ye S, Song S, Jiang N, et al: Polyphyllin I
attenuates cognitive impairments and reduces AD-like pathology
through CIP2A-PP2A signaling pathway in 3XTg-AD mice. FASEB J.
34:16414–16431. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Y, Liu X, Ma S, Zhang N, Yang D, Wang
L, Ye S, Zhang Q, Ruan J, Ma J, et al: ChK1 activation induces
reactive astrogliosis through CIP2A/PP2A/STAT3 pathway in
Alzheimer's disease. FASEB J. 36:e222092022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang Q, Wang Q, Zeng G, Li Q, Jiang T,
Zhang Z, Zheng W and Wang K: Overexpression of CIP2A in clear cell
renal cell carcinoma promotes cellular epithelial-mesenchymal
transition and is associated with poor prognosis. Oncol Rep.
34:2515–2522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Y, Gu TT and Zheng PS: CIP2A cooperates
with H-Ras to promote epithelial-mesenchymal transition in
cervical-cancer progression. Cancer Lett. 356:646–655. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seppälä M, Tervo S, Pohjola K, Laranne J,
Huhtala H, Toppila-Salmi S and Paavonen T: The association and
prognostic relevance of cancerous inhibitor of protein phosphatase
2A and inflammation in tongue squamous cell carcinoma. APMIS.
123:1007–1015. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nath S, Ohlmeyer M, Salathe MA, Poon J,
Baumlin N, Foronjy RF and Geraghty P: Chronic Cigarette smoke
exposure subdues PP2A activity by enhancing expression of the
oncogene CIP2A. Am J Respir Cell Mol Biol. 59:695–705. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin L, Si Y, Hong X, Liu P, Zhu B, Yu H,
Zhao X, Qin S, Xiong M, Liu Y, et al: Ethoxysanguinarine inhibits
viability and induces apoptosis of colorectal cancer cells by
inhibiting CIP2A. Int J Oncol. 52:1569–1578. 2018.PubMed/NCBI
|
|
26
|
House EL, Kim SY, Johnston CJ, Groves AM,
Hernady E, Misra RS and McGraw MD: Diacetyl vapor inhalation
induces mixed, granulocytic lung inflammation with increased
CD4+CD25+ T cells in the rat. Toxics. 9:3592021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
National Research Council Committee for
the Update of the Guide for the C and Use of Laboratory A, . The
National Academies Collection: Reports funded by National
Institutes of Health. Guide for the Care and Use of Laboratory
Animals National Academies Press (US) Copyright © 2011. National
Academy of Sciences; Washington (DC): 2011, PubMed/NCBI
|
|
28
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin J, Deng H, Zhang Y, Zou L, Fu Z and
Dai J: Effect of human umbilical cord-derived mesenchymal stem
cells on murine model of bronchiolitis obliterans like injury.
Pediatr Pulmonol. 56:129–137. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ueno-Iio T, Shibakura M, Iio K, Tanimoto
Y, Kanehiro A, Tanimoto M and Kataoka M: Effect of fudosteine, a
cysteine derivative, on airway hyperresponsiveness, inflammation,
and remodeling in a murine model of asthma. Life Sci. 92:1015–1023.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Foster MW, Gwinn WM, Kelly FL, Brass DM,
Valente AM, Moseley MA, Thompson JW, Morgan DL and Palmer SM:
Proteomic analysis of primary human airway epithelial cells exposed
to the respiratory toxicant diacetyl. J Proteome Res. 16:538–549.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Z, Ma L, Wen ZS, Cheng YX and Zhou GB:
Ethoxysanguinarine induces inhibitory effects and downregulates
CIP2A in lung cancer cells. ACS Med Chem Lett. 5:113–118. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wynn TA and Ramalingam TR: Mechanisms of
fibrosis: Therapeutic translation for fibrotic disease. Nat Med.
18:1028–1040. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kalluri R: EMT: When epithelial cells
decide to become mesenchymal-like cells. J Clin Invest.
119:1417–1419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Smith B and Bhowmick N: Role of EMT in
metastasis and therapy resistance. J Clin Med. 5:172016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Borthwick LA, Parker SM, Brougham KA,
Johnson GE, Gorowiec MR, Ward C, Lordan JL, Corris PA, Kirby JA and
Fisher AJ: Epithelial to mesenchymal transition (EMT) and airway
remodelling after human lung transplantation. Thorax. 64:770–777.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen XD, Tang SX, Zhang JH, Zhang LT and
Wang YW: CIP2A, an oncoprotein, is associated with cell
proliferation, invasion and migration in laryngeal carcinoma cells.
