Introduction
Thyroid carcinoma is the most common endocrine
malignancy, comprising 90% of endocrine cancers, but representing
~1% of whole-body malignant cancers (1,2). Thyroid
cancer is classified into three types: Well-differentiated;
poorly-differentiated; and undifferentiated (3). The most prevalent types of thyroid
cancer are well-differentiated [papillary thyroid cancer (PTC) and
follicular thyroid cancer (FTC)] thyroid carcinomas, which make up
~93% of all thyroid cancers (4,5). In
addition, the incidence of well-differentiated thyroid cancers
(WDTC) has increased dramatically in the past decade (6). Currently, surgical resection remains the
most effective treatment for this cancer. Therefore, finding an
effective natural medicine that has anti-WDTC effects is may be of
great importance.
There is increasing interest in herbal and botanical
remedies in oncotherapy. The botanical herb Prunella
vulgaris (PV), a common plant cultured in China, Korea, Japan
and Europe, has been established to have anti-inflammatory,
anti-oxidant, anti-allergic, anti-microbial and anti-viral
characteristics (7). PV has been
revealed to be rich in bioactive chemicals, including
polysaccharides, flavonoids, triterpenes and phenolic acid
(8). In China, this plant has a
history of over a thousand years (9)
as a traditional Chinese medicine for use in treating sore throat,
swelling of the thyroid gland, jaundice, fever, infectious
hepatitis, dermatosis, skin allergies and for accelerating wound
healing (10). Previous studies
identified that PV regulates cellular immunity via activating the
nuclear factor-B and mitogen-activated protein kinase (11), exhibiting anti-estrogenic properties
(12), inhibiting the gastric cancer
cell SGC-7901 growth in vivo (13), inducing the apoptosis related protein
of Raji cells (14) and suppressing
lung metastasis (15).
A number of published studies have demonstrated that
PV may affect the signal transduction, gene expression and
proliferation of lung, gastric and lymphatic tumor cells (13–15).
However, the function of PV on WDTC has not yet been reported. The
present study investigated the effect of PV on WDTC cell lines
(TPC-1 and FTC-133) and explored the activity of
apoptosis-associated signaling pathways. The present results show
that PV increases apoptosis in WDTC cells in vivo.
Materials and methods
PV preparation
PV was provided by Guiyang Xintian Pharmaceutical
Co., Ltd. (lot number JG141002; Guizhou, China) and was stored at
4°C in the dark. The cell culture medium contained PV at varying
concentrations [5, 10, 20 and 30% (v/v)] was diluted with
RPMI-1,640 medium supplemented with 10% fetal bovine serum
(FBS).
Cell lines and cell culture
The human thyroid papillary cancer cell line (TPC-1)
and follicular thyroid cancer cell line (FTC-133) were kindly
provided by Dr Ye Lei of Shanghai Rui Jin Hospital (Shanghai,
China). The cells were cultured in RPMI-1,640 medium supplemented
with 10% FBS in at atmosphere containing 5% CO2 at 37°C.
The cells were digested by 0.25% trypsin-0.01% EDTA.
Cell Counting Kit-8 (CCK-8) assay
The effect of PV on TPC-1 and FTC-133 cells was
measured by the CCK-8 assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). TPC-1 and FTC-133 were seeded at a density of
3×104 cells/ml in 96-well plates with 100 µl per well at
37°C for 24 h. Cells were then cultured with different
concentrations of PV [0, 5, 10, 20 and 30% (v/v)] for a number of
time periods (12, 24 and 36 h). Subsequently, 10 µl of CCK-8
reagent was added to each well, and the 96-well plates were
incubated at 37°C for 2 h. The absorbance of each well was
determined at 450 nm, using an automatic enzyme-linked
immunosorbent assay plate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The relative cell viability was calculated as
follows: Cell viability (%)=[mean optical density (OD) of
experimental group/mean OD of control group]x100. Experiments were
performed in triplicate. From the values obtained, the half-maximal
inhibitory concentration (IC50) for the respective
durations of treatment was deduced for TPC-1 and FTC-133 cells,
using curves obtained by plotting percentage inhibition against
concentration.
Hoechst 33342 staining
TPC-1 and FTC-133 cells in the logarithmic growth
phase were seeded onto 24-well plates. Following pre-incubation at
37°C for 24 h, cells were treated with PV at IC50.
Following 24 h, cells were washed twice with PBS and fixed with 500
µl of 4% paraformaldehyde, at 4°C for 10 min. The plate was then
washed with PBS and the cells were stained with Hoechst 33342
(Dingguo, Beijing, China) for 5 min at room temperature in the
dark. Subsequently, the cells were washed twice with PBS and
immediately observed under an inverted fluorescence microscope
(Shenzhen Coosway Optical Technology Co., Ltd., Shenzhen, China) at
magnification, ×200. Live cells exhibited dispersion and uniform
fluorescence in nuclei, while dead cells were not dyed by Hoechst
33342 staining. When apoptosis occurs, marked nuclear morphological
changes may be observed in the nucleus or cytoplasm, including blue
fluorescent-stained compact particulates. The cells with three or
more fluorescent DNA fragments were identified as apoptotic
cells.
