Introduction
Primary brain tumors are subdivided into benign and
malignant types and classified according to the World Health
Organization (WHO) Classification (1), ranging from e.g., WHO grade I meningioma
to WHO grade IV glioblastoma. Symptoms vary according to the
location of the tumor and include paralysis or sensory
disturbances, intracranial pressure, personality changes, epileptic
seizures and cognitive impairments, are possible. All these
symptoms may markedly affect health-related quality of life
(HRQOL). HRQOL is a multidimensional construct that examines the
subjective effects of diseases and therapy-associated symptoms on
the well-being of patients. This includes physical, psychological
and social functioning (2). During
previous decades, HRQOL has become an important ‘patient outcome’
consideration in oncology when evaluating treatment results
(3). In palliative cases, such as for
patients with glioblastoma multiforme (GBM), the stabilization of
HRQOL is the primary intention (4).
Understanding of the patterns of HRQOL is important
for patient information and shared decision-making. Patients need
to know what their HRQOL outcome will be during therapy, and also
during palliative care. Individual problems may be identified with
the assistance of QOL questionnaires prior to, during and
subsequent to therapy; therefore, supportive care may be initiated
(5). HRQOL surveys also tend to
improve communication between the patients and physicians (6). For an effective evaluation of treatment
outcomes, it is crucial to identify changes in HRQOL that occur as
result of the tumor, the therapy or other issues (3). It has previously been demonstrated that
the objective assessment of adverse events is often distinct from
the subjective experience of the patient (7).
In order to evaluate the information currently
available regarding HRQOL, patients with primary brain tumors who
were scheduled for radiotherapy were asked to complete QOL
questionnaires prior and subsequent to their treatment series.
Following radiotherapy, the objective adverse events were
classified and subsequently compared with the subjective data
obtained from the QOL questionnaires.
Materials and methods
Recruitment of patients
A total of 30 consecutive patients with primary
brain tumors were enrolled, following written informed consent
being obtained, between March 2009 and June 2010 at the Department
of Radiation Oncology, Martin Luther University Halle-Wittenberg
[Halle (Saale), Germany]. Patient characteristics and treatment
details are presented in Table I. The
patients were requested to complete the Core Questionnaire C30 and
the module questionnaire BN20, from the European Organization for
Research and Treatment of Cancer (EORTC). The study was approved by
the Ethics Review Committee of the Medical Faculty, Martin Luther
University Halle-Wittenberg.
 | Table I.Baseline characteristics of all 30
patients. |
Table I.
Baseline characteristics of all 30
patients.
| Characteristics | No. of patients
(%) |
|---|
| Age, years [median
(range)] | 64.8 (25.9–80.4) |
| Sex |
|
|
Female | 16 (53) |
| Male | 14 (47) |
| Primary tumor |
|
|
Glioblastoma | 10 (33) |
|
Astrocytoma WHO III | 3
(10) |
|
Oligodendroglioma | 1 (3) |
|
Neurocytoma | 1 (3) |
|
Craniopharyngioma | 1 (3) |
| Pituitary
adenoma | 2 (7) |
|
Meningioma | 11 (37) |
| Acoustic
neuroma | 1 (3) |
| Tumor type |
|
|
Benign | 16 (53) |
|
Malignant | 14 (47) |
| WHO grade |
|
| I | 15 (50) |
| II | 2 (7) |
| III | 3 (10) |
| IV | 10 (33) |
| Chemotherapy |
|
| Yes | 9
(30) |
| No | 21 (70) |
| Irradiation |
|
|
3D-conformal | 15 (50) |
|
Stereotactic | 15 (50) |
| Planning target
volume, ml [median (range)] | 106 (9–476) |
| Total dose, Gy
[median (range)] | 54
(45–60) |
| Duration of
radiotherapy, days [median (range)] | 42
(14–50) |
| Karnofsky performance
status prior to radiotherapy, % [median (range)] | 85
(60–100) |
| Karnofsky performance
status subsequent to radiotherapy, % [median (range)] | 80
(50–100) |
The following inclusion criteria were applied:
Sufficient compliance, understanding of patient information
describing the HRQOL study and the existence of a brain tumor
without previously administered radiotherapy. Patients who had
received prior irradiation or had insufficient cognitive skills to
complete the HRQOL questionnaire, as assessed by the treating
radiation oncologist, were excluded. The present study was
longitudinal, consisting of five questionnaires completed during a
12-month period: Prior to (t0), at the end of radiotherapy (t1) and
3 months (t2), 6 months (t3) and 12 months (t4) following the end
of radiotherapy. Questionnaires were distributed to patients at t0
and t1 in the Department of Radiation Oncology, Martin Luther
University Halle-Wittenberg. At subsequent time points, the
questionnaires were posted with a pre-stamped envelope. Other
information regarding the patients and their therapy was extracted
from medical records and anamnesis.
Questionnaires
For evaluation of QOL, the EORTC QLQ-C30
questionnaire version 3.0 (8) and the
QLQ-BN20 questionnaire (9) were used.
