Introduction
Prostate cancer (PCa) is one of the most prevalent
malignancies among men worldwide, and presents a significant public
health concern (1). It is the
second leading cause of cancer-associated mortality globally, with
its incidence varying widely across different geographical groups
and ethnic groups (1). Established
risk factors include advancing age, family history and genetic
predispositions, such as mutations in the BRCA1 and BRCA2 genes
(2). In addition, lifestyle
factors, including diet and obesity, are considered to contribute
to the risk of developing PCa (3).
Current therapeutic strategies for the management of PCa encompass
a range of options, including active surveillance, radical
prostatectomy, radiation therapy, androgen-deprivation therapy
(ADT) and, for advanced stages, chemotherapy and immunotherapy
(4). Despite these treatments,
effective management remains challenging due to variability in
disease progression and treatment responses among individuals
(5).
Prostate-specific antigen (PSA) testing is widely
used for the early diagnosis of PCa; however, it has several
limitations. Elevated PSA levels are not specific to PCa and may
also result from benign prostatic hyperplasia or prostatitis,
leading to false-positive results and unnecessary biopsies
(6). Furthermore, PSA does not
differentiate between indolent and aggressive forms of PCa, which
can complicate treatment decisions (7). The treatment of castration-resistant
PCa (CRPC) also presents considerable challenges. CRPC is
characterized by tumor progression despite the levels of
testosterone being at levels typical of castration, often resulting
in a poor prognosis and promoting a requirement for novel
therapeutic modalities (8).
Although advancements such as second-generation antiandrogens and
targeted therapies have been achieved, the heterogeneity of CRPC
leads to highly variable treatment responses among patients,
indicating that more personalized approaches are necessary
(9).
Advancements in multi-omics technologies, including
genomics, transcriptomics, proteomics and metabolomics, have
profoundly transformed cancer research by providing a comprehensive
view of the molecular landscape of tumors (10). Multi-omics approaches facilitate a
deeper understanding of tumor heterogeneity and the molecular
mechanisms underlying disease progression (11). Multi-omics methodologies are
increasingly applied in PCa research, yielding promising results
that enhance the understanding of PCa biology. For example, the
integration of genomic and epigenomic data has facilitated the
identification of novel therapeutic targets and biomarkers that may
assist in earlier diagnosis and the development of more effective
treatment strategies (12). In
addition, alterations in certain pathways, such as androgen
receptor (AR) signaling and metabolic reprogramming pathways, have
been demonstrated to play crucial roles in the progression of PCa
(13) (Fig. 1).
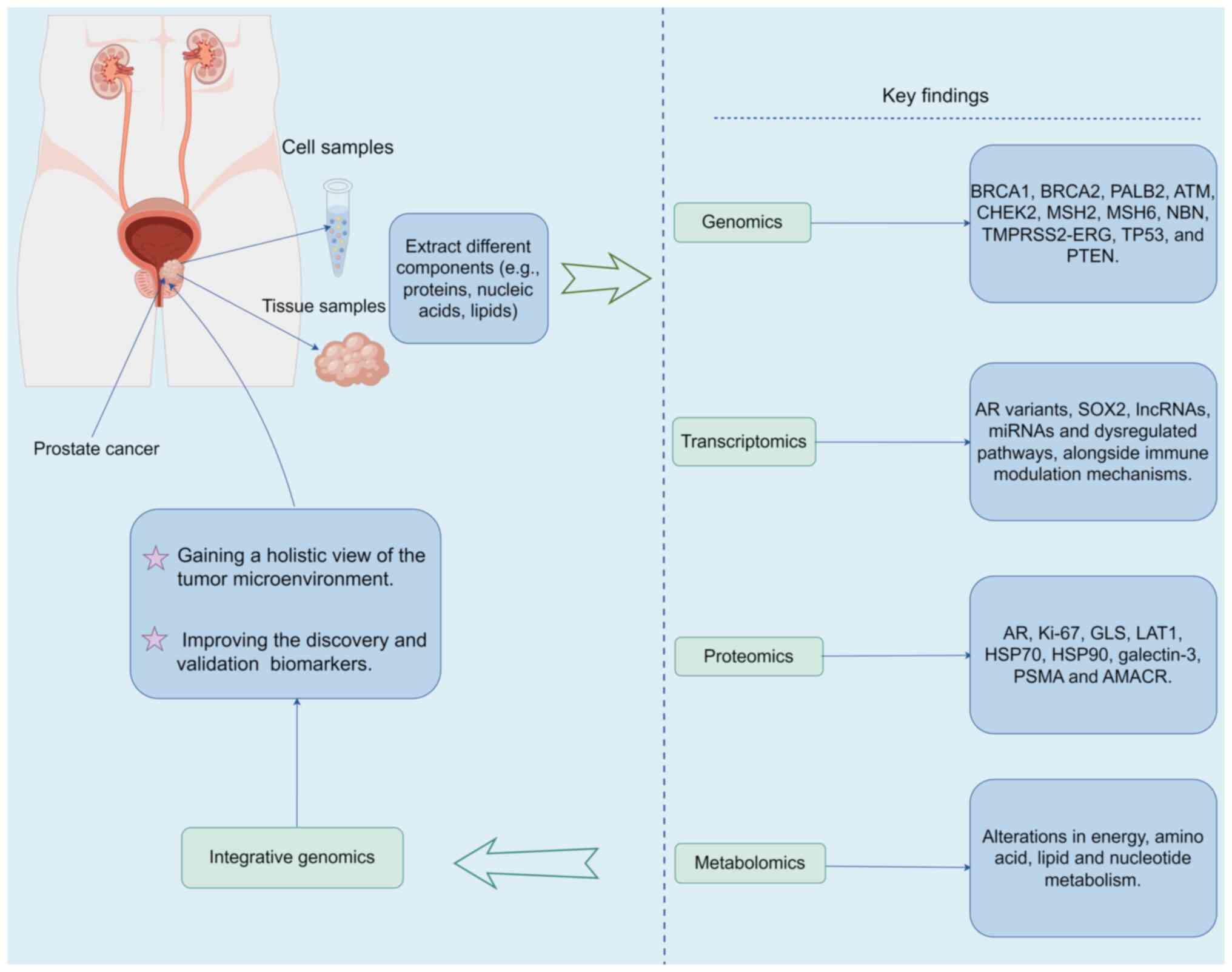 | Figure 1.Summary of the application of
multiple omics to PCa. Schematic illustrating numerous specific
biomarkers, signaling pathway changes and metabolic changes
associated with PCa that have been identified via multi-omics
studies. The figure was generated by Figdraw (www.figdraw.com; copyright code, ATAPU55533). PCa,
prostate cancer; PALB2, partner and localizer of BRCA2; ATM, DNA
mismatch repair gene; CHEK2, checkpoint kinase 2; MSH2/6, MutS
homolog 2/6; NBN, nibrin; TMPRSS2, transmembrane protease, serine
2; ERG, ETS-related gene; PTEN, phosphatase and tensin homolog
deleted on chromosome ten; AR, androgen receptor; SOX2, sex
determining region Y-box 2; lncRNAs, long non-coding RNAs; miRNAs,
microRNAs; GLS, glutaminase; LAT1, L-type amino acid transporter 1;
HSP70/90, heat shock protein 70/90; PSMA, prostate-specific
membrane antigen; AMACR, a-methylacyl-CoA racemase. |
The present review aims to explore the advances in
multi-omics technology applied to PCa research, emphasizing their
potential to transform diagnostic, prognostic and therapeutic
frameworks in the management of this disease. By synthesizing
current knowledge on multi-omics applications in PCa, the review
seeks to highlight how these innovations can offer new insights
into patient stratification and therapeutic targets, ultimately
leading to improved clinical outcomes. This exploration is not only
timely but also crucial for advancing the understanding and
management of PCa in an era increasingly driven by personalized
medicine.
Genomic research
Genomic research over the past decade has
fundamentally shifted the landscape of PCa diagnosis and treatment
strategies. The characterization of mutations, such as those in
BRCA1, BRCA2 and transmembrane protease, serine 2
(TMPRSS2)-ETS-related gene (ERG), has provided valuable insights
into the mechanisms driving PCa and has ushered in the era of
precision medicine. The implications for risk stratification and
treatment decisions are profound, as clinicians now have access to
tools that enable personalized therapy approaches. The ongoing
integration of genomic mapping into clinical practice continues to
hold promise for improving survival outcomes and the quality of
life for men diagnosed with PCa.
Key findings from the last decade in
PCa genomic studies
PCa has become a focus of intense genomic research
over the last decade, which has provided insights into its
pathogenesis and avenues for future therapeutic interventions. Key
findings have highlighted the roles of several important genes,
including BRCA1, BRCA2, partner and localizer of BRCA2 (PALB2), DNA
mismatch repair gene (ATM), checkpoint kinase 2 (CHEK2), MutS
homolog 2 (MSH2), MSH6, nibrin (NBN), TMPRSS2-ERG, TP53 and
phosphatase and tensin homolog deleted on chromosome ten (PTEN)
(Table I). The findings deepen
understanding of the biological behavior of PCa and pave the way
for the development of targeted therapies and personalized
treatment strategies. Future research is essential to explore the
therapeutic potential of targeting these genetic alterations, with
the goal of improving outcomes for patients with PCa.
 | Table I.Key findings from genomic studies on
PCa in the last decade. |
Table I.
Key findings from genomic studies on
PCa in the last decade.
| First author,
year | Gene | Samples | Major findings | Location | (Refs.) |
|---|
| Robinson et
al, 2015 | BRCA2, BRCA1,
ATM | Bone or soft tissue
tumor biopsies from 150 patients with mCRPC | Frequency of BRCA2,
BRCA1 and ATM aberrations in mCRPC is significantly higher compared
with that in primary PCa | United States | (14) |
| Chi et al,
2023 | BRCA2, BRCA1,
ATM | Matched tumor
tissue and circulating tumor DNA from patients with prostate cancer
screened in PROfound | BRCA1, BRCA2 and
ATM alterations were detected in both tumor tissue and circulating
tumor DNA, highlighting the potential for liquid biopsies to inform
treatment decisions | Various | (15) |
| Wokołorczyk et
al, 2021 | PALB2 | 5,472 unselected
cases of PCa and 8,016 controls | PALB2 mutations
predispose to an aggressive and lethal form of PCa | Poland | (16) |
| Karlsson et
al, 2021 | ATM | 5,560 cases and
3,353 controls of European ancestry | Carriers of
pathogenic ATM variants have an elevated risk of developing PCa and
increased risk of earlier-onset disease presentation | Europe | (17) |
| Alorjani et
al, 2023 | CHEK2 | 74 patients with
radical prostatectomy | CHEK2 mutation
frequency 1.4% supports the role of genetic variants of this gene
in the development of PCa | Jordan | (18) |
| Sharma et
al, 2020 | MSH2, MSH6 | 220 radical
prostatectomy specimens | MSH2 and MSH6
protein loss is associated with significantly elevated PSA levels
and helps predict tumor recurrence after radical prostatectomy | United States | (19) |
| Rusak et al,
2019 | NBN | 5,189 cases of PCa
and 6,152 controls | NBN mutation
predisposes to poor prognosis in PCa | Poland | (20) |
| Maxwell et
al, 2022 | TP53 | 31 PCa cases | Inherited
pathogenic TP53 variants predispose to aggressive PCa | United States | (21) |
| Imada et al,
2021 | PTEN | 1,832 cases of
PCa | PTEN somatic gene
mutation is associated with increased overall mortality in patients
with PCa | United States | (22) |
Robinson et al (14) conducted a comprehensive genomic
analysis of advanced PCa, which identified that alterations in
BRCA2 and ATM are particularly relevant to the aggressiveness and
therapeutic response of the disease. While BRCA1 and BRCA2
mutations are well-established as risk factors for hereditary
breast and ovarian cancer, they are increasingly being recognized
as being associated with an increased risk and poorer outcomes in
patients with PCa (15). PALB2 a
gene involved in DNA damage repair, has also been identified as a
significant risk factor for PCa. A study found that mutations in
PALB2 are associated with an elevated risk of developing PCa and
may impact survival rates, underscoring its importance in
hereditary cancer predisposition (16). ATM is a key gene in the cellular
response to DNA double-strand breaks, and its mutation predisposes
individuals to PCa, as discussed in the PRACTICAL Consortium study
(17). This mutation is considered
a key germline variant that is important to consider in the genetic
counseling of patients at risk. Similarly, CHEK2, which is involved
in the DNA damage response, has been shown to harbor germline
variants that contribute to cancer susceptibility, albeit with
varying penetrance (18).
