Introduction
Thyroid cancers are the most common malignancy of
endocrine organs (1). There were
62,980 estimated new cases of thyroid cancer in the United States
in 2014, as well as 1,890 cancer-associated mortalities (2). The frequency of ATC is increasing, and
it currently accounts for 2.5% of all cancers in the United States
(3). Thyroid cancer has become a
particular research focus as it encompasses several
histopathological tumor types with distinct levels of
differentiation (4). Thyroid
carcinomas originate from follicular and parafollicular cells
(5,6),
and are subdivided into well-differentiated papillary thyroid
carcinoma (PTC) and follicular thyroid carcinoma (FTC), poorly
differentiated carcinoma (PDC), and completely undifferentiated
anaplastic thyroid carcinoma (ATC) (4). ATC is responsible for 1.7% of all
thyroid cancer cases, however, is the most deadly of the
thyroid-derived tumors, with patients demonstrating a median
survival time of 5 months and a 20% 1-year survival rate (3,7).
MicroRNAs (miRs) are a class of endogenous noncoding
RNAs, which act as negative regulators of gene expression (8). Previous studies have indicated that a
large number of miRs are involved in almost every major cellular
function, including the process of oncogenesis (8–10). miRs
may play roles as tumor suppressors or oncogenes (11). The expression profiles of miRs have
been characterized in various histopathological types of thyroid
cancer, and a series of miRs exhibited differential expression
patterns (4). A series of miRs have
significant roles in ATC and their targets have been validated, for
example, miR-20a was upregulated in ATC and targets LIM domain
kinase 1 (12). Additionally, miRs
were considered as potential diagnostic markers for thyroid
carcinoma (13,14).
The expression of miR-146b in various types of
thyroid carcinoma has been summarized previously (15,16). Its
expression was markedly increased in PTC and PDC and slightly
increased in FTC (15,16); however, expression levels were not
consistent between various studies that investigated expression in
ATC. Visone et al (17) and
Braun et al (18) reported no
significant change in the expression levels of miR-146b (P<0.05)
in ATC. However, Nikiforova et al (4) reported that miR-146b was upregulated in
ATC compared with hyperplastic nodules, and Fassina et al
(19) reported that miR-146b was
upregulated in ATC compared with primary thyroid lymphoma and
multinodular goiter (19). Therefore,
the role of miR-146b in ATC remains to be fully elucidated.
p21 encodes a protein that binds to and
inhibits the activity of cyclin-dependent kinase 2 (CDK2) or CDK4
complexes, and functions as a regulator of cell cycle progression
at G1. p21 is the target of tumor suppressor protein p53 or
its isoform (20,21), and thus functions as a tumor
suppressor in a variety of types of cancer (22). It has been observed that p21 is
regulated by a variety of miRs during the promotion of progression
of the cell cycle or tumor growth, including miR-106b (11), miR-17 (23), miR-224 (24) and miR-663 (25).
In the present study, the effect of miR-146b on
proliferation was investigated in ATC cells, and the potential
targets of miR-146b were searched. It was concluded that miR-146b
may influence ATC proliferation through regulation of p21.
Materials and methods
Ethics statement
The present study was approved by The Ethics
Committee of the First Affiliated Hospital, Medical School of Xi'an
Jiaotong University (Xi'an, China), and no human/animal tissues
were used in the present study.
miR profile data collection and
analysis
miR profile data of ATC and matched non-tumor
controls were collected from the Gene Expression Omnibus (GEO)
database (www.ncbi.nlm.nih.gov/gds; GSE29265). During the study,
10 ATC samples and 10 patient-matched non-tumor samples were
utilized for additional miR analysis. The comparison of miR
profiles between 10 ATC and 10 non-tumor samples was performed by
studying the fold change and using the Stuent's t-test
method on the Limma package on R software version 3.0.3 (www.r-project.org). The cutoff for a significantly
differentially expressed miR was fold change>2 and
P<0.05.
