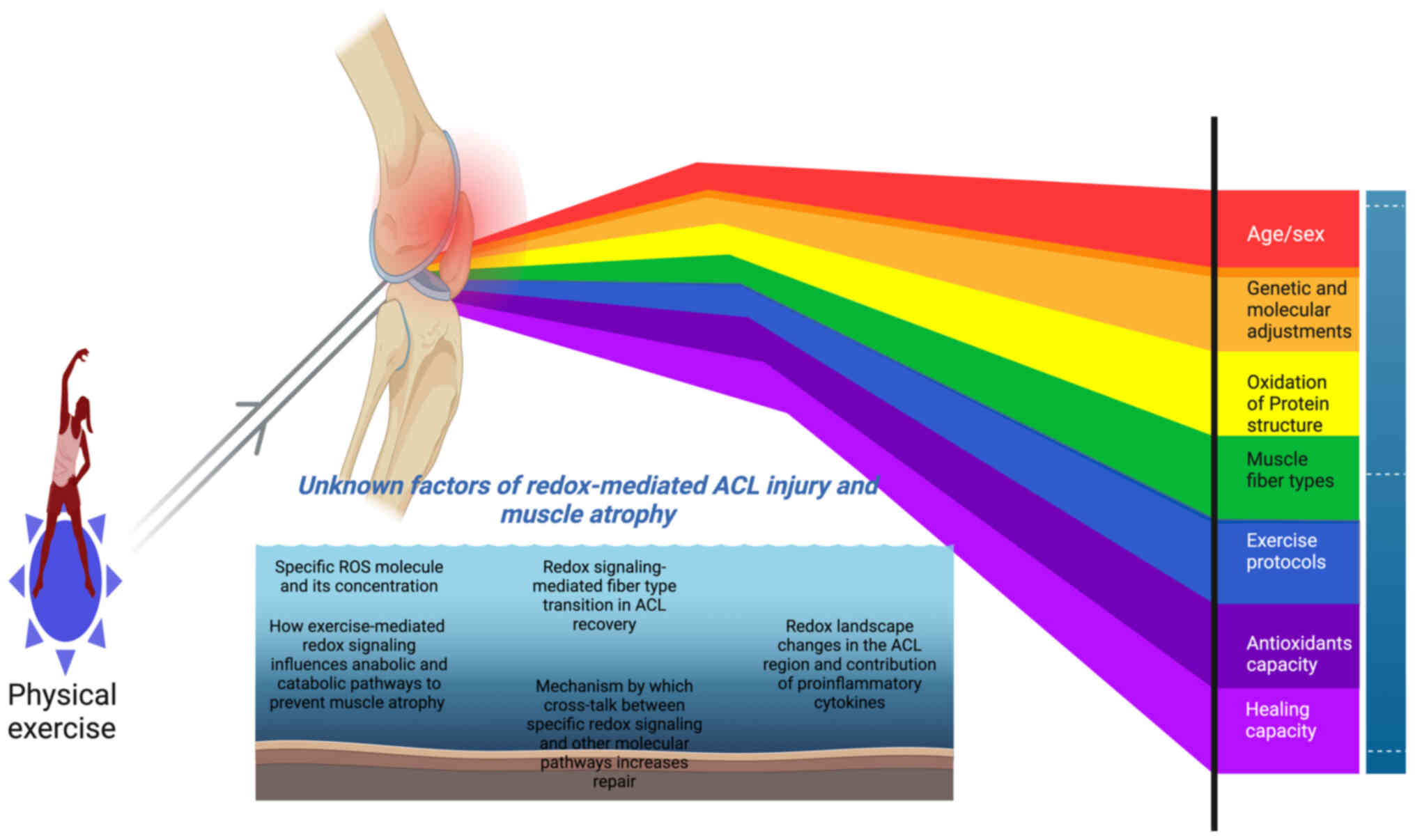
Introduction
Anterior cruciate ligament (ACL) injury is one of
the most prevalent injuries among athletes, the incidence of which
has doubled over the past two decades (1–5).
However, the underlying mechanism remains poorly understood.
Various risk factors, including age, sex and activity type, affect
the recovery time and the degree of injury (1,2). The
development of novel treatment strategies is crucial for improving
the overcomes for athletes with this type of injury. Current
treatment strategies, such as ACL reconstruction surgery, are often
inadequate, as they may be unable to fully restore the stability
and function of the knee when the injury is severe (3,4).
This leads to increased healthcare costs, estimated at 1–2 billion
dollars annually in the United States (4). Additionally, the pain level and
extended recovery times may limit the ability of athletes to return
to their sport. Treatment failure is often attributed to joint
injury and post-traumatic osteoarthritis (1,2);
however, the specific causes and mechanisms remain unclear. Several
meta-analyses suggest that strength and plyometric training have
the potential to prevent ACL injuries (2,6,7).
However, once an athlete has sustained an ACL injury, these types
of exercises are no longer effective. In addition, injury-related
biological processes impact muscle protein synthesis and strength
during the initial adaptation phase, contributing to muscle atrophy
in the ACL region (8).
Oxidative stress-induced muscle atrophy is a major
contributor to muscle weakness following ACL reconstruction.
Studies have shown that increased reactive oxygen species (ROS)
levels within muscle fibers increase the risk of injury and atrophy
(8,9). However, it remains unclear whether
oxidative stress is the primary cause of injury or if ACL
injury-induced muscle atrophy itself triggers oxidative stress. The
ACL is composed of several proteins, including collagen, elastin
and proteoglycans (10). These
proteins are highly sensitive to oxidative modifications that can
destabilize their scaffold structures; in particular, ROS have been
reported to affect the transcription, translation and
posttranslational modification of these proteins (11), thereby either impairing or
enhancing muscle protein synthesis. These effects may ultimately
lead to muscle atrophy or, in some cases, promote muscle protein
synthesis following ACL reconstruction.
The use of exercise therapy during the
rehabilitation of patients with ACL injury should be carefully
considered, since exercise protocols are associated with increased
ROS production (12).
Understanding the risk factors, such as individual genetic makeups
and molecular pathways that interact with redox molecules, may
provide novel insights into ACL injury. In addition, it may help to
expand the development of redox-based therapies for recovery.
Exercise-induced adaptation to prevent
muscle atrophy in ACL injury: Role of thiol-based redox
signaling
The production of ROS, particularly hydrogen
peroxide (H2O2), during exercise affects the
activation of various signaling pathways, enzymes and cell
membranes (13), thereby
influencing protein synthesis and muscle integrity. In particular,
the sulfhydryl group of cysteine (Cys) is considered a key target
of oxidation in several proteins across various tissues (14). For example, the oxidation of
Cys-299 and Cys-304 on the a subunit of AMP-activated protein
kinase (AMPK) has been shown to alter its kinase function (Fig. 1) (15). Notably, AMPK plays a crucial role
in meniscal degeneration during ACL injury and may increase the
risk of osteoarthritis (16).