The BN20 was developed specifically for patients with primary brain
tumors by the EORTC Quality of Life Group (9). The two questionnaires had previously
been validated by the EORTC (8,10). The
QLQ-C30 consisted of 30 questions associated with functions and
symptoms. Each question may be answered using 4 grades: 1, not at
all; 2, low; 3, moderate; and 4, high. There were two questions
concerning global QOL scored from 1 (very poor) to 7 (excellent).
According to the EORTC C30 scoring manual (11), the answers may be transformed into
scales ranging from 0 to 100. There are five functional scales
(physical, role, cognitive, emotional and social functioning), nine
symptom scales (fatigue, nausea and emesis, pain, dyspnea, sleep
disturbance, loss of appetite, constipation, diarrhea and financial
difficulties) and one scale for global QOL. The QLQ-BN20 consisted
of 20 questions; the responses may be transformed into 11 symptom
scales (future uncertainty, visual disorder, motor dysfunction,
communication deficit, headaches, seizures, drowsiness, alopecia,
itchy skin, weakness of legs and bladder control). For the
functional scales and global QOL, high scores represent a good
functioning and QOL. For the symptom scales, high scores indicate
the presence of severe symptoms.
Common Terminology Criteria for
Adverse Events (CTCAE)
Adverse events were also objectively assessed at t1,
using checklists based on the CTCAE (version 3) (12). The symptoms of alopecia, nausea,
headache and fatigue were evaluated. According to CTCAE, symptoms
are classified as grades 1–5: Grade 1, mild; grade 2, moderate;
grade 3, severe; grade 4, life-threatening or disabling adverse
event; and grade 5, mortality associated with adverse event. The
symptom of alopecia was classified from 0 to 2 as an exception, as
this toxicity cannot be severe or life-threatening. The objective
adverse events assessed using the CTCAE checklists could be
subsequently compared with the subjective responses from the QOL
questionnaires.
Assessment and statistical
analysis
The following five groups were formed: All patients
(n=30); malignant (n=14); benign (n=16); glioblastoma multiforme
(GBM; n=10); meningioma (n=11). GMB and meningioma were the two
largest subgroups (full patient characteristics are presented in
Table I). Data at each time point
were compared to t0 (prior to radiotherapy). The mean, median and
standard deviation values were calculated. Statistical evaluation
was performed using the non-parametric Mann-Whitney U test and
STATISTICA version 10 software (StatSoft, Inc., Tulsa, OK, USA).
Based on the multiple comparisons, P<0.01 were considered to
indicate a significant difference. Additionally, changes in
clinical significance over time were considered, according to Osoba
et al (13). This study
defined which magnitude of change in HRQOL scores, assessed using
EORTC questionnaires, corresponds to a change, noticed by the
patient as significant. According to Osoba et al (13), a deviation in an individual score by
5–10 points indicated a slight change, by 10–20 points indicated a
moderate change, and by >20 points represented a high clinical
relevance.
Results
Patient and treatment
characteristics
The median age was 64.6 years (range, 25.9–80.4
years). A total of 14 patients exhibited a malignant brain tumor
and 16 had a benign tumor. A total of nine patients also received
chemotherapy (temozolomide) during and subsequent to radiotherapy.
Detailed treatment characteristics are presented in Table I. During the 12-month follow-up
period, eight patients succumbed: Of those, two patients succumbed
after 3 months (t2), three patients after 6 months (t3) and
additional three patients after the 12-month period (t4). The
causes of mortalities were not analyzed; however, it was expected
that in patients with malignant brain tumors, including
glioblastoma, the mortalities were predominantly tumor-associated.
All patient and treatment characteristics are summarized in
Table I.
Questionnaire response rate
At t0 (prior to radiotherapy) and t1 (subsequent to
radiotherapy), all questionnaires were completed (100% response
rate). In the whole group, the response rate among surviving
patients was 78.6% at t2 (3 months), 81.5% at t3 (6 months) and
63,6% at t4 (12 months) which is predominantly >70% response
rate, the level which is typically aimed at achieving, and
acceptable, considering the palliative situation among the
malignant brain tumor patients. The poorest response rate was
achieved after 12 months in the malignant group (50%). At all time
points, there were higher response rates from the benign and
meningioma groups, compared with the malignant and GBM groups. The
response rates for all surveys are presented in Table II. The results were generated from
the proportion of questionnaires returned by patients who were
alive each of the time points.
 | Table II.Response rates to questionnaires among
surviving patients at all time points from t0 (prior to
radiotherapy) to t4 (12 months). |
Table II.
