Introduction
Giant cell tumor of the bone (GCTB) is a primary
intramedullary bone tumor with variable growth potential (1). GCTB does not normally exhibit the
invasive growth pattern of malignant sarcomas; however, patients
often require a second or third surgical resection owing to
recurrence and metastasis following inadequate surgical treatment
by an inexperienced orthopedic surgeon (2,3). At the
Department of Orthapedic Oncology Surgery, Beijing Jishuitan
Hospital (Beijing, China), >50% of patients with GCTB present
with recurrent or metastatic lesions following surgical treatment
at an orthopedic center not specialized in orthopedic oncology.
A diagnosis of GCTB is confirmed through analysis of
the clinical, radiological and histopathological manifestation of
the disease. Patients with GCTB often present with pain and
swelling at the site of the lesion. Occasionally, a pathological
fracture is the direct reason for the patients' attendance at the
orthopedic clinic (Fig. 1) (4). X-ray and computed tomography (CT) scans
may show eccentric lytic lesions with a cortical extension.
Recently, with the increasing popularity of novel imaging
techniques, including positron emission tomography (PET)/CT, the
accuracy of bone tumor diagnosis has markedly improved (5). However, owing to the high cost of PET/CT
for this supposedly benign tumor (and thus the reluctance of the
clinician to order it) there are few studies concerning how GCTB
manifests in PET/CT scans (this is particularly true in Asian
countries). In the present study, the PET/CT scans from
histologically confirmed GCTB patients treated in the Department of
Orthopedic Oncology Surgery of Beijing Jishuitan Hospital were
retrospectively reviewed to investigate the diagnostic value of
PET/CT for patients with GCTB.
Patients and methods
Patient eligibility
The study was approved by the Ethical Committee of
Beijing Jishuitan Hospital (Beijing, China). All patients enrolled
in this study underwent PET/CT scans between January 2006 and July
2015 and were confirmed to have a pathological and clinical
diagnosis of GCTB.
Demographic characteristics of
included patients
Data from 20 patients (12 men and 8 women; mean age,
33.5±15.7 years; age range, 12–45 years) with complete PET/CT scans
and a pathologically and clinically confirmed diagnosis were
analyzed.
18F-fludeoxyglucose
(18F-FDG) PET/CT scanning
Prior to chemotherapy, patients underwent
18F-FDG PET testing. These tests were performed using a
PET/CT scanner, which was a combined full-ring PET scanner and
spiral CT scanner. When the patient's blood glucose level reached
<11.1 mmol/l following a 6-h fast, 400 ml barium sulfate was
administered, followed by an intravenous injection of 450 MBq
18F-FDG. PET and CT images were acquired from the base
of the head to the middle of the thigh, with a wider range of
observations taken if the tumor was located below the thigh.
Pathological examinations
Fine-needle and intraoperative biopsies were
performed by senior attending surgeons and the chief of surgery,
and examined by an experienced specialist from the Department of
Pathology, who was provided with all patient information other than
the results of the patient's PET/CT scan. The diagnosis was
confirmed by observing the resected tumors or from the follow-up
clinical and radiological materials of the patient. Once the
pathological and clinical diagnosis was confirmed, the results were
recorded and analyzed at the end of the study.
Statistical analysis
The SUVmax of each PET/CT scan was recorded and
analyzed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). The mean
age and SUVmax were calculated and recorded as the mean ± standard
deviation (SD).
Results
PET/CT scans detected 28 lesions on the 20 patients.
The SUVmax of these lesions ranged between 1.8 and 18.6, and the
mean SUVmax was 9.2±3.8.
Of the 20 patients included in this study, 9 had
never received any surgical or adjuvant therapies prior to the
PET/CT scan. As prior therapy could have altered the avidity of
tumor cells (thus affecting the SUVmax in PET/CT tests), data from
those patients were analyzed separately, in addition to the overall
analysis. The mean SUVmax of those 9 patients was 9.2±2.9 (range,
5.2–13.7). Although the mean SUVmax value was the same as for the
20 patients overall, the range of values and the SD were
smaller.
