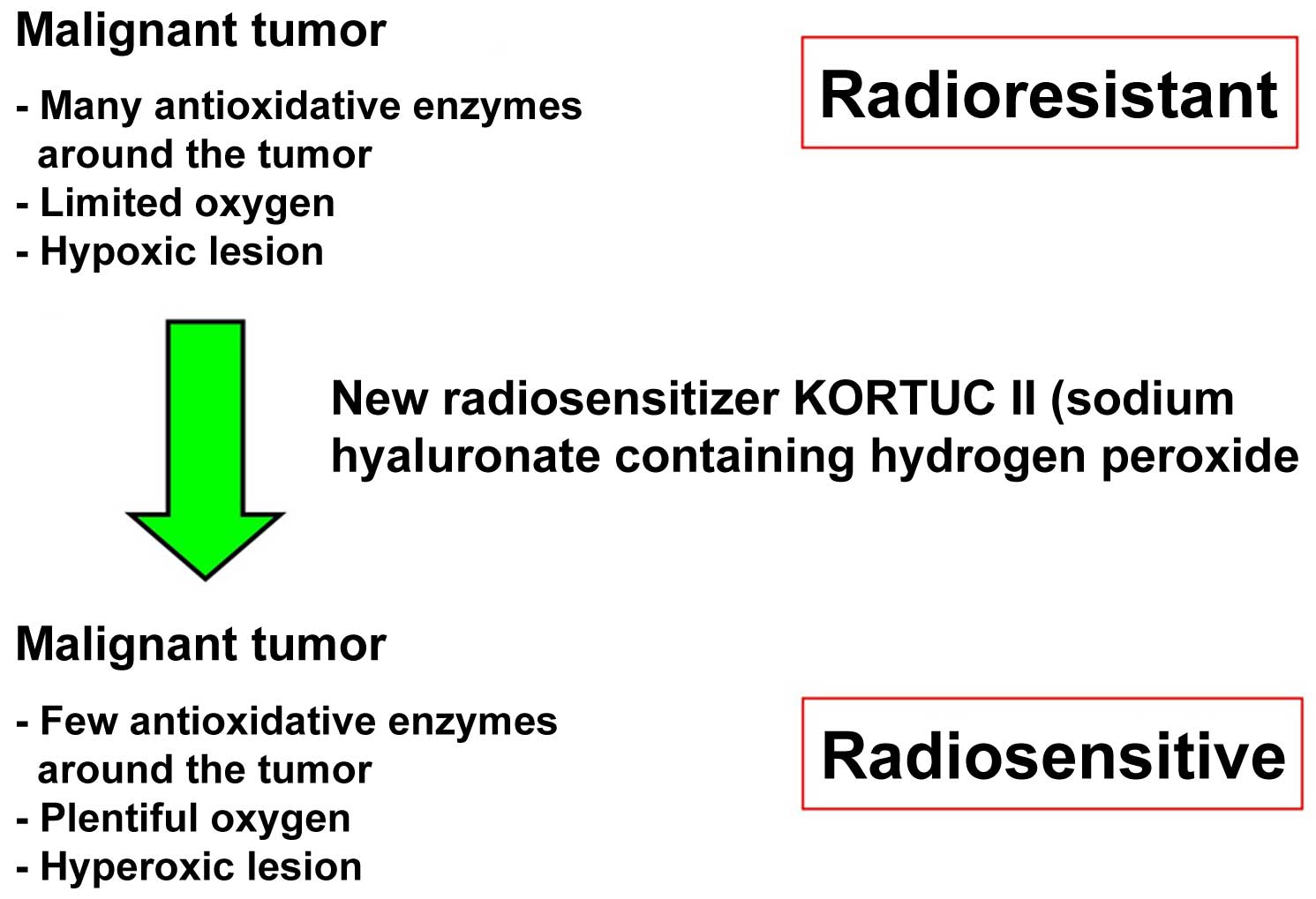
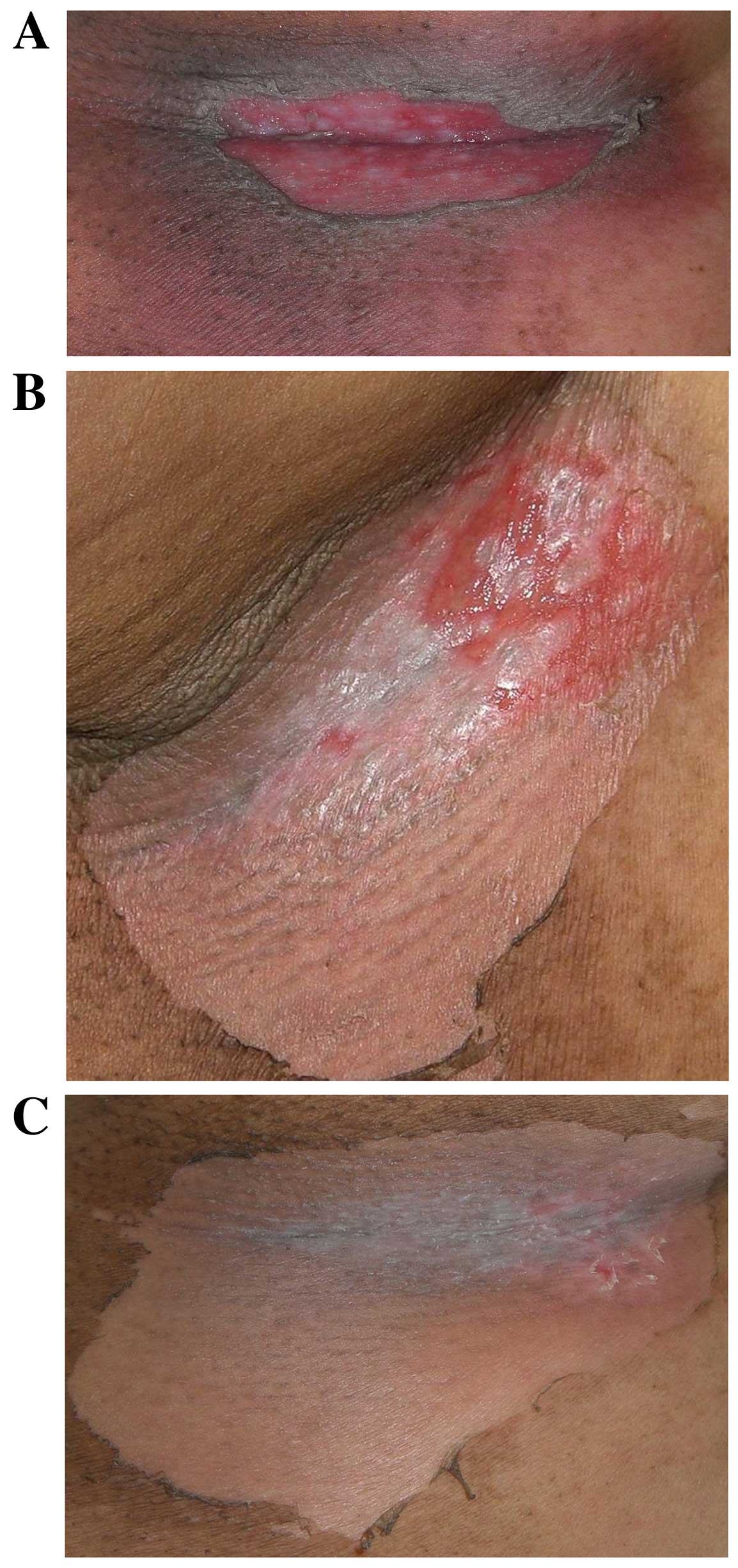
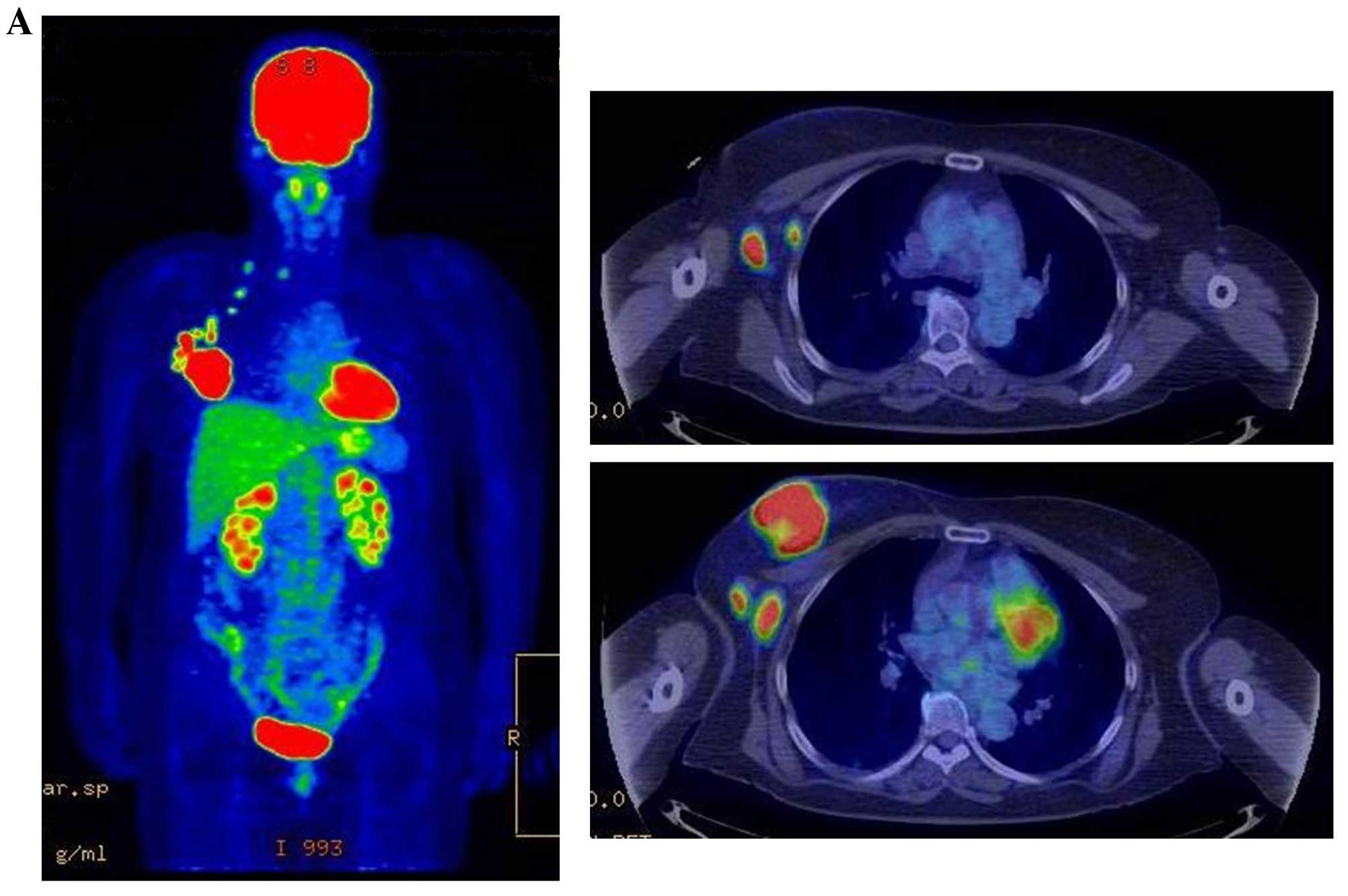
|
1
|
Ogawa Y, Kubota K, Ue H, Nishioka A,
Kariya S, Yokota N, Sasaki T, Suzuki K, Nakatani K, Yamanishi T, et
al: Development and clinical application of a new radio sensitizer
containing hydrogen peroxide and hyaluronic acid sodium for topical
tumor injection - a new enzyme-targeting radiosensitization
treatment, KORTUC II (Kochi Oxydol-Radiation Therapy for
Unresectable Carcinomas, Type II). Strahlenther Onkol. 183:100–101.
