Introduction
Genetic stability ensures the inheritance of the
correct genetic information and preserves the function of normal
physiological processes. However, cells living in a constantly
changing environment are influenced by various stresses, which may
alter DNA sequences and induce DNA damage (1). During the evolution of genes, cells have
developed a DNA damage response system including cell cycle
checkpoints, senescence and apoptosis (2). If that system is not able to repair DNA
damage, DNA replication, transcription and recombination may be
altered, leading to gene mutation and chromosomal rearrangements or
loss, which promotes the development of cancer (3). Wild-type p53-induced phosphatase 1
(Wipl) is an oncogene that negatively regulates the DNA damage
response system and serves a role in tumorigenesis, therapy and
prognosis in various types of human cancer (4).
Wip1 was originally identified as a target protein
in the p53-dependent response to ionizing radiation (5). Wip1 is a serine/threonine protein
phosphatase that is encoded by the protein phosphatase
magnesium-dependent 1 δ (gene, PPM1D) in the 17q22/q24 human
chromosomal region, and is a member of the protein phosphatase type
2C (PP2C) family (6). It is 605 amino
acids long and consists of a central phosphatase catalytic domain
and a non-functional region (7). Wip1
is a monomeric enzyme, similar to other members of the PP2C family,
the dephosphorylation of which requires catalysis by bivalent
cations, including magnesium and manganese ions (5).
Previous studies have revealed that Wip1
dephosphorylates several key DNA damage response proteins,
including p53, ataxia telangiectasia mutated, checkpoint kinase
(Chk) 1, Chk2, murine double minute 2 (Mdm2) and p38 mitogen
activated protein kinases (p38 MAPK), exercising negative feedback
loops that lead to cell cycle arrest, increased tumorigenesis and
the inhibition of apoptosis (Fig. 1)
(8–12). Among these loops, negative regulation
of p53 is vital. TP53 may be the most important tumor
suppressor gene, the mutation or depletion of which is present in
~50% of all human tumors (13).
However, Wip1 is not only able to directly dephosphorylate p53
protein at serine 15, but also indirectly inactivate p53 protein
through p38 MAPK and Mdm2 (8,14,15), which
attenuates the p53 function. Furthermore, dephosphorylation of p53
by Wip1 induces inappropriate re-initiation of mitosis and
uncontrolled polyploid progression that may be a potential
underlying mechanism of tumor progression (14). Previous studies have identified
additional Wip1 targets, including murine double minute X,
xeroderma pigmentosum complementation group A and C, nuclear factor
kappa B (NF-κB) and DNA methylation, resulting in the promotion of
proliferation, inhibition of inflammation and nucleotide excision
repair (9,16–19). On
the other hand, cytotoxic drugs, including cisplatin and
doxorubicin, are able to induce senescence and apoptosis in tumor
cells, an effect that is dependent on p53 signaling pathway in
vitro and in vivo (20,21). This
indicates that Wip1 phosphatase activity may mediate the
cytotoxicity of chemotherapeutic agents via targeting p53. The
present review summarizes the regulatory mechanisms and functions
of Wip1 as an oncogene in various types of cancer. In addition, the
potential role of Wip1 as a tumor biomarker and therapeutic target
in these cancer types was investigated.
