Introduction
The morbidity and mortality of prostate cancer is
increasing every year, with bone metastasis representing the
primary cause of disease-associated mortality in patients with
prostate cancer (1).
Epithelial-mesenchymal transition (EMT) is the primary mechanism of
prostate cancer metastasis to bone (2). Therefore, inhibition of this process in
prostate cancer cells is an important approach for the suppression
of prostate cancer progression.
The occurrence and development of prostate cancer
depends on paracrine interactions between matrix and epithelial
cells (3). Hepatocyte growth factor
(HGF) is a multifunctional growth factor produced by mesenchymal
cells, which promotes the migration and growth of epithelial cells
(including cancer cells) via EMT (3,4). The
levels of c-Met and HGF are significantly correlated, and it has
been demonstrated that c-Met expression is a strong prognostic
factor for histological tumor grade in patients with prostate
cancer (5). In addition, a number of
studies have demonstrated overexpression of c-Met in prostate
cancer cell models (6,7). Furthermore, inhibition of c-Met
expression has been observed to reduce invasion and metastasis in
prostate cancer (8).
Curcumin, a plant polyphenol extracted from the
turmeric rhizome, possesses antioxidant, anticoagulant,
anti-inflammatory, anti-atherosclerotic, lipid-lowering and
anti-aging properties (9,10). Previous studies have demonstrated that
curcumin is a highly effective anticancer agent (10,11). In
addition, curcumin has been revealed to inhibit the progression of
prostate cancer and bone metastasis (11). However, the effect of curcumin on EMT
in prostate cancer cells remains to be elucidated. In the present
study, the effect and mechanism of curcumin on HGF-induced EMT in
DU145 prostate cancer cells was investigated, in order to establish
a novel approach for the treatment of prostate cancer.
Materials and methods
Materials
Curcumin and dimethyl sulfoxide (DMSO; cat no.
CLS3085) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Trypsin and Dulbecco's modified Eagle's medium (DMEM)-high glucose
containing trypsin (cat no. SH30022.01B) and fetal bovine serum
(FBS; cat no. SH30084.03) were acquired from HyClone (GE Healthcare
Life Sciences, Logan, UT, USA). Antibodies against E-cadherin
(mouse monoclonal; cat no. sc-59905), vimentin (mouse monoclonal;
cat no. sc-373717), AKT (mouse polyclonal; cat no. sc-377457),
phosphorylated (p)-AKT (rabbit polyclonal; cat no. sc-33437),
extracellular signal-regulated kinase (ERK; mouse monoclonal; cat
no. sc-514302), p-ERK (T202/Y204; goat polyclonal; cat no.
sc-16982), Snail (goat polyclonal; cat no. sc-10433), c-Met (mouse
monoclonal; cat no. sc-8057) and β-actin (mouse monoclonal; cat no.
sc-8432) were obtained from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). HGF was acquired from Invitrogen (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and dissolved in
phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 4.3 mM
Na2HPO4.7 H2O and 1.4 mM
KH2PO4, pH 7.4), with 0.1% bovine serum
albumin. Rabbit anti-goat immunoglobulin G (IgG; cat no. sc-2768;
H+L)-horseradish peroxidase conjugated antibody and rabbit
anti-mouse IgG-R (cat no. sc-358922) were purchased from Santa Cruz
Biotechnology, Inc.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
cat no. C0009) were obtained from Beyotime Institute of
Biotechnology (Shanghai, China). RevertAid™ First Strand cDNA
Synthesis kit (cat no. N8080234) and Lipofectamine® 2000
Transfection Reagent (cat no. 11668030) were acquired from
Invitrogen (Thermo Fisher Scientific, Inc.).
Cell culture
The DU145 cell line was obtained from the cell
repository of the Chinese Academy of Sciences (Shanghai, China).
DU145 cells were cultured in DMEM-high glucose supplemented with
10% FBS, 100 U/ml penicillin (cat no. ST488-1; Beyotime Institute
of Biotechnology) and 100 U/ml streptomycin (cat no. ST488-2;
Beyotime Institute of Biotechnology), and incubated (cat no.
670190; Greiner Bio-One, Stonehouse, UK) in a humidified atmosphere
of 5% CO2 and 95% air at 37°C. Cells were cultured
without serum for ≥6 h prior to the commencement of the
experiments.
