Introduction
Papillary thyroid carcinoma is the most common type
of thyroid cancer, comprising ~80% of all thyroid epithelial
malignancies (1). Examination of
hematoxylin and eosin-stained tissue sections is considered to be
the gold standard for the differential diagnosis of thyroid
neoplasms (1). However, morphological
overlap is observed in certain cases, and the follicular variant of
papillary carcinoma (FVPC) is common (1). A number of critical characteristics of
this malignancy, including pale nuclei in papillary thyroid
carcinoma, are subjectively interpreted, and interobserver
variation among pathologists has been established (1). At present, an immunohistochemical panel
to address these challenges has not yet been developed, with a
limited contribution from the immunostaining markers galectin-3,
Hector Battifora mesothelial 1 (HBME-1) and cytokeratin-19 in
controversial follicular lesions (1,2).
Therefore, additional immunohistochemical and molecular methods
must be utilized.
Tight junctions (TJs) organize paracellular
permeability and have a critical function in apical cell-cell
adhesion and epithelial cell polarity (3). Numerous studies on the molecular
architecture of TJs have demonstrated that claudin protein family
members are important components of this structure (3). This family consists of 24 identified
members, each with a distinct distribution pattern (3,4). Claudins
1, 4, 5, 7, 8, 11, 14 and 19 are responsible for cell
impermeability claudins, and incremental increases in their
expression levels strengthens the density between epithelial cells
(5–8).
Claudins 2, 10 and 16 are pore-forming claudins, and their
increased expression levels reduce the density of epithelial cells
(9); other claudins possess the
ability to form paracellular anion/cation pores and water channels
(3,7).
Claudins 1, 4 and 7 are important members of the
claudin protein family. It has been demonstrated that their
expression levels are altered in numerous malignancies (10–30).
Follicular cells of the thyroid gland are arranged in a single
highly polarized layer and function as a barrier between the lumen
of the follicle, where thyroglobulin and thyroid hormones are
stored, and the extrafollicular space. Epithelial cell polarity and
follicular space entrenchment are due to the presence of tight
junctions (29). The present study
examined the expression levels of claudin 1, 4 and 7
immunohistochemically, and aimed to determine the role of the
expression of these TJ proteins in the differential diagnosis of
papillary carcinoma (PC) and follicular carcinoma (FC), follicular
adenoma (FA), dominant nodule of multinodular goiter (MNG-DN),
medullary carcinoma (MC) and anaplastic carcinoma (AC). The aim of
the current study was to identify the potential diagnostic role of
these markers in the follicular morphological mimics.
Materials and methods
Selection of patients
This retrospective study included 122 cases of
thyroid neoplasia that were histopathologically diagnosed using
thyroidectomy tissue at the University of Health Sciences, Antalya
Education and Research Hospital (Antalya, Turkey) between January
2010 and January 2014. The present study was performed using
pathologically stained tissue samples, and diagnostic values of the
claudins were evaluated. Therefore, the present study does not
include any prognostic or follow-up data. The study was approved by
the ethics committee of University of Health Sciences Antalya
Education and Research Hospital (#2017/024). Due to technical
reasons, 8 cases in which the immunohistochemical expression was
not eligible for evaluation were excluded. As a result, 114 cases
of thyroid neoplasia obtained from 90 female (79%) and 24 male
(21%) patients were enrolled into the present study. The average
age of patients was 44.50±13.60 years. Informed consent to use the
surgical specimens for scientific research was obtained from all
the patients. The expression levels of claudin 1, 4 and 7 were
examined in 29 FA, 18 MNG-DN, 47 PC, 10 FC, 5 MC and 5 AC cases
using immunohistochemical methods.