Acridine orange (AO)/ethidium bromide
(EB) staining
TPC-1 and FTC-133 cells were seeded onto 24-well
plates, then incubated at 37°C for 24 h to allow cell adherence.
Following incubation, TPC-1 and FTC-133 cells were treated with PV
at IC50, and the treated and untreated cells were washed
twice with PBS. A total of 200 µl AO/EB (Dingguo) dye mix (100
µg/ml AO and 100 µg/ml EB) was added to each well for 3 min. The
viability of the cells was examined using fluorescent microscopy
(Shenzhen Coosway Optical Technology Co., Ltd.) with 5 separate
fields of view at a magnification of ×200 to discriminate between
live, apoptotic and necrotic cells. AO (green fluorescence) stained
live and dead cells, whereas EB (red fluorescence) stained only the
dead cells. Apoptotic cells were identified by condensed and
fragmented nuclei. Necrotic cells were detected by uniformly orange
stained cell nuclei with EB. All the images were captured with a
fluorescent microscope equipped with a digital camera.
DNA extraction and fragmentation
assay
TPC-1 and FTC-133 cells were treated with PV at
IC50 at 37°C for 24 h. DNA was extracted from treated
and untreated cells using Takara Minibest Universal Genomic DNA
Extraction kit (Takara Biotechnology Co., Ltd., Dalian, China). The
genomic DNA samples were separated in 2% agarose gel by
electrophoresis and the gel was stained with Golden View (Dingguo)
and visualized under a UV Trans illuminator (Kodak, Rochester, NY,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The expression of the pro-apoptotic genes Bcl-2
associated X protein (Bax) and caspase-3, and the anti-apoptotic
gene B-cell lymphoma-2 (Bcl-2) were detected by RT-qPCR. The PTC-1
and FTC-133 cells were collected following treatment with 0% PV or
PV at IC50 at 37°C for 24 h. Total RNA was isolated from
cells by TRIzol, (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China) according to the manufacturer's protocol. The
total RNA was reverse transcribed into cDNA using a PrimeScript RT
reagent kit with gDNA Eraser (Perfect Real Time; Takara
Biotechnology Co., Ltd.). The resulting cDNA was quantified using a
RT-qPCR mRNA SYBR Green detection kit (Takara Biotechnology Co.,
Ltd.) to analyze the expression of apoptosis-associated genes using
gene-specific primers. Each reaction was performed in a total
volume of 20 µl (10 µl Premix, 0.8 µl forward primer, 0.8 µl
reverse primer, 2 µl cDNA 6.4 µl dH2O), using SYBR Green
PCR reagents (Takara Biotechnology Co., Ltd.) and incubated for 5
sec at 95°C, followed by 50 cycles of 95°C for 5 sec, 1 cycle of
60°C for 20 sec and 60°C for 30 sec. GAPDH was used as an internal
control. The primer sequences were designed using the program
primer BLAST as follows: GAPDH forward, 5′-TGAAGGTCGGAGTCAACGG-3′
and reverse, 5′-CTGGAAGATGGTGATGGGATT-3′; Bcl-2 forward,
5′-GGGTGGGAGGGAGGAAGAAT-3′ and reverse, 5′-TTCGCAGAGGCATCACATCG-3′;
Bax forward, 5′-CTCACCGCCTCACTCACCAT-3′ and reverse,
5′-TGTGTCCCGAAGGAGGTTTATT-3′; and caspase-3 forward,
5′-GAGTAGATGGTTTGAGCCTGAG-3′ and reverse,
5′-TGCCTCACCACCTTTAGAAC-3′. Following PCR, the threshold cycle (Cq)
value of each cell was recorded, and the dates were analyzed by the
comparative 2−ΔΔCq method (15).
Statistical analysis
The results are expressed as the mean ± standard
deviation of at least three independent experiments. Comparison
between groups was performed using one-way analysis of variance.
Student's t-test was performed to evaluate the significance of
differences in the mean value. P<0.05 was considered to indicate
a statistically significant difference. The statistical analyses
were performed using the SPSS 17.0 software for Windows (SPSS,
Inc., Chicago, IL, USA). GraphPad Prism 5 (GraphPad Software, Inc.,
La Jolla, CA, USA) was used for graphs.