Cell proliferation assay
DU145 cells (8×103 cells/ml) were seeded
into 96-well culture plates (cat no. CW0543; Beijing ComWin Biotech
Co., Ltd., Beijing, China), and incubated overnight in a humidified
atmosphere of 5% CO2 and 95% air at 37°C. Following 12 h
incubation, the medium was replaced with serum-free DMEM-high
glucose, and curcumin was added at various concentrations (0, 5,
10, 15, 20, 25, 30, 35, 40, 45 and 50 µmol/l). Blank (cell-free +
medium) and control (cells + medium, without curcumin) groups were
additionally evaluated. Following 48 h of incubation, the culture
medium was removed, and 5 mg/ml MTT was added to each well. The
plates were subsequently incubated for 4 h at 37°C. The culture
medium was replaced with 150 µl DMSO/well, and the absorbance (A)
at a wavelength of 490 nm was measured using 2104 EnVision®
Multilabel Reader (cat no. 2104-0010; PerkinElmer, Inc., Waltham,
MA, USA). Cell viability was calculated using the following
formula: Cell viability (%) = [(HGF A value - blank group A
value)]/[(control group A value - blank group A value)] × 100.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from the tissue samples using
TRIzol® reagent, according to the manufacturer's instructions
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Subsequently, cDNA was synthesized using a TaqMan Reverse
Transcription Reagents kit (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. The relative
expression levels of mRNA were determined using a Power SYBR® Green
PCR Master Mix kit (Thermo Fisher Scientific, Inc.) and normalized
to GAPDH. RT-PCR was performed using the Applied Biosystems 7500
Fast Dx Real-Time PCR Instrument (cat no. 4425757; Thermo Fisher
Scientific, Inc.) and the following gene-specific primers (Sangon
Biotech Co., Ltd., Shanghai, China): GAPDH sense,
5′-TGCCATCAACGACCCCTTCA-3′ and antisense,
5′-TGACCTTGCCCACAGCCTTG-3′; c-Met sense, 5′-GAGGCAGTGCAGCATGTAGT-3′
and antisense, 5′-GGTCAGAA-3′; Snail sense,
5′-TTACCTTCCAGCAGCCCTAC-3′ and antisense, 5′-GAG CA-3′; E-cadherin
sense, 5′-AGCTATCCTTGCACCTCAGC-3′ and antisense,
5′-CCCAGGAGTTTGAG-3′; and vimentin sense,
5′-CCAAACTTTTCCTCCCTGAACC-3′ and antisense,
5′-GTGATGCTGAGAAGTTTCGTTGA-3′. A control siRNA specific for the red
fluorescent protein, 5′-CCACTACCTGAGCACCCAG-3′, was used as negative
control (sc-37007; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA). All primers were designed using the National Center for
Biotechnology Information Primer-BLAST tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/).
PCR was performed under the following conditions: Denaturation at
50°C for 2 min, followed by 38 cycles of 95°C for 15 sec and 60°C
for 1 min. Gene expression was normalized to internal controls and
fold changes were calculated using relative quantification
(2−ΔΔCt) (12).
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
buffer (cat no. P0013; Beyotime Institute of Biotechnology)
containing 1 mmol/l phenylmethylsulfonyl fluoride (94:6 v/v; cat
no. ST506-2; Beyotime Institute of Biotechnology), and subsequently
placed on ice for 30 min. The supernatant was collected following
centrifugation at 13,600 × g for 10 min at 4°C. Protein
concentration was determined using Pierce BCA Protein Assay Kit
(cat no. 23227; Thermo Fisher Scientific, Inc.). Proteins were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis gels (10%; cat no. P0012A; Beyotime Institute of
Biotechnology) and subsequently transferred to a polyvinylidene
difluoride membrane (cat no. FFP39; Beyotime Institute of
Biotechnology) (12). The membrane
was immunoblotted with anti-β-actin (1:1,000), anti-c-Met (1:500),
anti-ERK1/2 (1:250), anti-p-ERK1/2 (1:250), anti-AKT (1:400),
anti-p-AKT (1:200), anti-Snai1 (1:400), anti-E-cadherin (1:500) and
anti-vimentin (1:500) at 4°C overnight. All antibodies were diluted
with 0.5% bovine serum albumin. Following incubation, the
corresponding secondary antibody conjugated with peroxidase
(1:1,000) and enhanced chemiluminescence reagents (BeyoECL Plus;
cat no. P0018; Beyotime Institute of Biotechnology) were applied,
and the blot was visualized (cat no. 121–2550; Beijing Liuyi
Biotech Co., Ltd., Beijing, China). The protein content was
assessed using LabWorks image acquisition and analysis software
(Biomagin Systems Pvt. Ltd., Battaramulla, Sri Lanka).