Deficiency in the DNA mismatch repair system,
involving genes such as MSH2 and MSH6, has been implicated in PCa.
Sharma et al (19)
highlighted that the loss of the proteins encoded by these genes is
associated with a distinct subset of aggressive tumors,
highlighting the importance of genomic stability in PCa. Hereditary
genes have been identified as contributors to PCA, but somatic
alterations are also crucial in PCa pathology. Inherited mutations
in NBN have been identified as a risk factor, with carriers of NBN
mutations being significantly more likely to develop PCa, which
highlights the importance of DNA double-strand break repair
mechanisms in cancer susceptibility (20). Furthermore, mutations in TP53 and
PTEN are well-documented in advanced PCa. As noted by Maxwell et
al (21), TP53 alterations are
significantly associated with poorer clinical outcomes, while PTEN
loss, associated with increased tumor aggressiveness, drives the
progression of PCa (22). The
interplay between these genetic alterations and their collective
impact on tumor biology and therapeutic resistance is an active
area of ongoing research.
Implications of genomic research for
risk stratification and treatment decisions
The integration of genomic data into clinical
practice has greatly improved risk stratification in PCa. Genomic
tests, such as the Decipher biopsy and Oncotype DX, utilize gene
expression profiles to improve the classification of patients into
risk groups, extending beyond traditional clinicopathological
parameters (23). This
stratification enables clinicians to identify patients at a higher
risk of aggressive disease and to tailor treatment approaches
accordingly. For instance, genomic profiling has been instrumental
in guiding decisions regarding active surveillance compared with
intervention. It has enabled patients with low genomic risk scores
to avoid overtreatment, and those with high risk scores to be
directed toward earlier and potentially more aggressive treatment
options, including intensity-modulated radiotherapy or radical
prostatectomy (24). However, the
application of genomic profiling to guide treatment decisions, such
as choosing between active surveillance and intervention, has
certain limitations. Patients classified at low genomic risk may
have aggressive disease characteristics not accounted for by
existing models, potentially leading to undertreatment. Conversely,
patients with high-risk scores may respond favorably to
conservative management, indicating that genomic scores do not
capture the full clinical complexity of PCa.
Genomic alterations also influence systemic
therapies. For example, BRCA1/BRCA2 mutations have been found to be
associated with sensitivity to therapies targeting DNA repair
mechanisms, including poly (ADP ribose) polymerase (PARP)
inhibitors (25). Similarly,
alterations in AR signaling pathways, such as mutations and
amplifications of the AR gene, can guide the use of AR-targeted
therapies such as abiraterone and enzalutamide (26). However, the presence of AR
signaling pathway alterations further complicates treatment
decisions, as their presence does not guarantee the enhanced
efficacy of AR-targeted therapies. Therefore, while genomic
research is propelling the evolution of personalized treatment
paradigms in PCa, clinicians must address the challenges associated
with variability in research outcomes and the limitations of
existing genomic tools to ensure optimal patient management.
Clinical applications of genomic
mapping
A notable case study demonstrates the clinical
application of genomic findings in a patient with metastatic CRPC
(mCRPC) harboring a BRCA2 mutation. Following genomic profiling,
the patient was treated with the PARP inhibitor olaparib, and
imaging revealed a significant reduction in tumor burden. This case
exemplifies how genomic data not only informs treatment eligibility
but also directly impacts clinical outcomes (27). While this case highlights the
potential for genomic data to inform therapy decisions and improve
clinical outcomes, questions remain concerning the generalizability
of such results, as individual patient responses can vary widely
due to genetic and phenotypic differences.
Another example comes from a cohort of patients
undergoing active surveillance, where genomic profiling revealed
the presence of the TMPRSS2-ERG fusion gene. Patients who tested
positive for this fusion exhibited higher rates of progression,
prompting a reconsideration of their management strategy. In
patients undergoing active surveillance, the presence of
TMPRSS2-ERG fusion and an increased genomic risk score were
significantly associated with a higher likelihood of disease
progression, demonstrating the potential for genomic data to alter
clinical management plans (28).
However, the reliability of the TMPRSS2-ERG fusion as a predictive
biomarker remains inconsistent across studies. For instance, a
meta-analysis found that the predictive significance of TMPRSS2-ERG
fusion in prostate cancer is not universally supported, with a
study showing no significant association between the fusion and
disease progression (29).
Additionally, a study by Álvarez-Garcia et al (30) highlighted the diversity of
mechanisms leading to TMPRSS2-ERG fusion and questioned its
clinical utility as a biomarker, emphasizing the need for further
research to clarify its role. The risk of over-treatment based on
genomic findings requires careful consideration.
Genomic mapping plays a critical role in the
stratification of patients based on treatment response in PCa. For
example, a study demonstrated that genomic alterations could guide
therapy choices, thereby enhancing personalized treatment
approaches (14). This
stratification enabled clinicians to optimize therapeutic regimens
and improve patient outcomes. However, the efficacy of such
stratification relies on a thorough understanding of the underlying
mechanisms and the potential impact of confounding factors on
treatment responses. Therefore, while genomic mapping holds
significant promise for improving PCa management, the ongoing
evaluation of its clinical utility and the robustness of its
predictive capabilities is essential to ensure that it translates
into improved patient care.
Transcriptomic advances
Advancements in transcriptomic research for PCa have
revolutionized the understanding of gene expression variability and
enabled the identification of promising biomarkers. These have
facilitated the generation of predictive models for treatment
response and paved the way for targeted therapies. As research
evolves, the integration of transcriptomic data with other omics
approaches is likely to yield further insights, further enhancing
the precision of PCa management and improving patient outcomes.
Role of transcriptomics in
understanding gene expression variability
Transcriptomics, the study of RNA transcripts
produced by the genome under specific circumstances, has markedly
improved the understanding of gene expression variability in PCa.
By employing high-throughput sequencing and microarray
technologies, the expression profiles of thousands of genes can be
analyzed simultaneously, revealing the molecular complexities of
PCa. This variability in gene expression is influenced by numerous
factors, including genetic mutations, epigenetic changes and the
tumor microenvironment (TME). Studies have demonstrated distinct
transcriptional subtypes of PCa that are associated with clinical
outcomes. For example, one landmark study comprehensively analyzed
gene expression profiles associated with different grades of
prostate tumors and discovered that specific gene sets associated
with cell cycle regulation, androgen response and immune response
significantly varied across these grades (31). In another study, the differential
expression of the MYC oncogene and its downstream targets was
implicated in PCa progression, which emphasizes the role of
oncogenic signaling pathways in the etiology of the disease
(32).
Transcriptomic analysis has led to the
identification of alternative splicing events and non-coding RNAs
that contribute to the complexity of gene regulation in PCa. For
example, the long non-coding RNA (lncRNA) PCAT-1 has been shown to
promote cell proliferation, and found to be upregulated in a subset
of aggressive PCa cases, suggesting its potential as a therapeutic
target (33). Thus,
transcriptomics provides critical insights into the dynamic
regulation of gene expression and its relevance to PCa
pathogenesis.
Identification of novel
biomarkers
Advances in transcriptomic research have provided
valuable insights into the molecular landscape of PCa and revealed
numerous novel therapeutic targets. Factors including AR variants
(34), sex determining region
Y-box 2 (SOX2) (35), lncRNAs,
microRNAs (miRNAs), dysregulated pathways and immune modulation
mechanisms, constitute promising areas for future drug development
(Table II). Consideration of
these findings together supports the clinical exploitation of these
targets to provide more personalized and effective treatment
strategies for patients with PCa. Furthermore, the continued
integration of transcriptomic data with clinical outcomes will be
essential for translating these discoveries into actionable
therapies.
 | Table II.Identification of new biomarkers for
PCa. |
Table II.
Identification of new biomarkers for
PCa.
| First author,
year | Biomarkers | Functions in
PCa | (Refs.) |
|---|
| Yang et al,
2024 | AR and its
variants | AR-V7, a splice
variant of AR mRNA with truncation of the ligand-binding domain, is
a biomarker for resistance to AR axis-targeted therapies | (34) |
| de Wet et
al, 2022 | SOX2 | SOX2 promotes PCa
metastasis, lineage plasticity and treatment resistance by
mediating the metabolic reprogramming of PCa cells | (35) |
| Fu et al,
2022 | PCGEM1 | lncRNA PCGEM1
promotes the progression of PCa by sponging miR-129-5p as a ceRNA
of CDT1 | (39) |
| Mu et al,
2022 | MALAT1 | lncRNA MALAT1
controls glucose metabolism and PCa progression by upregulating the
MYBL2-mTOR axis | (40) |
| Zeng et al,
2021 | miR-145 | miR-145 suppresses
the motility of PCa cells via the post-transcriptional
downregulation of CDH2 expression; miR-145-CDH2 may be a potential
target for intervention in PCa metastasis | (42) |
| Gui et al,
2017 | miR-221/222 | miR-221 and −222
function as oncogenes, promoting PCa cell proliferation and the
development of CRPC | (43) |
| Pungsrinont et
al, 2021 | PI3K-AKT-mTOR | Alterations in the
PI3K-AKT-mTOR pathway are associated with poor prognosis, driving
tumor growth and metastasis | (45) |
| Guan et al,
2017 | Wnt/β-catenin | Aberrant activation
of Wnt/β-catenin signaling pathway is associated with the
progression of PCa to an aggressive phenotype | (47) |
| Conley-LaComb et
al, 2013 | CXCL12/CXCR4 | Activation of
CXCL12 signaling through CXCR4 in PCa is driven by the loss of PTEN
and subsequent activation of Akt; Akt-associated CXCL12/CXCR4
signaling promotes PCa growth | (51) |
AR signaling is a cornerstone in the progression of
PCa. Transcriptomic studies have identified AR splice variants,
particularly AR splice variant 7 (AR-V7), which lack the
ligand-binding domain and are associated with resistance to ADT.
Notably, these studies indicate that targeting AR-V7 with small
molecule inhibitors or RNA-targeting therapies may improve
treatment outcomes in patients with CRPC (34,36).
SOX2 has also been implicated in promoting PCa stem cell properties
and tumor growth (37). High SOX2
expression is associated with a poor prognosis, suggesting the
potential of SOX2 as a therapeutic target. Therefore, inhibitors
targeting SOX2 signaling pathways may serve as novel strategies for
the treatment of advanced PCa (38).
Advances in transcriptomics have underscored the
role of lncRNAs in PCa pathogenesis. For example, lncRNA PCa gene
expression marker 1 (PCGEM1) is upregulated in prostate tumors and
is associated with cancer cell proliferation and migration
(39). In addition, silencing
PCGEM1 expression has been shown to reduce tumor growth in
vivo, supporting its potential as a therapeutic target
(39). Another lncRNA, metastasis
associated lung adenocarcinoma transcript 1 (MALAT1), has been
found to play an important role in the epithelial-mesenchymal
transition (EMT) of PCa cells (40). By regulating the splicing of its
target genes, MALAT1 attenuates EMT and potentially inhibits PCa
metastasis (41). miRNAs are key
regulatory molecules in gene expression that have been found to
contribute to tumorigenesis in PCa. For example, miR-145 suppresses
PCa cell proliferation by targeting multiple oncogenes (42). In addition, the loss of miR-145
expression is associated with advanced disease stages, suggesting
the potential of miR-145 as a therapeutic agent that could be
restored using miRNA mimic strategies. Furthermore, the miR-221/222
cluster is implicated in androgen resistance (43). Targeting this miRNA cluster has
demonstrated promising results in re-sensitizing CRPC cells to
androgen therapies (44),
highlighting its potential as an adjunct treatment option.
Transcriptomic analyses have revealed dysregulation
of the phosphoinositide 3-kinase-AKT-mTOR pathway in PCa, which is
frequently associated with poor prognosis, tumor growth and
metastasis (45). Therapeutic
strategies targeting this pathway, such as AKT inhibitors, have
shown some effectiveness and are being explored in clinical trials
(46), suggesting that this
pathway could be a viable target for novel PCa therapies. The
Wnt/β-catenin signaling pathway is another critical area of
interest identified through transcriptomic analysis. Aberrant
activation of this pathway is associated with the progression of
PCa to an aggressive phenotype (47). Targeting Wnt/β-catenin signaling
with small molecule inhibitors has been suggested to represent a
prospective therapeutic strategy, particularly in patients
exhibiting dysregulation of this pathway (48).