Response rates to questionnaires among
surviving patients at all time points from t0 (prior to
radiotherapy) to t4 (12 months).
|
| Patients who
responded at each time point (%) |
|---|
|
|
|
|---|
| Patient groups | t0 | t1 | t2 | t3 | t4 |
|---|
| All patients | 100 | 100 | 78.6 | 81.5 | 63.6 |
| Benign | 100 | 100 | 81.3 | 87.5 | 68.8 |
| Malignant | 100 | 100 | 75.0 | 72.7 | 50.0 |
| Meningioma | 100 | 100 | 90.9 | 90.9 | 72.7 |
| GBM | 100 | 100 | 78.8 | 62.5 | 66.7 |
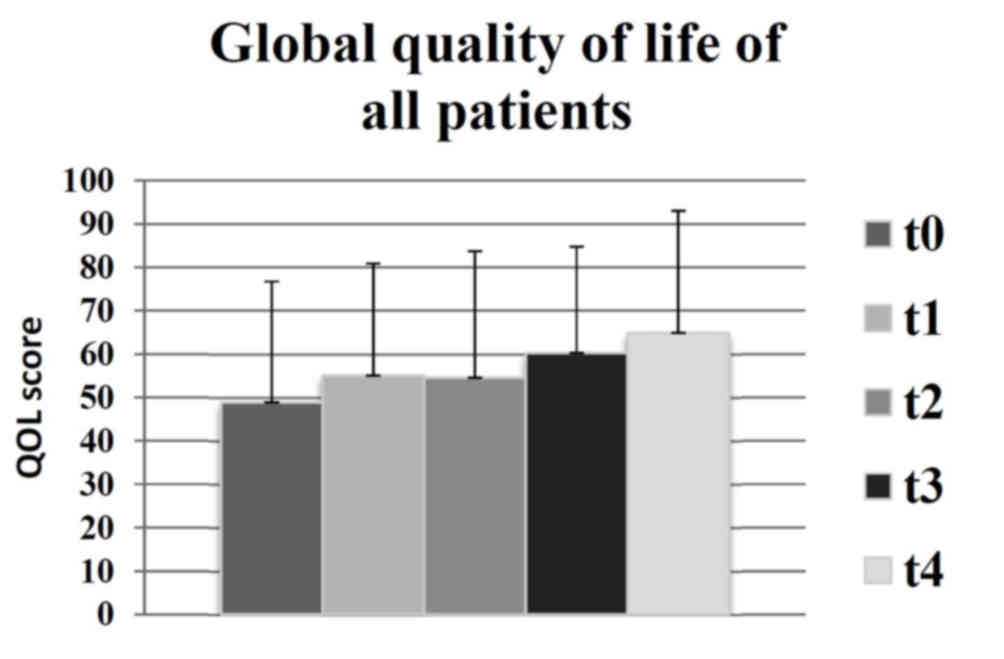
Changes to global QOL
There were no statistically significant changes
recorded in global QOL for any of the subgroups observed. However,
there was an increase of mean global QOL between t0 and t4, as
indicated in Fig. 1, which was of a
clinically significant extent, according to the definitions by
Osoba et al (13) (t0=49;
t4=65). Similar results were identified in the following groups:
Benign (t0=55; t4=66); meningioma (t0=54; t4=72); malignant (t0=42;
t4=61); GBM (t0=42; t4=67). Therefore, the results indicated that
there was a moderate increase in the mean global QOL for all
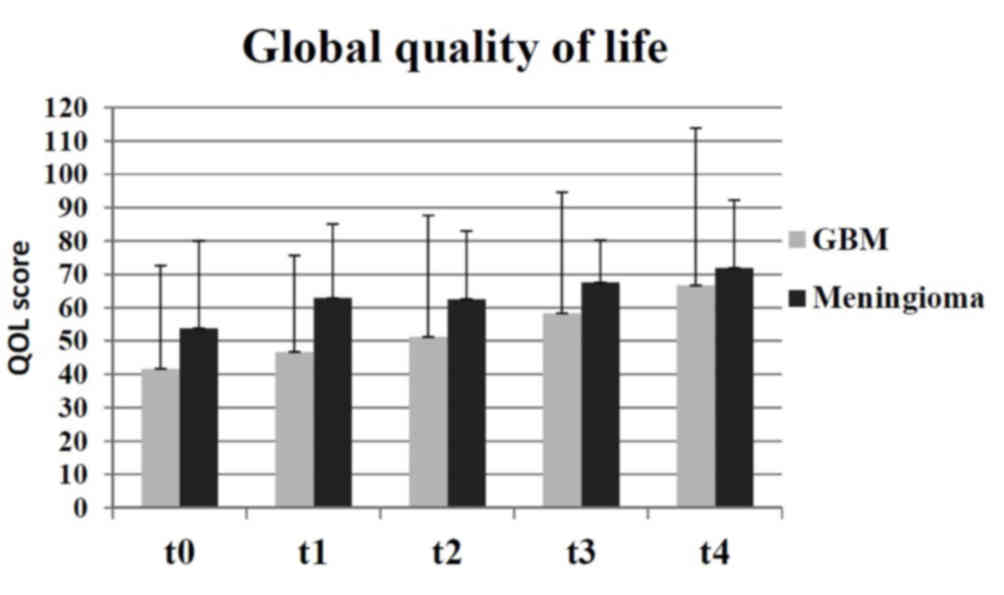
participating groups. Compared with the groups receiving palliative
therapy (malignant and GBM), the global QOL was higher in the
groups receiving curative therapy (benign and meningioma) during
duration of the present study (Fig.