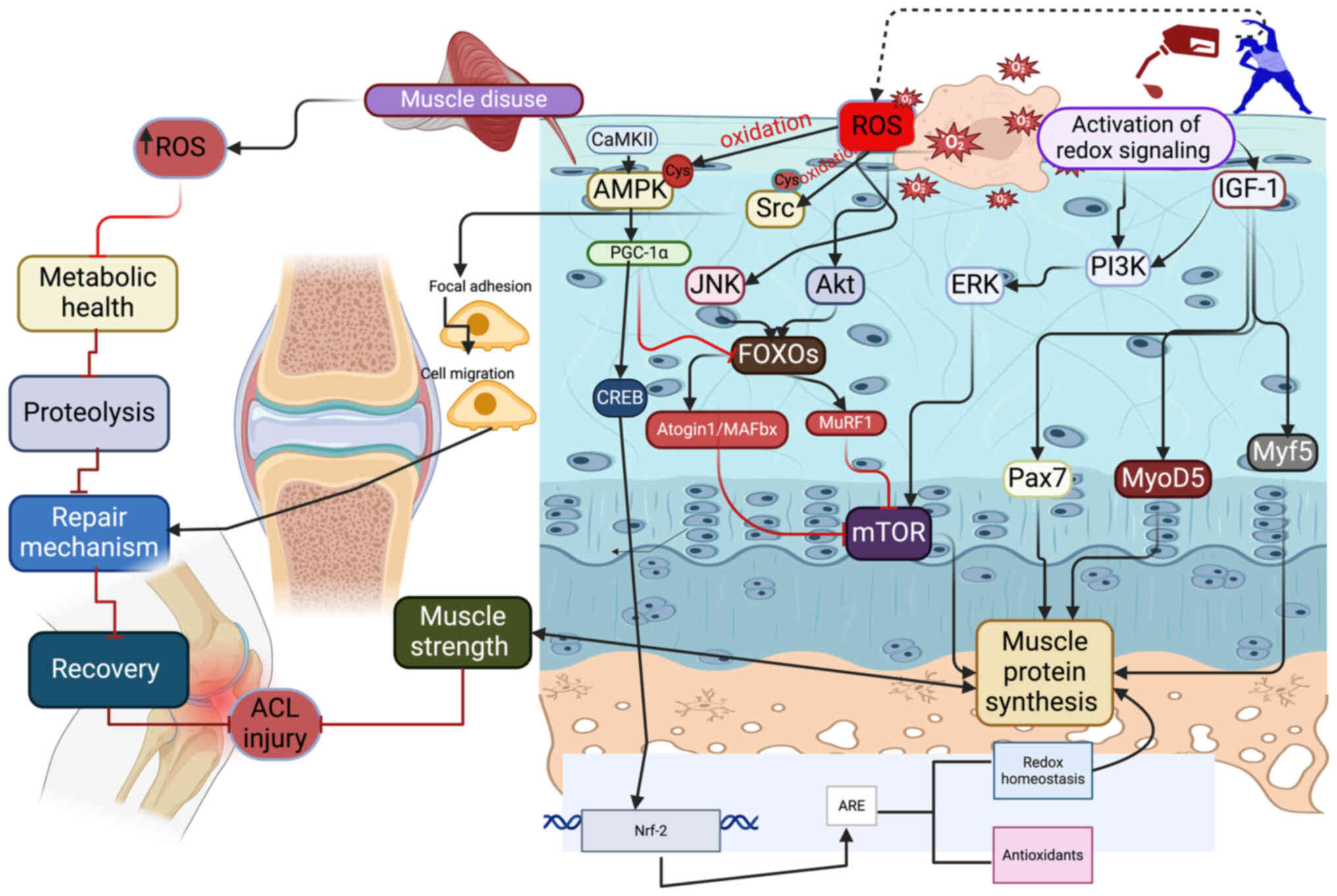 | Figure 1.Muscle disuse due to ACL injury
increases the levels of ROS, which in turn activate Akt, JNK and
FOXO to inhibit mTOR signaling and subsequent protein synthesis.
This affects metabolic health, increases proteolysis and inhibits
repair. Exercise-induced redox modifications of AMPK and Src can
improve tissue repair and remodeling, helping to prevent atrophy in
ACL injury. Additionally, the redox modification of IGF-1 due to
exercise can promote muscle protein synthesis via PI3K, ERK, mTOR,
Pax7, MyoD5 and Myf5. Physical exercise also induces CaMKII, which
in turn activates AMPK and PGC-1α, thus subsequently activating
CREB to upregulate Nrf-2. ACL, anterior cruciate ligament; ROS,
reactive oxygen species; JNK, c-Jun N-terminal kinase; FOXO,
forkhead transcriptional factor O; mTOR, mammalian target of
rapamycin; AMPK, AMP-activated protein kinase; IGF-1, insulin-like
growth factor 1; PI3K, phosphoinositide 3-kinase; ERK,
extracellular signal-regulated kinase; Pax7, paired box 7; MyoD5,
myoblast determination protein 1; Myf5, myogenic factor 5; CaMKII,
calmodulin-dependent protein kinase II; PGC-1α, peroxisome
proliferator-activated receptor γ coactivator-1 α; CREB, cAMP
response element-binding protein; Nrf-2, nuclear factor erythroid
2-related factor 2; MAFbx, F-Box/atrogin-1; MuRF1, muscle-specific
RING finger protein 1. |
The oxidation of insulin-like growth factor (IGF)
decreases its binding affinity to its receptors, potentially
affecting normal physiological processes such as meniscal repair
and promoting musculoskeletal health (17,18).
Furthermore, the oxidation of specific Cys residues on Src kinase
influences ACL regeneration by modulating focal adhesions (19), which are essential for cell
anchoring and migration in the ACL (20,21).
Therefore, redox signaling involving thiol groups may play a key
role in the recovery and healing of ACL injuries.
Thiol oxidation has been demonstrated to increase
the generation of H2O2 via reoxidation of the
active site of protein disulfide isomerase (22). This may increase the production of
ROS in muscles, thereby improving adaptation to exercise. In
addition, increased levels of H2O2 may
activate AMPK and peroxisome proliferator-activated receptor γ
coactivator-1 α (PGC-1α) to increase mitochondrial content, reduce
muscle damage and prevent atrophy in the context of ACL injury
(23).
Molecular pathways associated with the
regulation of redox balance in ACL recovery
Exercise has been suggested to improve ACL repair by
targeting redox-mediated molecular pathways. For example, the
exercise-induced oxidation of Kelch-like ECH-associated protein 1
(KEAP1) has been shown to activate nuclear factor erythroid
2-related factor 2 (Nrf-2), a major antioxidant regulator that
stimulates the transcription of antioxidant enzymes, including heme
oxygenase-1 and thioredoxin 1, to combat oxidative stress in the
ACL region (24). This effect
prevents oxidative stress-mediated chondrocyte injury and cartilage
degradation in the ACL, thereby reducing the risk of osteoarthritis
following ACL injury (25).
As chondrocytes are rich in mitochondria, the
increased oxygen demand induced by exercise may lead to hypoxic
conditions in the tissue, which in turn activates hypoxia-inducible
factor 1 and increases ROS production. However, this initial redox
perturbation can be counteracted by exercise-induced Nrf-2
signaling, which delays cartilage degradation and prevents
osteoarthritis in ACL-related injuries (26). Additionally, other pathways, such
as cAMP response element-binding protein-induced acetylation and
sirtuin 1-stimulated PGC-1α, have been demonstrated to contribute
to the upregulation and nuclear retention of Nrf-2 during exercise
(27).
The activation of Nrf-2 in fibroblasts can promote
extracellular matrix (ECM) production to facilitate tissue repair
in ACL injury (28). Furthermore,
the nuclear factor k-light-chain-enhancer of activated B cells
(NF-κB) pathway, another crucial pathway, has been suggested to
either increase the production of ROS by cyclooxygenase-2, NADPH
oxidase 2 (NOX2) and cytochrome p450, or reduce ROS levels via
modulation of the c-Jun kinase pathway (29). High-intensity exercise has been
shown to activate NF-κB signaling (29), leading to the increased production
of antioxidant enzymes and potentially promoting ACL repair via
calmodulin-dependent protein kinase II, mitogen-activated protein
kinase (MAPK) and signal transducer and activator of transcription
3 (STAT3) signaling pathways (Fig.
2) (30).
