Introduction
Infantile hemangiomas (IHs) are the most common
tumors of infancy and childhood, with an incidence of 2–3% in the
neonate, 10% in the baby after 1 year and up to 23% in premature
infants, with a slight female predominance (1). Hemangiomas are characterized
pathologically by endothelial cell hyperproliferation and may be
divided into the following three types, according to the depth of
their locations: Superficial hemangioma located in the papillary
dermis, deep hemangioma located in the reticular dermis and
subcutaneous tissue, and mixed hemangioma, which is a mixture of
superficial and deep components (2).
Deep hemangiomas present as blue or colorless masses, whereas
superficial hemangiomas often present as bright red lesions
(2). Blood flow in hemangiomas can be
observed by ultrasonography. The disease is self-limiting, which
often occurs in the neonatal period, and then enters the
proliferative phase, stops development around the age of 1 and
begins to regress (3,4). Parotid hemangioma is the most common
type of salivary gland hemangioma in children. Complications of
this condition may include ulceration, life-threatening airway
obstruction and the risk of delayed language acquisition due to ear
involvement. Patients should therefore be treated as soon a
possible (5). A number of clinical
studies have shown that propranolol is usually highly effective for
deep hemangiomas and that timolol maleate is usually highly
effective for superficial lesions (6). The present study reports the application
of topical timolol maleate combined with oral propranolol for
parotid IHs in the Department of Oral and Maxillofacial Surgery
(Hospital of Stomatology, China Medical University, Shenyang,
Liaoning, China), resulting in a clear curative effect.
Patients and methods
Patients
In total, 22 patients with mixed-type parotid gland
proliferating hemangioma were treated with topical timolol maleate
combined with oral propranolol between October 2012 and April 2014
at the Department of Oral and Maxillofacial Surgery in the Hospital
of Stomatology (Table I). The group
consisted of 9 boys and 13 girls, with a mean age of 4.7 months
(range, 2–9 months). The volume of these tumors varied between
3.5×4×0.5 and 7×8×3 cm, as measured by Doppler ultrasonography
(Philips iU22 Cart F 4D ultrasound system; Philips Electronics
Japan, Ltd., Tokyo Japan). The diagnosis of hemangioma was made
based on the medical history (age of onset), clinical presentations
and Doppler ultrasonography: A mixture of superficial and deep
components were observed and blood flow in the hemangiomas was
identified by ultrasonography. Data was collected from patient
files, including clinical characteristics, efficacy of treatment
and adverse reactions. The inclusion criteria for this study were
as follows: i) No infants were administered any treatment prior to
propranolol and timolol maleate.; ii) any other vascular
malformations were excluded, according to the classification and
nomenclature of vascular anomalies proposed by Waner and Suen
(7); and iii) normal chest X-ray,
electrocardiogram, blood coagulation, liver function, renal
function and blood routine examination results. The Ethical Review
Board of China Medical University approved the study and the
parents of all infants provided written informed consent for
publication.
 | Table I.Summary of treatment of IHs with
topical timolol maleate combined with oral propranolol. |
Table I.
Summary of treatment of IHs with
topical timolol maleate combined with oral propranolol.