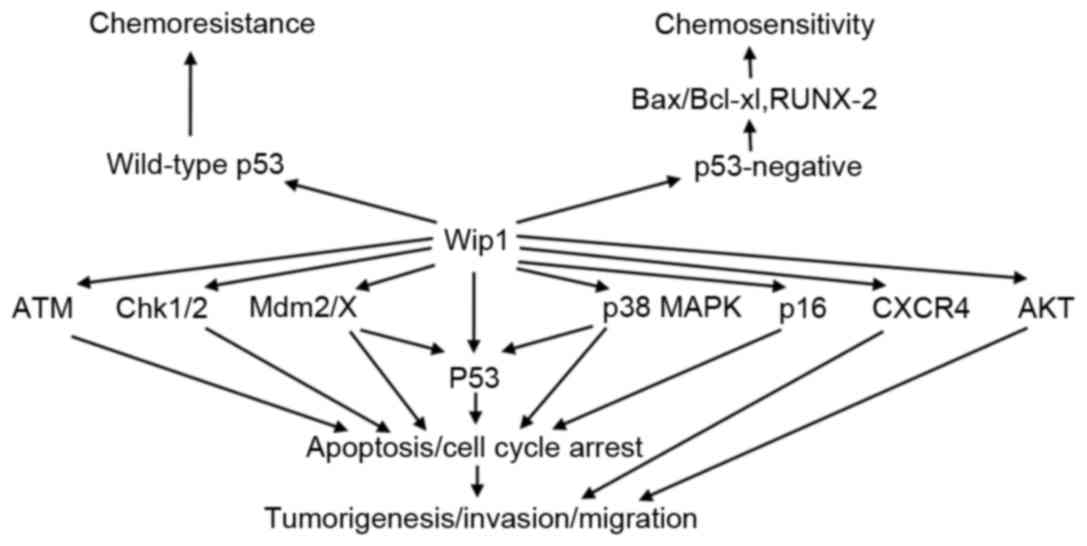 | Figure 1.Targets and functional consequences
of Wip1 signaling. Wip1 directly dephosphorylates target proteins
including ATM, Chk1/2, Mdm2/X, p53, p38 MAPK, p16, CXCR4 and AKT,
resulting in inhibition of apoptosis and cell cycle arrest, which
promotes tumorigenesis, invasion and migration. Wip1 leads to
chemoresistance in tumor cell with wild-type p53. However, Wip1
increases chemosensitivity in p53-negative tumor cells by
regulating Bax/Bcl-xl and RUNX-2. P38 MAPK, p38 mitogen activated
protein kinases; Chk1/2, checkpoint kinase 1/2; Mdm2/X, murine
double minute 2/X; CXCR4, C-X-C chemokine receptor type 4; AKT,
protein kinase B; Bax, B-cell lymphoma-2 associated X protein;
Bcl-xl, B-cell lymphoma-xl; RUNX-2, runt-related transcription
factor-2; ATM, ataxia telangiectasia mutated; Wip1, wild-type
p53-induced phosphatase 1. |
Wip1 in breast cancer
The role of Wip1 in breast cancer is the most
studied compared with all other types of human cancer (22). In 28% of primary breast cancer cases,
the amplification of the 17q22/q24 chromosomal region has been
demonstrated through cytogenetic analysis, a phenomenon that is
more common in high-grade breast cancer (23). In addition, a number of studies have
identified that the overexpression of Wip1 negatively regulates the
p53, p38 MAPK and p16 signaling pathways, which may lead to breast
cancer tumorigenesis, proliferation and poor prognosis (24,25).
Previous reports have demonstrated that the upregulation of Wip1
may reverse the induction of apoptosis by microRNA (miRNA/miR)-16
and miRNA-34a, which are tumor suppressors of breast cancer
(26,27). Therefore, high Wip1 expression levels
may be a predisposing factor for breast cancer. Spike and Wahl
(28) revealed that Wip1 regulated
chemosensitivity by controlling the p53 signaling pathway.
Downregulation of Wip1 enhanced the chemosensitivity of breast
cancer to adriamycin via targeting wild-type p53 and reducing cell
growth and cell survival; however, these effects were not present
in cell lines with mutant-type p53 (Table
I; Fig. 1) (29,30).
Although the occurrence of breast cancer is regulated by various
oncogenes, including ErbB2, Wnt1 and breast cancer susceptibility
protein type 1 and 2 (22), these
results suggested that Wip1 may be considered as a potential
biomarker for tumorigenesis and index of prognosis in patients with
breast cancer. In addition, decreasing its expression levels may
have a therapeutic effect during the chemotherapy of breast cancer
with wild-type p53.
 | Table I.Roles of Wip1 in various types of
cancer. |
Table I.