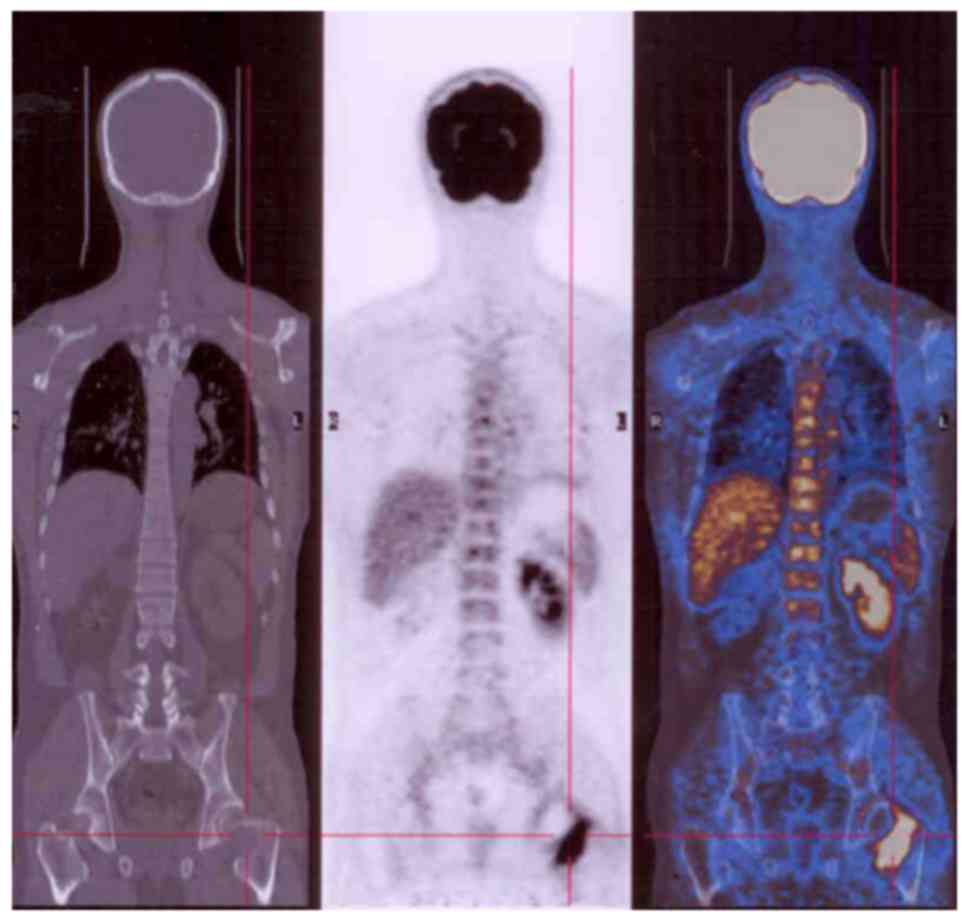
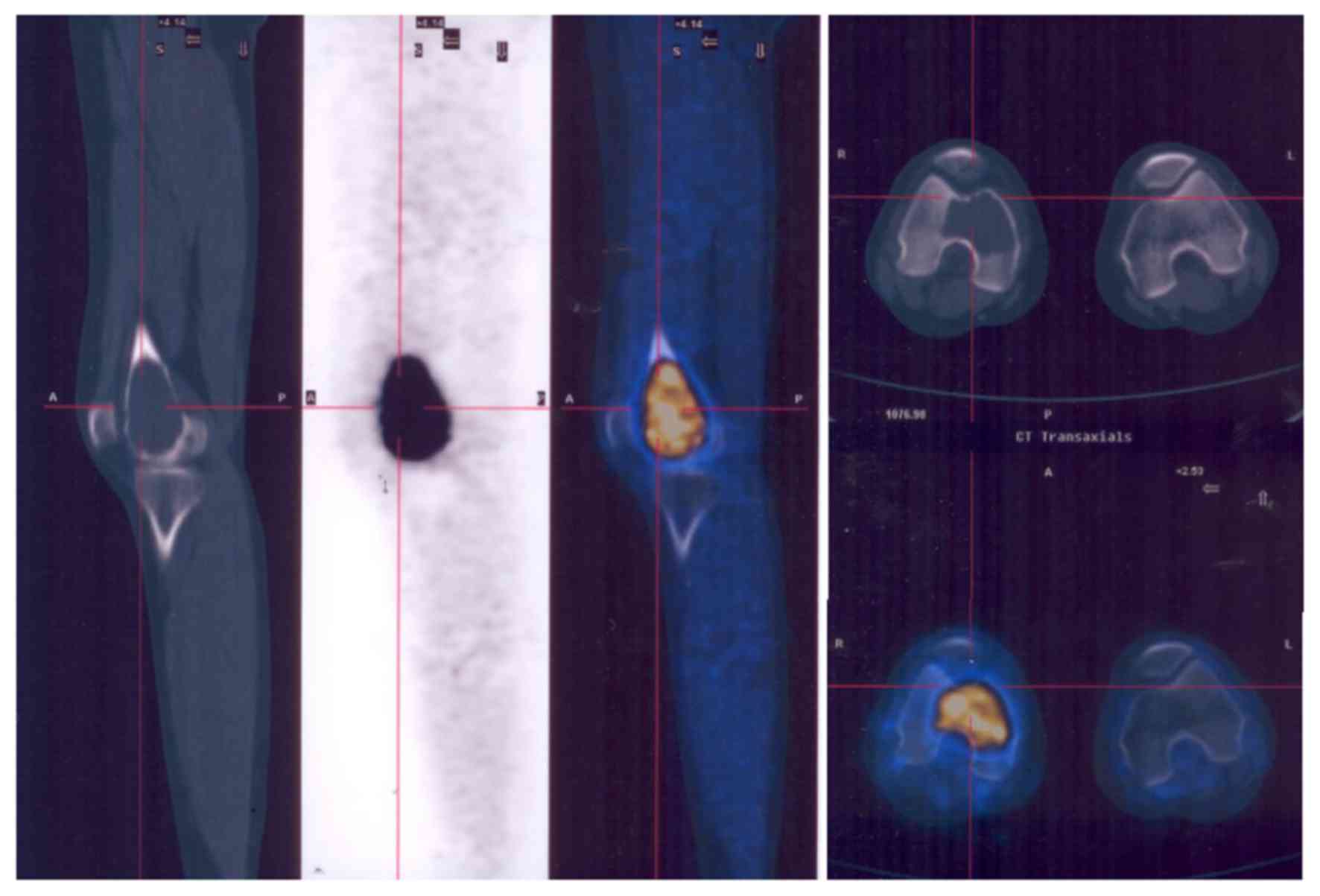
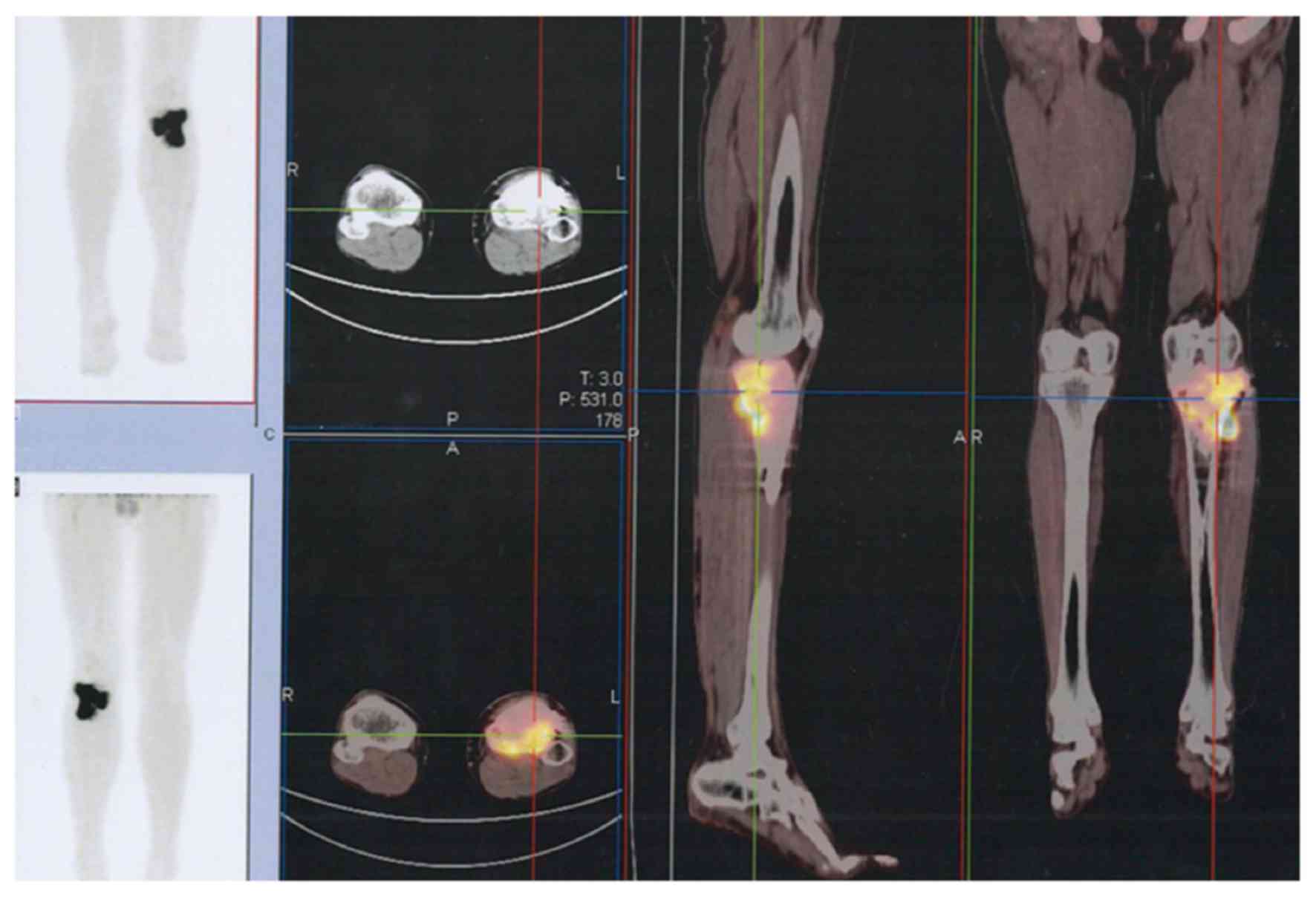
The majority of GCTBs were located in the
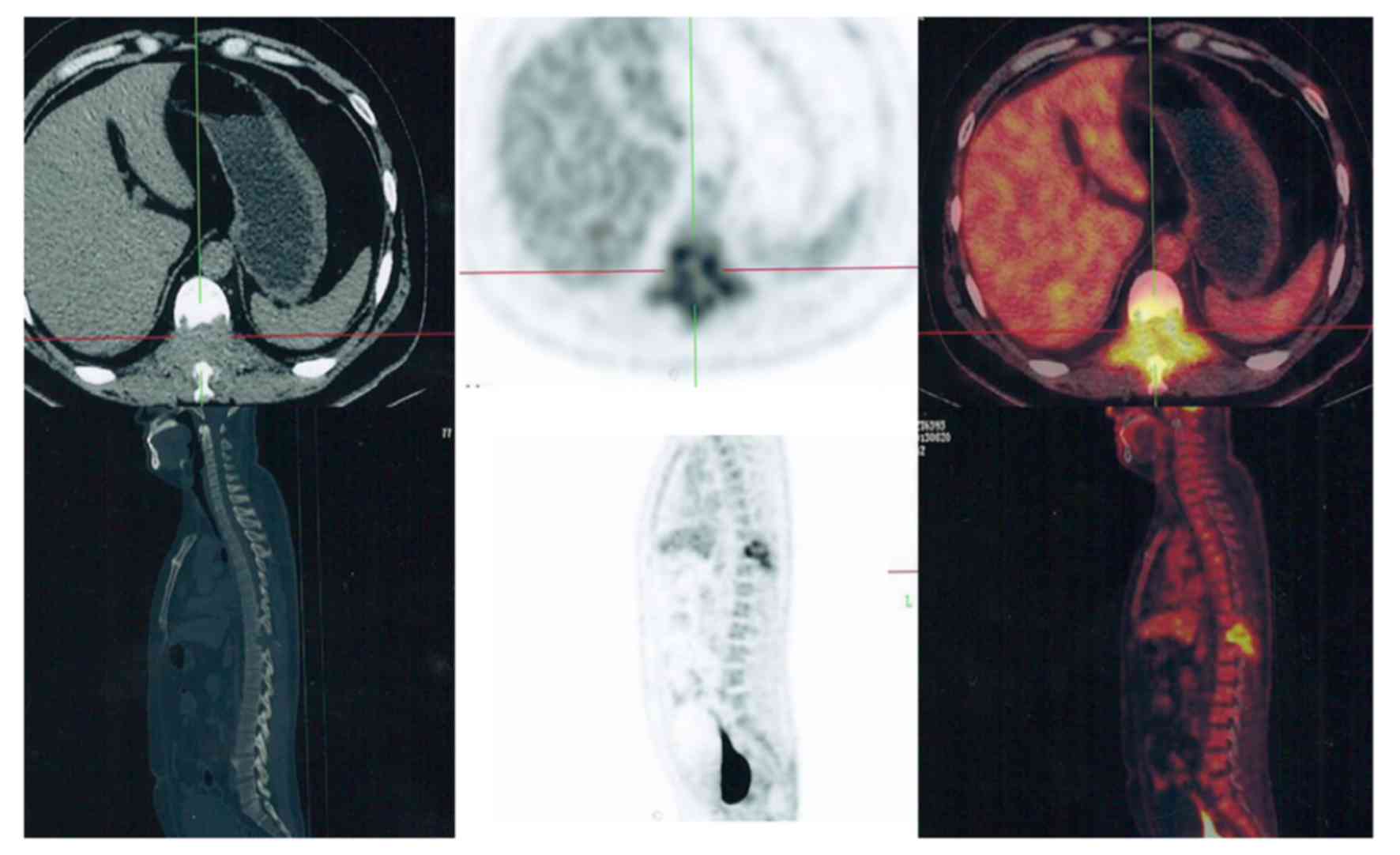
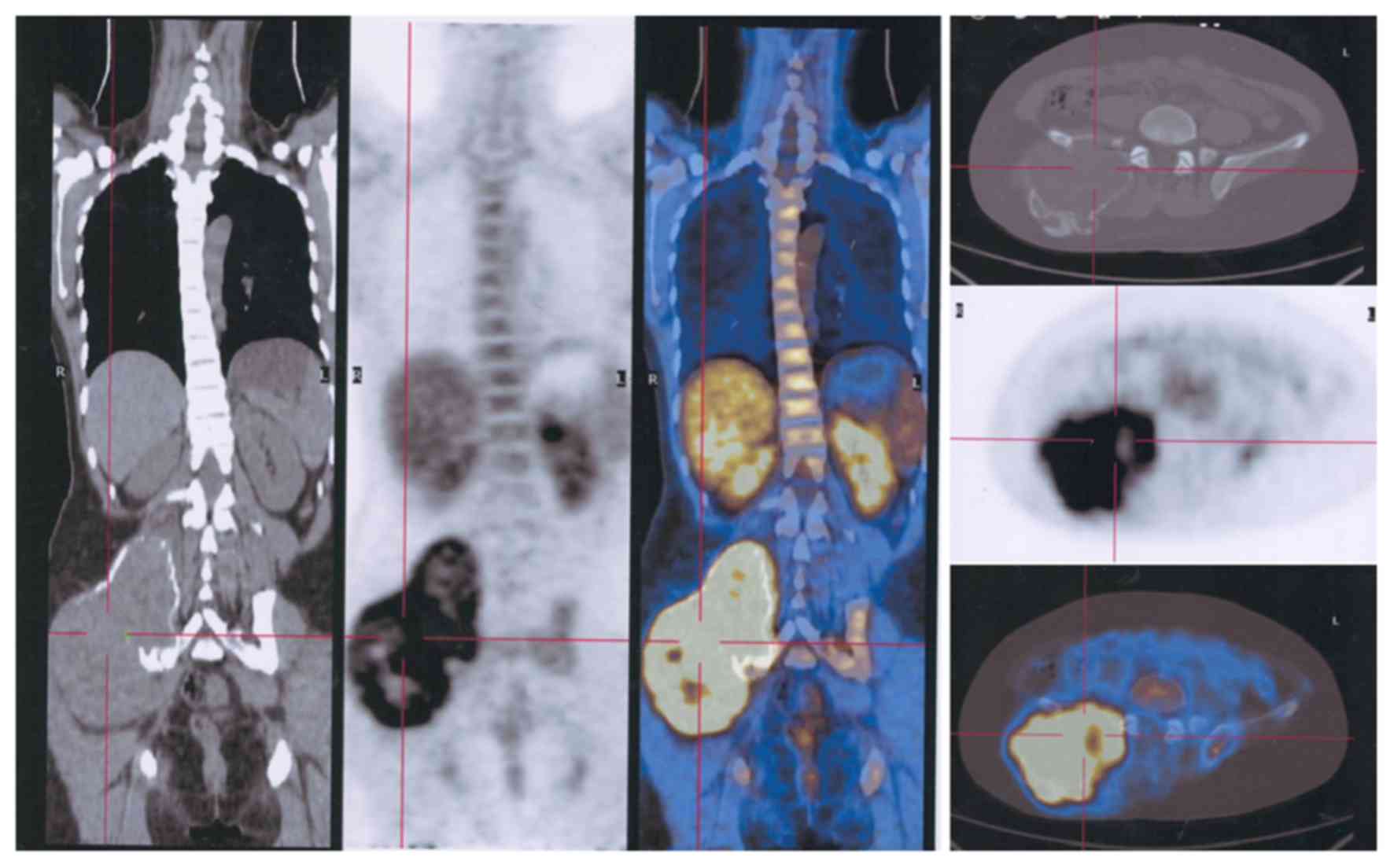
extremities (Fig. 2), but some spinal
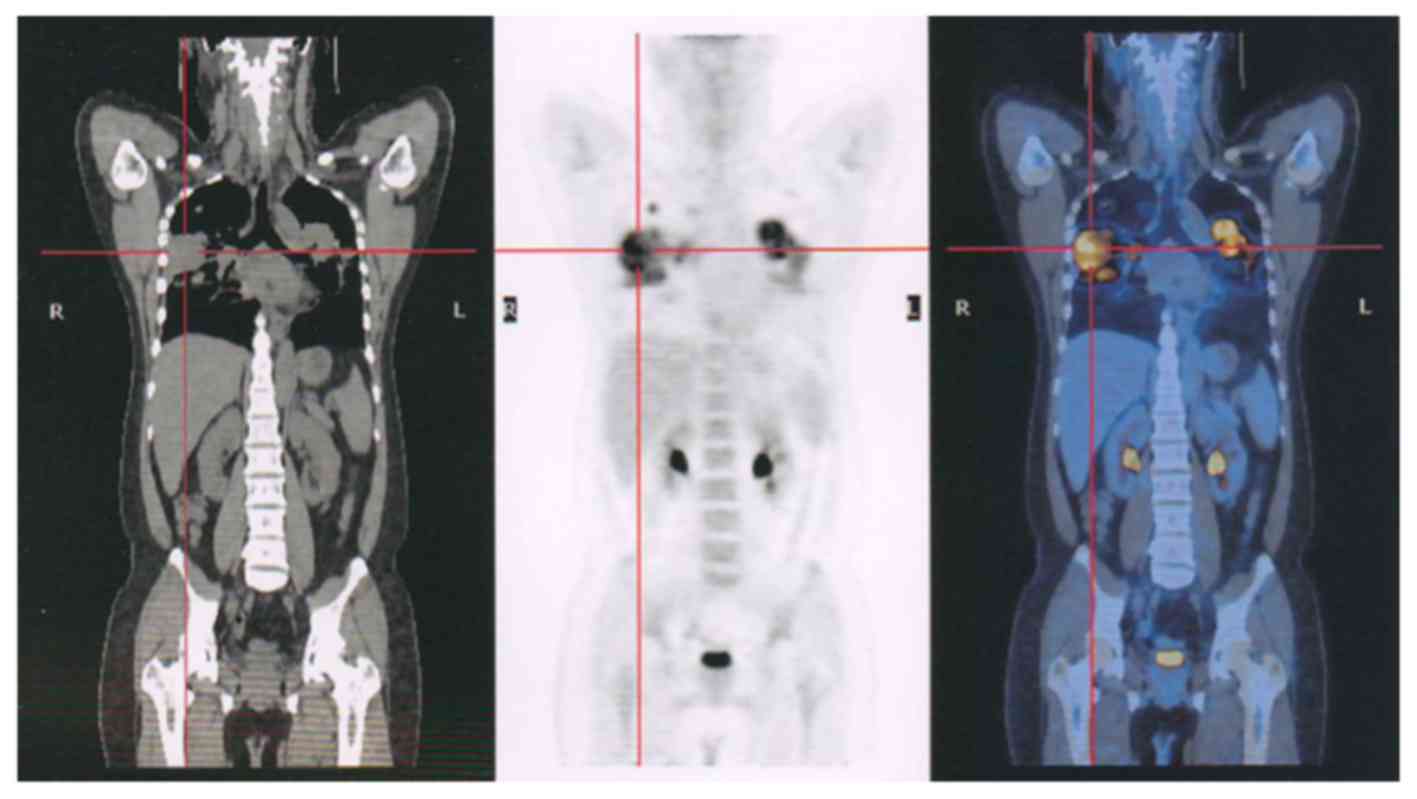
(Fig. 3) and pelvic (Fig. 4) lesions, in addition to several lung
metastases (Fig. 5), were observed.
To assess whether the PET/CT scans were affected by the location of
GCTB, SUVmax values were also analyzed according to their source