Scattering assay
DU145 cells (3×105 cells/ml) were seeded
into each well of a 24-well plate (cat no. 662102; Greiner
Bio-One), and incubated overnight at 37°C in an atmosphere of 5%
CO2. DU145 cells were pretreated with 15 µmol/l curcumin
for 2 h. HGF was added to each well at a final concentration of 33
ng/ml (12). Cells were subsequently
incubated at 37°C for 48 h. Representative images were captured at
magnification, ×20 using Eclipse TE2000-U inverted microscope
(Nikon, Corporation, Tokyo, Japan).
In vitro invasion assay
The in vitro invasion assay was performed as
described previously (13). A
Matrigel™ invasion assay was performed in HTS Transwell®-24 Well
Permeable Supports (Corning Life Sciences, Tewksbury, MA, USA).
Briefly, 25 µl Matrigel Basement Membrane Matrix (BD Biosciences,
Franklin Lakes, NJ, USA) was thawed at 4°C overnight and coated on
the Transwell insert membrane. The inserts were subsequently
incubated at 37°C for 15–30 min to set. Following Matrigel coating,
3×105 DU145 cells/ml in 200 µl minimal essential medium
(MEM) with 0.1% serum and 15 µmol/ml curcumin plus 33 ng/ml HGF, 33
ng/ml HGF or 0.1 ml DMSO was added to the upper chamber.
Serum-containing MEM (500 µl) was added to the lower chamber. The
cells were incubated at 37°C in an atmosphere of 5% CO2
for 48 h. Following incubation, the medium was aspirated, and the
non-invading cells were removed with a wet cotton swab. The cells
were washed using PBS, and fixed in 4% paraformaldehyde (cat no.
P1110; Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) at room temperature for 15 min. Fixed cells were
washed three times with PBS, and stained with toluidine blue (cat
no. 6586-04-5; Sigma-Aldrich) for 3–5 min. Excess stain was removed
by washing three times with distilled water for 15 sec (cat no.
Z329797; Sigma-Aldrich). The invading cells were visualized under
Eclipse TE2000-U inverted microscope at magnification, ×40.
Wound healing assay
DU145 cells (3×105 cells/ml) were seeded
into a 6-well plate (cat no. 657160; Greiner Bio-One) in
serum-containing medium, and incubated at 37°C in an atmosphere of
5% CO2 in order to form a confluent monolayer. The
monolayer was scratched using a sterile plastic pipette tip (cat
no. CLS4860; Sigma-Aldrich), and washed with PBS to remove cell
debris. Subsequently, fresh medium was added, and 15 µmol/ml
curcumin plus 33 ng/ml HGF, 33 ng/ml HGF or 0.1 ml DMSO was added
to each well. The scratched monolayer was incubated at 37°C in an
atmosphere of 5% CO2 for 48 h. Wound closure was
measured in 6 random high-power fields at magnification, ×200,
using Image-Pro® Express software version 6 (Media Cybernetics,
Inc., Rockville, MD, USA) and Eclipse TE2000-U inverted microscope
(13).
Statistical analysis
Experiments were performed at least in triplicate.
The data are presented as the mean ± standard deviation.
Statistical significance of differences between groups was
evaluated by analysis of variance and Student's t-test using SPSS
version 11.0 software (SPSS, Inc., Chicago, IL, USA) and GraphPad
Prism version 5.0 software (GraphPad Software, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Curcumin blocks HGF-induced scattering
of DU145 cells
In the present study, the effect of curcumin on
DU145 cell viability was examined. As demonstrated in Fig. 1A, curcumin induced DU145 cell death in
a concentration-dependent manner, while DU145 cell viability was
unaltered at concentrations of curcumin <15 µmol/l. Therefore,
15 µmol/l curcumin was used to treat cancer cells in all subsequent
experiments. Activation of HGF/c-Met signaling led to a marked
polarized cell morphology in DU145 cells (Fig. 1B). To determine if curcumin caused
this HGF-induced phenotypic change, cells were pretreated for 8 h
with various concentrations of curcumin (0, 5, 10, 15, 20, 25, 30,
35, 40, 45 and 50 µmol/l), prior to HGF stimulation. As shown in
Fig. 1B, curcumin blocked HGF-induced
cell scattering, but did not induce cell death under the conditions
used in the experiment.