Roles of Wip1 in various types of
cancer.
| Cancer type |
Overexpression/mutation level, % | Targets | Tumorigenesis |
Differentiation |
Chemoresistance | Prognosis |
|---|
| Breast | 28 | p53, p16, p38
MAPK | + | + | + | + |
| Glioma | 37.5 | Chk2, p53 | + | ND | ND | ND |
| Neuroblastoma | 56 | Mdm2, p53 | + | + | + | + |
|
Medulloblastoma | 64 | Mdm2, p53, CXCR4,
AKT | + | + | ND | + |
| Ovarian clear cell
carcinoma | 40 | Chk1, p53 | + | ND | + | + |
| Liver | 65 | P53 | + | + | ND | + |
| Bladder | ND | P53 | + | ND | + | ND |
| Kidney | 67 | ND | + | + | ND | + |
| Nasopharyngeal
carcinoma | 69 | ND | + | + | ND | + |
Wip1 in childhood glioma
Overexpression of Wip1 and gain-of-function
mutations of PPM1D have been detected in numerous types of
pediatric cancer, including glioma (31), neuroblastoma (32) and medulloblastoma (33).
Wip1 in glioma
Zhang et al (34) identified that carboxy terminal
truncating mutations of PPM1D occur in 37.5% of glioma
cases, and these gain-of-function PPM1D mutants suppressed
phosphorylation of Chk2 at threonine 68 and p53 at serine 15,
resulting in dysfunction of the DNA damage response network
(Table I) (34). This result may be associated with
predisposition to and the tumorigenesis of glioma.
Wip1 in neuroblastoma
Overexpression of Wip1 in neuroblastoma may repress
p53 function by two signaling pathways, one is the Wip1-p53
pathway, and the other is the Wip1-Mdm2-p53 pathway (35), resulting in tumorigenesis. In
addition, a previous report demonstrated that Wip1 was
significantly overexpressed in 56% of cancer tissues, and promoted
tumor progression to a higher stage, poor prognosis and
chemotherapy resistance (Table I)
(32). Therefore, these data suggest
that the inhibition of Wip1 expression levels may be a potential
therapeutic target. GSK2830371 is a Wip1 selective antagonist able
to significantly inhibit 96.5% of Wip1 activity in neuroblastoma
cell lines, which promotes p53 function and apoptotic responses
(32). Furthermore, GSK2830371 had a
synergistic effect on the antiproliferative properties of the
chemotherapeutic agents adriamycin and carboplatin (32).
Wip1 in medulloblastoma
Previous studies have reported that the
amplification and overexpression of Wip1 occurred in 64% of human
medulloblastomas, and it is more common in highly aggressive
medulloblastomas (36,37). Buss et al (37) also identified that high levels of Wip1
expression were associated with increased expression of Mdm2, which
may be an underlying mechanism of promoting medulloblastoma growth
via targeting p53 (37). In addition,
Pfister et al (38)
demonstrated that the upregulation of Wip1 expression was
associated with poor prognosis in medulloblastoma (Table I) (38).
Furthermore, the results of a previous study have revealed that
Wip1 is able to promote the progression and invasion of aggressive
medulloblastoma by regulating C-X-C chemokine receptor type 4 and
protein kinase B (Akt; Fig. 1)
(33). These data suggest that Wip1
serves an important role in tumorigenesis and cancer progression
and may be an indicator for the prognosis of medulloblastoma.
In pediatric types of cancer, overexpression of Wip1
contributes to a number of malignant characteristics, including
tumor progression, aggressive phenotype and poor prognosis
(32,34,37). In
addition, a Wip1 inhibitor may be a promising novel candidate for
targeted therapeutic strategies for these severe tumors.
Wip1 in ovarian clear cell carcinoma
A previous study identified that the amplification
and overexpression of the PPM1D gene occurred in ≥40% of
cases of ovarian clear cell carcinoma, which is higher compared
with other ovarian tumor subtypes (39). A gene knockdown study revealed the
viability of ovarian clear cell carcinoma cell lines depended on
the phosphatase activity of Wip1, indicating that the Wip1 and
PPM1D genes may be drivers of ovarian clear cell carcinoma
(40). Another previous study
reported that Wip1 is able to negatively regulate the Chk1 and p53
signaling pathway, resulting in tumorigenesis (41). However, cisplatin mediates tumor cell
DNA damage and apoptotic function through these signaling pathways,
suggesting that Wip1 may be responsible for the cisplatin
resistance of ovarian clear cell carcinoma (41). In addition, a recent study
demonstrated that Akt confers cisplatin resistance in part through
the regulation of PPM1D protein stability, preventing its
proteasomal degradation and consequently increasing its half-life
(Table I) (42). Accumulating evidence has indicated
that Wip1 is directly associated with tumor cell survival and
chemoresistance (43). Due to its
late diagnosis and the development of chemoresistance, ovarian
clear cell carcinoma is characterized by the poorest prognosis
among ovarian types of cancer (44).