A number of transcriptomic studies have focused on
the interaction between PCa cells and the TME, particularly the
immune landscape. These studies reveal that high expression levels
of immune checkpoint molecules, along with T cell exhaustion
markers, are associated with poor outcomes (49). Therefore, combining immune
checkpoint inhibitors with therapies that modulate the TME offers
the potential for new therapeutic approaches. In addition, the
transcriptomic identification of cytokines and chemokines that
facilitate tumor-immune cell interactions has uncovered promising
targets for therapeutic intervention (50); specifically, therapeutic agents
disrupting the chemokine C-X-C motif ligand 12/chemokine receptor 4
axis have been identified for further investigation, as they showed
promise in the reversal of immune suppression in PCa (51).
Impact of transcriptomics on the
development of targeted therapies for PCa
Insights gained from transcriptomic studies have
been pivotal in the development of targeted therapies for PCa. As
the molecular signatures of the disease are elucidated, it becomes
possible to explore and refine therapeutic strategies targeting
specific dysregulated pathways. A notable example is the AR, a
critical driver of PCa. Transcriptomic analyses have identified
mutations and splice variants of the AR gene that confer resistance
to standard ADTs (14). These
findings have facilitated the development of novel therapeutic
agents that target the wild-type receptor and its variants. Drugs
such as enzalutamide and abiraterone are now utilized for the
treatment of advanced PCa and have significantly improved patient
outcomes (52).
Transcriptomic profiling has identified pathways
involved in cell survival, apoptosis and proliferation that are
dysregulated in PCa, leading to the discovery of novel intervention
targets. For example, inhibitors targeting the cell cycle
regulatory protein CDK4/6 have shown promise in clinical trials,
particularly in tumors expressing high levels of the relevant
transcripts (53,54). However, the heterogeneity of
transcriptomic profiles across different tumor stages and patient
demographics may affect the reliability of these targets. In
addition, transcriptomic analyses of the TME have opened new
avenues for immunotherapy in PCa (55). By identifying expression profiles
associated with immune evasion, combinatorial therapeutic
strategies are being explored that integrate immune checkpoint
inhibitors with conventional treatments to harness the immune
system in PCa (56).
In summary, transcriptomics not only provides
information about the molecular mechanisms associated with PCa but
also impacts therapeutic approaches. By identifying novel
biomarkers, predicting treatment responses and uncovering novel
therapeutic targets, transcriptomic research lays the groundwork
for the continued advancement of targeted and personalized
therapies in PCa management.
Proteomic profiling
The integration of proteomics into PCa research has
yielded further insights into the molecular underpinnings of the
disease. Advances in analytical technologies such as mass
spectrometry (MS), and the identification of novel protein
biomarkers have improved the prospects for PCa diagnosis and
treatment. By leveraging proteomic data, clinicians can gain a
deeper understanding of tumor biology, paving the way for more
effective and personalized interventions tailored to individual
patients. Continued exploration of the proteomic landscape will
improve the current knowledge of the molecular mechanisms
associated with PCa and may also revolutionize therapeutic
strategies.
Techniques used in proteomics
Proteomics is an expansive field that focuses on the
large-scale study of proteins, particularly in terms of their
functions and structures. In the context of PCa, one of the most
critical proteomics techniques is MS. This technique sensitively
detects proteins and is key in the identification and
quantification of protein expression across different phases of PCa
progression.
The most frequently used form of MS is liquid
chromatography-tandem MS, which facilitates the separation and
identification of complex protein mixtures. Notably, it is able to
analyze protein post-translational modifications, which are crucial
in cancer biology (57).
Advancements in MS technology, including improved resolution,
sensitivity and speed, have greatly enhanced its ability to
identify low-abundance proteins that may serve as potential
biomarkers for the early diagnosis and monitoring of PCa (58).
Techniques such as two-dimensional gel
electrophoresis (2-DE) and affinity-based methods can be employed
alongside MS. 2-DE separates proteins based on their isoelectric
point and molecular weight, while affinity-based methods target
specific proteins or post-translational modifications (59). Furthermore, high-throughput
proteomic platforms enable the large-scale screening of tissue
samples, yielding valuable insights into the protein landscapes
associated with PCa pathology (60).
Protein biomarkers identified in
PCa
The exploration of proteomic alterations in PCa has
unveiled a plethora of potential biomarkers and therapeutic
targets. Elucidation of the specific roles of these proteins in
tumor biology offers opportunities for the development of novel
clinical applications, such as improved diagnostics or targeted
therapies (Table III). As
research advances, the integration of proteomic data into clinical
practice holds promise for personalized treatment strategies in PCa
management. Continuous collaboration between basic research and
clinical application is essential to translate these findings into
therapeutic interventions that improve patient outcomes.
 | Table III.Protein biomarkers identified in
PCa. |
Table III.
Protein biomarkers identified in
PCa.
| First author,
year | Proteins | Mechanism of
action | Clinical
application | (Refs.) |
|---|
| Aurilio et
al, 2020 | AR | AR plays a key role
in the progression of PCa and its resistance to endocrine
therapy | Targeting AR
signaling is a cornerstone of PCa treatment | (61) |
| Song et al,
2024 | Ki-67 | Ki-67 expression
level is a proliferation marker in PCa | Ki-67 is used
clinically as a prognostic marker for determining the
aggressiveness of prostate tumors | (63) |
| Xu et al,
2021 | GLS | GLS is a catalyst
in glutamine metabolism, which is essential for cellular
bioenergetics and anabolic processes in rapidly proliferating
cancer cells | Inhibitors of GLS,
such as CB-839, are being investigated in clinical trials for their
potential in treating advanced PCa | (64) |
| Xu et al,
2016 | LAT1 | LAT1 is pivotal for
the uptake of amino acids and other metabolites essential for tumor
growth and metabolism | LAT1 inhibitors
have shown the potential to disrupt amino acid supply and diminish
tumor growth | (68) |
| Hoter et al,
2019 | HSP70 and
HSP90 | Elevated levels of
HSPs, such as HSP70 and HSP90 in PCa, are associated with increased
tumor survival and resistance to chemotherapy | HSP90 inhibitors
disrupt client protein stability and promote apoptosis in cancer
cells | (70) |
| Souza et al,
2023 | Galectin-3 | Galectin-3 promotes
tumor growth and metastasis through its effects on the tumor
microenvironment | Galectin-3
inhibitors may reduce tumor growth and improve treatment
outcomes | (74) |
| Wang et al,
2022 | PSMA | PSMA regulates AR
activity and may contribute to tumor growth and progression via its
enzymatic activity | PSMA-targeted
radioligand therapy has demonstrated significant clinical efficacy,
providing a new option for patients with mCRPC | (76) |
| Fu et al,
2021 | AMACR | AMACR facilitates
the conversion of α-methylacyl-CoA to its racemic forms,
contributing to altered lipid metabolism in tumor cells | Elevated levels of
AMACR in prostate biopsy specimens are associated with cancer
presence and aggressiveness, helping to distinguish between benign
and malignant lesions | (79) |
The AR plays a pivotal role in PCa progression.
Alterations in protein expression, mutations and splice variants
such as AR-V7 are implicated in the development of CRPC. These
changes confer resistance to ADTs by promoting AR signaling, even
in low-androgen environments (61). Targeting AR signaling remains a
cornerstone of PCa treatment, with second-generation
anti-androgens, including enzalutamide and abiraterone, showing
efficacy against CRPC. Furthermore, circulating AR-V7 has been
identified in patients with PCa, and thus has emerged as a crucial
biomarker for predicting resistance to AR-targeted therapies,
thereby helping to guide treatment decisions towards alternative
therapies such as taxanes (62).
Ki-67 is a nuclear protein associated with cellular
proliferation that serves as a proliferation marker in various
types of cancer, including PCa. High Ki-67 expression is associated
with a poor prognosis and aggressive tumor behavior, indicating
that tumors with elevated Ki-67 are more likely to grow and evade
treatment (63). Ki-67 is used
clinically as a prognostic marker to determine the aggressiveness
of prostate tumors. Testing for Ki-67 levels, in conjunction with
other clinical parameters, can help to guide treatment strategies,
particularly in patients with intermediate-risk disease who may
benefit from more aggressive therapies (63).
Glutaminase (GLS) plays a critical role in glutamine
metabolism, which is essential for cellular bioenergetics and
anabolic processes in rapidly proliferating cancer cells. Elevated
GLS activity is associated with increased cancer cell proliferation
and survival, particularly under the nutrient deprivation
conditions common in solid tumors (64). Inhibitors of GLS, such as CB-839,
are being investigated in clinical trials for their potential in
treating advanced PCa, particularly in combination with
conventional therapies (65).
Targeting metabolic pathways offers a novel strategy, potentially
overcoming resistance mechanisms associated with traditional
hormonal therapies.
Nutrient transporters, such as L-type amino acid
transporter 1 (LAT1), have been demonstrated to serve an important
role in PCa cells (66). Other
notable nutrient transporters include the solute carrier family 1
member 5, which is involved in glutamine uptake and has been shown
to be upregulated in various cancers, including PCa (67). Additionally, the solute carrier
family 7 member 5 transporter, which facilitates the uptake of
large neutral amino acids, has also been identified as a key player
in cancer cell metabolism and proliferation. The activity of these
transporters is often influenced by the composition of the
microenvironment and can be targeted for therapeutic intervention
(67). These transporters are
pivotal for the uptake of amino acids and other metabolites
essential for tumor growth and metabolism, and the dysregulated
expression of these transporters contributes to the aggressive
behavior of PCa cells (68). The
inhibition of nutrient transporters appears to be a promising
therapeutic strategy; LAT1 inhibitors such as JPH203 have shown the
potential to disrupt amino acid supply and diminish tumor growth
(69). These strategies are being
explored in basic research and may provide a complementary
treatment method to existing therapies in the future.
Heat shock proteins (HSPs) are critical in protein
folding, which protects cells from stress-induced apoptosis
(70). In PCa, elevated levels of
HSPs, including HSP70 and HSP90, are associated with increased
tumor survival and resistance to chemotherapy (70). Their ability to stabilize oncogenic
proteins further increases cancer cell viability. Targeting HSPs is
being investigated as a therapeutic strategy for PCa. HSP90
inhibitors, such as tanespimycin, have been shown to disrupt the
stability of client proteins, such as mutant p53 and AR, and to
promote apoptosis in cancer cells (71). Clinical trials evaluating HSP
inhibitors, such as tanespimycin, are underway and aim to assess
their efficacy in conjunction with standard PCa treatments
(72). Other HSPs, such as HSP20,
HSP40, HSP60 and HSP80, are also being investigated for their roles
in cancer and potential therapeutic applications (73).
Galectin-3 is a member of the galectin family of
lectins, which is involved in various biological processes,
including cell proliferation, apoptosis and inflammation (74). Galectin-3 has been shown to promote
tumor growth and metastasis through its effects on the TME
(74). In addition, elevated serum
levels of galectin-3 have been linked to a poor prognosis in
patients with PCa (75).
Exploration of the potential of galectin-3 as a therapeutic target
indicates that galectin-3 inhibitors have the ability to reduce
tumor growth and improve treatment outcomes (75).
Prostate-specific membrane antigen (PSMA) is a
transmembrane protein that is expressed in higher levels in PCa
cells than in normal prostate tissue. It plays a role in the
regulation of AR activity and may also contribute to tumor growth
and progression via its enzymatic activity (76). PSMA-targeted therapies, such as
PSMA-617, a radiolabeled small molecule, have shown promise in the
imaging and treatment of advanced PCa. PSMA-targeted radioligand
therapy has demonstrated significant clinical efficacy, and
PSMA-617 has been approved by the FDA as a new radiotherapeutic
option for patients with mCRPC (77). Furthermore, PSMA can also be used
as a clinical biomarker for patient stratification (78).
α-Methylacyl-CoA racemase (AMACR) is an enzyme
implicated in fatty acid metabolism that has been found to be
upregulated in PCa cells. It facilitates the conversion of
α-methylacyl-CoA to its racemic forms, contributing to altered
lipid metabolism in tumor cells (79). AMACR has been explored as a
diagnostic marker in PCa, particularly for use in histopathological
assessments. This has revealed that elevated levels of AMACR in
prostate biopsy specimens are associated with the presence and
aggressiveness of cancer, making it a useful biomarker to assist
pathologists in distinguishing between benign and malignant lesions
(80).