 | Figure 1.Effects of curcumin on HGF-induced
scattering of DU145 cells. (A) Effect of curcumin on DU145 cell
viability. DU145 cells were incubated with various concentrations
of curcumin (0, 5, 10, 15, 20, 25, 30, 35, 40, 45 and 50 µmol/l)
for 48 h, prior to the detection of cell proliferation by thiazole
blue. Experiments were performed in triplicate. *P<0.05 vs.
control group. (B) Curcumin inhibited HGF-induced DU145 cell
scattering. Cells were pretreated with curcumin for 2 h, and
subsequently incubated with HGF for 48 h. Representative images
were captured at magnification, ×40 using Eclipse TE2000-U inverted
microscope. HGF, hepatocyte growth factor; Con, control; Cur,
curcumin. Scale bar, 10 µm. |
Curcumin attenuates HGF-induced
invasion and migration of DU145 cells
The present study additionally examined the effect
of curcumin on HGF-induced cell migration in wound closure assays.
Confluent DU145 cells were pretreated with 15 µmol/l curcumin for 2
h, while 0.1 ml DMSO/PBS was used as a control. Cell monolayers
were scratched using a pipette tip, and washed to remove any
debris. Next, fresh medium containing 0.5% serum with 15 µmol/l
curcumin was added. Cells were subsequently incubated with 33 ng/ml
HGF for 48 h, and HGF-induced cell migration was determined by
measuring wound closure. As shown in Fig.
2A, HGF induced significant cell migration, which was abrogated
in the presence of curcumin (Fig.
2A). The present study also determined the effect of curcumin
on HGF-induced invasion in Matrigel Transwell chamber invasion
assays. Compared with the control, HGF induced an ~2-fold increase
in the number of invasive cells. However, prior to treatment with
HGF, DU145 cells were pretreated with 15 µmol/l curcumin for 2 h,
which significantly reduced the number of HGF-induced invasive
cells. Consistent with the results obtained in the cell migration
assay (Fig. 2B), curcumin treatment
reduced HGF-induced DU145 cell invasion (Fig. 2C and D).
 | Figure 2.Curcumin inhibits HGF-induced DU145
cell invasion and migration. (A) In wound closure assays, confluent
DU145 cells were pretreated with 15 µmol/l curcumin for 2 h.
Dimethyl sulfoxide/phosphate-buffered saline was used as a control.
Cell monolayers were scratched using pipette tips and washed to
remove any debris, followed by the addition of fresh medium
containing 0.5% serum with 15 µmol/l curcumin. Cells were
subsequently incubated with 33 ng/ml HGF for 48 h, and HGF-induced
cell migration was determined by measuring wound closure. Scale
bar, 10 µm. (B) HGF-induced cell motility was determined by
measuring the wound closure in six random high-power fields at
magnification, ×200 using Image-Pro® Express software. Data are
presented as the mean ± standard deviation. *P<0.05 vs. control
group, #P<0.05 vs. HGF + curcumin group (C and D) For
Matrigel™ Transwell® chamber invasion assays, DU145 cells
(3×105 cells/ml) were plated on top of a
Matrigel™-coated 24-well Transwell® chamber, and cultured for 48 h
in Dulbecco's modified Eagle's medium containing 0.1% serum, 33
ng/ml HGF and 15 µmol/l curcumin. Invading cells were subsequently
fixed, stained with toluidine blue and counted in six random
high-power fields at magnification, ×200 using Image-Pro® Express
software. The experiment was performed in triplicate. *P<0.05
vs. control group, #P<0.05 vs. HGF + curcumin group.
HGF, hepatocyte growth factor; Con, control; Cur, curcumin. |
Curcumin suppresses HGF-induced EMT in
DU145 cells
Multiple molecules regarded as epithelial or
mesenchymal markers have been reported previously, and
downregulation of E-cadherin and upregulation of vimentin are known
to be markers of EMT (14). In the
present study, RT-qPCR and western blot analysis of the expression
of these markers were conducted to investigate whether the
abrogation of HGF-induced cell scattering, migration and invasion
observed in curcumin-treated DU145 cells was due to alterations in
the process of EMT. As shown in Fig.
