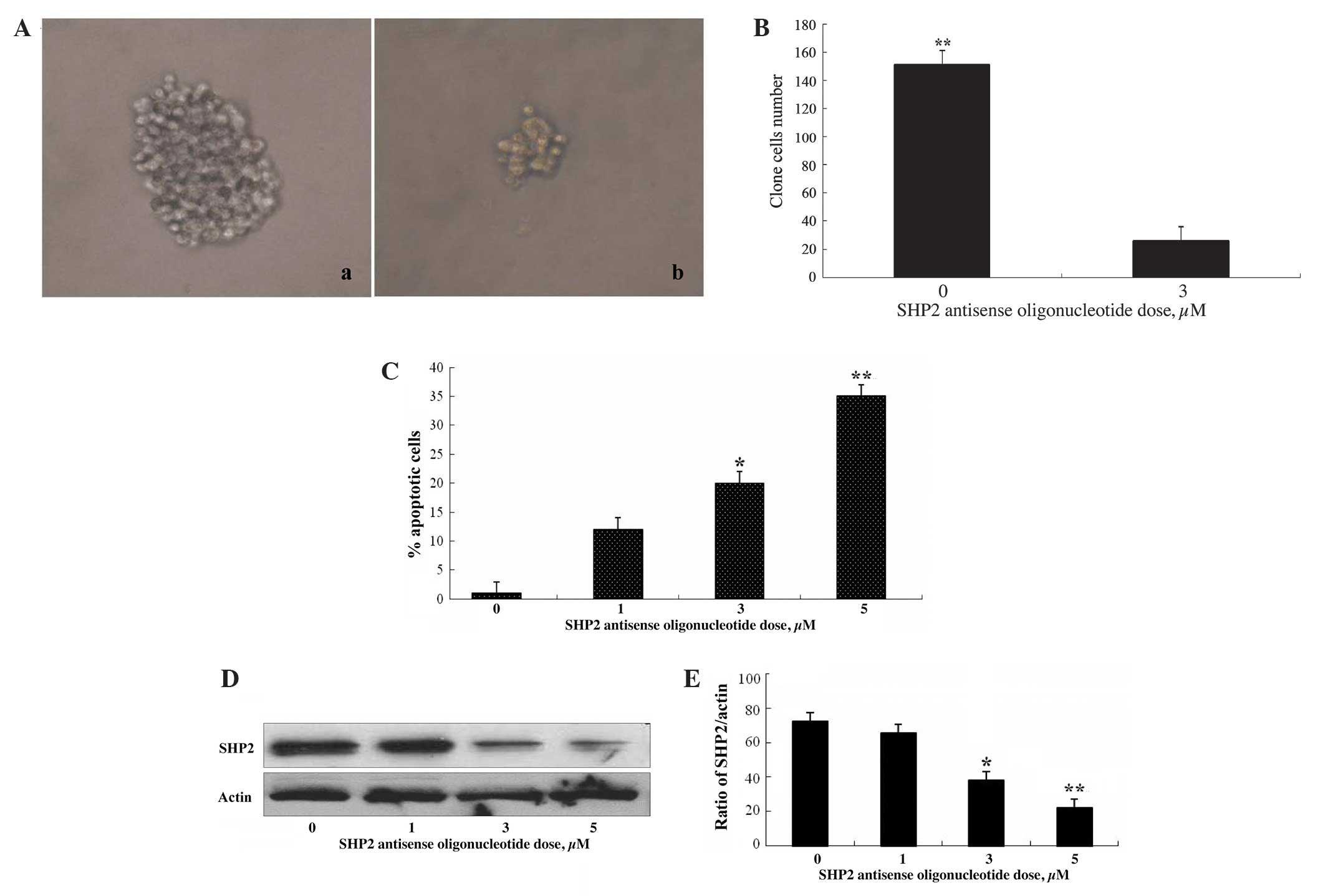
|
1
|
Grossmann KS, Rosário M, Birchmeier C and
Birchmeier W: The tyrosine phosphatase SHP2 in development and
cancer. Adv Cancer Res. 106:53–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrari E, Tinti M, Costa S, et al:
Identification of new substrates of the protein-tyrosine
phosphatase PTP1B by Bayesian integration of proteome evidence. J
Biol Chem. 286:4173–4185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Araki T, Chan G, Newbigging S, et al:
Noonan syndrome cardiac defects are caused by PTPN11 acting in
endocardium to enhance endocardial-mesenchymal transformation. Proc
Natl Acad Sci USA. 106:4736–4741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hagihara K, Zhang EE, Ke YH, et al: Shp2
acts downstream of SDF-1alpha/CXCR4 in guiding granule cell
migration during cerebellar development. Dev Biol. 334:276–284.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niihori T, Aoki Y, Ohashi H, et al:
Functional analysis of PTPN11/SHP-2 mutants identified in Noonan
syndrome and childhood leukemia. J Hum Gene. 50:192–202. 2005.