Therefore, Wip1 expression levels may be a biomarker of diagnosis
and index of prognosis. In addition, targeting Wip1 may improve
therapeutic outcomes in ovarian clear cell carcinoma.
Wip1 in liver cancer
miR-29c belongs to the miR-29 family, which are
established tumor suppressors (45).
A previous study demonstrated that miR-29c is downregulated in
liver cancer and may affect the apoptosis, tumorigenesis, and
prognosis of tumor cells (46).
Previous studies have revealed that the overexpression of miR-29c
inhibits cancer cell proliferation and metastasis, as well as
inducing cell cycle arrest (47,48). Wip1
was also revealed to be significantly upregulated in hepatocellular
carcinoma and may contribute to the development of this cancer
(49). Wang et al (49) were the first to investigate the
association between Wip1 and miR-29c, revealing an inverse
correlation. In addition, the overexpression of Wip1 may suppress
miR-29c-induced apoptosis and cell cycle arrest via
dephosphorylating wild-type p53 (49). Although mutations of p53 occur
in ~50% of human cancer cases, this rate is <30% for liver
cancer cases, the majority of which express wild-type p53
(50). These findings suggest that
Wip1 and miR-29c serve roles in the development of hepatocellular
carcinoma. Furthermore, a previous study demonstrated that Wip1 was
overexpressed in hepatocellular carcinoma tissues, compared with in
non-cancerous tissues, and high Wip1 expression levels were
associated with a more advanced tumor-node-metastasis stage, as
well as being a significantly independent prognostic factor
(51). Therefore, Wip1 not only
participates in tumorigenesis but also indicates poor prognosis in
liver cancer.
Wip1 in bladder cancer
Amplification and overexpression of Wip1 were also
identified in bladder cancer (52).
Wang et al (53) demonstrated
that RNA interference of PPM1D inhibited bladder cancer cell
proliferation and tumorigenesis in mice, potentially through
targeting of the p53, p38 MAPK and Akt signaling pathways. This
indicates that targeting PPM1D may be a potential
therapeutic strategy for the treatment of bladder cancer.
Furthermore, Lin et al (54)
revealed that the level of Wip1 expression in cisplatin-resistant
bladder cancer was high compared with the control tumor tissue
(54). In addition, loss of
homeodomain-interacting protein kinase-2 enhanced Wip1 expression,
which subsequently increased tumor cell viability in cell lines
with wild-type p53 during cisplatin treatment (54). These results suggest that Wip1
upregulation decreases the tumor response to cisplatin, resulting
in tumor cell-survival and resistance to apoptosis that is induced
by chemotherapeutic drugs. Therefore, Wip1 may lead to chemotherapy
resistance in bladder cancer. Conversely, previous studies have
revealed that chemotherapy resistance induced by Wip1 is dependent
on the presence of wild-type p53; however, in p53-negative cell
lines, Wip1 sensitizes tumor cells to chemotherapeutic drugs by
regulating the B-cell lymphoma-2 associated X protein:B-cell
lymphoma-extra large ratio and runt related transcription factor-2
and protects normal tissues (Fig. 1)
(55,56). Therefore, Wip1 inhibition is a
potential therapeutic target in bladder cancer with preserved
wild-type p53, but the reverse effect may occur in p53-negative
tumors; however, this remains to be elucidated.
Wip1 in kidney cancer
Two previous studies demonstrated that Wip1 is
amplified and overexpressed in kidney cancer, the pathological
types of which included clear cell type, granule cell type and
papillary cell type (57,58). These reports revealed that Wip1
expression levels are correlated with the clinical characteristics
of kidney cancer, including lymph node metastasis, distant
metastasis, Fuhrman grade, clinical stage and pathological
differentiation (57,58). In addition, these studies also
identified that patients with high levels of Wip1 expression had
significantly lower survival rates than those with low levels of
Wip1 expression, and the downregulation of Wip1 promoted apoptosis
and decreased migration and invasion in kidney cancer cell lines
(Table I) (57,58). These
results indicate that Wip1 may serve an important role in the
tumorigenesis and the progression of kidney cancer.
