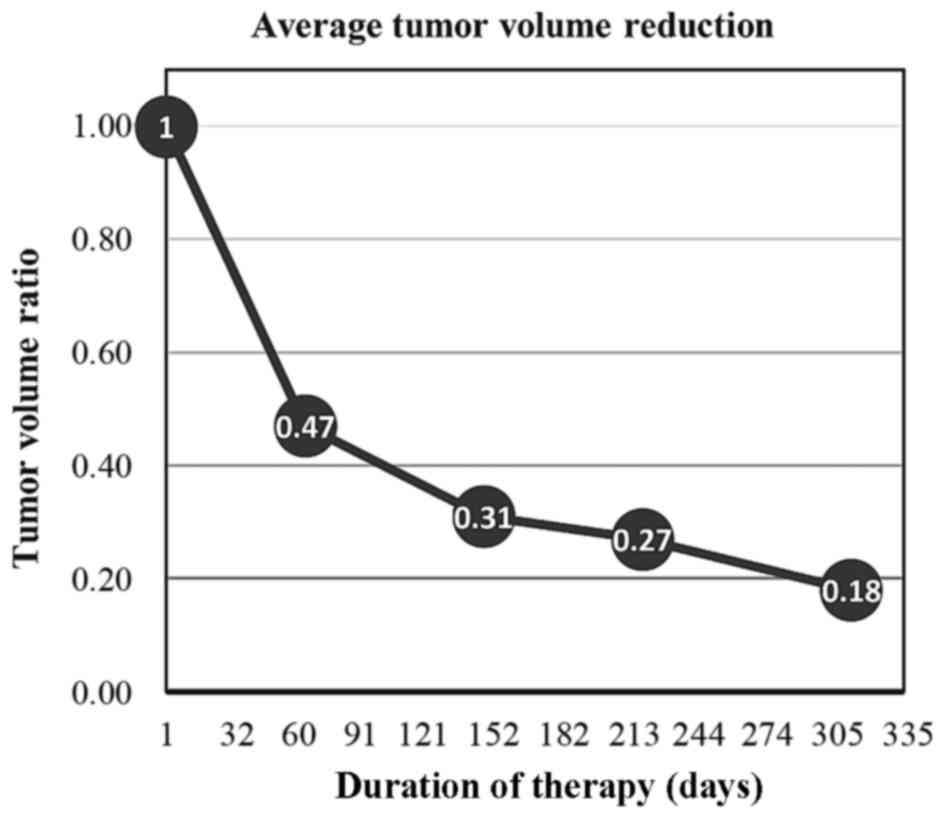
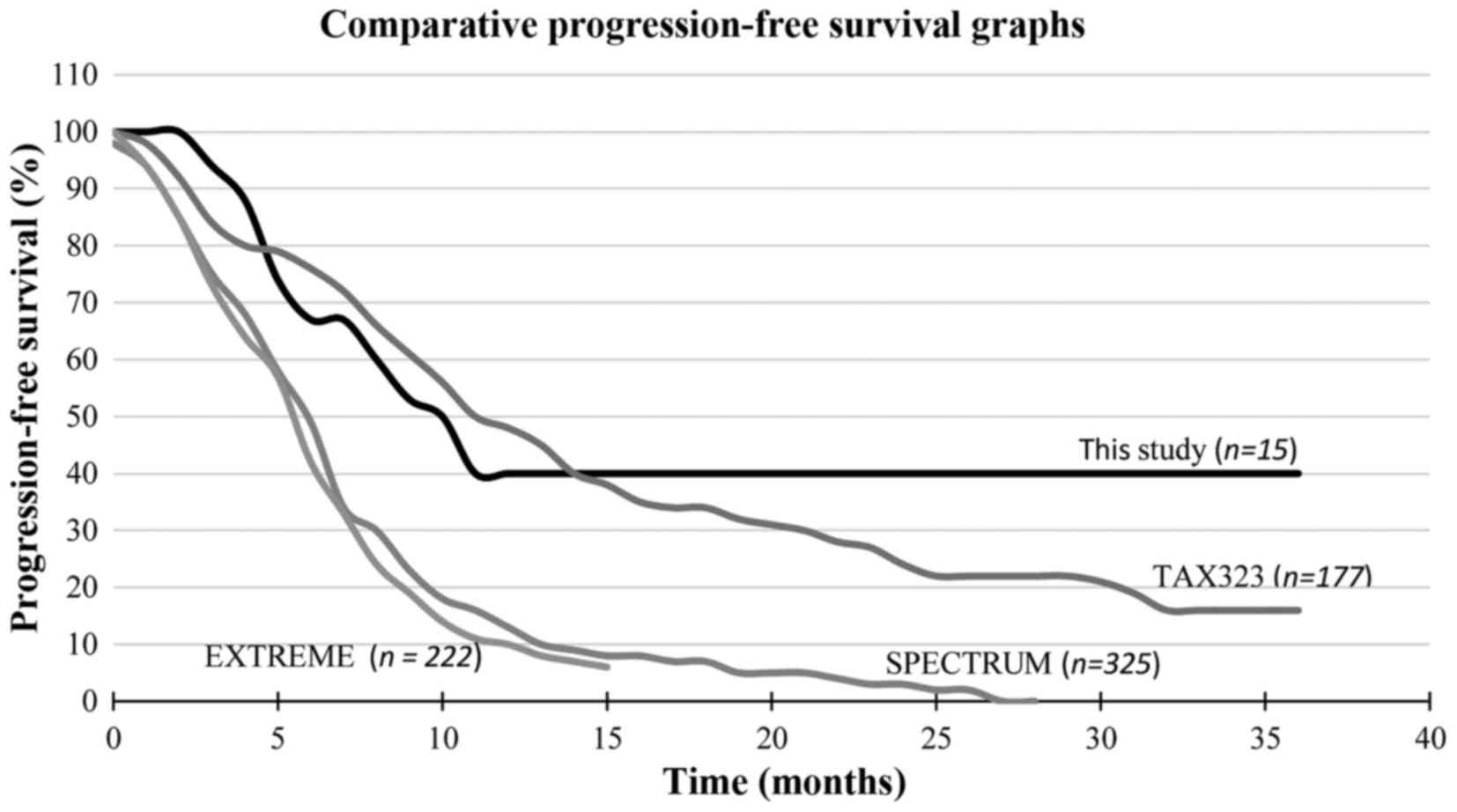
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Digonnet A, Hamoir M, Andry G, Haigentz M
Jr, Takes RP, Silver CE, Hartl DM, Strojan P, Rinaldo A, de Bree R,
et al: Post-therapeutic surveillance strategies in head and neck
squamous cell carcinoma. Eur Arch Otorhinolaryngol. 270:15692013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sanderson RJ and Ironside JAD: Squamous
cell carcinomas of the head and neck. BMJ. 325:822–827. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pulte D and Brenner H: Changes in survival
in head and neck cancers in the Late 20th and early 21st century: A
period analysis. Oncologist. 15:994–1001. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Molin Y and Fayette J: Current
chemotherapies for recurrent/metastatic head and neck cancer.
Anticancer Drugs. 22:621–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carvalho AL, Nishimoto IN, Califano JA and
Kowalski LP: Trends in incidence and prognosis for head and neck
cancer in the United States: A site-specific analysis of the SEER
database. Int J Cancer. 114:806–816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Péron J, Polivka V, Chabaud S, Poupart M,
Ceruse P, Ramade A, Girodet D, Zrounba P and Fayette J: An
effective and well-tolerated strategy in recurrent and/or
metastatic head and neck cancer: Successive lines of active
chemotherapeutic agents. BMC Cancer. 14:5042014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Posner MR: Docetaxel in squamous cell
cancer of the head and neck. Anticancer Drugs. 12 Suppl 1:S21–S24.
2001.PubMed/NCBI
|
|
9
|
Vermorken JB, Remenar E, van Herpen C,
Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss
JH, et al: Cisplatin, fluorouracil, and docetaxel in unresectable
head and neck cancer. N Engl J Med. 357:1695–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blanchard P, Bourhis J, Lacas B, Posner
MR, Vermorken JB, Hernandez JJ, Bourredjem A, Calais G, Paccagnella
A, Hitt R, et al: Taxane-cisplatin-fluorouracil as induction
chemotherapy in locally advanced head and neck cancers: An
individual patient data meta-analysis of the meta-analysis of