Tissue preparation and
immunohistochemical staining
Resection tissue samples were obtained following
thyroidectomy, placed in 10% formaldehyde immediately following the
procedure. Subsequently, pathologically sampled tumoral tissues
were embedded in paraffin. Immunohistochemical staining was applied
to resection tissue cross-sections containing nominal tumor samples
that were evaluated using hematoxylin and eosin staining. Briefly,
cross-sections of 4-µm thickness were prepared for
immunohistochemical staining by deparaffinization in an oven at
60°C for 2 h. Subsequently, tissue sections were immersed in xylene
for 30 min, gradient ethanol for 30 min (70% ethanol for 10 min,
96% ethanol for 10 min, 100% ethanol for 10 min used sequentially;
all steps were performed at room temperature) and washed with
distilled water. Next, the tissue sections were heated in a 10%
citrate buffer solution (#RE7113; Leica Microsystems, Inc., Milton
Keynes, UK) in the microwave at 800 W for 15 min and then at 400 W
for an additional 20 min. Tissue sections were allowed to cool at
room temperature for 20 min following heating. Endogenous
peroxidase activity was blocked with 3% hydrogen peroxide for 10
min. The tissue sections were incubated with primary antibodies
against claudin 1 (rabbit polyclonal; #ab15098; dilution, 1:200;
Abcam, Cambridge, MA, USA), claudin 4 (rabbit polyclonal; #ab15104;
dilution, 1:200; Abcam) and claudin 7 (rabbit polyclonal; #ab27487;
dilution, 1:200; Abcam) for 60 min at 30°C, and then washed with
PBS for 5 min at room temperature. The tissue sections were
subsequently incubated with Ready to Use Biotinylated
Goat-anti-rabbit Immunoglobulin secondary antibody (#BP-9100;
undiluted; Vector Laboratories, Burlingame, CA, USA) for 20 min at
30°C, washed with PBS for 5 min and incubated with the Peroxidase
Detection system Ready to Use conjugated antibody (#RE7110-K;
Novocastra; Leica Microsystems, Inc.) for 20 min at room
temperature. Tissue samples were then washed with PBS for 5 min,
incubated with chromogenic 3,3′-diaminobenzidine (Leica
Microsystems, Inc.) for 5 min, washed with tap water and
counterstained with hematoxylin; all steps were performed at room
temperature. The tissue samples were subsequently dehydrated in
100% ethanol, dried in an oven for 10 min at 60°C and mounted with
Entellan® mounting medium (Merck Millipore, Darmstadt,
Germany). The sections were visualized with a Nikon Eclipse Ci
Light microscope (Nikon Corporation, Tokyo, Japan). Positive
immunohistochemical staining of claudin 1, 4 and 7 in dermal
appendages from skin biopsies were used as a positive control,
whereas the primary antibodies were omitted for the negative
controls.
Evaluation of immunohistochemically
stained tissue sections
Claudin expression rates for the positive tumor
cells in the tissue specimens were independently evaluated by two
pathologists who were blinded to the patients' clinical features
and previous pathological diagnosis. Vascular structures,
fibroblasts, vessel endothelium, smooth-muscle cells of vessel
walls, lymphoid tissue, neural structures and adipocytes within the
cross-section exhibited no staining. The absence of claudin
expression in these non-epithelial structures was therefore used as
the negative internal control during immunohistochemical
evaluation. In the case of claudin expression, the staining was
membranous and accompanied by weak cytoplasmic staining. This
cytoplasmic weak staining was seen only in cases which had strong
membranous staining, therefore this cytoplasmic weak staining was
considered as non-specific and thus ignored; only the membranous
staining was evaluated. Claudin 1, 4 and 7 staining was assessed
using a previously described scoring method (10,11).