locations. The analysis revealed that the mean SUVmax of GCTB was
higher in the pelvis and spine than the extremities, however, the
difference was not significant (Table
I).
 | Table I.SUVmax of GCTB in different
locations. |
Table I.
SUVmax of GCTB in different
locations.
|
|
| SUVmax value |
|---|
|
|
|
|
|---|
| GCTB location | Patient no. | Lowest | Highest | Median | Mean ± SD |
|---|
| Femoral bone | 7 | 1.8 | 14.5 | 7.5 |
8.4±4.6 |
| Below knee | 8 | 3.9 | 14.4 | 7.8 |
8.3±3.5 |
| Spine | 4 | 7.6 | 13.7 | 10.0 | 10.4±2.7 |
| Pelvis | 3 | 5.2 | 18.6 | 12.6 | 12.1±6.7 |
| Lung | 4 | 4.5 | 10.8 | 9.9 |
8.8±2.9 |
Discussion
GCTB accounts for 5% of primary skeletal tumors and
21% of benign bone tumors (6,7). The tumor occurs primarily in the distal
femur, proximal tibia and distal radius (8–12), with
the incidence of GCTB in small bones being relatively rare
(13). Diagnostic parameters and
treatment measures of GCTB remain controversial among surgeons
(14). In general, GCTB is
metabolically benign and appears primarily at a single site;
however, a considerable portion of patients present with GCTB in
multiple locations. In the present study, 28 GCTB lesion sites in
20 patients were analyzed, 4 of which were GCTs that had
metastasized to the lung following the recurrence of GCTB in the
extremities (Figs. 5 and 6). The aggressiveness of GCTB can differ
between individuals, which could cause local destruction and
metastasis (15). Previous studies
have reported that GCTB metastasizes to the lymph nodes, liver,
soft tissue, brain, mediastinum, scalp, kidney and penis (16–19).
Histologically, the tumor consists of
osteoclast-like and spindle-shaped multinucleated giant cells
(20). These giant cells share the
same specific markers as osteoclasts, including tartrate-resistant
acid phosphatase (21), cathepsin K
(22), carbonic anhydrase II
(23), calcitonin receptor (24) and receptor activator of nuclear factor
κ-light-chain-enhancer of activated B cells (25), and are similarly capable of bone
resorption. Giant cells are, however, substantially larger than the
osteoclasts and contain hundreds of nuclei (26). Previous studies have provided
clinicians with diagnostic genetic markers of GCTB, including tumor
proteins 53, 63 and 73, kinectin, nebulin, ρ-associated coiled-coil
containing protein kinase 1, and sterile α-motif and leucine
zipper-containing kinase AZK (27–29).