2007.
|
|
2
|
Ogawa Y, Ue H, Tsuzuki K, Tadokoro M,
Miyatake K, Sasaki T, Yokota N, Hamada N, Kariya S, Hitomi J, et
al: New radiosensitization treatment (KORTUC I) using hydrogen
peroxide solution-soaked gauze bolus for unresectable and
superficially exposed neoplasms. Oncol Rep. 19:1389–1394.
2008.PubMed/NCBI
|
|
3
|
Ogawa Y, Kubota K, Ue H, Kataoka Y,
Tadokoro M, Miyatake K, Tsuzuki K, Yamanishi T, Itoh S, Hitomi J,
et al: Phase I study of a new radiosensitizer containing hydrogen
peroxide and sodium hyaluronate for topical tumor injection: A new
enzyme-targeting radiosensitization treatment, Kochi
Oxydol-Radiation Therapy for Unresectable Carcinomas, Type II
(KORTUC II). Int J Oncol. 34:609–618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miyatake K, Kubota K, Ogawa Y, Hamada N,
Murata Y and Nishioka A: Non-surgical care for locally advanced
breast cancer: Radiologically assessed therapeutic outcome of a new
enzyme-targeting radiosensitization treatment, Kochi
Oxydol-Radiation Therapy for Unresectable Carcinomas, Type II
(KORTUC II) with systemic chemotherapy. Oncol Rep. 24:1161–1168.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tokuhiro S, Ogawa Y, Tsuzuki K, Akima R,
Ue H, Kariya S and Nishioka A: Development of a novel
enzyme-targeting radiosensitizer (KORTUC) containing hydrogen
peroxide for intratumoral injection for patients with low linear
energy transfer-radioresistant neoplasms. Oncol Lett. 1:1025–1028.
2010.PubMed/NCBI
|
|
6
|
Hitomi J, Kubota K, Ogawa Y, Hamada N,
Murata Y and Nishioka A: Non-surgical therapy and radiologic
assessment of stage I breast cancer treatment with novel
enzyme-targeting radiosensitization: Kochi Oxydol-Radiation Therapy
for Unresectable Carcinomas, type II (KORTUC II). Exp Ther Med.
1:769–775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogawa Y, Kubota K, Ue H, Tadokoro M,
Matsui R, Yamanishi T, Hamada N, Kariya S, Nishioka A, Nakajima H,
et al: Safety and effectiveness of a new enzyme-targeting
radiosensitization treatment (KORTC II) for intratumoral injection
for low-LET radioresistant tumors. Int J Oncol. 39:553–560.
2011.PubMed/NCBI
|
|
8
|
Tsuzuki A, Ogawa Y, Kubota K, Tokuhiro S,
Akima R, Yaogawa S, Itoh K, Yamada Y, Sasaki T, Onogawa M, et al:
Evaluation of changes in tumor shadows and Microcalcifications on
mammography following KORTUC II, a new radiosensitization treatment
without any surgical procedure for elderly patients with Stage I
and II breast cancer. Cancers (Basel). 3:3496–3505. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aoyama N, Ogawa Y, Kubota K, Ohgi K,
Kataoka Y, Miyatake K, Tadokoro M, Yamanishi T, Ohnishi T, Hamada
N, et al: Therapeutic response to a new enzyme-targeting
radiosensitization treatment (KORTUC-SC) for patients with
chemotherapy-resistant supraclavicular lymph node metastasis. J
Cancer Res Ther. 1:215–219. 2013. View Article : Google Scholar
|
|
10
|
Aoyama N, Ogawa Y, Kubota K, Yasuoka M,
Takahashi M, Iwasa H, Miyatake K, Yamanishi T, Hamada N, Tamura T,
et al: Therapeutic response to a novel enzyme-targeting
radiosensitization treatment (Kochi Oxydol-Radiation Therapy for
Unresectable Carcinomas) in patients with recurrent breast cancer.
