Introduction
Bone metastasis is common in prostate cancer, and
the majority of patients with castration-resistant prostate cancer
(CRPC) present with radiographic evidence of bone disease and
associated symptoms. Up to 80% of patients develop associated
symptoms of bone disease, including bone pain, bone fractures or
even spinal cord compression. As prostate cancer has a particular
propensity to spread to the bone, common symptoms include
skeletal-related events (SREs), such as pain, fractures,
hypercalcemia and medullar compression, which result in significant
morbidity, worsen patient quality of life and comprise the most
frequent cause of mortality in these patients. Palliative treatment
for bone pain associated with metastasis includes non-steroidal
analgesics and opiates, surgery, bone-targeted therapies
(bisphosphonates and denosumab) and external beam radiotherapy. The
effect of the aforementioned treatments are limited to delaying the
onset of SREs (1,2).
Bone-targeting radiopharmaceuticals have also been
added to the treatment options available, as they have been shown
to be efficient in relieving metastatic bone pain, particularly in
patients with widespread bone disease (3). The radiopharmaceuticals most frequently
used for bone pain relief are β-emitting isotopes, such as
89Sr-chloride,
153Sm-ethylenediaminetetramethylene phosphonic acid and
186Re-etidronate.
Recently, an α-emitting isotope,
223Radium-dichloride (Ra-223), has been approved for the
treatment of patients with CRPC that develop painful bone
metastasis without evidence of visceral metastases. In general,
α-emitters are associated with a shorter range (<0.1 mm) and a
higher energetic radiation compared with β-particles and γ-rays,
which have a low Linear Energy Transfer (LET) radiation. In
addition, α-emitters exhibit a tissue penetration diameter of 2–10
cells, minimizing the damage to healthy hematopoietic tissue.
Furthermore, due to their high LET radiation, α-particles are
considered more lethal, inducing non-repairable double-stranded DNA
breaks in adjacent tumor cells (4).
Ra-223 is the only radiopharmaceutical that has been
demonstrated to increase overall survival (OS) in patients with
CRPC with bone metastasis in a phase III study. The aforementioned
study, the ALSYMPCA trial, is an international, randomized,
double-blinded, placebo-controlled study that was conducted in men
with symptomatic metastatic CRPC (5).
The inclusion criteria were symptomatic CRPC, no known visceral
metastasis, ≥2 bone metastases, and either docetaxel-pretreated
patients, patients that were unfit for docetaxel treatment or
patients that refused to receive chemotherapy. Patients were
randomized in a 2:1 ratio to receive Ra-223 injections at 50 kBq/kg
plus best standard of care or placebo plus best standard of care.
Best standard of care included secondary hormonal therapies
(second- or third-line hormonal maneuvers, including, but not
limited to, high-dose bicalutamide, prednisone and estrogens) or
external beam radiotherapy, but not cytotoxic chemotherapy or
radioisotopes. Patients were divided into groups according to total
bone alkaline phosphatase (BAP) levels, bisphosphonate use and
prior docetaxel treatment. The primary endpoint was OS, while the
secondary endpoints were: Time to i) first SRE, ii) total BAP
progression, iii) total BAP response, iv) total BAP normalization
and v) prostate-specific antigen (PSA) progression; vi) adverse
events; and vii) affected quality of life. The trial included 921
male patients (Ra-223, n=614; placebo, n=307). All patients
enrolled in the study received up to 6 intravenous injections of
Ra-223 or placebo, administered at 4-week intervals (5).
The trial was terminated following the
recommendation of an independent committee, due to early evidence
of Ra-223 benefit in OS. Ra-223 was shown to significantly improve
OS in patients with CRPC and bone metastases, as compared with the
placebo group [hazard ratio (HR), 0.70; 95% confidence interval
(CI), 0.58–0.83; median OS, 14.9 vs. 11.3 months; P<0.001]. A
significant beneficial effect was also observed in all patients
except for patients with an Eastern Cooperative Oncology Group
Performance Status of ≥2, possibly due to the limited number of
patients included in the study. Time to first SRE was also found to
be significantly prolonged in the in the Ra-223 group compared with
the placebo group (HR, 0.66; 95% CI, 0.52–0.83; median time to
first SRE, 15.6 vs. 9.8 months; P<0.001) (6). In addition, the quality of life of the
patients was markedly improved following treatment with Ra-223
(5–7),
with no differences in severe side-effects (grades 3–4) observed
between the Ra-223 and placebo groups (8).