Cell line
The FRO human anaplastic thyroid cancer cell line
has been described and authenticated previously (26). The FRO cell line was maintained in
RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
and 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.) at
37°C in an atmosphere of 5% CO2.
Cell transfection
FRO cells were seeded at 3×105
cells/wells into 6-well plates and incubated overnight at 37°C.
Transfection of miR-146b mimic, the anti-miR-146b, inactive control
cel-miR-67 (Dharmacon; GE Healthcare Life Sciences, Chalfont, UK)
or pMIR-Report vectors (Invitrogen; Thermo Fisher Scientific, Inc.)
was performed with Lipofectamine 2000® transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) using 300 nmol
of miR or 1 µg/ml DNA plasmid, respectively.
Cell proliferation
Cell proliferation assays were performed using a
Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). FRO cells were seeded into 24-well plates at
2×105 cells/well. Cells were incubated at 37°C in 10%
CCK-8 reagent, which was diluted in fresh Dulbecco's modified
Eagle's medium (Thermo Fisher Scientific, Inc.). Cell proliferation
was measured by microplate reader scanning at 450 nm according to
the manufacturer's protocol. Cell proliferation rates were
determined at 0, 24, 48 and 72 h following transfection.
Migration assays
Cell migration assays were performed using a
Transwell chamber (Corning Incorporated, Corning, NY, USA) with or
without Matrigel (Invitrogen; Thermo Fisher Scientific, Inc.). In
the Transwell assay, FRO cells 24, 48 or 72 h subsequent to
transfection, were trypsinized and seeded into chambers at a
density of 8×104 cells/well and cultured in RPMI-1640
medium with 2% FBS, while 600 ml 10% FBS RPMI-1640 was added to the
lower chamber. Following incubation at 37°C in an atmosphere of 5%
CO2 for 24 h, migrated cells were fixed with 100%
methanol for 30 min. Non-migrated cells were removed using cotton
swabs. Subsequently, cells on the bottom surface of the membrane
were stained by 0.1% crystal violet (Invitrogen; Thermo Fisher
Scientific, Inc.) for 20 min. Cell images were obtained under a
phase-contrast microscope (Olympus Corporation, Tokyo, Japan).
Western blotting
FRO cells were lysed with radioimmunoprecipitation
assay buffer (Thermo Fisher Scientific, Inc.) containing a mixture
of protease inhibitors from the Clean-Blot™ IP Detection kit
(Thermo Fisher Scientific, Inc.) for 30 min. The lysates were
subsequently centrifuged at 4°C and 8,050 × g for 15 min. The
protein concentrations were determined by bicinchoninic acid
protein assay kit (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. The proteins were boiled at 95°C for 5
min and subsequently stored at −70°C. A total of 50 µg protein was
electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gels
and transferred onto polyvinylidene difluoride (PVDF) membranes.
Subsequently, PVDF membranes were blocked for 1 h in 5% non-fat
milk at room temperature. Membranes were probed with rabbit
anti-human anti-p21 primary antibodies (catalog no., ab109199,
Abcam, Cambridge, MA, USA) at a dilution of 1:100 at 4°C overnight,
followed by three washes with Tris-buffered saline and Tween 20
(TBST). Goat anti-rabbit immunoglobulin G horseradish
peroxidase-conjugated secondary antibodies (catalog no., ab6721;
Abcam) were added at a dilution of 1:2,000, and incubated for 1 h
at room temperature. Following incubation, the membranes were
washed three times with TBST and enhanced chemiluminescence was
used for detecting antigens on X-ray film. β-actin (Immunocreate,
LLC, Birmingham, AL, USA) was used as a loading control. The fold
changes in gene expression were calculated by the equation
2−ΔΔCq (27).
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences between groups were assessed using the Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed on R
version 3.0.3 (www.r-project.org).