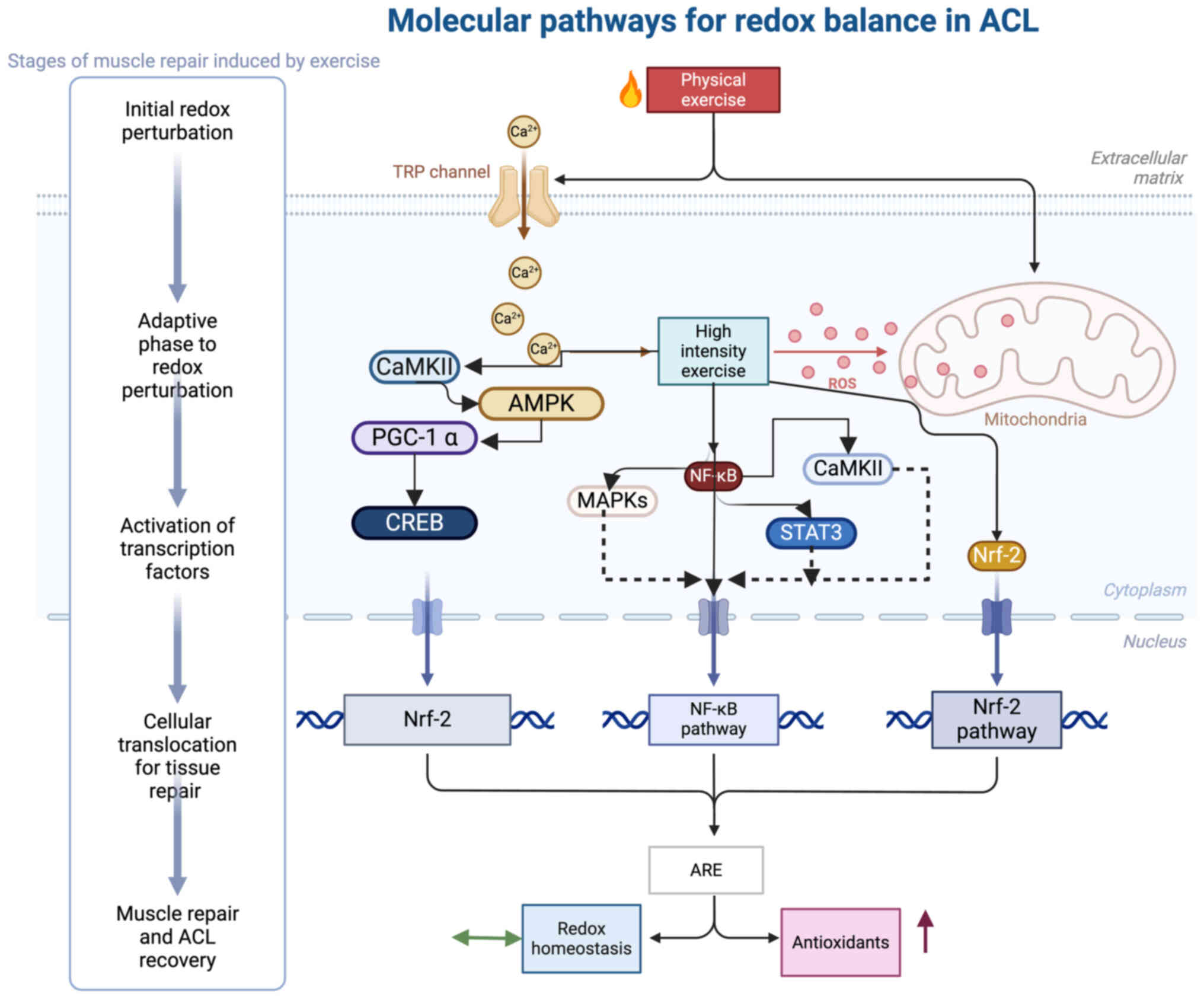 | Figure 2.Molecular pathways regulating the
redox system in ACL injuries during exercise. Physical exercise
induces CaMKII, which in turn activates AMPK and PGC-1α. The
subsequent activation of CREB upregulates Nrf-2. High-intensity
exercise promotes the production of ROS, which in turn activates
NF-κB signaling through MAPKs STAT3, thus triggering the Nrf-2
response. This results in an increased production of AREs and
antioxidants. An upward arrow (↑) indicates an increase in
antioxidants, while equivalence arrow (↔) represents a redox
balance. ACL, anterior cruciate ligament; CaMKII,
calmodulin-dependent protein kinase II; AMPK, a subunit of
AMP-activated protein kinase; PGC-1α, peroxisome
proliferator-activated receptor γ coactivator-1 α; CREB, cAMP
response element-binding protein; Nrf-2, nuclear factor erythroid
2-related factor 2; ROS, reactive oxygen species; NF-κB, nuclear
factor κ-light-chain-enhancer of activated B cells; MAPKs,
mitogen-activated protein kinases; STAT3, signal transducer and
activator of transcription 3; ARE, antioxidant response elements;
TRP, transient receptor potential. |
Targeting NF-κB expression has been indicated to
promote ACL repair by modulating the expression of various
metalloproteinases that play key roles in inflammation, cell
proliferation, differentiation and apoptosis in ACL fibroblasts
(31). Furthermore,
exercise-induced STAT3 signaling is critical for the regulation of
skeletal muscle growth and regeneration. STAT3 signaling has been
indicated to stimulate satellite cell expansion and facilitate the
repair of skeletal muscles via IL-6 (32). This regulation changes satellite
cell behavior and promotes myogenic lineage progression via
myogenic differentiation 1, which ultimately improves ACL recovery
(32).
Redox signaling mediates muscle atrophy in
ACL injury: Role of physical exercise
Although patients follow muscle strengthening
programs, muscle atrophy generally occurs in ACL injury (2,5,6).
Furthermore, the absence of countermeasures, such as exercise and
pharmacological interventions, can further increase atrophy in the
area affected by the ACL injury (7). Therefore, the identification of
molecular signaling pathways that may be targeted to restore the
health of muscles after ACL injury may help in the management of
this injury and prevent the predisposition to osteoarthritis.
Redox molecules are key catalytic regulators of
several molecular pathways during muscle strengthening. For
example, the mammalian target of the rapamycin (mTOR) pathway plays
a crucial role in protein synthesis, with ROS acting as the main
regulators of mTOR signaling (33,34).
In addition, ROS have been indicated to promote deactivation of the
mTOR signaling pathway via the activation of F-Box/atrogin-1
(MAFbx) and muscle-specific RING finger protein 1 (MuRF1) (35).
Forkhead transcriptional factor O (FOXO) protein,
which is also activated by ROS, is the main mediator of protein
turnover (33). Several signaling
pathways, including the extracellular signal-regulated kinase,
c-Jun N-terminal kinase, p38 MAPK and phosphoinositide 3-kinase
(PI3K)/Akt/mTOR pathways, are involved in FOXO-mediated protein
synthesis (36). These pathways
may collectively contribute to the amelioration of muscle mass and
strength in the ACL region following reconstruction.
Different aspects of exercise, such as its intensity
and duration, affect muscle strength gain. This may be due to their
impact on muscle protein synthesis and the associated molecular
pathways (11). For example,
studies have shown that the high intensity exercise-induced
production of ROS, including H2O2 and
ONOO−, enhances protein synthesis via the activation of
protein kinase B and mTOR, thus promoting muscle protein
translation and hypertrophy (11,37).
Consequently, this process may boost muscle strength during ACL
injury. Conversely, ROS can disrupt ribosomal activity and reduce
protein synthesis when muscles are not used sufficiently during ACL
injury. For example, prolonged muscle disuse during ACL injury can
increase ROS production, thereby inhibiting the proteolytic
activity of mammalian target of rapamycin complex 1, Akt and FOXO3,
and enhancing calpain activity to disrupt the contractile activity
of the muscle (37–39).
The initial adaptation to exercise disturbs muscle
protein synthesis and strength due to the elevation of ROS levels.