The findings on the aforementioned studies indicate
that Ra-223 should not merely be used to visualize bones, but also
as a systemic treatment strategy for patients with CRPC developing
bone metastasis, either as first-line or salvage therapy. Ra-223
had a significant benefit on survival, delaying the onset of SREs
with an acceptable safety profile. This effect had not been
previously recorded for other available radiopharmaceuticals
(4). The use of Ra-223 has been
approved for CRPC patients exhibiting symptomatic bone metastasis
without visceral disease, regardless of previous treatment with
docetaxel. In fact, the ALSYMPCA study demonstrated a benefit of
Ra-223 on OS compared with the placebo, regardless of prior
docetaxel treatment (hazard ratio, 0.71 vs. 0.74) (8–10).
The present study reports the case of a patient with
CRPC who met the inclusion criteria of the ALPSYMCA trial and was
treated with Ra-223.
Case report
A 70-year-old male patient, with no history of
concomitant diseases, was diagnosed with T3 Gleason 7 (11) prostate cancer by echography and
transrectal biopsy in November 1999 at Hospital Santa Creu I Sant
Pau (Barcelona, Spain). At that time, the patient had PSA levels of
12.5 ng/ml (normal range, <4 ng/ml) and a diagnosis of clinical
stage cT2aNx disease (12).
Therefore, a successful radical prostatectomy was performed in
January 2000. The histopathology report of formalin-fixed sections
revealed Gleason grade 7 left lobe adenocarcinoma with
extraprostatic invasion, non-affected surgical margins and negative
lymphatic nodes (pT3pN0).
Following definitive pathological staging, a bone
scan was performed in March 2001, which showed a total of 2
osteoblastic bone metastases in both ischiopubic branches.
Subsequent to the diagnosis of metastatic prostate cancer,
treatment with luteinizing hormone-releasing hormone (LH-RH)
analogues was initiated in March 2001. The patient achieved a
complete biochemical response (PSA level, 1.03 ng/ml). Hormonal
therapy was terminated in May 2004 due to stable disease, according
to Prostate Cancer Working Group (PCWG)-2 criteria (13), with undetectable PSA levels and a lack
of symptoms, within an intermittent therapy strategy.
In May 2005, the patient presented with a
biochemical relapse (PSA level, 77.0 ng/ml) and underwent complete
androgen blockade (CAB) with bicalutamide (50 mg) plus LH-RH
analogues, such as gosrelin acetate, until November 2006, when
treatment was terminated due to undetectable PSA levels.
In December 2007, PSA levels increased again (PSA
level, 105 ng/ml). As a result, CAB was re-initiated and continued
until October 2010, as the patient developed progressive disease
(PD), according to the PCWG-2 criteria. The patient presented with
bone pain, therefore, a bone scan was performed. The scan showed an
increase in the number and extension of pelvic metastases compared
with the bone scan performed in March 2001.
Between September and October 2010, PSA levels
increased from 66.8 to 75.6 ng/ml, and BAP levels reached 594 U/l
(normal range, 44–147 U/l). In November 2010, treatment with
ketoconazole (200 mg/8 h) was administered until July 2011, when
PSA increased to 408 ng/ml. A new bone scan showed PD with painful
pelvic lesions.
In July 2011, following the recurrence of PD, 10
cycles of docetaxel (75 mg/m2 every 3 weeks) were
administered between August 2011 and April 2012, with a good
clinical and biochemical response (PSA and BAP decreased to 4.01
ng/ml and 75 U/l, respectively). However, 3 months later, PSA and
BAP increased to 29.1 ng/ml and 203 U/l, respectively, and a bone
scan observed progression of bone metastasis (new PD). Docetaxel
rechallenge (75 mg/m2 every 3 weeks) was initiated in
July 2012; although a clinical response initially occurred, the
patient developed bone pain with radiological progression following
the fourth cycle. The patient also exhibited biochemical
progression (PSA, 317 ng/ml; BAP, 402 U/l). As the patient had
painful PD without visceral metastases, treatment with Ra-223 was
scheduled. A bone scan performed in February 2013 showed widespread
bone metastases on the bilateral ribs, dorsolumbar vertebrae,
pelvis, sacrum and proximal femurs (Fig.