3, vimentin expression was upregulated, while E-cadherin
expression was downregulated in response to HGF activation. These
HGF-induced effects were significantly suppressed by pretreatment
with curcumin (Fig. 3).
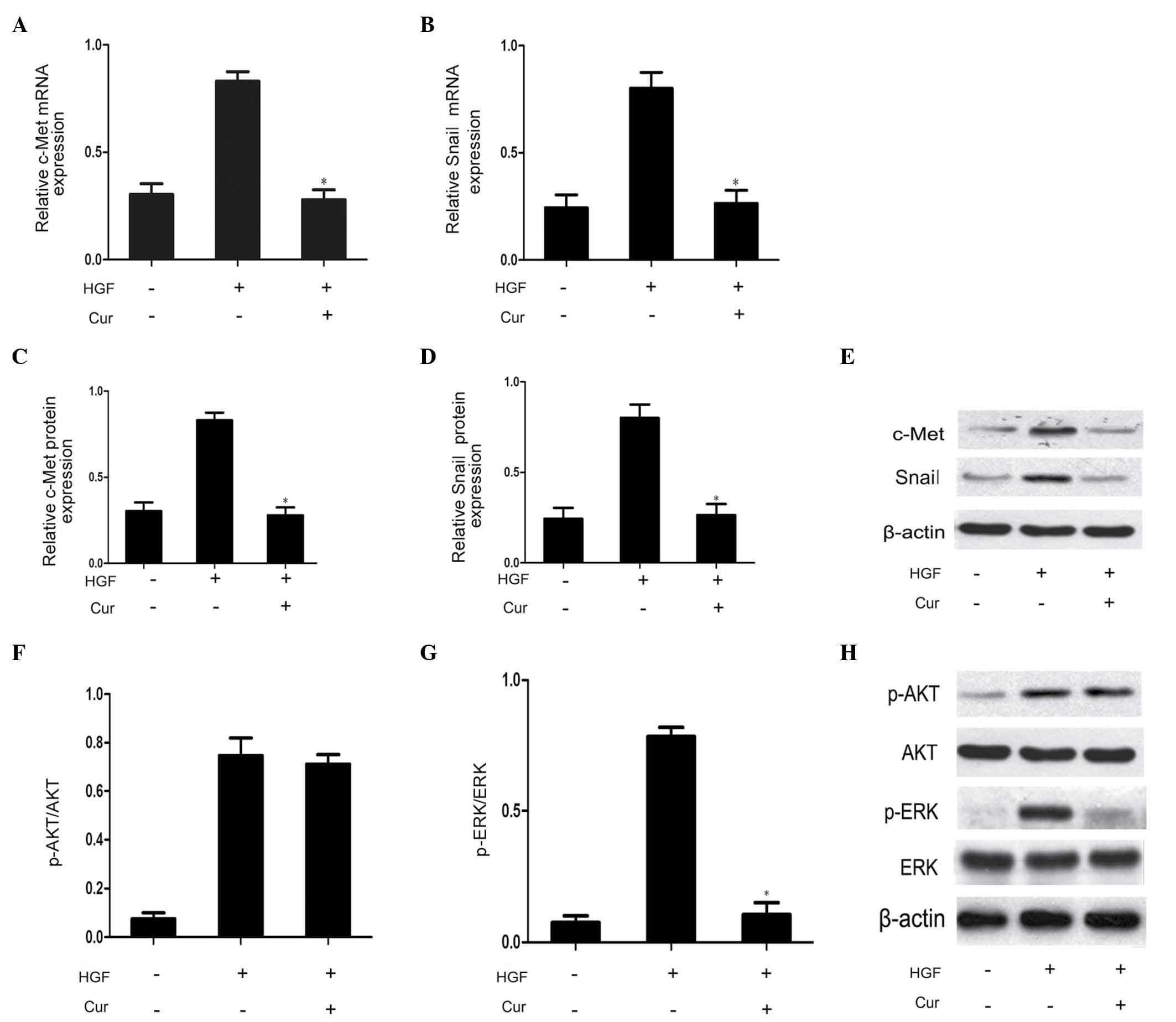
Curcumin has an inhibitory effect on
HGF/c-Met signaling pathway and Snail expression in DU145
cells
In order to determine the involvement of the
HGF/c-Met signaling pathway on the effects of curcumin on DU145
cell scattering and motility, DU145 cells were pretreated with
curcumin (10 µmol/l) for 2 h prior to HGF treatment for 48 h.