2).
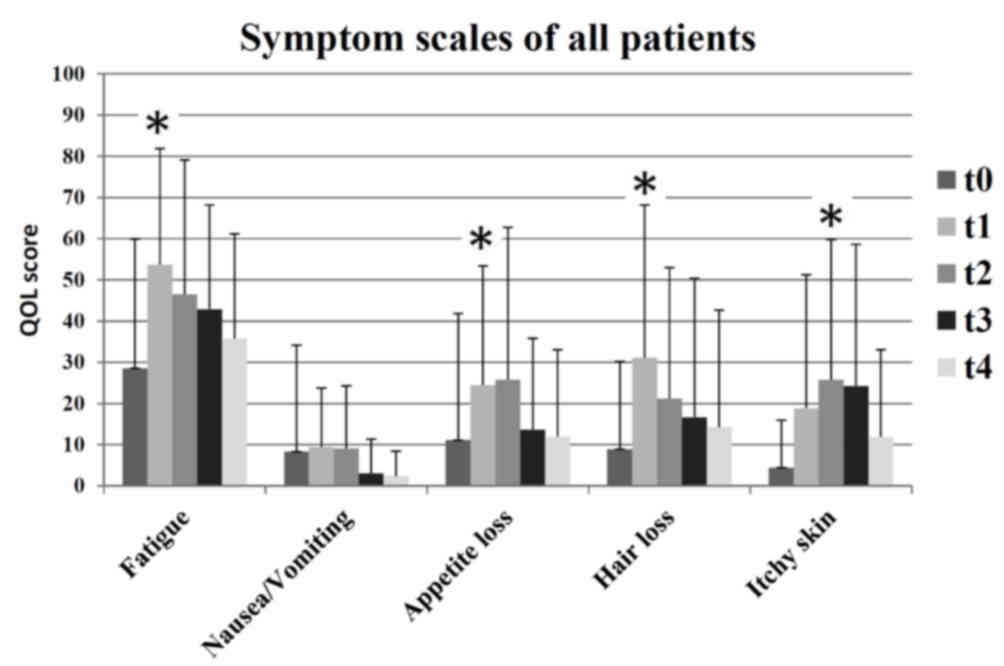
Subjective adverse events
At the end of radiotherapy (t1), patients
experienced significantly higher mean levels of fatigue (t0=29;
t1=54; P=0.002), appetite loss (t0=11; t1=24; P=0.008) and alopecia
(t0=9; t1=31; P=0.006) compared with the beginning of radiotherapy.
At 3 months after radiotherapy (t2), there was a significant
increase in reports of itchy skin (t0=4; t2=26; P=0.006). At the
end of radiotherapy (t1), patients exhibited increased levels of
itchy skin, however, this was not identified as significant (t0=4;
t1=19). Fig. 3 presents an overview
of the surveyed symptoms scales for all patients.
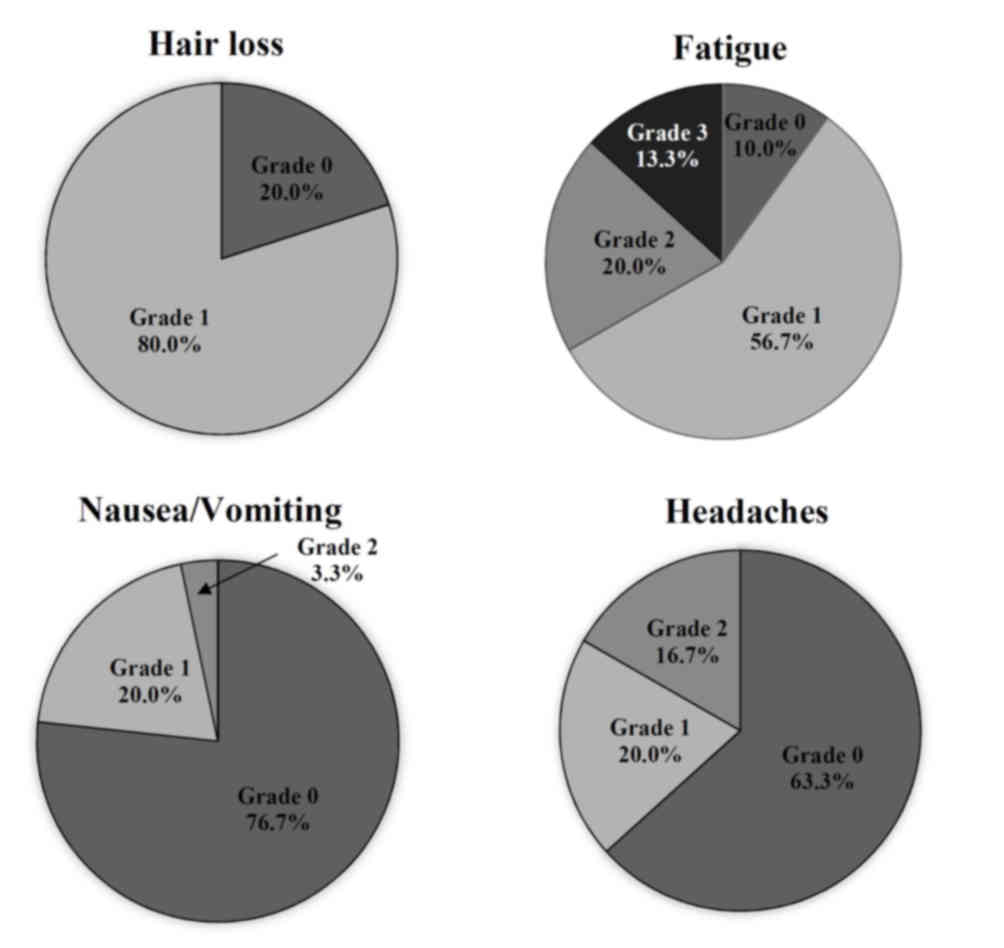
Objective adverse events
The objective evaluation of the side effects using
CTCAE following radiotherapy (t1) indicated certain types of
therapy-associated side effects in 93.3% of the participants. The
main issues that patients identified were fatigue and alopecia. In
addition, 80% of patients demonstrated alopecia grade 1 (thinned or
fragmentary). The results indicated that 90% of patients suffered
from fatigue; of those, 56.7% had mild fatigue and 13.3% had severe
fatigue. Overall, only two patients (6.7%) were asymptomatic
following radiotherapy at t1. There were specific differences
between the subgroups. All patients in the GBM group exhibited
alopecia grade 1; by contrast only 73% of patients with meningioma