1).
Between March and August 2013, the patient was
treated with 6 doses of Ra-223 (50 kBq/kg; 1 dose/4 weeks). The
administered doses were: 2.92 MBq (March 12), 3.64 MBq (April 8),
3.4 MBq (May 8), 3.3 MBq (June 6), 3.4 MBq (July 8) and 3.4 MBq
(August 5). During this period, PSA and BAP decreased from 317 to
80.1 ng/ml and from 402 to 81 U/l, respectively, and bone pain was
controlled. Furthermore, Ra-223 was well-tolerated without
associated bone marrow toxicity. However, 2 months after
administration of the final dose of Ra-223, PSA and BAP increased
to 314.8 ng/ml and 159 U/l, respectively, and bone pain worsened. A
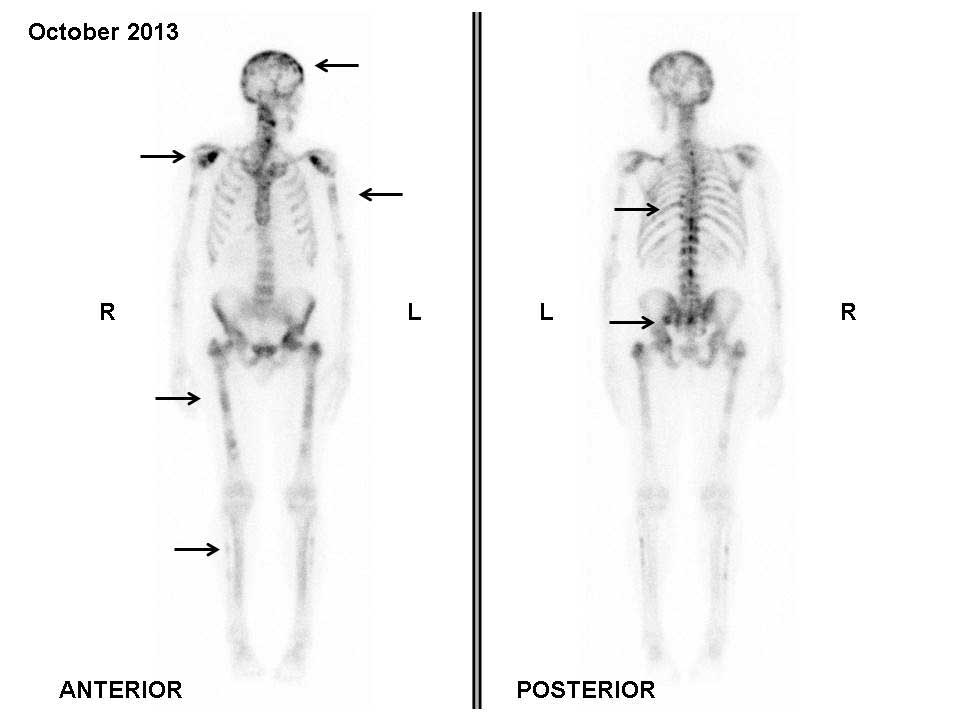
bone scan performed in October 2013 did not show any tracer uptake
in the previously observed metastatic lesions; however, PD was
evident due to the detection of tracer uptake by new lesions in the
backbone and head (Fig. 2).