Results
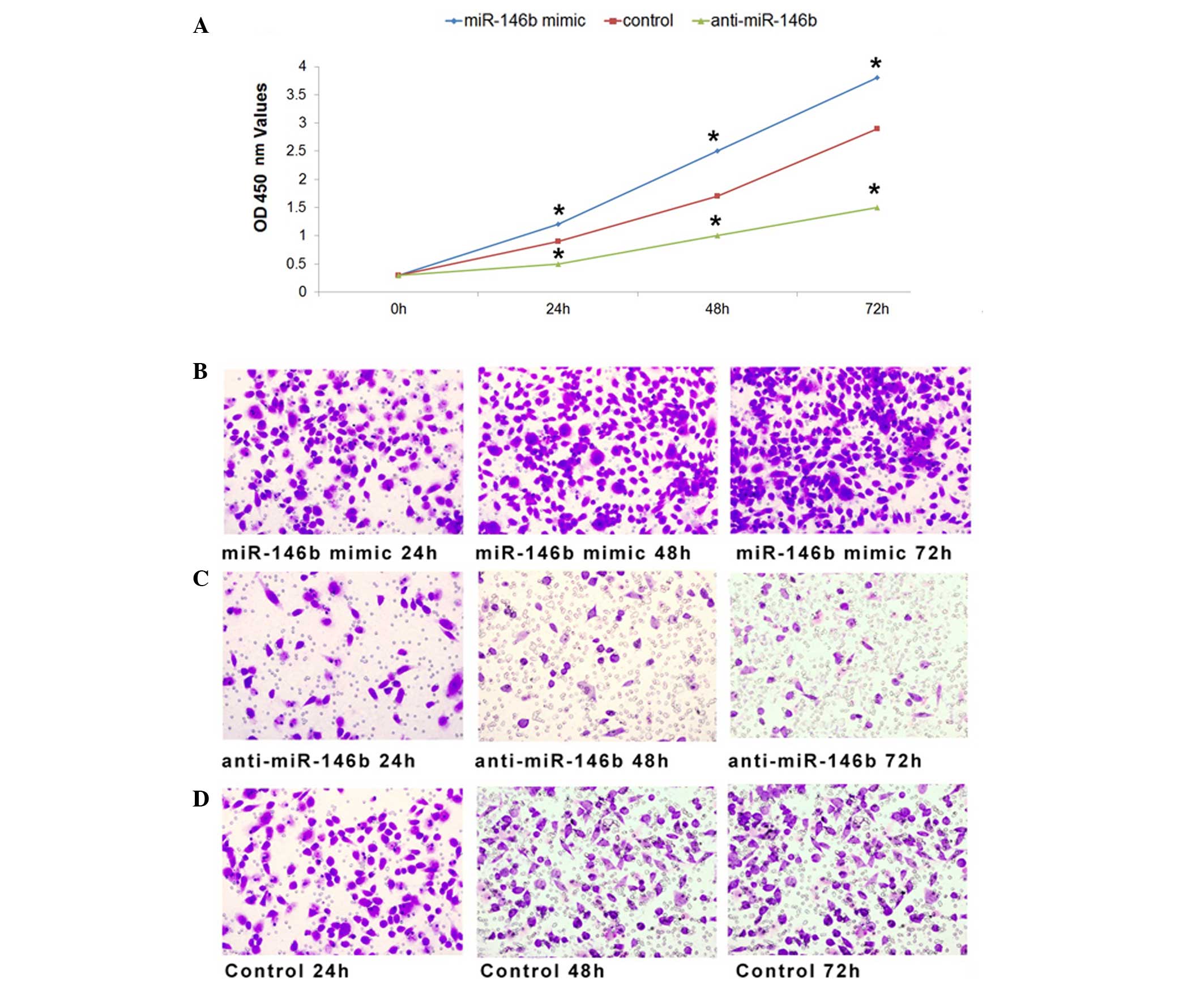
miR-146b affects the proliferation and
migration of ATC cells in vitro
To investigate the effects of miR-146b on the
proliferation of ATC cells, miR-146b mimic, anti-miR-146b or
inactive cel-mir-67 (control) was transfected into the FRO human
ATC cell line as previously described (26). The optical density at 450 nm was
measured 0, 24, 48 and 72 h subsequent to transfection as an
indicator of active cell number. As a result of miR-146b
overexpression following transfection of synthetic miR-146b RNA
duplexes, the cell proliferation rate increased compared with the
control at 24 h after transfection, and demonstrated a higher
proliferation rate at 48 and 72 h (Fig.