Progress in proteomics in PCa TME
research
The complexity of the TME presents considerable
challenges in PCa research. Proteomics has been vital in
characterizing the TME, which consists of cancer cells, stromal
cells and extracellular matrix components, all of which interact
dynamically to promote tumor growth and metastasis (81). A proteomic study has highlighted
the shifts in the protein composition of the TME in response to
oncogenic signaling and therapeutic interventions (82). Tumor-associated macrophages and
their secretory profiles have been found to be associated with
changes in the extracellular matrix composition, thereby
influencing tumor progression (82). These intricate interactions
underscore the potential of proteomic methodologies to elucidate
the cross-talk between PCa cells and the surrounding stromal
components. Spatial proteomics has been used to investigate the
heterogeneity of protein expression within the TME. Techniques such
as imaging mass cytometry have enabled the protein distributions in
tissue sections to be mapped, thereby revealing the spatial
organization of proteins involved in immune evasion and treatment
resistance (83). Such insights
are crucial for the development of novel therapeutic targets aimed
at remodeling the TME to inhibit tumor growth and improve patient
outcomes. While proteomics has been instrumental in elucidating
interactions within the TME in PCa, careful consideration of the
methodologies employed and the variability of findings is
essential. Understanding these nuances will be key in the
development of novel therapeutic targets aimed at modulating the
TME to inhibit tumor growth and, ultimately, improve patient
outcomes.
Metabolomics research
The application of metabolomics in PCa research is
enhancing the general understanding of tumor biology. By leveraging
cutting-edge technologies and integrating metabolomic data with
clinical outcomes, it is possible to identify key metabolic
alterations and potential biomarkers to inform early detection and
treatment strategies. As the complex metabolomic landscape of PCa
becomes more defined, the promise of personalized medicine becomes
increasingly tangible, ultimately benefiting patients through
improved diagnosis and tailored therapeutic approaches.
Overview of metabolomics methods and
technologies
Metabolomics is a powerful analytical approach
designed to characterize all the metabolites present in biological
samples, offering insights into metabolic changes associated with
various diseases, including cancer. In PCa research, several
methodologies are being employed to analyze metabolomic profiles
and elucidate the underlying biochemical pathways that contribute
to tumorigenesis and progression. The primary techniques utilized
in metabolomics include MS and nuclear magnetic resonance (NMR)
spectroscopy. MS, particularly when combined with chromatography
methods such as gas or liquid chromatography, allows for the
sensitive detection, identification and quantification of
metabolites in complex biological matrices. The high sensitivity
and resolution of modern mass spectrometers allow low-abundance
metabolites to be detected, which can be crucial in cancer
(84).
NMR spectroscopy is a non-destructive technique for
analyzing metabolites in a sample and providing structural
information. Although it is less sensitive than MS, NMR is valuable
for quantifying metabolites in intact tissues, preserving the
biological context of the sample (85). Furthermore, advanced bioinformatics
tools are essential in metabolomics research. These tools are used
for data processing, statistical analysis and the interpretation of
complex metabolomic datasets. Computational techniques such as
multivariate analysis, machine learning (ML) and pathway enrichment
analysis contribute to the identification of key metabolic pathways
disrupted in PCa (86).
Key metabolic alterations in PCa
Metabolomics has emerged as a powerful tool in
cancer research, offering insights into the biochemical mechanisms
of tumor biology. Key findings highlight alterations in energy,
amino acid, lipid and nucleotide metabolism, all of which
contribute to the aggressive nature of this malignancy (Table IV). In future research, a focus on
the integration of metabolomic data with genomic and proteomic
information is necessary to fully understand the complexities of
PCa biology and to refine therapeutic strategies.
 | Table IV.Key metabolic alterations in PCa. |
Table IV.
Key metabolic alterations in PCa.
| First author,
year | Metabolic
classification | Metabolic
alterations associated with PCa | (Refs.) |
|---|
| Chetta et
al, 2023 | Altered energy
metabolism | Levels of key
metabolites associated with energy metabolism are altered,
including increased lactic acid and decreased citric acid cycle
intermediates; this transformation supports the rapid proliferation
of PCa cells, and promotes their invasion and metastasis under
acidic conditions | (88) |
| Chen et al,
2024 | Amino acid
metabolism | Levels of
branched-chain amino acids such as leucine and valine are
significantly increased in PCa compared with normal tissue;
tryptophan metabolism, particularly its kynurenine pathway, is
implicated in immune evasion mechanisms employed by tumors | (90) |
| Zeković et
al, 2023 | Lipid metabolism
dysregulation | Levels of
unsaturated fatty acids, particularly arachidonic acid, are
elevated in PCa, suggesting a role in cancer progression | (91) |
| Škara et al,
2021 | Lipid metabolism
dysregulation | Cholesterol
metabolism, including increased levels of cholesterol and its
metabolites, promote tumorigenesis | (92) |
| Yun et al,
2015 | Nucleotide
metabolism | PCa cells show
heightened nucleotide synthesis, as evidenced by elevated levels of
purines and pyrimidines | (93) |
| Srihari et
al, 2018 | Carbohydrate
metabolism | Hexosamine
biosynthesis is elevated in PCa, and contributes to glycosylation
changes in proteins associated with oncogenic signaling | (94) |
One of the hallmarks of cancer is the switch from
oxidative phosphorylation to aerobic glycolysis, known as the
Warburg effect (87). In PCa
tissues, altered levels of key metabolites associated with energy
metabolism have been detected, including increased lactate and
decreased citric acid cycle intermediates (88). In addition to supporting rapid
proliferation, this shift influences the TME by promoting acidotic
conditions, facilitating invasion and metastasis (89).
PCa cells exhibit distinct patterns of amino acid
metabolism. Notably, increased levels of branched-chain amino acids
(BCAAs), including leucine and valine, have been identified in
patients with PCa compared with healthy controls (90). In addition, tryptophan metabolism,
particularly its kynurenine pathway, has been implicated in immune
evasion mechanisms employed by tumors (90). These alterations indicate potential
biomarkers and therapeutic targets, as the availability of amino
acids can influence tumor growth.
Lipid metabolism is notably altered in PCa, with
increased levels of specific fatty acids, sterols and
phospholipids. A previous review of metabolomic studies highlighted
that levels of unsaturated fatty acids, notably arachidonic acid,
are elevated in PCa, which suggests increased membrane fluidity and
a potential role in cancer progression (91). Furthermore, sterol metabolism,
particularly the accumulation of cholesterol and its metabolites,
contributes to membrane biogenesis and the activation of signaling
pathways that promote oncogenesis (92).
PCa cells exhibit heightened nucleotide synthesis,
as evidenced by elevated levels of purines and pyrimidines, which
supports the rapid proliferation of cancer cells (93). Targeting nucleotide metabolism
pathways is a novel therapeutic strategy that may hinder cancer
cell growth by disrupting the synthesis of nucleotides.
Metabolomic analyses have revealed alterations in
carbohydrate metabolism pathways, including increased glucose
uptake and metabolism. Elevated hexosamine biosynthesis in PCa has
been shown to contribute to glycosylation changes in proteins
associated with oncogenic signaling (94). Furthermore, changes in glycogen
metabolism have been documented, further emphasizing the shifts in
energy substrates utilized by cancer cells (94).
The identification of these metabolic alterations
has implications for the management of PCa. In particular,
metabolomic profiling may aid in risk stratification and the
development of personalized medicine approaches. For instance,
elevated BCAAs and lipid alterations may serve as potential
biomarkers for the early detection and monitoring of disease
progression (95). In addition,
targeting these metabolic pathways provides opportunities for
innovative treatment options, such as metabolic inhibitors that
disrupt cancer cell energetics and proliferation.
Integrating metabolomic features with
clinical data to improve diagnosis
The integration of metabolomic data with clinical
parameters has the potential to enhance diagnosis and
prognostication in PCa. By identifying associations between
metabolomic findings and clinical outcomes, it may be possible to
develop a more comprehensive understanding of the disease and
identify patterns that could inform personalized treatment
strategies. For example, studies have demonstrated that panels of
metabolites combined with clinical data, such as PSA levels,
Gleason scores and imaging findings, can be used to improve
diagnostic accuracy (96,97). In addition, a multi-analyte
metabolomic assay incorporating metabolomic signatures with
conventional markers was shown to provide improved results in the
prediction of malignancy in patients with elevated PSA levels
(98). Nevertheless, variability
in study design, sample sizes and metabolic profiling techniques
may affect the reliability and generalizability of these
findings.
The application of ML approaches to metabolomic
datasets is useful in the identification of novel metabolites as
potential prognostic markers. The classification of patients with
PCa based on their metabolomic profiles can contribute to the
prediction of disease progression, response to therapy and overall
survival (99). ML models can also
help to refine treatment decisions, allowing clinicians to tailor
interventions based on the metabolic signature of each individual
patient (100). However, while ML
models have the capacity to refine treatment decisions tailored to
specific metabolic signatures, concerns about overfitting and the
requirement for external validation should not be overlooked
(100).
As metabolomics continues to evolve, its integration
with other omics technologies, such as genomics, proteomics and
transcriptomics, may provide a holistic view of the tumor
landscape. This multi-omics approach is expected to yield deeper
insights into the molecular mechanisms associated with PCa, but the
complexities associated with data integration and interpretation
present ongoing challenges. Ultimately, addressing these issues
will be crucial in facilitating the development of more effective
diagnostic and therapeutic strategies.
Application of single-cell sequencing
The integration of single-cell sequencing technology
into PCa research has been transformative in offering valuable
insights into this heterogeneous disease. By dissecting the
cellular composition and functional characteristics of tumors at
unprecedented resolution, it is possible to identify critical
driver cell types and pathways associated with disease progression
and therapeutic resistance (101). The knowledge obtained by
single-cell sequencing has profound implications for the
development of personalized treatment strategies, and creates a
foundation for more effective and tailored therapeutic
interventions for patients with PCa.
Advances in single-cell technology in
PCa
Single-cell sequencing technology has emerged as a
groundbreaking tool for understanding the intratumoral
heterogeneity and cellular complexity of PCa (102). This technology enables individual
cells to be analyzed, thereby providing insights that are not
possible in bulk tissue analyses. Advancements in single-cell RNA
sequencing, single-cell DNA sequencing and single-cell assay for
transposase-accessible chromatin sequencing have markedly improved
the general understanding of PCa biology (102).
Single-cell RNA sequencing allows the transcriptomes
of thousands of individual cells to be profiled, leading to the
identification of distinct cellular populations within tumor
(103). For example, a recent
study has revealed the presence of previously unrecognized
subpopulations of tumor cells that exhibit unique gene expression
profiles associated with differential responses to therapy
(104). Furthermore, spatial
transcriptomics, which combines single-cell RNA sequencing with
spatial information, is a novel technique that can characterize the
spatial organization of tumor cells within the TME (105), which is critical for
understanding cellular interactions and signaling pathways in
PCa.
Advancements in droplet-based and microfluidic
technologies have enabled the high-throughput analysis of single
cells, which has major implications for large-scale studies of PCa
(106). Techniques such as 10×
Genomics Chromium and Seq-Well have made it feasible to examine
thousands of individual cells simultaneously, which greatly
increases throughput and reduces costs (105). These methods facilitate the
identification of rare cell populations, which may have critical
implications for tumor progression and treatment response.
Understanding tumor heterogeneity and
microenvironment
A critical finding from single-cell sequencing in
PCa is the extensive intratumoral heterogeneity that exists among
cancer cells. Different clones within a single tumor can exhibit
divergent genetic and phenotypic characteristics, contributing to
variable responses to therapy and disease progression (104). For instance, single-cell RNA
sequencing has identified distinct basal and luminal PCa subtypes,
with variations in metabolic and signaling pathways that can
influence outcomes following therapies such as ADT (105).
Single-cell technologies have been instrumental in
elucidating the role of the TME in PCa. The TME comprises not only
tumor cells but also stromal cells, immune cells and extracellular
matrix components, all of which profoundly influence cancer
progression (107). Single cell
RNA sequencing has facilitated the characterization of immune cell
populations infiltrating prostate tumors, providing insights into
how these cells may facilitate or inhibit tumor growth. For
example, a recent study identified a subset of immunosuppressive
macrophages that contribute to tumor immune evasion (108).
The identification of specific cell types that
contribute to cancer progression and treatment resistance is
crucial. Cancer stem cells (CSCs) have the ability to drive tumor
growth and metastasis (109).
Single-cell technologies have identified markers associated with
CSCs, highlighting their potential as targets for therapy (110). The insights obtained from
single-cell studies have profound implications for understanding
cancer cell plasticity. For example, the identification of hybrid
states, where tumor cells adopt features of different lineages,
helps to explain the emergence of treatment resistance and
metastasis (111).