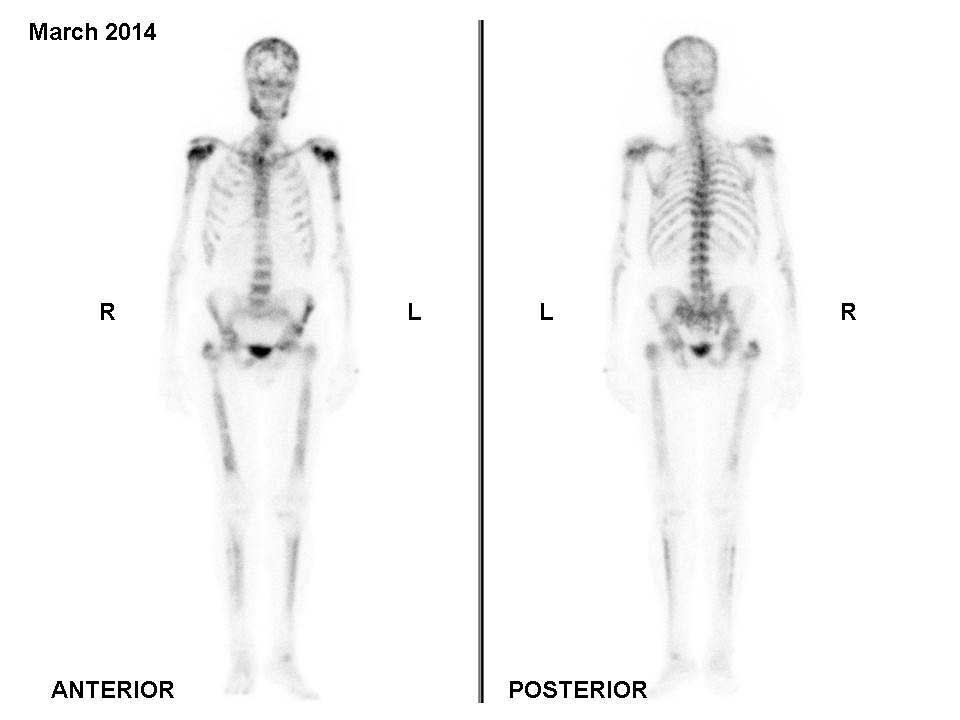
In November 2013, 4 cycles of cabazitaxel (20
mg/m2 every 3 weeks) were administered. A bone scan
performed in March 2014 revealed metastatic disease progression
(Fig. 3). Changes in the patient's
PSA and BAP levels over time are shown in Fig. 4, indicating that the patient is
currently in clinical and biochemical progression. The patient was
re-evaluated every 9 weeks with measurement of PSA levels. The
final follow-up was performed in March 2015, and due to symptomatic
deterioration, the patient was treated only for palliative relief
subsequent to that date. Written informed consent was obtained from
the patient and their family for publication of the present
study.
Discussion
Ra-223 is a bone-targeting calcium mimetic α-emitter
known to accumulate in areas of increased bone turnover (4–6). It has
been reported that α radiation has high LET that generates
double-stranded DNA breaks, resulting in elevated cytotoxicity and
greater biological effectiveness compared with β radiation
(7). At present, clinical experience
of Ra-223 is limited compared with β-emitting radiopharmaceuticals,
with knowledge of Ra-223 limited to findings from two phase I,
three phase II and one phase III clinical studies (4). Furthermore, in contrast to previous
studies based on β-emitter radiopharmaceuticals, clinical studies
assessing the effectiveness of Ra-223 in relieving bone pain used
primary endpoints of OS and secondary endpoints of time to the
first symptomatic skeletal-related event (9).
The dosage of Ra-223 depends on body weight and, due
to its higher biological effectiveness; administered doses are
smaller than those applicable to β-emitting radiopharmaceuticals. A
large multicentric double-blinded randomized phase III clinical
study showed that 6 doses of 50 kBq/kg administered once every 4
weeks resulted in a significant decrease in bone marker levels and
a significant delay in the onset of SREs, resulting in improved OS
and pain relief (6). Additional
clinical studies compared different dosages of Ra-223 or compared
Ra-223 with a placebo and achieved similar results (4–6,14–18).