Wip1 in nasopharyngeal carcinoma
Sun et al (59)
also observed the tumorigenic action of Wip1 in nasopharyngeal
carcinoma, leading to a more aggressive grade, distant metastasis
and a poorer prognosis (Table I).
Therefore, all the aforementioned evidence suggests that Wip1
overexpression promotes tumorigenesis in a number of solid tumors
and indicates that Wip1 is a potential molecular target for tumor
therapy.
Conclusion
Wip1 has received increasing attention since it was
first identified in 1997 (5). A
number of studies have demonstrated that Wip1 negatively regulates
various signaling pathways and feedback loops, particularly
p53-induced mechanisms, resulting in tumorigenesis of multiple
tissues and organs. In addition, Wip1 expression serves a critical
role in the progression, migration, invasion and apoptosis of
cancer. As an oncogene, its expression levels indicate a poor
prognosis of disease. Therefore, Wip1 may act as a potential tumor
biomarker, therapeutic target and index of prognosis in various
types of cancer.
In conclusion, a number of studies have demonstrated
that Wip1 is an attractive chemotherapeutic target. Its
overexpression and amplification increases chemotherapy resistance
in tumors with wild-type p53, but the reverse of this effect is
observed in p53-negative tumor cells. However, the underlying
mechanisms by which Wip1 affects chemotherapy remain to be
investigated.
Acknowledgements
The present study was supported by the Capital
Health Research and Development of Special Grants (grant no.
2011-2002-05).
References
|
1
|
Jackson SP and Bartek J: The DNA-damage
response in human biology and disease. Nature. 461:1071–1078. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harper JW and Elledge SJ: The DNA damage
response: Ten years after. Mol Cell. 28:739–745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lord CJ and Ashworth A: The DNA damage
response and cancer therapy. Nature. 481:287–294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Le Guezennec X and Bulavin DV: WIP1
phosphatase at the crossroads of cancer and aging. Trends Biochem
Sci. 35:109–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fiscella M, Zhang H, Fan S, Sakaguchi K,
Shen S, Mercer WE, Vande Woude GF, O'Connor PM and Appella E: Wip1,
a novel human protein phosphatase that is induced in response to
ionizing radiation in a p53-dependent manner. Proc Natl Acad Sci
USA. 94:6048–6053. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bulavin DV, Demidov ON, Saito S,
Kauraniemi P, Phillips C, Amundson SA, Ambrosino C, Sauter G,
Nebreda AR, Anderson CW, et al: Amplification of PPM1D in human
tumors abrogates p53 tumor-suppressor activity. Nat Genet.
31:210–215. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakagawa H, Wardell CP, Furuta M,
Taniguchi H and Fujimoto A: Cancer whole-genome sequencing: Present
and future. Oncogene. 34:5943–5950. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu X, Nguyen TA, Moon SH, Darlington Y,
Sommer M and Donehower LA: The type 2C phosphatase Wip1: An
oncogenic regulator of tumor suppressor and DNA damage response
pathways. Cancer Metastasis Rev. 27:123–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lowe J, Cha H, Lee MO, Mazur SJ, Appella E
and Fornace AJ Jr: Regulation of the Wip1 phosphatase and its
effects on the stress response. Front Biosci (Landmark Ed).
17:1480–1498. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shreeram S, Demidov ON, Hee WK, Yamaguchi
H, Onishi N, Kek C, Timofeev ON, Dudgeon C, Fornace AJ, Anderson
CW, et al: Wip1 phosphatase modulates ATM-dependent signaling
pathways. Mol Cell. 23:757–764. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu X, Nannenga B and Donehower LA: PPM1D
dephosphorylates Chk1 and p53 and abrogates cell cycle checkpoints.