Oncol Lett. 12:29–34. 2016.PubMed/NCBI
|
|
11
|
Danforth DN Jr, Zujewski J, O'Shaughnessy
J, Riseberg D, Steinberg SM, McAtee N, Noone M, Chow C, Chaudhry U,
Lippman M, et al: Selection of local therapy after neoadjuvant
chemotherapy in patients with Stage IIIA, B breast cancer. Ann Surg
Oncol. 5:150–158. 1988. View Article : Google Scholar
|
|
12
|
Lorvidhaya V, Kamnerdsupaphon P,
Chitapanarux I, Sukthomya V and Tonusin A: Cisplatin and
gemcitabine in patients with metastatic cervical cancer. Gan To
Kagaku Ryoho. 31:1057–1062. 2004.PubMed/NCBI
|
|
13
|
Hu XC, Zhang J, Xu BH, Cai L, Ragaz J,
Wang ZH, Wang BY, Teng YE, Tong ZS, Pan YY, et al: Cisplatin plus
gemcitabine versus paclitaxel plus gemcitabine as first-line
therapy for metastatic triple-negative breast cancer (CBCSG006): A
randomized, open-label, multicenter, phase 3 trial. Lancet Oncol.
16:436–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karasawa K, Saito M, Hirowatari H, Izawa
H, Furuya T, Ozawa S, Ito K, Suzuki T and Mitsuhashi N: The role of
chemoradiotherapy in patients with unresectable T4 breast tumors.
Breast Cancer. 20:254–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnson CJ, Graff R, Moran P, Cariou C and
Bordeaux S: Breast cancer stage, surgery, and survival statistics
for Idaho's national breast and cervical cancer early detection
program population, 2004–2012. Prev Chronic Dis. 12:E362015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mukai H, Watanabe T, Mitsumori M, Tsuda H,
Nakamura S, Masuda N, Yamamoto N, Shibata T, Sato A, Iwata H and
Aogi K: Final results of a safety and efficacy trial of
preoperative sequential chemoradiation therapy for the nonsurgical
treatment of early breast cancer: Japan Clinical Oncology Group
Study JCORG306. Oncology. 85:336–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yin Y, Zhang P, Xu BH, Zhang BL, Li Q,
Yuan P, Cai RG, Wang JY, Wang X and Xu XZ: Unfavorable pathological
complete response rate of neoadjuvant chemotherapy epirubicin plus
taxanes for locally advanced triple-negative breast cancer. J
Huazhong Univ Sci Technolog Med Sci. 33:262–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matuschek C, Bölke E, Roth S, Orth K, Lang
I, Bojar H, Janni JW, Audretsch W, Nestle-Kraemling C, Lammering G,
et al: Long-term outcome after neoadjuvant radiochemotherapy in
locally advanced noninflammatory breast cancer and predictive
factors for pathologic complete remission: Results of a
multivariate analysis. Strahlenther Onkol. 188:777–781. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Genet D, Lejeune C, Bonnier P, Aubard Y,
Venat-Bouvet L, Adjadj DJ, Martin J, Labourey JL, Benyoub A,
Clavère P, et al: Concomitant intensive chemoradiotherapy induction
in non-metastatic inflammatory breast cancer: Long-term follow-up.
Br J Cancer. 97:883–887. 2007.PubMed/NCBI
|
|
20
|
Bollet MA, Sigal-Zafrani B, Gambotti L,
Extra JM, Meunier M, Nos C, Dendale R, Campana F, Kirova YM, Diéras
V, et al: Pathological response to preoperative concurrent
chemo-radiotherapy for breast cancer: Result of phase II study. Eur
J Cancer. 42:2286–2295. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shaughnessy JN, Meena RA, Dunlap NE, Jain
D, Riley EC, Quillo AR and Dragun AE: Efficacy of concurrent
chemoradiotherapy for patients with locally recurrent or advanced
inoperable breast cancer. Clin Breast Cancer. 15:135–142. 2015.
View Article : Google Scholar : PubMed/NCBI
|