Single-cell sequencing has revolutionized PCa
research by enabling the exploration of cellular heterogeneity
within tumors (112). This
approach facilitates the identification of distinct cell
populations, including tumor-initiating cells and immune subsets,
thereby deepening understanding of the TME and cancer progression
(112). However, discrepancies in
findings across studies raise concerns about reproducibility. For
example, differences in sequencing techniques and data analysis
pipelines can lead to inconsistent interpretations of cellular
states and their functional roles (113). Furthermore, the resolution of
single-cell data may overlook the influence of cell-cell
interactions, limiting the scope of insights into tumor biology
(114). Thus, while single-cell
sequencing provides invaluable data for the advancement of PCa
research, careful consideration of methodological variations is
crucial for drawing robust and reliable conclusions.
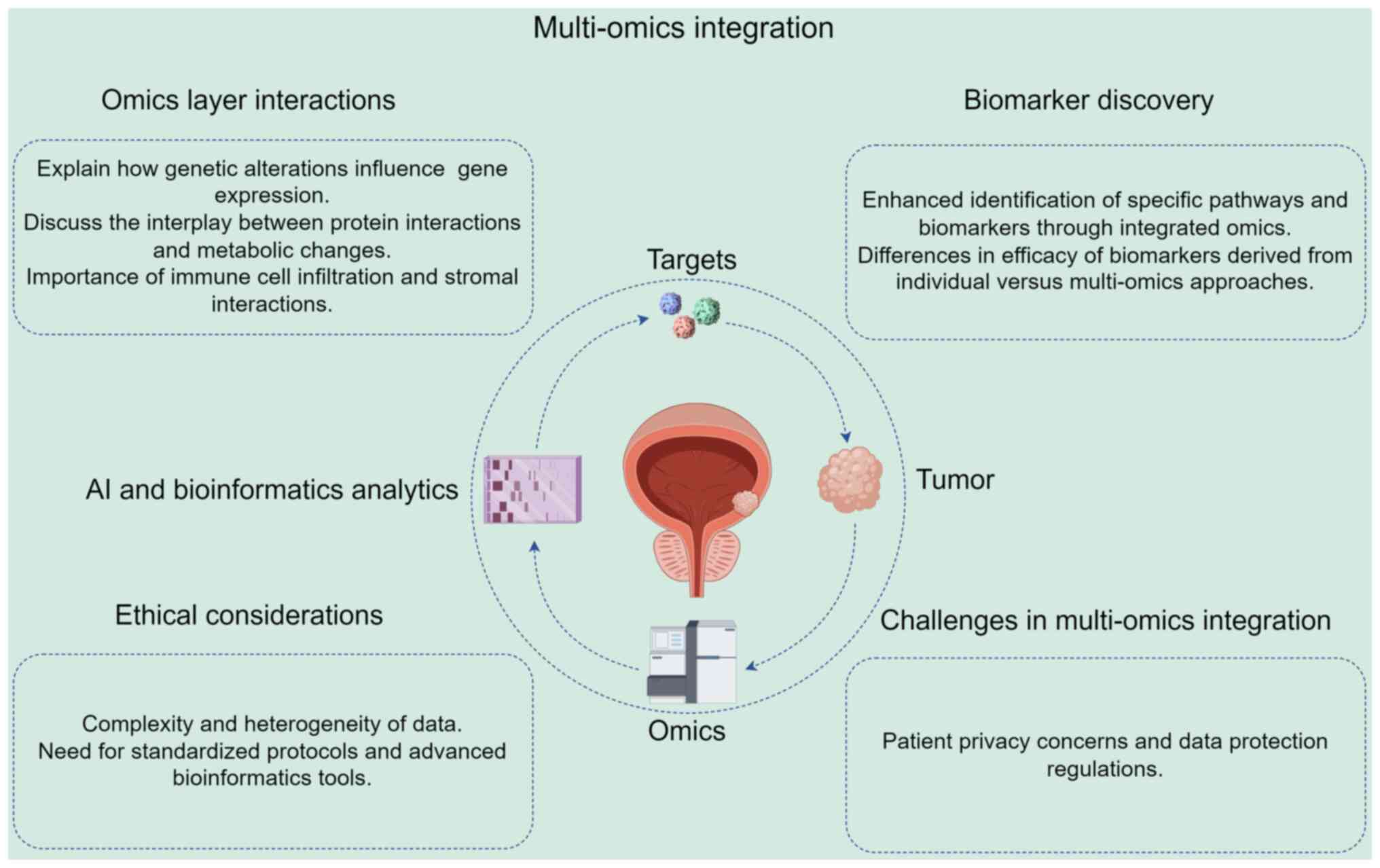
Synergy of multi-omics integration
While the integration of multi-omics approaches
presents unparalleled opportunities to advance the understanding of
PCa and improve biomarker discovery, it is critical to recognize
and overcome the associated challenges. Collaborations among
researchers, clinicians and bioinformaticians, along with the
implementation of standardized practices, are essential to unlock
the potential of multi-omics strategies in elucidating the
complexities of PCa and other malignancies.
Integrating data from various omics
techniques
The advent of multi-omics approaches, encompassing
genomics, transcriptomics, proteomics, metabolomics and
epigenomics, has provided substantial insights into cancer biology,
including PCa. By integrating diverse datasets from different omics
technologies, a holistic view of the TME can be gained, which
facilitates a deeper understanding of the molecular mechanisms
driving disease progression, metastasis and therapeutic
resistance.
One of the critical advantages of multi-omics
integration is its ability to elucidate the complex interplay
between genetic alterations, gene expression profiles,
post-transcriptional modifications, protein interactions and
metabolic changes within tumor cells. For example, the integration
of genomic and transcriptomic data can be used to identify key
driver mutations and their downstream effects on gene expression,
providing a more accurate delineation of cancer subtypes (115). This integrative approach also
allows specific pathways that might be targeted for therapeutic
intervention to be identified. Furthermore, the combination of
different omics data facilitates the discovery and validation of
biomarkers (Fig. 2). Biomarkers
derived through multi-omics approaches have the potential to be
more specific and sensitive, thereby improving the screening,
diagnosis and prognosis of PCa. For example, the integration of
metabolomic profiles with genomic data has revealed distinctive
metabolic signatures associated with tumor aggressiveness, which
improves the predictive capability of biomarkers beyond that of
individual omics datasets (116).
Multi-omics integration facilitates the
identification of biomarkers that reflect the complexity of
interactions between tumor heterogeneity and the TME (Fig. 2). By analyzing data from various
sources, it is possible to identify tumor cell-intrinsic markers
and markers associated with the TME, including immune cell
infiltration and stromal interactions. This comprehensive
perspective is critical for the development of targeted therapies
and personalized treatment strategies, offering insights that may
help to address variations in patient responses to therapies
(117).
Challenges of multi-omics data
integration
While the potential for multi-omics integration is
immense, there are notable challenges to overcome, particularly in
data standardization, integration methodologies and bioinformatics
analysis tools (11). The inherent
heterogeneity of omics data presents a major barrier; for example,
discrepancies in measurement techniques and data formats can
complicate integration efforts (118). The establishment of effective
standardization protocols is necessary to ensure comparability
across studies (119). Various
integration methods, including statistical learning techniques such
as multi-factor analysis and ML algorithms, and computational
frameworks such as Multi-Omics Factor Analysis, are crucial for
managing these data types effectively (119). However, the application of
advanced bioinformatics tools, including network-based approaches,
is able to elucidate biologically meaningful interactions despite
data complexity (86).
A primary obstacle to multi-omics integration is the
inherent complexity and heterogeneity of the generated data
(Fig. 2). Each omics technique
produces vast amounts of information, often with varying scales,
measures and corresponding analysis methods. This creates
substantial analytical and interpretative challenges, necessitating
sophisticated strategies to integrate and interpret the diverse
datasets (117). In addition, the
integration of multi-omics data requires robust bioinformatics
tools and expertise. Advanced statistical methods and ML algorithms
are essential for effective data processing, normalization and
integration. However, the development and implementation of these
tools has not kept up with the pace of data generation.
Consequently, it is urgently necessary for ongoing research to
focus on the improvement of computational methodologies for
handling multi-omics data, to enable the extraction of meaningful
biological insights (120).
Another challenge is the lack of standardized
protocols for multi-omics studies, leading to inconsistencies in
data collection, processing and analysis across different studies.
Standardization is required not only for methodological procedures
but also for data representation, which is essential for accurate
comparisons and meta-analyses in PCa research (121). Collaborative initiatives and
consortia focused on the establishment of standardized practices
may help to address these issues and promote the wider adoption of
multi-omics approaches in clinical settings. For example, the
Multi-Omics for Health and Disease consortium of the NIH National
Human Genome Research Institute is working to validate and enhance
generalizable multi-omics approaches to identify meaningful
biological changes related to health and disease in ancestrally
diverse populations (122).
Additionally, the National Microbiology Data Collaborative EDGE
initiative aims to develop data harmonization, integration,
visualization and analysis methods for multi-omics datasets
(122).
The integration of multi-omics data in clinical
settings poses ethical considerations and privacy concerns for
patients (123) (Fig. 2). A major challenge is ensuring the
anonymity and confidentiality of patient information, as genomic
data can be uniquely identifying (123). The implementation of stringent
data protection measures and adherence to regulatory guidelines is
essential for safeguarding sensitive information (124). Furthermore, discrepancies in data
integration methodologies can lead to inconsistent results,
complicating interpretations and clinical applications (125). For example, variations in study
cohorts and analytical frameworks may yield conflicting biological
insights, hindering reproducibility and eroding trust in
multi-omics approaches (126).
Addressing these challenges is essential for the ethical
advancement of PCa research.
Future perspectives in multi-omics
research
Multi-omics research has emerged as a cornerstone
for advancing the understanding and treatment of PCa, as it has
been key to the discovery of some of the complex molecular
underpinnings of PCa, thereby leading to improvements in clinical
outcomes. This section of the review aims to outline the future
perspectives on multi-omics research, focusing on its clinical
applications, the roles of artificial intelligence (AI) and ML, and
the importance of collaborative efforts in this field.
The landscape of PCa treatment is evolving due to
the use of multi-omics data, which has revealed novel therapeutic
avenues. One key advancement is the use of PARP inhibitors, which
has shown efficacy in patients with PCa harboring BRCA1/BRCA2
mutations (127). The TRIUMPH
trial, which investigated rucaparib monotherapy in patients with
metastatic hormone-sensitive prostate cancer harboring germline
homologous recombination repair gene mutations, showed some
clinical responses without concurrent ADT. However, the
pre-specified efficacy threshold was not met. At present, there is
a lack of other clinical trials reporting more encouraging results
for PARP inhibitors in PCa (128). Furthermore, the integration of
multi-omics approaches has deepened the understanding of targeted
therapies, such as AKT inhibitors, which have shown promising
outcomes in preclinical models and early-phase clinical trials,
warranting further exploration in larger cohorts (129). In addition, the role of
immunotherapy has garnered attention, particularly the use of
immune checkpoint inhibitors, which may provide synergistic
antitumor effects when used in combination with traditional
therapies (130). Collectively,
these advancements promote a shift in PCa management, emphasizing
personalized treatment strategies informed by comprehensive omics
analyses, which is ultimately paving the way for improved patient
outcomes and refined clinical practices. The clinical applicability
of multi-omics in PCa is substantial and continues to evolve as
more data and insights are gathered. A particularly promising
aspect is the development of personalized treatment plans that
harness the distinct molecular profiles of tumors (131).
Multi-omics approaches can improve strategies for
the screening and early detection of PCa. A combination of genomic,
proteomic and metabolomic markers can be utilized to improve the
sensitivity and specificity of current screening methods. A study
by Sinha et al (132)
demonstrated the ability of multi-omics to uncover biomarker
signatures predicting aggressiveness in prostate tumors,
potentially enabling earlier interventions and improved prognostic
outcomes. However, it is essential to validate these signatures in
larger, diverse cohorts and to explore their integration into
clinical settings as part of routine screenings.
AI and ML technologies are vital tools for analyzing
complex multi-omics data. Given the vast and intricate datasets
generated through genomic, transcriptomic and proteomic analyses,
conventional analytical methods often fail to extract meaningful
patterns. By leveraging AI and ML algorithms, it is possible to
analyze large-scale data more efficiently and effectively (133). For example, deep learning
techniques have been used to predict PCa outcomes from multi-omics
data, providing novel insights into tumor heterogeneity and
treatment responses (101). The
identification of specific AI frameworks that are able to integrate
high-dimensional data, such as whole-genome sequencing and RNA
expression profiles, will be key to the development of
comprehensive predictive models that account for individual
variations.