| Patient no. | Gender | Age at treatment
onset, months | Treatment duration,
weeks | Size of IH prior to
treatment, cma | Size of IH after
treatment, cma | Side effects | Follow-up,
months | Response |
|---|
| 1 | F | 3 | 20 | 2.5/2/0.5 | 0/0/0 | None | 6 | Excellent |
| 2 | M | 3 | 24 | 8/7/3 | 0/0/0 | None | 6 | Excellent |
| 3 | M | 2 | 24 | 4/3.5/1 | 0/0/0 | None | 10 | Excellent |
| 4 | F | 3 | 16 | 3.5/3/0.5 | 1/1.5/0 | None | 9 | Good |
| 5 | M | 7 | 16 | 3/4/1.5 | 1/0.5/0 | Diarrhea | 8 | Good |
| 6 | F | 6 | 28 | 7/6.5/2 | 0/0/0.5 | None | 10 | Excellent |
| 7 | F | 2.5 | 12 | 1.5/1/0.5 | 0.5/0/0 | None | 12 | Excellent |
| 8 | F | 8 | 20 | 3.5/2/1 | 2/1/0.5 | None | 6 | Good |
| 9 | M | 5 | 24 | 4.5/3.5/1.5 | 3/2/0.5 | None | 10 | Moderate |
| 10 | F | 6 | 16 | 5/3.5/2.5 | 1/1.5/1 | None | 8 | Excellent |
| 11 | F | 2.5 | 32 | 8/7/3 | 2/1.5/1 | None | 10 | Excellent |
| 12 | M | 3 | 12 | 2/3/0.5 | 1/0.5/0 | None | 9 | Excellent |
| 13 | M | 2 | 32 | 7/6.5/2 | 0/0/0 | None | 8 | Excellent |
| 14 | F | 4 | 28 | 5/4/1 | 1/0.5/0.5 | None | 6 | Excellent |
| 15 | M | 6 | 20 | 3/3/0.5 | 1/1.5/0 | None | 6 | Good |
| 16 | F | 9 | 24 | 4/4/2 | 1/0.5/1 | None | 8 | Excellent |
| 17 | F | 7 | 24 | 5/5/2 | 2/2/0.5 | None | 12 | Good |
| 18 | M | 5 | 12 | 2.5/2.5/0.5 | 1/0.5/0 | None | 12 | Excellent |
| 19 | M | 3 | 16 | 2/2/0.5 | 0/0/0 | None | 9 | Excellent |
| 20 | F | 4.5 | 20 | 2.5/1.5/1 | 0/0/0 | None | 8 | Excellent |
| 21 | F | 3.5 | 16 | 2/1.5/0.5 | 0.5/0.5/0 | None | 6 | Good |
| 22 | F | 9 | 28 | 4/4.5/2 | 2/2/0.5 | None | 7 | Moderate |
Treatment protocol
All patients were admitted to the hospital when the
hemangiomas were in the rapidly proliferating phase. Propranolol
(10 mg/tablet; Tianjin Lisheng Pharmaceutical Co. Ltd., Tianjin,
China) was prepared from a tablet into a suitable solution at a
single oral dose of 1.0–1.5 mg/kg/day (1.0 mg/kg for patients <3
months old and 1.5 mg/kg for patients >3 months old). A small
amount of 0.5% timolol maleate eye drop solution (25 mg/5 ml; Wuhan
Five King Pharmaceutical Co., Ltd., Wuhan, Hubei, China) was also
topically applied with medical cotton swabs to the area of the
lesion twice a day, every 12 h. For the first 3–5 days of
management, heart rate, blood pressure and blood glucose levels
were monitored in the inpatient ward. The patients who experienced
no adverse reactions were topically administered the drug by their
parents or guardians following discharge from the hospital. Close
observation was paid to the heart rate, blood pressure and other
vital signs during the treatment, and the parents were informed to
look for local redness and symptoms such as a loss of appetite,
nausea, vomiting, wheezing, shortness of breath or lethargy. Once
any symptom appeared, the drugs were stopped immediately and the
patient observed carefully. The patients were reexamined
periodically in order to observe the size and color of the
hemangiomas for assessment of the curative effect. The dose of the
drug was altered according to the change in the weight of the
patient and the degree of adverse reaction at the period of
follow-up. Treatment was continued until 1 year old, unless
complete resolution occurred (8). The
follow-up timeline ranged from 1–10 months (median, 6.4
months).
Evaluation of efficacy
The therapeutic outcome was evaluated by Doppler
ultrasonography. The results were measured by the hemisphere
measurement and visual changes on photography (9). The clinical effect of treatment was
graded on a 4-point scale proposed by Achauer et al
(10), based on improvements in tumor
volume, color and texture after treatment, as follows: I (poor),
tumor volume decreased by <25%; II (moderate), tumor volume
decreased by 26–50%; III (good), tumor volume decreased by 51–75%;
and IV (excellent), tumor volume decreased by 76–100%.