1A). Furthermore, FRO cell proliferation was inhibited when
miR-146b was downregulated compared with the control (Fig. 1A). Therefore, the results of the
present study indicated that miR-146b influenced the proliferation
of ATC cells.
Furthermore, the migration of FRO cells was
investigated following transfection with miR-146b mimic,
anti-miR-146b or inactive cel-mir-67. Compared with the negative
control, the migration of FRO cells was promoted by transfection
with miR-146b mimic (Fig. 1B), and
inhibited by transfection with anti-miR-146b (Fig. 1C). Taken together, the results of the
present study indicate that miR-146b exhibited a function in cell
proliferation and migration of ATC cells.
Potential targets of miR-146b in
ATC
In order to investigate the potential targets of
miR-146b in ATC, the miR profile data of ATC and patient-matched
non-tumor controls was searched online in the GEO database
(www.ncbi.nlm.nih.gov/gds; GSE29265).
Comparison of miR profiles in this database was performed through
fold change and t-test methods. In total, 215
differentially-expressed genes were obtained. Out of these
identified genes, p21 was focused on as it has been proven
to be a tumor suppressor targeted by p53 in a variety of types of
cancer (22). Compared with non-tumor
controls, the expression of p21 was significantly
downregulated in ATC samples (log fold change<-1; P=0.0039;
Table I). Therefore, the hypothesis
that miR-146b regulates ATC proliferation through p21 was
proposed and further investigated.
 | Table I.Differentially expressed target genes
of microRNA-146b in anaplastic thyroid carcinoma. |
Table I.
Differentially expressed target genes
of microRNA-146b in anaplastic thyroid carcinoma.
| Gene | logFC | P-value | Identification
method |
|---|
|
p21/CDKN1A | −1.39 | <0.001 | Reporter assay |
| MMP16 | 1.91 | <0.001 | Reporter assay |
| KLF7 | −0.87 | <0.001 | Predicted |
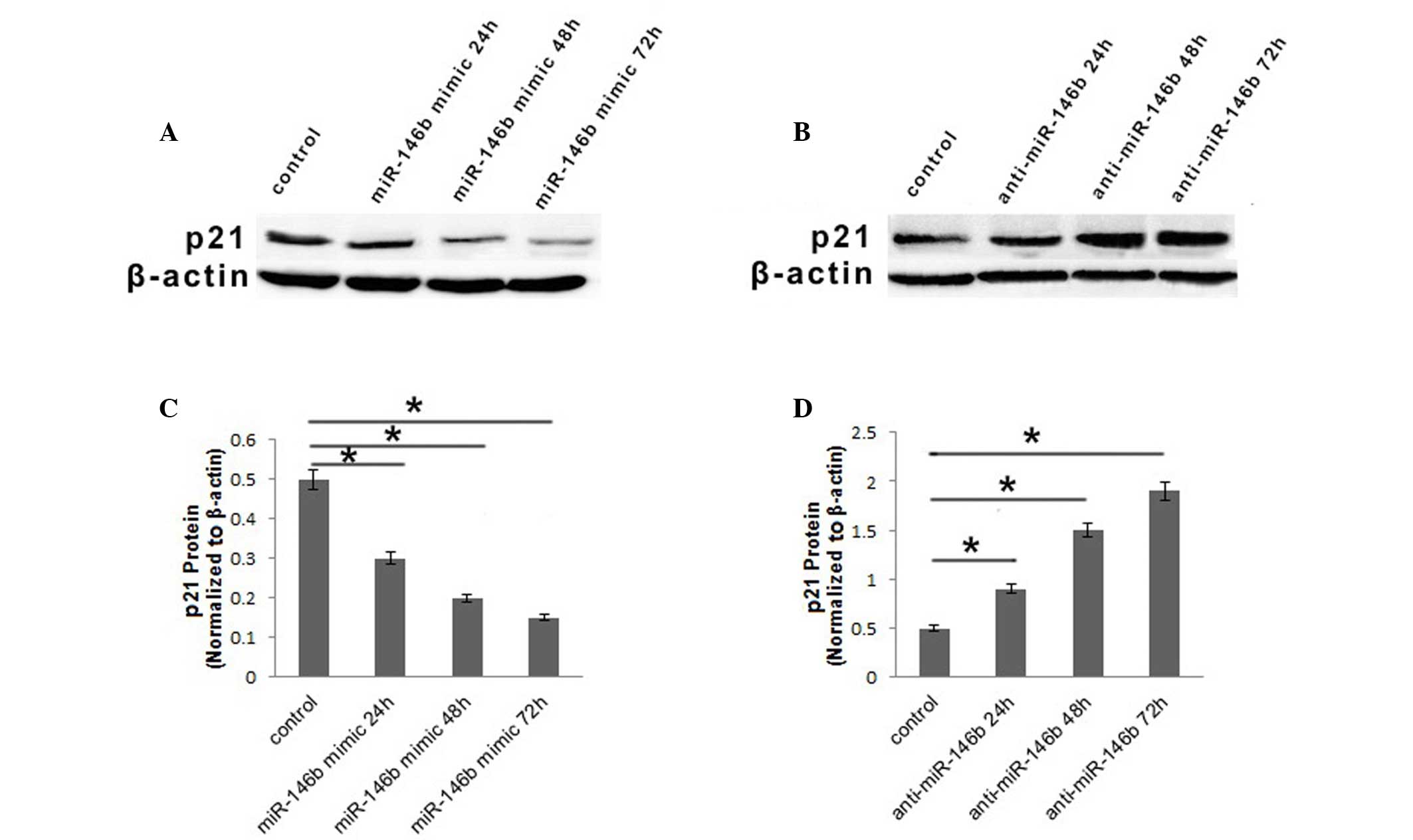
miR-146b influences the expression of
p21 in the FRO ATC cell line
As miR-146b had an effect on cell proliferation in
ATC cells and p21 was downregulated in ATC cells, the
present study aimed to determine whether miR-146b influenced ATC
cell proliferation via modulation of p21. The protein
