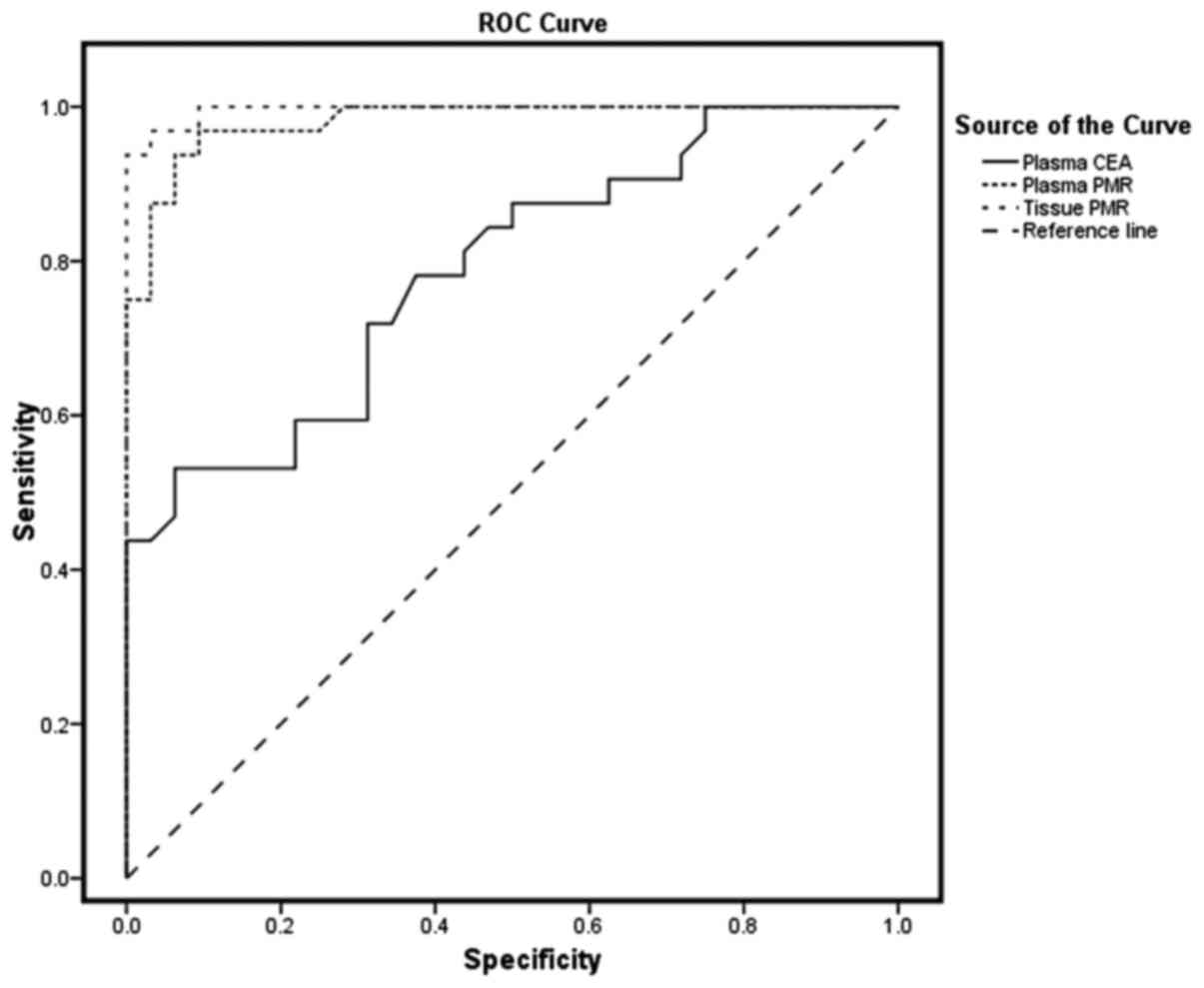
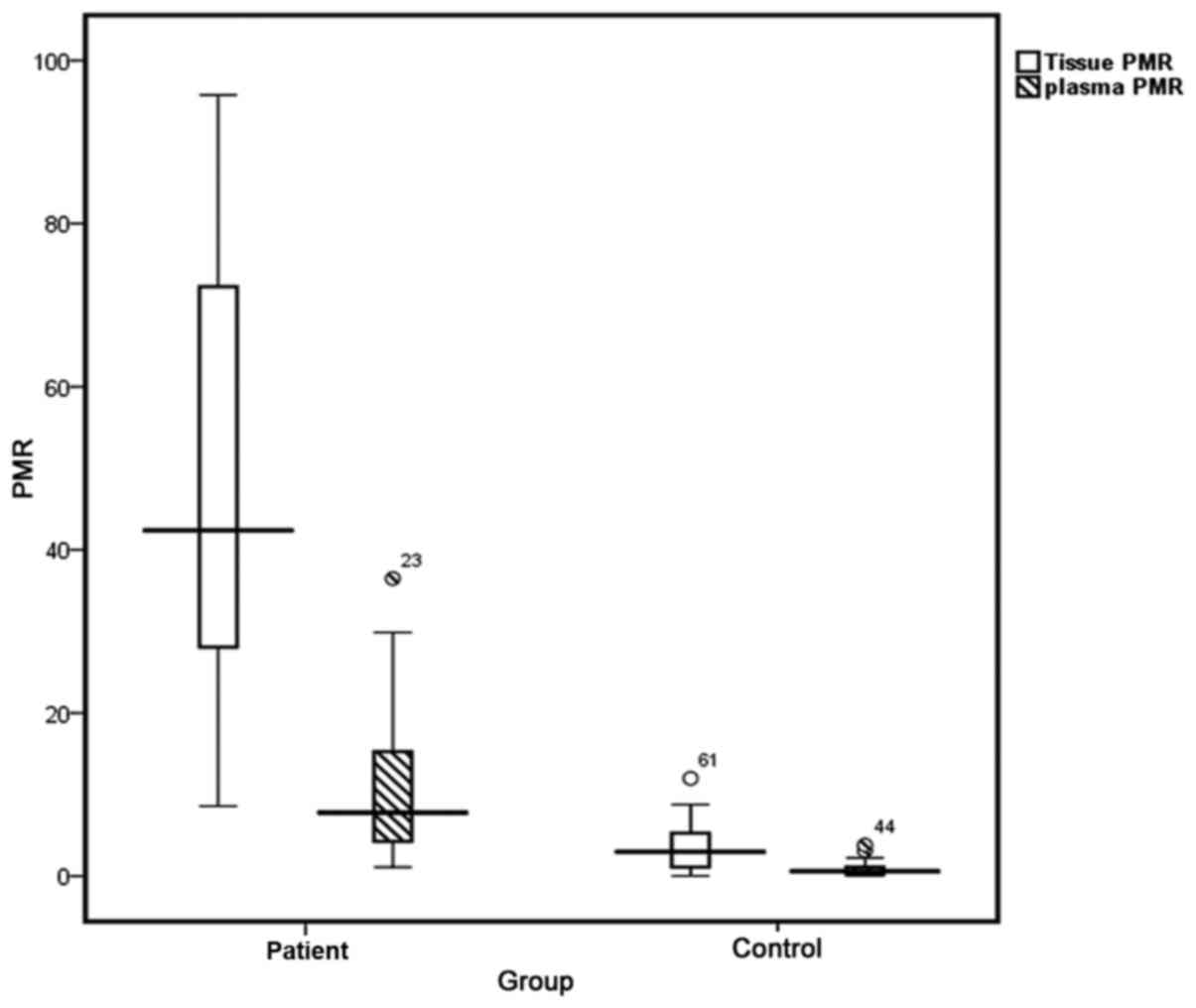
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pawa N, Arulampalam T and Norton JD:
Screening for colorectal cancer: Established and emerging
modalities. Nat Rev Gastroenterol Hepatol. 8:711–722. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stigliano V, Sanchez-Mete L, Martayan A
and Anti M: Early-onset colorectal cancer: A sporadic or inherited
disease? World J Gastroenterol. 20:12420–12430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohebbi M, Nourijelyani K, Mahmoudi M,
Moghadaszadeh B, Mohammad K, Zeraati H and Fotouhi A: Time of
occurrence and age distribution of digestive tract cancers in
northern Iran. Iranian J Publ Health. 37:8–19. 2008.
|
|
6
|
Smith RA, Cokkinides V and Eyre HJ:
American cancer society guidelines for the early detection of
cancer, 2006. CA Cancer J Clin. 56:11–25; quiz 49–50. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dawson MA and Kouzarides T: Cancer
epigenetics: From mechanism to therapy. Cell. 150:12–27. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Irizarry RA, Ladd-Acosta C, Wen B, Wu Z,
Montano C, Onyango P, Cui H, Gabo K, Rongione M, Webster M, et al:
The human colon cancer methylome shows similar hypo-and
hypermethylation at conserved tissue-specific CpG island shores.
Nat Genet. 41:178–186. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rakyan VK, Down TA, Balding DJ and Beck S:
Epigenome-wide association studies for common human diseases. Nat
Rev Genet. 12:529–541. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chan AO, Broaddus RR, Houlihan PS, Issa
JP, Hamilton SR and Rashid A: CpG island methylation in aberrant
crypt foci of the colorectum. Am J Pathol. 160:1823–1830. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fleischhacker M and Schmidt B: Circulating
nucleic acids (CNAs) and cancer-a survey. Biochim Biophys Acta.