exhibited alopecia grade 1 and 27% reported no alopecia. There were
higher grades of fatigue observed in the groups with palliative
therapy (7.2% grade 0; 50% grade 1; 21.4% grade 2; 21.4% grade 3;
0% grade 4) in comparison with the groups receiving curative
therapy (12.5% grade 0; 62.5% grade 1; 18.7% grade 2; 6.3% grade 3;
0% grade 4). Only a small number of patients exhibited nausea
(23.3%) or headaches (36,7%). The distribution of objective
toxicity grades is presented in Fig.
4.
Differences between the subgroups
Patients with benign brain tumors demonstrated
stable functional scales over time, revealing constant or improved
values after radiotherapy in the five types of function measured
using the EORTC QLQ-C30 questionnaire. However, in patients with
malignant tumors, the functional scales became poorer from t1 (end
of radiotherapy) and recovered slightly up until t4 (after 12
months). The cognitive function decreased in the GBM group 12
months following radiotherapy (t0=85; t4=58). Patients in the
meningioma group exhibited more stable cognitive functioning
(t0=88; t4=88). There was an increase in future uncertainty in the
GBM group after 12 months (t0=39; t4=58), whereas future
uncertainty continuously decreased in the meningioma group (t0=28;
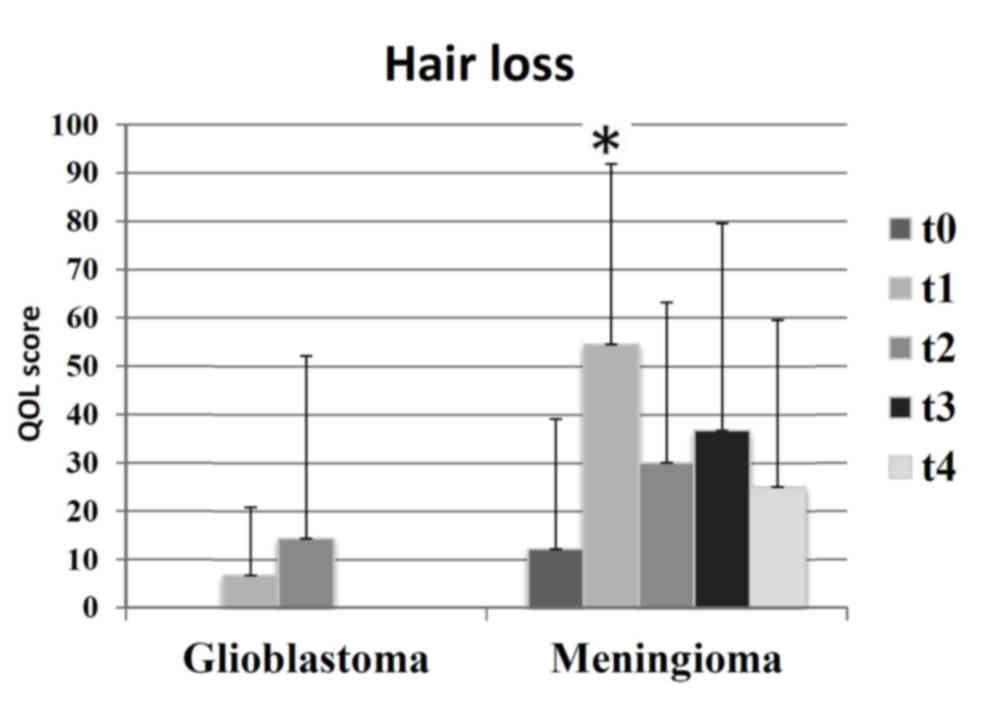
t4=17). Patients with meningioma experienced increased levels of
alopecia during the study period, as compared with patients with
GBM (Fig. 5).