expression of p21 in FRO cells was investigated using western
blotting following transfection with miR-146b mimic, anti-miR-146b
or inactive cel-mir-67. The protein level of p21 was downregulated
24 h subsequent to infection with miR-146b mimic, and then
decreased continuously, while 72 h subsequent to infection, it was
~30% of that observed in the control (Fig. 2A and B). Furthermore, when the FRO
cell line was infected with anti-miR-146b, the protein level of p21
was upregulated gradually at 24 and 48, and subsequently increased
3.8-fold compared with the control at 72 h (Fig. 2C and D). Based on the results of the
present study, it was concluded that miR-146b may influence
proliferation of ATC cells via regulation of p21.
Discussion
The role of miR-146b in ATC remains to be
elucidated. In order to characterize the role of miR-146b in ATC,
miR-146b was transfected into ATC cell lines and its impact on cell
proliferation and migration was investigated. In addition, the
potential mechanisms underling these phenomena were also
investigated.
In the present study, the role of miR146b in the
proliferation of ACT cells was described, and evidence to support
the participation of p21 in this process was presented.
p21, also known as Ras, is a potent cyclin-dependent kinase
inhibitor, which has an important role in cancer (28). The mechanism in which
RasG12V induces the arrest of cell growth serves as a
fail safe protection from malignant transformation (29). During this process in human mammary
epithelial cells, p21 was previously shown to be regulated
by a series of miRs, including miR-106b, miR-130b, miR-302a,
miR-302b, miR-302c, miR-302d, miR-512-3p and miR-515-3p, while miR
146a and miR-146b demonstrated a relatively weak rescue from
RasG12V-induced senescence (30). There is robust evidence that
p21 is the direct target of miR-106b during promotion of
cell cycle progression (11). Though
miR-146b exhibited varying seed sequences from miR-106b, the
expression level of p21 was observed to be regulated by
miR-146b in ATC cells in the present study. Therefore, it may be
assumed that there is an alternative mechanism that is responsible
for the regulation of miR-146b by p21 and its influence on
ATC cell proliferation.