With regard to safety, heart rate, systolic blood
pressure (SBP), diastolic blood pressure (DBP) and blood glucose
level were close monitored in the course of the 3-day
hospitalization period and were recorded 1 h after each dose. Local
side effects, including rashes, red spots, erosion and ulceration,
were also closely observed.
Statistical analysis
All data was entered into a database and processed
using SPSS software (version 18.0; SPSS Inc., Chicago, IL, USA).
The Kruskal-Wallis test was used to compare the differences in the
average heart rate, SBP and DBP prior to and following drug
administration. P<0.05 was used to indicate a statistically
significant difference.
Results
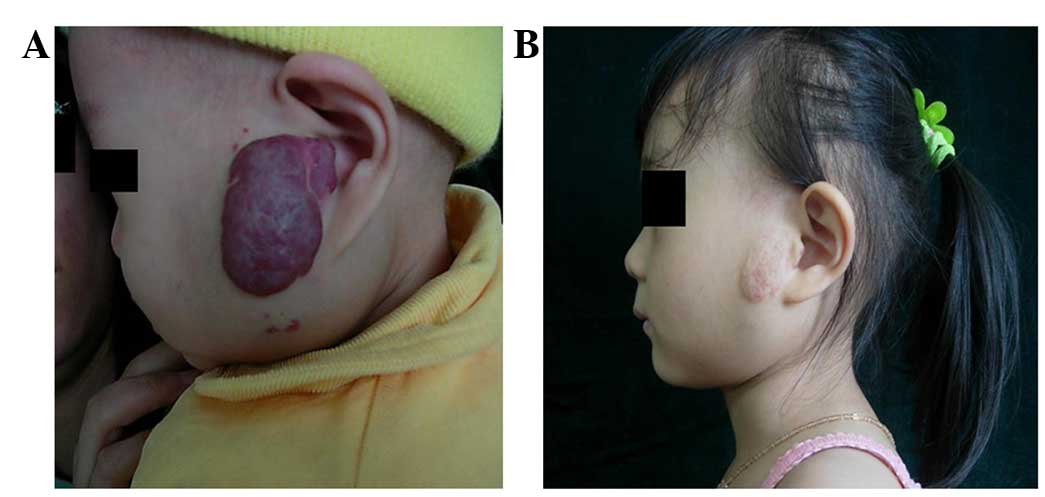
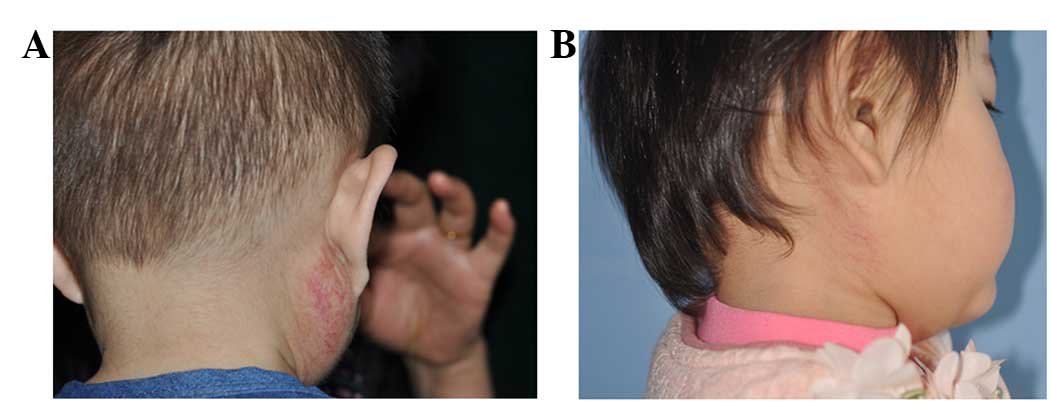
All the hemangiomas decreased in size following
treatment and in the majority of cases, the tumor color changed
from bright red to dull red. Efficacy scores was evaluated as
follows: IV (excellent), 14 cases (64%); III (good), 6 cases (27%);
II (moderate), 2 cases (9%); and I (poor), 0 cases (0%). The
parents were quite satisfied with the results. At 24 h
post-medication, the tension of the tumor surface of the majority
of children decreased and the texture became softer. In total, the
drug was administered for 12 weeks in 3 patients, for 16 weeks in 5
patients, for 20 weeks in 4 patients, for 24 weeks in 5 patients,
for 28 weeks in 3 patients and for 32 weeks in 2 patients, with a
mean time of 21.1 weeks. The tumor of 1 patient regressed
completely following 16 weeks of treatment. However, the tumor had
relapsed when the infant was followed up 6 weeks later and the
proliferation was subsequently effectively controlled by the
continuation of medication for ~10 weeks after treatment, until it
decreased in size. Typical cases are shown in Figs. 1 and 2.