1775:181–232. 2007.PubMed/NCBI
|
|
12
|
Shirahata A and Hibi K: Serum vimentin
methylation as a potential marker for colorectal cancer. Anticancer
Res. 34:4121–4125. 2014.PubMed/NCBI
|
|
13
|
deVos T, Tetzner R, Model F, Weiss G,
Schuster M, Distler J, Steiger KV, Grützmann R, Pilarsky C,
Habermann JK, et al: Circulating methylated SEPT9 DNA in plasma is
a biomarker for colorectal cancer. Clin Chem. 55:1337–1346. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herbst A, Rahmig K, Stieber P, Philipp A,
Jung A, Ofner A, Crispin A, Neumann J, Lamerz R and Kolligs FT:
Methylation of NEUROG1 in serum is a sensitive marker for the
detection of early colorectal cancer. Am J Gastroenterol.
106:1110–1118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee BB, Lee EJ, Jung EH, Chun HK, Chang
DK, Song SY, Park J and Kim DH: Aberrant methylation of APC, MGMT,
RASSF2A, and Wif-1 genes in plasma as a biomarker for early
detection of colorectal cancer. Clin Cancer Res. 15:6185–6191.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lind GE, Kleivi K, Meling GI, Teixeira MR,
Thiis-Evensen E, Rognum TO and Lothe RA: ADAMTS1, CRABP1, and NR3C1
identified as epigenetically deregulated genes in colorectal
tumorigenesis. Cell Oncol. 28:259–272. 2006.PubMed/NCBI
|
|
17
|
Lind GE, Danielsen SA, Ahlquist T, Merok
MA, Andresen K, Skotheim RI, Hektoen M, Rognum TO, Meling GI, Hoff
G, et al: Identification of an epigenetic biomarker panel with high
sensitivity and specificity for colorectal cancer and adenomas. Mol
Cancer. 10:852011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lind GE, Raiborg C, Danielsen SA, Rognum
TO, Thiis-Evensen E, Hoff G, Nesbakken A, Stenmark H and Lothe RA:
SPG20, a novel biomarker for early detection of colorectal cancer,
encodes a regulator of cytokinesis. Oncogene. 30:3967–3978. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bethge N, Lothe RA, Honne H, Andresen K,
Trøen G, Eknæs M, Liestøl K, Holte H, Delabie J, Smeland EB and
Lind GE: Colorectal cancer DNA methylation marker panel validated
with high performance in Non-Hodgkin lymphoma. Epigenetics.
9:428–436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bakowska JC, Jupille H, Fatheddin P,
Puertollano R and Blackstone C: Troyer syndrome protein spartin is
mono-ubiquitinated and functions in EGF receptor trafficking. Mol
Biol Cell. 18:1683–1692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eastman SW, Yassaee M and Bieniasz PD: A
role for ubiquitin ligases and Spartin/SPG20 in lipid droplet
turnover. J Cell Biol. 184:881–894. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsang HT, Edwards TL, Wang X, Connell JW,
Davies RJ, Durrington HJ, O'Kane CJ, Luzio JP and Reid E: The
hereditary spastic paraplegia proteins NIPA1, spastin and spartin
are inhibitors of mammalian BMP signalling. Hum Mol Genet.
18:3805–3821. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hooper C, Puttamadappa SS, Loring Z,
Shekhtman A and Bakowska JC: Spartin activates
atrophin-1-interacting protein 4 (AIP4) E3 ubiquitin ligase and
promotes ubiquitination of adipophilin on lipid droplets. BMC Biol.
8:722010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ciccarelli FD, Proukakis C, Patel H, Cross
H, Azam S, Patton MA, Bork P and Crosby AH: The identification of a
conserved domain in both spartin and spastin, mutated in hereditary
spastic paraplegia. Genomics. 81:437–441. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Connell JW, Lindon C, Luzio JP and Reid E:
Spastin couples microtubule severing to membrane traffic in
completion of cytokinesis and secretion. Traffic. 10:42–56. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sagona AP and Stenmark H: Cytokinesis and
cancer. FEBS Lett. 584:2652–2661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heresbach D, Manfredi S, D'Halluin PN,
Bretagne JF and Branger B: Review in depth and meta-analysis of
controlled trials on colorectal cancer screening by faecal occult
blood test. Eur J Gastroenterol Hepatol. 18:427–433. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lao VV and Grady WM: Epigenetics and
colorectal cancer. Nat Rev Gastroenterol Hepatol. 8:686–700. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bonetti Reggiani L, Barresi V, Bettelli S,
Domati F and Palmiere C: Poorly differentiated clusters (PDC) in
colorectal cancer: What is and ought to be known. Diagn Pathol.
