Article
Open Access
Protein expression under sustained activation of signal transducer and activator of transcription‑3 in diethylnitrosamine‑induced rat liver carcinogenesis
- Authors:
- Li‑Min Cui
- Kun Zhang
- Dong‑Jie Ma
- Shuang‑Ping Liu
- Xue‑Wu Zhang
-
View Affiliations / Copyright
Affiliations:
Department of Biochemistry and Molecular Biology, College of Medicine, Yanbian University, Yanji, Jilin 133002, P.R. China
-
Pages:
608-614
|
Published online on:
May 28, 2014
https://doi.org/10.3892/ol.2014.2194
- Expand metrics +
Metrics:
Total
Views: 0
(Spandidos Publications: | PMC Statistics:
)
Metrics:
Total PDF Downloads: 0
(Spandidos Publications: | PMC Statistics:
)
This article is mentioned in:
Abstract
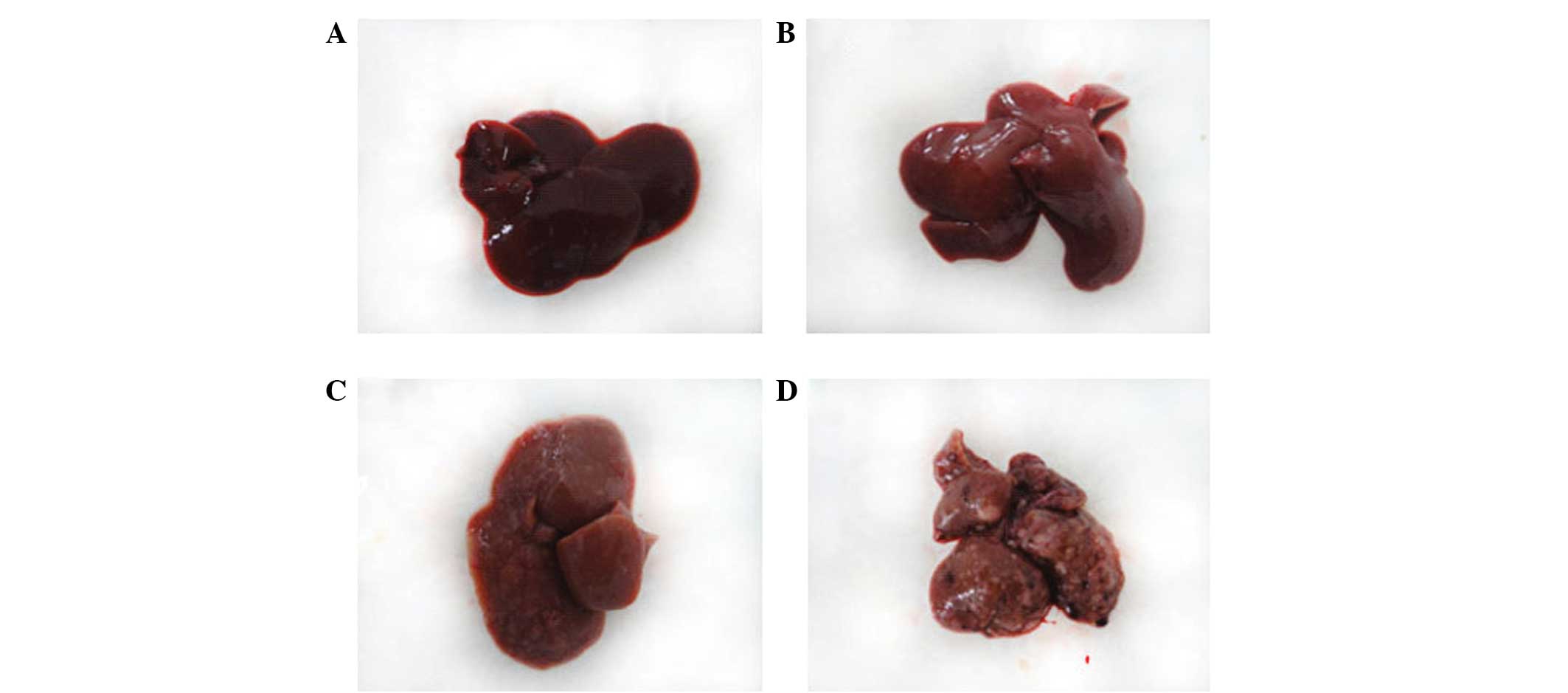