Results
Effects of PV on cell proliferation
and cell morphology
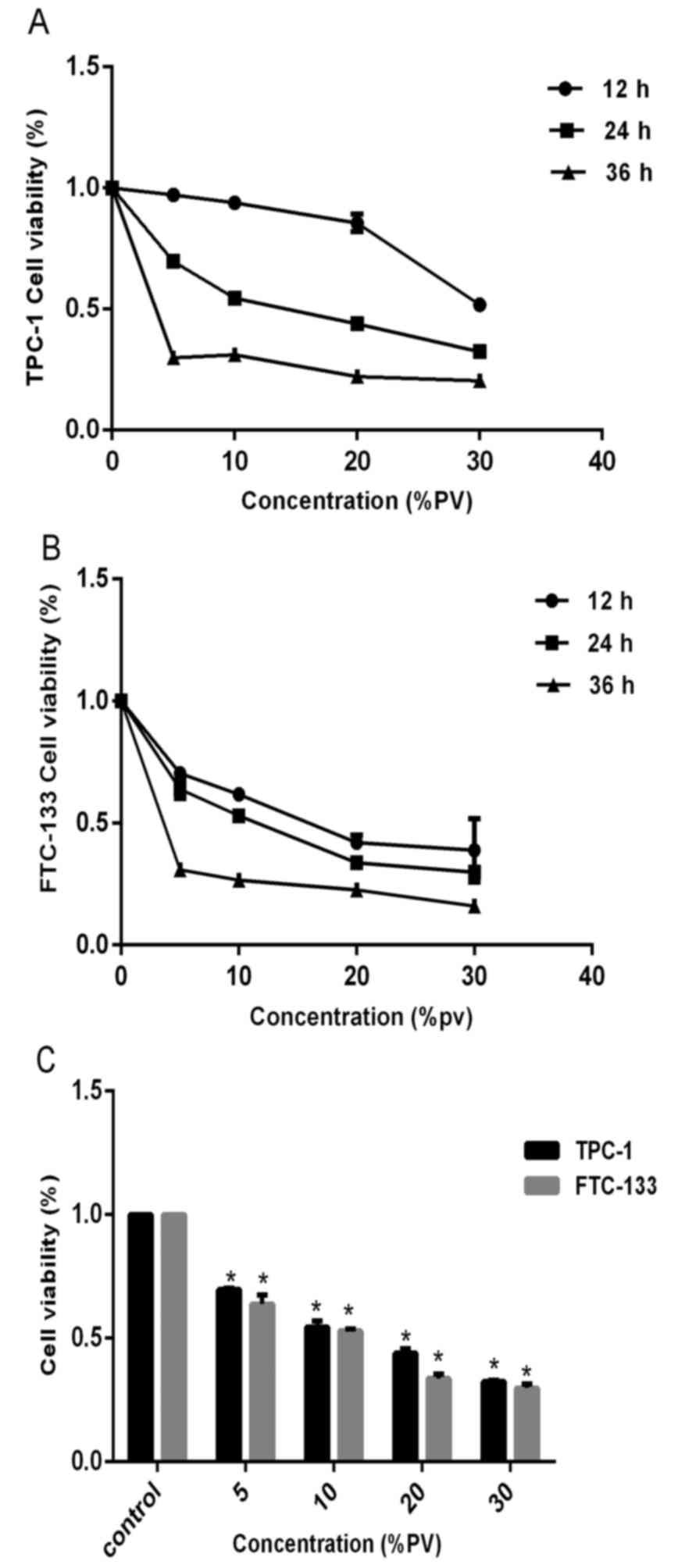
The effect of PV on cell viability was examined by
the CCK-8 assay. In order to investigate the anti-proliferative
effect of PV, TPC-1 and FTC-133 cells were cultured in the presence
of various concentrations of PV (0, 5, 10, 20 and 30%) for 12, 24
and 36 h. Subsequent to treatment, the cell viability presented
marked changes with different concentrations. The CCK-8 assay
showed that PV significantly inhibited the proliferation of TPC-1
and FTC-133 cells (P<0.05), compared with the control condition
(untreated cells). As the concentration increased, the cell
viability decreased at all time periods (Fig. 1A and B). Following 24 h treatment, the
IC50 values of PV were found to be 16.3 and 12.7% PV in
TPC-1 and FTC-133 cells, respectively (Fig. 1C). These data indicated that PV
significantly reduced the viability of WDTC cells in a dose- and
time-dependent manner (P<0.05).
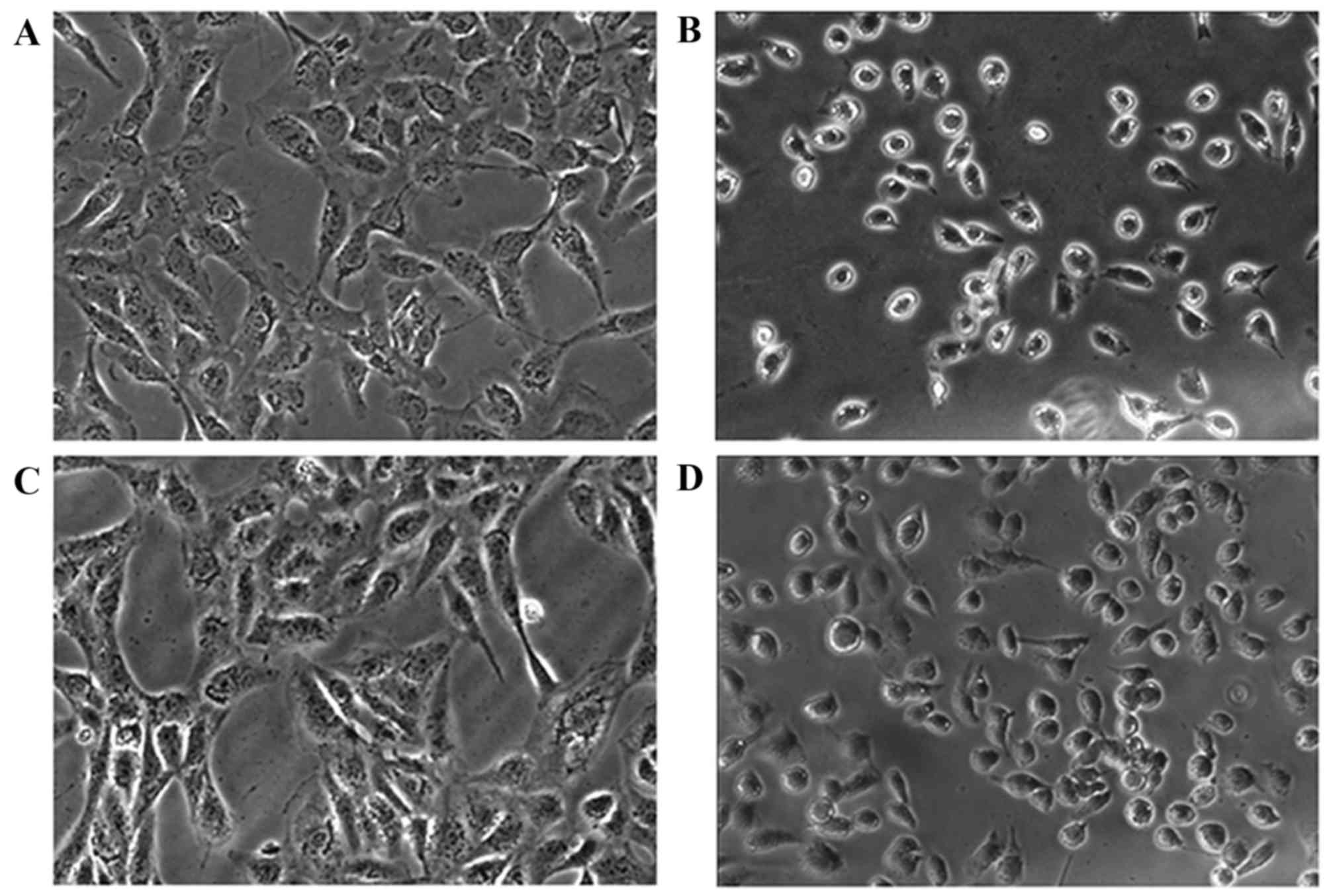