This is crucial in ACL injury, as it may exacerbate inflammation
and muscle degradation, and hinder recovery (39). In addition, other anabolic
signaling molecules, such as IGF-1, have been indicated to
accelerate protein synthesis through Akt activation during exercise
(40,41). For example, it has been shown that
4 weeks of aerobic exercise upregulate IGF-1, thereby promoting
protein synthesis and muscle growth via the PI3K/Akt signaling
pathway (40,41). This involves upregulation of the
expression of paired box 7, myoblast determination protein 1,
myogenic factor 5 and mTOR, and downregulation of the expression of
MuRF1 and MAFbx (42). As a
result, muscle atrophy following ACL injury is reduced, leading to
improved muscle strength. By contrast, extended exposure to low
levels of oxidants, such as H2O2, reduces Akt
phosphorylation and protein synthesis, thereby promoting
proteolysis and muscle wasting (41). Furthermore, oxidative stress is the
main regulator of muscle atrophy after ACL injury (43,44).
Therefore, the upregulation of antioxidants, such as manganese
superoxide dismutase, may help to attenuate atrophy and muscle
weakness following ACL injury (44).
Factors causing muscle atrophy in ACL injury
and the role of exercise-mediated redox signaling
Exercise is commonly prescribed to improve ACL
injuries. However, muscle atrophy can still occur, indicating that
an exercise program alone may not be sufficient to prevent muscle
atrophy after ACL injury. Other factors, such as age, sex, genetic
makeup and the molecular crosstalk induced by particular exercise
protocols, also require consideration (1,2)
(Fig. 3). Poor muscle regrowth
following ACL injury has been identified as a common factor that
delays the healing of an ACL injury (45). However, particular types of
exercise and the resulting fiber-type transitions have been
indicated to ameliorate this (45). For example, certain exercise types,
such as eccentric exercises, have been found to be particularly
effective in restoring muscle function after ACL injury, with ACL
rehabilitation programs involving eccentric exercises increasing
muscle strength by 30% compared with that achieved using concentric
exercises (45,46). Notably, one study demonstrated that
just 15 min of acute eccentric exercise improved muscle growth
without inducing muscle fiber injury (45). This may be due to the elastic
characteristics of the tendon unit, which protects the muscle from
damage (47).
Redox-mediated signaling, such as that involving the
mTOR pathway, is associated with muscle growth through the
phosphorylation of downstream targets, including p70S6k
(45). By contrast, over-training
may result in redox collapse, potentially reducing
p70S6k phosphorylation, inhibiting muscle growth and
contributing to the loss of muscle (48). In addition, elevated levels of ROS
and oxidative stress contribute to protein translation by
activating eukaryotic translation initiation factor 4E-binding
protein, a process possibly induced by p70S6k (48,49).
The phosphorylation levels of molecules from these pathways have
been indicated to vary during exercise according to its duration,
thereby affecting protein synthesis; for example, the activation of
p70S6k has been found to be elevated 6 h post-exercise
(50). However, other studies
found that protein synthesis was increased 24 h after a single bout
of exercise, suggesting that the type of exercise and the
experimental environment can affect muscle protein synthesis
(51,52).
Exercise-induced redox alterations have been
indicated to induce changes in fiber types. For example, a study
revealed that endurance performance shifts muscle fibers to a more
oxidative phenotype with higher oxygen consumption, thereby helping
to prevent atrophy in ACL injury (53). However, higher oxygen consumption
may disturb the oxidative environment and induce oxidative stress
and further injury (54). As a
result, a proteolytic event could be initiated, potentially
affecting muscle mass growth. Therefore, careful consideration of
the duration and intensity of endurance exercise is essential,
particularly following ACL injury.
Metabolic health in the ACL region is important in
promoting rapid recovery after ACL injury (55). In this context, exercise-induced
redox molecules, such as NAD+/NADH, can improve
mitochondrial respiration and muscle health by regulating
nicotinamide phosphoribosyltransferase (56). In addition, the exercise-mediated
activation of AKT and mTOR promotes the effective distribution of
glucose into the muscles around the ACL, aiding its recovery
(57). Furthermore, the redox
status of specific muscle fibers, influenced by different exercise
protocols, may improve muscle functions in the ACL region by
enhancing mitochondrial respiratory capacity, increasing muscle
capillarization and boosting maximal oxygen consumption (57). For example, performing aerobic
exercise for 2 weeks, ~30 min, has been indicated to alter the
redox status of muscle, resulting in improved muscle mass and
strength (57). In addition,
resistance-type exercises, comprising a 45-min session of upper and
lower body exercises three times per week, has been shown to
increase the NAD+ salvage capacity of skeletal muscle,
thereby improving protein synthesis and muscle strength following
ACL injury (58). Other studies
have shown that ACL injury impairs mitochondrial health and reduces
muscle size in the quadriceps (59,60).
This may be due to local redox collapse affecting the muscle
phenotype and mitochondrial content (60).
Association between proinflammatory
cytokines and redox molecules in muscle atrophy in ACL injury
Tissue breakdown following an ACL injury can trigger
the secretion of inflammatory cytokines, leading to muscle atrophy,
while the reduced expression of muscle growth factors commonly
results in a loss of muscle strength (45,46).
Studies have shown that patients who have undergone ACL
reconstruction exhibit elevated levels of cytokines and reduced
quadricep muscle strength, suggesting that cytokines contribute to
muscle atrophy (45,61). This finding may be attributed to
the initial redox flux in the injury sites increasing cytokine
synthesis and triggering catabolic processes in the muscle
(62). For example, the increased
production of ROS by NOXs or electron transport chains activates
the Nod-like receptor protein 3 inflammasome, thereby leading to
the activation of IL-1 and IL-18 (62). Furthermore, TNF-α activation
further increases ROS production (63), which initiates proteolytic events
in the muscle and results in muscle atrophy in ACL injury (45). However, while the initial bout of
exercise appears to trigger pro-inflammatory cytokines and
exacerbate redox homeostasis, the adaptive phase of the exercise
response has been indicated to induce anti-inflammatory cytokines
and antioxidants, ultimately improving muscle health (11). However, it is important to
determine whether exogenous supplements, such as vitamins C and E,
can help to mitigate the initial disruption of redox homeostasis
caused by exercise in ACL injury. One study demonstrated that
natural antioxidants, including vitamins C and E, as well as
certain polyphenols and terpenoids, effectively support tissue
regeneration and recovery in ACL injury (64). These biomolecules may regulate the
redox status by reversing inflammation in the tissue environment,
thereby creating a more favorable environment prior to surgical
implantation during ACL reconstruction (65).