No infants were withdrawn from the treatment study
due to side effects. In total, 22 infants exhibited a decreased
heart rate, blood pressure and rate of breathing after starting the
treatment. However, all these signs returned to normal after
propranolol had been administered for >12 h (P>0.05; Table II), and no patients required
additional treatment. No severe gastrointestinal adverse reactions
occurred.
 | Table II.Heart rate and blood pressure at
baseline and during the propranolol and timolol maleate treatment
initiation period (mean ± standard deviation). |
Table II.
Heart rate and blood pressure at
baseline and during the propranolol and timolol maleate treatment
initiation period (mean ± standard deviation).
| Parameter | Baseline | Day 1 | Day 2 | Day 3 |
|---|
| Heart rate (beats
per min) | 127.1±11.5 | 124.0±15.6 | 124.3±13.4 | 125.3±14.8 |
| SBP (mmHg) |
85.4±13.3 |
84.6±17.1 |
85.5±16.6 |
85.0±18.2 |
| DBP (mmHg) |
57.5±10.2 |
58.3±12.6 |
57.1±11.2 | 56.9±9.7 |
Discussion
Infantile parotid proliferating hemangiomas, which
are the most common parotid tumors, occurred around 1 year after
birth, accounting for >50% parotid gland tumors in infants
(11). Hemorrhage, ulceration,
infection and external auditory canal compression are the main
complications of the tumor. Facial hemangiomas can cause serious
psychological issues in the affected patients. Positive
intervention rather than observation should be therefore be
adopted, and appropriate treatment should be provided according to
the different growth phases of the hemangiomas (12).
At present, the treatment methods for IH are
conservative rather than surgical, and include as drug and laser
therapies. Common drugs for the treatment of hemangioma are
corticosteroids, and anticancer drugs, such as interferon and
imiquimod (13–18), but these methods lead to different
degrees of side effects. The only suitable treatment for early
superficial hemangiomas is laser therapy (19). With regard to the surgical treatment
of parotid region hemangiomas, there have been various proposals
and the optimal surgical duration is uncertain. The surgical
treatment must be selected carefully due to complications such as
temporary paralysis, recurrence, salivary fistula, hematoma,
auriculotemporal nerve syndrome, facial nerve injury and scarring
(20).
Hemangiomas can be divided into three types
according to the depth of their locations: Superficial hemangioma
located in the papillary dermis, deep hemangioma located in the
reticular dermis and subcutaneous tissue, and mixed hemangioma,
which is a mixture of superficial and deep components (21). In the Department of Oral and
Maxillofacial Surgery in the Hospital of Stomatology, personalized
treatment options are used based on the hemangioma site, size and
depth, among other factors, in order to obtain the best treatment
effect (22). Currently, drug
treatment is the preferred treatment of IHs. In 2008,
Léauté-Labrèze et al first reported the use propranolol for
the treatment of IH, and achieved good results (22). This study soon attracted the attention
of scholars from all over the world, which triggered a series of
associated studies on propranolol in the treatment of IH (8,11,22,23).