The CCK-8 assay indicated that PV may effectively
inhibit cell proliferation, and this result was confirmed by
observing cells under bright inverted microscopy. Following
incubation with IC50 PV for 24 h, cell morphology became
smaller in size and more rounded in shape, compared with the
control group, resulting in cells detaching from the surface of the
Petri dish. The images captured demonstrated that PV caused an
alteration in cellular morphology (Fig.
2).
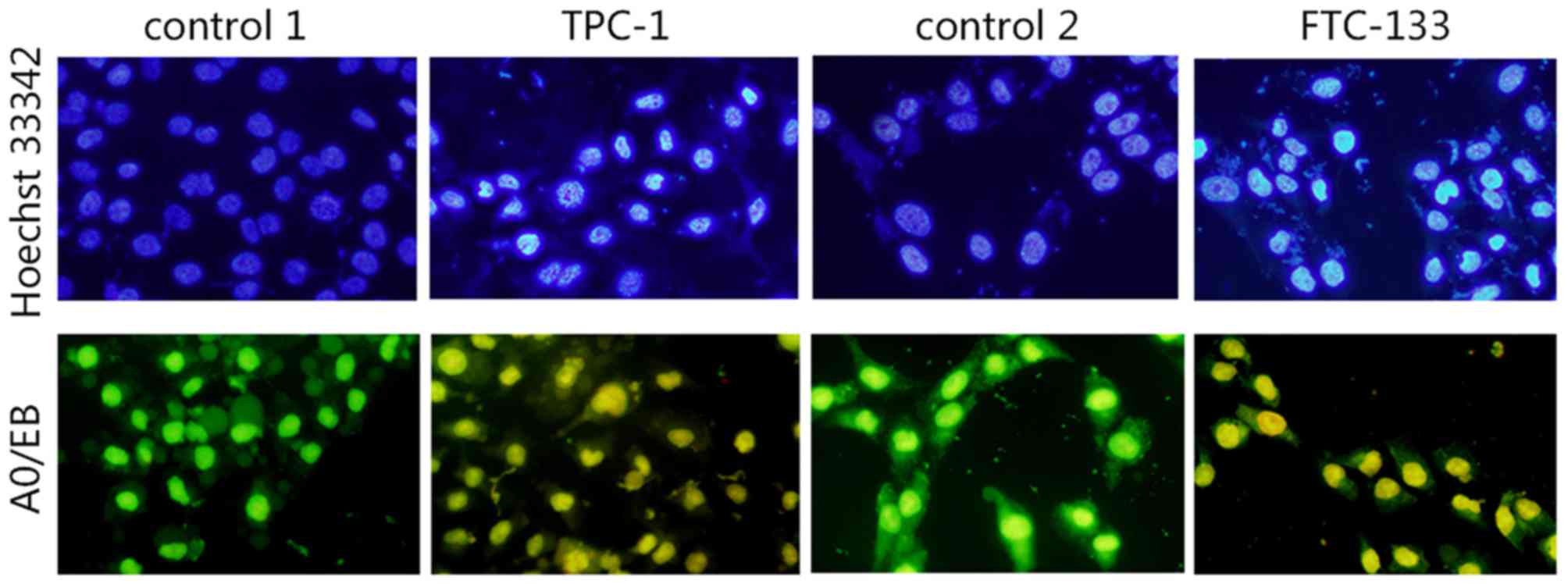
Hoechst 33342 staining
As shown in Fig. 3,
the cell nuclei of TPC-1 and FTC-133 cells dyed with Hoechst 33342
were uniform, round or oval, with uniformity in chromatin
distribution with a weak blue color. Following treatment with
IC50 PV for 24 h, more cells appeared to possess
apoptotic characteristics, with changes of nuclear morphometry,
including formation of round cell karyorrhexis particles, chromatin
condensation, particle shape distribution, bright blue nuclear
pyknosis and lobulated nuclear fragmentation.