Genes Dev. 19:1162–1174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujimoto H, Onishi N, Kato N, Takekawa M,
Xu XZ, Kosugi A, Kondo T, Imamura M, Oishi I, Yoda A and Minami Y:
Regulation of the antioncogenic Chk2 kinase by the oncogenic Wip1
phosphatase. Cell Death Differ. 13:1170–1180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hollstein M, Sidransky D, Vogelstein B and
Harris CC: p53 mutations in human cancers. Science. 253:49–53.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Crescenzi E, Raia Z, Pacifico F, Mellone
S, Moscato F, Palumbo G and Leonardi A: Down-regulation of
wild-type p53-induced phosphotase 1 (Wip1) plays a critical role in
regulating several p53-dependent functions in premature senescent
tumor cells. J Biol Chem. 288:16212–16224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu X, Ma O, Nguyen TA, Jones SN, Oren M
and Donehower LA: The Wip1 phosphatase acts as a gatekeeper in the
p53-Mdm2 autoregulatory loop. Cancer Cell. 12:342–354. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Lin L, Guo H, Yang J, Jones SN,
Jochemsen A and Lu X: Phosphorylation and degradation of MdmX is
inhibited by Wip1 phosphatase in the DNA damage response. Cancer
Res. 69:7960–7968. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nguyen TA, Slattery SD, Moon SH,
Darlington YF, Lu X and Donehower LA: The oncogenic phosphatase
Wip1 negatively regulates nucleotide excision repair. DNA Repair
(Amst). 9:813–823. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chew J, Biswas S, Shreeram S, Humaidi M,
Wong ET, Dhillion MK, Teo H, Hazra A, Fang CC, López-Collazo E, et
al: Wip1 phosphatase is a negative regulator of NF-kappaB
signaling. Nat Cell Biol. 11:659–666. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Filipponi D, Muller J, Emelyanov A and
Bulavin DV: Wip1 controls global heterochromatin silencing via
ATM/BRCA1-dependent DNA methylation. Cancer Cell. 24:528–541. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang BD, Broude EV, Dokmanovic M, Zhu H,
Ruth A, Xuan Y, Kandel ES, Lausch E, Christov K and Roninson IB: A
senescence-like phenotype distinguishes tumor cells that undergo
terminal proliferation arrest after exposure to anticancer agents.
Cancer Res. 59:3761–3767. 1999.PubMed/NCBI
|
|
21
|
te Poele RH, Okorokov AL, Jardine L,
Cummings J and Joel SP: DNA damage is able to induce senescence in
tumor cells in vitro and in vivo. Cancer Res. 62:1876–1883.
2002.PubMed/NCBI
|
|
22
|
Emelyanov A and Bulavin DV: Wip1
phosphatase in breast cancer. Oncogene. 34:4429–4438. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rennstam K, Ahlstedt-Soini M, Baldetorp B,
Bendahl PO, Borg A, Karhu R, Tanner M, Tirkkonen M and Isola J:
Patterns of chromosomal imbalances defines subgroups of breast
cancer with distinct clinical features and prognosis. A study of
305 tumors by comparative genomic hybridization. Cancer Res.
63:8861–8868. 2003.PubMed/NCBI
|
|
24
|
Demidov ON, Kek C, Shreeram S, Timofeev O,
Fornace AJ, Appella E and Bulavin DV: The role of the MKK6/p38 MAPK
pathway in Wip1-dependent regulation of ErbB2-driven mammary gland
tumorigenesis. Oncogene. 26:2502–2506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu E, Ahn YS, Jang SJ, Kim MJ, Yoon HS,
Gong G and Choi J: Overexpression of the Wip1 gene abrogates the
p38 MAPK/p53/Wip1 pathway and silences p16 expression in human
breast cancers. Breast Cancer Res Treat. 101:269–278. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Wan G, Mlotshwa S, Vance V,
Berger FG, Chen H and Lu X: Oncogenic Wip1 phosphatase is inhibited
by miR-16 in the DNA damage signaling pathway. Cancer Res.
70:7176–7186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang B, Li D and Kovalchuk O: p53 Ser15
phosphorylation and histone modifications contribute to IR-induced
miR-34a transcription in mammary epithelial cells. Cell Cycle.
12:2073–2083. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Spike BT and Wahl GM: P53, stem cells, and
reprogramming: Tumor suppression beyond guarding the genome. Genes
Cancer. 2:404–419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kong W, Jiang X and Mercer WE:
Downregulation of Wip-1 phosphatase expression in MCF-7 breast
cancer cells enhances doxorubicin-induced apoptosis through
p53-mediated transcriptional activation of Bax. Cancer Biol Ther.