11:312016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Susan JC, Harrison J, Paul CL and Frommer
M: High sensitivity mapping of methylated cytosines. Nucleic Acids
Res. 22:2990–2997. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grunau C, Clark SJ and Rosenthal A:
Bisulfite genomic sequencing: Systematic investigation of critical
experimental parameters. Nucleic Acids Res. 29:E65–E75. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clark SJ: Studying mammalian DNA
methylation: Bisulfite modification. CRC Press; Boca Raton: 2004,
View Article : Google Scholar
|
|
33
|
Ogino S, Kawasaki T, Brahmandam M, Cantor
M, Kirkner GJ, Spiegelman D, Makrigiorgos GM, Weisenberger DJ,
Laird PW, Loda M and Fuchs CS: Precision and performance
characteristics of bisulfite conversion and real-time PCR
(MethyLight) for quantitative DNA methylation analysis. J Mol
Diagn. 8:209–217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eads CA, Danenberg KD, Kawakami K, Saltz
LB, Blake C, Shibata D, Danenberg PV and Laird PW: MethyLight: A
high-throughput assay to measure DNA methylation. Nucleic Acids
Res. 28:E322000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Weisenberger DJ, Campan M, Long TI, Kim M,
Woods C, Fiala E, Ehrlich M and Laird PW: Analysis of repetitive
element DNA methylation by MethyLight. Nucleic Acids Res.
33:6823–6836. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jackson K, Yu MC, Arakawa K, Fiala E, Youn
B, Fiegl H, Müller-Holzner E, Widschwendter M and Ehrlich M: DNA
hypomethylation is prevalent even in low-grade breast cancers.
Cancer Biol Ther. 3:1225–1231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pfaffl MW: Quantification strategies in
real-time polymerase chain reactionApplied Microbiology. Filion M:
Caister Academic Press; Norfolk, UK: pp. 53–61. 2012
|
|
38
|
Widschwendter M, Siegmund KD, Müller HM,
Fiegl H, Marth C, Müller-Holzner E, Jones PA and Laird PW:
Association of breast cancer DNA methylation profiles with hormone
receptor status and response to tamoxifen. Cancer Res.
64:3807–3813. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Warren JD, Xiong W, Bunker AM, Vaughn CP,
Furtado LV, Roberts WL, Fang JC, Samowitz WS and Heichman KA:
Septin 9 methylated DNA is a sensitive and specific blood test for
colorectal cancer. BMC Med. 9:1332011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Grutzmann R, Molnar B, Pilarsky C,
Habermann JK, Schlag PM, Saeger HD, Miehlke S, Stolz T, Model F,
Roblick UJ, et al: Sensitive detection of colorectal cancer in
peripheral blood by septin 9 DNA methylation assay. PLoS One.
3:e37592008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kalmar A, Toth K, Wasserkort R, Sipos F,
Wichmann B, Valcz G, Tulassay Z and Molnar B: The detection of
methylated Septin 9 in tissue and plasma of colorectal neoplasia
and its relationship to the amount of free circulating DNA. Cancer
Res. 74:1850. 2014. View Article : Google Scholar
|
|
42
|
Leung WK, To KF, Man EP, Chan MW, Bai AH,
Hui AJ, Chan FK and Sung JJ: Quantitative detection of promoter
hypermethylation in multiple genes in the serum of patients with
colorectal cancer. Am J Gastroenterol. 100:2274–2279. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tan SH, Ida H, Lau QC, Goh BC, Chieng WS,
Loh M and Ito Y: Detection of promoter hypermethylation in serum
samples of cancer patients by methylation-specific polymerase chain
reaction for tumour suppressor genes including RUNX3. Oncol Rep.
18:1225–1230. 2007.PubMed/NCBI
|
|
44
|
Zhang H, Song YC and Dang CX: Detection of
hypermethylated spastic paraplegia-20 in stool samples of patients
with colorectal cancer. Int J Med Sci. 10:230–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yamashita K and Watanabe M: Clinical
significance of tumor markers and an emerging perspective on
colorectal cancer. Cancer Sci. 100:195–199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yamashita K, Waraya M, Kim MS, Sidransky
D, Katada N, Sato T, Nakamura T and Watanabe M: Detection of
methylated CDO1 in plasma of colorectal cancer; a PCR study. PLoS
One. 9:e1135462014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang SY, Lin M and Zhang HB: Diagnostic
value of carcinoembryonic antigen and carcinoma antigen 19-9 for
colorectal carcinoma. Int J Clin Exp Pathol. 8:9404–9409.
2015.PubMed/NCBI
|
|
48
|
Koga Y, Yamazaki N and Matsumura Y: Fecal
biomarker for colorectal cancer diagnosis. Rinsho Byori.
63:361–368. 2015.(In Japanese). PubMed/NCBI
|