According to this method, cases exhibiting membranous Claudin 1, 4
and 7 staining in >5% of cells were considered positive. Claudin
1, 4 and 7 expression were scored as follows; 0, staining in <5%
of the cells; 1, staining in 5–25% of the cells; 2, staining in
26–50% of the cells; 3, staining in >50% of the cells. Examples
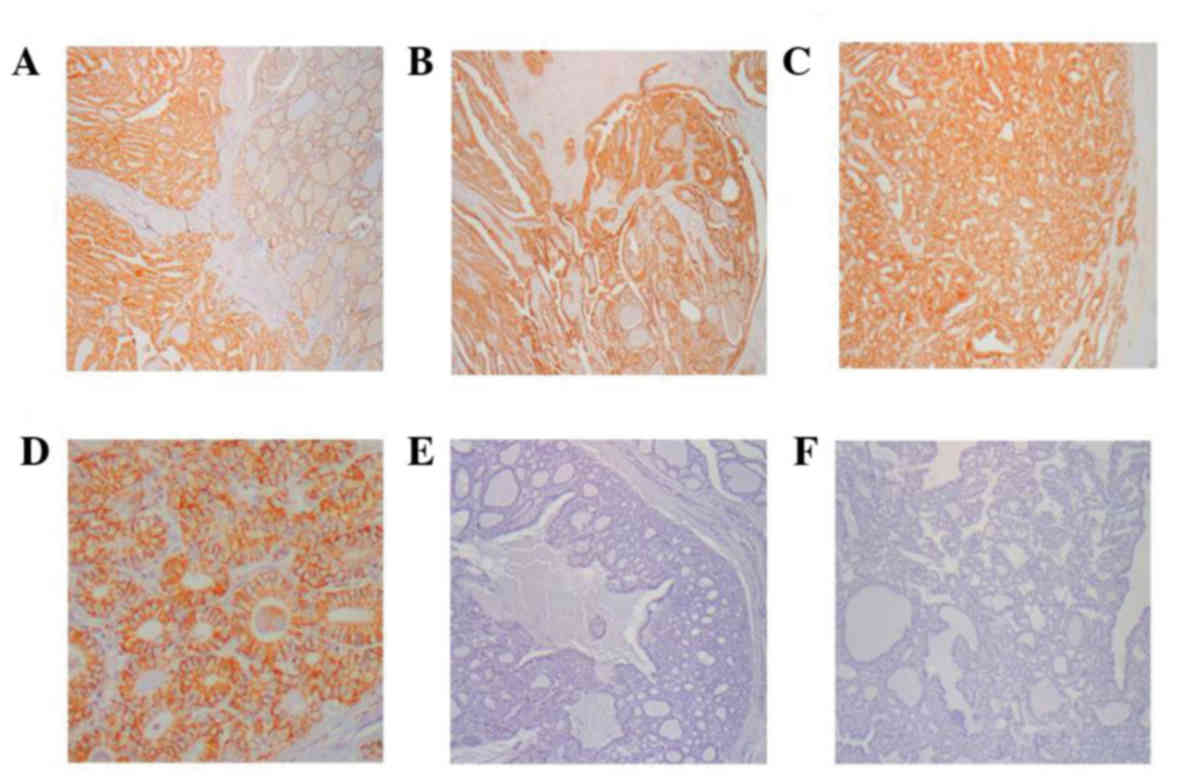
of immunohistochemical staining are presented in Fig. 1.
Statistical analysis
Statistical analyses were performed using SPSS
version 15.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Descriptive analyses were presented as the mean ± standard
deviation. The scores of claudin 1, 4 and 7 expression level in
benign and malignant neoplasms, as well as FA, MNG-DN, PC,
papillary microcarcinoma (PC-M) and FC, were identified.
Differences between the groups were analyzed using the
χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Histopathological distribution of
patients with thyroid neoplasm
A total of 114 patients were enrolled in the present
study, with benign thyroid neoplasm observed in 47 (41.0%) patients
and malignant thyroid neoplasm identified in 67 (59.0%) patients.
Benign thyroid neoplasms were present in 29 (62.0%) patients with
FA and 18 (8.1%) patients with MNG-DN. A total of 47 (70.0%)
patients with malignant thyroid neoplasms had PC and 14 (29.8%) PC
cases were PC-M.
Claudin 1 expression levels in
patients with thyroid neoplasms
Claudin 1 expression exhibited statistically
significant differences between malignant and benign thyroid
neoplasms (P<0.001; Table I).
Claudin 1 expression was detected in 6 (12.8%) benign cases, all of
which were FA, whereas no claudin 1 expression was observed in any
of the MNG-DN cases. A total of 49 (73.1%) malignant cases
exhibited positive claudin 1 expression. In 6/29 (20.7%) FA cases
and 1/10 (10.0%) FC cases, positive claudin 1 expression was
detected. No significant difference was identified in claudin 1
expression between FC and PC (P=0.653), whereas claudin 1
expression differed significantly between FC and PC (P<0.001).
In 9/10 (90.0%) FC cases, claudin 1 expression was not detected. In
32/33 (97.0%) non-microcarcinoma PC cases, and in all 14 PC-M
cases, claudin 1 expression was detected (Table II). No statistically significant
differences were identified between claudin 1 expression in PC-M
and in non-microcarcinoma PC (P=0.990). A total of 2/5 (40.0%) AC
cases and none of the 5 MC cases exhibited claudin 1
expression.
 | Table I.Comparison of claudin 1, 4 and 7
expression levels in malignant and benign lesions. |
Table I.