Propranolol is a non-selective β-adrenergic antagonist, whose
treatment effect on IH is verified. Compared with the traditional
corticosteroid therapy, it has better tolerability, less adverse
reactions and greater efficiency (23–25).
Timolol maleate is a non-selective potent β-adrenergic antagonist
(β1 and β2) that has been mainly used for the clinical treatment of
hypertension, angina pectoris, tachycardia and glaucoma (26). The predominant adverse effects include
hypotension, hypoglycemia, bronchospasm and local pruritus. Timolol
was first used for glaucoma in the United States in 1978. It is
safe for the treatment of the pediatric population, and has been
used for glaucoma as a first-line medicine by pediatric
ophthalmologists for >30 years (27). Timolol, as a type of ophthalmic drug,
causes little irritation to local tissues, and its effect can only
be observed in certain regions, with little systemic reaction, so
the application of timolol maleate as a topical medication is
extremely safe (28). Numerous
clinical studies have demonstrated that treatment with propranolol
is effective for both deep and superficial hemangiomas. In
addition, the treatment response to oral propranolol is better for
deep IHs than superficial IHs. Generally, timolol meleate is a
highly effective treatment for superficial lesions. Therefore,
combined treatment with oral propranolol and topical timolol
maleate has been developed for mixed and superficial lesions, and
oral propranolol is used to treat deep lesions (29).
No uniform safe dosage standard exists for the
treatment of hemangioma using propranolol, however, according to
domestic studies (30–33) and our considerable clinical
experience, we believe that 1.0 mg/kg propranolol for patients
<3 months old and 1.5 mg/kg propranolol for patients >3
months old is a safe dosage. An infant should, however, undergo a
detailed general examination prior to this prescription.
Furthermore, all infants should be hospitalized as an inpatient
under observation for 3–5 days after the initial treatment, and the
blood pressure, heart rate and adverse reactions should be closely
monitored.
In 2010, Guo and Ni (34) described the case of a 4-month-old
infant with superficial capillary hemangioma of the eyelid, and
became the first study to report a successful outcome subsequent to
the use of timolol solution; this success was subsequently
reinforced by a series of other studies. In 2011, Pope and
Chakkittakandiyil reported 6 cases of IHs treated with timolol in
which the symptoms improved significantly (35). Li et al reported 25 cases of
children with hemangiomas who were administered 0.5% topical
timolol maleate, resulting in a clear positive effect. According to
the related literature, the pharmacological effects of timolol are
8 times higher than propranolol (36). Therefore, the oral dose of propranolol
can be reduced while topically using timolol maleate, therefore
ensuring drug safety and reducing complications.
Despite the strong effects of propranolol and
timolol maleate for the treatment of IH, the mechanism of action
remains unknown (37).
Vasoconstriction may be responsible for the early effects on the
color of the hemangioma due to the decreased release of nitric
oxide. Growth arrest is attributable to the blocking of
proangiogenic signals, including vascular endothelial growth
factor, basic fibroblast growth factor, matrix metalloproteinases
and endothelial nitric oxide synthase (38).
In the present study, attention was focused on the
use of topical timolol maleate combined with oral propranolol for
the treatment of parotid IHs. Good results were achieved using this
combined treatment, which resulted in clear curative effects, with
reduced complications. On the whole, as a quicker, safe and
effective treatment, combined treatment with topical timolol
maleate and oral propranolol for the treatment of parotid IHs
should become the preferred therapeutic option.