Unlike the majority of non-malignant tumors, the
majority of GCTB lesions in the present study exhibited the
intensive uptake of 18F-FDG. This could be explained by
the cellular consistency of GCTB. Previous studies have
demonstrated that glucose transporter type 1 (GLUT-1) is
upregulated in human macrophages (30,31) and
that the avidity of 18F-FDG for GLUT-1 is positively
associated with GLUT-1 overexpression (32,33). In a
previous study, Hoshi et al suggested that the avidity of
18F-FDG was closely associated with strong hexokinase-2
activity in giant and spindle cells (34). It is possible that the high SUVmax of
GCTB obtained from the PET/CT scans is a result of GLUT-1 and
hexokinase-2 overexpression in macrophages and giant cells in the
tumor. The results of the present study suggest that, in order to
avoid excessive and unnecessary medical treatment, a diagnosis of
GCTB should be considered when PET/CT reveals the presence of a
bone lesion with intense 18F-FDG uptake that is
suggestive of a high-grade osseous sarcoma.
References
|
1
|
Enneking WF: Musculoskeletal tumor
surgery. Churchill Livingstone; New York, NY: 1983
|
|
2
|
Gong L, Liu W, Sun X, Sajdik C, Tian X,
Niu X and Huang X: Histological and clinical characteristics of
malignant giant cell tumor of bone. Virchows Arch. 460:327–334.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feigenberg SJ, Marcus RB Jr, Zlotecki RA,
Scarborough MT and Enneking WF: Whole-lung radiotherapy for giant
cell tumors of bone with pulmonary metastases. Clin Orthop Relat
Res. 1–208. 2002.
|
|
4
|
Ropars M, Kaila R, Cannon SR and Briggs
TW: Primary giant cell tumours of the digital bones of the hand. J
Hand Surg Eur Vol. 32:160–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tian R, Su M, Tian Y, Li F, Li L, Kuang A
and Zeng J: Dual-time point PET/CT with F-18 FDG for the
differentiation of malignant and benign bone lesions. Skeletal
Radiol. 38:451–458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lackman RD, Hosalkar HS, Ogilvie CM,
Torbert JT and Fox EJ: Intralesional curettage for grades II and
III giant cell tumors of bone. Clin Orthop Relat Res. 438:123–127.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kivioja AH, Blomqvist C, Hietaniemi K,
Trovik C, Walloe A, Bauer HC, Jorgensen PH, Bergh P and Follerås G:
Cement is recommended in intralesional surgery of giant cell
tumors: A Scandinavian Sarcoma Group study of 294 patients followed
for a median time of 5 years. Acta Orthop. 79:86–93. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niu X, Zhang Q, Hao L, Ding Y, Li Y, Xu H
and Liu W: Giant Cell Tumor of the Extremity: Retrospective
analysis of 621 Chinese patients from one institution. J Bone Joint
Surg Am. 94:461–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moon JC, Kim SR, Chung MJ and Lee YC:
Multiple pulmonary metastases from giant cell tumor of a hand. Am J
Med Sci. 343:171–173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Osaka S, Sugita H, Osaka E, Yoshida Y, Ryu
J, Hemmi A and Suzuki K: Clinical and immunohistochemical
characteristics of benign giant cell tumour of bone with pulmonary
metastases: Case series. J Orthop Surg (Hong Kong). 12:55–62. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim TS and Park JS: Metastasising
recurrent giant cell tumor: A case report. J Korean Bone Joint
Tumor Soc. 7:73–79. 2001.
|
|
12
|
Mirra JM: Giant cell tumorsBone tumors:
clinical, radiologic and pathologic correlations. Lea and Febiger.
Philadelphia, PA: pp. 941–1020. 1989
|
|
13
|
Averill RM, Smith RJ and Campbell CJ:
Giant cell tumors of the bones of the hand. J Hand Surg Am.
5:39–50. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balke M, Schremper L, Gebert C, Ahrens H,
Streitbuerger A, Koehler G, Hardes J and Gosheger G: Giant cell
tumor of bone: Treatment and outcome of 214 cases. J Cancer Res
Clin Oncol. 134:969–978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takeuchi A, Tsuchiya H, Niu X, Ueda T,
Jeon DG, Wang EH, Asavamongkolkul A, Kusuzaki K, Sakayama K and
Kang YK: The prognostic factors of recurrent GCT: A cooperative
study by the Eastern Asian Musculoskeletal oncology group. J Orthop
Sci. 16:196–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muheremu A and Niu X: Pulmonary metastasis
of giant cell tumor of bones. World J Surg Oncol. 12:2612014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jacopin S, Viehweger E, Glard Y, Launay F,
Jouve JL, Bouvier C and Bollini G: Fatal lung metastasis secondary
to index finger giant cell tumor in an 8-year-old child. Orthop
Traumatol Surg Res. 96:310–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kremen TJ Jr, Bernthal NM, Eckardt MA and
Eckardt JJ: Giant cell tumor of bone: Are we stratifying results
appropriately? Clin Orthop Relat Res. 470:677–683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Muheremu A, Huang Z and Niu X: Treatment
for giant cell tumor of the spine metastasizing to the lung: A
report of two cases and a literature review. Oncol Lett.