Comparison of claudin 1, 4 and 7
expression levels in malignant and benign lesions.
|
| Benign (n=47) | Malignant (n=67) |
|
|---|
|
|
|
|
|
|---|
| Claudin | Negative (%) | Positive (%) | Negative (%) | Positive (%) | P-value |
|---|
| Claudin 1 | 41 (87.2) | 6
(12.8) | 18 (26.9) | 49 (73.1) |
<0.001a |
| Claudin 4 | 37 (78.7) | 10 (21.3) | 42 (62.7) | 25 (37.3) |
0.068 |
| Claudin 7 | 1 (2.1) | 46 (97.9) | 6 (9.0) | 61 (91.0) |
0.135 |
 | Table II.Comparison of claudin 1, 4 and 7
expression levels in PC and FC. |
Table II.
Comparison of claudin 1, 4 and 7
expression levels in PC and FC.
|
| FC (n=10) | PC (n=33) |
|
|---|
|
|
|
|
|
|---|
| Claudin | Negative (%) | Positive (%) | Negative (%) | Positive (%) | P-value |
|---|
| Claudin 1 | 9 (90.0) | 1 (10.0) | 1 (3.0) | 32 (97.0) |
<0.001a |
| Claudin 4 | 7 (70.0) | 3 (30.0) | 18 (54.5) | 15 (45.5) |
0.480 |
| Claudin 7 | 0 (0.0) | 10 (100.0) | 0 (0.0) | 33 (100.0) |
1.000 |
Claudin 4 expression levels in
patients with thyroid neoplasms
No significant differences in claudin 4 expression
were observed between malignant and benign thyroid neoplasms
(P=0.068; Table I). A total of 10/47
(21.3%) benign thyroid neoplasms exhibited claudin 4 expression, of
which 3 cases were FA and the remaining were MNG-DN. A total of
25/67 (37.3%) malignant cases exhibited claudin 4 expression. In
3/29 (10.3%) FA cases, 7/18 (39.0%) MNG-DN cases and 3/10 (30.0%)
FC cases, claudin 4 expression was positive. No significant
differences in claudin 4 expression were identified among MNG-DN,
FA and FC groups (P=0.502). A total of 15/33 (45.5%) PC cases
exhibited claudin 4 expression, whilst no statistically significant
differences in claudin 4 expression were observed between PC and FC
(P=0.480; Table II). In addition,
claudin 4 expression was detected in 7/14 (50.0%) PC-M cases, and
no significant differences in claudin 4 expression were identified
between PC-M and non-microcarcinoma PC cases (P=0.775). No cases of
AC or MC exhibited claudin 4 expression.
Claudin 7 expression levels in
patients with thyroid neoplasms
Claudin 7 expression was identified in 107 (94%) of
the 114 analyzed cases. No significant differences in claudin 7
expression were observed between malignant and benign thyroid
neoplasms (P=0.135; Table I).
Negative claudin 7 expression was observed in 1/47 (2.1%) benign
cases and 6/67 (9.0%) malignant cases (Table I). The single negative benign case was
FA, and the 6 malignant cases were as follows: 1 PC-M, 1 AC and 4
MC. All 10 FC cases exhibited claudin 7 expression. No
statistically significant differences in claudin 7 expression were
identified among MNG-DN, FA and FC groups (P=0.470). FC and PC did
not exhibit any statistically significant differences, and all
cases in these two groups were positive for claudin 7 expression
(Table II). No significant
differences in claudin 7 expression were observed between PC-M and
PC cases (P=0.298), whereas 1/5 (20%) AC cases and 4/5 (80%) MC
cases exhibited claudin 7 expression.
Discussion
The diagnostic gold standard for the pathological
evaluation of thyroid nodules is hematoxylin and eosin staining
(1). However, morphological overlaps
between MNG-DN, FA and FC, as well as between PC and FC, are
common. In these cases, an objective consistent diagnosis based
solely on morphological assessment is occasionally impossible
(10). At present, no routine
immunohistochemical panel is in use to overcome these morphological
overlaps. Immunohistochemically, galectin-3, HBME-1 and
cytokeratin-19 provide a limited contribution to the assessment of
controversial neoplasms (1,2).