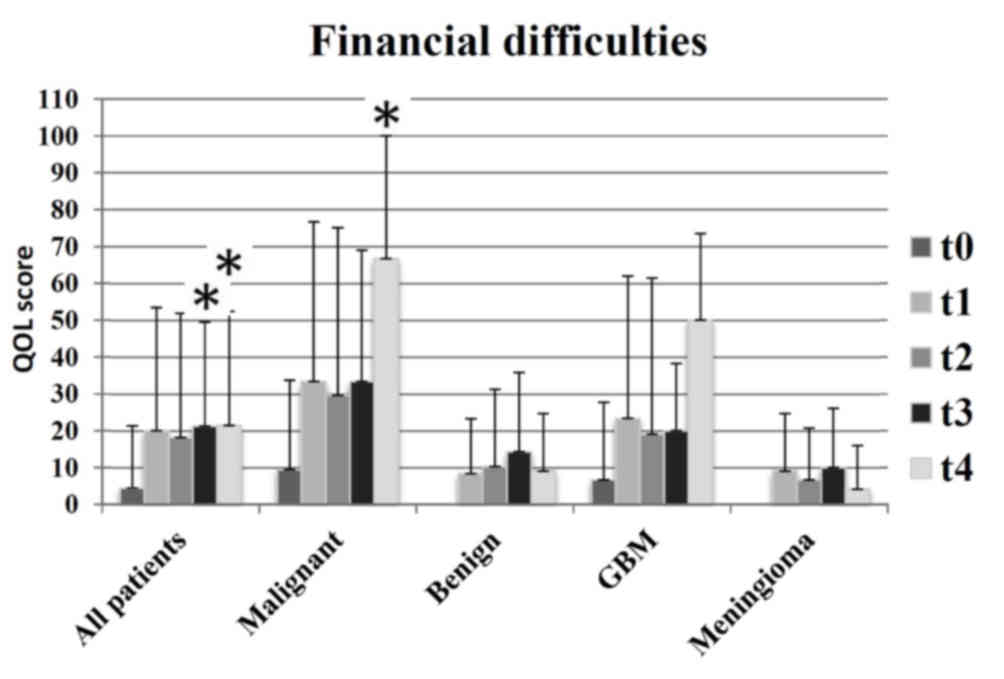
An additional distinction identified was associated
with financial difficulties (Fig. 6):
Patients in the meningioma and benign groups exhibited less
financial difficulties, compared with patients in the GBM and
malignant groups. In addition, financial difficulties decreased at
t4 (12 months following radiotherapy) in the groups receiving
curative therapy, and markedly increased in groups with palliative
therapy.
Discussion
The prospective evaluation of HRQOL in 30 patients
with brain tumors revealed that, despite radiotherapy and
associated side effects, there was no significant decrease in QOL
observed in any subgroup. There was a moderate increase in global
QOL 12 months following radiotherapy. The assessment scale for
global QOL consists of two questions and reveals only the
underlying trends of HRQOL. As HRQOL is a multidimensional concept,
its different domains, as measured with the EORTC questionnaires,
must be considered when evaluating the effects of a disease and its
treatment. There are intentions to develop a summary score that
integrates the majority of functional and symptom scales, and to
improve the evaluation of surveys of patient HRQOL (14); however, this approach may be limited
by the multidimensional concept of QOL.
Increased global QOL over time in the malignant and
GBM groups as observed during the present study may have been the
result of various effects. It is conceivable that a positive effect
of radiotherapy may increase HRQOL. An additional reason may be the
response shift (15,16): The personal assessment of patients may
change due to novel experiences, for instance if a patient
recovered from a poor health status (including recovery from
treatment side effects), and so patients may score an improved
HRQOL, in spite of deterioration of their condition. Also, patients
with similar tumor types and objective health status may estimate
their HRQOL in a different manner due to diverse expectations
(17). It is also possible that the
global QOL appears to increase due to selection effects. Patients
with low HRQOL may only provide data at the beginning but not at
later stages, due to mortality or non-response resulting from a
worsening of their general health. Non-response may lead to sample
distortion, and thus to an artificial improvement in the mean
HRQOL.
It is unclear whether the decrease in functional
scale in patients with malignant brain tumors is due to the cancer
therapy or the tumor itself. A study of long-term survivors with
low-grade gliomas suggested that the deterioration in QOL was not
due to previous radiotherapy or chemotherapy, but due to tumor
relapses (18). Therefore, the
results of the present study demonstrate certain similarities to
the previous studies, which may lead to the hypothesis that tumor
relapses may be the predominant reason for decreasing functional
scales during follow-up following radiotherapy.