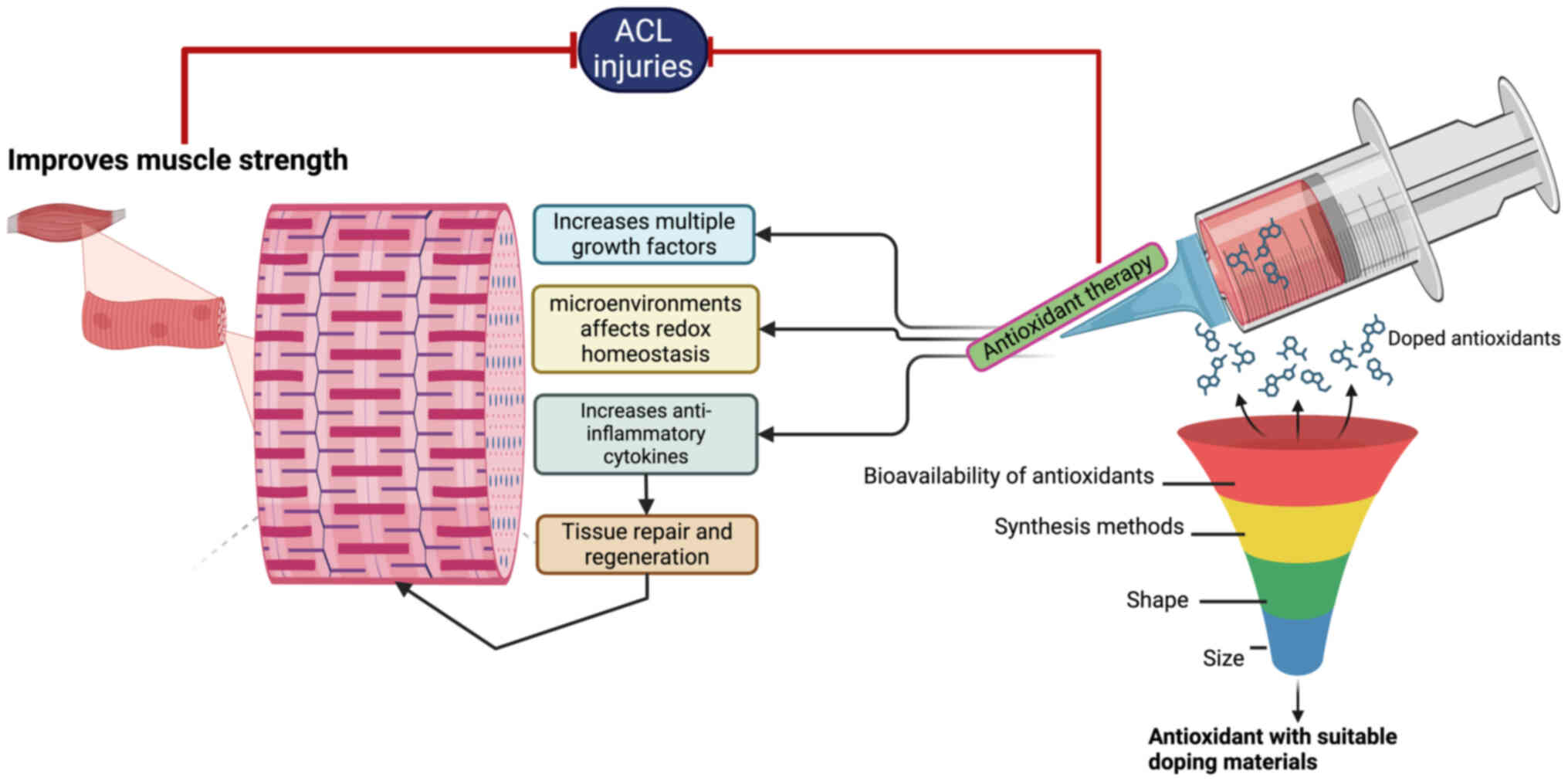
Targeted delivery of antioxidants affects
the tissue environment in ACL injury
When delivering antioxidants locally, it is
imperative to ensure that they are non-toxic to the surrounding
tissue. Otherwise, the antioxidants may exacerbate the ACL injury
during and after surgery. For example, specific doses of
antioxidants have been reported to activate multiple growth factors
and attenuate redox-related pathways that are critical for tissue
repair and regeneration (Fig. 4)
(66).
When administering antioxidants, achieving effective
delivery to the targeted tissues is of great importance (67). Biomaterials can help to preserve
the efficacy of antioxidants during their delivery to targeted
tissues. However, their biochemical properties, such as how they
interact with enzymes, and their biocompatibility and
biodegradability, may limit the advantages of these molecules
(68). The antioxidant dose can
influence redox homeostasis within the microenvironment of an ACL
injury. Therefore, the delivery of antioxidants using particular
biomaterials, such as coatings or scaffolds, can effectively
regulate the quantity of antioxidant delivered, improve redox
homeostasis around the ACL region, and promote the diffusion of the
antioxidant into the surrounding tissue, and increase
bioavailability (69). Studies
have demonstrated that nanoparticles, particularly gold
nanoparticles (AuNPs), are able to deliver antioxidants efficiently
(69,70). For example, superoxide dismutase
(SOD)-doped AuNPs can break the chain of free radical reactions by
inhibiting superoxide generation (69,71).
In addition, AuNPs doped with taurine can improve tissue repair in
the later phase of the healing process by reducing the expression
of IL-4 and IL-10 and upregulating myogenic regulatory factor 5,
thereby improving muscle recovery (69,70).
Also, a recent study reported that AuNPs alone were able to enhance
ACL graft performance in an ovine model. This improvement was
suggested to be due to the antioxidant and anti-inflammatory
properties of the AuNPs affecting the remodeling process following
ACL injury. However, the biological properties of AuNPs are
dependent upon their size, shape and method of synthesis (69,71).
Polyethylene terephthalate can be used to product
artificial ligaments that are commonly used for ACL reconstruction
(68). However, despite
polyethylene terephthalate having good mechanical properties, its
low biocompatibility reduces its efficiency. The fusion of
polyethylene glycol with appropriate antioxidants has been
demonstrated to restore the muscle structure, and ameliorate muscle
atrophy via the reduction of oxidative stress (72). Nanoemulsions infused with
antioxidants have exhibited the ability to decrease ROS formation
and promote muscle repair, which indicates that combining
antioxidants with appropriate delivery agents can yield favorable
outcomes after ACL reconstruction, with minimal non-specific side
effects (73).
Effect of redox-modifying drugs and exercise
on ACL injury
Redox-based drugs can help to mitigate oxidative
stress-induced muscle weakness associated with ACL injury and
atrophy. However, their efficacy remains unsatisfactory. For
example, antioxidants such as vitamin C and E have not been found
to improve muscle dysfunction following ACL surgery, suggesting
that they do not promote the synthesis of antioxidant enzymes, such
as SOD, catalase and glutathione peroxidase (GSH) (64). Therefore, combining physical
exercise with these supplements may be necessary to yield a more
positive effect on ACL-related injuries.
Targeting redox-sensitive proteins with
redox-modifying drugs may upregulate antioxidant response elements
(ARE) to enhance the production of antioxidants, thereby decreasing
ROS levels around or within the ACL region. For example, dimethyl
fumarate (DMF) and sulforaphane are redox-based drugs that target
the sulfhydryl groups of Cys residues, such as Cys151, Cys273 and
Cys288 in KEAP1. These drugs commonly interact with the Cys151
residue to disrupt the KEAP1-cullin 3 interaction, leading to the
accumulation of Nrf-2 in the nucleus along with ARE gene
activation. Combining these drugs with physical exercise may
further improve clinical outcomes and motoneuron inputs, as
exercise may modify their efficacy by boosting mitochondrial
function, reducing muscle fatigue and improving histopathology
through the promotion of Nrf-2 accumulation (74,75).
In addition, sulforaphane and curcumin have been shown to
ameliorate muscular dystrophy in mdx mice via the activation of
Nrf-2 (76,77), whereas running exercise alone,
without the activation Nrf-2 transcriptional activity, exhibits no
profound effect on muscle pathology (78). These findings highlight the
importance of combining these redox-based drugs with exercise to
optimize the activation of Nrf-2 and improve muscle recovery.
Other drugs, including ebselen and cadmium chloride,
have been indicated to interact with Cys273, Cys288, and C613
residues of KEAP1, causing conformational distortion of the DC
domain and dissociation of the KEAP1-Nrf-2 interaction, thereby
resulting in the nuclear accumulation of Nrf-2 (79). In addition, combining exercise with
ebselen increases muscle glucose uptake at rest by mimicking GSH
activity, suggesting that H2O2 plays a key
role in glucose uptake during exercise combined with ebselen
administration (80,81).