TJs are crucial intracellular joints in endothelial
cells and the epithelium (3). There
are two important functions that have been determined for TJs:
Paracellular permeability regulation and the maintenance of cell
polarization with window function (3,5,7,8). The
functions of TJs that are associated with cancer-cell biology
include epithelial paracellular permeability and the loss of cell
polarization (12,13). Claudin overexpression or loss of
expression varies depending on the type of cancer (3,14–16). In hepatocellular and renal cell
carcinoma, claudin 4 and 5 expression ceases, whereas claudin 3 and
4 overexpression is detected in various types of cancer, including
pancreatic ductal adenocarcinoma, and bladder, uterus, ovary and
breast cancer (3,14–16). A low
level of claudin 2 expression has been detected in breast and
bladder carcinomas, whereas claudin 1 and 7 expression, which is
not possible to detect in normal cervical squamous epithelium, is
increased in cervical neoplasia (17). Previous studies have revealed that
claudin 1 and 4 are overexpressed in nasopharyngeal carcinoma,
while claudin 7 is overexpressed in pancreatic ductal
adenocarcinoma (18,19).
The loss of claudin expression leads to the
suppression of TJ functions and serves a role in carcinogenesis by
inducing cancer cell proliferation, motility and invasiveness
(8). A previous study on
nanofibrillated cellulose (NFC) cell culture demonstrated that
claudin 1 expression levels were increased along with decreased
apoptosis in NFC cell lines following fluorouracil treatment
(20). Claudin 1 interacts with the
TJ protein zonula occludens 1 and affects other signaling pathways,
resulting in neoplastic transformation (21). In addition, a previous study
demonstrated that increased levels of claudin 1 expression prevent
NFC cell apoptosis (20). A possible
underlying mechanism for the role of claudins in neoplastic
transformation may occur via matrix metalloproteinases (MMPs). The
upregulation of claudin 1 expression levels in oral squamous cell
carcinoma enhances invasion via the activation of MMP-2 and MMP-1,
and the overexpression of claudins 3 and 4 in ovarian surface
epithelial cells promotes invasion by increasing MMP-2 activity
(22,23). Claudins may also promote neoplastic
transformation through their mixing ratios, as the barrier function
of TJs is controlled by a specific combination of claudins
(8). This hypothesis is concordant
with the observation that upregulated claudin 2 decreases the
tightness of TJ strands in Madin-Darby canine kidney cells, with
resultant cell leakage (24). In
invasive ductal carcinomas, as well as in head and neck and
metastatic breast cancers, claudin 7 expression has been observed
to be decreased (25–27), and lower expression levels of claudin
1, 4 and 7 have been detected in colorectal carcinoma (28).
Claudin expression levels in thyroid neoplasm have
been examined in relatively few studies. Abd El Atti and Shash
(11) examined claudin 1 expression
in PC, hyperplastic nodule and FA, but not in FC, MNG-DN, MC or AC,
identifying statistically significant positive expression of
claudin 1 in PC cases, as compared with hyperplastic nodule and FA
(11). Concordantly, the present
study detected claudin 1 expression in all cases but one; however,
claudin 1 expression in FA varied between Abd El Atti et al
(11) and the present study, despite
membranous staining in >5% of the neoplastic cells being
considered positive in each study. Claudin 1 expression was
identified in 75% of FA cases in the previous study (11), and in 20.7% of cases during the
current study. It was hypothesized that variation in the antibody
clones used for the immunohistochemical staining may underlie these
discrepancies between the study results. Tzelepi et al
(29) investigated the expression
levels of claudin 1, 4 and 7 in various thyroid neoplasms,
identifying claudin 1 expression in 15% of FA cases. This result,
as well as data from Hucz et al (30) on claudin 1 expression in FA, is
concordant with the results of the present study. Tzelepi et
al (29) also detected claudin 4
expression in 85% of FA cases, whereas this proportion was 10.3% in
the current study. In addition, this previous study identified
claudin 1, 4 and 7 expression to be 67, 80 and 33%, respectively,
in the FC group (29), whereas
proportions of 10, 30 and 100%, respectively, were observed in the
current study. Although the cut-off values for positive expression
were equal in the two studies, differences in the antibody clones
used for immunohistochemistry may again be hypothesized to have
produced the variation in the results of these studies. Due to this
inconsistency in FA and FC cases, further studies and larger sample
sizes are required. Tzelepi et al (29) detected similar claudin 1 expression
trends in PC cases, with higher rates of claudin 4 expression (88%)
and slightly lower rates of claudin 7 expression (73%) in PC cases,
as compared with the current study. The authors also identified the
expression rates of claudin 1, 4 and 7 to be 25, 25 and 13%,
respectively, in 8 AC cases, whereas the current study identified
these rates to be 40, 0 and 20%, respectively. Tzelepi et al
(29) identified claudin 1, 4 and 7
expression in 12.5, 87.5 and 25% of 8 MC cases, respectively,
whereas the present study observed these rates to be 0, 0 and 80%,
respectively. The low number of AC and MC cases are considered to
be a limitation of these two studies.