In general, the present study revealed that miR-146b
promotes ACT cell proliferation and inhibits p21. These
findings might improve our understanding on the pathogenesis of ACT
and provide potential target for future therapies.
Acknowledgements
The present study was supported by the Youth Program
of the National Natural Science Foundation of China (grant no.
81102056) and the Project of Science and Technology of Social
Development in Shaanxi Province (grant no. 2016SF-114).
References
|
1
|
Viola D, Valerio L, Molinaro E, Agate L,
Bottici V, Biagini A, Lorusso L, Cappagli V, Pieruzzi L, Giani C,
et al: Treatment of advanced thyroid cancer with targeted
therapies: Ten years of experience. Endocr Relat Cancer.
23:R185–R205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Likhterov I, Tuttle RM, Haser GC, Su HK,
Bergman D, Alon EE, Bernet V, Brett E, Cobin R, Dewey EH, et al:
Improving the adoption of thyroid cancer clinical practice
guidelines. Laryngoscope. April 14–2016.(Epub ahead of print).
View Article : Google Scholar
|
|
3
|
Smallridge RC, Ain KB, Asa SL, Bible KC,
Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal
MS, et al: American Thyroid Association Anaplastic Thyroid Cancer
Guidelines Taskforce: American Thyroid Association guidelines for
management of patients with anaplastic thyroid cancer. Thyroid.
22:1104–1139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nikiforova MN, Tseng GC, Steward D, Diorio
D and Nikiforov YE: MicroRNA expression profiling of thyroid
tumors: Biological significance and diagnostic utility. J Clin
Endocrinol Metab. 93:1600–1608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kebebew E: Hereditary non-medullary
thyroid cancer: World. J Surg. 32:678–682. 2008.
|
|
6
|
Vriens MR, Suh I, Moses W and Kebebew E:
Clinical features and genetic predisposition to hereditary
nonmedullary thyroid cancer. Thyroid. 19:1343–1349. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smallridge RC and Copland JA: Anaplastic
thyroid carcinoma: Pathogenesis and emerging therapies. Clin Oncol
(R Coll Radiol). 22:486–497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Azizian A, Gruber J, Ghadimi BM and
Gaedcke J: MicroRNA in rectal cancer. World J Gastrointest Oncol.
8:416–426. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Endzeliņš E, Melne V, Kalniņa Z,
Lietuvietis V, Riekstiņa U, Llorente A and Linē A: Diagnostic,
prognostic and predictive value of cell-free miRNAs in prostate
cancer: A systematic review. Mol Cancer. 15:412016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sheu SY, Grabellus F, Schwertheim S, Worm
K, Broecker-Preuss M and Schmid KW: Differential miRNA expression
profiles in variants of papillary thyroid carcinoma and
encapsulated follicular thyroid tumours. Br J Cancer. 102:376–382.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ivanovska I, Ball AS, Diaz RL, Magnus JF,
Kibukawa M, Schelter JM, Kobayashi SV, Lim L, Burchard J, Jackson
AL, et al: MicroRNAs in the miR-106b family regulate p21/CDKN1A and
promote cell cycle progression. Mol Cell Biol. 28:2167–2174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiong Y, Zhang L and Kebebew E: MiR-20a is
upregulated in anaplastic thyroid cancer and targets LIMK1. PLoS
One. 9:e961032014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen YT, Kitabayashi N, Zhou XK, Fahey TJ
III and Scognamiglio T: MicroRNA analysis as a potential diagnostic
tool for papillary thyroid carcinoma. Mod Pathol. 21:1139–1146.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vriens MR, Weng J, Suh I, Huynh N,
Guerrero MA, Shen WT, Duh QY, Clark OH and Kebebew E: MicroRNA
expression profiling is a potential diagnostic tool for thyroid
cancer. Cancer. 118:3426–3432. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lodewijk L, Prins AM, Kist JW, Valk GD,
Kranenburg O, Rinkes IH and Vriens MR: The value of miRNA in
diagnosing thyroid cancer: A systematic review. Cancer Biomark.