AO/EB staining to detect nuclear
changes
Microscopic evidence for apoptosis was obtained with
AO/EB staining, the fluorescent patterns of which depend on the
viability and membrane integrity of the cells. Significant changes
in morphology were observed following PV treatment for 24 h at
IC50, whereas such changes were not observed in the
control group (Fig. 3). Uniformly
green fluorescing nuclei with a highly organized cellular structure
indicated normal and viable cells (Fig.
3; control 1 and control 2). Orange to red fluorescing nuclei
with highly condensed or fragmented chromatin indicated apoptotic
cells (Fig. 3; TPC-1 and FTC-133).
The present results revealed the apoptosis inducing ability of PV
in TPC-1 and FTC-133 cell lines.
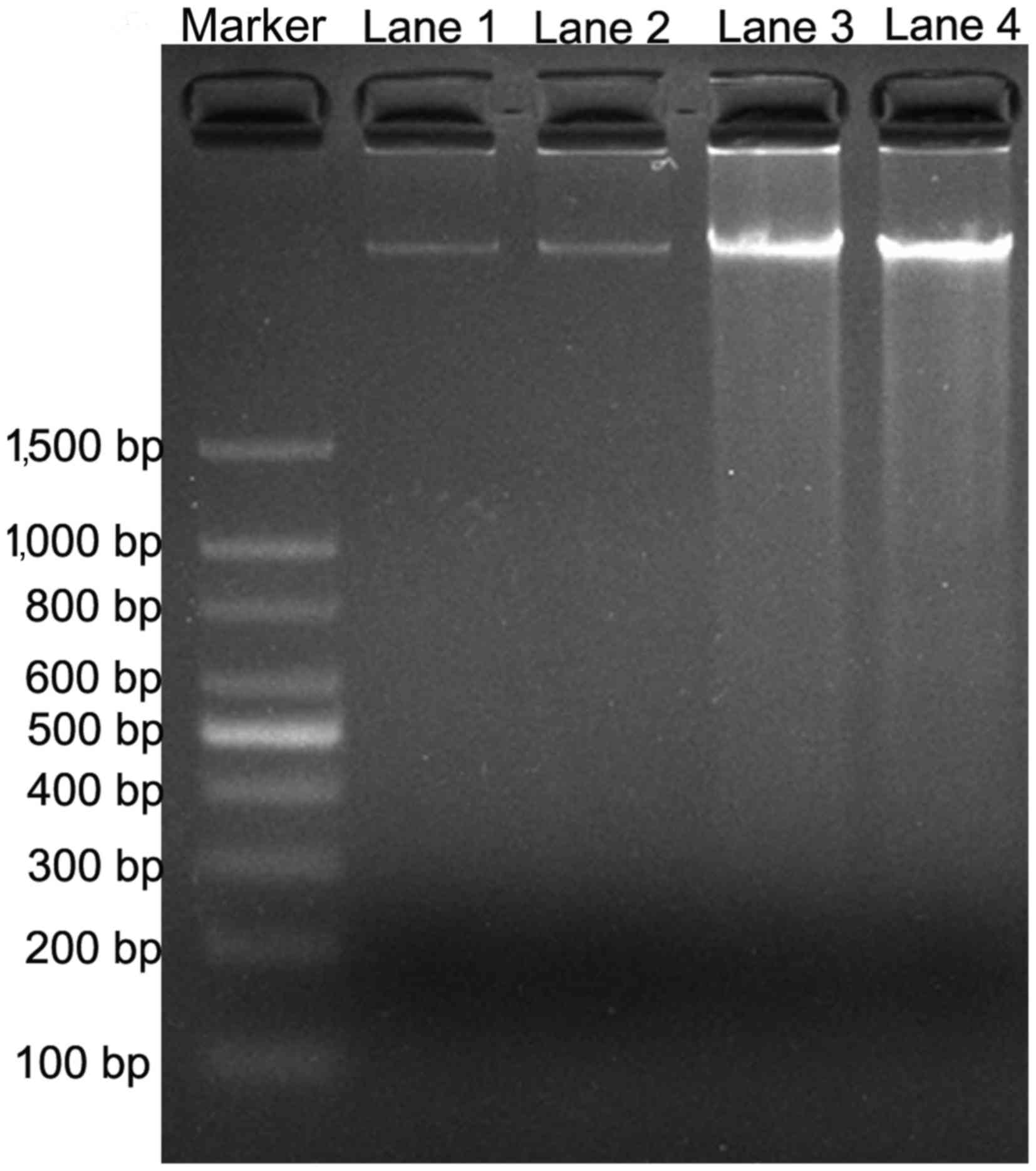
DNA fragmentation analysis by PV
The effect of PV on cell apoptosis was tested by the
DNA ladder formation assay. DNA fragmentation is generally
considered to be the hallmark of apoptosis (16). As shown in Fig. 4, DNA laddering was observed subsequent
to TPC-1 and FTC-133 cells being treated with IC50 PV
for 24 h, compared with the control groups. The results indicated
that PV was able to induce WDTC cell apoptosis.