References
|
1
|
Phung TL, Hochman M and Mihm MC: Current
knowledge of the pathogenesis of infantile hemangiomas. Arch Facial
Plast Surg. 7:319–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuan WL, Jin ZL, Wei JJ, Liu ZY, Xue L and
Wang XK: Propranolol given orally for proliferating infantile
haemangiomas: Analysis of efficacy and serological changes in
vascular endothelial growth factor and endothelial nitric oxide
synthase in 35 patients. Br J Oral Maxillofac Surg. 51:656–661.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mulliken JB and Glowacki J: Hemangiomas
and vascular malformations in infants and children: A
classification based on endothelial characteristics. Plast Reconstr
Surg. 69:412–422. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buckmiller LM, Richter GT and Suen JY:
Diagnosis and management of hemangiomas and vascular malformations
of the head and neck. Oral Dis. 16:405–418. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Enjolras O and Gelbert F: Superficial
hemangiomas: Associations and management. Pediatr Dermatol.
14:173–179. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu X, Tai M, Qin Z, Li K and Ge C:
Clinical analysis for treatment of 1,080 cases of infantile
hemangiomas with oral propranolol. Zhonghua Yi Xue Za Zhi.
94:1878–1881. 2014.(In Chinese). PubMed/NCBI
|
|
7
|
Waner M and Suen JY: Management of
congenital vascular lesions of the head and neck. Oncology
(Williston Park). 9:989–994, 997; discussion 998 passim.
1995.PubMed/NCBI
|
|
8
|
Malik MA, Menon P, Rao KL and Samujh R:
Effect of propranolol vs prednisolone vs. propranolol with
prednisolone in the management of infantile hemangioma: A
randomized controlled study. J Pediatr Surg. 48:2453–2459. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haggstrom AN, Drolet BA, Baselga E,
Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW,
Newell B, et al: Prospective study of infantile hemangiomas:
Clinical characteristics predicting complications and treatment.
Pediatrics. 118:882–887. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Achauer BM, Chang CJ and Vander Kam VM:
Management of hemangioma of infancy: Review of 245 patients. Plast
Reconstr Surg. 99:1301–1308. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mantadakis E, Tsouvala E, Deftereos S,
Danielides V and Chatzimichael A: Involution of a large parotid
hemangioma with oral propranolol: An illustrative report and review
of the literature. Case Rep Pediatr. 2012:3538122012.PubMed/NCBI
|
|
12
|
Zheng JW: Infantile hemangiomas: Seldom
wait and see. China Journal of Oral and Maxillofacial Surgery.
10:163–164. 2012.(In Chinese).
|
|
13
|
Bennett ML, Fleischer AB Jr, Chamlin SL
and Frieden IJ: Oral corticosteroid use is effective for cutaneous
hemangiomas: An evidence-based evaluation. Arch Dermatol.
137:1208–1213. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gangopadhyay AN, Sharma SP, Gopal SC,
Gupta DK, Panjawani K and Sinha JK: Local steroid therapy in
cutaneous hemangiomas. Indian Pediatr. 33:31–33. 1996.PubMed/NCBI
|
|
15
|
Muir T, Kirsten M, Fourie P, Dippenaar N
and Ionescu GO: Intralesional bleomycin injection (IBI) treatment
for haemangiomas and congenital vascular malformations. Pediatr
Surg Int. 19:766–773. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Omidvari S, Nezakatgoo N, Ahmadloo N,
Mohammadianpanah M and Mosalaei A: Role of intralesional bleomycin
in the treatment of complicated hemangiomas: Prospective clinical
study. Dermatol Surg. 31:499–501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Enjolras O, Brevière GM, Roger G, Tovi M,
Pellegrino B, Varotti E, Soupre V, Picard A and Leverger G:
Vincristine treatment for function- and life-threatening infantile
hemangioma. Arch Pediatr. 11:99–107. 2004.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ezekowitz RA, Mulliken JB and Folkman J:
Interferon alfa-2a therapy for life-threatening hemangiomas of
infancy. N Engl J Med. 326:1456–1463. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hochman A and Mascareno A: Management of