8:555–563. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pärssinen J, Alarmo EL, Karhu R and
Kallioniemi A: PPM1D silencing by RNA interference inhibits
proliferation and induces apoptosis in breast cancer cell lines
with wild-type p53. Cancer Genet Cytogenet. 182:33–39. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang CH, Jiao BH, Lu SK, Guo EK and Zhang
GY: The expression of proto-oncogene Wip1 in human glioblastoma
multiforme and cell lines. Chin J Neuro Oncol. 9:1–6. 2011.
|
|
32
|
Richter M, Dayaram T, Gilmartin AG, Ganji
G, Pemmasani SK, Van Der Key H, Shohet JM, Donehower LA and Kumar
R: Wip1 phosphatase as a potential therapeutic target in
neuroblastoma. PLoS One. 10:e01156352015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Buss MC, Remke M, Lee J, Gandhi K,
Schniederjan MJ, Kool M, Northcott PA, Pfister SM, Taylor MD and
Castellino RC: The Wip1 oncogene promotes progression and invasion
of aggressive medulloblastoma variants. Oncogene. 34:1126–1140.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang L, Chen LH, Wan H, Yang R, Wang Z,
Feng J, Yang S, Jones S, Wang S, Zhou W, et al: Exome sequencing
identifies somatic gain-of-function PPM1D mutations in brainstem
gliomas. Nat Genet. 46:726–730. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Barone G, Tweddle A, Shohet JM, Chesler L,
Moreno L, Pearson AD and Van Maerken T: MDM2-p53 interaction in
paediatric solid tumours: Preclinical rationale, biomarkers and
resistance. Curr Drug Targets. 15:114–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Northcott PA, Shih DJ, Peacock J, Garzia
L, Morrissy AS, Zichner T, Stütz AM, Korshunov A, Reimand J,
Schumacher SE, et al: Subgroup-specific structural variation across
1,000 medulloblastoma genomes. Nature. 488:49–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Buss MC, Read TA, Schniederjan MJ, Gandhi
K and Castellino RC: HDM2 promotes Wip1-mediated medulloblastoma
growth. Neuro Oncol. 14:440–458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pfister S, Remke M, Benner A, Mendrzyk F,
Toedt G, Felsberg J, Wittmann A, Devens F, Gerber NU, Joos S, et
al: Outcome prediction in pediatric medulloblastoma based on DNA
copy-number aberrations of chromosomes 6q and 17q and the MYC and
MYCN loci. J Clin Oncol. 27:1627–1636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hirasawa A, Saito-Ohara F, Inoue J, Aoki
D, Susumu N, Yokoyama T, Nozawa S, Inazawa J and Imoto I:
Association of 17q21-q24 gain in ovarian clear cell adenocarcinomas
with poor prognosis and identification of PPM1D and APPBP2 as
likely amplification targets. Clin Cancer Res. 9:1995–2004.
2003.PubMed/NCBI
|
|
40
|
Tan DS, Lambros MB, Rayter S, Natrajan R,
Vatcheva R, Gao Q, Marchiò C, Geyer FC, Savage K, Parry S, et al:
PPM1D is a potential therapeutic target in ovarian clear cell
carcinomas. Clin Cancer Res. 15:2269–2280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ali AY, Abedini MR and Tsang BK: The
oncogenic phosphatase PPM1D confers cisplatin resistance in ovarian
carcinoma cells by attenuating checkpoint kinase 1 and p53
activation. Oncogene. 31:2175–2186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ali AY, Kim JY, Pelletier JF, Vanderhyden
BC, Bachvarov DR and Tsang BK: Akt confers cisplatin
chemoresistance in human gynecological carcinoma cells by
modulating PPM1D stability. Mol Carcinog. 54:1301–1314. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ali AY, Farrand L, Kim JY, Byun S, Suh JY,
Lee HJ and Tsang BK: Molecular determinants of ovarian cancer
chemoresistance: New insights into an old conundrum. Ann NY Acad
Sci. 1271:58–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tan DS and Kaye S: Ovarian clear cell
adenocarcinoma: A continuing enigma. J Clin Pathol. 60:355–360.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bae HJ, Noh JH, Kim JK, Eun JW, Jung KH,
Kim MG, Chang YG, Shen Q, Kim SJ, Park WS, et al: MicroRNA-29c
functions as a tumor suppressor by direct targeting oncogenic SIRT1
in hepatocellular carcinoma. Oncogene. 33:2557–2567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y,
Jia WH and Zhuang SM: Effects of microRNA-29 on apoptosis,
tumorigenecity, and prognosis of hepatocellular carcinoma.