During the study time frame, no patients succumbed
to disease in the benign and meningioma groups, and the response
rates were ≥68.8%. Future uncertainty and headaches continuously
decreased. The increase in QOL in the benign and meningioma groups
appeared to be due to successful therapy. Functional scales of the
QLQ-C30 remained stable during radiotherapy. Similar results were
identified in a German study from 2013 (19), which evaluated 67 patients with
meningioma during radiotherapy. In this prior study, the functional
scales decreased shortly following radiotherapy and then normalized
after 12 months. The study by Henzel et al (19) indicated that pain decreased subsequent
to therapy, corresponding to the outcomes of the present study,
which recorded decreasing headaches in patients following
therapy.
At t1 (subsequent to radiotherapy) there were
certain detectable adverse events. In particular, fatigue increased
markedly (t0=29; t1=54); this may be a direct acute effect of
radiotherapy. Previous studies demonstrated that HRQOL decreased in
patients with glioma who suffered from fatigue (16,20). This
is concordant with the observation of increased levels of fatigue
in the GBM group (t0=37), compared with in the meningioma group
(t0=23), prior to radiotherapy.
The objective adverse events, measured using CTCAE,
were comparable with the subjective claims of the patients.
Predominant subjective and objective areas of relevance were
fatigue and alopecia. However, there was a distinct pattern of
occurrence of symptoms. All patients with GBM objectively exhibited
alopecia grade 1; however, only 73% of patients with meningioma
experienced alopecia grade 1, and 23% exhibited no alopecia. The
meningioma group reported higher scales of alopecia on the QLQ-BN20
compared with the GBM group, which may be due to a differing
standard of assessment for patients in evaluating their own HRQOL
(16). Participants with a palliative
diagnosis (GBM and malignant groups) considered alopecia to be a
minor issue; conversely, participants with a curative diagnosis
(meningioma and benign) considered alopecia a major issue. This
result demonstrates the need for simultaneous analyses of objective
and subjective adverse events in order to improve the HRQOL of
patients. Quinten et al (7)
identified that the simultaneous examination also assists in
predicting survival. Physicians may have different assessment
criteria for individual symptoms due to their practical experience
with patients with oncology. Therefore, there is a risk that the
effects of certain symptoms may not be recognized, which then leads
to a negative effect on HRQOL.
One year following radiotherapy, participants with
malignant brain tumors reported increased financial difficulties,
particularly for t4, while the financial problems in the benign and
meningioma groups were reversed. The reason may be that patients
with benign brain tumors go back to work, while patients with
malignant brain tumors have not.
A limitation of the present study was the low number
of participants (n=30). Data collection over longer time periods,
possibly with a larger number of participants or a multi-centric
study design, may produce more significant data. Due to the limited
sample, there were no additional subdivisions of the groups (sex,
age or total dose). In future studies, a subdivision may provide
more comprehensive results. A previous study suggested that
differences exist between male and female participants (21). Additional information concerning
depression and anxiety may also assist in improving the HRQOL of
patients. A previous study demonstrated that patients suffering
from depression and anxiety exhibited poorer HRQOL (22). Poorer HRQOL and depression are also
negative predictors of survival (23).
In conclusion, although there was no statistically
significant improvement in global QOL, the present study
demonstrated a moderately clinically relevant improvement to HRQOL.
Sub-features of HRQOL, such as headaches and visual disturbances,
may also be improved. It may be hypothesized that the simultaneous
collection of objective and subjective therapeutic effects are
important. Thus, the present study may assist in identifying
indications, which may be valuable for patient counseling.
Nevertheless, additional studies are necessary to improve the HRQOL
of patients with brain tumors. There is a need for tolerable and
effective therapies, intensive treatment of side effects and
additional efforts to assist patients to continue their daily
life.
Glossary
Abbreviations
Abbreviations:
|
HRQOL
|
health-related quality of life
|
|
GBM
|
glioblastoma multiforme
|
|
EORTC
|
European Organization for Research and
Treatment of Cancer
|
|
CTCAE
|
Common Terminology Criteria for
Adverse Events
|
References
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Velikova G, Coens C, Efficace F, Greimel
E, Groenvold M, Johnson C, Singer S, van de Poll-Franse L, Young T
and Bottomley A: Health-related quality of life in EORTC clinical
trials-30 years of progress from methodological developments to
making a real impact on oncology practice. EJC Suppl. 10:141–149.
2012. View Article : Google Scholar
|
|
3
|
Outcomes of cancer treatment for
technology assessment and cancer treatment guidelines. American
Society of Clinical Oncology. J Clin Oncol. 14:671–679. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jocham HR, Dassen T, Widdershoven G and
Halfens R: Quality of life in palliative care cancer patients: A
literature review. J Clin Nurs. 15:1188–1195. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Steinmann D, Vordermark D, Geinitz H,
Aschoff R, Bayerl A, Gerstein J, Hipp M, van Oorschot B, Wypior HJ
and Schäfer C: Proxy assessment of patients before and after
radiotherapy for brain metastases. Results of a prospective study
using the DEGRO brain module. Strahlenther Onkol. 189:47–53. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Velikova G, Booth L, Smith AB, Brown PM,
Lynch P, Brown JM and Selby PJ: Measuring quality of life in
routine oncology practice improves communication and patient
well-being: A randomized controlled trial. J Clin Oncol.