nasal hemangiomas. Arch Facial Plast Surg. 7:295–300. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hamou C, Diner PA, Dalmonte P, Vercellino
N, Soupre V, Enjolras O, Vazquez MP and Picard A: Nasal tip
haemangiomas: Guidelines for an early surgical approach. J Plast
Reconstr Aesthet Surg. 63:934–939. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng JW, Zhou GY, Wang YA and Zhang ZY:
Management of head and neck hemangiomas in China. Chin Med J
(Engl). 121:1037–1042. 2008.PubMed/NCBI
|
|
22
|
Léauté-Labrèze C, Dumas de la Roque E,
Hubiche T, Boralevi F, Thambo JB and Taïeb A: Propranolol for
severe hemangiomas of infancy. N Engl J Med. 358:2649–2651. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Price CJ, Lattouf C, Baum B, McLeod M,
Schachner LA, Duarte AM and Connelly EA: Propranlol vs
corticosteroids for infantile hemangiomas: A multicenter
retrospective analysis. Arch Dermatol. 147:1371–1376. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Storch CH and Hoeger PH: Propranolol for
infantile haemangiomas: Insights into the molecular mechanisms of
action. Br J Dermatol. 163:269–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ribatti D: The crucial role of vascular
permeability factor/vascular endothelial factor in angiogenesis: A
historical review. Br J Haematol. 128:303–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye XX, Jin YB, Lin XX, Ma G, Chen XD, Qiu
YJ, Chen H and Hu XJ: Topical timolol in the treatment of
periocular superficial infantile hemangiomas: A prospective study.
Zhonghua Zheng Xing Wai Ke Za Zhi. 28:161–164. 2012.(In Chinese).
PubMed/NCBI
|
|
27
|
Coppens G, Stalmans I, Zeyen T and
Casteels I: The safety andefficacy of glaucoma medication in the
pediatric population. J Pediatr Ophthalmol Strabismus. 46:12–18.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo S and Ni N: Topical treatment for
capillary hemangioma of the eyelid using beta-blocker solution.
Arch Ophthalmol. 128:255–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prey S, Voisard JJ, Delarue A, Lebbe G,
Taïeb A, Leaute-Labreze C and Ezzedine K: Safety of propranolol
therapy for severe infantile hemangioma. JAMA. 315:413–415. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pope E and Chakkittakandiyil A: Topical
timolol gel for infantile hemangiomas: A pilot study. Arch
Dermatol. 146:564–565. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu Y, Xue L, Wei J, Lei B, Liu Z and Wang
X: Oral propranolol treatment for infants with proliferating
hemangiomas: Clinical analysis of 35 cases. Zhongguo Kouqiang He
Mian Waike Zazhi. 10:159–162. 2012.(In Chinese).
|
|
32
|
Zheng JW, Zhou Q, Yang XJ, Wang YA, Fan
XD, Zhou GY, Zhang ZY and Suen JY: Treatment guideline for
hemangiomas and vascular malformations of the head and neck. Head
Neck. 32:1088–1098. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zheng JW: Comment on efficacy and safety
of propranolol in the treatment of parotid hemangioma. Cutan Ocul
Toxicol. 30:333–334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu DP, Cao RY and Tong S: Topical timolol
maleate for superficial infantile hemangiomas: an observational
study. J Oral Maxillofac Surg. 73:1089–1094. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chan H, Mckay C, Adam S and Wargon O: RCT
of timolol maleate gel for superficial infantile hemangiomas in
5-to 24-week-olds. Pediatrics. 131:e1739–e1747. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li G, Xu DP, Tong S, Xue L, Sun NN and
Wang XK: Oral Propranolol With Topical Timolol Maleate Therapy for
Mixed Infantile Hemangiomas in Oral and Maxillofacial Regions. J
Craniofac Surg. 27:56–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Storch CH and Hoeger PH: Propranolol for
infantile hemangiomas: Insights into the molecular mechanisms of
action. Br J Dermatol. 163:269–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Itinteang T, Brasch HD, Tan ST and Day DJ:
Expression of components of the renin-angiotensin system in
proliferating infantile hemangioma may account for the
propranolol-induced accelerated involution. J Plast Reconstr
Aesthet. 64:759–765. 2010. View Article : Google Scholar
|