Effects of PV on the expression of
apoptosis-associated proteins
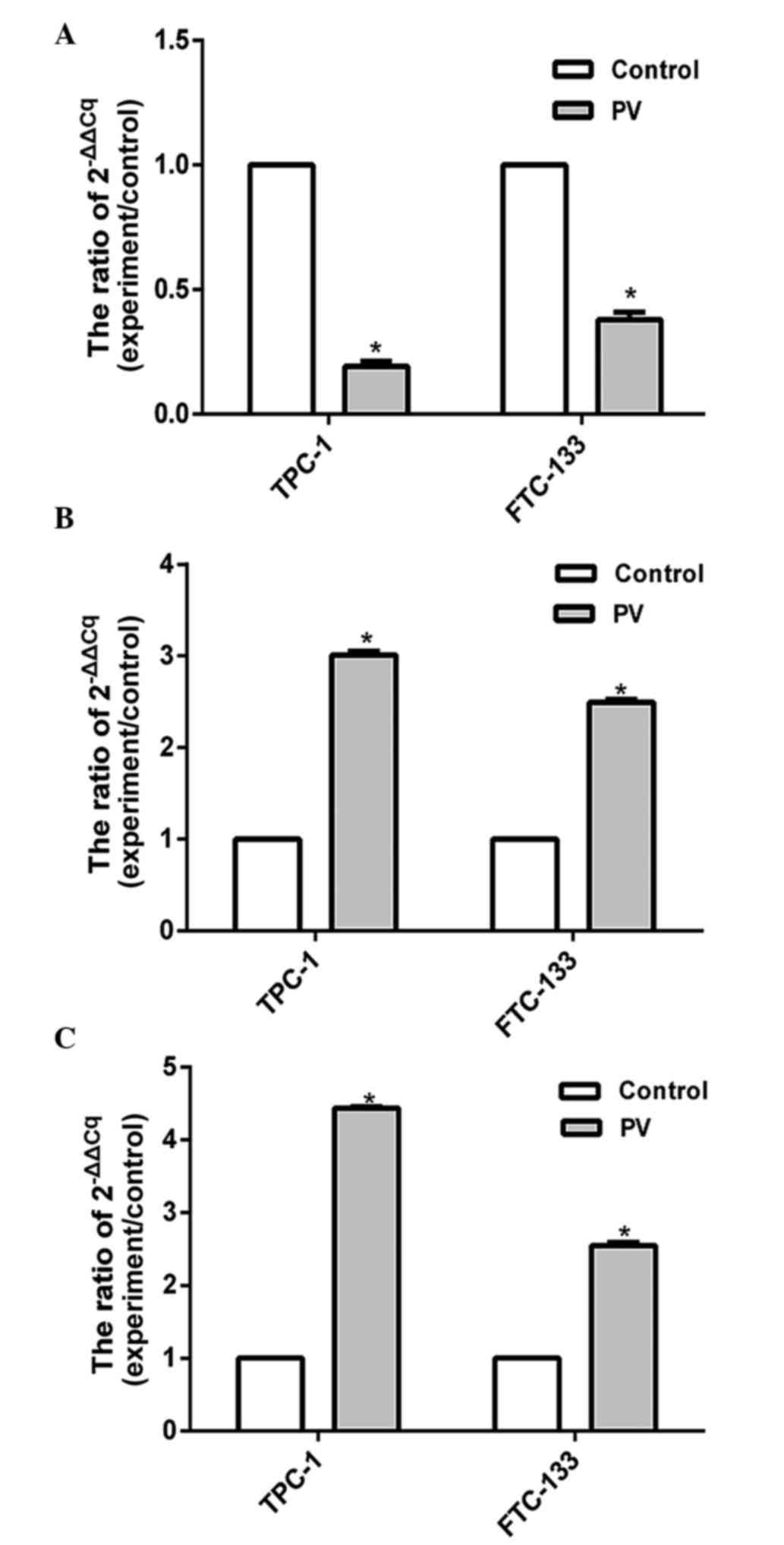
The expression levels of apoptosis-associated
proteins were assessed by RT-qPCR. The present results showed that
the expression level of Bcl-2 was significantly decreased
(P<0.05), while the levels of Bax and caspases-3 increased in
TPC-1 and FTC-133 cell lines treated with PV at IC50 for
24 h (Fig. 5). Previous results
demonstrated that the Bcl-2 protein family and the caspase cascade
perform key roles in mitochondria-mediated cell apoptosis (17), and the present findings indicated that
PV activates Bcl-2, Bax and caspase-3 apoptosis signaling pathways
in WDTC TPC-1 and FTC-133 cell lines that were treated with PV.