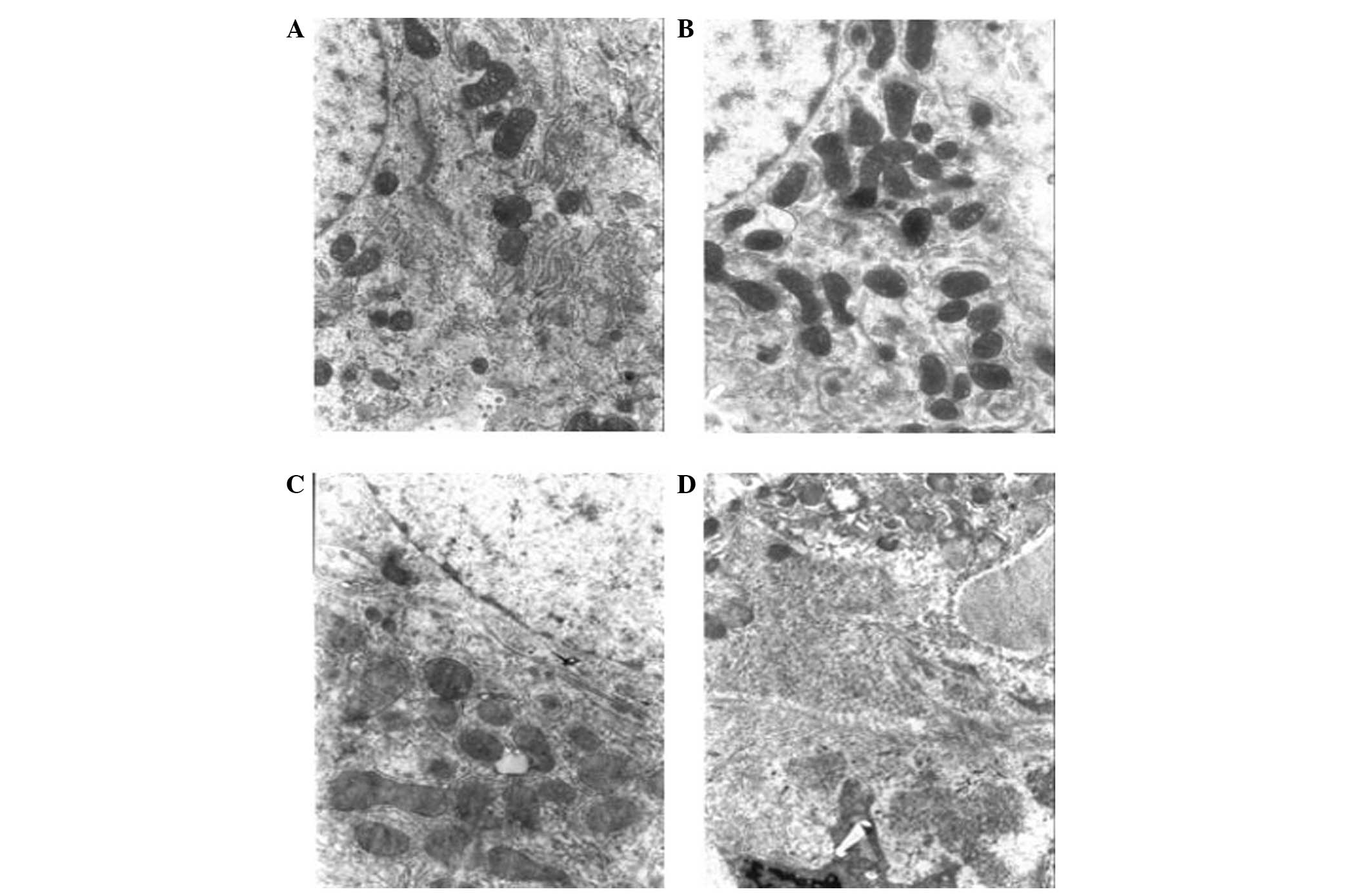
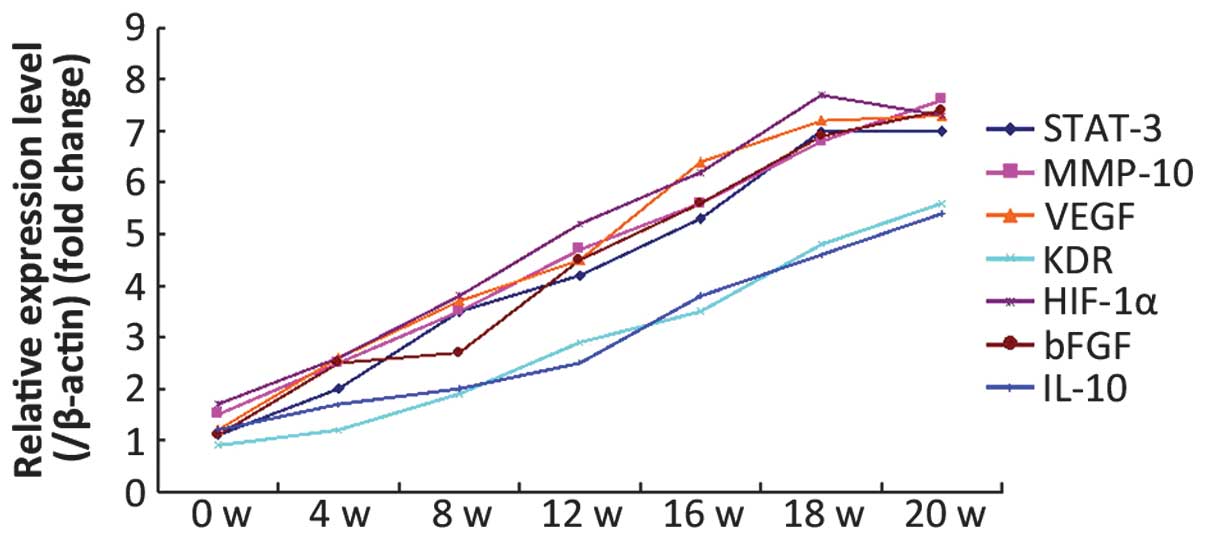
The aim of the present study was to investigate the expression of proteins associated with the sustained activation of the signal transducer and activator of transcription (STAT)‑3 pathway during diethylnitrosamine (DEN)‑induced rat liver carcinogenesis. DEN was intermittently administered to rats to induce liver cancer, and light and electron microscopy were used to observe the morphological changes in the liver during carcinogenesis. Western blotting and quantitative polymerase chain reaction (qPCR) were used to detect the expression of STAT‑3, phosphorylated (p)‑STAT‑3, matrix metalloproteinase (MMP)‑10, vascular endothelial growth factor (VEGF), kinase insert domain receptor (KDR), hypoxia inducible factor (HIF)‑1α, basic fibroblast growth factor (bFGF) and interleukin (IL)‑10, in order to investigate the association between STAT‑3 and p‑STAT‑3 expression and MMP‑10, VEGF, KDR, HIF‑1α, bFGF and IL‑10. The western blotting and qPCR results revealed that the expression of STAT‑3, p‑STAT‑3, MMP‑10, VEGF, KDR, HIF‑1α, bFGF and IL‑10 proteins gradually increased during carcinogenesis. Furthermore, the STAT‑3 and p‑STAT‑3 levels were found to positively correlate with MMP‑10, VEGF, KDR, HIF‑1α, bFGF and IL‑10 protein expression. During DEN‑induced rat liver carcinogenesis, STAT‑3 protein continually activated MMP‑10, VEGF, KDR, HIF‑1α, bFGF and IL‑10, and its expression was found to positively correlate with the expression of these proteins.