In the present case, a patient with CRPC treated
with 6 intravenous doses of Ra-223 (50 kBq/kg) achieved bone pain
relief, and a significant decrease in PSA and BAP levels. In
addition, 2 months after the administration of the final dose, a
bone scan showed improvement of the widespread bone metastases,
with tracer uptake normalization in the metastatic lesions of the
ribs and pelvis. PSA response is unusual under Ra-223 treatment,
whereas BAP response is common. Therefore, it has been recommended
that changes in BAP should be considered as a biomarker of response
to Ra-223 treatment; however, this only applies to patients with
elevated BAP levels at baseline. In the present patient, PD was
indicated by the appearance of new metastatic lesions located in
sites where no Ra-223 uptake had previously been reported. Notably,
a response to β-emitting isotopes is not commonly observed upon
imaging, however, partial responses may be observed with Ra-223. In
the present patient, pain relief was achieved, with normalization
of tracer uptake in the bone metastases that were previously
present, and a decrease in BAP and PSA levels during Ra-223
treatment.
Evaluating drugs for the treatment of prostate
cancer poses certain challenges. For example, measurable disease
occurs infrequently. As a result, the PCWG addressed this and
various other challenges in their consensus recommendations for the
conduct of clinical trials. Although the Response Evaluation
Criteria in Solid Tumors (RECIST) are considerably well-established
recommendations, they do not consider certain key characteristics
of prostate cancer; for example, post-therapeutic changes in PSA
levels were not addressed by RECIST (19).
Despite the developments made in imaging modalities,
interpreting the clinical significance of changes in the size or
intensity of bone metastases observed on bone scans is challenging.
In cases where a bone scan is the sole indicator of progression,
PCWG defines bone disease progression as the appearance of ≥2 new
lesions on a bone scan compared with a prior scan for trial entry.
When scan findings are suggestive of the appearance of new lesions
or a flare reaction, possibly due to trauma, it may prove useful to
confirm these results using other imaging modalities (13).
In addition, it should be taken into consideration
that the findings of scans can potentially appear worse before they
improve, resulting in the erroneous conclusion that the treatment
has failed when, in fact, it was too early to assess the effect of
the drug (20).
In conclusion, in the present study a patient with
CRPC was treated with Ra-223 without major toxicities. Following
the development of PD, the patient was treated with cabazitaxel.
The results of ongoing randomized trials are required to determine
whether Ra-223 or docetaxel rechallenge can be part of a long-term
treatment regime.
Acknowledgements
The authors thank Dr Sonia Maciá (Pivotal S.L.,
Madrid, Spain) for providing advice on how to handle and submit the
paper.
References
|
1
|
World Health Organization: Cancer Pain
Relief: With a Guide to Opioid Availability (2nd). WHO. Geneva,
Switzerland: 36–37. 1996.
|
|
2
|
National Comprehensive Cancer Network
(NCCN): NCCN Guidelines for Supportive Care: Adult cancer pain.
Version 2. 2013.http://www.nccn.org/professionals/physician_gls/pdf/pain.pdfAccessed.
January. 2016
|
|
3
|
Hamdy NA and Papapoulos SE: The palliative
management of skeletal metastases in prostate cancer: Use of
bone-seeking radionuclides and bisphosphonates. Semin Nucl Med.
31:62–68. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herranz Anido U, Fernández Calvo O, Afonso
Afonso FJ, de Rodríguez Martínez Llano S, Lázaro Quintela M, León
Mateos L, Vázquez Estévez S and Antón Aparicio LM: Radium-223
dichloride: A new paradigm in the treatment of prostate cancer.
Expert Rev Anticancer Ther. 15:339–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parker C, Nilsson S, Heinrich D, Helle SI,
O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, et
al: Alpha emitter radium-223 and survival in metastatic prostate
cancer. N Engl J Med. 369:213–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sartor O, Coleman R, Nilsson S, Heinrich
D, Helle SI, O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue
J, et al: Effect of radium-223 dichloride on symptomatic skeletal
events in patients with castration-resistant prostate cancer and
bone metastases: Results from a phase 3, double-blind, randomised
trial. Lancet Oncol. 15:738–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nilsson S, Franzén L, Parker C, Tyrrell C,
Blom R, Tennvall J, Lennernäs B, Petersson U, Johannessen DC, Sokal
M, et al: Two-year survival follow-up of the randomized,
double-blind, placebo-controlled phase II study of radium-223
chloride in patients with castration-resistant prostate cancer and
bone metastases. Clin Genitourin Cancer. 11:20–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nilsson S, Strang P, Aksnes AK, Franzèn L,
Olivier P, Pecking A, Staffurth J, Vasanthan S, Andersson C and
Bruland ØS: A randomized, dose-response, multicenter phase II study
of radium-223 chloride for the palliation of painful bone
metastases in patients with castration-resistant prostate cancer.