Discussion
Due to the advantages of limited side effects and
low toxicity of traditional Chinese medicines, there has been
increasing attention on the antitumor active ingredients extracted
from these medicines (18). PV is a
traditional Chinese medicine that has been shown to have potential
anti-inflammatory, anti-proliferative and apoptotic effects on
diverse cell systems (19). However,
the function of PV on WDTC has not been reported.
In order to explore the effect of PV on WDTC cell
lines, the present study investigated the anti-proliferative
activity of TPC-1 and FTC-133 cell lines following PV treatment. PV
showed anti-proliferative effects against TPC-1 and FTC-133 cell
lines. The CCK-8 assay showed concentration and time-dependent
cytotoxicity of PV. The morphological changes in TPC-1 and FTC-133
cells incubated for 24 h with IC50 included clumping of
cells with round morphology, retraction and shrinking of cells. The
present results demonstrated that PV has anti-proliferative
potential, which may be useful in deriving the novel anticancer
drug for WDTC in the future. Fragmented and condensed nuclei in
cells are a hallmark of apoptotic induction (20). The cell death pathway that is
preferential is apoptosis, although necrosis may also occurred to a
certain extent, and these inferences have been substantiated using
AO/EB fluorescent staining and Hoechst staining (20,21) The
present study showed that the nuclei morphology of papillary
thyroid cancer cells was changed significantly following
IC50 PV treatment at 37°C for 24 h (Fig. 3). The present results revealed that PV
inhibited the proliferation of WDTC cell lines by an apoptotic
process. DNA fragmentation is generally considered to be the
hallmark of apoptosis (22). The
present DNA fragmentation data supports earlier observation that PV
induced the DNA fragmentation and subsequent cellular damage
(Fig. 4).
Mitochondria are cellular organelles, associated
with execution of apoptosis (23).
This is achieved by regulating the expression of the anti-apoptotic
protein Bcl-2 and the pro-apoptotic protein Bax (24). Bcl-2 and Bax come from the Bcl-2
family, which may induce apoptosis by inhibiting oxygen free
radicals, controlling intracellular Ca2+ influx,
preventing the release of cytochrome c and inhibiting p53
and c-myc (25). The present study
also demonstrated that the caspase family of cysteine aspartic
proteases performs a role at the start and finish of cell
apoptosis, and the caspase-3 cascade is the key point (26). In order to explore the molecular
mechanism underlying PV-induced cell apoptosis, the expression of
Bax, caspase-3 and Bcl-2 was detected by RT-qPCR.
The results showed that the mRNA levels of Bcl-2,
Bax and caspase-3 were changed following PV treatment, compared
with the control group (Fig. 5). It
was also revealed that PV may improve the level of the
pro-apoptotic protein Bax and downregulate the anti-apoptotic
protein Bcl-2 expression, activating the caspase-3 cascade and
inducing apoptosis. This may be one of the molecular mechanisms
through which PV induces apoptosis on WDTC.
In summary, the present study demonstrated that PV
may induce apoptosis in WDTC TPC-1 and FTC-133 cell lines, which
was associated with the Bcl-2, Bax and caspase-3 signaling
pathways.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no. 81372863)
and the Innovation Scientists and Technicians Troop Construction
Projects of Zhengzhou City (grant no. 131PLJRC676).
References
|
1
|
Yin D, Wu W, Li M, Wang QE, Li H, Wang Y,
Tang Y and Xing M: DKK3 is a potential tumor suppressor gene in
papillary thyroid carcinoma. Endocr Relat Cancer. 20:507–514. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pacini F, Castagna MG, Brilli L and
Pentheroudakis G; ESMO Guidelines Working Group, : Thyroid cancer:
ESMO clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 23 Suppl 7:vii110–vii119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soares P, Lima J, Preto A, Castro P,
Vinagre J, Celestino R, Couto JP, Prazeres H, Eloy C, Máximo V and
Sobrinho-Simões M: Genetic alterations in poorly differentiated and
undifferentiated thyroid carcinomas. Curr Genomics. 12:609–617.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gruber JJ and Colevas AD: Differentiated
thyroid cancer: Focus on emerging treatments for radioactive
iodine-refractory patients. Oncologist. 20:113–126. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kartal K, Onder S, Kosemehmetoglu K,
Kilickap S, Tezel YG and Kaynaroglu V: Methylation status of TSHr
in well-differentiated thyroid cancer by using cytologic material.
BMC Cancer. 15:8242015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gunda V, Bucur O, Varnau J, Borre P
Vanden, Bernasconi MJ, Khosravi-Far R and Parangi S: Blocks to
thyroid cancer cell apoptosis can be overcome by inhibition of the
MAPK and PI3K/AKT pathways. Cell Death Dis. 5:e11042014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li C, Huang Q, Fu X, Yue XJ, Liu RH and
You LJ: Characterization, antioxidant and immunomodulatory
activities of polysaccharides from Prunella vulgaris Linn. Int J
Biol Macromol. 75:298–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gu X, Li Y, Mu J and Zhang Y: Chemical
constituents of Prunella vulgaris. J Environ Sci (China). 25 Suppl
1:S161–S163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo Q and Chen Y: Textual research on
original plant and dietotherapy history of Prunella vulgaris.