View Article : Google Scholar
|
|
6
|
Ogata T and Yoshida R: PTPN11 mutations
and genotype-phenotype correlations in Noonan and LEOPARD
syndromes. Pediatr Endocrinol Rev. 2:669–674. 2005.PubMed/NCBI
|
|
7
|
Oh ES, Gu H, Saxton TM, et al: Regulation
of early events in integrin signaling by protein tyrosine
phosphatase SHP-2. Mol Cell Biol. 19:3205–3215. 1999.PubMed/NCBI
|
|
8
|
Bowen ME, Ayturk UM, Kurek KC, et al: SHP2
regulates chondrocyte terminal differentiation, growth plate
architecture and skeletal cell fates. PLoS Genet. 10:e10043642014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hartman ZR, Schaller MD and Agazie YM: The
tyrosine phosphatase SHP2 regulates focal adhesion kinase to
promote EGF-induced lamellipodia persistence and cell migration.
Mol Cancer Res. 11:651–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiu W, Wang X, Romanov V, et al:
Structural insights into Noonan/LEOPARD syndrome-related mutants of
protein-tyrosine phosphatase SHP2 (PTPN11). BMC Struct Biol.
14:102014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oishi K, Zhang H, Gault WJ, et al:
Phosphatase-defective LEOPARD syndrome mutations in PTPN11 gene
have gain-of-function effects during Drosophila development.
Hum Mol Genet. 18:193–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan G, Kalaitzidis D, Usenko T, et al:
Leukemogenic Ptpn11 causes fatal myeloproliferative disorder via
cell-autonomous effects on multiple stages of hematopoiesis. Blood.
113:4414–4424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schneeberger VE, Luetteke N, Ren Y, et al:
SHP2E76K mutant promotes lung tumorigenesis in transgenic mice.
Carcinogenesis. 35:1717–1725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Furcht CM, Muñoz Rojas AR, Nihalani D and
Lazzara MJ: Diminished functional role and altered localization of
SHP2 in non small cell lung cancer cells with EGFR-activating
mutations. Oncogene. 32:2346–2355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sausgruber N, Coissieux MM, Britschgi A,
et al: Tyrosine phosphatase SHP2 increases cell motility in
triple-negative breast cancer through the activation of SRC-family
kinases. Oncogene. 34:2272–2278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Z, Fang H, Wang X, et al:
Overexpression of SHP2 tyrosine phosphatase promotes the
tumorigenesis of breast carcinoma. Oncol Rep. 32:205–212.
2014.PubMed/NCBI
|
|
17
|
Aceto N, Sausgruber N, Brinkhaus H, et al:
Tyrosine phosphatase SHP2 promotes breast cancer progression and
maintains tumor-initiating cells via activation of key
transcription factors and a positive feedback signaling loop. Nat
Med. 18:529–537. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang J, Jin MS, Kong F, et al: Increased
expression of tyrosine phosphatase SHP-2 in Helicobacter
pylori-infected gastric cancer. World J Gastroenterol.
19:575–580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong LB, Li GQ, Tian ZH, et al:
Expressions of Src homology 2 domain-containing phosphatase and its
clinical significance in laryngeal carcinoma. Genet Mol Res.
12:4207–4212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang HC, Chiang WF, Huang HH, et al:
Src-homology 2 domain-containing tyrosine phosphatase 2 promotes
oral cancer invasion and metastasis. BMC Cancer. 14:4422014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bard-Chapeau EA, Li S, Ding J, et al:
PTPN11/SHP2 acts as a tumor suppressor in hepatocellular
carcinogenesis. Cancer Cell. 19:629–639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai P, Guo W, Yuan H, et al: Expression
and clinical significance of tyrosine phosphatase SHP-2 in colon
cancer. Biomed Pharmacother. 68:285–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma N, Everingham S, Ramdas B, et al:
SHP2 phosphatase promotes mast cell chemotaxis toward stem cell
factor via enhancing activation of the Lyn/Vav/Rac signaling axis.
J Immunol. 192:4859–4866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Princen F, Bard E, Sheikh F, et al:
Deletion of Shp2 tyrosine phosphatase in muscle leads to dilated
cardiomyopathy, insulin resistance and premature death. Mol Cell
Biol. 29:378–388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Edwards JJ, Martinelli S, Pannone L, et
al: A PTPN11 allele encoding a catalytically impaired SHP2 protein
in a patient with a Noonan syndrome phenotype. Am J Med Genet A.
164A:2351–2355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Müller PJ, Rigbolt KT, Paterok D, et al:
Protein tyrosine phosphatase SHP2/PTPN11 mistargeting as a
consequence of SH2-domain point mutations associated with Noonan
Syndrome and leukemia. J Proteomics. 84:132–147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo H, Tang C, Yang X and Zhou X: The
tyrosine phosphatase SHP2: A key molecule linked both type 2
diabetes and cancers? Med Chem. 4:435–438. 2014.
|
|
28
|
Meng F, Zhao X and Zhang S: SHP-2
phosphatase promotes cervical cancer cell proliferation through
inhibiting interferon-β production. J Obstet Gynaecol Res.
39:272–279. 2013. View Article : Google Scholar : PubMed/NCBI
|