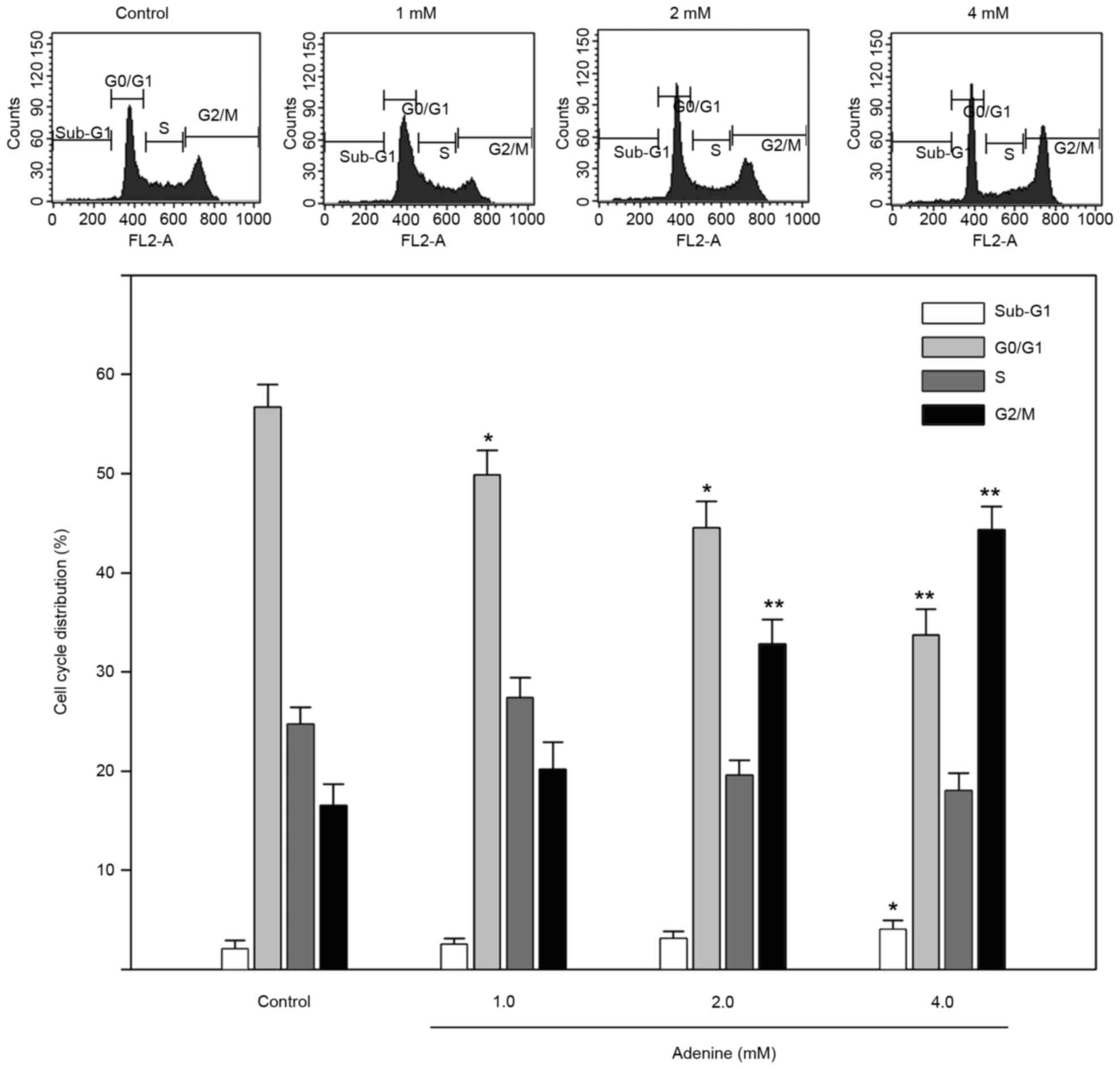
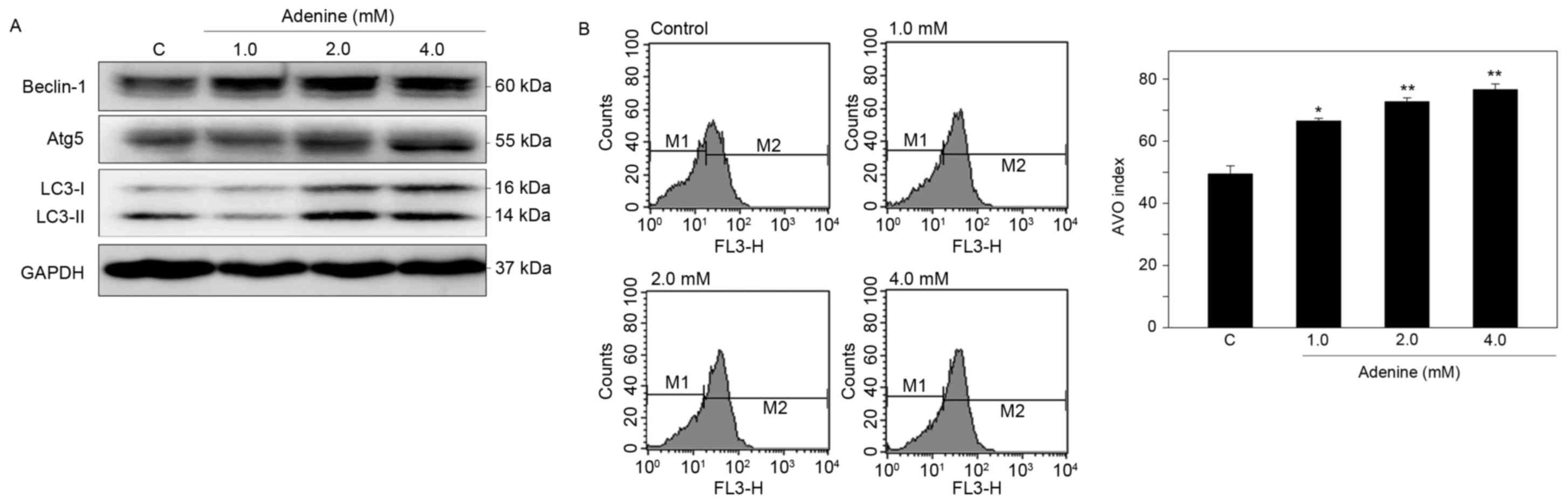
|
1
|
Clarkson B, Strife A, Wisniewski D, Lambek
CL and Liu C: Chronic myelogenous leukemia as a paradigm of early
cancer and possible curative strategies. Leukemia. 17:1211–1262.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Traganos F and Darzynkiewicz Z: Lysosomal
proton pump activity: Supravital cell staining with acridine orange
differentiates leukocyte subpopulations. Methods Cell Biol.
41:185–194. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vakana E, Altman JK, Glaser H, Donato NJ
and Platanias LC: Antileukemic effects of AMPK activators on
BCR-ABL-expressing cells. Blood. 118:6399–6402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sujobert P, Poulain L, Paubelle E,
Zylbersztejn F, Grenier A, Lambert M, Townsend EC, Brusq JM,
Nicodeme E, Decrooqc J, et al: Co-activation of AMPK and mTORC1
induces cytotoxicity in acute myeloid leukemia. Cell Rep.
11:1446–1457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bedi A, Zehnbauer BA, Barber JP, Sharkis
SJ and Jones RJ: Inhibition of apoptosis by BCR-ABL in chronic
myeloid leukemia. Blood. 83:2038–2044. 1994.PubMed/NCBI
|
|
6
|
Klionsky DJ, Meijer AJ and Codogno P:
Autophagy and p70S6 kinase. Autophagy. 1:59–61. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim I and He YY: Targeting the
AMP-activated protein kinase for cancer prevention and therapy.
Front Oncol. 3:1752013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vakana E and Platanias LC: AMPK in BCR-ABL
expressing leukemias. Regulatory effects and therapeutic
implications. Oncotarget. 2:1322–1328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Druker B, Okuda K, Matulonis U, Salgia R,
Roberts T and Griffin JD: Tyrosine phosphorylation of rasGAP and
associated proteins in chronic myelogenous leukemia cell lines.
Blood. 79:2215–2220. 1992.PubMed/NCBI
|
|
10
|
Gotoh A, Miyazawa K, Ohyashiki K, Tauchi
T, Boswell HS, Broxmeyer HE and Toyama K: Tyrosine phosphorylation
and activation of focal adhesion kinase (p125FAK) by BCR-ABL
oncoprotein. Exp Hematol. 23:1153–1159. 1995.PubMed/NCBI
|
|
11
|
Fernandes A, Azevedo MM, Pereira O,
Sampaio-Marques B, Paiva A, Correia-Neves M, Castro I and Ludovico
P: Proteolytic systems and AMP-activated protein kinase are
critical targets of acute myeloid leukemia therapeutic approaches.
Oncotarget. 6:31428–31440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Long YC and Zierath JR: AMP-activated
protein kinase signaling in metabolic regulation. J Clin Invest.