9:1321–1326. 2015.PubMed/NCBI
|
|
20
|
Werner M: Giant cell tumour of bone:
Morphological, biological and histogenetical aspects. Int Orthop.
30:484–489. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anazawa U, Hanaoka H, Shiraishi T, Morioka
H, Morii T and Toyama Y: Similarities between giant cell tumor of
bone, giant cell tumor of tendon sheath, and pigmented villonodular
synovitis concerning ultrastructural cytochemical features of
multinucleated giant cells and mononuclear stromal cells.
Ultrastruct Pathol. 30:151–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lindeman JH, Hanemaaijer R, Mulder A,
Dijkstra PD, Szuhai K, Bromme D, Verheijen JH and Hogendoorn PC:
Cathepsin K is the principal protease in giant cell tumor of bone.
Am J Pathol. 165:593–600. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kotake S, Sato K, Kim KJ, Takahashi N,
Udagawa N, Nakamura I, Yamaguchi A, Kishimoto T, Suda T and
Kashiwazaki S: Interleukin-6 and soluble interleukin-6 receptors in
the synovial fluids from rheumatoid arthritis patients are
responsible for osteoclast-like cell formation. J Bone Miner Res.
11:88–95. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Collier FM, Huang WH, Holloway WR, Hodge
JM, Gillespie MT, Daniels LL, Zheng MH and Nicholson GC:
Osteoclasts from human giant cell tumors of bone lack estrogen
receptors. Endocrinology. 139:1258–1267. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Atkins GJ, Kostakis P, Vincent C, Farrugia
AN, Houchins JP, Findlay DM, Evdokiou A and Zannettino AC: RANK
expression as a cell surface marker of human osteoclast precursors
in peripheral blood, bone marrow, and giant cell tumors of bone. J
Bone Miner Res. 21:1339–1349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Balke M, Campanacci L, Gebert C, Picci P,
Gibbons M, Taylor R, Hogendoorn P, Kroep J, Wass J and Athanasou N:
Bisphosphonate treatment of aggressive primary, recurrent and
metastatic giant cell tumour of bone. BMC Cancer. 10:4622010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cowan RW and Singh G: Giant cell tumor of
bone: A basic science perspective. Bone. 52:238–246. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Graziano V and De Laurenzi V: Role of p63
in cancer development. Biochim Biophys Acta. 1816:57–66.
2011.PubMed/NCBI
|
|
29
|
Babeto E, Conceição AL, Valsechi MC,
Junior P Peitl, de Campos Zuccari DA, de Lima LG, Bonilha JL, de
Freitas Calmon M, Cordeiro JA and Rahal P: Differentially expressed
genes in giant cell tumor of bone. Virchows Arch. 458:467–476.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fu Y, Maianu L, Melbert BR and Garvey WT:
Facilitative glucose transporter gene expression in human
lymphocytes, monocytes, and macrophages: A role for GLUT isoforms
1, 3, and 5 in the immune response and foam cell formation. Blood
Cells Mol Dis. 32:182–190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Malide D, Davies-Hill TM, Levine M and
Simpson IA: Distinct localization of GLUT-1, −3 and −5 in human
monocyte-derived macrophages: Effects of cell activation. Am J
Physiol. 274:E516–E526. 1998.PubMed/NCBI
|
|
32
|
Chung JH, Cho KJ, Lee SS, Baek HJ, Park
JH, Cheon GJ, Choi CW and Lim SM: Overexpression of Glut1 in
lymphoid follicles correlates with false-positive (18)F-FDG PET
results in lung cancer staging. J Nucl Med. 45:999–1003.
2004.PubMed/NCBI
|
|
33
|
Horiuchi C, Tsukuda M, Taguchi T, Ishiguro
Y, Okudera K and Inoue T: Correlation between FDG-PET findings and
GLUT1 expression in salivary gland pleomorphic adenomas. Ann Nucl
Med. 22:693–698. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hoshi M, Takada J, Oebisu N, Hata K,
Ieguchi M and Nakamura H: Overexpression of hexokinase-2 in giant
cell tumor of bone is associated with false positive in bone tumor
on FDG-PET/CT. Arch Orthop Trauma Surg. 132:1561–1568. 2012.
View Article : Google Scholar : PubMed/NCBI
|