In conclusion, claudin 1 may be a useful
immunohistochemical marker in histopathologically overlapping PC
and FC cases, favoring a PC diagnosis, as well as aiding the
subtyping of thyroid carcinoma. Further studies with larger sample
sizes are required in order to clarify the diagnostic utility of
claudin expression levels in differentiating between FA and FC.
References
|
1
|
Prasad ML, Pellegata NS, Huang Y, Nagraga
HN, De la chapelle A and Kloos RT: Galectin-3, Fibronectin-1, CI
TED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful for
the differential diagnosis of thyroid tumors. Mod Pathol. 18:48–57.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beesley MF and Mclaren KM: Cytokeratin-19,
galectin-3 immunohistochemistry in the differential diagnosis of
solitary thyroid nodule. Histopathology. 41:236–243. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsukita S, Furuse M and Itoh M:
Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol.
2:285–293. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rahner C, Mitic LL and Anderson JM:
Heterogenity in expression and subcellular localization of Claudins
2, 3, 4 and 5 in the rat liver, pancreas, and gut.
Gastroenterology. 120:411–422. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krause G, Winkler L, Mueller SL, Haseloff
RF, Piontek J and Blasig IE: Structure and function of claudins.
Biochim Biophys Acta. 1778:631–645. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ouban A and Ahmed AA: Claudins in human
cancer: A review. Histol Histopathol. 25:83–90. 2010.PubMed/NCBI
|
|
7
|
Will C, Fromm M and Müller D: Claudin
tight junction proteins: Novel aspects in paracellular transport.
Perit Dial Int. 28:577–584. 2008.PubMed/NCBI
|
|
8
|
Oliveira SS and Morgado-Diaz JA: Claudins:
Multifunctional players in epithelial tight junctions and their
role in cancer. Cell Mol Life Sci. 64:17–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bornholdt J, Friis S, Godiksen S, Poulsen
SS, Santoni-Rugiu E, Bisgaard HC, Lothe IM, Ikdahl T, Tveit KM,
Johnson E, et al: The level of claudin-7 is reduced as an early
event in colorectal carcinogenesis. BMC Cancer. 11:652011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Németh J, Németh Z, Tátrai P, Péter I,
Somorácz A, Szász AM, Kiss A and Schaff Z: High expression of
claudin-1 protein in papillary thyroid tumor and its regional lymph
node metastasis. Pathol Oncol Res. 16:19–27. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abd El Atti RM and Shash LS: Potential
diagnostic utility of CD56 and claudin-1 in papillary thyroid
carcinoma and solitary follicular thyroid nodules. J Egypt Natl
Canc Inst. 24:175–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martin TA and Jiang WG: Loss of tight
junction barrier function and its role in cancer metastasis.
Biochim Biophys Acta. 4:872–891. 2009. View Article : Google Scholar
|
|
13
|
Miyoshi J and Takai Y: Molecular
perspective on tight-junction assembly and epithelial polarity. Adv
Drug Deliv Rev. 57:815–855. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Michl P, Barth C, Buchholz M, Lerch MM,
Rolke M, Holzmann KH, Menke A, Fensterer H, Giehl K, Löhr M, et al:
Claudin-4 expression decreases invasiveness and metastatic
potential of pancreatic cancer. Cancer Res. 63:6265–6271.
2003.PubMed/NCBI
|
|
15
|
Rangel LB, Agarwal R, D'Souza T, Pizer ES,
Alò PL, Lancaster WD, Gregoire L, Schwartz DR, Cho KR and Morin PJ:
Tight junction proteins claudin-3 and claudin-4 are frequently
overexpressed in ovarian cancer but not in ovarian cystadenomas.