chemotherapy in head and neck cancer group. J Clin Oncol.
31:2854–2860. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guigay J, Fayette J, Dillies AF, Sire C,
Kerger JN, Tennevet I, Machiels JP, Zanetta S, Pointreau Y, Le Moal
L Bozec, et al: Cetuximab, docetaxel, and cisplatin as first-line
treatment in patients with recurrent or metastatic head and neck
squamous cell carcinoma: A multicenter, phase II GORTEC study. Ann
Oncol. 26:1941–1947. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Mello RA, Gerós S, Alves MP, Moreira F,
Avezedo I and Dinis J: Cetuximab plus platinum-based chemotherapy
in head and neck squamous cell carcinoma: A retrospective study in
a single comprehensive European cancer institution. PLoS One.
9:e866972014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vermorken JB, Stöhlmacher-Williams J,
Davidenko I, Licitra L, Winquist E, Villanueva C, Foa P, Rottey S,
Skladowski K, Tahara M, et al: Cisplatin and fluorouracil with or
without panitumumab in patients with recurrent or metastatic
squamous-cell carcinoma of the head and neck (SPECTRUM): An
open-label phase 3 randomised trial. Lancet Oncol. 14:697–710.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goldstein NE, Genden E and Morrison RS:
Palliative care for patients with head and neck cancer: ‘I would
like a quick return to a normal lifestyle’. JAMA. 299:1818–1825.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perri F, Muto P, Argenone A, Ionna F,
Longo F, Fulciniti F, Sandomenico F, Daponte A and Caponigro F:
Induction chemotherapy with docetaxel, cisplatin and capecitabine,
followed by combined cetuximab and radiotherapy in patients with
locally advanced inoperable squamous cell carcinoma of the head and
neck: A phase I–II study. Oncology. 84:251–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Felice F, Musio D and Tombolini V: Head
and neck cancer: Metronomic chemotherapy. BMC Cancer. 15:6772015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rangaraju RR, Sharma JB, Dewan AK, Anand
AK, Sheh R, Jena A and Chaturvedi AK: Palliative weekly
chemotherapy along with cetuximab in recurrent and metastatic head
and neck cancers: A retrospective analysis. Indian J Cancer.