AI applications can also assist in the
identification of novel biomarkers. For example, generative models
can be trained to discover previously unknown relationships among
different omics layers, thus revealing new avenues for therapeutic
intervention (134). Targeting
specific pathways implicated in PCa using these models may lead to
the identification of potential drug targets and facilitate the
development of targeted therapies. Collaborative efforts that merge
AI expertise with multi-omics research are also essential to ensure
these technologies are effectively applied to clinical
challenges.
Studies of multi-omics in PCa have revealed several
promising biomarkers that exhibit potential in the understanding
and management of this disease (135). These include circulating tumor
DNA, which is of particular interest due to the non-invasive method
of sample collection and its ability to provide real-time insights
into tumor dynamics and treatment responses, rendering it an
invaluable tool for monitoring disease progression and therapy
efficacy (136). Additionally, AR
splice variants, particularly AR-V7, are emerging as critical
indicators of resistance to ADTs, underscoring the necessity for
personalized approaches to treatment (34). Furthermore, studies on the
dysregulation of miRNAs, such as miR-145 (137) and the miR-221/222 cluster
(138), have highlighted their
role in tumorigenesis and their potential as therapeutic targets.
These biomarkers not only reflect the intricate mechanisms of PCa
biology but also create new avenues for innovative diagnostic and
therapeutic strategies. Ongoing research into these biomarkers is
essential for the development of tailored treatments to improve
clinical outcomes in PCa.
The future of multi-omics research in PCa is poised
for growth, embracing the tenets of precision medicine and
leveraging advanced computational technologies. Clinical
applications are likely become increasingly personalized and
effective, guided by new insights derived from AI and collaborative
research efforts. The validation of selected biomarkers and
comprehensive study of the interplay between different omics layers
is essential to ensure that findings are translated into actionable
clinical practices. By further exploring the complexities of PCa
using multi-omics, progress is being made in the tailoring of
treatments to the disease and the individual patient, ultimately
improving outcomes and quality of life for those affected by this
prevalent malignancy.
Conclusion
Multi-omics approaches have advanced the
understanding of PCa and associated diagnostic strategies by
enabling the more precise identification of molecular subtypes and
biomarkers. This approach is transformative as it not only aids the
early detection of PCa but also supports the development of
personalized treatment strategies. In the future, the integration
of multi-omics data holds great promise for improving patient
management, tailoring therapeutic interventions and ultimately
leading to improved clinical outcomes for patients with PCa.
However, continued research in this field will be essential to
fully realize the potential of these innovative methodologies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
LY and XS made key contributions to the conception
of the manuscript. YL and PS were responsible for literature
searching and analysis. LY, XS and PS were responsible for drafting
and writing the manuscript. Data authentication is not applicable.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patel VL, Busch EL, Friebel TM, Cronin A,
Leslie G, McGuffog L, Adlard J, Agata S, Agnarsson BA, Ahmed M, et
al: Association of genomic domains in BRCA1 and BRCA2 with prostate
cancer risk and aggressiveness. Cancer Res. 80:624–638. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ziglioli F, Patera A, Isgrò G, Campobasso
D, Guarino G and Maestroni U: Impact of modifiable lifestyle risk
factors for prostate cancer prevention: A review of the literature.
Front Oncol. 13:12037912023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Varaprasad GL, Gupta VK, Prasad K, Kim E,
Tej MB, Mohanty P, Verma HK, Raju GSR, Bhaskar L and Huh YS: Recent
advances and future perspectives in the therapeutics of prostate
cancer. Exp Hematol Oncol. 12:802023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wasim S, Lee SY and Kim J: Complexities of
prostate cancer. Int J Mol Sci. 23:142572022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Catalona WJ: Screening for prostate
cancer. Lancet. 343:14371994.PubMed/NCBI
|
|
7
|
Heijnsdijk EA, Wever EM, Auvinen A,
Hugosson J, Ciatto S, Nelen V, Kwiatkowski M, Villers A, Páez A,
Moss SM, et al: Quality-of-life effects of prostate-specific
antigen screening. N Engl J Med. 367:595–605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma Y, Liu Z, Yu W, Huang H, Wang Y and Niu
Y: Investigating high-risk factors, precise diagnosis, and
treatment of castration-resistant prostate cancer (CRPC). Comb Chem
High Throughput Screen. 27:2598–2608. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cai M, Song XL, Li XA, Chen M, Guo J, Yang
DH, Chen Z and Zhao SC: Current therapy and drug resistance in
metastatic castration-resistant prostate cancer. Drug Resist Updat.
68:1009622023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang N, Kandalai S, Zhou X, Hossain F and
Zheng Q: Applying multi-omics toward tumor microbiome research.
Imeta. 2:e732023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He X, Liu X, Zuo F, Shi H and Jing J:
Artificial intelligence-based multi-omics analysis fuels cancer
precision medicine. Semin Cancer Biol. 88:187–200. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sato G, Shirai Y, Namba S, Edahiro R,
Sonehara K, Hata T, Uemura M, Biobank Japan Project, Matsuda K,
Doki Y, et al: Pan-cancer and cross-population genome-wide
association studies dissect shared genetic backgrounds underlying
carcinogenesis. Nat Commun. 14:36712023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uo T, Sprenger CC and Plymate SR: Androgen
receptor signaling and metabolic and cellular plasticity during
progression to castration resistant prostate cancer. Front Oncol.
10:5806172020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Robinson D, Van Allen EM, Wu YM, Schultz
N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC,
Attard G, et al: Integrative clinical genomics of advanced prostate
cancer. Cell. 161:1215–1228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chi KN, Barnicle A, Sibilla C, Lai Z,
Corcoran C, Barrett JC, Adelman CA, Qiu P, Easter A, Dearden S, et
al: Detection of BRCA1, BRCA2, and ATM alterations in matched tumor
tissue and circulating tumor DNA in patients with prostate cancer
screened in PROfound. Clin Cancer Res. 29:81–91. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wokołorczyk D, Kluźniak W, Stempa K, Rusak
B, Huzarski T, Gronwald J, Gliniewicz K, Kashyap A, Morawska S,
Dębniak T, et al: PALB2 mutations and prostate cancer risk and
survival. Br J Cancer. 125:569–575. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karlsson Q, Brook MN, Dadaev T, Wakerell
S, Saunders EJ, Muir K, Neal DE, Giles GG, MacInnis RJ, Thibodeau
SN, et al: Rare germline variants in ATM predispose to prostate
cancer: A PRACTICAL consortium study. Eur Urol Oncol. 4:570–579.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alorjani M, Aburub M, Al-Trad B, Hamad MA,
AbuAlarja M, Bashir SA, Al-Batayneh K and Zoubi MA: The prevalence
of CHEK1 and CHEK2 mutations in prostate cancer: A Retrospective
cohort study. Med Arch. 77:8–12. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sharma M, Yang Z and Miyamoto H: Loss of
DNA mismatch repair proteins in prostate cancer. Medicine
(Baltimore). 99:e201242020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rusak B, Kluźniak W, Wokołorczykv D,
Stempa K, Kashyap A, Gronwald J, Huzarski T, Dębniak T, Jakubowska
A, Masojć B, et al: Inherited NBN mutations and prostate cancer
risk and survival. Cancer Res Treat. 51:1180–1187. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maxwell KN, Cheng HH, Powers J, Gulati R,
Ledet EM, Morrison C, Le A, Hausler R, Stopfer J, Hyman S, et al:
Inherited TP53 variants and risk of prostate cancer. Eur Urol.
81:243–250. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Imada EL, Sanchez DF, Dinalankara W,
Vidotto T, Ebot EM, Tyekucheva S, Franco GR, Mucci LA, Loda M,
Schaeffer EM, et al: Transcriptional landscape of PTEN loss in
primary prostate cancer. BMC Cancer. 21:8562021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goldberg H, Spratt D, Chandrasekar T,
Klaassen Z, Wallis CJD, Santiago-Jimenez M, Fishbane N, Davicioni
E, Noorani R, Ahmad AE, et al: Clinical-genomic characterization
unveils more aggressive disease features in elderly prostate cancer
patients with low-grade disease. Eur Urol Focus. 7:797–806. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ikeda S, Elkin SK, Tomson BN, Carter JL
and Kurzrock R: Next-generation sequencing of prostate cancer:
Genomic and pathway alterations, potential actionability patterns,
and relative rate of use of clinical-grade testing. Cancer Biol
Ther. 20:219–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kalampokis N, Zabaftis C, Spinos T,
Karavitakis M, Leotsakos I, Katafigiotis I, van der Poel H, Grivas
N and Mitropoulos D: Review on the role of BRCA mutations in
genomic screening and risk stratification of prostate cancer. Curr
Oncol. 31:1162–1169. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He Y, Xu W, Xiao YT, Huang H, Gu D and Ren
S: Targeting signaling pathways in prostate cancer: Mechanisms and
clinical trials. Signal Transduct Target Ther. 7:1982022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Bono J, Mateo J, Fizazi K, Saad F,
Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, et al:
Olaparib for metastatic castration-resistant prostate cancer. N
Engl J Med. 382:2091–2102. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kulda V, Topolcan O, Kucera R, Kripnerova
M, Srbecka K, Hora M, Hes O, Klecka J, Babuska V, Rousarova M, et
al: Prognostic significance of TMPRSS2-ERG fusion gene in prostate
cancer. Anticancer Res. 36:4787–4793. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song C and Chen H: Predictive significance
of TMRPSS2-ERG fusion in prostate cancer: A meta-analysis. Cancer
Cell Int. 18:1772018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Álvarez-Garcia V, Tawil Y, Wise HM and
Leslie NR: Mechanisms of PTEN loss in cancer: It's all about
diversity. Semin Cancer Biol. 59:66–79. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
LaTulippe E, Satagopan J, Smith A, Scher
H, Scardino P, Reuter V and Gerald WL: Comprehensive gene
expression analysis of prostate cancer reveals distinct
transcriptional programs associated with metastatic disease. Cancer
Res. 62:4499–4506. 2002.PubMed/NCBI
|
|
32
|
Itkonen HM, Urbanucci A, Martin SE, Khan
A, Mathelier A, Thiede B, Walker S and Mills IG: High OGT activity
is essential for MYC-driven proliferation of prostate cancer cells.
Theranostics. 9:2183–2197. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Prensner JR, Chen W, Han S, Iyer MK, Cao
Q, Kothari V, Evans JR, Knudsen KE, Paulsen MT, Ljungman M, et al:
The long non-coding RNA PCAT-1 promotes prostate cancer cell
proliferation through cMyc. Neoplasia. 16:900–908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang Y, Lv G, Xiu R, Yang H, Wang W, Yu P,
Zhang J, Ye L, Wang H and Tian J: Novel selective agents for the
degradation of AR/AR-V7 to treat advanced prostate cancer. Eur J
Med Chem. 271:1164002024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Wet L, Williams A, Gillard M, Kregel S,
Lamperis S, Gutgesell LC, Vellky JE, Brown R, Conger K, Paner GP,
et al: SOX2 mediates metabolic reprogramming of prostate cancer
cells. Oncogene. 41:1190–1202. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yi Q, Han X, Yu HG, Chen HY, Qiu D, Su J,
Lin R, Batist G and Wu JH: SC912 inhibits AR-V7 activity in
castration-resistant prostate cancer by targeting the androgen
receptor N-terminal domain. Oncogene. 43:1522–1533. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Verma P, Shukla N, Kumari S, Ansari MS,
Gautam NK and Patel GK: Cancer stem cell in prostate cancer
progression, metastasis and therapy resistance. Biochim Biophys
Acta Rev Cancer. 1878:1888872023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grimm D, Bauer J, Wise P, Krüger M,
Simonsen U, Wehland M, Infanger M and Corydon TJ: The role of SOX
family members in solid tumours and metastasis. Semin Cancer Biol.