Clin Cancer Res. 9:2567–2575. 2003.PubMed/NCBI
|
|
16
|
Soini Y: Expression of claudins 1, 2, 3,
4, 5 and 7 in various types of tumours. Histopathology. 46:551–560.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JW, Lee SJ, Seo J, Song SY, Ahn G,
Park CS, Lee JH, Kim BG and Bae DS: Increased expressions of
claudin-1 and claudin-7 during the progression of cervical
neoplasia. Gynecol Oncol. 97:53–59. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suren D, Yildirim M, Kaya V, Elal R,
Selcuk OT, Osma U, Yildiz M, Gunduz S and Sezer C: The expression
patterns of claudin 1, 4, and 7 and their prognostic significance
in nasopharyngeal carcinoma. J BUON. 20:212–217. 2015.PubMed/NCBI
|
|
19
|
Alikanoglu AS, Gunduz S, Demirpence O,
Suren D, Gunduz UR, Sezer C, Yildiz M and Yildirim M: Expression
pattern and prognostic significance of claudin 1, 4 and 7 in
pancreatic cancer. Asian Pac J Cancer Prev. 16:4387–4392. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JW, Hsiao WT, Chen HY, Hsu LP, Chen
PR, Lin MD, Chiu SJ, Shih WL and Hsu YC: Upregulated claudin-1
expression confers resistance to cell death of nasopharyngeal
carcinoma cells. Int J Cancer. 126:1353–1366. 2010.PubMed/NCBI
|
|
21
|
Resnick MB, Konkin T, Routhier J, Sabo E
and Pricolo VE: Claudin-1 is a strong prognostic indicator in stage
II colonic cancer: A tissue microarray study. Mod Pathol.
18:511–518. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oku N, Sasabe E, Ueta E, Yamamoto T and
Osaki T: Tight junction protein claudin-1 enhances the invasive
activity of oral squamous cell carcinoma cells by promoting
cleavage of laminin-5 gamma2 chain via matrix metalloproteinase
(MMP)-2 and membrane-type MMP-1. Cancer Res. 66:5251–5255. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Agarwal R, D'Souza T and Morin PJ:
Claudin-3 and claudin-4 expression in ovarian epithelial cells
enhances invasion and is associated with increased matrix
metalloproteinase-2 activity. Cancer Res. 65:7378–7385. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Furuse M, Furuse K, Sasaki H and Tsukita
S: Conversion of zonulae occludentes from tight to leaky strand
type by introducing claudin-2 into Madin-Darby canine kidney I
cells. J Cell Biol. 153:263–272. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kominsky SL, Argani P, Korz D, Evron E,
Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP and Sukumar S:
Loss of the tight junction protein claudin-7 correlates with
histological grade in both ductal carcinoma in situ and invasive
ductal carcinoma of the breast. Oncogene. 22:2021–2033. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Usami Y, Chiba H, Nakayama F, Ueda J,
Matsuda Y, Sawada N, Komori T, Ito A and Yokozaki H: Reduced
expression of claudin-7 correlates with invasion and metastasis in
squamous cell carcinoma of the esophagus. Hum Pathol. 37:569–577.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sauer T, Pedersen MK, Ebeltoft K and Næss
O: Reduced expression of claudin-7 in fine needle aspirates from
breast carcinomas correlate with grading and metastatic disease.
Cytopathology. 16:193–198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Süren D, Yıldırım M, Kaya V, Alikanoğlu
AS, Bülbüller N, Yıldız M and Sezer C: Loss of tight junction
proteins (Claudin 1, 4, and 7) correlates with aggressive behavior
in colorectal carcinoma. Med Sci Monit. 20:1255–1262. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tzelepi VN, Tsamandas AC, Vlotinou HD,
Vagianos CE and Scopa CD: Tight junctions in thyroid
carcinogenesis: Diverse expression of claudin-1, claudin-4,
claudin-7 and occludin in thyroid neoplasms. Mod Pathol. 21:22–30.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hucz J, Kowalska M, Jarzab M and Wiench M:
Gene expression of metalloproteinase 11, claudin 1 and selected
adhesion related genes in papillary thyroid cancer. Endokrynol Pol.
57:(Suppl A). S18–S25. 2006.(In Polish).
|