Eur J Cancer. 48:678–686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nilsson S, Larsen RH, Fosså SD, Balteskard
L, Borch KW, Westlin JE, Salberg G and Bruland OS: First clinical
experience with alpha-emitting radium-223 in the treatment of
skeletal metastases. Clin Cancer Res. 11:4451–4459. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nilsson S, Franzén L, Parker C, Tyrrell C,
Blom R, Tennvall J, Lennernäs B, Petersson U, Johannessen DC, Sokal
M, et al: Bone-targeted radium-223 in symptomatic,
hormone-refractory prostate cancer: A randomised, multicentre,
placebo-controlled phase II study. Lancet Oncol. 8:587–594. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Humphrey PA: Gleason grading and
prognostic factors in carcinoma of the prostate. Mod Pathol.
17:292–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
National Collaborating Centre for Cancer
(UK): TNM staging for prostate cancer. Prostate Cancer: Diagnosis
and Treatment. National Collaborating Centre for Cancer (UK).
(Cardiff). Appendix 2. 2008.
|
|
13
|
Sonpavde G, Pond GR, Armstrong AJ, Galsky
MD, Leopold L, Wood BA, Wang SL, Paolini J, Chen I, Chow-Maneval E,
et al: Radiographic progression by Prostate Cancer Working Group
(PCWG)-2 criteria as an intermediate endpoint for drug development
in metastatic castration-resistant prostate cancer. BJU Int.
114:E25–E31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sgouros G, Roeske JC, McDevitt MR, Palm S,
Allen BJ, Fisher DR, Brill AB, Song H, Howell RW, Akabani G, et al:
MIRD pamphlet no. 22 (abridged): Radiobiology and dosimetry of
alpha-particle emitters for targeted radionuclide therapy. J Nucl
Med. 51:311–328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lassmann M and Nosske D: Dosimetry of
Ra-223-chloride: Dose to normal organs and tissues. Eur J Nucl Med
Mol Imaging. 40:207–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pandit-Taskar N, Larson SM and
Carrasquillo JA: Bone-seeking radiopharmaceuticals for treatment of
osseous metastases, part 1: α therapy with 223Ra-dichloride. J Nucl
Med. 55:268–274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parker CC, Pascoe S, Chodacki A,
O'Sullivan JM, Germá JR, O'Bryan-Tear CG, Haider T and Hoskin P: A
randomized, double-blind, dose finding, multicenter, phase 2 study
of radium chloride (Ra 223) in patients with bone metastases and
castration-resistant prostate cancer. Eur Urol. 63:189–197. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carrasquillo JA, O'Donoghue JA,
Pandit-Taskar N, Humm JL, Rathkopf DE, Slovin SF, Williamson MJ,
Lacuna K, Aksnes AK, Larson SM, et al: Phase I pharmacokinetic and
biodistribution study with escalating doses of
223Ra-dichloride in men with castration-resistant
metastatic prostate cancer. Eur J Nucl Med Mol Imaging.
40:1384–1393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scher HI, Halabi S, Tannock I, Morris M,
Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ,
Dreicer R, et al: Design and end points of clinical trials for
patients with progressive prostate cancer and castrate levels of
testosterone: Recommendations of the Prostate Cancer Clinical
Trials Working Group. J Clin Oncol. 26:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carducci MA, Padley RJ, Breul J, Vogelzang
NJ, Zonnenberg BA, Daliani DD, Schulman CC, Nabulsi AA,
Humerickhouse RA, Weinberg MA, et al: Effect of endothelin-A
receptor blockade with atrasentan on tumor progression in men with
hormone-refractory prostate cancer: A randomized, phase II,
placebo-controlled trial. J Clin Oncol. 21:679–689. 2003.
View Article : Google Scholar : PubMed/NCBI
|