Compared with the HGF group, curcumin significantly suppressed
HGF-induced upregulation of c-Met expression, as well as Snail mRNA
and protein expression (Fig. 4A–E).
This observation indicated that curcumin reduced HGF/c-Met
signaling by direct inhibition of c-Met expression. The HGF/c-Met
signaling axis is a significant contributor to the promotion of EMT
via stimulation of AKT and ERK (3).
Therefore, the present study additionally investigated the effect
of curcumin on the phosphorylation of AKT and ERK. As demonstrated
in Fig. 4F–H, western blot analysis
revealed that curcumin pretreatment was able to effectively block
HGF-induced ERK phosphorylation. The rapidly accelerated
fibrosarcoma/mitogen-activated protein kinase (MAPK) kinase/ERK
signaling pathway has been demonstrated to be required for
HGF-induced EMT (3). By contrast,
HGF-mediated activation of AKT was not affected by curcumin
pretreatment in the present study. Therefore, MAPK signaling may be
involved in Snail expression. Thus, curcumin was capable of
blocking HGF-induced signaling via specific repression of
activation of the c-Met/ERK/Snail signaling pathway.
Discussion
EMT is a crucial step in the process of cancer cell
invasion and metastasis. During this process, the phenotype of
cancer cells undergoes alterations in cellular morphology, with
increased expression of mesenchymal markers such as vimentin and
decreased expression of epithelial markers such as E-cadherin
(15). These changes are associated
with decreased cell adhesion capacity, leading to enhanced invasion
and metastasis (15,16). Consequently, EMT inhibition represents
a significant target for the development of prostate cancer
treatments. In the present study, the effects of curcumin on the
HGF/c-Met signaling axis were investigated, and it was identified
that curcumin was capable of blocking c-Met expression, as well as
inhibiting EMT in DU145 cells.
HGF is a multifunctional protein, which has been
reported to increase the migration and invasion abilities of
prostate cancer cells (17). In the
present study, it was demonstrated that HGF induced migration and
invasion of DU145 prostate cancer cells, with concomitant
downregulation of E-cadherin expression and upregulation of
vimentin expression. HGF stimulates c-Met phosphorylation, which
activates MAPK signaling and increases Snail expression (18). Snail is a DNA-binding protein that
contains a zinc finger-like domain capable of binding to the E-box
domain of the E-cadherin promoter, thus inhibiting E-cadherin
expression (19). Therefore, it may
be speculated that HGF is able to induce EMT in DU145 prostate
cancer cells via the c-Met/ERK/Snail signaling pathway. c-Met is
known to be associated with prostate cancer cell differentiation
(17). Previous studies have revealed
that c-Met is overexpressed in poorly differentiated prostate
cancer, indicating that reduced expression of c-Met may stimulate
prostate cancer cell differentiation (20). Nishimura et al (17) reported that HGF was increased during
DU145 cell invasion and metastasis in a c-Met-dependent manner,
while this effect of HGF was not observed in LNcaP cell metastasis,
and these cells are known to be c-Met-deficient (17). Furthermore, Davies et al
(21) additionally indicated that
inhibition of c-Met expression by hammerhead ribozyme reduced the
invasion and metastasis of prostate cancer cells. Thus, inhibition
of prostate cancer cell invasion and metastasis via c-Met blockade
may represent a significant approach for the treatment of prostate
cancer.
The anticancer effects of curcumin have been widely
studied, and it is regarded as a promising drug candidate for the
treatment and prevention of cancer (22). It is considered that the mechanism
underlying the anticarcinogenic effects of curcumin involves the
induction of cancer cell apoptosis via regulation of the expression
of anti-oncogenes, oncogenes and the cell cycle (22). However, previous studies have revealed
that curcumin is able to inhibit cancer cell invasion and
metastasis via signaling changes. Chen et al (23) observed that curcumin was able to
inhibit lung cancer cell invasion and metastasis by upregulation of
E-cadherin expression, and stimulated the expression of the tumor
suppressor DnaJ-like heat shock protein HLJ1 via c-Jun N-terminal
kinase/JunD signaling. However, it has additionally been reported
that curcumin inhibited post-transcriptional expression of
hypoxia-inducible factor 1-α, leading to reversed hypoxia and
induction of liver cancer cell proliferation and migration
(24). Thus, the role of curcumin in
prostate cancer migration remains to be elucidated. In the present
study, it was demonstrated that preincubation of DU145 cells for 2
h with curcumin blocked HGF-induced cell scattering with associated
alterations in cell morphology (reduction in stress fibers and cell
flattening). This is consistent with the effects of the
naturally-occurring coumarin compound osthol, which has been
observed to inhibit HGF-induced migration and invasion in MCF-7
breast cancer cells (14).