NOX is an important contributor to the formation of
ROS. Therefore, inhibiting NOX may potentially mitigate the
consequences of oxidative stress. Redox drugs, such as GKT1 36901,
GKT137831 and VAS2870, have been shown to inhibit the activity of
NOX1, NOX4, NOX5 and dual oxidases. For example, GKT1 36901
inhibits NOX1/4, resulting in decreased vasodilation in the
collateral-dependent arterioles after exercise training (82). In addition, GLX481304 selectively
inhibits the activity of NOX2 and NOX4, thereby decreasing ROS
production in cardiomyocytes and improving the contractile
properties of the heart (83).
Therefore, combining these NOX inhibitors with exercise could be an
effective strategy for the management of exercise-induced ROS
production.
Xanthine oxidase (XO), a key source of ROS
production, contributes to skeletal muscle and cardiovascular
injury during high-intensity physical exercise. A study
demonstrated that XO inhibitors, such as allopurinol, reduce muscle
atrophy via inhibition of the p38 MAPK/MAFbx pathway (84). Similarly, febuxostat blocks XO
activity, which has been shown to improve left ventricular function
and strengthen the myocardial system during intense treadmill
exercise (85). Furthermore,
nitric oxide (NO) inhibitors, such as
NG-mono-methyl-l-arginine, reduce NO levels to improve
metabolic effects and protein synthesis, and regulate blood flow
during continuous and dynamic exercise, thereby mitigating muscle
atrophy associated with ACL injury (86,87).
Conclusion
Exercise is considered as a key approach for the
prevention of ACL injuries. However, the recommendation of exercise
for ACL injuries requires careful consideration, since exercise
intensity and duration are associated with the dysregulation of
redox signaling homeostasis. This can affect muscle protein
synthesis, metabolic health, the repair mechanism around the ACL
region and recovery rate. The present review highlights the
potential of exercise to ameliorate ACL injuries by inducing redox
signaling, thereby preventing muscle atrophy. Notably, redox
molecules induced by exercise can trigger crosstalk among several
molecular pathways in ACL injury to prevent muscle atrophy. In
addition, the exercise-induced regulation of redox status in muscle
fibers affects fiber type transitions, helping to prevent atrophy
following ACL injury. However, the rate and duration of
redox-related molecule expression during exercise may guide the use
of redox-based therapies in combination with exercise to enhance
recovery and prevent atrophy in ACL injury.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AT was responsible for conceptualization. YW, CG,
HZ, ZL and AT prepared and wrote the original draft of the
manuscript. Data authentication is not applicable. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Musahl V and Karlsson J: Anterior cruciate
ligament tear. N Engl J Med. 380:2341–2348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arundale AJH, Bizzini M, Giordano A,
Hewett TE, Logerstedt DS, Mandelbaum B, Scalzitti DA,
Silvers-Granelli H and Snyder-Mackler L: Exercise-based knee and
anterior cruciate ligament injury prevention. J Orthop Sports Phys
Ther. 48:A1–A42. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Friel NA and Chu CR: The role of ACL
injury in the development of posttraumatic knee osteoarthritis.
Clin Sports Med. 32:1–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mather RC III, Koenig L, Kocher MS, Dall
TM, Gallo P, Scott DJ, Bach BR Jr and Spindler KP; MOON Knee Group,
: Societal and economic impact of anterior cruciate ligament tears.
J Bone Joint Surg Am. 95:1751–1759. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herzog MM, Marshall SW, Lund JL, Pate V
and Spang JT: Cost of outpatient arthroscopic anterior cruciate
ligament reconstruction among commercially insured patients in the
United States, 2005–2013. Orthop J Sports Med.
27:23259671166847762017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taylor JB, Waxman JP, Richter SJ and
Shultz SJ: Evaluation of the effectiveness of anterior cruciate
ligament Injury Prevention Programme training components: A
systematic review and meta-analysis. Br J Sports Med. 49:79–87.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sugimoto D, Myer GD, Barber Foss KD, Pepin
MJ, Micheli LJ and Hewett TE: Critical components of neuromuscular
training to reduce ACL injury risk in female athletes:
meta-regression analysis. Br J Sports Med. 50:1259–1266. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kwon OS, Davi SM, White MS and Lepley LK:
The role of mitochondrial-derived reactive oxygen species in
non-invasive anterior cruciate ligament injury. FASEB J. 34:1.
2020. View Article : Google Scholar
|
|
9
|
Flück M, Viecelli C, Bapst AM, Kasper S,
Valdivieso P, Franchi MV, Ruoss S, Lüthi JM, Bühler M, Claassen H,
et al: Knee extensors muscle plasticity over a 5-years
rehabilitation process after open knee surgery. Front Physiol.
9:13432018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ogata Y, Mabuchi Y, Shinoda K, Horiike Y,
Mizuno M, Otabe K, Suto EG, Suzuki N, Sekiya I and Akazawa C:
Anterior cruciate ligament-derived mesenchymal stromal cells have a
propensity to differentiate into the ligament lineage. Regen Ther.
8:20–28. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Powers SK, Ji LL, Kavazis AN and Jackson
MJ: Reactive oxygen species: Impact on skeletal muscle. Compr
Physiol. 1:941–969. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Powers SK, Deminice R, Ozdemir M,
Yoshihara T, Bomkamp MP and Hyatt H: Exercise-induced oxidative
stress: Friend or foe? J Sport Health Sci. 9:415–425. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jackson MJ, Stretton C and McArdle A:
Hydrogen peroxide as a signal for skeletal muscle adaptations to
exercise: What do concentrations tell us about potential
mechanisms? Redox Biol. 35:1014842020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kehm R, Baldensperger T, Raupbach J and
Höhn A: Protein oxidation-Formation mechanisms, detection and
relevance as biomarkers in human diseases. Redox Biol.
42:1019012021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zmijewski JW, Banerjee S, Bae H, Friggeri
A, Lazarowski ER and Abraham E: Exposure to hydrogen peroxide
induces oxidation and activation of AMP-activated protein kinase. J
Biol Chem. 285:33154–33164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang Y, Huang H, Zhou G, Wang Q, Gao F, Li
C, Liu Y and Lin J: An animal model study on the gene expression
profile of meniscal degeneration. Sci Rep. 10:214692020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
LeRoith D, Holly JMP and Forbes BE:
Insulin-like growth factors: Ligands, binding proteins, and
receptors. Mol Metab. 52:1012452021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Katsuragawa Y, Saitoh K, Tanaka N, Wake M,
Ikeda Y, Furukawa H, Tohma S, Sawabe M, Ishiyama M, Yagishita S, et
al: Changes of human menisci in osteoarthritic knee joints.
Osteoarthritis Cartilage. 18:1133–1143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heppner DE, Dustin CM, Liao C, Hristova M,
Veith C, Little AC, Ahlers BA, White SL, Deng B, Lam YW, et al:
Direct cysteine sulfenylation drives activation of the Src kinase.
Nat Commun. 9:45222018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murray MM, Martin SD and Spector M:
Migration of cells from human anterior cruciate ligament explants
into collagen-glycosaminoglycan scaffolds. J Orthop Res.
18:557–564. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Min J, Reznichenko M, Poythress RH,
Gallant CM, Vetterkind S, Li Y and Morgan KG: Src modulates
contractile vascular smooth muscle function via regulation of focal
adhesions. J Cell Physiol. 227:3585–35492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ellgaard L, Sevier CS and Bulleid NJ: How
are proteins reduced in the endoplasmic reticulum? Trends Biochem
Sci. 43:32–43. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Irrcher I, Ljubicic V and Hood DA:
Interactions between ROS and AMP kinase activity in the regulation
of PGC-1alpha transcription in skeletal muscle cells. Am J Physiol
Cell Physiol. 296:C116–C123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen J, Zhou R, Feng Y and Cheng L:
Molecular mechanisms of exercise contributing to tissue
regeneration. Signal Transduct Target Ther. 7:3832022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sanada Y, Tan SJO, Adachi N and Miyaki S:
Pharmacological targeting of heme oxygenase-1 in osteoarthritis.