View References
|
1
|
Stefanatos RK and Vidal M: Tumor invasion
and metastasis in Drosophila: a bold past, a bright future. J Genet
Genomics. 38:431–438. 2011.
|
|
2
|
Uluer ET, Aydemir I, Inan S, Ozbilgin K
and Vatansever HS: Effects of 5-fluorouracil and gemcitabine on a
breast cancer cell line (MCF-7) via the JAK/STAT pathway. Acta
Histochem. 114:641–646. 2012.
|
|
3
|
Xiong H, Chen ZF, Liang QC, et al:
Inhibition of DNA methyltransferase induces G2 cell cycle arrest
and apoptosis in human colorectal cancer cells via inhibition of
JAK2/STAT3/STAT5 signalling. J Cell Mol Med. 13:3668–3679.
2009.
|
|
4
|
Constantinescu SN, Girardot M and Pecquet
C: Mining for JAK-STAT mutations in cancer. Trends Biochem Sci.
33:122–131. 2008.
|
|
5
|
Jenkins BJ, Grail D, Nheu T, et al:
Hyperactivation of Stat3 in gp130 mutant mice promotes gastric
hyperproliferation and desensitizes TGF-beta signaling. Nat Med.
11:845–852. 2005.
|
|
6
|
Haura EB, Turkson J and Jove R: Mechanisms
of disease: Insights into the emerging role of signal transducers
and activators of transcription in cancer. Nat Clin Pract Oncol.
2:315–324. 2005.
|
|
7
|
Sakamoto M, Hirohashi S and Shimosato Y:
Early stages of multistep hepatocarcinogenesis: adenomatous
hyperplasia and early hepatocellular carcinoma. J Third Mil Med
Univ. 22:172–178. 1991.
|
|
8
|
Zhang ZM, Wang G, Chen C, et al:
Pathologic and morphologic study on modified DEN-induced
hepatocarcinoma model in rats. Acat Academia Medicine Militaris
Tertiae. 29:1164–1167. 2007.
|
|
9
|
Munir S, Frøsig TM, Hansen M, Svane IM and
Andersen MH: Characterization of T-cell responses against IκBα in
cancer patients. Oncoimmunology. 1:1290–1296. 2012.
|
|
10
|
Lin L, Fuchs J, Li C, Olson V, Bekaii-Saab
T and Lin J: STAT3 signaling pathway is necessary for cell survival
and tumorsphere forming capacity in
ALDH+/CD133+ stem cell-like human colon
cancer cells. Biochem Biophys Res Commun. 416:246–251. 2011.
|
|
11
|
Minakhina S, Tan W and Steward R: JAK/STAT
and the GATA factor Pannier control hemocyte maturation and
differentiation in Drosophila. Dev Biol. 352:308–316. 2011.
|
|
12
|
Yen CY, Chen CH, Chang CH, et al: Matrix
metalloproteinases (MMP) 1 and MMP10 but not MMP12 are potential
oral cancer markers. Biomarkers. 14:244–249. 2009.
|
|
13
|
Saini S, Liu J, Yamamura S, Majid S,
Kawakami K, Hirata H and Dahiya R: Functional significance of
secreted Frizzled-related protein 1 in metastatic renal cell
carcinomas. Cancer Res. 69:6815–6822. 2009.
|
|
14
|
Aung PP, Oue N, Mitani Y, et al:
Systematic search for gastric cancer-specific genes based on SAGE
data: melanoma inhibitory activity and matrix metalloproteinase-10
are novel prognostic factors in patients with gastric cancer.
Oncogene. 25:2546–2557. 2006.
|
|
15
|
Goydos JS and Gorski DH: Vascular
endothelial growth factor C mRNA expression correlates with stage
of progression in patients with melanoma. Clin Cancer Res.
9:5962–5967. 2003.
|
|
16
|
Pandya NM, Dhalla NS and Santani DD:
Angiogenesis-a new target for future therapy. Vascul Pharmacol.
44:265–274. 2006.
|
|
17
|
Burdelya L, Kujawski M, Niu G, et al:
Stat3 activity in melanoma cells affects migration of immune
effector cells and nitric oxide-mediated antitumor effects. J
Immunol. 174:3925–3931. 2005.
|