Furthermore, the present study demonstrated that HGF stimulated
c-Met expression, as well as phosphorylation of AKT and ERK, and
additionally increased the protein expression levels of vimentin,
while curcumin suppressed HGF-induced EMT, cell migration and
invasion via repression of the c-Met/ERK signaling pathway in DU145
prostate cancer cells. However, the AKT signaling pathway was
unaffected by curcumin, even following a long preincubation when
c-Met was no longer maximally activated by HGF, and the remaining
c-Met was likely to be sufficient to induce phosphorylation of AKT.
In addition, the present study demonstrated that curcumin was able
to inhibit Snail expression, suggesting that ERK may regulate Snail
expression. Therefore, it may be speculated that curcumin inhibits
Snail expression via suppression of c-Met/ERK signaling, although
this remains to be confirmed.
In the present study, curcumin was additionally
revealed to suppress c-Met expression, although the exact
underlying mechanism remains to be elucidated. Seol et al
(25) observed that the transcription
factor activator protein-1 (AP-1) binds the c-Met gene promoter and
increases c-Met expression. Furthermore, previous studies have
indicated that increased fatty acid synthase (FAS) activity
maintains lipid rafts, which may aid to stabilize the levels of
c-Met (26). These studies suggest
that inhibited AP-1 and FAS expression are suppressed by c-Met
expression. Thus, it may be hypothesized that curcumin suppresses
c-Met expression via inhibition of AP-1 and FAS expression,
although this theory requires additional investigation in future
studies.
In conclusion, the present study has demonstrated
that curcumin may inhibit HGF-induced EMT via reduced c-Met
expression, and may attenuate associated signaling pathways. These
findings suggest that curcumin may be regarded as an inhibitor of
c-Met, the receptor tyrosine kinase for HGF, thus providing a
theoretical and experimental basis for the treatment of prostate
cancer, and a novel approach for the development of prostate cancer
drugs.
Acknowledgements
The present study was supported by the Medical
Foundation of Huizhou (Huizhou, China; grant no. 2014Y149) and The
Medical Research Foundation of Guangdong Province (Guangzhou,
China; grant no. A2014810).
References
|
1
|
Sturge J, Caley MP and Waxman J: Bone
metastasis in prostate cancer: Emerging therapeutic strategies. Nat
Rev Clin Oncol. 8:357–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Josson S, Gururajan M, Hu P, Shao C, Chu
GY, Zhau HE, Liu C, Lao K, Lu CL, Lu YT, et al: miR-409-3p/-5p
promotes tumorigenesis, epithelial-to-mesenchymal transition, and
bone metastasis of human prostate cancer. Clin Cancer Res.
20:4636–4646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parikh RA, Wang P, Beumer JH, Chu E and
Appleman LJ: The potential roles of hepatocyte growth factor
(HGF)-MET pathway inhibitors in cancer treatment. Onco Targets
Ther. 11:969–983. 2014.
|
|
4
|
Elliott BE, Hung WL, Boag AH and Tuck AB:
The role of hepatocyte growth factor (scatter factor) in
epithelial-mesenchymal transition and breast cancer. Can J Physiol
Pharmacol. 80:91–102. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Graveel CR, Tolbert D and Van de Woude GF:
MET: A critical player in tumorigenesis and therapeutic target.
Cold Spring Harb Perspect Biol. 5:a0092092013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Varkaris A, Corn PG, Gaur S, Dayyani F,
Logothetis CJ and Gallick GE: The role of HGF/c-Met signaling in
prostate cancer progression and c-Met inhibitors in clinical
trials. Expert Opin Investig Drugs. 20:1677–1684. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu P, Chung LW, Berel D, Frierson HF, Yang
H, Liu C, Wang R, Li Q, Rogatko A and Zhau HE: Convergent RANK- and
c-Met-mediated signaling components predict survival of patients
with prostate cancer: An interracial comparative study. PLoS One.