Oncol Rep. 38:1005–1012. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu X, Sun Z, Deng J, Liu J, Ma K, Si Y,
Zhang T, Feng T, Liu Y and Tan Y: Polyphyllin I inhibits invasion
and epithelial-mesenchymal transition via CIP2A/PP2A/ERK signaling
in prostate cancer. Int J Oncol. 53:1279–1288. 2018.PubMed/NCBI
|
|
41
|
Zhang Y, Huang P, Liu X, Xiang Y, Zhang T,
Wu Y, Xu J, Sun Z, Zhen W, Zhang L, et al: Polyphyllin I inhibits
growth and invasion of cisplatin-resistant gastric cancer cells by
partially inhibiting CIP2A/PP2A/Akt signaling axis. J Pharmacol
Sci. 137:305–312. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fujiki H, Sueoka E, Watanabe T, Komori A
and Suganuma M: Cancer progression by the okadaic acid class of
tumor promoters and endogenous protein inhibitors of PP2A, SET and
CIP2A. J Cancer Res Clin Oncol. 149:9425–9433. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nader CP, Cidem A, Verrills NM and Ammit
AJ: Protein phosphatase 2A (PP2A): a key phosphatase in the
progression of chronic obstructive pulmonary disease (COPD) to lung
cancer. Respir Res. 20:2222019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Marquardt JU, Gomez-Quiroz L, Arreguin
Camacho LO, Pinna F, Lee YH, Kitade M, Domínguez MP, Castven D,
Breuhahn K, Conner EA, et al: Curcumin effectively inhibits
oncogenic NF-κB signaling and restrains stemness features in liver
cancer. J Hepatol. 63:661–669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cai L, Ming D, Chen W, Zhao Y, Li Y, Sun
W, Pi Y, Jiang X and Li X: Silybin alleviated hepatic injury by
regulating redox balance, inflammatory response, and mitochondrial
function in weaned piglets under Paraquat-induced oxidative stress.
Antioxidants (Basel). 13:3242024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gardner A, Fisher AJ, Richter C, Johnson
GE, Moisey EJ, Brodlie M, Ward C, Krippner-Heidenreich A, Mann DA
and Borthwick LA: The critical role of TAK1 in accentuated
epithelial to mesenchymal transition in obliterative bronchiolitis
after lung transplantation. Am J Pathol. 180:2293–2308. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mohanakumar T, Sharma M, Bansal S,
Ravichandran R, Smith MA and Bremner RM: A novel mechanism for
immune regulation after human lung transplantation. J Thorac
Cardiovasc Surg. 157:2096–2106. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Farivar AS, Mackinnon-Patterson B, Woolley
S, Namkung J, Shimamoto A, Verrier ED and Mulligan MS: FR167653
reduces obliterative airway disease in rats. J Heart Lung
Transplant. 23:985–992. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Farivar AS, Woolley SM, Naidu BV, Fraga
CH, Byrne K, Thomas R, Salzman AL, Szabo CS and Mulligan MS: Poly
(ADP) ribose synthetase inhibition reduces obliterative airway
disease in rat tracheal allografts. J Heart Lung Transplant.
23:993–1002. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ohmori K, Takeda S, Miyoshi S, Minami M,
Nakane S, Ohta M, Sawa Y and Matsuda H: Attenuation of lung injury
in allograft rejection using NF-ĸB decoy transfection-novel
strategy for use in lung transplantation. Eur J Cardio-Thoracic
Surg. 27:23–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lei N, Peng B and Zhang JY: CIP2A
regulates cell proliferation via the AKT signaling pathway in human
lung cancer. Oncol Rep. 32:1689–1694. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Monga J, Suthar SK, Rohila D, Joseph A,
Chauhan CS and Sharma M: (+)-Cyanidan-3-ol inhibits epidermoid
squamous cell carcinoma growth via inhibiting AKT/mTOR signaling
through modulating CIP2A-PP2A axis. Phytomedicine. 101:1541162022.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Peng B, Chai Y, Li Y, Liu X and Zhang J:
CIP2A overexpression induces autoimmune response and enhances JNK
signaling pathway in human lung cancer. BMC Cancer. 15:8952015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Che Y, Zhang H, Li H and Wu X: CIP2A
interacts with AKT1 to promote the malignant biological behaviors
of oral squamous cell carcinoma by upregulating the
GSK-3β/β-catenin pathway. Exp Ther Med. 26:5142023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Son HE and Jang WG: Cip2A modulates
osteogenic differentiation via the ERK-Runx2 pathway in MG63 cells.
Biofactors. 47:658–664. 2021. View Article : Google Scholar : PubMed/NCBI
|