11:229–238. 2012.PubMed/NCBI
|
|
16
|
Fuziwara CS and Kimura ET: MicroRNA
deregulation in anaplastic thyroid cancer biology. Int J
Endocrinol. 2014:7434502014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Visone R, Pallante P, Vecchione A,
Cirombella R, Ferracin M, Ferraro A, Volinia S, Coluzzi S, Leone V,
Borbone E, et al: Specific microRNAs are downregulated in human
thyroid anaplastic carcinomas. Oncogene. 26:7590–7595. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Braun J, Hoang-Vu C, Dralle H and
Hüttelmaier S: Downregulation of microRNAs directs the EMT and
invasive potential of anaplastic thyroid carcinomas. Oncogene.
29:4237–4244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fassina A, Cappellesso R, Simonato F, Siri
M, Ventura L, Tosato F, Busund LT, Pelizzo MR and Fassan M: A
4-MicroRNA signature can discriminate primary lymphomas from
anaplastic carcinomas in thyroid cytology smears. Cancer
Cytopathol. 122:274–281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Löhr K, Möritz C, Contente A and
Dobbelstein M: p21/CDKN1A mediates negative regulation of
transcription by p53. J Biol Chem. 278:32507–32516. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rohaly G, Chemnitz J, Dehde S, Nunez AM,
Heukeshoven J, Deppert W and Dornreiter I: A novel human p53
isoform is an essential element of the ATR-intra-S phase
checkpoint. Cell. 122:21–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gupta R, Dong Y, Solomon PD, Wettersten
HI, Cheng CJ, Min JN, Henson J, Dogra SK, Hwang SH, Hammock BD, et
al: Synergistic tumor suppression by combined inhibition of
telomerase and CDKN1A. Proc Natl Acad Sci USA. 111:E3062–E3071.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Minami Y, Kohsaka S, Tsuda M, Yachi K,
Hatori N, Tanino M, Kimura T, Nishihara H, Minami A, Iwasaki N and
Tanaka S: SS18-SSX-regulated miR-17 promotes tumor growth of
synovial sarcoma by inhibiting p21WAF1/CIP1. Cancer Sci.
105:1152–1159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Zhu LJ, Yang YC, Wang ZX and Wang
R: MiR-224 promotes the chemoresistance of human lung
adenocarcinoma cells to cisplatin via regulating G1/S
transition and apoptosis by targeting p21(WAF1/CIP1). Br J Cancer.
111:339–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yi C, Wang Q, Wang L, Huang Y, Li L, Liu
L, Zhou X, Xie G, Kang T, Wang H, et al: MiR-663, a microRNA
targeting p21(WAF1/CIP1), promotes the proliferation and
tumorigenesis of nasopharyngeal carcinoma. Oncogene. 31:4421–4433.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schweppe RE, Klopper JP, Korch C,
Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow LA, Copland
JA, Smallridge RC and Haugen BR: Deoxyribonucleic acid profiling
analysis of 40 human thyroid cancer cell lines reveals
cross-contamination resulting in cell line redundancy and
misidentification. J Clin Endocrinol Metab. 93:4331–4341. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak and Schmittgen: Analysis of relative
gene expression data using real-time quantitative PCR and the
2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abbas T and Dutta A: p21 in cancer:
Intricate networks and multiple activities. Nature Review Cancer.
9:400–414. 2009. View
Article : Google Scholar
|
|
29
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Borgdorff V, Lleonart ME, Bishop CL,
Fessart D, Bergin AH, Overhoff MG and Beach DH: Multiple microRNAs
rescue from Ras-induced senescence by inhibiting p21(Waf1/Cip1).
Oncogene. 29:2262–2271. 2010. View Article : Google Scholar : PubMed/NCBI
|