8:e730812013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee RJ and Smith MR: Targeting MET and
vascular endothelial growth factor receptor signaling in
castration-resistant prostate cancer. Cancer J. 19:90–98. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghalandarlaki N, Alizadeh AM and
Ashkani-Esfahani S: Nanotechnology-applied curcumin for different
diseases therapy. Biomed Res Int. 2014:3942642014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dulbecco P and Savarino V: Therapeutic
potential of curcumin in digestive diseases. World J Gastroenterol.
19:9256–9270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dorai T, Diouri J, O'Shea O and Doty SB:
Curcumin inhibits prostate cancer bone metastasis by up-regulating
bone morphogenic protein-7 in vivo. J Cancer Ther.
5:369–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin XL, He XL, Zeng JF, Zhang H, Zhao Y,
Tan JK and Wang Z: FGF21 increases cholesterol efflux by
upregulating ABCA1 through the ERK1/2-PPARγ-LXRα pathway in THP1
macrophage-derived foam cells. DNA Cell Biol. 33:514–521. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang HY, Kao MC, Way TD, Ho CT and Fu E:
Diosgenin suppresses hepatocyte growth factor (HGF)-induced
epithelial-mesenchymal transition by down-regulation of Mdm2 and
vimentin. J Agric Food Chem. 59:5357–5363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hung CM, Kuo DH, Chou CH, Su YC, Ho CT and
Way TD: Osthole suppresses hepatocyte growth factor (HGF)-induced
epithelial-mesenchymal transition via repression of the
c-Met/Akt/mTOR pathway in human breast cancer cells. J Agric Food
Chem. 59:9683–9690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Książkiewicz M, Markiewicz A and Zaczek
AJ: Epithelial-mesenchymal transition: A hallmark in metastasis
formation linking circulating tumor cells and cancer stem cells.
Pathobiology. 79:195–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishimura K, Kitamura M, Takada S,
Nonomura N, Tsujimura A, Matsumiya K, Miki T, Matsumoto K and
Okuyama A: Regulation of invasive potential of human prostate
cancer cell lines by hepatocyte growth factor. Int J Urol.
5:276–281. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grotegut S, von Schweinitz D, Christofori
G and Lehembre P: Hepatocyte growth factor induces cell scattering
through MAPK/Egr-1-mediated upregulation of Snail. EMBO J.
25:3534–3545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Leenders G, van Balken B, Aalders T,
de Hulsbergen-van Kaa C, Ruiter D and Schalken P: Intermediate
cells in normal and malignant prostate epithelium express c-MET:
Implications for prostate cancer invasion. Prostate. 51:98–107.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Davies G, Watkins G, Mason MD and Jiang
WG: Targeting the HGF/SF receptor c-met using a hammerhead ribozyme
transgene reduces in vitro invasion and migration in
prostate cancer cells. Prostate. 60:317–324. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park W, Amin AR, Chen ZG and Shin DM: New
perspectives of curcumin in cancer prevention. Cancer Prev Res
(Phila). 6:387–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen HW, Lee JY, Huang JY, Wang CC, Chen
WJ, Su SF, Huang CW, Ho CC, Chen JJ, Tsai MF, et al: Curcumin
inhibits lung cancer cell invasion and metastasis through the tumor
suppressor HLJ1. Cancer Res. 68:7428–7438. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakulterdkiat T, Srisomsap C,
Udomsangpetch R, Svasti J and Lirdprapamongkol K: Curcumin
resistance induced by hypoxia in HepG2 cells is mediated by
multidrug-resistance-associated proteins. Anticancer Res.
32:5337–5342. 2012.PubMed/NCBI
|
|
25
|
Seol DW, Chen Q and Zarnegar R:
Transcriptional activation of the hepatocyte growth factor receptor
(c-met) gene by its ligand (hepatocyte growth factor) is mediated
through AP-1. Oncogene. 19:1132–1137. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duhon D, Bigelow RL, Coleman DT, Steffan
JJ, Yu C, Langston W, Kevil CG and Cardelli JA: The polyphenol
epigallocatechin-3-gallate affects lipid rafts to block activation
of the c-Met receptor in prostate cancer cells. Mol Carcinog.
49:739–749. 2010.PubMed/NCBI
|