49:1–5. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yossi S, Linot B, Peyraga G, Breheret R,
Laccourreye L and Capitain O: Feasibility and safety of dose-dense
modified docetaxel-cisplatin or carboplatin and 5-fluorouracil
regimen (mTPF) in locally advanced or metastatic head and neck
cancers: A retrospective monocentric study. Int J Clin Oncol.
20:1086–1092. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schena M, Barone C, Birocco N, Dongiovanni
D, Numico G, Colantonio I and Bertetto O: Weekly cisplatin
paclitaxel and continuous infusion fluorouracil in patients with
recurrent and/or metastatic head and neck squamous cell carcinoma:
A phase II study. Cancer Chemother Pharmacol. 55:271–276. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guntinas-Lichius O, Appenrodt S, Veelken F
and Krug B: Phase II study of weekly docetaxel and cisplatin in
patients with advanced recurrent and metastatic head and neck
cancer. Laryngoscope. 116:613–618. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho H, Nishiike S, Yamamoto Y, Takenaka Y,
Nakahara S, Yasui T, Hanamoto A and Inohara H: Docetaxel,
cisplatin, and fluorouracil for patients with inoperable recurrent
or metastatic head and neck squamous cell carcinoma. Auris Nasus
Larynx. 42:396–400. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin JT, Lai GM, Chang TH, Liu MT, Bi CP,
Wang JW and Chen MK: Chemotherapy with modified docetaxel,
cisplatin, and 5-fluorouracil in patients with metastatic head and
neck cancer. Adv Ther. 29:71–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Forastiere AA, Takasugi BJ, Baker SR, Wolf
GT and Kudla-Hatch V: High-dose cisplatin in advanced head and neck
cancer. Cancer Chemother Pharmacol. 19:155–158. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mantovani G, Ghiani M, Lai P, Maccio A,
Dessi D, Succu G, Massa D, Curreli L, Mulas C, Esu S, et al:
Clinical evaluation of two dosages and schedules of ifosfamide in
combination with cisplatin in neo-adjuvant chemotherapy of patients
with advanced (stage III–IV) head and neck squamous cell carcinoma:
A phase II randomized study. Oncol Rep. 5:1499–1505.
1998.PubMed/NCBI
|
|
26
|
Dhawan A, Ruwali M, Pant MC, Rahman Q and
Parmar D: Association of genetic variability in enzymes
metabolizing chemotherapeutic agents with treatment response in
head and neck cancer cases. Asia Pac J Clin Oncol. Jan
21–2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bussmann L, Busch CJ, Lörincz BB,
Rieckmann T, Block A and Knecht R: Perspectives in chemosensitivity
and chemoresistance assays and their implementation in head and
neck cancer. Eur Arch Otorhinolaryngol. 273:4073–4080. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ko EC, Genden EM, Misiukiewicz K, Som PM,
Kostakoglu L, Chen CT, Packer S and Kao J: Toxicity profile and
clinical outcomes in locally advanced head and neck cancer patients
treated with induction chemotherapy prior to concurrent
chemoradiation. Oncol Rep. 27:467–474. 2012.PubMed/NCBI
|
|
29
|
Posner MR, Hershock DM, Blajman CR,
Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM,
Cullen K, Ervin TJ, et al: Cisplatin and fluorouracil alone or with
docetaxel in head and neck cancer. N Engl J Med. 357:1705–1715.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pointreau Y, Garaud P, Chapet S, Sire C,
Tuchais C, Tortochaux J, Faivre S, Guerrif S, Alfonsi M and Calais
G: Randomized trial of induction chemotherapy with cisplatin and
5-fluorouracil with or without docetaxel for larynx preservation. J
Natl Cancer Inst. 101:498–506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
organization for research and treatment of cancer, national cancer
institute of the United states, national cancer institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
34
|
Mesía R, Vázquez S, Grau JJ, García-Sáenz
JA, Lozano A, García C, Carles J, Irigoyen A, Mañós M,
García-Paredes B, et al: A Phase 2 open label, single-arm trial to
evaluate the combination of cetuximab plus taxotere, cisplatin, and
5-flurouracil as an induction regimen in patients with unresectable
squamous cell carcinoma of the head and neck. Int J Radiat Oncol
Biol Phys. 94:289–296. 2016. View Article : Google Scholar : PubMed/NCBI
|