22:714–724. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quinten C, Maringwa J, Gotay CC,
Martinelli F, Coens C, Reeve BB, Flechtner H, Greimel E, King M,
Osoba D, et al: Patient self-reports of symptoms and clinician
ratings as predictors of overall cancer survival. J Natl Cancer
Inst. 103:1851–1858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aaronson NK, Ahmedzai S, Bergman B,
Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman
SB, de Haes JC, et al: The European organization for research and
treatment of cancer QLQ-C30: A quality-of-life instrument for use
in international clinical trials in oncology. J Natl Cancer Inst.
85:365–376. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chow R, Lao N, Popovic M, Chow E, Cella D,
Beaumont J, Lam H, Pulenzas N, Bedard G, Wong E, et al: Comparison
of the EORTC QLQ-BN20 and the FACT-Br quality of life
questionnaires for patients with primary brain cancers: A
literature review. Support Care Cancer. 22:2593–2598. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taphoorn MJ, Claassens L, Aaronson NK,
Coens C, Mauer M, Osoba D, Stupp R, Mirimanoff RO, van den Bent MJ
and Bottomley A; EORTC Quality of Life Group, and Brain Cancer,
NCIC and Radiotherapy Groups, : An international validation study
of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing
health-related quality of life and symptoms in brain cancer
patients. Eur J Cancer. 46:1033–1040. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fayers PM, Aaronson NK, Bjordal K,
Groenvold M, Curran D and Bottomley A; The EORTC Quality of Life
Group, : The EORTC QLQ-C30 Scoring Manual. 3rd. European
Organisation for Research and Treatment of Cancer; Brussels:
2001
|
|
12
|
Cancer Therapy Evaluation Program: Common
Terminology Criteria for Adverse Events, Version 3.0. DCTD, NCI,
NIH, DHHS. 2003.https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdfAugust
9–2006
|
|
13
|
Osoba D, Rodrigues G, Myles J, Zee B and
Pater J: Interpreting the significance of changes in health-related
quality-of-life scores. J Clin Oncol. 16:139–144. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giesinger JM, Kieffer JM, Fayers PM,
Groenvold M, Petersen MA, Scott NW, Sprangers MA, Velikova G and
Aaronson NK; EORTC Quality of Life Group, : Replication and
validation of higher order models demonstrated that a summary score
for the EORTC QLQ-C30 is robust. J Clin Epidemiol. 69:79–88. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schumacher J, Klaiberg A and Brähler E:
Diagnostische Verfahren zu Lebensqualität und Wohlbefinden. 1st.
Göttingen, Hogrefe: Verlag für Psychologie; pp. 9–24. 2003
|
|
16
|
Taphoorn MJ, Sizoo EM and Bottomley A:
Review on quality of life issues in patients with primary brain
tumors. Oncologist. 15:618–626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carr AJ, Gibson B and Robinson PG:
Measuring quality of life: Is quality of life determined by
expectations or experience? BMJ. 322:1240–1243. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okita Y, Narita Y, Miyahara R, Miyakita Y,
Ohno M and Shibui S: Health-related quality of life in long-term
survivors with Grade II gliomas: The contribution of disease
recurrence and Karnofsky Performance Status. Jpn J Clin Oncol.
45:906–913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Henzel M, Fokas E, Sitter H, Wittig A and
Engenhart-Cabillic R: Quality of life after stereotactic
radiotherapy for meningioma: A prospective non-randomized study. J
Neurooncol. 113:135–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aprile I, Chiesa S, Padua L, Di Blasi C,
Arezzo MF, Valentini V, DiStasio E and Balducci M: Occurrence and
predictors of the fatigue in high-grade glioma patients. Neurol
Sci. 36:1363–1369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sehlen S, Hollenhorst H, Schymura B,
Herschbach P, Aydemir U, Firsching M and Dühmke E: Psychosocial
stress in cancer patients during and after radiotherapy.
Strahlenther Onkol. 179:175–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lucchiari C, Botturi A, Silvani A,
Lamperti E, Gaviani P, Innocenti A, Finocchiaro CY, Masiero M and
Pravettoni G: Cognitive strategies and quality of life of patients
with high-grade glioma. Support Care Cancer. 23:3427–3435. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mainio A, Tuunanen S, Hakko H, Niemelä A,
Koivukangas J and Räsänen P: Decreased quality of life and
depression as predictors for shorter survival among patients with
low-grade gliomas: A follow-up from 1990 to 2003. Eur Arch
Psychiatry Clin Neurosci. 256:516–521. 2006. View Article : Google Scholar : PubMed/NCBI
|