Antioxidants (Basel). 10:4192021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu S, Zhang C, Ni L, Huang C, Chen D, Shi
K, Jin H, Zhang K, Li Y, Xie L, et al: Stabilization of HIF-1α
alleviates osteoarthritis via enhancing mitophagy. Cell Death Dis.
11:4812020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Y, Zhang X, Baker JS, Davison GW and
Yan X: Redox signaling and skeletal muscle adaptation during
aerobic exercise. iScience. 27:1096432024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hiebert P: The Nrf2 transcription factor:
A multifaceted regulator of the extracellular matrix. Matrix Biol
Plus. 10:1000572021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morgan MJ and Liu ZG: Crosstalk of
reactive oxygen species and NF-κB signaling. Cell Res. 21:103–115.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gallego-Selles A, Galvan-Alvarez V,
Martinez-Canton M, Garcia-Gonzalez E, Morales-Alamo D, Santana A,
Gonzalez-Henriquez JJ, Dorado C, Calbet JAL and Martin-Rincon M:
Fast regulation of the NF-κB signalling pathway in human skeletal
muscle revealed by high-intensity exercise and ischaemia at
exhaustion: Role of oxygenation and metabolite accumulation. Redox
Biol. 55:1023982022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang L, Xue R, Tang Z, Zhang J, Wang Y and
Sung KL: Role of NF-κB in the injury induced MMP expression and
activities in ACL. World Congress on Medical Physics and Biomedical
Engineering. Dössel O and Schlegel WC: IFMBE Proceedings Vol 4.
Springer; Berlin: 2009
|
|
32
|
Tierney MT, Aydogdu T, Sala D, Malecova B,
Gatto S, Puri PL, Latella L and Sacco A: STAT3 signaling controls
satellite cell expansion and skeletal muscle repair. Nat Med.
20:1182–1186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fang S, Wan X, Zou X, Sun S, Hao X, Liang
C, Zhang Z, Zhang F, Sun B, Li H and Yu B: Arsenic trioxide induces
macrophage autophagy and atheroprotection by regulating
ROS-dependent TFEB nuclear translocation and AKT/mTOR pathway. Cell
Death Dis. 12:882021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang Y, Kou J, Han X, Li X, Zhong Z, Liu
Z, Zheng Y, Tian Y and Yang L: ROS-dependent activation of
autophagy through the PI3K/Akt/mTOR pathway is induced by
hydroxysafflor yellow A-Sonodynamic therapy in THP-1 macrophages.
Oxid Med Cell Longev. 2017:85191692017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Edström E, Altun M, Hägglund M and Ulfhake
B: Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related
loss of skeletal muscle. J Gerontol A Biol Sci Med Sci. 61:663–674.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Klotz LO, Sánchez-Ramos C, Prieto-Arroyo
I, Urbánek P, Steinbrenner H and Monsalve M: Redox regulation of
FoxO transcription factors. Redox Biol. 6:51–72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lian D, Chen MM, Wu H, Deng S and Hu X:
The role of oxidative stress in skeletal muscle myogenesis and
muscle disease. Antioxidants (Basel). 11:7552022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Powers SK and Schrager M: Redox signaling
regulates skeletal muscle remodeling in response to exercise and
prolonged inactivity. Redox Biol. 54:1023742022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Keeble AR, Brightwell CR, Latham CM,
Thomas NT, Mobley CB, Murach KA, Johnson DL, Noehren B and Fry CS:
Depressed protein synthesis and anabolic signaling potentiate ACL
tear-resultant quadriceps atrophy. Am J Sports Med. 51:81–96. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feng L, Li B, Xi Y, Cai M and Tian Z:
Aerobic exercise and resistance exercise alleviate skeletal muscle
atrophy through IGF-1/IGF-1R-PI3K/Akt pathway in mice with
myocardial infarction. Am J Physiol Cell Physiol. 322:C164–C176.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tan PL, Shavlakadze T, Grounds MD and
Arthur PG: Differential thiol oxidation of the signaling proteins
Akt, PTEN or PP2A determines whether Akt phosphorylation is
enhanced or inhibited by oxidative stress in C2C12 myotubes derived
from skeletal muscle. Int J Biochem Cell Biol. 62:72–79. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bodine SC and Baehr LM: Skeletal muscle
atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am
J Physiol Endocrinol Metab. 307:E469–E484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Noehren B, Andersen A, Hardy P, Johnson
DL, Ireland ML, Thompson KL and Damon B: Cellular and morphological
alterations in the vastus lateralis muscle as the result of ACL
injury and reconstruction. J Bone Joint Surg Am. 98:1541–1547.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Latham CM, Balawender PJ, Thomas NT,
Keeble AR, Brightwell CR, Ismaeel A, Wen Y, Fry JL, Sullivan PG,
Johnson DL, et al: Overexpression of manganese superoxide dismutase
mitigates ACL injury-induced muscle atrophy, weakness and oxidative
damage. Free Radic Biol Med. 212:191–198. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lepley LK, Davi SM, Hunt ER, Burland JP,
White MS, McCormick GY and Butterfield TA: Morphology and anabolic
response of skeletal muscles subjected to eccentrically or
concentrically biased exercise. J Athl Train. 55:336–342. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lepley LK, Davi SM, Burland JP and Lepley
AS: Muscle atrophy after ACL injury: Implications for clinical
practice. Sports Health. 12:579–586. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Oberhofer K, Hosseini Nasab SH, Schütz P,
Postolka B, Snedeker JG, Taylor W and List R: The influence of
muscle-tendon forces on ACL loading during jump landing: A
systematic review. Muscles Ligaments Tendons J. 7:125–135. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Martin TD, Dennis MD, Gordon BS, Kimball
SR and Jefferson LS: mTORC1 and JNK coordinate phosphorylation of
the p70S6K1 autoinhibitory domain in skeletal muscle following
functional overloading. Am J Physiol Endocrinol Metab.
306:E1397–E1405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jones TW, Eddens L, Kupusarevic J, Simoes
DCM, Furber MJW, van Someren KA and Howatson G: Aerobic exercise
intensity does not affect the anabolic signaling following
resistance exercise in endurance athletes. Sci Rep. 11:107852021.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mitchell CJ, Churchward-Venne TA, Parise
G, Bellamy L, Baker SK, Smith K, Atherton PJ and Phillips SM: Acute
post-exercise myofibrillar protein synthesis is not correlated with
resistance training-induced muscle hypertrophy in young men. PLoS
One. 9:e894312014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Miller BF, Olesen JL, Hansen M, Døssing S,
Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj
JA, et al: Coordinated collagen and muscle protein synthesis in
human patella tendon and quadriceps muscle after exercise. J
Physiol. 567:1021–1033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kumar V, Atherton P, Smith K and Rennie
MJ: Human muscle protein synthesis and breakdown during and after
exercise. J Appl Physiol (1985). 106:2026–2039. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Plotkin DL, Roberts MD, Haun CT and
Schoenfeld BJ: Muscle fiber type transitions with exercise
training: Shifting perspectives. Sports (Basel). 9:1272021.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Meng Q and Su CH: The impact of physical
exercise on oxidative and nitrosative stress: Balancing the
benefits and risks. Antioxidants (Basel). 13:5732024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nyland J, Pyle B, Krupp R, Kittle G,
Richards J and Brey J: ACL microtrauma: Healing through nutrition,
modified sports training, and increased recovery time. J Exp
Orthop. 9:1212022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Amjad S, Nisar S, Bhat AA, Shah AR,
Frenneaux MP, Fakhro K, Haris M, Reddy R, Patay Z, Baur J and Bagga
P: Role of NAD+in regulating cellular and metabolic
signaling pathways. Mol Metab. 49:1011952021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shadiow J, Miranda ER, Perkins RK, Mazo
CE, Lin Z, Lewis KN, Mey JT, Solomon TPJ and Haus JM:
Exercise-induced changes to the fiber type-specific redox state in
human skeletal muscle are associated with aerobic capacity. J Appl.