Zhongguo Zhong Yao Za Zhi. 36:3057–3062. 2011.(In Chinese).
PubMed/NCBI
|
|
10
|
Hwang YJ, Lee EJ, Kim HR and Hwang KA:
NF-κB-targeted anti-inflammatory activity of Prunella vulgaris var.
lilacina in macrophages RAW 264.7. Int J Mol Sci. 14:21489–21503.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi JH, Han EH, Hwang YP, Choi JM, Choi
CY, Chung YC, Seo JK and Jeong HG: Suppression of PMA-induced tumor
cell invasion and metastasis by aqueous extract isolated from
Prunella vulgaris via the inhibition of NF-kappaB-dependent MMP-9
expression. Food Chem Toxicol. 48:564–571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Collins NH, Lessey EC, DuSell CD,
McDonnell DP, Fowler L, Palomino WA, Illera MJ, Yu X, Mo B, Houwing
AM and Lessey BA: Characterization of antiestrogenic activity of
the Chinese herb, prunella vulgaris, using in vitro and in vivo
(Mouse Xenograft) models. Biol Reprod. 80:375–383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao AG, Li T, You SF, Zhao HL, Gu Y, Tang
LD and Yang JK: Effects of Wei Chang An on expression of multiple
genes in human gastric cancer grafted onto nude mice. World J
Gastroenterol. 14:693–700. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang KJ, Zhang MZ, Wang QD and Liu WL:
The experimental research about the effect of Prunella vulgaris L.
on Raji cells growth and expression of apoptosis related protein.
Zhong Yao Cai. 29:1207–1210. 2006.(In Chinese). PubMed/NCBI
|
|
15
|
Feng L, Jia X, Zhu M, Chen Y and Shi F:
Chemoprevention by Prunella vulgaris L. extract of non-small cell
lung cancer via promoting apoptosis and regulating the cell cycle.
Asian Pac J Cancer Prev. 11:1355–1358. 2010.PubMed/NCBI
|
|
16
|
Vethakanraj HS, Babu TA, Sudarsanan GB,
Duraisamy PK and Kumar S Ashok: Targeting ceramide metabolic
pathway induces apoptosis in human breast cancer cell lines.
Biochem Biophys Res Commun. 464:833–839. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leibowitz B and Yu J: Mitochondrial
signaling in cell death via the Bcl-2 family. Cancer Biol Ther.
9:417–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hwang SM, Lee YJ, Lee YP, Yoon JJ, Lee SM,
Cha JD, Choi KM, Kang DG and Lee HS: Anti-proliferative effect of
an aqueous extract of Prunella vulgaris in vascular smooth muscle
cells. Evid Based Complement Alternat Med. 2013:9364632013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HI, Quan FS, Kim JE, Lee NR, Kim HJ,
Jo SJ, Lee CM, Jang DS and Inn KS: Inhibition of estrogen signaling
through depletion of estrogen receptor alpha by ursolic acid and
betulinic acid from Prunella vulgaris var. lilacina. Biochem
Biophys Res Commun. 451:282–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dhivya R, Jaividhya P, Riyasdeen A,
Palaniandavar M, Mathan G and Akbarsha MA: In vitro
antiproliferative and apoptosis-inducing properties of a
mononuclear copper (II) complex with dppz ligand, in two
genotypically different breast cancer cell lines. Biometals.
28:929–943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schmid I, Uittenbogaart C and Jamieson BD:
Live-cell assay for detection of apoptosis by dual-laser flow
cytometry using Hoechst 33342 and 7-amino-actinomycin D. Nat
Protoc. 2:187–190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahamad MS, Siddiqui S, Jafri A, Ahmad S,
Afzal M and Arshad M: Induction of apoptosis and antiproliferative
activity of naringenin in human epidermoid carcinoma cell through
ROS generation and cell cycle arrest. PLos One. 9:e1100032014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou WJ, Wang S, Hu Z, Zhou ZY and Song
CJ: Angelica sinensis polysaccharides promotes apoptosis in human
breast cancer cells via CREB-regulated caspase-3 activation.
Biochem Biophys Res Commun. 467:562–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hwang KT, Woo JW, Shin HC, Kim HS, Ahn SK,
Moon HG, Han W, Park IA and Noh DY: Prognostic influence of BCL2
expression in breast cancer. Int J Cancer. 131:E1109–E1119. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adams JM and Cory S: Bcl-2-regulated
apoptosis: Mechanism and therapeutic potential. Curr Opin Immunol.
19:488–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sabine VS, Faratian D, Kirkegaard-Clausen
T and Bartlett JM: Validation of activated caspase-3 antibody
staining as a marker of apoptosis in breast cancer. Histopathology.
60:369–371. 2012. View Article : Google Scholar : PubMed/NCBI
|