67((Pt 1)): 122–153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu Q, Wang F, Yang J, Sun W, Hu Z, Xu L,
Chu H, Wang X and Zhang W: Long non-coding RNA-PCGEM1 contributes
to prostate cancer progression by sponging microRNA miR-129-5p to
enhance chromatin licensing and DNA replication factor 1
expression. Bioengineered. 13:9411–9424. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mu X, Shen Z, Lin Y, Xiao J, Xia K, Xu C,
Chen B, Shi R, Zhu A, Sun X, et al: LncRNA-MALAT1 regulates cancer
glucose metabolism in prostate cancer via MYBL2/mTOR axis. Oxid Med
Cell Longev. 2022:86932592022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu X, Chen D, Yang F and Xing N: Quercetin
inhibits epithelial-to-mesenchymal transition (EMT) process and
promotes apoptosis in prostate cancer via downregulating lncRNA
MALAT1. Cancer Manag Res. 12:1741–1750. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zeng H, Huang Y, Liu Q, Liu H, Long T, Zhu
C and Wu X: MiR-145 suppresses the motility of prostate cancer
cells by targeting cadherin-2. Mol Cell Biochem. 476:3635–3646.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gui B, Hsieh CL, Kantoff PW, Kibel AS and
Jia L: Androgen receptor-mediated downregulation of microRNA-221
and −222 in castration-resistant prostate cancer. PLoS One.
12:e01841662017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ferreira M, Morais M, Medeiros R and
Teixeira AL: MicroRNAs as promising therapeutic agents against
prostate cancer resistant to castration-where are we now?
Pharmaceutics. 16:13472024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pungsrinont T, Kallenbach J and Baniahmad
A: Role of PI3K-AKT-mTOR pathway as a pro-survival signaling and
resistance-mediating mechanism to therapy of prostate cancer. Int J
Mol Sci. 22:110882021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Eberlein C, Williamson SC, Hopcroft L, Ros
S, Moss JI, Kerr J, van Weerden WM, de Bruin EC, Dunn S, Willis B,
et al: Capivasertib combines with docetaxel to enhance anti-tumour
activity through inhibition of AKT-mediated survival mechanisms in
prostate cancer. Br J Cancer. 130:1377–1387. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guan H, Liu C, Fang F, Huang Y, Tao T,
Ling Z, You Z, Han X, Chen S, Xu B and Chen M: MicroRNA-744
promotes prostate cancer progression through aberrantly activating
Wnt/β-catenin signaling. Oncotarget. 8:14693–14707. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang K, Wu R, Mei F, Zhou Y, He L, Liu Y,
Zhao X, You J, Liu B, Meng Q and Pei F: Phosphorylated
LASS2 inhibits prostate carcinogenesis via negative
regulation of Wnt/β-catenin signaling. J Cell Biochem. Apr
14–2021.(Epub ahead of print). View Article : Google Scholar
|
|
49
|
Marei HE, Hasan A, Pozzoli G and
Cenciarelli C: Cancer immunotherapy with immune checkpoint
inhibitors (ICIs): Potential, mechanisms of resistance, and
strategies for reinvigorating T cell responsiveness when resistance
is acquired. Cancer Cell Int. 23:642023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jia D, Zhao M, Zhang X, Cheng X, Wei Q,
Lou L, Zhao Y, Jin Q, Chen M and Zhang D: Transcriptomic analysis
reveals the critical role of chemokine signaling in the
anti-atherosclerosis effect of Xuefu Zhuyu decoction. J
Ethnopharmacol. 332:1182452024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Conley-LaComb MK, Saliganan A, Kandagatla
P, Chen YQ, Cher ML and Chinni SR: PTEN loss mediated Akt
activation promotes prostate tumor growth and metastasis via
CXCL12/CXCR4 signaling. Mol Cancer. 12:852013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hatano K and Nonomura N: Systemic
therapies for metastatic castration-resistant prostate cancer: An
updated review. World J Mens Health. 41:769–784. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
de Kouchkovsky I, Rao A, Carneiro BA,
Zhang L, Lewis C, Phone A, Small EJ, Friedlander T, Fong L, Paris
PL, et al: A phase Ib/II study of the CDK4/6 inhibitor ribociclib
in combination with docetaxel plus prednisone in metastatic
castration-resistant prostate cancer. Clin Cancer Res.
28:1531–1539. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Agarwal N, Castellano D, Alonso-Gordoa T,
Arranz Arija JA, Colomba E, Gravis G, Mourey L, Oudard S, Fléchon
A, González M, et al: A signal-finding study of abemaciclib in
heavily pretreated patients with metastatic castration-resistant
prostate cancer: Results from CYCLONE 1. Clin Cancer Res.
30:2377–2383. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tshering LF, Luo F, Russ S, Szenk M, Rubel
D, Tutuska K, Rail JG, Balázsi G, Shen MM and Talos F: Immune
mechanisms shape the clonal landscape during early progression of
prostate cancer. Dev Cell. 58:1071–1086.e8. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rebuzzi SE, Rescigno P, Catalano F,
Mollica V, Vogl UM, Marandino L, Massari F, Pereira Mestre R,
Zanardi E, Signori A, et al: Immune checkpoint inhibitors in
advanced prostate cancer: Current data and future perspectives.
Cancers (Basel). 14:12452022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shuken SR: An introduction to mass
spectrometry-based proteomics. J Proteome Res. 22:2151–2171. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Al-Daffaie FM, Al-Mudhafar SF, Alhomsi A,
Tarazi H, Almehdi AM, El-Huneidi W, Abu-Gharbieh E, Bustanji Y,
Alqudah MAY, Abuhelwa AY, et al: Metabolomics and proteomics in
prostate cancer research: Overview, analytical techniques, data
analysis, and recent clinical applications. Int J Mol Sci.
25:50712024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lee PY, Saraygord-Afshari N and Low TY:
The evolution of two-dimensional gel electrophoresis - from
proteomics to emerging alternative applications. J Chromatogr A.
1615:4607632020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhu Y, Weiss T, Zhang Q, Sun R, Wang B, Yi
X, Wu Z, Gao H, Cai X, Ruan G, et al: High-throughput proteomic
analysis of FFPE tissue samples facilitates tumor stratification.
Mol Oncol. 13:2305–2328. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Aurilio G, Cimadamore A, Mazzucchelli R,
Lopez-Beltran A, Verri E, Scarpelli M, Massari F, Cheng L, Santoni
M and Montironi R: Androgen receptor signaling pathway in prostate
cancer: from genetics to clinical applications. Cells. 9:26532020.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dai C, Dehm SM and Sharifi N: Targeting
the androgen signaling axis in prostate cancer. J Clin Oncol.
41:4267–4278. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Song Z, Zhou Q, Zhang JL, Ouyang J and
Zhang ZY: Marker Ki-67 is a potential biomarker for the diagnosis
and prognosis of prostate cancer based on two cohorts. World J Clin
Cases. 12:32–41. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xu L, Yin Y, Li Y, Chen X, Chang Y, Zhang
H, Liu J, Beasley J, McCaw P, Zhang H, et al: A glutaminase isoform
switch drives therapeutic resistance and disease progression of
prostate cancer. Proc Natl Acad Sci USA. 118:e20127481182021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jiang B, Zhang J, Zhao G, Liu M, Hu J, Lin
F, Wang J, Zhao W, Ma H, Zhang C, et al: Filamentous GLS1 promotes
ROS-induced apoptosis upon glutamine deprivation via insufficient
asparagine synthesis. Mol Cell. 82:1821–1835.e6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tampio J, Montaser AB, Järvinen J,
Lehtonen M, Jalkanen AJ, Reinisalo M, Kokkola T, Terasaki T, Laakso
M, Rysä J, et al: The L-type amino acid transporter 1 enhances drug
delivery to the mouse pancreatic beta cell line (MIN6). Eur J Pharm
Sci. 203:1069372024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chidley C, Darnell AM, Gaudio BL, Lien EC,
Barbeau AM, Vander Heiden MG and Sorger PK: A CRISPRi/a screening
platform to study cellular nutrient transport in diverse
microenvironments. Nat Cell Biol. 26:825–838. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xu M, Sakamoto S, Matsushima J, Kimura T,
Ueda T, Mizokami A, Kanai Y and Ichikawa T: Up-regulation of LAT1
during antiandrogen therapy contributes to progression in prostate
cancer cells. J Urol. 195:1588–1597. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Saito S, Ando K, Sakamoto S, Xu M, Yamada
Y, Rii J, Kanaoka S, Wei J, Zhao X, Pae S, et al: The LAT1
inhibitor JPH203 suppresses the growth of castration-resistant
prostate cancer through a CD24-mediated mechanism. Cancer Sci.
115:2461–2472. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hoter A, Rizk S and Naim HY: The multiple
roles and therapeutic potential of molecular chaperones in prostate
cancer. Cancers (Basel. 11:11942019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mori M, Hitora T, Nakamura O, Yamagami Y,
Horie R, Nishimura H and Yamamoto T: Hsp90 inhibitor induces
autophagy and apoptosis in osteosarcoma cells. Int J Oncol.
46:47–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Rastogi S, Joshi A, Sato N, Lee S, Lee MJ,
Trepel JB and Neckers L: An update on the status of HSP90
inhibitors in cancer clinical trials. Cell Stress Chaperones.
29:519–539. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tausif YM, Thekkekkara D, Sai TE,
Jahagirdar V, Arjun HR, Meheronnisha HK, Babu A and Banerjee A:
Heat shock protein paradigms in cancer progression: future
therapeutic perspectives. 3 Biotech. 14:962024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Souza DS, Macheroni C, Pereira GJS,
Vicente CM and Porto CS: Molecular regulation of prostate cancer by
Galectin-3 and estrogen receptor. Front Endocrinol (Lausanne).
14:11241112023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Keizman D, Frenkel M, Peer A, Rosenbaum E,
Sarid D, Leibovitch I, Mano R, Yossepowitch O, Wolf I, Geva R, et
al: Modified citrus pectin treatment in non-metastatic
biochemically relapsed prostate cancer: Long-term results of a
prospective phase II study. Nutrients. 15:35332023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang F, Li Z, Feng X, Yang D and Lin M:
Advances in PSMA-targeted therapy for prostate cancer. Prostate
Cancer Prostatic Dis. 25:11–26. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yan Y, Zhuo H, Li T, Zhang J, Tan M and
Chen Y: Advancements in PSMA ligand radiolabeling for diagnosis and
treatment of prostate cancer: A systematic review. Front Oncol.
14:13736062024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Bakht MK and Beltran H: Biological
determinants of PSMA expression, regulation and heterogeneity in
prostate cancer. Nat Rev Urol. 22:26–45. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Fu P, Bu C, Cui B, Li N and Wu J:
Screening of differentially expressed genes and identification of
AMACR as a prognostic marker in prostate cancer. Andrologia.
53:e140672021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Carswell BM, Woda BA, Wang X, Li C,
Dresser K and Jiang Z: Detection of prostate cancer by
alpha-methylacyl CoA racemase (P504S) in needle biopsy specimens
previously reported as negative for malignancy. Histopathology.
48:668–673. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Quail DF and Walsh DA: Revolutionizing
cancer research with spatial proteomics and visual intelligence.
Nat Methods. 21:2216–2219. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Osmulski PA, Cunsolo A, Chen M, Qian Y,
Lin CL, Hung CN, Mahalingam D, Kirma NB, Chen CL, Taverna JA, et
al: Contacts with macrophages promote an aggressive nanomechanical
phenotype of circulating tumor cells in prostate cancer. Cancer
Res. 81:4110–4123. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Hsieh WC, Budiarto BR, Wang YF, Lin CY,
Gwo MC, So DK, Tzeng YS and Chen SY: Spatial multi-omics analyses
of the tumor immune microenvironment. J Biomed Sci. 29:962022.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kowalczyk T, Ciborowski M, Kisluk J,
Kretowski A and Barbas C: Mass spectrometry based proteomics and
metabolomics in personalized oncology. Biochim Biophys Acta Mol
Basis Dis. 1866:1656902020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhong AB, Muti IH, Eyles SJ, Vachet RW,
Sikora KN, Bobst CE, Calligaris D, Stopka SA, Agar JN, Wu CL, et
al: Multiplatform metabolomics studies of human cancers with NMR
and mass spectrometry imaging. Front Mol Biosci. 9:7852322022.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li R, Li L, Xu Y and Yang J: Machine
learning meets omics: Applications and perspectives. Brief
Bioinform. 23:bbab4602022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ritterson Lew C, Guin S and Theodorescu D:
Targeting glycogen metabolism in bladder cancer. Nat Rev Urol.