Physiol (1985). 135:508–518. 2023. View Article : Google Scholar
|
|
58
|
de Guia RM, Agerholm M, Nielsen TS,
Consitt LA, Søgaard D, Helge JW, Larsen S, Brandauer J, Houmard JA
and Treebak JT: Aerobic and resistance exercise training reverses
age-dependent decline in NAD+salvage capacity in human
skeletal muscle. Physiol Rep. 7:e141392019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kuenze CM, Blemker SS and Hart JM:
Quadriceps function relates to muscle size following ACL
reconstruction. J Orthop Res. 34:1656–1662. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Davi SM, Ahn A, White MS, Butterfield TA,
Kosmac K, Kwon OS and Lepley LK: Long-Lasting impairments in
quadriceps mitochondrial health, muscle size, and phenotypic
composition are present after non-invasive anterior cruciate
ligament injury. Front Physiol. 13:8052132022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mendias CL, Lynch EB, Davis ME, Sibilsky
Enselman ER, Harning JA, Dewolf PD, Makki TA and Bedi A: Changes in
circulating biomarkers of muscle atrophy, inflammation, and
cartilage turnover in patients undergoing anterior cruciate
ligament reconstruction and rehabilitation. Am J Sports Med.
41:1819–1826. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxid Redox Signal. 20:1126–1167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ismail S, Sturrock A, Wu P, Cahill B,
Norman K, Huecksteadt T, Sanders K, Kennedy T and Hoidal J: NOX4
mediates hypoxia-induced proliferation of human pulmonary artery
smooth muscle cells: the role of autocrine production of
transforming growth factor-{beta}1 and insulin-like growth factor
binding protein-3. Am J Physiol Lung Cell Mol Physiol.
296:L489–L499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Barker T, Leonard SW, Trawick RH, Martins
TB, Kjeldsberg CR, Hill HR and Traber MG: Modulation of
inflammation by vitamin E and C supplementation prior to anterior
cruciate ligament surgery. Free Radic Biol Med. 46:599–606. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Barker T, Leonard SW, Trawick RH, Walker
JA and Traber MG: Antioxidant supplementation lowers circulating
IGF-1 but not F(2)-isoprostanes immediately following anterior
cruciate ligament surgery. Redox Rep. 14:221–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fadilah NIM, Phang SJ, Kamaruzaman N,
Salleh A, Zawani M, Sanyal A, Maarof M and Fauzi MB: Antioxidant
biomaterials in cutaneous wound healing and tissue regeneration: A
critical review. Antioxidants (Basel). 12:7872023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Comino-Sanz IM, López-Franco MD, Castro B
and Pancorbo-Hidalgo PL: The role of antioxidants on wound healing:
A review of the current evidence. J Clin Med. 10:35582021.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Multisanti CR, Zicarelli G, Caferro A,
Filice M, Faggio C, Vazzana I, Blahova J, Lakdawala P, Cerra MC,
Imbrogno S and Impellitteri F: From personal care to coastal
concerns: Investigating polyethylene glycol impact on Mussel's
antioxidant, physiological, and cellular responses. Antioxidants
(Basel). 13:7342024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Pinho RA, Haupenthal DPS, Fauser PE,
Thirupathi A and Silveira PCL: Gold nanoparticle-based therapy for
muscle inflammation and oxidative stress. J Inflamm Res.
15:3219–3234. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Thirupathi A, Guzzatti MFM, Corrêa MEAB,
Venturini LM, Casagrande LR, Lima IR, Da Costa C, De Pieri E,
Tietbohl LTW, Feuser PE, et al: Green synthesis of gold
nanoparticles with curcumin or açai in the tissue repair of palatal
wounds. Antioxidants (Basel). 12:15742023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tellechea E, Asensio AC, Ciaurriz P, Buezo
J, López-Gómez P, Urra M and Moran JF: A study of the interface of
gold nanoparticles conjugated to cowpea fe-superoxide dismutase.
Antioxidants (Basel). 11:20822022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Mongin C, Golden JH and Castellano FN:
Liquid PEG polymers containing antioxidants: A versatile platform
for studying oxygen-sensitive photochemical processes. ACS Appl
Mater Interfaces. 8:24038–24048. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
da Silva BD, do Rosário DKA, Neto LT,
Lelis CA and Conte-Junior CA: Antioxidant, antibacterial and
antibiofilm activity of nanoemulsion-based natural compound
delivery systems compared with non-nanoemulsified versions. Foods.
12:19012023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Timpani CA, Kourakis S, Debruin DA,
Campelj DG, Pompeani N, Dargahi N, Bautista AP, Bagaric RM, Ritenis
EJ, Sahakian L, et al: Dimethyl fumarate modulates the dystrophic
disease program following short-term treatment. JCI Insight.
8:e1659742023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bernardes D and de Oliveira ALR: Regular
exercise modifies histopathological outcomes of pharmacological
treatment in experimental autoimmune encephalomyelitis. Front
Neurol. 9:9502018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sun C, Yang C, Xue R, Li S, Zhang T, Pan
L, Ma X, Wang L and Li D: Sulforaphane alleviates muscular
dystrophy in mdx mice by activation of Nrf2. J Appl Physiol (1985).
118:224–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Pan Y, Chen C, Shen Y, Zhu CH, Wang G,
Wang XC, Chen HQ and Zhu MS: Curcumin alleviates dystrophic muscle
pathology in mdx mice. Mol Cells. 25:531–537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Bronisz-Budzynska I, Kozakowska M,
Podkalicka P, Kachamakova-Trojanowska N, Łoboda A and Dulak J: The
role of Nrf2 in acute and chronic muscle injury. Skelet Muscle.
10:352020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Baird L and Yamamoto M: The molecular
mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol.
40:e00099–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Noguchi N: Ebselen, a useful tool for
understanding cellular redox biology and a promising drug candidate
for use in human diseases. Arch Biochem Biophys. 595:109–112. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Margaritelis NV, Paschalis V, Theodorou
AA, Kyparos A and Nikolaidis MG: Redox basis of exercise
physiology. Redox Biol. 35:1014992020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sytha SP, Bray JF and Heaps CL: Exercise
induces superoxide and NOX4 contribution in endothelium-dependent
dilation in coronary arterioles from a swine model of chronic
myocardial ischemia. Microvasc Res. 150:1045902023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Szekeres FLM, Walum E, Wikström P and
Arner A: A small molecule inhibitor of Nox2 and Nox4 improves
contractile function after ischemia-reperfusion in the mouse heart.
Sci Rep. 11:119702021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sanchis-Gomar F, Pareja-Galeano H,
Perez-Quilis C, Santos-Lozano A, Fiuza-Luces C, Garatachea N, Lippi
G and Lucia A: Effects of allopurinol on exercise-induced muscle
damage: new therapeutic approaches? Cell Stress Chaperones.
20:3–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hou M, Hu Q, Chen Y, Zhao L, Zhang J and
Bache RJ: Acute effects of febuxostat, a nonpurine selective
inhibitor of xanthine oxidase, in pacing induced heart failure. J
Cardiovasc Pharmacol. 48:255–263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fryburg DA: NG-monomethyl-L-arginine
inhibits the blood flow but not the insulin-like response of
forearm muscle to IGF-I: Possible role of nitric oxide in muscle
protein synthesis. J Clin Invest. 97:1319–1328. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hickner RC, Fisher JS, Ehsani AA and Kohrt
WM: Role of nitric oxide in skeletal muscle blood flow at rest and
during dynamic exercise in humans. Am J Physiol. 273:H405–H410.
1997.PubMed/NCBI
|