Hepatology. 51:836–845. 2010.PubMed/NCBI
|
|
47
|
Wang H, Zhu Y, Zhao M, Wu C, Zhang P, Tang
L, Zhang H, Chen X, Yang Y and Liu G: miRNA-29c suppresses lung
cancer cell adhesion to extracellular matrix and metastasis by
targeting integrin β1 and matrix metalloproteinase 2 (MMP2). PLoS
One. 8:e701922013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ding DP, Chen ZL, Zhao XH, Wang JW, Sun J,
Wang Z, Tan FW, Tan XG, Li BZ, Zhou F, et al: miR-29c induces cell
cycle arrest in esophageal squamous cell carcinoma by modulating
cyclin E expression. Carcinogenesis. 32:1025–1032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang B, Li D, Sidler C, Rodriguez-Juarez
R, Singh N, Heyns M, Ilnytskyy Y, Bronson RT and Kovalchuk O: A
suppressive role of ionizing radiation-responsive miR-29c in the
development of liver carcinoma via targeting WIP1. Oncotarget.
6:9937–9950. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Oda T, Tsuda H, Scarpa A, Sakamoto M and
Hirohashi S: p53 gene mutation spectrum in hepatocellular
carcinoma. Cancer Res. 52:6358–6364. 1992.PubMed/NCBI
|
|
51
|
Li GB, Zhang XL, Yuan L, Jiao QQ, Liu DJ
and Liu J: Protein phosphatase magnesium-dependent 1 δ (PPM1D) mRNA
expression is a prognosis marker for hepatocellular carcinoma. PLoS
One. 8:e607752013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Koo SH, Kwon KC, Ihm CH, Jeon YM, Park JW
and Sul CK: Detection of genetic alterations in bladder tumors by
comparative genomic hybridization and cytogenetic analysis. Cancer
Genet Cytogenet. 110:87–93. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang W, Zhu H, Zhang H, Zhang L, Ding Q
and Jiang H: Targeting PPM1D by lentivirus-mediated RNA
interference inhibits the tumorigenicity of bladder cancer cells.
Braz J Med Bio Res. 47:1044–1049. 2014. View Article : Google Scholar
|
|
54
|
Lin J, Zhang Q, Lu Y, Xue W, Xu Y and Hu
X: Downregulation of HIPK2 increases resistance of bladder cancer
cell to cisplatin by regulating wip1. PLoS One. 9:e984182014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Goloudina AR, Mazur SJ, Appella E, Garrido
C and Demidov ON: Wip1 sensitizes p53-negative tumors to apoptosis
by regulating the Bax/Bcl-xl ratio. Cell Cycle. 11:1883–1887. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Goloudina AR, Tanoue K, Hammann A,
Fourmaux E, Le Guezennec X, Bulavin DV, Mazur SJ, Appella E,
Garrido C and Demidov ON: Wip1 promotes RUNX2-dependent apoptosis
in p53-negative tumors and protects normal tissues during treatment
with anticancer agents. Proc Natl Acad Sci USA. 109:E68–E75. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sun GG, Wang YD, Liu Q and Hu WN:
Expression of Wip1 in kidney carcinoma and its correlation with
tumor metastasis and clinical significance. Pathol Oncol Res.
21:219–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu S, Qi L, Han W, Wan X, Jiang S, Li Y,
Xie Y, Liu L, Zeng F, Liu Z and Zu X: Overexpression of Wip1 is
associated with biologic behavior in human clear cell renal cell
carcinoma. PLoS One. 9:e1102182014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sun GG, Zhang J, Ma XB, Wang YD, Cheng YJ
and Hu WN: Overexpression of wild-type p53-induced phosphatase1
confers poor prognosis of patients with nasopharyngeal carcinoma.
Pathol Oncol Res. 21:283–291. 2015. View Article : Google Scholar : PubMed/NCBI
|