116:1776–1783. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Denizot F and Lang R: Rapid colorimetric
assay for cell growth and survival. Modifications to the
tetrazolium dye procedure giving improved sensitivity and
reliability. J Immunol Methods. 89:271–277. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stankiewicz M, Jonas W, Hadas E, Cabaj W
and Douch PG: Supravital staining of eosinophils. Int J Parasitol.
26:445–446. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paglin S, Hollister T, Delohery T, Hackett
N, McMahill M, Sphicas E, Domingo D and Yahalom J: A novel response
of cancer cells to radiation involves autophagy and formation of
acidic vesicles. Cancer Res. 61:439–444. 2001.PubMed/NCBI
|
|
16
|
Hsu CY, Lin CH, Lin JT, Cheng YF, Chen HM
and Kao SH: Purine analogue ENERGI-F706 induces apoptosis of 786-O
renal carcinoma cells via 5′-adenosine monophosphate-activated
protein kinase activation. Mol Med Rep. 12:4566–4571. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sattler M and Griffin JD: Molecular
mechanisms of transformation by the BCR-ABL oncogene. Semin
Hematol. 40 (2 Suppl 2):S4–S10. 2003. View Article : Google Scholar
|
|
18
|
Skorski T, Kanakaraj P,
Nieborowska-Skorska M, Ratajczak MZ, Wen SC, Zon G, Gewirtz AM,
Perussia B and Calabretta B: Phosphatidylinositol-3 kinase activity
is regulated by BCR/ABL and is required for the growth of
Philadelphia chromosome-positive cells. Blood. 86:726–736.
1995.PubMed/NCBI
|
|
19
|
Cortez D, Reuther G and Pendergast AM: The
Bcr-Abl tyrosine kinase activates mitogenic signaling pathways and
stimulates G1-to-S phase transition in hematopoietic cells.
Oncogene. 15:2333–2342. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mace ML, Dahl J and Jabbour EJ: Which
tyrosine-kinase inhibitor to use first in chronic phase chronic
myelogenous leukemia? Expert Opin Pharmacother. 16:999–1007. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adekola K, Popat U and Ciurea SO: An
update on allogeneic hematopoietic progenitor cell transplantation
for myeloproliferative neoplasms in the era of tyrosine kinase
inhibitors. Bone Marrow Transplant. 49:1352–1359. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karnevi E, Said K, Andersson R and
Rosendahl AH: Metformin-mediated growth inhibition involves
suppression of the IGF-I receptor signalling pathway in human
pancreatic cancer cells. BMC Cancer. 13:2352013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng QY, Jin FS, Yao C, Zhang T, Zhang GH
and Ai X: Ursolic acid-induced AMP-activated protein kinase (AMPK)
activation contributes to growth inhibition and apoptosis in human
bladder cancer T24 cells. Biochem Biophys Res Commun. 419:741–747.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sauer H, Engel S, Milosevic N, Sharifpanah
F and Wartenberg M: Activation of AMP-kinase by AICAR induces
apoptosis of DU-145 prostate cancer cells through generation of
reactive oxygen species and activation of c-Jun N-terminal kinase.
Int J Oncol. 40:501–508. 2012.PubMed/NCBI
|
|
25
|
Borthakur G, Duvvuri S, Ruvolo V, Tripathi
DN, Piya S, Burks J, Jacamo R, Kojima K, Ruvolo P, Fueyo-Margareto
J, et al: MDM2 inhibitor, nutlin 3a, induces p53 dependent
autophagy in acute leukemia by AMP kinase activation. PLoS One.
10:e01392542015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Levine B and Kroemer G: Autophagy in
aging, disease and death: The true identity of a cell death
impostor. Cell Death Differ. 16:1–2. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Settembre C, Fraldi A, Medina DL and
Ballabio A: Signals from the lysosome: A control centre for
cellular clearance and energy metabolism. Nat Rev Mol Cell Biol.
14:283–296. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gozuacik D and Kimchi A: Autophagy as a
cell death and tumor suppressor mechanism. Oncogene. 23:2891–2906.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aredia F and Scovassi AI: Manipulation of
autophagy in cancer cells: An innovative strategy to fight drug
resistance. Future Med Chem. 5:1009–1021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang XH, Jackson S, Seaman M, Brown K,
Kempkes B, Hibshoosh H and Levine B: Induction of autophagy and
inhibition of tumorigenesis by beclin 1. Nature. 402:672–676. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yue Z, Jin S, Yang C, Levine AJ and Heintz
N: Beclin 1, an autophagy gene essential for early embryonic
development, is a haploinsufficient tumor suppressor. Proc Natl
Acad Sci USA. 100:15077–15082. 2003. View Article : Google Scholar : PubMed/NCBI
|