12:383–391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chetta P, Sriram R and Zadra G: Lactate as
key metabolite in prostate cancer progression: What are the
clinical implications? Cancers (Basel). 15:34732023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chen L, Xu YX, Wang YS and Zhou JL: Lipid
metabolism, amino acid metabolism, and prostate cancer: A crucial
metabolic journey. Asian J Androl. 26:123–134. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zeković M, Bumbaširević U, Živković M and
Pejčić T: Alteration of lipid metabolism in prostate cancer:
Multifaceted oncologic implications. Int J Mol Sci. 24:13912023.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Škara L, Huđek Turković A, Pezelj I,
Vrtarić A, Sinčić N, Krušlin B and Ulamec M: Prostate cancer-focus
on cholesterol. Cancers (Basel). 13:46962021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yun SJ, Yan C, Jeong P, Kang HW, Kim YH,
Kim EA, Lee OJ, Kim WT, Moon SK, Kim IY, et al: Comparison of mRNA,
protein, and urinary nucleic acid levels of S100A8 and S100A9
between prostate cancer and BPH. Ann Surg Oncol. 22:2439–2445.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Srihari S, Kwong R, Tran K, Simpson R,
Tattam P and Smith E: Metabolic deregulation in prostate cancer.
Mol Omics. 14:320–329. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Singh R and Mills IG: The interplay
between prostate cancer genomics, metabolism, and the epigenome:
Perspectives and future prospects. Front Oncol. 11:7043532021.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Penney KL, Tyekucheva S, Rosenthal J, El
Fandy H, Carelli R, Borgstein S, Zadra G, Fanelli GN, Stefanizzi L,
Giunchi F, et al: Metabolomics of prostate cancer gleason score in
tumor tissue and serum. Mol Cancer Res. 19:475–484. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Salciccia S, Capriotti AL, Laganà A, Fais
S, Logozzi M, De Berardinis E, Busetto GM, Di Pierro GB, Ricciuti
GP, Del Giudice F, et al: Biomarkers in prostate cancer diagnosis:
from current knowledge to the role of metabolomics and exosomes.
Int J Mol Sci. 22:43672021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Bansal N, Kumar M, Sankhwar SN and Gupta
A: Evaluation of prostate cancer tissue metabolomics: Would clinics
utilise it for diagnosis? Expert Rev Mol Med. 25:e262023.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lima AR, Carvalho M, Aveiro SS, Melo T,
Domingues MR, Macedo-Silva C, Coimbra N, Jerónimo C, Henrique R,
Bastos ML, et al: Comprehensive metabolomics and lipidomics
profiling of prostate cancer tissue reveals metabolic
dysregulations associated with disease development. J Proteome Res.
21:727–739. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Sirocchi C, Bogliolo A and Montagna S:
Medical-informed machine learning: Integrating prior knowledge into
medical decision systems. BMC Med Inform Decis Mak. 24 (Suppl
4):S1862024. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yu X, Liu R, Gao W, Wang X and Zhang Y:
Single-cell omics traces the heterogeneity of prostate cancer cells
and the tumor microenvironment. Cell Mol Biol Lett. 28:382023.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Murphy N, Shah P, Shih A, Khalili H, Liew
A, Zhu X and Lee A: Single-cell sequencing in genitourinary
malignancies. Adv Exp Med Biol. 1255:153–164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lu J, Sheng Y, Qian W, Pan M, Zhao X and
Ge Q: scRNA-seq data analysis method to improve analysis
performance. IET Nanobiotechnol. 17:246–256. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Song H, Weinstein HNW, Allegakoen P,
Wadsworth MH II, Xie J, Yang H, Castro EA, Lu KL, Stohr BA, Feng
FY, et al: Single-cell analysis of human primary prostate cancer
reveals the heterogeneity of tumor-associated epithelial cell
states. Nat Commun. 13:1412022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Feng DC, Zhu WZ, Wang J, Li DX, Shi X,
Xiong Q, You J, Han P, Qiu S, Wei Q and Yang L: The implications of
single-cell RNA-seq analysis in prostate cancer: unraveling tumor
heterogeneity, therapeutic implications and pathways towards
personalized therapy. Mil Med Res. 11:212024.PubMed/NCBI
|
|
106
|
Amirifar L, Besanjideh M, Nasiri R,
Shamloo A, Nasrollahi F, de Barros NR, Davoodi E, Erdem A, Mahmoodi
M, Hosseini V, et al: Droplet-based microfluidics in biomedical
applications. Biofabrication. 14:0220012022. View Article : Google Scholar
|
|
107
|
Xin S, Liu X, Li Z, Sun X, Wang R, Zhang
Z, Feng X, Jin L, Li W, Tang C, et al: ScRNA-seq revealed an
immunosuppression state and tumor microenvironment heterogeneity
related to lymph node metastasis in prostate cancer. Exp Hematol
Oncol. 12:492023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Peng G, Wang C, Wang H, Qu M, Dong K, Yu
Y, Jiang Y, Gan S and Gao X: Gankyrin-mediated interaction between
cancer cells and tumor-associated macrophages facilitates prostate
cancer progression and androgen deprivation therapy resistance.
Oncoimmunology. 12:21734222023. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Mengistu BA, Tsegaw T, Demessie Y, Getnet
K, Bitew AB, Kinde MZ, Beirhun AM, Mebratu AS, Mekasha YT, Feleke
MG and Fenta MD: Comprehensive review of drug resistance in
mammalian cancer stem cells: Implications for cancer therapy.
Cancer Cell Int. 24:4062024. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Hu WY, Hu DP, Xie L, Nonn L, Lu R, Abern
M, Shioda T and Prins GS: Keratin profiling by single-cell
RNA-sequencing identifies human prostate stem cell lineage
hierarchy and cancer stem-like cells. Int J Mol Sci. 22:81092021.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Muller L, Fauvet F, Chassot C, Angileri F,
Coutant A, Dégletagne C, Tonon L, Saintigny P, Puisieux A, Morel
AP, et al: EMT-driven plasticity prospectively increases cell-cell
variability to promote therapeutic adaptation in breast cancer.
Cancer Cell Int. 25:322025. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wei G, Zhu H, Zhou Y, Pan Y, Yi B and Bai
Y: Single-cell sequencing revealed metabolic reprogramming and its
transcription factor regulatory network in prostate cancer. Transl
Oncol. 44:1019252024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Nguyen AD, Haines C, Price MJ, Dalton TE,
Baëta CD, Hockenberry HA and Goodwin CR: Single-cell RNA sequencing
comparison of the human metastatic prostate spine tumor
microenvironment. STAR Protoc. 5:1028052024. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhang L, Lee M, Maslov AY, Montagna C,
Vijg J and Dong X: Analyzing somatic mutations by single-cell
whole-genome sequencing. Nat Protoc. 19:487–516. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zheng K, Hai Y, Xi Y, Zhang Y, Liu Z, Chen
W, Hu X, Zou X and Hao J: Integrative multi-omics analysis unveils
stemness-associated molecular subtypes in prostate cancer and
pan-cancer: Prognostic and therapeutic significance. J Transl Med.
21:7892023. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Nevedomskaya E and Haendler B: From omics
to multi-omics approaches for in-depth analysis of the molecular
mechanisms of prostate cancer. Int J Mol Sci. 23:62812022.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Ren S, Li J, Dorado J, Sierra A,
González-Díaz H, Duardo A and Shen B: From molecular mechanisms of
prostate cancer to translational applications: Based on multi-omics
fusion analysis and intelligent medicine. Health Inf Sci Syst.
12:62023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zhu Q, Zhao X, Zhang Y, Li Y, Liu S, Han
J, Sun Z, Wang C, Deng D, Wang S, et al: Single cell multi-omics
reveal intra-cell-line heterogeneity across human cancer cell
lines. Nat Commun. 14:81702023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Nabavizadeh A, Barkovich MJ, Mian A, Ngo
V, Kazerooni AF and Villanueva-Meyer JE: Current state of pediatric
neuro-oncology imaging, challenges and future directions.
Neoplasia. 37:1008862023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Huang J, Mao L, Lei Q and Guo AY:
Bioinformatics tools and resources for cancer and application. Chin
Med J (Engl). 137:2052–2064. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Chakraborty S, Sharma G, Karmakar S and
Banerjee S: Multi-OMICS approaches in cancer biology: New era in
cancer therapy. Biochim Biophys Acta Mol Basis Dis.
1870:1671202024. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhou Y, Xiao X, Dong L, Tang C, Xiao G and
Xu L: Cooperative integration of spatially resolved multi-omics
data with COSMOS. Nat Commun. 16:272025. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Mohr AE, Ortega-Santos CP, Whisner CM,
Klein-Seetharaman J and Jasbi P: Navigating challenges and
opportunities in multi-omics integration for personalized
healthcare. Biomedicines. 12:14962024. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Viana JN, Pilbeam C, Howard M, Scholz B,
Ge Z, Fisser C, Mitchell I, Raman S and Leach J: Maintaining
high-touch in high-tech digital health monitoring and multi-omics
prognostication: ethical, equity, and societal considerations in
precision health for palliative care. OMICS. 27:461–473. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Ramos-Lopez O, Martinez JA and Milagro FA:
Holistic integration of omics tools for precision nutrition in
health and disease. Nutrients. 14:40742022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ahmed Z: Practicing precision medicine
with intelligently integrative clinical and multi-omics data
analysis. Hum Genomics. 14:352020. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Messina C, Giunta EF, Signori A, Rebuzzi
SE, Banna GL, Maniam A, Buti S, Cattrini C, Fornarini G, Bauckneht
M, et al: Combining PARP inhibitors and androgen receptor
signalling inhibitors in metastatic prostate cancer: A quantitative
synthesis and meta-analysis. Eur Urol Oncol. 7:179–188. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Markowski MC, Sternberg CN, Wang H, Wang
T, Linville L, Marshall CH, Sullivan R, King S, Lotan TL and
Antonarakis ES: TRIUMPH: Phase II trial of rucaparib monotherapy in
patients with metastatic hormone-sensitive prostate cancer
harboring germline homologous recombination repair gene mutations.
Oncologist. 29:794–800. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Gasmi A, Roubaud G, Dariane C, Barret E,
Beauval JB, Brureau L, Créhange G, Fiard G, Fromont G, Gauthé M, et
al: Overview of the development and use of akt inhibitors in
prostate cancer. J Clin Med. 11:1602021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Noori M, Azizi S, Mahjoubfar A, Abbasi
Varaki F, Fayyaz F, Mousavian AH, Bashash D, Kardoust Parizi M and
Kasaeian A: Efficacy and safety of immune checkpoint inhibitors for
patients with prostate cancer: A systematic review and
meta-analysis. Front Immunol. 14:11810512023. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wei Z, Han D, Zhang C, Wang S, Liu J, Chao
F, Song Z and Chen G: Deep learning-based multi-omics integration
robustly predicts relapse in prostate cancer. Front Oncol.
12:8934242022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Sinha A, Huang V, Livingstone J, Wang J,
Fox NS, Kurganovs N, Ignatchenko V, Fritsch K, Donmez N, Heisler
LE, et al: The proteogenomic landscape of curable prostate cancer.
Cancer Cell. 35:414–427.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Ozaki Y, Broughton P, Abdollahi H, Valafar
H and Blenda AV: Integrating omics data and AI for cancer diagnosis
and prognosis. Cancers (Basel). 16:24482024. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Baydoun A, Jia AY, Zaorsky NG, Kashani R,
Rao S, Shoag JE, Vince RA Jr, Bittencourt LK, Zuhour R, Price AT,
et al: Artificial intelligence applications in prostate cancer.
Prostate Cancer Prostatic Dis. 27:37–45. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Bian X, Wang W, Abudurexiti M, Zhang X, Ma
W, Shi G, Du L, Xu M, Wang X, Tan C, et al: Integration analysis of
single-cell multi-omics reveals prostate cancer heterogeneity. Adv
Sci (Weinh). 11:e23057242024. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Fonseca NM, Maurice-Dror C, Herberts C, Tu
W, Fan W, Murtha AJ, Kollmannsberger C, Kwan EM, Parekh K, Schönlau
E, et al: Prediction of plasma ctDNA fraction and prognostic
implications of liquid biopsy in advanced prostate cancer. Nat
Commun. 15:18282024. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Armstrong L, Willoughby CE and McKenna DJ:
The suppression of the epithelial to mesenchymal transition in
prostate cancer through the targeting of MYO6 Using MiR-145-5p. Int
J Mol Sci. 25:43012024. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Yu B, Zuo X and Zhao C: Efficacy of
abiraterone combined with prednisone in castration-resistant
prostate cancer and its impact on miR-221/222 expression. Am J
Cancer Res. 14:4708–4716. 2024. View Article : Google Scholar : PubMed/NCBI
|